Abstract
Background: The US Food and Drug Administration has recently approved abaloparatide (ABL) for treatment of women with postmenopausal osteoporosis (PMO) at high risk of fracture. With increasing health care spending and drug prices, it is important to quantify the value of newly available treatment options for PMO. Objective: To determine cost-effectiveness of ABL compared with teriparatide (TPTD) for treatment of women with PMO in the United States. Methods: A discrete-event simulation (DES) model was developed to assess cost-effectiveness of ABL from the US health care perspective. The model included three 18-month treatment strategies with either placebo (PBO), TPTD, or ABL, all followed by additional 5-year treatment with alendronate (ALN). High-risk patients were defined as women with PMO ⩾65 years old with a prior vertebral fracture. Baseline clinical event rates, risk reductions, and patient characteristics were based on the Abaloparatide Comparator Trial in Vertebral Endpoints (ACTIVE) trial. Results: Over a 10-year period, the DES model yielded average total discounted per-patient costs of $10 212, $46 783, and $26 837 and quality-adjusted life-years (QALYs) of 6.742, 6.781, and 6.792 for PBO/ALN, TPTD/ALN, and ABL/ALN, respectively. Compared with TPTD/ALN, ABL/ALN accrued higher QALYs at lower cost and produced an incremental cost-effectiveness ratio (ICER) of $333 266/QALY relative to PBO/ALN. In high-risk women, ABL/ALN also had more QALYs and less cost over TPTD/ALN and yielded an ICER of $188 891/QALY relative to PBO/ALN. Conclusion and Relevance: ABL is a dominant treatment strategy over TPTD. In women with PMO at high risk of fracture, ABL is an alternative cost-effective treatment.
Keywords: economic analysis, cost-effectiveness analysis, cost-utility analysis, abaloparatide, teriparatide, postmenopausal women, osteoporosis
Introduction
Postmenopausal osteoporosis (PMO) is a systemic and progressive skeletal disorder characterized by reduced bone mass and weakening bone strength, leading to an increased risk of fracture.1 It is a major public health issue with substantial personal and societal burden.2 Osteoporosis is associated with significant morbidity and mortality following osteoporotic fractures, especially in the spine and hip.3 It was estimated that 15.4% of women in the United States have osteoporosis based on 2010 US Census and National Health and Nutrition Examination Survey data, with the prevalence of PMO increasing with age from 6.8% at age of 50 years to approximately 35% above the age of 80 years.4 In 2005, more than 2 million fractures were attributable to osteoporosis at a cost of 17 billion US dollars; hip fracture can lead to a substantial loss of healthy life-years in elderly people.5 By 2025, annual fractures and costs are projected to rise by almost 50%, with an increase of more than 87% in the age group between 65 and 74 years old.5
The treatment goal is to reduce the risk of the fragility fractures associated with osteoporosis. There are several therapeutic options to treat osteoporosis mostly comprising antiresorptive drugs, such as bisphosphonates and, more recently, denosumab.6 However, there are limitations with existing therapeutic options because they lead to a low turnover state where bone formation decreases in relation to the decrease in bone-remodeling activity.6 The anabolic drug stimulates processes and mechanisms associated with bone formation, which is ultimately improved, leading to an increase in bone mass.6 Most recently, the US Food and Drug Administration has approved the parathyroid hormone-related protein analog, abaloparatide (ABL), for daily subcutaneous injection for treatment of postmenopausal women with osteoporosis at high risk for fractures. It should be noted that because of the unknown relevance of the rodent osteosarcoma findings to humans, cumulative use of parathyroid hormone analogs, including ABL and teriparatide (TPTD), for more than 2 years during a patient’s lifetime is not recommended.
In the pivotal 18-month phase 3, double-blind, randomized controlled trial (Abaloparatide Comparator Trial In Vertebral Endpoints [ACTIVE]) of postmenopausal women with osteoporosis, ABL and TPTD significantly reduced the relative risk of vertebral fractures (86% and 80%, respectively). ABL further reduced the risk of nonvertebral fractures (43%) compared with placebo (PBO) and the risk of major osteoporotic fractures compared with TPTD (55%). Treatment-emergent adverse events were similar across treatment groups.7 In addition, the ACTIVExtend trial showed that the fracture risk reduction achieved with ABL-SC over the 18-month treatment in ACTIVE was sustained during the 24 months of alendronate (ALN) treatment after 18 months of active treatment.8,9
The objective of the current study was to determine the cost-effectiveness of the newly approved anabolic drug, ABL, relative to TPTD in the treatment of women with PMO from the US health care payer’s perspective.
Methods
Discrete-Event Simulation (DES) Approach in Economic Evaluation
An alternative to the Markov model approach, a health-state transition model in discrete time, discrete-event simulation (DES), is an event-driven model in continuous time at the patient level in which a series of clinically related events are sampled in discrete time for individual patients rather than moving patients through health states in the Markov model in predetermined time cycles in a deterministic (expected value of realized outcomes) context.10,11 The basic principle of DES models within health economic evaluation is that time-to-event (TTE) of all clinically related events are probabilistically sampled for each individual patient. All related costs—that is, treatment cost, potential costs of adverse events, inpatient and outpatient costs, and so on as well as patients’ quality-adjusted life-years (QALYs)—are recorded for each simulated patient. Then, a sample of 50 000 to 100 000 stochastically simulated patients is generated to estimate the average cost and QALYs per patient for each treatment group.10,11 Flexibility, the ability to reflect patient heterogeneity, increased precision, and better characterization of modeling uncertainty are advantages of the DES model and may be the reason why it is preferred to the Markov model.12
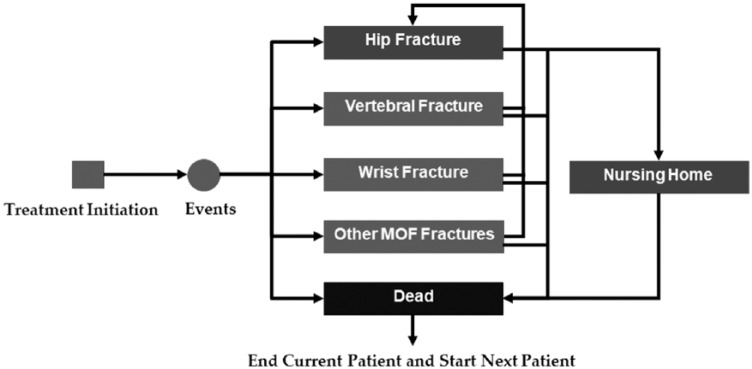
The development of the current DES model for cost-effectiveness analysis (CEA) of osteoporosis treatment was grounded in the Technical Support Document described by the UK NICE Decision Support Unit.10,12,13 Seven different clinical events are included in the model: hip; vertebral; wrist; other major osteoporotic fractures (MOFs), defined as any other MOF that is not wrist or vertebrae; death resulting from hip fracture; entering nursing home after hip fracture; and death resulting from causes other than hip fracture. In the DES model, a patient can experience a vertebral fracture, 1 wrist fracture, 1 other MOF, and multiple hip fractures. There is an increased risk of hip fracture following an initial fracture of the hip, vertebral or wrist fracture, or MOF (Table 1).14 In addition, for patients who experience hip fracture, there is a possibility that they would die from hip fracture or enter a nursing home (Figure 1).
Table 1.
DES Model Input Parameters for Natural History and Treatment Effects: Base-Case, Lower, and Upper Values and Distributions for Probabilistic Sensitivity Analysis.7-9,14-26
| DES Model Input Parameters | Base-Case Value | Lower Value | Upper Value | Distribution | References |
|---|---|---|---|---|---|
| Natural history | |||||
| Fracture rates | |||||
| Annual rate of hip fracture | 0.47% | N/A | N/A | Exponential | ACTIVE triala [7] |
| Annual rate of vertebral fracture | 2.9% | N/A | N/A | Exponential | ACTIVE trial [7] |
| Annual rate of wrist fracture | 1.4% | N/A | N/A | Exponential | ACTIVE trial [7] |
| Annual rate of other major osteoporotic fractures (MOFs) | 0.90% | N/A | N/A | Exponential | ACTIVE trial [7] |
| Average life expectancy | 18 years | 12.5 years | 23.5 years | Normal | [23] |
| Annual probability of nursing home after hip fracture | 12.2% | N/A | N/A | No change | [19, 24, 25] |
| Annual probability of death caused by hip fracture | 21.9% | N/A | N/A | No change | [26] |
| Hazard ratios of hip fracture following an initial fracture† | |||||
| Hip fracture | 2.30 | 1.50 | 3.70 | Log-normal | [14] |
| Vertebral fracture | 2.30 | 2.20 | 2.80 | Log-normal | [14] |
| Wrist fracture | 1.90 | 1.60 | 2.20 | Log-normal | [14] |
| Other MOFs | 2.00 | 1.70 | 2.30 | Log-normal | [14] |
| Treatment effects | |||||
| Fracture hazard ratios of abaloparatideb | |||||
| Hip fracture | 0.63 | 0.41 | 0.98 | Log-normal | ACTIVExtendc [9] |
| Vertebral fracture | 0.16 | 0.06 | 0.42 | Log-normal | ACTIVExtend [9] |
| Wrist fracture | 0.63 | 0.41 | 0.98 | Log-normal | ACTIVExtend [9] |
| Other MOFs | 0.42 | 0.25 | 0.70 | Log-normal | ACTIVExtend [9] |
| Duration of sustained treatment effects after active treatment (years) | 2.00 | N/A | N/A | No change | ACTIVExtend [8] |
| Offset time after period of sustained treatment effects (years)d | 3.00 | N/A | N/A | No change | [16-20] |
| Fracture hazard ratios of teriparatideb | |||||
| Hip fracture | 0.72 | 0.42 | 1.22 | Log-normal | ACTIVE trialc [7] |
| Vertebral fracture | 0.20 | 0.09 | 0.43 | Log-normal | ACTIVE trial [7] |
| Wrist fracture | 1.13 | 0.56 | 2.25 | Log-normal | ACTIVE trial [7] |
| Other MOFs | 0.67 | 0.39 | 1.14 | Log-normal | ACTIVE trial [7] |
| Duration of sustained treatment effects after active treatment (years) | 2.00 | N/A | N/A | No change | Assumed |
| Offset time after period of sustained treatment effects (years)d | 3.00 | N/A | N/A | No change | [16-20] |
| Fracture hazard ratios of alendronate (bisphosphonate)b | |||||
| Hip fracture | 0.62 | 0.40 | 0.98 | Log-normal | [18] |
| Vertebral fracture | 0.56 | 0.46 | 0.68 | Log-normal | [18] |
| Wrist fracture | 0.64 | 0.30 | 1.35 | Log-normal | [18] |
| Other MOFs | 0.80 | 0.67 | 0.97 | Log-normal | [18] |
| Offset time after period of sustained treatment effects (years) | 5.00 | N/A | N/A | No change | [15, 18-22] |
Abbreviation: DES, discrete-event simulation.
Based on the baseline FRAX score for hip fracture.
Lower and upper values were based on the 95% CI reported from relevant clinical trials/studies.
Risk reduction was assumed to be similar to the risk reduction for nonvertebral fractures.
Offset time after 43-month period of sustained treatment effects (18 months of treatment with abaloparatide-SC/teriparatide in ACTIVE trial, 1 month of reconsent, 24 months follow-up in ACTIVExtend trial.
Figure 1.
Structure of the discrete-event simulation model for osteoporosis treatment.
Abbreviation: MOF, major osteoporotic fracture.
First, on entry into the model, TTEs for hip, vertebral, and wrist fractures and other MOFs as well as time to hip fracture from an initial fracture of the hip, vertebral or wrist fracture, or other MOFs and death from causes other than hip fracture were sampled based on the annual fracture rates and average age of the PBO group in the ACTIVE trial7 for each individual patient. The random seed was set in such a way as to ensure that patients being simulated are identical for each treatment strategy, so the differences in outcomes are attributed to treatments and not to the differences in baseline risk for fracture. Second, to determine which clinical event would happen next, all TTEs were sorted from first to last occurrence. Third, for each fracture event, associated fracture cost and QALYs (based on its fracture-specific disutility multiplier) were added and recorded. The process continued until the patient died or reached the model time horizon, and all costs incurred (costs of treatment, potential adverse events, fracture events, nursing home) and QALYs gained were recorded for each patient over their stochastic time path.
TTEs for all the fractures were generated as follows10:
where U is a uniformly distributed random number between 0 and 1 and the fracture hazard ratios (HRs) or fracture risk reduction for the intervention were from the clinical trial and equal to 1 for the PBO group. The current DES model was developed using Microsoft Excel with the Visual Basic for Applications (VBA) programming language.
Treatment Efficacy and Real-World Adherence Rate
The current DES model for CEA compared 3 osteoporosis treatment strategies: (1) 18 months of PBO treatment followed by 5 years of bisphosphonate treatment with ALN (PBO/ALN), (2) 18 months of daily subcutaneous injection with TPTD followed by 5 years of treatment with ALN (TPTD/ALN), and (3) 18 months of daily subcutaneous injection with ABL followed by 5 years of treatment with ALN (ABL/ALN). In the DES model, real-world adherence rates for TPTD and ABL were assumed to be similar at 59.1%.27 The real-world adherence rates for TPTD and ALN were 59.1% and 35.1%, respectively. Treatment effects, in terms of HRs, and treatment costs were assumed to be reduced because of real-world nonadherence to the treatment strategies and adjusted based on Liu et al.15
The reductions in the risk of fracture at each fracture site in terms of HRs for TPTD and ABL were taken from the ACTIVE and ACTIVExtend clinical trials (Table 1). In addition, the ACTIVExtend trial showed that the fracture risk reduction achieved with ABL over the 18-month treatment in ACTIVE was sustained during the 24 months of ALN treatment after 18-month active treatment.9 The model assumed that a linear, gradual offset of fracture reduction benefits would last over the subsequent 3 years after the 24-month period of sustained fracture risk reduction for ABL (and TPTD)16-20 and over the subsequent 5 years after 5-year active treatment for ALN.15,18-22
Costs
The monthly cost of ABL was $1721 based on the wholesale acquisition cost (WAC) of one 3120-µg/1.56-mL pen-injector.28 The monthly cost of TPTD was $3569 taken from the 2018 WAC price from Redbook (using full dose as FDA-approved indication resulting in 13 pen-injectors per year at the cost of $3294.70 per one 600-µg/2.4-mL solution pen).28 The WAC price of ALN was $10/month.28 Estimated annual cost after a hip, vertebral, or wrist fracture or other MOF were derived from the literature29,30 (Table 2). Furthermore, patients who experienced subsequent hip fracture following an initial hip, vertebral, or wrist fracture or other MOF were assumed to incur an average incremental cost of $18 820.31 All costs were adjusted to 2017 US dollars based on the Consumer Price Index Medical Care Component.
Table 2.
DES Model Input Parameters for Costs and Health Utilities: Base-Case, Lower, and Upper Values, and Distributions for Probabilistic Sensitivity Analysis.a,15,28-34
| DES Model Input Parameters | Base-Case Value | Lower Value | Upper Value | Distribution | References |
|---|---|---|---|---|---|
| Costsb | |||||
| Monthly cost of abaloparatide | $1721 | $1377 | $2065 | Log-normal | [28] |
| Monthly cost of teriparatide | $3569 | $2855 | $4283 | Log-normal | [28] |
| Monthly cost of alendronate (oral bisphosphonate) | $10 | $8 | $12 | Log-normal | [28] |
| Average cost per hypercalcemia event | $208 | $166 | $249 | Log-normal | [15] |
| Average cost per nausea event | $100 | $80 | $120 | Log-normal | Assumed |
| Average annual cost of hip fracture | $32 687 | $26 150 | $39 224 | Log-normal | [29] |
| Average annual cost of vertebral fracture | $14 717 | $11 774 | $17 660 | Log-normal | [29] |
| Average annual cost of wrist fracture | $6169 | $4935 | $7403 | Log-normal | [30] |
| Average annual cost of other MOFs | $13 463 | $10 770 | $16 156 | Log-normal | [29] |
| Incremental cost of subsequent hip fracture | $18 820 | $15 056 | $22 584 | Log-normal | [31] |
| Annual cost of nursing-home care | $87 252 | $69 801 | $104 702 | Log-normal | [34] |
| Health utilitiesc | |||||
| Initial health utility | 0.806 | 0.725 | 0.887 | Beta | [32] |
| Post–hip fracture: first year (health utility decrement) | 0.797 | 0.717 | 0.877 | Beta | [32, 33] |
| Post–hip fracture: subsequent years (health utility decrement) | 0.900 | 0.810 | 0.990 | Beta | [32, 33] |
| Post–hip fracture: nursing home stay | 0.400 | 0.360 | 0.440 | Beta | [32, 33] |
| Post–vertebral fracture: first year (health utility decrement) | 0.820 | 0.738 | 0.902 | Beta | [32, 33] |
| Post–vertebral fracture: subsequent years (health utility decrement) | 0.931 | 0.838 | 1.00 | Beta | [32, 33] |
| Post–wrist fracture: first year (health utility decrement) | 0.981 | 0.883 | 1.00 | Beta | [32, 33] |
| Post–wrist fracture: subsequent years (health utility decrement) | 0.995 | 0.990 | 1.00 | Beta | [32, 33] |
| Post–other MOFs: first year (health utility decrement) | 0.753 | 0.678 | 0.828 | Beta | [32, 33] |
| Post–wrist fracture: subsequent years (health utility decrement) | 0.813 | 0.732 | 0.894 | Beta | [32, 33] |
Abbreviation: DES, discrete-event simulation; MOF, major osteoporotic fracture.
All drug costs were based on 2018 wholesale acquisition cost from the Online Redbook.28 All other costs were adjusted to 2017 US dollars based on the Consumer Price Index.
Lower and upper costs were assumed to be 20% of the mean (base-case) values.
Lower and upper health utilities were assumed to be 10% of the mean (base-case) values.
Health Utilities
The initial health utility of 0.806 was based on patient’s average age in the ACTIVE clinical trial with osteoporosis.32 In addition, Table 2 reports the specific health-disutility decrements (multipliers) during first and subsequent years for each fracture site derived from the studies of Brazier et al32 and Kanis et al.33 For example, the hip fracture health-disutility multiplier is 0.797 during the first year of the fracture; thus, if a patient’s current health utility is 0.800, then during the first year after hip fracture, her health utility would be 0.797 × 0.800 = 0.638.
Base-Case Analysis
The base-case time horizon was set at 10 years but the model can accommodate other time periods, including the lifetime of each individual patient. The baseline patient in the model was a 68.8-year-old woman with PMO based on the average patient who participated in the ACTIVE clinical trial.7 The model baseline fracture risk was taken directly from the ACTIVE clinical trial, where 23.8% and 31.0% of patients experienced vertebral and nonvertebral fractures within the past 5 years, respectively, and the baseline FRAX scores (ie, 10-year probability of fracture) were 4.84% for hip fracture and 13.15% for a MOF.7,35 The model applied a discount rate of 3% for both costs and QALYs.36 The DES model simulated 100 000 individual patients for each treatment strategy to estimate the average 10-year per-patient cost and QALYs.
Institutional review board (IRB) was not required as the current cost-effectiveness study was modeled using publicly available data.
Sensitivity Analysis
Probabilistic sensitivity analysis (PSA) was performed by generating 10 000 samples of model probabilistic parameters, and within each sample, 10 000 patients were simulated (ie, the model ran 100 000 000 simulations for each treatment strategy). The probabilistic parameters were created by varying (1) the fracture risk reduction in terms of HRs within their 95% CIs reported from the clinical trial in the assumed log-normal distribution, (2) treatment and fracture costs within ±20% of their mean (base-case) values in the log-normal distribution, and (3) fracture-specific health-utility decrements within ±10% of their base-case values in the β-distribution (Tables 1 and 2).
The incremental net monetary benefit, defined as (λ × ΔQALY) − ΔC, and its 95% CI were estimated and graphed at different willingness-to-pay thresholds (λ) based on the PSA simulation results. Treatment A is said to be cost-effective relative to treatment B when its net monetary benefit is positive.
One-way sensitivity analysis was specifically examined in women with high risk of fracture and defined as women with PMO who were 65 years or older with a prior vertebral fracture. In addition, we examined the annual cost of TPTD using 12 pen-injectors per year (instead of using full-dose 13 pen-injectors per year). We further performed 1-way sensitivity analysis by increasing the monthly cost of ABL by 110% and 130% of its base-case cost (noted that the current monthly treatment cost of ABL/ALN is 50% less than the TPTD/ALN), using a discount rate of 6%, using only ACTIVE trial without incorporating ACTIVExtend trial for sustained fracture risk reduction, and stratifying the patient population into different age categories: 50 to 54, 55 to 59, 60 to 64, <65, ⩾65, 65 to 69, 70 to 74, 75 to 79, 80 to 84, and ⩾85 years37,38 and with or without baseline prior fracture.
Results
Base-Case CEA Results
Over a 10-year time horizon and 100 000 simulated patients for each treatment strategy, the DES model yielded average total discounted per-patient costs of $10 212, $46 783, and $26 837 and QALYs per patient of 6.742, 6.781, and 6.792 for PBO/ALN, TPTD/ALN, and ABL/ALN, respectively (Table 3). Compared with TPTD/ALN, ABL/ALN was a dominant treatment strategy—that is, gained more QALYs and was less costly—and produced an incremental cost-effectiveness ratio (ICER) of $333 266/QALY relative to PBO/ALN.
Table 3.
Base-Case and High-Risk Subgroup Cost-effectiveness Results.
| Treatment Strategy | Total Discounted Cost | Total Discounted QALYs | ICER (vs PBO/ALN) | ICER (vs TPTD/ALN) |
|---|---|---|---|---|
| Base-case | ||||
| Placebo (PBO/ALN) | $10 212 | 6.742 | Reference | |
| Teriparatide (TPTD/ALN) | $46 783 | 6.781 | $951 016/QALY | Reference |
| Abaloparatide (ABL/ALN) | $26 837 | 6.792 | $333 266/QALY | Dominant treatment strategya |
| High-risk subgroup (⩾65 years, with prior vertebral fracture, 10-year time horizon)b | ||||
| Placebo (PBO/ALN) | $23 923 | 6.615 | Reference | |
| Teriparatide (TPTD/ALN) | $58 993 | 6.674 | $593 925/QALY | Reference |
| Abaloparatide (ABL/ALN) | $38 507 | 6.692 | $188 891/QALY | Dominant treatment strategya |
| High-risk subgroup (⩾65 years, with prior vertebral fracture, lifetime horizon)b | ||||
| Placebo (PBO/ALN) | $37 482 | 8.102 | Reference | |
| Teriparatide (TPTD/ALN) | $72 639 | 8.167 | $537 998/QALY | Reference |
| Abaloparatide (ABL/ALN) | $52 194 | 8.188 | $171 242/QALY | Dominant treatment strategya |
Abbreviations: ABL, abaloparatide; ALN, alendronate; ICER, incremental cost-effectiveness ratio; PBO, placebo; QALY, quality-adjusted life-year; TPTD, teriparatide.
Dominant treatment strategy means the treatment is more effective (more QALYs) and less costly.
Sensitivity Analysis Results
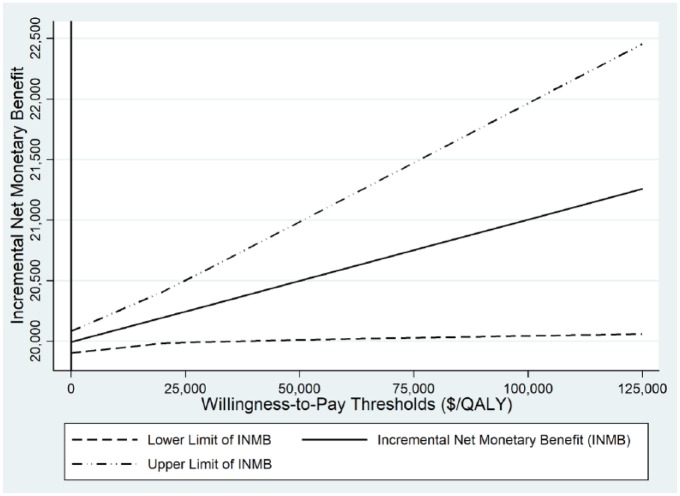
PSA showed that regardless of willingness-to-pay thresholds, ABL/ALN always resulted in a cost-effective treatment strategy, compared with TPTD/ALN (Figure 2).
Figure 2.
Incremental net monetary benefit of abaloparatide versus teriparatide.
Abbreviation: QALY, quality-adjusted life-year.
In 1-way sensitivity analyses, ABL/ALN was a dominant treatment strategy relative to TPTD/ALN in a high-risk subgroup of women with PMO age ⩾65 years and prior vertebral fracture as well as using 12 pen-injectors for the annual cost of TPTD. The ICERs of ABL/ALN versus PBO/ALN for the high-risk women with PMO were $188 891/QALY and $171 242/QALY at 10-year and lifetime time horizons, respectively (Table 3). ABL/ALN also remained a dominant treatment strategy even when assuming the same fracture risk reductions or same treatment cost (noted that the current monthly treatment cost of ABL is 50% less than the TPTD) for TPTD/ALN. In the assumed scenarios that the monthly cost of ABL would increase by 110% (ie, $3614) and 130% (ie, $3958), the resulting ICERs of ABL/ALN relative to TPTD/ALN were changed from dominant to $7936/QALY and $326 653/QALY, respectively. Using a discount rate of 6% instead of 3%, while ABL/ALN relative to TPTD/ALN remained a dominant treatment strategy, the ICER of ABL/ALN versus PBO/ALN was $387 769/QALY. Without incorporating data from the ACTIVExtend trial for sustained treatment effects, ABL/ALN remained dominant over TPTD/ALN and yielded an ICER of $523 006/QALY compared with PBO/ALN. Furthermore, ABL/ALN remained the dominant treatment strategy relative to TPTD/ALN across all age categories and with or without baseline prior fracture.
Discussion
The current CEA of ABL/ALN compared with TPTD/ALN using a DES model demonstrated that ABL/ALN is a dominant treatment strategy as compared with TPTD/ALN—that is, it produced more QALYs and cost less, regardless of any willingness-to-pay threshold. ABL is a new treatment option for women with PMO that may provide better health outcomes at a lower cost relative to TPTD. Even though our study reported the ICER of $333 266/QALY for ABL/ALN versus PBO/ALN, the comparison of an anabolic agent and no treatment was less relevant because health care payers would be more interested in clinical and economic evidence of a new treatment strategy relative to current treatment of the same anabolic therapeutic drug class—that is, TPTD.
Currently, there is only 1 recent report published online by the Institute for Clinical and Economic Review using a cohort Markov model that compared the cost-effectiveness of anabolic therapies (ABL and TPTD) with the antiresorptive bisphosphonate zoledronic acid.39 In their base-case analysis, the ICERs of ABL and TPTD relative to zoledronic acid were $333 892/QALY and $941 537/QALY, respectively.39 Nevertheless, there are several differences and limitations of this analysis. First, it is important to point out that anabolics should be compared with anabolics, not with antiresorptives for the following reasons: (1) they may be used for different fracture-risk profile—that is, patients who received bone-forming treatment were likely at higher fracture risk than those who were treated with bone-loss slowing agent40; (2) they are used for different treatment contexts over different timeframes—that is, bone-forming agents can improve compromised bone mass and structure in high fracture–risk patients allowing more rapid offset of fracture risk, whereas subsequent sequencing to slowing bone-loss agents may help maintain or augment gains in new bone and continue reducing fracture risk over the long-term41; and (3) there are no direct head-to-head clinical trials between the anabolic agents and antiresorptive agent zoledronic acid. Second, the report assumed perfect adherence rate and additional 10-year offset treatment effect for zoledronic acid, which may overestimate the actual benefit of zoledronic acid. Third, the Institute for Clinical and Economic Review has overlooked the onset of action; as a result, there is an overestimation of the clinical benefit associated with slow onset of action. This is particularly important because high-risk patients experience a 5 times elevation in fracture risk during the first year after an incident fracture and as high as a 17 times elevation in risk of a hip fracture during the first month after a wrist fracture and may not benefit from treatments with slower onset of action. Finally, rather than directly comparing an anabolic agent with an antiresorptive agent, it would be more appropriate to consider them as sequential therapies where patients at high fracture risk initially start bone-forming agents, followed by agents slowing bone loss as demonstrated in the ACTIVE and ACTIVExtend clinical trials.
The patient-level DES model used in the current study provided several advantages over the traditional cohort Markov model. The “memoryless” requirement in the Markov model structure means that it is difficult to keep track of past events/health conditions that may influence future events/health conditions; thus, it may underestimate health outcomes and costs.42 It is particularly important in osteoporosis treatment where prior fracture significantly increases risk of further fractures. In addition, it is challenging to model competing risks simultaneously in a Markov model—for example, multiple clinical events (fractures, adverse events, etc) could not be observed in 1 cycle time.43 The DES model can address these issues because its event-driven basis is much more flexible and natural than using transition health states.12 Furthermore, in 1-way sensitivity analyses, ABL remained a cost-effective treatment strategy across variation in all key parameters.
Limitations
As with any modeling approach, the current DES model for CEA of ABL/ALN has several limitations that are subject to availability and use of data and assumptions. First, the ACTIVE clinical trial for ABL was not powered to detect site-specific risk reduction for hip fracture between treatment arms. Thus, we assumed that the risk reduction for hip fracture would be similar to the risk reduction for nonvertebral fractures in the base-case. This assumption was reasonable and often implemented in CEA studies of osteoporosis treatments.15,44-46 In addition, our 1-way sensitivity analyses still indicated that ABL was a dominant treatment strategy relative to TPTD when assuming that risk reductions for all fracture types were similar. Second, in base-case analysis, the current DES model sampled TTEs based on the annual fracture rates and average age of the PBO group in the ACTIVE trial. Thus, the fracture rates might be different from real-world data, resulting in different cost-effectiveness results. In addition, because the model was based on average patient characteristics reported from the ACTIVE clinical trial, our cost-effectiveness results were not stratified by fracture risk factors such as weight, height, bone mineral density, tobacco and alcohol use, or prior fracture. Nevertheless, the DES model for CEA is flexible and able to accommodate alternative assumptions on patient population fracture risk. Furthermore, the DES model allowed for consideration of age and history of fractures, which are key predictors of future fracture risk. Third, the DES model assumed that time to fracture events followed an exponential distribution—that is, constant fracture hazard rates over time. As a result, the model may underestimate the actual fracture rates because they have been shown to increase with age over time; thus, the fracture risk reduction for ABL might also be underestimated. Moreover, the current model was limited to 1 non–hip fracture; as a result, it might underestimate the clinical and economic benefits of anabolic agents on non–hip fractures. The ABL cost-effectiveness findings would, therefore, likely be conservative estimates. Finally, in the current study, we assumed that poor medication adherence had direct negative consequences on treatment effects. However, healthy user bias as unmeasurable factor might also be a contributing factor to worse outcomes in those patients with poor medication adherence.47 Because of the model limitations, our cost-effectiveness results should be generalizable to populations of women similar to women with PMO in the ACTIVE trial and those who are at high risk of fracture, defined as 65 years or older with prior vertebral fracture.
Conclusion and Relevance
In conclusion, using direct head-to-head evidence from the ACTIVE and ACTIVExtend clinical trials, the current DES model demonstrated that ABL is a dominant treatment strategy because it produces more QALYs and costs less compared with TPTD regardless of the assumed QALY willingness-to-pay threshold.
Footnotes
Authors’ Note: The primary findings of this study were presented in part at the annual meetings of the Academy of Managed Care Pharmacy (AMCP) and International Society for Pharmacoeconomics and Outcomes Research (ISPOR) in October 2017 and May 2018.
Declaration of Conflicting Interests: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The authors declared the following potential conflict of interest with respect to the research, authorship, and/or publication of this article: Quang A. Le, Joel W. Hay, and Russell Becker received research grants and/or consulting fees from Radius Health, Inc. Yamei Wang is currently an employee of Radius Health, Inc.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by a research grant from Radius Health, Inc.
ORCID iD: Quang A. Le  https://orcid.org/0000-0002-4968-9334.
https://orcid.org/0000-0002-4968-9334.
References
- 1. NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001;285:785-795. doi: 10.1001/jama.285.6.785 [DOI] [PubMed] [Google Scholar]
- 2. Johnell O, Kanis J. Epidemiology of osteoporotic fractures. Osteoporos Int. 2005;16(suppl 2):S3-S7. doi: 10.1007/s00198-004-1702-6 [DOI] [PubMed] [Google Scholar]
- 3. Abrahamsen B, van Staa T, Ariely R, Olson M, Cooper C. Excess mortality following hip fracture: a systematic, epidemiological review. Osteoporos Int. 2009;20:1633-1650. doi: 10.1007/s00198-009-0920-3 [DOI] [PubMed] [Google Scholar]
- 4. Wright NC, Looker AC, Saag KG, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. 2014;29:2520-2526. doi: 10.1002/jbmr.2269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22:465-475. doi: 10.1359/jbmr.061113 [DOI] [PubMed] [Google Scholar]
- 6. Baron R, Hesse E. Update on bone anabolics in osteoporosis treatment: rationale, current status, and perspectives. J Clin Endocrinol Metab. 2012;97:311-325. doi: 10.1210/jc.2011-2332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Miller PD, Hattersley G, Riis BJ, et al. ; ACTIVE Study Investigators. Effect of abaloparatide vs placebo on new vertebral fractures in postmenopausal women with osteoporosis: a randomized clinical trial. JAMA. 2016;316:722-733. doi: 10.1001/jama.2016.11136 [DOI] [PubMed] [Google Scholar]
- 8. Cosman F, Miller PD, Williams GC, et al. Eighteen months of treatment with subcutaneous abaloparatide followed by 6 months of treatment with alendronate in postmenopausal women with osteoporosis: results of the ACTIVExtend Trial. Mayo Clin Proc. 2017;92:200-210. doi: 10.1016/j.mayocp.2016.10.009 [DOI] [PubMed] [Google Scholar]
- 9. Bone HG, Cosman F, Miller PD, et al. ACTIVExtend: 24 months of alendronate after 18 months of abaloparatide or placebo for postmenopausal osteoporosis. J Clin Endocrinol Metab. 2018;103:2949-2957. doi: 10.1210/jc.2018-00163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Davis S, Stevenson M, Tappenden P, Wailoo AJ. NICE DSU Technical Support Document 15: Cost-effectiveness modelling using patient-level simulation. Report by the Decision Support Unit. http://scharr.dept.shef.ac.uk/nicedsu/wp-content/uploads/sites/7/2016/03/TSD15_Patient-level_simulation.pdf. Accessed August 14, 2018. [PubMed]
- 11. Karnon J, Stahl J, Brennan A, Caro JJ, Mar J, Möller J. Modeling using discrete event simulation: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-4. Med Decis Making. 2012;32:701-711. doi: 10.1177/0272989X12455462 [DOI] [PubMed] [Google Scholar]
- 12. Caro JJ, Möller J, Getsios D. Discrete event simulation: the preferred technique for health economic evaluations. Value Health. 2010;13:1056-1060. doi: 10.1111/j.1524-4733.2010.00775.x [DOI] [PubMed] [Google Scholar]
- 13. Caro JJ, Möller J. Advantages and disadvantages of discrete-event simulation for health economic analyses. Expert Rev Pharmacoecon Outcomes Res. 2016;16:327-329. doi: 10.1586/14737167.2016.1165608 [DOI] [PubMed] [Google Scholar]
- 14. Klotzbuecher CM, Ross PD, Landsman PB, Abbott TA, III, Berger M. Patients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical synthesis. J Bone Miner Res. 2000;15:721-739. doi: 10.1359/jbmr.2000.15.4.721 [DOI] [PubMed] [Google Scholar]
- 15. Liu H, Michaud K, Nayak S, Karpf DB, Owens DK, Garber AM. The cost-effectiveness of therapy with teriparatide and alendronate in women with severe osteoporosis. Arch Intern Med. 2006;166:1209-1217. doi: 10.1001/archinte.166.11.1209 [DOI] [PubMed] [Google Scholar]
- 16. Lindsay R, Scheele WH, Neer R, et al. Sustained vertebral fracture risk reduction after withdrawal of teriparatide in postmenopausal women with osteoporosis. Arch Intern Med. 2004;164:2024-2030. doi: 10.1001/archinte.164.18.2024 [DOI] [PubMed] [Google Scholar]
- 17. Prince R, Sipos A, Hossain A, et al. Sustained nonvertebral fragility fracture risk reduction after discontinuation of teriparatide treatment. J Bone Miner Res. 2005;20:1507-1513. doi: 10.1359/JBMR.050501 [DOI] [PubMed] [Google Scholar]
- 18. Stevenson M, Jones LM, De Nigris E, Brewer N, Davis S, Oakley J. A systematic review and economic evaluation of alendronate, etidronate, risedronate, raloxifene and teriparatide for the prevention and treatment of postmenopausal osteoporosis. Health Technol Assess. 2005;9:1-160. doi: 10.3310/hta9220 [DOI] [PubMed] [Google Scholar]
- 19. Pham AN, Datta SK, Weber TJ, Walter LC, Colón-Emeric CS. Cost-effectiveness of oral bisphosphonates for osteoporosis at different ages and levels of life expectancy. J Am Geriatr Soc. 2011;59:1642-1649. doi: 10.1111/j.1532-5415.2011.03571.x [DOI] [PubMed] [Google Scholar]
- 20. Ström O, Jönsson B, Kanis JA. Intervention thresholds for denosumab in the UK using a FRAX®-based cost-effectiveness analysis. Osteoporos Int. 2013;24:1491-1502. doi: 10.1007/s00198-012-2115-6 [DOI] [PubMed] [Google Scholar]
- 21. Tosteson AN, Jönsson B, Grima DT, O’Brien BJ, Black DM, Adachi JD. Challenges for model-based economic evaluations of postmenopausal osteoporosis interventions. Osteoporos Int. 2001;12:849-857. doi: 10.1007/s001980170036 [DOI] [PubMed] [Google Scholar]
- 22. Black DM, Schwartz AV, Ensrud KE, et al. ; FLEX Research Group. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA. 2006;296:2927-2938. doi: 10.1001/jama.296.24.2927 [DOI] [PubMed] [Google Scholar]
- 23. Kochanek KD, Murphy SL, Xu J, Tejada-Vera B. National Vital Statistic Reports. Deaths: final data for 2014. http://www.cdc.gov/nchs/data/nvsr/nvsr65/nvsr65_04.pdf. Published June 30, 2016. Accessed August 14, 2018. [PubMed]
- 24. Schousboe JT, Nyman JA, Kane RL, Ensrud KE. Cost-effectiveness of alendronate therapy for osteopenic postmenopausal women. Ann Intern Med. 2005;142:734-741. doi: 10.7326/0003-4819-142-9-200505030-00008 [DOI] [PubMed] [Google Scholar]
- 25. Leibson CL, Tosteson AN, Gabriel SE, Ransom JE, Melton LJ. Mortality, disability, and nursing home use for persons with and without hip fracture: a population-based study. J Am Geriatr Soc. 2002;50:1644-1650. doi: 10.1046/j.1532-5415.2002.50455.x [DOI] [PubMed] [Google Scholar]
- 26. Brauer CA, Coca-Perraillon M, Cutler DM, Rosen AB. Incidence and mortality of hip fractures in the United States. JAMA. 2009;302:1573-1579. doi: 10.1001/jama.2009.1462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cheng LI, Durden E, Limone B, et al. Persistance and compliance with osteroporosis therapies among women in a commercially insured population in the United States. J Manag Care Spec Pharm. 2015;21:824-833, 833a. doi: 10.18553/jmcp.2015.21.9.824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Thompson Healthcare, Montvale. Drugtopics red book 2018. (online version). http://truvenhealth.com/Products/Micromedex/Product-Suites/Clinical-Knowledge/RED-BOOK. Accessed July 23, 2018. [Google Scholar]
- 29. O’Hanlon CE, Parthan A, Kruse M, et al. A model for assessing the clinical and economic benefits of bone-forming agents for reducing fractures in postmenopausal women at high, near-term risk of osteoporotic fracture. Clin Ther. 2017;39:1276-1290. doi: 10.1016/j.clinthera.2017.05.348 [DOI] [PubMed] [Google Scholar]
- 30. Tosteson AN, Melton LJ, III, Dawson-Hughes B, et al. ; National Osteoporosis Foundation Guide Committee. Cost-effective osteoporosis treatment thresholds: the United States perspective. Osteoporos Int. 2008;19:437-447. doi: 10.1007/s00198-007-0550-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Weaver J, Sajjan S, Lewiecki EM, Harris ST, Marvos P. Prevalence and cost of subsequent fractures among US patients with an incident fracture. J Manag Care Spec Pharm. 2017;23:461-471. doi: 10.18553/jmcp.2017.23.4.461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Brazier JE, Green C, Kanis JA; Committee of Scientific Advisors International Osteoporosis Foundation. A systematic review of health state utility values for osteoporosis-related conditions. Osteoporos Int. 2002;13:768-776. doi: 10.1007/s001980200107 [DOI] [PubMed] [Google Scholar]
- 33. Kanis JA, Johnell O, De Laet C, et al. A meta-analysis of previous fracture and subsequent fracture risk. Bone. 2004;35:375-382. doi: 10.1016/j.bone.2004.03.024 [DOI] [PubMed] [Google Scholar]
- 34. Genworth. Compare long-term care costs across the United States. https://www.genworth.com/aging-and-you/finances/cost-of-care.html. Accessed June 16, 2017.
- 35. McCloskey EV, Johansson H, Oden A, et al. The effect of abaloparatide-SC on fracture risk is independent of baseline FRAX fracture probability: a post hoc analysis of the ACTIVE study. J Bone Miner Res. 2017;32:1625-1631. doi: 10.1002/jbmr.3163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sanders GD, Neumann PJ, Basu A, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: Second Panel on Cost-Effectiveness in Health and Medicine. JAMA. 2016;316:1093-1103. doi: 10.1001/jama.2016.12195 [DOI] [PubMed] [Google Scholar]
- 37. Howden LM, Meyer JA. Age and sex composition: 2010. 2010 Census briefs. http://www.census.gov/prod/cen2010/briefs/c2010br-03.pdf. Accessed July 23, 2017.
- 38. Ettinger B, Black DM, Dawson-Hughes B, Pressman AR, Melton LJ., III Updated fracture incidence rates for the US version of FRAX. Osteoporos Int. 2010;21:25-33. doi: 10.1007/s00198-009-1032-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Institute for Clinical and Economic Review. Osteoporosis: final evidence report. https://icer-review.org/material/osteo-final-evidence-report. Accessed October 23, 2017.
- 40. Camacho PM, Petak SM, Binkley N, et al. AACE/ACE Postmenopausal Osteoporosis Clinical Practice Guidelines for the diagnosis and treatment of postmenopausal osteoporosis—2016. Endocr Pract. 2016;22(suppl 4):1-42. doi: 10.4158/EP161435.GL [DOI] [PubMed] [Google Scholar]
- 41. Rittmaster RS, Bolognese M, Ettinger MP, et al. Enhancement of bone mass in osteoporotic women with parathyroid hormone followed by alendronate. J Clin Endocrinol Metab. 2000;85:2129-2134. doi: 10.1210/jcem.85.6.6614 [DOI] [PubMed] [Google Scholar]
- 42. Briggs A, Sculpher M. An introduction to Markov modelling for economic evaluation. Pharmacoeconomics. 1998;13:397-409. doi: 10.2165/00019053-199813040-00003 [DOI] [PubMed] [Google Scholar]
- 43. Caro JJ. Pharmacoeconomic analyses using discrete event simulation. Pharmacoeconomics. 2005;23:323-332. doi: 10.2165/00019053-200523040-00003 [DOI] [PubMed] [Google Scholar]
- 44. Borgström F, Ström O, Marin F, Kutahov A, Ljunggren O. Cost effectiveness of teriparatide and PTH(1-84) in the treatment of postmenopausal osteoporosis. J Med Econ. 2010;13:381-392. doi: 10.3111/13696998.2010.499072 [DOI] [PubMed] [Google Scholar]
- 45. Murphy DR, Smolen LJ, Klein TM, Klein RW. The cost effectiveness of teriparatide as a first-line treatment for glucocorticoid-induced and postmenopausal osteoporosis patients in Sweden. BMC Musculoskelet Disord. 2012;13:213. doi: 10.1186/1471-2474-13-213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lundkvist J, Johnell O, Cooper C, Sykes D. Economic evaluation of parathyroid hormone (PTH) in the treatment of osteoporosis in postmenopausal women. Osteoporos Int. 2006;17:201-211. doi: 10.1007/s00198-005-1959-4 [DOI] [PubMed] [Google Scholar]
- 47. LaFleur J, Nelson RE, Sauer BC, Nebeker JR. Overestimation of the effects of adherence on outcomes: a case study in healthy user bias and hypertension. Heart. 2011;97:1862-1869. doi: 10.1136/hrt.2011.223289 [DOI] [PubMed] [Google Scholar]