Abstract
Introduction:
Memory deficit is an important issue in some psychiatric diseases either as a primary symptom or as a comorbid symptom. Factors that determine the decline or improvement of memory are an important subject to reduce the severity of these diseases.
Methods and materials:
In this study, 32 male Sprague-Dawley rats were randomly divided into 4 experimental groups: social (control), isolation, resocialization for 3 days, and resocialization for 7 days. Isolation occurred for 14 days. Resocialization groups were resocialized for 3 or 7 days after isolation. In the social group, there was no intervention with normal socializing among the rats. In the isolation group, rats were isolated with no resocialization. In all 4 groups, after performing the Y-maze, the rats’ brains were removed to assess oxidative stress status in the hippocampus and prefrontal cortex.
Results:
Y-maze performance improved after 3 and 7 days of resocialization. However, oxidative stress status for malondialdehyde, glutathione and nitrite/nitrate returned to normal levels except in 2 experiments after 7 days of resocialization. In addition, in 2 experiments, just glutathione in the prefrontal cortex and nitrite/nitrate in the hippocampus after 3 days of resocialization improved.
Conclusions:
A return to normal levels in all types of antioxidant markers in the resocialization groups is not the only factor for improving memory deficits resulting from isolation. Resocialization may also be activating other regulatory mechanisms besides an antioxidant defense.
Keywords: resocialization, isolation, malondialdehyde, glutathione and nitrite/nitrate
Introduction
Memory formation is a complex process that involves different parts of the brain. In some conditions, disturbed functioning in one or more parts of a brain region is enough to cause memory dysfunction. Impaired memory is defined as any disturbance at any stage of memory formation, including encoding and retrieving recently acquired information, as well as disturbances involved in transferring information to long-term storage. The hippocampus and prefrontal cortex (PFC) are 2 important brain regions that are well known for the proper functioning of working memory.1
Social isolation is a condition that has been shown to be associated with an impairment of memory. Because many people experience social isolation, the ability to recover from a memory impairment is important.2 Treating memory dysfunction can involve different strategies. In this study, we look at resocialization as a plausible intervention for social isolation-induced memory disruption.
Socialization is an important concept for many aspects of human development, such as language, cognition, and emotion. Without enough socialization in childhood, humans are unable to properly develop in and throughout adulthood.3 The ability to develop emotionally and cognitively enables humans to better communicate and succeed. Memory relies on developed emotional behaviors to properly function. One of the aims of this study was to approximate the importance of daily socialization for the maintenance of cognitive abilities. As investigated in this research, daily social interaction is needed to maintain normal brain functioning.
Following memory impairment, problems and difficulties may develop. Indeed, every aspect of life can be negatively influenced for someone having cognitive dysfunction. A wide range of symptoms can develop in a memory-disturbed person. These symptoms may be subtle or may present themselves as an advanced problem, such as those seen in Alzheimer disease.4 Therefore, the restoration of memory using any tool is critically important. In this study, we propose resocialization as an effective tool to restore memory.
Oxidative stress (OS) is defined as the production of excess free radicals in a diseased state. This condition mandates the production of necessary antioxidants. Excessive free radicals, in the case where there is a shortage of antioxidants, may react rapidly with other vital molecules, such as those necessary for memory formation. Also, the generation of excessive oxidized materials, such as those that occur as a consequence of lipid peroxidation, is the hallmark of excessive free radical formation. OS has been observed in a wide variety of neurodegenerative disorders.5 Apoptosis of neurons as a result of OS can occur via different mechanisms, such as those that may affect DNA integrity, protein function, and membrane lipid structure.6 These mechanisms ultimately lead to neuronal death.7
The improvement of short-term memory by enhancing oxidant defenses has been discussed8; however, the improvement of short-term memory in a Y-maze by decreasing OS in the hippocampus and PFC has not been studied. Another purpose of this study is to assess to what extent resocialization restores memory in a Y-maze task compared with an antioxidant therapy, such as vitamin E therapy.9
A social environment has profound effects on behavior and the nervous system. In many studies, it has been shown that various functions and the formation of certain structures in the nervous system are affected by social environments. Social environments vary across societies, families, friends, and even across natural environments. Emotional and cognitive abilities can decline if a subject is socially isolated.10 To reap the benefits that socialization has on cognitive function, an individual should engage in this behavior frequently in daily life.
The aim of this study is to show that social isolation for 14 days reduces memory performance in a Y-maze, and with resocialization, memory performance is restored back to normal. In this regard, it is important to know whether the level of OS has an effect on immediate memory retrieval or not.
Materials and Methods
Animal care
The experimental protocols followed in this study conform to the guidelines for the care and use of laboratory animals published by the National Institutes of Health (NIH Publication No. 85-23, revised 1996) and was further approved by the institutional ethical committee at Tehran University of Medical Sciences (reference code: 91-01-159-18022; Tehran, Iran).
Animals: A total of 32 male Sprague-Dawley rats 8 to 10 weeks (200-250 g) were kept in controlled conditions (controlled temperature [22°C ± 2°C] and humidity [50% ± 5%]) with ad libitum access to food and water. Animals were handled, trained, and tested during the light cycle. All experimental protocols were in accordance with the Animal Ethics Committee of Tehran University of Medical Sciences. Rats were randomly divided into 4 experimental groups: social (control), isolation, resocialization for 3 days, and resocialization for 7 days.
Social (control)
In this group, 2 rats were put together in one large cage for 14 days, plus 1 week for acclimatization in controlled conditions.
Isolation
Rats were placed in small individual cages for 14 days. Before isolation, rats were together for an acceptable time (14 days) to acclimate to the environment.11
Resocialization
Rats had a similar pretreatment as in the isolated group, where the rats were together for an acceptable time (14 days) to acclimate to the environment. Rats were then isolated for 14 days and then resocialized for either 3 or 7 days. The method we used was forced resocialization, meaning that rats had no choice for selecting their peers. Prior to isolation, 4 rats were together in one cage.
Preparation of tissues for assessing OS markers
Brains were immediately removed and frozen in liquid nitrogen and placed in a −70°C freezer. For the preparation of the final homogenized sample, tissue blocks from hippocampus and PFC were prepared and after weighing, they were homogenized with buffer phosphate (1:10).
Malondialdehyde assessment
Total malondialdehyde assessment (MDA) was measured according to thiobarbituric acid (TBA) reaction. Briefly, to perform this analysis, TBA 1% (Sigma-Aldrich Co.) and trichloroacetic acid 20% (Sigma-Aldrich Co.) were mixed together. Then, 100 µL of the homogenized sample was added to the above mixture. After 90 minutes of boiling, the final solution developed a reddish-brown color and maximum absorbance was measured at 532 nm with a spectrometer.12
Glutathione assessment
DTNB was used as the reaction substrate for estimating the amount of reduced glutathione. For performing this experiment, Tris buffer (0.2 mol; Sigma-Aldrich Co.), DTNB (0.01 mol; Sigma-Aldrich Co.), and methanol (1.58 mL; Sigma-Aldrich Co.) were used. The homogenized sample (100 µL) was added to the above mixture. The final solution developed a light yellow color and maximum absorbance was measured at 412 nm with a spectrometer.13
Nitrite/nitrate assessment
Total nitrite/nitrate was assessed according to Griess reaction. Briefly, to perform this experiment, n-1 (naphthyl) ethylenediamine (0.2%; Sigma-Aldrich Co.), sulfanilamide (2%; Sigma-Aldrich Co.), and acid phosphoric (5%; Sigma-Aldrich Co.) were mixed together. The homogenized sample (100 µL) was added to the above mixture. The final solution developed a pink color and maximum absorbance was measured at 540 nm with a spectrometer.14
Spontaneous alternation in Y-maze
Short-term spatial memory was assessed with a Y-maze apparatus. Briefly, a Y-maze apparatus has 3 arms that cross each other with 120° between them. Rats are expected to explore the new arms with a higher frequency than a recently explored arm. Returning to a previously explored arm was counted as a mistake. A lower frequency of exploration of the last previously explored arm is an indicator of better memory performance. The frequency of exploration of 3 consecutive new arms (A, B, and C) was considered as an actual alternation. The scores are calculated as follows: (actual alternation/maximal alternation − 2) × 100. Also, all numbers of entries were recorded.15
Statistics
Data were analyzed using SPSS version 22 and GraphPad Prism version 5. Univariate analysis of variance (ANOVA) with 2 factors (OS markers × resocialization) was done to investigate the equality of variance and if the inequality was significant, post hoc test of Tukey was done to assess the equality of means. Data were as represented as mean ± SEM and P < .05 is considered significant. *, #, and $ represent significance difference of mean data among different groups for the level of statistical significance of P < .05. * was used for groups that are adjacent (control (social) × isolation and resocialization for 3 days (Resoc for 3 days) × resocialization for 7 days (Resoc for 7 days)) and for those groups that are not adjacent, symbols # and $ were used.
Results
MDA level in hippocampus
To investigate memory impairment as a result of excess MDA production in the hippocampus, an ANOVA was performed among the 4 groups of study (F3,21 = 71.05, P < .0001 [column factor]). Rats in the control group had a lower level of MDA than in the isolation group (0.5975 ± 0.01638 vs 0.9475 ± 0.0715, P < .0003). Rats in the resocialization for 3 days group had a higher level of MDA than the control group (0.5975 ± 0.01638 vs 1.32 ± 0.04675, P < .0001). Rats in the resocialization for 7 days group had lower MDA levels than rats in resocialization for 3 days and in the isolation groups (44.95 ± 0.0211 vs 1.32 ± 0.04675, P < .0001 and 44.95 ± 0.0211 vs 0.9475 ± 0.0715, P < .0001, respectively; Figure 1).
Figure 1.
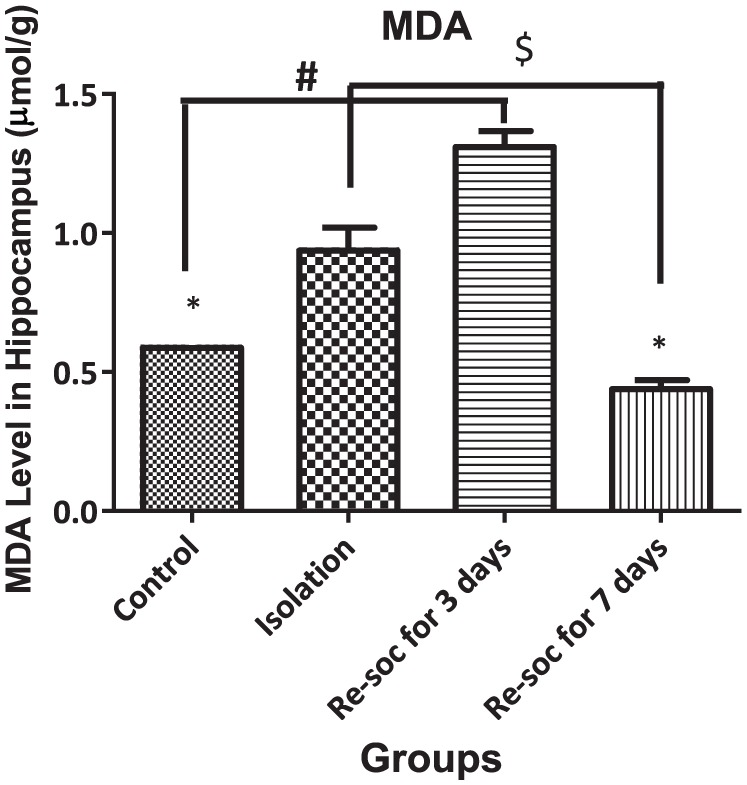
This figure shows MDA levels in the Hippocampus in all 4 groups. As the figure shows, after 7 days of resocialization, MDA levels returned to normal. Data were represented as mean ± SEM and P < .05 is considered significant (n = 8). * was used for groups that are adjacent (control × isolation and resocialization for 3 days (Resoc for 3 days) × resocialization for 7 days (Resoc for 7 days)) and for those groups that are not adjacent, the symbols # and $ were used. MDA indicates malondialdehyde.
MDA levels in the PFC
To investigate memory impairment as the result of excess MDA production in the PFC, an ANOVA was performed among the 4 groups of study (F3,21 = 64.4, P < .0001 [column factor]). Rats in the control group had lower levels of MDA than in the isolation group (0.2863 ± 0.07334 vs 0.96 ± 0.05004, P < .0001). Rats in the resocialization for 3 days group had higher levels of MDA than in the control group (0.2863 ± 0.07334 vs 1.288 ± 0.02836, P < .0001). Rats in resocialization for 7 days group had lower MDA levels than rats in the resocialization for 3 days and isolation groups (0.425 ± 0.08126 vs 1.288 ± 0.02836, P < .0001 and 0.425 ± 0.08126 vs 1.288 ± 0.02836, P < .001, respectively; Figure 2).
Figure 2.
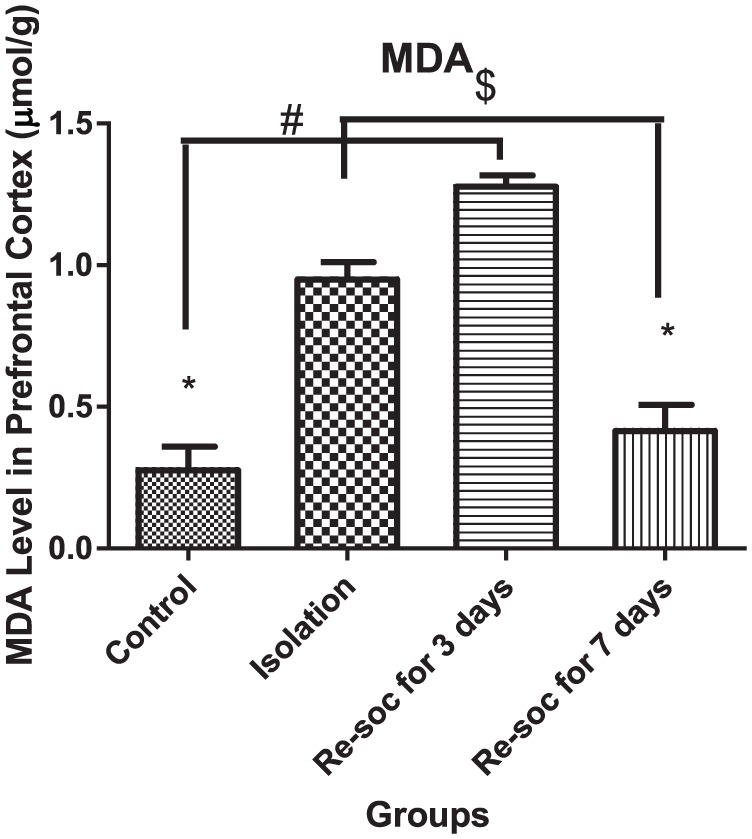
This figure shows MDA level in the prefrontal cortex in all 4 groups. As the figure shows, after 7 days of resocialization, MDA levels have normalized. Data were as represented as mean ± SEM and P < .05 is considered significant (n = 8). * was used for groups that are adjacent (control × isolation and resocialization for 3 days (Resoc for 3 days) × resocialization for 7 days (Resoc for 7 days)) and for those groups that are not adjacent, the symbols # and $ were used. MDA indicates malondialdehyde.
Glutathione level in hippocampus
For investigating memory improvement as a result of glutathione production in the hippocampus, an ANOVA was performed among the 4 experimental groups (F3,21 = 19.51, P < .0001 [column factor]). Rats in the control group had a higher level of glutathione than in the isolation group (3.895 ± 0.1675 vs 2.113 ± 0.06948, P < .0001). Rats in the resocialization for 7 days group had a higher level of glutathione than rats in resocialization for 3 days and isolation groups (4.074 ± 0.4309 vs 2.015 ± 0.07869, P < .0003; Figure 3).
Figure 3.
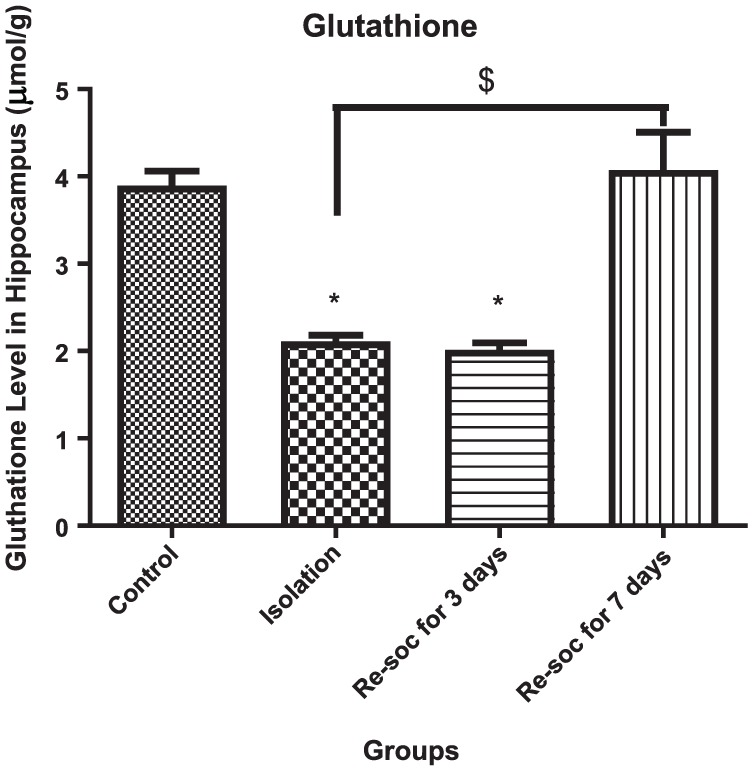
This figure shows the glutathione level in the hippocampus in all 4 groups. As the figure shows, after 7 days of resocialization, glutathione has normalized. Data were as represented as mean ± SEM and P < .05 is considered significant (n = 8). * was used for groups that are adjacent (control × isolation and resocialization for 3 days (Resoc for 3 days) × resocialization for 7 days (Resoc for 7 days)) and for those groups that are not adjacent, the symbols # and $ were used.
Glutathione level in PFC
For investigating memory improvement as a result of enough glutathione production in the PFC, an ANOVA was performed among the 4 groups of study (F3,21 = 12.84, P < .0001 [column factor]). Rats in the control group had a higher level of glutathione than the isolation group (2.449 ± 0.09927 vs 1.468 ± 0.1432, P < .0001). Rats in the resocialization for 7 days group had a higher level of glutathione than rats in the resocialization for 3 days and isolation groups (3.468 ± 0.2930 vs 2.210 ± 0.2721, P < .0072; Figure 4).
Figure 4.
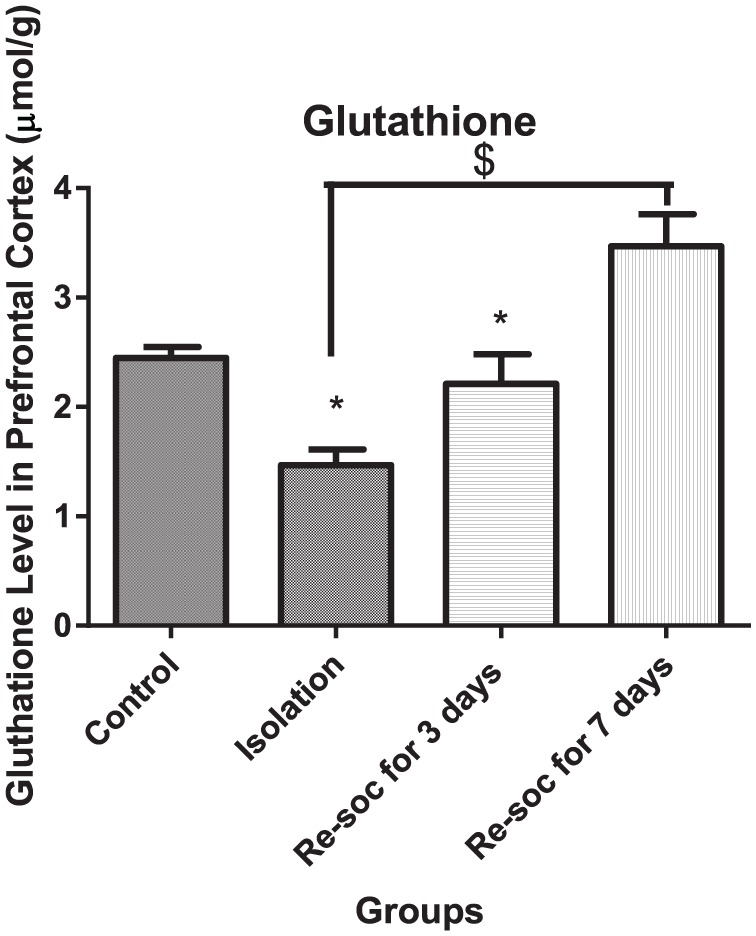
This figure shows glutathione levels in the prefrontal cortex in all 4 groups. As the figure shows, after 7 days of resocialization, glutathione has normalized. Also, after 3 days of resocialization, the level of glutathione is not significantly different from the control group. Data were as represented as mean ± SEM and P < .05 is considered significant (n = 8). * was used for groups that are adjacent (control × isolation and resocialization for 3 days (Resoc for 3 days) × resocialization for 7 days (Resoc for 7 days)) and for those groups that are not adjacent, the symbols # and $ were used.
Nitrite/nitrate in hippocampus
To investigate memory improvement as a result of enough nitrite/nitrate production in the hippocampus, an ANOVA was performed among the 4 groups of study (F3,21 = 12.45, P < .0001 [column factor]). Rats in the control group had a higher level of nitrite/nitrate than in the isolation group (17.27 ± 1.483 vs 9.231 ± 0.8847, P < .0004). Rats in the resocialization for 7 days group had a higher level of nitrite/nitrate than rats in the resocialization for 3 days and isolation groups (15.01 ± 2.438 vs 24.77 ± 1.748, P < .0058; Figure 5).
Figure 5.
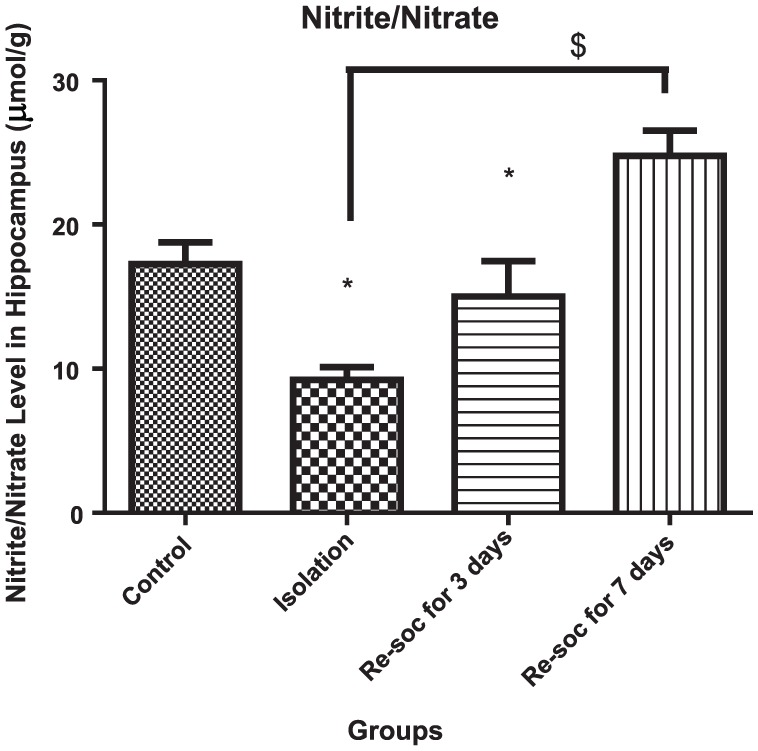
This figure shows the nitrite/nitrate level in the hippocampus in all 4 groups. As the figure shows, after 7 days of resocialization, nitrite/nitrate has normalized. Also, after 3 days of resocialization, nitrite/nitrate levels are not significantly different from the control group. Data were as represented as mean ± SEM and P < .05 is considered significant (n = 8). * was used for groups that are adjacent (control × isolation and resocialization for 3 days (Resoc for 3 days) × resocialization for 7 days (Resoc for 7 days)) and for those groups that are not adjacent, the symbols # and $ were used.
Nitrite/nitrate in PFC
For investigation of memory improvement as a result of enough nitrite/nitrate production in the PFC, an ANOVA was performed among the 4 experimental groups (F3,21 = 25.88, P < .0001 [column factor]). Rats in the control group had a higher level of nitrite/nitrate than the isolation and resocialization for 3 days rat groups (18.65 ± 1.591 vs 10.59 ± 0.3359, P < .0002 and 18.65 ± 1.591 vs 8.787 ± 0.8647, P < .0001, respectively). Rats in the resocialization for 7 days group had a higher level of nitrite/nitrate than rats in the resocialization for 3 days and isolation groups (8.787 ± 0.8647 vs 19.73 ± 1.136, P < .0001; Figure 6).
Figure 6.
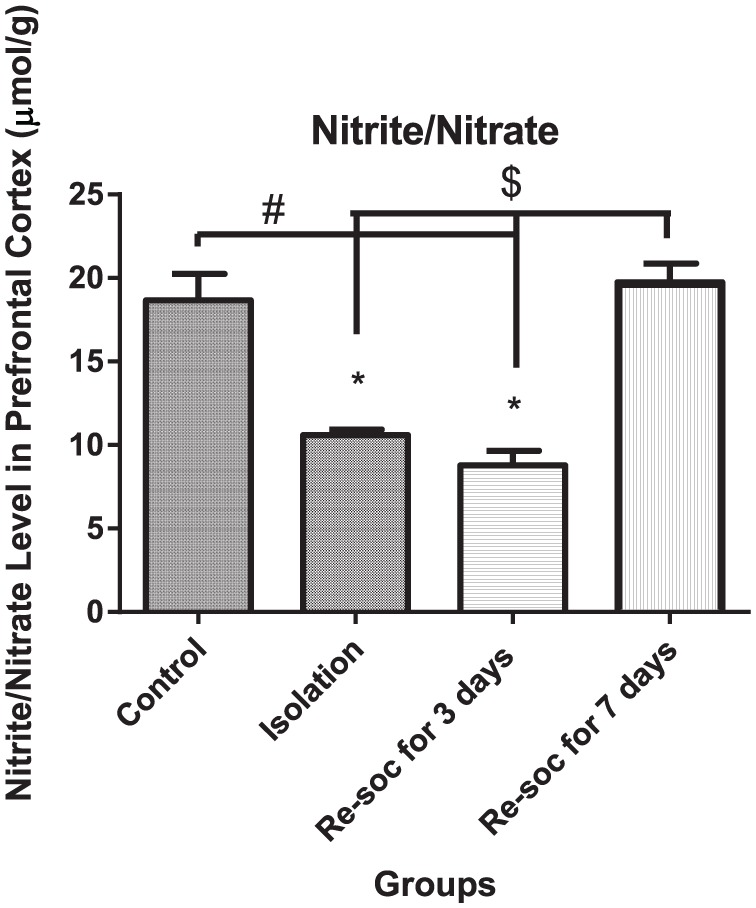
This figure shows the nitrite/nitrate level in the prefrontal cortex in all 4 groups. As the figure shows, after 7 days of resocialization, nitrite/nitrate has normalized. Data were as represented as mean ± SEM and P < .05 is considered significant (n = 8). * was used for groups that are adjacent (control × isolation and resocialization for 3 days (Resoc for 3 days) × resocialization for 7 days (Resoc for 7 days)) and for those groups that are not adjacent, the symbols # and $ were used.
The short-term memory (retrograde working memory) as assessed by Y-maze
Spontaneous alternation
For the investigation of memory performance among the different experimental groups, an ANOVA was performed (F3,21 = 31.53, P < .0001 [column factor]). Rats in the control group had a better performance in a Y-maze memory test than the isolation group (85.50 ± 2.533 vs 52.83 ± 2.934, P < .0001). Rats in the isolation group had a weaker performance in a Y-maze memory test than rats in the resocialization for 3 days and in resocialization for 7 days group (52.83 ± 2.934 vs 90.63 ± 3.128, P < .0001 and 52.83 ± 2.934 vs 85.00 ± 2.988, P < .0001, respectively; Figure 7).
Figure 7.
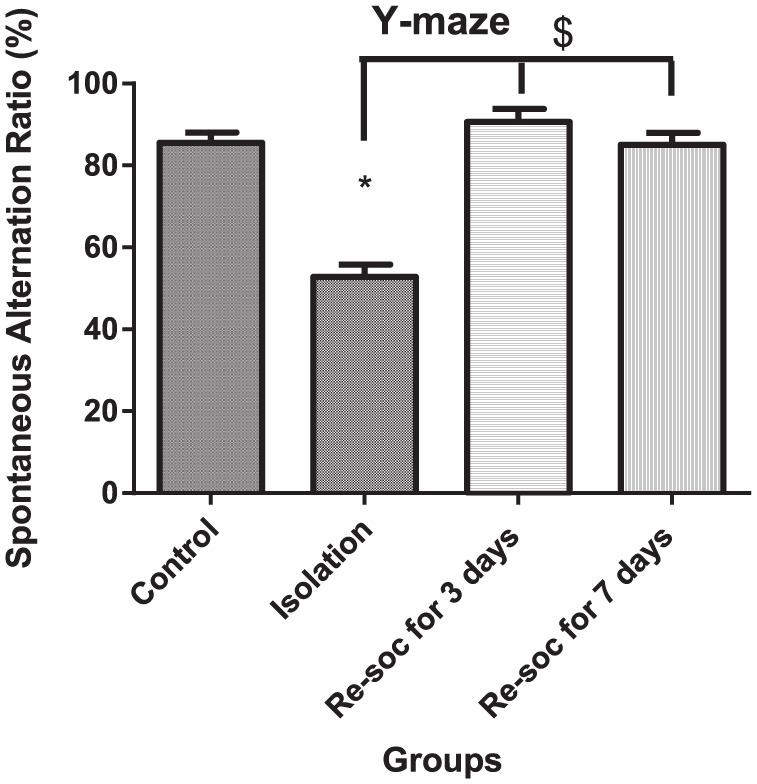
This figure shows spontaneous alternation in Y-maze. As marked by connectors, memory was retrieved after 3 days of resocialization. In this regard, other mechanisms other than oxidative stress status may be involved. Data were as represented as mean ± SEM and P < .05 is considered significant (n = 8). * was used for groups that are adjacent (control × isolation and resocialization for 3 days (Resoc for 3 days) × resocialization for 7 days (Resoc for 7 days)) and for those groups that are not adjacent, the symbols # and $ were used.
A number of entries
To investigate the number of entries into Y-maze arms among different groups of study, an ANOVA was performed among the 4 groups of the study (F3,21 = 6.676, P < .0024 [column factor]). Rats in the isolation group had more entries into Y-maze arms than the control group (8.375 ± 0.9989 vs 15.00 ± 2.009, P < .0105). Also, rats in resocialization for 3 days had more entries into the arms than rats in resocialization for 7 days (16.38 ± 1.899 vs 8.500 ± 1.225, P < .0036). Rats in the control group had fewer entries into Y-maze arms than rats in the resocialization for 3 days (8.375 ± 0.9989 vs 16.38 ± 1.899, P < .0022). Also, rats in the resocialization for 7 days group had fewer entries into Y-maze arms than rats in the isolation group (8.500 ± 1.225 vs 15.00 ± 2.009, P < .0153; Figure 8).
Figure 8.
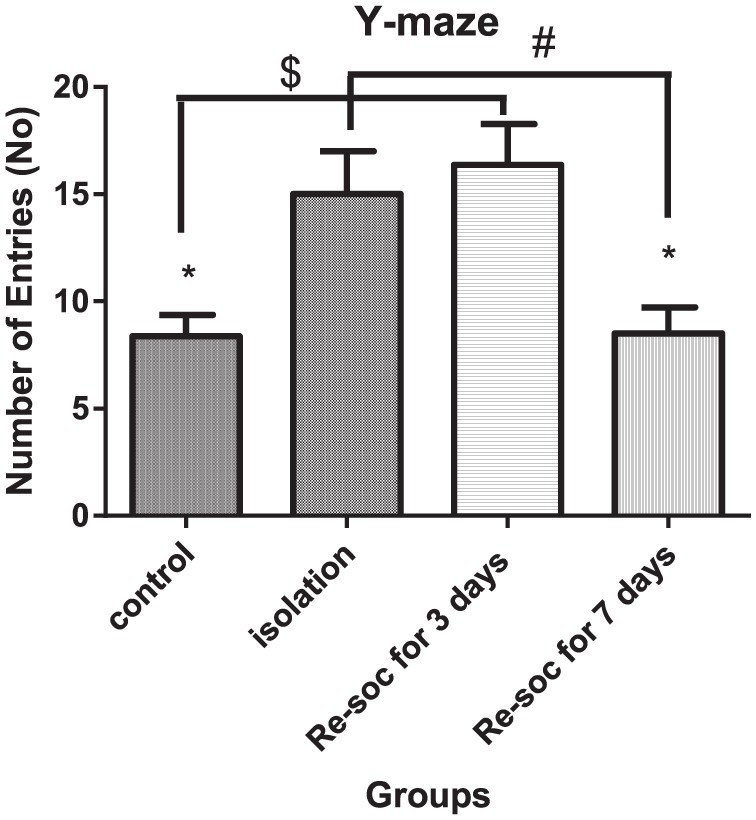
This figure shows a number of entries into Y-maze arms in Y-maze. More number of entries is associated with more memory disturbance. It is thought that more exploratory activity in rats with resocialization for 3 days is indicative of more effort to avoid wrong entries which means to enter to previously explored arms. Data were as represented as mean ± SEM and P < .05 is considered significant (n = 8). * was used for groups that are adjacent (control × isolation and resocialization for 3 days (Resoc for 3 days) × resocialization for 7 days (Resoc for 7 days)) and for those groups that are not adjacent, the symbols # and $ were used.
Discussion
In this experiment, we showed that the level of OS markers had little influence on short-term memory retrieval after 14 days of social isolation. This finding was highlighted by showing that after 3 days of resocialization, any memory deficit was restored to a normal state independent of all types of antioxidant defense. Also, after 7 days of resocialization, memory status in the Y-maze was equal to that of those in the 3 days of the resocialization group, so any improvement in the levels of antioxidant markers was not necessary to restore memory performance in the Y-maze.
Oxidative stress can cause memory impairment via different mechanisms. Age-related memory deficits occur because of the accumulation of oxidative damage and concurrent reduction in antioxidant defenses, which results in a reduction in acetylcholine.16 In childhood, OS can cause cognitive deficits through an overload of iron.17 Sleep deprivation also causes cognitive deficits through an increase in OS.18
Oxidative stress affects both short-term memory and long-term memory; for example, in one study, nicotine-induced both short-term and long-term memory impairment.19 Memory impairment can happen through changes in infrastructures of the synapse, such as alterations in synaptic plasticity.20
Oxidative stress is the cause of some important psychiatric disorders, such as schizophrenia and depression.21 The mechanism driving OS to produce such diseases is unclear, but treatment with antioxidants can improve symptoms in schizophrenia22 and bipolar disorder.23 This has importance because memory impairment, based on recent studies, can initiate psychiatric disorders. A depletion of glutathione is thought to impair spatial memory by weakening dopaminergic or glutamatergic action and this evidence comes from studies on schizophrenia.24
As our results indicate, memory in a Y-maze is not completely dependent on OS status and there may be another regulatory mechanism that operates in the absence of a good OS profile. In this experiment, MDA levels in the PFC were not reduced after 3 days of resocialization. Also, only glutathione in the PFC and nitrite/nitrate in the hippocampus returned to normal levels after 3 days of resocialization. Therefore, according to our research, short-term memory in a Y-maze is more dependent on glutathione in the PFC and nitrite/nitrate in the hippocampus along with MDA playing a weaker role in this regard. However, for maintenance of good short-term memory performance, OS seems to be necessary. Memory formation involves different stages that require that the PFC and hippocampus work in parallel to each. To understand the exact mechanism behind memory formation, more studies are recommended.
Based on previous studies, the stress from both long-term and short-term isolation increases the susceptibility of prefrontal and hippocampus disturbances in cognitive function.25 In this study, the behavioral paradigm altered memory processes assessed by a Y-maze. Overall, stress from isolation declines cognitive abilities. In this study, using our paradigm, we showed that memory declined in a Y-maze experiment and that the duration of isolation is important. It is possible that with a longer isolation period, memory may decline even more.
Social interaction vs social isolation is an important factor that alleviates the adverse effects of dementia-related induced social isolation.26 Studies have been well documented showing that social interaction is a necessary treatment for reducing the side effects of social isolation that occur as a consequence of dementia.27,28 In some studies, socialization with animals has successfully been used to improve memory in patients with dementia.29 Socialization is recommended for those affected patients who lose their memory gradually because dementia eventually detaches the person from society.30 However, in this study, social isolation was selected for inducing memory impairment as it is the cause of many behavioral abnormalities, such as memory impairment, anxiety-related disorders, and also emotionally related disorders.31 The mechanism that eventually leads to permanent memory loss is poorly understood. However, this study was designed in such a way that the mechanisms behind social interaction have been shown to improve memory. To investigate the benefits of social interaction and the mechanism that underlies this, we induced memory impairment in rats using social isolation and then resocialization for different durations. Social interaction of 3 days was selected to assess the prompt benefit of social interaction. Memory was recovered after this period, but OS that was assessed by measuring MDA, glutathione, and nitrite/nitrate was not improved. In this regard, this study describes that OS is not the only factor that needs to improve to see an improvement in memory retrieval. Based on previous studies, the amelioration of OS has been suggested as a new treatment for memory impairment.32 However, this study emphasizes the importance of social interaction, emphasizing that it is more effective than drug therapy. Social interaction is a necessary behavior for the maintenance of normal brain function and encompasses components such as emotion and cognition. This interaction of cognition and emotion on the maintenance of normal brain function is not well understood. In human society, many factors influence social interaction, such as culture, social class, social status, roles, groups, and social institutions, and they have been successfully used for the improvement of behavioral abnormalities, such as memory impairment.33
Memory that is assessed by a Y-maze involves two areas of the brain: the hippocampus and PFC. Memory formation is a multistage process. In each stage, some faculties of the brain are necessary for memory formation. Any type of memory requires a similar encoding process. However, for consolidation and storage of memories, especially spatial memories, different structures of the brain are necessary. In memory that is assessed by Y-maze (retrograde spatial working memory), the hippocampus is necessary to encode and retrieve the information of the main components of memory other than the surroundings. Spatial memory, in particular, appears to be much more confined to the hippocampus, particularly the right hippocampus, which seems to be able to create a mental map of space with special cells called place cells.34 The lateral PFC is essential for remembering contextual details of an experience rather than for memory formation.35 Therefore, both hippocampus and PFC are necessary parts of spatial working memory formation. The memory that is assessed by Y-maze is a type of memory that is most affected by Alzheimer disease.36
Based on the results of this study, OS is not a necessary factor for rapid memory retrieval. Although recent studies suggest that OS is a necessary factor for memory function, the result of this study highlighted the importance of other factors in memory retrieval besides OS. Different studies have well described the importance of OS in memory improvement.16,37 Most studies support the idea that OS can damage neuronal components and reduce memory.38 Based on these results, different studies have been designed to show the importance of OS in memory function in aged individuals.39,40 Also, many studies support the idea that antioxidant therapy is a necessary treatment for the improvement of long-term potentiation.20,41,42 A recent study suggests that cognitive training by improving OS will improve overall memory functioning in aged individuals.39 However, the importance of OS in rapid memory retrieval has not been supported by previous studies. For rapid memory retrieval, there are few studies and they cannot fully explain the precise mechanism. It has been suggested that mineralocorticoids and norepinephrine are necessary for rapid memory retrieval.43,44 Also, in another study, PFC activation was shown as an important factor for memory retrieval.45 However, there is a lack of studies to document the precise mechanism underlying rapid memory retrieval. This study suggests that OS is not mandatory for this purpose and social interaction may be more efficient for rapid memory retrieval. Previous studies have established the positive effects of social interaction on cognitive function. Social interaction involving discussing daily emotional and neutral events with other people can improve the accuracy of memory.46 This study also confirms that social interaction is necessary for proper memory function.
In recent studies, there has been great interest in elucidating the exact role of the hippocampus and PFC in various stages of memory formation and how they interact with each other. In this experiment, memory formation and retrieval were assessed. Therefore, it is important to know how the hippocampus and PFC contribute to memory formation and retrieval. In this experiment, we showed that hippocampus and PFC function in the Y-maze are related to each other, as a better score in 2 OS markers was obtained in the resocialization for 3 days group along with better performance in the Y-maze.
Generally, the hippocampus and its adjacent areas are involved in different memory-related tasks, but more specifically, the rapid formation of new memories requires the hippocampus.47 It has a close association with areas of the neocortex for the consolidation of memories.34 Aside from place cells, which are necessary for the formation of memories based on the spatial relationship of different objects, the hippocampus also has another type of cell called a time cell. The time cell relates to memories of related events that happen in sequential order in time.48 The hippocampus is not only capable of encoding memory but also can consolidate memories.49 The hippocampus has connections with the PFC for the consolidation of memories.50
The PFC is involved in the consolidation of memories and for the facilitation of the retrieval of memories. For the consolidation of memories, old memories need to be incorporated into new schemes. In this regard, the PFC has an important role. Frankland et al51 showed that destroying the medial PFC impairs the retrieval of distant memories, but not recent ones. Different parts of prefrontal areas do not have the same function in memory-related issues.52 It should be noted that for the retrieval of memories, new and old memories mix together in a context of memory formation. In this regard, the PFC plays a pivotal role.53
For the elucidation of the hippocampus and PFC in memory ability, different paradigms of experiments have been used. The prefrontal-hippocampus interconnection is useful for proper retrieval, but damage to the hippocampus does not disrupt memory retrieval.54
In this study, the role of different OS markers, such as MDA, glutathione, and nitrite/nitrate, was assessed in 2 regions of the brain to uncover the importance of each marker in the short term (3 days of resocialization) and long term (7 days of resocialization). We discovered that only 2 factors (glutathione in the PFC and nitrite/nitrate in the hippocampus) are necessary for short-term memory retrieval in the Y-maze. An interesting finding may be that rats after 3 days of resocialization had more exploratory activity (as was assessed by the number of entries in the Y-maze) to compensate for impaired memory.
Conclusions
Social isolation caused memory deficits in a Y-maze. Resocialization of rats was an effective treatment for the reinstatement of working memory. The restoration of antioxidant defense with resocialization was not the only factor that aided in the retrieval of memory in the Y-maze. Other factors were activated with resocialization to restore memory back to its normal state, as was shown by the fact that only 2 markers went back to a normal state after 3 days of resocialization. Therefore, it seems that an antioxidant defense is not the major factor for restoring memory deficits resulting from social isolation.
Footnotes
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: This work was carried out in collaboration between two authors. HF designed the study, performed the experiments and the statistical analysis. HF managed the literature search and prepared the first draft of the manuscript with assistance from MK. Both authors read and approved the final draft of the manuscript.
ORCID iD: Morteza Karimian  https://orcid.org/0000-0001-8015-1914
https://orcid.org/0000-0001-8015-1914
References
- 1. Kampa BM, Gundlfinger A, Letzkus JJ, Leibold C. Circuit mechanisms of memory formation. Neur Plast. 2011;2011:494675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment—beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256:240–246. [DOI] [PubMed] [Google Scholar]
- 3. Eisele M, Zimmermann T, Köhler M, et al. Influence of social support on cognitive change and mortality in old age: results from the prospective multicentre cohort study AgeCoDe. BMC Geriatr. 2012;12:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Williams JW, Plassman BL, Burke J, Benjamin S. Preventing Alzheimer’s disease and cognitive decline. Evid Rep Technol Assess. 2010;193:1–727. [PMC free article] [PubMed] [Google Scholar]
- 5. Sharma M, Gupta YK. Effect of chronic treatment of melatonin on learning, memory and oxidative deficiencies induced by intracerebroventricular streptozotocin in rats. Pharmacol Biochem Behav. 2001;70:325–331. [DOI] [PubMed] [Google Scholar]
- 6. Behl C, Moosmann B. Oxidative nerve cell death in Alzheimer’s disease and stroke: antioxidants as neuroprotective compounds. Biol Chem. 2002;383:521–536. [DOI] [PubMed] [Google Scholar]
- 7. Butterfield DA, Castegna A, Drake J, Scapagnini G, Calabrese V. Vitamin E and neurodegenerative disorders associated with oxidative stress. Nutr Neurosci. 2002;5:229–239. [DOI] [PubMed] [Google Scholar]
- 8. Harrison FE, Allard J, Bixler R, et al. Antioxidants and cognitive training interact to affect oxidative stress and memory in APP/PSEN1 mice. Nutr Neurosci. 2009;12:203–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kiasalari Z, Khalili M, Shafiee S, Roghani M. The effect of Vitamin E on learning and memory deficits in intrahippocampal kainate-induced temporal lobe epilepsy in rats. Indian J Pharmacol. 2016;48:11–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chang CH, Hsiao YH, Chen YW, Yu YJ, Gean PW. Social isolation-induced increase in NMDA receptors in the hippocampus exacerbates emotional dysregulation in mice. Hippocampus. 2015;25:474–485. [DOI] [PubMed] [Google Scholar]
- 11. Chan JN, Lee JC, Lee SS, et al. Interaction effect of social isolation and high dose corticosteroid on neurogenesis and emotional behavior. Front Behav Neurosci. 2017;11:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tukozkan N, Erdamar H, Seven I. Measurement of total malondialdehyde in plasma and tissues by high-performance liquid chromatography and thiobarbituric acid assay. Firat Tip Dergisi. 2006;11:88–92. [Google Scholar]
- 13. Khan RA, Khan MR, Sahreen S. Brain antioxidant markers, cognitive performance and acetylcholinesterase activity of rats: efficiency of Sonchus asper. Behav Brain Funct. 2012;8:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Miranda KM, Espey MG, Wink DA. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide. 2001;5:62–71. [DOI] [PubMed] [Google Scholar]
- 15. Mandillo S, Tucci V, Holter SM, et al. Reliability, robustness, and reproducibility in mouse behavioral phenotyping: a cross-laboratory study. Physiol Genomics. 2008;34:243–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Haddadi M, Jahromi SR, Sagar BK, Patil RK, Shivanandappa T, Ramesh SR. Brain aging, memory impairment and oxidative stress: a study in Drosophila melanogaster. Behav Brain Res. 2014;259:60–69. [DOI] [PubMed] [Google Scholar]
- 17. De Lima MN, Polydoro M, Laranja DC, et al. Recognition memory impairment and brain oxidative stress induced by postnatal iron administration. Eur J Neurosci. 2005;21:2521–2528. [DOI] [PubMed] [Google Scholar]
- 18. Miller RP. Nutrition in addiction recovery. Barre, MA: Many Hands Sustainability Center, 2010. [Google Scholar]
- 19. Hritcu L, Ciobica A, Gorgan L. Nicotine-induced memory impairment by increasing brain oxidative stress. Cent Eur J Biol. 2009;4:335–342. [Google Scholar]
- 20. Hu D, Serrano F, Oury TD, Klann E. Aging-dependent alterations in synaptic plasticity and memory in mice that overexpress extracellular superoxide dismutase. J Neurosci. 2006;26:3933–3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dean O, Bush AI, Berk M, Copolov DL, van den Buuse M. Glutathione depletion in the brain disrupts short-term spatial memory in the Y-maze in rats and mice. Behav Brain Res. 2009;198:258–262. [DOI] [PubMed] [Google Scholar]
- 22. Berk M, Copolov DL, Dean O, et al. N-acetyl cysteine as a glutathione precursor for schizophrenia—a double-blind, randomized, placebo-controlled trial. Biol Psychiatr. 2008;64:361–368. [DOI] [PubMed] [Google Scholar]
- 23. Berk M, Copolov DL, Dean O, et al. N-acetyl cysteine for depressive symptoms in bipolar disorder—a double-blind randomized placebo-controlled trial. Biol Psychiatr. 2008;64:468–475. [DOI] [PubMed] [Google Scholar]
- 24. Do KQ, Trabesinger AH, Kirsten-Kruger M, et al. Schizophrenia: glutathione deficit in cerebrospinal fluid and prefrontal cortex in vivo. Eur J Neurosci. 2000;12:3721–3728. [DOI] [PubMed] [Google Scholar]
- 25. Zlatkovic J, Todorovic N, Boskovic M, Pajovic SB, Demajo M, Filipovic D. Different susceptibility of prefrontal cortex and hippocampus to oxidative stress following chronic social isolation stress. Mol Cell Biochem. 2014;393:43–57. [DOI] [PubMed] [Google Scholar]
- 26. Hsiao YH, Chang CH, Gean PW. Impact of social relationships on Alzheimer’s memory impairment: mechanistic studies. J Biomed Sci. 2018;25:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang HX, Karp A, Winblad B, Fratiglioni L. Late-life engagement in social and leisure activities is associated with a decreased risk of dementia: a longitudinal study from the Kungsholmen project. Am J Epidemiol. 2002;155:1081–1087. [DOI] [PubMed] [Google Scholar]
- 28. Akanuma K, Meguro K, Meguro M, et al. Improved social interaction and increased anterior cingulate metabolism after group reminiscence with reality orientation approach for vascular dementia. Psychiatry Res. 2011;192:183–187. [DOI] [PubMed] [Google Scholar]
- 29. Richeson NE. Effects of animal-assisted therapy on agitated behaviors and social interactions of older adults with dementia. Am J Alzheimer’s Dis Other Demen. 2003;18:353–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jootun D, McGhee G. Effective communication with people who have dementia. Nurs Stand. 2011;25:40–46. [DOI] [PubMed] [Google Scholar]
- 31. Wallace DL, Han MH, Graham DL, et al. CREB regulation of nucleus accumbens excitability mediates social isolation-induced behavioral deficits. Nat Neurosci. 2009;12:200–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Farr SA, Poon HF, Dogrukol-Ak D, et al. The antioxidants alpha-lipoic acid and N-acetylcysteine reverse memory impairment and brain oxidative stress in aged SAMP8 mice. J Neurochem. 2003;84:1173–1183. [DOI] [PubMed] [Google Scholar]
- 33. Lareau A, Horvat EM. Moments of social inclusion and exclusion race, class, and cultural capital in family-school relationships. Sociol Educ. 1999;72:37–53. [Google Scholar]
- 34. Eichenbaum H. The hippocampus and declarative memory: cognitive mechanisms and neural codes. Behav Brain Res. 2001;127:199–207. [DOI] [PubMed] [Google Scholar]
- 35. Ofen N, Kao YC, Sokol-Hessner P, Kim H, Whitfield-Gabrieli S, Gabrieli JD. Development of the declarative memory system in the human brain. Nat Neurosci. 2007;10:1198–1205. [DOI] [PubMed] [Google Scholar]
- 36. Gotz J, Ittner LM. Animal models of Alzheimer’s disease and frontotemporal dementia. Nat Rev. 2008;9:532–544. [DOI] [PubMed] [Google Scholar]
- 37. Salim S. Oxidative stress and the central nervous system. J Pharmacol Exp Ther. 2017;360:201–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kruk-Slomka M, Boguszewska-Czubara A, Slomka T, Budzynska B, Biala G. Correlations between the memory-related behavior and the level of oxidative stress biomarkers in the mice brain, provoked by an acute administration of CB receptor ligands. Neur Plast. 2016;2016:9815092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pesce M, Tatangelo R, La Fratta I, et al. Aging-related oxidative stress: positive effect of memory training. Neuroscience. 2018;370:246–255. [DOI] [PubMed] [Google Scholar]
- 40. Dumont M, Wille E, Stack C, Calingasan NY, Beal MF, Lin MT. Reduction of oxidative stress, amyloid deposition, and memory deficit by manganese superoxide dismutase overexpression in a transgenic mouse model of Alzheimer’s disease. FASEB J. 2009;23:2459–2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mcdonald SR, Sohal RS, Forster MJ. Concurrent administration of coenzyme Q10 and α-tocopherol improves learning in aged mice. Free Radical Bio Med. 2005;38:729–736. [DOI] [PubMed] [Google Scholar]
- 42. Kamsler A, Avital A, Greenberger V, Segal M. Aged SOD overexpressing mice exhibit enhanced spatial memory while lacking hippocampal neurogenesis. Antioxid Redox Signal. 2007;9:181–189. [DOI] [PubMed] [Google Scholar]
- 43. Dorey R, Piérard C, Shinkaruk S, et al. Membrane mineralocorticoid but not glucocorticoid receptors of the dorsal hippocampus mediate the rapid effects of corticosterone on memory retrieval. Neuropsychopharmacology. 2011;36:2639–2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Murchison CF, Zhang XY, Zhang WP, Ouyang M, Lee A, Thomas SA. A distinct role for norepinephrine in memory retrieval. Cell. 2004;117:131–143. [DOI] [PubMed] [Google Scholar]
- 45. Rugg MD, Fletcher PC, Frith CD, Frackowiak RS, Dolan RJ. Differential activation of the prefrontal cortex in successful and unsuccessful memory retrieval. Brain. 1996;119:2073–2083. [DOI] [PubMed] [Google Scholar]
- 46. Kensinger EA, Choi HY, Murray BD, Rajaram S. How social interactions affect emotional memory accuracy: evidence from collaborative retrieval and social contagion paradigms. Mem Cognit. 2016;44:706–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Milner B, Squire LR, Kandel ER. Cognitive neuroscience and the study of memory. Neuron. 1998;20:445–468. [DOI] [PubMed] [Google Scholar]
- 48. MacDonald CJ, Lepage KQ, Eden UT, Eichenbaum H. Hippocampal “time cells” bridge the gap in memory for discontiguous events. Neuron. 2011;71:737–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jin J, Shie F-S, Liu J, et al. Prostaglandin E2 receptor subtype 2 (EP2) regulates microglial activation and associated neurotoxicity induced by aggregated α-synuclein. J Neuroinflammation. 2007;4:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Colgin LL. Oscillations and hippocampal-prefrontal synchrony. Curr Opin Neurobiol. 2011;21:467–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Frankland PW, Bontempi B, Talton LE, Kaczmarek L, Silva AJ. The involvement of the anterior cingulate cortex in remote contextual fear memory. Science (New York). 2004;304:881–883. [DOI] [PubMed] [Google Scholar]
- 52. Bonnici HM, Chadwick MJ, Lutti A, Hassabis D, Weiskopf N, Maguire EA. Detecting representations of recent and remote autobiographical memories in vmPFC and hippocampus. J Neurosci. 2012;32:16982–16991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Frankland PW, Bontempi B. The organization of recent and remote memories. Nat Rev Neurosci. 2005;6:119–130. [DOI] [PubMed] [Google Scholar]
- 54. Komorowski RW, Manns JR, Eichenbaum H. Robust conjunctive item-place coding by hippocampal neurons parallels learning what happens where. J Neurosci. 2009;29:9918–9929. [DOI] [PMC free article] [PubMed] [Google Scholar]


