Abstract
Human babesiosis is an emerging tick-borne disease caused by apicomplexan parasites of the genus Babesia. Clinical cases caused by Babesia duncani have been associated with high parasite burden, severe pathology, and death. In both mice and hamsters, the parasite causes uncontrolled fulminant infections, which ultimately lead to death. Resolving these infections requires knowledge of B. duncani biology, virulence, and susceptibility to anti-infectives, but little is known and further research is hindered by a lack of relevant model systems. Here, we report the first continuous in vitro culture of B. duncani in human red blood cells. We show that during its asexual cycle within human erythrocytes, B. duncani develops and divides to form four daughter parasites with parasitemia doubling every ∼22 h. Using this in vitro culture assay, we found that B. duncani has low susceptibility to the four drugs recommended for treatment of human babesiosis, atovaquone, azithromycin, clindamycin, and quinine, with IC50 values ranging between 500 nm and 20 μm. These data suggest that current practices are of limited effect in treating the disease. We anticipate this new disease model will set the stage for a better understanding of the biology of this parasite and will help guide better therapeutic strategies to treat B. duncani-associated babesiosis.
Keywords: parasitology, cell culture, infectious disease, inhibitor, erythrocyte, apicomplexa, Babesia duncani, human babesiosis, in vitro culture, red blood cells, tick-borne disease
Introduction
Human babesiosis is an emerging disease caused by apicomplexan Babesia parasites that are closely related to Plasmodium and Theileria species, the agents of malaria and theileriosis, respectively. Babesia microti, Babesia duncani, and Babesia divergens are responsible for most cases of babesiosis worldwide (1). Whereas B. microti is endemic in the Northeastern and Midwestern regions of the United States, and has further been reported worldwide, all confirmed cases of B. duncani have been found primarily in the Western United States (2, 3). A recent report based on available serological and/or molecular tests has suggested that B. duncani is also widespread in Canada (2, 3). On the other hand, cases of babesiosis in humans caused by B. divergens have so far been primarily reported in Europe, with some B. divergens-like cases reported in Japan and the United States (4, 5). Clinical symptoms in patients infected with these parasites are primarily the result of infection of the host red blood cells and can range from mild to severe. Mild to moderate symptoms include fever, fatigue, chills, sweats, anorexia, headache, myalgia, nausea, cough, and arthralgia, whereas in cases of severe babesiosis, complications include respiratory distress syndrome, pulmonary edema, congestive heart failure, renal failure, coma, and splenic rupture (6, 7). Patients with a weakened immune system or splenectomized patients are at greater risk of developing these symptoms and have a higher fatality rate (7). Although most cases of human babesiosis are caused by a tick bite, transfusion-transmitted babesiosis remains a major contributor to the increasing number of reported cases of the disease (8, 9). In the absence of a vaccine to prevent human babesiosis, drug combinations consisting primarily of antimalarials and antibiotics are the main arsenal used to treat infections (10, 11). However, drug failure is common, and some of the drugs are associated with major adverse events. In cases of drug failure, parasitemia can reach high levels, requiring exchange transfusion.
The first clinical cases of human babesiosis caused by B. duncani were reported in Washington State and California in 1991 and 1991–1993, respectively (12, 13). The majority of these cases were transmitted by an arthropod vector, whereas three cases were due to blood transfusion (14–16). B. duncani clinical isolates injected into hamsters and mice were found to cause a course of disease different from that caused by B. microti. Whereas B. microti infections in immunocompetent hosts start with an initial phase of high parasitemia, anemia, and splenomegaly and end with a decline in parasitemia and recovery (17, 18), infections with B. duncani result in a rapid increase in parasitemia, severe pathology, and no subsequent decrease in parasite burden. In experimental animal models, mortality rates associated with B. duncani vary, depending on mouse genotypes, with >95% mortality rates reported in C3H, A/J, AKR/N, and DBA/1J mice; 40–50% mortality in BALB/cJ, CBAJ and 129/J mice; and <10% mortality in C57BL/6 and C57BL/10 mice (19, 20). In Golden Syrian hamsters, B. duncani causes acute disease and death within 10 days of inoculation (21). Recent studies have identified Dermacentor albipictus as the main vector of B. duncani transmission, with the mule deer being the main mammalian host (22). The one-host life cycle of D. albipictus may account for the low incidence of B. duncani-associated babesiosis cases reported so far. However, the changing environmental factors that affect the habitat of the tick and its reservoir enhance the risk for increased transmission of this parasite to humans.
An important step toward a better understanding of the basic biological processes that control the development of Babesia parasites that infect humans is to develop a continuous in vitro culture system in human red blood cells. Whereas this has been successfully achieved in the case of B. divergens (23), a continuous in vitro culture system of B. microti or B. duncani in human red blood has not been developed to date. This constitutes an obstacle to the study of host–parasite interactions and the standardization of drug screening assays in vitro. Previous attempts to culture B. duncani in hamster red blood cells have been successful (21). However, such work requires permanent husbandry of hamsters and periodic collections of blood, causing unnecessary distress to the animals. Therapy of B. duncani infections has consisted of a combination of quinine and clindamycin or a drug mixture composed of both (6, 12, 15). However, to date, none of these drugs have been directly evaluated for their efficacy against B. duncani in vitro or in animal models.
Here, we report the first continuous in vitro culture of B. duncani in human red blood cells and assays to monitor parasitemia over time for the evaluation of parasite susceptibility to drugs recommended for the treatment of human babesiosis. Using these assays, we found that the parasite undergoes rapid development within human red blood cells, doubling its parasitemia every ∼22 h. Furthermore, we found that B. duncani has low susceptibility to atovaquone, clindamycin, azithromycin, and quinine.
Results
In vitro culture of B. duncani WA1 in human RBCs
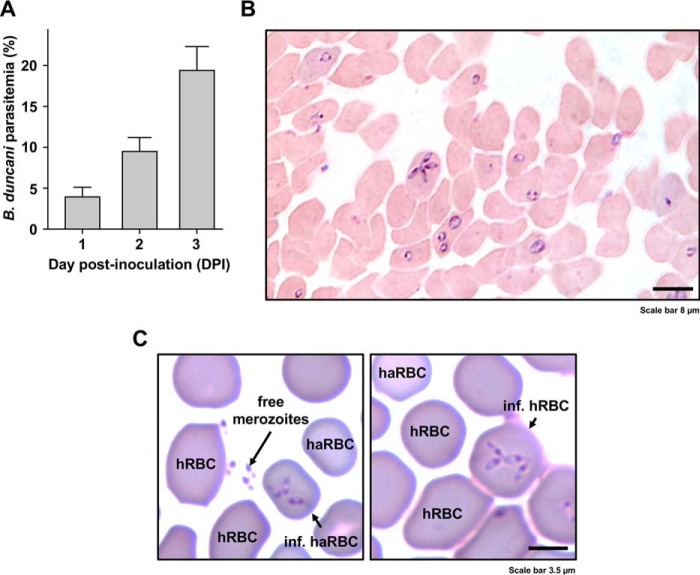
B. duncani was first cultured in hamster RBCs3 using freshly isolated RBCs from an infected hamster and incubation of the infected cells with uninfected hamster RBCs at 37 °C at 10% hematocrit and 2% parasitemia in hamster RBC growth medium (HaRGM). Under these conditions, the parasitemia reached ∼4% by day 1, ∼8% by day 2, and ∼16% by day 3 post-inoculation (Fig. 1A). Morphological analysis of infected hamster RBCs identified singly and multiply infected cells with ring form parasites as well as tetrads with four daughter parasites attached to each other at one end (Fig. 1B). To evaluate infection of human RBCs by B. duncani, a culture of the parasite in hamster RBCs at 5% parasitemia was split 1:2 into wells of a 24-well plate in the presence of human RBCs and human RBC growth medium (HuRGM). These mixed hamster/human RBC subcultures were first incubated for 2 days with daily replacements of HaRGM and the addition of fresh human RBCs at 5% hematocrit. 24 h post-inoculation, the parasitemia reached 9%, and both infected hamster and human RBCs could be detected (Fig. 1C). The mixed culture was then subcultured to 1% parasitemia into new wells with fresh human RBCs added to reach 5% hematocrit. Following incubation at 37 °C for an additional 3 days, the parasitemia increased overtime to reach 14% by day 4. Continuous growth in human RBCs was maintained in vitro in both 24-well plates and 25-cm2 tissue culture flasks with subcultures made every 3–4 days when the parasitemia reached 10–15%. The infectivity of B. duncani-infected human RBCs was demonstrated following infection of hamsters. The parasite caused fatal disease after 2 weeks of inoculation with parasitemia of 7–10% (not shown).
Figure 1.
Continuous in vitro culture of B. duncani in hamster RBCs and transfer to human RBCs. A, parasitemia expressed as the percentage of hamster RBCs infected by the parasite at 1, 2, and 3 days post-inoculation (DPI) in a representative experiment. Columns represent mean ± S.E. (error bars) of six biological replicates. B, micrograph of the intracellular development of cultured B. duncani in hamster RBCs in a Giemsa-stained smear prepared at 3 days post-inoculation. The parasitemia was 15% in this sample. C, transfer of B. duncani to human RBCs. B. duncani-infected hamster RBCs (haRBCs) were freshly harvested and maintained in culture in the presence of human RBCs (hRBCs). Left, free merozoites and infected haRBCs. Right, successful development of B. duncani in hRBCs. Human RBCs are distinguishable from haRBCs by their larger size and darker staining.
To confirm the infection of human RBCs by B. duncani, immunofluorescence analysis was conducted on samples from cultures of the parasite using an anti-Band3 mAb to detect the human erythrocyte anion exchanger (AE1, SLC4A1) followed by staining of the parasite DNA with 4′,6-diamidino-2-phenylindole (DAPI). As shown in Fig. 2A, Band3+, DAPI+ cells could be detected by confocal microscopy, indicating that indeed the infected cells are human RBCs. We have also conducted scanning EM (SEM) on in vitro cultured parasites to assess possible ultrastructural alterations of the host cells caused by the parasite. Whereas SEM identified infected RBCs by their distinctive protrusions and deformations (Fig. 2B), no significant differences in the size of the RBCs could be detected between uninfected and B. duncani-infected RBCs (Fig. 2C).
Figure 2.
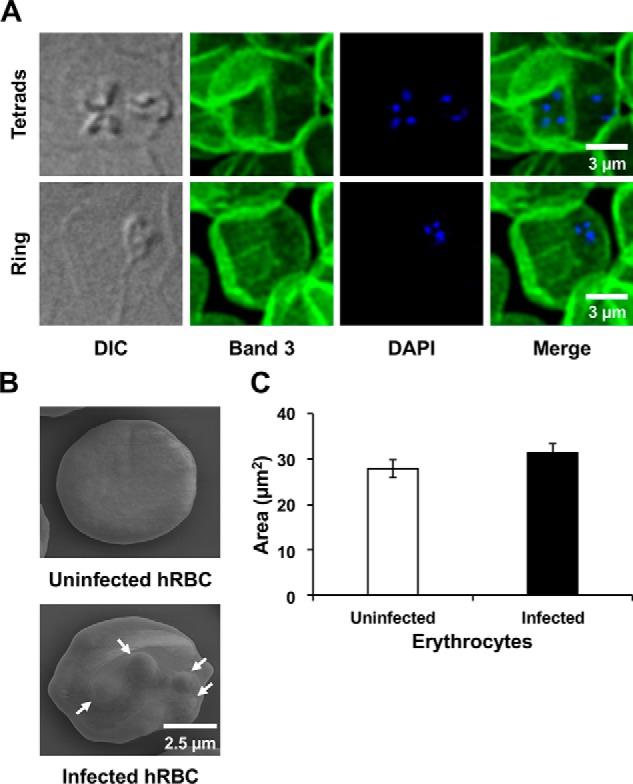
Confocal and scanning EM imaging of B. duncani-infected human RBCs. A, infected human RBCs were fixed and incubated with anti-Band3 mouse mAb (secondary antibody coupled to Alexa Fluor 488). Parasite DNA was stained with DAPI. B, representative images of uninfected and B. duncani-infected human RBCs visualized by scanning EM. Protrusions caused by the parasite are indicated by white arrows. C, quantification of the size of uninfected and B. duncani-infected human red blood cells from images collected by scanning EM. Data represent mean values of 20 uninfected and 19 infected erythrocytes. Error bars, S.E. The area of the examined erythrocytes was determined using Fiji. DIC, differential interference contrast.
B. duncani multiplication rate in human RBCs
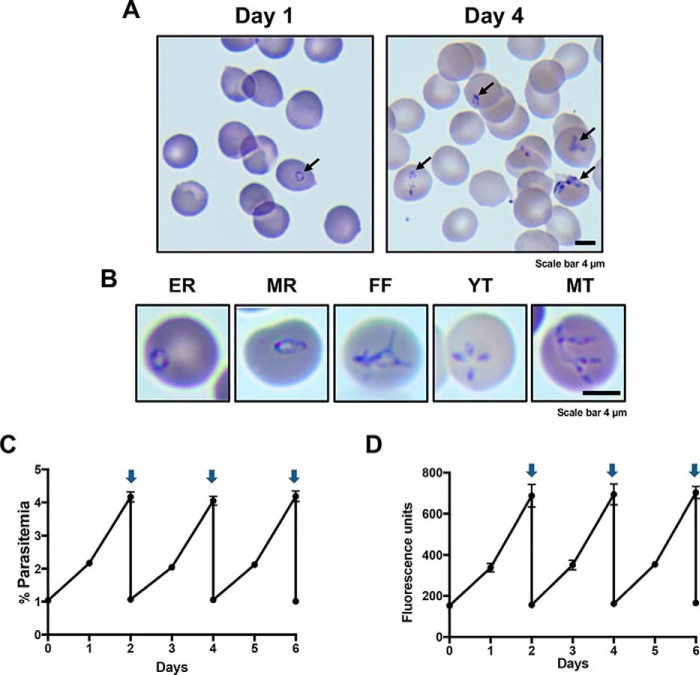
To determine the multiplication rate of B. duncani during its asexual development in human red blood cells, a culture of the parasite was initiated at 1% parasitemia in O+ type human RBCs at 5% hematocrit. The parasitemia was determined daily by light microscopy following Giemsa staining and a SYBR Green I–based fluorescence assay. As shown in Fig. 3A, B. duncani parasitemia increased to 10% by day 4. Under these culture conditions, the parasite develops asynchronously. Based on the morphology of the parasites, several stages indistinguishable from those observed in vivo can be identified. Ring form parasites ranged in size from 0.5 to 1 μm (early rings) and from 2 to 4 μm (mature rings) (Fig. 3B). The parasite then divides twice to produce “Maltese Cross” tetrads (21). These are often seen as four daughter parasites (merozoites) connected at one end by a filamentous structure (Fig. 3B). It is also common to see free individual or connected merozoites in the blood smears. Monitoring of parasite counts by light microscopy (Fig. 3C) and the SYBR Green I assay (Fig. 3D) showed that B. duncani parasitemia doubles every ∼22 h. With daily medium change, parasitemia levels above 20% can be reached in culture. However, after diluting cultures, we found that those initiated from cultures with parasitemia levels below 8% started their growth at a normal rate, whereas those initiated from a culture with higher parasitemia level showed a slow initial growth rate before parasitemia started doubling every ∼22 h.
Figure 3.
Continuous in vitro culture of B. duncani in human RBCs. A, representative images of Giemsa-stained blood smears of infected human RBCs at 1% parasitemia on day 1 and 8% parasitemia on day 4. B, representative images of the various stages identified in blood smears of B. duncani-infected human RBCs. EE, early rings; MR, mature rings; FF, filamentous forms; YT, young tetrads; MT, mature tetrads. C and D, growth of B. duncani over a 6-day period in human RBCs with culture dilution at day 2. Arrows indicate when cultures were diluted to 1% parasitemia. Parasitemia was determined by light microscopy and counting of 3,000–5,000 RBCs (C) or the SYBR Green I assay (D). Data represent mean values measured in triplicates. Error bars, S.D.
In vitro efficacy of currently recommended anti-babesiosis drugs against B. duncani
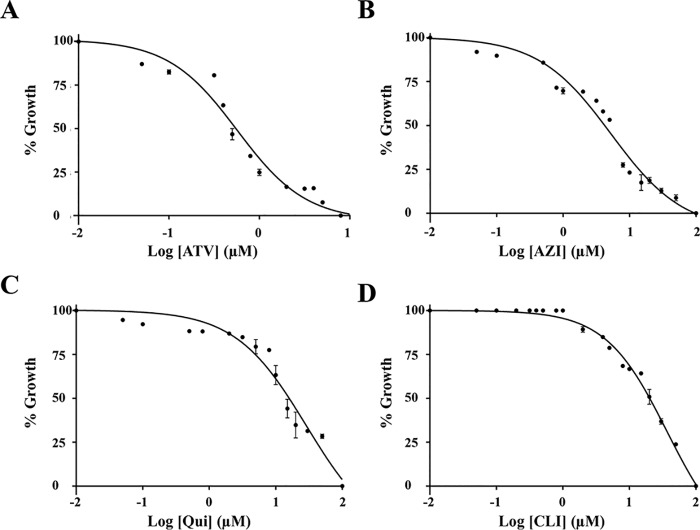
The development of the SYBR Green I assay as a tool to monitor B. duncani development within human RBCs made it possible to test the sensitivity of the parasite to the currently recommended anti-babesiosis drugs: atovaquone, azithromycin, clindamycin, and quinine. Dose-response assays identified the IC50 values of the compounds to be ∼500 nm for atovaquone, ∼5 μm for azithromycin, ∼12 μm for quinine, and ∼20 μm for clindamycin (Fig. 4).
Figure 4.
Evaluation of the in vitro drug susceptibility of B. duncani to atovaquone (ATV) (A), azithromycin (AZ) (B), clindamycin (CLI) (C), and quinine (QUI) (D) at concentrations up to 100 μm. Parasitemia was determined by the SYBR Green I assay at 60 h after the addition of the drugs. Wells with untreated infected erythrocytes and 0.1% DMSO vehicle were set as 100% growth, and wells with compounds at 100 μm in 0.1% DMSO were set as 0% growth. Each experiment was performed in triplicate, and data presented represent one of two biological replicates. GraphPad Prism (version 7.0a) was used to generate sigmoidal dose–response curves by fitting a nonlinear regression curve to the data. Each data point represents mean value measured in triplicates with error bars indicating S.D.
Discussion
Although the first reported case of human babesiosis caused by B. duncani was described in 1991 (13), the vector that transmits this parasite and the reservoir host have only recently been discovered (22). Furthermore, our understanding of the biological processes that control its pathophysiology and virulence remain unknown. Our study demonstrated the first successful continuous in vitro culture of B. duncani in human red blood cells. With this major development, we foresee major advances in our understanding of the biology, pathogenesis, and therapy of this parasite. All parasite forms (early and mature rings as well as tetrads) found in patient blood (13, 24) are also detected in cell culture. Our attempts to synchronize the parasite using sorbitol, a commonly used method for synchronizing Plasmodium falciparum cultures (25), did not result in synchronization of the blood stage development of the parasite (not shown). Scanning EM images of B. duncani-infected human erythrocytes show multiple protrusions. These results are consistent with previous studies of erythrocytes infected with the parasite collected from infected hamsters (26). We have successfully maintained a continuous culture of B. duncani in human O+ blood type for several months. These cultures can be stored as frozen stocks, which have been successfully used to initiate new cultures. The ease of handling and efficiency of this proposed method of culturing B. duncani allow for long-term studies and investigations that were previously not possible.
Whereas the IC50 values of atovaquone, azithromycin, clindamycin, and quinine in Plasmodium falciparum are in the low nanomolar range (27, 28), our study using the continuous in vitro culture of B. duncani in human RBCs showed that these drugs act with estimated IC50 values of 0.5, 5, 20, and 12 μm, respectively. These findings are also consistent with studies in B. microti using a short-term in vitro culture assay, where an effect with atovaquone was seen only at 1 and 10 μm, whereas azithromycin, clindamycin, and quinine had no effect on parasite development at these concentrations (11). Furthermore, studies of B. microti in mice showed that unlike atovaquone, which showed activity at a dose as low as 10 mg/kg, azithromycin and clindamycin at a dose as high as 50 mg/kg and quinine at a dose as high as 100 mg/kg had no effect on parasite development in vivo (11).
Unlike B. duncani, the bovine Babesia parasites B. bovis and B. divergens have been shown to be highly susceptible to atovaquone with IC50 values between 12 and 32 nm (29). It remains unknown whether the low susceptibility of B. duncani to atovaquone and other anti-babesiosis drugs is due to the molecular nature of their respective targets or to a general detoxification process unique to this parasite.
In conclusion, this novel in vitro culture sets the stage for advanced studies to elucidate the molecular mechanisms that control B. duncani metabolism, development, differentiation, transmission, and interaction with its host. The ease of harvesting B. duncani in vitro also facilitates the discovery of more effective therapies and the development of specific and sensitive diagnostic assays for early detection of active infection. Such assays could be used in epidemiological surveys to determine the incidence of duncani-babesiosis in North America and possibly other regions of the world.
Materials and methods
Parasite strain
B. duncani WA1 was originally isolated from the blood of the first reported case of babesiosis acquired in Washington State (13). The isolate was described as a novel Babesia species in 2006 (2) and deposited as cryostabilites from infected hamsters in ATCC (catalog no. PRA-302) and BEI Resources (catalog no. NR-12311) by Dr. Mark Eberhard (Centers for Disease Control and Prevention) in 2009. Cryostocks of low-passage in vitro cultures of NR-12311 in hamster RBCs were deposited by Dr. Patricia Holman (Texas A&M University) in BEI Resources in 2016.
HaRGM
HaRGM consisted of HL-1 medium (Lonza, catalog no. 344017) supplemented with 20% fetal bovine serum (ATCC, catalog no. 30-2020), 1% (v/v) HB 101 (Irvine Scientific, catalog no. T151), 10 μg/ml Albumax I (Thermo Fisher Scientific, catalog no. 11020021), 2 mm l-glutamine (ATCC, catalog no. 30-2214), 2× hypoxanthine/thymidine solution (Gibco, catalog no. 11067-030), 1× antibiotic/antimycotic solution (Gibco, catalog no. 15240-062), and 100 μg/ml gentamicin solution (Gibco, catalog no. 15750-060).
HuRGM
HuRGM consisted of HL-1 (Lonza, catalog no. 344017) or Claycomb (Sigma, catalog no. 51800C) medium supplemented with 20% human serum type A+ (Interstate Blood Bank, Inc.), 1% (v/v) HB 101 (Irvine Scientific, catalog no. T151), 200 mm l-glutamine (ATCC, catalog no. 30-2214), 2× hypoxanthine/thymidine solution (Gibco, catalog no. 11067-030), 1× antibiotic/antimycotic solution (Gibco, catalog no. 15240-062), and 100 μg/ml gentamicin solution (Gibco, catalog no. 15750-060).
Preparation of RBCs before culture
Hamster blood was collected from Golden Syrian hamsters (Harlan Laboratories, stock: HsdHan:AURA) by the peri-orbital route using heparin as anticoagulant according to protocols approved by the ATCC institutional animal care and use committee (IACUC). Type O+ human blood was obtained from Interstate Blood Bank, Inc. (Memphis TN). Blood was washed three times by centrifugation at 1800 rpm for 15 min at 4 °C in PBS, 15 mm EDTA solution. The plasma and buffy layer from the top of the RBC pellet were removed after each wash. After the last wash, RBCs were resuspended at a concentration of 50% hematocrit in Puck's saline glucose buffer with extra glucose (PSG+G; see supporting Materials and methods). RBCs were stored at 4 °C in PSG+G for up to 2 weeks before use.
Culture of B. duncani WA1 in vitro in hamster RBCs
A cryostock of B. duncani WA1 that had been passaged four times in vitro in hamster RBCs was thawed in a 37 °C water bath. A 0.5-ml aliquot of the blood was transferred to a 50-ml conical centrifuge tube, and 0.1 ml of a 12% (w/v) NaCl solution was added slowly in a dropwise fashion. Following a 5-min incubation at room temperature, 6 ml of 1.6% NaCl were added to the blood suspension in a dropwise fashion with gentle shaking. This was followed by the addition of 6 ml of HaRGM and centrifugation of the sample at 400 × g for 5 min at room temperature. The supernatant was removed carefully, and the RBC pellet was resuspended in 10 ml of HaRGM. The sample was centrifuged at 400 × g for 5 min, resuspended in 0.5 ml of warm (37 °C) HaRGM, and divided equally into two wells of a 24-well culture plate. Each well received 0.9 ml of HaRGM and 0.1 ml of fresh hamster donor RBCs in PSG+G. The plate was incubated at 37 °C under 2% O2, 5% CO2, 93% N2 atmosphere with 95% humidity using a modulator-incubator chamber (Billups-Rothenberg, Inc., catalog no. MIC-101). The culture was maintained for 16 days with replacements of HaRGM on a daily basis. 1–2-μl aliquots of blood were removed from the culture wells during each change of medium for microscopic examination of Giemsa-stained blood smears. Fresh hamster RBCs were added to the culture wells at a final hematocrit of 5% whenever the parasitemia reached a minimum of 1%.
Culture of B. duncani WA1 in human RBCs
HaRGM was removed from one well of the 16-day hamster RBC culture above that had reached 5% parasitemia. Hamster RBCs at the bottom of the well were split 1:2 into two wells of a 24-well plate. Each well received 0.9 ml of HuRGM and 0.1 ml of human donor RBCs in PSG+G. The plate was incubated at 37 °C under 2% O2, 5% CO2, 93% N2 atmosphere with 95% humidity using a modulator-incubator chamber. The culture was maintained for 2 days with daily replacements of HuRGM. Following the 2-day incubation, contents from one well were split 1:10 into 10 wells of a 24-well plate with 0.9 ml of HuRGM per well. Contents from the other well were transferred to a 25-cm2 tissue culture flask with 10 ml of HuRGM. The cultures were maintained for 3 days at 37 °C under 2% O2, 5% CO2, 93% N2 with 95% humidity using a modulator-incubator chamber. HuRGM was replaced on a daily basis, and fresh human RBCs were added at a hematocrit of 5%. Parasitemia was determined by examination of Giemsa-stained blood smears during each change of medium. Cultures were maintained for an additional 3 weeks with subcultures performed every 3–5 days into 25-cm2 flasks. For cryopreservation purposes, cultures were further expanded to 75 cm2 and frozen when parasitemia reached ≥10%. Cryostocks were prepared as described in the supporting Materials and methods and are available in BEI Resources (catalogue no. NR-50440).
Parasitemia determination
Parasitemia was determined in a minimum of 5000 RBCs/slide at ×1,000 magnification following staining of thin blood smears with Giemsa (Thermo Fisher Scientific; fixative (catalog no. 122-929), Solution I (catalog no. 122-937), and Solution II (catalog no. 122-952)). Parasite growth was also determined by the SYBR Green I assay as follows. 30 μl of culture was collected from each well of a 24-well plate and transferred into a Costar 96-well black plate and stored at −80 °C in a freezer for 1 h. Samples were then thawed and mixed with 30 μl of SYBR Green I lysis buffer consisting of 20 mm Tris, pH 7.4, 5 mm EDTA, 0.008% saponin, 0.08% Triton X-100, and 1× SYBR Green I (Molecular Probes, 10,000× solution in DMSO). The plate was incubated at room temperature in the dark for 1 h. Plates were then read on a BioTek SynergyMX fluorescence plate reader with an excitation of 497 nm and emission of 520 nm. Fluorescence readings were determined in triplicate wells.
Drug susceptibility assays
Experiments were performed to determine the susceptibility of B. duncani WA1 to increasing concentrations of atovaquone, azithromycin, clindamycin, and quinine. These assays were performed in triplicates in 96-well plates with a starting parasitemia of 1 and 5% hematocrit in 100 μl of culture medium. Cultures were incubated at 37 °C for 60 h in a chamber with a gas mixture of 2% O2, 5% CO2, and 93% N2. Cells were then collected and processed for Giemsa staining or SYBR Green I assay as described above. GraphPad Prism (version 7.0a) was used to generate sigmoidal dose–response curves by fitting a nonlinear regression curve to the data.
Indirect immunofluorescence assay
These assays were performed on coverslips following preparation of thin blood smears with 1–3 μl of B. duncani-infected human RBCs from culture. Blood smears were fixed in 1% formaldehyde at 37 °C for 30 min followed by three washes in PBS. Smears were then incubated for 30 min in a blocking buffer (5% fetal bovine serum, 5% normal goat serum, and 0.1% saponin) and rinsed once in wash buffer (0.5% fetal bovine serum, 0.5% normal goat serum, 0.05% saponin). The smears were incubated with mouse anti-Band3 (1:500) mAb (Sigma-Aldrich, catalog no. B9277) overnight at 4 °C. The next day, slides were washed three times with wash buffer and incubated with the secondary antibody goat anti-mouse coupled to Alexa Fluor 488 (1:500) at 37 °C for 1 h and rinsed three times in wash buffer. These coverslips were mounted using ProlongTM Gold Antifade Mountant with DAPI supplemented with DAPI (Invitrogen by Thermo Fisher Scientific) and further treated according to the manufacturer's instructions. Slides were visualized using a Leica TCS SP8 STED 3× confocal microscope (see below).
Animal studies
B. duncani WA1 strain was maintained in Golden Syrian hamsters according to protocols approved by the ATCC and the Yale Institutional Animal Care and Use Committee (IACUC). Rules for ending experiments in hamsters were to be enacted if animals showed any signs of distress or appeared moribund. Parasitemia was determined using standard methods of blood collection by the periorbital route (hamsters) followed by microscopic examination of Giemsa-stained blood smears.
Scanning EM
Infected cells were fixed with 2% paraformaldehyde and 2.5% glutaraldehyde in phosphate buffer for 30 min at the temperature they were cultured in and a further 30 min at 4 °C. They were spun and rinsed in phosphate-buffered saline, and drops of sample were placed onto coverslips for 30 min and then rinsed in PBS. This was replaced with 2.5% glutaraldehyde in 0.1 m sodium cacodylate buffer, pH 7.4 for a further 15 min at 4 °C. Samples were rinsed and post-fixed in 2% osmium tetroxide in 0.1 m sodium cacodylate buffer, pH 7.4, rinsed in buffer, and dehydrated through an ethanol series to 100%. The samples were dried using a Leica 300 critical point dryer with liquid carbon dioxide as transitional fluid. The coverslips were glued to aluminum stubs and sputter-coated with 5-nm platinum using a Cressington 208HR (Ted Pella) rotary sputter coater. Digital images were acquired in an FEI ESEM between 5 and 15 kV at a working distance of 12 mm.
Imaging on the Leica TCS SP8 STED 3X
The confocal images in Fig. 4 were acquired with a Leica TCS SP8 STED 3X microscope (Leica Microsystems) using a Leica HC PL APO CS2 ×100/1.40 numerical aperture oil immersion objective. The pinhole was set to 1 arbitrary unit. Alexa 488 was excited at 488 nm, and the HyD3 detector (detection window, 510–560 nm) was used for detection. DAPI was excited at 405 nm, and the HyD1 detector (detection window, 430–470 nm) was used for detection. Differential interference contrast images were acquired to visualize parasite and infected erythrocyte. Images were acquired with a scan speed of 1,000 Hz with unidirectional exposure and 6× line averaging in sequential scan mode. The image size was 51.67 × 51.67 μm (512 × 512 pixels).
Image processing
Raw confocal and SEM images were analyzed and prepared for presentation using Fiji. To determine the area of the red blood cells in the SEM images, we used the “Oval” or “Freehand selections” of Fiji (version 1.0) to trace around the plasma membrane of the red blood cell. The Huygens Professional Software (Scientific Volume Imaging) was used to deconvolve the confocal images. Fiji was then used to crop the confocal images. The Gaussian Blur filter was further applied (radius = 0.9) to smooth the images.
Author contributions
A. A., J. T., and C. B. M. data curation; A. A., J. T., and C. B. M. formal analysis; A. A., I. B., J. T., N. K., L. L., R. G., K. D., L. H., X. Y., M. G., X. L., and R. M. investigation; A. A., J. T., N. K., L. L., and C. B. M. visualization; A. A., I. B., J. T., N. K., L. L., R. G., K. D., L. H., X. Y., M. G., X. L., R. M., and C. B. M. methodology; A. A., I. B., J. T., N. K., L. L., R. G., K. D., L. H., X. Y., G. Z., M. G., X. L., R. M., and C. B. M. writing-original draft; A. A., J. T., N. K., G. Z., R. M., and C. B. M. writing-review and editing; G. Z., X. L., and C. B. M. conceptualization; C. B. M. resources; C. B. M. project administration.
Supplementary Material
Acknowledgments
We thank Dr. Patricia Holman for contributions to the initial efforts to establish an in vitro culture of B. duncani in hamster red blood cells. We also thank Dr. Maria Ciarleglio at the Yale Center for Analytical Sciences for help with statistical analyses.
The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This article contains supporting Materials and methods.
- RBC
- red blood cell
- DAPI
- 4′,6-diamidino-2-phenylindole
- SEM
- scanning EM
- HaRGM
- hamster RBC growth medium
- HuRGM
- human RBC growth medium
- IACUC
- institutional animal care and use committee.
References
- 1. Gelfand J. A., and Callahan M. V. (2003) Babesiosis: an update on epidemiology and treatment. Curr. Infect. Dis. Rep. 5, 53–58 10.1007/s11908-003-0065-z [DOI] [PubMed] [Google Scholar]
- 2. Conrad P. A., Kjemtrup A. M., Carreno R. A., Thomford J., Wainwright K., Eberhard M., Quick R., Telford S. R. 3rd, and Herwaldt B. L. (2006) Description of Babesia duncani n. sp. (Apicomplexa: Babesiidae) from humans and its differentiation from other piroplasms. Int. J. Parasitol. 36, 779–789 10.1016/j.ijpara.2006.03.008 [DOI] [PubMed] [Google Scholar]
- 3. Scott J. D., and Scott C. M. (2018) Human babesiosis caused by Babesia duncani has widespread distribution across Canada. Healthcare (Basel) 6, E49 10.3390/healthcare6020049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Leiby D. A. (2011) Transfusion-transmitted Babesia spp.: bull's-eye on Babesia microti. Clin. Microbiol. Rev. 24, 14–28 10.1128/CMR.00022-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zamoto-Niikura A., Tsuji M., Imaoka K., Kimura M., Morikawa S., Holman P. J., Hirata H., and Ishihara C. (2014) Sika deer carrying Babesia parasites closely related to B. divergens, Japan. Emerg. Infect. Dis. 20, 1398–1400 10.3201/eid2008.130061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kjemtrup A. M., and Conrad P. A. (2000) Human babesiosis: an emerging tick-borne disease. Int. J. Parasitol. 30, 1323–1337 10.1016/S0020-7519(00)00137-5 [DOI] [PubMed] [Google Scholar]
- 7. Vannier E. G., Diuk-Wasser M. A., Ben Mamoun C., and Krause P. J. (2015) Babesiosis. Infect. Dis. Clin. North Am. 29, 357–370 10.1016/j.idc.2015.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fang D. C., and McCullough J. (2016) Transfusion-transmitted Babesia microti. Transfus. Med. Rev. 30, 132–138 10.1016/j.tmrv.2016.04.002 [DOI] [PubMed] [Google Scholar]
- 9. Glanternik J. R., Baine I. L., Rychalsky M. R., Tormey C. A., Shapiro E. D., and Baltimore R. S. (2018) A cluster of cases of Babesia microti among neonates traced to a single unit of donor blood. Pediatr. Infect. Dis. J. 37, 269–271 10.1097/INF.0000000000001803 [DOI] [PubMed] [Google Scholar]
- 10. Krause P. J., Lepore T., Sikand V. K., Gadbaw J. Jr., Burke G., Telford S. R. 3rd, Brassard P., Pearl D., Azlanzadeh J., Christianson D., McGrath D., and Spielman A. (2000) Atovaquone and azithromycin for the treatment of babesiosis. N. Engl. J. Med. 343, 1454–1458 10.1056/NEJM200011163432004 [DOI] [PubMed] [Google Scholar]
- 11. Lawres L. A., Garg A., Kumar V., Bruzual I., Forquer I. P., Renard I., Virji A. Z., Boulard P., Rodriguez E. X., Allen A. J., Pou S., Wegmann K. W., Winter R. W., Nilsen A., Mao J., et al. (2016) Radical cure of experimental babesiosis in immunodeficient mice using a combination of an endochin-like quinolone and atovaquone. J. Exp. Med. 213, 1307–1318 10.1084/jem.20151519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Persing D. H., Herwaldt B. L., Glaser C., Lane R. S., Thomford J. W., Mathiesen D., Krause P. J., Phillip D. F., and Conrad P. A. (1995) Infection with a babesia-like organism in northern California. N. Engl. J. Med. 332, 298–303 10.1056/NEJM199502023320504 [DOI] [PubMed] [Google Scholar]
- 13. Quick R. E., Herwaldt B. L., Thomford J. W., Garnett M. E., Eberhard M. L., Wilson M., Spach D. H., Dickerson J. W., Telford S. R. 3rd, Steingart K. R., Pollock R., Persing D. H., Kobayashi J. M., Juranek D. D., and Conrad P. A. (1993) Babesiosis in Washington State: a new species of Babesia? Ann. Intern. Med. 119, 284–290 10.7326/0003-4819-119-4-199308150-00006 [DOI] [PubMed] [Google Scholar]
- 14. Bloch E. M., Herwaldt B. L., Leiby D. A., Shaieb A., Herron R. M., Chervenak M., Reed W., Hunter R., Ryals R., Hagar W., Xayavong M. V., Slemenda S. B., Pieniazek N. J., Wilkins P. P., and Kjemtrup A. M. (2012) The third described case of transfusion-transmitted Babesia duncani. Transfusion 52, 1517–1522 10.1111/j.1537-2995.2011.03467.x [DOI] [PubMed] [Google Scholar]
- 15. Herwaldt B. L., Kjemtrup A. M., Conrad P. A., Barnes R. C., Wilson M., McCarthy M. G., Sayers M. H., and Eberhard M. L. (1997) Transfusion-transmitted babesiosis in Washington State: first reported case caused by a WA1-type parasite. J. Infect. Dis. 175, 1259–1262 10.1086/593812 [DOI] [PubMed] [Google Scholar]
- 16. Kjemtrup A. M., Lee B., Fritz C. L., Evans C., Chervenak M., and Conrad P. A. (2002) Investigation of transfusion transmission of a WA1-type babesial parasite to a premature infant in California. Transfusion 42, 1482–1487 10.1046/j.1537-2995.2002.00245.x [DOI] [PubMed] [Google Scholar]
- 17. Cullen J. M., and Levine J. F. (1987) Pathology of experimental Babesia microti infection in the Syrian hamster. Lab. Anim. Sci. 37, 640–643 [PubMed] [Google Scholar]
- 18. Wozniak E. J., Lowenstine L. J., Hemmer R., Robinson T., and Conrad P. A. (1996) Comparative pathogenesis of human WA1 and Babesia microti isolates in a Syrian hamster model. Lab. Anim. Sci. 46, 507–515 [PubMed] [Google Scholar]
- 19. Moro M. H., David C. S., Magera J. M., Wettstein P. J., Barthold S. W., and Persing D. H. (1998) Differential effects of infection with a Babesia-like piroplasm, WA1, in inbred mice. Infect. Immun. 66, 492–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dao A. H., and Eberhard M. L. (1996) Pathology of acute fatal babesiosis in hamsters experimentally infected with the WA-1 strain of Babesia. Lab. Invest. 74, 853–859 [PubMed] [Google Scholar]
- 21. Thomford J. W., Conrad P. A., Telford S. R. 3rd, Mathiesen D., Bowman B. H., Spielman A., Eberhard M. L., Herwaldt B. L., Quick R. E., and Persing D. H. (1994) Cultivation and phylogenetic characterization of a newly recognized human pathogenic protozoan. J. Infect. Dis. 169, 1050–1056 10.1093/infdis/169.5.1050 [DOI] [PubMed] [Google Scholar]
- 22. Swei A., O'Connor K. E., Couper L. I., Thekkiniath J., Conrad P. A., Padgett K. A., Burns J., Yoshimizu M. H., Gonzales B., Munk B., Shirkey N., Konde L., Ben Mamoun C., Lane R. S., and Kjemtrup A. (2018) Evidence for transmission of the zoonotic apicomplexan parasite Babesia duncani by the tick Dermacentor albipictus. Int. J. Parasitol. S0020-7519(18)30243-1 10.1016/j.ijpara.2018.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rossouw I., Maritz-Olivier C., Niemand J., van Biljon R., Smit A., Olivier N. A., and Birkholtz L. M. (2015) Morphological and molecular descriptors of the developmental cycle of Babesia divergens parasites in human erythrocytes. PLoS Negl. Trop. Dis. 9, e0003711 10.1371/journal.pntd.0003711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Scholtens R. G., Braff E. H., Healey G. A., and Gleason N. (1968) A case of babesiosis in man in the United States. Am. J. Trop. Med. Hyg. 17, 810–813 10.4269/ajtmh.1968.17.810 [DOI] [PubMed] [Google Scholar]
- 25. Lambros C., and Vanderberg J. P. (1979) Synchronization of Plasmodium falciparum erythrocytic stages in culture. J. Parasitol. 65, 418–420 10.2307/3280287 [DOI] [PubMed] [Google Scholar]
- 26. Braga W., Venasco J., Willard L., and Moro M. H. (2006) Ultrastructure of Babesia WA1 (Apicomplexa: Piroplasma) during infection of erythrocytes in a hamster model. J. Parasitol. 92, 1104–1107 10.1645/GE-712R.1 [DOI] [PubMed] [Google Scholar]
- 27. Dahl E. L., and Rosenthal P. J. (2007) Multiple antibiotics exert delayed effects against the Plasmodium falciparum apicoplast. Antimicrob. Agents Chemother. 51, 3485–3490 10.1128/AAC.00527-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wilson D. W., Goodman C. D., Sleebs B. E., Weiss G. E., de Jong N. W., Angrisano F., Langer C., Baum J., Crabb B. S., Gilson P. R., McFadden G. I., and Beeson J. G. (2015) Macrolides rapidly inhibit red blood cell invasion by the human malaria parasite, Plasmodium falciparum. BMC Biol. 13, 52 10.1186/s12915-015-0162-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Paul A. S., Moreira C. K., Elsworth B., Allred D. R., and Duraisingh M. T. (2016) Extensive shared chemosensitivity between malaria and babesiosis blood-stage parasites. Antimicrob. Agents Chemother. 60, 5059–5063 10.1128/AAC.00928-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.