Short abstract
Glioblastoma multiforme (GBM) is the most common and malignant of the primary adult brain cancers. Ultrastructural and biochemical evidence shows that GBM cells exhibit mitochondrial abnormalities incompatible with energy production through oxidative phosphorylation (OxPhos). Under such conditions, the mitochondrial F0-F1 ATP synthase operates in reverse at the expense of ATP hydrolysis to maintain a moderate membrane potential. Moreover, expression of the dimeric M2 isoform of pyruvate kinase in GBM results in diminished ATP output, precluding a significant ATP production from glycolysis. If ATP synthesis through both glycolysis and OxPhos was impeded, then where would GBM cells obtain high-energy phosphates for growth and invasion? Literature is reviewed suggesting that the succinate-CoA ligase reaction in the tricarboxylic acid cycle can substantiate sufficient ATP through mitochondrial substrate-level phosphorylation (mSLP) to maintain GBM growth when OxPhos is impaired. Production of high-energy phosphates would be supported by glutaminolysis—a hallmark of GBM metabolism—through the sequential conversion of glutamine → glutamate → alpha-ketoglutarate → succinyl CoA → succinate. Equally important, provision of ATP through mSLP would maintain the adenine nucleotide translocase in forward mode, thus preventing the reverse-operating F0-F1 ATP synthase from depleting cytosolic ATP reserves. Because glucose and glutamine are the primary fuels driving the rapid growth of GBM and most tumors for that matter, simultaneous restriction of these two substrates or inhibition of mSLP should diminish cancer viability, growth, and invasion.
Keywords: therapies, gliomas, bioenergetics, Warburg
Introduction
Glioblastoma multiforme (GBM) carries the highest mortality rate among primary brain tumors and remains largely unmanageable. Life expectancy following diagnosis is only about 12 to 14 months. This has changed little for decades despite continuing research (Fisher and Buffler, 2005; Krex et al., 2007; Stupp et al., 2009; Lawrence et al., 2011; Ahmadloo et al., 2013; Alexander and Cloughesy, 2017). Indeed, a recent reevaluation found that overall survival for GBM is woefully similar to that reported by Cushing almost a century ago (Fatehi et al., 2018). A defining characteristic of GBM is the secondary structures of Scherer, which include diffuse parenchymal growth invasion over the subpial surface, along white matter tracks, and through the Virchow–Robin spaces (Scherer, 1940; Laws et al., 1993; Kleihues and Ohgaki, 1999; Zagzag et al., 2008; Shelton et al., 2010c). The highly invasive nature of GBM makes most current therapies ineffective (Chang et al., 2005; Krex et al., 2007; Cuddapah et al., 2014; Perry et al., 2017). Although the introduction of the toxic alkylating agent, temozolomide, has improved progression-free survival slightly, quality of life has also remained poor for most GBM patients especially for those receiving radiation alone (Taphoorn et al., 2005; Stupp et al., 2009; Flechl et al., 2012).
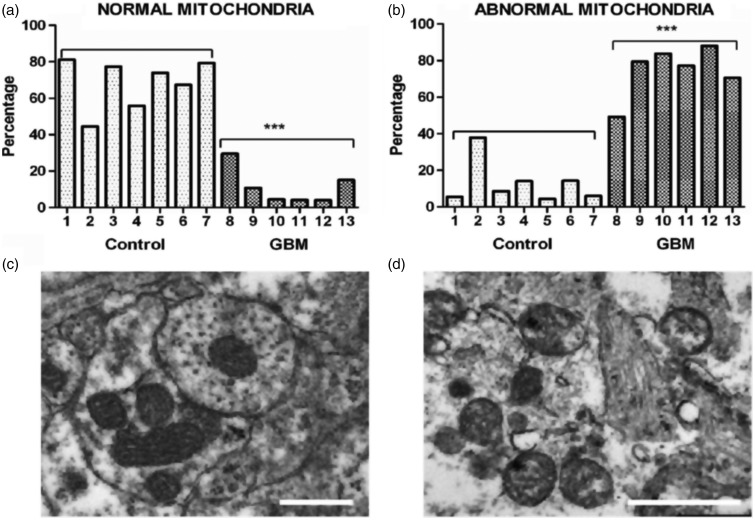
GBM contains a plethora of morphologically diverse neoplastic cell types that express neural, glial, and myeloid/mesenchymal markers (Rubinstein, 1972; Morantz et al., 1979; Wood and Morantz, 1979; Roggendorf et al., 1996; Seyfried, 2001; Yuan et al., 2004; Huysentruyt et al., 2011; Karsy et al., 2012). Also recognized are abnormalities in the number, structure, and function of mitochondria in GBM cells (Figure 1). Several groups have documented GBM cells with reduced or increased numbers of morphologically abnormal mitochondria with laminations and aberrant or absent cristae (Sipe et al., 1973; Scheithauer and Bruner, 1987; Oudard et al., 1997; Arismendi-Morillo and Castellano-Ramirez, 2008; Katsetos et al., 2013; Deighton et al., 2014; Feichtinger et al., 2014; Seyfried et al., 2017). Some mitochondria in human GBM cells contain few, if any, cristae (Arismendi-Morillo and Castellano-Ramirez, 2008). In murine GBM, the content and composition of cardiolipin, the signature lipid of the mitochondrial inner membrane that regulates oxidative phosphorylation (OxPhos), exhibit considerable deviations from the normal (Kiebish et al., 2008; Claypool and Koehler, 2012). A multitude of findings support the notion that OxPhos is defective in GBM (Lichtor and Dohrmann, 1986; Feichtinger et al., 2014; Bartesaghi et al., 2015; Dahlberg et al., 2017). Based on the concept that mitochondrial structure determines mitochondrial function (Lehninger, 1964), these multiple mitochondrial abnormalities, which are of genetic or environmental origin, would be expected to compromise effective energy production through OxPhos (Seyfried and Shelton, 2010; Seyfried et al., 2014, 2015).
Figure 1.
Morphological abnormalities in GBM mitochondria; (a) to (d) (distributed under a Creative Commons license) were reproduced from Deighton et al. (2014). The morphology of 150 mitochondria was assessed in six GBM samples and in seven peritumoural control samples using electron microscopy. (a) Percentage of normal mitochondria where cristae were visible throughout the mitochondria in peritumoural control and GBM samples (each bar represents one sample; ***p value = .0001); (b) Percentage of abnormal mitochondria where cristae were sparse and abnormal in peritumoural control and GBM samples (***p value = .0001). (c and d) Representative electron microscopy images of normal and abnormal mitochondria, respectively. The scale bars represent 0.5 µm. Cristolysis was significantly greater in mitochondria from GBM than from control brain. The possibility cannot be excluded that the abnormal GBM mitochondria shown in panel (d) originate from areas close to necrotic palisades and might not be representative of mitochondria in all types GBM cells.
GBM = glioblastoma multiforme.
In view of the documented abnormalities in GBM mitochondria, alternative energy source(s) to OxPhos must be in place to maintain cell viability. Accumulating evidence indicates that glucose and glutamine are the primary fuels used for driving the rapid growth of most tumors, including GBM (DeBerardinis et al., 2007; Yang et al., 2009). Abundant levels of glucose and glutamine are also available in cyst fluid that is often present in GBM and could be used as fuel for the growing tumor cells (Dahlberg et al., 2017). Other potential metabolic fuels in the tumor microenvironment, for example, acetate and branched-chain amino acids, are either not present in sufficient quantities to drive growth through fermentation or exert nonmetabolic effects that are yet to be understood (Jaworski et al., 2016; Seyfried et al., 2017). While amino acids other than glutamine could also be fuels, they would require energy for preparation prior to their catabolism (see later). Glucose drives tumor growth through aerobic fermentation (Warburg effect), whereas glutamine drives tumor growth through glutaminolysis (Rhodes et al., 1983; DeBerardinis and Cheng, 2010; Flavahan et al., 2013; Yang et al., 2017). Metabolism of glucose and glutamine is also responsible for the high antioxidant capacity of the tumor cells, thus making them resistant to chemo- and radiotherapies (Amores-Sanchez and Medina, 1999; Xu et al., 2005; Seyfried et al., 2017). It remains unclear, however, whether glutamine is used primarily as a substrate for citric acid cycle anaplerosis and lipid synthesis or if it is also used as a major substrate for energy production through mitochondrial substrate-level phosphorylation (mSLP). This review describes a framework for how provision of high-energy phosphates through mSLP could compensate for the loss of energy production through both glycolysis and OxPhos in GBM. The information reviewed is derived from a broad range of experimental materials including human glioma tissue, validated preclinical models of GBM, and cultured glioma cell lines. Advantages and disadvantages associated with some of the models systems were presented previously (Seyfried, 2012c; Seyfried et al., 2014).
Role of Glucose and Glycolysis in GBM Energy Metabolism
Warburg originally proposed that cancer originated from an irreversible damage to cellular respiration that was followed by a gradual increase in fermentation, that is, glucose metabolized through glycolysis, leading to excessive formation of lactate (Warburg, 1956a). The increased fermentation, which was present even in saturating partial pressures of oxygen, was necessary to compensate for a loss of energy through OxPhos. Aerobic fermentation is also a metabolic hallmark of GBM (Kirsch et al., 1972; Rhodes et al., 1983; Lichtor and Dohrmann, 1986; Oudard et al., 1997; Roslin et al., 2003). Despite the aforementioned evidence showing that mitochondria are abnormal in GBM tumor tissue, some investigators have claimed that mitochondria and OxPhos are normal in GBM and other brain tumor types based mostly, but not exclusively, on in vitro studies of oxygen consumption rates in tumor cells (Elstrom et al., 2004; Wise et al., 2008; Pike et al., 2011; Zhou et al., 2011; Janiszewska et al., 2012; Marin-Valencia et al., 2012; Ward and Thompson, 2012; Yang et al., 2014; Maximchik et al., 2016). It is not clear how these findings can be reconciled in light of the abnormalities reported in the number, structure, and function of GBM mitochondria obtained from GBM tissue sections (referenced earlier). Discrepancies between in vivo and in vitro studies related to mitochondrial structure and function were not discussed in most of these studies. Warburg also proposed that oxygen consumption could be similar in tumor cells and normal cells but that ATP output from respiration would be less in the former than in the latter.
Despite the widely held view that oxygen consumption in cultured cells is a biomarker for OxPhos activity, many studies have since established that oxygen consumption is not linked to OxPhos ATP output in various tumor cell lines (Arcos et al., 1969; Ramanathan et al., 2005; Hall et al., 2013; Leznev et al., 2013; Pacini and Borziani, 2016). It is not possible, however, to exclude a complete absence of OxPhos in tumor cells, as Hall et al. (2013) showed that coupling was reduced by about 50% even in an extreme case of V600EBRAF-induced OxPhos dysfunction in melanoma cells. It is important to emphasize, however, that a ∼50% reduction in OxPhos would dissipate the protonmotive force and cause reversal of the F0-F1 ATP synthase (Chinopoulos, 2011a, 2011b). The F0-F1 ATP synthase generally operates in forward mode (i.e., generating ATP) only when mitochondria are sufficiently polarized. It would not be possible for the F0-F1 ATP synthase to generate ATP under a loss of electron transport chain (ETC) operation on the order of 45% to 50% and would actually hydrolyze ATP, pumping protons out of the matrix. Reversal of the ATP synthase is what affords mSLP the critical role of providing ATP directly within the matrix when OxPhos becomes inhibited or impaired. These considerations are further addressed in the section below on, Role of Adenine Nucleotide Translocase and F0-F1 ATP synthase in GBM. Furthermore, the possibility of an inverse relationship between OxPhos efficiency and tumor aggression has been reported (Solaini et al., 2011). Warburg and Burk also described a similar phenomenon with respect to the degree of fermentation and tumor growth, that is, the more aggressive is the cancer, the greater is the fermentation (Warburg, 1956a; Burk et al., 1967). It is also documented that estimates of mitochondrial OxPhos efficiency can differ between in vivo and in vitro environments (Suarez et al., 1991; Kiebish et al., 2009). Viewed collectively, these studies indicate that oxygen consumption alone cannot be used as a measure of normal respiration in cultured cells including GBM cells.
Another misconception comes from findings that aerobic glycolysis is required not only for GBM cell proliferation but also for proliferation of nontransformed cells grown in vitro, thus giving the impression that aerobic glycolysis is a normal phenomenon of cell proliferation (Vander Heiden et al., 2009). This phenomenon does not, however, occur in proliferating nontransformed cells grown in vivo, for example, regenerating liver cells and normal colon cells, which use fatty acids and butyrate as fuel, respectively (Warburg, 1956a; Simek and Sedlacek, 1965; Hague et al., 1997; Thevananther, 2010). We also showed that the in vitro growth environment suppresses Complex I activity and forces normal astrocytes to ferment through a Crabtree-linked effect (Kiebish et al., 2009). This in vitro effect would obscure differences in energy metabolism between normal brain cells and GBM cells. Indeed, Warburg and Burk cautioned against generalizations made on cellular energy metabolism based solely on in vitro studies (Burk and Schade, 1956; Warburg, 1956a, 1956b). It is glucose and not lactate that primarily drives brain energy metabolism (Allen et al., 2005; Dienel, 2012; Nortley and Attwell, 2017), making it unlikely that lactate could serve as a major energy metabolite for neoplastic GBM cells with diminished OxPhos capacity. A continued production of lactate in the presence of saturating partial pressures of oxygen is indicative of an impaired Pasteur effect, which itself could be linked to respiratory impairment (Racker, 1974). Proliferation is the default state of metazoan cells and, together with fermentation, was the dominant phenotype of all cells in the alpha period before oxygen entered the atmosphere (Szent-Gyorgyi, 1977; Sonnenschein and Soto, 2000). Respiratory impairment or inhibition, arising from either genetic or environmental causes, could thus force cells into a fermentation metabolism with consequent proliferation (Seyfried and Shelton, 2010).
While it is well recognized that biochemical pathways are reprogramed in GBM and in other cancers (Vander Heiden et al., 2009; Ward and Thompson, 2012), it remains unclear how GBM cells would generate sufficient energy with the documented abnormalities in their mitochondria. A reanalysis of how tumor cells obtain energy and metabolites for growth can help elucidate the origin of energy synthesis in GBM. The rate of glycolysis is increased in GBM, which is necessary for supporting growth under hypoxic conditions that commonly occur in the interior of the tumor mass (Roslin et al., 2003), shuttling glycolytic intermediates for building blocks of new cells. The upregulation of this pathway is due in part to hypoxia-induced stabilization of hypoxia-inducing factor 1α (HIF-1α), which amplifies the transcription of genes encoding glucose transporters and glycolytic enzymes (Semenza, 2010). Oncogenic signaling pathways, including phosphoinositide 3-kinase, can activate HIF-1α under normoxic conditions (Plas and Thompson, 2005). Mutations in tumor suppressor proteins such as the von Hippel–Lindau tumor suppressor gene product (Kaelin, 2008), succinate dehydrogenase (SDH; Selak et al., 2005), and fumarate hydratase (FH) can also activate HIF-1α (King et al., 2006). Due to the cellular heterogeneity of GBM, it is likely that individual cells harbor one or more of these mutations (Soeda et al., 2015). It should also be recognized that the upregulation of oncogenes in GBM and most other cancers is necessary to facilitate substrate-level phosphorylation when OxPhos becomes unable to maintain the differentiated state (Seyfried et al., 2014). Relevant to this, dysregulated tumor cell growth and oncogene expression are often abolished when the cancer nucleus is placed in a cytoplasm containing normal mitochondria or when normal mitochondria replace tumor mitochondria in the cytoplasm (Seyfried, 2012b, 2012e; Kaipparettu et al., 2013; Seyfried, 2015). These findings indicate that normal mitochondrial functions can downregulate oncogenic signaling pathways restoring normal metabolic programing regardless of the genetic abnormalities present in the tumor cells. Indeed, recent findings of cancer driver mutations in normal cells seriously challenge the role of somatic mutations in the origin of cancer (Martincorena et al., 2018) including in the brain (Nishioka et al., 2018 and references therein).
The enhanced use of glucose is thought to provide essential intermediates for tumor cell growth and proliferation by diverting metabolites into the pentose phosphate pathway (Icard and Lincet, 2012), as well as providing intermediates for lipid synthesis, the hexosamine pathway, and the synthesis of uridine diphosphate glucose (Vander Heiden et al., 2009). More recently, it was shown that breast cancer cells could maintain rapid growth and proliferation by diverting 3-phosphoglycerate from glycolysis to generate serine and glycine through phosphoglycerate dehydrogenase (PHGDH; Possemato et al., 2011). Interestingly, suppression of PHGDH expression decreased cell proliferation but did not affect intracellular serine levels. On the other hand, reduced PHGDH expression was associated with reduced levels of α-ketoglutarate (Possemato et al., 2011), the main precursor for mSLP (see later). This mechanism also appears to operate in GBM (Liu et al., 2013). It should also be recognized that blood glucose levels are directly correlated with GBM growth, that is, the higher the blood glucose, the faster the growth and less the survival, while the lower the blood glucose, the slower the growth and the greater the survival (Seyfried et al., 2003; McGirt et al., 2008; Derr et al., 2009; Mayer et al., 2014; Tieu et al., 2015).
In tumor cells, glycolytic intermediates escape leak-down toward pyruvate formation and subsequent entry into the mitochondria, being shunted toward the aforementioned subsidiary pathways by the preferential expression of the M2 isoform of pyruvate kinase (PKM2). Pyruvate kinase (PK) catalyzes the rate-limiting, ATP-generating payoff step of glycolysis in which phosphoenolpyruvate (PEP) is converted to pyruvate (Mazurek et al., 2005). Multiple isoenzymes of PK exist in mammals. The type L is found in liver and kidneys, the type R in erythrocytes, type M1 in muscle and brain, and type M2 in embryonic and adult stem cells (Mazurek et al., 2005). GBM cells appear to switch from the PKM1 to PKM2 isoform (Desai et al., 2014). Unlike PKM1, which promotes glycolysis and rapid energy generation, PKM2 may attain a dimeric form that exhibits low affinity to its substrate PEP rendering it inactive at physiological PEP concentrations (Christofk et al., 2008). The high GBM cellular heterogeneity makes it difficult to deduce with certainty, which cells exhibit the dimeric versus tetrameric PKM2 form (Marin-Valencia et al., 2012; Desai et al., 2014; Soeda et al., 2015). Preferential expression of PKM2 over PKM1 is mediated by modulating exon splicing, promoted by the oncoprotein Myc (David et al., 2010). HIF-1α also induces transcription of PKM2, but not PKM1, and PKM2 serves as a cotranscriptional activator of HIF-1α, mediated by the proline hydroxylase PHD3 (Luo et al., 2011). A major paradox regarding PKM2 is that cells expressing it produce more glucose-derived pyruvate than PKM1-expressing cells, despite the diminished activity of the M2 isoenzyme compared with M1. One way to achieve this is through a proposed alternative glycolytic pathway involving an enzymatic activity not yet purified that dephosphorylates PEP to pyruvate, without generating ATP (Vander Heiden et al., 2010). Alternatively, the decreased enzymatic activity of PKM2 promoting accumulation of 3-phosphoglycerate may serve as an entry point for serine synthesis. In this pathway, serine dehydratase can directly convert serine to pyruvate (Ward and Thompson, 2012). Most recently though, PKM2 was reported to localize to the nucleus activating transcription of MEK5, a protein kinase that belongs to the MAP kinase kinase family involved in growth factor-stimulated cell proliferation, using PEP as a phosphate donor (Gao et al., 2012). The latter study highlighted an important link between altered metabolism and gene expression in carcinogenesis and created a precedent for considering roles of metabolic intermediates other than those participating in classical biochemical pathways.
The fate of pyruvate in GBM is mostly conversion to lactate, by lactate dehydrogenase (LDH), rather than entry into mitochondria (see later). LDH is a tetramer composed of two different subunits, LDHA and LDHB, which can assemble into five different combinations (Markert et al., 1975). Augmented expression of Myc-mediated LDHA is a hallmark of many tumors, the majority of which are highly glycolytic and are associated with a poor prognosis (Dang et al., 2008; Koukourakis et al., 2009). LDHA is the predominant isoform in GBM and most other malignant cancers (Moreno-Sanchez et al., 2009; Li et al., 2016). The association of LDHB with cancer is more complex (Doherty and Cleveland, 2013) and is not likely to play a major role in GBM growth. LDHA catabolizes pyruvate to lactate producing NAD+, which is necessary to drive glycolysis by GAPDH (Dawson et al., 1964; Li et al., 2016). The reaction catalyzed by the cytosolic malate dehydrogenase (MDH1) was also recently shown to contribute to NAD+ provision (Gaude et al., 2018). Furthermore, lactate acidifies the tumor microenvironment (Fantin et al., 2006; Gillies et al., 2008), which enhances the activity of several proinvasive factors (Gimenez-Roqueplo et al., 2008; Parks et al., 2013). Recent studies show, however, that it is carbonic anhydrase, and not LDH, that is mostly responsible for tumor-induced acidification (Swietach et al., 2014). Nonetheless, lactate efflux provokes a local inflammatory response attracting macrophages that secrete cytokines and growth factors promoting tumor cell growth and metastasis (Seyfried, 2001; Estrella et al., 2013). The importance of LDHA activity in acidifying the microenvironment and creating a local inflammatory response, together with the provision of NAD+ for GAPDH regarding tumor survival, is reflected by the fact that LDHA inhibitors are becoming promising antitumor agents (Billiard et al., 2013; Doherty and Cleveland, 2013; Li et al., 2016). Relevant to this, downregulation of LDHA in gliomas with mutations in isocitrate dehydrogenase (IDH) appeared responsible for slow growth and better prognosis (Chesnelong et al., 2014). This was probably mediated by increased methylation of the LDHA promoter because silencing of LDHA in brain tumor stem cells exhibiting mutations in IDH was associated with slower growth (Chesnelong et al., 2014). Fantin et al. (2006) and Li et al. (2016) also found that silencing LDHA could reduce tumor progression. Furthermore, Zhang et al. (2016) showed that additional epigenetic reprogramming, including immune-related genes, is likely to be involved in the pathogenesis of gliomas exhibiting mutations in IDH. IDH mutant glioma cells acquire resistance to natural killer (NK) cells through epigenetic silencing of NKG2D ligands.
Role of Pyruvate Dehydrogenase Complex and Pyruvate Carboxylase in GBM
Pyruvate has three fates following entry into the mitochondria in normal cells, (a) conversion to oxaloacetate by pyruvate carboxylase (PC), (b) conversion to acetyl-CoA by the pyruvate dehydrogenase complex (PDHC), and (c) transamination with glutamate to α-ketoglutarate and alanine by a mitochondrial alanine aminotransferase. In GBM cells, however, both PDHC and PC are usually downregulated (Cheng et al., 2011; Yuen et al., 2016). HIF-1α, Myc, and other tyrosine kinases activate pyruvate dehydrogenase kinases (PDKs), which phosphorylate and thus inactivate the mitochondrial PDHC (Papandreou et al., 2006). PC activity is suppressed in several cancer cell types (Chang and Morris, 1973), including glioma cells (Portais et al., 1993). Accordingly, PC is induced only when tumors grow in a glutamine-independent manner (Cheng et al., 2011). Inhibition of mitochondrial pyruvate catabolism is critical for tumor survival. Inhibition of PDK1 activates PDHC, with dichloroacetate, a drug used for the treatment of hereditary lactic acidosis. This then results in suppression of tumor growth in vitro and in vivo (Michelakis et al., 2008). In GBM, due to the high cellular heterogeneity, pyruvate metabolism through PDHC and PC exhibits cell-dependent variability (Marin-Valencia et al., 2012). However, the consensus is that PDHC, an NAD+ and CoASH-requiring enzyme complex, and PC, an ATP-hydrolyzing enzyme, are downregulated in cancer mitochondria. We discuss in the following sections how downregulation of PDHC free up CoASH for α-ketoglutarate dehydrogenase complex (KGDHC), a critical reaction by which mSLP produces high-energy phosphates through glutaminolysis.
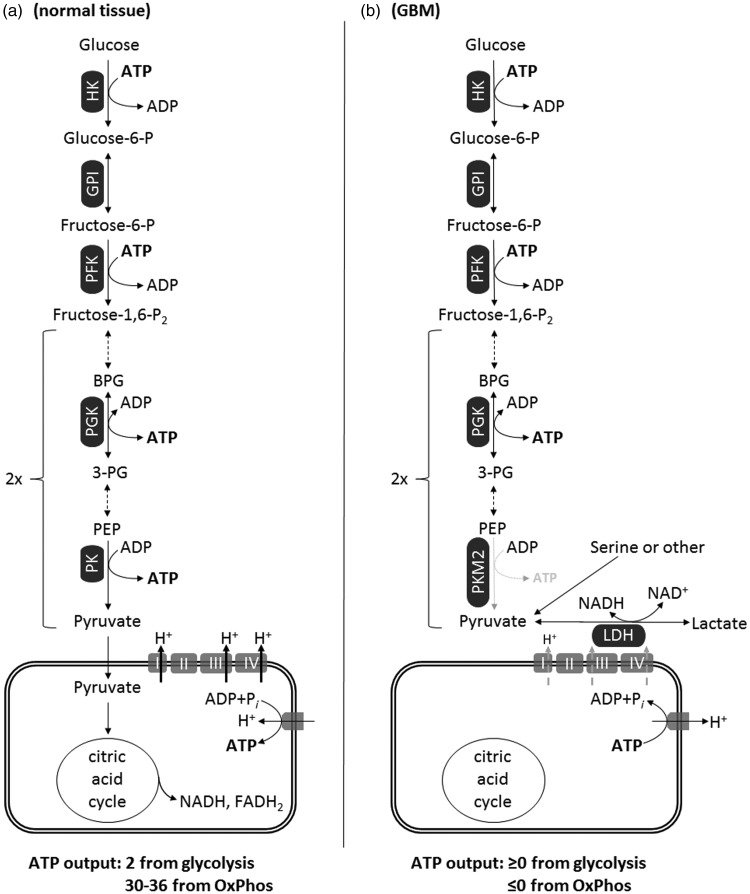
From the earlier considerations, it is evident that pyruvate metabolism by mitochondria is limited in GBM and that the high glycolytic rate may not generate adequate amounts of ATP due to a hindered payoff phase. This concept is depicted in Figure 2. Accordingly, the high glycolytic rate seen in GBM is needed more for rapid synthesis of growth metabolites than for ATP synthesis. Relevant to this, no alternative cytosolic pathways are known that could generate sufficient ATP to replace energy loss through glycolysis and OxPhos.
Figure 2.
Energetics of glycolysis and OxPhos in normal tissue (a) versus GBM (b). In GBM, the diminished activity of dimeric PKM2 to generate ATP yields a net ≥0 ATP output from glycolysis. Also, because GBM mitochondria exhibit a dysfunctional ETC and a reverse-operating F0-F1 ATP synthase, they are ATP consumers (≤0 ATP output). OxPhos in GBM mitochondria may operate at a diminished rate, and this is depicted by the gray-dashed arrows traversing complexes I, III, and IV implying diminished ability for proton expulsion.
FADH = reduced form of flavin adenine dinucleotide; GBM = glioblastoma multiforme; GPI = phospohexose isomerase; HK = hexokinase (any isoform); LDH = lactate dehydrogenase; PEP = phosphoenolpyruvate; PFK = phosphofructokinase; PGK = phosphoglycerate kinase; PK = pyruvate kinase; PKM2 = pyruvate kinase M2 isoform, dimeric; BPG = biphosphoglycerate; 3-PG = 3-phosphoglycerate.
Role of Citrate Metabolism in GBM
Acetyl-CoA is required for the cytosolic synthesis of fatty acids, cholesterol, and isoprenoid synthesis (Wakil et al., 1957). Acetyl-CoA cannot be directly exported to the cytoplasm but instead must first condense with oxaloacetate to form citrate through citrate synthase. Citrate is then converted to either isocitrate by aconitase (ACO2) or is exported to the cytosol where it is catabolized to acetyl-CoA and oxaloacetate by ATP-citrate lyase (ACL), at the expense of a high-energy phosphate bond provided by ATP. Acetyl-CoA is subsequently carboxylated to malonyl CoA also at the expense of a high-energy phosphate bond provided by ATP, initiating lipogenesis (Stryer, 1995). The importance of ACL is not only to catabolize citrate for the purpose of yielding acetyl-CoA, the building block for lipid synthesis in the cytoplasm, but also to decrease the concentration of citrate, which is a major negative allosteric modulator of phosphofructokinase, a rate-limiting step of glycolysis (Stryer, 1995). Akt facilitates the appearance of acetyl-CoA in the cytosol by phosphorylating and activating ACL (Berwick et al., 2002). Pharmacologic inhibition or silencing of ACL or acetyl-CoA carboxylase or fatty acid synthase decreases proliferation of cancer cells (Swinnen et al., 2006), and especially GBM (Lin et al., 2010; Lee et al., 2012; Poteet et al., 2013). It is important to mention, however, that ACL is likely to have little importance in GBM, as GBM cells can acquire fatty acids from the microenvironment, thus bypassing an essential need for fatty acid synthesis (Ta and Seyfried, 2015). However, many cancer cells exhibit a downregulation in the activity of PDHC, which results in diminished provision of acetyl-CoA in the mitochondrial matrix. In aggregate, it can be deduced that in GBM mitochondria, upregulations of ACL and acetyl-CoA carboxylase demand an adequate pool of cytosolic ATP.
Role of IDH in GBM
IDH exhibits three isoforms IDH1, NADP+-dependent isocitrate dehydrogenase (IDH2), and IDH3. IDH1 and IDH2 are homodimeric enzymes found in the cytoplasm and mitochondria, respectively, and produce NADPH. IDH1 and IDH2 are highly homologous but structurally and functionally distinct from the intramitochondrial IDH3, which produces NADH (Stryer, 1995). Many GBM cells exhibit reductive carboxylation of α-ketoglutarate to isocitrate by reversal of IDH and further isomerisation to citrate by ACO2, a pathway that was described in the early metabolic literature (Ochoa, 1948) and has been investigated as a way to produce citrate for the purpose of lipogenesis from α-ketoglutarate derived from glutamine (Wise et al., 2011). Furthermore, specific mutations in IDH1 and IDH2 are linked to tumorigenesis, in particular, GBM and acute myeloid leukemia (Yan et al., 2009), but not other tumors (Bleeker et al., 2009). It was shown that these mutations confer to IDH1 and IDH2 the ability to convert α-ketoglutarate to 2-hydroxyglutarate (2-HG) in a NADPH-dependent manner (Dang et al., 2009; Ohka et al., 2014). Conversion of α-ketoglutarate to 2-HG could lower ATP production, as α-ketoglutarate would be needed as a substrate for mSLP (see later). As also discussed earlier, reduced α-ketoglutarate levels and LDHA silencing could explain in part the more favorable prognosis for those GBM patients harboring IDH mutations (Chesnelong et al., 2014).
Role of Glutamine Metabolism in GBM
Substantial evidence exists documenting an important role for glutaminolysis and glutamine in cancer and specifically in GBM (Newsholme and Board, 1991; Medina, 2001; Yuneva, 2008; DeBerardinis and Cheng, 2010; Zhang et al., 2014; Liu et al., 2017; Maus and Peters, 2017; Oizel et al., 2017; Lemberg et al., 2018). Since the 1950s, it was recognized that tumors require large amounts of glutamine for growth and survival (hence the inclusion of glutamine in most culture media). The high-affinity glutamine transporter Slc1a5 (ASCT2) is upregulated in multiple types of cancer including GBM and has been implicated in mediating net glutamine uptake (Sidoryk et al., 2004; Fuchs and Bode, 2005). Even in the early studies, it was deduced that the high rate of glutamine consumption could not be fully accounted by protein synthesis because it exceeded the need for essential amino acids by an order of magnitude (Eagle et al., 1956). Several decades later, it was recognized that glutamine is a major energy source in tumor cells including GBM (Reitzer et al., 1979; Rossignol et al., 2004). The interconversion of glutamine and glutamate is bidirectional in normal cells, with glutamine synthetase catalyzing glutamine formation. In tumors, however, overexpression of glutaminases and suppression of glutamine synthetase favor the forward reaction toward glutamate (Perez-Gomez et al., 2005). Glutaminase activity correlates well with tumor growth rates in vivo (Knox et al., 1969), especially for GBM (Shelton et al., 2010b; Panosyan et al., 2016). Glutaminase activity is critically important for GBM, as glutaminase and glutamate levels are elevated following treatment with mTOR kinase inhibitor (Perez-Gomez et al., 2005). Glutamine-derived glutamate also facilitates GBM invasion (Takano et al., 2001). We also proposed that the most invasive cells of GBM are derived from neoplastic microglia/macrophages, which depend heavily on glutamine for growth (Huysentruyt et al., 2011). Moreover, a decrease in glutaminase activity diminishes growth rates of many tumor cells including the metastatic VM-M3 GBM model (Shelton et al., 2010b). Implantation of tumor tissue triggers a rapid increase in muscle glutamine output and a drop in muscle glutamine stores in rodents (Parry-Billings et al., 1991). A similar burden has been suggested to occur in human tumors (Souba, 1993). The consistency of high glutamine uptake in many tumors led to the development of glutamine PET tracers showing promise for the imaging of glutaminolysis (Wang et al., 2010; Qu et al., 2012). Although glutamine, like glucose, is a major energy metabolite for GBM, the mechanism by which glutamine generates energy remains unclear especially in tumor cells with abnormalities in the number, structure, and function of their mitochondria.
Some have suggested that GBM cells predominantly oxidize acetate rather than glutamine for growth (Marin-Valencia et al., 2012; Mashimo et al., 2014). As both acetate and glutamine pass through the common KGDHC → succinate-CoA ligase (SUCL) → SDH pathway (see later), it remains to be resolved how acetate, but not glutamine, is catabolized to citric acid cycle intermediates. Relevant to this, acetate has been implicated as an epigenetic regulator of posttranslational protein modification and might actually have therapeutic potential in cancer management (Jaworski et al., 2016). Thus, the role of acetate in GBM metabolism remains unclear.
The fate of glutamine in normal and tumor cells varies widely. An active synthesis of nitrogen-containing compounds, specifically nucleotides and nonessential amino acids (NEAAs), is required for tumor growth. Glutamine is the obligate nitrogen donor in at least three independent steps for purine synthesis including phosphoribosylpyrophosphate amidotranferase, phosphoribosylformylglycinamidine synthetase, and GMP synthetase. Glutamine is also important in two independent enzymatic steps for pyrimidine synthesis, carbamoyl phosphate synthetase II and CTP synthetase (Ahluwalia et al., 1990; Young and Ajami, 2001). Glutamine-derived glutamate is the primary nitrogen donor for the synthesis of NEAAs including asparagine (Ahluwalia et al., 1990). This is interesting because asparagine is considered a growth metabolite for GBM and other tumors (Panosyan et al., 2017; Knott et al., 2018). The glutamine:fructose-6-phosphate amidotransferase reaction transfers the amide nitrogen of glutamine to form glucosamine-6-phosphate, a precursor for N-linked and O-linked glycosylation needed for hexosamine synthesis (DeBerardinis and Cheng, 2010). Although some have considered that glutamine can be metabolized to lactate through the malic enzyme to produce NADPH for lipid biosynthesis (Qu et al., 2012), other findings indicate that little glutamine is metabolized to lactate in GBM and in other tumor cells (Ta and Seyfried, 2015).
In addition to the previous reactions, glutamine also participates in the synthesis of glutathione (GSH) through GSH cysteine ligase, thus playing a major role in the maintenance of cellular redox homeostasis (Amores-Sanchez and Medina, 1999; Le et al., 2012). GSH comprises glutamate, cysteine, and glycine. Glutamate contributes to the uptake of cystine (Sato et al., 1999). Cystine can then be converted to cysteine inside the cell and used in GSH synthesis (Stryer, 1995). In addition, glutamine induces synthesis of manganese superoxide dismutase (Aiken et al., 2008), another powerful antioxidant system in mitochondria of GBM (Ria et al., 2001; Shwetha et al., 2016). Hence, glucose and glutamine metabolism can protect GBM from oxidative stress, thus making GBM resistant to radio- and chemotherapy (Seyfried et al., 2017).
It is our view that glutamine not only provides nitrogen for synthesis of nucleotides and NEAAs but also provides α-ketoglutarate to serve as a precursor for ATP synthesis through substrate-level phosphorylation in the citric acid cycle. Relevant to this, it has been recently demonstrated that in fibroblasts from a patient with SUCL deficiency, glutamine oxidation was impaired (Chinopoulos et al., 2018). The availability of α-ketoglutarate may occur either through the glutamate dehydrogenase (GLUD1) reaction or through the transamination reaction (DeBerardinis and Cheng, 2010). Indeed, cells from GBM and other cancers rely on either GLUD1-mediated glutamate deamination (Sato et al., 1999) or transamination (Weinberg et al., 2010), thus providing α-ketoglutarate to the cycle. In at least one study, glutamate derived by transamination was suggested to be the major route (Moreadith and Lehninger, 1984). Furthermore, selective inhibition of glutamate transamination with aminooxyacetic acid hindered tumor growth in the absence of nonspecific toxicity of complete inhibition of glutamine metabolism involving GLUD1 (Wise et al., 2008). Oncogenic signals originating from Myc and perhaps also Ras induce all of the previously mentioned pathways involved in glutamine entry and ensuing glutaminolysis in tumor cells (DeBerardinis et al., 2007; Weinberg et al., 2010). Overall, it can be concluded that the mitochondria in GBM cells strongly depend on glutamine as a source of α-ketoglutarate that can be used for energy production through mSLP.
Role of the KGDHC in GBM
The KGDHC consists of multiple copies of three subunits: α-ketoglutarate dehydrogenase, dihydrolipoyl succinyltransferase, and dihydrolipoyl dehydrogenase. It catalyzes the conversion of α-ketoglutarate, CoASH, and NAD+ to succinyl-CoA, NADH, and CO2 and exhibits a high flux control coefficient in the generation of reducing equivalents and succinyl CoA (Sheu and Blass, 1999; Kiss et al., 2013). The PDHC and the KGDHC compete for the same pool of CoASH and NAD+ (Kiselevsky et al., 1990). Even a small decrease in KGDHC activity will lead to a considerable decrease in substrate-level phosphorylation in the mitochondrial matrix (Chinopoulos et al., 2010; Kiss et al., 2013, 2014). Hence, the diversion of α-ketoglutarate to 2-hydroxyglutarate could explain in part the improved overall survival of GBM patients containing the IDH mutations, as this diversion would reduce ATP synthesis through mSLP, thus reducing tumor growth.
Apart from tumor metabolism under ischemia or hypoxia, there is mounting evidence of pronounced conversion of α-ketoglutarate to succinate (Hochachka et al., 1975), implying that KGDHC remains operational (Chinopoulos, 2013). However, during anoxia, when the ability of Complex I to oxidize NADH to NAD+ is impaired, the question arises as to the origin of NAD+ for the KGDHC reaction. Under these conditions, we recently reported that mitochondrial diaphorases oxidize matrix NADH supplying NAD+ to KGDHC (Kiss et al., 2014; Ravasz et al., 2018). Furthermore, we showed that Complex III of the respiratory chain mediated reoxidation of the reducible substrates for the diaphorases using endogenous quinones (Kiss et al., 2014). Regarding KGDHC and cancer, it can be deduced that in hypoxia/anoxia, when the rate of NADH oxidation by Complex I is reduced, mitochondrial diaphorases use endogenous quinones to regenerate NAD+ for the KGDHC reaction. This raises the possibility that downstream provision of succinyl CoA by KGDHC maintains substrate-level phosphorylation yielding ATP or GTP in the mitochondrial matrix. We now propose that mSLP provides the majority of energy needed to drive GBM growth. This concept was originally suggested for the VM-M3 murine glioblastoma cells (Seyfried, 2012d).
Role of SUCL
SUCL is a heterodimeric enzyme, composed of an invariant α subunit encoded by SUCLG1 and a substrate-specific β subunit, encoded by either SUCLA2 or SUCLG2. This dimer combination results in either an ATP-forming (EC 6.2.1.5) or a GTP-forming SUCL (EC 6.2.1.4). The enzyme catalyzes the conversion of succinyl-CoA and ADP (or GDP) to CoASH, succinate, and ATP (or GTP). The ΔG’ for this reaction is 0.07 kJ/mol, and it is reversible (Li et al., 2013). When SUCL proceeds in the direction toward succinyl-CoA, this product may follow heme- or ketone body metabolism (Ottaway et al., 1981). On the other hand, the reaction proceeding toward ATP or GTP formation is termed substrate-level phosphorylation and can occur in the presence or absence of oxygen. Substrate-level phosphorylation during anoxia/ischemia rescues cells from cytosolic/nuclear ATP depletion. Moreover, this mechanism prevents mitochondria from reverting to ATP consumption by shifting the reversal potential (the mitochondrial membrane potential—ΔΨm—value at which there is no net transfer of ADP-ATP across the inner mitochondrial membrane) of the adenine nucleotide translocase (ANT) toward more negative values than the ΔΨm (Chinopoulos, 2013; Kiss et al., 2013, 2014). Relevant to this, matrix inorganic phosphate activates SUCL and thus contributes to the anaerobic provision of ATP synthesis in mitochondria (Phillips et al., 2009). GTP is a central regulator of cellular anabolism (Pall, 1985) and is used in mitochondria by the protein synthesis machinery, the PEP carboxykinase isoform 2, the GTP-AMP phosphotransferase, and by other GTP-binding proteins (Thomson, 1998). GTP-forming SUCL may support ATP formation in the matrix through the concerted action with a mitochondrial isoform of a nucleotide diphosphate kinase known as nm23-H4. This kinase complexes with either an ATP- or GTP-forming SUCL (Kadrmas et al., 1991; Kowluru et al., 2002) and plays a role in the maintenance of mtDNA (Suomalainen and Isohanni, 2010). Interestingly, nm23-H4 was recently reported to exhibit a moonlighting function involving intermembrane lipid transfer operated by cardiolipin (Schlattner et al., 2013). This property affords to the enzyme a regulatory role in lipid signaling of apoptosis. SUCL is not a critical enzyme in all tissues; for example, astrocytes in the human brain do not express SUCLA2 or SUCLG2 (Dobolyi et al., 2015a, 2015b); accordingly, these cells are expected not to exhibit SUCL activity. Finally, our recent studies show that ATP production through mSLP can support viability and growth in the VM-M3 glioblastoma cells (Flores et al., 2018).
Role of SDH
SDH, also known as Respiratory Complex II, is an integral mitochondrial inner membrane protein complex that oxidizes succinate to fumarate and transfers two electrons to coenzyme Q. SDH is composed of four subunits: SDHA, SDHB, SDHC, and SDHD. The assembly of SDH requires two factors, SDH Assembly Factor 1 (SDHAF1) and SDHAF2 (Bardella et al., 2011). Mutations in some of its subunits or its assembly factors were shown to cause paragangliomas and pheochromocytomas (Bardella et al., 2011). In these tumors, the resulting inhibition of SDH increases mitochondrial and cytosolic succinate levels, inhibiting α‑ketoglutarate-dependent prolyl hydroxylases (PHDs), thus causing stabilization of HIF-1α (Kurelac et al., 2011). HIF-1α mediates much of the genetic alterations of enzyme and transporter expressions mentioned earlier. In this sense, succinate has been considered a proinflammatory oncometabolite (Selak et al., 2005; Tannahill et al., 2013; Tretter et al., 2016). Succinate and fumarate also inhibit other α‑ketoglutarate-dependent dioxygenases, including the Jumonji‑C histone demethylases and the TET family of 5‑methylcytosine hydroxylases, resulting in genome-wide alterations of histone and DNA methylation and epigenetic dysregulation (Xiao et al., 2012). In other words, extranuclear mitochondrial abnormalities would underlie these nuclear epigenetic changes.
Although SDH mutations have not been reported in GBM, an increase in mRNA coding for SDH subunits has been shown in human GBM cells (Kim et al., 2015). This is most relevant regarding mSLP because the accumulation of succinate, as it may occur by high flux through SUCL, will on one hand mediate HIF-1α stabilization and inhibition of Jumonji‑C histone demethylases and TET 5‑methylcytosine hydroxylases aggravating epigenetic dysregulation, but on the other hand, it may push the reversible SUCL reaction toward ATP (or GTP) consumption. It is also interesting that the interaction of SIRT5 (a lysine desuccinylase) with cardiolipin can influence SDH activity and the efficiency of the ETC (Zhang et al., 2017b). The cardiolipin abnormalities we found in murine GBM might therefore influence SIRT5 function leading to enhancement of glutaminolysis and mSLP (Kiebish et al., 2008; Wang et al., 2018).
Role of FH
FH converts fumarate to malate in a reversible manner. Homozygous null mutations in the FH gene are associated with multiple cutaneous and uterine leiomyomatas and aggressive forms of renal cell cancer (Alam et al., 2003). Although fumarate has also been hypothesized to inhibit PHDs, stabilization of HIF-1α was not required for tumorigenesis in FH–/– mice (Adam et al., 2011). Fumarate is an electrophilic unsaturated dicarboxylic acid, and as such it has the ability to bind reactive thiol residues of proteins in a process termed protein succination (Alderson et al., 2006). Succination must not to be confused with succinylation, the latter being a posttranslational modification of lysine residues using succinyl-CoA (Weinert et al., 2013). Several targets of fumarate have now been identified, and they include the negative regulator of NRF2, Keap1 (Ooi et al., 2011), ACO2 (Ternette et al., 2013), and GSH (Sullivan et al., 2013). As such, fumarate has also achieved the status of an oncometabolite (Yang et al., 2012). As of October 2018, FH and its role in GBM have not yet been addressed. However, it is anticipated that accumulation of fumarate, as it may occur by high flux through SUCL performing substrate-level phosphorylation followed by the action SDH, will mediate excessive succinations, potentially contributing to tumor progression through increased glutaminolysis (Wang et al., 2018).
Role of Adenine Nucleotide Translocase and F0-F1 ATP Synthase in GBM
Both the ANT and the F0-F1 ATP synthase can catalyze reversible processes. The ΔΨm and the reversal potential (Erev) determine the directionality of these processes, the latter determined by the concentrations of the participating reactants (Chinopoulos, 2011a). Extramitochondrial adenine nucleotides can contribute to matrix adenine nucleotide pool only through the ANT and, by a minor fraction, through the ATP-Mg/Pi carrier (Aprille, 1993). As it has been extensively addressed in our laboratory, substrate-level phosphorylation substantiated by succinyl CoA ligase generating ATP assists respiration-impaired mitochondria exhibiting diminished values of ΔΨm to retain high matrix ATP levels, sparing cells from cytosolic/nuclear ATP consumption (Chinopoulos, 2011a, 2011b). This concept is inherently related to tumor metabolism generally and to GBM metabolism specifically, as the likelihood is high that GBM mitochondria exhibit diminished values of ΔΨm due to cristolysis and respiratory inhibition. Pinpointing the component(s) of the respiratory chain exhibiting defects leading to a decrease in respiratory capacity is a daunting task. This task is further complicated by the fact that the exact site of the defect in the respiratory chain will dictate whether a cell can be rescued by provision of substrates supporting mSLP (Chinopoulos, 2018). In any case, respiratory arrest and ensuing decrease in ΔΨm would result in consumption of matrix ATP by the reverse-operating F0-F1 ATP synthase. The generation of matrix ATP by succinyl CoA ligase is instrumental in rescuing cancer cells from cytosolic ATP consumption. On the same line of thought, the ATPase Inhibitory Factor 1 exhibits stark changes in expression among various types of human carcinomas (Sanchez-Arago et al., 2013) and has been linked to energy metabolism reprogramming, signaling the oncogenic phenotypes of cancer (Formentini et al., 2012). The critical information that can be obtained from this consideration is that substrate-level phosphorylation substantiated by succinyl CoA ligase generating ATP in the matrix assists respiration-impaired mitochondria that exhibit diminished values of ΔΨm in avoiding cytosolic ATP consumption, thus sparing cells from cytosolic/nuclear ATP depletion. This concept is inherently related to tumor metabolism because cancer mitochondria frequently exhibit diminished values of ΔΨm, a concept associated with the reverse operation of the F0-F1 ATP synthase and the consumption of matrix ATP.
From the above considerations, we have come to several insights regarding GBM metabolism from the earlier information: (a) The net balance of cytosolic ATP production is low despite having a high rate of glycolysis due to diminished production by the payoff phase substantiated by the reaction catalyzed by PKM2. (b) ATP is needed in the cytosol not only for maintenance of membrane ion pumps but also for ATP-consuming reactions of fatty acid synthesis. (c) The mitochondrial F0-F1 ATP synthase becomes an ATP consumer in pumping protons out of the matrix due to defects in the ETC. Consequently, little ATP is generated from either glycolysis or OxPhos, which increases the danger of mitochondria becoming net ATP consumers. (d) The branch of the citric acid cycle toward citrate formation becomes diminished due to lack of intramitochondrial catabolism of pyruvate. On the other hand, the rate of glutamine catabolism toward the citric acid cycle becomes high. (e) The obligate metabolites formed by this pathway are α-ketoglutarate, succinyl-CoA, and succinate. (f) This metabolite trio implies significant ATP (or GTP) synthesis through SUCL-mediated substrate-level phosphorylation. Maintenance of a high matrix ATP/ADP ratio also assures that the ANT provides ATP to the cytosol while also preventing the F0-F1 ATP synthase from draining cytosolic ATP reserves. The concomitant formation of succinate is efficiently shuttled outside the mitochondria but can also be metabolized to fumarate and malate, the latter supporting exchange of other metabolites across the inner mitochondrial membrane. An important question is whether other metabolites that converge on succinyl-CoA can also support ATP production in the mitochondrial matrix. This is addressed in the following section and in Figure 3. We also recognize that our views might be considered as a conjecture until additional evidence is obtained to support our position.
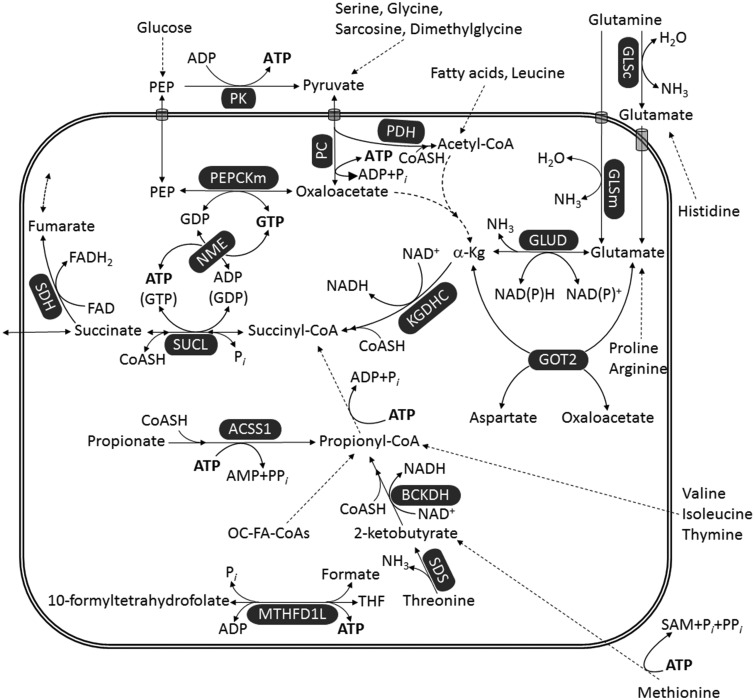
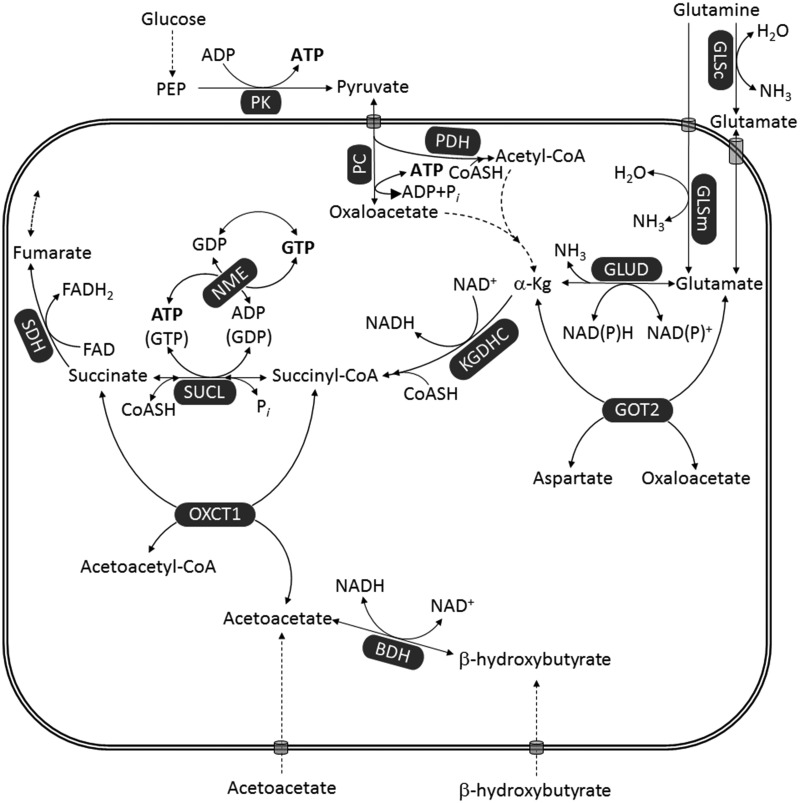
Figure 3.
Pathways leading to ATP (or GTP) generation at the substrate level. Dashed arrows imply multiple enzymatic steps (omitted for clarity). Reduction of NAD+ to NADH by PDH is omitted for uncluttering the figure.
α-Kg = α-ketoglutarate; ACSS1 = acetyl-coenzyme A synthetase 2-like, mitochondrial; BCKDH = branched-chain α-ketoacid dehydrogenase; GLSc = glutaminase, cytosolic; GLSm = glutaminase, mitochondrial; GLUD = glutamate dehydrogenase; GOT2 = aspartate aminotransferase; KGDHC = α-ketoglutarate dehydrogenase complex; MTHFD1L = monofunctional C1-tetrahydrofolate synthase; NME = nucleoside diphosphate kinase; OC-FA = odd-chain fatty acid; Pi = inorganic phosphate; PPi = pyrophosphate; PC = pyruvate carboxylase; PDH = pyruvate dehydrogenase; PEP = phosphoenolpyruvate; PEPCKm = mitochondrial phosphoenolpyruvate carboxykinase; PK = pyruvate kinase; SAM = S-adenosylmethionine; SDH = succinate dehydrogenase; SDS = serine dehydratase; SUCL = succinate-CoA ligase; THF = tetrahydrofolate.
Convergence of Metabolites Toward Succinyl-CoA and mSLP
There are a number of metabolites that catabolize toward the citric acid cycle through succinyl-CoA, thus leading to ATP (or GTP) formation through SUCL. Our review of the relevant biochemistry, however, shows that only glutamine catabolism can yield a net ATP (or GTP) production though mSLP. As shown in Figure 3, several metabolites eventually converge toward succinyl-CoA including the amino acids valine, isoleucine, methionine, histidine, threonine, glutamine, glutamate, and also propionate (Todesco et al., 1991; Laurent et al., 1995; Wong et al., 2006; Snyder et al., 2015) and thymine (Stryer, 1995), and odd-chain fatty acids (OC-FAs). Except for glutamate and glutamine, however, the catabolism of all other metabolites expends one or more high-energy phosphates during metabolic interconversions before becoming succinyl-CoA. Support for mSLP can also come from conversion to glutamate (such as proline and arginine) or from other metabolites that catabolize toward α-ketoglutarate (such as glycine, serine, sarcosine, dimethylglycine through pyruvate, and fatty acids or leucine through acetyl-CoA). However, the concentrations of these metabolites in the plasma or in the microenvironment are far lower than that of glutamine and are therefore unlikely to make a major contribution to mSLP (Yang et al., 2017). Dashed arrows in Figure 3 imply multiple enzymatic steps (omitted for the sake of clarity).
In addition to the pathways converging toward succinyl-CoA, there are two more ways for producing high-energy phosphates at the substrate level: first through the mitochondrial phosphoenolpyruvate carboxykinase, interconverting PEP and GDP to oxaloacetate and GTP (GTP, GDP, ADP, and ADP can interconvert through nucleoside diphosphate kinase isoforms) and second through the monofunctional C1-tetrahydrofolate (THF) synthase interconverting ADP, phosphate, and 10-formyltetrahydrofolate to ATP, formate, and THF. However, the mitochondrial phosphoenolpyruvate carboxykinase reaction is strongly favored toward PEP formation (thus consuming GTP) in a process called pyruvate recycling pathway (Freidmann et al., 1971; Rognstad and Katz, 1972; Cohen, 1987; Cerdan et al., 1990; Kunnecke et al., 1993; Bakken et al., 1997a, 1997b; Haberg et al., 1998; Chinopoulos, 2013). For this pathway, PEP enters mitochondria through a phosphate/PEP antiporter (McCoy and Doeg, 1975), a protein with isoforms in members C, D, and E of the solute carrier family 35 (Venter et al., 2001; Gerhard et al., 2004; Ota et al., 2004; Skarnes et al., 2011). Finally, carboxylation of pyruvate by PC yielding oxaloacetate is a thermodynamically reversible reaction; however, backflow toward pyruvate leading to ATP production may occur only at a very low rate (Freidmann et al., 1971; McClure et al., 1971a, 1971b; Barden et al., 1972). Consumption of one ATP in the reaction catalyzed by PC is also the reason why metabolites that converge to pyruvate (glycine, serine, sarcosine, and dimethylglycine) do not yield net ATP (or GTP) from mSLP. To date, the contribution of monofunctional C1-THF synthase on yielding ATP through substrate-level phosphorylation has not been adequately addressed. According to our analysis, glutamine would be the prime driver of mSLP and ATP production through SUCL. Evidence for our hypothesis was recently obtained from studies of the murine VM-M3 glioblastoma cells grown under hypoxia (Flores et al., 2018).
Interactions Between Oxidative Decarboxylation, Provision of Reducing Equivalents, Reductive Carboxylation, and mSLP
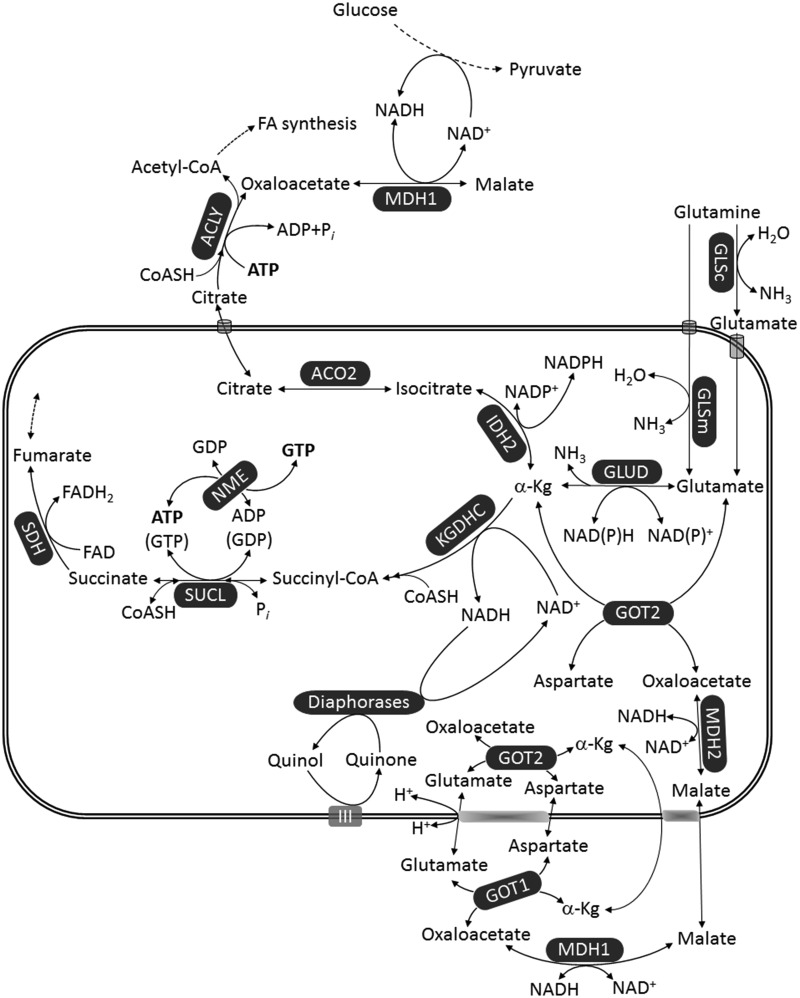
From the earlier considerations, it is evident that only catabolism of glutamine or glutamate may lead to net ATP (or GTP) production through mSLP; this is entirely consistent with the extremely high glutamine dependence of many tumors including GBM. However, this prompts considering the following two concepts: (a) reductive carboxylation of glutamine toward citrate that exits mitochondria and is destined for fatty acid synthesis has been well-documented to occur in cancers (Gaude et al., 2018), and especially in GBM (DeBerardinis et al., 2007; Ta and Seyfried, 2015) and (b) oxidative decarboxylation of glutamine/glutamate toward mSLP substantiated by SUCL demands stable provision of NAD+ for KGDHC. Reductive carboxylation versus oxidative decarboxylation of glutamine/glutamate will branch out at the level of α-ketoglutarate, depicted in Figure 4; in the former case, it will become isocitrate by the NADP+-dependent IDH2 and then citrate through reversal of ACO2; in the latter case, α-ketoglutarate will become succinyl-CoA by KGDHC and thus follow mSLP. Although both pathways start from α-ketoglutarate, they are not mutually exclusive; flux of glutamine/glutamate toward α-ketoglutarate is sufficiently high to support both pathways (Pike Winer and Wu, 2014; Yang et al., 2017). Interestingly, it was recently shown that in cancer cell lines, the degree of OxPhos defects conferred by mtDNA mutations dictates whether α-ketoglutarate follows oxidative decarboxylation or reductive carboxylation (Chen et al., 2018; Chinopoulos, 2018). On the other hand, regarding oxidative decarboxylation in the absence of OxPhos during which the ability of Complex I in providing NAD+ is impaired, it has been shown that intramitochondrial diaphorases can contribute to the matrix NAD+ pool (Kiss et al., 2014). This reaction was, however, demonstrated only in isolated mitochondria in artificial conditions. It is currently not known how mitochondria in situ regenerate NAD+ in the absence of OxPhos; it is not even known if a residual, minimal remaining function of Complex I during hypoxia/anoxia is sufficient for providing NAD+ for KGDHC for supporting mSLP, in view of the fact that overall respiration has ceased. Apart from mitochondrial diaphorases, there are other pathways that can adopt this role; as shown in Figure 4, the malate-aspartate shuttle can operate in reverse (Bremer and Davis, 1975; Chouchani et al., 2014) and lead to a decrease in the matrix NADH/NAD+ ratio. For example, conditions that can lead to excessive NADH oxidation in the cytosol may lead to increased formation of oxaloacetate in the same compartment that will in turn lead to transamination with glutamate to aspartate and α-ketoglutarate; this will cause the exchange of cytosolic α-ketoglutarate with mitochondrial malate, shifting the equilibrium of MDH2 toward NADH oxidation, thus yielding NAD+ in the matrix. Furthermore, salvage reactions that can lead to NAD+ formation could also play a role (Yang and Sauve, 2016). Moreover, there are other potential intramitochondrial reactions that can lead to a decrease in matrix NADH/NAD+ ratio, such as those participating in THF metabolism (Yang and Vousden, 2016). The group of Vamsi Mootha has published a compendium of 342 reactions utilizing NAD(P)H, termed the NAD(P)ome (Goodman et al., 2018). Hence, there are likely several mechanisms by which cancer cells can obtain NAD+ in low-oxygen, hyper-reducing conditions.
Figure 4.
Provision of NAD+ in the matrix of mitochondria in the absence of OxPhos. Enzyme annotations as in Figures 2 and 3.
α-Kg = α-ketoglutarate; ACO2 = aconitase; ACLY = ATP citrate lyase; FA = fatty acid; FAD = flavin adenine dinucleotide; GLSc = glutaminase, cytosolic; GLSm = glutaminase, mitochondrial; GLUD = glutamate dehydrogenase; GOT2 = aspartate aminotransferase; IDH2 = NADP+-dependent isocitrate dehydrogenase; KGDHC = α-ketoglutarate dehydrogenase complex; MDH = malate dehydrogenase; NME = nucleoside diphosphate kinase; SDH = succinate dehydrogenase; SUCL = succinate-CoA ligase.
The Warburg Theory Revisited
It is our view that SUCL can provision ATP (or GTP), thus bailing-in cancer mitochondria from a reverse-operating F0-F1 ATP synthase when ETC function(s) are impaired but can also assist glycolysis in providing high-energy phosphates for energy-consuming processes. This mechanism has been previously suggested and is pertinent to GBM and other solid tumors with hypoxic centers (Seyfried and Shelton, 2010; Seyfried, 2012d; Seyfried et al., 2017). Relevant to this, computer modeling of cancer metabolism predicted that a repression of SUCL would cause a significant reduction in growth rate, relative to known chemotherapeutic targets (Khazaei et al., 2012). It has also not escaped our attention that mSLP could represent the missing link in Warburg’s central theory that OxPhos insufficiency with compensatory fermentation is the origin of cancer. In addition to cytoplasmic substrate-level phosphorylation, that is, aerobic fermentation or the Warburg effect, mSLP could also compensate for insufficient or defective OxPhos, as we have described in this review. Unfortunately, the role of glutamine and mSLP was unknown to Warburg. Although most investigators have focused on aerobic fermentation (Warburg effect), we find it astonishing that almost all of the major reviews or previous studies on cancer energy metabolism have not addressed or even recognized the role of SUCL activity and mSLP, as a compensatory energy mechanism for deficient OxPhos. Based on our views, mSLP could become a key mechanism for energy production in tumor cells with defective respiration or for growth in hypoxic environments.
Confusion over the linkage of OxPhos to oxygen consumption and a general failure to recognize the role of mSLP as a compensatory energy mechanism have led many investigators to think that the ETC is normal in GBM and in other tumors. Further confusion could come in part from observations that GBM and other tumors might also use fatty acids and ketone bodies for growth (Bonuccelli et al., 2010; Baenke et al., 2013; Pascual et al., 2017; Xia et al., 2017; Jia et al., 2018). Fats and ketone bodies, however, are nonfermentable fuels and would require oxidative respiration for energy generation. Recent studies show, however, that fats can upregulate glycolysis and glutaminolysis through uncoupling mechanisms (Samudio et al., 2009; Valle et al., 2010; Vozza et al., 2014). Enhanced glucose and glutamine metabolism could therefore be an indirect effect of high-fat diets that are consumed in unrestricted amounts (Zhou et al., 2007). The numerous abnormalities in the number, structure, and function of GBM mitochondria mentioned earlier would be expected to alter the function of the ETC according to the predictions of Lehninger (1964) and Ferreria (2010). Little ATP produced from glycolysis might also be expected for those tumors (GBM) where the dimeric PKM2 expression predominates over PKM1 expression. In light of the PKM2 issue, the convergence of metabolites toward succinyl-CoA and mSLP could provision sufficient ATP to maintain growth of GBM and possibly other tumors in hypoxic environments. As glutamine would be the major metabolite for provisioning ATP synthesis through mSLP under normoxic or hypoxic conditions in tumor cells, the phenomenon could be considered a Warburg Q-Effect to distinguish this effect from that involving the aerobic fermentation of glucose. Both effects arise from compromised OxPhos. Emerging evidence indicates that the mitochondrial metabolic theory can explain better the hallmarks of cancer than can the somatic mutation theory (Seyfried and Shelton, 2010; Seyfried, 2012d; Seyfried et al., 2014; Seyfried, 2015). Recognition of mSLP as a second major compensatory energy mechanism for tumor cells with defective or insufficient OxPhos could have profound implications for managing most tumors including GBM.
Considerations for Controlling GBM Cell Growth
It is widely recognized that glucose and glutamine are the major energy metabolites that drive GBM growth and invasion. Glucose and glutamine become the major fermentable fuels for heterogeneous GBM cells due to the inability of OxPhos to meet energy demands and for growth in hypoxic environments. Recent findings indicate that glutamine is the major fuel for GBM cells with mesenchymal molecular subtype, whereas glucose in the major fuel for GBM cells with proliferative subtype (Oizel et al., 2017). According to our concepts based on the underlying biochemistry discussed earlier, control of GBM cell growth could involve the simultaneous targeting of substrate-level phosphorylation reactions in the cytoplasm (glycolysis) and in the mitochondria (glutaminolysis). Although we do not make any recommendations for treatment of GBM in patients, a parsimonious consideration would be to restrict availability of glucose and glutamine to the tumor cells to downregulate both glycolysis and glutaminolysis simultaneously. This approach was shown to kill GBM and HeLa cells in vitro (Shelton, 2010; Mathews et al., 2014), as would be predicted from ensemble modeling of cancer metabolism (Khazaei et al., 2012). No cell can grow without energy regardless of its genetic composition. Because ketone bodies can replace glucose as an energy source, prior studies show that glucose levels could be significantly reduced without harmful effects after first transitioning the body to therapeutic ketosis (Drenick et al., 1972; Seyfried et al., 2015; Seyfried et al., 2017). This fact was mentioned in the work of Cahill and Veech (2003) and was demonstrated in nine persons who received insulin injection after fasting for 2 months (Drenick et al., 1972). Indeed, the blood glucose level in one person in the insulin group reached as low as 9 mg/dl (0.5 mmol/l) without evidence of a hypoglycemic response (Drenick et al., 1972). These findings suggest that the glucose needed for GBM cell growth could be majorly restricted under therapeutic ketosis.
Ketone bodies also possess anti-inflammatory potential through reduction of reactive oxygen species and increase of GSH peroxidase activity in normal brain cells (Veech, 2004; Seyfried and Mukherjee, 2005). Some studies have shown that GBM and other malignant brain tumors may have a reduced ability to utilize ketone bodies due in part to diminished activity of succinyl-CoA:3-ketoacid coenzyme A transferase 1, which is essential for metabolism of ketone bodies (Fredericks and Ramsey, 1978; Zhou et al., 2007; Maurer et al., 2011). Glucose restriction under therapeutic ketosis or calorie restriction will downregulate the entire glycolytic pathway from glucose to pyruvate (Marsh et al., 2008). Glycolytic downregulation will not only deprive tumor cells of growth metabolites but will also reduce LDHA activity and lactate production, thus reducing angiogenesis and inflammation in the tumor microenvironment (Mukherjee et al., 2002, 2004; Marsh et al., 2008; Mulrooney et al., 2011; Urits et al., 2012; Husain et al., 2013; Vergati et al., 2017). Glucose restriction should also reduce the metabolites needed for serine-derived one-carbon metabolism, which would further reduce tumor cell antioxidant capacity and growth (Meiser and Vazquez, 2016; Semenza, 2017; Zhang et al., 2017a). We also found that calorie restriction and restricted ketogenic diets, which lower blood glucose and elevate blood ketone bodies are proapoptotic against murine glioblastoma and neural stem cell tumors (Mukherjee et al., 2002, 2004; Shelton et al., 2010a). Glucose targeting would also reduce metabolites generated through the pentose phosphate pathway that would be needed for the synthesis of GSH and growth metabolites.
From the biochemical point of view, catabolism of ketone bodies obligatorily bypasses mSLP. As shown in Figure 5, the ketone bodies acetoacetate and β-hydroxybutyrate enter mitochondria and eventually the citric acid cycle as succinate, bypassing mSLP. This is because formation of succinate is not through SUCL but through succinyl-CoA:3-ketoacid coenzyme A transferase 1. By doing so, catabolism of both acetoacetate and β-hydroxybutyrate does not lead to the formation of high-energy phosphates through mSLP. Furthermore, β-hydroxybutyrate is expected to decrease mSLP through glutamine/glutamate even further because it increases NADH/NAD+ ratio, thus hindering KGDHC operation (Kiss et al., 2013, 2014). It is also essential to target glucose and glutamine together, as glutamine can be synthesized from glucose-derived glutamate through the glutamine synthetase activity (Tardito et al., 2015). Hence, the simultaneous restriction of glucose and glutamine, while under therapeutic ketosis, could halt growth of GBM cells that are more dependent on substrate-level phosphorylation than on OxPhos for survival.
Figure 5.
Ketone bodies bypass mSLP. Enzyme annotations as for Figures 2, 3, and 4.
BDH = β-hydroxybutyrate dehydrogenase; FAD = flavin adenine dinucleotide; GLSc = glutaminase, cytosolic; GLSm = glutaminase, mitochondrial; GLUD = glutamate dehydrogenase; GOT2 = aspartate aminotransferase; KGDHC = α-ketoglutarate dehydrogenase complex; NME = nucleoside diphosphate kinase; OXCT1 = succinyl-CoA:3-ketoacid coenzyme A transferase 1; PC = pyruvate carboxylase; PDH = pyruvate dehydrogenase; PEP = phosphoenolpyruvate; PK = pyruvate kinase; SUCL = succinate-CoA ligase.
It is important to mention that the targeting of glutamine is more challenging than is the targeting of glucose for GBM growth control. Glutamine, like glucose, is an essential metabolite for the neoplastic cells of GBM. Unlike glucose, however, glutamine is also an essential metabolite for cells of the gut, the immune system, and for general physiological homeostasis (Souba, 1993; Newsholme, 2001; Young and Ajami, 2001). Consequently, glutamine targeting must be done carefully so as not to harm normal cell function or disrupt physiological homeostasis.
We recently proposed a press-pulse therapeutic strategy for managing most cancers including GBM while minimizing toxicity to normal cells and tissues (Seyfried et al., 2017). The press-pulse concept was developed from the field of paleobiology in showing that the extinction of organisms during prior evolutionary epochs occurred in the ecosystem only when a chronic press disturbance coincided with an acute pulse disturbance. In our adaption of this concept for the cancer problem, press therapies are designed to reduce systemic glucose availability while elevating blood levels of ketone bodies, which tumor cells cannot effectively use for energy generation. This approach is designed to pit the metabolic demands of normal cells against those of the mutated tumor cells, which are less capable than normal cells in adapting to metabolic stress from nutrient deprivation due to accumulation of tumor mutations (Seyfried and Mukherjee, 2005; Seyfried et al., 2017). Ketone body supplements could further reduce glucose levels while enhancing the respiratory energy metabolism in normal cells. Stress management techniques together with exercise could further reduce blood glucose levels while improving general health. The press therapies would be designed to work synergistically with acute pulse therapies to further target glucose and glutamine metabolism. Pulse therapies would involve drug cocktails and procedures that work synergistically with the press therapy to gradually degrade and eradicate the tumor cells. The drugs chosen would target simultaneously glycolysis in the cytoplasm and substrate-level phosphorylation in the mitochondria. Hence, diets, drugs, and procedures that restrict availability of glucose and glutamine could deprive GBM cells of the fuels necessary for growth while avoiding damage to normal cells of the brain and body.
Conclusions
We review evidence that describes abnormalities in the number, structure, and function of mitochondria in GBM. These abnormalities, together with expression of the PKM2 isoform, would compromise effective energy production through OxPhos and glycolysis, respectively. We also describe a framework for how provision of high-energy phosphates through mSLP in GBM could compensate for the loss of energy production through both glycolysis and OxPhos. A reliance on mSLP using glucose through cytoplasmic glycolysis and glutamine through glutaminolysis will cause the cancer cells to enter their default state of unbridled proliferation, that is, the state of existence for all cells prior the emergence of atmospheric oxygen (Szent-Gyorgyi, 1977; Sonnenschein and Soto, 2000; Seyfried and Shelton, 2010). The unbridled proliferation of GBM cells should not be viewed as a growth advantage but rather as a pathological phenotype arising from defective OxPhos with compensatory substrate-level phosphorylation occurring in the cytoplasm and in the mitochondria (Seyfried, 2012a; Seyfried et al., 2017). The simultaneous restriction of glucose and glutamine, while under therapeutic ketosis, will deprive GBM cells of substrates for growth and energy production. This information should have value in the clinic.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The authors would like to acknowledge support from the Foundation for Metabolic Cancer Therapies, the Claudia & Nelson Peltz Foundation, Crossfit Inc., Joseph Maroon, Joe Mercola, and Ellen Davis to T. N. S. and grants FIKP-61822-64888-EATV, VEKOP 2.3.3-15-2016-00012, NKFIH KH129567, and 2017-2.3.4-TET-RU-2017-00003 to C. C.
References
- Adam J., et al. (2011). Renal cyst formation in Fh1-deficient mice is independent of the Hif/Phd pathway: Roles for fumarate in KEAP1 succination and Nrf2 signaling. Cancer Cell, 20, 524–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahluwalia G. S., Grem J. L., Hao Z., Cooney D. A. (1990). Metabolism and action of amino acid analog anti-cancer agents. Pharmacol Ther, 46, 243–271. [DOI] [PubMed] [Google Scholar]
- Ahmadloo N., Kani A. A., Mohammadianpanah M., Nasrolahi H., Omidvari S., Mosalaei A., Ansari M. (2013). Treatment outcome and prognostic factors of adult glioblastoma multiforme. J Egypt Natl Canc Inst, 25, 21–30. [DOI] [PubMed] [Google Scholar]
- Aiken K. J., Bickford J. S., Kilberg M. S., Nick H. S. (2008). Metabolic regulation of manganese superoxide dismutase expression via essential amino acid deprivation. J Biol Chem, 283, 10252–10263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam N. A., et al. (2003). Genetic and functional analyses of FH mutations in multiple cutaneous and uterine leiomyomatosis, hereditary leiomyomatosis and renal cancer, and fumarate hydratase deficiency. Hum Mol Genet, 12, 1241–1252. [DOI] [PubMed] [Google Scholar]
- Alderson N. L., Wang Y., Blatnik M., Frizzell N., Walla M. D., Lyons T. J., Alt N., Carson J. A., Nagai R., Thorpe S. R., Baynes J. W. (2006). S-(2-Succinyl)cysteine: A novel chemical modification of tissue proteins by a Krebs cycle intermediate. Arch Biochem Biophys, 450, 1–8. [DOI] [PubMed] [Google Scholar]
- Alexander B. M., Cloughesy T. F. (2017). Adult glioblastoma. J Clin Oncol, 35, 2402–2409. [DOI] [PubMed] [Google Scholar]
- Allen N. J., Karadottir R., Attwell D. (2005). A preferential role for glycolysis in preventing the anoxic depolarization of rat hippocampal area CA1 pyramidal cells. J Neurosci, 25, 848–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amores-Sanchez M. I., Medina M. A. (1999). Glutamine, as a precursor of glutathione, and oxidative stress. Molecur Genet Met, 67, 100–105. [DOI] [PubMed] [Google Scholar]
- Aprille J. R. (1993). Mechanism and regulation of the mitochondrial ATP-Mg/P(i) carrier. J Bioenerg Biomembr, 25, 473–481. [DOI] [PubMed] [Google Scholar]
- Arcos J. C., Tison M. J., Gosch H. H., Fabian J. A. (1969). Sequential alterations in mitochondrial inner and outer membrane electron transport and in respiratory control during feeding of amino azo dyes; stability of phosphorylation. Correlation with swelling-contraction changes and tumorigenesis threshold. Cancer Res, 29, 1298–1305. [PubMed] [Google Scholar]
- Arismendi-Morillo G. J., Castellano-Ramirez A. V. (2008). Ultrastructural mitochondrial pathology in human astrocytic tumors: Potentials implications pro-therapeutics strategies. J Electron Microsc (Tokyo), 57, 33–39. [DOI] [PubMed] [Google Scholar]
- Baenke F., Peck B., Miess H., Schulze A. (2013). Hooked on fat: The role of lipid synthesis in cancer metabolism and tumour development. Dis Models Mech, 6, 1353–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakken I. J., Sonnewald U., Clark J. B., Bates T. E. (1997. a). [U-13C]glutamate metabolism in rat brain mitochondria reveals malic enzyme activity. Neuroreport, 8, 1567–1570. [DOI] [PubMed] [Google Scholar]
- Bakken I. J., White L. R., Aasly J., Unsgard G., Sonnewald U. (1997. b). Lactate formation from [U-13C]aspartate in cultured astrocytes: Compartmentation of pyruvate metabolism. Neurosci Lett, 237, 117–120. [DOI] [PubMed] [Google Scholar]
- Bardella C., Pollard P. J., Tomlinson I. (2011). SDH mutations in cancer. Biochim Biophys Acta, 1807, 1432–1443. [DOI] [PubMed] [Google Scholar]
- Barden R. E., Fung C. H., Utter M. F., Scrutton M. C. (1972). Pyruvate carboxylase from chicken liver. Steady state kinetic studies indicate a “two-site” ping-pong mechanism. J Biol Chem, 247, 1323–1333. [PubMed] [Google Scholar]
- Bartesaghi S., Graziano V., Galavotti S., Henriquez N. V., Betts J., Saxena J., Minieri V. A. D., Karlsson A., Martins L. M., Capasso M., Nicotera P., Brandner S., De Laurenzi V., Salomoni P. (2015). Inhibition of oxidative metabolism leads to p53 genetic inactivation and transformation in neural stem cells. Proc Natl Acad Sci U S A, 112, 1059–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berwick D. C., Hers I., Heesom K. J., Moule S. K., Tavare J. M. (2002). The identification of ATP-citrate lyase as a protein kinase B (Akt) substrate in primary adipocytes. J Biol Chem, 277, 33895–33900. [DOI] [PubMed] [Google Scholar]
- Billiard J., et al. (2013). Quinoline 3-sulfonamides inhibit lactate dehydrogenase A and reverse aerobic glycolysis in cancer cells. Cancer Metab, 1, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleeker F. E., Lamba S., Leenstra S., Troost D., Hulsebos T., Vandertop W. P., Frattini M., Molinari F., Knowles M., Cerrato A., Rodolfo M., Scarpa A., Felicioni L., Buttitta F., Malatesta S., Marchetti A., Bardelli A. (2009). IDH1 mutations at residue p.R132 (IDH1(R132)) occur frequently in high-grade gliomas but not in other solid tumors. Hum Mutat, 30, 7–11. [DOI] [PubMed] [Google Scholar]
- Bonuccelli G., Tsirigos A., Whitaker-Menezes D., Pavlides S., Pestell R. G., Chiavarina B., Frank P. G., Flomenberg N., Howell A., Martinez-Outschoorn U. E., Sotgia F., Lisanti M. P. (2010). Ketones and lactate “fuel” tumor growth and metastasis: Evidence that epithelial cancer cells use oxidative mitochondrial metabolism. Cell Cycle, 9, 3506–3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremer J., Davis E. J. (1975). Studies on the active transfer of reducing equivalents into mitochondria via the malate-aspartate shuttle. Biochim Biophys Acta, 376, 387–397. [DOI] [PubMed] [Google Scholar]
- Burk D., Schade A. L. (1956). On respiratory impairment in cancer cells. Science, 124, 270–272. [PubMed] [Google Scholar]
- Burk D., Woods M., Hunter J. (1967). On the significance of glucolysis for cancer growth, with special reference to Morris rat hepatomas. J Natl Cancer Inst, 38, 839–863. [PubMed] [Google Scholar]
- Cahill G. F., Jr., Veech R. L. (2003). Ketoacids? Good medicine? Trans Am Clin Climatol Assoc, 114, 149–161; discussion 162–163. [PMC free article] [PubMed] [Google Scholar]
- Cerdan S., Kunnecke B., Seelig J. (1990). Cerebral metabolism of [1,2-13C2]acetate as detected by in vivo and in vitro 13C NMR. J Biol Chem, 265, 12916–12926. [PubMed] [Google Scholar]
- Chang L. O., Morris H. P. (1973). Enzymatic and immunological studies on pyruvate carboxylase in livers and liver tumors. Cancer Res, 33, 2034–2041. [PubMed] [Google Scholar]
- Chang S. M., Parney I. F., Huang W., Anderson F. A., Jr., Asher A. L., Bernstein M., Lillehei K. O., Brem H., Berger M. S., Laws E. R., & Glioma Outcomes Project Investigators. (2005). Patterns of care for adults with newly diagnosed malignant glioma. JAMA, 293, 557–564. [DOI] [PubMed] [Google Scholar]
- Chen Q., Kirk K., Shurubor Y. I., Zhao D., Arreguin A. J., Shahi I., Valsecchi F., Primiano G., Calder E. L., Carelli V., Denton T. T., Beal M. F., Gross S. S., Manfredi G., D'Aurelio M. (2018). Rewiring of glutamine metabolism is a bioenergetic adaptation of human cells with mitochondrial DNA mutations. Cell Metab, 27, 1007–1025.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng T., Sudderth J., Yang C., Mullen A. R., Jin E. S., Mates J. M., DeBerardinis R. J. (2011). Pyruvate carboxylase is required for glutamine-independent growth of tumor cells. Proc Natl Acad Sci U S A, 108, 8674–8679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesnelong C., Chaumeil M. M., Blough M. D., Al-Najjar M., Stechishin O. D., Chan J. A., Pieper R. O., Ronen S. M., Weiss S., Luchman H. A., Cairncross J. G. (2014). Lactate dehydrogenase A silencing in IDH mutant gliomas. Neuro Oncol, 16, 686–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinopoulos C. (2011. a). Mitochondrial consumption of cytosolic ATP: Not so fast. FEBS Lett, 585, 1255–1259. [DOI] [PubMed] [Google Scholar]
- Chinopoulos C. (2011. b). The “B Space” of mitochondrial phosphorylation. J Neurosci Res, 89, 1897–1904. [DOI] [PubMed] [Google Scholar]
- Chinopoulos C. (2013). Which way does the citric acid cycle turn during hypoxia? The critical role of alpha-ketoglutarate dehydrogenase complex. J Neurosci Res, 91, 1030–1043. [DOI] [PubMed] [Google Scholar]
- Chinopoulos C. (2018). OXPHOS defects due to mtDNA mutations: Glutamine to the rescue! Cell Metab, 27, 1165–1167. [DOI] [PubMed] [Google Scholar]
- Chinopoulos C., Batzios S., Heuvel L. P., Rodenburg R., Smeets R., Waterham H. R., Turkenburg M., Ruiter J. P., Wanders R. J. A., Doczi J., Horvath G., Dobolyi A., Vargiami E., Wevers R. A., Zafeiriou D. I. (2018). Mutated SUCLG1 causes mislocalization of SUCLG2 protein, morphological alterations of mitochondria and an early-onset severe neurometabolic disorder. Mol Genet Metab. pii: S1096-7192(18)30607-3. doi: 10.1016/j.ymgme.2018.11.009. [DOI] [PubMed]
- Chinopoulos C., Gerencser A. A., Mandi M., Mathe K., Torocsik B., Doczi J., Turiak L., Kiss G., Konrad C., Vajda S., Vereczki V., Oh R. J., Adam-Vizi V. (2010). Forward operation of adenine nucleotide translocase during F0F1-ATPase reversal: Critical role of matrix substrate-level phosphorylation. FASEB J, 24, 2405–2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouchani E. T., et al. (2014). Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature, 515, 431–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christofk H. R., Vander Heiden M. G., Wu N., Asara J. M., Cantley L. C. (2008). Pyruvate kinase M2 is a phosphotyrosine-binding protein. Nature, 452, 181–186. [DOI] [PubMed] [Google Scholar]
- Claypool S. M., Koehler C. M. (2012). The complexity of cardiolipin in health and disease. Trends Biochem Sci, 37, 32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. M. (1987). Effects of insulin on perfused liver from streptozotocin-diabetic and untreated rats: 13C NMR assay of pyruvate kinase flux. Biochemistry, 26, 573–580. [DOI] [PubMed] [Google Scholar]
- Cuddapah V. A., Robel S., Watkins S., Sontheimer H. (2014). A neurocentric perspective on glioma invasion. Nat Rev Neurosci, 15, 455–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlberg D., Struys E. A., Jansen E. E., Morkrid L., Midttun O., Hassel B. (2017). Cyst fluid from cystic, malignant brain tumors: A reservoir of nutrients, including growth factor-like nutrients, for tumor cells. Neurosurgery, 80, 917–924. [DOI] [PubMed] [Google Scholar]
- Dang C. V., Kim J. W., Gao P., Yustein J. (2008). The interplay between MYC and HIF in cancer. Nat Rev Cancer, 8, 51–56. [DOI] [PubMed] [Google Scholar]
- Dang L., White D. W., Gross S., Bennett B. D., Bittinger M. A., Driggers E. M., Fantin V. R., Jang H. G., Jin S., Keenan M. C., Marks K. M., Prins R. M., Ward P. S., Yen K. E., Liau L. M., Rabinowitz J. D., Cantley L. C., Thompson C. B., Vander Heiden M. G., Su S. M. (2009). Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature, 462, 739–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David C. J., Chen M., Assanah M., Canoll P., Manley J. L. (2010). HnRNP proteins controlled by c-Myc deregulate pyruvate kinase mRNA splicing in cancer. Nature, 463, 364–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson D. M., Goodfriend T. L., Kaplan N. O. (1964). Lactic dehydrogenases: Functions of the two types rates of synthesis of the two major forms can be correlated with metabolic differentiation. Science, 143, 929–933. [DOI] [PubMed] [Google Scholar]
- DeBerardinis R. J., Cheng T. (2010). Q's next: The diverse functions of glutamine in metabolism, cell biology and cancer. Oncogene, 29, 313–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBerardinis R. J., Mancuso A., Daikhin E., Nissim I., Yudkoff M., Wehrli S., Thompson C. B. (2007). Beyond aerobic glycolysis: Transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci U S A, 104, 19345–19350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deighton R. F., Le Bihan T., Martin S. F., Gerth A. M., McCulloch M., Edgar J. M., Kerr L. E., Whittle I. R., McCulloch J. (2014). Interactions among mitochondrial proteins altered in glioblastoma. J Neurooncol, 118, 247–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derr R. L., Ye X., Islas M. U., Desideri S., Saudek C. D., Grossman S. A. (2009). Association between hyperglycemia and survival in patients with newly diagnosed glioblastoma. J Clin Oncol, 27, 1082–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai S., Ding M., Wang B., Lu Z., Zhao Q., Shaw K., Yung W. K., Weinstein J. N., Tan M., Yao J. (2014). Tissue-specific isoform switch and DNA hypomethylation of the pyruvate kinase PKM gene in human cancers. Oncotarget, 5, 8202–8210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienel G. A. (2012). Brain lactate metabolism: The discoveries and the controversies. J Cereb Blood Flow Metab, 32, 1107–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobolyi A., Bago A. G., Gal A., Molnar M. J., Palkovits M., Adam-Vizi V., Chinopoulos C. (2015. a). Localization of SUCLA2 and SUCLG2 subunits of succinyl CoA ligase within the cerebral cortex suggests the absence of matrix substrate-level phosphorylation in glial cells of the human brain. J Bioenerg Biomembr, 47, 33–41. [DOI] [PubMed] [Google Scholar]
- Dobolyi A., Ostergaard E., Bago A. G., Doczi T., Palkovits M., Gal A., Molnar M. J., Adam-Vizi V., Chinopoulos C. (2015. b). Exclusive neuronal expression of SUCLA2 in the human brain. Brain Struct Funct, 220, 135–151. [DOI] [PubMed] [Google Scholar]
- Doherty J. R., Cleveland J. L. (2013). Targeting lactate metabolism for cancer therapeutics. J Clin Invest, 123, 3685–3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drenick E. J., Alvarez L. C., Tamasi G. C., Brickman A. S. (1972). Resistance to symptomatic insulin reactions after fasting. J Clin Invest, 51, 2757–2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagle H., Oyama V. I., Levy M., Horton C. L., Fleischman R. (1956). The growth response of mammalian cells in tissue culture to L-glutamine and L-glutamic acid. J Biol Chem, 218, 607–616. [PubMed] [Google Scholar]
- Elstrom R. L., Bauer D. E., Buzzai M., Karnauskas R., Harris M. H., Plas D. R., Zhuang H., Cinalli R. M., Alavi A., Rudin C. M., Thompson C. B. (2004). Akt stimulates aerobic glycolysis in cancer cells. Cancer Res, 64, 3892–3899. [DOI] [PubMed] [Google Scholar]
- Estrella V., Chen T., Lloyd M., Wojtkowiak J., Cornnell H. H., Ibrahim-Hashim A., Bailey K., Balagurunathan Y., Rothberg J. M., Sloane B. F., Johnson J., Gatenby R. A., Gillies R. J. (2013). Acidity generated by the tumor microenvironment drives local invasion. Cancer Res, 73, 1524–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantin V. R., St-Pierre J., Leder P. (2006). Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell, 9, 425–434. [DOI] [PubMed] [Google Scholar]
- Fatehi M., Hunt C., Ma R., Toyota B. D. (2018). Persistent disparities in survival for patients with glioblastoma. World Neurosurg, 120, e511–e516. [DOI] [PubMed] [Google Scholar]
- Feichtinger R. G., Weis S., Mayr J. A., Zimmermann F., Geilberger R., Sperl W., Kofler B. (2014). Alterations of oxidative phosphorylation complexes in astrocytomas. Glia, 62, 514–525. [DOI] [PubMed] [Google Scholar]
- Ferreira L. M. (2010). Cancer metabolism: The Warburg effect today. Exp Mol Pathol, 89, 372–380. [DOI] [PubMed] [Google Scholar]
- Fisher P. G., Buffler P. A. (2005). Malignant gliomas in 2005: Where to GO from here? JAMA, 293, 615–617. [DOI] [PubMed] [Google Scholar]
- Flavahan W. A., Wu Q., Hitomi M., Rahim N., Kim Y., Sloan A. E., Weil R. J., Nakano I., Sarkaria J. N., Stringer B. W., Day B. W., Li M., Lathia J. D., Rich J. N., Hjelmeland A. B. (2013). Brain tumor initiating cells adapt to restricted nutrition through preferential glucose uptake. Nat Neurosci, 16, 1373–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flechl B., Ackerl M., Sax C., Dieckmann K., Crevenna R., Gaiger A., Widhalm G., Preusser M., Marosi C. (2012). Neurocognitive and sociodemographic functioning of glioblastoma long-term survivors. J Neurooncol, 109, 331–339. [DOI] [PubMed] [Google Scholar]
- Flores R. E., Brown A. K., Taus L., Khoury J., Glover F., Kami K., Sarangarajan R., Walshe T. E., Narain N. R., Kiebish M. A., Shelton L. M., Chinopoulos C., Seyfried T. N. (2018). Mycoplasma infection and hypoxia initiate succinate accumulation and release in the VM-M3 cancer cells. Biochim Biophys Acta, 1859, 975–983. [DOI] [PubMed] [Google Scholar]
- Formentini L., Sanchez-Arago M., Sanchez-Cenizo L., Cuezva J. M. (2012). The mitochondrial ATPase inhibitory factor 1 triggers a ROS-mediated retrograde prosurvival and proliferative response. Mol Cell, 45, 731–742. [DOI] [PubMed] [Google Scholar]
- Fredericks M., Ramsey R. B. (1978). 3-Oxo acid coenzyme A transferase activity in brain and tumors of the nervous system. J Neurochem, 31, 1529–1531. [DOI] [PubMed] [Google Scholar]
- Freidmann B., Goodman E. H., Jr., Saunders H. L., Kostos V., Weinhouse S. (1971). An estimation of pyruvate recycling during gluconeogenesis in the perfused rat liver. Arch Biochem Biophys, 143, 566–578. [DOI] [PubMed] [Google Scholar]
- Fuchs B. C., Bode B. P. (2005). Amino acid transporters ASCT2 and LAT1 in cancer: Partners in crime? Semin Cancer Biol, 15, 254–266. [DOI] [PubMed] [Google Scholar]
- Gao X., Wang H., Yang J. J., Liu X., Liu Z. R. (2012). Pyruvate kinase M2 regulates gene transcription by acting as a protein kinase. Mol Cell, 45, 598–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaude E., Schmidt C., Gammage P. A., Dugourd A., Blacker T., Chew S. P., Saez-Rodriguez J., O'Neill J. S., Szabadkai G., Minczuk M., Frezza C. (2018). NADH shuttling couples cytosolic reductive carboxylation of glutamine with glycolysis in cells with mitochondrial dysfunction. Mol Cell, 69, 581–593.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhard D. S., et al. (2004). The status, quality, and expansion of the NIH full-length cDNA project: The Mammalian Gene Collection (MGC). Genome Res, 14, 2121–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies R. J., Robey I., Gatenby R. A. (2008). Causes and consequences of increased glucose metabolism of cancers. J Nucl Med, 49(Suppl 2), 24S–42S. [DOI] [PubMed] [Google Scholar]
- Gimenez-Roqueplo A. P., Burnichon N., Amar L., Favier J., Jeunemaitre X., Plouin P. F. (2008). Recent advances in the genetics of phaeochromocytoma and functional paraganglioma. Clin Exp Pharmacol Physiol, 35, 376–379. [DOI] [PubMed] [Google Scholar]
- Goodman R. P., Calvo S., Mootha V. K. (2018). Spatiotemporal compartmentalization of hepatic NADH and NADPH metabolism. J Biol Chem, 293, 7508–7516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberg A., Qu H., Bakken I. J., Sande L. M., White L. R., Haraldseth O., Unsgard G., Aasly J., Sonnewald U. (1998). In vitro and ex vivo 13C-NMR spectroscopy studies of pyruvate recycling in brain. Dev Neurosci, 20, 389–398. [DOI] [PubMed] [Google Scholar]
- Hague A., Singh B., Paraskeva C. (1997). Butyrate acts as a survival factor for colonic epithelial cells: Further fuel for the in vivo versus in vitro debate. Gastroenterology, 112, 1036–1040. [DOI] [PubMed] [Google Scholar]
- Hall A., Meyle K. D., Lange M. K., Klima M., Sanderhoff M., Dahl C., Abildgaard C., Thorup K., Moghimi S. M., Jensen P. B., Bartek J., Guldberg P., Christensen C. (2013). Dysfunctional oxidative phosphorylation makes malignant melanoma cells addicted to glycolysis driven by the (V600E)BRAF oncogene. Oncotarget, 4, 584–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochachka P. W., Owen T. G., Allen J. F., Whittow G. C. (1975). Multiple end products of anaerobiosis in diving vertebrates. Comp Biochem Physiol B, 50, 17–22. [DOI] [PubMed] [Google Scholar]
- Husain Z., Huang Y., Seth P., Sukhatme V. P. (2013). Tumor-derived lactate modifies antitumor immune response: Effect on myeloid-derived suppressor cells and NK cells. J Immunol, 191, 1486–1495. [DOI] [PubMed] [Google Scholar]
- Huysentruyt L. C., Akgoc Z., Seyfried T. N. (2011). Hypothesis: Are neoplastic macrophages/microglia present in glioblastoma multiforme? ASN Neuro, 3, pii: e00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Icard P., Lincet H. (2012). A global view of the biochemical pathways involved in the regulation of the metabolism of cancer cells. Biochim Biophys Acta, 1826, 423–433. [DOI] [PubMed] [Google Scholar]
- Janiszewska M., Suva M. L., Riggi N., Houtkooper R. H., Auwerx J., Clement-Schatlo V., Radovanovic I., Rheinbay E., Provero P., Stamenkovic I. (2012). Imp2 controls oxidative phosphorylation and is crucial for preserving glioblastoma cancer stem cells. Genes Dev, 26, 1926–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworski D. M., Namboodiri A. M., Moffett J. R. (2016). Acetate as a metabolic and epigenetic modifier of cancer therapy. J Cell Biochem, 117, 574–588. [DOI] [PubMed] [Google Scholar]
- Jia D., Park J. H., Jung K. H., Levine H., Kaipparettu B. A. (2018). Elucidating the metabolic plasticity of cancer: Mitochondrial reprogramming and hybrid metabolic states. Cells, 7, pii: E21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadrmas E. F., Ray P. D., Lambeth D. O. (1991). Apparent ATP-linked succinate thiokinase activity and its relation to nucleoside diphosphate kinase in mitochondrial matrix preparations from rabbit. Biochim Biophys Acta, 1074, 339–346. [DOI] [PubMed] [Google Scholar]
- Kaelin W. G., Jr.(2008). The von Hippel-Lindau tumour suppressor protein: O2 sensing and cancer. Nat Rev Cancer, 8, 865–873. [DOI] [PubMed] [Google Scholar]
- Kaipparettu B. A., Ma Y., Park J. H., Lee T. L., Zhang Y., Yotnda P., Creighton C. J., Chan W. Y., Wong L. J. (2013). Crosstalk from non-cancerous mitochondria can inhibit tumor properties of metastatic cells by suppressing oncogenic pathways. PLoS One, 8, e61747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsy M., Gelbman M., Shah P., Balumbu O., Moy F., Arslan E. (2012). Established and emerging variants of glioblastoma multiforme: Review of morphological and molecular features. Folia Neuropathol, 50, 301–321. [DOI] [PubMed] [Google Scholar]
- Katsetos C. D., Anni H., Draber P. (2013). Mitochondrial dysfunction in gliomas. Semin Pediatr Neurol, 20, 216–227. [DOI] [PubMed] [Google Scholar]
- Khazaei T., McGuigan A., Mahadevan R. (2012). Ensemble modeling of cancer metabolism. Front Physiol, 3, 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiebish M. A., Han X., Cheng H., Chuang J. H., Seyfried T. N. (2008). Cardiolipin and electron transport chain abnormalities in mouse brain tumor mitochondria: Lipidomic evidence supporting the Warburg theory of cancer. J Lipid Res, 49, 2545–2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiebish M. A., Han X., Cheng H., Seyfried T. N. (2009). In vitro growth environment produces lipidomic and electron transport chain abnormalities in mitochondria from non-tumorigenic astrocytes and brain tumours. ASN Neuro, 1, pii: e00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Han J., Jang Y., Kim S. J., Lee M. J., Ryu M. J., Kweon G. R., Heo J. Y. (2015). High-capacity glycolytic and mitochondrial oxidative metabolisms mediate the growth ability of glioblastoma. Int J Oncol, 47, 1009–1016. [DOI] [PubMed] [Google Scholar]
- King A., Selak M. A., Gottlieb E. (2006). Succinate dehydrogenase and fumarate hydratase: Linking mitochondrial dysfunction and cancer. Oncogene, 25, 4675–4682. [DOI] [PubMed] [Google Scholar]
- Kirsch W. M., Schulz Q., Van Buskirk J., Nakane P. (1972). Anaerobic energy metabolism in brain tumors. Prog Exp Tumor Res, 17, 163–191. [DOI] [PubMed] [Google Scholar]
- Kiselevsky Y. V., Ostrovtsova S. A., Strumilo S. A. (1990). Kinetic characterization of the pyruvate and oxoglutarate dehydrogenase complexes from human heart. Acta Biochim Pol, 37, 135–139. [PubMed] [Google Scholar]
- Kiss G., Konrad C., Doczi J., Starkov A. A., Kawamata H., Manfredi G., Zhang S. F., Gibson G. E., Beal M. F., Adam-Vizi V., Chinopoulos C. (2013). The negative impact of alpha-ketoglutarate dehydrogenase complex deficiency on matrix substrate-level phosphorylation. FASEB J, 27, 2392–2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss G., Konrad C., Pour-Ghaz I., Mansour J. J., Nemeth B., Starkov A. A., Adam-Vizi V., Chinopoulos C. (2014). Mitochondrial diaphorases as NAD(+) donors to segments of the citric acid cycle that support substrate-level phosphorylation yielding ATP during respiratory inhibition. FASEB J, 28, 1682–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleihues P., Ohgaki H. (1999). Primary and secondary glioblastomas: From concept to clinical diagnosis. Neuro Oncol, 1, 44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knott S. R. V., et al. (2018). Asparagine bioavailability governs metastasis in a model of breast cancer. Nature, 554, 378–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox W. E., Horowitz M. L., Friedell G. H. (1969). The proportionality of glutaminase content to growth rate and morphology of rat neoplasms. Cancer Res, 29, 669–680. [PubMed] [Google Scholar]
- Koukourakis M. I., Giatromanolaki A., Winter S., Leek R., Sivridis E., Harris A. L. (2009). Lactate dehydrogenase 5 expression in squamous cell head and neck cancer relates to prognosis following radical or postoperative radiotherapy. Oncology, 77, 285–292. [DOI] [PubMed] [Google Scholar]
- Kowluru A., Tannous M., Chen H. Q. (2002). Localization and characterization of the mitochondrial isoform of the nucleoside diphosphate kinase in the pancreatic beta cell: Evidence for its complexation with mitochondrial succinyl-CoA synthetase. Arch Biochem Biophys, 398, 160–169. [DOI] [PubMed] [Google Scholar]
- Krex D., Klink B., Hartmann C., von Deimling A., Pietsch T., Simon M., Sabel M., Steinbach J. P., Heese O., Reifenberger G., Weller M., Schackert G., German Glioma N. (2007). Long-term survival with glioblastoma multiforme. Brain, 130, 2596–2606. [DOI] [PubMed] [Google Scholar]
- Kunnecke B., Cerdan S., Seelig J. (1993). Cerebral metabolism of [1,2-13C2]glucose and [U-13C4]3-hydroxybutyrate in rat brain as detected by 13C NMR spectroscopy. NMR Biomed, 6, 264–277. [DOI] [PubMed] [Google Scholar]
- Kurelac I., Romeo G., Gasparre G. (2011). Mitochondrial metabolism and cancer. Mitochondrion, 11, 635–637. [DOI] [PubMed] [Google Scholar]
- Laurent C., Simoneau C., Marks L., Braschi S., Champ M., Charbonnel B., Krempf M. (1995). Effect of acetate and propionate on fasting hepatic glucose production in humans. Eur J Clin Nutr, 49, 484–491. [PubMed] [Google Scholar]
- Lawrence Y. R., Blumenthal D. T., Matceyevsky D., Kanner A. A., Bokstein F., Corn B. W. (2011). Delayed initiation of radiotherapy for glioblastoma: How important is it to push to the front (or the back) of the line? J Neurooncol, 105, 1–7. [DOI] [PubMed] [Google Scholar]
- Laws E. R., Jr., Goldberg W. J., Bernstein J. J. (1993). Migration of human malignant astrocytoma cells in the mammalian brain: Scherer revisited. Int J Dev Neurosci, 11, 691–697. [DOI] [PubMed] [Google Scholar]
- Le A., Lane A. N., Hamaker M., Bose S., Gouw A., Barbi J., Tsukamoto T., Rojas C. J., Slusher B. S., Zhang H., Zimmerman L. J., Liebler D. C., Slebos R. J., Lorkiewicz P. K., Higashi R. M., Fan T. W., Dang C. V. (2012). Glucose-independent glutamine metabolism via TCA cycling for proliferation and survival in B cells. Cell Metab, 15, 110–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D. H., Lee T. H., Jung C. H., Kim Y. H. (2012). Wogonin induces apoptosis by activating the AMPK and p53 signaling pathways in human glioblastoma cells. Cell Signal, 24, 2216–2225. [DOI] [PubMed] [Google Scholar]
- Lehninger A. L. (1964). The mitochondrion: Molecular basis of structure and function. New York, NY: W.A. Benjamin, Inc. [Google Scholar]
- Lemberg K. M., Vornov J. J., Rais R., Slusher B. S. (2018). We’re not “DON” yet: Optimal dosing and prodrug delivery of 6-Diazo-5-oxo-L-norleucine. Mol Cancer Ther, 17, 1824–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leznev E. I., Popova I. I., Lavrovskaja V. P., Evtodienko Y. V. (2013). Comparison of oxygen consumption rates in minimally transformed BALB/3T3 and virus-transformed 3T3B-SV40 cells. Biochemistry (Mosc), 78, 904–908. [DOI] [PubMed] [Google Scholar]
- Li J., Zhu S., Tong J., Hao H., Yang J., Liu Z., Wang Y. (2016). Suppression of lactate dehydrogenase A compromises tumor progression by downregulation of the Warburg effect in glioblastoma. Neuroreport, 27, 110–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Wu F., Beard D. A. (2013). Identification of the kinetic mechanism of succinyl-CoA synthetase. Biosci Rep, 33, 145–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtor T., Dohrmann G. J. (1986). Respiratory patterns in human brain tumors. Neurosurgery, 19, 896–899. [DOI] [PubMed] [Google Scholar]
- Lin Y. C., Hung C. M., Tsai J. C., Lee J. C., Chen Y. L., Wei C. W., Kao J. Y., Way T. D. (2010). Hispidulin potently inhibits human glioblastoma multiforme cells through activation of AMP-activated protein kinase (AMPK). J Agric Food Chem, 58, 9511–9517. [DOI] [PubMed] [Google Scholar]
- Liu J., Guo S., Li Q., Yang L., Xia Z., Zhang L., Huang Z., Zhang N. (2013). Phosphoglycerate dehydrogenase induces glioma cells proliferation and invasion by stabilizing forkhead box M1. J Neurooncol, 111, 245–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Wang J., Li Y., Fan J., Chen L., Xu R. (2017). MicroRNA-153 regulates glutamine metabolism in glioblastoma through targeting glutaminase. Tumour Biol, 39, 1010428317691429. [DOI] [PubMed] [Google Scholar]
- Luo W., Hu H., Chang R., Zhong J., Knabel M., O'Meally R., Cole R. N., Pandey A., Semenza G. L. (2011). Pyruvate kinase M2 is a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell, 145, 732–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin-Valencia I., et al. (2012). Analysis of tumor metabolism reveals mitochondrial glucose oxidation in genetically diverse human glioblastomas in the mouse brain in vivo. Cell Metab, 15, 827–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markert C. L., Shaklee J. B., Whitt G. S. (1975). Evolution of a gene. Multiple genes for LDH isozymes provide a model of the evolution of gene structure, function and regulation. Science, 189, 102–114. [DOI] [PubMed] [Google Scholar]
- Marsh J., Mukherjee P., Seyfried T. N. (2008). Akt-dependent proapoptotic effects of dietary restriction on late-stage management of a phosphatase and tensin homologue/tuberous sclerosis complex 2-deficient mouse astrocytoma. Clin Cancer Res, 14, 7751–7762. [DOI] [PubMed] [Google Scholar]
- Martincorena I., Fowler J. C., Wabik A., Lawson A. R. J., Abascal F., Hall M. W. J., Cagan A., Murai K., Mahbubani K., Stratton M. R., Fitzgerald R. C., Handford P. A., Campbell P. J., Saeb-Parsy K., Jones P. H. (2018). Somatic mutant clones colonize the human esophagus with age. Science. Advance online publication. doi:10.1126/science.aau3879 [DOI] [PMC free article] [PubMed]
- Mashimo T., Pichumani K., Vemireddy V., Hatanpaa K. J., Singh D. K., Sirasanagandla S., Nannepaga S., Piccirillo S. G., Kovacs Z., Foong C., Huang Z., Barnett S., Mickey B. E., DeBerardinis R. J., Tu B. P., Maher E. A., Bachoo R. M. (2014). Acetate is a bioenergetic substrate for human glioblastoma and brain metastases. Cell, 159, 1603–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews E. H., Stander B. A., Joubert A. M., Liebenberg L. (2014). Tumor cell culture survival following glucose and glutamine deprivation at typical physiological concentrations. Nutrition, 30, 218–227. [DOI] [PubMed] [Google Scholar]
- Maurer G. D., Brucker D. P., Bahr O., Harter P. N., Hattingen E., Walenta S., Mueller-Klieser W., Steinbach J. P., Rieger J. (2011). Differential utilization of ketone bodies by neurons and glioma cell lines: A rationale for ketogenic diet as experimental glioma therapy. BMC Cancer, 11, 315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maus A., Peters G. J. (2017). Glutamate and alpha-ketoglutarate: Key players in glioma metabolism. Amino Acids, 49, 21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maximchik P. V., Kulikov A. V., Zhivotovsky B. D., Gogvadze V. G. (2016). Cellular energetics as a target for tumor cell elimination. Biochemistry (Mosc), 81, 65–79. [DOI] [PubMed] [Google Scholar]
- Mayer A., Vaupel P., Struss H. G., Giese A., Stockinger M., Schmidberger H. (2014). Strong adverse prognostic impact of hyperglycemic episodes during adjuvant chemoradiotherapy of glioblastoma multiforme. Strahlenther Onkol, 190, 933–938. [DOI] [PubMed] [Google Scholar]
- Mazurek S., Boschek C. B., Hugo F., Eigenbrodt E. (2005). Pyruvate kinase type M2 and its role in tumor growth and spreading. Semin Cancer Biol, 15, 300–308. [DOI] [PubMed] [Google Scholar]
- McClure W. R., Lardy H. A., Kneifel H. P. (1971. a). Rat liver pyruvate carboxylase. I. Preparation, properties, and cation specificity. J Biol Chem, 246, 3569–3578. [PubMed] [Google Scholar]
- McClure W. R., Lardy H. A., Wagner M., Cleland W. W. (1971. b). Rat liver pyruvate carboxylase. II. Kinetic studies of the forward reaction. J Biol Chem, 246, 3579–3583. [PubMed] [Google Scholar]
- McCoy G. D., Doeg K. A. (1975). Characterization of the phosphoenolpyruvate inhibition of mitochondrial protein synthesis. J Biol Chem, 250, 3510–3514. [PubMed] [Google Scholar]
- McGirt M. J., Chaichana K. L., Gathinji M., Attenello F., Than K., Ruiz A. J., Olivi A., Quinones-Hinojosa A. (2008). Persistent outpatient hyperglycemia is independently associated with decreased survival after primary resection of malignant brain astrocytomas. Neurosurgery, 63, 286–291. [DOI] [PubMed] [Google Scholar]
- Medina M. A. (2001). Glutamine and cancer. J Nutr, 131, 2539S–2542S. [DOI] [PubMed] [Google Scholar]
- Meiser J., Vazquez A. (2016). Give it or take it: The flux of one-carbon in cancer cells. FEBS J, 283, 3695–3704. [DOI] [PubMed] [Google Scholar]
- Michelakis E. D., Webster L., Mackey J. R. (2008). Dichloroacetate (DCA) as a potential metabolic-targeting therapy for cancer. Br J Cancer, 99, 989–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morantz R. A., Wood G. W., Foster M., Clark M., Gollahon K. (1979). Macrophages in experimental and human brain tumors. Part 2: Studies of the macrophage content of human brain tumors. J Neurosurg, 50, 305–311. [DOI] [PubMed] [Google Scholar]
- Moreadith R. W., Lehninger A. L. (1984). The pathways of glutamate and glutamine oxidation by tumor cell mitochondria. Role of mitochondrial NAD(P)+-dependent malic enzyme. J Biol Chem, 259, 6215–6221. [PubMed] [Google Scholar]
- Moreno-Sanchez R., Rodriguez-Enriquez S., Saavedra E., Marin-Hernandez A., Gallardo-Perez J. C. (2009). The bioenergetics of cancer: Is glycolysis the main ATP supplier in all tumor cells? Biofactors, 35, 209–225. [DOI] [PubMed] [Google Scholar]
- Mukherjee P., Abate L. E., Seyfried T. N. (2004). Antiangiogenic and proapoptotic effects of dietary restriction on experimental mouse and human brain tumors. Clin Cancer Res, 10, 5622–5629. [DOI] [PubMed] [Google Scholar]
- Mukherjee P., El-Abbadi M. M., Kasperzyk J. L., Ranes M. K., Seyfried T. N. (2002). Dietary restriction reduces angiogenesis and growth in an orthotopic mouse brain tumour model. Br J Cancer, 86, 1615–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulrooney T. J., Marsh J., Urits I., Seyfried T. N., Mukherjee P. (2011). Influence of caloric restriction on constitutive expression of NF-kappaB in an experimental mouse astrocytoma. PLoS One, 6, e18085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsholme E. A., Board M. (1991). Application of metabolic-control logic to fuel utilization and its significance in tumor cells. Adv Enzyme Regul, 31, 225–246. [DOI] [PubMed] [Google Scholar]
- Newsholme P. (2001). Why is L-glutamine metabolism important to cells of the immune system in health, postinjury, surgery or infection? J Nutr, 131, 2515S–2522S. [DOI] [PubMed] [Google Scholar]
- Nishioka M., Bundo M., Iwamoto K., Kato T. (2018). Somatic mutations in the human brain: Implications for psychiatric research. Mol Psychiatry. Advance online publication. doi:10.1038/s41380-018-0129-y [DOI] [PMC free article] [PubMed]
- Nortley R., Attwell D. (2017). Control of brain energy supply by astrocytes. Curr Opin Neurobiol, 47, 80–85. [DOI] [PubMed] [Google Scholar]
- Ochoa S. (1948). Biosynthesis of tricarboxylic acids by carbon dioxide fixation; enzymatic mechanisms. J Biol Chem, 174, 133–157. [PubMed] [Google Scholar]
- Ohka F., Ito M., Ranjit M., Senga T., Motomura A., Motomura K., Saito K., Kato K., Kato Y., Wakabayashi T., Soga T., Natsume A. (2014). Quantitative metabolome analysis profiles activation of glutaminolysis in glioma with IDH1 mutation. Tumour Biol, 35, 5911–5920. [DOI] [PubMed] [Google Scholar]
- Oizel K., Chauvin C., Oliver L., Gratas C., Geraldo F., Jarry U., Scotet E., Rabe M., Alves-Guerra M. C., Teusan R., Gautier F., Loussouarn D., Compan V., Martinou J. C., Vallette F. M., Pecqueur C. (2017). Efficient mitochondrial glutamine targeting prevails over glioblastoma metabolic plasticity. Clin Cancer Res, 23, 6292–6304. [DOI] [PubMed] [Google Scholar]
- Ooi A., Wong J. C., Petillo D., Roossien D., Perrier-Trudova V., Whitten D., Min B. W., Tan M. H., Zhang Z., Yang X. J., Zhou M., Gardie B., Molinie V., Richard S., Tan P. H., Teh B. T., Furge K. A. (2011). An antioxidant response phenotype shared between hereditary and sporadic type 2 papillary renal cell carcinoma. Cancer Cell, 20, 511–523. [DOI] [PubMed] [Google Scholar]
- Ota T., et al. (2004). Complete sequencing and characterization of 21,243 full-length human cDNAs. Nat Genet, 36, 40–45. [DOI] [PubMed] [Google Scholar]
- Ottaway J. H., McClellan J. A., Saunderson C. L. (1981). Succinic thiokinase and metabolic control. Int J Biochem, 13, 401–410. [DOI] [PubMed] [Google Scholar]
- Oudard S., Boitier E., Miccoli L., Rousset S., Dutrillaux B., Poupon M. F. (1997). Gliomas are driven by glycolysis: Putative roles of hexokinase, oxidative phosphorylation and mitochondrial ultrastructure. Anticancer Res, 17, 1903–1911. [PubMed] [Google Scholar]
- Pacini N., Borziani F. (2016). Oncostatic-cytoprotective effect of melatonin and other bioactive molecules: A common target in mitochondrial respiration. Int J Mol Sci, 17, 341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pall M. L. (1985). GTP: A central regulator of cellular anabolism. Curr Top Cell Regul, 25, 1–20. [DOI] [PubMed] [Google Scholar]
- Panosyan E. H., Lasky J. L., Lin H. J., Lai A., Hai Y., Guo X., Quinn M., Nelson S. F., Cloughesy T. F., Nghiemphu P. L. (2016). Clinical aggressiveness of malignant gliomas is linked to augmented metabolism of amino acids. J Neurooncol, 128, 57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panosyan E. H., Lin H. J., Koster J., Lasky J. L., III. (2017). In search of druggable targets for GBM amino acid metabolism. BMC Cancer, 17, 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papandreou I., Cairns R. A., Fontana L., Lim A. L., Denko N. C. (2006). HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab, 3, 187–197. [DOI] [PubMed] [Google Scholar]
- Parks S. K., Chiche J., Pouyssegur J. (2013). Disrupting proton dynamics and energy metabolism for cancer therapy. Nat Rev Cancer, 13, 611–623. [DOI] [PubMed] [Google Scholar]
- Parry-Billings M., Leighton B., Dimitriadis G. D., Curi R., Bond J., Bevan S., Colquhoun A., Newsholme E. A. (1991). The effect of tumour bearing on skeletal muscle glutamine metabolism. Int J Biochem, 23, 933–937. [DOI] [PubMed] [Google Scholar]
- Pascual G., Avgustinova A., Mejetta S., Martin M., Castellanos A., Attolini C. S., Berenguer A., Prats N., Toll A., Hueto J. A., Bescos C., Di Croce L., Benitah S. A. (2017). Targeting metastasis-initiating cells through the fatty acid receptor CD36. Nature, 541, 41–45. [DOI] [PubMed] [Google Scholar]
- Perez-Gomez C., Campos-Sandoval J. A., Alonso F. J., Segura J. A., Manzanares E., Ruiz-Sanchez P., Gonzalez M. E., Marquez J., Mates J. M. (2005). Co-expression of glutaminase K and L isoenzymes in human tumour cells. Biochem J, 386, 535–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry J. R., et al. (2017). Short-course radiation plus temozolomide in elderly patients with glioblastoma. N Engl J Med, 376, 1027–1037. [DOI] [PubMed] [Google Scholar]
- Phillips D., Aponte A. M., French S. A., Chess D. J., Balaban R. S. (2009). Succinyl-CoA synthetase is a phosphate target for the activation of mitochondrial metabolism. Biochemistry, 48, 7140–7149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike L. S., Smift A. L., Croteau N. J., Ferrick D. A., Wu M. (2011). Inhibition of fatty acid oxidation by etomoxir impairs NADPH production and increases reactive oxygen species resulting in ATP depletion and cell death in human glioblastoma cells. Biochim Biophys Acta, 1807, 726–734. [DOI] [PubMed] [Google Scholar]
- Pike Winer L. S., Wu M. (2014). Rapid analysis of glycolytic and oxidative substrate flux of cancer cells in a microplate. PLoS One, 9, e109916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plas D. R., Thompson C. B. (2005). Akt-dependent transformation: There is more to growth than just surviving. Oncogene, 24, 7435–7442. [DOI] [PubMed] [Google Scholar]
- Portais J. C., Schuster R., Merle M., Canioni P. (1993). Metabolic flux determination in C6 glioma cells using carbon-13 distribution upon [1-13C]glucose incubation. Eur J Biochem, 217, 457–468. [DOI] [PubMed] [Google Scholar]
- Possemato R., et al. (2011). Functional genomics reveal that the serine synthesis pathway is essential in breast cancer. Nature, 476, 346–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poteet E., Choudhury G. R., Winters A., Li W., Ryou M. G., Liu R., Tang L., Ghorpade A., Wen Y., Yuan F., Keir S. T., Yan H., Bigner D. D., Simpkins J. W., Yang S. H. (2013). Reversing the Warburg effect as a treatment for glioblastoma. J Biol Chem, 288, 9153–9164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu W., Oya S., Lieberman B. P., Ploessl K., Wang L., Wise D. R., Divgi C. R., Chodosh L. A., Thompson C. B., Kung H. F. (2012). Preparation and characterization of L-[5-11C]-glutamine for metabolic imaging of tumors. J Nucl Med, 53, 98–105. [DOI] [PubMed] [Google Scholar]
- Racker E. (1974). History of the Pasteur effect and its pathobiology. Mol Cell Biochem, 5, 17–23. [DOI] [PubMed] [Google Scholar]
- Ramanathan A., Wang C., Schreiber S. L. (2005). Perturbational profiling of a cell-line model of tumorigenesis by using metabolic measurements. Proc Natl Acad Sci U S A, 102, 5992–5997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravasz D., Kacso G., Fodor V., Horvath K., Adam-Vizi V., Chinopoulos C. (2018). Reduction of 2-methoxy-1,4-naphtoquinone by mitochondrially-localized Nqo1 yielding NAD(+) supports substrate-level phosphorylation during respiratory inhibition. Biochim Biophys Acta, 1859, 909–924. [DOI] [PubMed] [Google Scholar]
- Reitzer L. J., Wice B. M., Kennell D. (1979). Evidence that glutamine, not sugar, is the major energy source for cultured HeLa cells. J Biol Chem, 254, 2669–2676. [PubMed] [Google Scholar]
- Rhodes C. G., Wise R. J., Gibbs J. M., Frackowiak R. S., Hatazawa J., Palmer A. J., Thomas D. G., Jones T. (1983). In vivo disturbance of the oxidative metabolism of glucose in human cerebral gliomas. Ann Neurol, 14, 614–626. [DOI] [PubMed] [Google Scholar]
- Ria F., Landriscina M., Remiddi F., Rosselli R., Iacoangeli M., Scerrati M., Pani G., Borrello S., Galeotti T. (2001). The level of manganese superoxide dismutase content is an independent prognostic factor for glioblastoma. Biological mechanisms and clinical implications. Br J Cancer, 84, 529–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roggendorf W., Strupp S., Paulus W. (1996). Distribution and characterization of microglia/macrophages in human brain tumors. Acta Neuropathol, 92, 288–293. [DOI] [PubMed] [Google Scholar]
- Rognstad R., Katz J. (1972). Gluconeogenesis in the kidney cortex. Quantitative estimation of carbon flow. J Biol Chem, 247, 6047–6054. [PubMed] [Google Scholar]
- Roslin M., Henriksson R., Bergstrom P., Ungerstedt U., Bergenheim A. T. (2003). Baseline levels of glucose metabolites, glutamate and glycerol in malignant glioma assessed by stereotactic microdialysis. J Neurooncol, 61, 151–160. [DOI] [PubMed] [Google Scholar]
- Rossignol R., Gilkerson R., Aggeler R., Yamagata K., Remington S. J., Capaldi R. A. (2004). Energy substrate modulates mitochondrial structure and oxidative capacity in cancer cells. Cancer Res, 64, 985–993. [DOI] [PubMed] [Google Scholar]
- Rubinstein L. J. (1972). Tumors of the central nervous system. Washington, DC: Armed Forces Institute of Pathology. [Google Scholar]
- Samudio I., Fiegl M., Andreeff M. (2009). Mitochondrial uncoupling and the Warburg effect: Molecular basis for the reprogramming of cancer cell metabolism. Cancer Res, 69, 2163–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Arago M., Formentini L., Martinez-Reyes I., Garcia-Bermudez J., Santacatterina F., Sanchez-Cenizo L., Willers I. M., Aldea M., Najera L., Juarranz A., Lopez E. C., Clofent J., Navarro C., Espinosa E., Cuezva J. M. (2013). Expression, regulation and clinical relevance of the ATPase inhibitory factor 1 in human cancers. Oncogenesis, 2, e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H., Tamba M., Ishii T., Bannai S. (1999). Cloning and expression of a plasma membrane cystine/glutamate exchange transporter composed of two distinct proteins. J Biol Chem, 274, 11455–11458. [DOI] [PubMed] [Google Scholar]
- Scheithauer B. W., Bruner J. M. (1987). The ultrastructural spectrum of astrocytic neoplasms. Ultrastruct Pathol, 11, 535–581. [DOI] [PubMed] [Google Scholar]
- Scherer H. J. (1940). A critical review: The pathology of cerebral gliomas. J Neurol Psychiatry, 3, 147–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlattner U., Tokarska-Schlattner M., Ramirez S., Tyurina Y. Y., Amoscato A. A., Mohammadyani D., Huang Z., Jiang J., Yanamala N., Seffouh A., Boissan M., Epand R. F., Epand R. M., Klein-Seetharaman J., Lacombe M. L., Kagan V. E. (2013). Dual function of mitochondrial Nm23-H4 protein in phosphotransfer and intermembrane lipid transfer: A cardiolipin-dependent switch. J Biol Chem, 288, 111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selak M. A., Armour S. M., MacKenzie E. D., Boulahbel H., Watson D. G., Mansfield K. D., Pan Y., Simon M. C., Thompson C. B., Gottlieb E. (2005). Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell, 7, 77–85. [DOI] [PubMed] [Google Scholar]
- Semenza G. L. (2010). HIF-1: Upstream and downstream of cancer metabolism. Curr Opin Genet Dev, 20, 51–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza G. L. (2017). Hypoxia-inducible factors: Coupling glucose metabolism and redox regulation with induction of the breast cancer stem cell phenotype. EMBO J, 36, 252–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyfried T. N. (2001). Perspectives on brain tumor formation involving macrophages, glia, and neural stem cells. Perspect Biol Med, 44, 263–282. [DOI] [PubMed] [Google Scholar]
- Seyfried T. N. (2012. a). Nothing in cancer biology makes sense except in the light of evolution. In Cancer as a metabolic disease: On the origin, management, and prevention of cancer (pp. 261–275). Hoboken, NJ: John Wiley & Sons, Inc.
- Seyfried T. N. (2012. b). Cancer as a metabolic disease: On the origin, management, and prevention of cancer. In Cancer as a metabolic disease (pp. 133–144). Hoboken, NJ: John Wiley & Sons, Inc.
- Seyfried T. N. (2012. c). Cancer as a metabolic disease: On the origin, management and prevention of cancer. Hoboken, NJ: John Wiley & Sons, Inc. [Google Scholar]
- Seyfried T. N. (2012. d). Is mitochondrial glutamine fermentation a missing link in the metabolic theory of cancer? In Cancer as a metabolic disease: On the origin, management, and prevention of cancer (pp. 133–144). Hoboken, NJ: John Wiley & Sons, Inc.
- Seyfried T. N. (2012. e). Mitochondria: The ultimate tumor suppressor. In Cancer as a metabolic disease (pp. 195–205). Hoboken, NJ: John Wiley & Sons.
- Seyfried T. N. (2015). Cancer as a mitochondrial metabolic disease. Front Cell Dev Biol, 3, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyfried T. N., Flores R., Poff A. M., D'Agostino D. P., Mukherjee P. (2015). Metabolic therapy: A new paradigm for managing malignant brain cancer. Cancer Lett, 356, 289–300. [DOI] [PubMed] [Google Scholar]
- Seyfried T. N., Flores R. E., Poff A. M., D'Agostino D. P. (2014). Cancer as a metabolic disease: Implications for novel therapeutics. Carcinogenesis, 35, 515–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyfried T. N., Mukherjee P. (2005). Targeting energy metabolism in brain cancer: Review and hypothesis. Nutr Metab (Lond), 2, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyfried T. N., Sanderson T. M., El-Abbadi M. M., McGowan R., Mukherjee P. (2003). Role of glucose and ketone bodies in the metabolic control of experimental brain cancer. Br J Cancer, 89, 1375–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyfried T. N., Shelton L. M. (2010). Cancer as a metabolic disease. Nutr Metab (Lond), 7, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyfried T. N., Yu G., Maroon J. C., D'Agostino D. P. (2017). Press-pulse: A novel therapeutic strategy for the metabolic management of cancer. Nutr Metab (Lond), 14, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton L. M. (2010). Targeting energy metabolism in brain cancer. Doctoral Thesis (p. 228). O'Neil Library, Boston College, Chestnut Hill, MA.
- Shelton L. M., Huysentruyt L. C., Mukherjee P., Seyfried T. N. (2010. a). Calorie restriction as an anti-invasive therapy for malignant brain cancer in the VM mouse. ASN Neuro, 2, e00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton L. M., Huysentruyt L. C., Seyfried T. N. (2010. b). Glutamine targeting inhibits systemic metastasis in the VM-M3 murine tumor model. Inter J Cancer, 127, 2478–2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton L. M., Mukherjee P., Huysentruyt L. C., Urits I., Rosenberg J. A., Seyfried T. N. (2010. c). A novel pre-clinical in vivo mouse model for malignant brain tumor growth and invasion. J Neurooncol, 99, 165–176. [DOI] [PubMed] [Google Scholar]
- Sheu K. F., Blass J. P. (1999). The alpha-ketoglutarate dehydrogenase complex. Ann NY Acad Sci, 893, 61–78. [DOI] [PubMed] [Google Scholar]
- Shwetha S. D., Shastry A. H., Arivazhagan A., Santosh V. (2016). Manganese superoxide dismutase (MnSOD) is a malignant astrocytoma specific biomarker and associated with adverse prognosis in p53 expressing glioblastoma. Pathol Res Pract, 212, 17–23. [DOI] [PubMed] [Google Scholar]
- Sidoryk M., Matyja E., Dybel A., Zielinska M., Bogucki J., Jaskolski D. J., Liberski P. P., Kowalczyk P., Albrecht J. (2004). Increased expression of a glutamine transporter SNAT3 is a marker of malignant gliomas. Neuroreport, 15, 575–578. [DOI] [PubMed] [Google Scholar]
- Simek J., Sedlacek J. (1965). Effect of glucose administered in vivo or in vitro on the respiratory quotient of rat liver tissue after partial hepatectomy. Nature, 207, 761–762. [DOI] [PubMed] [Google Scholar]
- Sipe J. C., Herman M. M., Rubinstein L. J. (1973). Electron microscopic observations on human glioblastomas and astrocytomas maintained in organ culture systems. Am J Pathol, 73, 589–606. [PMC free article] [PubMed] [Google Scholar]
- Skarnes W. C., Rosen B., West A. P., Koutsourakis M., Bushell W., Iyer V., Mujica A. O., Thomas M., Harrow J., Cox T., Jackson D., Severin J., Biggs P., Fu J., Nefedov M., de Jong P. J., Stewart A. F., Bradley A. (2011). A conditional knockout resource for the genome-wide study of mouse gene function. Nature, 474, 337–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder N. W., Basu S. S., Worth A. J., Mesaros C., Blair I. A. (2015). Metabolism of propionic acid to a novel acyl-coenzyme A thioester by mammalian cell lines and platelets. J Lipid Res, 56, 142–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeda A., Hara A., Kunisada T., Yoshimura S., Iwama T., Park D. M. (2015). The evidence of glioblastoma heterogeneity. Sci Rep, 5, 7979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solaini G., Sgarbi G., Baracca A. (2011). Oxidative phosphorylation in cancer cells. Biochim Biophys Acta, 1807, 534–542. [DOI] [PubMed] [Google Scholar]
- Sonnenschein C., Soto A. M. (2000). Somatic mutation theory of carcinogenesis: Why it should be dropped and replaced. Mol Carcinog, 29, 205–211. [DOI] [PubMed] [Google Scholar]
- Souba W. W. (1993). Glutamine and cancer. Ann Surg, 218, 715–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stryer L. (1995). Biochemistry. New York, NY: W. H. Freeman & Company. [Google Scholar]
- Stupp R., et al. (2009). Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol, 10, 459–466. [DOI] [PubMed] [Google Scholar]
- Suarez R. K., Lighton J. R., Brown G. S., Mathieu-Costello O. (1991). Mitochondrial respiration in hummingbird flight muscles. Proc Natl Acad Sci U S A, 88, 4870–4873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan L. B., Martinez-Garcia E., Nguyen H., Mullen A. R., Dufour E., Sudarshan S., Licht J. D., DeBerardinis R. J., Chandel N. S. (2013). The proto-oncometabolite fumarate binds glutathione to amplify ROS-dependent signaling. Mol Cell, 51, 236–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suomalainen A., Isohanni P. (2010). Mitochondrial DNA depletion syndromes – Many genes, common mechanisms. Neuromuscul Disord, 20, 429–437. [DOI] [PubMed] [Google Scholar]
- Swietach P., Vaughan-Jones R. D., Harris A. L., Hulikova A. (2014). The chemistry, physiology and pathology of pH in cancer. Philos Trans R Soc Lond B Biol Sci, 369, 20130099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinnen J. V., Brusselmans K., Verhoeven G. (2006). Increased lipogenesis in cancer cells: New players, novel targets. Curr Opin Clin Nutr Metab Care, 9, 358–365. [DOI] [PubMed] [Google Scholar]
- Szent-Gyorgyi A. (1977). The living state and cancer. Proc Natl Acad Sci U S A, 74, 2844–2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ta N. L., Seyfried T. N. (2015). Influence of serum and hypoxia on incorporation of [(14)C]-D-glucose or [(14)C]-L-glutamine into lipids and lactate in murine glioblastoma cells. Lipids, 50, 1167–1184. [DOI] [PubMed] [Google Scholar]
- Takano T., Lin J. H., Arcuino G., Gao Q., Yang J., Nedergaard M. (2001). Glutamate release promotes growth of malignant gliomas. Nat Med, 7, 1010–1015. [DOI] [PubMed] [Google Scholar]
- Tannahill G. M., et al. (2013). Succinate is an inflammatory signal that induces IL-1beta through HIF-1alpha. Nature, 496, 238–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taphoorn M.J., Stupp R., Coens C., Osoba D., Kortmann R., van den Bent M. J., Mason W., Mirimanoff R. O., Baumert B. G., Eisenhauer E., Forsyth P., Bottomley A., European Organisation for Research and Treatment of Cancer Brain Tumour Group, EORTC Radiotherapy Group, & National Cancer Institute of Canada Clinical Trials Group. (2005). Health-related quality of life in patients with glioblastoma: A randomised controlled trial. Lancet Oncol, 6, 937–944. [DOI] [PubMed] [Google Scholar]
- Tardito S., Oudin A., Ahmed S. U., Fack F., Keunen O., Zheng L., Miletic H., Sakariassen P. O., Weinstock A., Wagner A., Lindsay S. L., Hock A. K., Barnett S. C., Ruppin E., Morkve S. H., Lund-Johansen M., Chalmers A. J., Bjerkvig R., Niclou S. P., Gottlieb E. (2015). Glutamine synthetase activity fuels nucleotide biosynthesis and supports growth of glutamine-restricted glioblastoma. Nat Cell Biol, 17, 1556–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ternette N., Yang M., Laroyia M., Kitagawa M., O'Flaherty L., Wolhulter K., Igarashi K., Saito K., Kato K., Fischer R., Berquand A., Kessler B. M., Lappin T., Frizzell N., Soga T., Adam J., Pollard P. J. (2013). Inhibition of mitochondrial aconitase by succination in fumarate hydratase deficiency. Cell Rep, 3, 689–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thevananther S. (2010). Adipose to the rescue: Peripheral fat fuels liver regeneration. Hepatology, 52, 1875–1876. [DOI] [PubMed] [Google Scholar]
- Thomson M. (1998). What are guanosine triphosphate-binding proteins doing in mitochondria? Biochim Biophys Acta, 1403, 211–218. [DOI] [PubMed] [Google Scholar]
- Tieu M. T., Lovblom L. E., McNamara M. G., Mason W., Laperriere N., Millar B. A., Menard C., Kiehl T. R., Perkins B. A., Chung C. (2015). Impact of glycemia on survival of glioblastoma patients treated with radiation and temozolomide. J Neurooncol, 124, 119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todesco T., Rao A. V., Bosello O., Jenkins D. J. (1991). Propionate lowers blood glucose and alters lipid metabolism in healthy subjects. Am J Clin Nutr, 54, 860–865. [DOI] [PubMed] [Google Scholar]
- Tretter L., Patocs A., Chinopoulos C. (2016). Succinate, an intermediate in metabolism, signal transduction, ROS, hypoxia, and tumorigenesis. Biochim Biophys Acta, 1857, 1086–1101. [DOI] [PubMed] [Google Scholar]
- Urits I., Mukherjee P., Meidenbauer J., Seyfried T. N. (2012). Dietary restriction promotes vessel maturation in a mouse astrocytoma. J Oncology, 264039, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valle A., Oliver J., Roca P. (2010). Role of uncoupling proteins in cancer. Cancers (Basel), 2, 567–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Heiden M. G., Cantley L. C., Thompson C. B. (2009). Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science, 324, 1029–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Heiden M. G., Locasale J. W., Swanson K. D., Sharfi H., Heffron G. J., Amador-Noguez D., Christofk H. R., Wagner G., Rabinowitz J. D., Asara J. M., Cantley L. C. (2010). Evidence for an alternative glycolytic pathway in rapidly proliferating cells. Science, 329, 1492–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veech R. L. (2004). The therapeutic implications of ketone bodies: The effects of ketone bodies in pathological conditions: Ketosis, ketogenic diet, redox states, insulin resistance, and mitochondrial metabolism. Prostaglandins Leukot Essent Fatty Acids, 70, 309–319. [DOI] [PubMed] [Google Scholar]
- Venter J. C., et al. (2001). The sequence of the human genome. Science, 291, 1304–1351. [DOI] [PubMed] [Google Scholar]
- Vergati M., Krasniqi E., Monte G. D., Riondino S., Vallone D., Guadagni F., Ferroni P., Roselli M. (2017). Ketogenic diet and other dietary intervention strategies in the treatment of cancer. Curr Med Chem, 24, 1170–1185. [DOI] [PubMed] [Google Scholar]
- Vozza A., Parisi G., De Leonardis F., Lasorsa F. M., Castegna A., Amorese D., Marmo R., Calcagnile V. M., Palmieri L., Ricquier D., Paradies E., Scarcia P., Palmieri F., Bouillaud F., Fiermonte G. (2014). UCP2 transports C4 metabolites out of mitochondria, regulating glucose and glutamine oxidation. Proc Natl Acad Sci U S A, 111, 960–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakil S. J., Porter J. W., Gibson D. M. (1957). Studies on the mechanism of fatty acid synthesis. I. Preparation and purification of an enzymes system for reconstruction of fatty acid synthesis. Biochim Biophys Acta, 24, 453–461. [DOI] [PubMed] [Google Scholar]
- Wang J. B., Erickson J. W., Fuji R., Ramachandran S., Gao P., Dinavahi R., Wilson K. F., Ambrosio A. L., Dias S. M., Dang C. V., Cerione R. A. (2010). Targeting mitochondrial glutaminase activity inhibits oncogenic transformation. Cancer Cell, 18, 207–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. Q., Wang H. L., Xu J., Tan J., Fu L. N., Wang J. L., Zou T. H., Sun D. F., Gao Q. Y., Chen Y. X., Fang J. Y. (2018). Sirtuin5 contributes to colorectal carcinogenesis by enhancing glutaminolysis in a deglutarylation-dependent manner. Nat Commun, 9, 545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburg O. (1956. a). On the origin of cancer cells. Science, 123, 309–314. [DOI] [PubMed] [Google Scholar]
- Warburg O. (1956. b). On the respiratory impairment in cancer cells. Science, 124, 269–270. [PubMed] [Google Scholar]
- Ward P. S., Thompson C. B. (2012). Metabolic reprogramming: A cancer hallmark even Warburg did not anticipate. Cancer Cell, 21, 297–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg F., Hamanaka R., Wheaton W. W., Weinberg S., Joseph J., Lopez M., Kalyanaraman B., Mutlu G. M., Budinger G. R., Chandel N. S. (2010). Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc Natl Acad Sci U S A, 107, 8788–8793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinert B. T., Scholz C., Wagner S. A., Iesmantavicius V., Su D., Daniel J. A., Choudhary C. (2013). Lysine succinylation is a frequently occurring modification in prokaryotes and eukaryotes and extensively overlaps with acetylation. Cell Rep, 4, 842–851. [DOI] [PubMed] [Google Scholar]
- Wise D. R., DeBerardinis R. J., Mancuso A., Sayed N., Zhang X. Y., Pfeiffer H. K., Nissim I., Daikhin E., Yudkoff M., McMahon S. B, Thompson C. B. (2008). Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc Natl Acad Sci U S A, 105, 18782–18787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise D. R., Ward P. S., Shay J. E., Cross J. R., Gruber J. J., Sachdeva U. M., Platt J. M., DeMatteo R. G., Simon M. C., Thompson C. B. (2011). Hypoxia promotes isocitrate dehydrogenase-dependent carboxylation of alpha-ketoglutarate to citrate to support cell growth and viability. Proc Natl Acad Sci U S A, 108, 19611–19616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong J. M., de Souza R., Kendall C. W., Emam A., Jenkins D. J. (2006). Colonic health: Fermentation and short chain fatty acids. J Clin Gastroenterol, 40, 235–243. [DOI] [PubMed] [Google Scholar]
- Wood G. W., Morantz R. A. (1979). Immunohistologic evaluation of the lymphoreticular infiltrate of human central nervous system tumors. J Natl Cancer Inst, 62, 485–491. [DOI] [PubMed] [Google Scholar]
- Xia S., et al. (2017). Prevention of dietary-fat-fueled ketogenesis attenuates BRAF V600E tumor growth. Cell Metab, 25, 358–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao M., Yang H., Xu W., Ma S., Lin H., Zhu H., Liu L., Liu Y., Yang C., Xu Y., Zhao S., Ye D., Xiong Y., Guan K. L. (2012). Inhibition of alpha-KG-dependent histone and DNA demethylases by fumarate and succinate that are accumulated in mutations of FH and SDH tumor suppressors. Genes Dev, 26, 1326–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R. H., Pelicano H., Zhou Y., Carew J. S., Feng L., Bhalla K. N., Keating M. J., Huang P. (2005). Inhibition of glycolysis in cancer cells: A novel strategy to overcome drug resistance associated with mitochondrial respiratory defect and hypoxia. Cancer Res, 65, 613–621. [PubMed] [Google Scholar]
- Yan H., Parsons D. W., Jin G., McLendon R., Rasheed B. A., Yuan W., Kos I., Batinic-Haberle I., Jones S., Riggins G. J., Friedman H., Friedman A., Reardon D., Herndon J., Kinzler K. W., Velculescu V. E., Vogelstein B., Bigner D. D. (2009). IDH1 and IDH2 mutations in gliomas. N Engl J Med, 360, 765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C., Ko B., Hensley C. T., Jiang L., Wasti A. T., Kim J., Sudderth J., Calvaruso M. A., Lumata L., Mitsche M., Rutter J., Merritt M. E., DeBerardinis R. J. (2014). Glutamine oxidation maintains the TCA cycle and cell survival during impaired mitochondrial pyruvate transport. Mol Cell, 56, 414–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C., Sudderth J., Dang T., Bachoo R. M., McDonald J. G., DeBerardinis R. J. (2009). Glioblastoma cells require glutamate dehydrogenase to survive impairments of glucose metabolism or Akt signaling. Cancer Res, 69, 7986–7993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Venneti S., Nagrath D. (2017). Glutaminolysis: A hallmark of cancer metabolism. Annu Rev Biomed Eng, 19, 163–194. [DOI] [PubMed] [Google Scholar]
- Yang M., Soga T., Pollard P. J., Adam J. (2012). The emerging role of fumarate as an oncometabolite. Front Oncol, 2, 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M., Vousden K. H. (2016). Serine and one-carbon metabolism in cancer. Nat Rev Cancer, 16, 650–662. [DOI] [PubMed] [Google Scholar]
- Yang Y., Sauve A. A. (2016). NAD(+) metabolism: Bioenergetics, signaling and manipulation for therapy. Biochim Biophys Acta, 1864, 1787–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young V. R., Ajami A. M. (2001). Glutamine: The emperor or his clothes? J Nutr, 131, 2449S–2459S. [DOI] [PubMed] [Google Scholar]
- Yuan X., Curtin J., Xiong Y., Liu G., Waschsmann-Hogiu S., Farkas D. L., Black K. L., Yu J. S. (2004). Isolation of cancer stem cells from adult glioblastoma multiforme. Oncogene, 23, 9392–9400. [DOI] [PubMed] [Google Scholar]
- Yuen C. A., Asuthkar S., Guda M. R., Tsung A. J., Velpula K. K. (2016). Cancer stem cell molecular reprogramming of the Warburg effect in glioblastomas: A new target gleaned from an old concept. CNS Oncol, 5, 101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuneva M. (2008). Finding an “Achilles' heel” of cancer: The role of glucose and glutamine metabolism in the survival of transformed cells. Cell Cycle, 7, 2083–2089. [DOI] [PubMed] [Google Scholar]
- Zagzag D., Esencay M., Mendez O., Yee H., Smirnova I., Huang Y., Chiriboga L., Lukyanov E., Liu M., Newcomb E. W. (2008). Hypoxia- and vascular endothelial growth factor-induced stromal cell-derived factor-1alpha/CXCR4 expression in glioblastomas: One plausible explanation of Scherer’s structures. Am J Pathol, 173, 545–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Fan J., Venneti S., Cross J. R., Takagi T., Bhinder B., Djaballah H., Kanai M., Cheng E. H., Judkins A. R., Pawel B., Baggs J., Cherry S., Rabinowitz J. D., Thompson C. B. (2014). Asparagine plays a critical role in regulating cellular adaptation to glutamine depletion. Mol Cell, 56, 205–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K., Xu P., Sowers J. L., Machuca D. F., Mirfattah B., Herring J., Tang H., Chen Y., Tian B., Brasier A. R., Sowers L. C. (2017. a). Proteome analysis of hypoxic glioblastoma cells reveals sequential metabolic adaptation of one-carbon metabolic pathways. Mol Cell Proteomics, 16, 1906–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Rao A., Sette P., Deibert C., Pomerantz A., Kim W. J., Kohanbash G., Chang Y., Park Y., Engh J., Choi J., Chan T., Okada H., Lotze M., Grandi P., Amankulor N. (2016) IDH mutant gliomas escape natural killer cell immune surveillance by downregulation of NKG2D ligand expression. Neuro Oncol, 18, 1402–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Bharathi S. S., Rardin M. J., Lu J., Maringer K. V., Sims-Lucas S., Prochownik E. V., Gibson B. W., Goetzman E. S. (2017. b). Lysine desuccinylase SIRT5 binds to cardiolipin and regulates the electron transport chain. J Biol Chem, 292, 10239–10249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W., Mukherjee P., Kiebish M. A., Markis W. T., Mantis J. G., Seyfried T. N. (2007). The calorically restricted ketogenic diet, an effective alternative therapy for malignant brain cancer. Nutr Metab (Lond), 4, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Zhou Y., Shingu T., Feng L., Chen Z., Ogasawara M., Keating M. J., Kondo S., Huang P. (2011). Metabolic alterations in highly tumorigenic glioblastoma cells: Preference for hypoxia and high dependency on glycolysis. J Biol Chem, 286, 32843–32853. [DOI] [PMC free article] [PubMed] [Google Scholar]