Summary/Abstract
In this chapter we describe the use of stopped flow fluorescence spectroscopy to analyze the kinetic mechanisms of protein mediated cholesterol transfer to, from, and between model membranes. These assays allow for the detection of protein-membrane interactions that may occur during cholesterol transfer by simply modifying donor or acceptor concentrations, membrane composition, or buffer properties, and analyzing resultant transfer rates.
Keywords: Collisional transfer, diffusional transfer, cholesterol, cholesterol trafficking, FRET, stopped flow, fluorescence, model membranes, small unilamellar vesicles
1. Introduction
Much is currently known about cholesterol synthesis and metabolism, about its extracellular transport in plasma lipoproteins and about the uptake of cholesterol-rich low density lipoprotein (LDL) via receptor- mediated endocytosis of the LDL receptor. Intracellular trafficking of cholesterol is crucial for maintaining cholesterol homeostasis, yet many questions remain regarding the mechanisms of intracellular cholesterol transport.
Cholesterol is extremely hydrophobic, and its transport has been shown to occur via vesicular and protein mediated mechanisms (1). Several of the processes involved in intracellular sterol transport, however, are still unclear. The lysosomal storage disorder Niemann Pick type C (NPC) disease presents a valuable model for understanding some of these processes, specifically with regards to the egress of LDL-derived cholesterol from the endosomal/lysosomal system. In NPC disease, LDL-derived cholesterol accumulates in the late endosomal/lysosomal (LE/LY) compartment, leading to lysosomal and cellular dysfunction. Studies have identified two endo/lysosomal proteins, NPC1 and NPC2, which are necessary for normal transport of cholesterol out of the LE/LY compartment. Questions remain, however, regarding the mechanism(s) by which these proteins mediate LE/LY cholesterol egress. Given the critical nature of normal LE/LY cholesterol egress in maintaining cellular and whole-body cholesterol homeostasis, elucidating the mechanism(s) by which this transport occurs is of great fundamental importance.
In the present chapter, we describe an in vitro approach that utilizes purified NPC2 protein and model membranes to determine whether the mechanism of protein mediated cholesterol transport involves protein-membrane interactions. Two of the three assays described utilize the intrinsic fluorescence of tryptophan residues in the cholesterol-binding NPC2 protein to monitor movement of cholesterol from the protein to membranes, and from membranes to the protein. The third approach detailed below employs the fluorescent cholesterol analog dehydroergosterol (DHE) and its fluorescence resonance energy transfer (FRET) partner dansyl, as part of dansyl-phosphotidylethanolamine (Dansyl-PE), in two independent vesicle populations for determining the mechanism of protein-mediated cholesterol transport between membranes. All three approaches can also be utilized to elucidate the transport mechanism of sterol binding proteins other than NPC2, however it is important to note that variations in the protocols may be necessary. In particular, if cholesterol binding does not modulate the intrinsic tryptophan fluorescence of the protein of interest, fluorescent cholesterol analogs such as DHE or cholestatrienol (CTE) can be instead utilized in a FRET pairing to monitor cholesterol transport, in lieu of changes in tryptophan fluorescence.
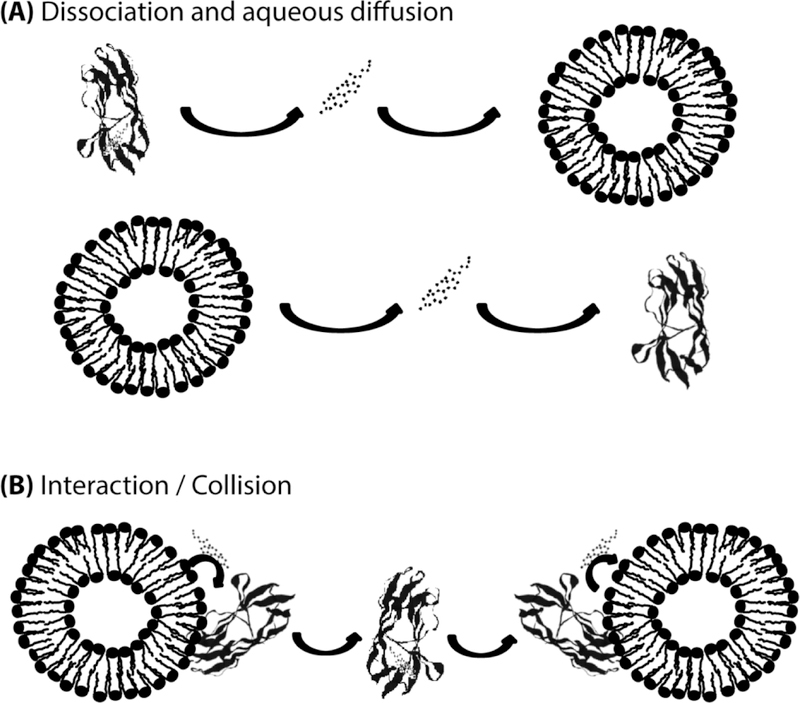
The basis of the three assays is similar; all are used to distinguish between the presence of a diffusional versus collisional mechanism of cholesterol transport. In short, if transfer involves the diffusion of sterol through an aqueous medium, the rate limiting step is dissociation of sterol from protein or membrane (Fig. 1A), which is unaffected by the acceptor. Thus, no change in the rate of sterol transfer would be observed as the concentration of donor or acceptor (either protein or membrane) increases (2,3). In contrast, the rate of sterol transfer for a collisional mechanism is proportional to the frequency of collisions between the protein and membrane (Fig. 1B), which is determined by the donor and acceptor concentrations (2,3). Therefore, if protein-membrane interactions are integral to the transfer of cholesterol between protein and membrane, the rate of transfer will increase as either the concentration of protein or the concentration of model membranes increases.
Figure 1. Potential mechanisms of cholesterol transport between protein and membranes.
(A) Aqueous diffusion of cholesterol between protein and membrane, in which the rate of sterol transfer is limited by the dissociation of cholesterol from the protein. (B) In a collisional mechanism, the rate of sterol transfer is proportional to the number of interactions between protein and membrane.
Factors beyond donor and acceptor concentration can also elicit changes in sterol transfer rates, and these properties can be exploited in the outlined assays as an additional means of distinguishing between collisional versus diffusional mechanisms of cholesterol transport. One such property is the phospholipid composition of membranes; if changes in sterol transfer rates are observed between membranes with varying phospholipid compositions, it is likely that the protein is directly interacting with membranes to transfer sterol. In the case of NPC2, as shown below, rates of sterol transfer from the protein to membranes increase dramatically with the incorporation of lyso-bis phosphotidic acid (LBPA), a unique LE/LY phospholipid, in zwitterionic phosphotidylcholine acceptor membranes, supporting a collisional transfer mechanism (4,5). Buffer properties like pH and ionic strength can also affect rates of diffusional sterol transfer by proteins. For instance, the solubility of hydrophobic compounds decreases as ionic strength of the aqueous medium increase. Thus, sterol transfer rates would decrease as the salt concentration of the buffer increases for a diffusional, but not a collisional transfer mechanism (4,6).
There are important advantages to utilizing stopped flow mixing in determining mechanisms of sterol transfer by proteins. First, rates of sterol transfer between protein and membranes can be quite rapid and may otherwise be undetectable in instances where either manual mixing is used, or with instrumentation that has considerable dead time prior to data acquisition. Methods that require physical separation of protein from membrane, such as centrifugation or column separation, are also unlikely to be able to detect rates of sterol movement; rather, these methods provide only the equilibrium distribution of the sterol between the protein and the membrane, i.e. the amount of ligand that can transfer given certain concentrations of protein and membranes, but not the rate at which the ligand transfers. Using stopped- flow mixing, on the other hand, we have for example been able to detect cholesterol transfer rates from the NPC2 protein to membranes containing LBPA of approximately 5.0 s−1 (half-time of about 0.14 seconds) (4). In the case of NPC2, we are able to use unmodified protein (e.g. no tags or labels) and the native ligand, cholesterol, thereby obtaining rates of sterol transfer that are likely to be physiologically relevant. Thus, in contrast to the stopped flow-based assays outlined in this chapter, equilibrium methods cannot be used to determine the mechanism(s) by which a sterol binding protein transfers ligand.
2. Materials
Sodium citrate buffer: 20 mM sodium citrate, 150 mM NaCl, pH 5.0 (see Note 1)
Delipidated protein (see Note 2)
Cholesterol (Sigma Chemical Company, St. Louis, MO, USA). Resuspend in DMSO to a concentration between 500 µM and 2 mM, and store at −20°C protected from light. (see Note 3 )
Egg phosphatidylcholine (EPC) (Avanti Polar Lipids, Alabaster, AL, USA). Store at −20°C.
Dehydroergosterol (DHE). (Avanti Polar Lipids, Alabaster, AL, USA). Protect from light and store at −20°C.
Dansyl-phosphotidylethanolamine (Dansyl-PE) (Avanti Polar Lipids, Alabaster, AL, USA). Protect from light and store at −20°C.
Nitrogen evaporator
Lyophilizer
Water bath
Sonicator equipped with a ½” diameter tapped bio horn (probe) with a removal flat tip.
Ultracentrifuge and fixed angle rotor.
Stopped flow spectrofluorometer and accompanying software
299 nm Long Pass filter for detection of tryptophan fluorescence (used with excitation wavelength of 280 nm).
370 nm Narrow Band filter for detection of DHE fluorescence (used with excitation wavelength of 323 nm).
515 nm Long Pass filter for detection of Dansyl-PE fluorescence (used in a FRET pair with the DHE excitation wavelength of 323 nm).
A suitable assay for determining phospholipid concentration of vesicles via quantification of inorganic phosphate (7).
3. Methods
All procedures are performed at room temperature unless otherwise noted.
3.1. Membrane vesicle preparation.
In a clean, dry, glass tube, prepare 1 mL of a 3 mM lipid solution in chloroform. Refer to specific transfer assay for necessary phospholipid compositions.
Dry the preparation under nitrogen.
Lyophilize overnight to remove trace solvent.
Hydrate the dried film in 3 mL buffer (citrate buffer or other, see Section 2) to generate a 1mM suspension of phospholipid.
Sonicate under nitrogen using a ½” diameter flat tip probe for 45 minutes at 4°C in order to avoid overheating, and thus degradation, of the lipid suspension. (On a Branson Sonifier 450, set the duty cycle to 70% and output control to 3) (see Note 4).
Ultracentrifuge the sonicated supernatant at 105,000 g for 45 min in order to remove titanium residue and any remaining multi-lamellar vesicles (MLVs).
Carefully remove the supernatant containing the SUVs and transfer to a brown vial. Keep on ice for immediate use or store at 4°C for up to one week (for EPC vesicles).
Determine phospholipid concentration of vesicles via quantification of inorganic phosphate (see Note 5).
3.2. Cholesterol transfer from protein to membranes.
Transfer assays are performed using an SX20 Stopped Flow Spectrofluorometer (Applied Photophysics, UK), with a 20 µL SF cell volume and 1.1 ms deadtime, equipped with a water bath chamber. Settings may need to be adjusted if alternate equipment is used.
Prepare 100 mol% EPC SUVs (or other compositions, as desired) as per Section 3.1.
Turn on stopped flow lamp and allow it to warm up.
Set water bath to 25°C or desired temperature, and allow temperature to equilibrate.
Set the excitation wavelength on the stopped flow to 280 nm and equip with a 299 nm long pass filter. Initially, set the monochromater slits to 0.5/0.5.
Prepare 100 µM, 200 µM and 500 µM samples of EPC SUVs in buffer. Keep on ice while not in use.
Prepare a 5 µM holo-protein sample by incubating 5 µM protein in buffer with 5 µM cholesterol (final concentration) using the DMSO stock solution, for at least 20 min at room temperature. Final DMSO level should always be kept at less than 1 % (v/v). Keep on ice while not in use.
Load holo-protein preparation (donor) and buffer into the sample syringes in order to obtain blank readings. (see Note 6)
Trigger mixing of the donor with the buffer solutions. Acquire tryptophan fluorescence readings (arbitrary units) in triplicate over a period of 200 seconds. Adjust instrument settings to ensure absence of photobleaching (see Note 7)
Load holo-protein preparation (donor) and SUVs (acceptor) into the sample syringes.
Trigger mixing. Acquire tryptophan fluorescence readings in triplicate over a period of 200 seconds (Fig. 2).
Average the readings and fit curves using a single exponential function. If unavoidable photobleaching remained during blank readings be sure to subtract these curves prior to obtaining transfer rates via curve fitting.
Repeat steps 9 – 11 using increasing concentrations of acceptor SUVs. If the rate of transfer increases as the concentration of acceptor SUVs increases, as in Fig. 3, the protein is likely directly interacting with membranes to transfer sterol, as the rate of collisional transfer is proportional to the product of the donor and acceptor concentration (2,3). If instead the rate of transfer remains constant as the concentration of acceptor SUVs increases, the protein is likely utilizing a diffusional mechanism as the rate limiting step in this mode of transfer is the off rate of the cholesterol from the protein (2,3) into the aqueous phase.
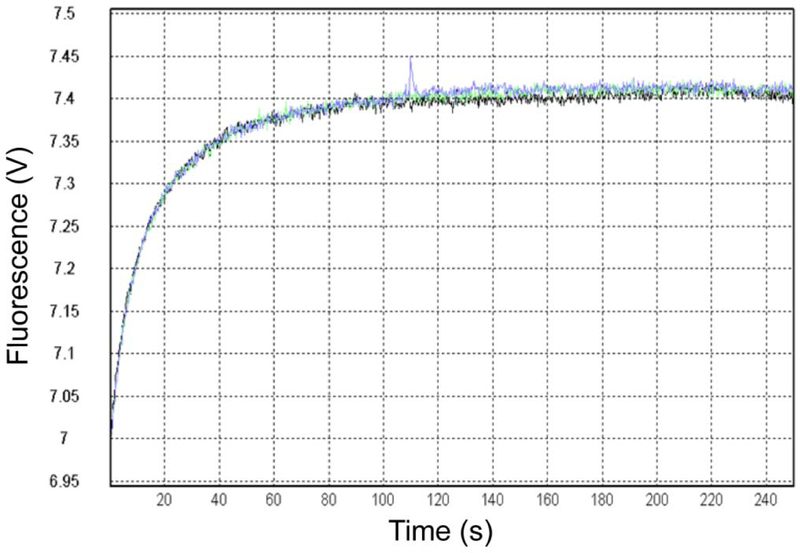
Figure 2. Transfer of cholesterol from NPC2 to membranes.
Example acquisition, in triplicate, monitoring tryptophan dequenching over time as 1 µM cholesterol is transferred from 1 µM NPC2 to 250 µM EPC SUVs. Curves were fit with a single exponential function.
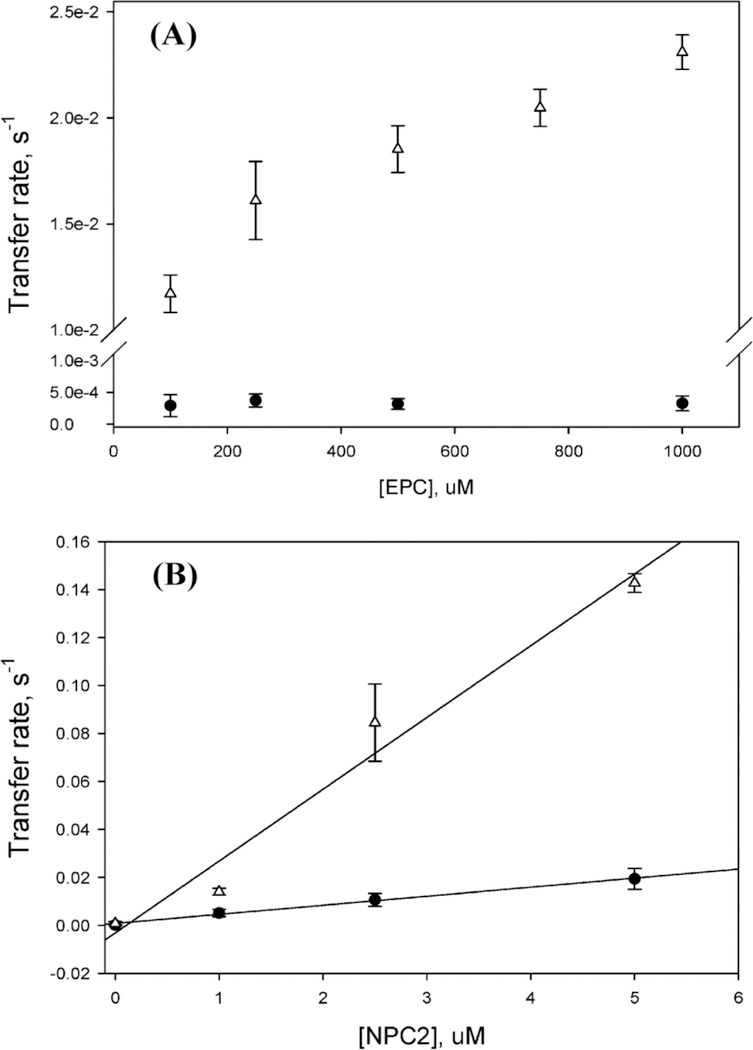
Figure 3. Transfer of cholesterol from membranes to NPC2.
Rates of NPC2 tryptophan quenching were determined for the transfer of 2.5 µM cholesterol from 250 µM SUVs to increasing concentrations of apo-NPC2 protein. The observed increase in transfer rate from donor membranes to NPC2 as the protein acceptor concentration increases indicates the presence of protein-membrane interactions. Reprinted with permission from Xu et al (2008) Regulation of sterol transport between membranes and NPC2. Biochemistry 47(42):11134–11143. Copyright 2016 American Chemical Society.
3.3. Cholesterol transfer from membranes to protein.
Prepare 90 mol% EPC, 10 mol% cholesterol SUVs (or other sterol compositions, as desired) as per Section 3.1.
Turn on stopped flow lamp and allow it to warm up.
Set water bath to 25°C or desired temperature, and allow temperature to equilibrate.
Set the excitation wavelength on the stopped flow to 280 nm and equip with a 299 nm long pass filter
Prepare 1 µM, 2 µM, 5 µM 10 µM and 20 µM of your delipidated protein (apo-protein) in buffer. Keep on ice while not in use.
Prepare a 200 µM EPC/cholesterol SUV sample in buffer (see Note 8). Keep on ice while not in use.
Load apo-protein preparation (acceptor) and buffer into the sample syringes in order to obtain blank readings. (see Note 6)
Trigger mixing. Acquire tryptophan fluorescence readings (arbitrary units) in triplicate over a period of at least 200 seconds. Adjust instrument settings to ensure absence of photobleaching (see Note 7)
Load cholesterol containing SUVs (donor) and apo-protein (acceptor) into the sample syringes.
Trigger mixing. Acquire fluorescence readings in triplicate over a period of at least 200 seconds.
Average the readings and fit curves using exponential fitting (see Note 9). If photobleaching was unavoidable during blank readings be sure to subtract these curves prior to obtaining transfer rates via curve fitting.
Repeat steps 9 – 11 with increasing concentrations of donor SUVs; evaluate the results as described above (under Section 3.2, step 12), to determine whether a collisional mechanism or diffusional mechanism of transfer is employed by the protein to extract cholesterol from membranes.
3.4. Intermembrane transfer of sterol.
Prepare two populations of SUVs as per Section 3.1; donor vesicles contain 75 mol% EPC and 25 mol% DHE; acceptor vesicles contain 97 mol% EPC and 3 mol% Dansyl-PE, serving as an energy transfer quencher of DHE fluorescence.
Turn on stopped flow lamp and allow it to warm up.
Set water bath to 25°C or desired temperature, and allow temperature to equilibrate.
Set the excitation wavelength on the stopped flow to 323 nm and equip with a 370 nm narrow band filter
Prepare a 100 µM sample of DHE-containing (donor) SUVs in buffer. Keep on ice while not in use.
Prepare 100 µM, 200 µM and 500 µM samples of Dansyl-PE containing (acceptor) SUVs in buffer. Keep on ice while not in use.
Prepare at least three additional samples of the 100 µM, 200 µM and 500 µM Dansyl-PE containing (acceptor) SUVs with 2 µM apo-protein.
Finally, prepare at least three additional samples of 500 µM Dansyl-PE containing (acceptor) SUVs with 2 µM, 5 µM and 10 µM apo-protein.
Load DHE containing (donor) SUVs and buffer into the sample syringes in order to obtain blank readings.
Trigger mixing. Acquire relative DHE fluorescence readings (arbitrary units) in triplicate over a period of at least 200 seconds and up to 1000 seconds. Adjust instrument settings to ensure absence of DHE photobleaching (see Note 10)
Rinse drive syringes and sample chamber well with buffer. Change to a 515 nm cut on filter.
Load Dansyl-PE containing (acceptor) SUVs and buffer into the sample syringes in order to obtain blank readings.
Trigger mixing. Acquire relative Dansyl fluorescence readings (arbitrary units) in triplicate over a period of at least 200 seconds and up to 1000 seconds. Further adjust instrument settings if necessary to ensure absence of Dansyl photobleaching.
Load DHE containing (donor) SUVs and Dansyl-PE containing (acceptor) SUVs into the sample syringes in order to determine spontaneous rates of DHE (cholesterol) transfer between membranes, i.e. in the absence of protein.
Trigger mixing and acquire relative Dansyl fluorescence readings in triplicate over a period of at least 200 seconds and up to 1000 seconds. (see Note 11)
Average the readings and fit curves using exponential fitting. If photobleaching was unavoidably present during blank readings, be sure to subtract these curves prior to obtaining transfer rates via curve fitting. (see Note 12)
Repeat steps 14 – 16 with increasing concentrations of acceptor vesicles. The spontaneous rate of DHE transfer between membranes occurs via a diffusional mechanism and therefore should not change as acceptor vesicle concentration increases (Fig. 4A) (5,8,9).
Load DHE containing (donor) SUVs and 100 µM Dansyl-PE containing (acceptor) SUVs, together with 2 µM apo-protein into the sample syringes.
Trigger mixing. Acquire Dansyl fluorescence readings in triplicate over a period of at least 200 seconds.
Average the readings and fit curves using exponential fitting (see Note 9). If photobleaching was unavoidable during blank readings be sure to subtract theses curves prior to obtaining transfer rates via curve fitting.
Repeat steps 18 – 20 with increasing concentrations of Dansyl-PE (acceptor) SUVs, each containing 2 µM apo-protein, to determine whether protein-membrane interactions are present during transfer (Fig. 4A).
Rinse drive syringes and sample chamber well with buffer.
Load DHE containing (donor) SUVs and Dansyl-PE containing (acceptor) SUVs, together with 2 µM protein into the sample syringes.
Trigger mixing. Acquire relative Dansyl fluorescence readings in triplicate over a period of at least 200 seconds.
Average the readings and fit curves using exponential fitting (see Note 9). If photobleaching was unavoidable during blank readings, be sure to subtract these curves prior to obtaining transfer rates via curve fitting.
Repeat steps 23 – 25 with Dansyl-PE (acceptor) SUVs containing increasing concentrations of protein to determine whether a collisional mechanism or diffusional mechanism of transfer is employed by the protein to transport sterol between membranes. (Fig. 4B) (see Note 13).
Figure 4. Transfer of DHE between two membrane populations.
(A) Transfer of DHE from 50 µM donor SUVs (75/25 EPC/DHE) to increasing concentrations of acceptor SUVs (97/3 EPC/Dansyl-PE) in the presence (Δ) of 1 µM NPC2 suggests a collisional mechanism of transfer. In the absence (●) of NPC2, no change in transfer rates is observed, demonstrating the characteristics of a diffusional transfer mechanism . (B) Variable transfer rates of DHE from 50 μM donor SUV (50/25/25 EPC/LBPA/DHE) to 250 μM Dansyl-PE containing acceptor SUV with (Δ) or without (●) LBPA in the presence of increasing concentrations of NPC2 is indicative of a collisional transfer mechanism. Reprinted with permission from Xu et al (2008) Regulation of sterol transport between membranes and NPC2. Biochemistry 47(42):11134–11143. Copyright 2016 American Chemical Society.
4. Notes
Sodium citrate buffer was employed in our studies to mimic the pH of the endo/lysosomal system, where the soluble NPC2 protein resides. Modify the buffer as necessary to reflect the pH of the inherent cellular compartment of the protein of interest.
Acetone precipitation is an effective way to delipidate purified NPC2 protein samples (10). Avoid acetone contamination of your sample, which can be detected by a peak at approximately 400 nm (Fig. 5), by ensuring the sample has been sufficiently dried. Conversely, be careful not to overdry the pellet prior to resuspending in buffer, or it will be difficult to solubilize the protein. Further note that we tended to experience significant losses in protein yield following precipitation. Thus, in instances where little purified protein is available it may be possible to bypass this step; we did observe similar kinetics between samples that were and were not delipidated following purification.
The suggested cholesterol stock concentration of 500 µM to 2 mM is based on maintaining a final DMSO concentration of at least <1% (v/v) in holo-protein preparations. Thus, the stock concentration of cholesterol will need to be determined based on the concentrations of holo- protein to be used in transfer assays, most notably the membrane to protein assays where increasing concentrations of protein are utilized.
When sonicating the lipid suspension for production of SUVs, the suspension needs to be kept at a temperature that is higher than the phase transition temperature of the membrane lipid component with the lowest melting point. If this cannot be achieved with probe sonication, a water bath sonicator may also be used. Ultra-centrifugation will still be necessary in order to remove multi-lamellar vesicles (MLVs).
If re-using an SUV preparation after several days of storage at 4°C, it is advisable to ultracentrifuge the sample and repeat the phospholipid quantification assay to account for aggregation of some of the vesicles.
Although we acquire blank readings for both donor and acceptor, it is especially critical to ensure that tryptophan fluorescence of the protein alone remains steady over the assay period.
If photobleaching is present, first reduce the monochromater slits; we have acquired data successfully at 0.1/0.1 settings. Alternatively, we have also experienced success by modifying the excitation wavelength by up to 10 nm. If some decrease in signal intensity over time remains after adjustments have been made, be sure to subtract these curves from the final data sets prior to determining transfer rates.
When using the protein as an acceptor (i.e when assaying the rate of sterol transfer from membrane to protein), it is crucial to measure the equilibrium distribution of sterol between protein and phospholipid membranes prior to deciding on the concentrations of membranes and protein to be used in the transfer assays. The relative partition coefficient will indicate the amount of acceptor protein needed to obtain the minimum1:1 relative partition ratio of membrane (donor) to protein (acceptor). A relative partition of 1:1 (and 1: >1) is required to ensure that unidirectional movement of sterol is monitored in the transfer assays. If relative partition is not determined and insufficient acceptor protein is used in the transfer assay, it is possible that back- transfer will obscure the true rate of sterol movement from membrane to protein (5,11,12,13).
When cholesterol or DHE are incorporated into donor membranes, it may be necessary to use a double exponential function to fit curves due to flip-flop of the sterol in membranes. This flip- flop will be observed as the kslow while actual transfer of sterol from donor to acceptor will be the kfast (3).
We tended to experience photobleaching often with DHE, and have had success by reducing the excitation wavelength to between 270 and 300 nm, in addition to reducing the monochromater slits.
Intermembrane transfer of DHE can either be measured by DHE quenching or by the sensitized emission of Dansyl fluorescence, as described. To monitor the former, replace the 370 nm narrow band filter and repeat the assay. Rates of transfer should be the same regardless of the fluorescence monitored.
The spontaneous rate of sterol transfer between membranes is slow; reported rates are in the range of 0.0003 s−1 (5,8,9).
In instances of limited protein supply, we have instead kept the concentration of protein in the assay constant while increasing the concentration of acceptor Dansyl-PE containing vesicles, as previously outlined in the protocol.
Figure 5. Acetone contamination in delipidated protein sample.
Pure acetone (black trace) and a delipidated NPC2 protein sample in buffer (gray trace) were excited at 280 nm and emission spectrum were acquired. Acetone contamination in the protein sample indicates insufficient drying of the protein pellet following precipitation.
Acknowledgements
This work was supported by the Ara Parseghian Medical Research Foundation (J.S.), the American Heart Association (L.M. and J.S) and the National Institute of Health (J.S.) (GM 115866).
References
- 1.Soccio RE, Breslow JL (2004) Intracellular cholesterol transport. Arterioscler Thromb Vasc Biol 24(7):1150–1160 [DOI] [PubMed] [Google Scholar]
- 2.Roseman MA, Thompson TE (1980) Mechanism of the spontaneous transfer of phospholipids between bilayers. Biochemistry 19(3):439–444 [DOI] [PubMed] [Google Scholar]
- 3.Storch J, Kleinfeld AM (1986) Transfer of long-chain fluorescent free fatty acids between unilamellar vesicles. Biochemistry 25(7):1717–1726 [DOI] [PubMed] [Google Scholar]
- 4.Cheruku S, Xu Z, Dutia R et al. (2006) Mechanisms of cholesterol transfer from the Niemann-Pick Type C2 protein to model membranes supports a role in lysosomal cholesterol transport. J Biol Chem 281(42):31594–31604 [DOI] [PubMed] [Google Scholar]
- 5.Xu Z, Farver W, Kodukula S, Storch J (2008) Regulation of sterol transport between membranes and NPC2. Biochemistry 47(42):11134–11143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim HK and Stoch J (1992) Free fatty acid transfer from rat liver fatty acid-binding protein to phospholipid vesicles. J Biol Chem 267(1):77–82 [PubMed] [Google Scholar]
- 7.Gomori G (1942) A modification of colorimetric phosphorus determination for use with photoelectric colorimeter. J Lab Clin Med 27:955 [Google Scholar]
- 8.Backer JM and Dawidowicz EA (1981) Mechanism of cholesterol exchange between phospholipid vesicles. Biochemistry 20:3805–3810 [DOI] [PubMed] [Google Scholar]
- 9.Neufeld EB, Cooney AM, Pitha J et al. (1996) Intracellular trafficking of cholesterol monitored with a cyclodextrin. J. Biol. Chem 271:21604–21613 [DOI] [PubMed] [Google Scholar]
- 10.Liou HL, Dixit SS, Xu S et al. (2006) NPC2, the protein deficient in Niemann–Pick C2 disease, consists of multiple glycoforms that bind a variety of sterols. J Biol Chem 281(48):36710–36723 [DOI] [PubMed] [Google Scholar]
- 11.Nichols JW and Pagano RE (1981) Kinetics of soluble lipid monomer diffusion between vesicles. Biochemistry 20(10):2783–2789 [DOI] [PubMed] [Google Scholar]
- 12.Storch J and Bass NM (1990) Transfer of fluorescent fatty acids from liver and heart fatty acid- binding proteins to model membranes. J Biol Chem 265:7827–7831 [PubMed] [Google Scholar]
- 13.Herr FM, Li E, Weinberg RB et al. (1999) Differential mechanisms of retinoid transfer from cellular retinol binding proteins types I and II to phospholipid membranes. J Biol Chem 274(14):9556–9563 [DOI] [PubMed] [Google Scholar]