Abstract
Congenital hypothyroidism is the most common neonatal endocrine disorder and is primarily caused by developmental abnormalities otherwise known as thyroid dysgenesis (TD). We performed whole exome sequencing (WES) in a consanguineous family with TD and subsequently sequenced a cohort of 134 probands with TD to identify genetic factors predisposing to the disease. We identified the novel missense mutations p.S148F, p.R114Q and p.L177W in the BOREALIN gene in TD-affected families. Borealin is a major component of the Chromosomal Passenger Complex (CPC) with well-known functions in mitosis. Further analysis of the missense mutations showed no apparent effects on mitosis. In contrast, expression of the mutants in human thyrocytes resulted in defects in adhesion and migration with corresponding changes in gene expression suggesting others functions for this mitotic protein. These results were well correlated with the same gene expression pattern analysed in the thyroid tissue of the patient with BOREALIN-p.R114W. These studies open new avenues in the genetics of TD in humans.
Introduction
Thyroid Dysgenesis (TD) occurs in 80–85% of congenital hypothyroidism (CH), the most common neonatal endocrine disorder while the remaining 15–20% are owing to inherited defects of thyroid hormone synthesis called dyshormonogenesis (1). TD includes a large spectrum of developmental anomalies with or without hypothyroidism varying from the absence of thyroid tissue (athyreosis), the presence of ectopic tissue to hypoplasia of an orthotopic gland or hemiagenesis (2). Ectopic thyroid is the most common cause of CH (60%), followed by athyreosis (20%). In contrast, thyroid hypoplasia, hemithyroid and asymmetric thyroid lobes are rare. In human development, the median anlage invaginates from the floor of the foregut starting towards embryonic day 22 (E22) and expresses NKX2-1, PAX8 and FOXE1 (E32-33) (3,4). From E26 onwards, the ultimobranchial bodies develop from the fourth pharyngeal pouch on each side (5). The median and lateral anlagen actively migrate and then fuse, at E44 at the definitive pretracheal position (6). The cells differentiate into thyrocytes expressing thyroglobulin (TG, from 8GW, Gestational Week) and producing T4 (from 11GW) or into C-cells expressing calcitonin (7). The functional unit of the thyroid is represented by the follicles where thyroid hormone synthesis takes place. Defects in any step of thyroid development (such as specification, proliferation, migration, growth, organization, differentiation, and survival) may result in a congenital anomaly and/or impaired hormonogenesis, leading to variable degrees of hypothyroidism. However, TD is not always associated with hypothyroidism (8,9). Mutations in six of genes PAX8, NKX2-1, FOXE1, NKX2-5, TSHR, GLIS3 have been reported in individuals with CH and TD, demonstrating the genetic heterogeneity of this pathology (10–13). These mutations account for less than 5% of patients with TD. TD is usually considered as a sporadic disease, although a prevalence of 2% of familial cases support the Mendelian inheritance but do not exclude other modes of inheritance such as multigenetic, multifactorial as well as epigenetics (2,14,15). To overcome the limitations posed by the rarity of new mutated genes associated with TD, we performed WES (Whole Exome Sequencing) for siblings with congenital hypothyroidism and TD and thereby identified mutations in BOREALIN/CDCA8 as a single-gene responsible for TD. By Sanger sequencing of the BOREALIN gene, we analysed 134 TD cases. We identified two more mutations in patients with CH and TD.
Borealin is a major component of the Chromosomal Passenger Complex (CPC) along with Survivin, Aurora B kinase (AURKB) and INCENP (inner centromere protein) (16,17). The CPC has well-described functions in chromosome segregation and cytokinesis. Interestingly, Borealin is highly expressed during early embryogenesis and this has been attributed to its role in mitosis. Specific roles for Borealin in development and particularly in thyroid development and TD have not been documented. We describe for the first time mutations in BOREALIN in TD and the involvement for this protein in migration and adhesion of human thyrocytes.
Results
Mutations in Borealin and clinical data
Family F1 was a consanguineous French family originating from Sri Lanka and of Tamil ethnicity. Parents were first cousins (I-1, I-2) and had four children sharing three different pathologies (Fig. 1A). One daughter died at four months of leprechaunism (II-2), while two children, one daughter (II-3) and one son (II-4), had hemolytic and uremic syndrome (HUS). Finally, two siblings had TD, one with ectopic thyroid and CH (II-3) and the other one with hemiagenesis and no CH (II-1) (18). The parents (I-1, I-2) were euthyroid but display thyroid morphogenesis alteration: asymmetric thyroid lobes in the mother and nodules in the father. The son (II-4) was euthyroid and has a normally formed and shaped thyroid gland of normal size. Using the recessive model of transmission, the WES revealed mutations potentially responsible for the three pathologies. Firstly, a homozygous frameshift mutation in the sibling with leprechaunism (II-2) was found in INSR (p.G5AfsX60). Secondly, homozygous codon-stop mutation was identified in ADAMSTS13 (p.R1119X) in two children with HUS (II-3, II-4). These two mutated genes (INSR, ADAMTS13) correlated well with the clinical phenotype (19,20). Finally, a missense homozygous mutation was found in CDCA8/BOREALIN (c.443C > T, p.S148F, rs546751848) in two daughters with TD (II-1, II-3) (Table 1). Parents carried the three mutations in the three different genes at the heterozygous state. By WES, the recessive model for TD found two mutated genes. Only the BOREALIN variant was confirmed by Sanger sequencing whereas the other was not. The euthyroid son (II-4) was carrier of the heterozygous BOREALIN mutation. Patients II-1 and II-3 were born at term and II-3 had CH detected by the systematic CH neonatal screening performed in France with initial high TSH level (150 μU/ml), confirmed at day 11 of life: TSH: 382 μU/ml (reference values: 0.3–7), free T3: 2.8 pmol/l (reference values: 2.6–10.8) and free T4: 4 pmol/l (reference values: 9.5–25). Treatment by L-Thyroxine was started at day 12 (8 micrograms/kg/day). TD as thyroid ectopy for II-3 and hemiagenesis for II-1 was diagnosed on scintigraphy and ultrasound respectively. Thyroid ultrasound revealed a defect in thyroid development: an asymmetric thyroid (right lobe: 9.88 cm3, left lobe: 5.49 cm3 according to thyroid ultrasound) for the mother (I-1). Furthermore, thyroid ultrasound showed nodules (measuring 2 mm and 5 mm) in the father (I-2). No BOREALIN mutation was found in the two others consanguineous families in our cohort.
Figure 1.
Molecular genetics. (A) Pedigrees of families with Borealin mutations. Family F1. Characterization of a homozygous missense mutation by WES, c.443C>T, p.S148F, in a consanguineous family F1. Familial pedigree with four children including two siblings with TD and the homozygous mutation (m/m). Parents are heterozygous (m/+) for the mutation. The mother has an asymmetric thyroid lobes and the father has nodules. Mutation and familial segregation are confirmed by Sanger sequencing. Family F2. Characterization of a heterozygous missense mutation, c.530T>G, p.R114Q, identified by Sanger sequencing in the mother and her daughter. The daughter has CH with ectopy and the mother had an asymmetric thyroid and developed later a papillary thyroid cancer. Family F3. Characterization of a heterozygous missense mutation, c.341G>A, p.L177W, found by Sanger sequencing in a girl with CH due to athyreosis and congenital heart defect and in the mother. The mother has nodules (in light gray). Affected individuals are indicated by solid black/gray symbols (black for thyroid ectopy, thyroid hemiagenesis, athyreosis, asymmetric thyroid and light gray for nodules). (B) Schematic representation of the Borealin protein with domain structure. (C). Multiple sequence alignment of Borealin proteins. For the three missense mutations (in gray) conservation across evolution of altered amino acid residues is shown.
Table 1.
Summary of thyroid phenotypes associated with BOREALIN mutations
| p.S148F | p.S148F | p.S148F | p.S148F | p.R114Q | p.R114Q | p.L177W | p.L177W | |
|---|---|---|---|---|---|---|---|---|
| F1, I-1 | F1, I-2 | F1, II-1 | F1, II-3 | F2, I-1 | F2, II-1 | F3, I-1 | F3, II-1 | |
| Shape of the thyroid | Asymmetric thyroid | Nodules | Hemiagenesis thyroid | Ectopic thyroid | Asymmetric thyroid and papillary thyroid cancer | Ectopic thyroid | Nodules | Athyreosis |
| Size of the thyroid | AbN | N | AbN | N | AbN | N | N | AbN |
| Thyroid function | N | N | N | CH | N | CH | N | CH |
N, normal; AbN, Abnormal; CH, congenital hypothyroidism.
In family F2, we identified a heterozygous mutation of BOREALIN, c.341G > A, p.R114Q (rs35565540), in a daughter with CH due to the ectopic thyroid gland (II-1) (Fig 1A and Table 1). CH was diagnosed due to systematic neonatal screening (TSH: 126 μU/ml) and confirmed the first days of life: TSH: 75 μU/ml, free T4: 15.1 pmol/l, free T3: 3 pmol/l. Her mother carried the same heterozygous mutation. She had an euthyroid asymmetric thyroid (right lobe 5.89 cm3, left lobe 3.87cm3) and developed papillary thyroid cancer in the left lobe, diagnosed by thyroid ultrasound as a malignant nodule of 1.5 cm3 and finally confirmed by cytological and histological analysis after surgery. The father was euthyroid with a normally shaped and of normal size thyroid gland and did not carry this mutation. We sequenced the cDNA of the white blood cells and we found the mutation at the heterozygous state in the daughter and in the mother (Supplementary Material, Fig. S1A). No autosomal monoallelic expression of the mutation p.R114Q of BOREALIN was identified in the cDNA of the thyroid tissue of the F2-I-1 by Sanger sequencing (21) (Supplementary Material, Fig. S1B).
In family F3, we identified a heterozygous mutation of BOREALIN, c.530T > G, p.L177W (rs140856315), in a proband with CH and athyreosis (Fig 1A and Table 1). CH was diagnosed during the neonatal period with high TSH levels (200 μU/ml) at diagnosis. Treatment with L-Thyroxine was started at 3 weeks of life. The patient had also a congenital heart defect (double outlet right ventricle with a ventricular septal defect), necessitating surgical correction. The mother carried the same heterozygous mutation (I-1) and she was euthyroid. However, thyroid ultrasound has shown three nodules in the right thyroid lobe for the mother (I-1), two of them measuring 3 mm and one measuring 9 mm. Thyroid ultrasound (size and shape) was normal for the father (I-2) and the brother (II-2), who had also normal thyroid function tests. The brother did not carry the mutation. Father’s DNA was not available.
Mutations Borealin-114, 148 and 177 are situated in a central unstructured region of Borealin that interacts with Shugoshin and HP1 proteins to enhance targeting to the centromere (Fig. 1B) (22,23). Moreover, the three mutations altered amino acid residues that are conserved from chicken to human (Fig. 1C). On ExAC (Exome Aggregation Consortium) database, the mutation Borealin-114 has a reported allele frequency estimated at 0.001368 (genotype frequency in 1000Genomes: 0.001), Borealin-148 an allele frequency at 0.0001411 (genotype frequency in 1000Genomes: 0.00039936) and Borealin-177 a allele frequency at 0.0001153 (unreported genotype frequency). Mutation Borealin-114 (p.R114Q) is predicted to be benign according to Polyphen and SIFT in silico prediction tools, while mutations Borealin-148 (p.S148F) and Borealin-177 (p.L177W) are predicted to be possibly damaging by Polyphen and deleterious by SIFT.
Borealin localizes in thyroid during development
Borealin is mainly expressed in organ development during mitosis (24). Adult tissues expressing Borealin with high proliferation rate are the testis, colon and skin. Borealin expression in others adult tissues is weaker (Human Protein Atlas available from www.proteinatlas.org (25)). By quantitative PCR, we observed mRNA encoding BOREALIN expression in human thyroid at 8 and 12GW (Fig 2A). Low expression was found in adult thyroid tissue. By immunofluorescence, at 8GW, BOREALIN was colocalized with E-cadherin-expressing cells (a marker of epithelial cells) undergoing mitosis (Fig. 2B). At 12GW, while the thyroid was thoroughly differentiated, we observed a clear colocalization of BOREALIN in the nucleus of some TG-expressing cells surrounding colloid in thyroid follicles. Thus, BOREALIN is expressed in human thyroid tissue during development. BOREALIN was also found expressed by immunocytochemistry in human primary thyrocytes in culture, obtained from adult thyroid control tissues (Supplementary Material, Fig. S2, see Plasmids, cells culture and transfection in Materials and Methods).
Figure 2.
Borealin expression during thyroid development. (A) Gene expression by quantitative PCR of Borealin in thyroid tissue at 8GW, 12GW and in adult thyroid, reported to one thyroid tissue at 8GW and Peptidylpropyl isomerase A. Experiments with three tissues by stage. (B) Immunohistofluorescence of the Borealin (A, E, in red), Ecadherin (B, in green) and TG (F, in green) in thyroid tissue at 8 and 12GW. In C, co-staining of Borealin and E-cadherin. In G, co-staining of Borealin and TG. Boxes indicating enlarged areas (D, H).
Mutations in Borealin do not disturb the mitosis
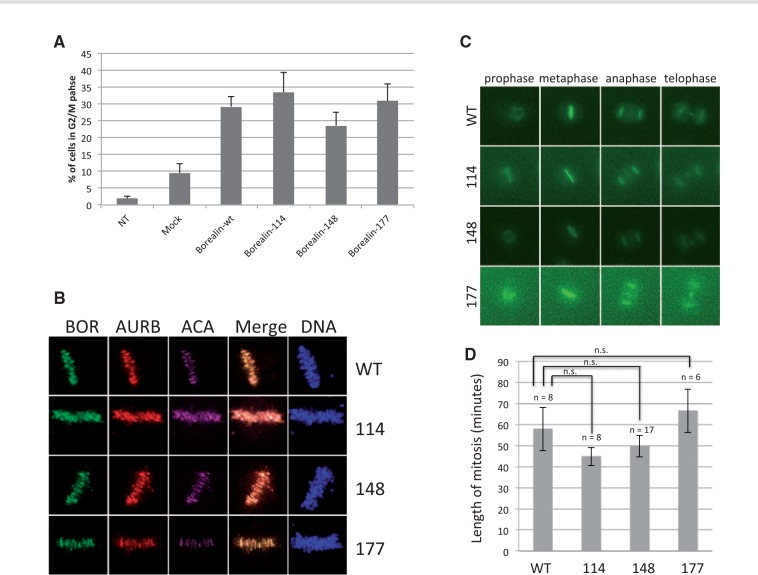
To understand the role of Borealin mutations identified in patients with TD, we studied its known role as a regulator during mitosis. We transfected an immortalized thyroid epithelial cell line, Nthy, with pFlagBorealin wild type (wt) or mutated (114, 148 and 177 mutants) and pSuperBorealin (sh) to inhibit endogenous Borealin to only study the effects of Borealin transfected wt or mutated, as previously done (26) (Supplementary Material, Fig. S3). After propidium iodide (PI) staining, we quantified cell cycle distributions by flow cytometry (Fig. 3A). Expression of Borealin mutants had no effect on the percent of cells in G2/M compared to Borealin-wt (Fig. 3A). Borealin mutants localized normally to centromeres, midzone and midbody, structures where CPC coordinates important mitotic events (Fig. 3B and Supplementary Material, Fig. S4). In addition, overexpression of GFP-tagged Borealin mutants in combination with Borealin shRNA had no effect on the duration of mitosis in HeLa cells (Supplementary Material, Figs 3C and D). Therefore, the three Borealin mutations found in TD patients do not appear to affect the mitotic functions of the protein.
Figure 3.
Effects on mitosis of Borealin mutants. (A). G2/M phases in Nthy transfected with Borealin-wt, Borealin-114, Borealin-148 and Borealin-177. Borealin transfection in cells lead to increase the mitosis. The percentage of cells was determined by flow cytometry. Values are represented as mean ± SEM from three independent measurements. NT, non-transfected; Mock, cells transfected with empty vector; Borealin-wt, Borealin-114, Borealin-148 and Borealin-177 were cells transfected with sh-vector and with vector containing Borealin-wt or mutated. (B) Localization of Borealin mutants during metaphase. HeLa M cells were transiently transfected with GFP-tagged wild-type and mutant Borealin. Single z-planes of representative transfected cells are shown. BOR, Borealin, AURB, Aurora B kinase, ACA, anti-centromere autoantibodies. (C) Time lapse. Examples of cells progressing through mitosis after transfection with wild-type and mutant Borealin-GFP along with Borealin shRNA. (D) Length of mitosis. GFP-positive cells as described in C were tracked from prophase to telophase. Average + SEM are shown.
Borealin mutations decrease cell migration and cell spreading
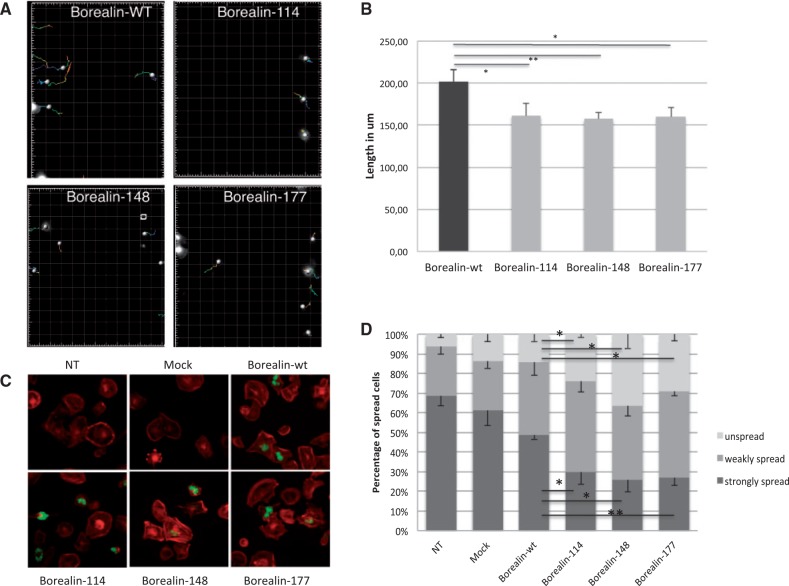
Two patients with the Borealin mutations 114 and 148 had an ectopic thyroid gland and two parents had an asymmetric thyroid gland. Migration of progenitor cells is critical for normal thyroid development (27). Interestingly, we observed that expression of Borealin mutants in a thyroid cell line altered migration and adhesion of these cells, potentially explaining the developmental defects observed. For these studies, we tracked Nthy cells transfected with sh and Borealin-GFP vector (containing Borealin-wt or Borealin-114, Borealin-148, Borealin-177) by microscopy during a time lapse of 8 hours following a wound-healing assay (Fig. 4A). We observed a significant decrease in the length of migration path of Nthy cells with three Borealin mutants compared to Borealin-wt (decrease of 20% for three mutants compared to wt, P < 0.05) (Fig. 4B). Therefore, Borealin mutants alter the migration of Nthy cells. To understand the altered migration ability, we analysed the adhesion of Nthy cells transfected with Borealin-Flag. Cell spreading was observed by immunofluorescence after staining of the actin cytoskeleton with phalloidin (Fig. 4C). Significantly more Nthy cells transfected with Borealin mutants remained unspread compared to Borealin-wt Nthy (respectively with Borealin-114, Borealin-148 and Borealin-177: increase of 74%, 168%, 213% P < 0.05) (Fig. 4D). Furthermore, we observed a weaker spreading of Nthy cells with mutants compared to Borealin-wt Nthy (respectively with Borealin-114, Borealin-148 and Borealin-177: decrease of 36%, 45%, 42%, P < 0.05). Phalloidin experiments showed the same effect for the three mutations at the heterozygous as for homozygous state (Supplementary Material, Fig. S5).
Figure 4.
Modifications in migration and spreading of cells by Borealin mutants. (A) Tracking of GFP-Nthy transfected with Borealin-wt, Borealin-114, Borealin-148 and Borealin-177 during 8 h after wound healing assay on time-lapse video microscopy as described in "Materials and Methods." (B) The length of migration in um was measured by quantifying the total distance that the positively transfected cells (GFP) moved from the edge of the wound toward the center of the wound in 8 h. Mean of the length of the migration from four independent experiments. *P = 0.04, 0.0036, 0.0202 respectively for Nthy with Borealin-114, Borealin-148 and Borealin-148 in comparison with Nthy with Borealin-wt, calculated by t test. (C) Analysis of spreading by immunofluorescence. Staining of Borealin-Flag (in green) and filamentous actin (in red) showed decreased spreading of Borealin-114, Borealin-148, Borealin-177 Nthy compared to Borealin-wt Nthy (NT, no transfected; mock, empty vectors). (D) The percentage of spread and unspread Nthy was estimated by counting Nthy for each cell line (light gray, unspread; medium gray, weakly spread; dark gray, strongly spread). The graph represents the mean five independent experiments. *P< 0.05, P < 0.01 calculated by t test.
The defect in spreading caused by the three Borealin mutants may explain the decrease in cell migration (28).
Impact of Borealin mutations on the expression of genes involved in adhesion/migration
Transcriptome analysis of human thyroid bearing the Borealin-114 mutation
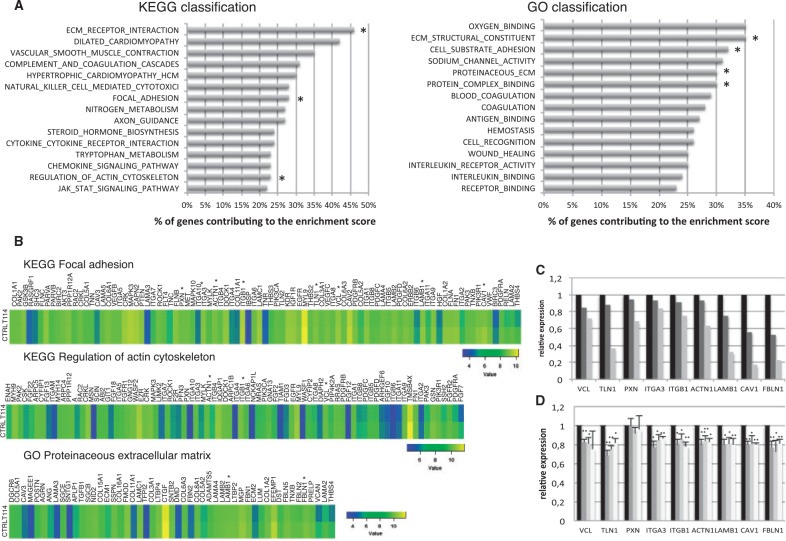
The only known role for Borealin is as part of the CPC, where it influences proper localization of the complex during mitosis. The effects on cell adhesion and migration were not predicted from its known role in mitosis. Borealin does bind to DNA, however the importance of this interaction has not been investigated (29). Given the effects observed with our Borealin mutants, we used transcriptome analysis to determine whether corresponding gene expression changes may explain the effects on cell behavior. We performed transcriptome analysis of thyroid tissue of the mother with asymmetric thyroid lobes (I-1) of the pedigree F2, carrying the mutation 114 compared to three thyroid adult tissues as controls. By an unbiased analysis with GSEA software (Gene Set Enrichment Analysis, Broad Institute), the whole gene expression pattern revealed a decrease in the following pathways: "Focal Adhesion", "Regulation of actin cytoskeleton", "ECM receptor interaction" (Extracellular Matrix) in the KEGG classification and "Proteinaceous extracellular matrix", "Cell substrate adhesion", "Protein complex binding", and "Extracellular Matrix structural constituent" in the GO (Gene Ontology) classification in the mutated Borealin-114 thyroid tissue in comparison with thyroid controls (Fig. 5A). In these pathways, we interestingly observed a decreased expression of VCL, TLN1, TLN2, PXN, CAV1, ITGA3 and ITGB1, directly implicated in focal adhesion compared to thyroid control tissues (Fig. 5B). Decreased expression was also observed for LAMB1, LAMA4, LAMC1, which encode laminins, extracellular matrix glycoproteins implicated in cell adhesion, migration and differentiation. Furthermore, we observed a diminution of ACTN1, a cytoskeletal protein involved in adherens junctions and FBLN1, a glycoprotein protein of the extracellular matrix, playing a role in cell migration and adhesion (Figs 5B and C). VCL, TLN1, PXN, ITGA3, ITGB1, ACTN1, LAMB1, CAV1 and FBLN1 genes, with decreased expression found by transcriptome analysis were validated by quantitative PCR on the same tissues (Fig. 5C).
Figure 5.
Transcriptome analysis of thyroid tissue with Borealin-114 and quantitative PCR in transfected HPT. (A) Genes with decreased expression in transcriptome analysis of the thyroid tissue with Borealin-114 compared to thyroid controls. On the left, KEGG Pathway analysis of expressed mRNAs. Top fifteen enriched down-regulated pathways. On the right, GO enrichment analysis of expressed mRNAs. Top fifteen enriched down-regulated pathways. The bar plot represents the percentage of genes contributing to the enrichment score. *Pathways involved in adhesion and/or migration. (B) Examples of pathways of interest with decreased expression genes in thyroid tissue with Borealin-114 (T114) compared to thyroid tissues control (CTRL): KEGG Focal adhesion, KEGG Regulation of actin cytoskeleton, GO Proteinaceous extracellular matrix. Colour scale for each pathway is shown. From left to right, gene expression difference between control tissues and T114 is increased. *Genes involved in adhesion and/or migration and validated in HPT. (C) Gene expression of Borealin-114 tissue compared to controls in trancriptome analysis and validated by quantitative PCR (black, thyroid controls; medium gray, transcriptome results of Borealin-114 tissue; light gray, validation by quantitative PCR in Borealin-114 tissue). (D) Genes involved in adhesion and/or migration in transfected HPT analysed by quantitative PCR 48 h post-transfection (black, Borealin-wt HPT, dark gray, Borealin-114 HPT; medium gray, Borealin-148 HPT; light gray, Borealin-177 HPT). The graph represents the mean of four independent experiments. *P < 0.05, **P<0.01 calculated by t-test.
Adhesion and migration gene expression in transfected human primary thyrocytes
By quantitative PCR, we analysed gene expression of human primary thyrocytes (HPT) transfected with Borealin-wt or Borealin mutants (114 or 148 or 177) (Fig. 5D). We found a significantly lower expression of VCL in HPT transfected with mutants Borealin-114 and Borealin-148 and a trend in Borealin-177 compared to Borealin-wt. We also observed a significant decrease in TLN1, ITGA3, ITGB1, ACTN1, LAMB1, CAV1 and FBLN1 expression with three mutants (Borealin-114 or Borealin-148 or Borealin-177) compared to HPT transfected with Borealin-wt. No difference was observed with PXN gene expression. These results were confirmed by the strong decrease in protein expression of TLN1 and slight decrease of VCL in transfected HPT of Borealin mutants compared to Borealin-wt (Supplementary Material, Fig. S6). We observed also a decrease of this same set of genes involved in the adhesion and/or the migration in Nthy transfected with Borealin mutants compared to Borealin-wt (Supplementary Material, Fig. S7). Our results in HPT confirmed the results of the transcriptome analysis of the thyroid with Borealin-114. Overall, our results obtained using human thyroid tissue derived from a patient with Borealin mutation or transfected cells indicate a novel involvement of Borealin in cell adhesion and migration. Furthermore, the mutations identified alter adhesion and migration in a manner that may be important in normal thyroid development.
Discussion
We describe here for the first time the impact of BOREALIN mutations as a genetic cause of TD in humans. We have shown that BOREALIN is expressed in thyroid tissue during development and localizes in thyrocytes. Through functional studies in human thyrocytes, we have demonstrated that BOREALIN is involved in the adhesion and in the migration of these cells.
Mutations in Borealin are linked to TD
To date, no Borealin mutation has been linked to human pathology. We identified three Borealin mutations in patients with different types of TD: ectopic thyroid gland, hemithyroid, athyreosis and asymmetric thyroid lobes. BOREALIN-114, 148 and 177 mutations are localized to an unstructured domain implicated in centromere targeting of the CPC. This region binds to Shugoshin, which in turn binds to phosphorylated histone H2A localized to the kinetochore. Of note, L177 also is in a putative chromo shadow domain (CSD) binding motif that has been implicated in binding HP1 protein also to the enhance centromere targeting. However, our imaging experiments show that BOREALIN-114, 148 and 177 are still able to localize to the inner centromere suggesting that the central domain of BOREALIN has alternative functions relevant to TD. Furthermore, cells progressed normally through mitosis upon overexpression of the mutants suggesting no mitotic defects. Phosphorylation of Borealin is critical for its normal function, raising the possibility that p.S148F disrupts phosphorylation. However, phosphorylation of this site has not been reported and is not indicated in phosphor-proteome databases. In any case, no significant effects on mitosis were observed between Nthy or HeLa transfected with Borealin-wt or mutants. Borealin knock out mice died very early during development due to its crucial function during mitosis (24). We can hypothesize that the mutations described here were not in a known crucial domain for the function of Borealin and cannot be loss of function alleles. Any major effects on mitosis would be expected to be lethal or associated with more syndromic features.
Mutations in Borealin altered cell migration
The three BOREALIN mutations identified in patients with TD led to defective migration of transfected Nthy. Migration of progenitors is a critical step during thyroid development. Any perturbation during the migration process can lead to TD (27,30,31).The mechanism is not very well understood but different concomitant actions are crucial for normal thyroid development including active migration of progenitor cells, the effects of surrounding tissues and vessels organization, and the neck elongation (4). Collective cell migration during morphogenesis is also important for thyroid tissue development. Molecular mechanisms of collective migration implicate cell motility, cell-cell adhesion, signalling and ECM (cell-extracellular matrix) remodeling allowing the integrity of cell-cell junctions, cell polarization, organization of the actin cytoskeleton generating traction and protrusion, and the guidance by chemical and physical signals of surrounding environment (32,33).
Accordingly, the decrease in migration and cell adhesion observed in our thyrocytes models are likely to explain the developmental defects in the affected families. Indeed, the decrease in migration shown by tracking of transfected Nthy shows the impact of Borealin mutations on this mechanism. Moreover, the defect in spreading of Nthy transfected with Borealin mutants and the decrease of adhesion molecules TLN1, ITGA3, ITGB1 led to defective adhesion in cells expressing Borealin mutants. We also observed a decrease in the FBLN1 and LAMB1 ECM proteins whose remodeling determines migration. These decrease in molecules involved in the adhesion and in the ECM were obtained by experiments in transfected HPT and by trancriptome analysis of thyroid tissue from the patient with Borealin-114. Indeed, all our experiments to unravel the Borealin function in adhesion/migration were performed in human thyroid context. Therefore, we can speculate that Borealin is necessary for the integrity of adhesion allowing the proper migration of thyrocytes. Nevertheless, the understanding of the link between the Borealin and the molecules involved in adhesion and/or migration remains to be explored and will be the focus of our further work.
Nkx2-1 and Pax8 expression in murine and human stem cells are sufficient to drive development of thyroid follicular cells (34–36). However, very few mutations in these transcription factor genes account for TD. The major difference between the Nkx2-1 and Pax8 driven stem cells and the thyroid organ development is that migration is at play for the latter. Therefore, it is not unexpected to find that migration alteration is responsible for TD.
Phenotypic variability of Borealin mutations
BOREALIN mutated patients had a range of TD, from asymmetric thyroid to ectopic thyroid gland and athyreosis while all three mutants caused a decrease in migration and spreading in our transfection studies. The spectrum of TD showed clearly a variable disease expressivity and penetrance with a dominant mode of inheritance. These types of effects could previously be found for other transcription factors involved in TD such as PAX8 and NKX2-1; affected patients presented sometimes a phenotypic variability, as parents (10,11). These data confirmed that TD, despite different types, represents the same disease and is modulated by epigenetic and environment factors (14,15,37–39). This is in line with recent results on a model of human disease, glaucoma, where it has been shown that the same TEK mutation gave different ocular disorders (40). Hemiagenesis could be linked to the BOREALIN mutation as some authors have considered hemiagenesis as the retarded migration of a hypoplastic thyroid and/or an earlier defect of the lateralization leading to mislobulation (41). This developmental defect could also explain thyroid asymmetry. Furthermore, alterations in cell adhesion may also contribute to this thyroid dysgenesis.
Moreover, heterozygous and homozygous state in transfected cells gave the same adhesion disorder independently of the BOREALIN mutations. Thus, there is no difference in severity of functional effect of BOREALIN mutations. In a way, we found the mutation thanks to a very informative consanguineous family. But the fact is that these BOREALIN mutations are active and explain the phenotype in the heterozygous state.
Monoallelic expression in the thyroid tissue could be one additional explanation for the phenotypic variability (21). However, this could be excluded for the p.R114Q on the thyroid of the mother (Family F2) but we could not test this hypothesis for the remaining patients as no thyroid tissue could be obtained under the ethical rules applied in our country.
To conclude, the WES, performed in a consanguineous family with TD, allowed us to identify a new gene involved in TD, BOREALIN. Two other mutations of BOREALIN in two distinct families with TD were also found. Our experiments showed an impact of Borealin mutants on cell adhesion and migration. These findings broaden the understanding of TD and more particularly highlight the integrity of cell adhesion required for the proper thyroid development. TD is a multigenetic and multifactorial disorder and this new gene should be considered for testing in patients with TD and their families.
Materials and methods
Subjects
Three consanguineous families with at least one child affected by TD and one non-affected child were first analysed. Subsequently, 134 patients with CH and TD were included in the study (69 with ectopic thyroid gland, 31 with athyreosis, 3 with thyroid in situ of normal size, 6 with hypoplastic thyroid gland and 25 with hemiagenesis; 43 boys and 91 girls). The study was approved by the institutional review board.
WES and Sanger sequencing
Genomic DNA was isolated from whole blood. Exome capture and sequencing were performed at the Genomics platform of the Imagine Institute. WES libraries were prepared from 3 µg of genomic DNA per individual, sheared by ultrasonication (Covaris S220 Ultrasonicator, Woburn, USA). Exome capture was performed using the SureSelect Human All Exon V6 kit (Agilent Technologies, Santa Clara, USA). The resulting libraries were sequenced on a HiSeq 2500 HT (Illumina, San Diego, USA) according to the manufacturer’s recommendations. Paired-end (2x130) 76 bp reads were generated and mapped on the human genome reference. More than 97% of the exome was covered at least 30x. Raw data were analysed as described, using an in-house software system (Polyquery) (42). Variant prioritization followed the detailed strategy: (i) selection of functional (protein altering) variants (removal of intergenic and 3’/5’ UTR variants, non-splice related intronic variants, synonymous variants) (ii) variants with a frequency below 1% in public databases (dbSNP, 1000 genomes, EVS, ExAC), (release date April, 2016), and (iii) variants previously identified in less than five individuals from the 7670 in-house exomes. The entire coding exon of CDCA8/Borealin (NM_001256875.1) was amplified by PCR, and DNA sequencing was performed (3500xL Genetic Analyzer, Thermo Fisher Scientific, Waltham, USA). Primers sequences and PCR conditions are available on request. Sequencing of coding and exons-introns boundary regions of NKX2-1, PAX8, FOXE1, TSHR and NKX2-5 was negative for all patients.
Plasmids, cells culture and transfection
The vectors pSuper, pSuperBorealin (sh), pFlag-tagged-humanBorealin, phumanBorealin-tagged-GFP were already described by William Taylor (26). The mutant clones of Borealin were generated by a PCR-based site-directed mutagenesis method as described previously, using the Stratagene Quikchange® kit (Agilent Technologies, Santa Clara, USA) (38). Nthy (Nthy-ori 3.1) cells, which are immortalized human thyroid cell lines, were culture as previously described (43). Normal human thyroid tissue specimens were collected at the Cochin University Hospital, Paris, in accordance with local and national ethical requirements. Informed consent was obtained from all donors. Human Primary thyrocytes (HPT) from human thyroid tissues were prepared as previously described and cultured with DMEM/F-12, glutaMAX supplement, MEM Non-Essential Amino Acids solution, Penicillin-Streptomycin (Thermo Fisher Scientific, Waltham, USA), supplemented with six nutritional factors 1U/L bovine TSH (Sigma–Aldrich, Saint-Louis, USA), 10mg/L human insulin (Roche Applied Science, Penzberg, Germany), 10 mg/l somatostatin (Sigma–Aldrich, Saint-Louis, USA), 6 mg/l human transferrine (Roche Applied Science, Penzberg, Germany), 10 − 8M hydrocortisone (Roche Applied Science, Penzberg, Germany), and 10 mg/L glycyl-histidyl-lysine acetate (Sigma–Aldrich, Saint-Louis, USA) (44).
Nthy were plated at 1.25 × 105/well in a 6-well plate 24 h before transfection and 1.105/well in a 12-well plate for HPT. Nthy and HPT were transfected using XtremeGENE-HP-DNA, as recommended by the manufacturer (Roche Applied Science, Penzberg, Germany) to co-transfect cells transiently with 700 ng in Nthy, 280 ng in HPT of pSuperBorealin and with 300 ng in Nthy, 120 ng in HPT of wild type or mutated BOREALIN (vectors containing Borealin tagged with Flag or with GFP). After 24 h, cells were used for the spreading and migration assays and after 48 h for quantitative RT-PCR and flow cytometer test.
HeLa M cells (45) used for localization and time-lapse studies were grown in a humidified atmosphere of 10% CO2 at 37°C in 10% Hyclone Cosmic Calf Serum (GE Healthcare, Little Chalfont, UK) in Dulbecco’s minimal essential medium DMEM (Mediatech, Manassas, USA) supplemented with penicillin/streptomycin. Cells were transfected using polyethylenimine (46).
Tissue sample
After approval by the Institutional Ethics Committee of the experimental design and protocols, embryonic and adult thyroid tissues were obtained from either elective termination of pregnancy or thyroid surgery. Tissue samples were snap-frozen and stored at −80 °C before RNA analysis. For immunohistochemical studies, tissues were fixed by immersion in 3.7% buffered formalin and then embedded in paraffin. Subsequently, 4 µm-thick sections were mounted on StarFrost adhesive slides (Knittel Glaser, Braunschweig, Germany) and processed for immunohistochemistry.
RNA extraction and quantitative RT-PCR
Total RNA of cells or thyroid tissue was isolated using the Qiagen RNeasy Microkit or Minikit (Qiagen, Valencia, USA). Maxima First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, Waltham, USA) was used for reverse transcription of 250 ng of each RNA sample. The synthesized cDNA was diluted to 1/20, and 5 µl was used per PCR reaction. Each reaction consisted of TaqMan Universal PCR Master Mix or SybrGreen PCR Master Mix (Thermo Fisher Scientific, Waltham, USA) and primers. Peptidylpropyl isomerase A was used as an endogenous control. Real-time PCR was performed using the QuantStudio 3 Real-Time PCR System (Thermo Fisher Scientific, Waltham, USA). The data were analysed using the comparative cycle threshold method and presented as the fold change in gene expression, normalized for a calibrator whose value equaled 1. Primer sequences and PCR conditions are available on request.
Immunofluoresence staining
Immunohistochemistry was already described (31). The primary antibodies were used at the following dilutions: rabbit anti-Borealin (1/1000, given by William Taylor), rabbit anti-Ecadherin (1/100, Becton Dickinson, Franklin Lakes, USA), and mouse anti-TG (1/100, Dako-Cytomation, Glostrup, Denmark). The used fluorescent secondary antibodies were Alexa Fluor 594 goat anti-rabbit and Alexa Fluor 488 goat anti-mouse antibodies (1/400, Thermo Fisher Scientific, Waltham, USA). Photographs were taken using a fluorescence microscope (Leitz DMRB; Leica, Wetzlar, Germany) and digitalized using a chilled 3CCD camera (C5810; Hamamatsu Photonics, Japan). In order to analyse localization in mitosis, HeLa cells were transfected with wild type or mutant Borealin-GFP constructs. Cells were released from a single thymidine block and fixed with 2% formaldehyde in PBS at 10.5 hours post thymidine release. Cells were permeabilized with 150mM NaCl, 10 mM Tris (pH 7.7), 0.1% Triton-X-100 (v/v) and 0.1% BSA (w/v) and then blocked in 0.1% BSA in PBS. Staining was carried as previously described (17) and included antibodies to Aurora B (Santa Cruz Biotechnology, Dallas, TX, USA) and human ACA (Antibodies Inc, Davis, CA, USA) to mark the position of the centromere. Primary antibodies were detected by staining with Alexa Fluor-conjugated secondary antibodies (Thermo Fisher Scientific, Waltham, USA). Nuclei were stained with Hoechst 33342 before cells were analysed using a Leica SP8 confocal microscope.
Flow cytometer
Two days after transfection, Nthy were fixed with PFA4% (paraformaldehyde solution) 10 min at −20 °C and permeabilised using TBS 1X with 0.1% triton then incubated with 10μg/ml of Brilliant Violet 605™ anti-mouse Ki-67 antibody (1/20, Biolegends, San Diego, USA) or anti-FLAG M2-FITC antibody (1/100, Sigma–Aldrich, Saint-Louis, USA) during 30 min at RT. After washing with PBS 1X, cells were incubated with PI/RNase staining buffer (propidium iodide, Becton Dickinson, Franklin Lakes, USA) during 15 min at RT before flow cytometric analysis (FACS Aria II, Becton Dickinson, Franklin Lakes) and FlowJo software (FlowJo LLC, Ashland, USA).
Time lapse
To analyse progression through mitosis, HeLa cells were transfected with wild type or mutant Borealin fused to GFP. Live cells were tracked at 37 °C using an environmental chamber attached to an Olympus inverted IX81 microscope. Images were captured with a Photometrics Coolsnap HQ2 camera every 12 min for 16–18 hours depending on the experiment.
Cell migration assay
Nthy were plated in Culture-Inserts on micro-slides (IBIDI, Martinsried, Germany) and transfected under the same conditions as above but adapting the quantity of vectors. One day after transfection, the insert was taken off and slides were incubated at 37 °C in growth medium for 8 h. Phase contrast and fluorescence images were taken every 8 min until the wound was closed (approximately 12 h). Only the GFP expressing cells were tracked. The length of migration was calculated measuring the distance moved toward the center of the wound in 8 h using Imaris Software allowing imaging-based computerized motility analysis method. The cells transfected with WT BOREALIN were therefore compared with the GFP-cells transfected with BOREALIN mutants.
Cell adhesion assay
Nthy were transfected as described above. 24 h after transfection, cells were trypsinized and plated on glass slides previously coated with polylysine. After 1 h 30 min incubation at 37 °C, cells were fixed with PFA4%. Borealin-Flag was detected by staining with mouse anti-Flag M2 antibody (1/1000, Sigma–Aldrich, Saint-Louis, USA) followed by Alexa Fluor 488 goat anti-mouse antibodies (1/400, Thermo Fisher Scientific, Waltham, USA). Filamentous actin was stained with rhodamine-coupled phalloidin (1/400, Sigma–Aldrich, Saint-Louis, USA).
Microarray and analysis
For gene expression analysis, 200 ng of RNA was used for the thyroid tissue with Borealin-114 and three thyroid tissue controls. Thyroid tissue control samples obtained from normal part after thyroidectomy for nodules or tumour. The Human Transcriptome Array 2.0 (HTA 2.0; manufactured by Affymetrix Inc., Santa Clara, USA) was employed in this study. HTA 2.0 covers the global profiling of full-length transcripts, containing more than 40,000 non-coding and 245,000 coding transcripts in the human genome; each transcript is accurately identified by specific exon or exon-exon splice junction probes. RMA method was used to normalize arrays using the Bioconductor affy package in R (47). Normalized expression values were then Log2-transformed before statistical analysis were performed. All results of the thyroid tissue with Borealin-114 were compared with the mean of the three thyroid tissue controls.
Statistics
Results are presented as mean ± SEM for the number of experiments indicated in the Figure legends. Statistical analysis of continuous data was performed with two-tailed Student’s t test. P < 0.05 was considered statistically significant. Statistical analysis was performed using GraphPad Prism4 (GraphPad Software).
Supplementary Material
Supplementary Material is available at HMG online.
Supplementary Material
Acknowledgements
The authors thank the patients and families for their kind participation.
The authors thank URC-CIC Paris Centre (Sandra Colas and Emilie Ervilus) for the implementation and the monitoring of the study and the Necker’s CRB. The authors greatly acknowledge Beatrice Durel and Pierre Bourdoncle of the Cochin Imaging Facility, Franck Letourneur of the Cochin Transcriptomic Facility. We thank Flora Silbermann for her help during this project. We thank Dr Virginie Scotet and Dr Emmanuelle Genin for discussion about the genetic analysis (Inserm U1078, Brest, France). We thank the Fédération Parisienne pour le Dépistage et la Prévention des Handicaps de l'Enfant (FPDPHE). We thank Prof. Paul Czernichow for his long-standing support of our projects in that field.
Conflict of Interest statement. None declared.
Funding
This study was funded by National clinical research funding "Programme Hospitalier de Recherche Clinique" ClinicalTrial.gov: NCTO1916018. AC, MG, MP were supported by EDF, SANDOZ SAS, Merck Serono France and Princess Grace Foundation. WRT was supported by NIH R15 GM100440-01. AS was supported by the European Society for Pediatric Endocrinology Clinical Fellowship Grant, Belgian Kids Fund for Pediatric Research and Onassis Foundation Grants.
This work was awarded the Henning Andersen award at the European Society for Pediatric Endocrinology meeting in Paris September 2016.
References
- 1. Stoupa A., Kariyawasam D., Carré A., Polak M. (2016) Update of Thyroid Developmental Genes. Endocrinol. Metab. Clin. North. Am., 45, 243–254. [DOI] [PubMed] [Google Scholar]
- 2. Léger J., Marinovic D., Garel C., Bonaıïti-Pellié C., Polak M., Czernichow P. (2002) Thyroid developmental anomalies in first degree relatives of children with congenital hypothyroidism. J. Clin. Endocrinol. Metab., 87, 575–580. [DOI] [PubMed] [Google Scholar]
- 3. Damante G., Tell G., Di Lauro R. (2001) A unique combination of transcription factors controls differentiation of thyroid cells. Prog. Nucleic. Acid. Res. Mol. Biol., 66, 307–356. [DOI] [PubMed] [Google Scholar]
- 4. Fagman H., Nilsson M. (2010) Morphogenesis of the thyroid gland. Mol. Cell. Endocrinol., 35–54. [DOI] [PubMed] [Google Scholar]
- 5. Johansson E., Andersson L., Örnros J., Carlsson T., Ingeson-Carlsson C., Liang S., Dahlberg J., Jansson S., Parrillo L., Zoppoli P., et al. (2015) Revising the embryonic origin of thyroid C cells in mice and humans. Development, 142, 3519–3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Trueba S.S., Augé J., Mattei G., Etchevers H., Martinovic J., Czernichow P., Vekemans M., Polak M., Attié-Bitach T. (2005) PAX8, TITF1, and FOXE1 gene expression patterns during human development: new insights into human thyroid development and thyroid dysgenesis-associated malformations. J. Clin. Endocrinol. Metab., 90, 455–462. [DOI] [PubMed] [Google Scholar]
- 7. Szinnai G., Lacroix L., Carré A., Guimiot F., Talbot M., Martinovic J., Delezoide A.L., Vekemans M., Michiels S., Caillou B., et al. (2007) Sodium/iodide symporter (NIS) gene expression is the limiting step for the onset of thyroid function in the human fetus. J. Clin. Endocrinol. Metab., 92, 70–76. [DOI] [PubMed] [Google Scholar]
- 8. Maiorana R., Carta A., Floriddia G., Leonardi D., Buscema M., Sava L., Calaciura F., Vigneri R. (2003) Thyroid hemiagenesis: prevalence in normal children and effect on thyroid function. J. Clin. Endocrinol. Metab., 88, 1534–1536. [DOI] [PubMed] [Google Scholar]
- 9. Stoppa-Vaucher S., Lapointe A., Turpin S., Rydlewski C., Vassart G., Deladoëy J. (2010) Ectopic thyroid gland causing dysphonia: imaging and molecular studies. J. Clin. Endocrinol. Metab., 95, 4509–4510. [DOI] [PubMed] [Google Scholar]
- 10. Carré A., Szinnai G., Castanet M., Sura-Trueba S., Tron E., Broutin-L’Hermite I., Barat P., Goizet C., Lacombe D., Moutard M.L., et al. (2009) Five new TTF1/NKX2.1 mutations in brain-lung-thyroid syndrome: rescue by PAX8 synergism in one case. Hum. Mol. Genet., 18, 2266–2276. [DOI] [PubMed] [Google Scholar]
- 11. Ramos H.E., Carré A., Chevrier L., Szinnai G., Tron E., Cerqueira T.L., Léger J., Cabrol S., Puel O., Queinnec C., et al. (2014) Extreme phenotypic variability of thyroid dysgenesis in six new cases of congenital hypothyroidism due to PAX8 gene loss-of-function mutations. Eur. J. Endocrinol., 171, 499–507. [DOI] [PubMed] [Google Scholar]
- 12. Sura-Trueba S., Aumas C., Carre A., Durif S., Leger J., Polak M., de Roux N. (2009) An inactivating mutation within the first extracellular loop of the thyrotropin receptor impedes normal posttranslational maturation of the extracellular domain. Endocrinology, 150, 1043–1050. [DOI] [PubMed] [Google Scholar]
- 13. Senée V., Chelala C., Duchatelet S., Feng D., Blanc H., Cossec J.C., Charon C., Nicolino M., Boileau P., Cavener D.R., et al. (2006) Mutations in GLIS3 are responsible for a rare syndrome with neonatal diabetes mellitus and congenital hypothyroidism. Nat. Genet., 38, 682–687. [DOI] [PubMed] [Google Scholar]
- 14. Castanet M., Lyonnet S., Bonaïti-Pellié C., Polak M., Czernichow P., Léger J. (2000) Familial forms of thyroid dysgenesis among infants with congenital hypothyroidism. N. Engl. J. Med., 343, 441–442. [DOI] [PubMed] [Google Scholar]
- 15. Castanet M., Polak M., Bonaïti-Pellié C., Lyonnet S., Czernichow P., Léger J.. AFDPHE (Association Française pour le Dépistage et la Prévention des Handicaps de l’Enfant). (2001) Nineteen years of national screening for congenital hypothyroidism: familial cases with thyroid dysgenesis suggest the involvement of genetic factors. J. Clin. Endocrinol. Metab., 86, 2009–2014. [DOI] [PubMed] [Google Scholar]
- 16. Gassmann R., Carvalho A., Henzing A.J., Ruchaud S., Hudson D.F., Honda R., Nigg E.A., Gerloff D.L., Earnshaw W.C. (2004) Borealin: a novel chromosomal passenger required for stability of the bipolar mitotic spindle. J. Cell. Biol., 166, 179–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bekier M.E., Mazur T., Rashid M.S., Taylor W.R. (2015) Borealin dimerization mediates optimal CPC checkpoint function by enhancing localization to centromeres and kinetochores. Nat. Commun., 6, 6775.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McLean R., Howard N., Murray I.P. (1985) Thyroid dysgenesis in monozygotic twins: variants identified by scintigraphy. Eur. J. Nucl. Med., 346–348. [DOI] [PubMed] [Google Scholar]
- 19. Taylor S.I., Marcus-Samuels B., Ryan-Young J., Leventhal S., Elders M.J. (1986) Genetics of the insulin receptor defect in a patient with extreme insulin resistance. J. Clin. Endocr. Metab., 62, 1130–1135. [DOI] [PubMed] [Google Scholar]
- 20. Furlan M., Robles R., Galbusera M., Remuzzi G., Kyrle P.A., Brenner B., Krause M., Scharrer I., Aumann V., Mittler U., et al. (1998) Von Willebrand factor-cleaving protease in thrombotic thrombocytopenic purpura and the hemolytic-uremic syndrome. New. Eng. J. Med., 339, 1578–1584. [DOI] [PubMed] [Google Scholar]
- 21. Magne F., Ge B., Larrivée-Vanier S., Van Vliet G., Samuels M.E., Pastinen T., Deladoëy J. (2016) Demonstration of autosomal monoallelic expression in thyroid tissue assessed by whole-exome and bulk RNA sequencing. Thyroid, 26, 852–859. [DOI] [PubMed] [Google Scholar]
- 22. Tsukahara T., Tanno Y., Watanabe Y. (2010) Phosphorylation of the CPC by Cdk1 promotes chromosome bi-orientation. Nature, 467, 719–723. [DOI] [PubMed] [Google Scholar]
- 23. Liu X., Song Z., Huo Y., Zhang J., Zhu T., Wang J., Zhao X., Aikhionbare F., Zhang J., Duan H., et al. (2014) Chromatin protein HP1 interacts with the mitotic regulator borealin protein and specifies the centromere localization of the chromosomal passenger complex. J. Biol. Chem., 289, 20638–20649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yamanaka Y., Heike T., Kumada T., Shibata M., Takaoka Y., Kitano A., Shiraishi K., Kato T., Nagato M., Okawa K., et al. (2008) Loss of Borealin/DasraB leads to defective cell proliferation, p53 accumulation and early embryonic lethality. Mech. Dev., 125, 441–450. [DOI] [PubMed] [Google Scholar]
- 25. Uhlen M., Oksvold P., Fagerberg L., Lundberg E., Jonasson K., Forsberg M., Zwahlen M., Kampf C., Wester K., Hober S., et al. (2010) Towards a knowledge-based Human Protein Atlas. Nat. Biotechnol., 28, 1248–1250. [DOI] [PubMed] [Google Scholar]
- 26. Kaur H., Bekier M.E., Taylor W.R. (2010) Regulation of Borealin by phosphorylation at serine 219. J. Cell. Biochem., 111, 1291–1298. [DOI] [PubMed] [Google Scholar]
- 27. De Felice M., Ovitt C., Biffali E., Rodriguez-Mallon A., Arra C., Anastassiadis K., Macchia P.E., Mattei M.G., Mariano A., Schöler H., et al. (1998) A mouse model for hereditary thyroid dysgenesis and cleft palate. Nat. Genet., 19, 395–398. [DOI] [PubMed] [Google Scholar]
- 28. Ferreira A.R., Felgueiras J., Fardilha M. (2015) Signaling pathways in anchoring junctions of epithelial cells: cell-to-cell and cell-to-extracellular matrix interactions. J. Recept. Signal. Transduct. Res., 35, 67–75. [DOI] [PubMed] [Google Scholar]
- 29. Klein U.R., Nigg E.A., Gruneberg U. (2006) Centromere targeting of the chromosomal passenger complex requires a ternary subcomplex of Borealin, Survivin, and the N-terminal domain of INCENP. Mol. Biol. Cell, 17, 2547–2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Carré A., Hamza R.T., Kariyawasam D., Guillot L., Teissier R., Tron E., Castanet M., Dupuy C., El Kholy M., Polak M. (2014) A novel FOXE1 mutation (R73S) in Bamforth-Lazarus syndrome causing increased thyroidal gene expression. Thyroid, 24, 649–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Carre A., Rachdi L., Tron E., Richard B., Castanet M., Schlumberger M., Bidart J.M., Szinnai G., Polak M. (2011) Hes1 is required for appropriate morphogenesis and differentiation during mouse thyroid gland development. PLoS One, 6, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Friedl P., Gilmour D. (2009) Collective cell migration in morphogenesis, regeneration and cancer. Nat. Rev. Mol. Cell. Biol., 10, 445–457. [DOI] [PubMed] [Google Scholar]
- 33. Scarpa E., Mayor R. (2016) Collective cell migration in development. J. Cell. Biol., 212, 143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Antonica F., Kasprzyk D.F., Opitz R., Iacovino M., Liao X.H., Dumitrescu A.M., Refetoff S., Peremans K., Manto M., Kyba M., et al. (2012) Generation of functional thyroid from embryonic stem cells. Nature, 2012 491, 66–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ma R., Latif R., Davies T.F. (2013) Thyroid follicle formation and thyroglobulin expression in multipotent endodermal stem cells. Thyroid, 23, 385–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ma R., Latif R., Davies T.F. (2015) Human embryonic stem cells form functional thyroid follicles. Thyroid, 25, 455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Castanet M., Sura-Trueba S., Chauty A., Carré A., de Roux N., Heath S., Léger J., Lyonnet S., Czernichow P., Polak M. (2005) Linkage and mutational analysis of familial thyroid dysgenesis demonstrate genetic heterogeneity implicating novel genes. Eur. J. Hum. Genet., 13, 232–239. [DOI] [PubMed] [Google Scholar]
- 38. Carré A., Castanet M., Sura-Trueba S., Szinnai G., Van Vliet G., Trochet D., Amiel J., Léger J., Czernichow P., Scotet V., et al. (2007) Polymorphic length of FOXE1 alanine stretch: evidence for genetic susceptibility to thyroid dysgenesis. Hum. Genet., 122, 467–476. [DOI] [PubMed] [Google Scholar]
- 39. Abu-Khudir R., Magne F., Chanoine J.P., Deal C., Van Vliet G., Deladoëy J. (2014) Role for tissue-dependent methylation differences in the expression of FOXE1 in nontumoral thyroid glands. J. Clin. Endocrinol. Metab., 99, E1120–E1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Souma T., Tompson S.W., Thomson B.R., Siggs O.M., Kizhatil K., Yamaguchi S., Feng L., Limviphuvadh V., Whisenhunt K.N., Maurer-Stroh S., et al. (2016) Angiopoietin receptor TEK mutations underlie primary congenital glaucoma with variable expressivity. J. Clin. Invest., 126, 2575–2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nilsson M., Fagman H. (2013) Mechanisms of thyroid development and dysgenesis: an analysis based on developmental stages and concurrent embryonic anatomy. Curr. Top. Dev. Biol., 106, 123–170. [DOI] [PubMed] [Google Scholar]
- 42. Gordon C.T., Petit F., Kroisel P.M., Jakobsen L., Zechi-Ceide R.M., Oufadem M., Bole-Feysot C., Pruvost S., Masson C., Tores F., et al. (2013) Mutations in endothelin 1 cause recessive auriculocondylar syndrome and dominant isolated question-mark ears. Am. J. Hum. Genet., 93, 1118–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lemoine N.R., Mayall E.S., Jones T., Sheer D., McDermid S., Kendall-Taylor P., Wynford-Thomas D. (1989) Characterisation of human thyroid epithelial cells immortalised in vitro by simian virus 40 DNA transfection. Br. J. Cancer, 60, 897–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Weyemi U., Caillou B., Talbot M., Ameziane-El-Hassani R., Lacroix L., Lagent-Chevallier O., Al Ghuzlan A., Roos D., Bidart J.M., Virion A., et al. (2010) Intracellular expression of re- active oxygen species-generating NADPH oxidase NOX4 in normal and cancer thyroid tissues. Endocr. Relat. Cancer, 17, 27–37. [DOI] [PubMed] [Google Scholar]
- 45. Tiwari R.K., Kusari J., Sen G.C. (1987) Functional equivalents of interferon-mediated signals needed for induction of an mRNA can be generated by double-stranded RNA and growth factors. Embo. J., 6, 3373–3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fukumoto Y., Obata Y., Ishibashi K., Tamura N., Kikuchi I., Aoyama K., Hattori Y., Tsuda K., Nakayama Y., Yamaguchi N. (2010) Cost-effective gene transfection by DNA compaction at pH 4.0 using acidified, long shelf-life polyethylenimine. Cytotechnology, 62, 73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gautier L., Cope L., Bolstad B.M., Irizarry R.A. (2004) affy—analysis of Affymetrix GeneChip data at the probe level. Bioinformatics, 20, 307–315. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.