To the Editor,
The gut microbiome is a dynamic population of microorganisms that modulate both local and systemic inflammatory responses [1–3]. Antibiotic (ATB)-induced alterations in the intestinal microbiota have been characterized with expansion in the relative abundance of specific species having been noted [4]. Interest in the effect of the microbiome and ATB-induced dysbacteriosis in oncologic treatment outcomes has heightened with the widespread use of immune checkpoint inhibitors (ICI). Previous reports suggest a potential relevance of microbiologic factors in the clinical activity of these drugs [1, 3, 5]. Derosa et al. [1] recently demonstrated that ATB use within 30 days of beginning ICI had a negative impact on progression-free survival (PFS) and overall survival (OS) in patients with renal cell carcinoma (RCC) and non-small-cell lung cancer (NSCLC) treated with ICI. Whether ATB use is associated with PFS and OS in patients with other advanced cancers treated on ICI remains unclear.
We recently published on the development of a prognostic scoring system for patients with advanced cancer enrolled in ICI phase I clinical trials [6]. Among those patients, we investigated the impact of ATB use on outcomes across tumor types in patients treated on phase I ICI trials at the MD Anderson Center from January 2013 to November 2015. Groups were compared for rate of primary progressive disease (PD), PFS, and OS. OS and PFS were estimated by the Kaplan–Meier method, and statistical significance was determined by the log-rank test.
A total of 172 patients were analyzed (105 CTLA-4 based; 67 PD-1 based). Median age was 60.0 years, 49% were female. Of all patients analyzed, 38.9% were treated with ICI as a single-agent, and the remaining received combination therapy (26.7% with radiation, 18.6% with targeted therapy, and 15.7% with additional immunotherapy agents).
Most common tumor types treated included RCC (14.5%), NSCLC (12.2%), melanoma (9.4%), sarcoma (9.4%), and gastrointestinal stromal tumors (5.8%). Twenty-six patients were not assessable for response; 1 patient experienced a CR and 13 patients achieved a PR (ORR = 9.5%), 57 had PD and 75 had SD as the best response. Overall, 57 patients used ATB—54 while on trial (all 54 used PO, 23 used IV as well), 19 patients in the 30-day before treatment and 14 patients 30–60 days preceding first treatment. The most commonly used ATB were quinolones (n = 39), β-lactams (n = 26), and tetracyclines (n = 7).
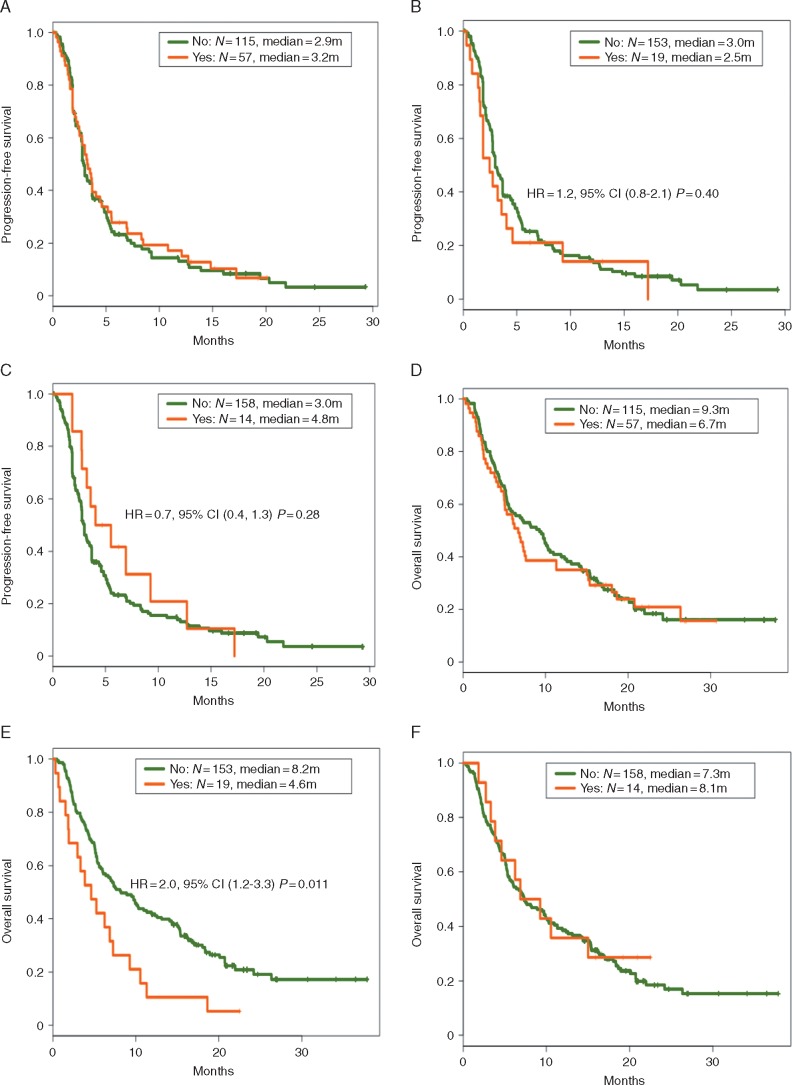
There was no difference in the rate of primary PD with ATB use within 30 days of ICI (43% without ATB, 25% with ATB; P = 0.22) or with ATB use within 30–60 days of ICI (41% without ATB, 23% with ATB, P = 0.20). In addition, no difference was observed in PFS with ATB at all time points (Figure 1A–C). OS was significantly decreased in patients that received ATB in the 30-day before ICI initiation (Figure 1E).
Figure 1.
PFS and OS in patients treated with immune checkpoint inhibitors (ICI) in phase I clinical trials by antibiotic (ATB) use. There was no difference in PFS in patients that used ATB compared with those who did not, regardless of timing of ATB use (A, use during ICI use; B, use within 30 days of ICI; C, use 30–60 days before ICI). OS was significantly worse in patients that used ATB within 30 days of ICI use (E); no difference in OS for patients that used ATB during ICI use (D) or 30–60 days before ICI (F).
Our findings identify no significant difference in PFS or the rate of primary PD in patients treated on phase I ICI trials who had concurrent ATB use compared with those who did not use ATB. However, as previously reported with RCC and NSCLC, ATB use within 30 days of initiating ICI was associated with worse OS. Our results highlight the complexity of the microbiome–immune system interactions and suggest that ATB use within 30 days prior enrolling on ICI phase I trials may impact survival. Future studies are warranted to elucidate the relationship of ATB use with survival in a histology-dependent manner as these findings may have significant implications for future ICI drug development.
Acknowledgments
Funding
The University of Texas MD Anderson Cancer Center is supported by the National Institutes of Health Cancer Center Support Grant CA016672. The University of Texas MD Anderson Cancer Center clinical trials program is supported in part by Cancer Prevention Research Institute of Texas Grant RP110584 and National Center for Advancing Translational Sciences Grant UL1 TR000371 (Center for Clinical and Translational Sciences).
Disclosure
VS receives research funding for clinical trials from LOXO oncology Roche/Genentech, Novartis, Bayer, GSK, Nanocarrier, Vegenics, Northwest Biotherapeutics, Berghealth, Incyte, Fujifilm, Pharmamar, D3, Pfizer, Multivir, Amgen, Abbvie, and Blueprint medicines. All remaining authors have declared no conflicts of interest.
References
- 1. Derosa L, Hellmann MD, Spaziano M. et al. Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non-small-cell lung cancer. Ann Oncol 2018; 29(6): 1437–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gilbert JA, Blaser MJ, Caporaso JG. et al. Current understanding of the human microbiome. Nat Med 2018; 24(4): 392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vétizou M, Pitt JM, Daillère R. et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 2015; 350(6264): 1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Modi SR, Collins JJ, Relman DA.. Antibiotics and the gut microbiota. J Clin Invest 2014; 124(10): 4212–4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Routy B, Le Chatelier E, Derosa L. et al. Gut microbiome influences efficacy of PD-1–based immunotherapy against epithelial tumors. Science 2018; 359(6371): 91. [DOI] [PubMed] [Google Scholar]
- 6. Sen S, Hess K, Hong DS. et al. Development of a prognostic scoring system for patients with advanced cancer enrolled in immune checkpoint inhibitor phase 1 clinical trials. Br J Cancer 2018; 118(6): 763. [DOI] [PMC free article] [PubMed] [Google Scholar]