Abstract
Background. Increasing evidence suggests that vigorous physical activity (VPA) in youth may yield greater health benefits than moderate (MPA) or moderate-to-vigorous physical activity (MVPA). The purpose of this review was to assess the relationship between PA intensity and body composition, cardiorespiratory fitness (CRF), and cardiometabolic (CM) biomarkers in youth. Methods. We conducted a systematic review of observational studies examining PA intensity and selected health outcomes in youth aged 6 to 18 years. Forty-five articles were selected for final review. Results. VPA was more strongly associated with reduced body fat and central adiposity compared with MPA and/or MVPA. Additionally, VPA was more strongly associated with increased CRF when compared with lower intensities. Findings were inconclusive between all PA intensity levels and CM biomarkers, and several significant relationships observed for VPA were attenuated when controlling for CRF. Conclusions. A potential VPA dose is identified as yielding favorable health benefits in adiposity and fitness. While CM biomarkers were not consistently associated with PA intensity level, the literature suggests VPA may yield health benefits above those received from MPA for reduced adiposity and improved CRF. This review highlights the need for longitudinal observational and experimental studies to determine optimal VPA dose for CM health in youth.
Keywords: vigorous physical activity, intensity, youth, biomarkers
‘Accumulating evidence suggests that VPA [vigorous physical activity] may be more beneficial than moderate PA (MPA) for several health outcomes among youth.’
Among youth, substantial evidence has documented that physical activity (PA) is associated with several favorable health outcomes, including body composition, fitness, and an optimal cardiometabolic (CM) profile.1,2 On the weight of this evidence, the US Department of Health and Human Services (USDHHS) developed PA guidelines, which recommend that youth engage in 60 minutes of moderate-to-vigorous PA (MVPA) daily.3 Evidence from several observational studies supports that 60 minutes of MVPA per day is a sufficient dose to elicit reductions in obesity, increases in fitness, and improvements in CM biomarkers indicative of chronic disease.4-7 However, only one-fourth of youth report achieving this 60-minute MVPA recommendation,8 with objective assessments indicating considerably fewer.9
Accumulating evidence suggests that VPA may be more beneficial than moderate PA (MPA) for several health outcomes among youth.10-12 This has been observed in adult populations, where a PA intensity dose-response relationship has been demonstrated for favorable health outcomes.13-15 As a result, PA recommendations for adults include a specific dose for VPA.9 However, among youth no specific dose for VPA exists in the current PA guidelines.3
While previous reviews have assessed the influence of PA intensity on health outcomes among youth, many included self-reported measures of PA.11,12 Considering the ubiquitous use and superiority of objectively measured PA tools and more recently published studies, an updated review of studies evaluating the association between PA intensity and health outcomes among youth is warranted. Thus, the purpose of this review is to summarize and evaluate the scientific literature on studies assessing the impact of PA intensity on adiposity, cardiorespiratory fitness (CRF) and CM biomarkers in the youth population. Our hypothesis is that the studies in this review will demonstrate that VPA is a stronger predictor of adiposity, CRF, and CM biomarkers compared with lesser PA intensities. As such, the aim of this study is to provide an evidence base from which VPA-specific recommendations may be developed for youth.
Methods
Four electronic research databases were searched (Web of Science, PubMed-Medline, CINAHL Complete, and Physical Education Index) to identify peer-reviewed articles assessing the effect of PA intensity on health outcomes in youth. Search terms were categorized into 4 main domains: activity, age group, mode of measurement, and health outcome. The following keywords were used in varying combinations: activity (eg, “physical activity” OR “exercise” OR “sport” OR “dance”) AND youth (eg, “child” OR “adolescent” OR “teen”) AND objective monitoring (eg, “accelerometer” OR “heart rate monitor”) AND health outcomes (eg, “BMI” OR “adiposity” OR “fitness” OR “performance” OR “blood pressure” OR “cholesterol” OR “triglycerides” OR “glucose” OR “insulin”). Age group was limited to youth aged 6 to 18 years. There were no restrictions for publication date. In order to be eligible for analysis the studies must have satisfied the following inclusion criteria:
Observational study design (eg, cohort or cross-sectional)
Utilized objective measures of PA (eg, accelerometers or heart rate monitors)
Published in peer-reviewed journals in the English language
Intervention studies were not included, as the focus of this review is on the benefits of PA incorporated into daily activity as opposed to the effects of acute changes in PA over a limited duration. In addition, all studies must have independently evaluated and reported the relationship between VPA and health outcomes of interest. For example, studies that assessed MVPA but did not evaluate VPA separately were excluded. Moreover, accelerometer validation studies as well as those assessing PA as an outcome variable were excluded. Studies drawing from the same cohort were included if they reported different outcomes.
Data Extraction
Data were extracted by the primary author (MG) from all selected articles, including sample size, participant demographics (age, sex, race/ethnicity, country), and the identified cohort when applicable. Information on PA assessment methods was also gathered, which included details regarding the assessment tool (accelerometer model) and PA intensity cut-points (eg, accelerometer counts). Statistical results describing the association between VPA and selected health outcomes were extracted to examine the magnitude and directionality of the relationship. For studies that also assessed the relationship between MPA and/or MVPA for the selected health outcomes, similar statistical information was extracted for comparison purposes.
Two additional reviewers (SM and CB) interpreted the statistical data to determine the independent associations of PA intensities (MPA, MVPA, VPA) with health outcomes. In the case where partial correlations and regression data were both reported, results from regression models were chosen to represent the relationship between PA intensity and the health outcome of interest. Studies only reporting unadjusted bivariate associations were removed from the review, as established covariates were not adjusted for, to reduce bias in the findings.
Results
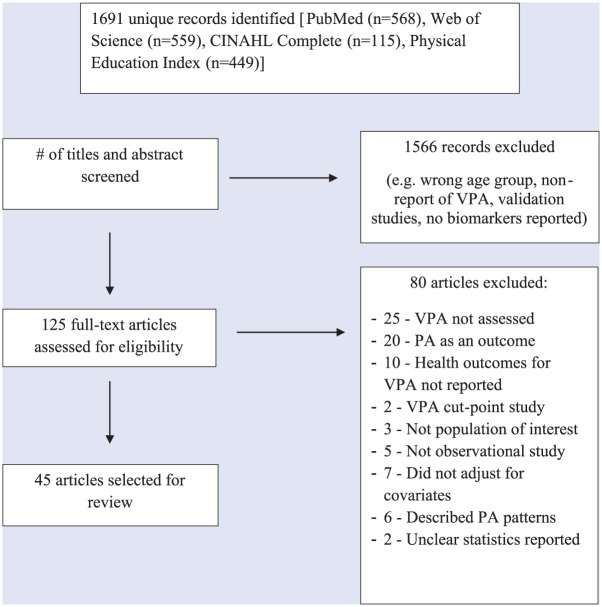
A flowchart describing the study selection process is depicted in Figure 1. Initial searches for all databases yielded a total of 1691 records. Titles and abstracts were examined, and of these, 1566 were excluded yielding 125 articles eligible for the initial review. After a full-text review, 46 articles were eliminated for failing to meet inclusion criteria such as it did not assess VPA (54%; n = 25) or did not assess health outcomes of interest (22%, n = 10), resulting in 79 articles for further review. Of these, an additional 34 articles were removed for reasons including assessment of PA intensity as an outcome variable (59%; n = 20) or failing to adjust for covariates (20.6%; n = 7), resulting in 45 articles eligible for the final review.
Figure 1.
Flowchart of study selection and ascertainment processes.
Demographics
Participant demographics are presented in Table 1. Geographical locations varied across the studies with 7 conducted in the United States,16-22 4 in Canada,23-26 and 33 in Europe.27-57 One study was conducted internationally7 and the final study did not specify location.58 Sample sizes varied considerably ranging from 36 to 6539 participants.7,38 A majority of articles (~96%) used a sample that included children and adolescents of both sexes and the remaining studies consisted of female-only16 or male-only57 samples. Several individual studies were derived from numerous large-scale studies, including the European Youth Heart Study (EYHS; n = 6)31,32,41,42,44,46 and Healthy Lifestyle in Europe by Nutrition in Adolescence (HELENA; n = 6).33,35,36,39,40,54
Table 1.
Sample Demographics From Articles Reporting the Relationship Between Moderate Physical Activity, Moderate-to-Vigorous Physical Activity, and Vigorous Physical Activity and Measures of Body Composition, Cardiorespiratory Fitness, and Cardiometabolic Biomarkers in Youth.
| Authors (Year) | N | Females (n) | Males (n) | Average Age (Years) | Race/Ethnicity | Average Weight Status | Location |
|---|---|---|---|---|---|---|---|
| Aires et al (2010)26 | 111 | 62 | 49 | 14.5 | NR | All: 22.24 kg/m2
%OWOB: Males: 38.8% Females: 27.4% |
Portugal (1 school) |
| Alhassan and Robinson (2008)15 | 208 | 208 | 0 | 9.4 | African American | All: 20.7 kg/m2 | United States (RCT subsample) |
| Blaes et al (2011)27 | 187 | 101 | 86 | 9.1 | European | Males: 17.6 kg/m2
Females: 17.1 kg/m2 |
France (16 elementary school classes) |
| Butte et al (2007)16 | 897 | 456 | 441 | NW: Males: 10.7 Females: 10.7 OW: Males: 11.1 Females: 10.9 |
Hispanic | %OW: Males: 56% Females: 49.6% |
United States—VIVA la Familia Study |
| Carson et al (2014)25 | 315 | 187 | 128 | 12.2 | NR | All: 18.5 kg/m2
Males: 18.7 kg/m2 Females: 18.4 kg/m2 |
Canada—Healthy Hearts Prospective Cohort Study of PA and CM Health in Youth |
| Chaput et al (2012)22 | 550 | 251 | 299 | 9.6 | European Canadian | %OW: Males: 20% Females: 17% %OB: Males: 22% Females: 23% |
Canada, Quebec—QUALITY study |
| Danielsen et al (2011)28 | 86 | 38 | 48 | 11.0 | European | NW: BMImean: 17.12kg/m2 OB: BMImean: 28.41kg/m2 |
Norway—Treatment program for families with obese children |
| Dencker et al (2010)48 | 468 | 222 | 246 | 6.7 | European | Males: 15.9 kg/m2
Females: 16.0 kg/m2 |
Denmark—Copenhagen School Child Intervention Study subsample (46 preschool classes in 18 schools) |
| Dencker et al (2008)59 | 225 | 101 | 124 | Males: 9.8 Females: 9.8 |
European | Males: 17.4 kg/m2
Females: 17.5 kg/m2 |
Sweden (4 schools, grades 3-4) |
| Dencker et al (2008)49 | 158 | 76 | 82 | Males: 9.9 Females: 9.8 |
European | All: 17.4 kg/m2 | Sweden (4 schools, grades 3-4) |
| Dencker et al (2007)50 | 226 | 101 | 125 | Males: 9.9 Females: 9.7 |
European | All: 17.4 kg/m2 | Sweden (4 schools, grades 3-4) |
| Dencker et al (2006)51 | 248 | 108 | 140 | Males: 9.9 Females: 9.7 |
European | All: 17.4 kg/m2 | Sweden (4 schools, grades 3-4) |
| Denton et al (2013)29 | 1292 | 654 | 638 | 9.7 | European | All: 17.2 kg/m2 | Denmark, Estonia, Portugal, Norway—EYHS subsample |
| Dubose et al (2015)57 | 72 | 61% | 39% | 9.5 | European American: 60% | All: BMImean: 22.1 kg/m2 %OW: 16% %OB: 45% |
United States |
| Ekelund et al (2007)30 | 9-10 y: 1008 15-16 y: 738 |
9-10 y: 504 15-16 y: 404 |
9-10 y: 504 15-16 y: 334 |
9-10; 15-16 | European | 9-10 y: Males: 17.4 kg/m2 Females: 17.2 kg/m2 15-16 y: Males: 20.8 kg/m2 Females: 20.8 kg/m2 |
Denmark, Estonia, Portugal—EYHS subsample |
| Ekelund et al (2004)31 | 409 | 224 | 185 | 11 | European | %OW: Males: 18.3% Females: 18.8% %OB: Males: 3.2% Females: 4.9% |
England (Northwest; 8 schools) |
| España-Romero et al (2010)32 | 254 | 132 | 122 | 12.5-17.5 | European | NR | Spain—HELENA study subsample |
| Fairclough et al (2012)55 | 409 | 224 | 185 | 11 | European | %OW: Males: 18.3% Females: 18.8% %OB Males: 3.2% Females: 4.9% |
England (Northwest; 8 schools) |
| Gaya et al (2009)33 | 163 | 97 | 66 | 13.91-14.02 | European | NR | Portugal |
| Gutin et al (2005)17 | 421 | 225 | 196 | 16.2 | European American (48.9%) African American (51.1%) |
All: 23.1 kg/m2 | United States (high schools in Augusta, GA) |
| Hay et al (2012)23 | 605 | 357 | 248 | 12.1 | Canadian | OWOB: All: 26% |
Canada—Healthy Hearts Prospective Cohort Study of PA and CM Health in Youth Study subsample |
| Jiménez-Pavón et al (2013)34 | 2200 | 1184 | 1016 | 14.7 | European | All: 21.1 kg/m2 | Europe—HELENA Study sample |
| Jiménez-Pavón et al (2013)36 | 2025 | 987 | 1038 | 7.6 | European | %BF All: 16.9 kg/m2 Males: 16.9 kg/m2 Females: 16.9 kg/m2 |
Europe—IDEFICS study sample |
| Jiménez-Pavón et al (2013)35 | 1053 | 554 | 499 | 14.9 | European | All: 21.4 kg/m2 | Europe—HELENA Study sample |
| Katzmarzyk et al (2015)7 | 6539 | 3554 | 2985 | 10.4 | Males: 18.4 kg/m2
Females: 18.4 kg/m2 %OB: All: 12.4% (5.2% - 24.6%) Males: 15.5% Females: 9.9% |
Australia, Brazil, Canada, China, Colombia, Finland, India, Kenya, Portugal, South Africa, United Kingdom, United States—ISCOLE Study | |
| Kennedy et al (2012)37 | 36 | 16 | 20 | 6.7 | European | Males: 0.27 (BMI z-score) Females: 0.40 (BMI z-score) |
Scotland—SGA follow-up study |
| Lätt et al (2015)56 | 136 | 0 | 136 | 11.9 (baseline) | European | 20.4 kg/m2 (baseline) 21.5 kg/m2 (2-y follow-up) |
Estonia |
| Mark and Janssen (2011)18 | 1165 | 557 | 608 | 12.9 | All %NHW 26.1% %NHB 34.1% %Hispanic 36.6% %Other 3.3% |
%BF All: 29.1% | United States—NHANES 2003-2004 |
| Martínez-Gómez et al (2010)52 | 192 | 94 | 98 | Males: 14.7 Females: 15.0 |
European | Males: 21.8 kg/m2
Females: 21.7 kg/m2 |
Spain—AFINOS Study subsample |
| Martínez-Gómez et al (2010)53 | 1808 | 964 | 844 | CRF UH: 14.7 H: 14.6 |
European | CRF UH: 22.5 kg/m2 H: 20.2 kg/m2 |
Europe—HELENA Study sample |
| Martínez-Gómez et al (2009)54 | 202 | 103 | 99 | Males: 14.7 Females: 14.9 |
European | Males: 22.2 kg/m2
Females: 21.8 kg/m2 |
Spain—AFINOS Study subsample |
| Moliner-Urdiales et al (2010)38 | 363 | 180 | 183 | Males: 14.7 Females: 14.8 |
European | All: 21.2 kg/m2 | Spain—HELENA Study subsample |
| Moliner-Urdiales et al (2009)39 | 365 | 182 | 183 | 14.8 | European | All: 21.2 kg/m2 | Spain—HELENA Study subsample |
| Moore et al. (2013)19 | 285 | 160 | 125 | Middle school | %NHW 30% %AA 49% %Other 21% |
All %NW 52% %OWOB 48% |
United States (3 middle schools in North Carolina) |
| Ortega et al (2010)40 | 9 y: 557 15 y: 518 |
9 y: 288 15 y: 280 |
9 y: 269 15 y: 238 |
9.5 15.6 |
European | 9 y males: 17.2 kg/m2
9 y females: 17.3 kg/m2 15 y males: 20.7 kg/m2 15 y females: 21.2 kg/m2 |
Sweden—EYHS subsample |
| Ortega et al (2007)41 | 9 y: 557 15 y: 517 |
9 y: 288 15 y: 279 |
9 y: 269 15 y: 238 |
9.5 15.6 |
European | 9 y males: 17.2 kg/m2
9 y females: 17.3 kg/m2 15 y males: 20.7 kg/m2 15 y females: 21.2 kg/m2 |
Sweden—EYHS |
| Patrick et al (2004)20 | 878 | 471 | 407 | 12.7 | A/PI: 3.4% AA: 6.6% NA: 0.7% Hispanic: 13.1% NHW: 57.1% Other: 18.3% |
All: 23.6 kg/m2
%OW: 18.1% %OB: 27.6% |
United States—PACE+ study subsample (RCT 1 y intervention study in California) |
| Radtke et al (2013)42 | 52 | 28 | 24 | 14.5 | European | All: 19.8 kg/m2 | Switzerland (1 elementary school) |
| Rizzo et al (2008)43 | 613 | 352 | 261 | 15.5 | European | All BMI: 20.5 kg/m2 %NW 90% %OW 9% %OB 1% |
Sweden, Estonia—EYHS subsample |
| Rowlands et al (2006)44 | 76 | 38 | 38 | Males: 9.1 Females: 9.0 |
European | Males: 18.0 kg/m2
Females: 17.5 kg/m2 |
Wales (8 schools) |
| Ruiz et al (2006)45 | 780 | 401 | 379 | 9.5 | European | All: 16.9 kg/m2 | Sweden, Estonia—EYHS subsample |
| Steele et al (2009)46 | 1862 | 1042 | 820 | Males: 10.2 Females: 10.3 |
European Non-white: Males: 3.8% Females: 4% |
Males: 17.9 kg/m2
%OW: 15.9% %OB 3.9% Females: 18.4 kg/m2 %OW: 19.4% %OB 6.0% |
United Kingdom—Norfolk–SPEEDY study (92 rural and urban primary schools) |
| Tanha et al (2011)47 | 223 | 100 | 123 | 9.8 | European | Males: 17.4 kg/m2
Females: 17.5 kg/m2 |
Sweden (4 schools, grades 3-4) |
| Treuth et al (2005)21(p200) | 229 | 130 | 99 | ES: Males: 9.3 Females: 9.2 MS: Males: 12.3 Females: 11.8 HS: Males: 15.9 Females: 15.4 |
%White: Males: 71% Females: 74% %AA: Males: 29% Females: 24% |
ES: Males: 19.98 kg/m2 Females: 19.81 kg/m2 MS: Males: 21.79 kg/m2 Females: 23.02 kg/m2 HS: Males: 22.59 kg/m2 Females: 24.23 kg/m2 |
United States (1 elementary school and 1 combined middle/high school in Maryland) |
| Wittmeier et al (2008)24 | 251 | 121 | 130 | 10 | NR | All: 18.5 kg/m2
%OWOB: 29.5% |
Canada (4 schools, grades 3-5) |
Abbreviations: y, year; N, population size; n, sample size; NR, not reported; OW, overweight; OB, obese; RCT, randomized control trial; NW, normal weight; BMI, body mass index; PA, physical activity; CM, cardiometabolic; %BF, percent body fat; SGA, small for gestational age; NHW, non-Hispanic white; NHB, non-Hispanic black; CRF, cardiorespiratory fitness; UH, unhealthy; H, healthy; A/PA, Asian/Pacific Islander; AA, African American; NA, Native American; ES, elementary school; MS, middle school; HS, high school.
Cohorts: QUALITY, Quebec Adiposity and Lifestyle Investigation in Youth; EYHS, European Youth Heart Study; HELENA, Healthy Lifestyle in Europe by Nutrition in Adolescence; IDEFICS, Identification and prevention of Dietary- and lifestyle-induced health EFfects In Children and infantS; AFINOS, La Actividad Fı’sica como Agente Preventivo del Desarrollo de Sobrepeso, Obesidad, Alergias, Infecciones y Factores de Riesgo Cardiovascular en Adolescentes: Physical Activity as a Preventive Agent of the Development of Overweight, Obesity, Infections, Allergies and Factors of Cardiovascular Risk in Adolescents; PACE+, Patient-Centered Assessment and Counseling for Exercise Plus Nutrition Project; SPEEDY, Sport, Physical Activity and Eating Behaviour, Environmental Determinants in Young People;
Outcome Variables
Because of varied methods of statistical reporting, results were categorized according to the directionality and statistical significance of the relationships for comparison within each intensity level. Magnitudes of the associations are listed in Table 2, which includes all reported relationships regardless of statistical significance. Outcomes are presented separately by sex, age, PA measurement modality, and/or analytical method employed within a study. A representation of proportional directionality of associations between PA and all health outcomes are summarized in Tables 3-5.
Table 2.
Reported Relationships Between Moderate Physical Activity, Moderate-to-Vigorous Physical Activity, and Vigorous Physical Activity and Measures of Body Composition, Cardiorespiratory Fitness, and Cardiometabolic Biomarkers in Youth.
| Author (Year) | Sample Size and Characteristics | BMI/Body Composition |
CRF |
CM Biomarkers |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MPA | MVPA | VPA | MPA | MVPA | VPA | MPA | MVPA | VPA | ||
| Aires (2010) | n = 111 Age: 11-18 y Sex: Both |
Logistic regression OR: 1.01 (0.98-1.05) |
Logistic regression OR: 1.00 (0.98-1.02) |
Logistic regression OR: 0.63 (0.33-1.21) |
— | — | — | — | — | — |
| Alhassan (2008) | n = 261 Age: 8-10 y Sex: Females Race/Ethnicity: AA |
Partial correlation BMI: N/R |
Partial correlation BMI: rs = 0.18 |
Partial correlation BMI: rs = −0.28*** |
— | — | — | Partial correlation Insulin: rs= −0.23*** |
Partial correlation SBP: −0.04 (ns) TC: −0.01(ns) LDL: 0.007 (ns) HDL: −0.01 (ns) TG: −0.04 (ns) Insulin: rs = −0.21** Glucose: −0.05 (ns) |
Partial correlation Insulin: −0.13 (ns) |
| Blaes (2011) | n = 187 Age: 6-12 y Sex: Both |
Multivariate stepwise regressiona
Males: %BF β = 0.02 (ns) (min of VPA) Females: %BF: N/R because correlations were nonsignificant |
— | Multivariate stepwise regressiona
Males: %BF: β = −0.29* (min of VPA) β = −0.05** (bout of VPA ≤15 sec) Females: %BF: N/R because correlations were nonsignificant |
— | — | — | — | — | — |
| Butte (2007) VIVA LA FAMILIA Study |
n = 897 Age: 4-19 y Sex: Both Race/Ethnicity: Hispanic |
Partial correlation %BF: r = −0.07* WC: Model 1: r = −0.05 (ns) Model 2: r = −0.01 (ns) GEE modelsa %BF β = −0.13* |
Partial correlation 5-minute bout accumulated minutes %BF: r = −0.10** WC: Model 1: r = −0.07* Model 2: r = −0.03 (ns) GEE modelsa %BF: β = −0.001** Partial correlation 10-minute bout accumulated minutes %BF: r = −0.10** WC: Model 1: r = −0.06 (ns) Model 2: r = −0.01 (ns) GEE models %BF: β = −0.001** |
Partial correlation %BF: r = −0.03 (ns) WC: Model 1: r = −0.02 (ns) Model 2: r = −0.01 (ns) |
Partial correlation VO2peak: Model 1: r = 0.09* Model 2: r = 0.06 (ns) |
Partial correlation 5-minute bout accumulated minutes VO2peak: Model 1: r = 0.16*** Model 2: r = 0.12** GEE modelsa %BF: β = 0.02** Partial correlation 10-minute bout accumulated minutes VO2peak: Model 1: r = 0.16*** Model 2: r = 0.12** GEE modelsa %BF: β = 0.03* |
Partial correlation VO2peak: Model 1: r = 0.11** Model 2: r = 0.10** |
Partial correlation Insulin: Model 1: r = −0.07* Model 2: r = −0.05 (ns) |
Partial correlation 5-minute bout accumulated minutes Insulin: Model 1: r = −0.08 (ns) Model 2: r = −0.06 (ns) GEE modelsa β = −0.02** Partial correlation 10-minute bout accumulated minutes Insulin: Model 1: r = −0.05 (ns) Model 2: r = −0.03 (ns) GEE modelsa β = −0.04(ns) Ordinal logistic regression: MetS: OR = 0.94* |
Partial correlation Insulin: Model 1: r = −0.03(ns) Model 2: r = −0.03 (ns) |
| Carson (2014) | n = 315 Age = 9.15 y Sex: Both |
Regression BMI z-score: Q1: reference Q2: β = −0.001 (ns) Q3: β = 0.05 (ns) Q4: β = −0.04 (ns) WCb: Q1: reference Q2: β = 0.02 (ns) Q3: β = 0.002 (ns) Q4: β = −0.02 (ns) |
— | Regression BMI z-score: Q1: reference Q2: β = −0.13* Q3: β = −0.15* Q4: β = −0.10 (ns) WCb: Q1: reference Q2: β = −0.02* Q3: β = −0.03* Q4: β = −0.02 (ns) |
Regression VO2max: Q1: reference Q2: β = −0.30 (ns) Q3: β = 0.04 (ns) Q4: β = 0.43 (ns) |
— | Regression VO2max: Q1: reference Q2: β = 0.81 (ns) Q3: β = 1.39 (ns) Q4: β = 1.99* |
Regression SBP: Q1: reference Q2: β = −1.32 (ns) Q3: β = 1.64 (ns) Q4: β = −0.90 (ns) |
— | Regression SBP: Q1: reference Q2: β = −1.06 (ns) Q3: β = −1.63 (ns) Q4: β = −2.41 (ns) |
| Chaput (2012) QUALITY Study |
n = 550 Age: 8- 10 y Sex: Both Race/Ethnicity: Caucasian |
Hierarchichal regression MPA min/da: %BF: Model 1: β = −0.12*** Model 2: β = −0.11*** Model 3: β= −0.06 (ns) Waist/height ratio: Model 1: β = −0.11*** Model 2: β = −0.10*** Model 3: β = −0.10** |
Hierarchichal regression MVPA min/da: %BF: Model 1: β = −0.09*** Model 2: β = −0.08*** Model 3: β = −0.05* Waist/height ratio: Model 1: β = −0.08*** Model 2: β = −0.08*** Model 3: β = −0.07*** |
Hierarchichal regression VPA min/da: %BF: Model 1: β = −0.20*** Model 2: β = −0.19*** Model 3: β = −0.11** Waist/height ratio: Model 1: β = −0.19*** Model 2: β = −0.18*** Model 3: β = −0.16*** |
— | — | — | — | — | — |
| Danielsen (2011) | n = 86 Age: 7-13 y Sex: Both |
— | — | — | — | — | — | — | — | Hierarchical regressiona
HbA1c: β = 0.21 (ns) IR: β = −0.20 (ns) TC: β = 0.10 (ns) LDL: β = 0.02 (ns) HDL: β = 0.17 (ns) TG: β = −0.19 (ns) |
| Dencker (2010) Copenhagen School Child Intervention Study |
n = 468 Age: 6-7 y Sex: Both |
— | — | — | Partial correlation VO2peak: Males: r = 0.28* Females: r = 0.14* Regressiona VO2peak: Males: β = 0.28*** Females: β = 0.15* |
Partial correlation VO2peak: Males: r = 0.27* Females: r = 0.12 Regressiona VO2peak: Males: β = 0.26*** Females: N/R (ns) |
Partial correlation VO2peak: Males: r = 0.16* Females: r = 0.07 Regressiona VO2peak: Males: β = 0.14* Females: N/R (ns) |
— | — | — |
| Dencker (2008) | n = 225 Age: 8-11 y Sex: Both |
Partial correlation %BFb: r = −0.09 (ns) Abdominal fat: r = −0.07 (ns) BF distribution: r = −0.05 (ns) |
Partial correlation %BFb: r = −0.22* Abdominal fat: r = −0.18* BF distribution: r = −0.12 (ns) |
Partial correlation %BFb: r = −0.40* Abdominal fat: r = −0.35* BF distribution: r = −0.22* |
Partial correlation VO2peak: r = 0.14* |
Partial correlation VO2peak: r = 0.25* |
Partial correlation VO2peak: r = 0.38* |
|||
| Dencker (2008) | n =172 Age: 8-11 y Sex: Both |
— | — | Regressiona
TBF: β = −0.011*** %BF: β = −0.008*** AFM: β = −0.011*** AFM/TBF: β = −0.0005* |
— | — | — | — | — | — |
| Dencker (2007) | n = 248 Age: 8-11 y Sex: Both |
— | — | — | — | — | Regressiona
abs VO2peak: β = 0.09* VO2peak/kg of body mass: β = 0.10* VO2peak/LBM: β = 0.13* |
— | — | — |
| Dencker (2006) | n = 248 Age: 8-11 y Sex: Both |
N/R (ns) | — | Regression %BF: r2 = 0.21 |
— | — | — | — | — | — |
| Denton (2013) HAPPY Study | n = 135 Age:10 -14 y Sex: Both |
— | — | — | Regression All: N/R (ns) Males: N/R (ns) Females: β = 0.27* |
— | Regression All: N/R (ns) Males: β = −0.50* Females: N/R (ns) |
— | — | — |
| Dubose (2015) | n = 72 Age: 7-11 y Sex: Both |
Univariate correlation WC: −0.14 (ns) Regression WC: β = −0.039 (ns) |
Univariate correlation WC: −0.15 (ns) Regression WC: β = −0.399 (ns) |
Univariate correlation WC: −0.21 (ns) Regression WC: β = −0.333 (ns) |
— | — | — | Univariate correlation MAP: −0.19 (ns) HDL: 0.21 (ns) TG: −0.05 (ns) Glucose: 0.05 (ns) MetS score: −0.18 (ns) Regression SBP: β = 0.018 (ns) DBP: β = −0.054* MAP: β = −0.029 (ns) HDL: β = 0.057 (ns) TG: β = −0.175 (ns) Glucose: β = 0.012 (ns) MetS score: β = −0.014 (ns) |
Univariate correlation MAP: −0.19 (ns) HDL: 0.17 (ns) TG: −0.03 (ns) Glucose: 0.04 (ns) MetS score: −0.16 (ns) Regression SBP: β = 0.010 (ns) DBP: β = −0.045* MAP: β = −0.027 (ns) HDL: β = 0.042 (ns) TG: β = −0.138 (ns) Glucose: β = 0.012 (ns) MetS score: β = −0.012 (ns) |
Univariate correlation MAP: −0.11 (ns) HDL: −0.15 (ns) TG: 0.11 (ns) Glucose: 0.04 (ns) MetS score: −0.01 (ns) Regression SBP: β = −0.213 (ns) DBP: β = −0.008* MAP: β = −0.076 (ns) HDL: β = −0.276 (ns) TG: β = 0.417 (ns) Glucose: β = 0.003 (ns) MetS score: β = 0.0013 (ns) |
| Ekelund (2007) EYHS | n = 1709 Age: 9-10 y and 15-16 y Sex: Both |
Partial correlation WC: r = −0.06* Skinfolds: r = −0.06** Regression WC: β = 0.006 (ns) |
— | Partial correlation WC: r = −0.11*** Skinfolds: r = −0.06** Regression WC: β = −0.01 (ns) |
Partial correlation CRF: r = 0.08*** |
— | Partial correlation CRF: r = 0.13*** |
Partial correlation SBP: r = −0.10*** DBP: r = −0.08** Glucose: r = −0.08** HDL: r = 0.2 (ns) TG: r = −0.04 (ns) Insulin: r = −0.07** |
— | Partial correlation SBP: r = −0.06* DBP: r = −0.08** Glucose: r = −0.08** HDL: r = −0.01(ns) TG: r = −0.04 (ns) Insulin: r = −0.07** Regression |
| Regression DBP: β = −0.12*** SBP: β = −0.16*** Glucose: β = −0.11*** HDL: β = 0.02 (ns) TG: β = −0.05* Insulin: β = -0.08** MetS risk score: β = −0.06*** |
DBP: β = −0.07** SBP: β = −0.07** Glucose: β = −0.10** HDL: β = −0.004 (ns) TG: β = −0.03 (ns) Insulin: β = −0.08** MetS risk score: β = −0.05*** |
|||||||||
| Ekelund (2004) EYHS | n = 1292 Age: 9-10 y Sex: Both |
— | Regression BMI: N/R (ns)%BFa: β = −0.0019* |
Regression BMI: (ns) %BFa: β = −0.0034* |
— | — | — | — | — | — |
| España-Romera (2010) HELENA Study |
n = 254 Age: 12.5-17.5 y Sex: Both |
Regression Low CRF: WC: β = −0.01 (ns) Waist/height ratio: β = 0.01 (ns) High CRF: WC: β = 0.01 (ns) Waist/height ratio: β = 0.02 (ns) |
Regression Low CRF: WC: β = −0.07 (ns) Waist/height ratio: β = −0.08 (ns) High CRF: WC: β = −0.06 (ns) Waist/height ratio: β = −0.07 (ns) |
Regression Low CRF: WC: β = −0.18* Waist/height ratio: β = −0.20* High CRF: WC: β = −0.15* Waist/height ratio: β = −0.17* |
— | — | — | — | — | — |
| Fairclough (2012) | n = 409 Age::10-11 y Sex: Both |
Binomial logistic regression Males: ORNW= 0.94 (95% CI: 0.89-1.0) Females: ORNW= 0.96 (95% CI: 0.90-1.02) |
— | Binomial logistic regression Males: ORNW= 1.13 (95% CI: 1.03-1.23) Females: ORNW= 1.13 (95% CI: 1.02-1.25) |
— | — | — | — | — | — |
| Gaya (2009) | n = 163 Age: 11-17 y Sex: Both |
— | — | — | — | — | — | Regressiona
SBP: β = −0.25* DBP: (ns) |
— | Regression SBP: (ns) DBP: (ns) |
| Gutin (2005) | n = 421 Age: 16 y Sex: Both |
Regression %BFc: β = −0.64 (ns) |
Regression %BFc: β = −0.16 (ns) |
Regression %BFc: β = −4.19** |
Regression CRF: β = 4.80** |
Regression CRF: β = 5.63** |
Regression CRF: β = 6.17** |
— | — | — |
| Hay (2012) 2008 Healthy Hearts Prospective Cohort Study of PA and CM Health in Youth |
n = 605 Age: 9- 17 y Sex: Both |
Regressiona
WC: β = 0.003 (ns) BMI z-score: β = 0.04 (ns) |
— | Regressiona
WC: β = −0.11 (ns) BMI z-score: β = −0.15** Multiple logistic regression Highest VPA to low tertile: ORowob: 0.43 (95% CI: 0.27-0.68) |
Regressiona
VO2max: β = 0.02 (ns) |
— | Regressiona
VO2max: β = 0.17** |
Regressiona
SBP: β = 0.04 (ns) |
— | Regression SBP: β = −0.19** Highest VPA to low tertile: %high SBP: OR = 0.36 (95% CI: 0.19-0.66) |
| Jiménez-Pavón (2013) | n = 2200 Age: 14.7 (1.2) y Sex: Both |
Regression Males: BMI: β = −0.02 (ns) Skinfolds: β = −0.04 (ns) %BF: β = −0.05 (ns) WC: β = −0.05 (ns) FFM: β = −0.04 (ns) FFM (BIA): β = −0.04 (ns) Females: BMI: β = −0.01 (ns) Skinfolds: β = −0.04 (ns) %BF: β = −0.04 (ns) WC: β = −0.03 (ns) FFM: β = 0.03 (ns) FFM (BIA): β = 0.01 (ns) |
Regression Males: BMI: β = −0.11** Skinfolds: β = −0.16*** %BF: β = −0.17*** WC: β = −0.11*** FFM: β = 0.03 (ns) FFM (BIA): β = 0.02 (ns) Females: BMI: β = −0.064* Skinfolds: β = −0.115*** %BF: β = −0.110*** WC: β = −0.092** FFM: β = 0.05 (ns) FFM (BIA): β = 0.03 (ns) |
Regression Males: BMI: β = −0.20*** Skinfolds: β = −0.26*** %BF: β = −0.26*** WC: β = −0.18*** FFM: β = 0.10** FFM (BIA): β = 0.06* Females: BMI: β = −0.142*** Skinfolds: β = −0.178*** %BF: β = −0.176*** WC: β = −0.146*** FFM: β = 0.08** FFM (BIA): β = 0.06* |
— | — | — | — | — | — |
| Jiménez-Pavón (2013) | n = 2025 Age: 6-9 y Sex: Both |
— | — | — | — | — | — | Logistic regression for CVD risk score (only in 6- 9-y-olds) Males: Q1: ORCVDscore = 5.4 (95% CI: 2.05-14.20) Q2: OR = 2.1 (95% CI: 0.76-5.58) Q3: OR = 2.0 (95% CI: 0.75-5.31) Q4: OR = 1.2 (95% CI: 0.42-3.20) Q5: ref (highest total PA) Females: Q1: OR = 3.7 (95% CI: 1.21-11.29) Q2: OR = 4.5 (95% CI: 1.69-11.77) Q3: OR = 5.3 (95% CI: 2.12-13.42) Q4: OR = 1.9 (95% CI: 0.66-5.38) Q5: ref (highest total PA) |
Logistic regression for CVD risk score (only in 6-9-y olds) Males: Q1: OR = 4.4 (95% CI: 95% CI: 1.62-11.71) Q2: OR = 2.38 (95% CI: 0.91-6.23) Q3: OR = 2.06 (95% CI: 0.78-5.48) Q4: OR = 1.29 (95% CI: 0.47-3.56) Q5: ref (highest total PA) Females: Q1: OR = 6.0 (95% CI: 1.86-19.05) Q2: OR = 7.1 (95% CI: 2.47-20.15) Q3: OR= 5.23 (95% CI: 1.81-15.13) Q4: OR = 3.5 (95% CI: 1.17-10.26) Q5: ref (highest total PA) |
Logistic regression for CVD risk score (only in 6- 9-y olds) Males: Q1: OR = 2.7 (95% CI: 1.06-6.80) Q2: OR = 1.7 (95% CI: 0.66-4.16) Q3: OR = 1.2 (95% CI: 0.44-2.99) Q4: OR = 0.73 (95% CI: 0.26-2.04) Q5: ref (highest total PA) Females: Q1: OR: 5.9 (95% CI: 2.20-15.76) Q2: OR = 2.9 (95% CI: 1.02-7.96) Q3: OR = 1.9 (95% CI: 0.64-5.51) Q4: OR = 1.8 (95% CI: 0.63-5.36) Q5: ref (highest total PA) |
| Jiménez-Pavón (2013) HELENA Study |
n = 1053 Age: 12.5-17.5 y Sex: Both |
Partial correlations Males: BMI: r = −0.05 (ns) WC: r = 0.03 (ns) TBF: r = −0.01 (ns) Females: BMI: r = −0.01 (ns) WC: r = −0.07 (ns) TBF: r = −0.12** |
Partial correlations Males: BMI: r = −0.04 (ns) WC: r = 0.02 (ns) TBF: r = −0.10 (ns) Females: BMI: r = −0.10 (ns) WC: r = −0.12* TBF: r = −0.20** |
Partial correlations Males: BMI: r = −0.02 (ns) WC: r = −0.04 (ns) TBF: r = −0.19** Females: BMI: r = −0.19** WC: r = −0.17** TBF: r = −0.26** |
Partial correlations Males: CRF: 0.13** Females: CRF: −0.03 (ns) |
Partial correlations Males: CRF: 0.17** Females: CRF: 0.06 (ns) |
Partial correlations Males: CRF: 0.20** Females: CRF: 0.19** |
Partial correlations Males: Insulin: r = 0.05 (ns) INS: r = −0.04 IR: r = 0.06 (ns) Glucose: r = 0.01 (ns) Females: Insulin: r = −0.09 (ns) INS: r = 0.11** IR: r = 0.09 (ns) Glucose: r = −0.07 Regression Model 3: Males: Insulin: β = 0.07 (ns) INS: β = −0.06 (ns) IR: β = 0.08 (ns) Stratified by CRF: N/R (ns) Females: Insulin: β = −0.08 (ns) INS: β = 0.08 (ns) IR: β = −0.08 (ns) Stratified by CRF: Low CRF Insulin: β = −0.2* INS: β = 0.24** IR: β = −0.20* Middle to high CRF Insulin: β = −0.07 (ns) INS: β = 0.07 (ns) IR: β = −0.08 (ns) |
Partial correlations Males: Insulin: r = 0.04 (ns) INS: r = 0.05 (ns) IR: r = −0.03 (ns) Glucose: r = 0.02 (ns) Females: Insulin: r = −0.14* INS: r = 0.15** IR: r = −0.12* Glucose: r = −0.04 (ns) Regression Model 3: Males: Insulin: β = −0.02 (ns) INS: β = 0.02 (ns) IR: β = −0.02 (ns) Stratified by CRF: N/R (ns) Females: Insulin: β= -0.06 (ns) INS: β = 0.06 (ns) IR: β = −0.07 (ns) Stratified by CRF: Low CRF Insulin: β = −0.22* INS: β = 0.26** IR: β = −0.21* Middle to high CRF Insulin: β = −0.08 (ns) INS: β = 0.08 (ns) IR: β = −0.08 (ns) |
Partial correlations Males: Insulin: r = 0.12* INS: r = 0.13** IR: r = −0.18* Glucose: r = −0.04 (ns) Females: Insulin: r = 0.15** INS: r = 0.16** IR: r = −0.13* Glucose: r = −0.01 (ns) Regression Model 3: Males: Insulin: β = −0.11 (ns) INS: β = 0.11 (ns) IR: β = −0.11 (ns) Stratified by CRF: N/R (ns) Females: Insulin: β = −0.001 (ns) INS: β = −0.002 (ns) IR: β = −0.003 (ns) Stratified by CRF: Low CRF Insulin: β = −0.17* INS: β = 0.21* IR: β = −0.16* Middle to high CRF Insulin: β = −0.05 (ns) INS: β = 0.04 (ns) IR: β = −0.04 (ns) |
| Katzmarzyk (2015) | n = 6539 Age: 9-11 y Sex: Both |
— | Odds ratio Obesity: 0.49* ROC analysis Obesity thresholds: All: 55 min/d* Boys: 65 min/d* Girls: 49 min/d* |
Odds ratio Obesity: 0.41* ROC analysis Obesity thresholds: All: 14 min/d* Boys: 20 min/d* Girls: 11 min/d* |
— | — | — | — | — | — |
| Kennedy (2012) | n = 36 Age: 6.7 y Sex: Both |
Partial correlations Minutes /day: BMI z-score: r = 0.06 (ns) FMI z-score: r = −0.28 (ns) LMI z-score: r = 0.40* %Time in MPA: N/R |
Partial correlations Minutes/day: N/R %Time in MVPA: BMI z-score: r = −0.07 (ns) FMI z-score: r = −0.26 (ns) LMI z-score: r = 0.35* |
Partial correlations Minutes/day: BMI z-score: r = 0.06 (ns) FMI z-score: r = −0.02 (ns) LMI z-score: r = 0.18 (ns) %Time in VPA: N/R |
— | — | — | — | — | — |
| Lätt (2015) | n = 136 Age: 10-12 y at baseline Sex: Males |
Partial correlation BMI: r = −0.152 (ns) ROC analysis NW vs OW: 46 min/d (ns) NW vs OB: 46 min/d (ns) |
Partial correlation BMI: r = −0.260** ROC analysis NW vs OW: 59 min/d* NW vs OB: 55 min/d** |
Partial correlation BMI: r = −0.317*** ROC analysis NW vs OW: 14 min/d** NW vs OB: 10 min/d*** |
— | — | — | — | — | — |
| Mark (2011) 2003-2004 NHANES |
n = 1165 Age: 8-17 y Sex: Both |
— | Regression Model 3: Trunk fat %tile (Q1 reference)a: Low activity Q2: β = −6.97 (ns) Q3: β = −9.70 (ns) Q4 (low): β = −16.18 (ns) Q4 (high): β = −22.57* Ptrend = .029 |
Regression Model 3: Trunk fat %tile (Q1 reference)a: Low activity Q2: β = −4.34 (ns) Q3: β = −6.57 (ns) Q4 (low): β = −6.92 (ns) Q4 (high): β = −6.28 (ns) Ptrend = .41 |
— | — | — | — | — | — |
| Martínez-Gómez (2010) AFINOS Study |
n = 192 Age: 13-17 y Sex: Both |
Partial correlations BMI: r = −0.004 (ns) SF: r = 0.07 (ns) WC: r = 0.05 (ns) |
Partial correlations BMI: r = −0.01 (ns) SF: r = −0.01 (ns) WC: r = 0.03 (ns) |
Partial correlations BMI: r = −0.11 (ns) SF: r = −0.27*** WC: r = −0.08 (ns) |
Partial correlation CRF: r = 0.08 (ns) |
Partial correlation CRF: r = 0.25*** |
Partial correlation CRF: r = 0.48*** |
Partial correlations Glucose: r = 0.04 (ns) Insulin: r = 0.04 (ns) IR: r = 0.04 (ns) |
Partial correlations Glucose: r = 0.01 (ns) Insulin: r = 0.02 (ns) IR: r = 0.02 (ns) |
Partial correlations Glucose: r = −0.04 (ns) Insulin: r = −0.04 (ns) IR: r = −0.05 (ns) |
| Martínez-Gómez (2010) HELENA Study |
n =1808 Age: 12.5- 17.5 y Sex: Both |
— | — | — | Logistic regression All: ORhealthy CRF = 1.71*** (95% CI: 1.39-2.12) Males: ORhealthy CRF = 1.81*** (95% CI: 1.31-2.49) Females: ORhealthy CRF = 1.68*** (95% CI: 1.28-2.21) |
Logistic regression All: ORhealthy CRF = 1.96*** (95% CI: 1.58-2.43) Males: ORhealthy CRF = 2.13*** (95% CI: 1.54-2.94) Females: ORhealthy CRF = 1.90*** (95% CI: 1.45-2.51) |
Logistic regression All: ORhealthy CRF = 2.20*** (95% CI: 1.74-2.71) Males: ORhealthy CRF = 2.30*** (95% CI: 1.64-3.14) Females: ORhealthy CRF = 2.32*** (95% CI: 1.75-3.06) |
— | — | — |
| Martínez-Gómez (2009) AFINOS Study |
n = 202 Age: 13-17 y Sex: Both |
Partial correlations BMI: r = 0.02 (ns) %BF: r = −0.01 (ns) WC: r = 0.03 (ns) |
Partial correlations BMI: r = 0.02 (ns) %BF: r = −0.08 (ns) WC: r = 0.01 (ns) |
Partial correlations BMI: r = −0.03 (ns) %BF: r = −0.29*** WC: r = −0.06 (ns) |
Partial correlation CRF: N/R (ns) |
Partial correlation CRF: r = 0.280*** |
Partial correlation CRF: r = 0.484*** |
Partial correlations SBP: r = 0.03 (ns) DBP: r = 0.05 (ns) TC: r = 0.06 (ns) HDL: r = 0.08 (ns) TG: r = −0.05 (ns) Glucose: r = −0.01 (ns) Insulin: r = −0.02 (ns) |
Partial correlations SBP: r = 0.06 (ns) DBP: r = −0.05 (ns) TC: r = 0.08 (ns) HDL: r = 0.11 (ns) TG: r = −0.11 (ns) Glucose: r = −0.02 (ns) Insulin: −0.03 (ns) Regression MetS: CCCHS: β = −0.08 (ns) ACLS: β = −0.09 (ns) EYHS: β = −0.11 (ns) |
Partial correlations SBP: r = 0.04 (ns) DBP: r = −0.05 (ns) TC: r = 0.05 (ns) HDL: r = 0.18* TG: r = −0.19** Glucose: r = −0.06 (ns) Insulin: r = −0.09 (ns) Regression MetS: |
|
Regression MetS: CCCHS: β = −0.04 (ns) ACLS: β = −0.08 (ns) EYHS: β = −0.05 (ns) |
CCCHS: β = −0.19* ACLS: β = −0.16* EYHS: β = −0.24* MetS (additionally controlled for fitness): CCCHS: β = −0.03 (ns) ACLS: β = −0.03 (ns) EYHS: β = −0.09 (ns) |
|||||||||
| Moliner-Urdiales (2010) HELENA Study |
n = 363 Age: 12.5-17.5 y Sex: Both |
Regressiona
FFM (DXA) a Males: β = 0.11 (ns) Females: β = −0.01 (ns) |
Regressiona
FFM(DXA)a Males: β = 0.08 (ns) Females: β = 0.02 (ns) |
Regressiona
FFM(DXA)a Males: β = 0.04 (ns) Females: β = 0.05 (ns) |
— | — | — | — | — | — |
| Moliner-Urdiales (2009) HELENA Study |
n = 365 Age: 12.5-17.5 y Sex: Both |
Regressiona
TBF(DXA): β = −0.10 (ns) TBF(BodPod): β = −0.06 (ns) SF: β = −0.09 (ns) WC: β = −0.03 (ns) |
Regressiona
TBF(DXA): β = −0.20*** TBF(BodPod): β = −0.14** SF: β = −0.19*** WC: β = −0.10 (ns) |
Regressiona
TBF(DXA): β = −0.26*** TBF(BodPod): β = −0.22*** SF: β = −0.24*** WC: β = −0.17** |
— | — | — | — | — | — |
| Moore (2013) | n = 285 Age: Middle school Sex: Both |
— | — | — | Regression Model 4 CRF: β = −0.03 (ns) |
— | Regression Model 4 CRF: β = −0.22* |
— | — | — |
| Ortega (2010) EYHS |
n = 1075 Age: 9-10 y and 15-16 y Sex: Both |
Regression All: β = 0.13** Low CRF: β = −0.002 (ns) High CRF: β = 0.27*** |
Regression All: β = 0.10* Low CRF: β = −0.10 (ns) High CRF: β = 0.23** |
Regression All: β = 0.01 (ns) Low CRF: β = −0.09 (ns) High CRF: β = 0.10* |
— | — | — | — | — | — |
| Ortega (2007) EYHS |
n = 1074 Age: 9.5 (0.3)y and 15.6 (0.4) y Sex: Both |
Logistic regression Overweight: Ref = High PA Low PA: OROW = 2.0 (95% CI: 0.9-4.4) Middle PA: OROW = 1.7 (95% CI: 0.7-3.7) High WC: Ref = High PA Low PA: ORHi_WC = 1.3 (95% CI: 0.7-2.4) Middle PA: ORHi_WC = 1.1 (95% CI: 0.6-2.0) |
Logistic regression Overweight: Ref = High PA Low PA: OROW = 2.1 (95% CI: 0.9-4.3) Middle PA: OROW = 1.0 (95% CI: 0.5-2.3) High WC: Ref = High PA Low PA: ORHi_WC = 1.3 (95% CI: 0.6-2.8) Middle to high PA level: ORHi_WC = 0.6 (95% CI: 0.3-1.5) |
Logistic regression Overweight: Ref = High PA Low to high PA level: OROW = 4.1*** (95% CI: 1.8-9.5) Middle to high PA level: OROW = 1.9 (95% CI: 0.8-4.7) High WC: Ref = High PA Low PA: ORHi_WC = 2.1* (95% CI: 1.1-3.9) Middle PA: ORHi_WC = 1.4 (95% CI: 0.8-2.7) |
— | — | — | — | — | — |
| Patrick (2004) PACE+ Study |
n = 878 Age: 11-15 y Sex: Both |
Logistic regression Males: N/R (ns) Females: N/R (ns) |
— | Logistic regression Males: ORat risk plus OW = 0.92*** (95% CI: 0.89-0.95) Females: ORat risk plus OW = 0.93** (95% CI: 0.89-0.97) |
— | — | — | — | — | — |
| Radtke (2013) | n = 52 Age: 14.5 (0.7) y Sex: Both |
— | — | — | — | Regression Maximum power output: β = 0.01 (ns) MVPAlow vs high = P > .05 VO2peak: β = 0.06 (ns) MVPAlow_tertile vs high_tertile = P > .05 |
Regression Maximum power output: β = 0.25* VPAlow vs high: P = .01 VO2peak: β = 0.12 (ns) VPAlow_tertile vs high_tertile = P > .05 |
— | — | — |
| Rizzo (2008) EYHS |
n = 613 Age: 15.5 y Sex: Both |
— | — | — | — | — | — | Regression Glucose: β = −0.09* Insulin: β = −0.11** IR: β = −0.11** MPAlow_tertile vs high_tertile = P = .02 MPAlow vs. middle = P > .05 |
Regression Glucose: β = −0.12** Insulin: β = −0.14*** IR: β = −0.14*** |
Regression Glucose: β = −0.15*** Insulin: β = −0.16*** IR: β = −0.16*** VPAlow_tertile vs high_tertile = P = .02 VPAlow vs middle = P = .05 |
| Rowlands (2006) | n = 76 Age: 8-11 y Sex: Both |
— | Partial correlation Males: MVPAlow_tertile vs upper two tertiles = P > .05 Females: MVPAlow_tertile vs high_ tertiles = P < .05 |
Partial correlation Males: r = −0.22 (ns) VPAlow_tertile vs upper two tertiles = P < .05 Females: r = −0.28 (ns) VPAlow_tertile vs upper two tertiles = P > .05 |
— | — | — | — | — | — |
| Ruiz (2006) EYHS |
n = 780 Age: 9-10 y Sex: Both |
Regression %BF: β = 0.018 (ns) |
Regression %BF: β = −0.011 (ns) |
Regression %BF: β = −0.081* Time spent in VPA VPA2nd_quintile vs 5th_quintile = P < .001 |
Regression CRF: β = 0.087** |
Regression CRF: β = 0.108** |
Regression CRF: β = 0.124*** Time spent in VPA VPA1st_quintile vs. 5th_quintile = P <.001 VPA2nd_quintile vs 4th_quintile = P = .018 |
— | — | — |
| Steele (2009) SPEEDY Study |
n = 1862 Age: 9-10 y Sex: Both |
Regression WC:a Model 1: β = −0.048** Model 2: β = −0.039* Model 3: β = −0.034 (ns) FMIb: Model 1: β = −0.002** Model 2: β = −0.002* Model 3: β = −0.002 (ns) BMI: Model 1: β = −0.003 (ns) Model 2: β = −0.001 (ns) Model 3: β = −0.0004 (ns) Standardized coefficients: WC: β = −0.49(ns) FMI: β = −0.022(ns) BMI: β = −0.006(ns) |
Regression WC:a Model 1: β = −0.053*** Model 2: β = −0.042*** Model 3: β = −0.044*** FMIb: Model 1: β = −0.003*** Model 2: β = −0.002*** Model 3: β = −0.002*** BMI: Model 1: β = −0.005*** Model 2: β = −0.00** Model 3: β = −0.003** Standardized coefficients: WC: β = −1.09*** FMI: β = −0.059*** BMI: β = −0.094** Time spent in MVPA Odds of OWOB OROWOB MVPA least active quartile vs. most active quintile = Ptrend < .01 |
Regression WC: a Model 1: β = −0.12*** Model 2: β = −0.098*** Model 3: β = −0.10*** FMIb: Model 1: β = −0.006*** Model 2: β = −0.005*** Model 3: β = −0.06*** BMI: Model 1: β = −0.013*** Model 2: β = −0.01*** Model 3: β = −0.01*** Standardized coefficients: WC: β = −1.32*** FMI: β = −0.075*** BMI: β = −0.15*** Time spent in VPA WC and FMI VPA least active quartile vs. most active quintile = Ptrend < .001 |
— | — | — | — | — | — |
| Tanha (2011) | n = 223 Age: 7.9-11.1 y Sex: Both |
— | GLM-derived partial correlation %BF: r = −0.32*** AFM: r = −0.29*** AFM/TBF: r = −0.21** |
GLM-derived partial correlation %BF: r = −0.38*** AFM: r = −0.34*** AFM/TBF: r = −0.23*** |
— | GLM-derived partial correlation VO2peak: r = 0.32*** |
GLM-derived partial correlation VO2peak: r = 0.28*** |
— | GLM-derived partial correlation SBP: r = −0.10 (ns) DBP: r = −0.12 (ns) MAP: r = −0.13 (ns) CVD risk score: Males: r = −0.29** Females: r = −0.28** |
GLM-derived partial correlation SBP: r = −0.14* DBP: r = −0.08 (ns) MAP: r = −0.12 (ns) CVD risk score: Males: r = −0.33*** Females: r = −0.32** |
| Treuth (2005) | n = 229 Age: 7-19 y Sex: Both |
Partial correlation Males: ES: BMI: rs = 0.14 (ns) TBF: rs = −0.05 (ns) %BF: rs = −0.02 (ns) MS: BMI: rs = 0.02 (ns) TBF: rs = −0.07 (ns) %BF: rs = −0.04 (ns) HS: BMI: rs = 0.01 (ns) TBF: rs = 0.02 (ns) %BF: rs = −0.01 (ns) Females: ES: BMI: rs = 0.14 (ns) TBF: rs = −0.07 (ns) %BF: rs = −0.04 (ns) MS: BMI: rs = 0.10 (ns) TBF: rs = −0.07 (ns) %BF: rs = −0.08 (ns) HS: |
— | Partial correlation Males: ES: BMI: rs = 0.12 (ns) TBF: rs = 0.01 (ns) %BF: rs = 0.01 (ns) MS: BMI: rs = −0.06 (ns) TBF: rs = −0.17 (ns) %BF: rs = −0.11 (ns) HS: BMI: rs = −0.21 (ns) TBF: rs = −0.16 (ns) %BF: rs = −0.17 (ns) Females: ES: BMI: rs = 0.20 (ns) TBF: rs = 0.01 (ns) %BF: rs = 0.04 (ns) MS: BMI: rs = 0.30* TBF: rs = 0.21 (ns) %BF: rs = 0.18 (ns) |
— | — | — | — | — | — |
| BMI: rs = 0.04 (ns) TBF: rs = −0.13 (ns) %BF: rs = −0.14 (ns) |
HS: BMI: rs = 0.02 (ns) TBF: rs = −0.11 (ns) %BF: rs = −0.12 (ns) |
|||||||||
| Wittmeier (2008) | n = 251 Age: 8-10 y Sex: Both |
Partial correlation BMI: r = −0.076 (ns) %BF: r = −0.113 (ns) Time spent in MPA (Q1-Q4) %BF Q1 (<15min) vs Q3 and Q4 (>30-45min): P = .009** BMI Q1 (<15min) vs Q4 (>45min): P = .016* Regressiona N/R (ns) Logistic regression BMI OW ≤45 min vs >45 min(ref): ORBMI_OW = 1.52 (ns) ≤30 min vs 45 min(ref): ORBMI_OW = 1.49 (ns) ≤15 min vs 45 min(ref): ORBMI_OW = 2.04 (ns) %BF (≥20%) ≤45 min vs >45 min(ref):: OROW = 3.15*** |
Partial correlation BMI: r = −0.123 (ns) %BF: r = −0.143* Logistic regression ≤60 min vs >60 min (ref): ORBMI_OW = 1.96 (ns) ≤30 min vs 60 min(ref): ORBMI_OW = 2.08 (ns) %BF (≥20%) ≤60 min vs >60 min(ref): OROW = 3.36*** ≤30 min vs 60 min(ref): Logistic regression ≤60 min vs >60 min (ref): ORBMI_OW = 1.96 (ns) ≤30 min vs 60 min(ref): ORBMI_OW = 2.08 (ns) |
Partial correlation BMI: r = −0.197** %BF: r = −0.173** Time spent in VPA (Q1-Q4) %BF Q1 (<5min) vs Q3 and Q4 (>10-15min): P = .002** BMI Q1 (<5min) vs Q4 (>15min): P = .011* Regressiona BMI: β = −0.21*** %BF: β = −0.17** Logistic regression ≤15 min vs >15 min(ref): ORBMI_OW = 4.45** ≤10 min vs >15 min(ref): ORBMI_OW = 4.63** ≤5 min vs >15 min(ref): ORBMI_OW = 5.21** %BF (≥20%) ≤15 min vs >15 min(ref): OROW = 3.23** ≤10 min vs >15 min(ref): |
||||||
| ≤30 min vs 45 min(ref): OROW = 3.51*** ≤15 min vs 45 min(ref): OROW = 4.20*** %BF (≥25%) ≤45 min vs >45 min(ref): OROW = 1.97 (ns) ≤30 min vs 45 min(ref): OROW = 2.16* ≤15 min vs 45 min(ref): OROW = 3.04** |
%BF (≥20%) ≤60 min vs >60 min(ref): OROW = 3.36*** ≤30 min vs 60 min(ref): OROW = 4.15*** %BF (≥25%) ≤60 min vs >60 min(ref): OROW = 2.71* ≤30 min vs 60 min(ref): OROW = 3.03** |
OROW = 3.26** ≤5 min vs >15 min(ref): OROW = 4.03*** %BF (≥25%) ≤15 min vs >15 min(ref): OROW = 2.23 (ns) ≤10 min vs >15 min(ref): OROW = 2.58* ≤5 min vs >15 min(ref): OROW = 2.90* |
— | — | — | — | — | — | ||
Abbreviations: PA, physical activity; BMI, body mass index; CRF, cardiorespiratory fitness; CM, cardiometabolic; y, year; MPA, moderate physical activity; MVPA, moderate to vigorous physical activity; VPA, vigorous physical activity; n, sample size; OR, odds ratio; AA, African-American; N/R, not reported; r, Pearson partial correlation; SBP, systolic blood pressure; ns, nonsignificant; TC, total cholesterol; LDL, low-density lipoprotein; HDL, high-density lipoprotein; TG, triglycerides; %BF, body fat percentage; β, standardized regression coefficient; min, minute; sec, second; WC, waist circumference; GEE, generalized estimating equation; VO2peak, peak volume of oxygen consumption; MetS, metabolic syndrome; min/d, minutes per day; HbA1c, glycated hemoglobin; IR, insulin resistance; TBF, total body fat; AFM, abdominal fat mass; abs, absolute; kg, kilogram; LBM, lean body mass; DBP, diastolic blood pressure; ow, overweight; ob, obese; VO2max, maximum volume of oxygen consumption; FFM, fat-free mass; BIA, bioelectrical impedance analysis; CVD, cardiovascular disease; Q, quartile; TBF, total body fat; INS, insulin sensitivity; FMI, fat mass index; LMI, lean mass index; DXA, dual energy x-ray absorptiometry; SF, subcutaneous fat; MAP, mean arterial pressure; ROC, receiver operating characteristic.
Cohorts: GEMS, Stanford Girls Health Enrichment Multisite Studies; QUALITY, Quebec Adiposity and Lifestyle Investigation in Youth; HAPPY, Health and Physical Activity Promotion in Youth; EYHS, European Youth Heart Study; HELENA; Healthy Lifestyle in Europe by Nutrition in Adolescence; NHANES, National Health and Nutrition Examination Survey; AFINOS, La Actividad Fı’sica como Agente Preventivo del Desarrollo de Sobrepeso, Obesidad, Alergias, Infecciones y Factores de Riesgo Cardiovascular en Adolescentes: Physical Activity as a Preventive Agent of the Development of Overweight, Obesity, Infections, Allergies and Factors of Cardiovascular Risk in Adolescents; ACLS, Aerobic Center Longitudinal Study; PACE+, Patient-Centered Assessment and Counseling for Exercise Plus Nutrition Project; SPEEDY, Sport, Physical Activity and Eating Behaviour, Environmental Determinants in Young People
Standardization not reported.
Log-transformed.
Unstandardized.
p < 0.05.
p < 0.01.
p < 0.001.
Table 3.
Summary of Articles Reporting Beneficial, Not Beneficial, or No Association Between Physical Activity Intensity and Body Mass Index/Body Composition Outcomes in Youth.
| Beneficial | Not Beneficial | No Association | |
|---|---|---|---|
| Body mass index | |||
| MPA | 1/14 (7.1%)55 | 13/14 (92.9%)21,23-26,34,35,37,41,46,52,54-56 | |
| MVPA | 5/13 (38.5%)7,15,34,46,56 | 8/13 (61.5%)24,26,31,35,37,41,52,54 | |
| VPA | 12/19 (63.2%)7,15,20,23-25,34,35,41,46,55,56 | 1/19 (5.3%)21 | 8/19 (42.1%)21,26,31,35,37,44,52,54 |
| Fat mass index | |||
| MPA | 2/2 (100.0%)37,46 | ||
| MVPA | 2/2 (100.0%)37,46 | ||
| VPA | 2/2 (100.0%)37,46 | ||
| Total body fat | |||
| MPA | 2/5 (40.0%)18,35 | 3/5 (60.0%)21,35,39 | |
| MVPA | 2/2 (100.0%)35,39 | 1/2 (50.0%)35 | |
| VPA | 3/4 (75.0%)18,35,39 | 1/4 (25.0%)21 | |
| Percent body fat | |||
| MPA | 2/11 (18.2%)16,22 | 9/11 (81.8%)17,21,24,27,30,34,45,49,54 | |
| MVPA | 7/10 (70.0%)16,22,24,34,47,49,55 | 3/10 (30.0%)17,45,54 | |
| VPA | 12/15 (80.0%)17,22,24,27,31,34,45,47,49,51,54,59 | 3/15 (20.0%)16,21,30 | |
| Waist circumference | |||
| MPA | 1/13 (7.7%)46 | 1/13 (7.7%)40 | 12/13 (92.3%)16,23,25,30,32,34,35,39,40,52,54,57 |
| MVPA | 3/10 (30.0%)34,35,46 | 1/10 (10.0%)40 | 8/10 (80.0%)16,32,35,39,40,52,54,57 |
| VPA | 8/13 (61.5%)23,25,32,34,35,39,40,46 | 1/13 (7.7%)40 | 6/13 (46.2%)16,30,35,52,54,57 |
| Waist/height ratio | |||
| MPA | 1/2 (50.0%)22 | 1/2 (50.0%)32 | |
| MVPA | 1/2 (50.0%)22 | 1/2 (50.0%)32 | |
| VPA | 2/2 (100.0%)22,32 | ||
| Subcutaneous fat | |||
| MPA | 2/2 (100.0%)39,52 | ||
| MVPA | 1/2 (50.0%)39 | 1/2 (50.0%)52 | |
| VPA | 2/2 (100.0%)39,52 | ||
| Abdominal fat mass | |||
| MPA | |||
| MVPA | 1/1 (100.0%)47 | ||
| VPA | 1/1 (100.0%)47 | ||
| Fat-free mass | |||
| MPA | 1/1 (100.0%)38 | ||
| MVPA | 1/1 (100.0%)38 | ||
| VPA | 1/1 (100.0%)38 | ||
| Lean mass index | |||
| MPA | 1/1 (100.0%)37 | ||
| MVPA | 1/1 (100.0%)37 | ||
| VPA | |||
Abbreviations: MPA, moderate physical activity; MVPA, moderate to vigorous physical activity; VPA, vigorous physical activity.
Table 4.
Summary of Articles Reporting Beneficial, Not Beneficial, or No Association Between Physical Activity Intensity and Cardiorespiratory Fitness Outcomes in Youth.
| Beneficial | Not Beneficial | No Association | |
|---|---|---|---|
| Cardiorespiratory fitness | |||
| MPA | 5/7 (71.4%)17,29,30,45,53 | 3/7 (42.9%)29,52,54 | |
| MVPA | 5/5 (100.0%)17,45,52-54 | ||
| VPA | 6/7 (85.7%)17,30,45,52-54 | 1/7 (14.3%)29 | 1/7 (14.3%)29 |
| VO2peak | |||
| MPA | 3/3 (100.0%)16,48,49 | 1/3 (33.3%)16 | |
| MVPA | 4/5 (80.0%)16,47-49 | 2/5 (40.0%)42,48 | |
| VPA | 5/6 (83.3%)16,47-50 | 2/6 (33.3%)42,48 | |
| VO2max | |||
| MPA | 2/2 (100.0%)23,25 | ||
| MVPA | |||
| VPA | 2/2 (100.0%)23,25 | ||
| Wmax | |||
| MPA | |||
| MVPA | 1/1 (100.0%)42 | ||
| VPA | 1/1 (100.0%)42 | ||
| Heart rate | |||
| MPA | 1/1 (100.0%)19 | ||
| MVPA | |||
| VPA | 1/1 (100.0%)19 | ||
Abbreviations: VO2peak, peak volume of oxygen; VO2max, maximal volume of oxygen; Wmax, maximal power output; MPA, moderate physical activity; MVPA, moderate to vigorous physical activity; VPA, vigorous physical activity.
Table 5.
Summary of Articles Reporting Beneficial, Not Beneficial, or No Association Between Physical Activity Intensity and Cardiometabolic Biomarker Outcomes in Youth.
| Beneficial | Not Beneficial | No Association | |
|---|---|---|---|
| CVD/MetS risk score | |||
| MPA | 2/4 (50.0%)30,36 | 2/4 (50.0%)54,57 | |
| MVPA | 3/5 (60.0%)16,36,47 | 2/5 (40.0%)54,57 | |
| VPA | 4/5 (80.0%)30,36,47,54 | 1/5 (20.0%)57 | |
| Systolic BP | |||
| MPA | 2/6 (33.3%)30,33 | 4/6 (66.7%)23,25,54,57 | |
| MVPA | 4/4 (100%)15,47,54,57 | ||
| VPA | 3/7 (42.9%)23,30,47 | 4/7 (57.1%)25,33,54,57 | |
| Diastolic BP | |||
| MPA | 2/4 (50.0%)30,57 | 2/4 (50.0%)33,54 | |
| MVPA | 1/3 (33.3%)57 | 2/3 (66.7%)47,54 | |
| VPA | 2/5 (40.0%)30,57 | 3/5 (60.0%)33,47,54 | |
| Total cholesterol | |||
| MPA | 1/1 (100.0%)54 | ||
| MVPA | 2/2 (100.0%)15,54 | ||
| VPA | 2/2 (100.0%)28,54 | ||
| HDL-cholesterol | |||
| MPA | 3/3 (100.0%)30,54,57 | ||
| MVPA | 3/3 (100.0%)15,54,57 | ||
| VPA | 1/4 (25.0%)54 | 3/4 (75.0%)28,30,57 | |
| LDL-cholesterol | |||
| MPA | |||
| MVPA | 1/1 (100.0%)15 | ||
| VPA | 1/1 (100.0%)28 | ||
| Triglycerides | |||
| MPA | 1/3 (33.3%)30 | 2/3 (66.7%)54,57 | |
| MVPA | 3/3 (100.0%)15,54,57 | ||
| VPA | 1/4 (25.0%)54 | 3/4 (75.0%) 28,30,57 | |
| Glucose | |||
| MPA | 2/6 (33.3%)30,43 | 4/6 (66.7%)35,52,54,57 | |
| MVPA | 1/6 (16.7%)43 | 5/6 (83.3%)15,35,52,54,57 | |
| VPA | 2/6 (33.3%)30,43 | 4/6 (66.7%)35,52,54,57 | |
| Insulin | |||
| MPA | 5/8 (62.5%)15,16,30,36,43 | 4/8 (50.0%)16,35,52,54 | |
| MVPA | 3/7 (42.9%)15,36,43 | 4/7 (57.1%)16,35,52,54 | |
| VPA | 2/7 (28.6%)30,36 | 5/7 (71.4%)15,16,35,52,54 | |
| Insulin resistance | |||
| MPA | 2/4 (50.0%)36,43 | 2/4 (50.0%)35,52 | |
| MVPA | 2/4 (50.0%)36,43 | 2/4 (50.0%)35,52 | |
| VPA | 2/5 (40.0%)36,43 | 3/5 (60.0%)28,35,52 | |
Abbreviations: CVD, cardiovascular disease; MetS, metabolic syndrome; MPA, moderate physical activity; MVPA, moderate to vigorous physical activity; VPA, vigorous physical activity; BP, blood pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Body Composition
Body Mass Index
Nineteen studies examined the link between PA intensity and body mass index (BMI).* Eleven of the 19 associations tested indicated that VPA was significantly, negatively correlated with weight status, and the magnitude was greater than the relationship between MPA or MVPA and BMI for both sexes.† Two studies45,57 demonstrated that VPA was more strongly associated with BMI only among boys,45,57 with MVPA being a stronger predictor among only girls.45
The remaining 8 associations from 7 studies did not demonstrate a significant relationship between PA and weight status, regardless of intensity.22,27,32,36,38,53,55 Despite these nonsignificant findings, there were several noticeable trends between PA intensity and weight status. Three of these 8 associations found VPA to be a stronger negative predictor of weight status,27,32,53 whereas 4 reported MPA to be either more strongly related to weight status than VPA or of equal magnitude with VPA.36,38,55 Mixed results were found for the remaining study as there were no consistent trends observed.22
Body Fat
Seventeen studies addressed PA intensity and its connection with percent body fat (%BF).‡ Of these studies, 13 (~76%) demonstrated that VPA was negatively associated with %BF and at a greater magnitude than the relationship between MPA or MVPA and %BF.§ One of the 17 studies17 found that MPA was more strongly associated with %BF than VPA while another study31 found MPA and VPA similarly associated in magnitude. For 1 report25 that employed various analytical techniques (eg, partial correlation, logistic and mean regression), each technique observed a different PA intensity more strongly and negatively predicting percent body fat. The remaining study found a nonsignificant relationship between PA intensity and percent body fat.22
Two studies38,47 assessed the relationship between fat mass index (FMI) and PA intensity. One study found significant, negative connections between the 2 variables for both VPA and MVPA, with a strong association reported with VPA.47 Although the other study reported nonsignificant findings, VPA was more strongly associated with FMI than MPA or MVPA.38
Central Adiposity
Seventeen studies assessed the relationship between PA intensity and a measure of central adiposity‖ with 2 studies addressing 2 separate central adiposity measures,33,50 resulting in a total of 17 associations. Eleven of the 17 associations were statistically significant,¶ of which 10 demonstrated that VPA was a stronger negative predictor for central adiposity compared with MPA or MVPA.# Of these, one study36 found this relationship only among females. The remaining association reported MVPA as a stronger negative predictor compared to VPA.19
Six of the 17 associations were nonsignificant for all PA intensities with central adiposity.17,24,31,53,55,58 Albeit nonsignificant, 4 of these 6 relationships reported a stronger link between VPA and central adiposity compared with MPA or MVPA.24,31,53,55 The remaining studies found MVPA to be a stronger negative predictor of central adiposity compared with MPA or VPA.17,58
Ortega et al41 showed that when the association between PA intensity and central adiposity was stratified by CRF level, findings were contradictory. Among youth with a high CRF level, MPA and MVPA yielded significant positive associations with central adiposity, but this relationship was reversed and nonsignificant for those with low CRF levels.41
Age and Sex
Eight articles presented information on sex differences in the relationship between PA intensity and markers of body composition, which yielded mixed findings.21,22,28,35,36,39,45,56 For youth younger than 12 years, Blaes et al28 indicated that a significant, inverse correlation was observed between VPA and %BF for males, but not females. In contrast, Rowlands et al45 reported females exhibiting a stronger relationship between VPA and %BF than males, although nonsignificant. Still, Fairclough et al56 showed equal odds of normal weight status from VPA engagement for both sexes.
Similar evidence was presented for youth older than 12 years. Two articles reported stronger correlations observed between VPA and body composition markers for males than females.35,39 Significant inverse relationships between VPA and body composition markers was observed for females but not males in another study,36 and the last article reported similar findings irrespective of sex.21 Interestingly, Treuth et al22 found that while all non-significant, elementary and middle school-aged females exhibited stronger relationships between VPA and markers of body composition than males.22 However, this observation was switched in high school–age youth.
Cardiorespiratory Fitness
Sixteen studies assessed the link between PA intensity and CRF.** Fifteen of the 16 studies reported a significant, direct relationship between PA intensity and CRF with the remaining study demonstrating mixed findings.30 Ten articles demonstrated that VPA was more strongly associated with CRF compared with MPA or MVPA.†† Three of the 15 studies found MPA or MVPA to be more strongly and positively associated with CRF compared with VPA.17,48,49 Interestingly, 2 studies found VPA to be a significant negative predictor of CRF.20,30
Age and Sex
In youth aged 6 to 7 years, Dencker et al49 found that MPA was significantly related to CRF for both males and females, but only males retained this significance as intensity increased. Similarly, age at 12 (±2) years showed a significant direct correlation between MPA and CRF in females only, while a significant inverse relationship existed between VPA and CRF in males only.30 For youth older than 12 years, strength of relationship between VPA and CRF was similar for both sexes. However, males displayed stronger relationships at lower PA intensities.36,54
Cardiometabolic Biomarkers and Disease Risk Scores
Cardiovascular Biomarkers
Seven articles evaluated the association between varying levels of PA intensity and cardiovascular biomarkers (systolic blood pressure [SBP; n = 7],24,26,31,34,48,55,58 diastolic blood pressure [DBP; n = 5],31,34,48,55,58 and mean arterial pressure [MAP; n = 1]).48 Overall, findings from the 7 studies did not suggest that any PA intensity was more strongly related to cardiovascular markers. Among studies assessing SBP as an outcome, 2 studies24,48 reported that VPA was a significant, negative predictor of SBP of a greater magnitude than MPA and/or MVPA, while 2 other studies31,34 reported that MPA was significantly and more strongly, negatively associated with SBP than VPA and/or MVPA. The remaining studies reported a nonsignificant correlation between PA at all intensities and SBP.26,55,58
Of the 5 studies evaluating DBP as an outcome, 2 reported a significant result, one of which31 indicated MPA as a stronger negative predictor of DBP than VPA and/or MVPA, and the other58 having significant negative associations for all PA intensities. The 3 remaining articles yielded nonsignificant observations between DBP and PA intensity, with 1 study48 finding MVPA to be more strongly and inversely related with DBP than VPA, while another55 finding the association to be equivalent across MPA, MVPA, and VPA. In the final study, it could not be determined which PA intensity potentially resulted in a stronger influence on DBP as only the significance (P value) of the association was reported.34 The 2 studies examining the link between PA intensity and MAP found both to be nonsignificant with relatively equal magnitudes of association.48,58
Metabolic Biomarkers
Nine studies assessed the relationship between PA intensity and metabolic biomarkers (total cholesterol [TC; n = 3],16,29,55 high-density lipoprotein cholesterol [HDL-C; n = 5],16,29,31,55,58 low-density lipoprotein cholesterol [LDL-C; n = 2],16,29 triglycerides [TGs; n = 5],16,29,31,55,58 glucose [n = 6],16,31,44,53,55,58 insulin [n = 7],16,17,31,36,44,53,55 insulin resistance [IR; n = 4],29,36,44,53 and insulin sensitivity [INS; n = 1]).36 Of the 5 studies evaluating lipids (TC, HDL-C, LDL-C, TG) as an outcome,16,29,31,55,58 2 studies assessed the influence of a single PA intensity and found nonsignificant associations for both MVPA16 and VPA.29 Among the 3 remaining studies, 1 study found that VPA was significantly directly associated with HDL-C and significantly inversely related to TGs, and more strongly linked with these biomarkers than MPA and/or MVPA.55 The second study found a significant inverse correlation between MPA and TGs, greater in magnitude when compared to MPA and/or VPA.31 The last study reported nonsignificant associations among all PA intensities.58
Of the 6 articles that assessed the relationship between PA intensity and glucose,16,31,36,44,55,58 one study assessed the influence of a single PA intensity and found a non-significant association for MVPA.16 Two of the 5 remaining studies demonstrated that PA intensity was significantly and negatively associated with fasting glucose levels,31,44 with 1 study44 finding a stronger inverse correlation for VPA, and the other31 observing a stronger connection between MPA and glucose levels. The remaining 3 studies reported nonsignificant relationships between PA intensity and glucose.53,55,58 Of these nonsignificant findings, 1 study55 observed VPA to have a stronger negative association with glucose levels compared with MPA and MVPA, and the others had conflicting findings.53,58
Seven studies measured insulin16,17,31,36,44,53,55 as an outcome with only 3 observing a significant and negative association between PA intensity and insulin.16,31,44 Of these significant findings, 1 article44 reported that VPA was inversely and more strongly related to insulin than MPA or MVPA, while the others showed a stronger relationship for insulin and MPA16 or displayed similar findings between MPA and VPA.31 Two of the 7 studies observed a nonsignificant association between PA intensity and insulin, with 1 study55 demonstrating VPA as a stronger negative predictor of insulin whereas the other17 found MPA to have a stronger relationship.17 The remaining 2 studies reported mixed findings between PA intensity and insulin: one found nonsignificant direct correlations for MPA and MVPA whereas VPA demonstrated a nonsignificant inverse correlation,53 and the other exhibited conflicting results between sexes when stratified by CRF.36 Among females with low CRF, MVPA exerted a significant and stronger negative influence on insulin levels compared with MPA and VPA. This relationship in females became nonsignificant with increased CRF for all PA intensity levels. Conversely, for males, regardless of the level of CRF, PA intensity was not significantly associated with insulin levels.36
Four studies assessed the link between PA intensity and IR29,36,44,53 with 1 study assessing the influence of a single PA intensity and found a nonsignificant relationship with VPA.29 Of the remaining 3 articles, only 1 significant association was reported, which indicated that VPA was a stronger negative predictor of IR compared with MPA and MVPA.44 Another found conflicting results between the sexes when stratified by CRF.36 Among females with low CRF, MVPA displayed a significant and stronger inverse association with IR compared with MPA and VPA; however, higher levels of CRF yielded nonsignificant findings for all PA intensities. Among males, nonsignificant relationships were found for all PA intensities and IR.36 In the remaining report,53 the influence of PA intensity on IR was conflicting as VPA demonstrated an inverse relationship with IR, whereas a positive relationship was found for MPA and MVPA.
Only 1 study examined the link between PA intensity and INS and found that the results differed by sex when stratified by CRF level.36 The findings of this study suggested that MVPA significantly and positively influenced INS compared with MPA and VPA only among females with low CRF. This relationship was nonsignificant among females with moderate to high CRF. No association existed among males between any PA intensity and INS regardless of CRF level.
Cardiovascular Disease and Metabolic Syndrome Risk Scores
Five articles evaluated the association between PA intensity and cardiovascular disease (CVD)37,48 and/or metabolic syndrome (MetS)31,55,58 risk score, of which all but one demonstrated significant relationships.58 Two studies found VPA was more strongly inversely associated with disease risk score than MPA and/or MVPA.48,55 However, the correlations with MPA and MVPA with MetS risk score were nonsignificant for one of these studies.55 In this report, the magnitude of association between VPA and MetS risk score was substantially attenuated and became nonsignificant after further adjustment for CRF.55 Another article found that across all quintiles of PA (highest PA level as referent), MVPA demonstrated a significant negative and stronger influence on CVD risk score compared with MPA and VPA, however, only occurring among females.37 In males, MPA was a significant negative predictor of CVD risk score compared with MVPA and VPA, although only among those with the lowest level of PA.37 The remaining study showed both MPA and VPA to be significantly and negatively associated with MetS risk score, with impact appearing to be similar in magnitude.31
Age and Sex
Three articles reported outcomes for PA intensity and CM biomarkers by sex.36,37,48 Tanha et al48 showed that males and females, aged 7.9 to 11.1 years, had relatively similar, significant correlations between VPA and CVD risk score, as well as MVPA and CVD risk score. The 2 other articles, both by Jiménez-Pavón et al,36,37 found mixed results. In youth aged 6 to 9 years, females had higher CVD risk scores than males across all PA intensities.37 While CVD risk for both sexes generally declined with increasing PA quintiles, it increased with increasing MPA in females. Additionally, the odds ratio retained significance for females as PA quintiles increased, whereas males lost significance.37
In the second report concerning youth older than 12 years, Jiménez-Pavón et al36 noted that partial correlations yielded stronger significant findings for females regarding the connection between VPA and insulin and INS. However, males had a stronger, significant correlation between VPA and IR, and a stronger nonsignificant correlation between VPA and glucose.36 When intensity decreased, these relationships for males became nonsignificant, but females retained significance, although strength was reduced but still stronger than respective results for males. When reporting regression coefficients, stronger nonsignificant associations for females were demonstrated concerning MPA and MVPA versus insulin, INS, and IR, with males showing a stronger nonsignificant relationship between VPA and these biomarkers.36
Discussion
The purpose of this review was to systematically examine the current literature on the associations between PA intensity and body composition, CRF, and CM biomarkers in youth. Major findings of this study were (1) VPA was more strongly negatively associated with body composition, and more strongly positively associated with CRF compared with MPA or MVPA, (2) no specific PA intensity appeared to consistently and more strongly associate with CM biomarkers, and (3) among the majority of studies demonstrating that VPA had a stronger relationship with adiposity and CRF outcomes, participants often engaged in at least 10 minutes of VPA, potentially suggesting that 10 minutes of VPA may be a sufficient dose for youth.
In a previous review of PA intensity and health outcomes related to adiposity and fitness, which examined 17 observational studies published between 1999 and 2009, authors noted that a shorter duration of VPA yielded a stronger association with body fat than a longer duration of MPA.11 A more recent review remarked that every minute of VPA yields the same improvements in body composition as 2 or 3 minutes of MPA.12 In our expanded and updated review, among the studies that found VPA significantly related to BMI, body composition, and central adiposity compared with other PA intensities, a majority reported participants engaging in an average of at least 10 minutes of VPA.‡‡ Considering that the “dose” of VPA was relatively modest in many of these studies, as well as the sporadic nature of PA in youth with brief intermittent bouts of VPA,61 it is conceivable that VPA may be more attainable simply because a health-improving dose is less time-consuming to attain. Therefore, it could be suggested that VPA should be promoted independent of MPA as a behavior. Additionally, Martinez-Gomez et al62 and Katzmarzyk et al7 conducted receiver operating characteristic analyses on activity dose and identified that 17 and 20 minutes of VPA in boys and 9 and 11 minutes of VPA in girls, respectively, mitigated the risk of being overfat and/or obese, highlighting possible sex differences in VPA recommendations.7,62
In line with previous studies, we found that VPA was consistently more strongly associated with CRF compared to MPA and/or MVPA. This finding has also been demonstrated among exercise training studies with intensities greater than 70% of age-predicted maximum heart rate (vigorous intensity) eliciting significant changes in CRF.3 A prior review by Ortega et al63 on fitness and health outcomes in youth discussed the importance of VPA to increase CRF for favorable changes in adiposity and CM profile. Additionally, among adults substantial evidence indicates that there is a dose-response relationship between PA intensity and CRF, with the greatest improvement in CRF found among very high intensities (90% to 100% VO2max).64 Importantly, CRF has been demonstrated to be a strong predictor of morbidity65,66 and mortality67-69 among adults, and some evidence indicates that CRF tracks modestly from childhood to adolescence to adulthood.70-72 As a result, it is reasonable to suggest that increasing CRF among youth should be a public health priority; as such, including a dose of PA that may elicit improvements in CRF (ie, VPA) in the PA guidelines is warranted. Among the studies in this review that found VPA to be more strongly associated with CRF, a majority described youth engaging in at least 10 minutes of VPA (range 12-46 minutes), suggesting this dose of VPA may be a good starting point for youth.36,43,46,53-55,60
In contrast to the previous findings of this review, associations between PA intensity and CM biomarkers were equivocal. We did not find a PA intensity (MPA, MVPA, or VPA) that was strongly associated with any of the CM biomarkers consistently when compared with other intensities. VPA did not display a strong relationship with MetS/CVD risk score in youth, and in fact became nonsignificant after controlling for CRF.55 This finding was echoed for insulin and IR, with higher levels of CRF negating any significant influence of VPA on these CM biomarkers.36 It is possible that the conflicting findings in our review may be due to the inherent healthiness of children and adolescents. The review by Owens et al12 suggested that VPA was associated with a more favorable blood lipid profile and glucose-insulin profile. Conversely, inconclusiveness of findings between PA intensity and CM biomarkers was demonstrated in a previous review6 that examined BP, plasma lipid and lipoproteins, and MetS. However, authors noted that favorable changes in blood lipids and lipoproteins post-exercise intervention were more likely to occur in high-risk participants, indicating that the initial health status of the study population may greatly affect the observed association.6
In addition, Gutin and Owens10 in their review of both observational and intervention studies identified fitness and fatness as mediating factors of the relationship between PA and CM biomarkers among youth, noting that PA interventions conducted among overweight and obese youth had a beneficial effect on several CM biomarkers. Conversely, the evidence was limited for PA interventions targeting nonobese youth.10 However, there is evidence demonstrating the emergence of obesity-related complications in early childhood that includes the premature manifestation of CM risk factors.73-76 Ferreira et al77 noted in their longitudinal study following 450 participants from adolescence to young adulthood that increases in body fat, declines in CRF, and a greater shift from time spent in VPA to light-to-moderate intensity PA were characteristic of MetS at the age of 36 years. As such, the findings of our review provide additional support for weight control and improving CRF in youth as a preventive measure for future CM disease.
There are several strengths to this review that warrant attention. Few reviews in the past have solely focused on objective PA measurement, which has now become more standardized in PA assessment. We also included CM biomarkers in addition to body composition and CRF, which are often not collectively examined despite their connections. Additionally, this is the first review to identify a possible VPA dose for youth. As with any study, there are also limitations that are worth mentioning. We chose not to examine intervention studies as done in prior reviews,10-12 narrowing our focus to observational studies. Even though not limited to objective measurement tools, we felt these previous reviews provided sufficient support for the changes that high-intensity structured exercise could make over the course of an intervention, and chose instead to focus on larger observational studies that gathered data on VPA when incorporated in normal daily activity. Nonetheless, this review highlights the importance of further conducting PA interventions focused on intensity in youth for future review.
An additional limitation in the observational studies we reviewed also involves variation in method of PA measurement. All articles used accelerometry to measure PA intensity, but accelerometer model, length of wear, and VPA cutpoints varied widely. The Actigraph GT1M model was primarily used (33.3%; n = 15)§§ or one of its predecessors (ActiGraph 7164, MTI 7164, or CSA 7164; 43.9%; n = 18),‖‖ but even when using the same model, VPA cutpoints applied were inconsistent (eg, 3000 to 4012 counts/min; Actigraph GT1M).20,34 Considering that youth primarily engage in very short periods of VPA at a time, a 60-second or even a 10-second epoch may not adequately differentiate VPA as it is combined with periods of rest or light PA.61,78
In conclusion, while there are certainly benefits to MPA, as evidenced in this review, VPA was a stronger predictor of improved body composition, particularly for reduced body fat and central adiposity, and increased fitness compared with MPA and/or MVPA. These patterns were observed even when findings were nonsignificant. From our results, it appears that a dose of 10 minutes of VPA/day may be sufficient to elicit favorable changes in these health outcomes, with possible differences for male and female youth. However, these suggestions need to be confirmed with rigorously randomized controlled exercise training studies.
Footnotes
References
- 1. Strong WB, Malina RM, Blimkie CJ, et al. Evidence based physical activity for school-age youth. J Pediatr. 2005;146:732-737. doi: 10.1016/j.jpeds.2005.01.055. [DOI] [PubMed] [Google Scholar]
- 2. Hallal PC, Victora CG, Azevedo MR, Wells JCK. Adolescent physical activity and health: a systematic review. Sports Med. 2006;36:1019-1030. doi: 10.2165/00007256-200636120-00003. [DOI] [PubMed] [Google Scholar]
- 3. US Department of Health and Human Services. Physical activity guidelines for Americans. 2008. http://www.health.gov/paguidelines. Accessed December 15, 2015.
- 4. Laguna M, Ruiz JR, Lara MT, Aznar S. Recommended levels of physical activity to avoid adiposity in Spanish children. Pediatr Obes. 2013;8:62-69. doi: 10.1111/j.2047-6310.2012.00086.x. [DOI] [PubMed] [Google Scholar]
- 5. Mark AE, Janssen I. Dose-response relation between physical activity and blood pressure in youth. Med Sci Sports Exerc. 2008;40:1007-1012. doi: 10.1249/MSS.0b013e318169032d. [DOI] [PubMed] [Google Scholar]
- 6. Janssen I, LeBlanc A. Systematic review of the health benefits of physical activity and fitness in school-aged children and youth. Int J Behav Nutr Phys Act. 2010;7:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Katzmarzyk PT, Barreira TV, Broyles ST, et al. Physical activity, sedentary time, and obesity in an international sample of children. Med Sci Sports Exerc. 2015;47:2062-2069. doi: 10.1249/MSS.0000000000000649. [DOI] [PubMed] [Google Scholar]
- 8. Fakhouri T, Hughes J, Burt V, Song M, Fulton J, Ogden C. Physical activity in U.S. youth aged 12-15 years, 2012. NCHS Data Brief. 2014;(141):1-8. [PubMed] [Google Scholar]
- 9. Troiano RP, Berrigan D, Dodd KW, Mâsse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40:181-188. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 10. Gutin B, Owens S. The influence of PA on CM biomarkers in youths—a review. Pediatr Exerc Sci. 2011;23:169-185. [DOI] [PubMed] [Google Scholar]
- 11. Parikh T, Stratton G. Influence of intensity of physical activity on adiposity and cardiorespiratory fitness in 51-8 year olds. Sports Med. 2011;41:477-488. [DOI] [PubMed] [Google Scholar]
- 12. Owens S, Galloway R, Gutin B. The case for vigorous physical activity in youth [published online July 15, 2015]. Am J Lifestyle Med. 2015. doi: 10.1177/1559827615594585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bailey BW, Borup P, LeCheminant JD, Tucker LA, Bromley J. Examining the relationship between physical activity intensity and adiposity in young women. J Phys Act Health. 2015;12:764-769. doi: 10.1123/jpah.2013-0441. [DOI] [PubMed] [Google Scholar]
- 14. Irving BA, Davis CK, Brock DW, et al. Effect of exercise training intensity on abdominal visceral fat and body composition. Med Sci Sports Exerc. 2008;40:1863-1872. doi: 10.1249/MSS.0b013e3181801d40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tremblay A, Simoneau JA, Bouchard C. Impact of exercise intensity on body fatness and skeletal muscle metabolism. Metabolism. 1994;43:814-818. [DOI] [PubMed] [Google Scholar]
- 16. Alhassan S, Robinson TN. Objectively measured physical activity and cardiovascular disease risk factors in African American girls. Ethn Dis. 2008;18:421-426. [PMC free article] [PubMed] [Google Scholar]
- 17. Butte NF, Puyau MR, Adolph AL, Vohra FA, Zakeri I. Physical activity in nonoverweight and overweight Hispanic children and adolescents. Med Sci Sports Exerc. 2007;39:1257-1266. doi: 10.1249/mss.0b013e3180621fb6. [DOI] [PubMed] [Google Scholar]
- 18. Gutin B, Yin Z, Humphries MC, Barbeau P. Relations of moderate and vigorous physical activity to fitness and fatness in adolescents. Am J Clin Nutr. 2005;81:746-750. [DOI] [PubMed] [Google Scholar]
- 19. Mark AE, Janssen I. Influence of movement intensity and physical activity on adiposity in youth. J Phys Act Health. 2011;8:164-173. [DOI] [PubMed] [Google Scholar]
- 20. Moore JB, Beets MW, Barr-Anderson DJ, Evenson KR. Sedentary time and vigorous physical activity are independently associated with cardiorespiratory fitness in middle school youth. J Sports Sci. 2013;31:1520-1525. doi: 10.1080/02640414.2013.793378. [DOI] [PubMed] [Google Scholar]
- 21. Patrick K, Norman GJ, Calfas KJ, et al. Diet, physical activity, and sedentary behaviors as risk factors for overweight in adolescence. Arch Pediatr Adolesc Med. 2004;158:385-390. [DOI] [PubMed] [Google Scholar]
- 22. Treuth MS, Hou N, Young DR, Maynard LM. Accelerometry-measured activity or sedentary time and overweight in rural boys and girls. Obes Res. 2005;13:1606-1614. [DOI] [PubMed] [Google Scholar]
- 23. Chaput JP, Lambert M, Mathieu ME, Tremblay MS, O’ Loughlin J, Tremblay A. Physical activity vs. sedentary time: independent associations with adiposity in children. Pediatr Obes. 2012;7:251-258. doi: 10.1111/j.2047-6310.2011.00028.x. [DOI] [PubMed] [Google Scholar]
- 24. Hay J, Maximova K, Durksen A, et al. Physical activity intensity and cardiometabolic risk in youth. Arch Pediatr Adolesc Med. 2012;166:1022-1029. doi: 10.1001/archpediatrics.2012.1028. [DOI] [PubMed] [Google Scholar]
- 25. Wittmeier KD, Mollard RC, Kriellaars DJ. Physical activity intensity and risk of overweight and adiposity in children. Obesity (Silver Spring). 2008;16:415-420. doi: 10.1038/oby.2007.73. [DOI] [PubMed] [Google Scholar]
- 26. Carson V, Rinaldi RL, Torrance B, et al. Vigorous physical activity and longitudinal associations with cardiometabolic risk factors in youth. Int J Obes (Lond). 2014;38:16-21. doi: 10.1038/ijo.2013.135. [DOI] [PubMed] [Google Scholar]
- 27. Aires L, Silva P, Silva G, Santos MP, Ribeiro JC, Mota J. Intensity of physical activity, cardiorespiratory fitness, and body mass index in youth. J Phys Act Health. 2010;7:54-59. [DOI] [PubMed] [Google Scholar]
- 28. Blaes A, Baquet G, Fabre C, Van Praagh E, Berthoin S. Is there any relationship between physical activity level and patterns, and physical performance in children? Int J Behav Nutr Phys Act. 2011;8:122. doi: 10.1186/1479-5868-8-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Danielsen Y, Júlíusson P, Nordhus I, et al. The relationship between life-style and cardio-metabolic risk indicators in children: the importance of screen time. Acta Paediatr. 2011;100:253-259. doi: 10.1111/j.1651-2227.2010.02098.x. [DOI] [PubMed] [Google Scholar]
- 30. Denton SJ, Trenell MI, Plötz T, Savory LA, Bailey DP, Kerr CJ. Cardiorespiratory fitness is associated with hard and light intensity physical activity but not time spent sedentary in 10-14 year old schoolchildren: the HAPPY study. PLoS One. 2013;8(4):e61073. doi: 10.1371/journal.pone.0061073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ekelund U, Anderssen SA, Froberg K, Sardinha LB, Andersen LB, Brage S. Independent associations of physical activity and cardiorespiratory fitness with metabolic risk factors in children: the European youth heart study. Diabetologia. 2007;50:1832-1840. doi: 10.1007/s00125-007-0762-5. [DOI] [PubMed] [Google Scholar]
- 32. Ekelund U, Sardinha LB, Anderssen SA, et al. Associations between objectively assessed physical activity and indicators of body fatness in 9-to 10-y-old European children: a population-based study from 4 distinct regions in Europe (the European Youth Heart Study). Am J Clin Nutr. 2004;80:584-590. [DOI] [PubMed] [Google Scholar]
- 33. España-Romero V, Ortega FB, Ruiz JR, et al. Role of cardiorespiratory fitness on the association between physical activity and abdominal fat content in adolescents: the HELENA study. Int J Sports Med. 2010;31:679-682. doi: 10.1055/s-0030-1261935. [DOI] [PubMed] [Google Scholar]
- 34. Gaya AR, Alves A, Aires L, Martins CL, Ribeiro JC, Mota J. Association between time spent in sedentary, moderate to vigorous physical activity, body mass index, cardiorespiratory fitness and blood pressure. Ann Hum Biol. 2009;36:379-387. doi: 10.1080/03014460902817976. [DOI] [PubMed] [Google Scholar]
- 35. Jiménez-Pavón D, Fernández-Vázquez A, Alexy U, et al. Association of objectively measured physical activity with body components in European adolescents. BMC Public Health. 2013;13(1):667. doi: 10.1186/1471-2458-13-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jiménez-Pavón D, Ruiz JR, Ortega FB, et al. Physical activity and markers of insulin resistance in adolescents: role of cardiorespiratory fitness levels—the HELENA study. Pediatr Diabetes. 2013;14:249-258. doi: 10.1111/pedi.12000. [DOI] [PubMed] [Google Scholar]
- 37. Jiménez-Pavón D, Konstabel K, Bergman P, et al. Physical activity and clustered cardiovascular disease risk factors in young children: a cross-sectional study (the IDEFICS study). BMC Med. 2013;11:172. doi: 10.1186/1741-7015-11-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kennedy K, Shepherd S, Williams JE, Ahmed SF, Wells JC, Fewtrell M. Activity, body composition and bone health in children. Arch Dis Child. 2013;98:204-207. doi: 10.1136/archdischild-2012-302823. [DOI] [PubMed] [Google Scholar]
- 39. Moliner-Urdiales D, Ortega FB, Vicente-Rodriguez G, et al. Association of physical activity with muscular strength and fat-free mass in adolescents: the HELENA study. Eur J Appl Physiol. 2010;109:1119-1127. doi: 10.1007/s00421-010-1457-z. [DOI] [PubMed] [Google Scholar]
- 40. Moliner-Urdiales D, Ruiz JR, Ortega FB, et al. Association of objectively assessed physical activity with total and central body fat in Spanish adolescents: the HELENA study. Int J Obes (Lond). 2009;33:1126-1135. doi: 10.1038/ijo.2009.139. [DOI] [PubMed] [Google Scholar]
- 41. Ortega FB, Ruiz JR, Hurtig-Wennlöf A, et al. Cardiovascular fitness modifies the associations between physical activity and abdominal adiposity in children and adolescents: the European Youth Heart Study. Br J Sports Med. 2010;44:256-262. doi: 10.1136/bjsm.2008.046391. [DOI] [PubMed] [Google Scholar]
- 42. Ortega FB, Ruiz JR, Sjöström M. Physical activity, overweight and central adiposity in Swedish children and adolescents: the European Youth Heart Study. Int J Behav Nutr Phys Act. 2007;4:61. doi:10.1186/1479- 5868-4-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Radtke T, Kriemler S, Eser P, Saner H, Wilhelm M. Physical activity intensity and surrogate markers for cardiovascular health in adolescents. Eur J Appl Physiol. 2013;113:1213-1222. doi: 10.1007/s00421-012-2542-2. [DOI] [PubMed] [Google Scholar]
- 44. Rizzo NS, Ruiz JR, Oja L, Veidebaum T, Sjöström M. Associations between physical activity, body fat, and insulin resistance (homeostasis model assessment) in adolescents: the European Youth Heart Study. Am J Clin Nutr. 2008;87:586-592. [DOI] [PubMed] [Google Scholar]
- 45. Rowlands AV, Eston RG, Powell SM. Total physical activity, activity intensity and body fat in 8 to 11 year old boys and girls. J Exerc Sci Fit. 2006;4:96-102. [Google Scholar]
- 46. Ruiz JR, Rizzo NS, Hurtig-Wennlöf A, Ortega FB, Wärnberg J, Sjöström M. Relations of total physical activity and intensity to fitness and fatness in children: the European Youth Heart Study. Am J Clin Nutr. 2006;84:299-303. [DOI] [PubMed] [Google Scholar]
- 47. Steele RM, van Sluijs EM, Cassidy A, Griffin SJ, Ekelund U. Targeting sedentary time or moderate- and vigorous-intensity activity: independent relations with adiposity in a population-based sample of 10-y-old British children. Am J Clin Nutr. 2009;90:1185-1192. [DOI] [PubMed] [Google Scholar]
- 48. Tanha T, Wollmer P, Thorsson O, et al. Lack of physical activity in young children is related to higher composite risk factor score for cardiovascular disease. Acta Paediatr. 2011;100:717-721. doi: 10.1111/j.1651-2227.2011.02226.x. [DOI] [PubMed] [Google Scholar]
- 49. Dencker M, Bugge A, Hermansen B, Andersen LB. Objectively measured daily physical activity related to aerobic fitness in young children. J Sports Sci. 2010;28:139-145. doi: 10.1080/02640410903460726. [DOI] [PubMed] [Google Scholar]
- 50. Dencker M, Thorsson O, Karlsson MK, et al. Body fat related to daily physical activity and insulin concentrations in non-diabetic children. Clin Physiol Funct Imaging. 2008;28:211-215. doi: 10.1111/j.1475-097X.2007.00787.x. [DOI] [PubMed] [Google Scholar]
- 51. Dencker M, Thorsson O, Karlsson MK, et al. Gender differences and determinants of aerobic fitness in children aged 8-11 years. Eur J Appl Physiol. 2007;99:19-26. doi: 10.1007/s00421-006-0310-x. [DOI] [PubMed] [Google Scholar]
- 52. Dencker M, Thorsson O, Karlsson MK, et al. Daily physical activity related to body fat in children aged 8-11 years. J Pediatr. 2006;149:38-42. doi: 10.1016/j.jpeds.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 53. Martinez-Gomez D, Eisenmann JC, Wärnberg J, et al. Associations of physical activity, cardiorespiratory fitness and fatness with low-grade inflammation in adolescents: the AFINOS study. Int J Obes (Lond). 2010;34:1501-1507. doi: 10.1038/ijo.2010.114. [DOI] [PubMed] [Google Scholar]
- 54. Martinez-Gomez D, Ruiz JR, Ortega FB, et al. Recommended levels and intensities of physical activity to avoid low-cardiorespiratory fitness in European adolescents: the HELENA study. Am J Hum Biol. 2010;22:750-756. doi: 10.1002/ajhb.21076. [DOI] [PubMed] [Google Scholar]
- 55. Martínez-Gómez D, Eisenmann JC, Moya JM, Gómez-Martínez S, Marcos A, Veiga OL. The role of physical activity and fitness on the metabolic syndrome in adolescents: effect of different scores. The AFINOS study. J Physiol Biochem. 2009;65:277-289. doi: 10.1007/BF03180580. [DOI] [PubMed] [Google Scholar]
- 56. Fairclough SJ, Boddy LM, Ridgers ND, Stratton G. Weight status associations with physical activity intensity and physical self-perceptions in 10- to 11-year-old children. Pediatr Exerc Sci. 2012;24:100-112. [DOI] [PubMed] [Google Scholar]
- 57. Lätt E, Mäestu J, Ortega FB, Rääsk T, Jürimäe T, Jürimäe J. Vigorous physical activity rather than sedentary behaviour predicts overweight and obesity in pubertal boys: a 2-year follow-up study. Scand J Public Health. 2015;43:276-282. doi: 10.1177/1403494815569867. [DOI] [PubMed] [Google Scholar]
- 58. Dubose KD, McKune AJ, Brophy P, Geyer G, Hickner RC. The relationship between physical activity and the metabolic syndrome score in children. Pediatr Exerc Sci. 2015;27:364-371. doi: 10.1123/pes.2014-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Martinez-Gomez D, Tucker J, Heelan KA, Welk GJ, Eisenmann JC. Associations between sedentary behavior and blood pressure in young children. Arch Pediatr Adolesc Med. 2009;163:724-730. [DOI] [PubMed] [Google Scholar]
- 60. Dencker M, Thorsson O, Karlsson MK, Lindén C, Wollmer P, Andersen LB. Daily physical activity related to aerobic fitness and body fat in an urban sample of children. Scand J Med Sci Sports. 2008;18:728-735. doi: 10.1111/j.1600-0838.2007.00741.x. [DOI] [PubMed] [Google Scholar]
- 61. Welk GJ, Corbin CB, Dale D. Measurement issues in the assessment of physical activity in children. Res Q Exerc Sport. 2000;71(2 suppl.):S59-S73. [PubMed] [Google Scholar]
- 62. Martinez-Gomez D, Ruiz JR, Ortega FB, et al. Recommended levels of physical activity to avoid an excess of body fat in European adolescents. Am J Prev Med. 2010;39:203-211. [DOI] [PubMed] [Google Scholar]
- 63. Ortega FB, Ruiz JR, Castillo MJ, Sjöström M. Physical fitness in childhood and adolescence: a powerful marker of health. Int J Obes (Lond). 2008;32:1-11. doi: 10.1038/sj.ijo.0803774. [DOI] [PubMed] [Google Scholar]
- 64. Wenger HA, Bell GJ. The interactions of intensity, frequency and duration of exercise training in altering cardiorespiratory fitness. Sports Med. 1986;3:346-356. [DOI] [PubMed] [Google Scholar]
- 65. Ross R, Katzmarzyk PT. Cardiorespiratory fitness is associated with diminished total and abdominal obesity independent of body mass index. Int J Obes Relat Metab Disord. 2003;27:204-210. doi: 10.1038/sj.ijo.802222. [DOI] [PubMed] [Google Scholar]
- 66. Wei M, Gibbons LW, Mitchell TL, Kampert JB, Lee CD, Blair SN. The association between cardiorespiratory fitness and impaired fasting glucose and type 2 diabetes mellitus in men. Ann Intern Med. 1999;130:89-96. [DOI] [PubMed] [Google Scholar]
- 67. Blair SN, Kohl HW, Paffenbarger RS, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA. 1989;262:2395-2401. [DOI] [PubMed] [Google Scholar]
- 68. Blair SN, Kampert JB, Kohl HW, 3rd, et al. Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. JAMA. 1996;276:205-210. doi: 10.1001/jama.1996.03540030039029. [DOI] [PubMed] [Google Scholar]
- 69. Wei M, Kampert JB, Barlow CE, et al. Relationship between low cardiorespiratory fitness and mortality in normal-weight, overweight, and obese men. JAMA. 1999;282:1547-1553. [DOI] [PubMed] [Google Scholar]
- 70. Sorić M, Jembrek Gostović M, Gostović M, Hočevar M, Mišigoj-Duraković M. Tracking of BMI, fatness and cardiorespiratory fitness from adolescence to middle adulthood: the Zagreb Growth and Development Longitudinal Study. Ann Hum Biol. 2014;41:238-243. doi: 10.3109/03014460.2013.851739. [DOI] [PubMed] [Google Scholar]
- 71. Malina RM. Tracking of physical activity and physical fitness across the lifespan. Res Q Exerc Sport. 1996;67(3 suppl):S48-S57. [DOI] [PubMed] [Google Scholar]
- 72. Janz KF, Dawson JD, Mahoney LT. Tracking physical fitness and physical activity from childhood to adolescence: the Muscatine study. Med Sci Sports Exerc. 2000;32:1250-1257. [DOI] [PubMed] [Google Scholar]
- 73. Corvalan C, Uauy R, Kain J, Martorell R. Obesity indicators and cardiometabolic status in 4-y-old children. Am J Clin Nutr. 2009;91:166-174. doi: 10.3945/ajcn.2009.27547 [DOI] [PubMed] [Google Scholar]
- 74. Weiss R, Dziura J, Burgert TS, et al. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med. 2004;350:2362-2374. doi: 10.1056/NEJMoa031049. [DOI] [PubMed] [Google Scholar]
- 75. Weiss R, Kaufman FR. Metabolic complications of childhood obesity: identifying and mitigating the risk. Diabetes Care. 2008;31(suppl 2):S310-S316. doi: 10.2337/dc08-s273. [DOI] [PubMed] [Google Scholar]
- 76. Lee JM, Okumura MJ, Davis MM, Herman WH, Gurney JG. Prevalence and determinants of insulin resistance among U.S. adolescents: a population-based study. Diabetes Care. 2006;29:2427-2432. doi: 10.2337/dc06-0709. [DOI] [PubMed] [Google Scholar]
- 77. Ferreira I, Twisk JWR, van Mechelen W, Kemper HCG, Stehouwer CDA. Development of fatness, fitness, and lifestyle from adolescence to the age of 36 years: determinants of the metabolic syndrome in young adults: the Amsterdam Growth and Health Longitudinal Study. Arch Intern Med. 2005;165:42-48. doi: 10.1001/archinte.165.1.42. [DOI] [PubMed] [Google Scholar]
- 78. Aibar A, Bois JE, Zaragoza J, Generelo E, Julián JA, Paillard T. Do epoch lengths affect adolescents’ compliance with physical activity guidelines? J Sports Med Phys Fitness. 2014;54:326-334. [PubMed] [Google Scholar]