Abstract
Glucosidase I (GI) removes the outermost glucose from protein-linked Glc3Man9GlcNAc2 (G3M9) in the endoplasmic reticulum (ER). Individuals with congenital disorders of glycosylation MOGS-CDG bear mutations in the GI-encoding gene (gls1). Although GI absence has been reported to produce lethality in Schizosaccharomyces pombe yeasts, here we obtained two viable Δgls1 mutants, one with a very sick but not lethal phenotype (Δgls1-S) and the other with a healthier one (Δgls1-H). The sick strain displayed only G3M9 as an ER protein–linked oligosaccharide, whereas the healthier strain had both G3M9 and Man9GlcNAc2. The lipid-linked oligosaccharide patterns of the two strains revealed that the most abundantly formed glycans were G3M9 in Δgls1-S and Glc2Man9GlcNAc2 in Δgls1-H, suggesting reduced Alg10p glucosyltransferase activity in the Δgls1-H strain. A mutation in the alg10+ gene was indeed observed in this strain. Our results indicated that abrogated G3M9 deglucosylation was responsible for the severe defects observed in Δgls1-S cells. Further studies disclosed that the defects could not be ascribed to disruption of glycoprotein entrance into calnexin-folding cycles, inhibition of the oligosaccharyltransferase by transfer reaction products, or reduced proteasomal degradation of misfolded glycoproteins. Lack of triglucosylated glycoprotein deglucosylation neither significantly prevented glycan elongation in the Golgi nor modified the overall cell wall monosaccharide composition. Nevertheless, it resulted in a distorted cell wall and in the absence of underlying ER membranes. Furthermore, Golgi expression of human endomannosidase partially restored normal growth in Δgls1-S cells. We propose that accumulation of G3M9-bearing glycoproteins is toxic and at least partially responsible for defects observed in MOGS-CDG.
Keywords: endoplasmic reticulum (ER), N-linked glycosylation, Schizosaccharomyces pombe, yeast, glycoprotein, CDG-IIb, congenital disorders of glycosylation, glucosidase I, glycan, MOGS-CDG
Introduction
N-Glycans have multiple functions in the secretory pathway as they influence protein folding in the ER5 and, after being remodeled in the Golgi, mediate interactions among cells and with the environment. It is not surprising then that genetic defects resulting in either their absence in normally occupied glycoprotein sites or in formation of aberrant N-glycan structures have a dramatic impact on many intra- and extracellular functions.
N-Glycosylation is one of the most frequent post-translational modifications of proteins following the secretory pathway. It starts with the initial en bloc transfer of glycan G3M96 (Fig. 1A) from a step-by-step prebuilt dolichol pyrophosphate derivative (Dol-PP) to Asn residues in the consensus sequence Asn-Xaa-Ser/Thr (Xaa≠Pro) in proteins entering the ER. The reaction is catalyzed by the oligosaccharyltransferase (OST), a multimeric membrane complex (Fig. 1B) (1–3). Addition of glycans facilitates per se protein folding as it provides bulky hydrophilic groups that help keeping folding intermediates in solution. N-Glycan processing by glycosidases and glucosyltransferases starts in the ER immediately after glycan transfer. Glycan remodeling plays a determining role in the mechanism of quality control of glycoprotein folding (4). Briefly, the glycan G3M9 transferred to Asn residues is first deglucosylated by GI, a type II membrane protein with a luminal hydrolytic domain that removes the outermost Glc of the glycan (residue n, Fig. 1A). The G2M9 thus produced is then deglucosylated by glucosidase II (GII) that successively generates G1M9 and M9 by removing the remaining glucose residues (l and m, Fig. 1A). G1M9 may be regenerated from M9 by the UDP-Glc:glycoprotein glucosyltransferase (UGGT), a protein conformation sensor that adds a Glc (residue l, Fig. 1A) exclusively to proteins that have not yet acquired their native conformation (5, 6). Monoglucosylated glycan-bearing glycoproteins produced either by de-glucosylation of the transferred glycan or by re-glucosylation by UGGT may interact with calnexin (CNX) and/or calreticulin, two ER-resident lectin chaperones that specifically recognize monoglucosylated structures. Glycoprotein–lectin interactions enhance folding efficiency and allow participation of a protein-disulfide protein isomerase (ERp57) in the folding process. In addition, they prevent Golgi exit of folding intermediates and irreparably misfolded glycoproteins (Fig. 1B). Cycles of glucosylation and deglucosylation catalyzed by the opposite activities of UGGT and GII continue until proper folding is achieved (Fig. 1B) (4). Folded glycoproteins pursue their transit through the secretory pathway where their glycans are further modified in the Golgi. Irreversibly misfolded glycoproteins are demannosylated and eventually retrotranslocated to the cytosol to be degraded in proteasomes in a process known as ER-associated degradation (ERAD).
Figure 1.
N-Glycosylation, N-glycan processing, and quality control of glycoprotein folding in the ER. A, synthesis and structure of glycan G3M9 transferred to proteins in N-glycosylation. The glycan is built in the ER membrane by the step by step addition of single monosaccharides to dolichol-P following the order of letters (a, b, c, d …) by the indicated ALG glycosyltransferases. It starts at the cytoplasmic side of the ER membrane, and once the structure Dol-PP-M5 is formed the glycan flips to the lumen of the ER where synthesis is completed. Arm A (residues d, f, g, and l–n), arm B (residues h and I), arm C (residues j and k) of the glycan, sites of GI and GII trimming in the ER after transference, and site of expressed Golgi human endomannosidase trimming are indicated. B, glycan transfer, N-glycan processing and ER quality control of glycoprotein folding. The last three steps of Dol-PP-G3M9 synthesis are shown. The OST complex transfers the glycan to the consensus sequence NX(S/T)(X≠P) of proteins entering into the ER through the Sec61 translocon. GI removes Glc n and GII removes Glc m. Monoglucosylated glycans interact with CNX, which facilitates glycoprotein folding. GII removes Glc l and folded glycoproteins exit the ER. Unfolded proteins are recognized by UGGT, which adds back Glc l. Glycoproteins that fail to properly fold after several reglucosylation–deglucosylation cycles are demannosylated by ER α-mannosidases (αMAN), recognized then by lectin OS9, and retrotranslocated to the cytosol for degradation in proteasomes (ERAD). Monosaccharide symbols follow the Symbol Nomenclature for Glycans (SNFG) system (49).
The congenital disorders of glycosylation (CDG) are a group of more than 100 human inherited syndromes, most of them (70%) produced by deficiencies in protein N-glycosylation pathways (7). The clinical features of these disorders involve many organ systems but are especially common in the nervous, hepatic, visual, and immune systems. Patients display pathologies that include delayed growth, developmental disabilities, low muscle tone, visual problems, and incomplete brain development. Type I CDG are caused by defects in the biosynthesis of lipid-linked oligosaccharides (LLO) and their transfer to proteins, whereas type II CDG are caused by defects in the glycan processing of protein-linked oligosaccharides (NLO). The first type results in glycoprotein hypoglycosylation (normally occupied glycosylation sites are empty), but the remaining glycans have almost normal structures, and in the second type there is a normal occupation of glycosylation sites, but the attached glycans have abnormal structures (7). Regarding the type II diseases, MOGS-CDG (also known as CDG-IIb) is produced by mutations in GI, the enzyme responsible for the first step in N-glycan processing (Fig. 1). The clinical course of the disease is progressive and characterized by multiple neurological complications, severe hypogammaglobulinemia, hepatomegaly, hypoventilation, feeding problems, seizures, and sometimes a fatal outcome (8, 9).
Contrary to the more commonly used yeast Saccharomyces cerevisiae, which lacks UGGT, the fission yeast Schizosaccharomyces pombe has conserved all components of the early N-glycosylation steps and the quality control of glycoprotein folding mechanism present in mammalian cells, and it has also been extensively used as a model organism to study those processes (4, 10). In an attempt to understand the role of GI in the molecular bases of MOGS-CDG, we obtained two haploid strains of the fission yeast S. pombe lacking the gene coding for GI (Δgls1). Surprisingly, one of the strains showed a severe growth defect, and the other displayed an almost normal growth rate. We then analyzed NLO and LLO patterns produced by both strains. Our results suggest that a suppression of the mutant phenotype is caused by a simultaneous mutation in the alg10+ gene in the strain that displayed a relieved growth rate. As the protein encoded by that gene is responsible for the addition of the outermost glucose to the Dol-PP derivative, we concluded that accumulation of three Glc moieties in the newly synthesized glycoproteins is the main cause of the growth defect in GI mutants. We propose that at least part of the defects observed in MOGS-CDG patients are caused by the accumulation of triglucosylated glycoproteins.
Results
Knockout of GI-encoding gene produces severe morphological and growth defects in the fission yeast S. pombe
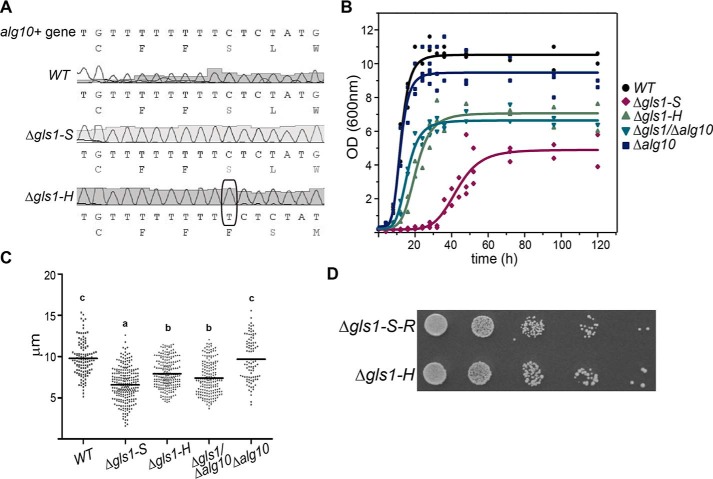
According to an analysis of a genome-wide set of gene deletions in the fission yeast S. pombe, deletion of the GI-encoding gene (Δgls1 mutants) is apparently lethal (11). This is in contrast to what was reported for S. cerevisiae, in which mutants lacking the GI-encoding gene are viable (12) but in agreement with the report of patients in which mutations in GI-encoding gene result in MOGS-CDG with severe and sometimes lethal pathologies (8, 13). In an attempt to understand the role of GI in MOGS-CDG, we induced meiosis and sporulation in a heterozygous Δgls1/+ diploid S. pombe strain. We were able to obtain two viable Δgls1 haploid strains that arose from spores of the same tetrad (Fig. 2A). One of the strains (Δgls1-S) showed a very sick but not lethal growth defect. Surprisingly, the other strain (Δgls1-H), although genetically also a Δgls1 knockout mutant, displayed a much healthier phenotype (Fig. 2B). We compared the growth-rate parameters in rich liquid medium of both Δgls1 mutants with that of WT and recorded that although the sick Δgls1-S strain had all the growing parameters altered, i.e. an 8 times longer lag phase (32 h versus 4 h, p = 0.005), a 4-fold increase in the duplication time at the middle of the exponential phase (p = 0.027), and half the maximal A600 nm at the stationary phase (p = 0.045), the same growth parameters of the healthier Δgls1-H strain were significantly improved, with a shorter lag time (8 h), a duplication time twice as fast than that of Δgls1-S strain (p = 0.045), and only a moderate reduction in the cell density at the stationary phase (Table S1).
Figure 2.

Lack of GI-encoding gene (gls1+) produces severe growth and morphological defects in S. pombe. A, genomic characterization of S. pombe Δgls1-S and Δgls1-H strains. PCRs were performed using primers that hybridize inside and outside the knockout insertion cassette (NatMX fw and gls1–3′NC, respectively) and the indicated boiled yeast sister colonies as DNA template. B, growth of S. pombe WT, Δgls1-S, and Δgls1-H in solid media. WT and mutant cells were grown up to A600 nm = 1, and drops (5 μl) of 10-fold dilutions were plated on YES. Plates were incubated 72 h at 28 °C. C, reversion of the sick phenotype of Δgls1-S cells by complementation with gls1+ gene. Competent Δgls1-S cells were transformed either with vector alone or with vector carrying gls1+ gene. Leu+ arising transformants were streaked in MM supplemented with adenine and uracil and grown for 72 h. D, morphology of Δgls1-S and Δgls1-H mutants. Yeast cultures at A600 nm = 1 were observed under a transmission microscope with a ×100 objective. Bar, 10 μm.
Complementation of the sick Δgls1-S mutant with gene gls1+ restored a normal growth phenotype, indicating that the absence of such gene was responsible for the observed defect in the mutant (Fig. 2C). The Δgls1-S mutants cells observed under the microscope showed an aberrant morphology, with a reduced size and a rounded shape that tended to clump when compared with both WT and the healthier Δgls1-H cells, suggesting that some kind of suppression had occurred in the last strain (Fig. 2D) (14).
NLO and LLO produced by Δgls1 mutants
To understand the phenotypic differences between both Δgls1 sick and healthier mutants, we first inspected the differences in the NLOs produced in vivo in the ER by WT, Δgls1-S, and Δgls1-H strains. Cells were labeled for 15 min with [14C]Glc in the absence of any glucosidase inhibitor and in the presence of 5 mm DTT to preclude glycoprotein ER exit. WT cells rapidly trimmed protein-linked glycans from the transferred G3M9 structure to M9 by the sequential action of GI and GII (Fig. 3A and see Fig. 1). The severely affected Δgls1-S cells showed the N-glycan pattern expected for a Δgls1 mutant, as these mutant cells are unable to hydrolyze the transferred G3M9 glycans and thus accumulate such structures (Fig. 3B). However, the healthier Δgls1-H cell glycan pattern showed a further N-glycan processing to M9 (Fig. 3C), which was delayed when GI and GII were partially inhibited upon addition of castanospermine (CST) and N-methyl-deoxynojirimycin (NM-DNJ) (Fig. 3D).
Figure 3.
NLOs were synthesized in vivo by S. pombe mutants WT (A), Δgls1-S (B), and Δgls1-H (C). S. pombe exponentially growing cultures were washed and labeled with 5 mm [14C]Glc for 15 min in the presence of 5 mm DTT and 3.5 mm kifunensine (an ER α-mannosidase inhibitor). D, Δgls1-H cells were additionally labeled in the presence of the glucosidase inhibitors CST and NM-DNJ. Endo H–sensitive N-glycans were purified, run on paper chromatography in 1-propanol/nitromethane/water (5:2:4), eluted, and further resolved by HPLC. Positions of standards are indicated by arrows.
Δgls1-H strain showed a significant amount of glucose-free NLOs containing nine Man units similar to those produced by WT cells. This result cannot be explained by either a partial activity of GI, as the knockout strain has a large coding-sequence deleted, or by the presence of another GI-like activity in the ER (15). We hypothesized that the unexpected N-glycan pattern observed in Δgls1-H strain could be explained by an incomplete LLO synthesis. Thus, we analyzed the LLO pattern synthesized in vivo by WT and Δgls1-H mutant cells. As expected, almost all WT LLOs had a G3M9 structure (Fig. 4A). However, in Δgls1-H mutant cells G2M9 was the main LLO present (Fig. 4B). The pattern resembled that produced by mutants in the gene coding the glucosyltransferase that adds the outermost Glc to the glycan in Dol-PP–G3M9 biosynthesis (Δalg10 mutants, Fig. 1) (16). Thus, the NLO pattern produced by Δgls1-H mutants in which a large amount of M9 glycans was produced is most probably due to a partial defect in the biosynthesis of the LLO that is processed to G1M9 and M9 by GII upon transfer to proteins. The remaining G3M9 in glycoproteins of Δgls1-H mutants could be explained by the small amount of G3M9 LLO in this strain that is transferred to proteins but could not be trimmed as it lacks a functional GI. As expected, Δgls1-S mutants only displayed G3M9 LLO structures, the same as WT cells (Fig. 4C).
Figure 4.
Dol-PP-linked oligosaccharide (LLO) in vivo synthesized by S. pombe mutants. WT (A), Δgls1-H (B), and Δgls1-H (C) exponentially growing mutant cells were labeled with [14C]Glc for 15 min in the presence of cycloheximide and puromycin. Glycans obtained by mild acid hydrolysis were analyzed by paper chromatography in 1-propanol/nitromethane/water (5:2:4), eluted and further solved by HPLC. Positions of standards are indicated by arrows.
Mutation in alg10+ gene significantly improves the growth and morphological defects of S. pombe GI mutants
As the LLO pattern produced by Δgls1-H mutants resembled that present in Δalg10 mutants (16), we compared the sequence of the alg10+ gene in Δgls1-S and Δgls1-H strains. We obtained the whole alg10 gene (including introns and 5′ and 3′ UTR regions) by PCR from genomic DNA extracted from WT and both Δgls1 mutants using a high-fidelity polymerase. Upon DNA sequencing, we found an insertion of a thymidine at position 477 from the start codon in the alg10 gene obtained from Δgls1-H DNA. The mutation was absent in alg10+ sequences obtained from WT and Δgls1-S DNAs, which were identical to the annotated sequence in the PomBase database (Fig. 5A) (17, 18). The insertion in mutant Δgls1-H produces a sequence frameshift that results in a premature stop codon. A 183-amino acid protein was then probably synthesized instead of the 445-amino acid normal one. This confirms that in the mutant with the healthier phenotype an additional mutation in gene alg10+ had arisen spontaneously. As in mutant Δgls1-H, a very small amount of G3M9 LLO is still formed (Fig. 4B), and the mutation probably does not completely abolish the alg10+ gene product activity.
Figure 5.
Mutation in alg10+ gene suppresses the growth defects of Δgls1-S mutants. A, sequence profile of alg10 gene in Δgls1-S and Δgls1-H mutants. The complete alg10 gene was obtained by PCR from genomic DNA purified from WT and both Δgls1 mutants. The products were sequenced and compared with the annotated sequence in PomBase. B, growth profile of S. pombe mutant strains in liquid-rich YES media. Triplicate cultures of each plotted mutant were started at A600 nm = 0.2 and grown at 28 °C for 5 days. Aliquots were taken every 4 h and plotted. Data were analyzed using R software. C, morphology of WT, Δalg10, and Δgls1 mutants. Cells were grown in liquid media to an A600 nm = 1 and photographed with a bright field microscope. Cell lengths (at least 180 for each culture) were measured using ImageJ software. Statistical analyses were done using one-way ANOVA and Bonferroni's multiple comparison tests. Different letters (a–c) indicate statistically significant (p < 0.0001) differences. D, reversion of the growth defect in Δgls1-S mutants after several passages. Δgls1-S and Δgls1-H mutants were grown in liquid YES media until saturation, diluted 1:100 in fresh media, and grown again several times. Finally 5 μl of 10-fold serial dilutions of the cultures were plated in solid-rich media and grown for 72 h at 28 °C. Δgls1-S-R states for the reverted Δgls1-S strain after several passages.
To confirm that a deletion of alg10+ gene can indeed improve most of the growth defects of Δgls1-S mutants, we constructed a S. pombe Δgls1/Δalg10 double mutant strain by a genetic cross using as parental strains Δgls1-S′ (Δgls1-S strain in which the mating type had been converted to h− to allow mating) (Table 1) and a strain in which the gene alg10+ had been deleted (Δalg10). We verified the genotype of the double mutant by antibiotic resistance and by PCR (Fig. S1). We then compared the growth rate in rich medium of the double mutant with that of WT and single Δalg10, Δgls1-S, and Δgls1-H mutants. The Δgls1/Δalg10 double mutant showed a growth curve very similar to that of the single mutant Δgls1-H, with all its growing parameters significantly improved compared with those of Δgls1-S strain: a shorter lag time, a duplication time at the mid-exponential phase more than twice faster than that of the Δgls1-S strain, and a maximum OD at 600 nm that was about 65% that of the WT strain (Fig. 5B, Fig. S2, and Table S1). However, the WT or Δalg10 mutant strain growth parameters were not completely restored.
Table 1.
S. pombe strains used in this work
| Strain (nickname) | Genotype | Origin |
|---|---|---|
| ADm (WT) | h−, ade6-M210, leu1-32, ura4-D18 | Our stock (31) |
| Δgls1::KanMX/+ diploid | h+/h+, ade6-M210/ade6-M216, leu1-32/leu1-32, ura4-D18/ura4-D18, Δgls1::KanMX/+ | Bioneer |
| Δgls1::NatMX/+ diploid | h+/h+, ade6-M210/ade6-M216, leu1-32/leu1-32, ura4-D18/ura4-D18, Δgls1::NatMX/+ | This work |
| ΔGI-10A (Δgls1-S) | h+, leu1-32, ade6-M216, ura4-D18, Δgls1::NatMX4 | This work |
| ΔGI-10D (Δgls1-H) | h+, leu1-32, ade6-M216, ura4-D18, Δgls1::NatMX4 | This work |
| ΔGI-210m1A (Δgls1-S′) | h−, leu1-32, ade6-M210, ura4-D18, Δgls1::NatMX4 | This work |
| SPAC56F8.06c (Δalg10) | h+, leu1-32, ade6-M216, ura4-D18, Δalg10::KanMX4 | Bioneer |
| ΔGIA10–18 (Δgls1/Δalg10) | h+, leu1-32, ade6-M210, ura4-D18, Δgls1::NatMX4, Δalg10::KanMX4 | This work |
| Sp61IIα (Δgls2α) | h−, leu1-32, ade6-M210, ura4-D18, ade1, Δgls2α::ura4+ | Our stock (43) |
| SpAC227.11c (Δyos9) | h+, leu1-32, ade6-M210, ura4-D18, Δyos9::KanMX6 | Bioneer |
| Sp61A (Δalg6) | h−, leu1-32, ade1, ade6-M210, ura4-D18, Δalg6::ura4+ | Our stock (45) |
The aberrant morphology observed in Δgls1-S yeast mutants was also restored to a much normal one when an additional mutation in its alg10+ gene was introduced (Fig. S3). Although Δgls1-S strain cultures had mostly small-rounded cells, the Δgls1-H and Δgls1/Δalg10 mutants had rod-shaped cells similar to WT and Δalg10 cells, although still a bit shorter. To quantify the improvement, we calculated the average cell length of the Δgls1/Δalg10 double mutant in liquid culture and compared it with those of WT, Δalg10, and Δgls1 strains. Length measurements of Δgls1/Δalg10, WT, Δalg10, and Δgls1 cells indicated that the double mutant significantly increased its length with respect to the Δgls1-S ones and that there were no statistically significant differences between Δgls1-H and Δgls1/Δalg10 cells (Fig. 5C). Taken together, these results indicate that the Δgls1 defects were suppressed or at least significantly improved by a simultaneous mutation in the alg10+ gene.
As Δgls1-H arose spontaneously as a suppressor mutant during meiosis and spore formation, we wondered whether suppression was also a survival strategy during vegetative growth. We grew Δgls1-S mutants up to stationary phase and initiated a new culture at least five times. Comparison of the growth rate of the resulting culture with that of Δgls1-H mutants suggested that defects in the “true” Δgls1 mutants are so severe that cells tend to revert by suppression after several passages, as reverted Δgls1-S (Δgls1-S-R) and Δgls1-H cells showed the same growth rates (Fig. 5D).
Lack of G3M9 NLO deglucosylation and not the absence of GI is the main cause of the sick phenotype of Δgls1 cells
Results reported above indicate that mutation of GI-encoding gene produces a very sick phenotype, that the mutant is unable to process G3M9 NLO, and that its growth defects can be at least partially suppressed by a simultaneous mutation in the alg10+ gene. A mutation in the alg10+ gene is expected to result in the accumulation of G2M9 LLO in the ER membrane, which eventually may be transferred to proteins by OST, although maybe less efficiently than G3M9. Therefore, we determined the LLO and the NLO patterns produced in vivo by Δgls1/Δalg10 S. pombe double mutants (Fig. 6). The LLO pattern showed that the main LLO produced in vivo was G2M9, plus a small amount of G1M9. This LLO pattern was similar to that produced by the Δgls1-H strain except for the total absence of G3M9 in the double mutant. This result was consistent with a complete absence of alg10p activity (compare Fig. 6A with 4C). Accordingly, no G3M9 NLO was observed in Δgls1/Δalg10 mutants, even though these cells lack GI (Fig. 6B). Moreover, almost all NLOs were M9, suggesting that GII had trimmed efficiently the middle and innermost glucoses of the transferred G2M9 glycans. The largest NLO detected was G2M9 upon addition of glucosidase inhibitors (CST and NM-DNJ), thus indicating that such a structure was the one transferred in Δgls1/Δalg10 mutants (Fig. 6C).
Figure 6.
NLOs and LLOs produced by S. pombe Δgls1/Δalg10 double mutant. Cells were labeled in vivo with [14C]Glc for 15 min. NLOs and LLOs were purified and analyzed by paper chromatography in 1-propanol/nitromethane/water (5:2:4), eluted, and further resolved by HPLC. Positions of standards are indicated by arrows.
Our results confirm that cells with a mutation in alg10+ genes transfer glycans with the structure G2M9 to proteins and that such defect can suppress the sick phenotype observed in mutants lacking GI. Results also indicate that the presence of triglucosylated glycans in proteins is probably the cause of the severe growth defects observed in the “true” Δgls1-S mutant cells. The fact that the phenotype of the Δgls1/Δalg10 double mutant is so similar to that of Δgls1-H strain would suggest that the amount of G3M9 produced by the last strain is too small to produce the toxicity observed in Δgls1-S strain. Our results do not rule out, however, the possibility that GI plays an additional role in the ER, as bypassing its necessity for further glycan processing by simultaneously mutating the alg10 gene did not fully restore the WT phenotype.
Sick phenotype of Δgls1-S mutants is not due to the impossibility of their glycoproteins to interact with CNX or enter quality control cycles in the ER
Cellular effects produced in Δgls1-S mutants could be due to the lack of interaction of their triglucosylated glycoproteins with CNX. To test whether the absence of lectin–glycoprotein interactions could be the cause of the sick phenotype observed in Δgls1-S mutants, we compared the growth rates of WT, Δgls1-S, and Δgls2α mutant cells. As the last ones lack the GII catalytic subunit, they are unable to deglucosylate G2M9 glycans and, the same as in Δgls1-S cells, their glycoproteins cannot interact with CNX. However, Δgls2α S. pombe mutants grew normally when compared with WT cells and much better than Δgls1-S mutants (Fig. 7). These results indicate that the absence of CNX–glycoprotein interaction does not affect growth rate and therefore cannot be the cause of the sick phenotype observed in Δgls1-S mutants.
Figure 7.

Comparison of the growth phenotype of Δgls1-S cells with those of S. pombe mutants whose glycoproteins are unable to interact with CNX or display a defective ERAD pathway. WT and the indicated mutant cells were grown up to A600 nm = 1, and drops (5 μl) of 10-fold dilutions were plated on YES. Plates were incubated for 72 h at 28 °C.
S. pombe cells that lack key components of the ERAD machinery grow normally
OS9 (Yos9p in yeasts), a key component of the ERAD machinery, is a lectin that upon recognition of ER-demannosylated glycans drives irreparably misfolded glycoproteins to proteasomal degradation (Fig. 1B). Mutants lacking Yos9p substantially delay misfolded glycoprotein degradation (19, 20). We hypothesized that if irreversibly misfolded glycoproteins bearing three Glc moieties in Δgls1-S cells were not demannosylated in the ER or were not recognized by Yos9p, then S. pombe Δyos9 mutants would present growth defects similar to those in Δgls1-S cells. Comparison of yeast WT, Δyos9, and Δgls1-S mutant cell growth rates indicated that a limitation in the degradation of misfolded glycoproteins did not result in noticeable growth defects (Fig. 7).
OST transfer activity is not significantly inhibited in cells unable to deglucosylate G3M9 NLOs
Our results point to the accumulation of triglucosylated NLOs to be responsible for the severe defects observed in Δgls1-S yeast cells. Such accumulation could inhibit the OST complex, as those glycans are the product of its transfer reaction, thus reducing the glycan-transfer rate. Therefore, this would result in a protein hypoglycosylation similar to that observed when the N-glycosylation inhibitor tunicamycin is added to cells (21). To test this hypothesis, we analyzed whether protein hypoglycosylation occurred in S. pombe Δgls1 mutants. We tested the glycosylation status of CNX, a resident ER glycoprotein with a single glycosylation site, that at nearly stationary phase normally presents soluble and membrane-bound isoforms (22). Upon treatment of cell extracts with Endo H, membrane CNX isoform of all strains tested (WT, Δgls1-S, Δgls1-H, Δgls1/Δalg10, and Δalg10) showed an ∼2-kDa faster migrating band (Fig. 8A). Only Δalg10, Δgls1-H, and Δgls1/Δalg10 mutants showed a hypoglycosylation pattern, with a double band corresponding to glycosylated and nonglycosylated CNX in the absence of Endo H treatment. No double band was observed in the Δgls1-S mutants in the absence of Endo H, thus indicating that CNX was originally fully glycosylated in Δgls1-S mutants and that the OST ability to transfer pre-assembled glycans to proteins was not affected in that strain (Fig. 8A).
Figure 8.
OST protein glycosylation activity is not affected in Δgls1-S mutants. A, glycosylation status of CNX in S. pombe Δgls1 mutants. Cell extracts were prepared from S. pombe cultures grown up to A600 nm = 1, treated or not with Endo H, solved by SDS-PAGE 12%, and analyzed by Western blotting using rabbit anti CNX serum 1:10,000 as primary antibody and anti-rabbit HRP 1:30,000 as secondary antibody. B, expression of L. major STT3D in S. pombe mutants. WT or Δgls1-S mutants were transformed with HA-epitope–tagged L. major STT3D. Total-cell extracts and ER-enriched fractions were obtained, and 50 μg of each submitted to a 12% SDS-PAGE followed by immunoblot using rat anti-HA 1:5000 (Roche Applied Science) followed by anti-rat conjugated to HRP 1:5000 (Sigma). C, reversion of CNX hypoglycosylation in Δalg6 mutants expressing L. major STT3D. Cell extracts were prepared from the indicated S. pombe strains and analyzed as in A. D, L. major STT3D overexpression does not suppress Δgls1-S growth defect. WT or Δgls1-S mutants transformed with L. major STT3 were grown in EMM medium supplemented with adenine and uracil up to A600 nm = 1. Drops (5 μl) of 10-fold dilutions were plated on solid MMAU an incubated for 72 h at 28 °C.
The possibility remained, however, that other glycoproteins different from CNX had affected their glycosylation degree by OST in the Δgls1-S mutant. We reasoned that if this were the case, OST overexpression in that mutant would relieve the hypoglycosylation that may occur in any ER glycoprotein. Mammalian and yeast OST are multisubunit complexes whose catalytic subunit is STT3 (23). We expressed Leishmania major STT3D, a single-subunit OST of broad-range specificity (24). This OST would not be inhibited by the accumulation of triglucosylated proteins as its natural substrate is M7, the main glycan transferred in those parasites (25). The protein was expressed correctly in the ER, as its expression was enriched in microsomes when compared with total-cell extracts (Fig. 8B). Moreover, L. major STT3D was able to correct CNX hypoglycosylation observed in an Δalg6 mutant (Alg6p is the glycosyltransferase responsible of the addition of the first Glc in G3M9 LLO synthesis, and its absence results in M9 transfer to proteins and in a severe protein hypoglycosylation) (Fig. 8C) (26). However, no changes in the growth defects of Δgls1-S were observed upon L. major STT3D expression in this strain (Fig. 8D). This suggests that an inhibition of the N-glycosylation reaction is not the cause of the phenotypic defects observed in the Δgls1-S mutants.
Persistence of three Glc units in the transferred glycan neither precludes glycoprotein extension in the Golgi nor affects S. pombe cell-wall monosaccharide composition
We wondered whether the continued presence of Glc residues in the arm A of the glycan could preclude glycan extension with Man and Gal residues in the Golgi, thus reducing the size of protein-linked galactomannans at the cell surface or somehow affecting cell-wall synthesis. We compared the size of NLOs produced by mutant Δgls1-S that conserved triglucosylated glycoproteins with that of Δgls2α mutant, which displayed instead diglucosylated glycoproteins. We labeled cells with [14C]Glc for 45 min in the absence of DTT, chased them with Glc for 150 min, and isolated NLOs from total-cell glycoproteins. As expected, after 45 min labeling NLOs obtained from Δgls2α mutants were mainly G2M9 and those obtained from Δgls1-S were G3M9, consistent with the absence of GII and GI activities in the ER, respectively. After 150 min chase, both mutants were able to extend their NLOs in the secretory pathway, and almost no differences were observed between GI- and GII-lacking mutant patterns (Fig. 9). Moreover, the cell wall polysaccharide composition of both mutants was indistinguishable from each other (Fig. S4) and from that of WT cells (27) as they produced mainly Glc, but also Man and Gal. As growth rate differences between both strains are evident (Fig. 7), these results indicate that the impossibility of trimming Glc units in the ER did not preclude NLO elongation and that it is not the main cause of the growth impairment of Δgls1-S cells.
Figure 9.
Glycan extension of glycoproteins in S. pombe mutants lacking either GI or GII. Cultures of S. pombe mutants Δgls2α (A and C) or Δgls1-S (B and D) were labeled for 45 min with 5 mm Glc in the presence of 300 μCi/ml [14C]Glc (C and D) and chased for 150 min with 100 mm Glc. NLOs were purified and analyzed by paper chromatography in 1-propanol/nitromethane/water (5:2:4), eluted, and further solved by HPLC. Positions of standards are indicated by arrows.
Cells lacking GI exhibit a thick cell wall and lack a visible underlying ER
It has been reported that S. cerevisiae mutants in the OST catalytic subunit STT3 exhibit a diffused cell wall with loss of the outer mannoprotein layer (28). Transmission electron microscopy (TEM) of WT, Δgls1-S, and Δgls2α mutants showed that the cell wall of Δgls1-S is thicker than the others, has a feathered appearance, and lacks the characteristic three-layered structure typical of WT cells (Fig. 10, A–C) (29). Quantification of the electron-density profiles (Fig. 10, D–F) was shown to be relatively homogeneous in the Δgls1-S mutant. This result correlates with the diffuse appearance of its cell wall. Interestingly, this profile appears to be specific for cells lacking GI and bearing triglucosylated glycoproteins, as cells lacking GII and thus bearing diglucosylated glycoproteins display a cell wall identical to that of WT cells. Moreover, TEM comparison of ER structures localized below the plasma membrane of WT, Δgls1-S, and Δgls2α mutants showed a defined ER structure in the WT and Δgls2α strains that was absent in Δgls1-S mutants, thus suggesting that the ER structure may be altered or distorted in the last strain or that the contact sites between the ER and the plasma membrane are lost (Fig. 10, A–C).
Figure 10.
Δgls1-S mutants exhibit changes in the cell wall and in the underlying ER structures. A–C, transmission electron microscopy images of S. pombe WT (A), Δgls1-S (B), and Δgls2α (C) mutants. Cells were grown to A600 nm = 1 (± 0.2) and fixed with 2.5% glutaraldehyde in 0.1 m PBS supplemented with ruthenium red. The cell wall (CW) is indicated with a bracket and its underlying ER with an arrowhead. The arrows in A–C correspond to sections used to measure TEM signal intensities (bar, 0.1 μm). D–F, representative intensity profiles of a WT (D), Δgls1-S (E), and Δgls2α (F) S. pombe cell wall TEM images. Nine cells for each genotype were analyzed, and three cross-section intensity profiles per cell were plotted, showing an identical behavior. D–F, displayed are the characteristic profiles obtained from sections indicated by the arrows in A–C, respectively. A.U., arbitrary units.
Continued presence of triglucosylated glycoproteins results in cell death
To understand the severe growth defect in cells unable to remove the glucoses from NLOs, we wondered whether there was a loss of viability at a particular phase in the fission yeast growth curve. Dead cells or cells with a reduced metabolism cannot efficiently exclude the fluorescent dye phloxine B (Fig. S5A). We thus analyzed the ability of individual S. pombe cells of WT, Δgls1-S, Δgls1-H, Δgls1/Δalg10, and Δalg10 cultures to exclude phloxine B by flow cytometry (Fig. S5B), and we calculated the proportion of cells excluding it at all phases of the growth curve (Fig. 11A). The proportion of live and metabolically active cells in Δgls1-S cultures was markedly reduced at all growth phases when compared with that in WT ones (p < 0.001). Δgls1-S cell viability reached its maximum at the exponential phase, but the value did not exceed 30% that of WT cells. The proportion of live cells in Δgls1-S cultures dramatically dropped in the stationary phase. On the contrary, Δgls1-H and Δgls1/Δalg10 mutants showed a proportion of metabolically active cells that was significantly higher than the Δgls1-S ones (p < 0.05) at all stages of the growth curve. They were 45–60% (Δgls1-H) and 30–50% (Δgls1/Δalg10) that of WT cell values (Fig. 11A). Results obtained using the exclusion of a vital dye as an indication of vital activity were compared with the ability to generate new colonies (% CFU) of each strain's cells (Fig. 11B). The CFU percentage values were reduced at all stages in the Δgls1-S strain. The decrease was especially severe at the stationary phase. Conversely, the Δgls1-H and Δgls1/Δalg10 mutant CFU percentage values were quite similar to those of WT cells, and this fact was consistent with the observed suppressed phenotype of the mutants.
Figure 11.

Viability of S. pombe mutants at different phases of the growth curve. A, percentage of cells able to exclude the vital dye phloxine B. The indicated cell strains were grown in rich media, and aliquots were taken (triplicates for each growth phase) and incubated with the dye phloxine B. Fluorescence of 10,000 cells of each sample was analyzed by flow cytometry. Each bar represents the percentage of fluorescent cells. Mean averages that are significantly different from each other (p < 0.0001) are indicated with different letters and those that are not (p > 0.05) are indicated with the same letter. B, percentage of S. pombe mutant cells able to form a colony at the different growth phases. Cells were grown in YES, and aliquots (triplicates) were taken at the different growth phases, diluted, and plated in YEA. After at least 5 days at 28 °C, colonies were counted. Each bar represents the percentage of arisen colonies considering 100% the calculated theoretical number of cells plated (A600 nm = 1 was considered to contain 2.2 × 107 cells/ml). Asterisks indicate a significant difference (p < 0.0001) of Δgls1-S with respect to all other strains.
Expression of human endomannosidase relieves the Δgls1-S mutant sick phenotype
Mammalian cells display an endomannosidase in the Golgi whose expression levels are enhanced in MOGS-CDG patients (13). The enzyme cleaves the bond between Man f and Man g (Fig. 1A) provided either one, two, or three Glc units are present in the glycan (30). In addition to protein-linked M8, it yields Glc1–3Man depending on the substrate hydrolyzed. Although about 80% of glycans in MOGS-CDG patients are then normally processed in the Golgi, they still present a very sick pathology. As S. pombe lacks any endomannosidase activity, we expressed human endomannosidase (hEM) as a GFP fusion (schematized in Fig. 12A) in S. pombe Δgls1-S and Δgls2α mutants. The GFP-hEM clone was active in S. pombe as microsomes obtained from the transformed Δgls2α strain were able to release a disaccharide from glucose-labeled G1M9 (Fig. 12B). The rationale for the use of cells lacking GII to test for endomannosidase activity was to prevent removal of the single Glc from such substrate, in addition to the disaccharide Glc-Man, a fact that would obscure results (Fig. 1A). The active hEM expressed in both Δgls1-S and Δgls2α mutants presented a typical Golgi pattern localization for fission yeasts when observed by confocal microscopy (Fig. 12C). Those GFP-fluorescent membrane vesicle-like structures distributed within the cell did not correspond to endocytic or degradation vesicles as they did not co-localize with the endocytic pathway-specific dye FM464 (Fig. 12C). We then compared the growth rate of Δgls1-S mutants expressing hEM with those of cells transformed with WT gls1+ or with an empty vector. Results obtained showed that expression of hEM in the Golgi of Δgls1-S mutants significantly relieved the sick phenotype but not as well as expression of WT GI (Fig. 12D).
Figure 12.
Expressed Golgi human endomannosidase is functional in S. pombe and partially rescues the growth defect of Δgls1-S mutants. A, schematic representation of the hEM fused to green fluorescent protein (GFP-hEM). TMD, transmembrane domain. B, endomannosidase activity in microsomal fractions of Δgls2α S. pombe mutants. Release of [14C]Glc-Man from 2000 cpm of [14C-Glc]G1M9 was measured using 100 μg of microsomal fractions obtained from Δgls2α S. pombe mutants expressing or not GFP-hEM for 30 min at 37 °C. The reaction was stopped with methanol, and released Glc-Man disaccharides were separated from the substrate on paper chromatography using 2-propanol/acetic acid/H2O (25:4:9). C, GFP-hEM shows a typical Golgi distribution, and it is excluded from endocytic and vacuolar compartments. Confocal images of Δgls1-S and Δgls2α expressing GFP-hEM that were stained with FM4-64 dye were obtained by confocal microscopy using 488 nm argon and 543 nm He-Ne lasers, respectively. Scale bar, 5 μm. D, relief of the growth defect of Δgls1-S mutants expressing GFP-hEM. Δgls1-S strains transformed either with empty vector, GFP-hEM, or gls1+ were grown up to A600 nm = 1, and drops (5 μl) of 10-fold dilutions were plated on minimal medium lacking leucine. Growth of different strains was compared after a 6-day incubation at 28 °C.
Discussion
In an attempt to understand the molecular basis of the pathology observed in individuals with mutations in the GI-encoding gene and thus bearing MOGS-CDG (also known as CDG-IIb), we reproduced in the fission yeast S. pombe the genetic defect of the disease. We obtained two mutants: Δgls1-S mutant had a severe growth defect and an aberrant morphology, and Δgls1-H mutant had an almost normal phenotype (Fig. 2). We characterized the NLOs of both strains, and surprisingly, in the healthier Δgls1-H cells most of the NLOs were fully trimmed M9 species (Fig. 3). We speculated that these NLOs could have been formed upon GII deglucosylation of an incomplete glycan transferred to proteins. Analysis of LLOs present in the Δgls1-H strain showed that G2M9 was the main species (Fig. 4B). This indicated that the LLO had not reached the complete G3M9 structure, so we speculated that a suppressing mutation had arisen in the enzyme Alg10p responsible for the addition of the outermost glucose to the LLO (Fig. 1). The sequence of alg10 gene in Δgls1-H mutants showed indeed an insertion that produced a frameshift in the coding sequence (Fig. 5A). The growth and morphological defects observed in Δgls1-S mutants were relieved in the double knockout strain Δgls1/Δalg10 (Fig. 5, B and C, and Table S1). Taken together, our results indicate that the slow growth and aberrant morphology observed in the nonsuppressed Δgls1-S mutants were due to lack of ER G3M9 NLO deglucosylation. This metabolic defect is, apparently, extremely deleterious for cells as shown by the difficulty of maintaining an unsuppressed Δgls1-S yeast strain: after several rounds of culture replication, the strain tended to suppress the growth defect and started growing as the healthier Δgls1-H (Fig. 5D).
Cellular effects of mutations in GI could be in the ER and/or Golgi and/or cell surface. Our results indicate that the phenotypic defects in Δgls1-S mutants are not likely due to a hindered entrance of glycoproteins into the ER quality control of a glycoprotein-folding mechanism as yeast mutants lacking GII activity (Δgls2α mutants) presented a normal growth rate, although they accumulated protein-linked G2M9 structures also unable to enter into such cycles (Fig. 7) (31). A hypothetical impairment of misfolded triglucosylated glycoproteins to be recognized by the ERAD machinery does not seem to be the problem in Δgls1-S mutants either, as lack of the OS9 lectin (Δyos9 mutants), which is a central player in the ERAD process as it recognizes demannosylated misfolded glycoproteins and drives them to proteasomal degradation, did not result in relevant growth defects. It may be speculated that increased amounts of triglucosylated glycoproteins in the ER would result in a reaction product inhibition of the OST, thus leading to a severe glycoprotein hypoglycosylation. This appeared not to be the case either as CNX, an ER resident glycoprotein with a single glycosylation site, did not show detectable amounts of hypoglycosylation in Δgls1-S mutant cells (Fig. 8A). The possibility existed, however, that hypoglycosylation of other protein(s) may be responsible for the observed phenotypes. Expression in the ER of Δalg6 S. pombe mutants of L. major STT3, a subunit with a broad range of substrate specificity that was able to replace the whole OST complex in S. cerevisiae (24, 32), was able to revert the hypoglycosylation produced in such a strain. However, its expression did not result in a reversion of the defects in the Δgls1-S mutants caused by accumulation of G3M9 NLOs (Fig. 8D), indicating that either there is no OST reaction product inhibition when G3M9 NLOs are not trimmed or that it is not severe enough to result in the observed toxicity.
We next explored the possibility that the presence of three Glc units could prevent the Golgi extension of protein-linked glycans by addition of Man and Gal. A size comparison of NLOs produced by mutant Δgls1-S with those of Δgls2α mutant showed that both strains were capable of extending their protein-linked glycans (Fig. 9) and had normal cell wall monosaccharide compositions (Fig. S4), even though they have very different growth and morphological phenotypes (Fig. 7). As we evaluated the bulk of cell wall glycoproteins, we cannot exclude the possibility that a particular glycoprotein displaying an essential functionality could be affected in Δgls1-S mutants by an impaired glycan elongation. Studies on the morphology of WT, Δgls1-S, and Δgls2α cells by TEM showed an altered cell wall ultrastructure only in Δgls1-S cells, which lacked the three-layered structure present in WT and Δgls2α cells (Fig. 10). Interestingly, this phenotype resembles that of S. cerevisiae cells bearing mutations in the catalytic subunit of the OST, which exhibit a diffused cell wall with loss of the outer mannoprotein layer present in WT cells (28). The S. pombe ER structure below the plasma membrane was clearly observed in WT and Δgls2α cells by TEM. However, no such structures were ever observed in Δgls1-S mutants. The clear differences observed in ER structures of the strains make attractive the possibility that accumulation of triglucosylated glycoproteins in cells lacking GI somehow alters the secretory pathway subcellular structure. This hypothesis is currently under investigation.
Previous reports showed that Caenorhabditis elegans mutants lacking GI did not display any visible morphological or behavioral phenotypes, but life span was greatly reduced (33). We thus investigated whether there was a loss of viability in a particular phase of the fission yeast cell growth curve. With both parameters analyzed, the proportion of cells that exclude the vital fluorescent dye phloxine B and the proportion of cells able to form colony units showed a drastic reduction of viability at all phases of the growing curve, although the effects were more severe at the early stationary phase (Fig. 11).
Mammalian cells have a Golgi endomannosidase that removes G3M from protein-linked G3M9 (Fig. 1) (34). Expression of the enzyme is enhanced in fibroblasts of patients displaying the MOGS-CDG syndrome thus allowing synthesis of complex type NLOs in the Golgi, more than 80% of which display structures similar to those present in healthy cells (13). Nevertheless, affected individuals still display extremely severe phenotypes. It is unknown whether these are caused by the ∼16% of remaining noncanonical NLOs or, alternatively, by the extended occurrence of triglucosylated glycans. Expression of a functional Golgi hEM in the Golgi of Δgls1-S significantly relieved the sick phenotype of the cells, although not as well as expressing GI (Fig. 12). This indicates that the toxic effect exerted by triglucosylated glycoproteins takes place in the Golgi (or ahead in the secretory pathway), that endomannosidase expression has not completely removed the harmful structures, or alternatively, that the extended occurrence of triglucosylated glycoproteins in the ER, the Golgi, and beyond results in growth defects.
In conclusion, we demonstrated that the inability to deglucosylate protein-linked G3M9 glycans in the ER is extremely toxic to the fission yeast. Although we were unable to exactly pinpoint either the cause or the subcellular site of toxicity, we propose that the persistence of those structures may be, at least in part, responsible for the severe pathology observed in MOGS-CDG patients.
Experimental procedures
Materials
Yeast extract and Malt extract were from Britania (Buenos Aires, Argentina). Bactopeptone and yeast nitrogen base (YNB) were from Difco (Detroit, MI). Endo H, protease inhibitors, dithiothreitol (DTT), amino acids, and supplements for culture media were from Sigma. [14C]Glc (301 Ci/mol) was from PerkinElmer Life Sciences. NM-DNJ was from Toronto Biochemicals. Enzymes used for general DNA procedures were from New England Biolabs (Ipswich, MA), and LR clonase was from Invitrogen. Geneticin (G418) was from InVivoGen (Carlsbad, CA), and nourseothricin was from WERNER BioAgents (Jena, Germany).
Strains and media
Escherichia coli DH5α and JA226 were used for cloning purposes and DB3.1 to amplify Gateway plasmids bearing the ccdB gene. Bacteria were grown at 37 °C in Luria broth medium (LB: 0.5% NaCl, 1% tryptone, and 0.5% yeast extract), supplemented with 200 μg/ml ampicillin or 50 μg/ml kanamycin as needed. S. pombe strains were grown at 28 °C in low adenine-rich media YE (0.5% yeast extract, 3% glucose) when testing for ade6 genotype or in YES medium (YE medium supplemented with 75 mg/liter adenine). S. pombe were selected in EMM minimal medium (35, 36) supplemented with adenine 75 mg/liter, leucine 250 mg/liter, or uracil 75 mg/liter as required. Geneticin was added to YES media at 200 μg/ml for kanMX marker selection, and nourseothricin was added to YES media at 100 μg/ml for NatMX marker selection. When double selection for geneticin and auxotrophic markers was needed, NH4Cl was replaced by 0.37% monosodium l-glutamate as the nitrogen source in EMM. Malt extract medium (3% Bacto Malt Extract, pH 5.5, supplemented with adenine, uracil, and leucine) was used for matings. The S. pombe strains used in this work are summarized in Table 1.
DNA and cloning procedures
General DNA procedures were as described previously (37). Yeast DNA extraction was performed as described in Ref. 38.
The alg10+ gene in S. pombe WT, Δgls1-H, and Δgls1-S strains was amplified in two parts using genomic DNA of each strain as a template and the primer pairs alg10–5′NC (5′-CCAAACTTCCTGCCAACAAC-3′)/alg10rev (5′-CAATCCCCACTTAGCTGAAG-3′) and alg10fw (5′-CTGGCTTGTAGGCGTAATTG-3′)/alg10–3′NC (5′-GGTGCTCGTTGATTTTTGGT-3′) in a high-fidelity PCR using KOD (Merck). Both PCR products obtained from each mutant were purified by QIAquick gel extraction kit (Qiagen) and sequenced.
The gateway pDONR201 plasmid containing clone 28/H08 (S. pombe gls1+ gene, which was obtained from RIKEN DNA Bank (39)) was transferred to the pREP41-ccdb2 Gateway-compatible S. pombe destination expression vector (kindly provided by Dr. M. Yoshida, Chemical Genetics Laboratory, RIKEN DNA Bank, Japan) by the LR recombination reaction (Invitrogen) as indicated by the manufacturer. Recombinant expression plasmid was amplified and recovered from JA226 E. coli strain. S. pombe competent Δgls1-S cells were then electroporated with the pREP41-GI episomal-replicating constructs and selected in EMM supplemented with adenine and uracil.
L. major STT3D was amplified from plasmid pRS425-STT3D (kindly provided by Dr. Markus Aebi, ETH, Zurich, Switzerland) (24) in-frame with a sequence coding the hemagglutinin (HA) epitope tag using the following primers that provide at both ends the attB sites for Gateway recombination: attB1-STT3D fw (5′-GGGGACAAGTTTGTACAAAAAAGCAGGCTGGATGGGCAAGCGGAAGGG-3′) and attB2+stop STT3D rev (5′-GGGGACCACTTTGTACAAGAAAGCTGGGTtcaAGCGTAATCTGGAACGTC-3′). The 2600-bp fragment was introduced in pDONR-201ccdB vector by recombination using BP clonase (Invitrogen) and the plasmids recovered from DH5α E. coli strain. Positive clones were identified by colony PCR using primers attb-STT3fw and pDONR201a (5′-CATCAGAGATTTTGAGACAC-3′) and sequenced. The insert was then transferred to a pREP1-ccdB (Riken) (39) destination vector using LR clonase as described above. The resulting expression vector pREP1-STT3D-HA was sequenced and electroporated into WT and Δgls1-S yeast cells. Transformants were selected in EMM lacking leucine.
Human Golgi endomannosidase fused to GFP was amplified with KOD polymerase from plasmid pSV-GFP-hEM (34) using primers pREP3X-XhoI-fEGFP (5′-TTCTCACTTTCTGACTTATAGTCGCTTTGTTAAATGGCCTCGAGATGGTGAGCAAGGGCGAGGAG-3) and hEM-SmaI-pREP3x (5′-AACCCTAGCAGTACTGGCAAGGGAGACATTCCTTTTACCCGGGTTAAGAAACAGGCAGCTGGCG-3′) that introduce ∼40 bp of flanking homology to S. pombe expression vector pREP3X. The insert was cloned by gap repair in S. pombe by transforming competent WT cells with 5 μg of the insert plus 1 μg of pREP3X previously opened with SmaI and XhoI restriction enzymes (40, 41). Transformants selected in EMM lacking leucine were screened by colony PCR to detect the presence of plasmid pREP3X-GFP-hEM produced by homologous recombination. Total DNA obtained from positive cells was used to transform E. coli, and plasmids were recovered and sequenced (41). The same clone was introduced into Δgls1-S and Δgls2α S. pombe strains.
Genetic procedures
S. pombe transformations by electroporation
S. pombe electrocompetent cells were prepared by extensively washing exponentially growing cultures twice with 1 volume of sterile water, twice with 1 m sorbitol, and finally resuspended at a 1:100 of the initial volume in 1 m sorbitol. Plasmidic supercoiled DNA (0.2 μg) or linear DNA (0.5–1 μg) were electroporated at 1.5 kV, 200 ohm, 25 microfarads with a Gene Pulser II (Bio-Rad). Cells were recovered in 0.5 m sorbitol in YES for 1 h at 28 °C, harvested, and plated in the appropriate selective media.
Construction of Δgls1::NatMX haploid strains
The geneticin resistance cassette of the heterozygous Δgls1::KanMX/+ diploid S. pombe strain purchased from Bioneer (Korea) was exchanged by the nourseotricine resistance cassette according to Sato et al. (42) as follows: primers MD1 (5′-CGGATCCCCGGGTTAATTAAGGCG-3′) and MD2 (5′-GAATTCGAGCTCGTTTAAACACTGGATGGCGGCGTTAGTATCG-3′) were used in a PCR using KOD (Merck) to obtain NatMX6 (nourseothricin-resistant marker) flanked by promoter PTEF and terminator TTEF using plasmid pCR2.1-Nat as a template (kindly provided by Takashi Toda, London Research Institute, UK). This DNA fragment was gel-purified and used to transform electrocompetent Δgls1::KanMX/+ diploid S. pombe mutants. Nourseothricin-resistant Δgls1::NatMX/+ diploid S. pombe colonies were tested for sensitivity to geneticin, and homologous recombination was verified by PCR using primers gls1–5′NC B (5′-GCTCCAAATGTTTTACG CAG-3′) and ClonNAT rev (5′-CGAGACGACCACGAAGC-3′). To obtain haploid Δgls1::NatMX strains, sporulation was induced by transformation of the nourseothricin-resistant diploid strain with plasmid pON177 (Bioneer) according to company instructions followed by selection of uracil phototrophs. Spore micromanipulations were carried out with a Manual Micromanipulator (Singer Instruments Co). Δgls1::NatMX genotypes of the spores were determined by antibiotic resistance and also by colony PCR with the primers ClonNAT fw (5′-CTTCGTGGTCGTCTCGTAC-3) and gls1–3′NC B (5′-AAATACGAAACGCAGTTCGC-3′). Δgls1-S and Δgls1-H haploid nourseothricin mutants were obtained from the dissection of the same tetrad (Table 1).
Construction of S. pombe double mutants
S. pombe Δalg10 and Δyos9 mutants were purchased from Bioneer (Korea). S. pombe Δgls2α strain construction was described previously (43). Double mutants were obtained using standard genetic techniques for mating, sporulation, tetrad dissection, and analysis, as described previously (27, 35). Relevant genotypes were determined by antibiotic resistance or auxotrophic growth in the appropriate media and also by colony PCR with the primers described below. To construct ΔGIA10-18 strain, first haploid Δgls1-S was mated with ADm to obtain ΔGI210m-1A. This h− strain was in turn mated with strain Δalg10 (SPAC56F8.06c), and the diploids obtained were sporulated and tetrads dissected by micromanipulation. The genotype of the resulting spores that were both geneticin- and nourseothricin-resistant was confirmed by PCR using the primer pairs ClonNAT fw/gls1–3′NC B for Δgls1::NatMX and Spalg10s 5′NC (5′-CCAAACTTCCTGCCAACAAC-3′)/KanMXrev2 (5′-CGCTACCTTTGCCATGTTTCAG-3′) for Δalg10::KanMX, respectively. Mating types were determined by PCR as described (44).
Analysis of NLO and LLOs synthesized in vivo
S. pombe cultures in exponential growth phase were harvested, extensively washed with 1% YNB medium without glucose, and resuspended in 2 volumes (v/w) of the same medium. For NLO analysis, 0.5 ml of cells were preincubated with the mannosidase inhibitor 3.5 mm kifunensine for 30 min and with 5 mm DTT for 5 min and pulsed for 15 min in 5 mm Glc with 300 μCi/ml [14C]Glc at 28 °C. When GI and GII inhibitors CST and NM-DNJ were used, they were added 60 min prior to labeling at 8.5 and 5 mm, respectively. Purification and protease digestion of glycoproteins and Endo H–sensitive NLO purification were performed as described previously (10). Glycans were run on paper chromatography in Whatman 1 papers and 1-propanol/nitromethane/H2O (5:2:4) as solvent, and peaks were identified by standards run in parallel. To improve the resolution of the glycan species, the identified glycans were eluted from the papers and resolved by HPLC using a TSK-GEL Amide-80 column (4.6 mm inner diameter × 25 cm; Tosoh Bioscience, Tokyo, Japan) with a mobile phase of H2O/CH3CN in a linear gradient from 35:65 to 55:45 over 65 min and a flow rate of 0.75 ml/min at room temperature as reported (45). For the analysis of LLO, 1 ml of washed cells were pulsed for 15 min in 5 mm Glc with 300 μCi/ml [14C]Glc at 28 °C in the presence of 50 μg/ml puromycin and 75 μg/ml cycloheximide. Cells were precipitated with 5 ml of methanol, and the pellet was extracted three times with CHCl3/methanol (3:2). The pellet was then washed once with methanol and once with water, resuspended in 2 ml of water and 1 ml of glass beads, vortexed for 10 min, and centrifuged. The resulting pellet was washed four times with water and then extracted with CH3CN/methanol/water (1:1:0.3). The extracted material was dried and boiled for 15 min in 1 ml of 0.02 n HCl. Once cold, 3 ml of CHCl3 and 2 ml of methanol were added and centrifuged. The upper phase was recovered, dried, and analyzed as described for NLO.
Analysis of glycan extension in the secretory pathway and cell wall composition of GI and GII mutants
S. pombe cultures in exponential growth phase were harvested and washed with 1% YNB medium without glucose, and the pellet was resuspended in 2 volumes (v/w) of the same medium. Cells (1 ml) were labeled for 45 min in 5 mm Glc with 300 μCi/ml [14C]Glc. Half of each labeled sample was washed with 20 volumes of 1% YNB, centrifuged, and chased for 150 min in 0.5 ml of 1% YNB containing 100 mm glucose, whereas the other half was processed immediately. Glycoprotein purification and glycan pattern analysis of all samples were performed as described above for NLOs. To determine cell wall monosaccharide composition, the water-insoluble material obtained after protease digestion of labeled glycoproteins was heated for 150 min at 100 °C in 2 n NaOH in a final volume of 2.5 ml, precipitated with 3 ml of methanol, and washed three times with 75% methanol. Insoluble material was heated for 4 h at 100 °C in 1 n HCl, dried at room temperature, and submitted to paper chromatography in Whatman 1 paper with butanol/pyridine/H2O (10:3:3) as solvent.
Purification of total-cell protein extracts, microsomal fractions, and protein deglycosylation
Yeast whole-cell extracts were obtained from 20 ml of exponentially growing cultures at A600 nm = 2. Cells were washed once with water, harvested, and broken with glass beads (0.5 mm inner diameter) by 10 repetitive cycles of 1-min vortexing followed by 1 min on ice in 0.1 m HEPES, pH 7.2, 1% Triton X-100, 5 mm EDTA with protease inhibitors (100 μm phenylmethylsulfonyl fluoride, 10 μm l-1-tosylamido-2-phenylethyl chloromethyl ketone, 10 μm N-p-tosyl-l-lysine chloromethyl ketone, 10 μm leupeptin, 10 μm pepstatin, and 10 μm E64), and cleared by centrifugation at 20,000 × g for 20 min. Microsomal (ER and Golgi-enriched) fractions were obtained as described previously (31). To measure S. pombe endomannosidase activity, Δgls2α microsomal fractions were resuspended in 100 mm phosphate buffer, pH 6.8, 0.25 m sucrose, 1 mm EDTA, and protease inhibitors. Protein concentrations were determined by protein assay as described by Bio-Rad. To obtain deglycosylated extracts, 50 μg of whole-cell proteins were denaturalized with 0.5% SDS and 40 mm DTT at 100 °C, and treated with 1 milliunit of Endo H overnight at 37 °C in 50 mm triethanolamine, pH 5.5.
Endomannosidase activity assays
Endomannosidase activity of 100 μg of total protein from microsomal fractions of S. pombe Δgls2α cells was determined by incubation with 2000 cpm of [glucose-14C]Glc1Man9GlcNAc in 50 μl of 50 mm sodium phosphate buffer, pH 6.8, 0.5% Triton X-100 for 30 min at 37 °C. Reactions were stopped by the addition of 50 μl of methanol, incubated for 5 min at 60 °C, and centrifuged for 5 min at 15,000 × g. Released [14C]Glc-Man and the remaining nonhydrolyzed substrate of the supernatant were separated by ascending paper chromatography in Whatman 1 using 2-propanol/acetic acid/H2O (25:4:9) as solvent. The disaccharide nature of the released radioactivity was confirmed by ascending paper chromatography using 1-butanol/ethanol/H2O (10:1:2) as solvent and sucrose, maltose, and Glc as standard.
Immunoblottings
Total S. pombe proteins (50 μg) were run in SDS-PAGE and electrotransferred to Immobilon-P membranes (Millipore) 1.5 h at 100 mA. For detection of the CNX glycosylation status, the resolving gel was 12%, and the membrane was incubated first with previously obtained rabbit anti-S. pombe CNX antibody (1:10,000) (46) and then with anti-rabbit antibody (1:3000) conjugated to HRP (Sigma). For STT3D-HA detection, the gel was 9%, and the membrane was incubated with rat anti-HA (1:5000) (Roche Applied Science) followed by anti-rat conjugated to HRP (1:5000) (Sigma). Bands were revealed using West Pico SuperSignal chemiluminescent substrate (ThermoFisher Scientific, Waltham, MA) following the instructions of the manufacturer. Membranes were developed using an Image Quant Las4000 luminescent image analyzer.
Transmission electron microscopy
WT or Δgls1-S cells were grown to A600 nm = 1 (±0.2) and fixed overnight at room temperature using 2.5% glutaraldehyde in 0.1 m phosphate buffer, pH 7.4, supplemented with 0.01% ruthenium red to increase electronic contrast in the cell wall. This step was followed by post-fixation in 1% osmium tetroxide for 1 h. Samples were stained overnight at 4 °C in 2% uranyl acetate and dehydrated with ethanol. Then they were embedded in Epoxy resin (Durcupan), and 60–70-nm sections were cut. Images were obtained using a transmission electron microscope (TEM-Zeiss-EM109T) and photographed with a Gatan ES1000W digital camera. Images with a ×85,000 magnification of 9–12 cells per genotype were taken randomly. The signal intensities along the cell wall's cross-sections were obtained using ImageJ software.
Subcellular localization of expressed hEM
Δgls1-S or Δgls2α cells transformed with Golgi hEM fused to GFP were grown to A600 nm = 1 in EMM lacking leucine. Cells (200 μl) were harvested and resuspended in 500 μl of YES. Images were obtained using a Zeiss confocal microscope LSM 710, with a ×63 Zeiss Plan-Apochromat objective (NA 1.4). At least 10 images were taken randomly per genotype. For processing images, a smooth filter was set using ImageJ software to improve the quality. For endocytic pathway staining, 20 μl of cultures were incubated with 0.1 mg/ml FM4-64 at 30 °C for 90 min and washed with PBS.
Growth, morphology, and survival of S. pombe mutants
Growth of S. pombe mutants in solid media was performed as follows: cultures of to-be-compared strains at the early stationary phase were diluted to a final concentration of 2 × 106 cells/ml. Tenth dilution series in YES were performed, and drops of 5 μl of each dilution were spotted in solid YES, in triplicate. Plates were incubated at 28 °C, and images were taken every 24 h with an Olympus C-610 digital camera for 5 days.
To perform growth curves of S. pombe mutants in liquid media, a pre-culture of each S. pombe strain at the end of the exponential phase was used to start three 50-ml cultures of YES at A600 nm = 0.2 in 250-ml flasks (time 0), which were grown for 5 days at 28 °C with agitation at 250 rpm. Aliquots were taken every 4 h to monitor OD at 600 nm. Data were analyzed with R (47) and a logistic adjustment was used.
Survival of S. pombe mutants at different stages of the growth curve was scored by counting colony-forming units from dilutions of aliquots taken at each time point plated on YES and incubated for 7 days at 28 °C. The proportion of colony formation units in a population was considered as the percentage of survival at a given time point. Cell growth curves were performed in independent triplicates for each mutant. ANOVA statistical analysis was done using Bonferroni's test.
Survival was also measured by the ability to exclude the fluorescent dye phloxine B (disodium 2′,4′,5′,7′-tetrabromo-4,5,6,7-tetrachlorofluorescein) as follows: for each time point of the growing curve 1.4 × 107 cells were incubated in 1 ml of a final volume of YES with 5 μg/ml phloxine B for 2 h at 28 °C with shaking as described (48). An amount of 10,000 cells were then analyzed by flow cytometry using a FACSCalibur flow cytometer (BD Biosciences) with an argon laser (excitation at 488 nm). Emission at 546–550 nm (filter FL2) was monitored for phloxine B–stained cells that were compared with a nonstained culture. As a control of stained death-cell fluorescence intensity, cultures in exponential growth were heated 1 h at 65 °C and analyzed by flow cytometry as described above. ANOVA statistical analysis was done using Bonferroni's test.
To measure the cell length of mutant yeasts, images of cultures in exponential phase (A600 nm = 1) were acquired using an Olympus BX60 light transmission microscope with a UPlanFI ×100 oil objective (NA = 1.30) and an Olympus DP71 camera. Cell lengths (at least 180 for each mutant) were measured with ImageJ software. Statistical analyses were done using Bonferroni's multiple comparison test.
Author contributions
G. L. G., A. P., and C. D. conceptualization; G. L. G. and C. D. data curation; G. L. G. and C. D. formal analysis; G. L. G., A. V., S. I. A., E. E., A. P., and C. D. investigation; G. L. G., A. V., S. I. A., and C. V. methodology; A. P. and C. D. writing-review and editing; C. D. supervision; C. D. funding acquisition; C. D. writing-original draft; C. D. project administration.
Supplementary Material
Acknowledgments
We thank Dr. Markus Aebi (ETH, Zurich, Switzerland), Dr. Takashi Toda (London Research Institute, United Kingdom), and Dr. M. Yoshida (Chemical Genetics Laboratory, RIKEN DNA Bank, Japan) for providing us with plasmids pRS425-STT3D, pCR2.1-Nat, and pREP41-ccdb2, respectively.
This work was supported in part by the National Research Council (CONICET, Argentina) Grant PIP-11220150100759. The authors declare that they have no conflicts of interest with the contents of this article.
This article contains Figs. S1–S5 and Table S1.
In glycan structures, Glc is abbreviated as G; Man is M; and GlcNAc is omitted. Thus, G3M9 is Glc3Man9GlcNAc2; G2M9 is Glc2Man9GlcNAc2; and M9 is Man9GlcNAc2, for example.
- ER
- endoplasmic reticulum
- CDG
- congenital disorders of glycosylation
- CNX
- calnexin
- Endo H
- endo-β-N-acetylglucosaminidase H
- GI
- glucosidase I
- GII
- glucosidase II
- LLO
- lipid-linked oligosaccharide
- NLO
- protein-linked oligosaccharide
- UGGT
- UDP-Glc:glycoprotein glucosyltransferase
- OST
- oligosaccharyltransferase
- ERAD
- ER-associated degradation
- ANOVA
- analysis of variance
- Dol-PP
- dolichol pyrophosphate
- NM-DNJ
- N-methyl-deoxynojirimycin
- TEM
- transmission electron microscopy
- hEM
- human endomannosidase
- fw
- forward
- HRP
- horseradish peroxidase
- CST
- castanospermine.
References
- 1. Aebi M. (2013) N-Linked protein glycosylation in the ER. Biochim. Biophys. Acta 1833, 2430–2437 10.1016/j.bbamcr.2013.04.001 [DOI] [PubMed] [Google Scholar]
- 2. Mohorko E., Glockshuber R., and Aebi M. (2011) Oligosaccharyltransferase: the central enzyme of N-linked protein glycosylation. J. Inherit. Metab. Dis. 34, 869–878 10.1007/s10545-011-9337-1 [DOI] [PubMed] [Google Scholar]
- 3. Parodi A. J. (2000) Protein glucosylation and its role in protein folding. Annu. Rev. Biochem. 69, 69–93 10.1146/annurev.biochem.69.1.69 [DOI] [PubMed] [Google Scholar]
- 4. D'Alessio C., Caramelo J. J., and Parodi A. J. (2010) UDP-GlC:glycoprotein glucosyltransferase-glucosidase II, the ying-yang of the ER quality control. Semin. Cell Dev. Biol. 21, 491–499 10.1016/j.semcdb.2009.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sousa M. C., Ferrero-Garcia M. A., and Parodi A. J. (1992) Recognition of the oligosaccharide and protein moieties of glycoproteins by the UDP-Glc:glycoprotein glucosyltransferase. Biochemistry 31, 97–105 10.1021/bi00116a015 [DOI] [PubMed] [Google Scholar]
- 6. Caramelo J. J., Castro O. A., Alonso L. G., De Prat-Gay G., and Parodi A. J. (2003) UDP-Glc:glycoprotein glucosyltransferase recognizes structured and solvent accessible hydrophobic patches in molten globule-like folding intermediates. Proc. Natl. Acad. Sci. U.S.A. 100, 86–91 10.1073/pnas.262661199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Freeze H. H., Schachter H., and Kinoshita T. (2015) in Essentials of Glycobiology (Varki A., Cummings R. D., Esko J. D., Stanley P., Hart G. W., Aebi M., Darvill A. G., Kinoshita T., Packer N. H., Prestegard J. H., Schnaar R. L., and Seeberger P. H., eds) pp. 569–582, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY: [PubMed] [Google Scholar]
- 8. De Praeter C. M., Gerwig G. J., Bause E., Nuytinck L. K., Vliegenthart J. F., Breuer W., Kamerling J. P., Espeel M. F., Martin J. J., De Paepe A. M., Chan N. W., Dacremont G. A., and Van Coster R. N. (2000) A novel disorder caused by defective biosynthesis of N-linked oligosaccharides due to glucosidase I deficiency. Am. J. Hum. Genet. 66, 1744–1756 10.1086/302948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sadat M. A., Moir S., Chun T. W., Lusso P., Kaplan G., Wolfe L., Memoli M. J., He M., Vega H., Kim L. J. Y., Huang Y., Hussein N., Nievas E., Mitchell R., Garofalo M., et al. (2014) Glycosylation, hypogammaglobulinemia, and resistance to viral infections. N. Engl. J. Med. 370, 1615–1625 10.1056/NEJMoa1302846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fernández F. S., Trombetta S. E., Hellman U., and Parodi A. J. (1994) Purification to homogeneity of UDP-glucose:glycoprotein glucosyltransferase from Schizosaccharomyces pombe and apparent absence of the enzyme from Saccharomyces cerevisiae. J. Biol. Chem. 269, 30701–30706 [PubMed] [Google Scholar]
- 11. Kim D. U., Hayles J., Kim D., Wood V., Park H. O., Won M., Yoo H. S., Duhig T., Nam M., Palmer G., Han S., Jeffery L., Baek S. T., Lee H., Shim Y. S., et al. (2010) Analysis of a genome-wide set of gene deletions in the fission yeast Schizosaccharomyces pombe. Nat. Biotechnol. 28, 617–623 10.1038/nbt.1628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Herscovics A. (1999) Processing glycosidases of Saccharomyces cerevisiae. Biochim. Biophys. Acta 1426, 275–285 10.1016/S0304-4165(98)00129-9 [DOI] [PubMed] [Google Scholar]
- 13. Völker C., De Praeter C. M., Hardt B., Breuer W., Kalz-Füller B., Van Coster R. N., and Bause E. (2002) Processing of N-linked carbohydrate chains in a patient with glucosidase I deficiency (CDG type IIb). Glycobiology 12, 473–483 10.1093/glycob/cwf050 [DOI] [PubMed] [Google Scholar]
- 14. Piel M., and Tran P. T. (2009) Cell shape and cell division in fission yeast. Curr. Biol. 19, R823–R827 10.1016/j.cub.2009.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hossain T. J., Harada Y., Hirayama H., Tomotake H., Seko A., and Suzuki T. (2016) Structural analysis of free N-glycans in α-glucosidase mutants of Saccharomyces cerevisiae: lack of the evidence for the occurrence of catabolic α-glucosidase acting on the N-glycans. PLoS One 11, e0151891 10.1371/journal.pone.0151891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Burda P., and Aebi M. (1998) The ALG10 locus of Saccharomyces cerevisiae encodes the α-1,2 glucosyltransferase of the endoplasmic reticulum: the terminal glucose of the lipid-linked oligosaccharide is required for efficient N-linked glycosylation. Glycobiology 8, 455–462 10.1093/glycob/8.5.455 [DOI] [PubMed] [Google Scholar]
- 17. Wood V., Harris M. A., McDowall M. D., Rutherford K., Vaughan B. W., Staines D. M., Aslett M., Lock A., Bähler J., Kersey P. J., and Oliver S. G. (2012) PomBase: a comprehensive online resource for fission yeast. Nucleic Acids Res. 40, D695–D699 10.1093/nar/gkr853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McDowall M. D., Harris M. A., Lock A., Rutherford K., Staines D. M., Bähler J., Kersey P. J., Oliver S. G., and Wood V. (2015) PomBase 2015: updates to the fission yeast database. Nucleic Acids Res. 43, D656–D661 10.1093/nar/gku1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Buschhorn B. A., Kostova Z., Medicherla B., and Wolf D. H. (2004) A genome-wide screen identifies Yos9p as essential for ER-associated degradation of glycoproteins. FEBS Lett. 577, 422–426 10.1016/j.febslet.2004.10.039 [DOI] [PubMed] [Google Scholar]
- 20. Szathmary R., Bielmann R., Nita-Lazar M., Burda P., and Jakob C. A. (2005) Yos9 protein is essential for degradation of misfolded glycoproteins and may function as lectin in ERAD. Mol. Cell 19, 765–775 10.1016/j.molcel.2005.08.015 [DOI] [PubMed] [Google Scholar]
- 21. Fernandez F., Jannatipour M., Hellman U., Rokeach L. A., and Parodi A. J. (1996) A new stress protein: synthesis of Schizosaccharomyces pombe UDP–Glc:glycoprotein glucosyltransferase mRNA is induced by stress conditions but the enzyme is not essential for cell viability. EMBO J. 15, 705–713 10.1002/j.1460-2075.1996.tb00406.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Núñez A., Dulude D., Jbel M., and Rokeach L. A. (2015) Calnexin is essential for survival under nitrogen starvation and stationary phase in Schizosaccharomyces pombe. PLoS One 10, e0121059 10.1371/journal.pone.0121059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wild R., Kowal J., Eyring J., Ngwa E. M., Aebi M., and Locher K. P. (2018) Structure of the yeast oligosaccharyltransferase complex gives insight into eukaryotic N-glycosylation. Science 359, 545–550 10.1126/science.aar5140 [DOI] [PubMed] [Google Scholar]
- 24. Nasab F. P., Schulz B. L., Gamarro F., Parodi A. J., and Aebi M. (2008) All in one: Leishmania major STT3 proteins substitute for the whole oligosaccharyltransferase complex in Saccharomyces cerevisiae. Mol. Biol. Cell 19, 3758–3768 10.1091/mbc.e08-05-0467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Parodi A. J. (1993) N-Glycosylation in trypanosomatid protozoa. Glycobiology 3, 193–199 10.1093/glycob/3.3.193 [DOI] [PubMed] [Google Scholar]
- 26. Fanchiotti S., Fernández F., D'Alessio C., and Parodi A. J. (1998) The UDP-Glc:glycoprotein glucosyltransferase is essential for Schizosaccharomyces pombe viability under conditions of extreme endoplasmic reticulum stress. J. Cell Biol. 143, 625–635 10.1083/jcb.143.3.625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bredeston L. M., Marino-Buslje C., Mattera V. S., Buzzi L. I., Parodi A. J., and D'Alessio C. (2017) The conundrum of UDP-Glc entrance into the yeast ER lumen. Glycobiology 27, 64–79 10.1093/glycob/cww092 [DOI] [PubMed] [Google Scholar]
- 28. Chavan M., Suzuki T., Rekowicz M., and Lennarz W. (2003) Genetic, biochemical, and morphological evidence for the involvement of N-glycosylation in biosynthesis of the cell wall β1,6-glucan of Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. U.S.A. 100, 15381–15386 10.1073/pnas.2536561100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Osumi M. (2012) Visualization of yeast cells by electron microscopy. J. Electron Microsc. 61, 343–365 10.1093/jmicro/dfs082 [DOI] [PubMed] [Google Scholar]
- 30. Lubas W. A., and Spiro R. G. (1987) Golgi endo-α-d-mannosidase from rat liver, a novel N-linked carbohydrate unit processing enzyme. J. Biol. Chem. 262, 3775–3781 [PubMed] [Google Scholar]
- 31. D'Alessio C., Fernández F., Trombetta E. S., and Parodi A. J. (1999) Genetic evidence for the heterodimeric structure of glucosidase II. The effect of disrupting the subunit-encoding genes on glycoprotein folding. J. Biol. Chem. 274, 25899–25905 10.1074/jbc.274.36.25899 [DOI] [PubMed] [Google Scholar]
- 32. Kelleher D. J., and Gilmore R. (2006) An evolving view of the eukaryotic oligosaccharyltransferase. Glycobiology 16, 47R–62R 10.1093/glycob/cwj066 [DOI] [PubMed] [Google Scholar]
- 33. Katoh T., Takase J., Tani Y., Amamoto R., Aoshima N., Tiemeyer M., Yamamoto K., and Ashida H. (2013) Deficiency of α-glucosidase I alters glycoprotein glycosylation and lifespan in Caenorhabditis elegans. Glycobiology 23, 1142–1151 10.1093/glycob/cwt051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hardt B., Völker C., Mundt S., Salska-Navarro M., Hauptmann M., and Bause E. (2005) Human endo-α1,2-mannosidase is a Golgi-resident type II membrane protein. Biochimie 87, 169–179 10.1016/j.biochi.2004.11.004 [DOI] [PubMed] [Google Scholar]
- 35. Alfa C., Fantes P., Hyams J., McLeod M., and Wabrik E. (1993) Experiments with Fission Yeast: A Laboratory Manual, 188 pp., Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 36. Moreno S., Klar A., and Nurse P. (1991) Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 194, 795–823 10.1016/0076-6879(91)94059-L [DOI] [PubMed] [Google Scholar]
- 37. Sambrook J., and Russell D. W. (2001) Molecular Cloning: A Laboratory Manual, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 38. Hoffman C. S., and Winston F. (1987) A 10-minute DNA preparation from yeast efficiently releases autonomous plasmids for transformation of Escherichia coli. Gene 57, 267–272 10.1016/0378-1119(87)90131-4 [DOI] [PubMed] [Google Scholar]
- 39. Matsuyama A., Arai R., Yashiroda Y., Shirai A., Kamata A., Sekido S., Kobayashi Y., Hashimoto A., Hamamoto M., Hiraoka Y., Horinouchi S., and Yoshida M. (2006) ORFeome cloning and global analysis of protein localization in the fission yeast Schizosaccharomyces pombe. Nat. Biotechnol. 24, 841–847 10.1038/nbt1222 [DOI] [PubMed] [Google Scholar]
- 40. Chino A., Watanabe K., and Moriya H. (2010) Plasmid construction using recombination activity in the fission yeast Schizosaccharomyces pombe. PLoS One 5, e9652 10.1371/journal.pone.0009652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hagan I. M., Carr A. M., Grallert A., and Nurse P. (2016) Fission Yeast: A Laboratory Manual (Hagan I. M., Carr A. M, Grallert A., and Nurse P., eds) 490 pp., Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 42. Sato M., Dhut S., and Toda T. (2005) New drug-resistant cassettes for gene disruption and epitope tagging in Schizosaccharomyces pombe. Yeast 22, 583–591 10.1002/yea.1233 [DOI] [PubMed] [Google Scholar]
- 43. Soussilane P., Soussillane P., D'Alessio C., Paccalet T., Fitchette A. C., Parodi A. J., Williamson R., Plasson C., Faye L., and Gomord V. (2009) N-Glycan trimming by glucosidase II is essential for Arabidopsis development. Glycoconj. J. 26, 597–607 10.1007/s10719-008-9201-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. D'Alessio C., Trombetta E. S., and Parodi A. J. (2003) Nucleoside diphosphatase and glycosyltransferase activities can localize to different subcellular compartments in Schizosaccharomyces pombe. J. Biol. Chem. 278, 22379–22387 10.1074/jbc.M300892200 [DOI] [PubMed] [Google Scholar]
- 45. Stigliano I. D., Alculumbre S. G., Labriola C. A., Parodi A. J., and D'Alessio C. (2011) Glucosidase II and N-glycan mannose content regulate the half-lives of monoglucosylated species in vivo. Mol. Biol. Cell 22, 1810–1823 10.1091/mbc.e11-01-0019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Stigliano I. D., Caramelo J. J., Labriola C. A., Parodi A. J., and D'Alessio C. (2009) Glucosidase II β subunit modulates N-glycan trimming in fission yeasts and mammals. Mol. Biol. Cell 20, 3974–3984 10.1091/mbc.e09-04-0316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. R-Core-Team. (2013) R: A language and environment for statistical computing R Foundation for Statistical Computing, Vienna, Austria [Google Scholar]
- 48. Roux A. E., Quissac A., Chartrand P., Ferbeyre G., and Rokeach L. A. (2006) Regulation of chronological aging in Schizosaccharomyces pombe by the protein kinases Pka1 and Sck2. Aging Cell 5, 345–357 10.1111/j.1474-9726.2006.00225.x [DOI] [PubMed] [Google Scholar]
- 49. Varki A., Cummings R. D., Aebi M., Packer N. H., Seeberger P. H., Esko J. D., Stanley P., Hart G., Darvill A., Kinoshita T., Prestegard J. J., Schnaar R. L., Freeze H. H., Marth J. D., Bertozzi C. R., et al. (2015) Symbol nomenclature for graphical representations of glycans. Glycobiology 25, 1323–1324 10.1093/glycob/cwv091 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.