Abstract
Retinol palmitate oral administration is convenient, but it is difficult to assess/monitor its nutritional status in preterm infants and literature is controversial about the administration route and the effectiveness of vitamin A supplementation. We primarily evaluated retinol plasma levels to assess the vitamin A nutritional status in preterm infants (<1500 g; 32 weeks) after 28 days of oral supplementation (3000 IU/kg/day, retinol palmitate drops), in addition to vitamin A standard amount as suggested by European Society of Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) guidelines. We then observed the rate of typical preterm pathologies in the supplemented group (31 newborns) and in 10 matching preterm infants, hospitalized in neonatal intensive care unit (NICU) in the same period, who received neither vitamin A supplementation nor parents allowed plasma sampling. Oral integration resulted in constant retinol plasma concentration around the desired level of 200 ng/mL, but without statistical increase during the study period. Due to the complexity of vitamin A metabolism and the immaturity of preterm infant’s organs, retinol supplementation may had first saturated other needy tissues; therefore, plasmatic measures may not be consistent with improved global vitamin A body distribution. Therefore, achieving a constant retinol concentration is a valuable result and supportive for oral administration: decreasing levels, even after parenteral/enteral supplementation, were reported in the literature. In spite of favourable trend and no adverse events, we did not report statistical difference in co-morbidities. This investigation confirms the necessity to perform further trials in preterm newborns, to find an index reflecting the complex nutritional retinol status after oral administration of vitamin A, highlighting its effectiveness/tolerability in correlated preterm infant’s pathologies.
Keywords: oral administration, plasma concentration, preterm newborn, vitamin A
Introduction
The Neonatal Adequate Care for Quality of Life (NEO-ACQUA) Study Group recently reported that approximately 552,000 infants are born in Italy each year, of whom 1% of them are very low birth weight (VLBW) infants, with a birth weight under 1500 g or a gestational age (GA) under 30 weeks. Survival rates for these infants improved strikingly by the mid-1990s due to specific and advanced antenatal and postnatal care.1 Whereas mortality has decreased, co-morbidities are exceptionally common: bronchopulmonary dysplasia (BPD),2 retinopathy of prematurity (ROP),3 necrotizing enterocolitis (NEC)4 and sepsis especially late-onset sepsis (LOS)5 are typical pathologies related to a preterm birth. Much research has focused on reducing the incidence of preterm infant morbidity and vitamin A (or retinol) supplementation has been proposed in order to try to decrease the incidence of the described pathologies with disputed results.6–9
Vitamin A is responsible for the growth and differentiation and integrity of epithelial cells; in particular, it plays an important role in the development of the lungs and retina.7–9 Maternal retinol levels increase during gestation,10,11 and vitamin A is transferred across the placenta to foetus, mainly during the third trimester. As a result, preterm infants have limited hepatic stores, lower plasma concentrations of vitamin A and retinol-binding protein (RBP) than term infants.6,10,11 World Health Organization (WHO) indicates the limit for low serum retinol as ⩽0.70 μmol/L (⩽200 ng/mL).12 Nevertheless, it is worth noting that the adequate dosage and route of vitamin A supplementation to maintain sustained plasma level are still controversial issues. Literature reported that early high-dose intramuscular (IM) vitamin A supplementation improved retinal sensitivity at 36 weeks’ post-menstrual age (PMA) in preterm infants at risk of ROP.8 High-dose oral vitamin A supplementation demonstrated to reduce the incidence of BPD or death at 36 weeks PMA in extremely low birth weight (ELBW) infants, with positive benefit-risk ratio.9 In developing countries, supplementing newborn infants with vitamin A, within 48 h of birth, demonstrated to reduce infant mortality by almost a quarter, and morbidity from gastrointestinal diseases.9,13
On the contrary, other authors doubted efficacy of the therapy and in particular, of the administration mode, an increased incidence of sepsis, possibility linked to repeated injections of vitamin A, with no clear evidence of reduction of chronic lung disease (CLD) has been evidenced.14–16
Different results in the reported studies may depend on the dissimilar level of competence of preterm newborns in metabolizing vitamin A, a micronutrient owning a complex metabolic pathway. After oral administration, in particular, vitamin A is absorbed by duodenal mucosal as micelles able to pass the gut lumen; it is mainly stored into liver as retinyl esters, while in blood, as retinol, is carried by the RBP to the target organs. Preterm infants are at risk for vitamin A, not only because of the lack of supply during the last week of pregnancy but also due to the immaturity of the developing metabolic pathway, resulting in low serum level of retinol and RBP thus leading to limited hepatic reserve.17,18 Therefore, supplementation is recommended, but the administration route is tricking point: parenteral administration of retinol presents problems, such as photodegradation and adsorption of the vitamin to the plastic of the intravenous administration set, while IM supplementation is very painful.7,10
Oral administration is convenient for preterm infants who had achieved the enteral feeding capability, and plasma retinol concentrations are typically measured to assess vitamin A levels; nevertheless, it is not easy to assess vitamin A real availability. The first aim of this study was to evaluate oral supplementation of 3000 IU/kg/day retinol palmitate drops for 28 days and relevant plasma vitamin A nutritional status in preterm infants in addition to the standard amount suggested by European Society of Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) guidelines.19,20 The secondary aim was to observe the pattern of typical preterm pathologies (BPD, NEC, ROP, LOS) in the supplemented group and in a matching group of 10 preterm infants who complied with the inclusion/exclusion criteria, hospitalized in neonatal intensive care unit (NICU) in the same period, who received neither vitamin A supplementation nor the parents allowed their baby’s plasma sampling for research purposes.
Subjects and methods
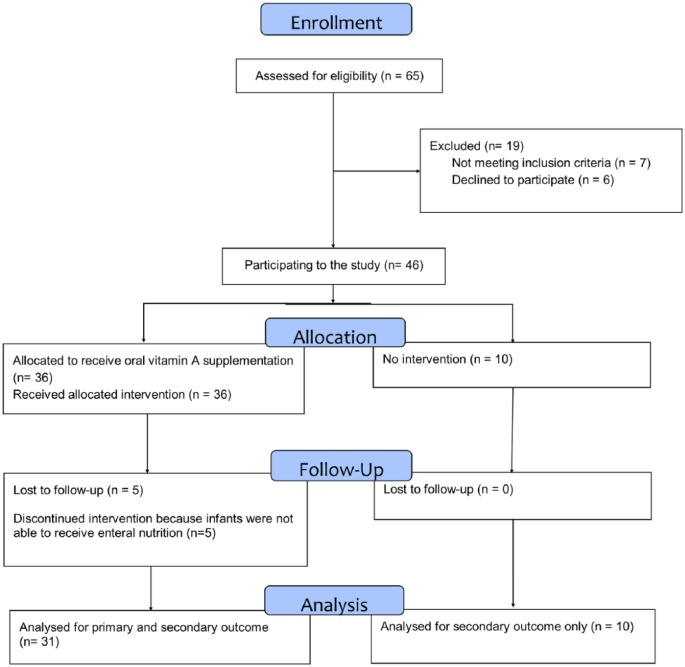
In this open, prospective study, 65 consecutive preterm infants (from June 2014 to May 2016, GA <32 weeks or birth weight <1500 g) referred to the Neonatal Intensive Care Unit of the IRCCS Policlinico S. Matteo, Pavia, Italy, were enrolled into this investigation. Both the parents had signed an informed consent form. The study was approved by the Ethics Committee of the Institution and registered at ClinicalTrials.gov (NCT02102711). A total of 24 infants were excluded from the study: 7 did not meet inclusion criteria, 6 declined to participate and the parents of other 6 newborns failed to sign informed consent, and 5 patients were lost to follow-up, as they were no longer able to receive enteral nutrition. Eligibility criteria: infants able to receive a minimum enteral feeding as 20 mL/kg/day of breast/formula milk within 1 week of life, for the subsequent 4 weeks. All the preterm infants received routine suitable nutrition adequate to their body weight, clinical condition and mode of feeding. Within their daily nutrient supply, routine dose of vitamin A, included in 700–1500 IU/kg/day range, as reported in the guidelines,20 was administered, depending also on the source of nutrition: either parenteral or enteral feeding as fortified breast milk or formula milk for preterm infants.
We analysed data from 31 patients who completed the study receiving vitamin integration and retinol plasma monitoring and from 10 infants who complied with the same inclusion/exclusion criteria, but received neither additional vitamin A supplementation nor the parents allowed their baby’s plasma sampling. During the hospitalization, the infants were simply scored for the following pathologies: BPD, ROP, LOS and NEC.
A total of 31 infants received 3000 IU/kg/day of retinol palmitate drops (VISPO – Biotrading, Marsala, Italy), just before the first morning feed, in a 4-week period (Figure 1). The supplementation was given in addition to the standard vitamin A dose, calculated based on infant’s body weight, GA and in accordance with the requirements of ESPGHAN guidelines.19,20 Infants with congenital malformations of the liver, kidney or bowel were excluded. Blood samples were collected before the first day of vitamin A supplementation, basal and after 14 and 28 days of oral administration. Plasma was obtained by centrifuging blood (3000 g; 10 min), and it was stored at −80°C and analysed within 2 months. Retinol plasma concentrations were measured by high-performance liquid chromatography (HPLC) with the fluorimetric detection method;21 this method involved sample protein precipitation and extraction with an ethanol–chloroform mixture. The dynamic range of the assay was 37.5–1200 ng/mL. The lower limit of quantification (LOQ) was 37.5 ng/mL.
Figure 1.
Consort flow chart.
The analytical method has been validated according to European Medicines Agency (EMA) guidelines.22
Statistics
Statistical analysis was performed in 31 preterm infants who fully completed the study period. To summarize quantitative variable, when normally distributed, mean and standard deviation (SD) were used, otherwise, if not normally distributed, median and interquartile range (IQR, 25th–75th percentile) were used. Differences between preterm infants or control were evaluated with t test for independent data or Mann–Whitney test. To analyse retinol plasma concentrations over time, a regression model for repeated measures was used. All tests were two-sided and P < 0.05 was considered statistically significant. Data analysis was performed using the STATA statistical package (release 14.1, 2015, Stata Corporation, College Station, Texas, USA).
Number and percentage in each category were used for qualitative measures and compared using the chi-square test or Fisher’s exact test.
Results
Tables 1 and 2 report demographic data and clinical pattern of the infants of the two groups until they were discharged from the hospital. No statistical difference was found at baseline in the descriptive variables, between the two groups. Details on infants’ nutrition: in the studied group, 13 infants started with parenteral nutrition: 9 gradually switching into increasing doses of preterm formula milk and 4 into fortified breast milk; 11 received preterm formula milk and 4 received fortified breast milk. In the matching group, 13 infants started with parenteral nutrition: 4 gradually switching into increasing doses of preterm formula milk; 2 into fortified breast milk; 1 received preterm formula milk; and 3 received fortified breast milk.
Table 1.
Demographic and hospital stay data.
| Vitamin A supplemented N = 31 |
Not supplemented n = 10 |
P | |
|---|---|---|---|
| Gestational age, mean (SD) | 29 weeks (2.33) | 29.5 weeks (2.47) | 0.418 |
| Maternal age, mean (SD) | 33 years (7) | 32.5 years (5.7) | 0.562 |
| Sex, number (%) | 0.152 | ||
| Male | 16 (51.6) | 8 (80) | |
| Female | 15 (48.4) | 2 (20) | |
| Mode of delivery, number (%) | 0.378 | ||
| Vaginal delivery | 5 (16) | 3 (30) | |
| Caesarean section | 26 (84) | 7 (70) | |
| Anthropometric data at birth | |||
| Weight (g), mean (SD) | 1134 (327) | 1172 (165) | 0.860 |
| Length (cm), mean (SD) | 36.5 (3.4) | 37.0 (1.2) | 0.665 |
| Head circumference (cm), mean (SD) | 25.8 (2.5) | 26.2 (1.6) | 0.559 |
| Apgar score at 1′ and 5′, mean (SD) | 5.1 (2.4) and 7.2 (2.2) | 5.9 (1.9) and 7.9 (1.1) | 0.283 0.240 |
| Stay in NICU, days, median (IQR, 25–75) | 57 (44–107) | 70.5 (51.3–93.0) | 0.867 |
| Oxygen therapy, days, median (IQR, 25–75) | 26 (10–56) | 20.5 (9.8–47.3) | 0.972 |
| Mechanical ventilation, days, median (IQR, 25–75) | 3 (1–7) | 6 (2–10.8) | 0.292 |
| CPAP, days, median (IQR, 25–75) | 9.5 (4–33) | 11 (3.8–36.3) | 0.531 |
| IVH, number (%) | 0 grade ⩾III | 1 (10) grade III | 0.244 |
SD: standard deviation; NICU: neonatal intensive care unit; IQR: interquartile range; CPAP: continuous positive airway pressure; IVH: intraventricular haemorrhage.
Table 2.
Co-morbidities.
| BPD number (%) | ROP number (%) | NEC number (%) | Sepsis number (%) | |
|---|---|---|---|---|
| Vitamin A supplemented N = 31 |
13 (41.9) | 9 (29) grade I | 0 (0) | 1 (3) |
| Not supplemented N = 10 |
5 (50) | 3 (30) grade I | 0 (0) | 1 (10) |
| P | 0.775 | 0.953 | – | 0.245 |
BPD: bronchopulmonary dysplasia; ROP: retinopathy of prematurity; NEC: necrotizing enterocolitis.
Retinol concentrations
Figure 2 reports retinol plasma concentrations at basal, on day 14 and on day 28 for the supplemented group.
Figure 2.
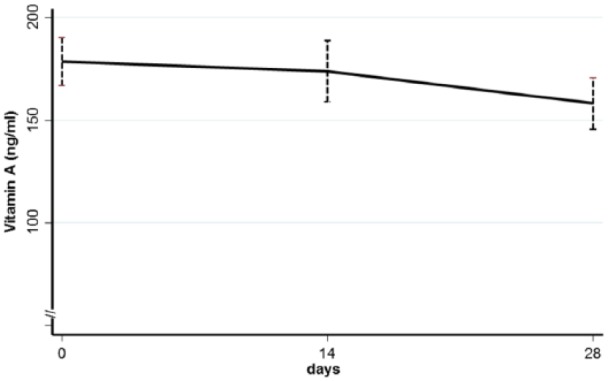
Mean and standard error (bars) of plasma retinol concentrations at baseline, after 14 and 28 days of supplementation in the 31 newborns who properly completed the study. Concentration resulted statistically constant during the whole study period, around the goal of 200 ng/mL.
Mean concentrations of vitamin A were about 200 ng/mL, stable for the studied period: no statistical difference was reported in mean values from basal to day 28.
Clinical outcome: BPD, NEC, ROP and LOS
Among the 31 infants receiving vitamin A supplementation, 13 (41.9%) suffered from BPD, 9 (29.0%) from degree 1 ROP and 1 (3.2%) from LOS. None had NEC episodes.
Among the 10 control infants, 5 (50%) suffered from BPD, 3 (30%) from degree 1 ROP and 1 (10%) from LOS; none had NEC episodes. Differences between the two groups were not statistically significant.
We did not report any adverse event related to vitamin A supplementation.
Discussion
A scientific debate is ongoing about possible beneficial effects of vitamin A supplementation, in VLBW preterm newborns, regarding administration route and effectiveness and different evidences and clinical trial report controversial results.23,24 Vitamin A is an essential factor for growth, from embryonic life to postnatal development, for morphogenesis, and epithelial cell differentiation.7 Vitamin A transfer reaches its highest and most effective mother–foetus transport during the last trimester of pregnancy;6,10,11 for this reason, preterm infants, in particular VLBW and ELBW ones, have vitamin deficiency.7,10,11
After oral administration, vitamin A is present in different forms (retinol ester, retinyl esters bound to chylomicrons, to lipoproteins, free retinol and retinol bound to RBP and others); moreover, the kinetics of retinol can vary greatly depending on the status of tissue saturation.17 In the gut lumen, its uptake by enterocytes depends on a specific carrier protein, cellular RBP type 2, the availability of which may be limited in the preterm infant. Retinol is the predominant circulating form of vitamin A in blood, and in response to tissue demand, it is released from the liver in a 1:1 ratio with its carrier protein, RBP. This complex combines in blood with transthyretin, itself or its active metabolites are delivered to target tissues via a specific membrane or nuclei receptors.17 About 90% of the body’s reserve of vitamin A is stored in the liver as retinyl esters; other sites of major vitamin A storage include the eye and the lung.8
Literature demonstrated the complexity of monitoring vitamin A global nutritional status in preterm infants, using different biological parameters as indexes of shortage.7,10,11,25,26 The fact that a univocal method for assessing vitamin A status has not been properly defined makes difficult to determine the optimal oral intake of vitamin A in VLBW, ELBW and critical newborns. Our study demonstrated that plasma levels were at constant steady state, around desired concentration, during the 28 days of oral supplementation. This finding is important and supportive of the effectiveness of oral administration in VLBW preterm infants. Some papers previously highlighted that the already low retinol levels of the preterm newborns even decreased despite enteral or parenteral vitamin A supplementation.11,12 Moreover, measurements of plasma retinol concentrations may not represent the vitamin A status, perhaps because it does not quantify other body compartments concentration. It is possible that supplementation of vitamin A first had provided primary need for tissue saturation. Nevertheless, monitoring plasma retinol concentrations is an indirect, but useful measure of global vitamin A neonatal availability, recommended by WHO to avoid the depletion.8,10,12,26,27 Furthermore, oral vitamin A is absorbed by duodenal mucosal cells after solubilizing into micelles in the lumen of the intestine, which in the preterm newborn is not fully developed with impaired intestinal barrier function that may prevent the optimal absorption of micronutrients.18 A recent study by Schmiedchen suggested an increased renal retinol excretion in vitamin A supplemented VLBW infants, possibly due to an uncompleted structural development of the nephrons or the impossibility to fully associate retinol, RBP with transthyretin, a high weight complex that prevents an excessive glomerular filtration and subsequent urinary retinol loss. The impaired development of important organs, such as bowel kidney, are important conditions avoiding the option of an optimal bioavailability of retinol. In our study, oral vitamin A supplementation demonstrates a positive trend maintaining constant plasma concentration, nevertheless did not exert in significant difference in co-morbidities, as reported in Table 2. On the other hand, we did not report any adverse event related to vitamin A supplementation. In fact, the supplementation of vitamin A in high doses may expose the preterm infants to risk of toxicity, and it is important to balance effective dosage dose avoiding side effects. At present, doses of 2000–3000 IU/kg/day have been suggested for preterm infants7 and they resulted in avoiding vitamin A side effects. These are described as bulging fontanelle, nausea/vomiting, signs of increased intracranial pressure, skin lesion and altered laboratory parameters.9 The paucity of the study groups may have prevented us from reaching our aims. Nevertheless, other papers that evaluated larger population had shown controversial results about oral vitamin A supplementation.16 Wardle et al.6 demonstrated that oral supplementation with high doses of vitamin A in ELBW infants does not significantly alter the incidence of CLD. Other papers demonstrated the efficacy of oral high-dose oral vitamin A supplementation, in reducing the incidence of BPD or death in ELBW infants.9,15 Mactier and Weaver11 in a review properly evidenced that the relationship between vitamin A concentration and its functional status is not unequivocal in preterm infants, highlighting the need to define, with good quality research, the optimal intake and mode of delivery of vitamin A for preterm infants with different feeding competence.
The present investigation, in agreement with other papers, confirms the necessity to perform further trials in preterm newborns, to find an index reflecting the complex nutritional retinol status after oral administration of vitamin A, certainly highlighting its effectiveness/tolerability in correlated pathologies.
Acknowledgments
We thank Claudia Cova for her technical assistance during the whole study, from Neonatal Unit and NICU and SRL, Fondazione IRCCS Policlinico S. Matteo, Pavia, Italy. We thank all the nurses of the wards of the NICU for precisely administering the supplementation. We thank all the families who volunteered to participate in this study. We thank Biotrading, Marsala, Italy, who freely supplied vitamin A drops (VISPO drops). We thank Karen Doyle, a native English speaker, who peer reviewed the manuscript. F.G. and I.M. contributed equally to this work. Trial registration: NCT02102711.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Ethical approval: The study was conducted in accordance with the Helsinki Declaration for investigations in human subjects. The Ethics Committee of the IRCCS Fondazione IRCCS Policlinico S. Matteo, Pavia, Italy, approved the study.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
Informed consent: Parental/guardian written consent was obtained.
ORCID iD: Francesca Garofoli  https://orcid.org/0000-0003-3710-966X
https://orcid.org/0000-0003-3710-966X
References
- 1. Cavallo MC, Gugiatti A, Fattore G, et al. (2015) Cost of care and social consequences of very low birth weight infants without premature-related morbidities in Italy. Italian Journal of Pediatrics 19: 41–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Borghesi A, Massa M, Campanelli R, et al. (2009) Circulating endothelial progenitor cells in preterm infants with bronchopulmonary dysplasia. American Journal of Respiratory and Critical Care Medicine 180(6): 540–546. [DOI] [PubMed] [Google Scholar]
- 3. Piermarocchi S, Bini S, Martini F, et al. (2017) Predictive algorithms for early detection of retinopathy of prematurity. Acta Ophthalmologica 95: 158–164. [DOI] [PubMed] [Google Scholar]
- 4. Namachivayam K, MohanKumar K, Arbach D, et al. (2015) All-trans retinoic acid induces TGF-β2 in intestinal epithelial cells via RhoA- and p38α MAPK-mediated activation of the transcription factor ATF2. PLoS ONE 10(7): e0134003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wynn JL. (2016) Defining neonatal sepsis. Current Opinion in Pediatrics 28: 135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wardle SP, Hughes A, Chen S, et al. (2001) Randomised controlled trial of oral vitamin A supplementation in preterm infants to prevent chronic lung disease. Archives of Disease in Childhood (Fetal and Neonatal Edition) 84: F9–F13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yang SN. (2014) Nutritional approach to the prevention of complications of prematurity with emphasis on vitamin A supplementation. Pediatrics and Neonatology 55: 331–332. [DOI] [PubMed] [Google Scholar]
- 8. Mactier H, McCulloch DL, Hamilton R, et al. (2012) Vitamin A supplementation improves retinal function in infants at risk of retinopathy of prematurity. The Journal of Pediatrics 160: 954–959. [DOI] [PubMed] [Google Scholar]
- 9. Meyer S, Gortner L. (2014) Early postnatal additional high-dose oral vitamin A supplementation versus placebo for 28 days for preventing bronchopulmonary dysplasia or death in extremely low birth weight infants. Neonatology 105(3): 182–188. [DOI] [PubMed] [Google Scholar]
- 10. Weinman AR, Jorge SM, Martins AR, et al. (2007) Assessment of vitamin A nutritional status in newborn preterm infants. Nutrition 23: 454–460. [DOI] [PubMed] [Google Scholar]
- 11. Mactier H, Weaver LT. (2005) Vitamin A and preterm infants: What we know, what we don’t know, and what we need to know. Archives of Disease in Childhood (Fetal and Neonatal Edition) 90: F103–F108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. World Health Organization (2011) Serum retinol concentrations for determining the prevalence of vitamin A deficiency in populations (Vitamin and Mineral Nutrition Information System, WHO/NMH/NHD/MNM/11.3). Geneva: World Health Organization; Available at: http://www.who.int/vmnis/indicators/retinol.pdf [Google Scholar]
- 13. Rahmathullah L, Tielsch JM, Thulasiraj RD, et al. (2003) Impact of supplementing newborn infants with vitamin A on early infant mortality: Community based randomized trial in southern India. British Medical Journal 327: 254–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chabra S, Mayock DE, Zerzan J, et al. (2013) Vitamin A status after prophylactic intramuscular vitamin A supplementation in extremely low birth weight infants. Nutrition in Clinical Practice 28: 381–386. [DOI] [PubMed] [Google Scholar]
- 15. Uberos J, Miras-Baldo M, Jerez-Calero A, et al. (2014) Effectiveness of vitamin A in the prevention of complications of prematurity. Pediatrics and Neonatology 55: 358–362. [DOI] [PubMed] [Google Scholar]
- 16. Gawronski CA, Gawronski KM. (2016) Vitamin A supplementation for prevention of bronchopulmonary dysplasia: Cornerstone of care or futile therapy? Annals of Pharmacotherapy 50: 680–684. [DOI] [PubMed] [Google Scholar]
- 17. Alpers DH. (2011) Vitamins as drugs: The importance of pharmacokinetics in oral dosing. Current Opinion in Gastroenterology 27: 146–151. [DOI] [PubMed] [Google Scholar]
- 18. Schwartz E, Zelig R, Parker A, et al. (2017) Vitamin A supplementation for the prevention of bronchopulmonary dysplasia in preterm infants: An update. Nutrition in Clinical Practice 32: 346–353. [DOI] [PubMed] [Google Scholar]
- 19. Koletzko B, Poindexter B, Uauy R. (2014) Recommended nutrient intake levels for stable, fully enterally fed very low birth weight infants. World Review of Nutrition and Dietetics 110: 297–299. [DOI] [PubMed] [Google Scholar]
- 20. Agostoni C, Buonocore G, Carnielli VP, et al. (2010) Enteral nutrient supply for preterm infants: Commentary from the European Society of Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. Journal of Pediatric Gastroenterology and Nutrition 50: 85–91. [DOI] [PubMed] [Google Scholar]
- 21. Taibi G, Nicotra CMA. (2002) Development and validation of a fast and sensitive chromatographic assay for all-trans retinol and tocopherols in human serum and plasma using liquid-liquid extraction. Journal of Chromatography B: Analytical Technologies in the Biomedical and Life Sciences 780: 261–267. [DOI] [PubMed] [Google Scholar]
- 22. European Medicines Agency (2011) EMEA/CHMP/EWP/192217/2009 Rev. 1 Corr. 2** Committee for Medicinal Products for Human Use (CHMP) EMA, 21 July 2011. European Medicines Agency; Available at: www.ema.europa.eu/contact [Google Scholar]
- 23. Darlow BA, Graham PJ. (2011) Vitamin A supplementation to prevent mortality and short- and long-term morbidity in very low birthweight infants. Cochrane Database of Systematic Reviews 10: CD000501. [DOI] [PubMed] [Google Scholar]
- 24. Darlow BA, Graham PJ, Rojas-Reyes MX. (2016) Vitamin A supplementation to prevent mortality and short- and long-term morbidity in very low birth weight infants. Cochrane Database of Systematic Reviews 8: CD000501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schmiedchen B, Longardt AC, Bührer C, et al. (2014) The relative dose response test based on retinol-binding protein is not suitable to assess vitamin A status in very low birth weight infants. Neonatology 105: 155–160. [DOI] [PubMed] [Google Scholar]
- 26. Longardt AC, Schmiedchen B, Raila J, et al. (2014) Characterization of the vitamin A transport in preterm infants after repeated high-dose vitamin A injections. European Journal of Clinical Nutrition 68: 1300–1304. [DOI] [PubMed] [Google Scholar]
- 27. Schmiedchen B, Longardt AC, Loui A, et al. (2016) Effect of vitamin A supplementation on the urinary retinol excretion in very low birth weight infants. European Journal of Pediatrics 175: 365–372. [DOI] [PubMed] [Google Scholar]