Abstract
Purpose: Manganism is a cognitive disorder take places in peoples are exposed to environmental manganese pollution. Overexposure to manganese ion (Mn2+) mainly influences central nervous system and causes symptoms that increase possibility of hippocampal damages.
Methods: In this study rats were administrated by two different doses of MnCl2 and behavioral and physiological consequences were evaluated. We also investigated effects of E. Amoenum on Mn2+-imposed toxicity by behavioral, biochemical, immunoblotting and histological studies on hippocampus tissue.
Results: Results showed metal overexposure increases oxidative stress mainly by lipid peroxidation and reactive oxygen species overproduction. Histological studies and caspase 3 analyses by immunoblotting revealed Mn2+ induced apoptosis from mitochondrial-dependent pathway in the presence of low metal dose. This study provides evidence that oral administration of E. amoenum extract inhibited manganese neurotoxicity by oxidative stress attenuation and apoptosis reduction that lead to improved depression like behavior. Plant extract also increased catecholamine content in Mn2+ treated hippocampus.
Conclusion: As molecular and pathophysiological effects of E. amoenum, it could be considered as a pre-treatment for Parkinson and Parkinson like disorders in high-risk people.
Keywords: Catecholamine, Cognitive disorder, Depression like behavior, Hippocampus, Manganism, Mitochondria dysfunction
Introduction
Manganese (Mn) plays key role in mammalian brain development and function as a trace element.1 Mn2+ is cofactor of important enzymes such as glutamine synthetase, pyruvate decarboxylase, serine/threonine protein phosphatase I, Mn-superoxide dismutase and arginase, which are required for neurotransmitter synthesis, metabolism and antioxidant defense system.2 It is also the fourth most widely used heavy metal in the industry such as textile bleaching, leather tanning and iron, steel, potassium permanganate, hydroquinone, glass and ceramics production.2,3 By considering high application of Mn2+ in today’s life, exposing to this poisonous metal is predictable especially in miners and factory workers.4 Previous studies confirmed Mn2+ overdose causes Parkinson like disorder known as manganism that accompanied by tremors, odd movements, mask like face, and body stiffness that first observed in miners.3 The risk of Mn2+ exposure is not limited to miners or welders. The environmental accessibility and high Mn2+ concentration in water or food represent a source of contamination for the general population.4 As previous study, systemic injected Mn2+ mainly accumulated in central nervous system (CNS) and damaged it.5 Our prior results also showed acute dose of Mn2+ causes reduction of catecholamine level in the brain tissue by unknown mechanism.6 The main theories for damaging mechanisms of heavy metals are mitochondrial dysfunction and oxidative stress.7 Plants, especially Echium amoenum, are important source of antioxidants and cell protectants can be used for CNS toxicity.8 E. amoenum Fisch, a common traditional herbal medicine, is widely used as an effective treatment for tranquillizer, diaphoretic, cough, sore throat and pneumonia.8,9 Dried violet-blue petals of E. amoenum have been recently recognized as an important source of phenolic compounds like rosmarinic acid, cyaniding and delphinidin that could be extracted by water solvents.10 By considering molecular composition, it is believed that this plant has antibacterial, antioxidant, analgesic, anxiolytic, antidepressant and immunomodulatory properties.10-13 It has been shown that E. amoenum aqueous extract is effective treatment for obsessive-compulsive disorder and pancreatitis.14,15 Neuroprotective effects of cyanidin 3-glucoside, the most common anthocyanin in petals of this plant, have been investigated previously and results showed it can inhibit inflammation by blocking the c-Jun and NF-κB factors translocation into the nucleus.16 Therefor its possible E. amoenum aqueous extract prevents toxic effects induced by heavy metals such as Mn2+ in CNS.
The main goal of this study is evaluation of the MnCl2 toxicity in the rat hippocampus by biochemical analysis, behavioral assessment and histological studies. Hippocampus tissue plays an important role in hippocampal-dependent learning and memory, depressive-like behaviors and cognitive disorders similar to manganism, therefore we investigated Mn2+ toxicity in hippocampus tissue.17,18 Two different doses of metal were used to dose dependent assessment of physiological and biochemical parameters. This study also estimated E. amoenum aqueous extract improving effects on the neurotoxicity imposed by high dose of MnCl2.
Materials and Methods
2,7 dichlorofluoresc indiacetate (DCFHDA), thiobarbituric acid, and 5,5′-Dithiobis (2-nitrobenzoic acid) riboflavin and nitro blue tetrazolium were purchased from Sigma Chemical Company. All other solvents and chemicals were of the highest grade-commercially available.
Experimental design and plant extraction
In vivo study was conducted on experimental animals and using adult male Wistar rats weighing 250-300 g obtained from the animal house of martyr portal. Animals with average age of 4.5-6 months were selected. Testing was carried out at temperature of 20-25 centigrade degree and that day duration was 12 hours and dark period was 12 hours. Municipal tap water was used as drinking water and animal feed as nutrition (compressed feed). We have 4 experimental groups in this study and 8 rats in each group. The first group was daily injected by physiological saline (0.9 % NaCl) for 15 days, the second group was daily injected by 10 mg/kg MnCl2 in saline as vehicle during 15 days, third group was injected daily by 15 mg/kg MnCl2 in saline for 15 days and fourth group was administrated by 15 mg/kg MnCl2 + 5 mg/kg E. amoenum extract for same time duration. MnCl2 and extract doses determined according to previous study19 and some experiments (data not shown). Although there are many methods for plant extract preparing, but few scientific reports are available in the literature on lyophilized extracts of fresh violet petals of E. amoenum, as this type of extraction causes better antioxidant and cell protective potential in rats.20 Sample was collected after approval of agricultural experts. The fresh violet petals of E. amoenum were thoroughly washed with tap water and its juice was obtained using a blender. After obtaining the juice, it was lyophilized to get the dry powder using freeze dryer. We used 5 mg/kg of plant extract in saline for orally administration of rats before 15 mg/kg MnCl2 intraperitoneally injection.
Behavioral assessment
Forced swimming test (FST)
As previous standard protocol,21 rats were placed in a transparent plexiglass cylinder (20 cm diameter and 50 cm height) filled with warm water (25 °C and 30 cm depth). The classical procedure uses a two-day protocol. The first day of habituation, the rats were forced to swim for 15 min; 24 h after, on the test day three categories of behavioral activity (climbing, swimming and immobility) were recorded during the 5 min test period. Immobilization time considered as time between introduction of a rat into the pool and making only those movements necessary to keep its head above water without struggling. Immediately after each experience rats were dried and kept warm before returning to their home cage.
Sucrose preference test (SPT)
SPT estimates hedonia (pleasure-seeking) or its deficient (anhedonia) by monitoring preference of rats to sucrose water.22 Rats were placed in individual cages with food and water. At first, rats were adapted to having two water bottles in the cage lid for 72 hours and their position was randomly changed as many times as possible to avoid a place preference. The bottles were fitted with ball-bearing sipper tubes to prevent fluid leak. After this acclimation, rats had the free choice of either bottle for water drinking. Then one of the bottles filled with 1% sucrose solution during 48 hours test. Water and sucrose solution intake was measured daily. The locations of two bottles were switched daily to reduce side bias. Sucrose preference was calculated as follows: 100 * [sucrose consumption (g)/(sucrose consumption (g) + water intake (g))] and averaged over the 2 days of testing.
Reactive oxygen species (ROS) measurement
ROS generation was measured according to the methods of Keston and Brandt23 and Lebel et al.24 with some modifications. The method used to measure the oxidative conversion of DCFH-DA to dichlorofluorescin (DCFH) as a fluorescent compound. Hippocampus homogenates were diluted 1:10 in buffer to obtain a concentration of 5 mg tissue/ml. Then the homogenates were pipetted into 24-well plates (0.45 ml/ well) and allowed to warm to room temperature for 5 min. At that time, 5 µl of DCFH-DA (10 µM final concentration) was added to each well and the plates preincubated for 15 min at room temperature to allow the DCFH-DA to be incorporated into any membrane-bound vesicles and the diacetate group cleaved by esterases. After the preincubation, 50 µl of the appropriate concentration of Fe2+ was added to the wells. After 30 min, DCFH-DA converted into non-fluorescent DCFH, which reacts with ROS to form the fluorescent product DCF. DCF fluorescence was determined at 485 nm excitation and 530 nm emission using a (Perkin Elmer luminescence spectrometer LS 55) fluorescence spectrophotometer. The slit width was 5 nm for both excitation and emission. Background fluorescence (conversion of DCFH to DCF in the absence of homogenate) was corrected by the inclusion of parallel blanks. ROS content was expressed as DCF fluorescence/mg protein/min in comparison with control. Protein concentration in homogenates was determined using Bradford method25 and did not differ between groups.
Lipid peroxidation (LPO) measurement
Hippocampus tissue samples were used for measurement of lipid peroxidation as a marker of oxidative stress.26 Tissue was separated after anesthesia and homogenized with ice cold buffer containing 0.15 M KCl to obtain 1:10 (w/v) homogenates. Aliquots of homogenate (1 ml) were incubated at 37°C for 3 h in a shaker. Then, 1ml of 10% aqueous trichloroacetic acid (TCA) was added and mixed. The mixture was then centrifuged at 800 g for 10 min. Then, supernatant (1 ml) was mixed with 1ml of 0.67% thiobarbituric acid and placed in a boiling water bath for 10 min. The mixture was cooled and diluted with 1ml distilled water. The absorbance of the solution was then read using spectrophotometer at 532 nm. In this process malondialdehyde (MDA) as a final product of lipid peroxidation reacts with tiobarbitouric acid (TBA) (this reaction completes in 100°C) and releases TBARS that absorbs 532 nm light.27 Results were expressed as percent of MDA production compared to the control.
Antioxidant enzymes assay
Superoxide dismutase (SOD)
SOD activity was measured spectrophotometrically according to the riboflavin/nitro blue tetrazolium (NBT/RF) assay method.28 This indirect method involves the inhibition of NBT reduction. In the NBT/RF method, SOD competes with NBT for O2- generated by the RF under illumination. The 1.5 ml reaction mixture (50 mM KH2PO4, pH 7.8, 0.1 mM EDTA), 2 mM riboflavin and 57 μM NBT) were used. Since generation of O2- radicals in the NBT/RF assay is driven by light, samples were subsequently illuminated from above for 15 min by 4 fluorescence tubes (40 W, 30 cm distance) giving 199 μmol photons m2 s−1. Afterwards, absorbance was measured at 560 nm. Fifty percent inhibition was calculated by regression using the linear part of a natural semi-log curve after which the specific activity was calculated. One unit of SOD activity was defined as the amount of enzyme that inhibits 50% of NBT photochemical reduction.
Catalase (CAT)
Catalase activity was measured following the method of Aebi with minor modifications.29 The principle of this method was based on the hydrolyzation of H2O2 and decreased absorbance at 240 nm. The conversion of H2O2 into water and 1/2 oxygen per minute at 25 °C and phosphate buffer (pH 7) was considered to be the enzyme reaction velocity.
Catecholamine measurement
24 hours after last treatment rats were anesthetized with ketamine/xylazine and hippocampus was separated from sculpture. Fresh tissue or tissue that had been frozen in liquid nitrogen and stored at −80 °C were homogenized in cold 0.05 N HClO4 containing dihydroxybenzylamine as internal standard. The supernatant after 15 min centrifugation at 12000 g was processed according to Felice et al., except that 0.1 N HClO4 was used to elute the amines from the alumina.30 Catecholamine converts to fluorescent substance in alkaline environment and in the presence of ascorbic acid and iodine as a strong oxidant.30 Fluorescence studies were carried out on a Perkin Elmer luminescence spectrometer LS 55. The excitation wavelength was set at 405 nm and the emission spectra were recorded in 515 nm. Excitation and emission slit were both set at 5 nm.
Western blotting analysis
Caspase 3 and caspase 9 have important role in mitochondria dependent and independent apoptosis pathway respectively so were selected to be quantified via western blot by using specific antibodies (rat specific anti-Caspase-3 and anti-Caspase-9 antibodies purchased from Abcam company). Western blotting was carried out according to our previous study,31 after SDS-PAGE, the proteins were transferred onto PVDF membrane actively at 140 V for 1.5-2 h in the transfer buffer. After completion of the transfer and blocking, membrane was probed with the primary and secondary specific antibodies and was washed four times in TBST (50 mM Tris, pH 7.5, 150 mM NaCl, 0.05 % Tween 20) between incubations. Bands containing specific proteins were visualized using an ECL detection system according to the manual. Anti β-actin (1:1,000) (Cell Signaling Technology) was used as a housekeeping control. The density was calculated through ImageJ 1.46r; Java 1.6.0_20 software for each band.
Statistical evaluation
All values were expressed as the mean ± standard error of mean (S.E.M). Data was analyzed using one-way ANOVA followed by the post-hoc Duncan multiple range test for analysis of biochemical data using SPSS version 11. Differences were considered significant at p < 0.05.
Results and Discussion
Extensive application of the Mn2+in industry and its pollution in the environment, expose human and animal to the manganese neurotoxicity.4 This study examines toxic effects of MnCl2 on Wistar rats as a suitable model. Therefore we administrated rats by 10 and 15 mg/kg MnCl2 intraperitoneally and assessed behavioral parameters of depression such as body weight dynamic, sucrose preference and immobilizing time in forced swimming test. We also measured ROS, LPO and oxidative stress barriers such as catalase and SOD for examine the role of Mn2+ in oxidative damages in rat brain. Catecholamine as an important neurotransmitter in health and normal application of brain was measured. MnCl2 role in cell death was estimated by measuring of the apoptosis involved proteins and tissue sections analysis. We also assessed Echium amoenum extract effects on Mn2+-induced neurotoxicity as an important traditional medicine grows in northern part of Iran.9,10 This plant has been recognized as an important source of phenolic compounds like rosmarinic acid, cyanidin and delphinidin which potentially have chemoprotective effects against toxic metals that are increasing in life today.4,13 By considering chemical nature of antioxidants and biocompatibility, saline was used in extraction process. Manganese toxicity upon overexposure (manganism) was reported to be accompanied by Parkinson and depression like behavior assigns in miners and welders.2,4 By considering cognitive disorder as main problem in patients suffer from manganism, its predictable hippocampus is one of the affected tissues in CNS that has not been studied previously.17,18
Behavioral assessment
Body weight
Body weight dynamics is a sensitive indicator for chemical toxicants.32 Previous studies have shown that administration of toxic nanoparticles such as AgNPs and ZnNPs significantly decreased body weight growth rate in rats.32,33 Therefore to investigate whether exposure to MnCl2 could be considered as a global health issue, we monitored the mortality rate, food consumption, water intake, and body weight dynamic of experimental groups during study. Results showed water/food and survival of rats were not changed significantly in treated rats during experiment. But body weight progressive curve was affected by both doses of MnCl2 and metal treated animals showed a slow increase in body weight. The rats were injected by MnCl2 revealed growing body weight with a mean ± S.E.M. of 304.7 ± 15.2 g in 10 mg/kg Mn2+-treated rats, 297.2 ± 10.4 in 15 mg/kg Mn2+-treated rats and 323.4 ± 12.9 g in control after 15 days. As Figure 1, slop of the body growing curve is dose dependent and has an inverse relationship with Mn2+ dose. Interestingly E. amoenum extract improved Mn2+ effect, as Figure 1 showed. Body weight of rats that received plant extract in addition to high dose of Mn2+, increases near to the control, 320 ± 13.1 g at the end of experiment.
Figure 1.
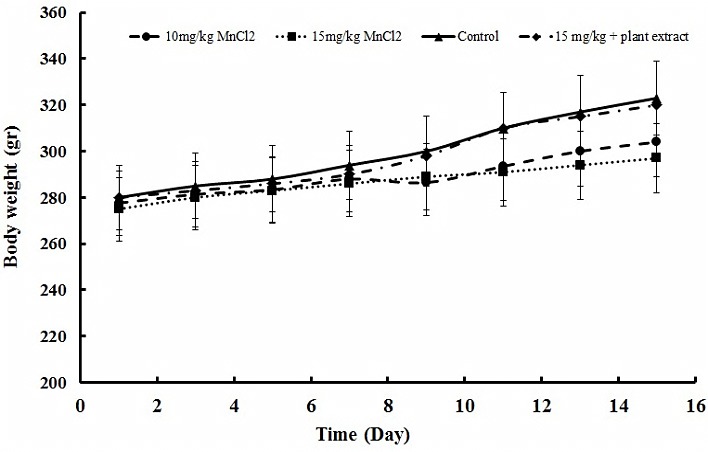
Body weight dynamic during the experimental period. Results showed weight loss in manganese treated rats that improved by oral administration of E. amoenum extract. Each data indicates the mean ± S.E.M.
Forced swimming test (FST)
FST was done to assess depressive-like behavior21 as a result of MnCl2 injection. Recorded immobility time for 10 mg/kg MnCl2 treated rats is 80.54 ± 4.65 sec, for 15 mg/kg is 101.34 ± 6.43 sec and in control is 15.20 ± 3.23 sec. Therefore Mn2+ treatment significantly increased immobility time compared to the control that refer to depression in Mn2+treated rats dose dependently. Figure 2 revealed daily treatment of rats that received 15 mg/kg Mn2+ with plant extract reduced immobilization time significantly from 80.54 ± 4.65 sec to 20.22 ± 3.43 sec that approved aqueous extract of E. amoenum relieved depression like behaviors signs. Plant extract slightly increased immobility time in control rats but it’s not significant (P<0.05).
Figure 2.
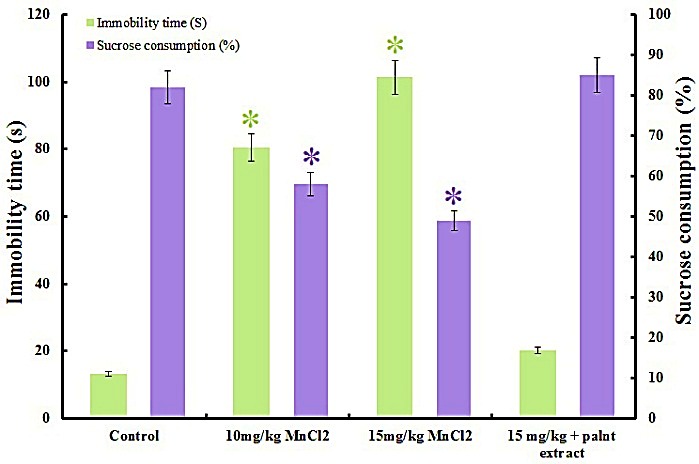
Depression like behavior test. Increased immobilization time and decreased sucrose preference confirmed depression like behavior in rats that received manganese dose dependently. Results showed E. amoenum extract compensates metal toxic effects. Each value indicates the mean ± S.E.M. Asterisk symbols showed significant changes by P<0.05.
Sucrose preference test (SPT)
Sucrose preference test is one of the most commonly used assays for depression in rodents,22 so we used this test to anhedonia evaluation in Mn2+ or/and plant extract treated rats and control. Results showed sucrose consumption decreased significantly in Mn2+ received rats dose dependently (Figure 2). Sucrose intake in rats received 10 mg/kg is 58.02 ± 5.13 %, in rats administrated with 15 mg/kg is 49.32 ± 4.29 % and in control rats is 82.16 ± 8.56 %. Decreased sucrose preference refers to depression like behavior in rats as a result of Mn2+injection especially in rats that received high dose. Figure 2 also showed decreased sucrose consumption was improved by plant extract in rats that received high dose of metal (85.45 ± 4.76 %). Control rats which received an equal dose of plant extract don’t show significant differences in sucrose intake (data not shown).
Oxidative stress in hippocampus tissue
Noticed depression like behavior and previous studies approved one of the important targets for metals toxicity is CNS.5-7 To investigate whether the administration of toxic doses of Mn2+ creates oxidative stress in hippocampus tissue, we studied ROS content and oxidative barrier enzymes activity in four experimental groups.
ROS measurement
ROS are chemically reactive molecules mainly contain super oxide, peroxide and hydroxyl groups that basically are produced by mitochondria during normal metabolism in low concentration but acute stress increases it by causing mitochondrial dysfunction.34 Therefore, ROS measurement will give a useful report from oxidative state of the hippocampus tissue related to experimental groups. Figure 3 revealed Mn2+ induced high DCF fluorescence intensity that refers to ROS overproduction in hippocampus tissue. 10 mg/kg MnCl2 administration raised ROS level more than 2.5 folds and by increasing metal dose to 15 mg/kg, ROS elevated to more than 3 folds rather than control. Therefore MnCl2 administration causes harsh oxidative stress in hippocampus tissue by overproduction of ROS molecules (Figure 3). Previous studies showed heavy metals damaged mitochondria and interrupted respiratory chain that lead to reactive molecules production.35 ROS content of MnCl2 treated hippocampus decreased significantly by plant extract. The antioxidant capacity of phenolic compounds in plant extract is attributed to their ability in metal ions and ROS molecules chelating, so protect cell compounds from oxidation.31 Apoptosis and necrosis in living system are main outcomes of ROS overproduction.36 Plant effects on ROS level of control rats are not significant that approved E. amoenum possibly doesn’t affect healthy function of mitochondrial.
Figure 3.
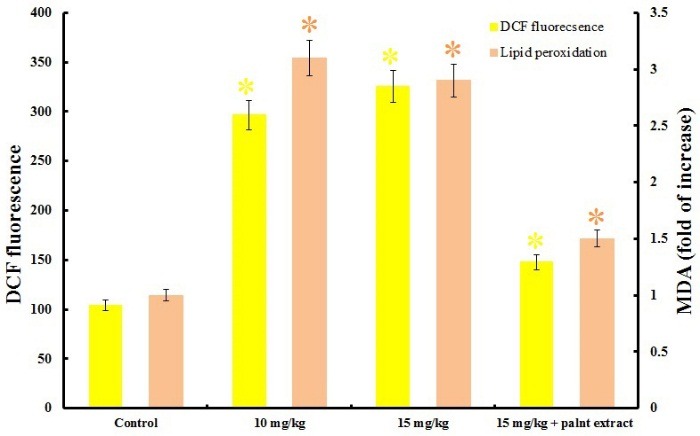
Oxidative stress investigation. Rising of the DCF fluorescence in hippocampus related to metal treated rats refer to ROS overproduction. Increased ROS molecules causes lipid peroxidation that showed by high malondialdehyde content. Interestingly increased oxidative stress attenuated by E. amoenum extract. Each data indicates the mean ± S.E.M. Asterisk symbols showed significant changes by P<0.05.
Lipid peroxidation (LPO) evaluation
LPO is the most popular indicator of oxidative stress can be used as a marker of cell membrane injuries also.26,37 Overproduced reactive molecules attack to the cell membrane lipids and damage it by lipid peroxidation.38 Malondialdehyde (MDA) is the most known secondary products of lipid peroxidation could be evaluated by thiobarbituric acid method.27 The rats which received both doses of metal showed increased MDA about 3 folds than control, that were initially caused by increased free radicals (Figure 3). As Figure 3, plant extract treatment reduces MDA level (about 2 folds) in hippocampus tissue of rats that received 15 mg/kg MnCl2. Therefore MDA level increased in the presence of MnCl2 dose dependently that refers to elevated lipid peroxidation as a result of metal toxicity (Figure 3). Plant extract contains antioxidant molecules that diminished ROS level and consequently reduced MDA in metal treated rats.
SOD and catalase activity measurement
Hippocampus tissue samples were used for measurement of superoxide dismutase (SOD) and catalase (CAT) activities as the most popular antioxidants barrier in biological systems. Since the Mn2+ treated rats produce an oxidative stress which could be exhausted by the antioxidative ability of SOD and CAT.39 Therefore, we estimated both enzyme activities in four experimental groups. Activities of both enzymes were increased in rats who received 10 and 15 mg/kg MnCl2 (Figure 4). SOD and CAT activity that induced by Mn2+, were reduced by plant extract near to the control. As Figure 4, 15 mg/kg MnCl2 causes activation of SOD to 7.8 unit and catalase to 1.35 mmol/min but plant extract decreased it to 2.3 unit and 0.56 mmol/min respectively, while activity of enzymes in control rats are 2.1 unit and 0.52 mmol/min for SOD and catalase respectively. Difference between control group, rats that received 15 mg/kg MnCl2 + plant extract and normal rats that received plant extract are not significant (P<0.05). By considering ROS overproduction in Mn2+ administrated rats, increased activity of the SOD and catalase (Figure 3) refers to an adaptation of hippocampus cells to neutralize the extra produced oxidant compounds.40 The increased SOD activity possibly resulted from SOD overexpression that is regulated by Mn2+ as essential cofactor of this enzyme.2
Figure 4.
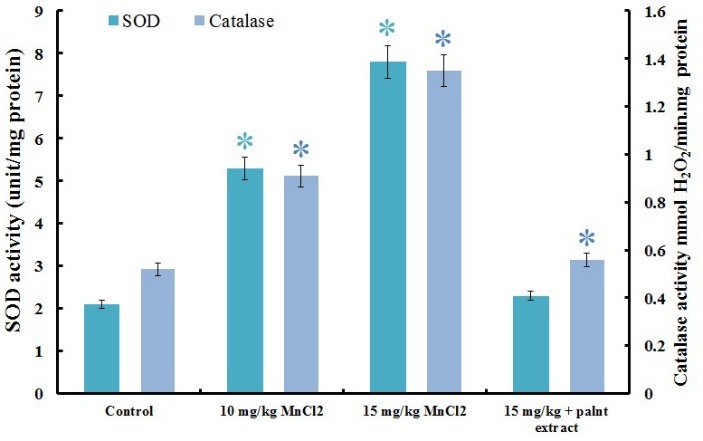
Antioxidant enzyme assessment. Oxidative stress imposed by manganese toxicity causes improving of the superoxide dismutase and catalase enzymes activity in hippocampus tissue as an adaptation to toxic stress. Antioxidant enzymes activity decreased in rats that received plant extract. Each value indicates the mean ± S.E.M. Asterisk symbols showed significant changes by P<0.05.
Catecholamine content of hippocampus tissue
Catecholamines, including dopamine and norepinephrine, are most the important neurotransmitters that mediate a variety of functions in CNS, such as motor control, cognition, emotion, memory processing, and endocrine modulation.41 Dysfunctions in catecholamine neurotransmission are related to some neuropsychiatric disorders specially Parkinson disease and epilepsy.31 Similar neuropsychiatric signs in Parkinson disease and manganism possibly are caused by equal molecular events.2 Therefore catecholamine content of hippocampus tissue was compared between experimental groups as follow: 10 mg/kg MnCl2, 142.43 ± 12.52 ng/mg protein; 15 mg/kg MnCl2, 91.45 ± 4.52 ng/mg protein; 15 mg/kg MnCl2 + plant extract, 250.45 ± 12.34 ng/mg protein and control rats, 210.32 ± 10.23 ng/mg protein. Decreased catecholamine may be caused by increased dopaminergic cell death in the presence of metal ions.7 Diminished catecholamine was returned near to (even more than) the control by plant extraction treatment, while these kinds of neurotransmitters have dual action (Neurotoxic and neuroprotective) and according to previous experiments, high doses of catecholamine induces apoptosis in the neurons.42 Control rats that received plant extract showed increase in catecholamine content (224.41 ± 14.29 ng/mg protein) but it’s not significant and does not accompanied with abnormal neurobehaviours. Relieving effects of E. amoenum in molecular level especially catecholamine rising, finally lead to improved depression like behavior in rats treated by toxic doses of metal as discussed above.
Caspase 9 and caspase 3 analysis
Raised oxidative stress and reduced catecholamine possibly cause cell death in metal treated hippocampus. Catecholamine level of brain is important in healthy function and survival of neurons and decreased catecholamine lead to neurodegeneration in some neurological disease.42 ROS overproduction was caused by mitochondrial dysfunction or/and inefficient antioxidant barrier that lead to mitochondrial-dependent and –independent apoptosis with different molecular mechanisms.43 Caspase 9 involves in mitochondrial-independent and caspase 3 participates in mitochondrial-dependent apoptosis.44,45 Our experiments revealed in rats that received 10 mg/kg MnCl2 only caspase 9 increased significantly but in rats treated by 15 mg/kg MnCl2 both of the caspase 3 and caspase 9 increased in hippocampus (Figure 5). These results confirmed more sensitivity of the mitochondria against metal toxicity. Manganese overexposing well documented to result in a disrupted Fe2+homeostasis that lead to mitochondrial dysfunction.44 As Figure 5, increased expression of the caspase 3 and 9 that induced by 15 mg/kg of metal was improved by oral administration of plant extract.
Figure 5.
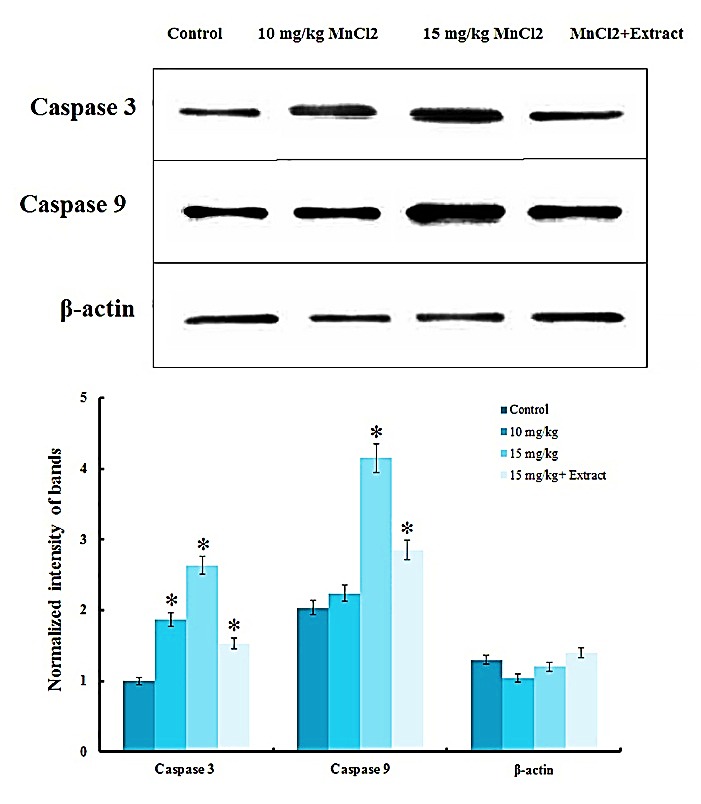
Immunoblotting studies. Up-regulation of caspase 3 and 9 during manganese intoxication refers to increased apoptosis in metal received rats. E. amoenum extraction significantly decreased neurodegeneration in hippocampus tissue. The intensity of bands was quantified by ImageJ software. The data were expressed as mean ± S.E.M of three independent experiments. Asterisk (*) was used to denote statistical significance (P<0.05).
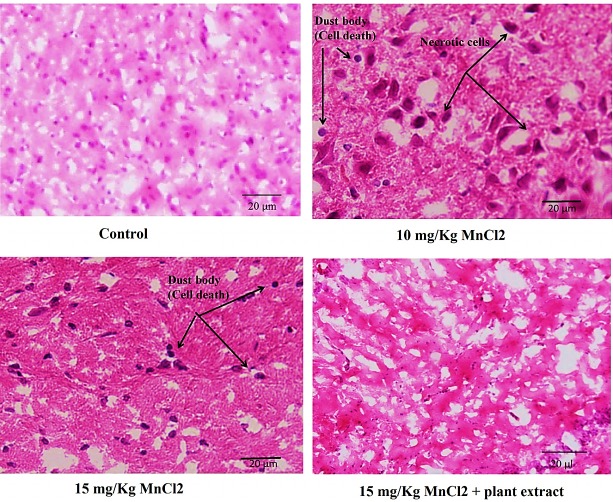
Histological studies
The biological significance and toxicological importance of any changes which are found between tissue section in control and experimental groups have been considered as biochemical results confirmation. Therefore after the end of experimental time course, rats were anesthetized and brain tissue separated from scalp. Tissue samples were treated by formalin for fixation and stained by hematoxilin-eosin method and then studied by light microscope.46 Results showed presence of necrotic and apoptotic cells in tissues were administrated by 10 and 15 mg/kg MnCl2 (Figure 6). Early apoptotic nuclei have a condensed appearance that frequently seen in MnCl2 administrated tissue especially in 15 mg/kg MnCl2 received rats. Increased apoptosis in the metal treated rats accompanied by decreased level of the catecholamine may be due to catecholamine positive role in cell survival or catecholamine producing cell death in Mn2+neurotoxicity. Histology results also were confirmed by elevated level of caspase 3 and 9 in intoxicated rats. As Figure 6, amounts of condensed apoptotic and deformed necrotic cells reduced in tissues related to rats received plant extract+15 mg/kg MnCl2 that accompanied by decreased caspases and improved behavioral abnormalities also.
Figure 6.
Histological studies. Hematoxylin/eosin staining of hippocampus sections revealed presence of the apoptotic and necrotic cells in MnCl2 treated rat hippocampus rather than control. Result showed oral administration of E. amoenum extraction significantly decreased apoptotic and necrotic cells in hippocampus.
Conclusion
Pathophysiological signs of manganism in human and animal models suggest hippocampus as a possible affected tissue. Our biochemical results approved hash oxidative damages in the presence of MnCl2 doses that attenuated by plant extract. Increased expression of the caspase 9 in low dose of Mn2+ revealed, metal toxicity causes mitochondrial dysfunction at first and then induces oxidative damages that lead to mitochondrial independent apoptosis in the presence of high metal dose (caspase 3 upregulation). Despite the prevalent use of E. amoenum as an antidepressant, there are no pharmacological data to support such effects. Our molecular and biochemical studies confirmed E. amoenum extract inhibited apoptosis from both described pathways possibly by ROS molecules scavenging, mitochondrial dysfunction improving and metal ions trapping. All of the identified beneficial effects or possibly uncharacterized mechanisms lead to decreased depressive behaviors. Investigated therapeutic effects of E. amoenum on Mn2+ neurotoxicity revealed this plant could be considered in antidepressant drug design and as supplement against all of the metals toxicity or oxidative damages. By considering physiological and molecular similarities between manganism and Parkinson disease and also E. amoenum role in catecholamine overproduction, this plant could be used as natural co-treatment in associated diseases.
Ethical Issues
All the experimental works were approved by the Ethical Committee of Isfahan University of Medical Science (Isfahan, Iran) and conform to the European Communities Council Directive of 24 November 1986 (86/609/EEC).
Conflict of Interest
All of the Authors have no conflict of interest to declare.
References
- 1.O’Neal SL, Zheng W. Manganese Toxicity Upon Overexposure: a Decade in Review. Curr Environ Health Rep. 2015;2(3):315–28. doi: 10.1007/s40572-015-0056-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kwakye GF, Paoliello MM, Mukhopadhyay S, Bowman AB, Aschner M. Manganese-induced parkinsonism and parkinson's disease: Shared and distinguishable features. Int J Environ Res Public Health. 2015;12(7):7519–40. doi: 10.3390/ijerph120707519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takeda A. Manganese action in brain function. Brain Res Brain Res Rev. 2003;41(1):79–87. doi: 10.1016/S0165-0173(02)00234-5. [DOI] [PubMed] [Google Scholar]
- 4.Long Z, Jiang YM, Li XR, Fadel W, Xu J, Yeh CL. et al. Vulnerability of welders to manganese exposure--a neuroimaging study. Neurotoxicology. 2014;45:285–92. doi: 10.1016/j.neuro.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oulhote Y, Mergler D, Barbeau B, Bellinger DC, Bouffard T, Brodeur ME. et al. Neurobehavioral function in school-age children exposed to manganese in drinking water. Environ Health Perspect. 2014;122(12):1343–50. doi: 10.1289/ehp.1307918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peres TV, Schettinger MR, Chen P, Carvalho F, Avila DS, Bowman AB. et al. Manganese-induced neurotoxicity: a review of its behavioral consequences and neuroprotective strategies. BMC Pharmacol Toxicol. 2016;17(1):57. doi: 10.1186/s40360-016-0099-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yousefi Babadi V, Sadeghi L, Amraie E, Rezaei M, Malekirad AA, Abarghouei Nejad M. Manganese toxicity in the central nervous system: Decreeing of catecholamine in rat’s brains. Health. 2013;5(12):2146–9. doi: 10.4236/health.2013.512292. [DOI] [Google Scholar]
- 8.Belyaeva EA, Sokolova TV, Emelyanova LV, Zakharova IO. Mitochondrial electron transport chain in heavy metal-induced neurotoxicity: Effects of cadmium, mercury, and copper. ScientificWorldJournal. 2012;2012:136063. doi: 10.1100/2012/136063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Safaeian L, Haghjoo Javanmard S, Ghanadian M, Seifabadi S. Cytoprotective and antioxidant effects of echium amoenum anthocyanin-rich extract in human endothelial cells (huvecs) Avicenna J Phytomed. 2015;5(2):157–66. [PMC free article] [PubMed] [Google Scholar]
- 10.Ranjbar A, Khorami S, Safarabadi M, Shahmoradi A, Malekirad AA, Vakilian K. et al. Antioxidant activity of iranian echium amoenum fisch & c.A. Mey flower decoction in humans: A cross-sectional before/after clinical trial. Evid Based Complement Alternat Med. 2006;3(4):469–73. doi: 10.1093/ecam/nel031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nadi F. Bioactive compound retention in Echium amoenum Fisch. & C. A. Mey. petals: Effect of fluidized bed drying conditions. Int J Food Prot. 2017;20:2249–60. doi: 10.1080/10942912.2016.1233436. [DOI] [Google Scholar]
- 12.Abolhassani M. Antibacterial effect of borage (echium amoenum) on staphylococcus aureus. Braz J Infect Dis. 2004;8(5):382–5. doi: 10.1590/S1413-86702004000500008. [DOI] [PubMed] [Google Scholar]
- 13.Rabbani M, Sajjadi SE, Vaseghi G, Jafarian A. Anxiolytic effects of echium amoenum on the elevated plus-maze model of anxiety in mice. Fitoterapia. 2004;75(5):457–64. doi: 10.1016/j.fitote.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 14.Hosseini N, Abolhassani M. Immunomodulatory properties of borage (Echium amoenum) on BALB/c mice infected with Leishmania major. J Clin Immunol. 2011;31(3):465–71. doi: 10.1007/s10875-010-9502-6. [DOI] [PubMed] [Google Scholar]
- 15.Abed A, Minaiyan M, Ghannadi A, Mahzouni P, Babavalian MR. Effect of echium amoenum fisch. Et mey a traditional iranian herbal remedy in an experimental model of acute pancreatitis. ISRN Gastroenterol. 2012;2012:141548. doi: 10.5402/2012/141548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sayyah M, Boostani H, Pakseresht S, Malaieri A. Efficacy of aqueous extract of echium amoenum in treatment of obsessive-compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(8):1513–6. doi: 10.1016/j.pnpbp.2009.08.021. [DOI] [PubMed] [Google Scholar]
- 17.Munoz-Espada AC, Watkins BA. Cyanidin attenuates PGE2 production and cyclooxygenase-2 expression in LNCaP human prostate cancer cells. J Nutr Biochem. 2006;17(9):589–96. doi: 10.1016/j.jnutbio.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 18.Sarnyai Z, Sibille EL, Pavlides C, Fenster RJ, McEwen BS, Toth M. Impaired hippocampal-dependent learning and functional abnormalities in the hippocampus in mice lacking serotonin(1A) receptors. Proc Natl Acad Sci U S A. 2000;97(26):14731–6. doi: 10.1073/pnas.97.26.14731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robison G, Zakharova T, Fu S, Jiang W, Fulper R, Barrea R. et al. X-ray fluorescence imaging of the hippocampal formation after manganese exposure. Metallomics. 2013;5(11):1554–65. doi: 10.1039/c3mt00133d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deepa P, Kannappan N. Comparative in vitro antioxidant studies of aqueous solution of formulated poly herbal formulation with marketed preparation. Der Pharm Lett. 2012;4(5):1515–7. [Google Scholar]
- 21. Yankelevitch-Yahav R, Franko M, Huly A, Doron R. The forced swim test as a model of depressive-like behavior. J Vis Exp 2015(97). [DOI] [PMC free article] [PubMed]
- 22.Overstreet DH. Modeling depression in animal models. Methods Mol Biol. 2012;829:125–44. doi: 10.1007/978-1-61779-458-2_7. [DOI] [PubMed] [Google Scholar]
- 23.Keston AS, Brandt R. The fluorometric analysis of ultramicro quantities of hydrogen peroxide. Anal Biochem. 1965;11:1–5. doi: 10.1016/0003-2697(65)90034-5. [DOI] [PubMed] [Google Scholar]
- 24.LeBel CP, Ischiropoulos H, Bondy SC. Evaluation of the probe 2',7'-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem Res Toxicol. 1992;5(2):227–31. doi: 10.1021/tx00026a012. [DOI] [PubMed] [Google Scholar]
- 25.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 26.Niki E. Lipid peroxidation products as oxidative stress biomarkers. Biofactors. 2008;34(2):171–80. doi: 10.1002/biof.5520340208. [DOI] [PubMed] [Google Scholar]
- 27. Buege JA, Aust SD. Microsomal lipid, Peroxidation. In: Flesicher S, Packer L, editors. Methods in Enzymology. New-York: Academic Press; 1978. [DOI] [PubMed]
- 28.Oyanagui Y. Reevaluation of assay methods and establishment of kit for superoxide dismutase activity. Anal Biochem. 1984;142(2):290–6. doi: 10.1016/0003-2697(84)90467-6. [DOI] [PubMed] [Google Scholar]
- 29.Mittal M, Flora SJ. Vitamin E supplementation protects oxidative stress during arsenic and fluoride antagonism in male mice. Drug Chem Toxicol. 2007;30(3):263–81. doi: 10.1080/01480540701380075. [DOI] [PubMed] [Google Scholar]
- 30.Felice LJ, Felice JD, Kissinger PT. Determination of catecholamines in rat brain parts by re- verse-phase ion-pair liquid chromatography. J Neurochem. 1978;31(6):1461–5. doi: 10.1111/j.1471-4159.1978.tb06573.x. [DOI] [PubMed] [Google Scholar]
- 31.Sadeghi L, Rizvanov AA, Salafutdinov Salafutdinov, II II, Dabirmanesh B, Sayyah M, Fathollahi Y. et al. Hippocampal asymmetry: Differences in the left and right hippocampus proteome in the rat model of temporal lobe epilepsy. J Proteomics. 2017;154:22–9. doi: 10.1016/j.jprot.2016.11.023. [DOI] [PubMed] [Google Scholar]
- 32.Jacquier M, Crauste F, Soulage CO, Soula HA. A predictive model of the dynamics of body weight and food intake in rats submitted to caloric restrictions. PLoS One. 2014;9(6):e100073. doi: 10.1371/journal.pone.0100073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yin N, Yao X, Zhou Q, Faiola F, Jiang G. Vitamin E attenuates silver nanoparticle-induced effects on body weight and neurotoxicity in rats. Biochem Biophys Res Commun. 2015;458(2):405–10. doi: 10.1016/j.bbrc.2015.01.130. [DOI] [PubMed] [Google Scholar]
- 34.Gao L, Laude K, Cai H. Mitochondrial pathophysiology, reactive oxygen species, and cardiovascular diseases. Vet Clin North Am Small Anim Pract. 2008;38(1):137–55, vi. doi: 10.1016/j.cvsm.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharma B, Singh S, Siddiqi NJ. Biomedical implications of heavy metals induced imbalances in redox systems. Biomed Res Int. 2014;2014:640754. doi: 10.1155/2014/640754. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36.Fu PP, Xia Q, Hwang HM, Ray PC, Yu H. Mechanisms of nanotoxicity: generation of reactive oxygen species. J Food Drug Anal. 2014;22(1):64–75. doi: 10.1016/j.jfda.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barrera G. Oxidative stress and lipid peroxidation products in cancer progression and therapy. ISRN Oncol. 2012;2012:137289. doi: 10.5402/2012/137289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pernot F, Heinrich C, Barbier L, Peinnequin A, Carpentier P, Dhote F. et al. Inflammatory changes during epileptogenesis and spontaneous seizures in a mouse model of mesiotemporal lobe epilepsy. Epilepsia. 2011;52(12):2315–25. doi: 10.1111/j.1528-1167.2011.03273.x. [DOI] [PubMed] [Google Scholar]
- 39.Zhan CD, Sindhu RK, Pang J, Ehdaie A, Vaziri ND. Superoxide dismutase, catalase and glutathione peroxidase in the spontaneously hypertensive rat kidney: Effect of antioxidant-rich diet. J Hypertens. 2004;22(10):2025–33. doi: 10.1097/00004872-200410000-00027. [DOI] [PubMed] [Google Scholar]
- 40.Vermeij WP, Alia A, Backendorf C. Ros quenching potential of the epidermal cornified cell envelope. J Invest Dermatol. 2011;131(7):1435–41. doi: 10.1038/jid.2010.433. [DOI] [PubMed] [Google Scholar]
- 41.Kobayashi K. Role of catecholamine signaling in brain and nervous system functions: New insights from mouse molecular genetic study. J Investig Dermatol Symp Proc. 2001;6(1):115–21. doi: 10.1046/j.0022-202x.2001.00011.x. [DOI] [PubMed] [Google Scholar]
- 42.Noh JS, Kim EY, Kang JS, Kim HR, Oh YJ, Gwag BJ. Neurotoxic and neuroprotective actions of catecholamines in cortical neurons. Exp Neurol. 1999;159(1):217–24. doi: 10.1006/exnr.1999.7144. [DOI] [PubMed] [Google Scholar]
- 43.Tai YK, Chew KC, Tan BW, Lim KL, Soong TW. Iron mitigates dmt1-mediated manganese cytotoxicity via the ask1-jnk signaling axis: Implications of iron supplementation for manganese toxicity. Sci Rep. 2016;6:21113. doi: 10.1038/srep21113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang C, Youle RJ. The role of mitochondria in apoptosis. Annu Rev Genet. 2009;43:95–118. doi: 10.1146/annurev-genet-102108-134850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brentnall M, Rodriguez-Menocal L, De Guevara RL, Cepero E, Boise LH. Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biol. 2013;14:32. doi: 10.1186/1471-2121-14-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dhandapani S, Subramanian VR, Rajagopal S, Namasivayam N. Hypolipidemic effect of cuminum cyminum l. On alloxan-induced diabetic rats. Pharmacol Res. 2002;46(3):251–5. doi: 10.1016/s1043-6618(02)00131-7. [DOI] [PubMed] [Google Scholar]