Abstract
Background:
Regional nerve blocks are commonly used to manage postoperative pain after arthroscopic shoulder procedures. The interscalene brachial plexus block (ISB) is commonly used; however, because of the reported side effects of ISB, the use of a suprascapular nerve block (SSNB) has been described as an alternative strategy with fewer reported side effects.
Purpose:
To examine the efficacy of SSNB for pain control after shoulder arthroscopy compared with ISB as well as anesthesia without a nerve block.
Study Design:
Systematic review; Level of evidence, 1.
Methods:
Three databases (PubMed, MEDLINE, and EMBASE) were searched on April 20, 2018, to systematically identify and screen the literature for randomized controlled trials (RCTs). A meta-analysis of standard mean differences (SMDs) was performed to pool the estimated effects of the nerve blocks.
Results:
The search identified 14 RCTs that included 1382 patients, with a mean age of 54 years (SD, 13 years). The mean follow-up time was 3 days (range, 24 hours to 6 weeks). Postoperative pain control was significantly more effective in the SSNB groups compared with the control groups within 1 hour (SMD, –0.76; 95% CI, –1.45 to –0.07; P = .03) and 4 to 6 hours (SMD, –0.81; 95% CI, –1.53 to –0.09; P = .03) postoperatively. However, pain control was significantly less effective in the SSNB groups compared with ISB within 1 hour (SMD, 0.87; 95% CI, 0.28 to 1.46; P = .004). No major complications were noted in the SSNB groups, and minor complications such as hoarseness and prolonged motor block were significantly less common for SSNB compared with ISB.
Conclusion:
Although not more efficacious than ISB in terms of pain control for patients undergoing shoulder arthroscopy, SSNB provides significantly improved pain control in comparison with analgesia without a nerve block. Moreover, few major and minor complications are associated with SSNB reported across the literature.
Keywords: nerve block, regional, suprascapular, shoulder, arthroscopy
Shoulder arthroscopy is a commonly performed procedure that many providers prefer to open approaches because it involves less soft tissue dissection, shorter duration of hospital stay, and improved cosmesis. However, arthroscopic surgery of the shoulder can be associated with moderate to severe early postoperative pain that can interfere with recovery and rehabilitation.9 Management of this pain is often accomplished by use of opioids; however, their use is often associated with side effects such as nausea, vomiting, respiratory depression, dysphoria, and hormonal effects including the levels of luteinizing hormone and testosterone.32,45 Controlling postoperative pain while minimizing opioid administration is particularly important, because poor pain control is thought to be responsible for more than 60% of unplanned or prolonged hospitalizations. Additionally, achieving good pain control is an important factor in determining patient-reported postoperative satisfaction.5
To manage postoperative pain, regional nerve blocks are commonly used. Among the various types of nerve blocks, the interscalene brachial plexus block (ISB) is considered the gold standard, as it has consistently been shown to significantly reduce postoperative pain.18,29 Moreover, ISB can be used to provide surgical anesthesia, rather than just the postoperative anesthesia than is provided by other types of blocks. However, complications ranging from the serious (such as accidental epidural anesthesia, vertebral artery injection, paralysis of the phrenic nerve, pneumothorax, and brachial plexus injury)26 to the unpleasant (such as extended motor block after the procedure) have been reported following the use of ISB.11,31 Furthermore, ISB has relative contraindications; for example, it is contraindicated in patients with severe chronic obstructive pulmonary disease because of phrenic nerve issues.11 Because of these issues with ISB, the use of a suprascapular nerve block (SSNB) has been described as an alternative strategy with fewer reported side effects. The suprascapular nerve is thought to innervate approximately 70% of the shoulder joint, capsule, subacromial space, acromioclavicular joint, and coracoacromial ligament, with the remaining 30% thought to be innervated by the lateral pectoral and axillary nerves.10
The purpose of this systematic review and meta-analysis was to examine the efficacy of SSNB for analgesia outcomes after shoulder arthroscopy in adult patients and to identify the complication rate from such blocks. Secondarily, the study assessed the efficacy of SSNB compared with ISB and non–nerve block controls.
Methods
Search Strategy
The PubMed (MEDLINE), Ovid (MEDLINE), and EMBASE databases were searched for literature addressing the use of SSNB for shoulder arthroscopy from database inception until April 20, 2018. The search terms “nerve block,” “regional anesthesia,” “regional block,” “suprascapular,” “shoulder,” and “arthroscopy” were used (Appendix Table A1).
Study Screening
Two reviewers (J.K., M.M.) independently screened the titles, abstracts, and full-text version of the articles. Any disagreements were discussed between reviewers and the senior author (O.R.A.) to determine study inclusion when necessary. The references of the included studies were then screened for additional articles that may have eluded the initial search strategy.
Assessment of Study Eligibility
The research question and eligibility criteria were determined a priori. The inclusion criteria included therapeutic studies written in English, studies evaluating live human participants, studies evaluating the use of SSNB (either alone or in combination with an axillary nerve block), and randomized controlled trials (RCTs) that reported any outcomes, including pain, opioid consumption, length of hospital stay, and cost. Commentaries, cadaveric studies, animal studies, conference papers, book chapters, review articles, and technical reports were excluded.
Data Abstraction
Two reviewers collected data in duplicate and recorded them in a Microsoft Excel spreadsheet (version 2007). Data regarding year of publication, author, location of study, procedures undergone by the patients, type of block used, study design, age, sex, sample size, pain scores, opioid consumption (expressed as morphine equivalents), side effects and complications (nausea, pneumothorax, prolonged motor block, phrenic nerve palsy, and hoarse voice), patient satisfaction and hospital length of stay, and level of evidence were recorded. An attempt was made to contact the authors of any study whose article contained insufficient data.
Quality Assessment
The Grading of Recommendations Assessment, Development and Evaluation (GRADE) system was used to assess the methodological quality of the included studies.12 The GRADE method begins by initially assigning RCTs as high-quality evidence. Thereafter, the studies are evaluated for 5 factors related to the quality of evidence: risk of bias, inconsistency, indirectness, imprecision, and publication bias.3,17 Risk of bias assesses the limitations of the included studies on the basis of randomization, allocation concealment, blinding, incomplete outcome reporting, selective reporting, and other biases.17 Inconsistency refers to the level of similarity of results throughout the included studies by assessing variation in the intervention groups and the point estimates and, if meta-analyses are performed, the amount of overlap in CIs, the magnitude of I 2, and statistical tests for heterogeneity.13 Indirectness assesses the relevance of study outcomes and the applicability of the studies to the interventions and populations of interest.14 Imprecision assesses the sample size of the analysis as well as certainty of the effect estimate.15 Publication bias refers to the cumulative assessment of the direction of findings in each study, as well as funding sources, funnel plot analysis for each outcome, and study sample sizes.16 Ultimately, the final overall body of literature on the topic is scored and reported as high, moderate, low, or very low quality, and this score relates to the overall confidence in the pooled effect estimate.
Assessment of Agreement
To assess the interreviewer agreement, a kappa (κ) statistic was calculated for the title, abstract, and full-text screening stages. Agreement was categorized a priori as follows: κ of ≥0.61 was considered substantial agreement; 0.21 to 0.60, moderate agreement; and ≤0.20, slight agreement.28
Statistical Analysis
Descriptive statistics including means, proportions, ranges, 95% CIs, standard deviations, and κ values were calculated by use of Minitab statistical software (version 17). Given the nonuniform nature of reporting for opioid consumption, length of stay, and patient satisfaction in the studies included in this systematic review, the results for these secondary outcomes are presented as a narrative summary.
A meta-analysis was conducted evaluating postoperative pain in order to compare the use of SSNB with ISB and with controls. A decision was made a priori that a minimum of 3 trials would be needed for any outcome to be pooled. Standardized mean differences (SMDs) were used to account for differences in pain outcome scales. Pooled analyses were performed by use of the Review Manager (RevMan) software 5.3 (The Nordic Cochrane Centre, The Cochrane Collaboration, 2012). P < .05 was considered to be significant. The I 2 test was used to assess heterogeneity across the reported results of the included studies. The proportions were then combined using a random effects model (I 2 < 30%, which was considered as low statistical heterogeneity).33 A sensitivity analysis was conducted to account for differences with respect to block technique, specifically to account for studies that used a combination of axillary nerve blocks with SSNB.
Results
Search Strategy
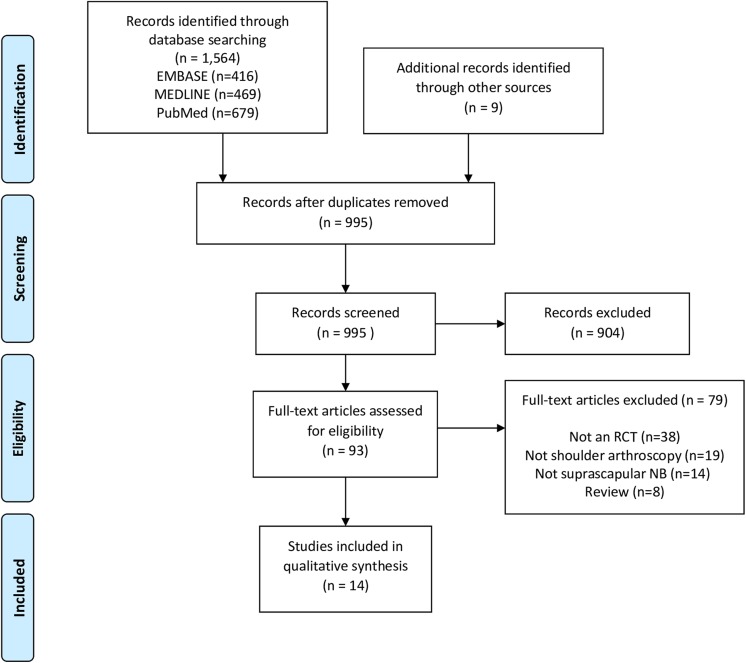
The initial search of the 3 databases resulted in 1564 total studies. Of these, 569 studies were removed as duplicates, resulting in 995 studies. A systematic screening approach resulted in 14 available full-text articles for review (Figure 1). Substantial agreement was achieved among reviewers at the title (κ = 0.90; 95% CI, 0.82 to 0.98), abstract (κ = 0.93; 95% CI, 0.79 to 1.00), and full-text (κ = 1.00; 95% CI, 1.00 to 1.00) screening stages.
Figure 1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram of the search strategy for articles assessing the use of suprascapular nerve block for pain control after shoulder arthroscopy. NB, nerve block; RCT, randomized controlled trial.
Study Characteristics
The present review of 14 RCTs included a total of 1382 patients, with a mean age of 53.8 years (SD, 13.1 years). The mean follow-up time was 3.1 days (range, 24 hours to 6 weeks) (Table 1). Every study investigated SSNB; 11 studies additionally investigated ISB, and 3 studies examined a combined axillary nerve block and SSNB (Table 2).
TABLE 1.
Characteristics of Included Studies and Patientsa
| Study | Country | Treatment Group | Control Groups | Sample Sizes, n | Age, y, Mean (SD) | % Female | Follow- up | Surgery |
|---|---|---|---|---|---|---|---|---|
| Aliste et al1 (2018) | Chile | Infraclavicular SSNB | ISB | Treatment: 20 Control: 20 |
Treatment: 57.9 (9.3) Control: 50.6 (8.0) |
50 | 24 h | Arthroscopic shoulder surgery |
| Auyong et al2 (2018) | USA | SSNB | Control A: ISB Control B: Supraclavicular nerve block |
Treatment: 60 Control A: 61 Control B: 62 |
Treatment: 55 (14) Control A: 54 (13) Control B: 55 (14) |
37 | 24 h | Outpatient arthroscopic rotator cuff or Bankart repair |
| Desroches et al6 (2016) | France | SSNB | Preoperative ISB | Treatment: 28 Control: 25 |
60.8 (8.7) | 38 | 7 d | Rotator cuff repair |
| Dhir et al7 (2016) | Canada | Combined SSNB and axillary nerve blocks | ISB | Treatment: 30 Control: 29 |
Treatment: 51.3 (14.2) Control: 46.5 (14.5) |
19 | 7 d | Shoulder arthroscopy |
| Ikemoto et al21 (2010) | Brazil | SSNB | Control A: ISB Control B: No nerve block |
Treatment: 15 Control A: 15 Control B: 15 |
Treatment: 57 (45-69)b
Control A: 54 (39-65)b Control B: 57 (47-76)b |
71 | 48 h | Arthroscopic rotator cuff repair |
| Jeske et al23 (2011) | Germany and Italy | SSNB | Control A: Subacromial infiltration of local anesthesia Control B: Placebo |
Treatment: 15 Control A: 15 Control B: 15 |
61.9 (8.2) | 47 | 6 wk | Arthroscopic subacromial decompression |
| Kumara et al27 (2016) | India | SSNB | ISB | Treatment: 30 Control: 30 |
NR | NR | 24 h | Shoulder arthroscopy |
| Lee et al30 (2015) | South Korea | SSNB | Placebo | Treatment: 15 Control: 15 |
Treatment: 48.9 (11.7) Control: 51.6 (10.6) |
30 | 24 h | Arthroscopic rotator cuff repair |
| Neuts et al36 (2018) | Belgium | SSNB | ISB | Treatment: 50 Control: 50 |
Treatment: 51 (10) Control: 54 (10) |
52 | 48 h | Arthroscopic shoulder surgery |
| Ovesen et al37 (2014) | Denmark | SSNB | Control A: ISB Control B: Subacromial bursa block Control C : No nerve block |
Treatment: 23 Control A: 22 Control B: 22 Control C: 24 |
Treatment: 48.95 Control A: 48.70 Control B: 54.77 Control C: 48.79 |
62 | 24 h | Arthroscopic subacromial decompression |
| Park et al38 (2016) | South Korea | Blind SSNB, axillary nerve block, and patient-controlled analgesia | Control A: Blind SSNB and patient-controlled analgesia Control B: Only patient-controlled analgesia |
Treatment: 37 Control A: 36 Control B: 33 |
Treatment: 61.3 (10.8) Control A: 59.2 (11.5) Control B: 63.3 (9.1) |
50 | 48 h | Arthroscopic rotator cuff repair |
| Pitombo et al40 (2013) | Brazil | SSNB and axillary nerve blocks | ISB | Treatment: 34 Control: 34 |
Treatment: 55.03 (13.04) Control: 52.21 (15.39) |
57 | 48 h | Shoulder arthroscopy |
| Singelyn et al47 (2004) | France | SSNB | Control A: ISB Control B: Intra-articular local anesthetic Control C: No nerve block |
Treatment: 30 Control A: 30 Control B: 30 Control C: 30 |
Treatment: 52 (14) Control A: 54 (15) Control B: 50 (14) Control C: 53 (17) |
55 | 24 h | Arthroscopic acromioplasty |
| Wiegel et al49 (2017) | Germany | SSNB | ISB | Treatment: 164 Control: 165 |
Treatment: 53 (13) Control: 55 (13) |
38 | 24 h | Shoulder arthroscopy |
aISB, interscalene block; NR, not reported; SSNB, suprascapular nerve block.
bAge expressed as mean (range).
TABLE 2.
Details of the Nerve Blocks and Anesthesia Useda
| Study | Anesthesia | Treatment Technique | Control Technique | Analgesia Used |
|---|---|---|---|---|
| Aliste et al1 (2018) | GA | 10 mL of 1% lidocaine. Lateral decubitus position. The US transducer was applied cephalad and parallel to the scapular spine to obtain a view of the suprascapular fossa. With an in-plane technique and a lateral-to-medial direction, the block needle was advanced until its tip was located in the floor of the suprascapular fossa, ventral to the fascia of the supraspinatus muscle. | 20 mL of 1% lidocaine. The US transducer was applied on the lateral side of the neck at the level of the cricoid cartilage to obtain a view of the brachial plexus. The block needle was advanced until its tip was positioned under the prevertebral fascia between the 2 most superficial hypoechoic structures. | In the PACU, all patients received acetaminophen 1 g IV, ketoprofen 100 mg IV, and patient-controlled analgesia (1-mg bolus doses of morphine with a lockout interval of 8 min). Afterward, all patients continued to receive acetaminophen 1 g by mouth every 8 h and ketoprofen 100 mg by mouth every 12 h as well as patient-controlled morphine. |
| Auyong et al2 (2018) | GA | 15 mL of 0.5% ropivacaine. The suprascapular nerve was traced laterally as it branched away from the superior trunk or C5 nerve root in the supraclavicular fossa on the anterior lateral portion of the neck. The injection endpoint was immediately beneath the suprascapular nerve. | 15 mL of 0.5% ropivacaine. ISB: US probe was used. The needle was inserted with in-plane technique into the interscalene grove. The injection endpoint was posterior to the brachial plexus at this level. Supraclavicular block: US probe was used. An in-plane technique was used to reach the endpoint at the superior portion of the brachial plexus that corresponds to the superior and middle trunks. |
In the PACU, the opioid algorithm was as follows immediately following surgery: (1) for NRS pain score of 4-6, the patient received 25 μg of fentanyl IV; (2) for NRS score 7-10, the patient received 50 μg of fentanyl IV. Oral oxycodone, if necessary, was dosed using the following criteria: (1) for NRS score 4-6, the patient received 5 mg; (2) for NRS score 7-10, the patient received 10 mg. |
| Desroches et al6 (2016) | GA | 10 mL of 0.75% ropivacaine. The needle was inserted at the midpoint of the line connecting the anterolateral edge of the acromion and the superomedial angle of the scapula. The needle was advanced at an angle of 30° to contact the base of the coracoid process, and the anesthetic was injected slowly. | Both neurostimulation at 0.8 mA and US guidance were used. Posterior and anterior diffusions were checked during the injection. If diffusion was insufficient, the needle was moved for correct diffusion. 20-mL bolus of 0.75% of ropivacaine. |
In the recovery room: 1 g of acetaminophen, 100 mg of ketoprofen, 100 mg of tramadol IV were given. If VAS >3, the patient received 3 mg of morphine IV; 5 min later, if VAS >3, the patient received another 3 mg of morphine IV. |
| Dhir et al7 (2016) | GA | The needle was inserted with dual guidance along the long axis. 15 mL of 0.5% ropivacaine was injected in the supraspinatus fossa after stimulation of supraspinatus and/or infraspinatus was observed. | US guidance with nerve stimulation assistance was used for lateral-to-medial in-plane ISB block with 20 mL of 0.5% ropivacaine. Target was C6 in interscalene groove, which was confirmed with deltoid motor response. | In the PACU, all patients received ketorolac and acetaminophen, plus opioids as needed. Patients were prescribed oral opioids and instructed to take them every 4-6 h as needed when discharged. |
| Ikemoto et al21 (2010) | GA | Two-thirds of 2 mg/kg of 0.5% ropivacaine was used; the remaining third was applied in the subacromial space. | 2 mg/kg of 0.5% ropivacaine. | After the procedure, simple analgesics, opioid analgesics, and anti-inflammatory agents were administered as requested by the patient. |
| Jeske et al23 (2011) | GA | 10 mL of 0.1% ropivacaine. The scapular spine and acromion were palpated, and the total length between them was divided into 2 equal halves. The needle was placed 2 cm proximal and medial to this point and positioned laterally and caudally. Stimulation current was used to confirm motor response of the infraspinatus and supraspinatus. | See technique for treatment group; the placebo group received 10 mL of 0.9% saline and the subacromial infiltration group received 20 mL of 1% ropivacaine. | Postoperative analgesia consisted of 75 mg of diclofenac 4 h postoperatively for at least 48 h, in combination with 40 mg of pantoprazole. If VAS >3, patients received subcutaneous or oral morphine. If patients were pain free, then nonsteroidal anti-inflammatory agents were discontinued. |
| Kumara et al27 (2016) | GA | The scapula was divided into 4 quadrants created by the spine of the scapula and a vertical line parallel to the spine. The upper outer quadrant was then bisected, and 2 mL of 1% lignocaine was injected 2.5 cm along the plane of bisection. After location was confirmed with electrophysiological stimulation and negative aspiration, a 22-gauge needle was used to inject 15 mL of 0.5% bupivacaine with 75 μg of clonidine. An additional 5 mL of 0.5% bupivacaine was infiltrated subcutaneously over the shoulder to block cutaneous sensory branches of C3 and C4 spinal nerves. |
The brachial plexus was approached at the level of C6, with the needle angled at 60° from the sagittal plane. The needle was introduced with an electrophysiological probe. After confirmation of location with electrical stimulation and negative aspiration, 20 mL of 0.5% bupivacaine and 75 μg of clonidine were injected. |
VAS was administered immediately upon admission to the PACU and then at 30 min, 1 h, 2 h, 4 h, 6 h, and 8 h. If VAS ≥4, then 75 mg of diclofenac was given via the intramuscular route. |
| Lee et al30 (2015) | GA | The suprascapular ligament and nerve were exposed via electrocautery through the anterior portal. An 18-gauge needle was inserted perpendicularly, 7 cm medial to the lateral margin of the acromion and above the previously located transverse suprascapular ligament. 10 mL of 0.5% ropivacaine was then injected, | Same as the treatment protocol but with 10 mL of saline. | Patient-controlled analgesia was provided, consisting of 1 μg/kg of fentanyl with a lockout time of 1 h and a maximum dose of 700 μg. |
| Neuts et al36 (2018) | GA | 10 mL 0.75 ropivacaine. Lateral decubitus position. A US-guided, in-plane, medial to lateral approach was used. The needle was positioned in the concave depression under the supraspinatus fascia. | 20 mL of 0.75% ropivacaine. US guided, in-plane technique through the middle scalene muscle was used. The tip of the needle was placed anterosuperior to the C6 root without making contact with neural structures. | Postoperative pain management included IV paracetamol (15 mg/kg 4 times a day), ketorolac (0.5 mg/kg 3 times a day), and patient-controlled intravenous analgesia with piritramide (bolus dose = 2 mg and lockout interval = 12 min). |
| Ovesen et al37 (2014) | GA | 20 mL of bupivacaine. The needle was introduced 1 cm cephalad to the middle of the spine of the scapulae and advanced parallel to the blade until the bony floor of the fossa supraspinatus reached. | ISB: 30 mL of ropivacaine. The block was performed by use of Winnie landmarks (palpating the interscalene groove at the level of the cricoid cartilage (C6 vertebra). A Stimuplex needle was connected to a peripheral nerve-stimulator introduced into the plexus sheath. Bursal block: 10 mL bupivacaine and 5 mL morphine were injected into subacromial space. |
All patients had 1 g of paracetamol 4 times a day and 600 mg of ibuprofen 3 times a day. If VAS >3, patients received 3-5 mg nicomorphine hydrochloride IV followed by 5 mg ketobemidone. |
| Park et al38 (2016) | GA | A line was drawn connecting the medial area of the acromion to the medial end of the spine of the scapula. The needle was inserted parallel to the vertebral column 2 cm medial and 2 cm cephalad to the midpoint of the previous line. 10 mL of 0.75% ropivacaine was injected with repeated withdrawal. | Same as treatment group. | All patients were administered pregabalin 75 mg, aceclofenac 100 mg, tramadol 37.5 mg, and acetaminophen 325 mg the night before the procedure. The patient-controlled analgesia consisted of 80 mL of saline with fentanyl 0.5 mg, ketorolac 180 mg, and ondansetron 12 mg in a time-release injection for 48 h. |
| Pitombo et al40 (2013) | GA | The puncture location was 2 cm medial to the posterior edge of the acromion and 2 cm cranial to the upper border of the scapular spine. After muscle response was confirmed via a neurostimulator, 15 mL of 0.33% levobupivacaine with 1:200,000 epinephrine was injected. |
The nerve block protocol was not detailed. After observation of motor response to neurostimulator, 30 mL of 0.33% levobupivacaine with 1:200,000 epinephrine was injected. |
Postoperative analgesia consisted of 2 g of dipyrone IV every 6 h. Pain was assessed by use of the VAS immediately in the PACU and 6 h, 12 h, and 24 h after nerve blockade. If VAS scores were ≥3, then a single dose of IV morphine at 0.04 mg/kg was used as rescue analgesia. |
| Singelyn et al47 (2004) | GA | A 5-cm, 21-gauge intramuscular needle was introduced 1 cm cephalad to the midpoint of the scapular spine and advanced. 10 mL of 0.25% bupivacaine with 1:200,000 epinephrine was injected. | Intra-articular anesthetic: 20 mL of 0.25% bupivacaine with 1:200,000 epinephrine was administered after skin closure at the end of the procedure. ISB: The needle was introduced into the plexus sheath with a peripheral stimulator. 20 mL of 0.25% bupivacaine with 1:200,000 epinephrine was injected. |
If VAS was >30, the patient received 2 g of IV propacetamol followed by 5 mg (if <60 kg body weight) or 10 mg (if >60 kg body weight) of subcutaneous morphine if VAS remained unchanged after 30 min. |
| Wiegel et al49 (2017) | GA | The needle was advanced through the inferior belly of the omohyoid and superficial to the prevertebral fascia and then visualized with US. 10 mL of 1% ropivacaine was injected. | US was used to identify the superior trunk of the brachial plexus. 20 mL of 0.75% ropivacaine was injected between the lateral aspect of the brachial plexus and middle scalene. | If the postoperative pain NRS score was >3, then 3 mg of IV piritramide was administered. |
aGA, general anesthesia; ISB, interscalene block; IV, intravenous; NRS, numerical rating scale; PACU, postanesthesia care unit; US, ultrasound; VAS, visual analog scale.
Study Quality
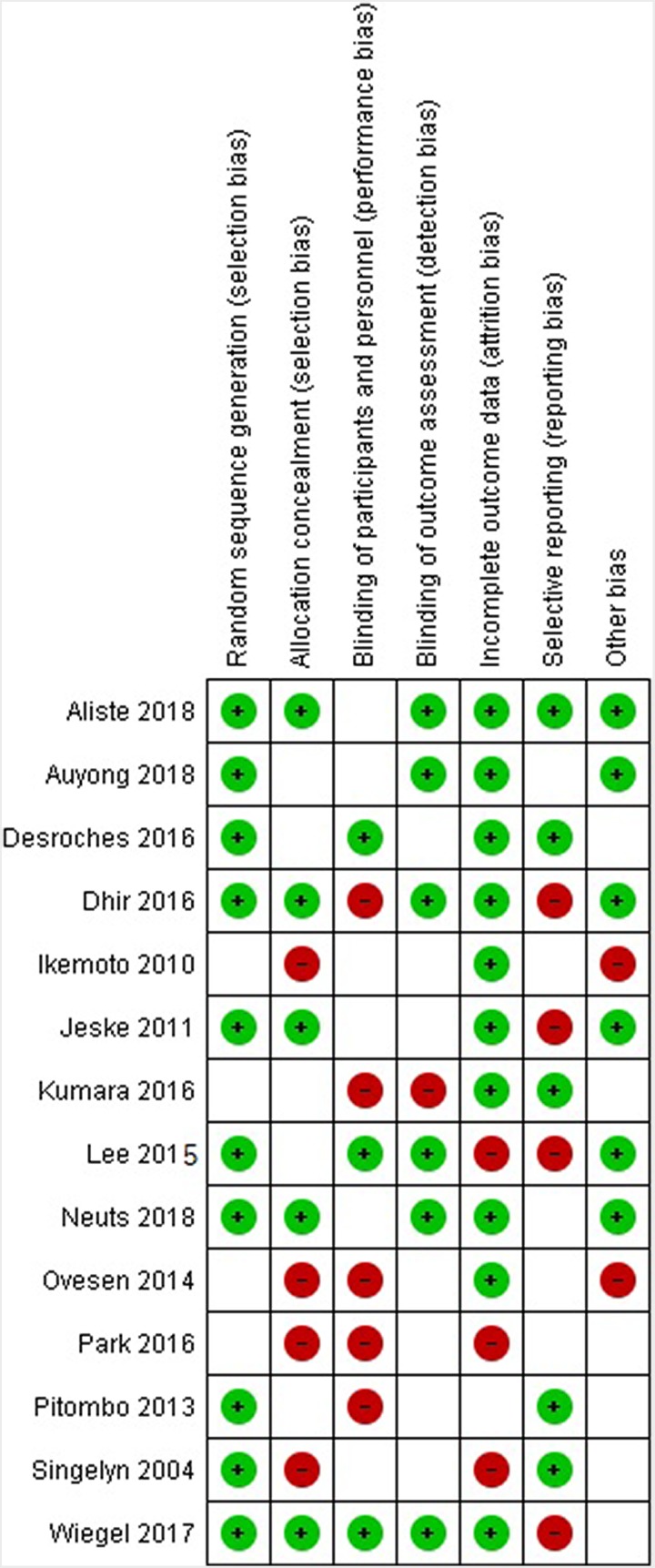
Overall, study quality as assessed with the GRADE criteria was moderate.12 Blinding of the patients or the outcome assessors was not consistently practiced in the studies. Additionally, selective reporting was an issue with several studies. Details of potential bias in each study are presented in Figure 2.
Figure 2.
Risk of bias assessment summary: suprascapular nerve block for pain control after shoulder arthroscopy. Green “+” circles indicate low risk of bias, empty cells indicate unclear risk of bias, and red “−” circles indicate high risk of bias.
Pain Scores
Every study reported on postoperative pain scores and outcomes associated with the various nerve blocks. Eight studies reported postoperative pain using visual analog scale (VAS) scores, a continuous scale comprising a horizontal or vertical line, 10 cm (100 mm) in length.20 Five studies reported postoperative pain scores using numerical rating scale (NRS) scoring, a numeric version of the VAS in which the patient selects the whole number (0-10) best reflecting the intensity of his or her pain20 (Table 3).
TABLE 3.
Outcomesa
| Study | Pain Outcome | Opioid Consumption Outcomes | Length of Stay Outcomes | Complications, No. of Patients/Total Patients Included | Other Outcomes |
|---|---|---|---|---|---|
| Aliste et al1 (2018) |
NRS Pain Scores
ISB group displayed significantly lower PACU pain scores at 30 min (difference of the medians, –4; 99% CI, –6 to –3). Although pain scores at 1, 2, and 3 h were lower in the ISB group, the upper bounds of the 99% CIs did not exceed the equivalence margin. |
Patients in the ISB group required less cumulative IV morphine at 24 h (difference of the means, –6.1; 95% CI, –10.5 to –1.6). | Not assessed. | ISB: hemidiaphragm paralysis, 18/20; Horner syndrome, 4/20; hoarseness, 2/20; paresthesia, 1/20. SSNB: paresthesia, 2/20. |
ISB resulted in a shorter mean (SD) performance time than the SSNB: 9.9 (4.6) vs 17.9 (10.1) min, respectively; P = .003. No difference in patient satisfaction at 24 h. |
| Auyong et al2 (2018) |
NRS Pain Scores
No difference in mean [SD] PACU pain scores at initial presentation (SSNB, 2.0 [3.0]; ISB, 2.0 [2.9]) or 1 h postoperatively (SSNB, 2.6 [2.7]; ISB, 2.1 [2.6]). |
Less intraoperative fentanyl with SSNB group (23 [30] μg) compared with ISB (36 [42] μg). Similar total PACU opioid consumption (SSNB, 52 [59] μg; ISB, 51 [85] μg). |
No significant difference between SSNB (98 [34] min) and ISB (102 [35] min). | ISB: subjective dyspnea, 4/61; Horner syndrome, 18/61; hoarseness, 14/61. SSNB: subjective dyspnea, 1/60; Horner syndrome, 5/60; hoarseness, 5/60. |
Satisfaction at 24-h assessment was at least 95% for each group, and there was no evidence of group differences. |
| Desroches et al6 (2016) |
VAS Pain Scores, Mean (SD)
Preoperative, 6.5 (2); postoperative, 2.4 (2.3) (P = .003). Mean 24-h postoperative pain: SSNB, 2.9 (2); ISB, 2.9 (2.1). One-way analysis of variance (changes in VAS according to sex, age, working status, preoperative VAS scores), P = .11, not significant. |
No significant difference in percentage of patients taking opium-like analgesics in the recovery room (P = .55), on postoperative day 1 (P = .67), or on postoperative day 2 (P = 1). | No difference in length of stay in recovery room because all patients were released 2 h after surgery. | No symptoms of neuropathy at 6 mo. 1 case of pneumothorax in control group (ISB). | No difference in cost between ISB and SSNB. Mean duration to perform SSNB was shorter (2 min vs 12 min for ISB), and SSNB did not require ultrasonography. |
| Dhir et al7 (2016) |
NRS Pain Scores
Higher scores for SSNB and ANB (5.45) in PACU compared with ISB (1.8) (P < .001). NRS scores were comparable at 6 h (4.0 vs 2.35, P = .064). Pain control was superior in the SSNB and ANB (3.92) group at 24 h compared with ISB (6.35) (P < .05). No difference in pain scores at 7-day follow-up (2.08 vs 2.76, P = .315). |
Significantly less opioid use intraoperatively and in PACU for interscalene block group. Unable to analyze opioid use post-discharge due to inconsistent data collection. | Not assessed. | Significant numbness and tingling in ISB group at 6 h (P < .001) and significantly less nausea and vomiting in SSNB and ANB group at 24 h (P = .028). However, no difference in nausea, vomiting, or numbness at 7 d. | Satisfaction was higher in ISB group at 6 h (P = .02), but there was no difference at later time periods. |
| Ikemoto et al21 (2010) |
VAS Pain Scores
No statistical difference across groups. At 0, 8, 16, and 24 h, mean (range) pain scores in the SSNB group were 5.8 (0-10), 5.5 (0-10), 5.8 (2-10), and 5.1 (2-8). ISB group, 6.2 (0-10), 5.6 (0-10), 4.5 (0-8), 3.8 (0-9). Control group, 6.0 (0-10), 5.4 (0-10), 4.6 (0-7), 4.3 (0-10). |
In SSNB, consumption was 3.1 ampoules of analgesics, 1.05 ampoules of anti-inflammatory agents, and 1.2 ampoules of opioids. In ISB group, 5.4 ampoules of analgesics, 2.7 ampoules of anti-inflammatory agents, and 0.8 ampoules of opioids. In control group, 4.2 ampoules of analgesics, 2.2 ampoules of anti-inflammatory agents, and 1 ampoule of morphine. |
Not assessed. | Not reported. | None. |
| Jeske et al23 (2011) |
VAS Pain Scores
VAS both with rest and activity was significantly lower in SSNB group (rest, 0.4; activity, 0.6) compared with both placebo (rest, 2.4; activity, 3.9) and subacromial infiltration (rest, 3.1; activity, 4.9) at 6 h after surgery (P < .001). |
Not assessed. | Not assessed. | No complications (neurovascular, infectious, or traumatic) observed. | Patient-reported satisfaction was significantly higher at 2 d (P < .001) and 14 d (P < .005) for SSNB group compared with placebo and subacromial infiltration groups. No differences in satisfaction after 6 wk. |
| Kumara et al27 (2016) |
VAS Pain Scores
ISB group had significantly lower VAS pain scores at 30 min, 1 h, and 2 h postoperatively (2.03, 1.67, 1.93) than SSNB group (4.1, 3.27, 2.53) (P = .001, .001, .002). |
Not assessed because only diclofenac was used in postoperative analgesia. | Not assessed. | Not assessed. | None. |
| Lee et al30 (2015) |
VAS Pain Scores
VAS scores at 1, 3, 6, 12, 18, and 24 h did not differ significantly between SSNB group (6.9, 4.9, 3.9, 3.3, 2.9, 2.4) and placebo group (6.9, 5.2, 4.2, 3.6, 3.0, 2.5). |
Number of boluses and total amount of fentanyl were significantly less in treatment group (P < .05). | No significant difference in length of stay (P > .05). | Incidence of nausea and vomiting was not significantly different between groups (P > .05). | None. |
| Neuts et al36 (2018) |
NRS Pain Scores
During the first 4 h after surgery, the difference in mean NRS at rest between SSNB and ISB was higher than 2.0, thereby suggesting analgesic inferiority of SSNB. After 8 h, the difference became inconclusive. During the night and after 24 h, the intergroup difference was very small, and the confidence interval included 0, thus resulting in a conclusion of noninferiority. |
Piritramide consumption was significantly higher in the SSNB group in the PACU (P = .004), from discharge to the ward until 4 h after surgery (P < .001), and from 4 to 8 h after surgery (P = .017). No difference from 8 to 24 h after surgery (P > 0.300). Mean total IV piritramide used during first 24 h after surgery was slightly higher with ISB compared with SSNB, 17.77 (14.62) mg vs 13.85 (12.50) mg, (P = .1889). |
Not assessed. | In the PACU, 14 patients (28%) in the ISB group experienced dyspnea vs 4 patients (8.3%) in the SSNB group. | Quality of sleep on the first postoperative night was similar in the ISB and SSNB groups (mean NRS, respectively, 4.62 and 4.71; P = .908). Overall patient satisfaction with pain therapy was also similar in the ISB and SSNB groups (mean NRS, respectively, 8.68 and 8.28; P = .131). |
| Ovesen et al37 (2014) |
VAS Pain Scores, Mean [SD]
Significantly lower scores in ISB compared with SSNB in PACU (0.09 [0.43] vs 0.096 [1.73], P = .037) and at 4 h (0.68 [1.25] vs 1.70 [1.66], P = .036) postoperatively. No difference between ISB (3.09 [2.49]) and SSNB (3.21 [2.51]) at 24 h. No significant difference between SSNB and control at 0, 4, and 24 h (0.96 [1.73], 1.70 [1.66], 3.21 [2.51] vs 1.30 [2.2], 1.5 [1.35], 2.45 [2.33]). |
No significant difference in total morphine (mg per 24 h) consumption between SSNB (3.65 [7.71]) and ISB (2.0 [4.7]) or control (5.67 [10.46]). | Not assessed. | SSNB: Nausea/vomiting, 1/23; “dead arm,” 1/23; local tenderness, 3/23. ISB: Nausea/vomiting, 1/22; “dead arm,” 5/22; local tenderness, 4/22. |
None. |
| Park et al38 (2016) |
VAS Pain Score, Mean
VAS scores in the group with PCA, SSNB, and ANB (6.4, 4.1) and the group with PCA and SSNB (7.2, 5.1) were significantly lower than the group with only PCA (7.9, 6.2) (P < .01) at 1 and 6 h postoperatively. The group with PCA, SSNB, and ANB (5.6, 4.0) had significantly lower VAS scores than the group with PCA and SSNB (6.6, 4.9) at 12 and 36 h (P < .01). |
Not assessed. | Not assessed. | Groups with nerve blocks had no neurological complications. | None. |
| Pitombo et al40 (2013) | No pain scores were measured. Mean duration of analgesia was significantly higher in the axillary and suprascapular nerve block group (P < .05). |
Rescue morphine consumption was significantly higher in the suprascapular and axillary nerve block group in the PACU (P < .05), but not at later times postblock. | Not assessed. | No complications like pneumothorax, accidental epidural analgesia injection, or adverse effects were observed. No statistically significant difference in rates of nausea and vomiting (P = .961). |
Discomfort with motor paralysis 24 h postblock was significantly lower in the axillary and suprascapular block group (P < .05). Significantly less motor block in the suprascapular and axillary nerve block group compared with ISB (P < .05). |
| Singelyn et al47 (2004) |
VAS Pain Scores, Mean
SSNB group had lower VAS scores during rest at 4 h and 24 h postoperatively (1.9, 1.1) compared with intra-articular injection (4.0, 3.0) (P < .001). VAS scores during movement were significantly lower (3.5) than intra-articular injection (6.1) at 24 h postoperatively (P < .001). ISB group showed significantly lower VAS scores (1.3, 1.3) during movement in PACU and 4 h postoperatively than SSNB (5.4, 3.5) (P < .01). |
Morphine use was significantly lower in the ISB group compared with SSB, intra-articular injection, and control groups (P < .01). | Not assessed. | Side effects included sedation, local tenderness, and nausea/vomiting. Incidence of nausea/vomiting was significantly lower in the ISB group than in the control group (P < .05). | Patient satisfaction at 24 h was significantly higher in the ISB group (P < .01). |
| Wiegel et al49 (2017) |
NRS Pain Scores
SSNB group pain scores were noninferior to ISB group (P < .0001). Number of patients reporting significant pain (NRS >3) was not significantly different between ISB (25%) and SSNB (30%) groups (P = .37). |
Piritramide use in PACU did not differ significantly between ISB and SSNB groups (P = .99). | Not assessed. | ISB group had significantly higher incidence of side effects, including hoarseness (P < .05), Horner syndrome (P < .001), and dyspnea (P < .05). | SSNB group reported significantly higher satisfaction (P < .001). SSNB group also demonstrated significantly higher grip strength 24 h after operation (P < .001). |
aANB, axillary nerve block; ISB, interscalene block; IV, intravenous; NRS, numerical rating scale; PACU, postanesthesia care unit; PCA, patient-controlled analgesia; SSNB, suprascapular nerve block; VAS, visual analog scale.
SSNB Versus Control
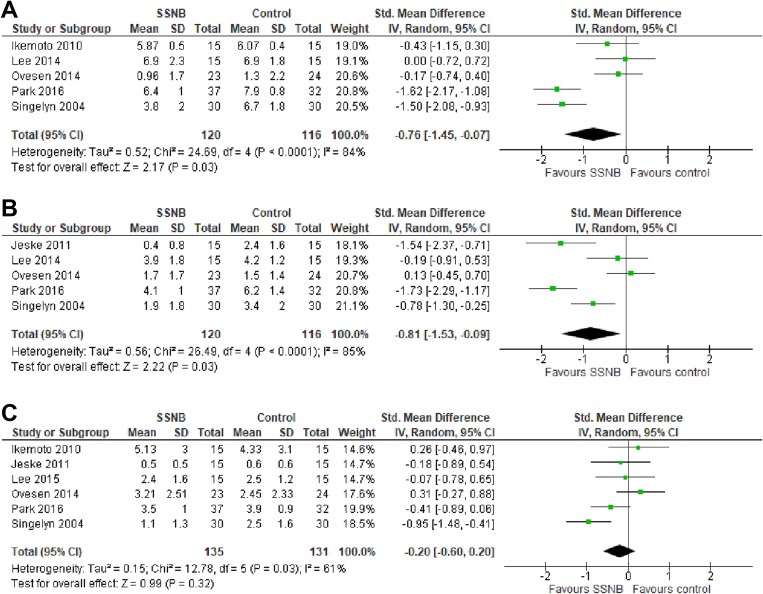
Six studies21,23,30,37,38,47 assessed the efficacy of SSNB in controlling pain postoperatively compared with a control group that did not involve nerve blocks. Three of these studies23,38,47 found significantly reduced postoperative pain scores in the SSNB group compared with controls, while the remaining 3 studies21,30,37 reported no significant difference. Pain control within 1 hour postoperatively was compared in 5 studies,21,30,37,38,47 with significantly reduced pain identified in the SSNB groups (SMD, –0.76; 95% CI, –1.45 to –0.07; P = .03; I 2 = 84%) (Figure 3A). Five studies21,30,37,38,47 compared pain control between 4 and 6 hours postoperatively, reporting significantly improved pain control in the SSNB groups (SMD, –0.81; 95% CI, –1.53 to –0.09; P = .03; I 2 = 85%) (Figure 3B). At 24 hours postoperatively, no significant difference was noted in pain control between patients who received SSNB versus controls (SMD, –0.20; 95% CI, –0.60 to 0.20; P = .32; I 2 = 61%) (Figure 3C).21,23,30,37,38,47 These results indicate that SSNB is efficacious at improving pain control compared with control treatments in the early postoperative period; however, the effect may abate beyond 24 hours postoperatively.
Figure 3.
Forest plots of standard mean difference between suprascapular nerve block (SSNB) group and control group for pain scores (A) within 1 hour (B), at 4 to 6 hours, and (C) at 24 hours postoperatively.
SSNB Versus ISB
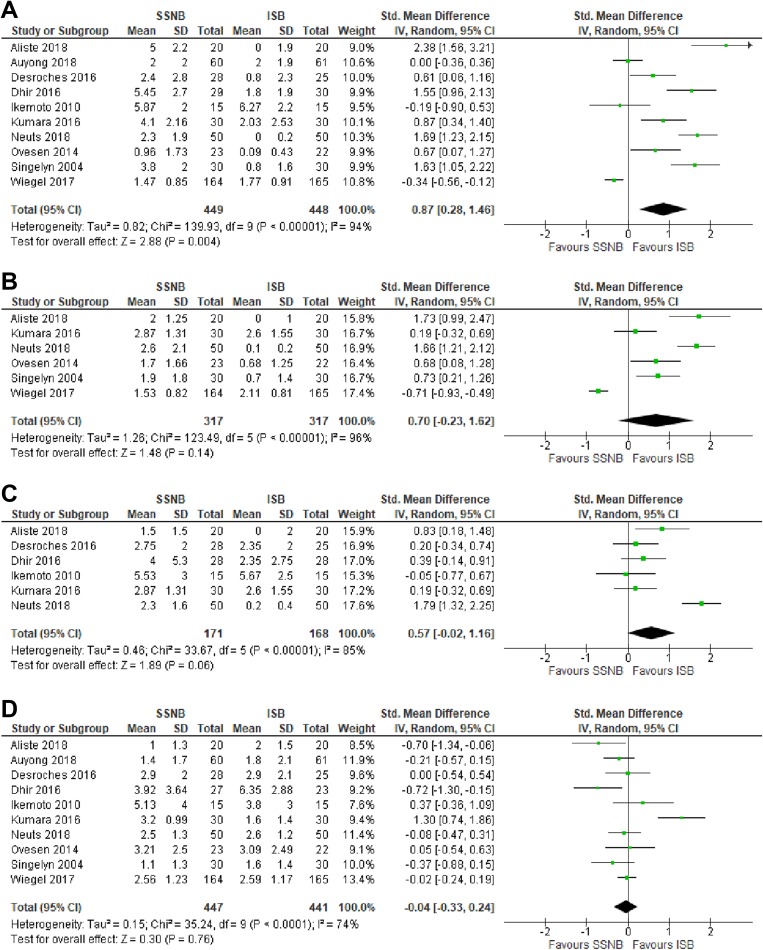
Ten studies compared SSNB versus ISB.# All 10 studies reported pain scores while patients were recovering in the postanesthesia care unit (PACU). Pooled VAS and NRS scores from these studies revealed significantly less effective pain control in the SSNB group compared with the ISB group (SMD, 0.87; 95% CI, 0.28 to 1.46; P = .004; I 2 = 94%) (Figure 4A). Six studies1,27,36,37,47,49 compared postoperative pain control at 3 to 4 hours postoperatively, with no significant difference found between the SSNB and ISB groups (SMD, 0.70; 95% CI, –0.23 to 1.62; P = .14; I 2 = 96%) (Figure 4B). Postoperative pain at 6 to 8 hours was compared in 6 studies,1,6,7,21,27,36 with no significant difference found between the SSNB and ISB groups (SMD, 0.57; 95% CI, –0.02 to 1.16; P = .06; I 2 = 85% ) (Figure 4C). Pain at 24 hours postoperatively was reported in all 10 studies, with no significant difference found between SSNB and ISB (SMD, –0.04; 95% CI, –0.33 to 0.24; P = .76; I 2 = 74%) (Figure 4D).
Figure 4.
Forest plots of standard mean difference between suprascapular nerve block (SSNB) group and interscalene brachial plexus block (ISB) group for pain scores (A) while patients were recovering in the postanesthesia care unit and (B) 3 to 4 hours, (C) 6 to 8 hours, and (D) 24 hours postoperatively.
Opioid Use
Twelve studies examined opioid use postoperatively.
SSNB Versus ISB
Ten studies** assessed the difference in opioid consumption in patients with SSNB compared with ISB. Three of these studies6,40,47 assessed the consumption in terms of percentage of patients requiring opioids. Two studies6,40 reported a higher percentage of PACU patients requiring morphine in the SSNB group (39% and 53%) compared with the ISB group (12% and 12%) (P = .03 and P = .009). Conversely, no significant difference was found in patients requiring morphine at 24 hours postoperatively in the SSNB group (4% and 15%) compared with the ISB group (20% and 41%) (P = .08 and P = .16). Two studies36,49 assessed the use of postoperative piritramide. One study36 identified higher consumption in the SSNB compared with the ISB group within 8 hours of surgery (P = .004); however, there was no difference at 24 hours (P = .30). The other study49 found no difference in the percentage of patients in the SSNB or ISB groups who required opioids in the PACU (7.3% and 7.3%, not significant). Three studies2,7,47 assessed the quantity of opioids required in the PACU. Dhir et al7 and Singelyn et al47 reported significantly less morphine consumption by the ISB group (mean of 5.82 ME [morphine equivalents] and 0.5 ± 1.5 mg, respectively) compared with the SSNB group (mean of 13.97 ME and 4 ± 5 mg, respectively) (P < .001). In contrast, Auyong et al2 found no significant difference in PACU opioid consumption between the SSNB group (0.52 ± 0.59 mg) and the ISB group (0.51 ± 0.85 mg).
SSNB Versus Placebo
Three studies21,30,37 examined opioid use in SSNB compared with control groups. Lee et al30 compared SSNB and placebo injection, reporting that significantly fewer fentanyl boluses were required for the SSNB group. Ikemoto et al21 and Ovesen et al37 similarly compared consumption of opioids in control groups (1.2 ampoules of opioids and 5.67 ± 10.46 mg of morphine, respectively) and SSNB groups (1.0 ampoule of opioids and 3.65 ± 7.71 mg of morphine, respectively); however, the differences did not meet the threshold of significance.
Length of Stay
There were 3 studies2,6,30 that assessed length of stay outcomes. No significant difference was found in length of stay between the SSNB, ISB, and control groups.
Patient Satisfaction
A total of 7 studies1,2,7,23,36,47,49 reported on patient satisfaction. Two studies, using a numeric rating scale from 0 to 100, found that ISB resulted in higher satisfaction at 6 hours (92 ± 14 vs 78 ± 2.7; P = .02)7 and 24 hours (87 ± 12 vs 82 ± 17; P = .01) postoperatively.47 Another study49 reported higher satisfaction in the SSNB group, with 91% satisfied or highly satisfied with the block, compared with 79% of patients who received the ISB (P = .01). One study23 found that when compared with placebo, SSNB resulted in significantly higher patient satisfaction at 48 hours (97 ± 5 vs 83 ± 12; P < .001) and at 14 days (95 ± 6 vs 79 ± 13; P = .001) postoperatively. Three other studies1,2,36 reported no significant difference in patient satisfaction between the SSNB and ISB groups at 24 hours postoperatively.
Complications
Twelve studies†† reported on complications and side effects arising from the use of SSNB. Out of 257 cases for which the presence or absence of pneumothorax was reported, no cases of pneumothorax were associated with SSNB, whereas 1 case of pneumothorax was reported with the use of ISB (0.39%).6 No other major complications, such as neurovascular impairment, cardiovascular collapse, or seizure, were reported following the use of SSNB. ISB was associated with increased rates of hoarseness (identified in a combined rate of 24/338 cases [7.1%]) and Horner syndrome (identified in a combined rate of 34/338 cases [10%]) when compared with SSNB (combined rates of 5/262 [1.9%] and 5/262 [1.9%], respectively).49 Prolonged motor block (deficits in motor function of the hand and wrist at 24 hours) was also a concern with ISB (combined rate of 29/199 [14.6%]), with significantly higher rates than SSNB (combined rate of 3/198 [1.5%]).7,40,49 Motor block at 24 hours was assessed by use of a 0 to 2 scale (0, complete; 1, incomplete; 2, none) in 2 of the studies,7,40 whereas 1 study49 used grip strength as the surrogate for motor block and used the Modified Medical Research Council Scale to measure hand muscle strength.43
Three studies37,40,47 compared symptoms of nausea and vomiting between SSNB and ISB. One study47 found that the incidence of nausea and vomiting was lower in the ISB group, whereas the other 2 studies37,40 identified no significant difference. One study30 compared nausea and vomiting associated with SSNB and placebo and found no significant difference.
Discussion
The most significant finding of the present systematic review is that SSNB resulted in significantly improved pain control in the first 24-hour postoperative period compared with non–nerve block control groups. However, patients who received SSNB demonstrated significantly greater pain and increased opioid consumption compared with patients receiving ISB in the early postoperative period. SSNB may be associated with fewer major (pneumothorax, Horner syndrome) and minor (prolonged motor block, hoarseness) complications than ISB. The studies included in this review were from Asia, Europe, South America, and Canada.
As discussed, ISB has historically been considered the gold standard for regional pain blocks for pain control following shoulder arthroscopy. As well as providing postoperative anesthesia, ISB can be used to provide surgical anesthesia for patients undergoing shoulder arthroscopy. This may explain the improved pain control in the immediate postoperative period in patients receiving ISB. However, SSNB has been investigated recently given its theoretical efficacy along with the possibility of a reduced complication risk. The present meta-analysis of RCTs found that up to 24 hours postoperatively, SSNB significantly reduced pain in patients undergoing shoulder arthroscopy compared with those who received no nerve block. However, SSNB provided inferior pain control compared with ISB, particularly in the short-term period (within 6 hours postoperatively). At 24 hours postoperatively, no difference was found in pain control between the SSNB and the ISB groups. The 30% of the joint and capsule that is innervated by the lateral pectoral and axillary nerves rather than the suprascapular nerve may explain this imperfect early pain control in the SSNB groups. Moreover, the suprascapular nerve rarely has cutaneous innervation, and therefore the SSNB does not provide analgesia for the pain from skin incisions.
It is thought that the 30% of the shoulder joint that is not innervated by the suprascapular nerve is likely mostly innervated by the axillary nerve.41 It has therefore been hypothesized that the combination of an SSNB with an axillary nerve block would provide patients with effective pain control postoperatively.7 The axillary nerve block in combination with SSNB was found to provide improved postoperative pain control compared with SSNB alone in 1 study.38 Dhir et al7 found that the combination of axillary and suprascapular nerve blocks provided improved pain control, even compared with ISB, at 24 hours postoperatively; however, ISB was superior immediately postoperatively when the patients were still in the PACU. Although effective, the combination of both SSNB and an axillary nerve block has the disadvantage of taking twice as much time as a single nerve block and would therefore only be practical in centers that have dedicated “block rooms” outside the operating room.
The SSNB can be guided by use of anatomic landmarks alone (blind) or with the assistance of modalities for a guided approach.19,22 Electrophysiology-guided SSNB has been shown to provide more effective blocks than blind techniques.24,50 Ultrasound guidance has been reported to improve the accuracy and efficacy of suprascapular nerve blocks by allowing the provider to visualize the suprascapular nerve with high-resolution transducers.39 The technique for SSNB reported in the studies we reviewed typically involved identification of the midpoint of the spine of the scapula with the patient in lateral decubitus position. The needle was then introduced approximately 1 cm cephalad to this landmark and advanced parallel to the blade until the floor of the supraspinatus fossa was reached. Anywhere from 10 to 20 mL of lidocaine, ropivacaine, or bupivacaine was then injected. One study25 that compared the blind SSNB with both the electrophysiology-guided SSNB and the ultrasound-guided block found that the latter two guided blocks may provide improved pain relief over the blind techniques. Furthermore, the anterior approach to the suprascapular nerve was uniquely used by 1 study included in this review.49 Such an approach would not have been possible before the availability of ultrasound guidance. Another unique approach was described in the study by Lee et al,30 who used an arthroscopically guided approach to the suprascapular nerve. Such approaches must be considered when interpreting the results from these studies.
The technique for ISB reported in the studies we reviewed involved introduction of the needle through the middle of the scalene muscle at the level of C6 with or without ultrasound guidance and/or neurostimulation. Anywhere from 10 to 20 mL of lidocaine, ropivacaine, or bupivacaine was then injected. Whereas ISB can provide excellent postoperative pain control after arthroscopic shoulder surgery,46 1 study34 found that 16% of patients reported immediate block side effects, with more than 4% reporting persistent neurological complications. The rates of persistent neurological complications following ISB ranged from to 2.5% to 4.2% in other large trials.4,48 Moreover, a rebound phenomenon of increased pain after 12 hours postoperatively has been reported following ISB.35 While increased pain and prolonged neurological deficits are important complications to consider, the major complications such as cardiac arrest, pneumothorax, respiratory distress, phrenic nerve palsy, and central nervous toxicity are key factors that cause surgeons and anesthesiologists to consider other modalities for postoperative pain control.34,42 Another group of patients who may not receive complete benefit from ISB includes those with obesity. In a study of 528 patients receiving ISB, Schroeder et al44 found that those with an increased body mass index required longer times to complete the block and reported inferior pain control postoperatively. SSNB has been proposed as a safe and efficacious alternative to ISB for postoperative pain control following shoulder surgery.8 Similarly, the present review found that fewer instances of major complications such as pneumothorax, moderate complications including Horner syndrome, and minor complications such as hoarseness and prolonged motor block occur in patients receiving SSNB compared with ISB.
Strengths and Limitations
Strengths of this systematic review include the assessment of RCTs, which inherently have less bias. Additionally, the GRADE system was used for assessment of study quality, considering a range of quality measures to ensure the accurate and comprehensive assessment of the overall body of evidence. However, this systematic review was limited to the quality of the individual studies included. The most significant source of bias identified was related to the selective and incomplete reporting of outcomes. For example, studies that assessed multiple time intervals and reported only those that entailed significant results contributed to an overestimation of the overall effect of an intervention. Other bias inherent to the present review relates to the inability to blind the operator or clinician to the technique being performed because of the nature of the interventions. Techniques to blind the outcome assessors, such as concealment of the block sites, were used in some studies but not all.7 Furthermore, heterogeneity was found across the included studies in terms of nerve block techniques as well as outcome measures and timing of assessment, which precluded the pooling of many of the secondary outcomes.
Another limitation relates to the techniques with which the blocks were performed in the individual studies, particularly the strategies used for landmarking. In 1 of the studies6 included in this review, ultrasound guidance was used to perform the ISB, but landmarks alone were used for SSNB. In another study,6 ISB was carried out via neurostimulation, whereas SSNB was performed using only a landmark-based technique. Such differences in landmarking may have contributed to differences noted between blocks within the individual studies. Further, we could not account for the potential differences in the skill level of the anesthesiologists performing the nerve blocks across the included studies. We excluded non–English language studies, and this may have left out some studies that would have changed the results. We found significant statistical heterogeneity across studies, measured using the I 2 statistic for several of the assessed SMDs which reduced our confidence in the pooled results. However, we combined the rates using a random-effects model in a meta-analysis of proportions to account for these differences. All nerve blocks and studies assessing them should have a reported failure rate to allow proper assessment of the success of the block. Failure rates were poorly defined across the included studies, with only 1 study49 reporting the criteria used to categorize a block failure. Further large-scale RCTs comparing the efficacy and complication rates of SSNB and ISB are indicated in order to elucidate definitive conclusions.
Conclusion
Although not more efficacious than ISB in terms of pain control, the use of SSNB provides patients undergoing shoulder arthroscopy with significantly improved pain control compared with patients receiving analgesia without a nerve block. Moreover, SSNB is a safe procedure with few major and minor complications reported.
Appendix
TABLE A1.
Detailed Search Strategy
| EMBASE: 416 Studies | MEDLINE: 469 Studies | PubMed: 679 Studies | |||
|---|---|---|---|---|---|
| Strategy | No. of Studies Identified | Strategy | No. of Studies Identified | Strategy | No. of Studies Identified |
| 1) arthroscopy.mp. or arthroscopy/ or shoulder arthroscopy/ | 32,006 | 1) arthroscopy.mp. or Arthroscopy/ | 25,720 | 1) arthroscop* | 33,666 |
| 2) shoulder surgery/ or shoulder/ or shoulder.mp. | 87,311 | 2) Shoulder Joint/ or Shoulder/ or shoulder.mp. | 67,877 | 2) shoulder | 70,181 |
| 3) nerve block.mp. or nerve block/ | 29,830 | 3) nerve block.mp. or Nerve Block/ | 21,771 | 3) ((nerve block) OR local OR regional OR suprascapular) | 1,053,052 |
| 4) suprascapular.mp. | 1693 | 4) regional block.mp. or Pain, Postoperative/ or Anesthetics, Local/ | 61,767 | 4) 1 AND 2 AND 3 | 679 |
| 5) local anesthetic agent/ or regional anesthesia/ or regional block.mp. | 43,757 | 5) suprascapular.mp. | 1247 | ||
| 6) 3 or 4 or 5 | 67,414 | 6) 3 or 4 or 5 | 77,326 | ||
| 7) 1 and 2 and 6 | 416 | 7) 1 and 2 and 6 | 469 | ||
Footnotes
One or more of the authors has declared the following potential conflict of interest or source of funding: G.A. receives royalties from ConMed Linvatec, Inspace Orthospace, Exactech, and Wright Medical. O.R.A. is part of the speakers’ bureau for ConMed and the Arthroscopy Association of Canada. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
References
- 1. Aliste J, Bravo D, Finlayson RJ, Tran DQ. A randomized comparison between interscalene and combined infraclavicular-suprascapular blocks for arthroscopic shoulder surgery. Can J Anaesth. 2018;65(3):280–287. [DOI] [PubMed] [Google Scholar]
- 2. Auyong DB, Hanson NA, Joseph RS, Schmidt BE, Slee AE, Yuan SC. Comparison of anterior suprascapular, supraclavicular, and interscalene nerve block approaches for major outpatient arthroscopic shoulder surgery. Anesthesiology. 2018;129(1):47–57. [DOI] [PubMed] [Google Scholar]
- 3. Balshem H, Helfand M, Schünemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401–406. [DOI] [PubMed] [Google Scholar]
- 4. Candido KD, Sukhani R, Doty R, et al. Neurologic sequelae after interscalene brachial plexus block for shoulder/upper arm surgery: the association of patient, anesthetic, and surgical factors to the incidence and clinical course. Anesth Analg. 2005;100(5):1489–1495. [DOI] [PubMed] [Google Scholar]
- 5. Chung F, Ritchie E, Su J. Postoperative pain in ambulatory surgery. Anesth Analg. 1997;85(4):808–816. [DOI] [PubMed] [Google Scholar]
- 6. Desroches A, Klouche S, Schlur C, Bauer T, Waitzenegger T, Hardy P. Suprascapular nerve block versus interscalene block as analgesia after arthroscopic rotator cuff repair: a randomized controlled noninferiority trial. Arthroscopy. 2016;32(11):2203–2209. [DOI] [PubMed] [Google Scholar]
- 7. Dhir S, Sondekoppam RV, Sharma R, Ganapathy S, Athwal GS. A comparison of combined suprascapular and axillary nerve blocks to interscalene nerve block for analgesia in arthroscopic shoulder surgery: an equivalence study. Reg Anesth Pain Med. 2016;41(5):564–571. [DOI] [PubMed] [Google Scholar]
- 8. Fernandes MR, Barbosa MA, Sousa ALL, Ramos GC. Suprascapular nerve block: important procedure in clinical practice, part II. Rev Bras Reumatol. 2012;52(4):616–622. [PubMed] [Google Scholar]
- 9. Fontana C, Di Donato A, Di Giacomo G, et al. Postoperative analgesia for arthroscopic shoulder surgery: a prospective randomized controlled study of intraarticular, subacromial injection, interscalenic brachial plexus block and intraarticular plus subacromial injection efficacy. Eur J Anaesthesiol. 2009;26(8):689–693. [DOI] [PubMed] [Google Scholar]
- 10. Fredrickson MJ, Krishnan S, Chen CY. Postoperative analgesia for shoulder surgery: a critical appraisal and review of current techniques. Anaesthesia. 2010;65(6):608–624. [DOI] [PubMed] [Google Scholar]
- 11. Fujimura N, Namba H, Tsunoda K, et al. Effect of hemidiaphragmatic paresis caused by interscalene brachial plexus block on breathing pattern, chest wall mechanics, and arterial blood gases. Anesth Analg. 1995;81(5):962–966. [DOI] [PubMed] [Google Scholar]
- 12. Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383–394. [DOI] [PubMed] [Google Scholar]
- 13. Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 7. Rating the quality of evidence—inconsistency. J Clin Epidemiol. 2011;64(12):1294–1302. [DOI] [PubMed] [Google Scholar]
- 14. Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 8. Rating the quality of evidence—indirectness. J Clin Epidemiol. 2011;64(12):1303–1310. [DOI] [PubMed] [Google Scholar]
- 15. Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 6. Rating the quality of evidence—imprecision. J Clin Epidemiol. 2011;64(12):1283–1293. [DOI] [PubMed] [Google Scholar]
- 16. Guyatt GH, Oxman AD, Montori V, et al. GRADE guidelines: 5. Rating the quality of evidence—publication bias. J Clin Epidemiol. 2011;64(12):1277–1282. [DOI] [PubMed] [Google Scholar]
- 17. Guyatt GH, Oxman AD, Vist G, et al. GRADE guidelines: 4. Rating the quality of evidence—study limitations (risk of bias). J Clin Epidemiol. 2011;64(4):407–415. [DOI] [PubMed] [Google Scholar]
- 18. Hadzic A, Williams BA, Karaca PE, et al. For outpatient rotator cuff surgery, nerve block anesthesia provides superior same-day recovery over general anesthesia. Anesthesiology. 2005;102(5):1001–1007. [DOI] [PubMed] [Google Scholar]
- 19. Harmon D, Hearty C. Ultrasound-guided suprascapular nerve block technique. Pain Physician. 2007;10(6):743–746. [PubMed] [Google Scholar]
- 20. Hawker G, Mian S, Kendzerska T, French M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res (Hoboken). 2011;63(suppl 11):S240–S252. [DOI] [PubMed] [Google Scholar]
- 21. Ikemoto RY, Murachovsky J, Prata Nascimento LG, et al. Prospective randomized study comparing two anesthetic methods for shoulder surgery. Rev Bras Ortop. 2010;45(4):395–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jerosch J, Saad M, Greig M, Filler T. Suprascapular nerve block as a method of preemptive pain control in shoulder surgery. Knee Surg Sports Traumatol Arthrosc. 2008;16(6):602–607. [DOI] [PubMed] [Google Scholar]
- 23. Jeske H-C, Kralinger F, Wambacher M, et al. A randomized study of the effectiveness of suprascapular nerve block in patient satisfaction and outcome after arthroscopic subacromial decompression. Arthroscopy. 2011;27(10):1323–1328. [DOI] [PubMed] [Google Scholar]
- 24. Karataş GK, Meray J. Suprascapular nerve block for pain relief in adhesive capsulitis: comparison of 2 different techniques. Arch Phys Med Rehabil. 2002;83(5):593–597. [DOI] [PubMed] [Google Scholar]
- 25. Ko SH, Kang BS, Hwang CH. Ultrasonography- or electrophysiology-guided suprascapular nerve block in arthroscopic acromioplasty: a prospective, double-blind, parallel-group, randomized controlled study of efficacy. Arthroscopy. 2013;29(5):794–801. [DOI] [PubMed] [Google Scholar]
- 26. Krone S. Analgesic effects of low-dose ropivacaine for interscalene brachial plexus block for outpatient shoulder surgery—a dose-finding study. Reg Anesth Pain Med. 2001;26(5):439–443. [DOI] [PubMed] [Google Scholar]
- 27. Kumara AB, Gogia AR, Bajaj JK, Agarwal N. Clinical evaluation of post-operative analgesia comparing suprascapular nerve block and interscalene brachial plexus block in patients undergoing shoulder arthroscopic surgery. J Clin Orthop Trauma. 2016;7(1):34–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159. [PubMed] [Google Scholar]
- 29. Laurila PA, Löppönen A, Kanga-Saarela T, Flinkkilä T, Salomäki TE. Interscalene brachial plexus block is superior to subacromial bursa block after arthroscopic shoulder surgery. Acta Anaesthesiol Scand. 2002;46(8):1031–1036. [DOI] [PubMed] [Google Scholar]
- 30. Lee JJ, Yoo Y-S, Hwang J-T, et al. Efficacy of direct arthroscopy-guided suprascapular nerve block after arthroscopic rotator cuff repair: a prospective randomized study. Knee Surg Sports Traumatol Arthrosc. 2015;23:562–566. [DOI] [PubMed] [Google Scholar]
- 31. Lenters TR, Davies J, Matsen FA. The types and severity of complications associated with interscalene brachial plexus block anesthesia: local and national evidence. J Shoulder Elbow Surg. 2007;16(4):379–387. [DOI] [PubMed] [Google Scholar]
- 32. Manchikanti L, Fellows B, Ailinani H, Pampati V. Therapeutic use, abuse, and nonmedical use of opioids: a ten-year perspective. Pain Physician. 2010;13(5):401–435. [PubMed] [Google Scholar]
- 33. Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22(4):719–748. [PubMed] [Google Scholar]
- 34. Misamore G, Webb B, McMurray S, Sallay P. A prospective analysis of interscalene brachial plexus blocks performed under general anesthesia. J Shoulder Elbow Surg. 2011;20(2):308–314. [DOI] [PubMed] [Google Scholar]
- 35. Nam YS, Jeong JJ, Han SH, et al. An anatomic and clinical study of the suprascapular and axillary nerve blocks for shoulder arthroscopy. J Shoulder Elbow Surg. 2011;20(7):1061–1068. [DOI] [PubMed] [Google Scholar]
- 36. Neuts A, Stessel B, Wouters PF, et al. Selective suprascapular and axillary nerve block versus interscalene plexus block for pain control after arthroscopic shoulder surgery. Reg Anesth Pain Med. 2018;43(7):738–744. [DOI] [PubMed] [Google Scholar]
- 37. Ovesen J, Falstie-Jensen T, Christensen C. A comparison of subacromial bursae block, suprascapular nerve block and interscalene brachial plexus block after arthroscopic shoulder surgery. Pain Stud Treat. 2014;2(3):107–112. [Google Scholar]
- 38. Park JY, Bang JY, Oh KS. Blind suprascapular and axillary nerve block for post-operative pain in arthroscopic rotator cuff surgery. Knee Surg Sports Traumatol Arthrosc. 2016;24(12):3877–3883. [DOI] [PubMed] [Google Scholar]
- 39. Peng PWH, Wiley MJ, Liang J, Bellingham GA. Ultrasound-guided suprascapular nerve block: a correlation with fluoroscopic and cadaveric findings. Can J Anesth. 2010;57(2):143–148. [DOI] [PubMed] [Google Scholar]
- 40. Pitombo PF, Meira Barros R, Matos MA, Pinheiro Módolo NS. Selective suprascapular and axillary nerve block provides adequate analgesia and minimal motor block: comparison with interscalene block. Braz J Anesthesiol. 2013;63(1):45–51. [DOI] [PubMed] [Google Scholar]
- 41. Price DJ. Combined suprascapular and axillary nerve blockade. Arthroscopy. 2008;24(11):1316–1317. [DOI] [PubMed] [Google Scholar]
- 42. Robaux S, Bouaziz H, Boisseau N, Raucoules-Aimé M, Laxenaire MC; S.O.S. Regional Hot Line Service. Persistent phrenic nerve paralysis following interscalene brachial plexus block. Anesthesiology. 2001;95(6):1519–1521. [DOI] [PubMed] [Google Scholar]
- 43. Schreuders TAR. Sensible manual muscle strength testing to evaluate and monitor strength of the intrinsic muscles of the hand: a commentary. J Hand Ther. 2001;14(4):273–278. [DOI] [PubMed] [Google Scholar]
- 44. Schroeder K, Andrei A-C, Furlong MJ, Donnelly MJ, Seungbong H, Becker AM. Efeito perioperatório do índice de massa corporal elevado no bloqueio do nervo periférico: uma análise de 528 bloqueios interescalênicos guiados por ultrassom. Rev Bras Anestesiol. 2012;62(1):33–38. [Google Scholar]
- 45. Seyfried O, Hester J. Opioids and endocrine dysfunction. Br J Pain. 2012;6(1):17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shin S-W, Byeon G-J, Yoon J-U, et al. Effective analgesia with ultrasound-guided interscalene brachial plexus block for postoperative pain control after arthroscopic rotator cuff repair. J Anesth. 2014;28(1):64–69. [DOI] [PubMed] [Google Scholar]
- 47. Singelyn FJ, Lhotel L, Fabre B. Pain relief after arthroscopic shoulder surgery: a comparison of intraarticular analgesia, suprascapular nerve block, and interscalene brachial plexus block. Anesth Analg. 2004;99(2):589–592. [DOI] [PubMed] [Google Scholar]
- 48. Webb BG, Sallay PI, McMurray SD, Misamore GW. Comparison of interscalene brachial plexus block performed with and without steroids. Orthopedics. 2016;39(6):e1100–e1103. [DOI] [PubMed] [Google Scholar]
- 49. Wiegel M, Moriggl B, Schwarzkopf P, Petroff D, Reske AW. Anterior suprascapular nerve block versus interscalene brachial plexus block for shoulder surgery in the outpatient setting: a randomized controlled patient- and assessor-blinded trial. Reg Anesth Pain Med. 2017;42(3):310–318. [DOI] [PubMed] [Google Scholar]
- 50. Wilbourn AJ. Nerve conduction studies: types, components, abnormalities, and value in localization. Neurol Clin. 2002;20(2):305–338. [DOI] [PubMed] [Google Scholar]