Abstract
Purpose
Lutein, RR-zeaxanthin, and RS-zeaxanthin (L-Z) are antioxidants which can reduce endoplasmic reticulum stress (ERS) and oxidative stress (OS), and ameliorate neurodegenerative diseases. However, their treatment effect in the Pde6brd10 (rd10) mouse model of retinitis pigmentosa (RP) and the underlying cellular mechanisms have not been studied. ERS is an important factor which causes photoreceptor apoptosis. The aim of the current project is to test the treatment effect of L-Z in rd10 mice and to investigate the underlying molecular mechanisms of ERS.
Methods
L-Z (Lutemax 2020, 10 mg/kg) diluted in sunflower oil (SFO, 1 mg/ml) or the same volume of SFO was administrated via gavage from postnatal day 6 (P6) to P20 daily in L-Z group (n=5) or SFO group (n=6) of rd10 mice. At P21, electroretinography (ERG) was performed to show the functional change of retinas. 78 kDa glucose-regulated protein (GRP78) and endoplasmic reticulum protein 29 (ERp29) were tested by western blot and immunostaining.
Results
The ERG amplitudes were larger in the L-Z group than those of the SFO group in all flash luminances of dark-adapted and light-adapted ERG (all p < 0.01). Western blot revealed that GRP78 in the retinas of the L-Z group was significantly downregulated compared to that of the SFO group (p < 0.01). Meanwhile, the retinal ERp29 protein was significantly upregulated in the L-Z treatment group than that of the SFO group (p < 0.01).
Conclusions
L-Z provide protection to the photoreceptors of rd10 mouse model of RP, which is probably associated with the reduction of ERS.
1. Introduction
Retinitis pigmentosa (RP) is an inherited retinal degenerative disease which affects one in 3500-5000 individuals [1]. The common clinical symptoms of RP include night blindness and progressive vision loss from peripheral to central vision due to the degeneration of rod and cone photoreceptors, which could eventually lead to irreversible blindness [2]. However, the exact pathologic mechanism of the photoreceptor death has not been fully understood. It was found that the death of cone as well as rod may be due to OS [3] and ERS [4]. As RP is a kind of disease resulting from mutant genes, increasing knowledge of the causative genes with the associated biochemical pathogenesis have been gained, and gene therapy has emerged as one of the most potential treatments for RP [5, 6]. Recently, the U.S. Food and Drug Administration has approved the first gene therapy for a type of RP, Leber congenital amaurosis, which is caused by a mutant RPE65 gene [7, 8]. However, RP is highly genetically heterogeneous with over 200 mutations linked to more than 60 human genes [9, 10]. Until now, it is still not feasible to correct all RP mutations. Therefore, it is necessary to study pharmacological intervention that targets the common and major cellular signaling pathways of the pathogenesis of RP to control retinal degeneration [11].
Although RP is genetically heterogeneous, apoptosis is widely recognized as the general final pathway for the death of photoreceptors [12]. ERS, caused by excessive misfolded proteins in the ER, could activate the ER unfolded protein response (UPR) and the apoptotic pathways [13–15], which has been associated with neurodegenerative disorders, including Alzheimer's disease and Parkinson's disease [16, 17]. These diseases cause the accumulation of misfolded proteins and ERS, which could eventually lead to the apoptosis of neuronal cells [18]. In addition, a lot of studies provide evidence that ERS is present in many types of photoreceptor degenerations, such as light-induced retinopathy [19], experimental retinal detachment [20], achromatopsia, and RP [21]. In addition, the interventions that ameliorate ERS render protection to photoreceptors and reduce the rate of photoreceptor death [22].
L-Z, the two major xanthophylls, are the oxygenated forms of carotenoids [23] that are usually accumulated in the central retina of human eyes [24], with the protective effect in human and animal models of retinal degeneration [25–31]. However, the mechanism of L-Z in RP has not been clearly elucidated. The main mechanism of the protective effect of L-Z is attributed to the antioxidant ability [32, 33], while the reduction of ERS might be another related mechanism. We previously showed that L-Z ameliorated the ERS in the protection against light-induced retinopathy [34]. The rd10 mouse is a RP model with clear characteristics [35–38], which carries a missense point mutation in exon 13 of the rod phosphodiesterase 6B (Pde6b) gene [36, 37]. The mutations in the same gene also cause human RP [39, 40]. In the present study, the protective effect of L-Z on the retinas of rd10 mice was investigated. Furthermore, the involvement of ERS was also explored to explain the protective effect of L-Z on the retinal degeneration of rd10 retinas.
2. Materials and Methods
2.1. Animals and Experimental Design
The breeding pairs of rd10 mice (Stock Number: 004297) were purchased from the Jackson Laboratory (Bar Harbor, ME). All animal procedures were approved by the Institutional Animal Care and Use Committee of the Cleveland Clinic Foundation and were performed in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research.
L-Z (Lutemax 2020, 10 mg/kg of body weight, OmniActive Health Technologies, Mumbai, India) dissolved in SFO (1 mg/ml, W530285, Sigma, St. Louis, MO) or the same volume of SFO was administered by daily oral gavage from P6 to P20 to rd10 mice in L-Z treatment group (n=5) or SFO group (n=6), respectively. This dose of L-Z was used based on our preliminary data of different doses (data not shown), which is the highest dose without significant adverse effect on the growth of body weight. The mice in each litter were randomly assigned to the L-Z group and the SFO group. At P21, ERG was performed to show the change of retinal function. The order of the mice in the same litter for ERG test was random. 78 kDa glucose-regulated protein (GRP78) and endoplasmic reticulum protein 29 (ERp29) were tested by western blot and immunostaining. The number of the animals used was conservative enough for obtaining the statistical power of 0.8 or higher.
2.2. Electroretinography (ERG)
Before ERG test, the mice were taken out from a cabinet in which the mice were kept for overnight dark adaptation, and were anesthetized with ketamine (80 mg/kg) and xylazine (16 mg/kg) dissolved in saline solution. The pupils were dilated with 1% cyclopentolate HCl, 2.5% phenylephrine HCl, and 1% tropicamide. The corneal surface was anesthetized with 0.5% proparacaine HCl. During the ERG test, the mouse was placed onto a platform with a heating pad in a grounded Faraday cage to keep the body temperature of the anesthetized mouse at 37°C and to reduce electromagnetic interference. The amplitudes of a-wave and b-wave were tested in a series of flash luminances to obtain the luminance-response functions of dark-adapted and light-adapted ERG. In dark-adapted session of ERG test, the flash luminance ranged from -3.6 to 2.1 log cd s/m2. In light-adapted session of ERG test, the flash luminance ranged from -0.8 to 1.9 log cd s/m2. The light-adaptation time was 7 minutes before the first light-adapted ERG was recorded. The a-wave and b-wave amplitudes were used for the evaluation of the retinal function. The a-wave amplitude was measured from the baseline to the amplitude at 8 ms after the onset of flash stimulation. The b-wave amplitude was measured from the a-wave trough to the b-wave peak, using the published procedure [34, 41–46].
2.3. Western Blotting
The same protocol in our previous study [34] was used. Briefly, the retinas were extracted from mouse eyeballs with a cut on the cornea, and were homogenized. The proteins were obtained in the supernatant after centrifugation of the lysates at 16,000 x g for 25 minutes at 4°C and measured by Pierce 660 nm Protein Assay Reagent (Thermo Scientific, Rockford, IL). Equal amounts of protein (15 μg) of each extract in Laemmli Sample buffer were heated for 7 minutes and then electrophoresed on 8-16% gradient sodium dodecyl sulfate- (SDS-) polyacrylamide gel. Afterwards, the proteins were transferred to a polyvinylidene difluoride (PVDF) membrane. The membrane was blocked and then incubated with primary antibodies (anti-GRP78/BiP, 1:300, ab21685 and anti-ERp29, 1:2500, ab11420, Abcam, Cambridge, MA) in Tris buffered saline with Tween 20 (TBST) for 16 hours at 4°C. After washing 3 times with TBST, the membrane was incubated with secondary antibody goat-anti-rabbit Immunoglobulin G- (IgG-) horseradish peroxidase (HRP) (1:7000, sc-2004, Santa Cruz, CA) in TBST for 1 hour at 25°C. The membrane was then washed 3 times with TBST. Finally, the antigen-antibody complexes were detected by the enhanced chemilu minescence-2 (ECL-2, Thermo Scientific, Rockford, CA).
2.4. Immunostaining
The eyeballs were harvested and fixed in 4% paraformaldehyde (PFA) with 1X phosphate buffer saline (PBS, pH 7.4) for 20 minutes and then a hole was punched on the cornea. The eyes were put back to the 4% PFA fixative for additional 4 hours. Afterwards, the eyeballs were immersed in three graded sucrose solutions in 1X PBS in the following order/time: 10% for 1 hour, 20% for 1 hour, and 30% overnight. The eyeballs were then embedded in optimum temperature cutting compound (OCT), flash frozen on dry ice, and stored in −80°C freezer for at least overnight. The eyeballs in OCT were sectioned with a cryostat (Leica CM 1850, Buffalo Grove, IL) to 8 micron sections. Protein expression in situ was tested by immunofluorescence staining. The sections were incubated in 1X PBS containing 5% normal goat serum, 1% bovine serum albumin (BSA), and 0.5% Triton X-100 for 1 hour to block nonspecific binding, followed by incubation with primary antibodies (anti-GRP78/BiP, 1:400, ab21685 and anti-ERp29, 1:500, ab11420, Abcam, Cambridge, MA) overnight at 4°C. After three washes with 1X PBS, the sections of retinas were incubated with secondary antibody goat anti-rabbit IgG H&L Alexa Fluo® 555 (1:600, ab150086, Abcam, Cambridge, MA) for 2 hours at room temperature. After washing with 1X PBS, sections were mounted with VECTASHIELD mounting medium with 4′,6-diamidino-2-phenylindole (DAPI, Vector Laboratories, Burlingame, CA) and examined under a fluorescent microscope (BX61, Olympus, Tokyo, Japan).
2.5. Measurement of ONL Thickness
The DAPI-stained images obtained in the above procedure were used for the measurement of the thickness of outer nuclear layer (ONL). The locations for the measurement of ONL thickness were at 200 μm from the edges of the optic disc on both sides of the optic nerve head. ImageJ 1.48v software (National Institutes of Health, Bethesda, MD) was used for the measurement. In each retina, four sections were used to obtain the average of ONL thickness.
2.6. Statistical Analysis
For the analysis of ERG data, two-way repeated measures analysis of variance (ANOVA) was performed. For the analysis of the other data, one-way ANOVA was performed.
3. Results
3.1. The Effect of L-Z on Retinal Function of rd10 Mice
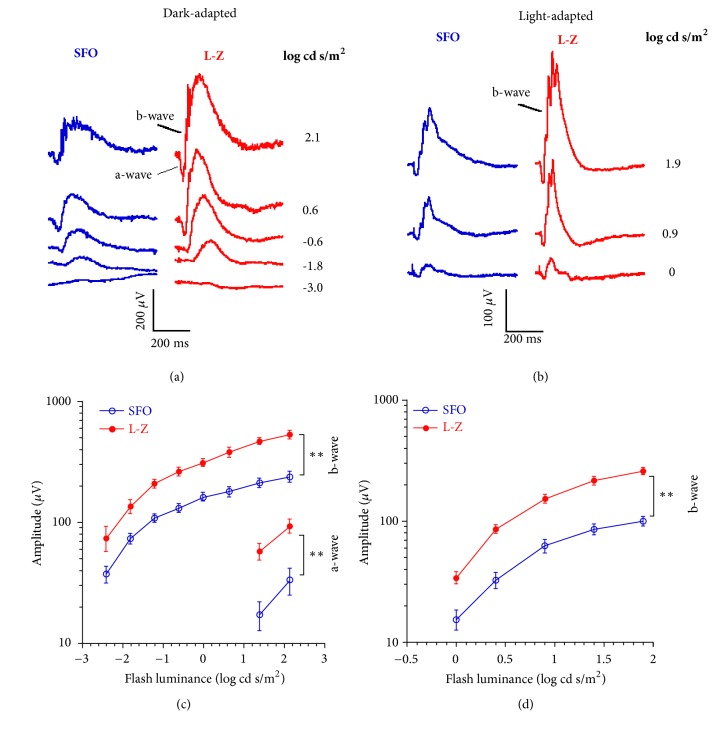
The retinal function of mice was tested by ERG (Figure 1). The ERG waveform typically consists of a-wave followed by b-wave. In dark-adapted ERG, the a-wave is the response from rod or rod/cone photoreceptors, and the b-wave is mainly from bipolar cells of the rod or rod/cone pathways, which is also affected by the output of rod or rod/cone photoreceptors. The b-wave of the light-adapted ERG is from bipolar cells of the cone pathway, which is also affected by the output of cone photoreceptors. Our ERG data showed that the a-wave and b-wave amplitudes of the dark-adapted ERG were significantly larger in the L-Z treatment group than those in the SFO group under the flash luminances we used (all p < 0.01). In addition, the b-wave amplitudes of the light-adapted ERG of the L-Z treatment group were significantly higher than those in the SFO group under all of the flash luminances tested (all p < 0.01).
Figure 1.
ERG results obtained from rd10 mice at P21 treated with L-Z (n = 5) or SFO (n = 6). (a) Typical dark-adapted ERG waveforms from both groups at P21. (b) Typical light-adapted ERG waveforms from both groups at P21. (c) Luminance-response curves of a-wave and b-wave in dark-adapted ERG at P21. (d) Luminance-response curves of b-wave in light-adapted ERG at P21. Under both dark-adapted and light-adapted conditions, the ERG a-wave and b-wave amplitudes were significantly higher in the L-Z treatment group than those in the SFO group at P21 under all of the luminances tested. ∗∗ p < 0.01.
3.2. The Effect of L-Z on ERS in rd10 Mice
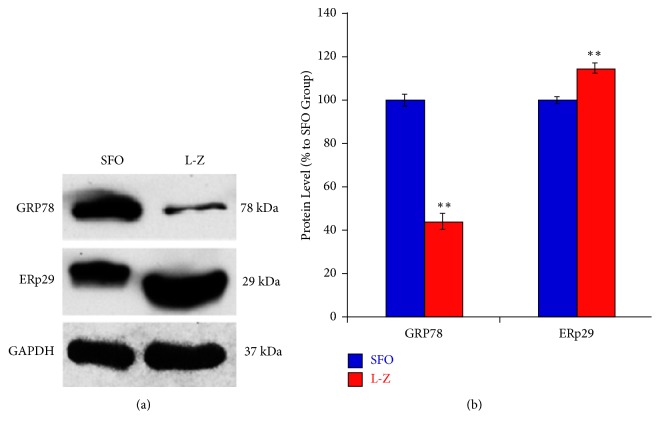
GRP78 acts as the main ER chaperon of the UPR, while the role of ERp29 has not been clearly elucidated in ERS. They were tested by western blot and immunostaining in this study. Our western blot data (Figure 2) revealed that GRP78 in the retina of the L-Z treatment group was significantly downregulated compared to that in the SFO group (p < 0.01). Meanwhile, the retinal ERp29 was significantly upregulated in the L-Z treatment group than that of the SFO group (p < 0.01).
Figure 2.
Western blot results of ER stress protein markers (GRP78 and ERp29) in the L-Z treatment group and the SFO group. (a) Representative images of western blot for GPR78. (b) Average protein expression of GRP78 (n = 3) in both L-Z-treated and SFO groups. ∗∗ p < 0.01 versus SFO group. The GRP78 was significantly downregulated in the L-Z group than that of the SFO group, while the ERp29 was significantly upregulated in the L-Z group compared to that of the SFO group.
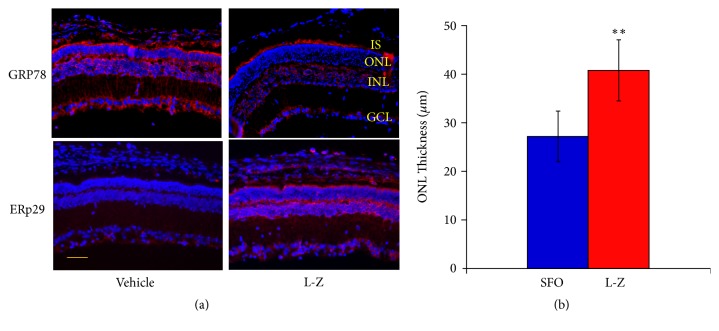
Immunostaining of GRP78 showed that GRP78 protein was expressed mainly in the inner segment (IS), inner nuclear layer (INL), outer plexiform layer (OPL) and ganglion cell layer (GCL) in the SFO group. This result is consistent with the finding of another study [47] and our previous studies [34, 41, 42]. On the other hand, there was a marked downregulation of GRP78 in the above retinal layers in the L-Z treatment group compared to the SFO group (Figure 3). The immunostaining of ERp29 revealed that there was a weak expression of ERp29 presented only in the GCL of the SFO group. However, ERp29 expression was upregulated in IS, OPL, INL and GCL in the retina of the L-Z group compared to the SFO group (Figure 3).
Figure 3.
Immunostaining results of GRP78 and ERp29 in the L-Z treatment group and the SFO group. (a) Representative images (20x) of retinal immunostaining for GRP78 and ERp29. Scale bar indicates 50 μm. The GRP78 was markedly downregulated in several retinal layers in the L-Z group than that of the SFO group, while the ERp29 was markedly upregulated in some retinal layers in the L-Z group compared to that of the SFO group. (b) The ONL thickness (mean ± SD) based on the DAPI staining in these images was significantly increased in the L-Z group than in the SFO group. IS: inner segment; ONL: outer nuclear layer; INL: inner nuclear layer; GCL: ganglion cell layer. n = 3. ∗∗ p < 0.01 versus SFO group.
3.3. The Effect of L-Z on ONL Thickness in rd10 Mice
The ONL thickness (mean ± SD) was measured in the DAPI staining of immunostaining images, which showed significant increase of ONL thickness in the L-Z group than in the SFO group (p < 0.01).
4. Discussion
The present study revealed the protective effect of L-Z on photoreceptor degeneration of rd10 mice, an animal model of retinitis pigmentosa. The ERG results in our study showed that L-Z treatment improved rod and cone functions in rd10 mice, rendering protective effect on both rod and cone photoreceptors.
rd10 mice have long been established as an animal model of RP in several studies. It was observed that at 2 weeks of age, the photoreceptors of rd10 mice already show minor photoreceptor degeneration [48]. By 8 weeks of age, most of the photoreceptors disappear [49]. The progressive photoreceptor degeneration in this strain of mice is attributed to a homozygous point mutation in Pde6b gene [36, 37]. Some mutations in Pde6b gene have also been found in human RP patients [50, 51]. The photoreceptor degeneration in rd10 mice leads to deterioration of the retinal function. The a-wave and b-wave of ERG are still detectable at 3-week-old mice, but these components are not recordable at the age of 5 weeks [52].
The antioxidants used for the treatment in this study are L-Z, which can neutralize reactive oxygen species to counter oxidative damage in retinal cells in many eye disorders [23, 53–56]. In addition, our previous study showed that L-Z reduce ERS in light-induced retinopathy [34].
Endoplasmic reticulum (ER) is the organelle for protein folding and assembly. When the cellular homeostasis is interfered, the protein misfolding or unfolding induces ERS and activates the UPR, which can reduce ERS by increasing ER protein folding capacity, increasing degradation of misfolded proteins, and suppressing the translation of proteins. However, under persistent ERS, the UPR signaling can cause cell death by activating the intrinsic pathway of apoptosis [57–61]. ERS is associated with many retinal diseases, including RP. The amelioration of ERS can reduce photoreceptor degeneration in rd10 mice [41, 42, 62, 63]. In this study, our result illustrated that administration of L-Z reduced the ERS, which was indicated by the downregulation of GRP78, a marker of ERS [64–66].
While our GRP78 test verified that L-Z reduced the ERS, we are also interested in the expression of endoplasmic reticulum protein 29 (ERp29) which was investigated in recent retinal studies [67, 68]. ERp29, ubiquitously expressed in the ER membrane among various tissues and cell types, is an ER luminal protein in all mammals [69, 70]. It plays a pivotal role in modulating the folding and transportation of proteins during ERS [67]. GRP78 is one of the main targets of ERp29 [69]. In addition, ERp29 could also regulate other proteins, such as p38, p58IPK and heat shock protein 27 (Hsp27), for cell survival or apoptosis during ERS [71, 72].
According to previous studies, the change of ERp29 level has at least two phases of either upregulation or downregulation during ERS [68, 73]. Zhang et al. reported that ERp29 expression is beneficial to cellular viability. Overexpression of ERp29 in ARPE-19 cell line with cigarette smoke extract- (CSE-) triggered ERS reduced the number of apoptotic cells, while the inhibition of ERp29 upregulated CSE-triggered CCAAT/enhancer-binding protein homologous protein (CHOP) expression and induced cell apoptosis [68]. The ERS induced by 6-hour, 24-hour and 10-day CSE administration upregulated ERp29. However, during 3-week CSE administration, the expression of ERp29 was downregulated while the GRP78 was still upregulated. Furthermore, a peak level of ERp29 expression was induced by a medium level of CSE in their study [68]. In the study of Park et al., ERp29 was downregulated 1 day after spinal injury and upregulated around 7 days after spinal injury [73]. Therefore, it is possible that the direction of the change of ERp29 level is associated with the level or duration of ERS. In our study, ERp29 was upregulated while the GRP78 was downregulated after the L-Z treatment, which implies that ERp29 may reduce ERS and protect retinal cells. Our result of the SFO group is consistent with the result in the 3-week CSE-induced ERS experiment of Zhang et al. [68]. In the SFO group of rd10 retinas, it is possible that there is long-term ERS. It is not surprising that the high level of GRP78 and the low level of ERp29 were shown in this group. After L-Z treatment, the oxidative stress was reduced in the rd10 retinas, which reduced the ERS indicated by the reduction of GRP78 level [74–78]. In the meantime, the ERp29 level was increased due to the change of ERS level, which in turn downregulated the expression of GRP78 and finally reduced the photoreceptor apoptosis.
5. Conclusions
In conclusion, L-Z prevent photoreceptors from degeneration in rd10 retinas, which is possibly associated with the reduction of ERS. Future studies are necessary to clarify the details of the cellular pathways in which L-Z provide the protective effect against retinal degeneration. Our study reveals the possible target of ERS in photoreceptors for the treatment of retinal degenerations.
Acknowledgments
This research was funded by OmniActive Health Technologies Ltd., India Research Grant, NIH P30EY025585 and Research to Prevent Blindness. In addition, we would like to thank Professor Zuoming Zhang, the Fourth Military Medical University, Xi'an, China, for sending Weiming Yan, who is now a resident in the Department of Ophthalmology, Fuzhou General Hospital of Chinese PLA, to Cleveland Clinic Foundation to participate in this work.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
Authors' Contributions
Minzhong Yu was in charge of the project conception, designing the experiments, applying for the main research grant, organizing and supervising the project, performing of some of the experiments, analyzing and interpreting all of the data, drafting and revising the manuscript. Weiming Yan interpreted all of the data, drafted and revised the manuscript. Craig Beight performed some of the experiments.
References
- 1.Daiger S. P., Sullivan L. S., Bowne S. J. Genes and mutations causing retinitis pigmentosa. Clinical Genetics. 2013;84(2):132–141. doi: 10.1111/cge.12203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verbakel S. K., van Huet R. A., Boon C. J., et al. Non-syndromic retinitis pigmentosa. Progress in Retinal and Eye Research. 2018;66:157–186. doi: 10.1016/j.preteyeres.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Komeima K., Rogers B. S., Lu L., Campochiaro P. A. Antioxidants reduce cone cell death in a model of retinitis pigmentosa. Proceedings of the National Acadamy of Sciences of the United States of America. 2006;103(30):11300–11305. doi: 10.1073/pnas.0604056103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Griciuc A., Aron L., Ueffing M. ER stress in retinal degeneration: A target for rational therapy? Trends in Molecular Medicine. 2011;17(8):442–451. doi: 10.1016/j.molmed.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Schön C., Sothilingam V., Mühlfriedel R., et al. Gene Therapy Successfully Delays Degeneration in a Mouse Model of PDE6A-Linked Retinitis Pigmentosa (RP43) Human Gene Therapy. 2017;28(12):1180–1188. doi: 10.1089/hum.2017.156. [DOI] [PubMed] [Google Scholar]
- 6.Dias M. F., Joo K., Kemp J. A., et al. Molecular genetics and emerging therapies for retinitis pigmentosa: Basic research and clinical perspectives. Progress in Retinal and Eye Research. 2018;63:107–131. doi: 10.1016/j.preteyeres.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Takkar B., Bansal P., Venkatesh P. Leber’s Congenital Amaurosis and Gene Therapy. The Indian Journal of Pediatrics. 2018;85(3):237–242. doi: 10.1007/s12098-017-2394-1. [DOI] [PubMed] [Google Scholar]
- 8.Kumaran N., Michaelides M., Smith A. J., Ali R. R., Bainbridge J. W. Retinal gene therapy. British Medical Bulletin. 2018;126(1):13–25. doi: 10.1093/bmb/ldy005. [DOI] [PubMed] [Google Scholar]
- 9.Ali M. U., Rahman M. S. U., Cao J., Yuan P. X. Genetic characterization and disease mechanism of retinitis pigmentosa; current scenario. 3 Biotech. 2017;7(4) doi: 10.1007/s13205-017-0878-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simpson D. A., Clark G. R., Alexander S., Silvestri G., Willoughby C. E. Molecular diagnosis for heterogeneous genetic diseases with targeted high-throughput DNA sequencing applied to retinitis pigmentosa. Journal of Medical Genetics. 2011;48(3):145–151. doi: 10.1136/jmg.2010.083568. [DOI] [PubMed] [Google Scholar]
- 11.Yang H., Fu Y., Liu X., et al. Role of the sigma-1 receptor chaperone in rod and cone photoreceptor degenerations in a mouse model of retinitis pigmentosa. Molecular Neurodegeneration. 2017;12(1) doi: 10.1186/s13024-017-0202-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cottet S., Schorderet D. F. Mechanisms of apoptosis in retinitis pigmentosa. Current Molecular Medicine. 2009;9(3):375–383. doi: 10.2174/156652409787847155. [DOI] [PubMed] [Google Scholar]
- 13.Sano R., Reed J. C. ER stress-induced cell death mechanisms. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 2013;1833(12):3460–3470. doi: 10.1016/j.bbamcr.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sovolyova N., Healy S., Samali A., Logue S. E. Stressed to death - Mechanisms of ER stress-induced cell death. biological chemistry. 2014;395(1):1–13. doi: 10.1515/hsz-2013-0174. [DOI] [PubMed] [Google Scholar]
- 15.Szegezdi E., Logue S. E., Gorman A. M., Samali A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Reports. 2006;7(9):880–885. doi: 10.1038/sj.embor.7400779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu T., Zhang Y., He J., et al. Bajijiasu Ameliorates β-Amyloid-Triggered Endoplasmic Reticulum Stress and Related Pathologies in an Alzheimer’s Disease Model. Cellular Physiology and Biochemistry. 2018;46(1):107–117. doi: 10.1159/000488414. [DOI] [PubMed] [Google Scholar]
- 17.Zeng X.-S., Jia J.-J., Kwon Y., Wang S.-D., Bai J. The role of thioredoxin-1 in suppression of endoplasmic reticulum stress in Parkinson disease. Free Radical Biology & Medicine. 2014;67:10–18. doi: 10.1016/j.freeradbiomed.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 18.Cai Y., Arikkath J., Yang L., Guo M., Periyasamy P., Buch S. Interplay of endoplasmic reticulum stress and autophagy in neurodegenerative disorders. Autophagy. 2016;12(2):225–244. doi: 10.1080/15548627.2015.1121360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shimazawa M., Sugitani S., Inoue Y., Tsuruma K., Hara H. Effect of a sigma-1 receptor agonist, cutamesine dihydrochloride (SA4503), on photoreceptor cell death against light-induced damage. Experimental Eye Research. 2015;132:64–72. doi: 10.1016/j.exer.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 20.Yan Q., Zhu H., Wang F.-H., et al. Inhibition of TRB3 Protects Photoreceptors against Endoplasmic Reticulum Stress-Induced Apoptosis after Experimental Retinal Detachment. Current Eye Research. 2016;41(2):240–248. doi: 10.3109/02713683.2015.1006371. [DOI] [PubMed] [Google Scholar]
- 21.Chan P., Stolz J., Kohl S., Chiang W.-C., Lin J. H. Endoplasmic reticulum stress in human photoreceptor diseases. Brain Research. 2016;1648:538–541. doi: 10.1016/j.brainres.2016.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shirai Y., Mori A., Nakahara T., Sakamoto K., Ishii K. Deferiprone Protects against Photoreceptor Degeneration Induced by Tunicamycin in the Rat Retina. Biological & Pharmaceutical Bulletin. 2015;38(7):1076–1080. doi: 10.1248/bpb.b15-00185. [DOI] [PubMed] [Google Scholar]
- 23.Jia Y.-P., Sun L., Yu H.-S., et al. The pharmacological effects of lutein and zeaxanthin on visual disorders and cognition diseases. Molecules. 2017;22(4, article no. 610) doi: 10.3390/molecules22040610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vishwanathan R., Schalch W., Johnson E. J. Macular pigment carotenoids in the retina and occipital cortex are related in humans. Nutritional Neuroscience. 2016;19(3):95–101. doi: 10.1179/1476830514Y.0000000141. [DOI] [PubMed] [Google Scholar]
- 25.Mozaffarieh M., Sacu S., Wedrich A. The role of the carotenoids, lutein and zeaxanthin, in protecting against age-related macular degeneration: a review based on controversial evidence. Nutrition Journal . 2003;2, article 20 doi: 10.1186/1475-2891-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li B., Rognon G. T., Mattinson T., et al. Supplementation with macular carotenoids improves visual performance of transgenic mice. Archives of Biochemistry and Biophysics. 2018;649:22–28. doi: 10.1016/j.abb.2018.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miranda M., Arnal E., Ahuja S., et al. Antioxidants rescue photoreceptors in rd1 mice: relationship with thiol metabolism. Free Radical Biology & Medicine. 2010;48(2):216–222. doi: 10.1016/j.freeradbiomed.2009.10.042. [DOI] [PubMed] [Google Scholar]
- 28.Kim S. R., Nakanishi K., Itagaki Y., Sparrow J. R. Photooxidation of A2-PE, a photoreceptor outer segment fluorophore, and protection by lutein and zeaxanthin. Experimental Eye Research. 2006;82(5):828–839. doi: 10.1016/j.exer.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 29.Berson E. L., Rosner B., Sandberg M. A., et al. Clinical trial of lutein in patients with retinitis pigmentosa receiving vitamin A. JAMA Ophtalmology. 2010;128(4):403–411. doi: 10.1001/archophthalmol.2010.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scripsema N. K., Hu D.-N., Rosen R. B. Lutein, Zeaxanthin, and meso-Zeaxanthin in the Clinical Management of Eye Disease. Journal of Ophthalmology. 2015;2015 doi: 10.1155/2015/865179.865179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mewborn C., Lindbergh C., Robinson T., et al. Lutein and Zeaxanthin Are Positively Associated with Visual–Spatial Functioning in Older Adults: An fMRI Study. Nutrients. 2018;10(4):p. 458. doi: 10.3390/nu10040458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palombo P., Fabrizi G., Ruocco V., et al. Beneficial long-term effects of combined oral/topical antioxidant treatment with the carotenoids lutein and zeaxanthin on human skin: A double-blind, placebo-controlled study. Skin Pharmacology and Physiology. 2007;20(4):199–210. doi: 10.1159/000101807. [DOI] [PubMed] [Google Scholar]
- 33.Shinojima A., Sawa M., Sekiryu T., et al. A multicenter randomized controlled study of antioxidant supplementation with lutein for chronic central serous chorioretinopathy. Ophthalmologica. 2017;237(3):159–166. doi: 10.1159/000455807. [DOI] [PubMed] [Google Scholar]
- 34.Yu M., Yan W., Beight C. Lutein and Zeaxanthin Isomers Protect against Light-Induced Retinopathy via Decreasing Oxidative and Endoplasmic Reticulum Stress in BALB/cJ Mice. Nutrients. 2018;10(7):p. 842. doi: 10.3390/nu10070842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gargini C., Terzibasi E., Mazzoni F., Strettoi E. Retinal organization in the retinal degeneration 10 (rd10) mutant mouse: a morphological and ERG study. Journal of Comparative Neurology. 2007;500(2):222–238. doi: 10.1002/cne.21144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang B., Hawes N., Pardue M., et al. Two mouse retinal degenerations caused by missense mutations in the β-subunit of rod cGMP phosphodiesterase gene. Vision Research. 2007;47(5):624–633. doi: 10.1016/j.visres.2006.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chang B., Hawes N. L., Hurd R. E., Davisson M. T., Nusinowitz S., Heckenlively J. R. Retinal degeneration mutants in the mouse. Vision Research. 2002;42(4):517–525. doi: 10.1016/S0042-6989(01)00146-8. [DOI] [PubMed] [Google Scholar]
- 38.Starr C. R., Pitale P. M., Gorbatyuk M. Translational attenuation and retinal degeneration in mice with an active integrated stress response. Cell Death & Disease. 2018;9(5) doi: 10.1038/s41419-018-0513-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McLaughlin M. E., Sandberg M. A., Berson E. L., Dryja T. P. Recessive mutations in the gene encoding the β–subunit of rod phosphodiesterase in patients with retinitis pigmentosa. Nature Genetics. 1993;4(2):130–134. doi: 10.1038/ng0693-130. [DOI] [PubMed] [Google Scholar]
- 40.McLaughlin M. E., Ehrhart T. L., Berson E. L., Dryja T. P. Mutation spectrum of the gene encoding the beta subunit of rod phosphodiesterase among patients with autosomal recessive retinitis pigmentosa. Proceedings of the National Acadamy of Sciences of the United States of America. 1995;92(8):3249–3253. doi: 10.1073/pnas.92.8.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kang K., Tarchick M. J., Yu X., Beight C., Bu P., Yu M. Carnosic acid slows photoreceptor degeneration in the Pde6b rd10 mouse model of retinitis pigmentosa. Scientific Reports. 2016;6(1) doi: 10.1038/srep22632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kang K., Yu M. Protective effect of sulforaphane against retinal degeneration in the Pde6rd10 mouse model of retinitis pigmentosa. Current Eye Research. 2017;42(12):1684–1688. doi: 10.1080/02713683.2017.1358371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu M., Kang K., Bu P., et al. Deficiency of CC chemokine ligand 2 and decay-accelerating factor causes retinal degeneration in mice. Experimental Eye Research. 2015;138:126–133. doi: 10.1016/j.exer.2015.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu M., Zou W., Peachey N. S., McIntyre T. M., Liu J. A novel role of complement in retinal degeneration. Investigative Ophthalmology & Visual Science. 2012;53(12):7684–7692. doi: 10.1167/iovs.12-10069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu M., Sturgill-Short G., Ganapathy P., Tawfik A., Peachey N. S., Smith S. B. Age-related changes in visual function in cystathionine-beta-synthase mutant mice, a model of hyperhomocysteinemia. Experimental Eye Research. 2012;96(1):124–131. doi: 10.1016/j.exer.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu M., Narayanan S. P., Wang F., Morse E., MacKlin W. B., Peachey N. S. Visual abnormalities associated with enhanced optic nerve myelination. Brain Research. 2011;1374:36–42. doi: 10.1016/j.brainres.2010.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nookala S., Gandrakota R., Wohabrebbi A., et al. In search of the identity of the XAP-1 antigen: A protein localized to cone outer segments. Investigative Ophthalmology & Visual Science. 2010;51(5):2736–2743. doi: 10.1167/iovs.09-4286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pennesi M. E., Michaels K. V., Magee S. S., et al. Long-term characterization of retinal degeneration in rd1 and rd10 mice using spectral domain optical coherence tomography. Investigative Ophthalmology & Visual Science. 2012;53(8):4644–4656. doi: 10.1167/iovs.12-9611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jae S. A., Ahn K. N., Kim J. Y., Seo J. H., Kim H. K., Goo Y. S. Electrophysiological and Histologic Evaluation of the Time Course of Retinal Degeneration in the. The Korean Journal of Physiology & Pharmacology. 2013;17(3):p. 229. doi: 10.4196/kjpp.2013.17.3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shen S., Sujirakul T., Tsang S. H. Next-generation sequencing revealed a novel mutation in the gene encoding the beta subunit of rod phosphodiesterase. Ophthalmic Genetics. 2014;35(3):142–150. doi: 10.3109/13816810.2014.915328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Novel mutations in PDE6B causing human retinitis pigmentosa. International Journal of Ophthalmology. 2016 doi: 10.18240/ijo.2016.08.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rösch Sarah, Johnen Sandra, Müller Frank, Pfarrer Christiane, Walter Peter. Correlations between ERG, OCT, and Anatomical Findings in the rd10 Mouse. Journal of Ophthalmology. 2014;2014:10. doi: 10.1155/2014/874751.874751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arunkumar R., Calvo C. M., Conrady C. D., Bernstein P. S. What do we know about the macular pigment in AMD: the past, the present, and the future. Eye (Basingstoke) 2018:1–13. doi: 10.1038/s41433-018-0044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rinsky B. Characterizing the effect of supplements on the phenotype of cultured macrophages from patients with age-related macular degeneration. Mol Vis. 2017;23:889–899. [PMC free article] [PubMed] [Google Scholar]
- 55.Yanai R., Chen S., Uchi S., et al. Attenuation of choroidal neovascularization by dietary intake of ω-3 long-chain polyunsaturated fatty acids and lutein in mice. PLoS ONE. 2018;13(4):p. e0196037. doi: 10.1371/journal.pone.0196037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Neelam K., Goenadi C. J., Lun K., Yip C. C., Au Eong K.-G. Putative protective role of lutein and zeaxanthin in diabetic retinopathy. British Journal of Ophthalmology. 2017;101(5):551–558. doi: 10.1136/bjophthalmol-2016-309814. [DOI] [PubMed] [Google Scholar]
- 57.Kroeger H., Chiang W., Felden J., Nguyen A., Lin J. H. ER stress and unfolded protein response in ocular health and disease. FEBS Journal. doi: 10.1111/febs.14522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shaheen A. Effect of the unfolded protein response on ER protein export: a potential new mechanism to relieve ER stress. Cell Stress and Chaperones. 2018;23(5):797–806. doi: 10.1007/s12192-018-0905-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Saito A., Imaizumi K. The broad spectrum of signaling pathways regulated by unfolded protein response in neuronal homeostasis. Neurochemistry International. 2017 doi: 10.1016/j.neuint.2017.06.012. [DOI] [PubMed] [Google Scholar]
- 60.Zhang S. X., Sanders E., Fliesler S. J., Wang J. J. Endoplasmic reticulum stress and the unfolded protein responses in retinal degeneration. Experimental Eye Research. 2014;125:30–40. doi: 10.1016/j.exer.2014.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jing G., Wang J. J., Zhang S. X. ER Stress and Apoptosis: A New Mechanism for Retinal Cell Death. Journal of Diabetes Research. 2012;2012:11. doi: 10.1155/2012/589589.589589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Osada H., Okamoto T., Kawashima H., et al. Neuroprotective effect of bilberry extract in a murine model of photo-stressed retina. PLoS ONE. 2017;12(6):p. e0178627. doi: 10.1371/journal.pone.0178627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li G.-Y., Fan B., Jiao Y.-Y. Rapamycin attenuates visible light-induced injury in retinal photoreceptor cells via inhibiting endoplasmic reticulum stress. Brain Research. 2014;1563:1–12. doi: 10.1016/j.brainres.2014.02.020. [DOI] [PubMed] [Google Scholar]
- 64.Liu H., Qian J., Wang F., et al. Expression of two endoplasmic reticulum stress markers, GRP78 and GADD153, in rat retinal detachment model and its implication. Eye. 2010;24(1):137–144. doi: 10.1038/eye.2009.20. [DOI] [PubMed] [Google Scholar]
- 65.Feng J., Chen X., Sun X., Wang F., Sun X. Expression of endoplasmic reticulum stress markers GRP78 and CHOP induced by oxidative stress in blue light-mediated damage of A2E-containing retinal pigment epithelium cells. Ophthalmic Research. 2014;52:224–233. doi: 10.1159/000363387. [DOI] [PubMed] [Google Scholar]
- 66.Li J., Wang J. J., Yu Q., Wang M., Zhang S. X. Endoplasmic reticulum stress is implicated in retinal inflammation and diabetic retinopathy. FEBS Letters. 2009;583(9):1521–1527. doi: 10.1016/j.febslet.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McLaughlin T., Falkowski M., Wang J. J., Zhang S. X. Retinal Degenerative Diseases. Vol. 1074. Cham: Springer International Publishing; 2018. Molecular Chaperone ERp29: A Potential Target for Cellular Protection in Retinal and Neurodegenerative Diseases; pp. 421–427. (Advances in Experimental Medicine and Biology). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huang C., Wang J. J., Jing G., et al. Erp29 attenuates cigarette smoke extract–induced endoplasmic reticulum stress and mitigates tight junction damage in retinal pigment epithelial cells. Investigative Ophthalmology & Visual Science. 2015;56(11):6196–6207. doi: 10.1167/iovs.15-16795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gao J., Zhang Y., Wang L., et al. Endoplasmic reticulum protein 29 is involved in endoplasmic reticulum stress in islet beta cells. Molecular Medicine Reports. 2016;13(1):398–402. doi: 10.3892/mmr.2015.4527. [DOI] [PubMed] [Google Scholar]
- 70.Sargsyan E., Baryshev M., Backlund M., Sharipo A., Mkrtchian S. Genomic organization and promoter characterization of the gene encoding a putative endoplasmic reticulum chaperone, ERp29. Gene. 2002;285(1-2):127–139. doi: 10.1016/S0378-1119(02)00417-1. [DOI] [PubMed] [Google Scholar]
- 71.Gao D., Bambang I. F., Putti T. C., Lee Y. K., Richardson D. R., Zhang D. ERp29 induces breast cancer cell growth arrest and survival through modulation of activation of p38 and upregulation of ER stress protein p58 IPK. Laboratory Investigation. 2012;92(2):200–213. doi: 10.1038/labinvest.2011.163. [DOI] [PubMed] [Google Scholar]
- 72.Chen S., Zhang D. Friend or foe: Endoplasmic reticulum protein 29 (ERp29) in epithelial cancer. FEBS Open Bio. 2015;5:91–98. doi: 10.1016/j.fob.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Park S., Hwang H., Lee Y., You K., Shin K., Kwon O. Expression of endoplasmic reticulum chaperone ERP29 in the injured spinal cord. Korean Journal of Biological Sciences. 2010;7(3):265–269. doi: 10.1080/12265071.2003.9647714. [DOI] [Google Scholar]
- 74.Cao S. S., Kaufman R. J. Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease. Antioxidants & Redox Signaling. 2014;21(3):396–413. doi: 10.1089/ars.2014.5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chong W. C., Shastri M. D., Eri R. Endoplasmic reticulum stress and oxidative stress: A vicious nexus implicated in bowel disease pathophysiology. International Journal of Molecular Sciences. 2017;18(4) doi: 10.3390/ijms18040771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Patel S., Sharma D., Kalia K., Tiwari V. Crosstalk between endoplasmic reticulum stress and oxidative stress in schizophrenia: The dawn of new therapeutic approaches. Neuroscience & Biobehavioral Reviews. 2017;83:589–603. doi: 10.1016/j.neubiorev.2017.08.025. [DOI] [PubMed] [Google Scholar]
- 77.Amodio Giuseppina, Moltedo Ornella, Faraonio Raffaella, Remondelli Paolo. Targeting the Endoplasmic Reticulum Unfolded Protein Response to Counteract the Oxidative Stress-Induced Endothelial Dysfunction. Oxidative Medicine and Cellular Longevity. 2018;2018:13. doi: 10.1155/2018/4946289.4946289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu A.-X., He W.-H., Yin L.-J., et al. Sustained endoplasmic reticulum stress as a cofactor of oxidative stress in decidual cells from patients with early pregnancy loss. The Journal of Clinical Endocrinology & Metabolism. 2011;96(3):E493–E497. doi: 10.1210/jc.2010-2192. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.