Abstract
Depression after traumatic brain injury (TBI) is common but the mechanisms by which TBI causes depression are unknown. TBI decreases glutamate transporters GLT-1 and GLAST and allows extravasation of thrombin. We examined the effects of thrombin on transporter expression in primary hippocampal astrocytes. Application of a PAR-1 agonist caused down-regulation of GLT-1, which was prevented by inhibition of Rho kinase (ROCK). To confirm these mechanisms in vivo, we subjected mice to closed-skull TBI. Thrombin activity in the hippocampus increased one day following TBI. Seven days following TBI, expression of GLT-1 and GLAST was reduced in the hippocampus, and this was prevented by administration of the PAR-1 antagonist SCH79797. Inhibition of ROCK attenuated the decrease in GLT-1, but not GLAST, after TBI. We measured changes in glutamate levels in the hippocampus seven days after TBI using an implanted biosensor. Stress-induced glutamate levels were significantly increased following TBI and this was attenuated by treatment with the ROCK inhibitor fasudil. We quantified depressive behavior following TBI and found that inhibition of PAR-1 or ROCK decreased these behaviors. These results identify a novel mechanism by which TBI results in down-regulation of astrocyte glutamate transporters and implicate astrocyte and glutamate transporter dysfunction in depression following TBI.
Keywords: Astrocyte, depression, glutamate transporter, thrombin, traumatic brain injury
Introduction
Traumatic brain injury (TBI) affects 1.7 million people annually in the United States, with associated medical costs of approximately $76 billion.1 Depression following TBI is common, with an estimated prevalence of up to 77%.2,3 Depression exacerbates adverse outcomes following TBI, including greater physical disability, lower quality of life, poor psychosocial functioning, and suicidal ideation.4 The mechanisms by which TBI results in depression are not known. Current approaches to treatment of depression after TBI, including antidepressants or cognitive behavioral therapy, have limited efficacy.5–7
Pre-clinical and clinical data implicate glutamate signaling and astrocyte function in the cellular mechanisms of major depressive disorder (MDD). Astrocyte glutamate transporters, GLT-1 and GLAST are responsible for more than 90% of glutamate uptake in most brain regions.8,9 In rodent models of depression, astrocyte glutamate transporters are down-regulated in animals exhibiting depressive behaviors compared to controls.10,11 Pharmacologic blockade of astrocyte glutamate transporters causes anhedonic behaviors in rodents.12,13 Clinical studies indicate that cerebrospinal fluid (CSF), serum, and plasma levels of glutamate are increased in depressed patients compared to healthy controls.14,15 Treatment with the N-methyl-D-aspartate receptor antagonist Ketamine results in significant antidepressant effects in patients with MDD.16 Like MDD, TBI also results in loss of astrocyte glutamate transporters and changes in glutamate signaling.17 A link between altered glutamate homeostasis and glutamate transporter dysregulation in the mechanisms of depression after TBI has not been investigated.
Compromise of the blood–brain barrier (BBB) after TBI allows the extravasation of serum proteases including thrombin into the brain parenchyma.18,19 The effects of thrombin in the CNS are mediated by protease-activated receptors (PARs) 1, 3, and 4, all of which are expressed by astrocytes.20 We have previously shown that exposing cortical astrocytes to thrombin causes down-regulation of the astrocyte glutamate transporter GLAST, resulting in compromise of glutamate uptake.21 Pre-clinical and clinical research suggests that TBI and MDD share common pathways in the dysregulation of glutamate homeostasis. Accordingly, we tested the hypothesis that TBI produces an increase in brain thrombin levels, and a decrease in astrocyte glutamate transporters via activation of the PAR-1 receptor, resulting in elevated glutamate levels and the development of depressive behaviors.
Materials and methods
All animal protocols and procedures were approved by the Northwestern University Institutional Animal Care and Use Committees (USDA registration # 33R0129; PHS assurance # A328301)) and were carried out in accordance with the guidelines outlined in the Guide for the Care and Use of Laboratory Animals published by National Institute of Health (DHEW publication NIH 85-23-2985). All experiments were carried out in compliance with ARRIVE guidelines. All efforts were made to minimize suffering.
Closed-skull midline TBI
Mice were subjected to closed-skull midline impact using an electromagnetic impact device (MyNeurolab, Zeiss Instruments, St. Louis, MO) as described previously.22 Briefly, adult male CD1 (Charles River) mice (25–30 g) were induced with 4.2% isoflurane in oxygen at a FiO2 of 100%, and endotracheal intubation was performed using an 18-gauge angiocatheter. Mice were mechanically ventilated with a mouse ventilator (MiniVent, Hugo Sachs Elektronik, March-Hugstetten, Germany) using lung protective ventilation strategies as we have previously described.23 The mouse head was secured on a custom mold, and a concave 3 mm stainless steel disk was secured immediately posterior to bregma on the exposed skull surface. Mice were then transferred to the stereotactic apparatus (remaining intubated and under 1.5 ∼ 2% isoflurane anesthesia), and a midline controlled skull impact was delivered using a 3 mm impounder tip at 5.00 ± 0.02 m/s velocity and a deformation depth of 3 mm. Mice with skull fracture or visible hemorrhage were excluded from the study. Rectal temperature was maintained at 37.0 ± 0.1℃ using a computer controlled heating pad (Physitemp, NJ) throughout surgery until recovery from anesthesia. Sham-operated animals underwent the same procedures without impact. All animals were monitored for at least 4 h after surgery and thereafter daily. Animals were assigned randomly for drug treatment groups. A total of 22 mice were excluded from the study due to skull fractures or failure to successfully extubate following TBI or sham operation.
Measurement of BBB breakdown
Animals underwent transcardiac perfusion with cold PBS followed by 4% paraformaldehyde. Cryopreserved brains were sent to Neuroscience Associate (Knoxville, TN). All the brains (n = 5 each group, total 10 brains) were embedded in one gelatin mode, and 40 µm thick sections were prepared. Brain sections were blocked by 10% normal goat serum, and incubated with Alexafluo 488-conjugated goat anti-mouse IgG antibody (Molecular Probes, Invitrogen). Whole brain section fluorescence microscopy was performed using a stage-motorized Zeiss 700 confocal laser scanning microscope system (Zeiss).
BBB compromise was measured by extravasation of immunoglobulin in brain parenchyma. The impact site extends from Bregma 0 to Bregma −3.5 mm, and sections at Bregma −1.75 mm were used for analysis. Quantification of IgG-immunoreactive cells was performed using ImageJ software, version 1.4 (NIH) by an investigator blinded to treatment group as previously described.24 Briefly, immunofluorescent images from independent sections were converted to 8-bit gray scale. The background was set using threshold command for each channel. All images in the same series were processed using the same analysis parameters. The numbers shown in the graphs represent the average number of pixels per image for each group.
Measurement of thrombin activity
For in vivo thrombin activity measurements, mice were perfused transcardially with saline. Brain samples were homogenized and thrombin activities were measured using the thrombin-specific chromogenic substrate, S2238 (Anaspec, Fremont, CA, USA).25 The final concentration of S2238 was 0.3 mmol/L in phosphate-buffered saline (PBS), and absorption of supernatant at 405 nm was measured 30 min later.
Double immunofluorescent labeling
Standard free-floating immunostaining technique was performed on 40 µm sections as previously described.21,26 The following primary antibodies were used: goat anti-cleaved thrombin (1:100, Santa Cruz), rabbit anti- PAR-1 (1:200, Santa Cruz), rabbit anti-GFAP (1:500; DAKO), mouse anti-NeuN (1:500, Chemicon), rabbit anti-GLAST (1:500, Abcam), rabbit-anti-GLT-1 (1:500, Abcam), with corresponding secondary antibodies. For qualitative analysis of the localization of cleaved thrombin immunolabeling after TBI, we performed triple immunofluorescent staining with the following primary antibodies: mouse anti-GFAP (1:400, ab4648; Abcam), rabbit anti-von Willebrand Factor (1:400, ab6944; Abcam), goat anti-cleaved thrombin (S-19, 1:200, sc-8204, SantaCruz). Corresponding Alexa Fluor secondary antibodies were used (Invitrogen). DAPI (Sigma) was used for nuclear staining. Photo-bleaching was prevented by Prolong-Gold mounting medium (Invitrogen). Sections lacking primary antibodies served as a negative control. Confocal microscopy was performed using a Zeiss LSM510 mega microscope system (Zeiss).21
RNA preparation
Hippocampal tissue was dissected and further subdivided to CA1, CA3, and DG subregions. Tissues were snap frozen and stored at −80℃. Before RNA extraction, frozen tissues were incubated in RNAlater ICE (Invitrogen) solution over night at 4℃. Samples were then homogenized in Trizol (Invitrogen) buffer with a 5 mm stainless steel bead (Qiagen) by Tissue lyser LT (Qiagen) at 50 s−1 oscillation for 1 min. The lysates were then used to obtain total RNA following the manufacturer’s protocol (Purelink RNA mini kit, Invitrogen). To eliminate genomic DNA contamination, lysates were treated with Purelink DNase (Invitrogen) followed by on-column protocol. RNA quantities and qualities were determined using UV spectrophotometer at 260 nm (ND-1000, Nano Drop Technologies, Inc.).
Reverse transcription and quantitative real-time PCR
Total RNA (1 µg) was used to generate cDNA using SuperScript VILO reverse transcriptase kit (Invitrogen). Taqman gene expression master mix (Invitrogen) was used in a 20 µl reaction. Commercially available premade Taqman primer probes were used to determine the transcription levels of glutamate transporter subunits; GLAST/EAAT1 (Mm00600697_m1), GLT1/EAAT2 (Mm00441457_m1), EAAT3 (Mm00436590_m1), EAAT4 (Mm01173279_m1). EAAT5 was not studied as it is predominantly expressed in the retina. GAPDH (Mm99999915_g1) was used as an endogenous control. For negative controls for the qPCR reaction, cDNA or primers were omitted. Relative concentrations of the transcripts of interest were calculated with comparison to a standard curve prepared from dilutions of cDNA from pooled samples.
Western blot
Animals underwent transcardiac perfusion with cold PBS. Hippocampal tissue was snap frozen and stored at −80℃ until biochemical analysis. Tissue homogenates were prepared by sonication in PBS with protease inhibitor cocktail (1 µg/ml leupeptin, 1 µg/ml pepstatin A, 1 µg/ml aprotinin, 100 µM sodium orthovanadate, and 1 mM phenylmethane sulfonyl fluoride, 2 mM EDTA). Total protein concentration was measured by BCA assay (Pierce, Rockford, IL).
Hippocampal tissue was collected at 1 h, one day, and seven days post-injury as described above and used for Western blotting analysis.24,27 Briefly, 25 µg of protein was run on SDS polyacrylamide gel electrophoresis and transferred onto PVDF membrane. The blot was then probed with the following antibodies: rabbit anti-GLAST (1:1000, Abcam), rabbit-anti-GLT-1 (1:1000, Abcam), rabbit anti-GFAP (1:10,000, DAKO), rabbit anti-phospho-MYPT1Thr696 (1:1000, EMD Millipore). Beta-actin (1:10,000, Sigma-Aldrich) was used as an endogenous control. Immune complexes were detected with the appropriate HRP-conjugated secondary antibodies and visualized using SuperSignal Pico Substrate (Thermo Scientific). Protein bands were quantified by densitometric analysis using Carestream Molecular Imaging Software (Version 5.0.2.30; Carestream). The data (n = 4–6 each group) presented reflect the density of target protein divided by the density of the β-actin endogenous control in each sample, and are expressed as a fold of control treatments.
Neurobehavioral outcome assessments
Behavioral testing was carried out by an examiner blinded to the treatment groups.
Tail suspension test
The tail suspension test (TST) assesses depression-like behavior in mice and is based on the observation that mice develop an immobile posture when placed in an inescapable stressor when hung by their tails.28,29 The test was performed on day 7 post-injury as described previously.30 Each mouse was suspended at a height of 50 cm using adhesive tape placed approximately 1 cm from the tip of its tail. The immobility time was recorded during the 5-min test period. The definition of immobility was passive hanging and lack of motion. Immobile time was recorded and analyzed by EthoVision software (Noldus).
Forced swim test
The forced swim test (FST) was initially developed for screening of anti-depressant drugs.31 When mice are forced to swim in a restricted space, they will cease attempts to escape and adopt a characteristic immobile posture, which can be identified and timed. Following TST, mice were introduced into 20 cm-diameter glass jars containing 23–25℃ tap water to 20 cm depth. Immobile time was recorded during the last 4 min of the 6 min-test and analyzed by EthoVision software.32
Sucrose preference test
Mice were housed singly and allowed ad libitum access to drink from two bottles for 12 h, one filled with 1% (w/v) sucrose solution and another with water. The consumption of water and sucrose solution was estimated simultaneously by weighing the bottles before and after the test. Sucrose preference was calculated as a percentage of consumed sucrose solution of the total fluid intake.33
Primary hippocampal astrocyte cell culture
Primary hippocampal astrocyte cultures were prepared from Sprague-Dawley one to three days old rat pups (Charles River, Wilmington, MA) as we have previously described.21 Hippocampi were isolated and cleaned of meninges in Mg2+ and Ca2+-free Hank’s balanced buffered salt solution (HBSS) (HyClone, Logan, UT). After digestion with trypsin, the cell suspension was filtered through a 40 -µm filter, centrifuged, and resuspended in High Glucose DMEM supplemented with 10% fetal bovine serum and 1% penicillin and streptomycin (HyClone). Cells were then plated in 75 cm2 flasks and cultured in a humidified incubator at 37℃ in 5% CO2, with media changed every two to three days. After 13 to 14 days in culture, astrocyte cultures were prepared by shaking flasks at 200 r/min for 24 h, and media containing floating cells were removed and replaced. When confluent, cells were lifted from the flasks with 0.05% trypsin/0.2% EDTA and plated at a density of 1 × 105 cells/mL.
Cell treatments
Twenty-four hours prior to experiments, media were changed to serum-free, phenol red-free DMEM supplemented with 1% N2 supplement (Gibco, Invitrogen, Carlsbad, CA), 1% penicillin and streptomycin (HyClone). Cells were treated with either PBS, thrombin from human plasma (≥2000 NIH U/mg, Sigma Aldrich, St. Louis, MO), PAR-1 (TFLLR-NH2), PAR-4 (GYPGQV-NH2) (Tocris, Minneapolis, MN) or PAR-3 agonist peptide (AP) (Anaspec, Fremont, CA). The Rho kinase inhibitors, Y-27632 (EMD Millipore, Billerica, MA) or Fasudil (SelleckChem, Houston, TX), or diluent were administered to the cells 90 min prior to thrombin treatment; 24 h later, cell lysates were analyzed.
Quantification of glutamate clearance from primary astrocyte cultures
Glutamate uptake by hippocampal astrocytes seeded onto 6- and 12-well plates was measured using enzymatic quantification as we have previously described.21 Briefly, 24 h after thrombin treatment, astrocytes were washed with HBSS supplemented with 10 mM D-glucose. Medium was then removed and 1 or 2 mL per well, for 12- and 6-well plates, respectively, of 200 µM L-glutamate diluted in HBBS + D-Glucose was added. After 20 min in the CO2 incubator at 37℃, samples of cell medium were collected and the residual glutamate concentrations in cell medium were measured in duplicate using the enzymatic quantification kit according to the manufacturer’s instructions (Amplex® Red Glutamic Acid/Glutamate Oxidase Assay Kit, Invitrogen); 5 µl of supernatant and 45 µl reaction buffer were transferred into 96-well microplates, mixed with 50 µl substrate mixture containing 100 mM Amplex Red, 0.25 U/ml horseradish peroxidase, 0.08 U/ml L-glutamate oxidase, 0.5 U/ml L-glutamate-pyruvate transaminase, 200 µl L-alanine, 1 × reaction buffer, and incubated at 37℃ for 30 min. Detection of fluorescence reaction was determined at 590 nm after excitation at 530 nm using a microplate reader (Spectramax M5, Molecular Devices). Glutamate clearance was calculated from residual glutamate concentration by subtracting the measured extracellular amounts of glutamate from the amount of glutamate initially added to the cells, and normalized using protein content in the wells determined by the bicinchoninic acid (BCA) assay (Pierce, Rockford, IL). To determine the role of individual glutamate transporters, the assay was performed in the presence of GLT1 inhibitor, dihydrokainate,34 or the GLAST inhibitor, UCPH-101.35 In the following experiments, glutamate uptake was normalized using the values in the control cells as 100%.
Intraventricular drug administration
We used a block randomization method to allocate mice to each treatment group. To determine whether decrease in the expression of GLT1 and GLAST after TBI is due to activation of PAR-1, mice were administered selected agents (PAR-1 agonist peptide, SFLLRN-NH2, or scrambled peptide RLLFT-NH2, Tocris Minneapolis, MN) via intraventricular (icv) infusion into the right lateral ventricle for 24 h using a brain infusion kit (Alzet, Durect, Cupertino, CA). Animals were anesthetized with isofluorane, and the mouse head was secured on a stereotactic apparatus. The mouse skull was carefully exposed, and a small hole was made with a 25-gauge needle above the right lateral ventricle (AP: −2.18 mm; ML: +3 mm; DV: −3 mm). Based on our in vitro results and published work,36 20 µM peptides were infused for 24 h.
Other drug administration
The selective PAR-1 antagonist SCH79797 (25 µg/kg; Tocris) was administered via intraperitoneal (IP) injection 3 h after TBI.36,37 The selective ROCK inhibitor, fasudil (HA-1077) was administered (25 mg/kg/day, Selleck Chemicals, Houston, TX) subcutaneously via osmotic mini-pump (Alzet, model 2001, 1 ul/h, 7 day delivery) beginning immediately after induction of TBI.38,39
Real-time measurement of change in L-glutamate levels in vivo
Mice were anesthetized using 2% isofluorane and surgically implanted indwelling cannula for later biosensor implantation following the manufacture’s protocol (Pinnacle Technology, Lawrence, KS).40 Briefly, three holes were made with a 25 G needle and a headmount placed on top of a screw within each hole. All parts were sealed securely with dental acrylic resin. A cannula for biosensor insertion (ID ∼ 400 µm) (BASi, West Lafayette, IN) was implanted in the temporal cortex (A/P: −2.0 mm, M/L: 2.5 mm, D/V: −1.5 mm). Accuracy of the sensor placement was confirmed through visual examination of brain tissue after sacrifice at the end of experiments. The L-glutamate biosensor was inserted in the guide cannula at six-day recovery following TBI or sham surgery. Amperometric biosensor readings were initiated at the onset of the “light-off” period, which coincided with the insertion of the biosensor. The biosensor was then allowed to stabilize for 16 h. After this baseline was established, the animals underwent a stressful stimulus by placement in tail suspension for 15 min. Mice were then returned to the reading cage for 1 h for recovery from stress. The biosensor measures L-glutamate in the extracellular fluid of the brain of a conscious, freely moving (tethered) animal. All biosensors were explanted through the guide cannula following the end of the experiment and immediately post-calibrated by stepwise addition of L-glutamate (10 µM) at regular intervals in a 37℃ bath containing 100 mM PBS (pH 7.4) buffer solution. Amperometric changes in individual biosensor readings were used to calculate concentration changes in vivo.
Statistical analysis
Quantitative data are presented as mean ± SEM. Western blot data were analyzed using Kruskal–Wallis test and Dunn’s post hoc analysis. Glutamate concentration measurements were analyzed with repeated measures one-way analysis of variance (ANOVA) and post hoc adjustments using the Tukey’s test. Other data were analyzed using one-way ANOVA followed by Bonferroni’s post hoc adjustments for three or more groups and one-tailed Student’s t-test for two group comparisons. All statistical tests were performed using GraphPad Prism, Version 5.0 for Mac (GraphPad Software, San Diego, CA). A p value < 0.05 was considered statistically significant.
Results
TBI significantly increased BBB permeability and thrombin activity at one-day recovery
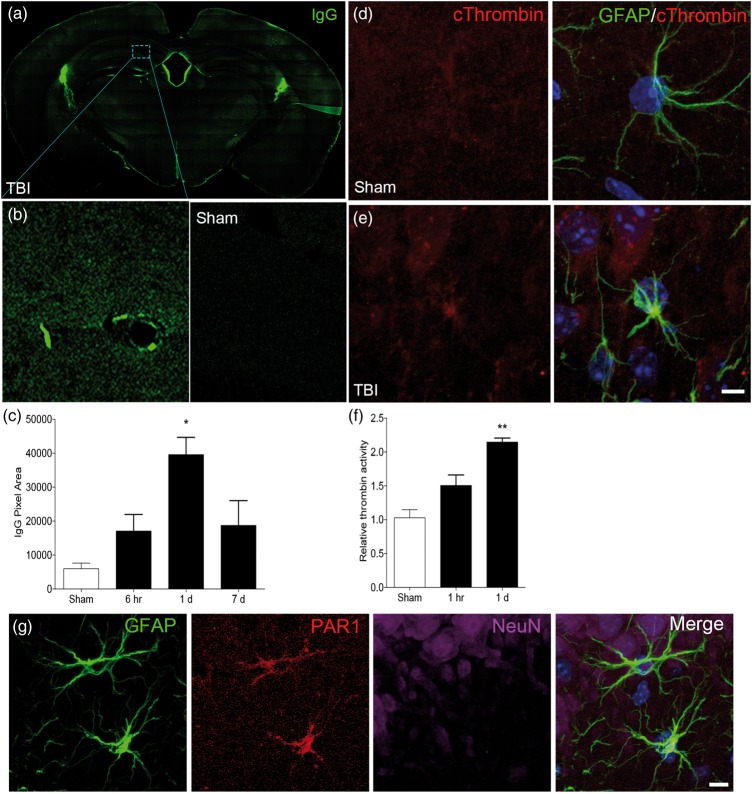
The closed head injury model used for this study creates a diffuse injury, and neuroinflammation involving both hippocampus and cortex, resulting in vestibulomotor and cognitive dysfunction.26,41 We first quantified changes in permeability of the BBB and thrombin activity following TBI (Figure 1). At 1 day following TBI, there was a significant increase in fluorescent IgG immunolabeling compared to sham controls (p < 0.05, by one-way ANOVA) (Figure 1(a) to (c)). We used triple immunolabeling methods for a qualitative assessment of changes in expression of cleaved thrombin in hippocampus after TBI (Figure 1(d) and (e)). We selected a one-day recovery window based on the results of the BBB experiments. Expression of the active (cleaved) form of thrombin was increased compared to sham controls (Figure 1(d) and (e)) and co-localized with the astrocyte marker GFAP. To characterize the localization of cleaved thrombin expression in more detail, we performed triple immunofluorescent studies in hippocampal sections with antibodies to GFAP, cleaved thrombin, and the endothelial marker vWF (Supplemental Figure 1). Qualitative analysis of these images showed a pattern of increase in cleaved thrombin around blood vessels at one-day recovery and confirmed its co-localization with the astrocyte marker, GFAP.
Figure 1.
Traumatic brain injury compromises blood–brain barrier integrity and leads to activation of thrombin. (a, b) Blood–brain barrier breakdown was measured by IgG immunolabeling. Representative images of bregma −1.75 mm coronal brain sections show increased immunoreactivity for IgG in the brains of TBI-exposed animals compared to sham. (b) Higher magnification of the CA1 region of the hippocampus in TBI and sham-operated brains. (c) Quantification of IgG pixels shows a significant increase in immunoreactive cells at one-day recovery following TBI. (d, e) Triple immunolabeling for the astrocyte marker GFAP (green), cleaved thrombin (red), and the nuclear marker DAPI (blue) shows increased expression of cleaved thrombin in hippocampal astrocytes following TBI (e) compared to sham (d). (f) Thrombin activity is increased in hippocampus at one day after TBI. (g) Triple immunolabeling in the hippocampus for the astrocyte marker GFAP (green), thrombin receptor, PAR-1 (red), and the neuronal marker NeuN (magenta) shows co-localization of GFAP-immunoreactive astrocyte (green) and PAR-1 (red), suggesting activated thrombin may act on PAR1 on astrocytes. D, E, G; Bar = 50 µm. (n = 4–5 per group; *p < 0.05, **p < 0.01 vs. sham).
To confirm the results of the immunohistochemical studies, we measured serial changes in thrombin activity over one-day recovery after TBI (Figure 1(f)). Thrombin activity was increased from 1 h post-injury and reached a 2-fold increase in the hippocampus (p < 0.05 vs. Sham) at 24 h. We have previously shown that thrombin acts through PAR-1 in cortical astrocytes.21 To confirm the in vivo expression profile of PAR-1, we performed triple immunolabeling (Figure 1(g)). PAR-1 immunofluorescence co-localized with the astrocyte marker GFAP, but not the neuronal marker NeuN (Figure 1(g)).
TBI decreased mRNA and protein expression of GLAST and GLT1 in the hippocampus and increased depressive behaviors
Transcript changes at one-day recovery
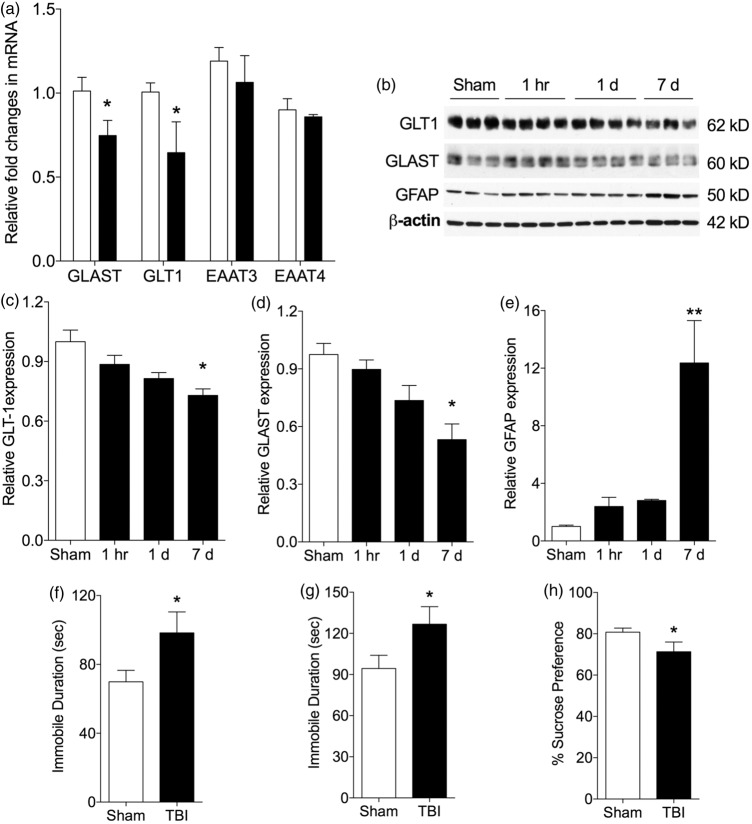
We measured expression of transcripts for GLAST, GLT1, EAAT3, and EAAT4 following TBI or sham operation (Figure 2). At one-day recovery, GLAST and GLT1 transcript expression was significantly decreased in the CA1 subregion of hippocampus compared to sham controls (p < 0.05) (Figure 2(a)). There was no significant change in levels for EAAT3 and EAAT4. GLT1 transcript expression was also significantly decreased in CA3 region (p < 0.05) (data not shown). In the DG, there was no significant change in any glutamate transporters (data not shown).
Figure 2.
TBI causes down-regulation of GLT1 and GLAST mRNA and protein in the hippocampus, and increases depressive behaviors. (a) Decrease in mRNA for GLAST and GLT1 in hippocampus at one-day recovery following TBI with no intergroup difference for EAAT3 and EAAT4 (p < 0.05, vs. sham; n = 5–6 per group; open column, sham; dark column, TBI). (b) Representative Western blot images (b) and quantification (c, d, e) showing a decrease in protein levels of GLT1, GLAST, and increase in GFAP in hippocampus over seven-days recovery after TBI (n = 3–4 per group; *p < 0.05, **p < 0.01 vs. sham). (f–h) Quantification of depressive behavior at seven-day recovery showed a significant increase in depressive behaviors following TBI as measured by immobile time during (f) tail suspension, and (g) forced swim, and (H) by decrease in consumption of sucrose water. (n = 10–12; *p < 0.05 vs. sham). EAAT3, excitatory amino acid transporter 3; EAAT4, excitatory amino acid transporter 4.
Protein changes at one- and seven-day recovery
We then used Western blotting to quantify changes in GLAST and GLT1 protein expression following TBI (Figure 2(b) to (d)). Since EAAT3 and EAAT4 transcript expression did not change in any regions, we focused on GLAST and GLT1 expression. In the hippocampus, levels of GLAST and GLT1 expression gradually deceased from day 1 onward and reached significance by day 7. In contrast, expression of the astrocyte marker GFAP increased significantly by day 7 after TBI (Figure 2(e)).
Behavioral measures at seven-day recovery
We measured depressive-like behaviors by using TST, FST, and sucrose preference tests at seven-day recovery after TBI (Figure 2(f) to (h)). Following TBI, mice showed a significant increase in immobile time during tail suspension and FSTs and reduced preference for consumption of 1% sucrose water compared to sham animals (p < 0.05 by Student’s t-test, n = 12–13).
PAR-1 activation contributes to the reduction in the expression of GLT1 and GLAST and the development of depressive behaviors after TBI
We have previously shown that thrombin-induced activation of the PAR-1 receptor results in decreased expression of glutamate transporters in primary cortical astrocytes.21 To confirm that this response occurred in the hippocampus, we exposed hippocampal astrocytes to thrombin (1 U/mL) or to PAR1- (20 µM, 40 µM), PAR3- (100 µM), or PAR4 (100 µM)-APs for 24 h (Supplemental Figure 2). We quantified expression levels of GLT1 and GLAST protein. PAR-1 and PAR-3 APs significantly decreased GLT1 expression compared to control. Neither thrombin, nor PAR-APs treatment significantly affected GLAST expression. We examined the effect of transporter downregulation on astrocyte glutamate uptake. DHK, a selective GLT1 inhibitor, significantly blocked astrocyte glutamate uptake, whereas UCPH-01, a selective GLAST inhibitor, did not significantly affect astrocyte glutamate uptake. Both thrombin and PAR-1AP treatments tended to decrease glutamate uptake compared to control but did not reach statistical significance.
Role of PAR-1 activation and ROCK at seven-day recovery following TBI
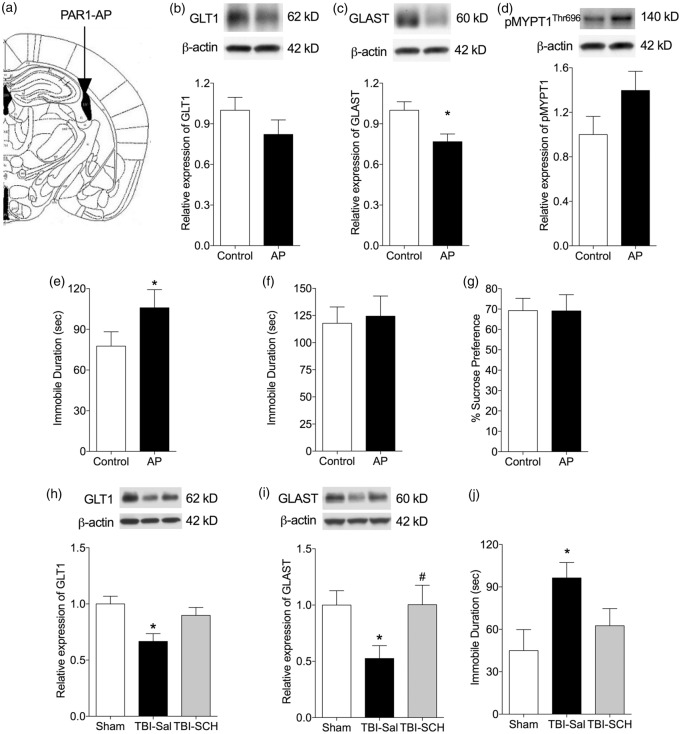
To determine whether the GLT1 and GLAST decrease is mediated by PAR-1 receptor in vivo, we infused a PAR-1 AP into the right lateral ventricle for 24 h and measured relative GLT1 and GLAST expression in the hippocampus at seven-day recovery (Figure 3(a)). SFLLRN-NH2, a selective PAR-1 AP, reduced GLT1 (0.82 ± 0.1 vs. scrambled peptide, n = 10) and GLAST (0.76 ± 0.05, p < 0.05 vs. scrambled peptide, n = 10) expression in hippocampus (Figure 3(b) and (c)).
Figure 3.
Thrombin acts on the PAR-1 receptor to decrease the expression of GLT1 and GLAST and to cause depressive behaviors. (a) Atlas shows in vivo PAR-1AP injection site. (b–d) Representative Western blots and quantification of the effect of intraventricular (icv) administration of PAR-1 agonist peptide (AP) on levels of GLT1, GLAST, and phospho-MYPT1. Hippocampal levels of GLT1 (b), GLAST (c) phospho-MYPT1 (d) were quantified seven days following exposure to PAR-1AP (20 µM icv for 24 h) or control peptide. There was a trend toward a decrease in GLT1 and increase in phospho-MYPT1 in the PAR-1AP infused mice but this did not reach significance. The decrease in GLAST in the PAR-1 AP treated mice was significant. (e–g) Quantification of depressive-like behaviors on day 7–9 of recovery showed an increase in immobile time in the FST (E), but not TST (f) or sucrose preference (g) test (n = 10; *p < 0.05, vs. control peptide). (h–j) The PAR-1 inhibitor SCH79797 25 (mg/kg, i.p.) was administered 3 h after TBI and the expression of GLT1 and GLAST examined in the hippocampus by Western blot. (h, i) Treatment with SCH79797 attenuated the decrease in levels of GLT1 and GLAST 1 day after TBI (n = 7–8 per group; *p < 0.05 vs. sham; #p < 0.05 vs. TBI-Saline). (j) Quantification of depressive-like behavior on day 7 of recovery showed an increase in immobile time in saline-treated TBI mice in the TST. Immobile time in mice treated with SCH79797 following TBI was not significantly different from sham controls. (n = 7–9 per group; *p < 0.05 vs. sham). AP, PAR-1 agonist peptide.
We previously showed that PAR-1 activation and ROCK signaling result in reduced expression of glutamate transporters in cortical astrocytes in vitro.21 Therefore, we next tested whether the ROCK pathway is involved in the in vivo mechanisms of transporter downregulation (Figure 3(d)). Infusion of the PAR-1 AP SFLLRN-NH2 AP infusion tends to increase ROCK activation in the hippocampus (1.18 ± 0.15 vs. scrambled peptide, n = 10) (Figure 3(d)).
Next, we tested whether activation of PAR-1 resulted in an increase in depressive behavior at seven-day recovery. SFLLRN-NH2 AP-infused mice showed a significant increase in freezing time (105.9 ± 13.4), vs. scrambled peptide (77.7 ± 10.6, p < 0.05, n = 10) during FST (Figure 3(e)). There was no significant difference in TST or sucrose preference testing between PAR1 AP and scramble peptide-infused mice (Figure 3(f) and (g)).
Effect of PAR-1 antagonist at one- and seven-day recovery following TBI
To determine the contribution of this signaling pathway to the decrease in glutamate transporters following TBI, we treated mice with SCH79797, a specific PAR-1 antagonist (25 ug/kg, IP) at 3 h following TBI (Figure 3(h) to (j)). At one-day recovery following TBI, the level of both GLAST and GLT1 expression was significantly decreased in hippocampus compared to sham controls. Administration of SCH79797 prevented the decrease of GLAST (1.004 ± 0.17 vs. TBI-Sal 0.52 ± 0.11; p < 0.05 n = 8) and GLT1 (0.89 ± 0.07 vs. TBI-Sal 0.66 ± 0.06, n = 8) (Figure 3(h) and (i)). At seven-day recovery following TBI, there was a significant increase in immobile time (96.4 ± 10.8 vs. 45.0 ± 14.8 s, p < 0.01 by one-way ANOVA and Bonferroni’s multiple comparisons test, n = 7–9) during TST compared to the sham control (Figure 3(j)). Immobile time in mice treated with SCH79797 following TBI (62.6 ± 12.1 s, n = 8) was not significantly different from sham controls.
Inhibition of ROCK signaling prevents the loss of GLT1 and GLAST both in vitro and in vivo, and reduces depressive-like behavior after TBI
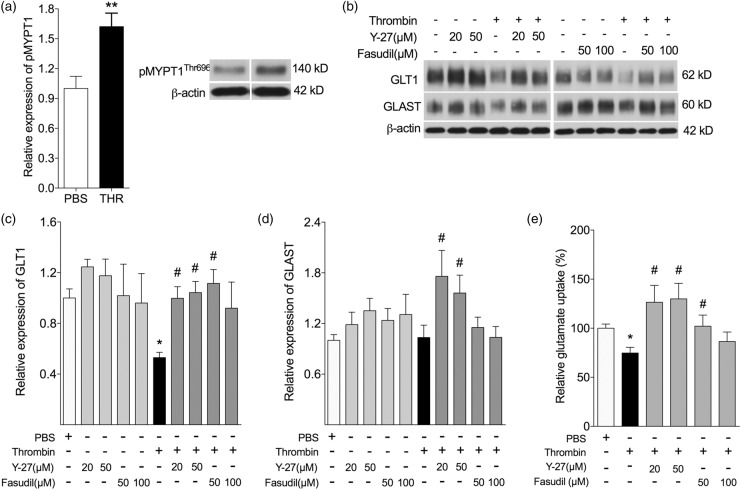
Role of ROCK in vitro at one-day recovery after exposure to thrombin
We have previously shown that activation of ROCK is associated with a decrease in glutamate transporter expression in cortical astrocytes.21 To examine this mechanism in the hippocampus, we treated hippocampal astrocytes with vehicle or thrombin (1 U/mL), in the absence or presence of ROCK inhibitors, Y-27632 (20 or 50 µM) or Fasudil (50 or 100 µM), for 24 h. We used Western blot to quantify expression levels (fold of control) of phosphorylated MYPT1Thr696 (p-MYPT1), GLT1, and GLAST, and used an enzymatic assay to quantify glutamate uptake (% of Control) after exposure of astrocytes to an excess of glutamate (Figure 4). Exposure to thrombin resulted in a significant increase increased phosphorylation of MYPT1 at 24 h after treatment compared to vehicle control (p < 0.01) (Figure 4(a)). Thrombin treatment significantly decreased GLT1 expression compared to control (p < 0.01) (Figure 4(b) and (c)). Inhibition of ROCK with Y-27632 or Fasudil in the presence of thrombin significantly attenuated the thrombin-induced down-regulation of GLT1 (Figure 4(c)). Thrombin alone did not significantly affect GLAST expression in hippocampal astrocytes (Figure 4(b) and (d)). ROCK inhibition significantly induced the expression of GLAST in the presence of thrombin compared to thrombin treatment alone (Figure 4(b) and (d)). Thrombin treatment (74.7 ± 5.80%) significantly decreased glutamate uptake compared to control (p < 0.05). ROCK inhibition with 20 or 50 µM Y-27632 (127 ± 17.3%, p < 0.05; 130 ± 15.9%, p < 0.05, respectively) or 50 µM Fasudil (102 ± 11.4%, p < 0.05) in the presence of thrombin significantly increased glutamate uptake compared to thrombin treatment alone (Figure 4(e)).
Figure 4.
Inhibition of Rho signaling prevents GLT1 loss in in vitro. (a–e) Hippocampal astrocytes were treated with vehicle, or thrombin (1 U/mL) in the presence or absence of increasing doses of ROCK inhibitors Y-27632 and Fasudil, for 24 h. (a) Thrombin treatment significantly increased phosphorylation of MYPT1 (pMYPT1). (b–d) Exposure to thrombin resulted in a significant decrease in GLT1 (b, c) with no significant change in GLAST (b, d). Inhibition of ROCK with Y-27632 or Fasudil prevented the decrease in GLT1 (c), but not GLAST (d) caused by thrombin. (e) Thrombin treatment significantly decreased glutamate uptake. Inhibition of ROCK with Y-27632 or Fasudil, in the presence of thrombin significantly increased glutamate uptake compared to thrombin treatment alone. *p < 0.05, **p < 0.01 compared to control; #p < 0.05 compared to thrombin alone (n = 7–12 per experiment, each performed in duplicate or triplicate).
Role of ROCK at seven-day recovery following TBI
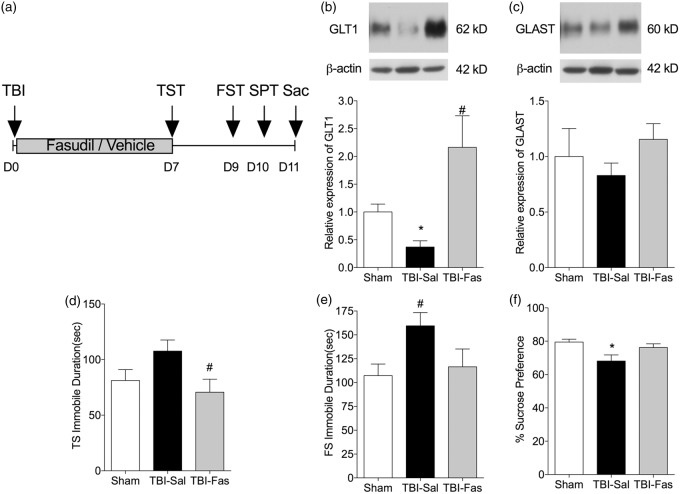
The results of the in vitro studies identified ROCK as the primary pathway mediating thrombin-induced down regulation of GLT1 in hippocampal astrocytes. Therefore, we next treated mice with subcutaneous infusion of fasudil (25 mg/kg/day for seven days) and measured changes in hippocampal GLT1 and GLAST protein levels (Figure 5). Mice treated with fasudil were significantly protected against the decrease in GLT1 caused by TBI (TBI-Fas 2.16 ± 0.56 vs. TBI-Sal 0.37 ± 0.14; p < 0.01, n = 4) (Figure 5(b)). There was a similar trend for GLAST but this did not reach significance (Figure 5(c)).
Figure 5.
Inhibition of Rho signaling prevents GLT1 and GLAST loss in in vivo.(a) Schematic drawing of in vivo experimental paradigm. (b, c) Decrease in GLT1 and GLAST levels in hippocampal homogenates after seven-day recovery following TBI. The decrease in GLT1 is prevented by treatment with Fasudil (25 µg/kg/day for seven days, s.c.). (d–f) Behavioral tests of depressive behaviors following TBI. Administration of fasudil following TBI attenuates the increase of immobile time in tail suspension (d), forced swim (e) and the decrease in sucrose consumption (f). *p < 0.05, compared to sham control; #p < 0.05, compared to TBI-saline group. n = 4–6 animals per group for Western blot and n = 16 animals for behavioral tests; Fas: fasudil; THR: thrombin; Sal: saline; TS: tail suspension test; FS: forced swim test.
We next tested whether preventing astrocyte glutamate transporters loss by inhibition of Rho signaling can attenuate depressive behaviors after TBI (Figure 5(d) to (f)). At seven-day recovery following TBI, there was an increase in immobile time during TST in saline-treated TBI group (107.7 ± 10.0 s) compared to the sham control (81.2 ± 9.8 s) (Figure 5(d)). Immobile time in mice treated with fasudil following TBI (70.7 ± 11.6 s) was not significantly different from sham controls (p > 0.05 by ANOVA and Bonferroni’s multiple comparisons test n = 16–18). We found similar results for FST (Figure 5(e)) and sucrose preference tests (Figure 5(f)). At nine-day recovery following TBI, there was an increase in immobile time during FST in saline-treated TBI group (159.5 ± 13.9 s) compared to the sham control (107.2 ± 12.1 s, p < 0.05, n = 16–18) (Figure 5(e)). Immobile time in mice treated with fasudil following TBI (116.5 ± 18.67 s, n = 12) was not significantly different from sham controls. At 10-day recovery following TBI, there was a reduced consumption of sucrose solution in the saline-treated TBI group (68.1 ± 3.7%) compared to the sham control (79.49 ± 1.7%, p < 0.05, Kruskal–Wallis and Dunn’s multiple comparisons) (Figure 5(f)). Sucrose solution consumption in mice treated with fasudil following TBI (76.2 ± 2.2%) was not significantly different from sham controls.
Decrease in astrocyte glutamate transporter expression is associated with an increase in extracellular glutamate in response to stress at 7–10-day recovery following TBI
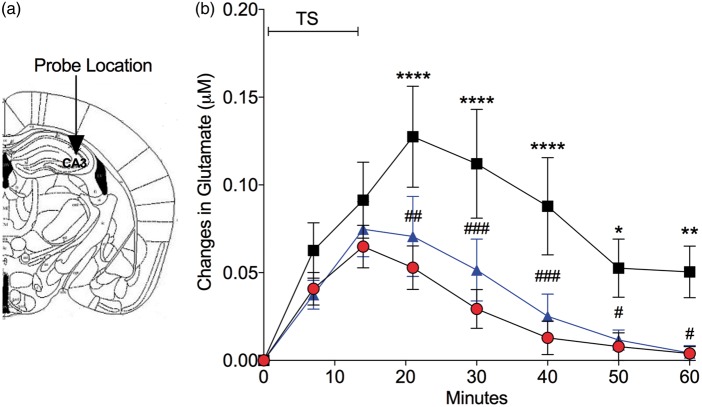
To determine if the decrease in astrocyte glutamate transporters following TBI resulted in an increase in extracellular glutamate, we measured changes in hippocampal glutamate levels in real time (Figure 6). We exposed mice to TBI or sham operation and administered the ROCK inhibitor fasudil via subcutaneous infusion. Following implantation of a glutamate biosensor into CA3 region of hippocampus (Figure 6(a)) on day 7 to 10 following TBI or sham procedure, mice were subject to a stressful stimulus (tail suspension) for 15 min and allowed to recover for 45 min. We concurrently recorded L-glutamate changes in real-time using biosensors implanted in the hippocampus (Figure 6(b)). In sham-operated mice, L-glutamate levels increased compared to baseline at 15 min and recovered to basal levels at 45 min after the completion of TS. Mice subject to TBI showed a significant relative increase in L-glutamate levels at 20 min of measurement (5 min after the completion of TS) and the level remained elevated at the conclusion of the monitoring period. In contrast, mice treated with fasudil to prevent glutamate transporter loss after TBI, showed a significantly attenuated elevation in L-glutamate (by repeated measures two-way ANOVA followed by Tukey’s multiple comparisons) in response to TS and recovered to basal levels by the same time as sham controls.
Figure 6.
Following TBI glutamate levels in the hippocampus are increased in response to stress and glutamate clearance is prolonged. Mice were implanted in CA3 on day 7 following TBI with a biosensor to detect L-glutamate. (a) Atlas shows the region for in vivo glutamate measurement. Concentrations of L-glutamate on the y-axis are based on individual post-calibration values after removal of the biosensor. (b) Real-time relative changes in L-glutamate concentration measured in the CA3 region of hippocampus in response to tail suspension (TS). Individual points represent changes in L-glutamate concentration relative to the basal level in each mouse during 15 min of TS and a 45-min recovery period. Note that mice treated with saline after TBI (black squares) show a greater increase in glutamate compared to sham controls (red circles) and do not fully return to basal levels at the end of the monitoring period. Administration of fasudil (25 µg/kg/day for seven days, s.c.) significantly attenuated the increase in glutamate levels in hippocampus of mice after TBI (blue triangles) similar to the glutamate levels of sham animals. (n = 8 mice per group). *p < 0.05, **p < 0.01 ****p < 0.0001, compared to sham control, and #p < 0.05, ##p < 0.001, ###p < 0.0005, compared to TBI-Saline group, by repeated measures two-way ANOVA and Tukey’s post hoc analysis.
Discussion
The mechanisms by which TBI leads to depression are not known, and are likely to involve multiple brain regions and neurotransmitters. Here, we provide evidence that thrombin, acting via PAR-1 and ROCK causes a decrease in the astrocyte glutamate transporters GLT-1 and GLAST in the hippocampus following TBI. This decrease is linked both to an increase in extracellular glutamate in response to stress, and to an increase in depressive-like behavior following TBI. Depression is one of the numerous psychological complications of TBI.4,42 Current approaches to treatment of depression after TBI, including antidepressants or cognitive behavioral therapy have limited efficacy. Our results identify a novel mechanism by which TBI results in down-regulation of astrocyte glutamate transporters, and suggest a potential pathway for investigation as a therapeutic target for preventing depression following TBI.
Compromise of astrocyte uptake of glutamate has not previously been linked to depression after TBI. Numerous clinical and pre-clinical studies identify dysfunction in glutamatergic signaling as one of the mechanisms of MDD. Serum and plasma levels of glutamate are elevated in patients with MDD.15 Postmortem studies of cerebral cortical and hippocampal samples in patients with MDD show a decrease of both astrocyte glutamate transporters.43,44 In animal models of depression, GLAST and GLT-1 are downregulated in animals exhibiting depressive behaviors,10,11 and blockade of glutamate uptake causes anhedonic behaviors in rats.12
Disruption of the BBB has been proposed as an initial step in the mechanisms of epileptogenesis but its contribution to the mechanisms of depression after TBI is not established.45 We have previously shown, in resected human tissue of patients with intractable epilepsy, that the BBB is compromised and that this is associated with reduction in immunoreactivity for both glutamate transporters along with an increase in cleaved thrombin and reactive astrogliosis.21 In the present study, we found a similar pattern after TBI, with an increase in thrombin activity and GFAP expression accompanied by a decrease in transporter mRNA and protein levels in the hippocampus. This increase in thrombin activity is consistent with previous results in mild TBI.46 There is indirect evidence of an association between BBB permeability and MDD, including an increase in CSF-to-serum ratio of albumin in MDD patients, but this has not been studied in depression after TBI.47,48
Previous studies have shown a decrease in astrocyte glutamate transporter expression in the cortex after TBI, although the mechanisms of this decrease are not established.17,49 The effects of thrombin in the CNS are mediated by PARs 1, 3 and 4, all of which are expressed by astrocytes.50 Knockout of PAR-1 results in a decrease in cerebral edema, microglial activation and neuronal injury following cerebral ischemia or hemorrhage.51,52 Our results also point to a role for PAR-1 in the mechanisms by which thrombin causes a decrease in hippocampal glutamate transporters, as we have previously shown in the cortex,21 and which contributes to the development of depressive behaviors.
The effects of thrombin on glutamate transporter levels and depressive behavior in the present study were prevented by inhibition of ROCK, and other studies have identified an important role for ROCK in BBB integrity and astrogliosis. Inhibition of Rho signaling reduces endothelial oxidative stress, decreases cerebral edema and improves functional outcome after ischemic injury.53 Fasudil decreases actin stress fiber formation and GFAP expression by astrocytes, which may prevent astrogliosis and improve astrocyte function.54,55 The clinical implications of the effects of these treatments are not clear. The administration of each inhibitor was delayed until after TBI to provide some clinical relevance for these experiments. The duration of each treatment varied depending on the route of administration. The PAR-1 antagonist SCH79797 was delivered by a single IP injection 3 h after TBI, a time window selected to be clinically relevant. Both the PAR-1 AP and the ROCK inhibitor Fasudil were administered by osmotic infusion (icv and SQ, respectively). These pumps were placed immediately after TBI but the drugs would not reach a therapeutic level until at least 3 h of recovery.
Other pathways are known to regulate GLT-1 and GLAST expression. Glutamate transporter expression is regulated in C6 glioma cells by protein kinase C.56 In cortical astrocytes, the reduction in GLT-1 produced by hypoxia is prevented by inhibition of NFκB.57 Up-regulation of GLT-1 by ketamine requires the brain-derived neurotrophic factor receptor TrkB.11 Thrombin signaling also occurs via JAK-STAT and Src pathways.58,59 We cannot exclude a role for these pathways in the effects of thrombin following TBI. However, our results are consistent with previous data showing a major role for ROCK on GLT-1 and GLAST expression and function, including the increase in GLAST in vitro following treatment with the ROCK inhibitor Fasudil.54
These studies have a number of limitations. Cultured astrocytes predominantly express GLAST with much lower expression of GLT-1 unless co-cultured with neurons. However, the effects of PAR-1 activation and ROCK signaling in vitro on transporter expression were recapitulated in the in vivo experiments following TBI. A decrease in transporter expression may not lead to a compromise of the ability of astrocytes to clear glutamate as we observed in the in vitro experiments. Our in vivo measurement of glutamate levels following TBI provides direct evidence of the effect of transporter loss on the capacity of astrocytes to buffer glutamate. We have focused on the hippocampus but the symptoms of depression are due to abnormal function of a network, not a single brain region.60 Additionally, augmenting glutamate signaling including the administration of the N-methyl-d-aspartate receptor co-agonist D-cycloserine following TBI may improve cognitive outcome, suggesting that the increase in glutamate resulting from transporter loss may not be entirely detrimental.61 Last, these studies were carried out only with male mice and cannot be extrapolated to females given the evidence for sex differences in response to stress.62
Pre-clinical and clinical research suggest that TBI and MDD share common pathways in the disturbance of the regulation of glutamate homeostasis, but this mechanism has not previously been investigated as one of the factors which cause depression after TBI. The results of this study suggest that selective modulation of PAR-1 signaling to preserve glutamate transporter function after TBI may be a novel approach to preventing this significant public health problem.
Supplementary Material
Acknowledgements
The authors thank Ms. Allison Rusie for expert technical assistance.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: These studies were funded by the Ruth D. & Ken M Davee Pediatric Neurocritical Care Program and by the Lyndsey Whittingham Foundation.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
CP, AH and SHR performed all experiments and contributed to the analysis of the data, their interpretation and the draft of the paper. MW conceived the research project, supervised CP, AH and SHR, and is responsible for the data and the finalization of the paper.
Supplementary material
Supplementary material for this paper can be found at the journal website: http://journals.sagepub.com/home/jcb
References
- 1.Faul M, Xu L, Wald MM, et al. Traumatic brain injury in the United States: emergency department visits, hospitalizations and deaths 2002–2006, Atlanta, GA: Center for Disease Control and Prevention, National Center for Injury Prevention and Control, 2010. [Google Scholar]
- 2.Rosenthal M, Christensen BK, Ross TP. Depression following traumatic brain injury. Arch Phys Med Rehabil 1998; 79: 90–103. [DOI] [PubMed] [Google Scholar]
- 3.Dikmen SS, Bombardier CH, Machamer JE, et al. Natural history of depression in traumatic brain injury. Arch Phys Med Rehabil 2004; 85: 1457–1464. [DOI] [PubMed] [Google Scholar]
- 4.Bombardier CH, Fann JR, Temkin NR, et al. Rates of major depressive disorder and clinical outcomes following traumatic brain injury. JAMA 2010; 303: 1938–1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jorge RE, Acion L, Burin DI, et al. Sertraline for preventing mood disorders following traumatic brain injuryy: a randomized clinical trial. JAMA Psychiatry 2016; 73: 1041–1047. [DOI] [PubMed] [Google Scholar]
- 6.Fann JR, Bombardier CH, Vannoy S, et al. Telephone and in-person cognitive behavioral therapy for major depression after traumatic brain injury: a randomized controlled trial. J Neurotrauma 2015; 32: 45–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ashman TA, Cantor JB, Gordon WA, et al. A randomized controlled trial of sertraline for the treatment of depression in persons with traumatic brain injury. Arch Phys Med Rehabil 2009; 90: 733–740. [DOI] [PubMed] [Google Scholar]
- 8.Danbolt NC, Storm-Mathisen J, Kanner BI. An [Na++ K+]coupled L-glutamate transporter purified from rat brain is located in glial cell processes. Neuroscience 1992; 51: 295–310. [DOI] [PubMed] [Google Scholar]
- 9.Regan MR, Huang YH, Kim YS, et al. Variations in promoter activity reveal a differential expression and physiology of glutamate transporters by glia in the developing and mature CNS. J Neurosci 2007; 27: 6607–6619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gomez-Galan M, De Bundel D, Van Eeckhaut A, et al. Dysfunctional astrocytic regulation of glutamate transmission in a rat model of depression. Mol Psychiatry 2013; 18: 582–594. [DOI] [PubMed] [Google Scholar]
- 11.Liu WX, Wang J, Xie ZM, et al. Regulation of glutamate transporter 1 via BDNF-TrkB signaling plays a role in the anti-apoptotic and antidepressant effects of ketamine in chronic unpredictable stress model of depression. Psychopharmacology 2016; 233: 405–515. [DOI] [PubMed] [Google Scholar]
- 12.Bechtholt-Gompf AJ, Walther HV, Adams MA, et al. Blockade of astrocytic glutamate uptake in rats induces signs of anhedonia and impaired spatial memory. Neuropsychopharmacology 2010; 35: 2049–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.John CS, Smith KL, Van’t Veer A, et al. Blockade of astrocytic glutamate uptake in the prefrontal cortex induces anhedonia. Neuropsychopharmacology 2012; 37: 2467–2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Altamura C, Maes M, Dai J, et al. Plasma concentrations of excitatory amino acids, serine, glycine, taurine and histidine in major depression. Eur Neuropsychopharmacol 19955 (Suppl71–75. [DOI] [PubMed] [Google Scholar]
- 15.Mauri MC, Ferrara A, Boscati L, et al. Plasma and platelet amino acid concentrations in patients affected by major depression and under fluvoxamine treatment. Neuropsychobiology 1998; 37: 124–129. [DOI] [PubMed] [Google Scholar]
- 16.Zarate CA, Jr, Singh JB, Carlson PJ, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 2006; 63: 856–864. [DOI] [PubMed] [Google Scholar]
- 17.Goodrich GS, Kabakov AY, Hameed MQ, et al. Ceftriaxone treatment after traumatic brain injury restores expression of the glutamate transporter, GLT-1, reduces regional gliosis, and reduces post-traumatic seizures in the rat. J Neurotrauma 2013; 30: 1434–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barzo P, Marmarou A, Fatouros P, et al. Acute blood-brain barrier changes in experimental closed head injury as measured by MRI and Gd-DTPA. Acta Neurochir Suppl 1997; 70: 243–246. [DOI] [PubMed] [Google Scholar]
- 19.Tomkins O, Shelef I, Kaizerman I, et al. Blood brain barrier disruption in post-traumatic epilepsy. J Neurol Neurosurg Psych 2008; 79: 774–777. [DOI] [PubMed] [Google Scholar]
- 20.Wang H, Ubl JJ, Reiser G. Four subtypes of protease-activated receptors, co-expressed in rat astrocytes, evoke different physiological signaling. Glia 2002; 37: 53–63. [DOI] [PubMed] [Google Scholar]
- 21.Piao C, Ralay Ranaivo H, Rusie A, et al. Thrombin decreases expression of the glutamate transporter GLAST and inhibits glutamate uptake in primary cortical astrocytes via the Rho kinase pathway. Exp Neurol 2015; 273: 288–300. [DOI] [PubMed] [Google Scholar]
- 22.Venkatesan C, Chrzaszcz M, Choi N, et al. Chronic up regulation of activated microglia immunoreactive for galectin-3/Mac-2 and nerve growth factor following diffuse axonal injury. J Neuroinflammation 2010; 7: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wainwright M, Rossi J, Schavocky J, et al. Protein kinase involved in lung injury susceptibility: evidence from enzyme isoform genetic knockout and in vivo inhibitor treatment. Proc Natl Acad Sci U S A 2003; 100: 6233–6238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Piao CS, Loane DJ, Stoica BA, et al. Combined inhibition of cell death induced by apoptosis inducing factor and caspases provides additive neuroprotection in experimental traumatic brain injury. Neurobiol Dis 2012; 46: 745–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gong Y, Xi G, Hu H, et al. Increase in brain thrombin activity after experimental intracerebral hemorrhage. Acta Neurochir Suppl 2008; 105: 47–50. [DOI] [PubMed] [Google Scholar]
- 26.Chrzaszcz M, Venkatesan C, Dragisic T, et al. Minozac treatment prevents increased seizure susceptibility in a mouse ‘two-hit’ model of closed skull traumatic brain injury and electroconvulsive shock-induced seizure. J Neurotrauma 2010; 27: 1283–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ralay Ranaivo H, Hodge JN, Choi N, et al. Albumin induces upregulation of matrix metalloproteinase-9 in astrocytes via MAPK and reactive oxygen species-dependent pathways. J Neuroinflammation 2012; 9: 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steru L, Chermat R, Thierry B, et al. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology 1985; 85: 367–370. [DOI] [PubMed] [Google Scholar]
- 29.Cryan JF, Mombereau C, Vassout A. The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev 2005; 29: 571–625. [DOI] [PubMed] [Google Scholar]
- 30.Piao CS, Stoica BA, Wu J, et al. Late exercise reduces neuroinflammation and cognitive dysfunction after traumatic brain injury. Neurobiol Dis 2013; 54: 252–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther 1977; 229: 327–336. [PubMed] [Google Scholar]
- 32.Ghasemi M, Montaser-Kouhsari L, Shafaroodi H, et al. NMDA receptor/nitrergic system blockage augments antidepressant-like effects of paroxetine in the mouse forced swimming test. Psychopharmacology 2009; 206: 325–333. [DOI] [PubMed] [Google Scholar]
- 33.Strekalova T, Spanagel R, Bartsch D, et al. Stress-induced anhedonia in mice is associated with deficits in forced swimming and exploration. Neuropsychopharmacology 2004; 29: 2007–2017. [DOI] [PubMed] [Google Scholar]
- 34.Anderson CM, Swanson RA. Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia 2000; 32: 1–14. [PubMed] [Google Scholar]
- 35.Huynh TH, Shim I, Bohr H, et al. Structure-activity relationship study of selective excitatory amino acid transporter subtype 1 (EAAT1) inhibitor 2-amino-4-(4-methoxyphenyl)-7-(naphthalen-1-yl)-5-oxo-5,6,7,8-tetrahydro-4H-chrom ene-3-carbonitrile (UCPH-101) and absolute configurational assignment using infrared and vibrational circular dichroism spectroscopy in combination with ab initio Hartree-Fock calculations. J Med Chem 2012; 55: 5403–5412. [DOI] [PubMed] [Google Scholar]
- 36.Itzekson Z, Maggio N, Milman A, et al. Reversal of trauma-induced amnesia in mice by a thrombin receptor antagonist. J Mol Neurosci 2014; 53: 87–95. [DOI] [PubMed] [Google Scholar]
- 37.Manaenko A, Sun X, Kim CH, et al. PAR-1 antagonist SCH79797 ameliorates apoptosis following surgical brain injury through inhibition of ASK1-JNK in rats. Neurobiol Dis 2013; 50: 13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Satoh S, Hitomi A, Ikegaki I, et al. Amelioration of endothelial damage/dysfunction is a possible mechanism for the neuroprotective effects of Rho-kinase inhibitors against ischemic brain damage. Brain Res Bull 2010; 81: 191–195. [DOI] [PubMed] [Google Scholar]
- 39.Bye N, Christie KJ, Turbic A, et al. Rho kinase inhibition following traumatic brain injury in mice promotes functional improvement and acute neuron survival but has little effect on neurogenesis, glial responses or neuroinflammation. Exp Neurol 2016; 279: 86–95. [DOI] [PubMed] [Google Scholar]
- 40.Naylor E, Aillon DV, Gabbert S, et al. Simultaneous real-time measurement of EEG/EMG and L-glutamate in mice: a biosensor study of neuronal activity during sleep. J Electroanal Chem 2011; 656: 106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Laskowitz DT, Wang H, Chen T, et al. Neuroprotective pentapeptide CN-105 is associated with reduced sterile inflammation and improved functional outcomes in a traumatic brain injury murine model. Sci Rep 2017; 7: 46461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perry DC, Sturm VE, Peterson MJ, et al. Association of traumatic brain injury with subsequent neurological and psychiatric disease: a meta-analysis. J Neurosurg 2016; 124: 511–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Choudary PV, Molnar M, Evans SJ, et al. Altered cortical glutamatergic and GABAergic signal transmission with glial involvement in depression. Proc Natl Acad Sci U S A 2005; 102: 15653–15658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Medina A, Burke S, Thompson RC, et al. Glutamate transporters: a key piece in the glutamate puzzle of major depressive disorder. J Psychiatr Res 2013; 47: 1150–1156. [DOI] [PubMed] [Google Scholar]
- 45.Friedman A, Kaufer D, Heinemann U. Blood-brain barrier breakdown-inducing astrocytic transformation: novel targets for the prevention of epilepsy. Epilepsy Res 2009; 85: 142–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Itsekson-Hayosh Z, Shavit-Stein E, Katzav A, et al. Minimal traumatic brain injury in mice: protease-activated receptor 1 and thrombin-related changes. J Neurotrauma 2016; 33: 1848–1854. [DOI] [PubMed] [Google Scholar]
- 47.Najjar S, Pearlman DM, Devinsky O, et al. Neurovascular unit dysfunction with blood-brain barrier hyperpermeability contributes to major depressive disorder: a review of clinical and experimental evidence. J Neuroinflammation 2013; 10: 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gudmundsson P, Skoog I, Waern M, et al. The relationship between cerebrospinal fluid biomarkers and depression in elderly women. Am J Geriatr Psychiatry 2007; 15: 832–838. [DOI] [PubMed] [Google Scholar]
- 49.Yi JH, Pow DV, Hazell AS. Early loss of the glutamate transporter splice-variant GLT-1v in rat cerebral cortex following lateral fluid-percussion injury. Glia 2005; 49: 121–133. [DOI] [PubMed] [Google Scholar]
- 50.Luo W, Wang Y, Reiser G. Protease-activated receptors in the brain: receptor expression, activation, and functions in neurodegeneration and neuroprotection. Brain Res Rev 2007; 56: 331–345. [DOI] [PubMed] [Google Scholar]
- 51.Wang J, Jin H, Hua Y, Keep RF, Xi G. Role of protease-activated receptor-1 in brain injury after experimental global cerebral ischemia. Stroke 2012; 43: 2476–2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wan S, Cheng Y, Jin H, et al. Microglia activation and polarization after intracerebral hemorrhage in mice: the role of protease-activated receptor-1. Transl Stroke Res 2016; 7: 478–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gibson CL, Srivastava K, Sprigg N, et al. Inhibition of Rho-kinase protects cerebral barrier from ischaemia-evoked injury through modulations of endothelial cell oxidative stress and tight junctions. J Neurochem 2014; 129: 816–826. [DOI] [PubMed] [Google Scholar]
- 54.Lau CL, O’Shea RD, Broberg BV, et al. The Rho kinase inhibitor Fasudil up-regulates astrocytic glutamate transport subsequent to actin remodelling in murine cultured astrocytes. Br J Pharmacol 2011; 163: 533–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kesherwani V, Tarang S, Barnes R, et al. Fasudil reduces GFAP expression after hypoxic injury. Neurosci Lett 2014; 576: 45–50. [DOI] [PubMed] [Google Scholar]
- 56.Gonzalez MI, Susarla BT, Robinson MB. Evidence that protein kinase Calpha interacts with and regulates the glial glutamate transporter GLT-1. J Neurochem 2005; 94: 1180–1188. [DOI] [PubMed] [Google Scholar]
- 57.Dallas M, Boycott HE, Atkinson L, et al. Hypoxia suppresses glutamate transport in astrocytes. J Neurosci 2007; 27: 3946–3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huang C, Ma R, Sun S, et al. JAK2-STAT3 signaling pathway mediates thrombin-induced proinflammatory actions of microglia in vitro. J Neuroimmunol 2008; 204: 118–125. [DOI] [PubMed] [Google Scholar]
- 59.Liu DZ, Ander BP, Xu H, et al. Blood-brain barrier breakdown and repair by Src after thrombin-induced injury. Ann Neurol 2010; 67: 526–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang T, Wang K, Qu H, et al. Disorganized cortical thickness covariance network in major depressive disorder implicated by aberrant hubs in large-scale networks. Sci Rep 2016; 6: 27964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sta Maria NS, Reger ML, Cai Y, et al. D-cycloserine restores experience-dependent neuroplasticity after traumatic brain injury in the developing rat brain. J Neurotrauma 2017; 34: 1692–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rincon-Cortes M, Grace AA. Sex-dependent effects of stress on immobility behavior and VTA dopamine neuron activity: modulation by ketamine. Int J Neuropsychopharmacol 2017; 20: 823–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.