Abstract
Butenolides are an emerging family of signaling molecules in Streptomyces. They control complex physiological traits, such as morphological differentiation and antibiotic production. However, how butenolides regulate these processes is poorly investigated because of obstacles in obtaining these signaling molecules. This study reports the identification of a butenolide-type signaling system for nikkomycin biosynthesis in Streptomyces ansochromogenes with distinct features. We identified a gene cluster, sab, consisting of three genes, sabAPD, for butenolide biosynthesis and two regulator genes, sabR1 and sabR2, and characterized three butenolides (SAB1, -2, and -3) by heterologous expression of sabAPD. sabA disruption abolished nikkomycin production, which could be restored by the addition of SABs or by deletion of sabR1 in ΔsabA. Electrophoretic mobility-shift assays and transcriptional analyses indicated that SabR1 indirectly represses the transcription of nikkomycin biosynthetic genes, but directly represses sabA and sabR1. In the presence of SABs, the SabR1 transcriptional regulator dissociated from its target genes, verifying that SabR1 is the cognate receptor of SABs. Genome-wide scanning with the conserved SabR1-binding sequence revealed another SabR1 target gene, cprC, whose transcription was strongly repressed by SabR1. Intriguingly, CprC positively regulated the pleiotropic regulatory gene adpA by binding to its promoter and, in turn, activated nikkomycin biosynthesis. This is the first report that butenolide-type signaling molecules and their cognate receptor SabR1 can regulate adpA via a newly identified activator, CprC, to control nikkomycin production. These findings pave the way for further studies seeking to unravel the regulatory mechanism and functions of the butenolide signaling system in Streptomyces.
Keywords: molecular genetics, receptor, transcription regulation, antibiotics, signal transduction, secondary metabolism, butenolide signaling molecules, gene cluster, nikkomycin, Streptomyces
Introduction
Improper use of antibiotics as well as the horizontal transfer of antibiotic resistance genes has led to the continuous appearance of antibiotic-resistant pathogens and the loss of antibiotic native efficiency. Most commercially important natural antibiotics are produced by Streptomyces. Their biosynthesis is precisely controlled by cluster-situated regulators (CSRs)4 or global regulators, most of which can respond to small molecules including hormone-like signaling molecules, also known as autoregulators, and some specialized metabolites (1). Defining these signal transmission pathways is desirable to improve the efficiency of antibiotic production yield and to identify new gene clusters to discover previously unknown bioactive products of interest.
Although signaling molecules are widely distributed in Streptomyces, their biosynthesis is usually under stringent control, resulting in low yields in producing strains. This impedes the discovery of new signaling molecules and elucidation of their function. Since the first γ-butyrolactone (GBL) signaling molecule, A-factor, was discovered in Streptomyces griseus, only 33 autoregulators have been identified in Streptomyces (1–3). They are classified into five groups, GBLs, furans, butenolides, PI factor, and N-methylphenylalanyl-dehydrobutyrine diketopiperazine, based on their structures. GBL is the largest family of autoregulators, and its regulatory mechanism and biosynthesis have been intensively investigated, whereas studies on other family of autoregulators are very rare (4). Recently, a butenolide signaling molecule, avenolide, was found to be essential for biosynthesis of the clinically important antibiotic avermectin, inspiring more interest in this family of regulators (5). Seven butenolides associated with antibiotic biosynthesis have been discovered so far, including avenolide from Streptomyces avermitilis and Streptomyces albus (3, 5) and SRBs from Streptomyces rochei (6). Butenolide contains a five-member ring backbone, but with an unsaturated bond at C3–C4 and diverse side chains at C3, C4, or C5, which confer unique activities.
Signaling molecules and their receptors can exert considerable impact on the onset and production of antibiotic biosynthesis. A typical autoregulator system has been exemplified in S. griseus and portrayed as a pyramid-like network. A-factor and its receptor ArpA constitute the apex of the pyramid, controlling a series of diverse downstream pathways via a pivot regulator, AdpA. ArpA directly represses the transcription of adpA, whereas binding of A-factor to ArpA results in derepression of adpA transcription and consequently the expression profile switch of numerous target genes of AdpA (7). Subsequently, more autoregulators and their cognate receptors have been characterized. They have intimate association with antibiotic production and morphological differentiation in various Streptomyces species, and some of these are involved in the AdpA regulatory network, a pivotal pleiotropic regulator widespread in Streptomyces. Although the target genes of AdpA and their regulation have been comprehensively illustrated in many Streptomyces species, how AdpA expression itself is maintained under delicate and precise control is poorly investigated (8, 9). Signaling molecules taking part in the AdpA regulatory cascade provide an important mechanism for cells to respond to environmental and physiological changes, and also signal amplification can be achieved via AdpA transmission (10).
Streptomyces ansochromogenes 7100 produces nikkomycin under the control of global regulators, such as WblA, AdpA, GBL receptor-like regulator SabR (11–13), and the pathway-specific regulator SanG (14, 15). AdpA positively regulates nikkomycin biosynthesis via binding to the promoter region of sanG, but negatively regulates oviedomycin production by repressing ovmZ/ovmW (16). An autoregulator biosynthetic gene cluster sab (KF170348) was revealed in this strain by genome mining (17). BLAST search suggested that the homolog of sabA, the core gene in sab, is widely distributed in Streptomyces. What autoregulators may be synthesized by sab and how they coordinate with AdpA to regulate the secondary metabolism in S. ansochromogenes are of great interest. In this work, we report the characterization of a novel butenolide signal transduction pathway, in which signal input is transmitted to nikkomycin biosynthesis via a newly discovered activator, CprC, of adpA.
Results
Characterization of butenolide signal molecules (SABs) triggering nikkomycin production
It is well-known that AfsA-like proteins are key enzymes in autoregulator biosynthesis. By genome mining of S. ansochromogenes, an autoregulator biosynthetic gene cluster (sab) was identified (Fig. 1A), and sab is located about 1.87 megabases away from the nikkomycin biosynthetic gene cluster (nik) in the chromosome of S. ansochromogenes. In sab cluster, sabA encodes an AfsA-like enzyme (31% identity with AfsA from S. griseus), whereas sabP and sabD encode phosphatase and dehydrogenase enzymes, respectively, with putative tailoring functions. sabR1 and sabR2 encode TetR family regulators. SabR1 belongs to the GBL receptor family and shows 40% identity with ScbR from S. coelicolor (18), and SabR2 shows 31% identity with pseudo-GBL receptor JadR2 from S. venezuelae (19). sabR1 and sabR2 are situated downstream of sabD in sab cluster, implying their potential correlation with the signal molecules as receptors.
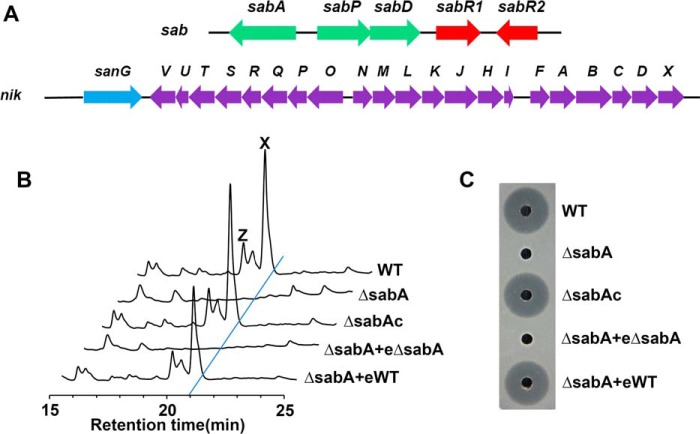
Figure 1.
Effect of sabA disruption on nikkomycin production. A, genetic organization of nik gene cluster and sab gene cluster in S. ansochromogenes 7100. B, HPLC analysis of nikkomycin in different strains. C, nikkomycin bioassays against C. albicans with fermentation filtrates of different strains. WT, S. ansochromogenes 7100. ΔsabA, sabA disruption mutant. ΔsabAc, sabA complementary strain. eΔsabA, the extract of ΔsabA fermentation filtrate. eWT, the extract of WT strain fermentation filtrate. X, nikkomycin X. Z, nikkomycin Z.
To understand what kind of signal molecules can be synthesized by sab and whether they can affect nikkomycin biosynthesis, a sabA disruption mutant (ΔsabA) was constructed. Nikkomycin was determined by HPLC analysis and bioassays against Candida albicans. No nikkomycin was detected in the culture supernatant of ΔsabA after 5 days' incubation. When a copy of sabA with its promoter region was introduced into ΔsabA, nikkomycin production was almost restored to the level of the WT strain. Furthermore, nikkomycin in ΔsabA was restored with the addition of ethyl acetate extracts of WT, but not with the extract of ΔsabA as expected (Fig. 1, B and C). These results indicated that compounds synthesized by SabA are closely related to nikkomycin biosynthesis.
Signaling molecules usually work at nanomolar concentrations, and it is difficult to obtain a large enough quantity from native producer strains for structural determination. To overcome this problem, heterologous expression of sabAPD was carried out in Escherichia coli and in Streptomyces, respectively, and the products were detected by HPLC (Fig. 2, A and B). It was shown that nikkomycin production in ΔsabA was restored by the addition of extracts from E. coli C41 containing sabAPD (CpAPD) or S. coelicolor M1146 containing sabAPD (MpAPD) but not recovered by the addition of extracts from strains E. coli C41/pET23b and S. coelicolor M1146/pKC1139 as controls (data not shown). Clearly, sabAPD were expressed in both E. coli C41 and S. coelicolor M1146, and the resulting products triggered nikkomycin production.
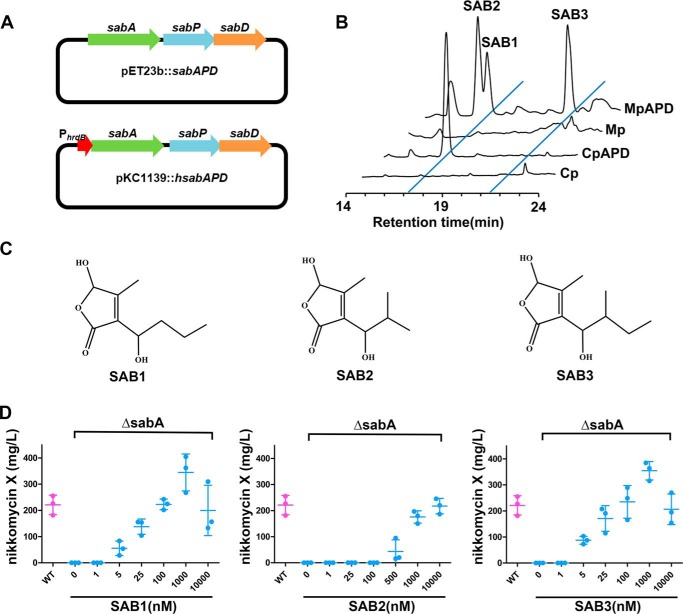
Figure 2.
Characterization of structure and bioactivity of SABs. A, plasmids used for expression of sabAPD in E. coli and Streptomyces. B, HPLC analysis of SABs after expression of sabAPD in S. coelicolor M1146 and E. coli C41. MpAPD, S. coelicolor M1146 containing sabAPD. Mp, S. coelicolor M1146 containing pKC1139 as control. CpAPD, E. coli C41 containing sabAPD. Cp, E. coli C41 containing pET23b as control. C, structures of SABs. D, nikkomycin X production of ΔsabA with the addition of various concentrations of SAB1, SAB2, and SAB3.
Compounds inducing nikkomycin production were isolated from CpAPD and MpAPD strains and subsequently purified using HPLC by tracking the activity inducing nikkomycin biosynthesis in ΔsabA. A total of 30 mg of purified SAB1 was gained from 3 liters of culture broth of CpAPD. High-resolution electrospray ionization MS showed a molecular ion peak at m/z 187.0964 [M + H]+ (Fig. S1A), and the molecular formula was deduced as C9H14O4. By comparing the NMR spectroscopic data with those of known compounds (compounds 1 and 3 in S. antibioticus) (20), the only difference indicated was in the side chain at C3. On 1H NMR, one methyl group signal (δH 0.79; δC 15.0) at C2′ in compound 3 was absent, and CH was changed to CH2 (δH 1.8/1.6; δC 38.2) (Fig. S1 and Table S1). Further analyses of the 1H-1H COSY, HSQC, and HMBC confirmed the side chain at C3 in SAB1 to be hydroxyl-butyl, and the structure of SAB1 was thus determined as 5-hydroxy-3-(1′-hydroxyl-butyl)-4-methyl-2(5H)-furanone (Fig. 2C), a novel member of butenolide autoregulators in Streptomyces.
HPLC analysis revealed that two other molecules, SAB2 and -3, were produced by MpAPD in addition to SAB1 (Fig. 2B). A total of 20 mg of purified SAB2 and 10 mg of SAB3 was gained from MpAPD extract. [M + H]+ ions m/z 187.0968 for SAB2 and 201.1124 for SAB3 were observed on high-resolution electrospray ionization MS, corresponding to molecular formulae of C9H14O4 and C10H16O4, respectively (Figs. S2 and S3). SAB2 shares the same molecular formula with SAB1, but there is a different retention time on HPLC (Fig. 2B), suggesting that it is an isomer of SAB1. NMR spectroscopic data of SAB2 and SAB3 were consistent with those of compounds 1 and 3 isolated from S. antibioticus DSM40725 (Figs. S2 and S3) (20). Thus, SAB2 was determined as 5-hydroxy-3-(1′-hydroxy-2′-methylpropyl)-4-methyl-2(5H)-furanone and SAB3 as 5-hydroxy-3-(1′-hydroxy-2′-methylbutyl)-4-methyl-2(5H)-furanone (Fig. 2C). Compounds SAB1, -2, and -3 all contain a 4-methyl-5-hydroxybutenolide ring as the core structure but attached with a different side chain at the C3 position. To characterize the native SABs in S. ansochromogenes, the extract of S. ansochromogenes was analyzed by LC-electrospray ionization-MS. It was shown that native SAB1, -2, and -3 were all present in S. ansochromogenes, and SAB2 and SAB3 are dominant (Fig. S4).
To confirm that purified SABs are functional in vivo, SAB1, -2, or -3 was added into ΔsabA at concentrations ranging from 1 nm to 10 μm, and nikkomycin production was detected after incubation for 5 days. HPLC showed that nikkomycin production was elicited in ΔsabA with increasing concentration of SAB1, -2, or -3. SAB1 and SAB3 were effective at 5 nm, and nikkomycin production was fully restored to WT strain levels at 100 nm. In contrast, the minimum effective concentration of SAB2 required for eliciting nikkomycin biosynthesis was 500 nm (Fig. 2D), suggesting that the side chain of SABs is key in structure–activity relationships.
SABs and their receptor SabR1 coordinately regulate nikkomycin biosynthesis
Autoregulators usually perform their regulatory functions on secondary metabolism and morphological differentiation through specific receptor proteins. By multiple-sequence alignment and phylogeny evolution analysis, SabR1 appears to belong to GBL receptor family.
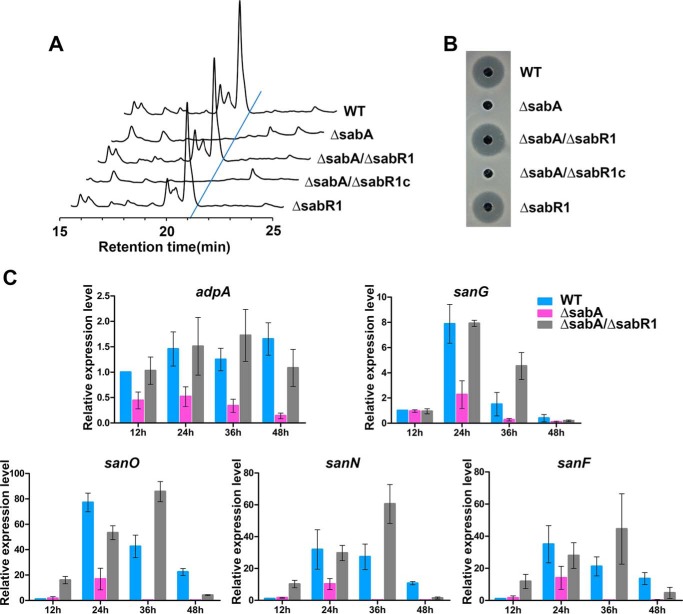
To verify this hypothesis, sabR1 was disrupted in WT S. ansochromogenes to generate ΔsabR1 and also in SABs-defective mutant (ΔsabA) to generate ΔsabA/ΔsabR1. Nikkomycin production was not affected in ΔsabR1, where SABs are present. However, nikkomycin production was abolished in ΔsabA due to the absence of SABs, which could be reversed by further disruption of sabR1 in ΔsabA. The complementation of sabR1 in ΔsabA/ΔsabR1 (ΔsabA/ΔsabR1c) completely repressed nikkomycin biosynthesis (Fig. 3, A and B). Accordingly, qRT-PCR revealed that the transcription of key regulatory genes adpA and sanG for nikkomycin biosynthesis as well as the structural genes sanF, sanN, and sanO in nik cluster was reduced in ΔsabA, but restored in ΔsabA/ΔsabR1 (Fig. 3C). These experiments clearly demonstrated that the inhibition of nikkomycin production in ΔsabA resulted from SabR1-dependent repression of the transcription of nikkomycin biosynthetic genes. Similarly, in the absence of SABs, SabR1 repressed the transcription of sabR1, sabR2, and sabA in ΔsabA but not in the WT strain, where SABs are present (Fig. S5). Taken together, it was suggested that SABs are associated with SabR1 to regulate nikkomycin biosynthesis.
Figure 3.
Effect of sabR1 disruption on nikkomycin production. A, HPLC analysis of fermentation filtrates in different strains. B, nikkomycin bioassays against C. albicans with fermentation filtrates of different strains. WT, S. ansochromogenes 7100. ΔsabA, sabA disruption mutant. ΔsabA/ΔsabR1, disruption mutant of sabA and sabR1. ΔsabA/ΔsabR1c, sabR1 complementary strain of ΔsabA/ΔsabR1. ΔsabR1, disruption mutant of sabR1. C, qRT-PCR transcriptional analyses of adpA, sanG, sanO, sanN, and sanF in WT, ΔsabA, and ΔsabA/ΔsabR1 strains. Error bars, S.D. calculated from three independent experiments.
To verify the recognition of SABs by SabR1, electrophoretic mobility-shift assays (EMSAs) were performed using SabR1-His6 and potential target gene promoter regions as probes. Complexes of SabR1 with the upstream regions of sabA, sabR1, or sabR2 were formed in a concentration-dependent manner (Fig. 4A), whereas binding of SabR1 to the upstream regions of sanF, sanG, or adpA was not observed (data not shown), implying that SabR1 regulates nikkomycin biosynthesis indirectly. When SABs were added into the EMSA reaction mixture, the complex could not be formed, demonstrating that sabA, sabR1, and sabR2 are the direct targets of SabR1, and SABs can interact with SabR1 to cause its dissociation from the target DNA (Fig. 4B). Thus, SabR1 was determined as the cognate receptor of SABs in vitro.
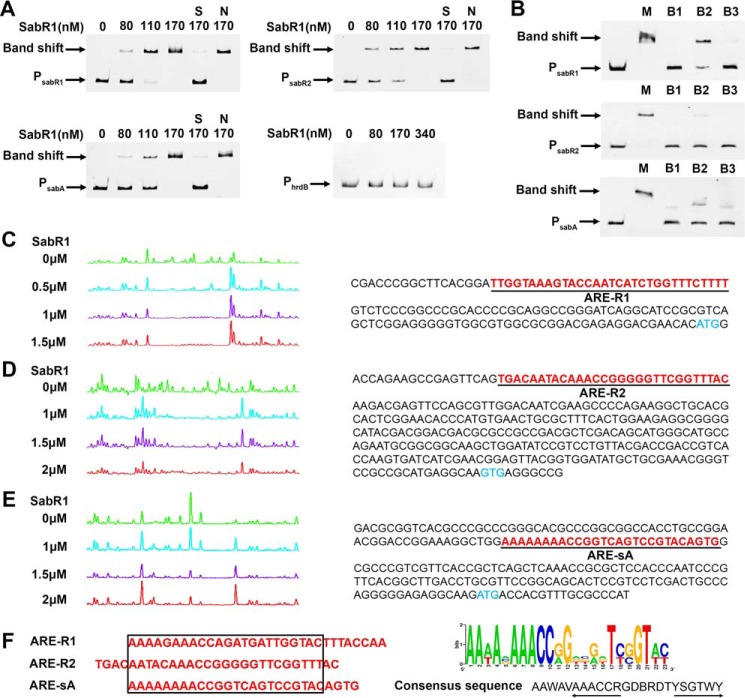
Figure 4.
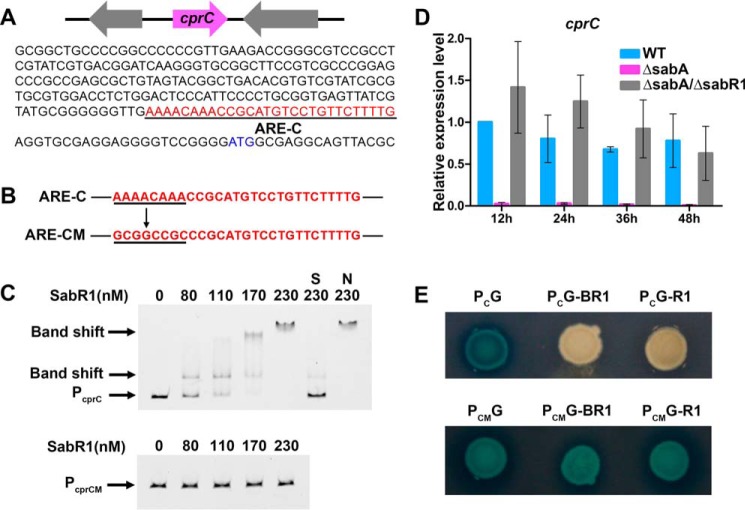
EMSAs and DNase I footprinting of SabR1 binding to the promoter regions of sabR1, sabR2, and sabA. A, EMSAs of different concentrations of SabR1 binding to the fluorescently labeled promoter regions of sabR1 (PsabR1), sabR2 (PsabR2) and sabA (PsabA). Each lane contains 50 ng of labeled probes and 1 μg of poly(dI-dC). S, unlabeled specific probe (30-fold) was added; N, unlabeled nonspecific probe PhrdB (30-fold) was added. B, EMSAs of SabR1 (110 nm) binding to unlabeled PsabR1, PsabR2, and PsabA in the absence or presence of SAB1, -2, and -3. Each lane contains 20-ng probes. The lanes marked with M were added with methanol as control. The lanes marked with B1, B2, and B3 were added with SAB1 (5 μm), SAB2 (25 μm), and SAB3 (5 μm), respectively. C, determination of SabR1-binding site on PsabR1 (ARE-R1) and the nucleotide sequences of PsabR1. D, determination of SabR1-binding site on PsabR2 (ARE-R2) and the nucleotide sequences of PsabR2. E, determination of SabR1-binding site on PsabA (ARE-sA) and the nucleotide sequences of PsabA. The blue letters represent the translational start sites. The underlined red letters are binding sites of SabR1. F, alignment of SabR1-binding sequences and the sequence logo of conserved bases. The sequences in the black box are consensus nucleotides, and 6-bp inverted repeats are indicated by arrows. The sequence logo was created using the WebLogo program (version 2.8.2; Department of Plant and Microbial Biology, University of California, Berkeley (http://weblogo.berkeley.edu/)). (Please note that the JBC is not responsible for the long-term archiving and maintenance of this site or any other third party hosted site.) The height of each letter is proportional to the frequency of the base appearance.
To identify the precise binding sequences of SabR1 on its target genes, DNase I footprinting experiments were performed. The results showed that SabR1 protected regions from −137 to −111 bp relative to the sabA translation start site (tss), −118 to −88 bp relative to the sabR1 tss, and −282 to −254 bp relative to the sabR2 tss (Fig. 4, C–E). Analysis of the three binding sites using the MEME program (21) revealed a conserved consensus sequence (5′-AAWAVAAACCRGDBRDTYSGTWY-3′) (Fig. 4F). In agreement with EMSAs, this consensus sequence was not found in the promoters of sanF, sanG, and adpA, confirming that SabR1 regulates nikkomycin biosynthesis indirectly.
cprC is directly repressed by SabR1 and positively regulates nikkomycin biosynthesis
As mentioned above, SabR1 regulates nikkomycin production indirectly via unknown intermediates. One candidate is sabR2, a target gene of SabR1, encoding a TetR family regulator, which is potentially an intermediate protein between SabR1 and nikkomycin biosynthetic genes. However, sabR2 showed no impact on nikkomycin production after its disruption (Fig. S6), implying that SabR1 exerts its regulatory function on nikkomycin biosynthesis through other means. To explore more SabR1 potential target genes, S. ansochromogenes genome scanning was undertaken using the conserved 23-bp SabR1 binding consensus. This revealed another TetR-family regulator gene, cprC, whose encoded protein CprC shows 68% identity with CprA and 70% identity with CprB from S. coelicolor (22). The promoter region of cprC (PcprC) contains a SabR1 conserved binding motif 5′-AAAACAAACCGCATGTCCTGTTCTTTTG-3′ (Fig. 5A). EMSA experiments confirmed that PcprC could be recognized by SabR1 to form a complex. When PcprC was mutated to 5′-GCGGCCGCCCGCATGTCCTGTTCTTTTG-3′ at the specific sites shown in boldface type, SabR1 no longer bound the mutated PcprC (Fig. 5, B and C). Thus, cprC is confirmed as a direct target gene of SabR1.
Figure 5.
SabR1 repressed the transcription of cprC by directly binding to its promoter region. A, sequence of cprC promoter region (PcprC). The underlined red letters are the predicted binding site of SabR1 on PcprC (ARE-C). B, the underlined ARE-C site in PcprC was mutated to generate the mutant promoter region (PcprCM) of cprC. C, EMSA of SabR1 binding to fluorescently labeled PcprC and PcprCM. Each lane contains 50 ng of labeled probes and 1 μg of poly(dI-dC). S, unlabeled specific probe PcprC (30-fold) was added; N, unlabeled nonspecific probe PhrdB (30-fold) was added. D, qRT-PCR transcriptional analysis of cprC in WT, ΔsabA, and ΔsabA/ΔsabR1. Error bars, S.D. calculated from three independent experiments. E, GusA activity in derivatives of S. coelicolor M1146 containing various promoters fused with gusA. PCG and PCMG, S. coelicolor M1146 containing PcprC or PcprCM fused with gusA. PCG-BR1 and PCMG-BR1, strains PCG and PCMG containing PhrdB fused with sabR1. PCG-R1 and PCMG-R1, strains PCG and PCMG containing sabR1 with its own promoter.
Analyses by qRT-PCR showed that the transcription of cprC was dramatically reduced in ΔsabA but restored in ΔsabA/ΔsabR1, suggesting that SabR1 is a repressor of cprC (Fig. 5D). Along with the EMSA results, it was verified that SabR1 represses the transcription of cprC by directly binding to the promoter region, which was also further illustrated by the expression of gusA encoding a β-glucuronidase reporter system (Fig. 5E). It can be hypothesized that the decreased transcription of cprC may lead to abolition of nikkomycin production in ΔsabA.
To ascertain the effect of CprC on nikkomycin production, disruption of cprC was performed to generate ΔcprC. The yield of nikkomycin in ΔcprC was notably decreased compared with that in WT, suggesting that CprC is an important activator for nikkomycin production. In addition, cprC was complementarily expressed in ΔsabA under the control of hrdB promoter (PhrdB), and the resulting strain (ΔsabA/cprCoe) efficiently restored nikkomycin production (Fig. 6). Thus, CprC was confirmed to be an activator for nikkomycin biosynthesis as the target of SabR1.
Figure 6.
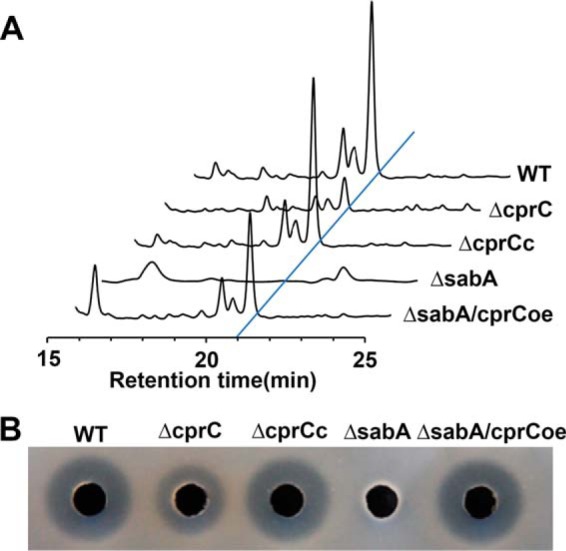
Effect of cprC disruption and overexpression on nikkomycin production. A, HPLC analysis of nikkomycin production in different strains. B, nikkomycin bioassays of fermentation filtrates from different strains. WT, S. ansochromogenes 7100. ΔcprC, disruption mutant of cprC. ΔcprCc, cprC complementary strain of ΔcprC. ΔsabA, sabA disruption mutant. ΔsabA/cprCoe, cprC overexpression strain in ΔsabA.
CprC directly activates the transcription of adpA
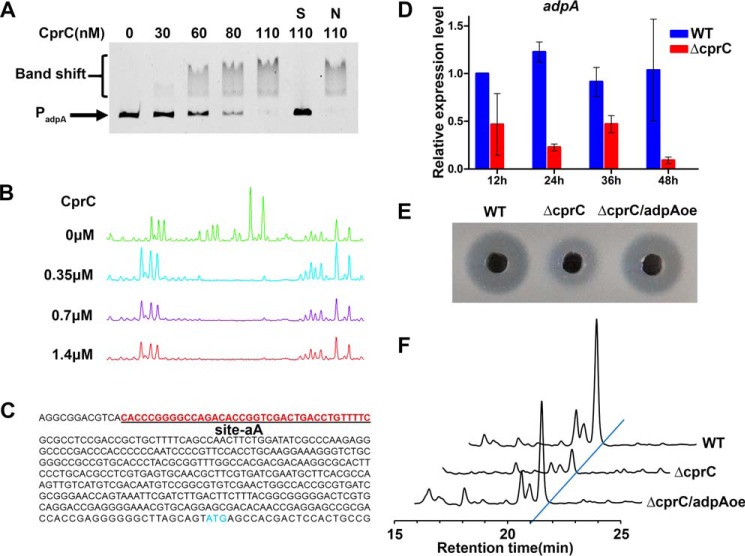
To understand how CprC activates nikkomycin biosynthesis, EMSAs were performed using purified CprC-His6 and the promoters of potential target genes, adpA, sanG, and sanF. It was shown that CprC binds to the upstream region of adpA (Fig. 7A), but not to the promoter regions of sanG and sanF (data not shown). DNase I footprinting experiments revealed that the protected sequence on adpA promoter by CprC is 5′-CACCCGGGGCCAGACACCGGTCGACTGACCTGTTTTC-3′, from −406 to −369 bp relative to the tss of adpA (Fig. 7, B and C). Therefore, adpA is verified to be a target gene of CprC.
Figure 7.
CprC activates the transcription of adpA by directly binding to its promoter region. A, EMSA of CprC binding to the fluorescently labeled promoter region of adpA (PadpA). Each lane contains 50 ng of labeled probes and 1 μg of poly(dI-dC). S, unlabeled specific probe PadpA (30-fold) was added; N, unlabeled nonspecific probe PhrdB (30-fold) was added. B, DNase I footprinting of CprC-binding site on PadpA (site-aA). C, the nucleotide sequence of PadpA. Blue letters, translational start sites. Underlined red letters, binding sites of CprC. D, qRT-PCR transcriptional analysis of adpA in WT and ΔcprC. Error bars, S.D. calculated from three independent experiments. E, nikkomycin bioassays of fermentation filtrates from different strains. F, HPLC analyses of nikkomycin in different strains. WT, S. ansochromogenes 7100. ΔcprC, disruption mutant of cprC. ΔcprC/adpAoe, adpA overexpressed in ΔcprC.
Analyses by qRT-PCR revealed that the transcription of adpA was significantly decreased in ΔcprC (Fig. 7D), confirming that CprC is an activator of adpA in S. ansochromogenes. Remarkably, upon adpA overexpression under the control of PhrdB in ΔcprC (ΔcprC/adpAoe), nikkomycin production was restored compared with ΔcprC (Fig. 7, E and F), demonstrating that CprC activation on adpA transcription is an important route in regulating nikkomycin biosynthesis.
Taken together, these findings enable us to propose a plausible regulatory model of the SABs-SabR1 signaling system in S. ansochromogens. sabR1, encoding a receptor of signaling molecule, is situated in a minicluster sab, in which sabAPD are signaling molecule biosynthetic genes. SabR1 can repress the transcription of cprC, which can be relieved by signaling molecules synthesized by SabAPD. CprC is newly identified as an activator of adpA, whereas AdpA positively regulates nikkomycin production by activating the cluster-situated regulatory gene sanG. In this complicated regulatory system, CprC and AdpA are key factors that transmit butenolide signals to secondary metabolism and thereby regulate nikkomycin biosynthesis (Fig. 8).
Figure 8.
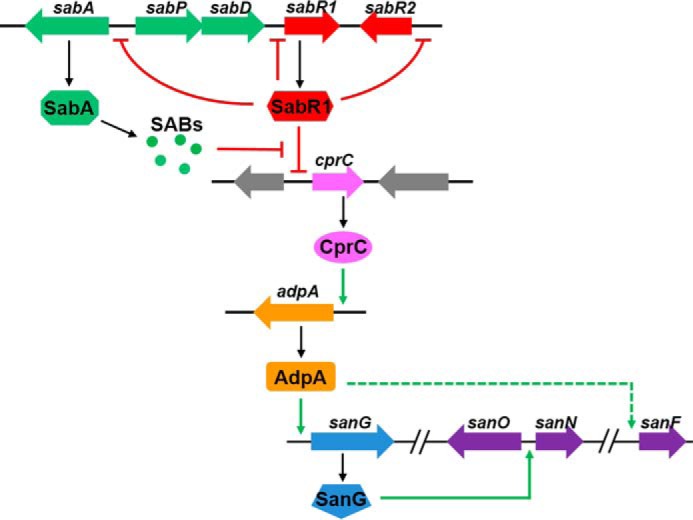
A plausible model for the roles of SabR1 and its ligands (SABs) in regulation of nikkomycin biosynthesis. Signal molecules SABs synthesized by sab exert regulatory functions via the cognate receptor SabR1. SabR1 can repress the transcription of cprC and other target genes (sabR1, sabR2, and sabA). CprC is a new activator of adpA, and AdpA positively regulates nikkomycin production by activating the cluster-situated regulatory gene sanG. In WT strain, binding of SABs to SabR1 causes the dissociation of SabR1 from cprC promoter and increases crpC transcription, which in turn activates adpA transcription to trigger nikkomycin biosynthesis. When sabA is disrupted or SABs are absent, binding of SabR1 to cprC would result in the repression of cprC and consequently cause the transcriptional reduction of adpA and nikkomycin biosynthetic genes.
Discussion
The dramatic rise in drug-resistant pathogens creates an urgent need for effective alternatives. Bacteria of the genus Streptomyces are a particularly abundant source of antibiotics, and their signaling systems are appealing for their critical roles in the regulation of secondary metabolism, particularly in antibiotic biosynthesis. The discovery of new signaling molecules and elucidation of their regulatory roles would have great significance for their use. In this work, a novel member of the butenolide autoregulators, SAB1, along with two analogues, SAB2 and SAB3, were characterized. A cascade regulation of nikkomycin biosynthesis mediated by SABs and its receptor SabR1 via a newly discovered adpA activator CprC was revealed for the first time, which may be applicable for other Streptomyces, as SabA and CprC homologs are widely distributed. Moreover, butenolide regulation of global regulator AdpA indicates their potential pleiotropic effect. These findings provide new insights into the butenolide signaling system and also facilitate the application of signaling molecules in natural product discovery.
Genome sequencing has revealed the presence of a large number of cryptic secondary metabolic gene clusters in Streptomyces. Such pathways are usually under stringent control by a variety of different regulatory mechanisms, in which signaling molecules might play important roles. A lack of specific signaling molecules, such as γ-butyrolactones and butenolides, may be a factor causing the silence of gene clusters (23). Characterization of signaling molecules or autoregulators is essential to evaluate their significance in antibiotic production and morphological differentiation. However, this task is hindered by the low yield of autoregulators in producing strains, which makes their study by large-scale fermentation rather burdensome and inefficient (1). It has been evidenced that AfsA and its homologs as well as tailoring enzymes are responsible for the biosynthesis of various signaling molecules, such as GBLs, furans, and some butenolides (4, 6, 24). In this study, heterologous expression of afsA homologue sabA and two tailoring enzyme genes, sabP and sabD, of S. ansochromogenes significantly improved the signal molecule yield, and three different butenolides were characterized. SAB1, SAB2, and SAB3 share a common ring skeleton but with different side chains at the C-3 position. In addition, more than one configurational isomer of SABs was visible on NMR spectra. SAB1 and SAB3 are more effective than SAB2 in nikkomycin production, reflecting the structure–activity relationship. Thus, heterologous expression of the butenolide biosynthetic genes proved to be an effective and feasible approach for producing higher yield and diverse structures of signal molecules, which can confer on them different receptor binding affinity and regulatory activity on antibiotic biosynthesis. Interestingly, genome mining revealed that the homologs of SabA are present in Streptomyces lavenduligriseus, Streptomyces achromogenes, Streptomyces sp. NRRL S-31, and so on, suggesting that SAB-like autoregulators may exist widely in Streptomyces.
Autoregulators usually function via binding to cognate receptor proteins, and their effects can be transmitted in cascade fashion to specific pathways by a series of regulators. The well-exemplified representative signaling system is GBLs. For butenolides, a total of 10 members (including SAB1, -2, and -3 identified in this study) of this family of signaling molecules from five Streptomyces species have been discovered to date, but their signal transduction system has been the subject of little investigation. In S. avermitilis, avenolide receptors AvaR1 and AvaR2 were verified, and AvaR2 directly repressed the transcription of aveR, a CSR activator gene for avermectin production (25–27). Recently, four compounds structurally resembling avenolide were found in Streptomyces albus strain J1074 with different avenolide-like activity (3). In S. rochei, SRB1 and SRB2 activated the production of lankacidin and lankamycin but showed a negative effect on morphological differentiation. Their receptor, SrrA, controls two SARP regulators, SrrY and SrrZ, but the exact molecular mechanisms of these regulators activating antibiotic production and morphological differentiation still remain obscure (28, 29). Moreover, four butenolide compounds were isolated from S. antibioticus DSM40725, but their activities on antibiotic biosynthesis have not yet been reported in detail (20). It is noteworthy that the regulation mediated by the above butenolides, avenolide or SRBs, is AdpA-independent. Interestingly, our work indicated that SabR1 does not target to the CSRs, but can regulate adpA via an activator CprC, and the established signaling system might be significant for elucidating complicated regulatory network in antibiotic biosynthesis.
CprC, a new activator of adpA discovered in this work, is a member of the TetR-family regulators. BLAST searches revealed that its homologs are widespread in Streptomyces, but only CprA and CprB have been studied. CprA and CprB are two ArpA-like proteins in S. coelicolor and possess regulatory functions in both secondary metabolism and morphogenesis in S. coelicolor A3 (2). CprA stimulates actinorhodin and undecylprodigiosin biosyntheses and sporulation, whereas CprB represses actinorhodin biosynthesis and sporulation (22). However, their mechanism of action has not been revealed. Here we demonstrated that CprC directly binds to the adpA promoter region to activate its transcription, which may be also applicable for CprC homologs in other Streptomyces species. Interestingly, a signal binding domain at the C terminus of these proteins is present, but how they coordinate with small molecules to influence the expression of target genes is unclear. It will be of interest to verify the ligand of CprC and dissect the coordinated regulatory network in future studies.
Experimental procedures
Bacterial strains, plasmids, and growth conditions
Bacteria strains and plasmids used in this study are listed in Table 1. S. ansochromogenes and its derivative strains were grown at 28 °C on MS medium agar for sporulation and in SP medium for nikkomycin production (11). E. coli strains were grown in lysogeny broth medium at 37 °C containing corresponding antibiotics (16).
Table 1.
Bacterial strains and plasmids used in this study
| Strains/plasmids | Relevant characteristics | Source/references |
|---|---|---|
| Strains | ||
| S. ansochromogenes | ||
| 7100 | WT strain | Ref. 16 |
| ΔsabA | sabA disruption mutant | This work |
| ΔsabAc | sabA complementary strain of ΔsabA | This work |
| ΔsabR1 | sabR1 disruption mutant | This work |
| ΔsabA/ΔsabR1 | sabA and sabR1 disruption mutant | This work |
| ΔsabA/ΔsabR1c | sabR1 complementary strain of ΔsabA/ΔsabR1 | This work |
| ΔsabR2 | sabR2 disruption mutant | This work |
| ΔsabA/sabR2oe | sabR2 overexpression strain of ΔsabA | This work |
| ΔcprC | cprC disruption mutant | This work |
| ΔcprCc | cprC complementary strain of ΔcprC | This work |
| ΔsabA/cprCoe | cprC overexpression strain of ΔsabA | This work |
| ΔcprC/adpAoe | adpA overexpression strain of ΔcprC | This work |
| S. coelicolor | ||
| M1146 | act−, red−, cpk−, cda−, SCP1−, SCP2− | Ref. 33 |
| MpAPD | M1146 derivative containing PhrdB-sabAPD fusion plasmid for SABs production | This work |
| PCG | M1146 derivative containing PcprC-gusA fusion plasmid | This work |
| PCMG | M1146 derivative containing PcprCM-gusA fusion plasmid | This work |
| PCG-R1 | M1146 derivative containing PcprC-gusA fusion plasmid and plasmid containing sabR1 with its own promoter | This work |
| PCG-BR1 | M1146 derivative containing PcprC-gusA fusion plasmid and PhrdB-sabR1 fusion plasmid | This work |
| PCMG-R1 | M1146 derivative containing PcprCM-gusA fusion plasmid and plasmid containing sabR1 with its own promoter | This work |
| PCMG-BR1 | M1146 derivative containing PcprCM-gusA fusion plasmid and PhrdB-sabR1 fusion plasmid | This work |
| E. coli | ||
| JM109 | F′, proA+B+, lacIq, Δ(lacZ)M15/Δ(lac-proAB), gyrA96, recA1, relA1, endA1, hsdR17 | Invitrogen |
| C41(DE3) | F−, ompT, gal dcm hsd SB (rB−, mB−) (DE3) | Lucigen |
| ET12567/pUZ8002 | dam− dcm− hsdM− pUZ8002 | Refs. 34 and 52 |
| CpAPD | C41 derivative containing pET23b::sabAPD for SABs production | This work |
| Candida albicans CGMCC2.4159 | Indicator strain for nikkomycin bioactivity | CGMCC |
| Plasmids | ||
| pSET152 | aac (3)IV, lacZ, reppMB1*attφC31, oriT | Ref. 32 |
| pKC1139 | aac (3)IV, E. coli-Streptomyces shuttle plasmid contains a Streptomyces temperature-sensitive origin of replication eptomyces temperature-sensitive origin of replication | Ref. 35 |
| pIJ10500 | HygR, a derivative of pMS82 containing φBT1 integrase gene | Ref. 32 |
| pET23b | Expression vector | Novagen |
| pGUS | Plasmid containing gusA | Ref. 36 |
| pKC1139AD | pKC1139 derivative used for disruption of sabA | This work |
| pSET152::sabA | pSET152 containing intact sabA with its putative promoter used for complement of sabA | This work |
| pET23b::sabAPD | pET23b containing intact sabAPD | This work |
| pKC1139::hsabAPD | pKC1139 containing sabAPD and the promoter PhrdB | This work |
| pKC1139R1D | pKC1139 derivative used for disruption of sabR1 | This work |
| pSET152::sabR1 | pSET152 containing intact sabR1 with its putative promoter used for complement of sabR1 | This work |
| pET23b::sabR1 | pET23b containing SabR1 coding region | This work |
| pKC1139R2D | pKC1139 derivative used for disruption of sabR2 | This work |
| pSET152::hsabR2 | pSET152 containing sabR2 and the promoter PhrdB | This work |
| pKC1139CD | pKC1139 derivative used for disruption of cprC | This work |
| pSET152::cprC | pSET152 containing intact cprC with its putative promoter used for complement of cprC | This work |
| pSET152::hcprC | pSET152 containing intact cprC and PhrdB | This work |
| pET23b::cprC | pET23b containing cprC coding region | This work |
| pIJ10500::cgusA | pIJ10500 containing gusA and the promoter PcprC | This work |
| pIJ10500::cmgusA | pIJ10500 containing gusA and the promoter PcprCM | This work |
| pSET152::hsabR1 | pSET152 containing sabR1 and PhrdB | This work |
| M13 | Cloning vector | Stratagene |
| pPsabR1 | PsabR1 was inserted into plasmid M13 | This work |
| pPsabR2 | PsabR2 was inserted into plasmid M13 | This work |
| pPsabA | PsabA was inserted into plasmid M13 | This work |
| pPadpA | PadpA was inserted into plasmid M13 | This work |
| pSET152::hadpA | pSET152 containing adpA and PhrdB | This work |
Construction of disruption and complementation mutants
Gene disruption mutants were constructed via homologous recombination. The plasmids for disruption, complementation, and overexpression were first constructed in E. coli JM109 with appropriate primers for individual genes as shown in Table S2 and then conjugally transferred into Streptomyces through ET12567/pUZ8002.
To inactivate sabA, the fragment corresponding to the upstream region of sabA was amplified using the primer pairs sabALF/R, and the resulting product was digested with EcoRI/XbaI. The DNA fragment corresponding to the downstream region of sabA was amplified using primer pair sabARF/R and followed by XbaI/HindIII digestion. The two fragments were then ligated into the EcoRI/HindIII-digested sites of pKC1139 to generate plasmid pKC1139AD. Primers sabAcF/R were used to verify the mutant ΔsabA. To complement sabA, a 1.55-kb XbaI/BamHI-digested fragment containing the intact sabA and its own promoter region was inserted into pSET152 to give plasmid pSET152::sabA. Primers sabAcF/R were used to confirm the complementary strain ΔsabAc. The construction of disruption strains of sabR1, sabR2, and cprC and the complement strains of sabR1 and cprC was performed as mentioned above using appropriate primer pairs as shown in Table S2.
To overexpress cprC in ΔsabA, a 714-bp fragment containing the coding sequence of cprC was amplified using primers cprCoF/R, and the constitutive promoter PhrdB of S. coelicolor was amplified using primer pair PhrdBF/R. After digestion with NdeI/EcoRI and XbaI/NdeI, respectively, the two fragments were inserted into the EcoRI/XbaI-digested sites of pSET152 to generate pSET152::hcprC. Primers PhrdBF and cprCoR were used to confirm the strain ΔsabA/cprCoe. The overexpression strains of sabR2 in ΔsabA and adpA in ΔcprC were constructed as mentioned above using appropriate primer pairs as shown in Table S2.
Expression and purification of SabR1 and CprC
SabR1 coding region was amplified by PCR using primers SabR1F/R (Table S2). After digestion with NdeI and XhoI, the amplified fragments were inserted into the same sites of pET-23b to give plasmid pET23b::sabR1. The plasmid pET23b::cprC for expression and purification of CprC-His6 was constructed as mentioned above with suitable primers as shown in Table S2. Plasmids pET23b::sabR1 and pET23b::cprC were transferred into E. coli C41 for expression of SabR1-His6 and CprC-His6. Protein purification was performed with a nickel-nitrilotriacetic acid–agarose column as described previously (30).
Construction of plasmids pET23b::sabAPD and pKC1139::hsabAPD
To construct the plasmids for heterologous expression of sabAPD, a 0.96-kb fragment containing sabA was amplified using the primer pairs sabAF/R, and a 1.56-kb fragment containing sabPD was amplified using the primer pairs sabPDF/R. The two fragments were digested with NdeI/XbaI and XbaI/EcoRI, respectively, and then cloned into the NdeI/EcoRI-digested sites of pET23b to generate plasmid pET23b::sabAPD. It was transferred into E. coli C41 to generate CpAPD. A 2.5-kb fragment containing sabAPD digested with NdeI/EcoRI from pET23b::sabAPD and PhrdB of S. coelicolor digested with XbaI/NdeI were inserted into the EcoRI/XbaI-digested sites of pKC1139 to generate plasmid pKC1139::hsabAPD, which was transferred into S. coelicolor M1146 to generate MpAPD.
Purification and structural analyses of SABs
Seed broth (60 ml) of CpAPD grown in lysogeny broth medium at 37 °C for 12 h was inoculated into 3 liters of M9 medium. After incubation at 37 °C for 4 h, isopropyl 1-thio-β-d-galactopyranoside was added at a final concentration of 0.1 mm and further incubated at 28 °C for 4 h, followed by another 12 h at 37 °C with an airflow rate of 3 liters/min and agitation speed of 200 rpm. The culture filtrate was extracted twice with equal volumes of ethyl acetate, and the organic phase was dried and redissolved in methanol. SAB1 was isolated from the extract by three rounds of HPLC separation (Zorbax, SB-C18, 9.4 × 250 mm, 5 μm) with a flow rate of 3 ml/min at 210-nm detection wavelength. The elution gradient was set as follows: 31% methanol for 15 min on first HPLC, followed by a linear gradient of 5–20% acetonitrile in 40-min elution on second HPLC, and finally 5–100% methanol in a 30-min linear gradient elution.
Seed broth (60 ml) of MpAPD grown in YEME medium at 28 °C for 48 h was inoculated into 3 liters of modified AlaMM medium (24) (supplemented with 10 g/liter mannitol and 3 g/liter casaminoacids, pH 6.0) with an airflow rate of 0.8 liter/min and agitation speed of 200 rpm. After incubation at 28 °C for 5 days, the culture filtrate was processed with the same procedure as that of CpAPD. To separate SAB1, -2, and -3, the elution profile was set as follows: 10–40% methanol for 30 min and then 40–60% methanol for 10 min. SAB1, -2, and -3 were eluted at 25.7–27 min, 24.2–25.7 min, and 34.7–36.2 min, respectively. The collected fractions of SAB1 were further purified with the elution profile of 14% acetonitrile in 20 min, SAB2 with a linear gradient of 14–16% acetonitrile in 20 min, and SAB3 with a linear gradient of 22–23% acetonitrile in 20 min.
Mass spectral analysis was performed on a triple quadrupole LC/MS/MS system (Agilent 1260/6460) in positive mode with an Agilent ZORBAX SB-C18 column (3.5 μm, 2.1 × 100 mm). 1H and 13C NMR spectra as well as 1H-1H COSY, 1H-13C HMBC, and 1H-13C HSQC were recorded on a 500-MHz Bruker spectrometer using CDCl3 as solvent.
EMSAs and DNase I footprinting
The EMSAs and DNase I footprinting assays were performed as described (16, 31). All probes for EMSAs were amplified by PCR using the corresponding primer pairs listed in Table S2. For EMSAs with unlabeled probes, SabR1 was incubated with 20-ng probes in a 20-μl reaction mixture at 25 °C for 30 min, and then the samples were loaded on 4% (w/v) native polyacrylamide gels for electrophoresis. The gel was stained with SYBR Gold nucleic acid gel stain for 30 min and photographed under UV transillumination using Quantity One. EMSAs with fluorescently labeled probes were carried out as follows. To obtain the fluorescently labeled DNA, the promoter regions of target genes were individually inserted into the EcoRV site of plasmid M13. The resulting plasmids were used as templates for probe amplification using fluorescently labeled primers FAM-F/HEX-R. 50-ng labeled probes were incubated with SabR1 in a 20-μl reaction mixture containing 1 μg of poly(dI-dC) at 25 °C for 30 min. The fluorescently labeled DNA was detected by Tanon-5200 Multi. For competition assays, 1.5 μg of unlabeled specific probe or the nonspecific probe PhrdB was added into the binding reaction mixture. The EMSAs of CprC binding to DNA probes were performed similarly as mentioned above with appropriate probes.
RNA isolation and real-time quantitative PCR
Total RNA was isolated from cultures of S. ansochromogenes and its derivative strains at various time points. RNA isolation, genomic DNA removal, reverse transcription, and qRT-PCR were performed as described previously (30). All of the primers used were listed in Table S2. 23S rRNA of S. ansochromogenes was used as internal control.
Bioassay and HPLC analysis of nikkomycin
The bioassay and HPLC of nikkomycin in culture filtrates of S. ansochromogenes and its derivative strains was carried out as described previously (12).
Bioassays of SABs
The activity of SABs in vivo was determined by the ability to restore nikkomycin production in ΔsabA. Different concentrations of SABs were added into 50-ml cultures of ΔsabA at the beginning of fermentation. After incubation for 5 days, nikkomycin production was detected by HPLC as described above.
gusA transcriptional fusion assays
To confirm the binding activity of SabR1 to PcprC, two reporter plasmids containing PcprC or PcprCM-gusA fusions were constructed. PcprC was first amplified using the primers PGcprCF/PGcprCR. To obtain the template of PcprCM (mutant of PcprC at the binding motif), two fragments amplified by PCR using PcprCF/PcprCMR and PcprCMF/PcprCR were digested with NotI and then ligated by T4-ligase. The resulting mixture was diluted 10 times and then used as the template of PcprCM. PcprCM was amplified using the primers PGcprCF/PGcprCR. gusA was amplified from pGUS using primer pair gusA-F/R. Then the PcprC or PcprCM digested with SpeI/NdeI and gusA with NdeI/XhoI were cloned into the SpeI/XhoI-digested sites of pIJ10500 to generate plasmid pIJ10500::cgusA and pIJ10500::cmgusA. Then the two constructs were respectively introduced and integrated into the ΦBT1 attB site of S. coelicolor M1146. Subsequently, pSET152::sabR1 and pSET152::hsabR1 were respectively introduced into the two S. coelicolor M1146 derivatives containing different reporter constructs and integrated into the ΦC31 attB site. GusA activity was detected as described previously (32).
Author contributions
W. W., J. Z., and H. T. conceptualization; W. W. and J. Z. data curation; W. W., X. L., and Y. T. software; W. W. formal analysis; W. W., X. L., D. L., Y. L., and Y. T. investigation; W. W. and H. T. writing-original draft; J. Z. and H. T. supervision; J. Z. and H. T. funding acquisition; J. Z., Y. L., Y. T., and H. T. writing-review and editing; H. T. resources; H. T. validation; H. T. project administration.
Supplementary Material
Acknowledgments
We are grateful to Drs Guomin Ai and Jinwei Ren (Institute of Microbiology, Chinese Academy of Sciences, Beijing, China) for assistance with MS and NMR spectroscopy. We thank Prof. Mervyn J. Bibb (Department of Molecular Microbiology, John Innes Centre, Norwich, UK) for providing the plasmid pGUS. We thank Prof. Clive Robinson (Institute for Infection and Immunity, St George's, University of London, London, UK) for the critical reading and helpful suggestions in preparation of the manuscript.
This work was supported by Ministry of Science and Technology of China Grant 2015CB150600 and National Natural Science Foundation of China Grants 31571281, 31771378, and 31470206. The authors declare that they have no conflicts of interest with the contents of this article.
This article contains Tables S1 and S2 and Figs. S1–S6.
- CSR
- cluster-situated regulator
- ARE
- autoregulator element
- qRT-PCR
- quantitative real-time PCR
- GBL
- γ-butyrolactone
- HSQC
- heteronuclear single-quantum correlation
- HMBC
- heteronuclear multiple-bond correlation
- EMSA
- electrophoretic mobility-shift assay
- tss
- translation start site.
References
- 1. Niu G., Chater K. F., Tian Y., Zhang J., and Tan H. (2016) Specialised metabolites regulating antibiotic biosynthesis in Streptomyces spp. FEMS Microbiol. Rev. 40, 554–573 10.1093/femsre/fuw012 [DOI] [PubMed] [Google Scholar]
- 2. Sidda J. D., Poon V., Song L., Wang W., Yang K., and Corre C. (2016) Overproduction and identification of butyrolactones SCB1–8 in the antibiotic production superhost Streptomyces M1152. Org. Biomol. Chem. 14, 6390–6393 10.1039/C6OB00840B [DOI] [PubMed] [Google Scholar]
- 3. Nguyen T. B., Kitani S., Shimma S., and Nihira T. (2018) Butenolides from Streptomyces albus J1074 act as external signals to stimulate avermectin production in Streptomyces avermitilis. Appl. Environ. Microbiol. 84, e02791–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kato J. Y., Funa N., Watanabe H., Ohnishi Y., and Horinouchi S. (2007) Biosynthesis of γ-butyrolactone autoregulators that switch on secondary metabolism and morphological development in Streptomyces. Proc. Natl. Acad. Sci. U.S.A. 104, 2378–2383 10.1073/pnas.0607472104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kitani S., Miyamoto K. T., Takamatsu S., Herawati E., Iguchi H., Nishitomi K., Uchida M., Nagamitsu T., Omura S., Ikeda H., and Nihira T. (2011) Avenolide, a Streptomyces hormone controlling antibiotic production in Streptomyces avermitilis. Proc. Natl. Acad. Sci. U.S.A. 108, 16410–16415 10.1073/pnas.1113908108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Arakawa K., Tsuda N., Taniguchi A., and Kinashi H. (2012) The butenolide signaling molecules SRB1 and SRB2 induce lankacidin and lankamycin production in Streptomyces rochei. Chembiochem 13, 1447–1457 10.1002/cbic.201200149 [DOI] [PubMed] [Google Scholar]
- 7. Horinouchi S., and Beppu T. (2007) Hormonal control by A-factor of morphological development and secondary metabolism in Streptomyces. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 83, 277–295 10.2183/pjab.83.277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu G., Chater K. F., Chandra G., Niu G., and Tan H. (2013) Molecular regulation of antibiotic biosynthesis in streptomyces. Microbiol. Mol. Biol. Rev. 77, 112–143 10.1128/MMBR.00054-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang W., Ji J., Li X., Wang J., Li S., Pan G., Fan K., and Yang K. (2014) Angucyclines as signals modulate the behaviors of Streptomyces coelicolor. Proc. Natl. Acad. Sci. U.S.A. 111, 5688–5693 10.1073/pnas.1324253111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Higo A., Hara H., Horinouchi S., and Ohnishi Y. (2012) Genome-wide distribution of AdpA, a global regulator for secondary metabolism and morphological differentiation in Streptomyces, revealed the extent and complexity of the AdpA regulatory network. DNA Res. 19, 259–273 10.1093/dnares/dss010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lu C., Liao G., Zhang J., and Tan H. (2015) Identification of novel tylosin analogues generated by a wblA disruption mutant of Streptomyces ansochromogenes. Microb. Cell Fact. 14, 173 10.1186/s12934-015-0359-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pan Y., Liu G., Yang H., Tian Y., and Tan H. (2009) The pleiotropic regulator AdpA-L directly controls the pathway-specific activator of nikkomycin biosynthesis in Streptomyces ansochromogenes. Mol. Microbiol. 72, 710–723 10.1111/j.1365-2958.2009.06681.x [DOI] [PubMed] [Google Scholar]
- 13. Pan Y., Wang L., He X., Tian Y., Liu G., and Tan H. (2011) SabR enhances nikkomycin production via regulating the transcriptional level of sanG, a pathway-specific regulatory gene in Streptomyces ansochromogenes. BMC Microbiol. 11, 164 10.1186/1471-2180-11-164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. He X., Li R., Pan Y., Liu G., and Tan H. (2010) SanG, a transcriptional activator, controls nikkomycin biosynthesis through binding to the sanN-sanO intergenic region in Streptomyces ansochromogenes. Microbiology 156, 828–837 10.1099/mic.0.033605-0 [DOI] [PubMed] [Google Scholar]
- 15. Liu G., Tian Y., Yang H., and Tan H. (2005) A pathway-specific transcriptional regulatory gene for nikkomycin biosynthesis in Streptomyces ansochromogenes that also influences colony development. Mol. Microbiol. 55, 1855–1866 10.1111/j.1365-2958.2005.04512.x [DOI] [PubMed] [Google Scholar]
- 16. Xu J., Zhang J., Zhuo J., Li Y., Tian Y., and Tan H. (2017) Activation and mechanism of a cryptic oviedomycin gene cluster via the disruption of a global regulatory gene, adpA, in Streptomyces ansochromogenes. J. Biol. Chem. 292, 19708–19720 10.1074/jbc.M117.809145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhong X., Tian Y., Niu G., and Tan H. (2013) Assembly and features of secondary metabolite biosynthetic gene clusters in Streptomyces ansochromogenes. Sci. China. Life. Sci. 56, 609–618 10.1007/s11427-013-4506-0 [DOI] [PubMed] [Google Scholar]
- 18. Takano E., Chakraburtty R., Nihira T., Yamada Y., and Bibb M. J. (2001) A complex role for the γ-butyrolactone SCB1 in regulating antibiotic production in Streptomyces coelicolor A3(2). Mol. Microbiol. 41, 1015–1028 [DOI] [PubMed] [Google Scholar]
- 19. Xu G., Wang J., Wang L., Tian X., Yang H., Fan K., Yang K., and Tan H. (2010) “Pseudo” γ-butyrolactone receptors respond to antibiotic signals to coordinate antibiotic biosynthesis. J. Biol. Chem. 285, 27440–27448 10.1074/jbc.M110.143081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Braun D., Pauli N., Séquin U., and Zähner H. (1995) New butenolides from the photoconductivity screening of Streptomyces antibioticus (Waksman and Woodruff) Waksman and Henrici 1948. FEMS Microbiol. Lett. 126, 37–42 10.1111/j.1574-6968.1995.tb07387.x [DOI] [PubMed] [Google Scholar]
- 21. Bailey T. L., Boden M., Buske F. A., Frith M., Grant C. E., Clementi L., Ren J., Li W. W., and Noble W. S. (2009) MEME SUITE: tools for motif discovery and searching. Nucleic Acids Res. 37, W202–W208 10.1093/nar/gkp335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Onaka H., Nakagawa T., and Horinouchi S. (1998) Involvement of two A-factor receptor homologues in Streptomyces coelicolor A3(2) in the regulation of secondary metabolism and morphogenesis. Mol. Microbiol. 28, 743–753 [DOI] [PubMed] [Google Scholar]
- 23. Li Y., and Tan H. (2017) Biosynthesis and molecular regulation of secondary metabolites in microorganisms. Sci. China Life Sci. 60, 935–938 10.1007/s11427-017-9115-x [DOI] [PubMed] [Google Scholar]
- 24. Corre C., Song L., O'Rourke S., Chater K. F., and Challis G. L. (2008) 2-Alkyl-4-hydroxymethylfuran-3-carboxylic acids, antibiotic production inducers discovered by Streptomyces coelicolor genome mining. Proc. Natl. Acad. Sci. U.S.A. 105, 17510–17515 10.1073/pnas.0805530105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhu J., Sun D., Liu W., Chen Z., Li J., and Wen Y. (2016) AvaR2, a pseudo γ-butyrolactone receptor homologue from Streptomyces avermitilis, is a pleiotropic repressor of avermectin and avenolide biosynthesis and cell growth. Mol. Microbiol. 102, 562–578 10.1111/mmi.13479 [DOI] [PubMed] [Google Scholar]
- 26. Sultan S. P., Kitani S., Miyamoto K. T., Iguchi H., Atago T., Ikeda H., and Nihira T. (2016) Characterization of AvaR1, a butenolide-autoregulator receptor for biosynthesis of a Streptomyces hormone in Streptomyces avermitilis. Appl. Microbiol. Biotechnol. 100, 9581–9591 10.1007/s00253-016-7781-4 [DOI] [PubMed] [Google Scholar]
- 27. Zhu J., Chen Z., Li J., and Wen Y. (2017) AvaR1, a butenolide-type autoregulator receptor in Streptomyces avermitilis, directly represses avenolide and avermectin biosynthesis and multiple physiological responses. Front. Microbiol. 8, 2577 10.3389/fmicb.2017.02577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Arakawa K., Mochizuki S., Yamada K., Noma T., and Kinashi H. (2007) γ-Butyrolactone autoregulator-receptor system involved in lankacidin and lankamycin production and morphological differentiation in Streptomyces rochei. Microbiology 153, 1817–1827 10.1099/mic.0.2006/002170-0 [DOI] [PubMed] [Google Scholar]
- 29. Suzuki T., Mochizuki S., Yamamoto S., Arakawa K., and Kinashi H. (2010) Regulation of lankamycin biosynthesis in Streptomyces rochei by two SARP genes, srrY and srrZ. Biosci. Biotechnol. Biochem. 74, 819–827 10.1271/bbb.90927 [DOI] [PubMed] [Google Scholar]
- 30. Zou Z., Du D., Zhang Y., Zhang J., Niu G., and Tan H. (2014) A γ-butyrolactone-sensing activator/repressor, JadR3, controls a regulatory mini-network for jadomycin biosynthesis. Mol. Microbiol. 94, 490–505 10.1111/mmi.12752 [DOI] [PubMed] [Google Scholar]
- 31. Li Y., Li J., Tian Z., Xu Y., Zhang J., Liu W., and Tan H. (2016) Coordinative modulation of chlorothricin biosynthesis by binding of the glycosylated intermediates and end product to a responsive regulator ChlF1. J. Biol. Chem. 291, 5406–5417 10.1074/jbc.M115.695874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li J., Li Y., Niu G., Guo H., Qiu Y., Lin Z., Liu W., and Tan H. (2018) NosP-regulated nosiheptide production responds to both peptidyl and small-molecule ligands derived from the precursor peptide. Cell Chem. Biol. 25, 143–153.e4 10.1016/j.chembiol.2017.10.012 [DOI] [PubMed] [Google Scholar]
- 33. Gomez-Escribano J. P., and Bibb M. J. (2011) Engineering Streptomyces coelicolor for heterologous expression of secondary metabolite gene clusters. Microb. Biotechnol. 4, 207–215 10.1111/j.1751-7915.2010.00219.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Paget M. S., Chamberlin L., Atrih A., Foster S. J., and Buttner M. J. (1999) Evidence that the extracytoplasmic function σ factor σE is required for normal cell wall structure in Streptomyces coelicolor A3(2). J. Bacteriol. 181, 204–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bierman M., Logan R., O'Brien K., Seno E. T., Rao R. N., and Schoner B. E. (1992) Plasmid cloning vectors for the conjugal transfer of DNA from Escherichia coli to Streptomyces spp. Gene 116, 43–49 10.1016/0378-1119(92)90627-2 [DOI] [PubMed] [Google Scholar]
- 36. Myronovskyi M., Welle E., Fedorenko V., and Luzhetskyy A. (2011) β-Glucuronidase as a sensitive and versatile reporter in actinomycetes. Appl. Environ. Microbiol. 77, 5370–5383 10.1128/AEM.00434-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.