Abstract
Proteins of the cyclin M family (CNNMs; also called ancient conserved domain proteins, or ACDPs) are represented by four integral membrane proteins that have been proposed to function as Mg2+ transporters. CNNMs are associated with a number of genetic diseases affecting ion movement and cancer via their association with highly oncogenic phosphatases of regenerating liver (PRLs). Structurally, CNNMs contain an N-terminal extracellular domain, a transmembrane domain (DUF21), and a large cytosolic region containing a cystathionine-β-synthase (CBS) domain and a putative cyclic nucleotide–binding homology (CNBH) domain. Although the CBS domain has been extensively characterized, little is known about the CNBH domain. Here, we determined the first crystal structures of the CNBH domains of CNNM2 and CNNM3 at 2.6 and 1.9 Å resolutions. Contrary to expectation, these domains did not bind cyclic nucleotides, but mediated dimerization both in crystals and in solution. Analytical ultracentrifugation experiments revealed an inverse correlation between the propensity of the CNBH domains to dimerize and the ability of CNNMs to mediate Mg2+ efflux. CNBH domains from active family members were observed as both dimers and monomers, whereas the inactive member, CNNM3, was observed only as a dimer. Mutational analysis revealed that the CNBH domain was required for Mg2+ efflux activity of CNNM4. This work provides a structural basis for understanding the function of CNNM proteins in Mg2+ transport and associated diseases.
Keywords: magnesium, transporter, crystal structure, dimerization, biophysics, structural biology, X-ray crystallography, analytical ultracentrifugation, CNNM, cyclic nucleotide-binding homology domain
Introduction
Magnesium (Mg2+) is the most abundant divalent cation inside cells and essential for a wide variety of biochemical processes, such as energy metabolism, maintenance of genomic stability, protein synthesis, and over 600 enzymatic reactions (1). In humans, abnormal Mg2+ handling is linked to different pathologies, including osteoporosis, diabetes, hypertension, neurological disorders, and immunodeficiency (2, 3). Mg2+ is unique among divalent cations in that it has the smallest ionic radius and largest hydrated radius; therefore, the transport of Mg2+ across the membrane requires the action of Mg2+ channels and transporters, including CNNM3 proteins (4).
Originally called cyclin M due to specious sequence similarity with the cyclin family, CNNMs are a conserved family of four integral membrane proteins implicated in maintaining Mg2+ homeostasis (5). CNNM2 and CNNM4 possess Mg2+ efflux activity and have been proposed to facilitate renal/intestinal (re)absorption of Mg2+ as they localize to the basolateral membrane of renal/intestinal epithelial cells (6, 7). CNNM mutations are associated with a number of genetic diseases affecting Mg2+ homeostasis. Mutations in CNNM2 were found in patients with familial dominant hypomagnesemia accompanied by low Mg2+ serum level and symptoms such as muscle weakness, tremor, and headaches (6). Mutations in CNNM4 are implicated in Jalili syndrome, characterized by recessive amelogenesis imperfecta and cone-rod dystrophy (8, 9).
More recently, CNNM-associated Mg2+ transport was found to be regulated by the binding of phosphatases of regenerating liver (PRLs), which are potent oncogenes with strong association with metastatic cancers (10). When PRL binds CNNM, intracellular Mg2+ level is increased, thereby promoting tumor progression and cellular proliferation (11, 12). Despite a clear association with Mg2+ transport, it is still debated whether CNNM proteins are themselves Mg2+ transporters or whether they regulate other proteins that transport Mg2+ (13, 14).
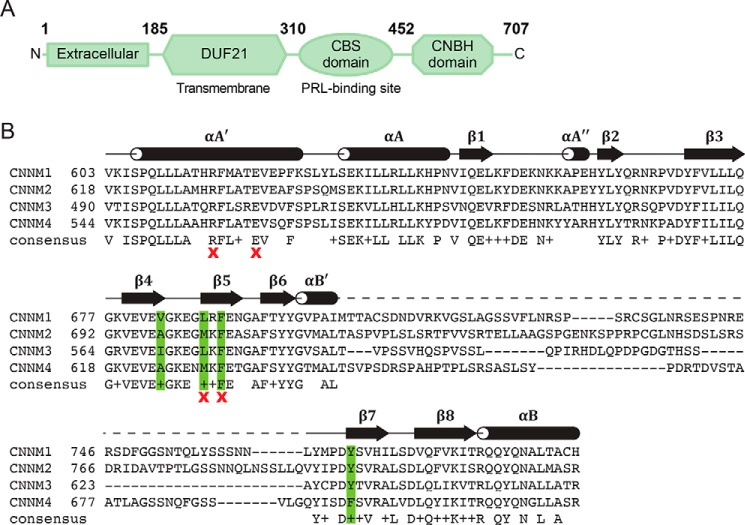
Structurally, CNNMs contain an N-terminal extracellular domain, a transmembrane domain (domain of unknown function 21; DUF21), and a large cytosolic region containing a cystathionine-β-synthase (CBS-pair) domain and a cyclic nucleotide–binding homology (CNBH) domain (Fig. 1A) (15). Currently, only the CBS-pair domains have been characterized structurally. The CBS-pair domain is the site of PRL binding (16–18) and required for Mg2+ efflux (19). The CBS-pair domains dimerize and are likely a site of regulation through ATP-Mg2+ binding. Nucleotide binding induces a conformational change of the dimer from a twisted to a flat disc-like structure (20).
Figure 1.
CNNM domain organization. A, CNNM consists of four domains: the extracellular domain, DUF21, CBS domain, and CNBH domain. Residues are numbered according to CNNM3. B, sequence alignment of CNBH domains of human CNNM proteins. Secondary structure corresponds to the crystal structure of the CNNM3 CNBH domain. The dashed line corresponds to a disordered region that was not observed in the electron density map. Hydrophobic residues involved in the dimerization interface are highlighted in green. Positions tested by mutagenesis for dimerization or efflux activity are marked by a red X.
Whereas CBS-pair domains have been extensively characterized, little is known about the CNNM C termini other than the sequence similarity to cyclic nucleotide–binding domains (5). The CNBH domains of CNNMs are well-conserved across isoforms with the exception of a large variable loop (Fig. 1B). The similarity to cyclic nucleotide–binding domains suggested that CNNMs could be regulated by cyclic nucleotide binding. The CNBH domain does not interact with CBS-pair; nor does it interact with PRLs (18). One mutation in the domain has been linked to Jalili syndrome (21).
Here, we carried out structural and functional characterization of the CNBH domain of CNNM. We determined the structures of CNBH domains from CNNM2 and CNNM3. We found that, contrary to expectations, the CNBH domains do not bind cyclic nucleotides. Rather, the domains exist as dimers both in the crystal and in solution. Efflux measurements with CNBH mutants showed that deletion of the CNNM4 CNBH domain abrogated activity in an Mg2+ efflux assay but that the dimerization was not required. Our results suggest that CNBH dimerization may function to inhibit and thus regulate CNNM activity across the different isoforms.
Results
Structural determination of CNBH domain of CNNM3 and CNNM2
To gain insight into CNNM proteins, we endeavored to determine the three-dimensional structures of their CNBH domains. We first obtained crystals of the CNBH domain from human CNNM3 (residues 453–707) that diffracted to ∼3 Å. Attempts to solve the structure by molecular replacement were unsuccessful; thus, we chose to label the protein with selenomethionine for experimental phasing. This required the introduction of additional methionine residues by site-directed mutagenesis, as there is only one methionine in the CNNM3 CNBH domain and its location was predicted to be in a mobile region. Seven sites (Ile-516, Arg-535, Thr-591, Ala-623, Leu-651, Val-669, and Ile-670) were selected based on the sequence alignment of CNNM proteins from different species. Mutants with three or more additional methionines were screened for expression and solubility. Several mutants produced a significant amount of soluble protein, but the best-diffracting crystals were obtained with a mutant with six artificially introduced methionines (Fig. S1).
The structure of the selenomethionine-labeled CNNM CNBH mutant was solved using the single-wavelength anomalous dispersion (SAD) method to 1.9 Å (Table 1 and Fig. 2A). Four of the seven selenomethionines were observed in the electron density and allowed phasing of the diffraction data. The crystal contained two CNBD domains in the asymmetric unit, which could be superposed with an all-heavy atom root mean square deviation (RMSD) of 0.4 Å. Notably, close to 50% of the crystallized protein was not observed in the electron density maps. Specifically, the N terminus (residues 453–488), a long internal loop (residues 592–623), and the C terminus (residues 655–707) were disordered in both molecules (Fig. 1B).
Table 1.
Statistics of data collection and refinement
| CNNM3 | CNNM2 | |
|---|---|---|
| Data collection | ||
| X-ray source | CHESS A1 | CLS 08ID-1 |
| Wavelength (Å) | 0.9779 | 1.0332 |
| Space group | P42212 | P3221 |
| Cell dimensions | ||
| a, b, c (Å) | 101.28, 101.28, 77.12 | 110.58, 110.58, 84.60 |
| α, β, γ (degrees) | 90.0, 90.0, 90.0 | 90.0, 90.0, 120.0 |
| Resolution (Å) | 50–1.90 (1.93–1.90)a | 50–2.60 (2.69–2.60) |
| Rmeas (%) | 9 (286) | 10 (146) |
| Rpim (%) | 2 (53) | 4 (48) |
| I/σI | 44.2 (1.8) | 19.8 (0.8) |
| Completeness (%) | 99.9 (100) | 96.5 (70.9) |
| Redundancy | 28.7 (27.5) | 8.7 (5.4) |
| CC½ | 0.662 | 0.609 |
| Refinement | ||
| Resolution (Å) | 32.5–1.90 | 47.9–2.60 |
| No. of reflections | 32,014 | 14,399 |
| Rwork/Rfree | 0.219/0.239 | 0.219/0.251 |
| No. of atoms | ||
| Protein | 2073 | 2332 |
| Water | 70 | 23 |
| B-Factors | ||
| Protein | 61.6 | 53.9 |
| Water | 54.2 | 26.8 |
| RMSDs | ||
| Bond lengths (Å) | 0.004 | 0.002 |
| Bond angles (degrees) | 0.70 | 0.48 |
| Ramachandran statistics (%) | ||
| Most favored regions | 97.2 | 96.7 |
| Additional allowed regions | 2.8 | 3.3 |
| Disallowed regions | 0.0 | 0.0 |
| PDB code | 6DFD | 6DJ3 |
a Values for the highest-resolution shell are shown in parentheses.
Figure 2.
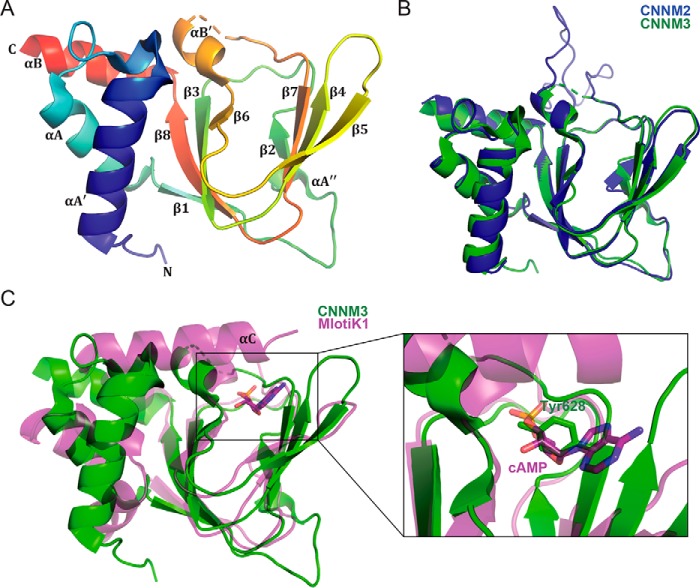
Crystal structures of CNBH domain of CNNM proteins. A, cartoon representation of CNNM3 CNBH domain, colored blue (N terminus) to red (C terminus). A disordered loop of 31 amino acids is indicated by a dashed line. The CNNM3 CNBH domain structure shows the typical fold of a cyclic nucleotide–binding domain: a wide antiparallel β-roll capped by an α-helical bundle. B, overlay of cartoon representations of CNBH domains of CNNM2 (blue) and CNNM3 (green). C, structural overlay of the CNBH domain of CNNM3 (green) with the cyclic nucleotide–binding domain of the bacterial K+ channel from Mesorhizobium loti (magenta; PDB code 1VP6). The M. loti K+ channel has an additional C-terminal helix (αC) that contacts the bound cAMP ligand. In CNNM3, a tyrosine side chain blocks the nucleotide-binding site.
We subsequently used this structure to design a construct of the CNBH domain of human CNNM2 as longer fragments had not crystallized. Residues 724–767, corresponding to the missing loop in the CNNM3 structure, were deleted. This loop shows little conservation between isoforms and is not predicted to form a regular secondary structure. Crystals of CNNM2 (residues 585–822, Δ724–767) were obtained, and the structure was solved by molecular replacement (Table 1). The CNNM2 crystals also contained two molecules in the asymmetric unit. The CNBH domains from CNNM2 and CNNM3 were very similar, with an RMSD value of 0.6 Å for 121 Cα atoms (Fig. 2B).
Crystal structures of CNBH domain of CNNM3 and CNNM2
As expected, the CNBH domain structures are similar to structures of cyclic nucleotide–binding domains (22). The domain contains an eight-stranded antiparallel β-roll that is capped by an α-helical bundle on the side (Fig. 2A). For consistency with the literature on cyclic nucleotide–binding domains, we labeled the two helices preceding the β-roll as αA′ and αA, the helix between β6 and β7 as αB′, and the helix following the β-roll as αB. The CNNM CNBH domains contain an additional single-turn α-helix (αA″) located between the β1 and β2 strands (Fig. 1B).
A major difference between the cyclic nucleotide–binding and CNBH domains is the large loop that was disordered in CNNM3 and partially deleted in CNNM2. In cyclic nucleotide–binding domains, the loop is 5 residues long and connects the αB′ helix and β7 strand (Fig. 2A). In CNNMs, the loop varies between 30 and 70 residues in length (Fig. 1B) and likely requires contacts with other domains in the full-length protein to fold properly. In one of the two CNNM2 molecules, weak electron density for the loop could be modeled due to crystal contacts that reduced the loop's mobility. The very high B-factors and the absence of a regular secondary structure suggest that its fold is not physiologically relevant (Fig. 2B).
A structural similarity search using the CNNM3 CNBH domain and the DALI database (23) identified the most structurally similar protein as the regulatory subunit of cAMP-dependent protein kinase (PDB code 2QVS) with an RMSD of 1.9 Å for 120 Cα atoms (Z-score of 15.1). Other top hits were a regulatory subunit of cGMP-dependent protein kinase (PDB code 5DYL, Z-score 14.0), cyclic nucleotide–binding protein (PDB code 5DLI, Z-score 14.0), and the cyclic nucleotide–binding domain of MlotiK1 potassium ion channel protein (PDB code 3CO2, Z-score 13.5). We used the MlotiK1 cyclic nucleotide–binding domain for detailed structural comparisons with the CNBH domains because it is from an ion channel, and structures are available for both ligand-free and nucleotide-bound forms (24).
Overall structural similarity to the cyclic nucleotide–binding domain of MlotiK1 is very high, particularly in the β-roll region, which is the site of nucleotide binding (Fig. 2C). The differences largely include slightly different orientations of helices and varied loop conformation. A number of loops are significantly longer in CNNMs. The main difference is the absence of helix αC in the CNBH domain of CNNM3. In cyclic nucleotide–binding domains, this C-terminal helix closes on the bound nucleotide, providing additional contacts and increased affinity (Fig. 2C). This region is unstructured in CNNM3 despite being present in the crystallized protein. The amino acid sequence does not contain any predicted secondary structure elements and shows low sequence conservation among CNNM family members, strongly suggesting that the region does not form a helix in CNNMs.
A structural alignment of the CNNM3 CNBH structure with the nucleotide-bound structure of MlotiK1 (PDB code 1VP6) suggests that CNNM3 should not bind nucleotides. CNNM3 contains a tyrosine residue (Tyr-628) in the middle of the putative nucleotide-binding site that would sterically clash with a bound ligand (Fig. 2C). This residue is an alanine in MlotiK1 but a tyrosine or phenylalanine in all four CNNM members, suggesting that none of the isoforms bind cyclic nucleotides.
CNBH domains of CNNMs do not bind cyclic nucleotides
To test this hypothesis, we used thermal shift assays (TSAs), also known as differential scanning fluorimetry, with purified CNBH domains of CNNM1, CNNM2, CNNM3, and CNNM4 (Fig. 3A and Table S1). Protein stability is typically increased upon ligand binding, resulting in a higher melting (denaturation) temperature (25), as illustrated by a positive control that binds cAMP (Fig. S2) (26). None of the melting temperatures of CNBH domains changed significantly upon the addition of cAMP or cGMP. Similar results were obtained either in the presence or absence of Mg2+ ions.
Figure 3.
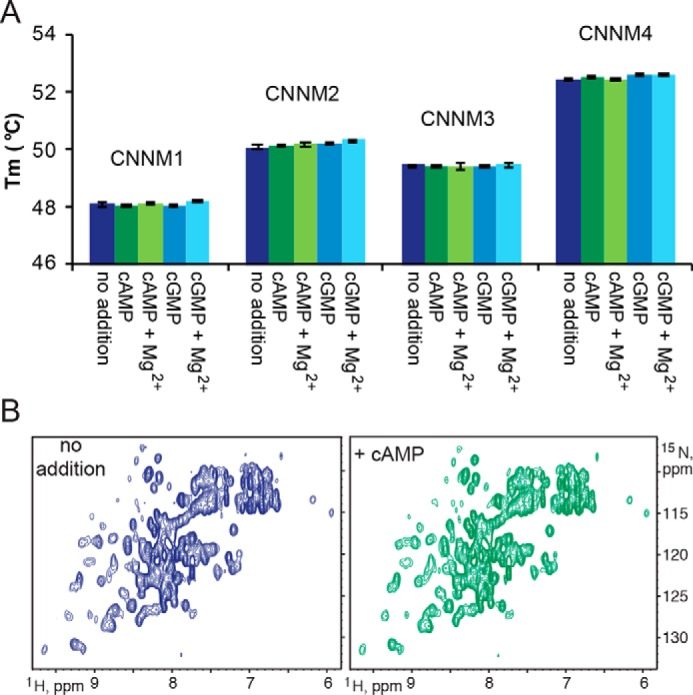
CNBH domains of CNNM proteins do not bind cyclic nucleotides. A, thermal shift assays of the denaturation of the CNBH domains of CNNM1–4 in the presence and absence of 1 mm cyclic nucleotides and 1 mm Mg2+. Each experiment was performed in triplicates. Error bars, S.E. B, two-dimensional 1H-15N correlation spectra of CNNM4 CNBH domain without nucleotide or 3 mm cAMP. No significant shifts were observed, indicating no ligand binding.
As a more sensitive alternative, we used NMR to detect binding of cAMP to CNNM4 (Fig. 3B). NMR is very sensitive to molecular interactions, allowing for detection of even low-affinity binding in the millimolar range. The 1H-15N correlation spectrum of the 15N-labeled CNNM4 CNBH domain showed good dispersion of signals, characteristic of a well-folded protein. Even at a cAMP concentration of 3 mm, no significant spectral changes were observed. Assuming a detection limit of 10% binding, this yields a lower bound on the affinity of 30 mm. Together, the TSA experiments and crystal structures demonstrate that CNBH domains of CNNM proteins do not bind cyclic nucleotides.
CNBH domains form dimers in solution
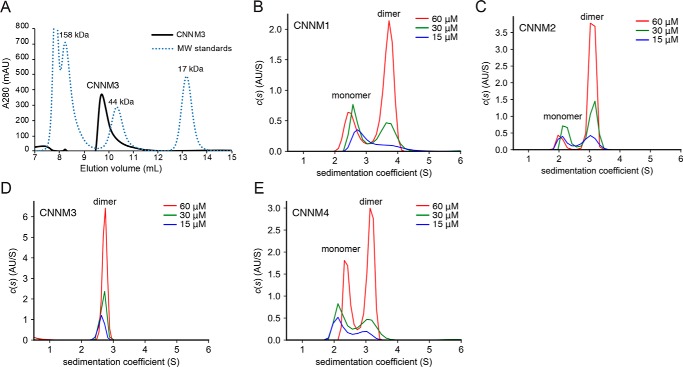
During purification, the CNNM3 CNBH domain was observed to elute as a dimer on size exclusion chromatography (Fig. 4A). To improve the resolution of monomers and dimers, which were poorly separated by size exclusion chromatography, we turned to sedimentation velocity analytical ultracentrifugation (AUC) (Fig. 4 (B–E), Fig. S3, and Table S2). AUC analysis at different protein concentrations (15–60 μm) revealed a single, dimeric species for the CNNM3 CNBH domain. On the other hand, the CNNM1, CNNM2, and CNNM4 CNBH domains displayed a mixture of monomer and dimer species in a ratio that varied with protein concentration. At the same concentration, the ratio of dimer to monomer was higher for CNNM2 than that of CNNM1 and CNNM4, with CNNM4 having the least propensity toward dimerization.
Figure 4.
CNBH domains of CNNM proteins dimerize in solution. A, gel-filtration chromatography of the CNBH domain of CNNM3. The protein elutes as dimer of 47.8 kDa relative to gel-filtration standards (γ-globulin, 158 kDa; ovalbumin, 44 kDa; myoglobin, 17 kDa). B–E, sedimentation velocity analytical ultracentrifugation experiments of CNBH domains at three protein concentrations (15, 30, and 60 μm). The CNNM3 CNBH domain sediments as a dimer at all concentrations, whereas the domains from CNNM1, CNNM2, and CNNM4 sediment as mixtures of monomer and dimer forms (see Table S2).
Mutagenesis identifies the dimerization interface
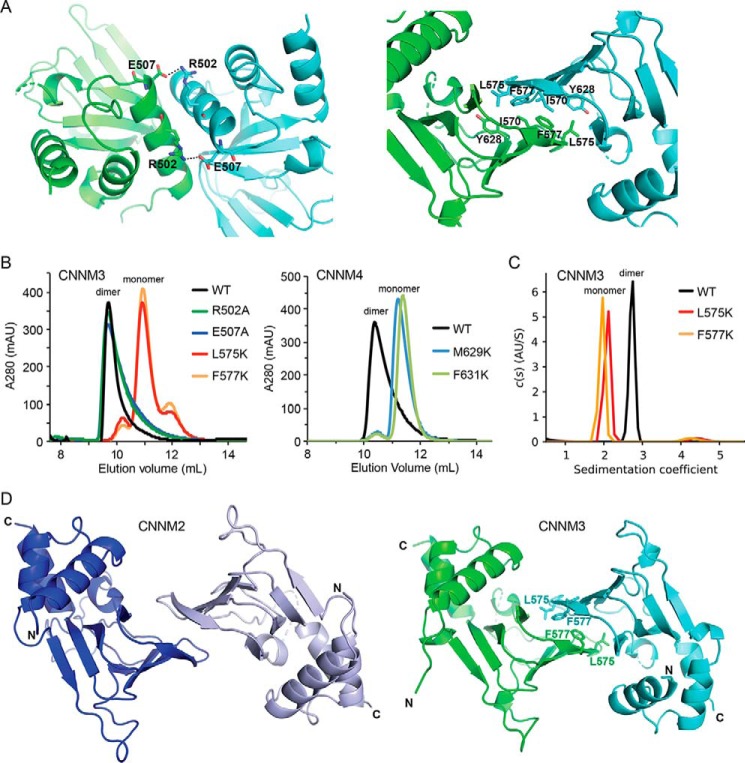
Analysis of crystal contacts revealed two protein–protein interfaces that could be responsible for CNBH dimerization: one mediated by ionic interactions between the N-terminal helices and one involving hydrophobic contacts between the β-roll elements (Fig. 5A). To identify the interface responsible for dimerization, we generated four mutants in the CNNM3 CNBH domain and analyzed them by gel-filtration chromatography and AUC. Mutating hydrophobic residue Leu-575 or Phe-577 disrupted dimerization, whereas the loss of either charged residue in the N-terminal helix had no effect (Fig. 5B). Analogous CNNM4 mutants also disrupted dimerization. The WT domain eluted as a broad peak consisting mostly of dimers, whereas the M629K and F631K mutants eluted as monomers (Fig. 5B). AUC analysis confirmed that the CNNM3 CNBH mutants L575K and F577K were both monomeric (Fig. 5C).
Figure 5.
Identification of the dimer interface. A, two alternative dimerization interfaces observed in the CNNM3 crystals. Left, mediated by ionic interactions. Right, mediated by hydrophobic contacts. B, gel-filtration chromatograms of WT and mutant CNBH domains. Four mutants were generated: R502A and E507A to disrupt the first interface and L575K and F577K to disrupt the second. The CNNM3 L575K and F577K mutants and analogous CNNM4 M629K and F631K mutants eluted as monomers. C, analytical ultracentrifugation of CNNM3 CNBH domain, confirming that the L575K and F577K mutants behave as monomers at 60 μm. D, comparison of the CNNM2 (left) and CNNM3 (right) CNBH dimers. The dimer interface is formed by a β-roll structure with a buried surface area of 1125 and 1547 Å2 for CNNM2 and CNNM3, respectively. The locations of the CNNM3 residues mutated to disrupt dimerization are indicated.
The β-roll dimerization surface is conserved in both CNNM2 and CNNM3 structures (Fig. 5D). The hydrophobic residues Ile-570, Leu-575, Phe-577, and Tyr-628 in CNNM3 are highly conserved across all four CNNM isoforms (Fig. 1B), which suggests that all of the CNBH domains can dimerize in the same fashion. The weaker dimerization of CNNM2 and CNNM4 could be related to the substitutions of alanine and methionine at positions 570 and 575 of CNNM3 or smaller buried surface areas (Fig. 5E). Finally, we note that the dimerization further explains the inability of CNBH domains to bind nucleotides; the intermolecular contacts at the dimer interface overlap with the putative ligand-binding site.
CNNM4 CNBH domain is essential for function
To assess the role of the CNBH domain in CNNM function, we measured CNNM-dependent Mg2+ efflux in a cellular assay with WT and mutant CNNM4 (7). Deletion of the CNBH domain completely blocked CNNM4-associated Mg2+ efflux but had no effect on protein expression or localization (Fig. 6). To test the role of CNBH dimerization, we assayed the two CNNM4 point mutants that prevent CNBH dimerization. The mutations, M629K and F631K, had divergent effects on the cellular Mg2+ efflux. F631K showed significant impairment, but M629K showed close-to-WT efflux, indicating that CNBH dimerization is not required for Mg2+ efflux. This is consistent with the observation that the CNBH domain from the most active CNNM isoform has the least tendency to form dimers.
Figure 6.
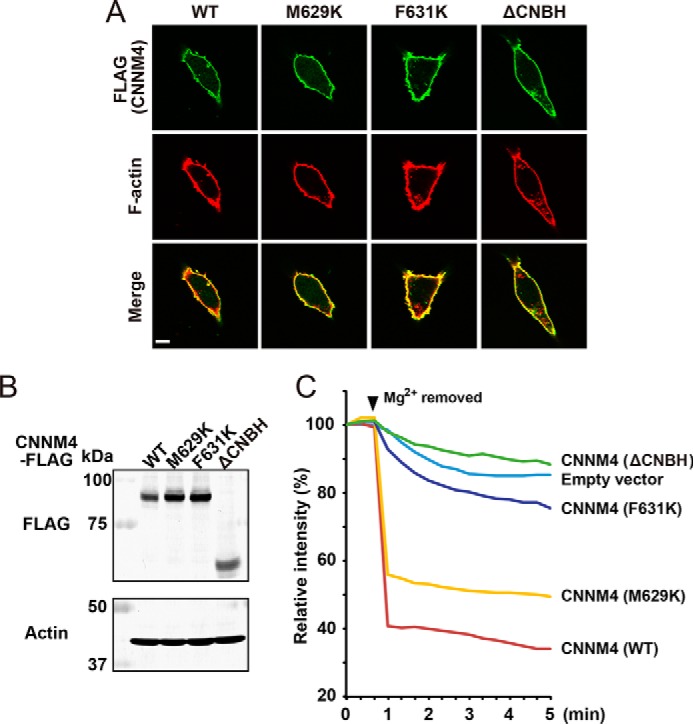
CNBH domain is not required for CNNM4 expression or localization but is required for Mg2+ efflux. A, immunofluorescence images of HEK293 cells with anti-FLAG (green) and rhodamine-phalloidin (red) showing that CNNM4 WT and mutant proteins are properly colocalized with F-actin adjacent to the cell membrane. The mutations, M629K and F631K, prevent CNBH dimerization. Bar, 10 μm. B, Western blotting showing equal expression of CNNM proteins in lysates of HEK293 cells transfected with the indicated constructs. C, Mg2+ efflux assays showing that deletion of the CNBH domain (ΔCNBH) blocks activity. HEK293 cells transfected with the indicated constructs were loaded with Magnesium Green and then subjected to Mg2+ depletion at the indicated time point (arrowhead). The mean relative fluorescence intensities of 10 cells are shown in the graph.
Discussion
A wide variety of presumptive Mg2+ transporters exist in bacterial and eukaryotic cells, but the precise molecular function of many is under debate (27). Atomic structures are known for only two bacterial transporters: CorA and MgtE (28). CNNM proteins show the greatest sequence similarity to DUF21 domain proteins, a large family of putative Mg2+ transporters found in bacteria, plants, and animals. Bacterial DUF21 domain proteins have architectures similar to CNNM proteins, consisting of an integral membrane domain of three transmembrane helices, followed by a CBS-pair domain and a small C-terminal globular protein domain similar in size to CNNM CNBH domains. Crystal structures of the C-terminal domains of several bacterial DUF21 proteins show that they also form dimers (PDB entries 3DED, 2OAI, and 2R8D).
The CNBH domain of CNNM proteins are structurally similar to many cyclic nucleotide–binding domains of cyclic nucleotide-gated channels (29–31), but our results demonstrate the inability of these domains to bind cAMP or cGMP. Although co-purification with bacterial cAMP (22) or binding to noncanonical cyclic nucleotides (not tested) is still possible (32), the three-dimensional structures strongly suggest otherwise. The first reason is the presence of a large aromatic residue (Tyr-628 in CNNM3) in the middle of the typical nucleotide-binding site (Fig. 2C). This is similar to the related domain in the KCNH (ether-à-go-go K+) channel, which does not bind nucleotide due to blockage of the β-roll binding pocket by an adjacent three-residue β-strand motif (33). Second, CNBH domains lack the C-terminal αC helix typically involved in cyclic nucleotide binding (Fig. 2C). Normally, upon nucleotide binding, the αB and αC helices rotate toward the β-roll, allowing the αC helix to interact with the base moiety and cap the binding pocket (29). In the case of CNNM, the absence of the αC helix prevents the stable binding of the nucleotide. Finally, the would-be nucleotide-binding surface is heavily involved in the intermolecular interaction of the dimer interface (Fig. 5). Major conformational changes would be required for a nucleotide to bind.
Instead, the function of the CNBH domain appears to be dimerization. Cyclic nucleotide–binding domains mediate dimerization in many proteins. The best-understood example is catabolite gene activator protein, which contains a cAMP-binding domain that is responsible for protein dimerization (34). The cyclic nucleotide–binding domains of the superfamily of voltage-gated K+ channel (which includes KCNH channels) form dimers (35). Like CNNM proteins, KCNH CNBH domains do not bind nucleotides but rather use the β-roll structure to interact with another protein domain, regulating the channel in a cyclic nucleotide–independent manner (36).
CNNM CNBH domains may play a similar role in regulating CNNM activity. CNNM isoforms show large differences in Mg2+ efflux activity, where CNNM4 possesses the highest activity, CNNM2 shows intermediate activity, CNNM1 shows weak activity, and CNNM3 is inactive (19). The differences in efflux activity inversely correlate with the propensity of CNBH domains to dimerize (Fig. 4). Whereas the CNBH domain is essential for Mg2+ efflux activity, dimerization is not required as the M629K mutation that disrupts the dimer interface has only a modest effect on Mg2+ efflux (Fig. 6C).
Although the exact molecular function of CNNM proteins remains still unclear, the structural and functional characterization of their C-terminal CNBH domains is a valuable piece of the puzzle. However, fully understanding the connections between CNNM and Mg2+ metabolism and cancer will require additional functional studies and ultimately structural elucidation of the full-length protein.
Experimental procedures
Cloning of CNNM C-terminal domains
Human CNNM1 CNBH domain (residues 569–798), CNNM2 CNBH domain (residues 585–824), CNNM2 CNBHcryst domain (residues 585–822 with a deletion of residues 724–767), CNNM3 CNBH domain (residues 453–707), and CNNM4 CNBH domain (residues 513–728) were codon-optimized for Escherichia coli (Bio Basic Inc., Markham, Canada) and subcloned into BamHI and NotI sites of pGEX-6P-1 vector (Amersham Biosciences) with an N-terminal GST tag. Mutagenesis was performed using the QuikChange site-directed/multi-mutagenesis kit (Agilent).
Expression and purification of recombinant proteins
All constructs were verified by DNA sequencing and transformed into E. coli strain BL21 (DE3). Cultures were grown at 37 °C in Luria broth to an optical density of 0.8 and induced with 1 mm isopropyl 1-thio-β-d-galactopyranoside for 4 h at 30 °C. Cell pellet was obtained by centrifuging at 5000 × g for 20 min. The pellet was resuspended in buffer A (50 mm HEPES, 500 mm NaCl, 5% glycerol, 5 mm β-mercaptoethanol, pH 7.5) and lysed by sonication. Cellular debris was removed by centrifugation at 44,000 × g for 45 min at 4 °C. The supernatant was loaded onto Pierce GSH-agarose resin, washed with buffer A, and eluted with buffer A containing 20 mm GSH. The GST tag was removed by overnight incubation with PreScission Protease, leaving an N-terminal Gly-Pro-Leu-Gly-Ser extension. The protein was further purified by a Superdex-75 size-exclusion column (GE Healthcare) in HPLC buffer (20 mm HEPES, 100 mm NaCl, 3 mm TCEP, pH 7.5). The final purified protein was concentrated to around 10 mg/ml (measured by NanoDrop), and the purity was verified by SDS-PAGE. For selenomethionine labeling, the plasmid was transformed into a methionine auxotroph strain DL41 (DE3), and the protein was produced using LeMaster medium. The expression and purification protocols were the same as for the native protein. For 15N labeling, the cells were grown in M9 minimal medium supplemented with 15N-ammonium chloride as the sole source of nitrogen. The expression and purification protocols were the same as for the native protein.
Crystallization
Crystals of selenomethionine-labeled CNNM3 CNBH domain with amino acid substitutions I516M, R535M, T591M, A623M, L651M, V669M, and I670M were obtained by equilibrating 0.6 μl of protein (10 mg/ml) with 0.6 μl of reservoir solution (0.8 m succinic acid, pH 7.0) in a hanging-drop vapor diffusion system incubated at 22 °C. Crystals of CNNM2 CNBHcryst domain were obtained by equilibrating 2 μl of protein (10 mg/ml) with 2 μl of reservoir solution (0.1 m BisTris, pH 6.0, 0.5 m sodium citrate, pH 7.0) in a hanging-drop vapor diffusion system incubated at 22 °C.
Data collection, structure determination, and refinement
The crystals were cryoprotected by soaking in mother liquor supplemented with 30% ethylene glycol, picked up in a nylon loop, and flash-cooled in a N2 cold stream. The CNNM3 CNBH domain SAD data set from a single crystal was collected using a single-wavelength (0.9779 Å) regime at beamline A1 of the Cornell High-Energy Synchrotron Source (CHESS) using an ADSC Quantum-210 CCD detector (Area Detector Systems Corp.). Data processing and scaling were performed with HKL-2000 (37). The starting phases were obtained by selenium-SAD using PHENIX (38). The resulting model was extended manually with the help of the program Coot (39) and was improved by several cycles of refinement using the program REFMAC5 (40) followed by TLS refinement (41). The CNNM2 CNBH domain diffraction data set was collected on beamline 08ID-1 with a Rayonix MX300 CCD detector at the Canadian Macromolecular Crystallography Facility (CMCF) of the Canadian Light Source (CLS). Data processing and scaling were performed with HKL-2000 (37). The CNNM2 CNBH domain structure was solved by molecular replacement using Phaser with the CNNM3 CNBH domain structure as a search model (42). Refinement was carried out by phenix.refine (43). Crystallographic data collection and structure refinement statistics are shown in Table 1. The final models have good stereochemistry with no outliers in the Ramachandran plot computed using PROCHECK (44). Structural images were prepared with PyMOL, Version 2.0 (Schroedinger LLC, New York).
Thermal shift assays
Each reaction contained 20 μl of solution with 25 μm CNBH domain, 1× Protein Thermal ShiftTM dye (Life Technologies), HPLC buffer with and without nucleotides and MgCl2. Samples were heated from 25 to 99 °C at a rate of 1 °C/min, and fluorescence signals were monitored by the StepOne Plus quantitative real-time PCR system (Life Technologies, Inc.). Data were analyzed using Thermal Shift software (Life Technologies). The maximum change of fluorescence with respect to temperature was used to determine the melting temperature (Tm). Each sample was performed in triplicates, and S.E. was calculated for each Tm measured. Positive control protein (lpg1496-KLAMP1) was expressed and purified as described (26).
NMR spectroscopy
1H-15N heteronuclear single-quantum correlation spectroscopy was performed on a Bruker 600-MHz spectrometer using 0.15 mm 15N-labeled CNNM4 CNBH domain in 90% HPLC buffer and 10% D2O. Testing for cNMP binding was carried by acquiring TROSY spectra before and after the addition of 3 mm cAMP. NMR data were acquired at 40 °C. NMR spectra were processed using NMRPipe (45) and analyzed with SPARKY (46).
Analytical ultracentrifugation
Sedimentation velocity AUC experiments were performed at 20 °C using a Beckman Coulter XL-I analytical ultracentrifuge using an An-60Ti rotor at 98,000 × g (35,000 rpm) for 18 h with scans performed every 60 s. A double-sector cell, equipped with a 12-mm Epon centerpiece and sapphire windows, was loaded with 380 and 400 μl of sample and HPLC buffer, respectively. Samples at concentrations ranging from 15 to 60 μm were monitored with UV at 280 nm. The data were analyzed with Sedfit version 1501b (47) using a continuous c(s) distribution. Numerical values for the solvent density, viscosity, and the partial specific volume were determined using Sednterp (48). Buffer density and viscosity were calculated to be 1.0039 g/cm3 and 0.01026 millipascals·s, respectively (20 mm HEPES, 100 mm NaCl, 3 mm TCEP, pH 7.5). Partial specific volumes for CNBH domains of CNNM1–4 were calculated to be 0.7172, 0.7419, 0.7416, and 0.7350 cm3/g, respectively. Frictional ratio (f/f0) values of CNNM2 and CNNM3 CNBH domains were calculated using US-SOMO (49) to be 1.29 and 1.21, and the value for CNNM1 and CNNM4 was estimated to be 1.29 based on similarity to CNNM2. Residual and c(s) distribution graphs were plotted using GUSSI (50).
Size-exclusion chromatography
Analytical gel-filtration experiments were carried out on an Akta Purifier HPLC system (GE Healthcare) using a 100-μl sample volume on a Superdex 75 Increase 10/300 GL column (GE Healthcare) with a flow rate of 0.8 ml/min at 4 °C using HPLC buffer. Gel-filtration standards were purchased from Bio-Rad (catalogue no. 151-1901).
Constructs used in mammalian culture cells
Human CNNM4 inserted into mammalian expression vector (pCMV tag-4A) was generated in the previous study (7). M629K, F631K, and ΔCNBH (Δ512–775) mutants were generated using the QuikChange site-directed mutagenesis kit (Agilent).
Mg2+-efflux assays
Mg2+-imaging analyses with Magnesium Green were performed as described in the previous study (7, 16). Transfected HEK293 cells were incubated under growth media supplemented with 40 mm Mg2+ until use, to avoid potential decrease of intracellular Mg2+ levels by the expressed proteins. Then the cells were incubated with Mg2+-loading buffer (78.1 mm NaCl, 5.4 mm KCl, 1.8 mm CaCl2, 40 mm MgCl2, 5.5 mm glucose, 5.5 mm HEPES-KOH, pH 7.4), including 2 μm Magnesium Green-AM (Invitrogen), for 30 min at 37 °C. The cells were rinsed once with loading buffer and viewed using a microscope (IX81 (Olympus) equipped with an ORCA-Flash 4.0 CMOS camera (Hamamatsu) and a USH-1030L mercury lamp (Olympus)). Fluorescence was measured every 20 s (excitation at 470–490 nm and emission at 505–545 nm) under the control of the Metamorph software (Molecular Devices). Then the buffer was changed to remove Mg2+ buffer (MgCl2 in the loading buffer was replaced with 60 mm NaCl). The data are presented as line plots of the mean fluorescence of 10 cells. After imaging analyses, cells were fixed with PBS containing 3.7% formaldehyde and subjected to immunofluorescence microscopy to confirm protein expression.
Immunofluorescence microscopy
Cells cultured on coverslips were stained and observed according to the previous study (7). Cells were fixed with 3.7% formaldehyde in PBS for 20 min and permeabilized with 0.2% Triton X-100 in PBS for 5 min. After blocking with PBS containing 3% fetal bovine serum and 10% BSA (blocking buffer) for 1 h, cells were incubated for 1 h with rabbit anti-FLAG antibody (Sigma F7425) diluted in blocking buffer. Cells were washed three times with PBS and incubated for 30 min with Alexa 488–conjugated anti-rabbit IgG (Invitrogen A-11034) and rhodamine-phalloidin (for F-actin visualization, Wako 165-21641) diluted in blocking buffer. After three washes with PBS, coverslips were mounted on slides and observed with a confocal scanning laser microscope (FLUOVIEW FV1000, Olympus).
Author contributions
Y. S. C. and G. K. designed the experiments, cloned the constructs, performed NMR experiments, and solved the crystal structures. Y. S. C. and R. F. performed TSA and AUC experiments. Y. S. C. performed gel-filtration experiments. Y. F. and Y. S. C. performed the Mg2+ efflux assays. Y. S. C., G. K., and K. G. wrote the manuscript. H. M. and K. G. oversaw the research.
Supplementary Material
Acknowledgments
We thank Véronique Sauvé and Jean-François Trempe for assistance with crystallographic data collection, Nadeem Siddiqui for advice on AUC data processing, and Alexei Gorelik for advice on crystallographic data processing. Crystallographic data were acquired at the Canadian Light Source (CLS) and at the Macromolecular Diffraction (MacCHESS) facility at the Cornell High-Energy Synchrotron Source (CHESS). CHESS is supported by National Science Foundation award DMR-0225180 and National Institutes of Health/NCRR Grant RR-01646.
This work was supported by Natural Sciences and Engineering Research Council of Canada (NSERC) Grant RGPIN-2014-04686 (to K. G.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This article was selected as one of our Editors' Picks.
This article contains Tables S1 and S2 and Figs. S1–S3.
The atomic coordinates and structure factors (codes 6DFD and 6DJ3) have been deposited in the Protein Data Bank (http://wwpdb.org/).
- CNNM
- cyclin M
- CNBH
- cyclic nucleotide–binding homology
- PRL
- phosphatase of regenerating liver
- CBS
- cystathionine-β-synthase
- RMSD
- root mean square deviation
- PDB
- Protein Data Bank
- AUC
- analytical ultracentrifugation
- TSA
- thermal shift assay
- GST
- glutathione S-transferase
- BisTris
- bis(2-hydroxyethyl)iminotris(hydroxymethyl)methane
- DUF21
- domain of unknown function 21
- SAD
- single-wavelength anomalous dispersion.
References
- 1. de Baaij J. H., Hoenderop J. G., and Bindels R. J. (2015) Magnesium in man: implications for health and disease. Physiol. Rev. 95, 1–46 10.1152/physrev.00012.2014 [DOI] [PubMed] [Google Scholar]
- 2. Li F. Y., Chaigne-Delalande B., Kanellopoulou C., Davis J. C., Matthews H. F., Douek D. C., Cohen J. I., Uzel G., Su H. C., and Lenardo M. J. (2011) Second messenger role for Mg2+ revealed by human T-cell immunodeficiency. Nature 475, 471–476 10.1038/nature10246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rude R. K. (1998) Magnesium deficiency: a cause of heterogeneous disease in humans. J Bone Miner. Res. 13, 749–758 10.1359/jbmr.1998.13.4.749 [DOI] [PubMed] [Google Scholar]
- 4. Jahnen-Dechent W., and Ketteler M. (2012) Magnesium basics. Clin. Kidney J. 5, i3–i14 10.1093/ndtplus/sfr163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang C. Y., Shi J. D., Yang P., Kumar P. G., Li Q. Z., Run Q. G., Su Y. C., Scott H. S., Kao K. J., and She J. X. (2003) Molecular cloning and characterization of a novel gene family of four ancient conserved domain proteins (ACDP). Gene 306, 37–44 10.1016/S0378-1119(02)01210-6 [DOI] [PubMed] [Google Scholar]
- 6. Stuiver M., Lainez S., Will C., Terryn S., Günzel D., Debaix H., Sommer K., Kopplin K., Thumfart J., Kampik N. B., Querfeld U., Willnow T. E., Němec V., Wagner C. A., Hoenderop J. G., et al. (2011) CNNM2, encoding a basolateral protein required for renal Mg2+ handling, is mutated in dominant hypomagnesemia. Am. J. Hum. Genet. 88, 333–343 10.1016/j.ajhg.2011.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yamazaki D., Funato Y., Miura J., Sato S., Toyosawa S., Furutani K., Kurachi Y., Omori Y., Furukawa T., Tsuda T., Kuwabata S., Mizukami S., Kikuchi K., and Miki H. (2013) Basolateral Mg2+ extrusion via CNNM4 mediates transcellular Mg2+ transport across epithelia: a mouse model. PLoS Genet. 9, e1003983 10.1371/journal.pgen.1003983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Parry D. A., Mighell A. J., El-Sayed W., Shore R. C., Jalili I. K., Dollfus H., Bloch-Zupan A., Carlos R., Carr I. M., Downey L. M., Blain K. M., Mansfield D. C., Shahrabi M., Heidari M., Aref P., Abbasi M., Michaelides M., Moore A. T., Kirkham J., and Inglehearn C. F. (2009) Mutations in CNNM4 cause Jalili syndrome, consisting of autosomal-recessive cone-rod dystrophy and amelogenesis imperfecta. Am. J. Hum. Genet. 84, 266–273 10.1016/j.ajhg.2009.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Polok B., Escher P., Ambresin A., Chouery E., Bolay S., Meunier I., Nan F., Hamel C., Munier F. L., Thilo B., Mégarbané A., and Schorderet D. F. (2009) Mutations in CNNM4 cause recessive cone-rod dystrophy with amelogenesis imperfecta. Am. J. Hum. Genet. 84, 259–265 10.1016/j.ajhg.2009.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Saha S., Bardelli A., Buckhaults P., Velculescu V. E., Rago C., St. Croix B., Romans K. E., Choti M. A., Lengauer C., Kinzler K. W., and Vogelstein B. (2001) A phosphatase associated with metastasis of colorectal cancer. Science 294, 1343–1346 10.1126/science.1065817 [DOI] [PubMed] [Google Scholar]
- 11. Funato Y., Yamazaki D., Mizukami S., Du L., Kikuchi K., and Miki H. (2014) Membrane protein CNNM4-dependent Mg2+ efflux suppresses tumor progression. J. Clin. Invest. 124, 5398–5410 10.1172/JCI76614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hardy S., Uetani N., Wong N., Kostantin E., Labbé D. P., Bégin L. R., Mes-Masson A., Miranda-Saavedra D., and Tremblay M. L. (2015) The protein tyrosine phosphatase PRL-2 interacts with the magnesium transporter CNNM3 to promote oncogenesis. Oncogene 34, 986–995 10.1038/onc.2014.33 [DOI] [PubMed] [Google Scholar]
- 13. Funato Y., Furutani K., Kurachi Y., and Miki H. (2018) CrossTalk proposal: CNNM proteins are Na+/Mg2+ exchangers playing a central role in transepithelial Mg2+ (re)absorption. J. Physiol. 596, 743–746 10.1113/JP275248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Arjona F. J., and de Baaij J. H. F. (2018) CrossTalk opposing view: CNNM proteins are not Na+/Mg2+ exchangers but Mg2+ transport regulators playing a central role in transepithelial Mg2+ (re)absorption. J. Physiol. 596, 747–750 10.1113/JP275249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. de Baaij J. H., Stuiver M., Meij I. C., Lainez S., Kopplin K., Venselaar H., Müller D., Bindels R. J., and Hoenderop J. G. (2012) Membrane topology and intracellular processing of cyclin M2 (CNNM2). J. Biol. Chem. 287, 13644–13655 10.1074/jbc.M112.342204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gulerez I., Funato Y., Wu H., Yang M., Kozlov G., Miki H., and Gehring K. (2016) Phosphocysteine in the PRL-CNNM pathway mediates magnesium homeostasis. EMBO Rep. 17, 1890–1900 10.15252/embr.201643393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang H., Kozlov G., Li X., Wu H., Gulerez I., and Gehring K. (2017) PRL3 phosphatase active site is required for binding the putative magnesium transporter CNNM3. Sci. Rep. 7, 48 10.1038/s41598-017-00147-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Giménez-Mascarell P., Oyenarte I., Hardy S., Breiderhoff T., Stuiver M., Kostantin E., Diercks T., Pey A. L., Ereño-Orbea J., Martínez-Chantar M. L., Khalaf-Nazzal R., Claverie-Martin F., Müller D., Tremblay M. L., and Martínez-Cruz L. A. (2017) Structural basis of the oncogenic interaction of phosphatase PRL-1 with the magnesium transporter CNNM2. J. Biol. Chem. 292, 786–801 10.1074/jbc.M116.759944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hirata Y., Funato Y., Takano Y., and Miki H. (2014) Mg2+-dependent interactions of ATP with the cystathionine-beta-synthase (CBS) domains of a magnesium transporter. J. Biol. Chem. 289, 14731–14739 10.1074/jbc.M114.551176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Corral-Rodríguez M. Á., Stuiver M., Abascal-Palacios G., Diercks T., Oyenarte I., Ereño-Orbea J., de Opakua A. I., Blanco F. J., Encinar J. A., Spiwok V., Terashima H., Accardi A., Müller D., and Martínez-Cruz L. A. (2014) Nucleotide binding triggers a conformational change of the CBS module of the magnesium transporter CNNM2 from a twisted towards a flat structure. Biochem. J. 464, 23–34 10.1042/BJ20140409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Topçu V., Alp M. Y., Alp C. K., Bakır A., Geylan D., and Yilmazoğlu M. Ö. (2017) A new familial case of Jalili syndrome caused by a novel mutation in CNNM4. Ophthalmic Genet. 38, 161–166 10.3109/13816810.2016.1164192 [DOI] [PubMed] [Google Scholar]
- 22. Clayton G. M., Silverman W. R., Heginbotham L., and Morais-Cabral J. H. (2004) Structural basis of ligand activation in a cyclic nucleotide regulated potassium channel. Cell 119, 615–627 10.1016/j.cell.2004.10.030 [DOI] [PubMed] [Google Scholar]
- 23. Holm L., and Rosenström P. (2010) Dali server: conservation mapping in 3D. Nucleic Acids Res. 38, W545–549 10.1093/nar/gkq366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pessoa J., Fonseca F., Furini S., and Morais-Cabral J. H. (2014) Determinants of ligand selectivity in a cyclic nucleotide-regulated potassium channel. J. Gen. Physiol. 144, 41–54 10.1085/jgp.201311145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lo M. C., Aulabaugh A., Jin G., Cowling R., Bard J., Malamas M., and Ellestad G. (2004) Evaluation of fluorescence-based thermal shift assays for hit identification in drug discovery. Anal. Biochem. 332, 153–159 10.1016/j.ab.2004.04.031 [DOI] [PubMed] [Google Scholar]
- 26. Wong K., Kozlov G., Zhang Y., and Gehring K. (2015) Structure of the Legionella effector, lpg1496, suggests a role in nucleotide metabolism. J. Biol. Chem. 290, 24727–24737 10.1074/jbc.M115.671263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schäffers O. J. M., Hoenderop J. G. J., Bindels R. J. M., and de Baaij J. H. F. (2018) The rise and fall of novel renal magnesium transporters. Am. J. Physiol. Renal Physiol. 314, F1027–F1033 10.1152/ajprenal.00634.2017 [DOI] [PubMed] [Google Scholar]
- 28. Payandeh J., Pfoh R., and Pai E. F. (2013) The structure and regulation of magnesium selective ion channels. Biochim. Biophys. Acta 1828, 2778–2792 10.1016/j.bbamem.2013.08.002 [DOI] [PubMed] [Google Scholar]
- 29. Zagotta W. N., Olivier N. B., Black K. D., Young E. C., Olson R., and Gouaux E. (2003) Structural basis for modulation and agonist specificity of HCN pacemaker channels. Nature 425, 200–205 10.1038/nature01922 [DOI] [PubMed] [Google Scholar]
- 30. Flynn G. E., Black K. D., Islas L. D., Sankaran B., and Zagotta W. N. (2007) Structure and rearrangements in the carboxy-terminal region of SpIH channels. Structure 15, 671–682 10.1016/j.str.2007.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kesters D., Brams M., Nys M., Wijckmans E., Spurny R., Voets T., Tytgat J., Kusch J., and Ulens C. (2015) Structure of the SthK carboxy-terminal region reveals a gating mechanism for cyclic nucleotide-modulated ion channels. PLoS One 10, e0116369 10.1371/journal.pone.0116369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. VanSchouwen B., and Melacini G. (2016) Regulation of HCN ion channels by non-canonical cyclic nucleotides. in Non-canonical Cyclic Nucleotides, pp. 123–133, Springer, New York: [DOI] [PubMed] [Google Scholar]
- 33. Brelidze T. I., Carlson A. E., Sankaran B., and Zagotta W. N. (2012) Structure of the carboxy-terminal region of a KCNH channel. Nature 481, 530–533 10.1038/nature10735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Weber I. T., and Steitz T. A. (1987) Structure of a complex of catabolite gene activator protein and cyclic AMP refined at 2.5 Å resolution. J. Mol. Biol. 198, 311–326 10.1016/0022-2836(87)90315-9 [DOI] [PubMed] [Google Scholar]
- 35. James Z. M., and Zagotta W. N. (2018) Structural insights into the mechanisms of CNBD channel function. J. Gen. Physiol. 150, 225–244 10.1085/jgp.201711898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Haitin Y., Carlson A. E., and Zagotta W. N. (2013) The structural mechanism of KCNH-channel regulation by the eag domain. Nature 501, 444–448 10.1038/nature12487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Otwinowski Z., and Minor W. (1997) [20] Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 10.1016/S0076-6879(97)76066-X [DOI] [PubMed] [Google Scholar]
- 38. Adams P. D., Afonine P. V., Bunkóczi G., Chen V. B., Davis I. W., Echols N., Headd J. J., Hung L. W., Kapral G. J., Grosse-Kunstleve R. W., McCoy A. J., Moriarty N. W., Oeffner R., Read R. J., Richardson D. C., et al. (2010) PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 10.1107/S0907444909052925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Emsley P., Lohkamp B., Scott W. G., and Cowtan K. (2010) Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 10.1107/S0907444910007493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Murshudov G. N., Skubák P., Lebedev A. A., Pannu N. S., Steiner R. A., Nicholls R. A., Winn M. D., Long F., and Vagin A. A. (2011) REFMAC5 for the refinement of macromolecular crystal structures. Acta Crystallogr. D Biol. Crystallogr. 67, 355–367 10.1107/S0907444911001314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Painter J., and Merritt E. A. (2006) Optimal description of a protein structure in terms of multiple groups undergoing TLS motion. Acta Crystallogr. D Biol. Crystallogr. 62, 439–450 10.1107/S0907444906005270 [DOI] [PubMed] [Google Scholar]
- 42. McCoy A. J., Grosse-Kunstleve R. W., Adams P. D., Winn M. D., Storoni L. C., and Read R. J. (2007) Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 10.1107/S0021889807021206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Afonine P. V., Grosse-Kunstleve R. W., Echols N., Headd J. J., Moriarty N. W., Mustyakimov M., Terwilliger T. C., Urzhumtsev A., Zwart P. H., and Adams P. D. (2012) Towards automated crystallographic structure refinement with phenix.refine. Acta Crystallogr. D Biol. Crystallogr. 68, 352–367 10.1107/S0907444912001308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Laskowski R. A., MacArthur M. W., Moss D. S., and Thornton J. M. (1993) PROCHECK: a program to check the stereochemical quality of protein structures. J. Appl. Crystallogr. 26, 283–291 10.1107/S0021889892009944 [DOI] [Google Scholar]
- 45. Delaglio F., Grzesiek S., Vuister G. W., Zhu G., Pfeifer J., and Bax A. (1995) NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR 6, 277–293 [DOI] [PubMed] [Google Scholar]
- 46. Goddard T., and Kneller D. (2008) SPARKY 3, University of California, San Francisco [Google Scholar]
- 47. Schuck P. (2000) Size-distribution analysis of macromolecules by sedimentation velocity ultracentrifugation and lamm equation modeling. Biophys. J. 78, 1606–1619 10.1016/S0006-3495(00)76713-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Laue T. (1993) Computer-aided interpretation of analtical sedimentation data for proteins. In Analytical Ultracentrifugation in Biochemistry and Polymer Science, pp. 90–125, Royal Society of Chemistry, London [Google Scholar]
- 49. Brookes E., Demeler B., and Rocco M. (2010) Developments in the US-SOMO bead modeling suite: new features in the direct residue-to-bead method, improved grid routines, and influence of accessible surface area screening. Macromol. Biosci. 10, 746–753 10.1002/mabi.200900474 [DOI] [PubMed] [Google Scholar]
- 50. Brautigam C. A. (2015) Calculations and publication-quality illustrations for analytical ultracentrifugation data. Methods Enzymol. 562, 109–133 10.1016/bs.mie.2015.05.001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.