Abstract
LRRK2 mutation is the most common inherited, autosomal dominant cause of Parkinson’s disease (PD) and has also been observed in sporadic cases. Most mutations result in increased LRRK2 kinase activity. LRRK2 is highly expressed in brain regions that receive dense, convergent innervation by dopaminergic and glutamatergic axons, and its levels rise developmentally coincident with glutamatergic synapse formation. The onset and timing of expression suggests strongly that LRRK2 regulates the development, maturation and function of synapses. Several lines of data in mice show that LRRK2-G2019S, the most common LRRK2 mutation, produces an abnormal gain of pathological function that affects synaptic activity, spine morphology, persistent forms of synapse plasticity and behavioral responses to social stress. Effects of the mutation can be detected as early as the second week of postnatal development and can last or have consequences that extend into adulthood and occur in the absence of dopamine loss. These data suggest that the generation of neural circuits that support complex behaviors is modified by LRRK2-G2019S. Whether such alterations impart vulnerability to neurons directly or indirectly, they bring to the forefront the idea that neural circuits within which dopamine neurons eventually degenerate are assembled and utilized in ways that are distinct from circuits that lack this mutation and may contribute to non-motor symptoms observed in humans with PD.
The relevance of brain development and critical periods to Parkinson’s disease
Waves of protein-based signaling networks become activated and inactivated during development in a tightly orchestrated and genetically controlled process that produces appropriately wired neural circuits [1]. As young vertebrates interact with the world, a collaboration between behavioral experience and synaptic activity during an early, restricted postnatal period (a so-called critical period) shapes and refines nascent neural circuits in ways that become permanent and are essential for normal function. Disease-causing mutations in genes that are expressed during this period can have a strong impact on relationships between circuits, activity and environment, and it is well accepted that changes in these interactions contribute directly to the development and severity of nervous system disorders in which major disease-defining symptoms arise at early ages (e.g. autism) or by late adolescence (e.g. schizophrenia) [2–4]. A growing body of work studying patients and their relatives suggests that disease-causing gene mutations can significantly alter the structure and function of the brain at early stages in ways that may contribute to later-onset disorders, including Parkinson’s disease (PD) [5]. While it is possible that such structural/functional changes and disease-defining symptoms sit in parallel, unrelated pathways, it is far more likely that early structural and functional changes are part of the disease progression. Thus, a complete understanding of the consequences of a gene mutation on neural circuits will provide key insights into disease onset and progression. Recent work supports the idea that the most common PD-related mutation, LRRK2-G2019S, increases neural activity at a time and in a manner that would be anticipated to alter striatal and other neural circuits [6,7]. Here, we review evidence from mouse models, showing that LRRK2-G2019S alters the development and function of striatal circuits in a manner that may contribute directly to the onset of the disease later in life or affect indirectly the nature and expression of both motor and non-motor symptoms.
LRRK2 hyperactivation and PD
LRRK2 encodes a multidomain 280 kDa protein with a catalytic core domain that supports both GTPase and kinase activities. Most pathogenic mutations are found within the catalytic core (Figure 1A). The G2019S mutation, which sits in the kinase domain, increases kinase activity by ∼2-fold, whereas the next most common site of mutation R1141G/C/H lies in the GTPase domain and increases substrate phosphorylation several-fold [8]. Based on this and studies of additional mutations, the source of pathogenicity is thought to be due to hyperactivation of the kinase. Mechanisms driving LRRK2 pathogenicity may be central to other forms of PD. For example, a PD-causing mutation in VPS35 (D620N) increases LRRK2-mediated Rab phosphorylation substrates [9], and accumulation of extracellular α-synuclein aggregates is enhanced in the mouse brain and in neuron cultures expressing LRRK2-G2019S [10].
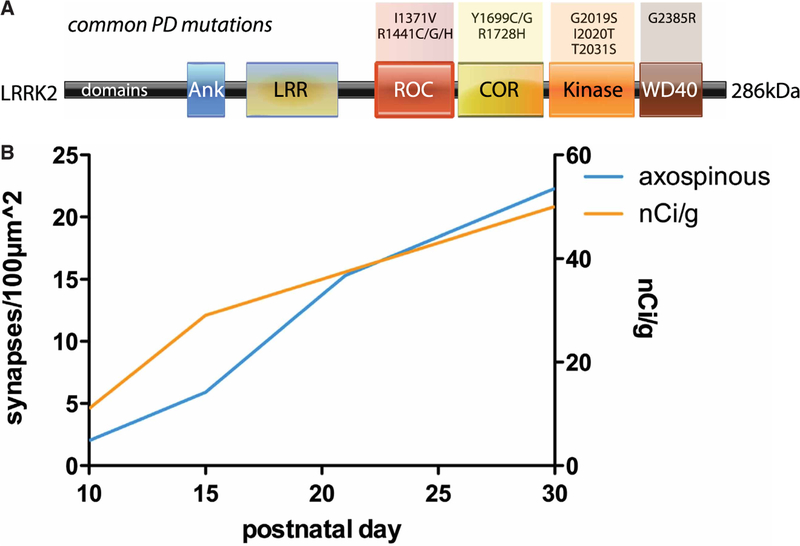
Figure 1. LRRK2 structure and its expression during synaptogenesis.
(A) Schematic showing principal protein domains in LRRK2 and sites of common PD-causing mutations. (B) LRRK2 levels rise over a time course that is shared by the generation of corticostriatal synapses. Estimates of synapse density over time (blue) are plotted on the left-hand Y-axis from data published by Sharpe and Tepper [11]; development of asymmetric synapses on dendritic spines serves as a proxy for the generation of corticospinal synapses. Estimates of LRRK2 mRNA levels over time (orange) are plotted on the right-hand Y-axis and are based on in situ hybridization data published in Westerlund et al. [12].
LRRK2 expression, localization and onset are concurrent with synapse development
LRRK2 is expressed at the right place and time to strongly influence the development of corticostriatal connections. In situ hybridization and immunohistochemical studies using verified antibodies show that within the brain, LRRK2 is most highly expressed in cerebral cortex and dorsal striatum [12–17]. Its expression levels rise postnatally over a time course that closely matches the generation of glutamatergic corticostriatal synapses [11,18] (Figure 1B). They show no such correlation with the development of thalamocortical or GABAergic synapses, suggesting that the influence of LRRK2 on synaptic circuits may be selective. Expression levels reach a maximum and level off [12,16] when experience is shaping connections [19–21]. This pattern of expression is typical for proteins that influence the development of neural circuits.
Cellular and functional data provide additional support that LRRK2 regulates synapses. Although immunocytochemical localization of endogenous LRRK2 has proved to be particularly difficult, biochemical data support that LRRK2 is present within synaptic fractions [15,22,23] and can be targeted to membranes in a regulated manner [24]. Neither the pace of synaptogenesis nor density of synapses appears to be affected by either Lrrk2 deletion or mutation, but there is compelling evidence showing that LRRK2 regulates synapse function following synapse assembly [6,7,25].
LRRK2 and synaptic vesicle recycling
Functional studies in neurons lacking LRRK2 or having reduced LRRK2 expression generally agree that synaptic vesicle endocytosis is impaired [23,26–28]. The mechanisms driving impaired endocytosis are not yet clear, but Rab5b and potentially EndoA and Synaptojanin1 could be relevant LRRK2 kinase substrates [26,28,29] (Figure 2). LRRK2-G2019S and LRRK2-R1441C/G both appear to decrease synaptic vesicle endocytosis [28–30]. Since either loss or gain of kinase function reduces the efficiency or degree of endocytosis, the data suggest that LRRK2 kinase activity may be deployed in a cyclical fashion [26]. The mechanism, however, must also account for the fact that LRRK2 kinase inhibitors can rescue endocytic defects observed in neurons expressing LRRK2-G2019S with no detectable impact on endocytosis in wild-type (WT) control neurons [29]. Based on this, the endocytic defects seen with LRRK2-G2019S or R1441C/G may be regulating a pathway distinct from the decreased endocytosis observed following the loss of the entire, 280 kDa, multifunctional LRRK2 protein (and which can be rescued by expressing full-length WT LRRK2) [26].
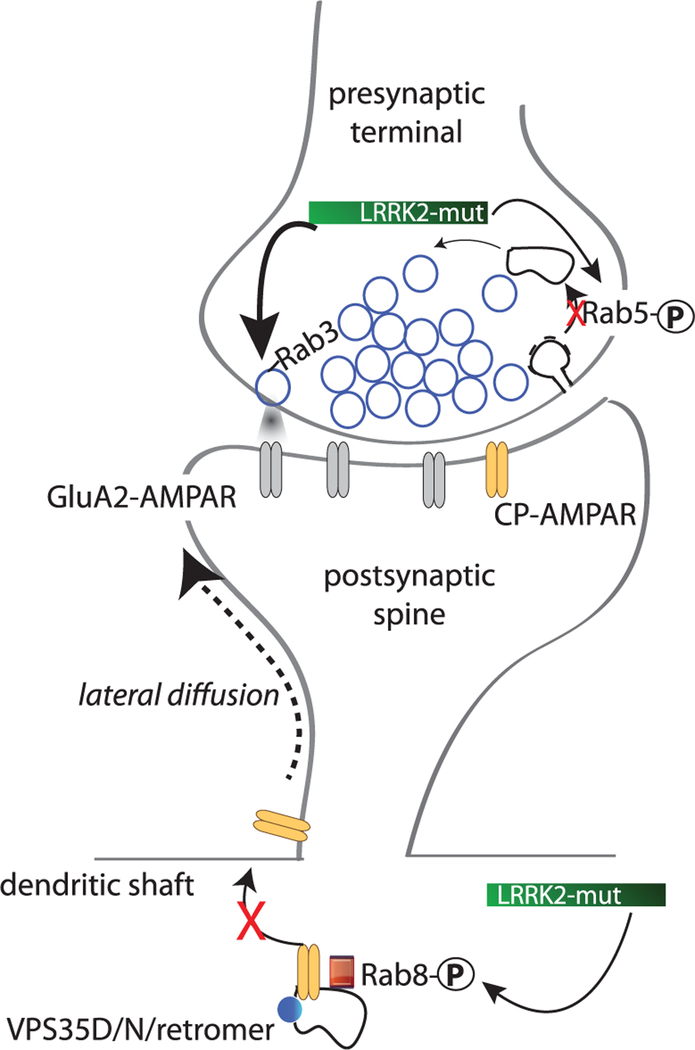
Figure 2. Potential pre- and postsynaptic mechanisms by which mutant LRRK2 can influence synaptic function.
Cartoon depicts a glutamatergic synapse and highlights possible sites of LRRK2-G2019S interaction/interference with the normal processes of presynaptic vesicle recycling and insertion of new CP-AMPA receptor subunits into the plasma membrane. CP: calcium permeable; LRRK2-mut: PD-causing LRRK2 mutations, particularly G2019S.
Several studies support that cortical neurons expressing LRRK2-G2019S can also display increased exocytosis in neuron cultures or in preparations from young mice (Figure 2), an effect that is normalized by the addition of an LRRK2 kinase inhibitor [29,31]. This effect likely contributes to the increased spontaneous glutamatergic activity that is observed in postnatal day (P) 21–P28 striatal spiny projection neurons (SPNs) [6,7]. Additionally, P15 striatal SPNs lacking LRRK2 show increased EPSC amplitudes compared with WT neurons likely due to increased postsynaptic exocytosis and surface expression of AMPA receptors. In forebrain homogenates, the same study shows that LRRK2 binds to protein kinase A (PKA) regulatory subunit RIIβ and in cultured hippocampal neurons appears to negatively regulate PKA localization to synapses. In the absence of LRRK2, PKA activity was increased, and PKA-mediated phosphorylation and surface expression of GluA1 were enhanced. The R1441C mutation appears to reduce LRRK2/RIIβ binding, but it is not known whether or not LRRK2 kinase activity is involved in this mechanism [32]. PKA can also phosphorylate LRRK2, an action that may be important for LRRK2 localization and actions on particular substrates [33,34], but see also [35]. Both G2019S and R1441C/G mutations serve to increase the phosphorylation of LRRK2 substrate Rab proteins [8], making it also likely that decreased postsynaptic exocytosis also involves dysfunctional vesicle targeting and fusion (Figure 2).
Alpha-synuclein concentrates in presynaptic vesicles where it modulates endo- and exocytosis, suggesting a possible point of mechanistic convergence with LRRK2. However, the effects of PD-associated mutation or overexpression of α-synuclein reduce synaptic vesicle release [36–38] or alter vesicle tethering [39] depending on the nerve terminal type, effects that are not at all similar to those observed with LRRK2 mutation. Perhaps more revealing is that in the presence of increased levels of α-synuclein, repetitive stimulation in a lamprey nerve preparation nearly eliminates endocytosis [40], suggesting that only certain kinds of activity may share convergent mechanisms.
LRRK2 and neural activity
Consistent with the effects of LRRK2-G2019S on presynaptic vesicle recycling and postsynaptic glutamate receptor expression, mutant LRRK2 alters synaptic activity recorded in cultured cortical neurons [41] and in acute striatal slices [6,7]. While there are some differences in approach or interpretation, the datasets from different laboratories are largely in agreement that SPNs expressing one or two copies of a knockin G2019S mutation show a developmentally transient increase in glutamatergic excitatory postsynaptic currents (EPSCs) that declines to WT levels by young adulthood [6,7]. The increase in activity is kinase-dependent, action potential driven, largely derived from the cerebral cortex, and observed in both dopamine receptor 1 (D1R)- and dopamine receptor 2 (D2R)-expressing SPNs [7]. Increased spontaneous EPSCs result from a gain in pathological function; activity in Lrrk2-D2017A (kinase-dead) mutants was no different from WT, and LRRK2 kinase inhibitors administered acutely reduced activity to WT levels (but not below WT levels) [7].
The G2019S mutation also affects dopaminergic actions in striatum. Striatal slices from 3-month-old mice expressing Lrrk2-G2019S showed increased peak dopamine levels detected in response to sustained stimulation compared with a decline in WT slices using fast scan voltammetry [6]. At 12 months, this effect was no longer evident in slices [6], and in vivo, microdialysis showed reduced extracellular dopamine in Lrrk2-G2019S striatum in the absence of stimulation [42]. Also at 12 months, mice overexpressing Lrrk2-G2019S showed reduced peak dopamine levels that were reduced further by repetitive stimulation [43]. It is not yet clear how Lrrk2-G2019S drives age-dependent changes in dopamine levels detected at baseline and in response to sustained stimulation, particularly since LRRK2 is expressed at very low levels in dopaminergic neurons. However, the low levels of LRRK2 do appear to confer a cell autonomous effect on DA release [29] and it seems likely that the striatum as a major postsynaptic target for dopamine fibers has a retrograde impact on DA release.
Increases in cortical glutamatergic activity onto SPNs might be predicted to drive changes in synapse formation. On a small scale, local stimulation of SPN dendrites in 2-week-old mice can prompt the genesis of new dendritic spines, and on a larger scale, silencing activity of either D1R- or D2R-expressing SPNs produces a circuit-wide imbalance that decreases or increases (respectively) the density of cortical synapses onto both D1R- and D2R SPNs [44]. However, measurements of pre- or postsynaptic protein levels, synapse or dendritic spine density have revealed no differences over the course of development that can be related to LRRK2 or mutant LRRK2 [6,7,32]. Adult Lrrk2 knockout mice also show no differences in dendritic spine density in dorsal striatum [42]. Taken together, these data indicate that it is unlikely that normal LRRK2 is necessary and sufficient for proper synaptogenesis. However, at P15, Lrrk2 knockout SPNs have thinner dendritic protrusions and decreased EPSC amplitudes relative to WT SPNs, a developmentally transient phenotype that suggests LRRK2 expression may help to regulate glutamatergic synapse maturation [32]. Consistent with this idea, LRRK2-G2019S SPNs have larger dendritic spine heads and increased EPSC amplitudes at P21 [7]. Thus, it appears that the increased spontaneous EPSCs recorded in neurons expressing knockin Lrrk2-G2019S result from either enhanced presynaptic release, a decrease in the population of immature, postsynaptically silent (NMDAR-only) synapses, or both.
Synapse plasticity
The dramatic effects of the G2019S mutation on spontaneous glutamatergic activity in SPNs in mice are developmentally transient and not evident by adulthood. Nevertheless, the developmental timing suggests that this transient effect could mediate lasting changes in striatal circuit function. To test this, we asked whether a persistent form of synapse plasticity, long-term potentiation (LTP), would be altered in SPNs of adult Lrrk2-G2019S knockin mice. The data showed that while LTP can be readily elicited from SPNs in WT dorsal striatum, it was not only abolished in G2019S SPNs, but an LTP induction protocol produced long-term depression (LTD) instead [45]. When responses were compared between D1R and D2R-expressing SPNs, it was confirmed that LTP was absent in both SPN types, but that only D2R SPNs showed a robust LTD, consistent with previous work indicating that LTD is more readily elicited in D2R SPNs [46]. The impact of LRRK2-G2019S on LTP was evident at P21 and remained at P70 [45]. How LRRK2-G2019S interferes with LTP is not known, but likely involves its regulation of Rab proteins [8], since Rabs can regulate AMPA receptor insertion at synapses [47]. This idea gains strength from recent work, showing that PD-causing mutations in VPS35 enhance the kinase activity of LRRK2-G2019S ([9]; Figure 2) and also fail to sustain LTP in hippocampal neurons via a mechanism that appears to impair GluA1 insertion into the postsynaptic membrane [48,68]. It is also possible that LRRK2-G2019S impedes normal PKA function (see above [32]; Figure 2), since PKA-mediated phosphorylation of GluA1 is necessary for LTP [49]. No matter the underlying mechanism, the data suggest that rules for recruiting and modifying striatal circuits are different in neurons expressing LRRK2-G2019S.
In adult hippocampal slices from mice overexpressing (OE) either Lrrk2-WT or -G2019S, LTP is normal, but LTD is reduced selectively in G2019S OE neurons. While the mechanism is not fully understood, increased AMPA-to-NMDA ratios in G2019S OE neurons suggest that AMPA receptors fail to internalize in response to an LTD stimulus [50]. Although mechanisms underlying LTP and LTD in hippocampus are different from those in the dorsal striatum, the data from the two regions suggest that basic mechanisms regulating bidirectional changes in synapse strength are disrupted by LRRK2-G2019S in a cell-type specific manner.
Stress, experience and behavior
Bidirectional synapse plasticity is essential for acquiring and supporting the full range of learned behaviors. The absence of one arm of plasticity–LTP–in Lrrk2-G2019S knockin SPNs and LTD in Lrrk2-G2019S OE hippocampal neurons strongly suggests that learning, memory and the performance of particular behaviors will be altered by G2019S. Since depression is a common early symptom in PD [51,52] and psychological stress can promote the onset of depression and exacerbate PD symptoms [53–55], we recently tested the effect of the Lrrk2-G2019S knockin mutation in chronic social defeat stress (CSDS), a validated depression model in male mice [56,57]. The data showed that following 10 days of CSDS in which 2- to 3-month-old mice were exposed for brief periods to physical subordination by a novel aggressor mouse, ∼40% of WT mice were ‘resilient’ to the stress-experience and spent more time approaching and interacting with a novel social target mouse during a subsequent social interaction test. The remaining WT mice that had undergone CSDS were ‘susceptible’ to the social stress paradigm and spent more time avoiding the novel mouse [45]. This proportion of resilient and susceptible mice is consistent with previous work [57]. In stark contrast, all but one mouse in two separate cohorts of eight Lrrk2-G2019S mice each were resilient to CSDS (94% resilient), a result that strongly suggests that the mutation alters responsiveness to stress. Importantly, in the absence of CSDS, there were no differences between Lrrk2-G2019S and WT mice in exploratory behavior or social interaction.
Lack of self-care is associated with depression in humans, and self-care in mice can be measured using a sucrose splash test [58]. The data showed that Lrrk2-G2019S mice spent significantly more time grooming in the splash test than WT mice post-CSDS. In the absence of CSDS, there were no differences between WT and Lrrk2-G2019S mice in this or other anxiety-related tests, consistent with findings from other studies of Lrrk2-G2019S knockin and knockout mice [42,59], indicating that it was the response to CSDS that produces strikingly distinct behavioral outcomes [45].
While the mechanisms driving this different reaction to social stress are not fully understood, the data point towards an inability of LRRK2-G2019S synapses in the nucleus accumbens (NAc), a brain region involved in reward and depression [60–62], to undergo appropriate, behaviorally driven synaptic adaptations to CSDS that may involve AMPA receptor function and/or trafficking. Previous studies have established that a variety of synaptic and other cellular adaptations in the NAc are critical for the expression of both resilience and susceptibility to CSDS [56,63,64]. In WT mice, glutamatergic synapses in the NAc contain a mixture of both calcium-permeable (CP) and calcium-impermeable AMPAR subunits at baseline. WT mice that are susceptible to CSDS exhibit a significant incorporation of CP-AMPARs in comparison with those that are resilient [64,65]. In contrast, in Lrrk2-G2019S knockin mice, glutamatergic synapses in the NAc mostly lack CP-AMPARs at baseline and, following CSDS, fail to incorporate them [45]. This apparent inability to synaptically incorporate CP-AMPARs cannot be attributed to differences between genotypes in the expression levels of GluA1 or GluA2, as the major AMPAR subunit subtypes in striatum are detected at normal levels by immunoblot or immunostaining [45]. As discussed above, cellular data suggest that these differences in AMPA receptor trafficking could be driven by altered Rab activity [8,47] or by changes in the regulation of PKA [32] or a combination of both.
These data indicate a strong effect of the G2019S mutation on a complex behavior in adult mice. This stands in contrast with several studies showing at most mild hyperkinesia in young adult mice (10–12 months) expressing endogenous or transgenic LRRK2-G2019S [6,42,59,66,67]. It may be the case that robust behavioral phenotypes may emerge only following a significant or stressful challenge, such as that imposed by CSDS. This may speak to the two-hit theory of PD, wherein genetic susceptibility is one component that, together with adverse environmental factors, predisposes the brain to PD. It will also be interesting to test these behaviors in R1441C/G/H mice where based on the strong effect of these mutations on LRRK2 kinase activity one would predict an even more robust response.
Findings showing Lrrk2-G2019S knockin mice to be highly resilient to CSDS are unexpected because depression and anxiety are commonly seen in patients with PD [51,52]. It is possible that the mice are showing an adaptive behavior in response to the mutation that could become maladaptive in older animals. In this context, it is interesting that a recent paper suggests that mice older than 10 months of age and overexpressing human LRRK2-G2019S can display anxiety and depression-like behaviors compared with non-transgenic mice expressing normal levels of endogenous LRRK2 [67]. A possible (and logical) mechanism for an age-related transition sits with dopamine, serotonin, or other neurochemical systems: mice expressing LRRK2-G2019S display an age-related decline in dopamine release and SPNs appear to respond to dopamine differently in Lrrk2-G2019S knockin compared with WT neurons [6,42,43].
Conclusion
Despite evidence that PD-linked mutant LRRK2 alters early postnatal synaptic structure and function in developing striatal circuits, and that aberrant behavioral phenotypes occur in Lrrk2 knockin mutants and overexpressors at early and late adult ages, respectively, a causal link between early increases in corticostriatal activity and later behavioral abnormalities remains elusive. One possible link may lie in lasting changes in synaptic plasticity; perhaps, the disrupted synaptic potentiation and depression associated with G2019S expression has lasting consequences rendering insufficient synaptic changes in response to environmental cues. Regardless, continued investigation into the precise mechanisms by which LRRK2-G2019S affects synaptic form and function at all ages will likely shed light on the etiology of PD.
Acknowledgments
Funding
This work was supported by National Institute of Mental Health MH110727 (G.W.H.), MH104491 (G.W.H. and D.L.B.), MH103455 (D.L.B.), National Institute of Neurological Disorders and Stroke F31NS096892 (B.A.M.-A.) and National Science Foundation (A.H.).
Abbreviations
- AMPAR
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid-type glutamate receptor
- CP
calcium permeable
- CSDS
chronic social defeat stress
- D1R
dopamine receptor 1
- D2R
dopamine receptor 2
- EPSCs
excitatory postsynaptic currents
- LTD
long-term depression
- LTP
long-term potentiation
- NAc
nucleus accumbens
- PD
Parkinson’s disease
- PKA
protein kinase A
- SPNs
spiny projection neurons
- WT
wild type
Footnotes
Competing Interests
The Authors declare that there are no competing interests associated with the manuscript.
References
- 1.Benson DL, Colman DR and Huntley GW (2001) Molecules, maps and synapse specificity. Nat. Rev. Neurosci 2, 899–909 10.1038/35104078 [DOI] [PubMed] [Google Scholar]
- 2.Gulsuner S, Walsh T, Watts AC, Lee MK, Thornton AM, Casadei S et al. (2013) Spatial and temporal mapping of de novo mutations in schizophrenia to a fetal prefrontal cortical network. Cell 154, 518–529 10.1016/j.cell.2013.06.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marin O (2016) Developmental timing and critical windows for the treatment of psychiatric disorders. Nat. Med 22, 1229–1238 10.1038/nm.4225 [DOI] [PubMed] [Google Scholar]
- 4.Berger JM, Rohn TT and Oxford JT (2013) Autism as the early closure of a neuroplastic critical period normally seen in adolescence. Biol. Syst. Open Access 2, 118 10.4172/2329-6577.1000118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Helmich RC, Thaler A, van Nuenen BF, Gurevich T, Mirelman A, Marder KS et al. (2015) Reorganization of corticostriatal circuits in healthy G2019S LRRK2 carriers. Neurology 84, 399–406 10.1212/WNL.0000000000001189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Volta M, Beccano-Kelly DA, Paschall SA, Cataldi S, MacIsaac SE, Kuhlmann N et al. (2017) Initial elevations in glutamate and dopamine neurotransmission decline with age, as does exploratory behavior, in LRRK2 G2019S knock-in mice. eLife 6, e28377 10.7554/eLife.28377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matikainen-Ankney BA, Kezunovic N, Mesias RE, Tian Y, Williams FM, Huntley GW et al. (2016) Altered development of synapse structure and function in striatum caused by Parkinson’s disease-linked LRRK2-G2019S mutation. J. Neurosci 36, 7128–7141 10.1523/JNEUROSCI.3314-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steger M, Tonelli F, Ito G, Davies P, Trost M, Vetter M et al. (2016) Phosphoproteomics reveals that Parkinson’s disease kinase LRRK2 regulates a subset of Rab GTPases. eLife 5, e12813 10.7554/eLife.12813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mir R, Tonelli F, Lis P, Macartney T, Polinski NK, Martinez TN et al. (2018) The Parkinson’s disease VPS35[D620N] mutation enhances LRRK2-mediated Rab protein phosphorylation in mouse and human. Biochem. J 475, 1861–1883 10.1042/BCJ20180248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Volpicelli-Daley LA, Abdelmotilib H, Liu Z, Stoyka L, Daher JP, Milnerwood AJ et al. (2016) G2019S-LRRK2 expression augments α-synuclein sequestration into inclusions in neurons. J. Neurosci 36, 7415–7427 10.1523/JNEUROSCI.3642-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharpe NA and Tepper JM (1998) Postnatal development of excitatory synaptic input to the rat neostriatum: an electron microscopic study. Neuroscience 84, 1163–1175 10.1016/S0306-4522(97)00583-6 [DOI] [PubMed] [Google Scholar]
- 12.Westerlund M, Belin AC, Anvret A, Bickford P, Olson L and Galter D (2008) Developmental regulation of leucine-rich repeat kinase 1 and 2 expression in the brain and other rodent and human organs: implications for Parkinson’s disease. Neuroscience 152, 429–436 10.1016/j.neuroscience.2007.10.062 [DOI] [PubMed] [Google Scholar]
- 13.Davies P, Hinkle KM, Sukar NN, Sepulveda B, Mesias R, Serrano G et al. (2013) Comprehensive characterization and optimization of anti-LRRK2 (leucine-rich repeat kinase 2) monoclonal antibodies. Biochem. J 453, 101–113 10.1042/BJ20121742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.West AB, Cowell RM, Daher JP, Moehle MS, Hinkle KM, Melrose HL et al. (2014) Differential LRRK2 expression in the cortex, striatum, and substantia nigra in transgenic and nontransgenic rodents. J. Comp. Neurol 522, 2465–2480 10.1002/cne.23583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mandemakers W, Snellinx A, O’Neill MJ and de Strooper B (2012) LRRK2 expression is enriched in the striosomal compartment of mouse striatum. Neurobiol. Dis 48, 582–593 10.1016/j.nbd.2012.07.017 [DOI] [PubMed] [Google Scholar]
- 16.Giesert F, Hofmann A, Burger A, Zerle J, Kloos K, Hafen U et al. (2013) Expression analysis of Lrrk1, Lrrk2 and Lrrk2 splice variants in mice. PLoS ONE 8, e63778 10.1371/journal.pone.0063778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taymans JM, Van den Haute C and Baekelandt V (2006) Distribution of PINK1 and LRRK2 in rat and mouse brain. J. Neurochem 98, 951–961 10.1111/j.1471-4159.2006.03919.x [DOI] [PubMed] [Google Scholar]
- 18.Tepper JM, Sharpe NA, Koos TZ and Trent F (1998) Postnatal development of the rat neostriatum: electrophysiological, light- and electron-microscopic studies. Dev. Neurosci 20, 125–145 10.1159/000017308 [DOI] [PubMed] [Google Scholar]
- 19.Ibarra GR, Rodriguez JA, Paratcha GC and Azcurra JM (1995) Permanent alteration of muscarinic acetylcholine receptor binding in rat striatum after circling training during development. Brain Res 705, 39–44 10.1016/0006-8993(95)01076-9 [DOI] [PubMed] [Google Scholar]
- 20.Ibarra GR, Paratcha GC, Wolansky MJ and Azcurra JM (1996) Co-alteration of dopamine D2 receptor and muscarinic acetylcholine receptor binding in rat striatum after circling training. Neuroreport 7, 2491–2494 10.1097/00001756-199611040-00018 [DOI] [PubMed] [Google Scholar]
- 21.Simonetti T, Lee H, Bourke M, Leamey CA and Sawatari A (2009) Enrichment from birth accelerates the functional and cellular development of a motor control area in the mouse. PLoS ONE 4, e6780 10.1371/journal.pone.0006780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Biskup S, Moore DJ, Celsi F, Higashi S, West AB, Andrabi SA et al. (2006) Localization of LRRK2 to membranous and vesicular structures in mammalian brain. Ann. Neurol 60, 557–569 10.1002/ana.21019 [DOI] [PubMed] [Google Scholar]
- 23.Piccoli G, Condliffe SB, Bauer M, Giesert F, Boldt K, De Astis S et al. (2011) LRRK2 controls synaptic vesicle storage and mobilization within the recycling pool. J. Neurosci 31, 2225–2237 10.1523/JNEUROSCI.3730-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berger Z, Smith KA and Lavoie MJ (2011) Membrane localization of LRRK2 is associated with increased formation of the highly active LRRK2 dimer and changes in its phosphorylation. Biochemistry 49, 5511–5523 10.1021/bi100157u [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hinkle KM, Yue M, Behrouz B, Dachsel JC, Lincoln SJ, Bowles EE et al. (2012) LRRK2 knockout mice have an intact dopaminergic system but display alterations in exploratory and motor coordination behaviors. Mol. Neurodegener 7, 25 10.1186/1750-1326-7-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matta S, Van Kolen K, da Cunha R, van den Bogaart G, Mandemakers W, Miskiewicz K et al. (2012) LRRK2 controls an EndoA phosphorylation cycle in synaptic endocytosis. Neuron 75, 1008–1021 10.1016/j.neuron.2012.08.022 [DOI] [PubMed] [Google Scholar]
- 27.Arranz AM, Delbroek L, Van Kolen K, Guimaraes MR, Mandemakers W, Daneels G et al. (2015) LRRK2 functions in synaptic vesicle endocytosis through a kinase-dependent mechanism. J. Cell Sci 128, 541–552 10.1242/jcs.158196 [DOI] [PubMed] [Google Scholar]
- 28.Shin N, Jeong H, Kwon J, Heo HY, Kwon JJ, Yun HJ et al. (2008) LRRK2 regulates synaptic vesicle endocytosis. Exp. Cell Res 314, 2055–2065 10.1016/j.yexcr.2008.02.015 [DOI] [PubMed] [Google Scholar]
- 29.Pan PY, Li X, Wang J, Powell J, Wang Q, Zhang Y et al. (2017) Parkinson’s disease-associated LRRK2 hyperactive kinase mutant disrupts synaptic vesicle trafficking in ventral midbrain neurons. J. Neurosci 37, 11366–11376 10.1523/JNEUROSCI.0964-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nguyen M and Krainc D (2018) LRRK2 phosphorylation of auxilin mediates synaptic defects in dopaminergic neurons from patients with Parkinson’s disease. Proc. Natl Acad. Sci. U.S.A 115, 5576–5581 10.1073/pnas.1717590115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Belluzzi E, Gonnelli A, Cirnaru MD, Marte A, Plotegher N, Russo I et al. (2016) LRRK2 phosphorylates presynaptic N-ethylmaleimide sensitive fusion (NSF) protein enhancing its ATPase activity and SNARE complex disassembling rate. Mol. Neurodegener 11, 1 10.1186/s13024-015-0066-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parisiadou L, Yu J, Sgobio C, Xie C, Liu G, Sun L et al. (2014) LRRK2 regulates synaptogenesis and dopamine receptor activation through modulation of PKA activity. Nat. Neurosci 17, 367–376 10.1038/nn.3636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li X, Wang QJ, Pan N, Lee S, Zhao Y, Chait BT et al. (2011) Phosphorylation-dependent 14–3-3 binding to LRRK2 is impaired by common mutations of familial Parkinson’s disease. PLoS ONE 6, e17153 10.1371/journal.pone.0017153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muda K, Bertinetti D, Gesellchen F, Hermann JS, von Zweydorf F, Geerlof A et al. (2014) Parkinson-related LRRK2 mutation R1441C/G/H impairs PKA phosphorylation of LRRK2 and disrupts its interaction with 14–3-3. Proc. Natl Acad. Sci. U.S.A 111, E34–E43 10.1073/pnas.1312701111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reynolds A, Doggett EA, Riddle SM, Lebakken CS and Nichols RJ (2014) LRRK2 kinase activity and biology are not uniformly predicted by its autophosphorylation and cellular phosphorylation site status. Front. Mol. Neurosci 7, 54 10.3389/fnmol.2014.00054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taylor TN, Potgieter D, Anwar S, Senior SL, Janezic S, Threlfell S et al. (2014) Region-specific deficits in dopamine, but not norepinephrine, signaling in a novel A30P α-synuclein BAC transgenic mouse. Neurobiol. Dis 62, 193–207 10.1016/j.nbd.2013.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Janezic S, Threlfell S, Dodson PD, Dowie MJ, Taylor TN, Potgieter D et al. (2013) Deficits in dopaminergic transmission precede neuron loss and dysfunction in a new Parkinson model. Proc. Natl Acad. Sci. U.S.A 110, E4016–E4025 10.1073/pnas.1309143110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nemani VM, Lu W, Berge V, Nakamura K, Onoa B, Lee MK et al. (2010) Increased expression of α-synuclein reduces neurotransmitter release by inhibiting synaptic vesicle reclustering after endocytosis. Neuron 65, 66–79 10.1016/j.neuron.2009.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vargas KJ, Schrod N, Davis T, Fernandez-Busnadiego R, Taguchi YV, Laugks U et al. (2017) Synucleins have multiple effects on presynaptic architecture. Cell Rep 18, 161–173 10.1016/j.celrep.2016.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Busch DJ, Oliphint PA, Walsh RB, Banks SM, Woods WS, George JM et al. (2014) Acute increase of α-synuclein inhibits synaptic vesicle recycling evoked during intense stimulation. Mol. Biol. Cell 25, 3926–3941 10.1091/mbc.e14-02-0708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beccano-Kelly DA, Kuhlmann N, Tatarnikov I, Volta M, Munsie LN, Chou P et al. (2014) Synaptic function is modulated by LRRK2 and glutamate release is increased in cortical neurons of G2019S LRRK2 knock-in mice. Front. Cell. Neurosci 8, 301 10.3389/fncel.2014.00301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yue M, Hinkle KM, Davies P, Trushina E, Fiesel FC, Christenson TA et al. (2015) Progressive dopaminergic alterations and mitochondrial abnormalities in LRRK2 G2019S knock-in mice. Neurobiol. Dis 78, 172–195 10.1016/j.nbd.2015.02.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li X, Patel JC, Wang J, Avshalumov MV, Nicholson C, Buxbaum JD et al. (2010) Enhanced striatal dopamine transmission and motor performance with LRRK2 overexpression in mice is eliminated by familial Parkinson’s disease mutation G2019S. J. Neurosci 30, 1788–1797 10.1523/JNEUROSCI.5604-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kozorovitskiy Y, Peixoto R, Wang W, Saunders A and Sabatini BL (2015) Neuromodulation of excitatory synaptogenesis in striatal development. eLife 4, e10111 10.7554/eLife.10111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matikainen-Ankney BA, Kezunovic N, Menard C, Flanigan M, Zhong Y, Russo SJ et al. (2018) Parkinson’s disease-linked LRRK2-G2019S mutation alters synaptic plasticity and promotes resilience to chronic social stress in young adulthood. J. Neurosci 38, 9700–9711 10.1523/JNEUROSCI.1457-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kreitzer AC and Malenka RC (2007) Endocannabinoid-mediated rescue of striatal LTD and motor deficits in Parkinson’s disease models. Nature 445, 643–647 10.1038/nature05506 [DOI] [PubMed] [Google Scholar]
- 47.Brown TC, Correia SS, Petrok CN and Esteban JA (2007) Functional compartmentalization of endosomal trafficking for the synaptic delivery of AMPA receptors during long-term potentiation. J. Neurosci 27, 13311–13315 10.1523/JNEUROSCI.4258-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Temkin P, Morishita W, Goswami D, Arendt K, Chen L and Malenka R (2017) The retromer supports AMPA receptor trafficking during LTP. Neuron 94, 74–82.e5 10.1016/j.neuron.2017.03.020 [DOI] [PubMed] [Google Scholar]
- 49.Esteban JA, Shi SH, Wilson C, Nuriya M, Huganir RL and Malinow R (2003) PKA phosphorylation of AMPA receptor subunits controls synaptic trafficking underlying plasticity. Nat. Neurosci 6, 136–143 10.1038/nn997 [DOI] [PubMed] [Google Scholar]
- 50.Sweet ES, Saunier-Rebori B, Yue Z and Blitzer RD (2015) The Parkinson’s disease-associated mutation LRRK2-G2019S impairs synaptic plasticity in mouse hippocampus. J. Neurosci 35, 11190–11195 10.1523/JNEUROSCI.0040-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gaig C, Vilas D, Infante J, Sierra M, Garcia-Gorostiaga I, Buongiorno M et al. (2014) Nonmotor symptoms in LRRK2 G2019S associated Parkinson’s disease. PLoS ONE 9, e108982 10.1371/journal.pone.0108982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ishihara L and Brayne C (2006) A systematic review of depression and mental illness preceding Parkinson’s disease. Acta Neurol. Scand 113, 211–220 10.1111/j.1600-0404.2006.00579.x [DOI] [PubMed] [Google Scholar]
- 53.Hemmerle AM, Herman JP and Seroogy KB (2012) Stress, depression and Parkinson’s disease. Exp. Neurol 233, 79–86 10.1016/j.expneurol.2011.09.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smith AD, Castro SL and Zigmond MJ (2002) Stress-induced Parkinson’s disease: a working hypothesis. Physiol. Behav 77, 527–531 10.1016/S0031-9384(02)00939-3 [DOI] [PubMed] [Google Scholar]
- 55.Austin KW, Ameringer SW and Cloud LJ (2016) An integrated review of psychological stress in Parkinson’s disease: biological mechanisms and symptom and health outcomes. Parkinson’s Dis 2016, 9869712 10.1155/2016/9869712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Christoffel DJ, Golden SA and Russo SJ (2011) Structural and synaptic plasticity in stress-related disorders. Rev. Neurosci 22, 535–549 10.1515/RNS.2011.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Golden SA, Covington III HE, Berton O and Russo SJ (2011) A standardized protocol for repeated social defeat stress in mice. Nat. Protoc 6, 1183–1191 10.1038/nprot.2011.361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Menard C, Pfau ML, Hodes GE, Kana V, Wang VX, Bouchard S et al. (2017) Social stress induces neurovascular pathology promoting depression. Nat. Neurosci 20, 1752–1760 10.1038/s41593-017-0010-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Volta M, Cataldi S, Beccano-Kelly D, Munsie L, Tatarnikov I, Chou P et al. (2015) Chronic and acute LRRK2 silencing has no long-term behavioral effects, whereas wild-type and mutant LRRK2 overexpression induce motor and cognitive deficits and altered regulation of dopamine release. Parkinsonism Relat. Disord 21, 1156–1163 10.1016/j.parkreldis.2015.07.025 [DOI] [PubMed] [Google Scholar]
- 60.Bosch-Bouju C, Larrieu T, Linders L, Manzoni OJ and Laye S (2016) Endocannabinoid-mediated plasticity in nucleus accumbens controls vulnerability to anxiety after social defeat stress. Cell Rep 16, 1237–1242 10.1016/j.celrep.2016.06.082 [DOI] [PubMed] [Google Scholar]
- 61.Carlezon WAJ, Duman RS and Nestler EJ (2005) The many faces of CREB. Trends Neurosci 28, 436–445 10.1016/j.tins.2005.06.005 [DOI] [PubMed] [Google Scholar]
- 62.Han MH and Nestler EJ (2017) Neural substrates of depression and resilience. Neurotherapeutics 14, 677–686 10.1007/s13311-017-0527-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Francis TC and Lobo MK (2017) Emerging role for nucleus accumbens medium spiny neuron subtypes in depression. Biol. Psychiatry 81, 645–653 10.1016/j.biopsych.2016.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vialou V, Robison AJ, Laplant QC, Covington IIIHE, Dietz DM, Ohnishi YN et al. (2010) Deltafosb in brain reward circuits mediates resilience to stress and antidepressant responses. Nat. Neurosci 13, 745–752 10.1038/nn.2551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lim BK, Huang KW, Grueter BA, Rothwell PE and Malenka RC (2012) Anhedonia requires MC4R-mediated synaptic adaptations in nucleus accumbens. Nature 487, 183–189 10.1038/nature11160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Longo F, Russo I, Shimshek DR, Greggio E and Morari M (2014) Genetic and pharmacological evidence that G2019S LRRK2 confers a hyperkinetic phenotype, resistant to motor decline associated with aging. Neurobiol. Dis 71, 62–73 10.1016/j.nbd.2014.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lim J, Bang Y, Choi JH, Han A, Kwon MS, Liu KH et al. (2018) LRRK2 g2019s induces anxiety/depression-like behavior before the onset of motor dysfunction with 5-HT1A receptor upregulation in mice. J. Neurosci 38, 1611–1621 10.1523/JNEUROSCI.4051-15.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Munsie LN, Milnerwood AJ, Seibler P, Beccano-Kelly DA, Tatarnikov I, Khinda J et al. (2015) Retromer-dependent neurotransmitter receptor trafficking to synapses is altered by the Parkinson’s disease VPS35 mutation p.D620N. Hum. Mol. Genetics 24, 1691–1703 10.1093/hmg/ddu582 [DOI] [PubMed] [Google Scholar]