Short abstract
Neuroinflammation plays an important role in the induction and maintenance of chronic pain. Orchestra of pattern-recognition receptor-induced pro-inflammatory and anti-inflammatory cytokines is critical for inflammation homeostasis. CD11b on macrophages could inhibit toll-like receptor (TLR) activation-induced inflammatory responses. However, the function of CD11b on microglia remains unknown. In the current study, we demonstrated that CD11b-deficient microglia cells produced more inflammatory cytokines, such as interleukin-6 and tumor necrosis factor alpha, while less anti-inflammatory cytokines. Signal transduction assay confirmed that nuclear factor-κB activation was increased in CD11b-deficient microglia cells, which resulted from decreased activation of Src. Inhibition of Src by PP1 increased inflammation in wild-type microglia cells significantly, but not in CD11b-deficient microglia cells. In vivo, CD11b-deficient mice were more susceptible to chronic constrictive injury-induced allodynia and hyperalgesia with significantly more inflammatory cytokines expression. All these results indicated that the regulatory function of CD11b-Src signal pathway on both inflammatory and anti-inflammatory cytokines in microglia cells is a potential target in neuropathic pain treatment.
Keywords: Neuropathic pain, neuroinflammation, CD11b, Src, TLR4
Introduction
Chronic pain is typically characterized by hyperalgesia, which is an increased response to noxious thermal or mechanical stimuli, as well as allodynia, nociceptive responses which occur to normally innocuous stimuli even light touch (known as mechanical allodynia).1–3 Inflammation is defined as homeostatic interaction between the immune system and injury tissues. Inflammatory mediators, such as misfolded proteins, aberrantly localized nucleic acids, reactive oxygen species (ROS), H+, adenosine-triphosphate (ATP) bradykinin, prostaglandins, nerve growth factor, and pro-inflammatory cytokines and chemokines, released locally after tissue injury can directly stimulate and cause sensitization of pain-sensing nociceptors located at nerve fibers of primary afferent neurons in peripheral tissue.4–8 The innate immune receptors in microglia cells can directly respond to damage-associated molecular patterns (DAMPs) or pathogen-associated molecular patterns (PAMPs).9,10 Abnormal activation of microglia cells leads to the sustained exposure of neurons to pro-inflammatory mediators and causes neuronal dysfunction or cell death. Thus, activation and regulation of microglia cells are critical for neuron system homeostasis.
Microglia expressed a board spectrum of pattern-recognition receptors (PRRs), including toll-like receptor (TLR) 2, TLR4, and TLR6. As there is a strong overlap between the signaling pathways induced by PAMPs and DAMPs, microglia may not be able to discriminate invading pathogens and misfolded or aberrant endogenous molecular patterns.11–13 Inappropriate activation of PRRs can lead to prolonged inflammation, which induces both pro-inflammatory cytokines, such as interleukin (IL)-6 and tumor necrosis factor alpha (TNF-α), and anti-inflammatory cytokines, such as IL-10 and transforming growth factor beta (TGF-β).9,10,14 Although the molecular regulatory mechanism of TLR signaling in macrophages has been under intensive research, it remains unknown whether there is a potential signal pathway orchestrating both inflammatory and anti-inflammatory cytokines. Thus, it is critical to determine the balance of PRRs signal transduction pathway in inflammatory response and immune disorders in order to maintain innate immune response.
CD11b, a member of integrin family, is highly expressed on monocytes/macrophages, common dendritic cells (DCs), and microglia cells.15,16 CD11b expressed on DCs inhibited Th17 differentiation and T-cell activation.17,18 Stromal microenvironment of spleen, liver, and lung can drive generation of a new population of CD11bhi regulatory DCs with higher IL-10 production, which could inhibit T-cell activation.19–21 CD11b on macrophages could also inhibit TLR activation-induced inflammatory responses by inhibiting inflammatory cytokines and increasing anti-inflammatory cytokine IL-10 by activating Src-spleen tyrosine kinase (Syk) signaling.22,23 However, the function of integrins on microglia, such as CD11b, remains unknown.
Therefore, we made the following assumptions and validate them by experiments: CD11b inhibits inflammatory cytokines IL-6 and TNF-α via Src/Syk pathway, and attenuates chronic constrictive injury (CCI)-induced allodynia by promoting TLR-triggered IL-10 expression. Here, we found that CD11b-deficient microglia produced more inflammatory cytokines, such as IL-6 and TNF-α, while less anti-inflammatory cytokines, such as IL-10. Signal transduction assay confirmed that CD11b inhibited TLR-signaling and inflammatory cytokines through Src/Syk. CD11b-deficient mice were more susceptible to CCI-induced hypersensitivity. Our results revealed the regulatory function of CD11b-Src signal pathway in orchestrating both inflammatory and anti-inflammatory cytokines in microglia cells, which outlines potential-targeted signal pathway in the treatment of neuropathic pain by Src activator.
Materials and methods
Animal preparation
Male C57 BL/6J mice (20–25 g) were purchased from the Laboratory Animal Center of the Second Military Medical University and caged in groups of four or five. Mice homozygous for Itgam (CD11b) deficiency (B6.129S4-Itgamtm1Myd/J; 003991; Jackson Laboratories, Bar Harbor, ME) were bred in pathogen-free conditions. Mice were maintained 12:12 h light/dark cycle with adequate food and water. All mice were placed in the experimental room 24 h before behavioral test for acclimatization. All experiments were reviewed and approved by the Institutional Animal Care and Use Committee of the Second Military Medical University (Shanghai, China) and conducted in accordance with the American Physiological Society’s Guiding Principles in the Care and Use of Animals.24
All male mice were randomly separated from animal center. On the day before experiment, the mice were randomly assigned to either the control group or one of the experiment groups, and sample size for each experiment was estimated by Mead’s resource equation, which are appropriate.
Primary cultures of microglia
Microglia were isolated from primary mixed glial cell cultures prepared from the whole brain of new born mice on day 14 using the “shaking off” method as previously described.5,6 The isolated cells (1 × 106) were plated into six-well plate in high-glucose Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, 2 mM L-glutamine and 1% penicillin/streptomycin. After 10 days in culture, plates were gently shaken to detach microglial cells to culture media. The culture media were then plated in a fresh six-well plate for 30 min. The suspensory glial cells were then removed via changing culture media. The purity of the cultures (99%) was determined by anti-CD11b immunostaining (BD Biosciences, Franklin Lakes, NJ).
CCI model
CCI to the sciatic nerve in mice was performed as previously described.25,26 Briefly, mice were anesthetized with 5% chloral hydrate (0.8 ml/100 g), and an incision was made just below the right hipbone, parallel to the sciatic nerve. The sciatic nerve was isolated and loosely ligated with three ligatures (4-0 silk), around the nerve distal. A brief twitch can be observed in the right hind limb. Then, the incision was closed and mice were placed on the constant temperature mattress until they recovered from anesthesia. In sham-operated mice, the right sciatic nerve was exposed without ligation.
Behavioral tests for mechanical thresholds
Mechanical allodynia, measured as paw withdraw threshold assessed by von Frey monofilaments (Stoelting, Wood Dale, IL), was carried out prior to CCI surgery and 1, 3, 7, and 14 days after CCI surgery. Briefly, mice were placed in individual plastic boxes (20 × 20 × 15 cm) on a metal mesh floor and allowed to acclimate for approximately 1 h before testing. Beginning with 0.16 g and ending with 4.0 g, we used the von Frey filament to touch the plantar surface of the hind paw, which was held for 6–8 s. In the event of the absence of paw withdrawal, the next stronger stimulus was chosen, while a weaker stimulus was applied in the opposite situation. This was repeated 10 times, and the stimulation strength was determined as the average corresponding to a 50% response rate. The measurements were repeated five times and performed between 11 a.m. and 3 p.m.26,27
Behavioral tests for thermal hypersensitivity
Thermal hyperalgesia was assessed by testing the paw withdrawal latency using the Hargreaves radiant heat apparatus (IITC Life Science, Woodland Hills, CA). Specifically, mice were placed beneath the same plastic cages upon an elevated glass floor. With mouse standing relatively still, a radiant heat source beneath the glass floor was aimed at the plantar surface of the hind paw and was moved away immediately when mice lifted up or licked the hind paw. The stimulus also shut off automatically after 20 s to prevent tissue damage.3 This was repeated four times for each hind paw at 5-min intervals, and the average was taken as the paw withdrawal latency.
Real-time PCR
Total RNAs from L5 DRGs were isolated using an Ultraspec RNA isolation kit (Biotecx, Houston, TX). Triplicate real-time PCR samples were preceded as previously reported on the ABI Prism 7000 sequence detection system. The primers for TNF-α, IL-6, and IL-1β (sTable 1) were according to previously reported.22,23 All real-time RT-PCR experiments were performed at least three times, and the mean ± SEM values are presented.
Immunoblot assay
Cells pretreated as described above were lysed with RIPA buffer (Cell Signaling Technology, Beverly, MA) supplemented with protease inhibitor cocktail. Protein concentrations of the extracts were measured with BCA assay. Phosphor-specific antibodies against p65(Ser536), p38(Thr180/Tyr182), ERK(Thr202/Tyr204), JNK(Thr183/Tyr185), TAK1(Thr184/187), IKKα/β(Ser176/180), c-jun(Ser63), phosphor-Src(Tyr416), phosphor-Syk(Tyr352), and β-actin antibodies were from Cell Signaling Technology. Inhibitors for PP1, R406, and U0126 were from Calbiochem (San Diego, CA).
Immunostaining
Mice were deeply anesthetized with sodium pentobarbital (65 mg/kg intraperitoneal injection) and perfused transcardially with 40 ml ice-cold phosphate-buffered saline (PBS) and 60 ml 4% paraformaldehyde (PFA). After perfusion, the spinal cord was exposed and the L4-L5 vertebrae were removed and postfixed in 4% PFA for 6 h; these samples were transferred to 30% sucrose in phosphate buffer for approximately two days. The spinal cord was removed from the sucrose, blocked in optimum cutting temperature compound, and incubated at −20°C for approximately 20 min. The tissues were sectioned at a 16-µm thickness on a freezing microtome. All samples were incubated in blocking solution containing 1% bovine serum albumin, 5% donkey serum (Abcam), and 0.3% Triton X-100 at room temperature for 2 h. The sections were then incubated with the following primary antibodies at room temperature overnight: phosphor-specific antibodies against p65(Ser536), pERK(Thr202/Tyr204), and goat anti-ionized calcium-binding adapter Molecule 1 (IBA-1) (1:200, Abcam). On the second day, the sections were washed four times with PBS, incubated with specific secondary antibodies for 2 h, and washed again as described above. Finally, the sections were rinsed and mounted on a gelatin-coated slide. The images of the sections were captured using a fluorescence microscope connected to a charge-coupled device spot camera.
Enzyme-linked immunosorbent assay
The production of TNF-α, IL-6, and IL-10 was measured using supernatant from 2 × 105 microglia cells upon lipopolysaccharide (LPS) (100 ng/ml) with a commercial enzyme-linked immunosorbent assay (ELISA) kit from R&D Systems (Minneapolis, MN), as described previously.26
Statistical analysis
Data are represented as mean ± SEM. The statistical significance of differences was analyzed using the PASW statistical program (SPSS Inc., Chicago, IL). Statistical analyses were performed using a Student’s t-test and one-way analysis of variance (ANOVA). For behavior responses, a two-way ANOVA with repeated measure ANOVAs was performed followed by the Holm-Sidak posthoc test for multiple comparisons. A value of P < 0.05 was considered statistically significant. As the predefined criteria, the outliers will be discarded in our study.
Results
Increased inflammatory cytokines and decreased anti-inflammatory cytokines in CD11b-deficient microglia cells
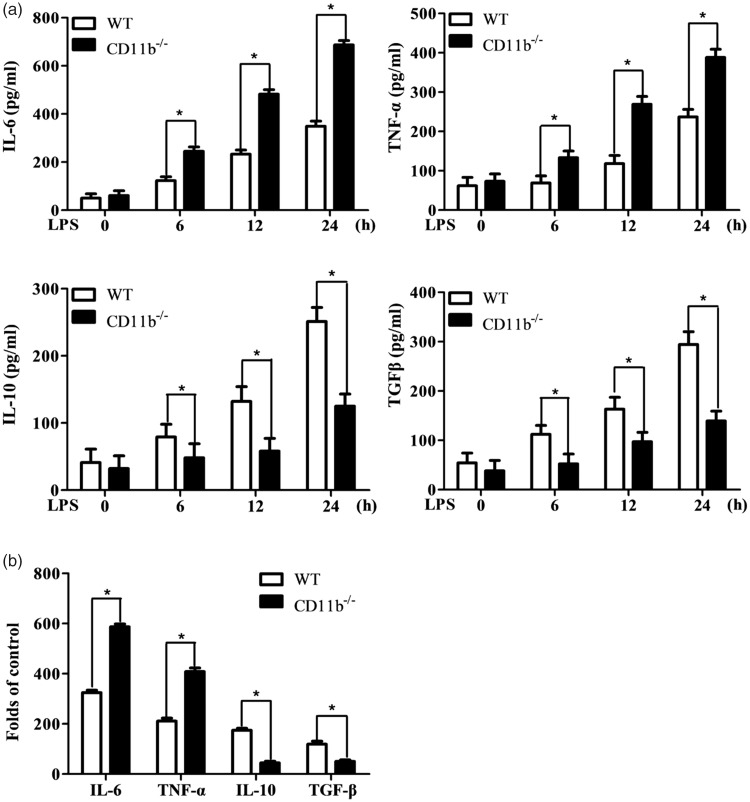
As the function of integrin in microglia remains elusive, we assayed TLR4-induced inflammatory and anti-inflammatory cytokines in CD11b-deficient microglia cells. The LPS stimulation-induced IL-6 and TNF-α production was significantly increased, while the production of IL-10 and TGF-β was decreased in CD11b-deficient microglia cells (Figure 1(a) and sFigure 1(A)). Accordingly, the mRNA expression of IL-6 and TNF-α was increased, while mRNA of IL-10 and TGF-β was decreased in CD11b-deficient microglia cells (Figure 1(b)). In accordance with previous report that CD11b promoted LPS-induced ROS production,22 CD11b-deficient microglia cells also produced less ROS production than control microglia cells (data not shown). Thus, CD11b inhibits TLR-induced inflammatory responses by the inhibition of inflammatory cytokines (IL-6 and TNF-α) and increasing anti-inflammatory cytokines (IL-10 and TGF-β) production in microglia cells.
Figure 1.
CD11b orchestrates inflammatory and anti-inflammatory cytokines in microglia cells. (a) ELISA of IL-6, TNF-α, IL-10, and TGF-β production in supernatant from microglia cells primed with LPS for 0, 6, 12, and 24 h. (b) Q-PCR assay of mRNA expression of IL-6, TNF-α, IL-10, and TGF-β in microglia cells primed with LPS for 6 h. Data are representative of three different experiments. Data are presented as mean ± SEM. *P < 0.01. WT: wild type; LPS: lipopolysaccharide; IL: interleukin; TGF-β: transforming growth factor beta; TNF-α: tumor necrosis factor alpha.
Increased TLR-signaling in CD11b-deficient microglia cells
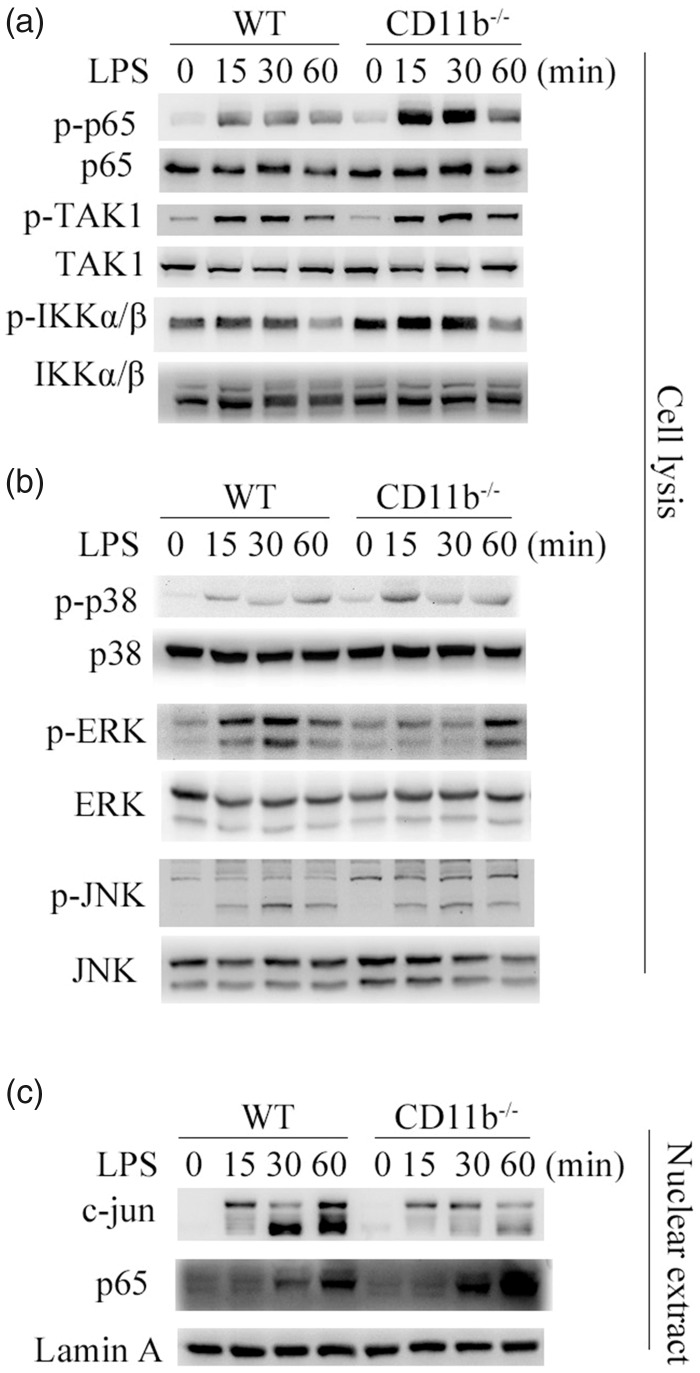
To further investigate how CD11b orchestrates TLR-induced inflammation, TLR-induced signal transduction was assayed. TLR4-induced nuclear factor-κB (NF-κB) activation was enhanced in CD11b-deficient microglia cells, which was proved by increased phosphorylation level of TAK1, IKKα/β. and p65 (Figure 2(a)). Furthermore, the activation of MAPK was reduced in CD11b-deficient microglia cells, as evidenced by potently decreased phosphorylation level of extracellular signal-regulated kinase 1 (ERK) (Figure 2(b)). However, the phosphorylation of JNK and p38 was not affected by CD11b deficiency (Figure 2(b)). To further confirm the activation of NF-κB and MAPK, we assayed the nuclear translocation of p65 and c-jun (Figure 2(c)). Accordingly, the nuclear translocation of p65 was also increased, while the nuclear translocation of c-jun was decreased upon on TLR4 activation in CD11b-deficient microglia cells. Thus, CD11b deficiency increased TLR4-induced NF-κB activation, but decreased ERK activation.
Figure 2.
CD11b orchestrates TLR signaling. (a and b) Immunoblot analysis of NF-κB and MAPK activation in microglia cells primed with LPS for 30 min by using antibodies as indicated. (c) Immunoblot analysis of the p65 and MAPK transcript factor c-jun in microglia cells stimulated with LPS for 30 min. Data are representative of three different experiments. WT: wild type; LPS: lipopolysaccharide.
Increased stability of myeloid differentiation factor 88 and decreased Src-Syk activation in CD11b-deficient microglia cells
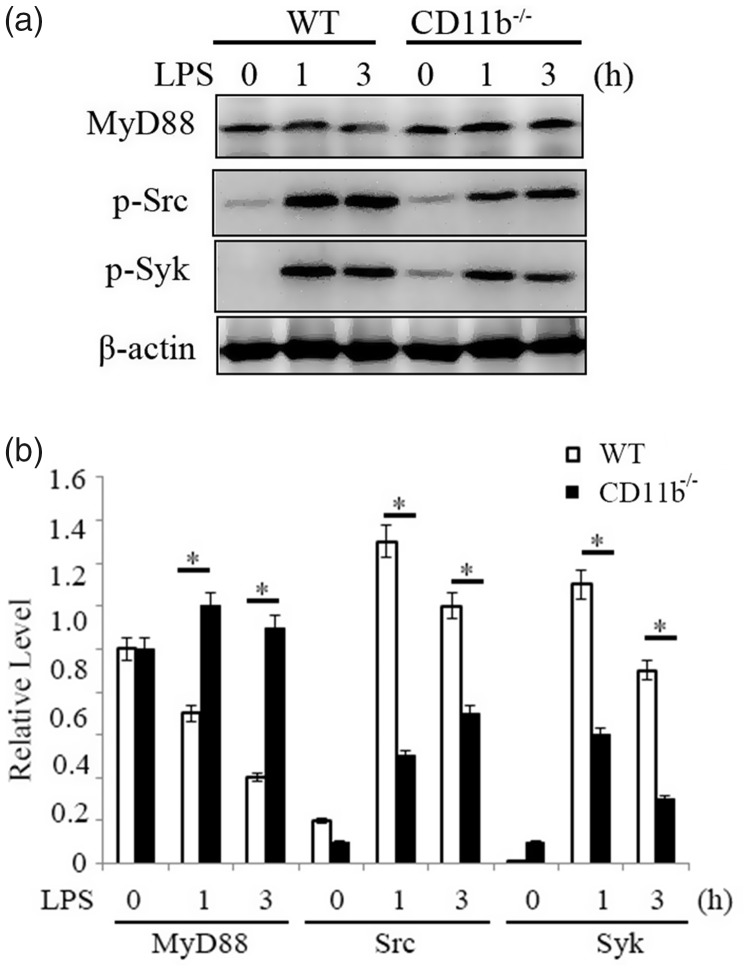
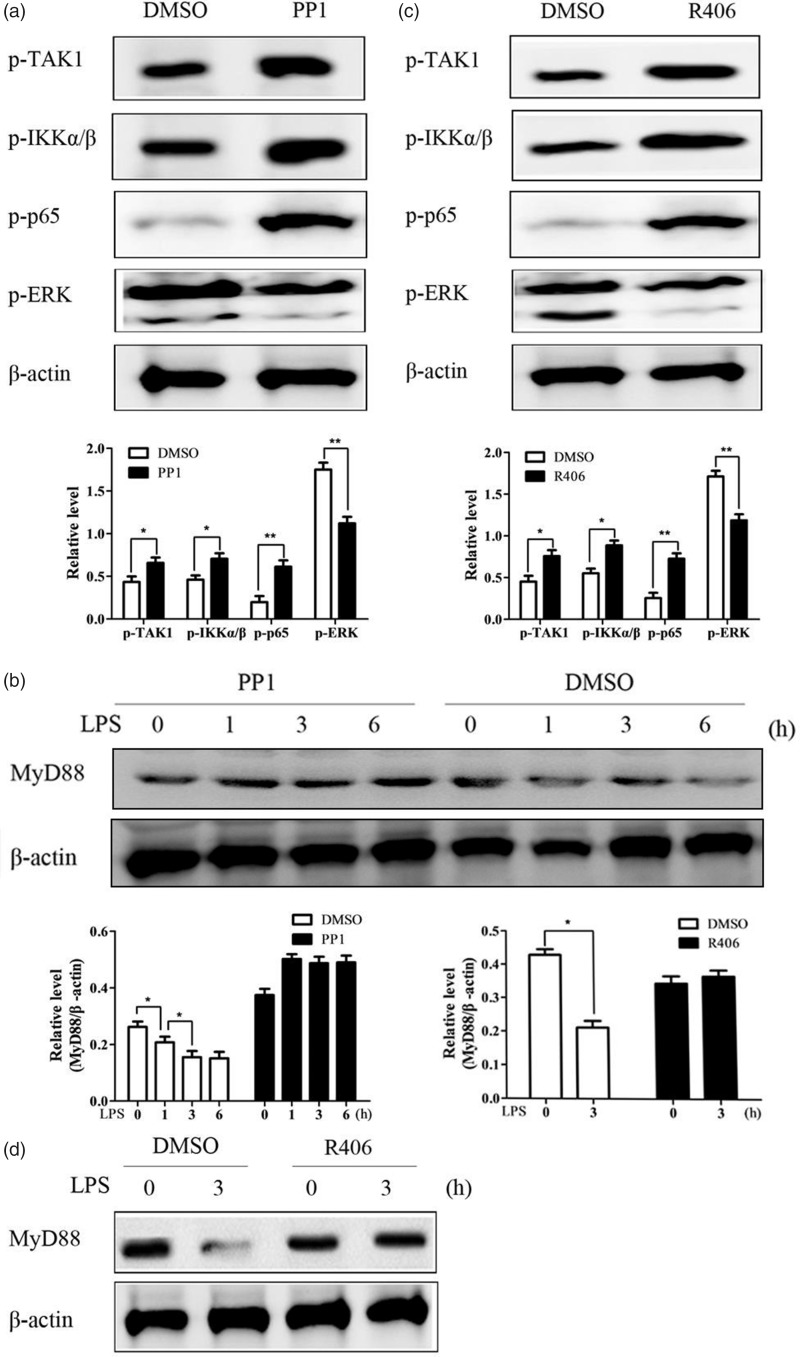
Myeloid differentiation factor 88 (MyD88) is a key adaptor protein of TLR signaling.9 Previous studies reported that CD11b promoted Casitas B lineage lymphoma proto-oncogene b (Cbl-b)-mediated degradation of MyD88 through the activation of Src, a major downstream signal molecule of CD11b.22,28 We assayed protein expression of MyD88 in CD11b-deficient and control microglia cells (Figure 3(a)). TLR4-ligation also induced time-dependent degradation of MyD88 in control microglia cells, while the expression of MyD88 in CD11b-deficient microglia cells was more stable. The results indicated that CD11b might inhibit inflammation through promoting degradation of MyD88.
Figure 3.
CD11b promotes MyD88 degradation. (a) Immunoblot analysis of MyD88 expression and Src/Syk activation in microglia cells primed with LPS for indicated time by using antibodies as indicated. (b) Quantification of bands strength. Data are representative of three different experiments. Data are presented as mean ± SEM. *P < 0.05. WT: wild type; LPS: lipopolysaccharide.
Previous studies showed tyrosine kinases including Src and Syk were major downstream signal molecules of CD11b.22,28 We investigated whether CD11b deficiency also affects the activation of Src and Syk in microglia cells (Figure 3(a)). TLR4-ligation-induced activation of Src and Syk was decreased by CD11b deficiency in microglia cells, as evidenced by decreased phosphorylation of the Src and Syk. These results indicate that CD11b may promote MyD88 degradation and inhibit TLR-induced inflammatory responses by activating Src-Syk.
Inhibition Src/Syk increases inflammatory cytokines production and decreases anti-inflammatory cytokines
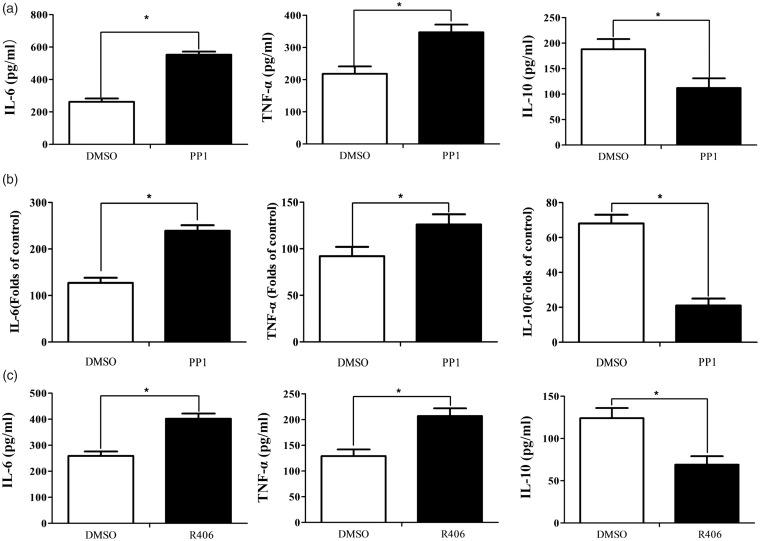
Then, we investigated whether the inhibition of Src could simulate the phenotype of increased inflammatory responses in CD11b-deficient microglia cells. Wild-type microglia cells pretreated with Src inhibitor PP1 produced more TLR4-induced IL-6 and TNF-α than control (Figure 4(a)). Interestingly, pretreatment of PP1 also decreased in anti-inflammatory cytokine IL-10 (Figure 4(a)). Accordingly, pretreatment of PP1 also increased the mRNA expression of IL-6 and TNF-α and decreased the mRNA expression of IL-10 (Figure 4(b) and sFigure 1B). Wild-type microglia cells pretreated with Syk inhibitor R406 produced more TLR4-induced inflammatory cytokines, IL-6 and TNF-α, than control (Figure 4(c)), whereas produced less anti-inflammatory cytokine IL-10 (Figure 4(c)). A combination of these results indicated that CD11b may inhibit inflammatory cytokines production and increase anti-inflammatory cytokines through Src/Syk signaling.
Figure 4.
Src/Syk orchestrates inflammatory and anti-inflammatory cytokines in microglia cells. (a) ELISA of IL-6, TNF-α, and IL-10 production in supernatant from microglia cells pretreated with Src inhibitor PP1 for 30 min and then primed with LPS for 12 h. (b) ELISA of IL-6, TNF-α, and IL-10 production in supernatant from microglia cells pretreated with Syk inhibitor R406 for 30 min and then primed with LPS for 12 h. (c) Q-PCR assay of mRNA expression of IL-6, TNF-α, and IL-10 in microglia cells pretreated with Src inhibitor PP1. Data are representative of three different experiments. Data are presented as mean ± SEM. *P < 0.01. IL: interleukin; TNF-α: tumor necrosis factor alpha; DMSO: dimethyl sulfoxide.
Src/Syk inhibits TLR signaling by increasing degradation of MyD88
To investigate how CD11b inhibits inflammatory cytokines production and increases anti-inflammatory cytokines, signal transduction in microglia cells pretreated with PP1 was assayed by immunoblot. TLR4-ligation-induced activation of NF-κB signal was increased by PP1 pretreatment, as shown by increased phosphorylation level of TAK1, IKKα/β, and p65 (Figure 5(a)). TLR4-ligation-induced activation of MAPK family indicated that PP1 pretreatment decreased the ERK activation (Figure 5(a)). MyD88, as the key adaptor protein of TLR-signaling, was found to increase the stability in CD11b-deficient microglia cells. We further investigated whether the expression level of MyD88 was also affected by PP1 pretreatment (Figure 5(b)). TLR4-ligation induced time-dependent downregulation of MyD88 expression in control pretreated microglia cells, whereas PP1 pretreatment blocked the downregulation of MyD88 expression.
Figure 5.
Src/Syk orchestrates TLR signaling. Immunoblot analysis of NF-κB and MAPK activation (a) and MyD88 expression (b) in microglia cells pretreated with Src inhibitor PP1 for 30 min and then primed with LPS for 30 min by using antibodies as indicated. Immunoblot analysis of NF-κB and MAPK activation (c) and MyD88 expression (d) in microglia cells pretreated with Syk inhibitor R406 for 30 min and then primed with LPS for 30 min by using antibodies as indicated. Data are representative of three different experiments. Data are presented as mean ± SEM. LPS: lipopolysaccharide; DMSO: dimethyl sulfoxide.
Accordingly, pretreatment of Syk inhibitor R406 also increased NF-κB signal (Figure 5(c)) and decreased ERK activation (Figure 5(c)). Accordingly, the stability of MyD88 protein was also stabilized by R406 pretreatment (Figure 5(e)).
CD11b orchestrates TLR responses through balancing NF-κB and ERK activation
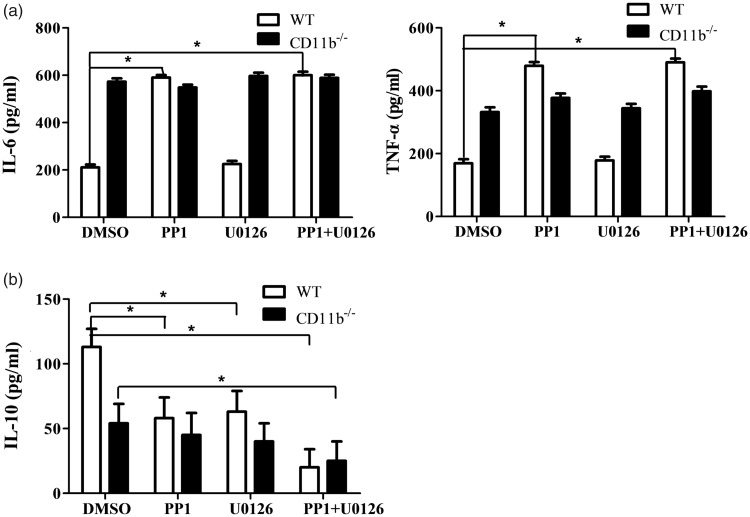
The aforementioned results indicated that CD11b inhibited TLR-ligation-induced signaling and inflammatory cytokines production through Src/Syk. Then, we wonder how CD11b-activated Src-Syk signaling could balance both inflammatory and anti-inflammatory cytokines. NF-κB and ERK activation was reported to be important for TLR-induced inflammatory and anti-inflammatory cytokines.9,10 As the above data indicated that CD11b regulated the activation of Src and ERK, CD11b-deficient or wild-type microglia cells were pretreated concurrent with Src inhibitor PP1 and ERK inhibitor U0126. PP1 pretreatment increased TLR-ligation-induced inflammatory cytokine production in wild-type microglia cells, but not in CD11b-deficient microglia cells (Figure 6(a) and sFigure 1C). Interestingly, both PP1 and U0126 pretreatment respectively decreased anti-inflammatory cytokine IL-10 production in wild-type microglia cells, but not in CD11b-deficient microglia cells (Figure 6(b)). PP1 and U0126 co-pretreatment almost abolished the IL-10 production in both wild-type and CD11b-deficient microglia cells. Furthermore, PP1 and U0126 could inhibit ERK activation vigorously in wild-type microglia cells, but not in CD11b–/– microglia cells (sFigure 2). These results indicated that CD11b might orchestrate TLR responses through balancing NF-κB and ERK activation.
Figure 6.
Src and ERK cooperate to orchestrate inflammatory and anti-inflammatory cytokines in microglia cells. ELISA of IL-6 and TNF-α (a), IL-10 (b) production in supernatant from WT or CD11b–/– microglia cells pretreated with Src inhibitor PP1 and ERK inhibitor U0126 respectively or in combination for 30 min and then primed with LPS for 12 h. Data are representative of three different experiments. Data are presented as mean ± SEM. *P < 0.01. WT: wild type; DMSO: dimethyl sulfoxide; IL: interleukin; TNF-α: tumor necrosis factor alpha.
CD11b-deficient mice were more susceptible to CCI-induced hyperalgesia and allodynia
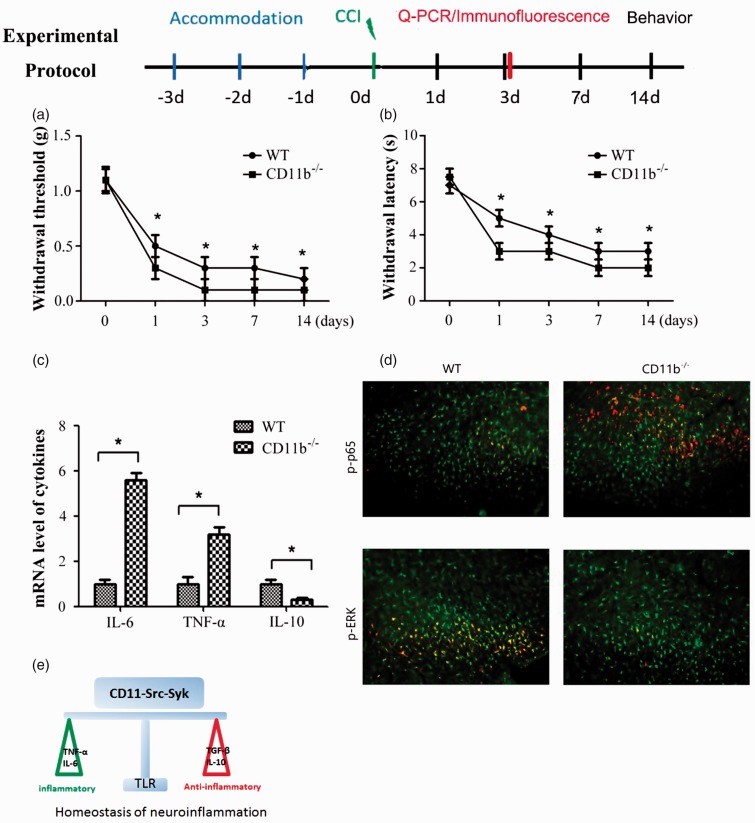
To address in vivo function and phenotype of increased inflammatory responses in CD11b-deficient microglia cells, CCI-induced animal neuropathic pain model was built in CD11b-deficient and wild-type mice. The CCI-induced mechanical allodynia was significantly increased and its effects lasted for more than 14 days in CD11b-deficient mice (Figure 7(a)). Accordingly, CD11b-deficient mice also showed more susceptible to the CCI-induced thermal hyperalgesia than wild-type mice (Figure 7(b)).
Figure 7.
CD11b suppresses CCI-induced mechanical and thermal allodynia. Mechanical (a) and thermal (b) allodynia was measured at indicated time. (c) Q-PCR analysis of IL-6, TNF-α, and IL-10 in spinal cord of WT or CD11b-deficient mice three days after CCI. (d) Immunofluorescence staining of phorspho-p65 and phorspho-p-ERK (as indicated by white arrows) in the WT or CD11b-deficient mice three days after CCI. Scale, 20 μm. (E) Schematic diagram of CD11b-activated Src-Syk signaling keeps homeostasis of neuroinflammation. Data are presented as mean ± SEM, n = 8 (n indicates the number of animals per group); *P < 0.01. WT: wild type; TLR: toll-like receptor; IL: interleukin; TNF-α: tumor necrosis factor alpha; TGF-β: transforming growth factor beta; CCI: chronic constrictive injury; Q-PCR: quantitative PCR.
To further determine whether the increased susceptibility of mechanism and thermal hyperalgesia of CD11b-deficient mice was related to increased inflammation, we measured the expression of inflammatory and anti-inflammatory cytokines. The expression of inflammatory cytokines IL-6 and TNF-α was significantly higher in the spinal cord of CD11b-deficient mice than wild-type mice (Figure 7(c)). Whereas the expression of anti-inflammatory cytokine IL-10 in the spinal cord was significantly lower in CD11b-deficient mice (Figure 7(c)). Accordingly, the activation of NF-κB was increased, while the activation of ERK was decreased in the spinal cord of CD11b-deficient mice compared with wild-type mice (Figure 7(d)). These results indicated the negative regulation of inflammation of CD11b in vivo in microglia cells.
Discussion
Emerging studies have shown that microglial–neuronal interactions play an important role in the genesis of pathological pain.11,13,29 Alteration of glial functions is a culprit causing aberrant neuronal activities in the pain signaling pathway. In present study, we found CD11b could inhibit NF-κB activation and inflammatory cytokines production through promoting MyD88 degradation, whereas CD11b promoted ERK activation and anti-inflammatory cytokines production by activating Src/Syk.
Increasing evidence suggests that neuroinflammation is an underlying cause of several central nervous system diseases, including Alzheimer’s disease, Parkinson’s disease, multiple sclerosis, and psychiatric disorders.30,31 Although acute inflammation produces transient central sensitization, chronic pain is associated with a long-lasting and sometimes permanent central sensitization that persists even after acute inflammation has been resolved.32 For example, TLR4 expressed in the spinal cord has been shown to mediate the transition from acute to persistent mechanical hypersensitivity after the resolution of inflammation in a rodent model of arthritis, where the DAMPs play critical function.33,34 During neuron injury, DAMPs released by injured cells, such as HMGB-1, mammalian dsDNA, and uric acid crystals could activate inflammatory responses via TLR4, nucleotide-binding oligomerization domain, or NLPR3 signaling.4,7,30,35 Our finding that CD11-b-Src signaling could balance inflammatory and anti-inflammatory cytokines induced by TLR4 activation may be also promising for other nervous inflammation-associated diseases. Pain and inflammation can also be dissociated in other conditions, for example, periodontal disease (which occurs as a result of chronic inflammation) is not normally associated with pain.30,34
PRRs play an essential role in recognizing specific components of microorganisms and triggering innate immune responses that eliminate the invading microorganisms.9,10,36 For neuron system, the activation of PRRs is mainly aimed to repair the damaged tissue upon DAMPs. However, inappropriate activation of PRRs can lead to prolonged inflammation or even autoimmune and inflammatory diseases, for example, persistent activation of microglia cells by amyloid-β protein leads to Alzheimer’s disease.9,10,36 The polymorphism of TNF-α, tumor necrosis factor receptor, and IL-1β genes has been found to be related to Alzheimer’s disease.37,38 On the other way, anti-inflammatory cytokine IL-10 could prevent inflammatory and autoimmune pathologies and function at different stages of an immune response via signal transducer and activator of transcription3.39,40 Including pro-inflammatory cytokines, such as IL-6 and TNF-α, macrophages, and myeloid DCs also produces large amounts of IL-10 during pathogens infection upon TLRs activation, which is critical in preventing overactivation of PRRs-induced inflammatory responses. Monocytes-induced inflammation and microglial-induced microgliosis were elegantly shown to be quite important for pain after peripheral nerve injury.41,42 Thus, the proper production of inflammatory and anti-inflammatory cytokine needs the cooperation of various signal pathways. Our finding that CD11b-activated Src/Syk could balance PRRs signal transduction and balance immune homeostasis of pro-inflammatory and anti-inflammatory cytokines may provide novel insight for maintaining innate response and inflammatory diseases.
There are several reports about the roles of Src/Syk in the regulation of TLR and other PRR signaling. Syk deficiency rescues the enhanced TLRs signaling in DAP12-deficient macrophages,43 and Src family member Lyn-deficient mice suffered from MyD88-dependent autoimmune disease44; however, the underlying mechanism is unclear. In accordance with recent report that neutrophils secreted high amounts of the anti-inflammatory cytokine IL-10 in a DAP12-Syk and MyD88-dependent manner, we found that CD11b attenuated CCI-induced allodynia by promoting TLR-triggered IL-10 expression, while inhibiting inflammatory cytokines IL-6 and TNF-α via Src/Syk pathway.
Conclusions
Taking our in vivo and in vitro results together, we suggest that CD11b attenuates CCI-induced allodynia by promoting TLR-triggered IL-10 expression while inhibiting inflammatory cytokines IL-6 and TNF-α via Src/Syk pathway.
Supplemental Material
Supplemental material for CD11b-activated Src signal attenuates neuroinflammatory pain by orchestrating inflammatory and anti-inflammatory cytokines in microglia by Mei Yang, Wenyun Xu, Yiru Wang, Xin Jiang, Yingke Li, Yajuan Yang and Hongbin Yuan in Molecular Pain
Acknowledgments
The authors thank professor Chaofeng Han for his capable assistance with the animal breeding and genotyping.
Authors’ contributions
MY and WX designed of the study and participated in the data analysis. YW and YY carried out the experiments. XJ, YL and HY participated in the manuscript preparation and interpretation of the results. All authors read and approved the final manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics Approval
All animal experiments were approved by the Experimental Animal Ethics Committee of Second Military Medical University.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was partly funded by the National Natural Science Foundation of China (81371253 and 81671079); Research Foundation of Shanghai Science and Technology Commission (17XD1424300/16411967300); and TCM Supported Project (15401900800) from Science and Technology Commission of Shanghai Municipality.
Supplemental Material
Supplemental material for this article is available online.
References
- 1.Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell 2009; 139: 267–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scholz J, Woolf CJ. The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci 2007; 10: 1361–1368. [DOI] [PubMed] [Google Scholar]
- 3.Streit WJ. Microglia as neuroprotective, immunocompetent cells of the CNS. Glia 2002; 40: 133–139. [DOI] [PubMed] [Google Scholar]
- 4.Fontaine M, Lepape A, Piriou V, Venet F, Friggeri A. Innate danger signals in acute injury: from bench to bedside. Anaesth Crit Care Pain Med 2016; 35: 283–292. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y, Zhang X, Wang C, Li G, Gu Y, Huang LY. Activation of P2X7 receptors in glial satellite cells reduces pain through downregulation of P2X3 receptors in nociceptive neurons. Proc Natl Acad Sci USA 2008; 105: 16773–16778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao X, Kim HK, Chung JM, Chung K. Reactive oxygen species (ROS) are involved in enhancement of NMDA-receptor phosphorylation in animal models of pain. Pain 2007; 131: 262–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grace PM, Strand KA, Galer EL, Urban DJ, Wang X, Baratta MV, Fabisiak TJ, Anderson ND, Cheng K, Greene LI, Berkelhammer D, Zhang Y, Ellis AL, Yin HH, Campeau S, Rice KC, Roth BL, Maier SF, Watkins LR. Morphine paradoxically prolongs neuropathic pain in rats by amplifying spinal NLRP3 inflammasome activation. Proc Natl Acad Sci USA 2016; 113: E3441–E3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang X, Chen Y, Wang C, Huang LY. Neuronal somatic ATP release triggers neuron-satellite glial cell communication in dorsal root ganglia. Proc Natl Acad Sci USA 2007; 104: 9864–9869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao X. Self-regulation and cross-regulation of pattern-recognition receptor signalling in health and disease. Nat Rev Immunol 2016; 16: 35–50. [DOI] [PubMed] [Google Scholar]
- 10.O’Neill LA, Golenbock D, Bowie AG. The history of toll-like receptors – redefining innate immunity. Nat Rev Immunol 2013; 13: 453–460. [DOI] [PubMed] [Google Scholar]
- 11.Miron VE. Microglia-driven regulation of oligodendrocyte lineage cells, myelination, and remyelination. J Leukoc Biol 2017; 101: 1103–1108. [DOI] [PubMed] [Google Scholar]
- 12.Lan X, Han X, Li Q, Yang QW, Wang J. Modulators of microglial activation and polarization after intracerebral haemorrhage. Nature Rev Neurol 2017; 13: 420–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colonna M, Butovsky O. Microglia function in the central nervous system during health and neurodegeneration. Annu Rev Immunol 2017; 35: 441–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marshak-Rothstein A. Toll-like receptors in systemic autoimmune disease. Nat Rev Immunol 2006; 6: 823–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kourtzelis I, Mitroulis I, von Renesse J, Hajishengallis G, Chavakis T. From leukocyte recruitment to resolution of inflammation: the cardinal role of integrins. J Leukocyte Biol 2017; 102: 677–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paolillo M, Serra M, Schinelli S. Integrins in glioblastoma: still an attractive target? Pharmacol Res 2016; 113: 55–61. [DOI] [PubMed] [Google Scholar]
- 17.Varga G, Balkow S, Wild MK, Stadtbaeumer A, Krummen M, Rothoeft T, Higuchi T, Beissert S, Wethmar K, Scharffetter-Kochanek K, Vestweber D, Grabbe S. Active MAC-1 (CD11b/CD18) on DCs inhibits full T-cell activation. Blood 2007; 109: 661–669. [DOI] [PubMed] [Google Scholar]
- 18.Ehirchiou D, Xiong Y, Xu G, Chen W, Shi Y, Zhang L. CD11b facilitates the development of peripheral tolerance by suppressing Th17 differentiation. J Exp Med 2007; 204: 1519–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang M, Tang H, Guo Z, An H, Zhu X, Song W, Guo J, Huang X, Chen T, Wang J, Cao X. Splenic stroma drives mature dendritic cells to differentiate into regulatory dendritic cells. Nat Immunol 2004; 5: 1124–1133. [DOI] [PubMed] [Google Scholar]
- 20.Li Q, Guo Z, Xu X, Xia S, Cao X. Pulmonary stromal cells induce the generation of regulatory DC attenuating T-cell-mediated lung inflammation. Eur J Immunol 2008; 38: 2751–2761. [DOI] [PubMed] [Google Scholar]
- 21.Tang H, Guo Z, Zhang M, Wang J, Chen G, Cao X. Endothelial stroma programs hematopoietic stem cells to differentiate into regulatory dendritic cells through IL-10. Blood 2006; 108: 1189–1197. [DOI] [PubMed] [Google Scholar]
- 22.Han C, Jin J, Xu S, Liu H, Li N, Cao X. Integrin CD11b negatively regulates TLR-triggered inflammatory responses by activating Syk and promoting degradation of MyD88 and TRIF via Cbl-b. Nat Immunol 2010; 11: 734–742. [DOI] [PubMed] [Google Scholar]
- 23.Hu X, Han C, Jin J, Qin K, Zhang H, Li T, Lin N, Cao X. Integrin CD11b attenuates colitis by strengthening Src-Akt pathway to polarize anti-inflammatory IL-10 expression. Sci Rep 2016; 6: 26252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983; 16: 109–110. [DOI] [PubMed] [Google Scholar]
- 25.Osikowicz M, Mika J, Makuch W, Przewlocka B. Glutamate receptor ligands attenuate allodynia and hyperalgesia and potentiate morphine effects in a mouse model of neuropathic pain. Pain 2008; 139: 117–126. [DOI] [PubMed] [Google Scholar]
- 26.Shimoyama M, Tanaka K, Hasue F, Shimoyama N. A mouse model of neuropathic cancer pain. Pain 2002; 99: 167–174. [DOI] [PubMed] [Google Scholar]
- 27.Sommer C, Schäfers M. M. Painful mononeuropathy in C57BL/Wld mice with delayed wallerian degeneration: differential effects of cytokine production and nerve regeneration on thermal and mechanical hypersensitivity. Brain Res 1998; 784: 154–162. [DOI] [PubMed] [Google Scholar]
- 28.Hirahashi J, Mekala D, Van Ziffle J, Xiao L, Saffaripour S, Wagner DD, Shapiro SD, Lowell C, Mayadas TN. Mac-1 signaling via Src-family and Syk kinases results in elastase-dependent thrombohemorrhagic vasculopathy. Immunity 2006; 25: 271–283. [DOI] [PubMed] [Google Scholar]
- 29.Costa FAM, Neto FL. Satellite glial cells in sensory ganglia: its role in pain. Rev Bras Anestesiol 2015; 65: 73–81. [DOI] [PubMed] [Google Scholar]
- 30.Guo H, Callaway JB, Ting JP. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med 2015; 21: 677–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci 2009; 32: 1–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chapman CR, Vierck CJ. The transition of acute postoperative pain to chronic pain: an integrative overview of research on mechanisms. J Pain 2017; 18: e359.e1–e359.e38. [DOI] [PubMed] [Google Scholar]
- 33.Kim D, Kim MA, Cho IH, Kim MS, Lee S, Jo EK, Choi SY, Park K, Kim JS, Akira S, Na HS, Oh SB, Lee SJ. A critical role of toll-like receptor 2 in nerve injury-induced spinal cord glial cell activation and pain hypersensitivity. J Biol Chem 2007; 282: 14975–14983. [DOI] [PubMed] [Google Scholar]
- 34.Tanga FY, Nutile-McMenemy N, DeLeo JA. The CNS role of toll-like receptor 4 in innate neuroimmunity and painful neuropathy. Proc Natl Acad Sci USA 2005; 102: 5856–5861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang CA, Chiang BL. Inflammasomes and human autoimmunity: a comprehensive review. J Autoimmun 2015; 61: 1–8. [DOI] [PubMed] [Google Scholar]
- 36.Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature 2007; 449: 819–826. [DOI] [PubMed] [Google Scholar]
- 37.Marei HE, Althani A, Suhonen J, El Zowalaty ME, Albanna MA, Cenciarelli C, Wang T, Caceci T. Common and rare genetic variants associated with Alzheimer’s disease. J Cell Physiol 2016; 231: 1432–1437. [DOI] [PubMed] [Google Scholar]
- 38.VanItallie TB. Alzheimer’s disease: innate immunity gone awry? Metab Clin Exp 2017; 69S: S41–S49. [DOI] [PubMed] [Google Scholar]
- 39.Engelhardt KR, Grimbacher B. IL-10 in humans: lessons from the gut, IL-10/IL-10 receptor deficiencies, and IL-10 polymorphisms. Curr Top Microbiol Immunol 2014; 380: 1–18. [DOI] [PubMed] [Google Scholar]
- 40.Shim JO, Hwang S, Yang HR, Moon JS, Chang JY, Ko JS, Park SS, Kang GH, Kim WS, Seo JK. Interleukin-10 receptor mutations in children with neonatal-onset Crohn’s disease and intractable ulcerating enterocolitis. Eur J Gastroenterol Hepatol 2013; 25: 1235–1240. [DOI] [PubMed] [Google Scholar]
- 41.Gu N, Peng J, Murugan M, Wang X, Eyo UB, Sun D, Ren Y, DiCicco-Bloom E, Young W, Dong H, Wu LJ. Spinal microgliosis due to resident microglial proliferation is required for pain hypersensitivity after peripheral nerve injury. Cell Rep 2016; 16: 605–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peng J, Gu N, Zhou L, B Eyo U, Murugan M, Gan WB, Wu LJ. Microglia and monocytes synergistically promote the transition from acute to chronic pain after nerve injury. Nat Commun. 2016; 7: 12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hamerman JA, Tchao NK, Lowell CA, Lanier LL. Enhanced Tolltoll-like receptor responses in the absence of signaling adaptor DAP12. Nat Immunol Nature immunology. 2005; 6: 579–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Silver KL, Crockford TL, Bouriez-Jones T, Milling S, Lambe T, Cornall RJ. MyD88-dependent autoimmune disease in Lyn-deficient mice. Eur J Immunol European journal of immunology. 2007; 37: 2734–2743. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material for CD11b-activated Src signal attenuates neuroinflammatory pain by orchestrating inflammatory and anti-inflammatory cytokines in microglia by Mei Yang, Wenyun Xu, Yiru Wang, Xin Jiang, Yingke Li, Yajuan Yang and Hongbin Yuan in Molecular Pain