Abstract
Reactions involving thiol biochemistry seem to play a crucial role in skeletal muscle fatigue. N-acetylcysteine amide (NACA) and L-ergothioneine (ERGO) are thiol-based antioxidants available for human use that have not been evaluated for effects on muscle fatigue.
Purpose:
To test the hypothesis that NACA and ERGO delay skeletal muscle fatigue.
Methods:
We exposed mouse diaphragm fiber bundles to buffer (CTRL), NACA, ERGO, or N-acetylcysteine (NAC; positive control). Treatments were performed in vitro using 10 mM for 60 min at 37°C. After treatment, we determined the muscle force–frequency and fatigue characteristics.
Results:
The force–frequency relationship was shifted to the left by ERGO and to the right by NACA compared with CTRL and NAC. Maximal tetanic force was similar among groups. The total force–time integral (FTI; N·s·cm−2) during the fatigue trial was decreased by NACA (420 ± 35, P < 0.05), unaffected by ERGO (657 ± 53), and increased by NAC (P < 0.05) compared with CTRL (581 ± 54). The rate of contraction (dF/dtMAX) during the fatigue trial was not affected by any of the treatments tested. NAC, but not NACA or ERGO, delayed the slowing of muscle relaxation (dF/dtMIN) during fatigue.
Conclusions:
In summary, NACA and ERGO did not delay skeletal muscle fatigue in vitro. We conclude that these antioxidants are unlikely to improve human exercise performance.
Keywords: N-ACETYLCYSTEINE, ANTIOXIDANTS, DIAPHRAGM, MUSCLE FORCE
Skeletal muscle fatigue limits exercise performance by healthy subjects and patients with chronic diseases. Recent evidence also suggests that fatigue of the diaphragm muscle contributes to failure to wean patients from mechanical ventilation (5). In a recent review (11), we proposed that reactions involving thiol biochemistry play a crucial role in skeletal muscle fatigue.
To our knowledge, N-acetylcysteine (NAC) is the only thiol-containing compound available to humans that consistently delays fatigue in vitro (6,10,19) and in vivo (18,26,33,36). However, the unpleasant smell, taste, and dose-dependent adverse reactions of NAC limit its wide-spread use as an ergogenic aid. Thus, it is important to seek other antioxidants with potential to delay fatigue and minimize adverse reactions. Increases in intracellular glutathione content seem to mediate the fatigue-sparing effects of NAC (10,35). However, the sulfur-containing compound L-2-oxothiazolidine-4-carboxylate (OTC) delays in vitro skeletal muscle fatigue independent of changes in glutathione content (10). Thus, our working hypothesis is that sulfur-based antioxidants delay muscle fatigue.
There are several sulfur-based antioxidants with potential to delay muscle fatigue that are available for humans. For example, N-acetylcysteine amide (NACA) was developed in the last decade as a modified form of NAC (3) in which an amide group neutralizes the negative charge of the carboxyl region. This modification increases NACA membrane transport such that lower concentrations of NACA than NAC are required to alter some cellular processes (14). Theoretically, NACA might improve exercise performance in humans at lower doses than NAC, lessening or eliminating adverse reactions, and therefore is a candidate for preclinical testing.
L-ergothioneine (ERGO) is a sulfur-based antioxidant found in human and animal diets (9,16). ERGO is membrane impermeable; transport to the intracellular space occurs through an organic cation transporter named OCTN1 (21,25). OCTN1 mRNA is expressed in skeletal muscle (21), and dietary ERGO accumulates in rodent muscles (29). These findings suggest that skeletal muscle expresses OCTN1 protein and transports ERGO to the sarcoplasm. Thus, ERGO supplementation has the potential to delay muscle fatigue. If ERGO delays fatigue, the advantage for human use is that ERGO’s sulfur atom is within an aromatic ring, which eliminates the characteristic sulfur odor (7).
In the current study, we tested the hypothesis that NACA and ERGO act on muscle to delay fatigue. NACA and ERGO effects were tested in vitro using murine diaphragm fiber bundles. Contractile function was measured in unfatigued muscle and during repetitive, fatiguing contractions. NAC served as a positive control (10).
METHODS
We studied a total of 26 male C57BL6 mice (6–8 wk old; Harlan, Indianapolis, IN). All animals were maintained in a 12:12-h dark–light cycle. Water and a commercial rodent chow were available ad libitum. The chow was prepared from natural ingredients that are known to contain ergothioneine (e.g., oats and corn) (9). The chow did not contain synthetic ergothioneine. Our experiments conformed to the guiding principles for use and care of laboratory animals of the American Physiological Society and adhered to the American College of Sports Medicine’s animal care standards. All procedures were approved by the Institutional Animal Care and Use Committee of the University of Kentucky.
On the day of the experiment, each animal was anesthetized using isoflurane (Aerrane; Baxter Healthcare, Deerfield, IL) and killed by exsanguination. The diaphragm muscle was quickly excised and placed in Krebs–Ringer solution (in mM: 137 NaCl, 5 KCl, 1 MgSO4, 1 NaH2PO4, 24 NaHCO3, and 2 CaCl2) bubbled continuously using 95%O2–5%CO2 to maintain a pH of ~7.4. We dissected a fiber bundle from the left hemidiaphragm and tied the associated rib and central tendon segments to a glass rod and force transducer (BG Series 100 g; Kulite, Leonia, NJ). The muscle fiber bundle was placed in a water-heated organ bath between platinum plate electrodes (Radinoti LLC, Monrovia, CA) and was electrically stimulated using supramaximal voltage (Grass S48; Quincy, MA). We positioned the fiber bundle at the optimal length for twitch force production (Lo). The temperature of the organ bath was then increased to 37°C, and the muscle was exposed to buffer alone (control), NAC, ERGO, or NACA for 60 min. All antioxidants were tested at 10 mM. At this concentration, NAC delays fatigue of rodent diaphragm in vitro without depressing maximal force (10,19). Like NAC, NACA and ERGO contain one sulfur atom per molecule. Thus, the concentrations of NACA and ERGO were matched to the optimal concentration of NAC for in vitro experiments (10 mM) (19). NAC was from Sigma-Aldrich (St. Louis, MO), NACA was provided by Dr. Patrick G. Sullivan (University of Kentucky) and Dr. Glenn A. Goldstein (David Pharmaceuticals, New York, NY), and ERGO was from Oxis International (Eugene, OR). All solutions were prepared on the day of the experiment and contained D-tubocurarine (0.025 mM).
After 60 min of equilibration with each antioxidant, we determined the force–frequency characteristics of unfatigued muscle using a standard protocol (19). One minute after the last stimulation, we initiated a fatigue protocol consisting of tetanic contractions every 2 s for 600 s (stimulus frequency = 40 Hz, train duration = 500 ms, pulse duration = 0.3 ms). The output of the force transducer was recorded by a computer using a sampling frequency of 1000 Hz and Minidigi 1A and AxoScope software (Axon Instruments, Molecular Devices, Sunnyvale, CA) for later analyses. After the fatigue protocol, we measured Lo and muscle weight to estimate cross-sectional area. We used a custom-developed MatLab application (Mathworks, Inc., Natick, MA) to calculate peak tetanic force, force–time integral (FTI), and maximal rates of force development (+dF/dtMAX) and relaxation (−dF/dtMIN) for each tetanus of the fatigue trial.
Data analysis.
Specific and relative force measurements from unfatigued muscles stimulated at 1–300 Hz were fitted with the Hill equation to determine the force–frequency relationship, maximal tetanic force (Po), and the frequency required to elicit 50% of Po. Tracings from fatigue protocols were analyzed for fatigue index (FI), where FI = [(Finitial - Ft)/Finitial] × 100; the subscript initial refers to specific force for the first contraction and t is force at 600 s, as described previously (10). The time to reach 50% of peak tetanic force (t50%) was determined manually. We analyzed dF/dtMAX and dF/dtMIN data from the eighth contraction of the fatigue protocol (16 s, approximately peak of dF/dtMAX and nadir of dF/dtMIN) to the end of the protocol (600 s) using Prism 5.0b software (GraphPad, La Jolla, CA). Data were fitted with a monoexponential function of the form Y = Y0 + (plateau - Y0)(1 – e-t/τ), where Y0 refers to baseline, plateau is the asymptote of the response, t refers to time, and τ is the time constant (time to reach 63% of the response from baseline to plateau).
Statistics.
We used one-way ANOVA to compare control, NACA, and ERGO. Post hoc analyses were performed using the Fisher LSD test. Because NAC was studied as a positive control, we used Student’s t-test to compare individual parameters between control and NAC-treated muscles. Statistics were calculated using Sigma Stat v. 3.05 (Sigma Stat; Systat Software, San Jose, CA), and significance was accepted at P < 0.05. Data are presented as mean ± SEM.
RESULTS
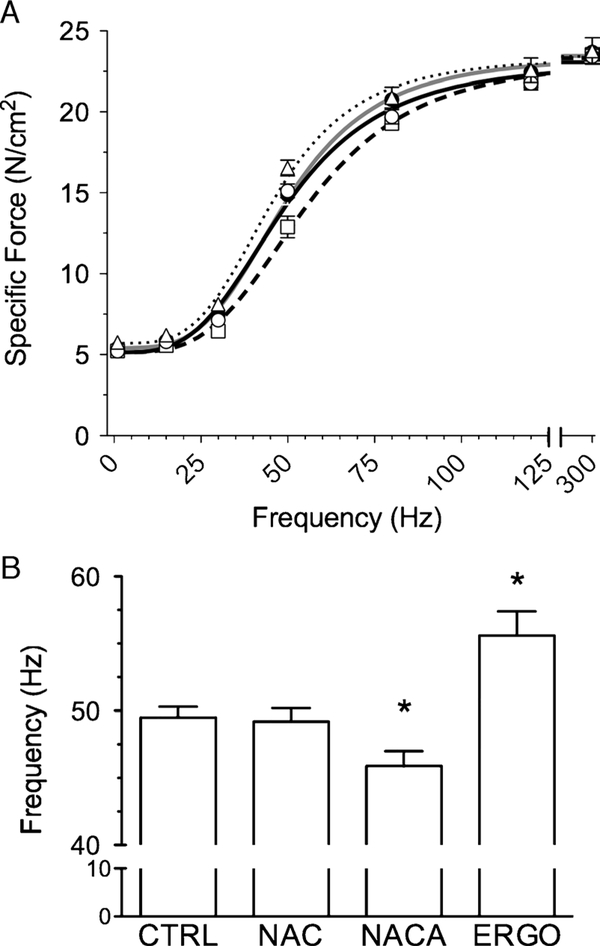
Force–frequency relationships of all experimental groups (Fig. 1A) were typical sigmoids with the NACA curve shifted rightward and the ERGO curve shifted leftward relative to control. A concern with pharmacologic probes is depression of Po at high intracellular concentrations (19). This did not occur with the current antioxidants. However, compared with control, NACA decreased and ERGO increased the stimulus frequency that elicited 50% Po (Fig. 1B); NAC did not. These data are consistent with the observed shifts in the force–frequency relationship.
FIGURE 1 —
Force–frequency characteristics of unfatigued diaphragm muscle bundles. A, Symbols are mean ± SEM for CTRL (closed circles), NAC (open circles), NACA (squares), and ERGO (triangles). Lines are best fit of mean data using Hill equation: CTRL (solid gray line), NAC (solid black line), NACA (long-dashed line), and ERGO (dotted line). B, Stimulus frequency that elicited 50% maximal tetanic force. Results obtained from curve fitting of individual muscle responses using Hill equation. * P < 0.05 versus control.
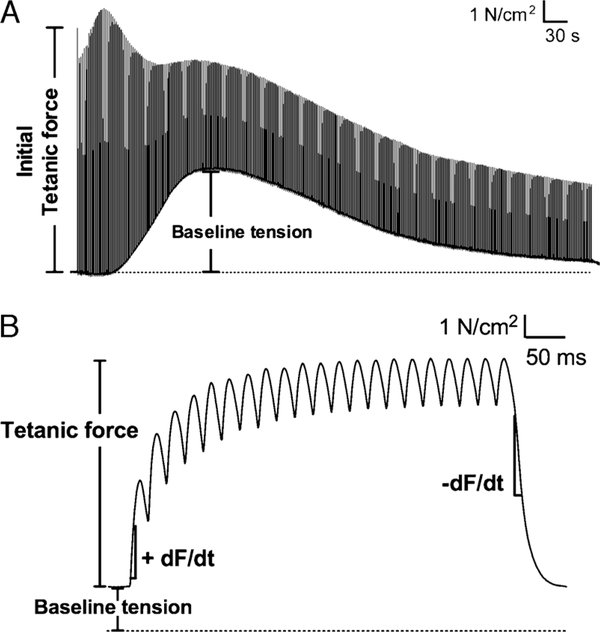
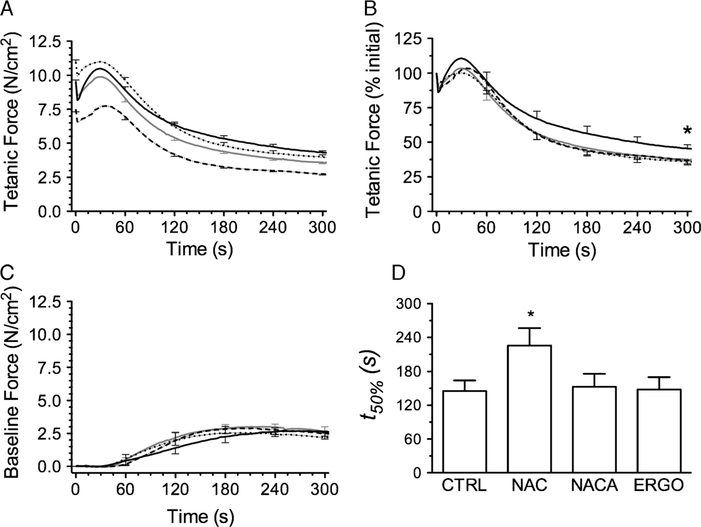
Representative tracings of muscle force during the fatigue trial are shown in Figure 2. As depicted, we evaluated fatigue using time-dependent changes in peak tetanic force, force sustained between tetanic stimulations (baseline force), and the rates of tetanic force development and relaxation. Grouped data showing changes in tetanic force and baseline force are shown in Figure 3. NACA decreased the initial tetanic force during fatiguing contractions (Fig. 3A) compared with all other groups—results were consistent with the rightward shift in the force–frequency relationship. Initial tetanic forces of NAC- and ERGO-treated muscles were not different from controls. Normalized for initial force, the fall in relative force was blunted by NAC (Fig. 3B), evidence of diminished fatigue. NACA and ERGO did not alter this relationship nor did any treatment affect changes in baseline force (Fig. 3C). NAC alone increased the t50% of specific force (Fig. 3D). NAC also decreased the diaphragm fatigue index at 600 s (64% ± 2%; P < 0.05) compared with control (70% ± 1%); NACA (69% ± 1%) and ERGO (71% ± 1%) did not.
FIGURE 2 —
Tracings of diaphragm muscle force during fatiguing contractions in vitro. Muscles were electrically stimulated after incubating in buffer (control) for 60 min. A, Representative recording of an entire fatigue protocol. B, Tracing from one tetanic contraction illustrating variables used to analyze muscle fatigue. dF/dt refers to the first derivative of force as a function of time. Data shown were collected at 1000 Hz.
FIGURE 3 —
Temporal changes in tetanic force and baseline tension during fatiguing contractions after exposure to sulfur-based compounds: CTRL (solid gray line), NAC (solid black line), NACA (long-dashed line), ERGO (dotted line). Error bars are shown at 60-s intervals. A, Initial tetanic force was decreased by NACA and increased by ERGO compared with control and NAC (P < 0.05). B, NAC increased tetanic force (%initial) at 300 s compared with CTRL (*P < 0.05). C, Sulfur-based compounds did not affect baseline force throughout the protocol (data from 300 to 600 s not shown). D, Time to reach 50% of initial tetanic force (t50%). *P < 0.05 versus CTRL.
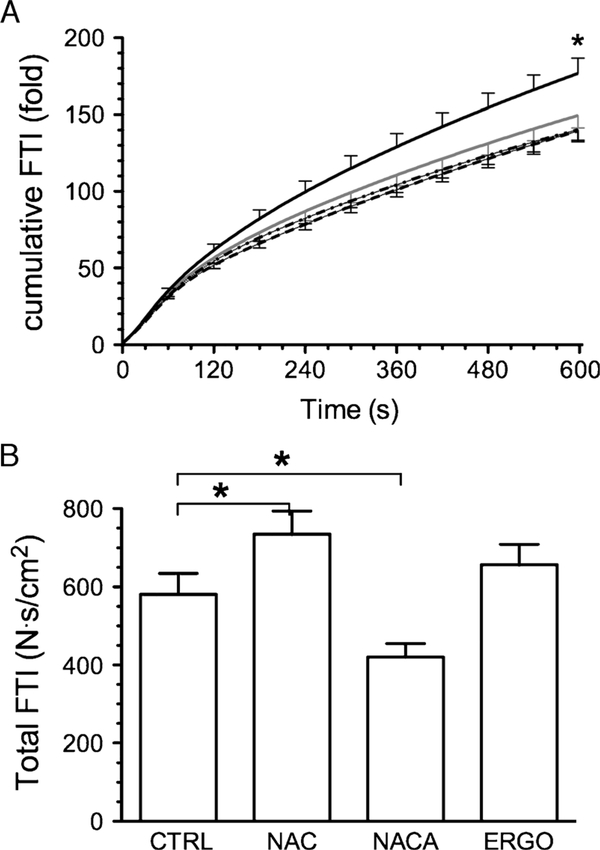
The initial FTI (N∙s∙cm−2) was decreased by NACA (2.91 ± 0.19), increased by ERGO (4.65 ± 0.19), and not changed by NAC (3.89 ± 0.17) compared with control (3.87 ± 0.27). When normalized to initial values, time-dependent increments in cumulative FTI did not differ among NACA, ERGO, and control groups but was increased by NAC (Fig. 4A). Total FTI accumulated during the fatigue trial was also increased by NAC (Fig. 4B). NACA decreased total FTI and ERGO had no effect.
FIGURE 4 —
Cumulative and total FTI during fatiguing contractions. A, Cumulative FTI during fatigue trials; data normalized for FTI of first tetanic contraction; solid gray line, CTRL; solid black line, NAC; long-dashed line, NACA; dotted line, ERGO. Error bars are shown at 60-s intervals. B, Total FTI represents sum of absolute FTI from all contractions of fatigue trial. *P < 0.05 control versus NAC.
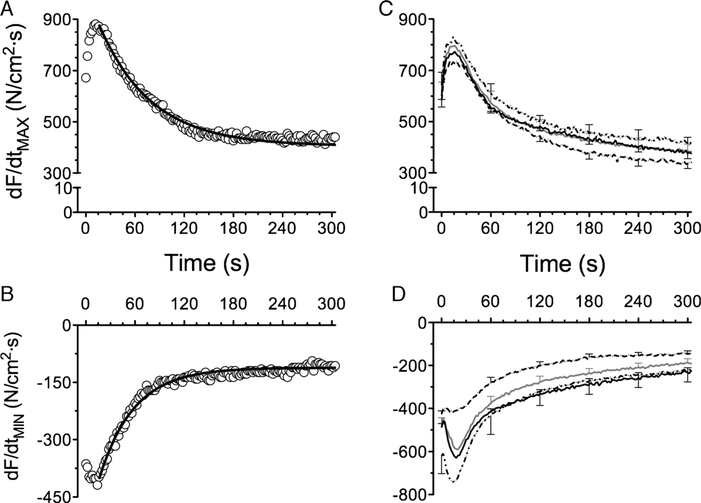
The dF/dtMAX increased during the first five contractions, reaching a peak between 15 and 20 s and decreasing thereafter in an exponential manner (Fig. 5). Accordingly, dF/dtMAX was well fitted by a first-order exponential function (Fig. 5A). Sulfur-containing antioxidants did not affect the profile of dF/dtMAX during the fatigue trial (Fig. 5C). The dF/dtMIN decreased in the first 15–20 s and increased subsequently with a profile that could be described by an exponential model (Fig. 5B). The drop in dF/dtMIN (Fig. 5D) reflects an increase in the rate of relaxation during the first 30 s of repetitive contractions. The subsequent increase in dF/dtMIN reflects slowing of relaxation during muscle fatigue (37). The time constant of increase in dF/dtMIN was prolonged by NAC (147.2 ± 28.4, P < 0.05) compared with control (88.3 ± 8.9 s) but was unchanged by NACA (72.3 ± 9.8 s) or ERGO (82.9 ± 14.7 s). Thus, NAC delayed the slowing of relaxation induced by fatigue.
FIGURE 5 —
Rate of contraction (dF/dtMAX) and relaxation (dF/dtMIN) during fatiguing contractions. Representative data from control muscle (open circles) and line of best fit using nonlinear regression (solid line) for dF/dtMAX (A) and dF/dtMIN (B). C, Sulfur-containing antioxidants did not alter dF/dtMAX. D, ERGO decreased dF/dtMIN (initial and nadir); NAC slowed the increase in dF/dtMIN as evidenced by a 1.6-fold increase in the time constant of the response (see text for further details). Data for panels (C) and (D) are mean ± SEM: control (solid gray line), NAC (solid black line), NACA (long-dashed line), ERGO (dotted line).
DISCUSSION
Our main observation was that NACA or ERGO treatment did not increase the fatigue index, t50%, or FTI of diaphragm fiber bundles. These variables were increased by NAC, which served as a positive control. NAC also delayed the slowing of relaxation and increased total FTI, two more original observations. These findings provide new information on sulfur-containing antioxidants and their potential efficacy as ergogenic aids.
NAC.
NAC is used as a cysteine donor to promote increases in intracellular glutathione (28,34). NAC promotes a rightward shift in the force–frequency relationship of rat diaphragm (6,19). However, we observed no effect of NAC on the force–frequency relationship of mouse diaphragm—findings consistent with our previous study (10). NAC also slows the decrease in isometric tetanic force in mouse and rat diaphragm preparations during repetitive contractions in vitro (6,10,19,30). Therefore, we used NAC as a positive control for fatigue effects of sulfur-containing antioxidants. Our analyses of FTI and dF/dtMIN provide new insights on the fatigue-sparing effects of NAC. NAC increased FTI, an index that is conceptually equivalent to work and determines the muscle metabolic rate during isometric contractions (20). Our findings in vitro are analogous to human data showing that NAC increases the work achieved during strenuous, fatiguing exercise (26,27). NAC also delayed the slowing of relaxation during fatigue. The rate of relaxation after isometric contraction is determined mainly by Ca2+ reuptake and cross-bridge detachment kinetics (12). Because NAC has no effect on the rate of sarcoplasmic reticulum Ca2+ reuptake in fatigued rat diaphragm (30), our data suggest NAC delays the slowing of cross-bridge detachment induced by fatigue.
NACA.
NAC contains a carboxyl group that is negatively charged at pH 7.4, which limits membrane transport and intracellular bioavailability. Atlas et al. (3) produced NACA by adding an amide group to NAC to neutralize the negative charge of carboxyl and create a membrane-permeable antioxidant. NAC and NACA have generally similar reactions with oxidants in cell-free experiments (2). In biological systems, the effects of NACA on cytosolic oxidants and glutathione are cell type–specific (14,39). In our study, NACA altered muscle function in the unfatigued state, depressing peak force at submaximal tetanic stimulus frequencies and shifting the force–frequency relationship rightward. NACA did not delay fatigue. Distinct muscle responses to NACA and NAC may be a consequence of their effects on glutathione metabolism and/or antioxidant properties, despite similarities in thiol biochemistry.
L-ergothioneine.
ERGO is a 2-thio-imidazole amino acid found in components of the human diet, mainly mushrooms and grains (9), and accumulates in tissues at millimolar concentrations (25,29). The biological role of ERGO is still unclear. ERGO scavenges agonist-induced oxidants in cells and tissues (17,23,25,31,32), but a lack of antioxidant effect has also been reported (9). Intracellular effects depend on transport of ERGO through OCTN1 (32), which is present in plasma membranes (15) and mitochondria (22). OCTN1 has a high affinity for ERGO (15), which undergoes bidirectional cotransport with Na+ through OCTN1 (40). Previous studies suggest a role for OCTN1-mediated ERGO transport in skeletal muscles. Rodent skeletal muscle expresses OCTN1 mRNA (21) and accumulates dietary ERGO (29). Moreover, ERGO delays palmitate-induced cell death in cultured muscle cells (23). ERGO effects on the diaphragm were inconsistent with an antioxidant action. ERGO promoted a leftward shift on the force–frequency relationship and had no effect on the rate of fatigue. Our protocol was designed to optimize ERGO bioavailability. At the concentration we used, OCTN1 is saturated in non-muscle cells, and cellular uptake of ERGO reaches a plateau within 30 min (15). The leftward shift suggests increased calcium release on electrical stimulation and/or an increase in calcium sensitivity of the contractile apparatus. These mechanisms could arise from a pro-oxidant action of ERGO (1). If ERGO had a pro-oxidant effect, diaphragm bundles would have fatigued faster. Yet, fatigue characteristics of ERGO and control muscles were generally similar. It seems that any potential effects of ERGO on calcium release or sensitivity were independent of changes in the muscle oxidant/antioxidant balance. Overall, our results suggest that exogenous ERGO supplementation does not delay the decline in muscle contractile function during repetitive contractions.
Sulfur biochemistry and muscle fatigue.
The sulfur atom of biological antioxidants can exist in several forms. Relevant to the current study, sulfur is present in the thiol (R–SH) or thiolate (R–S-) forms, depending on local pH and pKa of the sulfur-containing residue (4,8,16). In aromatic rings, sulfur can be present as thiol or thione (R2C = S) form. These three forms of sulfur have different biochemical reactivity: thiolate > thiol >> thione (8,13,16). Sulfur atoms in cytosolic NAC and NACA are predominantly in the thiol/thiolate form, whereas in cytosolic ERGO, sulfur is mostly in the thione form (17). NAC delayed fatigue, whereas ERGO or NACA did not. OTC is another sulfur-containing antioxidant that, through enzyme metabolism, increases cellular thiols (mostly cysteine and glutathione [38]). OTC delays diaphragm muscle fatigue in vitro (10). Glutathione, which contains sulfur in the thiol/thiolate form (28), does not increase time to fatigue in rats performing treadmill running (35) and swimming exercises (24). Therefore, the presence of a thiol group per se does not ensure that a sulfur-containing antioxidant will delay muscle fatigue.
CONCLUSIONS
We speculate that the inability of NACA and ERGO to delay muscle fatigue is a consequence of, respectively, the biochemistry of the carboxyl group and sulfur on each antioxidant. The existing literature suggests that reactions involving thiol/thiolate groups of sulfur-based antioxidants oppose skeletal muscle fatigue. However, our current findings show that the presence of thiol/thiolate groups in a compound per se is not sufficient to slow the fatigue process. Additional research is needed to identify the biochemical properties that render sulfur-containing antioxidants effective against fatigue. In contrast to NAC, which reproducibly delays fatigue in exercising humans (18,26,27,33,36), our current data suggest that NACA and ERGO supplementation is unlikely to increase human performance.
Acknowledgments
The authors thank Dr. Patrick Sullivan for scientific input on N-acetylcysteine amide.
This study was supported by a grant from the National Space Biomedical Research Institute (NSBRI/NASA Consortium MA00209). L. F. Ferreira was supported by postdoctoral fellowships from the American Heart Association – Great Rivers Affiliate (09POST2020082 and 0725334B). The authors were supported by funds from the National Institutes of Health to M. B. Reid (AR055974) and K. S. Campbell (HL 090749).
Footnotes
The authors have no conflict of interest regarding the outcomes of the study.
The results of our study do not constitute endorsement by the American College of Sports Medicine.
REFERENCES
- 1.Andrade FH, Reid MB, Westerblad H. Contractile response of skeletal muscle to low peroxide concentrations: myofibrillar calcium sensitivity as a likely target for redox-modulation. FASEB J. 2001;15(2):309–11. [DOI] [PubMed] [Google Scholar]
- 2.Ates B, Abraham L, Ercal N. Antioxidant and free radical scavenging properties of N-acetylcysteine amide (NACA) and comparison with N-acetylcysteine (NAC). Free Radic Res. 2008; 42(4):372–7. [DOI] [PubMed] [Google Scholar]
- 3.Atlas D, Melaned E, Offen D. Brain Targeted Low Molecular Weight Hydrophobic Antioxidant Compounds. 2002. Available from: US Patent Office. Application number 10/114,475; publication number US 2002/0107176 A1.
- 4.Atmaca G Antioxidant effects of sulfur-containing amino acids. Yonsei Med J. 2004;45(5):776–88. [DOI] [PubMed] [Google Scholar]
- 5.Carlucci A, Ceriana P, Prinianakis G, Fanfulla F, Colombo R, Nava S. Determinants of weaning success in patients with prolonged mechanical ventilation. Crit Care. 2009;13(3):R97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diaz PT, Brownstein E, Clanton TL. Effects of N-acetylcysteine on in vitro diaphragm function are temperature dependent. J Appl Physiol. 1994;77(5):2434–9. [DOI] [PubMed] [Google Scholar]
- 7.Dong KK, Damaghi N, Kibitel J, Canning MT, Smiles KA, Yarosh DB. A comparison of the relative antioxidant potency of L-ergothioneine and idebenone. J Cosmet Dermatol. 2007;6(3): 183–8. [DOI] [PubMed] [Google Scholar]
- 8.Eaton P Protein thiol oxidation in health and disease: techniques for measuring disulfides and related modifications in complex protein mixtures. Free Radic Biol Med. 2006;40(11):1889–99. [DOI] [PubMed] [Google Scholar]
- 9.Ey J, Schomig E, Taubert D. Dietary sources and antioxidant effects of ergothioneine. J Agric Food Chem. 2007;55(16): 6466–74. [DOI] [PubMed] [Google Scholar]
- 10.Ferreira LF, Gilliam LA, Reid MB. L-2-Oxothiazolidine-4-carboxylate reverses glutathione oxidation and delays fatigue of skeletal muscle in vitro. J Appl Physiol. 2009;107:211–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferreira LF, Reid MB. Muscle-derived ROS and thiol regulation in muscle fatigue. J Appl Physiol. 2008;104(3):853–60. [DOI] [PubMed] [Google Scholar]
- 12.Fitts RH. The cross-bridge cycle and skeletal muscle fatigue. J Appl Physiol. 2008;104(2):551–8. [DOI] [PubMed] [Google Scholar]
- 13.Forman HJ, Fukuto JM, Torres M. Redox signaling: thiol chemistry defines which reactive oxygen and nitrogen species can act as second messengers. Am J Physiol Cell Physiol. 2004;287(2): C246–56. [DOI] [PubMed] [Google Scholar]
- 14.Grinberg L, Fibach E, Amer J, Atlas D. N-acetylcysteine amide, a novel cell-permeating thiol, restores cellular glutathione and protects human red blood cells from oxidative stress. Free Radic Biol Med. 2005;38(1):136–45. [DOI] [PubMed] [Google Scholar]
- 15.Grundemann D, Harlfinger S, Golz S, et al. Discovery of the ergothioneine transporter. Proc Natl Acad Sci U S A. 2005;102(14): 5256–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hand CE, Honek JF. Biological chemistry of naturally occurring thiols of microbial and marine origin. J Nat Prod. 2005;68(2): 293–308. [DOI] [PubMed] [Google Scholar]
- 17.Hartman PE. Ergothioneine as antioxidant. Methods Enzymol. 1990;186:310–8. [DOI] [PubMed] [Google Scholar]
- 18.Kelly MK, Wicker RJ, Barstow TJ, Harms CA. Effects of N-acetylcysteine on respiratory muscle fatigue during heavy exercise. Respir Physiol Neurobiol. 2009;165(1):67–72. [DOI] [PubMed] [Google Scholar]
- 19.Khawli FA, Reid MB. N-acetylcysteine depresses contractile function and inhibits fatigue of diaphragm in vitro. J Appl Physiol. 1994;77(1):317–24. [DOI] [PubMed] [Google Scholar]
- 20.Klawitter PF, Clanton TL. Tension–time index, fatigue, and energetics in isolated rat diaphragm: a new experimental model. J Appl Physiol. 2004;96(1):89–95. [DOI] [PubMed] [Google Scholar]
- 21.Kobayashi D, Aizawa S, Maeda T, et al. Expression of organiccation transporter OCTN1 in hematopoietic cells during erythroid differentiation. Exp Hematol. 2004;32(12):1156–62. [DOI] [PubMed] [Google Scholar]
- 22.Lamhonwah AM, Tein I. Novel localization of OCTN1, an organic cation/carnitine transporter, to mammalian mitochondria. Biochem Biophys Res Commun. 2006;345(4):1315–25. [DOI] [PubMed] [Google Scholar]
- 23.Laurenza I, Colognato R, Migliore L, Del Prato S, Benzi L. Modulation of palmitic acid–induced cell death by ergothioneine: evidence of an anti-inflammatory action. Biofactors. 2008;33(4):237–47. [DOI] [PubMed] [Google Scholar]
- 24.Leeuwenburgh C, Ji LL. Glutathione and glutathione ethyl ester supplementation of mice alter glutathione homeostasis during exercise. J Nutr. 1998;128(12):2420–6. [DOI] [PubMed] [Google Scholar]
- 25.Markova NG, Karaman-Jurukovska N, Dong KK, Damaghi N, Smiles KA, Yarosh DB. Skin cells and tissue are capable of using L-ergothioneine as an integral component of their antioxidant defense system. Free Radic Biol Med. 2009;46(8):1168–76. [DOI] [PubMed] [Google Scholar]
- 26.McKenna MJ, Medved I, Goodman CA, et al. N-acetylcysteine attenuates the decline in muscle Na+,K+-pump activity and delays fatigue during prolonged exercise in humans. J Physiol. 2006; 576(Pt 1):279–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Medved I, Brown MJ, Bjorksten AR, McKenna MJ. Effects of intravenous N-acetylcysteine infusion on time to fatigue and potassium regulation during prolonged cycling exercise. J Appl Physiol. 2004;96(1):211–7. [DOI] [PubMed] [Google Scholar]
- 28.Meister A, Anderson ME. Glutathione. Annu Rev Biochem. 1983; 52:711–60. [DOI] [PubMed] [Google Scholar]
- 29.Melville DB, Horner WH, Lubschez R. Tissue ergothioneine. J Biol Chem. 1954;206(1):221–8. [PubMed] [Google Scholar]
- 30.Mishima T, Yamada T, Matsunaga S, Wada M. N-acetylcysteine fails to modulate the in vitro function of sarcoplasmic reticulum of diaphragm in the final phase of fatigue. Acta Physiol Scand. 2005;184(3):195–202. [DOI] [PubMed] [Google Scholar]
- 31.Obayashi K, Kurihara K, Okano Y, Masaki H, Yarosh DB.L-Ergothioneine scavenges superoxide and singlet oxygen and suppresses TNF-alpha and MMP-1 expression in UV-irradiated human dermal fibroblasts. J Cosmet Sci. 2005;56(1):17–27. [PubMed] [Google Scholar]
- 32.Paul BD, Snyder SH. The unusual amino acid L-ergothioneine is a physiologic cytoprotectant. Cell Death Differ. 2010;17(7):1134–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reid MB, Stokic DS, Koch SM, Khawli FA, Leis AA. N-acetylcysteine inhibits muscle fatigue in humans. J Clin Invest. 1994;94(6):2468–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sen CK. Nutritional biochemistry of cellular glutathione. J Nutr Biochem. 1997;8:660–72. [Google Scholar]
- 35.Sen CK, Atalay M, Hanninen O. Exercise-induced oxidative stress: glutathione supplementation and deficiency. J Appl Physiol. 1994;77(5):2177–87. [DOI] [PubMed] [Google Scholar]
- 36.Travaline JM, Sudarshan S, Roy BG, Cordova F, Leyenson V, Criner GJ. Effect of N-acetylcysteine on human diaphragm strength and fatigability. Am J Respir Crit Care Med. 1997;156(5): 1567–71. [DOI] [PubMed] [Google Scholar]
- 37.Vedsted P, Larsen AH, Madsen K, Sjogaard G. Muscle performance following fatigue induced by isotonic and quasi-isometric contractions of rat extensor digitorum longus and soleus muscles in vitro. Acta Physiol Scand. 2003;178(2):175–86. [DOI] [PubMed] [Google Scholar]
- 38.Williamson JM, Meister A. Stimulation of hepatic glutathione formation by administration of L-2-oxothiazolidine-4-carboxylate, a 5-oxo-L-prolinase substrate. Proc Natl Acad Sci U S A. 1981; 78(2):936–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu W, Abraham L, Ogony J, Matthews R, Goldstein G, Ercal N. Effects of N-acetylcysteine amide (NACA), a thiol antioxidant on radiation-induced cytotoxicity in Chinese hamster ovary cells. Life Sci. 2008;82(21–22):1122–30. [DOI] [PubMed] [Google Scholar]
- 40.Yabuuchi H, Tamai I, Nezu J, et al. Novel membrane transporter OCTN1 mediates multispecific, bidirectional, and pH-dependent transport of organic cations. J Pharmacol Exp Ther. 1999;289(2): 768–73. [PubMed] [Google Scholar]