Abstract
A flurry of research in recent years has revealed the molecular origins of many membrane less organelles to be the liquid phase separation of intrinsically disordered proteins (IDPs). Consequently, protein disorder has emerged as an important driver of intracellular compartmentalization, by providing specialized microenvironments chemically distinct from the surrounding medium. Though the importance of protein disorder and its relationship to intracellular phase behavior is clear, a detailed understanding of how such phase behavior can be predicted and controlled remains elusive. While research in IDPs has largely focused on the implications of structural disorder on cellular function and disease, another field, that of artificial protein polymers, has focused on the de novo design of protein polymers with controllable material properties. A subset of these polymers, specifically those derived from structural proteins such as elastin and resilin, are also disordered sequences that undergo liquid-liquid phase separation. This phase separation has been used in a variety of biomedical applications, and researchers studying these polymers have developed methods to precisely characterize and tune their phase behavior. Despite their disparate origins, both fields are complementary as they study the phase behavior of intrinsically disordered polypeptides. This perspective hopes to stimulate collaborative efforts by highlighting the similarities between these two fields and by providing examples of how such collaboration could be mutually beneficial.
Graphical abstract
1.0. Introduction
Membrane less organelles have fascinated biologists since the observation of the nucleolus almost two centuries ago1,2. Only in the last two decades, however, has the origin of these curious intracellular structures been elucidated. We now know that membrane less organelles are largely composed of intrinsically disordered proteins (IDPs) that phase separate3–7 from the aqueous interior of the cell. With the emergence of IDPs as a new and exciting field in biochemistry and cell biology, another field in materials science, that of protein polymers, has been quietly maturing for the past few decades. Behind this research lies the idea that repeat units composed of collections of amino acids can be used to create new classes of polymers with useful material properties 8–10. While research in membrane less organelles has focused on the consequences of disorder on cellular function and disease, research in protein polymers has focused on the bottom-up, de novo design of macromolecules with tunable material properties. Similar to IDPs that constitute membrane less organelles, many unstructured protein polymers exhibit aqueous phase behavior, highlighting the fact that structural disorder plays an essential role in their material properties11–15.
Given that both these classes of biomolecules are unstructured —disordered— macromolecules, we propose that there is a clear connection between the liquid-liquid demixing of protein polymers and membrane less organelles, yet there has been little crossover or communication between these two fields of research. This perspective hopes to stimulate collaborative efforts by highlighting the many commonalities shared by these two related fields.
2.0. A Historical Perspective
The membrane less organelle and protein polymer communities have predominantly coexisted as two separate communities with brief periods of intersection16. Although a full review of both fields is beyond the scope of this perspective, some of the most important discoveries that impacted both polymer science/protein polymer and membrane less organelles/IDPs are documented in Figure 1. As early as 1826, the connection was made between the similarity of synthetic rubber to ‘albumen-like’ material contained within cells17. Naegli’s observations of granules of various biologic materials is mentioned in the polymer chemistry literature, especially in regard to the separation of large polymers from solution18. By the time that membrane less organelles had first been robustly characterized by electron microscopy in 196219, polymer chemists had developed the experimental techniques to accurately determine the molecular weight of large, unstructured polymers 20, 21 and had a clear statistical understanding of their solution conformations21–24. This understanding enabled the rapid acceptance of these ‘nuages’ as disordered, high molecular weight, protein and nucleic acid condensates20–22, 25. Historically, the recognition that membrane less organelles contain unstructured proteins and that synthetic polymers are frequently unstructured was a moment in time when these fields could have overlapped productively, but this was not be, as the emergence of molecular and structural biology took biochemistry down a different road.
Figure 1. Significant events in the history of protein polymers and membrane less organelles.
Protein polymers are an offshoot of polymer science, both of which are denoted by the blue line. Membrane less organelles emerged from biochemistry and cell biology and are denoted by the red line. At various points in the 20th century, advancements made by polymer science and biochemistry and cell biology influenced one another, which is symbolically represented in the two lines crossing over one another. With time, however, both fields drifted away from each other, as graphically represented by the divergence of the parallel red and blue lines away from one another. With the recent discovery of myriad IDPs and protein polymers derived from IDP building blocks and their common features, the time is now right for renewed communication and collaboration between these two fields 1, 2, 16–26, 28–74.
Changing research motivations in the middle of the 20th century reduced the collaborative efforts between polymer science and biologists. This was driven in part the rise of molecular biology and recombinant protein expression that provided the ability to design, synthesize and purify proteins at the single residue level, and the emergence of x-ray crystallography as the dominant structural characterization technique of proteins. This led to the era of modern structural biology that naturally focused on the major class of known polypeptides that are folded and have a clearly defined function and are amenable to structural characterization by x-ray diffraction.26 Recombinant protein expression and site-directed mutagenesis reinforced the structure-function paradigm by enabling study of structure-function at the single residue level, providing an unprecedented understanding of how many proteins function. An unfortunate consequence of the structure-function era, however, was the fact that disordered elements within proteins or proteins that were largely unstructured became the “dark matter” of the cell, given their lack of structure and somewhat opaque —difficult to discern— function. As their structure could not be resolved with x-ray crystallography, their role was subsumed within the dominant protein structure-function paradigm as nonfunctional connecting segments. As we now know, these unstructured—intrinsically disordered— proteins are not an evolutionary dead end, but play an important role in biology, and in human health and disease 27.
3.0. Disorder and phase behavior are two features that unite protein polymers and IDPs.
The importance of disordered protein regions and their ability to affect function was eventually realized independently by researchers in the membrane less organelle and protein polymer fields. While there was limited communication between these fields, they are united by polypeptide sequences that: (1) can be described as intrinsically disordered and (2) rely on intrinsic disorder for liquid-liquid phase separation.
3.1. Disorder
Chain disorder is the first common ‘structural’ feature shared by many membrane less organelles and protein polymers. This is a negative description of protein structure —a lack of order— and therefore its precise meaning has been a source of constant debate11. Recently, there has been a concerted effort among researchers in the IDP field to clarify this definition, consolidating a variety of descriptions—from the more analytical ‘natively denatured’ to the more creative ‘dancing proteins’—under the umbrella term of ‘intrinsically disordered’ 3, 75. IDPs and intrinsically disordered regions (IDRs) within proteins are generally composed of tandem repeats of low complexity sequence domains which can be polymorphic76 or adopt multiple conformations in solution3. Mechanisms ranging from electrostatic repulsion to the presence of structurally disruptive amino acids (Gly-Pro), have been proposed, with a flexible chain conformation as the unifying feature70. Despite the challenge of predicting intrinsic disorder in a multidomain protein that contains other structured elements, prediction algorithms that account for mean net charge, hydropathy, and relative Gly/Pro content within a sliding window have enabled the identification hundreds of IDPs and IDRs77. This ability to broadly predict disorder has accelerated our understanding of the functional importance of IDPs on gene regulation78, intra-cellular communication79, and disease80 in many biological organisms. Intra-cellular IDPs have been implicated in the formation of membrane less organelles that appear to play a role in cell regulation and communication. While not all membrane less organelles require disorder, we know that many require disorder as a ‘structurally’ necessary feature for their formation73, 74, 81, 82.
In contrast to membrane less organelles, understanding the molecular origins of chain disorder in protein polymers was a more straightforward process. The sequence origins of disorder were readily identified during early work with tropoelastin and resilin, two native proteins that have provided much inspiration for the design of protein polymers53, 83. Tropoelastin, the soluble precursor to elastin, is perhaps the most extensively studied IDP, and characterization of its unstructured regions predates our current understanding of protein disorder by half a century83–86. The conformational flexibility of tropoelastin was experimentally verified in the 1960’s when the protein was observed to be highly disordered at low temperatures87. Subsequent sequencing of both animal and human tropoelastin led to the discovery that elastin is composed of alternating ordered, hydrophilic domains and disordered, hydrophobic domains59, 60, 88. Noting the conserved low sequence complexity tandem repeats, Urry pioneered the development of an even more reductionist version of the disordered hydrophobic domains of tropoelastin, which he termed elastin-like polypeptides (ELPs), that consist of polymers of VPGXG units, a pentapeptide65, 89 that recurs in the primary sequence of the hydrophobic, disordered domains of tropoelastin.
The evolution of resilin-like polypeptides followed a similar trajectory. Resilin was first noted for its remarkable mechanical properties in the 1960s53, 55 and although it would take another 40 years to derive a consensus amino acid sequence for Drosophila Rec-1 resilin90, it turned out to also be a highly disordered protein with a repetitive sequence motif91. The presence of a high degree of disorder was postulated as an important source of the elastomeric mechanical properties of elastin92 and resilin93, which motivated the production of recombinant mimics such as ELPs and resilin-like polypeptides (RLPs), in an effort to design protein polymers with similar properties94.
3.2. Phase Behavior
Chain disorder is a prerequisite to the second shared capability of membrane less organelles and protein polymers — aqueous liquid-liquid phase separation. Phase separation is a complicated balance of entropic and enthalpic forces which rely on both polymer chemistry and solvent quality in a given state. Thus, if an IDP does not phase separate under a certain set of physiological conditions that does not exclude phase separation under a different set. We note that though there are many examples of IDPs that do not phase separate, there are relatively few examples of proteins which phase separate that do not contain an obvious degree of disorder. As a result, there is a growing understanding in both the protein polymer community and membrane less organelle community that IDPs and IDRs are required to observe phase separation, although the exact amount remains unclear. To move the discussion forward, we accept the mounting evidence of permissive responsibility of intrinsic disorder in both protein polymers and membrane less organelles.
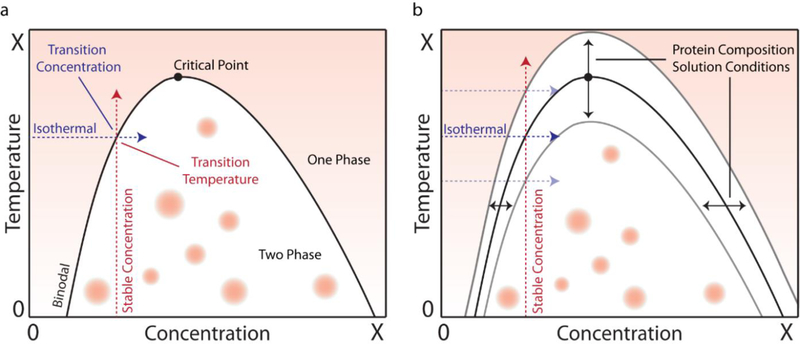
In the protein polymer community, phase behavior is generally studied as a function of temperature and is manifested as lower critical solution temperature (LCST) or upper critical solution temperature (UCST) phase behavior95, 96. Polymers with LCST behavior become immiscible with heat while UCST polymers become miscible with heat. In principle, both UCST and LCST phase behaviors can be observed in the same system97. Changes in temperature as a driving force for LCST or UCST phase transition, while eminently useful in the application of such polymers in an abiotic and technological context, are less relevant to natural biological conditions. In the largely isothermal environment of life, nature frequently uses other variables to drive phase behavior within cells such as changes in protein concentration98, post-translation modifications of key proteins82, or ligand binding induced coacervation99. What unites these myriad and seemingly disparate manifestations of aqueous phase behavior is the concept of the miscibility, specifically the miscibility of a two component solution consisting of polymer chains and solvent molecules100. All environmental variables that drive aqueous —LCST or UCST— phase behavior in a test tube or within a cell, at their core, simply do so by altering solvent quality between miscible and immiscible states. These observations suggest a testable hypothesis that membrane less organelle formation in cells is driven by the LCST or UCST phase behavior of their constituent IDP(s) in many, if not all, cases (Figure 2).
Figure 2. Phase Behavior.
(a) Polymers with upper critical solution temperature (UCST) phase behavior transition at a critical point that shifts along the phase boundary. At a constant concentration, a specific temperature is required for phase separation, and at a constant temperature, a specific concentration is required. (b) The shape of the phase diagram changes between systems—either narrowing, broadening, or shifting up and down—with protein sequence and solvent conditions.
Tropoelastin provides a concrete example of how the IDP field has influenced the development of protein polymers. As early as the mid-1950’s —much before formal recognition of IDPs as a unique class of proteins— it was observed that solubilized elastin reversibly phase separates into liquid-like coacervates, and it was postulated that their liquid nature was due to “loose skeins of random kinks or coils” within the protein83. Seminal work by Keeley and coworkers has shown through recombinant expression of different tropoelastin exons that the unstructured hydrophobic domains promote coacervation with their ordered counterparts responsible for the maturation of elastin fibers 13, 101–103. Concurrently, the rise of ELPs inspired by motifs in the unstructured regions of tropoelastin further suggested that the disordered domains were responsible for its coacervation in addition to their contributions to elastin’s material properties65, 101, 104. Their facile synthesis by recombinant expression, and the ability to tune their LCST phase behavior precisely by controlling their composition and sequence led to their widespread application in drug delivery and tissue engineering105–109. Similar to ELPs, minimalist resilin-like motifs have been used as repeat units for RLPs, and have been produced recombinantly to understand their dual —LCST and UCST— phase separation behavior, characterized by a low temperature UCST transition and a high temperature LCST phase transition under physiological conditions 91, 110.
Until recently, the phase behavior of stimulus-responsive protein polymers was only studied in the context of very specific repetitive amino acid motifs, as LCST phase behavior was thought to be restricted to specific sequences, such as (VPGXG)n and its close analogs, such as VPAXG and IPGVG 111, 112. However, recent research on both protein polymers and IDPs that form membrane less organelles clearly demonstrates that disorder, and not sequence, is the most important structural feature that drives aqueous phase behavior103, 113. We have, for example, recently shown that the periodic inclusion of structurally disruptive Gly and Pro residues within protein polymers inspired by elastin and resilin is a common feature of diverse class of protein polymers that exhibit LCST and UCST phase behavior94. On this Pro and Gly rich template, the remaining amino acid composition simply determines whether the polypeptide exhibits LCST or UCST phase separation and the window of temperature that this phase behavior occurs in; these observations then led us to postulate a set of simple design heuristics to encode LCST or UCST phase behavior in protein polymers94. Similarly, recent work on the nephrin intracellular domain confirmed that the overall amino acid composition and its propensity for disorder was a more important determinant of its intracellular phase behavior than its precise amino acid sequence7. Teasing out the relative importance of structural disorder on the phase behavior in membrane less organelles and protein polymers remains an open challenge, but the evidence that structural disorder in these systems promotes phase separation is compelling. Contextualizing protein polymers and membrane less organelles under the umbrella of intrinsic disorder and phase behavior has the potential to motivate collaboration between both fields.
4.0. Opportunities for Collaboration
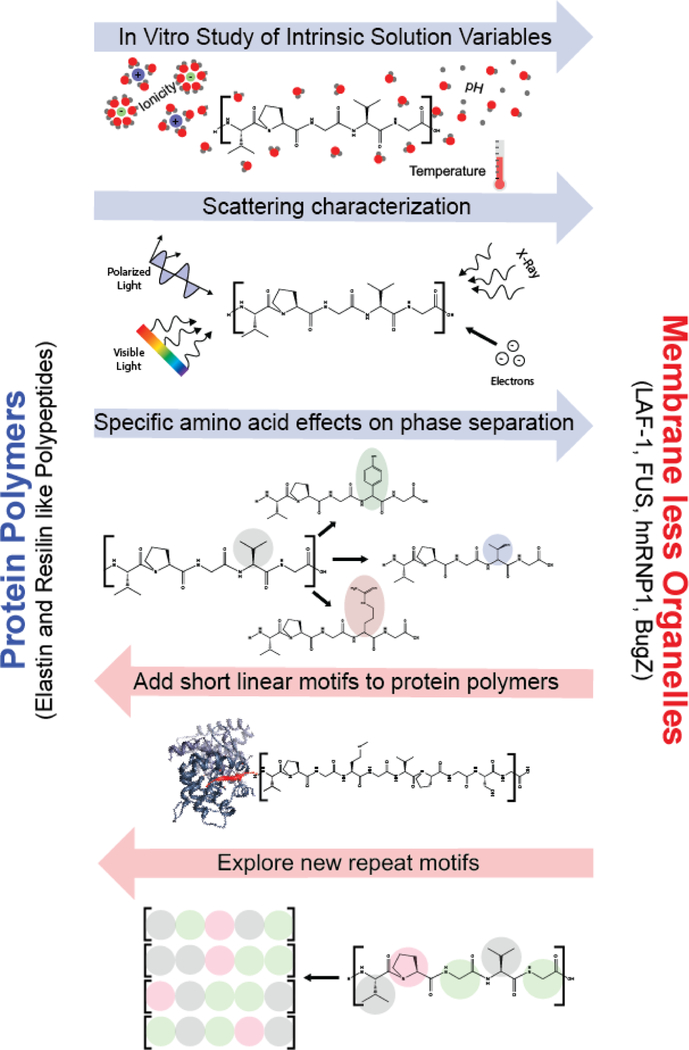
We believe that this is an opportune time for renewed collaboration between materials scientists working on protein polymers and biologists working on membrane less organelles/IDPs. This section highlights directions of inquiry explored in the development of protein polymers that could inform the study of IDPs and, conversely, questions explored by research into IDPs that could inform the protein polymer discipline (Figure 4).
Figure 4. Specific research opportunities for collaboration between researchers working on protein polymers and membrane less organelles.
Scientists studying protein polymers and membrane less organelles have many opportunities to contribute to each other’s disciplines. Protein polymer chemists have adapted the approaches and experimental methodologies of polymer chemistry to study the effect of chain architecture and solution conditions on phase separation. These same techniques could be easily adopted by the membrane less organelle/IDP community to provide new molecular insights into the origins and mechanisms of phase separation of these macromolecules. Likewise, the prevalence of phase separation across biology provides new inspiration for polymer chemists to expand the diversity of motifs that when polymerized will exhibit phase behavior. The recognition of bioactive sequences that are short and disordered (short linear motifs) provides new building blocks for protein polymers.
4.1. Protein Polymers can Inform Membrane less Organelles
A critical component in the development of protein polymers was the recognition that there is immense value in studying proteins outside of their biological context. Although our understanding of membrane less organelles must be relevant to their biological context, a limitation of solely studying them within cells is the complexity of the intracellular environment, coupled to the difficulty of controlling and modulating that environment. Detailed in vitro characterization of artificial peptide polymers has revealed that variations in the concentration of the polymer, the types of other cosolutes and their concentration can affect phase behavior by moving phase boundaries114, 115. Furthermore, we have shown that ligand binding to a protein fused to an LCST peptide polymer such as ELPs can dramatically impact phase behavior116, 117. This is especially relevant to IDPs because there can be considerable spatio-temporal changes in protein expression levels, pH, and ligand concentration within a cell and from cell to cell 118,which can greatly impact their phase behavior119. While the biological complexity of the intracellular environment cannot fully be recapitulated in an in vitro system, in vitro studies can be highly relevant to developing a deeper, physics driven understanding of aqueous demixing phase behavior within cells (Figure 3). For example, a systematic study of how ELP molecular architecture affects protein phase behavior65, 89, 105, informed the mechanistic study of elastin fiber formation —which we now know to be a multi-step process involving the maturation and crosslinking of phase separated coacervates102.
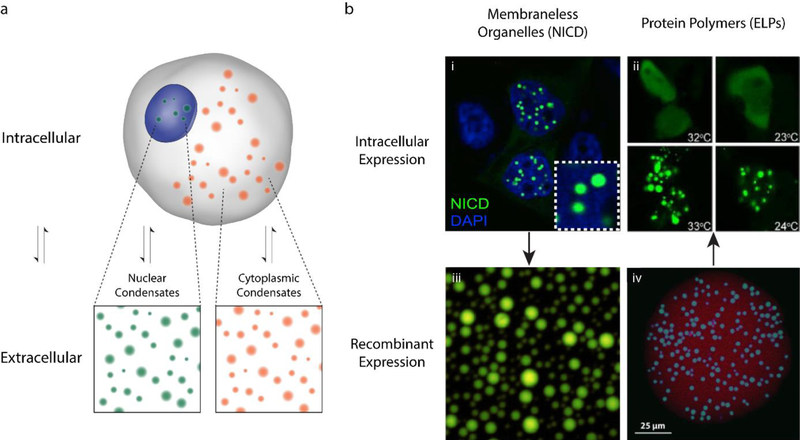
Figure 3. Intracellular and extracellular phase transitions.
(a) Phase behavior is a robust process that largely depends on a small set of variables which readily translate between intracellular and extracellular environments. (b) Recombinantly expressing IDPs responsible for the formation of membrane less organelles, such as (bi/iii) the nephrin intracellular domain (NICD)7, has allowed meaningful investigation into the conditions necessary for intracellular phase separation. Likewise, (bii/iv) elastin-like polypeptides (ELPs), disordered protein peptides historically studied in extracellular environments, retain their phase behavior when constitutively expressed in mammalian cells120, 121. This consistency in behavior opens the door for the de novo design of IDPs with specific intracellular function.
The ability to analyze protein polymers in a simple, well-defined solution environment is predicated on recombinant expression of these polymers and purifying them to homogeneity; fortunately, both ELPs and RLPs are amenable to over-expression in bacterial expression systems. The availability of large amounts of recombinant protein polymers has allowed understanding of their phase behavior within a thermodynamic framework and provides a rational basis for the design of stimulus responsive protein polymers. The phase separation of a polymer in a solvent can be described by three pairwise interactions: polymer-solvent, solvent-solvent, polymer-polymer. As with protein polymers, the phase separation of many IDPs can be mathematically described by the Flory interaction parameter, chi, which accounts for competition among these interactions as a function of temperature6, 82. Because this type of phase behavior is driven by the thermodynamic favorability of the polymer interacting with itself, the complex intracellular environment has to be distilled down to a set of intrinsic variables such as ionicity, pH, and viscosity that impact phase behavior of a single IDP. As demonstrated by the low sequence complexity domains from RNA/protein assemblies73, 81, 122, 123, single protein sequences are often sufficient to drive phase separation. Therefore, binary phase diagrams are potentially informative even in the context of the complex intracellular environment of membrane less organelles that may contain other components.
A critical issue facing the membrane less organelle community is explaining the relative importance of the many intrinsic —protein related — and extrinsic —environment related— variables that influence liquid-liquid phase separation. For example, it is clear that phase separation in some membrane less organelles is driven by ligand binding such as RNA73, which triggers coacervation of the IDP into a membrane less organelle with the cell7, 72, 124, 125. On the other hand, recent reports show that nature also uses many other triggers to drive phase separation within the cell; Sup35 makes pH sensitive droplets that is driven by modulation of the cloud point temperature by a shift in the pKa of the protein126 while TIA-1, exhibits Zn2+ dependent phase separation in the cell127. It is interesting that both pH and Zn2+ concentration have been independently used to drive LCST phase behavior in ELPs. We have previously shown that ELPs with ionizable residues exhibit a sharp pH dependent phase transition128, while protonation of the imidazole side chain of His-containing ELPs as a function of pH in a narrow range for 7.4 to 6.4 causes a drastic shift in the cloud point temperature that enables the LCST phase transition to isothermally triggered by a change of pH in this range. The LCST phase transition of these His-containing ELPs can also can be isothermally triggered by changing the Zn2+ concentration. It is notable that these results predate recent observations that these same mechanisms are used by IDPs to drive droplet formation within the cell127, 129, suggesting that stimulus responsive protein polymers have useful insights to offer the IDP community. What unites the seemingly disparate observations is the likely fact that all IDPs exist near phase boundaries and that Nature has found clever ways of crossing phase boundaries by an intracellular trigger such as binding of a ligand with RNA or metal ions, or by a change in pH.
Another benefit of recombinant expression of IDPs is that it provides enough pure material to allow detailed in vitro analysis by a variety of experimental techniques that can provide a mechanistic understanding of IDP function. In contrast, microscopy has been the dominant technique used to study membrane less organelles within the cell for over 100 years despite the fact that much of their behavior —such as their phase diagrams— cannot be easily elucidated by visible light microscopy in the complex environment of the cell. Biophysical and structural analysis techniques that provide detailed structural information have recently helped to inform work on membrane less organelles include circular dichroism81, NMR124, light scattering72, electron microscopy72, 123, 130, and rheology81.
Detailed biophysical studies of the aqueous phase separation of a large set of LCST and UCST protein polymers can provide useful insights of how amino acid sequence controls phase behavior that may translate to the sequence determinants of the coacervation behavior of IDPs. Conventional thinking suggests that phase behavior of stimulus responsive protein polymers is controlled to a first approximation by the average amino acid composition rather than the specific amino acid sequence7. For example, for a ~30kDa ELP, changing just 5% of the amino acids from valine to alanine—differentiated by a single methyl group—increases the concentration necessary for phase transition at 37˚C by more than an order of magnitude, suggesting that a simple post-translational modification —methylation— could easily trigger the phase behavior of an IDP in vivo106. Furthermore, the distribution of amino acids can also affect both the critical concentration and the architecture of the resultant coacervate in ELPs. This concept has been theoretically explored in the context of IDPs through atomistic simulations on the impact of the distributions of charged amino acids within a polymer chain on the radius of gyration131. In a similar vein, we recently produced a library of ELPs where two guest residues of differing hydrophilicities were precisely distributed throughout the polymer, ranging from alternating copolymer on one end, to blocky copolymers in the middle, and di-block copolymers on the other end of the mixing scale107. Alternating polymers, where the two “monomers” are perfectly mixed along the polymer chain, display classic coacervation phase behavior in response to temperature, whereas polymers with poor mixing of monomers in the polymer chain form of nanoparticles upon undergoing phase separation. These studies suggest that much work remains to be done to understand the role of amino acid composition and distribution on the phase separation behavior of IDPs that form membrane less organelles.
The primary utility of artificial LCST or UCST protein polymers for the IDP field is to provide simple but relevant models to understand how amino acid sequence controls the formation of membrane less organelles in the cell. For example, can the shape of a LCST or UCST phase diagram be predicted from the primary amino acid sequence, enabling de novo prediction of phase behavior of IDPs? Conversely, what lessons can we learn from IDPs to repurpose their intracellular phase separation to create novel compartments within cells? Could wild type IDPs be replaced with engineered versions, without affecting wild type functionality, but that are more resistant to pathogenic structural changes (i.e. liquid to amyloid transition) such as those seen in Alzheimer’s disease? These are just some examples of the new directions that could be explored through collaborative efforts between these two communities.
4.2. Membrane less Organelles can Inform Protein Polymers
Biological structures have long been a source of inspiration for polymer chemists, and IDPs that form membrane less organelles are no exception. In fact, developments in protein-based polymers are only a recent subset of the centuries old field of bio-inspired materials. Since the initial discovery of disordered protein polymers capable of phase separation, until recently, only a handful of sequences from IDPs have been used to create phase separating protein polymers11. This limited set of available sequences is due to researchers working on protein polymers placing a higher priority on manipulating the phase behavior of existing sequences for specific applications over the discovery of new sequences that exhibit phase behavior. Even elastin biopolymers, with their rich history of sequence modifications, have remained largely restricted to polymers of the pentapeptide motifs (VPGXG)n and (VPAXG)n. While working towards the development of design rules for IDPs with phase behavior, we have recently shown that the amino acid sequence space available to biopolymers with LCST or UCST is much larger than previously thought94. This finding is consistent with a growing body of literature on IDPs, which has further uncovered a diverse sequence space that exhibit aqueous phase behavior3, 7. These new sequences provide building blocks for the design of the next generation of stimulus-responsive protein polymers.
Studies on IDPs and membrane less organelles have also revealed the breadth of bioactive domains that can be encoded into IDPs without loss of phase behavior3–5, 132. In the field of LCST protein polymers, the dogma that perturbation of the repeat sequence is likely to abolish phase behavior has largely restricted the incorporation of proteins or peptides to the chain ends of the polymer or to periodic incorporation of very short peptide sequence such as RGD within the polymer sequence that were thought to be minimally disruptive133–137. There are two useful insights from IDPs that are relevant for the design of protein polymers that exhibit aqueous phase behavior that suggest that these restrictions are irrelevant. First, intrinsically disordered proteins have evolved to not only create unique biochemical environments within the cell, but also to actively interact with other macromolecules. These interactions are often controlled by short linear motifs (SLiMs), which, like the RGD peptide, do not rely on a unique three-dimensional structure for their activity and therefore can be easily encoded into disordered proteins132. Second, the entire length of an IDP is frequently not disordered and yet they still exhibit reversible phase behavior that is driven primarily by their intrinsically disordered regions73, 81, 82. These observations suggest that there is considerable flexibility in incorporating extrinsic —folded and functional— domains into artificial protein polymers that will retain their aqueous phase behavior, as long as a critical —but as yet undefined— fraction of the polypeptide chain is unstructured.
5.0. Conclusion
Despite their divergent origins, the underlying physics of membrane less organelles and protein polymers are likely to be similar. Researchers in both fields are working to understand the functional implications of phase separation driven by protein disorder. Until recently, IDPs responsible for membrane less organelles have only been studied within the confines of the intracellular environment through live cell microscopy and mutagenesis. While this approach has been highly informative, a detailed understanding of how intracellular phase behavior can be predicted and controlled remains elusive. On the other hand, the imperatives of industrial applications have driven biomaterial researchers to design disordered protein polymers with sharp, tunable phase boundaries. Reflective of the sentiment of Richard Feynman, “what I cannot create, I do not understand,” we propose that the insights discovered through the bottom-up design of protein polymers may provide the missing link to understanding intracellular phase behavior. Conversely, the diversity of IDPs that exhibit aqueous phase behavior provides inspiration for the design of new protein polymers. It is our hope that increased collaboration across these two fields will eventually lead to their coalescence.
References:
- [1].Valentin GG (1837) Repertorium für anatomie und physiologie: Kritische darstellung fremder und ergebnisse eigener forschung, Veit und comp. [Google Scholar]
- [2].Wagner R (1835) Ueber die Geschlechtswerkzeuge der Blutegel und über merkwürdige Eigenschaften ihrer Samenthierchen, Archiv für Anatomie, Physiologie und wissenschaftliche Medicin, 220–223.
- [3].van der Lee R, Buljan M, Lang B, Weatheritt RJ, Daughdrill GW, Dunker AK, Fuxreiter M, Gough J, Gsponer J, Jones DT, Kim PM, Kriwacki RW, Oldfield CJ, Pappu RV, Tompa P, Uversky VN, Wright PE, and Babu MM (2014) Classification of intrinsically disordered regions and proteins, Chem Rev 114, 6589–6631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Uversky VN (2017) Intrinsically disordered proteins in overcrowded milieu: Membrane-less organelles, phase separation, and intrinsic disorder, Curr Opin Struct Biol 44, 18–30. [DOI] [PubMed] [Google Scholar]
- [5].Uversky VN (2017) Protein intrinsic disorder-based liquid-liquid phase transitions in biological systems: Complex coacervates and membrane-less organelles, Adv Colloid Interface Sci 239, 97–114. [DOI] [PubMed] [Google Scholar]
- [6].Brangwynne Clifford P., Tompa P, and Pappu Rohit V. (2015) Polymer physics of intracellular phase transitions, Nature Physics 11, 899–904. [Google Scholar]
- [7].Pak CW, Kosno M, Holehouse AS, Padrick SB, Mittal A, Ali R, Yunus AA, Liu DR, Pappu RV, and Rosen MK (2016) Sequence Determinants of Intracellular Phase Separation by Complex Coacervation of a Disordered Protein, Mol Cell 63, 72–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ratner BD, and Bryant SJ (2004) Biomaterials: where we have been and where we are going, Annu Rev Biomed Eng 6, 41–75. [DOI] [PubMed] [Google Scholar]
- [9].Malafaya PB, Silva GA, and Reis RL (2007) Natural-origin polymers as carriers and scaffolds for biomolecules and cell delivery in tissue engineering applications, Adv Drug Deliv Rev 59, 207–233. [DOI] [PubMed] [Google Scholar]
- [10].Hu X, Cebe P, Weiss AS, Omenetto F, and Kaplan DL (2012) Protein-based composite materials, Materials Today 15, 208–215. [Google Scholar]
- [11].Rauscher S, and Pomes R (2012) Structural disorder and protein elasticity, Adv Exp Med Biol 725, 159–183. [DOI] [PubMed] [Google Scholar]
- [12].Balu R, Knott R, Cowieson NP, Elvin CM, Hill AJ, Choudhury NR, and Dutta NK (2015) Structural ensembles reveal intrinsic disorder for the multi-stimuli responsive bio-mimetic protein Rec1-resilin, Sci Rep 5, 10896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Muiznieks LD, Weiss AS, and Keeley FW (2010) Structural disorder and dynamics of elastin, Biochem Cell Biol 88, 239–250. [DOI] [PubMed] [Google Scholar]
- [14].Dicko C, Porter D, Bond J, Kenney JM, and Vollrath F (2008) Structural disorder in silk proteins reveals the emergence of elastomericity, Biomacromolecules 9, 216–221. [DOI] [PubMed] [Google Scholar]
- [15].Vrhovski B, and Weiss AS (1998) Biochemistry of tropoelastin, Eur J Biochem 258, 1–18. [DOI] [PubMed] [Google Scholar]
- [16].Morawetz H (2002) Polymers: The Origins and Growth of a Science, Dover Publications. [Google Scholar]
- [17].Faraday M (1826) Quarterly Journal of Science, Literature and the Arts, Royal Institution of Great Britain 21, 19. [Google Scholar]
- [18].Fox CJJ (1928) Die chemie der zellulose und three begleiter. By K. Hess. With a chapter by J. R. Katz, and an appendix by R. Haller. Pp. xx+836. Leipzig: Aeademische verlagsgesellschaft, m.b.H., 1928. Paper, 57 rm.; bound, 59 rm, Journal of the Society of Chemical Industry 47, 1165–1166. [Google Scholar]
- [19].Mahowald AP (1962) Fine structure of pole cells and polar granules inDrosophila melanogaster, Journal of Experimental Zoology 151, 201–215. [Google Scholar]
- [20].Kraemer EO, and Lansing WD (1933) The Molecular Weight of Linear Macromolecules by Ultracentrifugal Analysis. I. Polymeric ι-Hydroxydecanoic Acid1, Journal of the American Chemical Society 55, 4319–4326. [Google Scholar]
- [21].Stein RS, and Doty P (1946) A Light Scattering Investigation of Cellulose Acetate1, Journal of the American Chemical Society 68, 159–167. [Google Scholar]
- [22].Volkenstein MV (1951) Linear polymer as a mixture of rotamers, Doklady Akademii Nauk SSSR 78. [Google Scholar]
- [23].Flory PJ (1942) Thermodynamics of High Polymer Solutions, The Journal of Chemical Physics 10, 51–61. [Google Scholar]
- [24].Huggins ML (1942) Thermodynamic Properties of Solutions of Long-Chain Compounds, Annals of the New York Academy of Sciences 43, 1–32. [Google Scholar]
- [25].Mark H, and Meyer KH (1928) The Structure of the Crystallized Components of Cellulose., Chemische Berichte 61, 1939. [Google Scholar]
- [26].Fischer E (1894) Einfluss der Configuration auf die Wirkung der Enzyme, Berichte der deutschen chemischen Gesellschaft 27, 2985–2993. [Google Scholar]
- [27].Babu MM (2016) The contribution of intrinsically disordered regions to protein function, cellular complexity, and human disease, Biochem Soc Trans 44, 1185–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Berzelius J (1832) Jahresber. Fortsch. Phys. Wissensch 11, 44. [Google Scholar]
- [29].Demselben. (1839) Ueber den flüssigen Storax (Styrax liquidus), Annalen der Pharmacie 31, 265–277. [Google Scholar]
- [30].Kekulé A (1858) Ueber die Constitution und die Metamorphosen der chemischen Verbindungen und über die chemische Natur des Kohlenstoffs, Annalen der Chemie und Pharmacie 106, 129–159. [Google Scholar]
- [31].Pasteur L, and Paris S c. d. (1861) Leçons de chimie professées en 1860, Librairie de L. Hachette et Cie. [Google Scholar]
- [32].Kekuié A (1878) Nature 18, 210. [Google Scholar]
- [33].Ritter R (1890) Die Entwicklung der Geschlechtsorgane und des Darmes bei Chiromomus, Zeit. furr Wiss. Zool. Bd 50, 408–427. [Google Scholar]
- [34].Weismann A (1893) Das Keimplasma: eine Theorie der Vererbung, 1. [Google Scholar]
- [35].Fischer E, and Raske K (1906) Beitrag zur Stereochemie der 2.5-Diketopiperazine, Berichte der deutschen chemischen Gesellschaft 39, 3981–3995. [Google Scholar]
- [36].Hegner RW (1911) The Germ Cell Determinants in the Eggs of Chrysomelid Beetles, Science 33, 71–72. [DOI] [PubMed] [Google Scholar]
- [37].Nishikawa S, and Ono S (1913) Transmission of X-Rays through Fibrous, Lamellar and Granular Substances, Proceedings of the Tokyo Mathematico-Physical Society 2nd Series 7, 131–138_132. [Google Scholar]
- [38].Svedberg T, and Nichols JB (1923) Determination of Size and Distribution of Size of Particle by Centrifugal Methods, Journal of the American Chemical Society 45, 2910–2917. [Google Scholar]
- [39].Staudinger HSR; Johner H ; Luthy M ; Kern W ; Russidis D; Schweitzer O (1929) Justus Liebigs Annalen Der Chemi 474, 145. [Google Scholar]
- [40].Knoll M, and Ruska E (1932) Beitrag zur geometrischen Elektronenoptik. I, Annalen der Physik 404, 607–640. [Google Scholar]
- [41].Mark H (1932) Scientia 51. [Google Scholar]
- [42].Bernal JD, and Crowfoot D (1934) X-Ray Photographs of Crystalline Pepsin, Nature 133, 794–795. [Google Scholar]
- [43].Kuhn W (1934) Über die Gestalt fadenförmiger Moleküle in Lösungen, Kolloid-Zeitschrift 68, 2–15. [Google Scholar]
- [44].Fuller CS, Frosch CJ, and Pape NR (1940) X-Ray Examination of Polyisobutylene1, Journal of the American Chemical Society 62, 1905–1913. [Google Scholar]
- [45].Bloch F, Hansen WW, and Packard M (1946) The Nuclear Induction Experiment, Physical Review 70, 474–485. [Google Scholar]
- [46].Purcell EM, Torrey HC, and Pound RV (1946) Resonance Absorption by Nuclear Magnetic Moments in a Solid, Physical Review 69, 37–38. [Google Scholar]
- [47].Karush F (1950) Heterogeneity of the Binding Sites of Bovine Serum Albumin1, Journal of the American Chemical Society 72, 2705–2713. [Google Scholar]
- [48].Pauling L, Corey RB, and Branson HR (1951) The structure of proteins: Two hydrogen-bonded helical configurations of the polypeptide chain, Proceedings of the National Academy of Sciences 37, 205–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].André J, and Rouiller C (1957) THE ULTRASTRUCTURE OF THE VITELLINE BODY IN THE OOCYTE OF THE SPIDER TEGENARIA PARIETINA, The Journal of Biophysical and Biochemical Cytology 3, 977–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Saunders M, Wishnia A, and Kirkwood JG (1957) The Nuclear Magnetic Resonance Spectrum of Ribonuclease1, Journal of the American Chemical Society 79, 3289–3290. [Google Scholar]
- [51].Koshland DE (1958) Application of a Theory of Enzyme Specificity to Protein Synthesis, Proceedings of the National Academy of Sciences of the United States of America 44, 98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Alder BJ, and Wainwright TE (1959) Studies in Molecular Dynamics. I. General Method, The Journal of Chemical Physics 31, 459–466. [Google Scholar]
- [53].WEIS-FOGH T (1960) A Rubber-Like Protein in Insect Cuticle, Journal of Experimental Biology 37, 889–907. [Google Scholar]
- [54].Minsky M (1961) Microscopy apparatus, Google Patents. [Google Scholar]
- [55].Romanes GA (1962) The Cell and the Organism, Quarterly Journal of Experimental Physiology and Cognate Medical Sciences 47, 100–101. [Google Scholar]
- [56].De Rosier DJ, and Klug A (1968) Reconstruction of Three Dimensional Structures from Electron Micrographs, Nature 217, 130–134. [DOI] [PubMed] [Google Scholar]
- [57].Hart RG (1968) Electron Microscopy of Unstained Biological Material: The Polytropic Montage, Science 159, 1464–1467. [DOI] [PubMed] [Google Scholar]
- [58].Anfinsen CB (1973) Principles that Govern the Folding of Protein Chains, Science 181, 223–230. [DOI] [PubMed] [Google Scholar]
- [59].Foster JA, Bruenger E, Gray WR, and Sandberg LB (1973) Isolation and amino acid sequences of tropoelastin peptides, J Biol Chem 248, 2876–2879. [PubMed] [Google Scholar]
- [60].Sandberg LB, Gray WR, and Bruenger E (1972) Structural studies of alanine- and lysine-rich regions of porcine aortic tropoelastin, Biochimica et Biophysica Acta (BBA) - Protein Structure 285, 453–458. [DOI] [PubMed] [Google Scholar]
- [61].Waring GL, Allis CD, and Mahowald AP (1978) Isolation of polar granules and the identification of polar granule-specific protein, Dev Biol 66, 197–206. [DOI] [PubMed] [Google Scholar]
- [62].Karplus M, and McCammon JA (1979) Protein structural fluctuations during a period of 100 ps, Nature 277, 578–578. [DOI] [PubMed] [Google Scholar]
- [63].Strome S, and Wood WB (1983) Generation of asymmetry and segregation of germ-line granules in early C. elegans embryos, Cell 35, 15–25. [DOI] [PubMed] [Google Scholar]
- [64].Henderson R, Baldwin JM, Ceska TA, Zemlin F, Beckmann E, and Downing KH (1990) Model for the structure of bacteriorhodopsin based on high-resolution electron cryo-microscopy, J Mol Biol 213, 899–929. [DOI] [PubMed] [Google Scholar]
- [65].Urry DW, Gowda DC, Parker TM, Luan CH, Reid MC, Harris CM, Pattanaik A, and Harris RD (1992) Hydrophobicity scale for proteins based on inverse temperature transitions, Biopolymers 32, 1243–1250. [DOI] [PubMed] [Google Scholar]
- [66].Wootton JC (1994) Non-globular domains in protein sequences: Automated segmentation using complexity measures, Computers & Chemistry 18, 269–285. [DOI] [PubMed] [Google Scholar]
- [67].Frank J, Zhu J, Penczek P, Li Y, Srivastava S, Verschoor A, Radermacher M, Grassucci R, Lata RK, and Agrawal RK (1995) A model of protein synthesis based on cryo-electron microscopy of the E. coli ribosome, Nature 376, 441–444. [DOI] [PubMed] [Google Scholar]
- [68].Romero POZKCRV, J.E. ; Garner E ; Guilliot S ; Dunker AK (1998) Thousands of proteins likely to have long disordered regions, Pacific Symposium Biocomputing 3, 437–448. [PubMed] [Google Scholar]
- [69].Wright PE, and Dyson HJ (1999) Intrinsically unstructured proteins: re-assessing the protein structure-function paradigm, J Mol Biol 293, 321–331. [DOI] [PubMed] [Google Scholar]
- [70].Uversky VN (2002) Natively unfolded proteins: a point where biology waits for physics, Protein Sci 11, 739–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Brangwynne CP, Eckmann CR, Courson DS, Rybarska A, Hoege C, Gharakhani J, Julicher F, and Hyman AA (2009) Germline P granules are liquid droplets that localize by controlled dissolution/condensation, Science 324, 1729–1732. [DOI] [PubMed] [Google Scholar]
- [72].Li P, Banjade S, Cheng HC, Kim S, Chen B, Guo L, Llaguno M, Hollingsworth JV, King DS, Banani SF, Russo PS, Jiang QX, Nixon BT, and Rosen MK (2012) Phase transitions in the assembly of multivalent signalling proteins, Nature 483, 336–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Molliex A, Temirov J, Lee J, Coughlin M, Kanagaraj AP, Kim HJ, Mittag T, and Taylor JP (2015) Phase separation by low complexity domains promotes stress granule assembly and drives pathological fibrillization, Cell 163, 123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Uversky VN, Kuznetsova IM, Turoverov KK, and Zaslavsky B (2015) Intrinsically disordered proteins as crucial constituents of cellular aqueous two phase systems and coacervates, FEBS Lett 589, 15–22. [DOI] [PubMed] [Google Scholar]
- [75].Uversky VN (2014) Introduction to intrinsically disordered proteins (IDPs), Chem Rev 114, 6557–6560. [DOI] [PubMed] [Google Scholar]
- [76].Tompa P, and Fuxreiter M (2008) Fuzzy complexes: polymorphism and structural disorder in protein-protein interactions, Trends Biochem Sci 33, 2–8. [DOI] [PubMed] [Google Scholar]
- [77].Meng F, Uversky VN, and Kurgan L (2017) Comprehensive review of methods for prediction of intrinsic disorder and its molecular functions, Cell Mol Life Sci 74, 3069–3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Babu MM, van der Lee R, de Groot NS, and Gsponer J (2011) Intrinsically disordered proteins: regulation and disease, Curr Opin Struct Biol 21, 432–440. [DOI] [PubMed] [Google Scholar]
- [79].Wright PE, and Dyson HJ (2015) Intrinsically disordered proteins in cellular signalling and regulation, Nat Rev Mol Cell Biol 16, 18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Uversky VN, Oldfield CJ, and Dunker AK (2008) Intrinsically disordered proteins in human diseases: introducing the D2 concept, Annu Rev Biophys 37, 215–246. [DOI] [PubMed] [Google Scholar]
- [81].Elbaum-Garfinkle S, Kim Y, Szczepaniak K, Chen CC, Eckmann CR, Myong S, and Brangwynne CP (2015) The disordered P granule protein LAF-1 drives phase separation into droplets with tunable viscosity and dynamics, Proc Natl Acad Sci U S A 112, 7189–7194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Nott TJ, Petsalaki E, Farber P, Jervis D, Fussner E, Plochowietz A, Craggs TD, Bazett-Jones DP, Pawson T, Forman-Kay JD, and Baldwin AJ (2015) Phase transition of a disordered nuage protein generates environmentally responsive membraneless organelles, Mol Cell 57, 936–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Partridge SM, Davis HF, and Adair GS (1955) The chemistry of connective tissues. 2. Soluble proteins derived from partial hydrolysis of elastin, Biochem J 61, 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Smith DW, Weissman N, and Carnes WH (1968) Cardiovascular studies on copper deficient swine. XII. Partial purification of a soluble protein resembling elastin, Biochem Biophys Res Commun 31, 309–315. [DOI] [PubMed] [Google Scholar]
- [85].Cox BA, Starcher BC, and Urry DW (1974) Communication: Coacervation of tropoelastin results in fiber formation, J Biol Chem 249, 997–998. [PubMed] [Google Scholar]
- [86].Roberts S, Costa S, Schaal J, Simon JR, Dzuricky M, Quiroz FG, and Chilkoti A (2017) 2.5 Elastin-Like Polypeptides ☆, 90–108.
- [87].Urry DW, Starcher B, and Partridge SM (1969) Coacervation of solubilized elastin effects a notable conformational change, Nature 222, 795–796. [DOI] [PubMed] [Google Scholar]
- [88].Indik Z, Yeh H, Ornstein-Goldstein N, Sheppard P, Anderson N, Rosenbloom JC, Peltonen L, and Rosenbloom J (1987) Alternative splicing of human elastin mRNA indicated by sequence analysis of cloned genomic and complementary DNA, Proc Natl Acad Sci U S A 84, 5680–5684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Urry DW, Long MM, Cox BA, Ohnishi T, Mitchell LW, and Jacobs M (1974) The synthetic polypentapeptide of elastin coacervates and forms filamentous aggregates, Biochimica et Biophysica Acta (BBA) - Protein Structure 371, 597–602. [DOI] [PubMed] [Google Scholar]
- [90].Elvin CM, Carr AG, Huson MG, Maxwell JM, Pearson RD, Vuocolo T, Liyou NE, Wong DC, Merritt DJ, and Dixon NE (2005) Synthesis and properties of crosslinked recombinant pro-resilin, Nature 437, 999–1002. [DOI] [PubMed] [Google Scholar]
- [91].Balu R, Dutta NK, Choudhury NR, Elvin CM, Lyons RE, Knott R, and Hill AJ (2014) An16-resilin: an advanced multi-stimuli-responsive resilin-mimetic protein polymer, Acta Biomater 10, 4768–4777. [DOI] [PubMed] [Google Scholar]
- [92].Tamburro AM, Bochicchio B, and Pepe A (2005) The dissection of human tropoelastin: from the molecular structure to the self-assembly to the elasticity mechanism, Pathol Biol (Paris) 53, 383–389. [DOI] [PubMed] [Google Scholar]
- [93].Qin G, Hu X, Cebe P, and Kaplan DL (2012) Mechanism of resilin elasticity, Nat Commun 3, 1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Quiroz FG, and Chilkoti A (2015) Sequence heuristics to encode phase behaviour in intrinsically disordered protein polymers, Nat Mater 14, 1164–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Gandhi A, Paul A, Sen SO, and Sen KK (2015) Studies on thermoresponsive polymers: Phase behaviour, drug delivery and biomedical applications, Asian Journal of Pharmaceutical Sciences 10, 99–107. [Google Scholar]
- [96].Klouda L, and Mikos AG (2008) Thermoresponsive hydrogels in biomedical applications, Eur J Pharm Biopharm 68, 34–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Tian HY, Yan JJ, Wang D, Gu C, You YZ, and Chen XS (2011) Synthesis of thermo-responsive polymers with both tunable UCST and LCST, Macromol Rapid Commun 32, 660–664. [DOI] [PubMed] [Google Scholar]
- [98].Weber SC, and Brangwynne CP (2015) Inverse size scaling of the nucleolus by a concentration-dependent phase transition, Curr Biol 25, 641–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Gianni S, Dogan J, and Jemth P (2014) Deciphering the mechanisms of binding induced folding at nearly atomic resolution: The Phi value analysis applied to IDPs, Intrinsically Disord Proteins 2, e970900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Rubinstein M, and Colby RH (2003) Polymer Physics, Oxford University Press. [Google Scholar]
- [101].Bellingham CM, Lillie MA, Gosline JM, Wright GM, Starcher BC, Bailey AJ, Woodhouse KA, and Keeley FW (2003) Recombinant human elastin polypeptides self-assemble into biomaterials with elastin-like properties, Biopolymers 70, 445–455. [DOI] [PubMed] [Google Scholar]
- [102].Yeo GC, Keeley FW, and Weiss AS (2011) Coacervation of tropoelastin, Adv Colloid Interface Sci 167, 94–103. [DOI] [PubMed] [Google Scholar]
- [103].Reichheld SE, Muiznieks LD, Keeley FW, and Sharpe S (2017) Direct observation of structure and dynamics during phase separation of an elastomeric protein, Proceedings of the National Academy of Sciences of the United States of America 114, E4408–E4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Urry DW, Long MM, Cox BA, Ohnishi T, Mitchell LW, and Jacobs M (1974) The synthetic polypentapeptide of elastin coacervates and forms filamentous aggregates, Biochim Biophys Acta 371, 597–602. [DOI] [PubMed] [Google Scholar]
- [105].Chilkoti A, Dreher MR, and Meyer DE (2002) Design of thermally responsive, recombinant polypeptide carriers for targeted drug delivery, Advanced Drug Delivery Reviews 54, 1093–1111. [DOI] [PubMed] [Google Scholar]
- [106].McDaniel JR, Radford DC, and Chilkoti A (2013) A unified model for de novo design of elastin-like polypeptides with tunable inverse transition temperatures, Biomacromolecules 14, 2866–2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].MacEwan SR, and Chilkoti A (2010) Elastin-like polypeptides: biomedical applications of tunable biopolymers, Biopolymers 94, 60–77. [DOI] [PubMed] [Google Scholar]
- [108].Nettles DL, Chilkoti A, and Setton LA (2010) Applications of elastin-like polypeptides in tissue engineering, Adv Drug Deliv Rev 62, 1479–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Rodriguez-Cabello JC, Arias FJ, Rodrigo MA, and Girotti A (2016) Elastin-like polypeptides in drug delivery, Adv Drug Deliv Rev 97, 85–100. [DOI] [PubMed] [Google Scholar]
- [110].Lyons RE, Lesieur E, Kim M, Wong DC, Huson MG, Nairn KM, Brownlee AG, Pearson RD, and Elvin CM (2007) Design and facile production of recombinant resilin-like polypeptides: gene construction and a rapid protein purification method, Protein Eng Des Sel 20, 25–32. [DOI] [PubMed] [Google Scholar]
- [111].Tatham AS, and Shewry PR (2002) Comparative structures and properties of elastic proteins, Philos Trans R Soc Lond B Biol Sci 357, 229–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Wright ER, McMillan RA, Cooper A, Apkarian RP, and Conticello VP (2002) Thermoplastic Elastomer Hydrogels via Self-Assembly of an Elastin-Mimetic Triblock Polypeptide, Advanced Functional Materials 12, 149–154. [Google Scholar]
- [113].Lin Y, Currie SL, and Rosen MK (2017) Intrinsically disordered sequences enable modulation of protein phase separation through distributed tyrosine motifs, J Biol Chem. [DOI] [PMC free article] [PubMed]
- [114].Cho Y, Zhang Y, Christensen T, Sagle LB, Chilkoti A, and Cremer PS (2008) Effects of Hofmeister anions on the phase transition temperature of elastin-like polypeptides, J Phys Chem B 112, 13765–13771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Meyer DE, and Chilkoti A (2004) Quantification of the effects of chain length and concentration on the thermal behavior of elastin-like polypeptides, Biomacromolecules 5, 846–851. [DOI] [PubMed] [Google Scholar]
- [116].Hassouneh W, Nunalee ML, Shelton MC, and Chilkoti A (2013) Calcium binding peptide motifs from calmodulin confer divalent ion selectivity to elastin-like polypeptides, Biomacromolecules 14, 2347–2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Kim B, and Chilkoti A (2008) Allosteric actuation of inverse phase transition of a stimulus-responsive fusion polypeptide by ligand binding, J Am Chem Soc 130, 17867–17873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].VanHook AM (2015) Intracellular pH gradient guides cells, Science Signaling 8, ec106–ec106. [Google Scholar]
- [119].Christensen T, Hassouneh W, Trabbic-Carlson K, and Chilkoti A (2013) Predicting transition temperatures of elastin-like polypeptide fusion proteins, Biomacromolecules 14, 1514–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Simon JR, Carroll NJ, Rubinstein M, Chilkoti A, and Lopez GP (2017) Programming molecular self-assembly of intrinsically disordered proteins containing sequences of low complexity, Nat Chem 9, 509–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Pastuszka MK, Janib SM, Weitzhandler I, Okamoto CT, Hamm-Alvarez S, and Mackay JA (2012) A tunable and reversible platform for the intracellular formation of genetically engineered protein microdomains, Biomacromolecules 13, 3439–3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Kato M, Han TW, Xie S, Shi K, Du X, Wu LC, Mirzaei H, Goldsmith EJ, Longgood J, Pei J, Grishin NV, Frantz DE, Schneider JW, Chen S, Li L, Sawaya MR, Eisenberg D, Tycko R, and McKnight SL (2012) Cell-free formation of RNA granules: low complexity sequence domains form dynamic fibers within hydrogels, Cell 149, 753–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Hennig S, Kong G, Mannen T, Sadowska A, Kobelke S, Blythe A, Knott GJ, Iyer KS, Ho D, Newcombe EA, Hosoki K, Goshima N, Kawaguchi T, Hatters D, Trinkle-Mulcahy L, Hirose T, Bond CS, and Fox AH (2015) Prion-like domains in RNA binding proteins are essential for building subnuclear paraspeckles, J Cell Biol 210, 529–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Fromm SA, Kamenz J, Noldeke ER, Neu A, Zocher G, and Sprangers R (2014) In vitro reconstitution of a cellular phase-transition process that involves the mRNA decapping machinery, Angew Chem Int Ed Engl 53, 7354–7359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Banani SF, Rice AM, Peeples WB, Lin Y, Jain S, Parker R, and Rosen MK (2016) Compositional Control of Phase-Separated Cellular Bodies, Cell 166, 651–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Franzmann TM, Jahnel M, Pozniakovsky A, Mahamid J, Holehouse AS, Nuske E, Richter D, Baumeister W, Grill SW, Pappu RV, Hyman AA, and Alberti S (2018) Phase separation of a yeast prion protein promotes cellular fitness, Science 359. [DOI] [PubMed] [Google Scholar]
- [127].Rayman JB, Karl KA, and Kandel ER (2018) TIA-1 Self-Multimerization, Phase Separation, and Recruitment into Stress Granules Are Dynamically Regulated by Zn(2), Cell Rep 22, 59–71. [DOI] [PubMed] [Google Scholar]
- [128].Mackay JA, Callahan DJ, Fitzgerald KN, and Chilkoti A (2010) Quantitative model of the phase behavior of recombinant pH-responsive elastin-like polypeptides, Biomacromolecules 11, 2873–2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Kedersha N, Ivanov P, and Anderson P (2013) Stress granules and cell signaling: more than just a passing phase?, Trends Biochem Sci 38, 494–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Jiang H, Wang S, Huang Y, He X, Cui H, Zhu X, and Zheng Y (2015) Phase transition of spindle-associated protein regulate spindle apparatus assembly, Cell 163, 108–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Das RK, and Pappu RV (2013) Conformations of intrinsically disordered proteins are influenced by linear sequence distributions of oppositely charged residues, Proc Natl Acad Sci U S A 110, 13392–13397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Van Roey K, Uyar B, Weatheritt RJ, Dinkel H, Seiler M, Budd A, Gibson TJ, and Davey NE (2014) Short linear motifs: ubiquitous and functionally diverse protein interaction modules directing cell regulation, Chem Rev 114, 6733–6778. [DOI] [PubMed] [Google Scholar]
- [133].Glassman MJ, Avery RK, Khademhosseini A, and Olsen BD (2016) Toughening of Thermoresponsive Arrested Networks of Elastin-Like Polypeptides To Engineer Cytocompatible Tissue Scaffolds, Biomacromolecules 17, 415–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Betre H, Setton LA, Meyer DE, and Chilkoti A (2002) Characterization of a Genetically Engineered Elastin-like Polypeptide for Cartilaginous Tissue Repair, Biomacromolecules 3, 910–916. [DOI] [PubMed] [Google Scholar]
- [135].Girotti A, Reguera J, Rodriguez-Cabello JC, Arias FJ, Alonso M, and Matestera A (2004) Design and bioproduction of a recombinant multi(bio)functional elastin-like protein polymer containing cell adhesion sequences for tissue engineering purposes, J Mater Sci Mater Med 15, 479–484. [DOI] [PubMed] [Google Scholar]
- [136].Welsh ER, and Tirrell DA (2000) Engineering the Extracellular Matrix: A Novel Approach to Polymeric Biomaterials. I. Control of the Physical Properties of Artificial Protein Matrices Designed to Support Adhesion of Vascular Endothelial Cells, Biomacromolecules 1, 23–30. [DOI] [PubMed] [Google Scholar]
- [137].Heilshorn SC, DiZio KA, Welsh ER, and Tirrell DA (2003) Endothelial cell adhesion to the fibronectin CS5 domain in artificial extracellular matrix proteins, Biomaterials 24, 4245–4252. [DOI] [PubMed] [Google Scholar]