Abstract
Dasatinib, a second-generation BCR-ABL1 tyrosine kinase inhibitor, is approved for the treatment of chronic myeloid leukemia and Philadelphia chromosome-positive acute lymphoblastic leukemia, both as first-line therapy and after imatinib intolerance or resistance. While generally well tolerated, dasatinib has been associated with a higher risk for pleural effusions. Frequency, risk factors, and outcomes associated with pleural effusion were assessed in two phase 3 trials (DASISION and 034/Dose-optimization) and a pooled population of 11 trials that evaluated patients with chronic myeloid leukemia and Philadelphia chromosome-positive acute lymphoblastic leukemia treated with dasatinib (including DASISION and 034/Dose-optimization). In this largest assessment of patients across the dasatinib clinical trial program (N=2712), pleural effusion developed in 6-9% of patients at risk annually in DASISION, and in 5-15% of patients at risk annually in 034/Dose-optimization. With a minimum follow up of 5 and 7 years, drug-related pleural effusion occurred in 28% of patients in DASISION and in 33% of patients in 034/Dose-optimization, respectively. A significant risk factor identified for developing pleural effusion by a multivariate analysis was age. We found that overall responses to dasatinib, progression-free survival, and overall survival were similar in patients who developed pleural effusion and in patients who did not. clinicaltrials.gov identifier 00481247; 00123474.
Introduction
Dasatinib is a potent second-generation BCR-ABL1 tyrosine kinase inhibitor (TKI) approved at 100 mg once daily (QD) as first-line therapy in patients with chronic myeloid leukemia in chronic phase (CML-CP), and in patients with CML-CP who are resistant to or intolerant of prior therapy.1 Dasatinib is also approved at 140 mg QD in patients with accelerated or blast phase CML (CML-AP/BP) or Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL) with resistance to or intolerance of prior therapy.2–4
Although fluid retention has been associated with TKIs used to treat CML, pleural effusions, specifically with exudate characteristics, have been reported more commonly with dasatinib.1,5,6 The exact mechanisms behind treatment-related pleural effusion remain to be elucidated; however, it has been suggested that immune mechanisms may play a role, based on reports of association with lymphocytosis and the presence of lymphocyte-dominant exudates and chyle accumulate.7–9 Alternatively, pleural effusion (with or without exudates) may also occur through inhibition of platelet-derived growth factor receptor-β or SRC-family kinases.10,11 In clinical practice, pleural effusion observed with dasatinib therapy remains a concern for both patients and prescribers.
Risk factors for pleural effusion in dasatinib-treated patients have been described in previous reports, including advanced age, advanced disease, heart disease, preexisting hypertension, hypercholesterolemia, autoimmune disease, rash, drug dose and schedule, and the presence of lymphocytosis.10,12–14 A correlation with plasma trough level, drug exposure, treatment duration, and depth of response to treatment has been suggested, but not confirmed.15
Here, we present an analysis of the proportion of patients with pleural effusion by grade, and efficacy outcomes in these patients, in dasatinib clinical trials. Additionally, we report the results of multivariate analyses of dasatinib clinical trial data, exploring factors associated with the development of pleural effusion in dasatinib-treated patients.
Methods
Patient populations
DASISION (CA180-056)
In the phase 3 dasatinib versus imatinib study in treatment-naive CML patients (DASISION [CA180-056]; clinicaltrials.gov identifier 00481247), 519 patients with newly diagnosed CML-CP were randomized to receive either 100 mg QD dasatinib (n=259) or 400 mg QD imatinib (n=260).16–18 The primary endpoint was the proportion of patients with confirmed complete cytogenetic responses (CCyR) by 12 months. Patients included in this report had a minimum of 5 years of follow up.19
034/Dose-optimization (CA180-034)
In the phase 3 dose-optimization study in imatinib-resistant or -intolerant CML-CP patients (CA180-034; clinicaltrials.gov identifier 00123474), 670 patients with CML-CP intolerant of or resistant to imatinib were randomized to receive dasatinib 100 mg QD (n=167), 140 mg QD (n=167), 50 mg twice daily (BID; n=168), or 70 mg BID (n=168).20–22 A subset of patients modified their dose over the course of the study; however, analyses of data were performed according to each patient’s initial randomization. The primary endpoint was the percentage of patients with major cytogenetic response (MCyR) after a minimum follow up of 6 months. Patients included in this report had a minimum of 7 years of follow up.23
Pooled population of patients with Ph+ leukemia
Patients (N=2712) with Ph+ leukemia who were treated with first- or second-line dasatinib 15 mg to 240 mg QD in 1 of 11 phase 1, 2, or 3 trials were included in these analyses (Online Supplementary Table S1).16,19,21,23–31 DASISION and 034/Dose-optimization were analyzed separately and as part of the pooled population for this report. In total, 324 newly diagnosed patients treated with dasatinib 100 mg QD (DASISION [n=258], CA180-363 [n=66]), and 2388 patients with CML (CML-CP [n=1294], advanced phases of CML [n=958]) or Ph+ ALL (n=136) previously treated with imatinib were included. Previously treated patients received dasatinib at daily doses ranging from 15 mg to 240 mg administered once or in divided doses daily.
Assessments
Pleural effusions were monitored continuously in treated patients who received at least 1 dose of study drug (DASISION [n=258], 034/Dose-optimization [n=662], pooled population [n=2712]). Pleural effusion by first onset is presented by year for patients at risk (the number of patients who were treated within a year and did not have pleural effusion). Effusions were graded according to the Common Terminology Criteria for Adverse Events Version 3.032 in DASISION and 034/Dose-optimization. Additional description of assessments and statistical analyses can be found in the Online Supplementary Material.
Each study protocol was approved by all institutional review boards, ethics committees, and national competent authorities at participating sites.
Results
Incidence of pleural effusion
DASISION
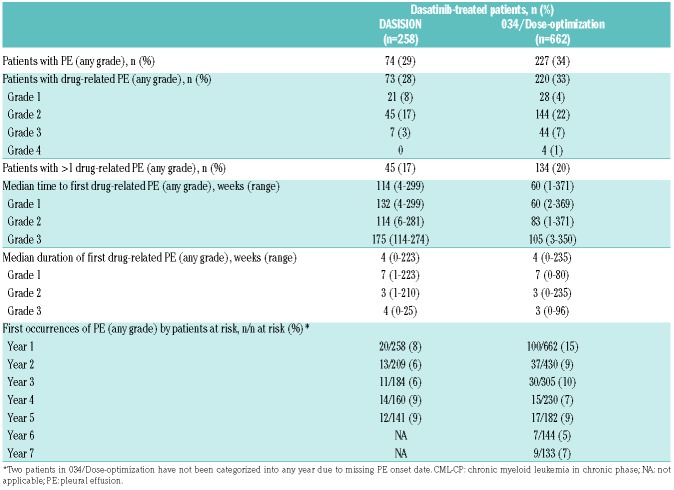
With a minimum of 5 years of follow up, 73 (28%) patients in DASISION reported drug-related pleural effusion (Table 1). One case of pleural effusion was not attributed to dasatinib by the investigator (Grade 2) and occurred >30 days after the last study dose was given. Most drug-related pleural effusions were Grade 1 (8%) or Grade 2 (17%), and no Grade 4 events were reported. The median duration of all first cases of drug-related pleural effusion was 4 weeks. The median daily dose of dasatinib for patients who developed drug-related pleural effusion was 100 mg, similar to the overall dasatinib-treated population.19
Table 1.
Summary of pleural effusion events in dasatinib-treated patients with CML-CP in DASISION and 034/Dose-optimization.
New cases of pleural effusion (any grade) occurred in 8% of patients receiving dasatinib in the first year of the study, and 6-9% each remaining year up to 5 years, suggesting a steady but continuous risk over time (Table 1). The proportion of patients with a recurrent (>1) drugrelated pleural effusion (any grade) was 62% (n=45/73). Of these, 16 patients had 2 separate events, 6 had 3 separate events, 4 had 4 separate events, and 19 had ≥5 separate events. Twelve patients discontinued due to recurrent pleural effusion. Of these patients, 9 had ≥3 recurring events. Dose interruptions and dose reductions due to pleural effusion occurred in 62% and 41% of patients, respectively.
034/Dose-optimization
With a minimum follow up of 7 years, drug-related pleural effusion (any grade) occurred in 33% of patients (Table 1). Although the median dose of dasatinib for patients with drug-related pleural effusion (any grade) was 100 mg daily (range 0-180 mg), pleural effusion rates varied across dosing groups in 034/Dose-optimization, with 28% (n=46/165) of patients experiencing any-grade pleural effusion in the 100 mg QD group and 35% (n=174/497) in the other groups. The median duration of first pleural effusion was 4 weeks. The majority of drug-related pleural effusions were Grade 1/2, and Grade 3/4 drug-related pleural effusions were reported in 48 (7%) patients in the total treated population. Of these, 8 occurred in the 100 mg QD group and 40 in the remaining groups. First occurrences of pleural effusion (any grade) occurred in 15% of dasatinib-treated patients in the first year of the study, and in 5-10% per year up to 7 years. Recurrent (>1) drug-related pleural effusion (any grade) occurred in 61% (n=134/220). Of these, 54 patients had 2 separate events, 31 had 3 separate events, 16 had 4 separate events, and 33 had ≥5 separate events. In all 034/Dose-optimization dose groups, discontinuation due to recurrent pleural effusion occurred in 46 (7%) patients.
The cumulative incidence rate of pleural effusion was lower in the 100 mg QD group than in dose groups and increased over time (at 2 years 15% vs. 24%, respectively; at 7 years 27% vs. 36%, respectively). Similarly, the cumulative incidence rate of Grade 3/4 pleural effusion was lower in the 100 mg QD group than in the other dose groups (at 7 years 5% vs. 9%, respectively), and increased over time (at 2 years 2% vs. 4%, respectively; at 5 years 4% vs. 7%, respectively). Within year 7 of the study, new cases of pleural effusion occurred in 5% (2/42) of patients at risk treated with dasatinib 100 mg QD and in 8% (7/91) of patients at risk in the other treatment arms. The cumulative rates of drug-related pleural effusion over time for the 100 mg QD arm were 14% at 2 years, 24% at 5 years, and 28% at 7 years. For the other treatment arms, the cumulative rates of drug-related pleural effusion over time were 24% at 2 years, 32% at 5 years, and 35% at 7 years.
The incidence of pleural effusion was also lower in imatinib-intolerant and imatinib-resistant patients receiving 100 mg QD than in the other dose groups. Pleural effusion (any grade) was reported in 19% of patients who were imatinib-intolerant in the 100 mg QD arm and in 43% of imatinib-intolerant patients in the other dose groups, while pleural effusion (any grade) was reported in 31% of imatinib-resistant patients in the 100 mg QD arm and in 35% in the other dose groups. Pleural effusions were managed with dose interruptions in 44% of patients.
Pooled population of patients with Ph+ leukemia
Eleven dasatinib clinical trials of patients with Ph+ leukemia, including the DASISION and 034/Dose-optimization trials, were pooled for this analysis to include the largest number of dasatinib-treated patients possible. Pleural effusion of any grade from any cause occurred in 946 patients (35%), 553 (34%) with CML-CP and 393 (36%) with CML-AP/BP or Ph+ ALL. Grade 3/4 pleural effusions were reported in 223 (8.2%) patients, 119 (7%) with CML-CP, and 104 (10%) with CML-AP/BP or Ph+ ALL. Deaths due to pleural effusion were reported in 5 (<1%) patients, all with CML-AP/BP or Ph+ ALL (4 were not receiving the currently approved dose of 140 mg QD dasatinib1). Drug-related pleural effusion of any grade occurred in 538 (33%) patients with CML-CP and 345 (32%) with CML-AP/BP or Ph+ ALL (883 [33%] patients total). Grade 3/4 drug-related pleural effusion episodes were reported in 114 (7%) patients with CML-CP and 93 (9%) patients with CML-AP/BP or Ph+ ALL (207 [8%] patients in total). One drug-related death was reported (<1%) in a patient with CML-AP/BP or Ph+ ALL.
Risk factors for pleural effusion
Based on risk factors for pleural effusion in dasatinib-treated patients described in literature,9,11–13 retrospective multivariate analyses were performed to investigate the association between pleural effusion and baseline hypertension, age, and lymphocytosis in patients treated with dasatinib, as well as additional variables of interest including sex, region, dosing schedule (034/Dose-optimization only), baseline Euro (Hasford) risk scores (DASISION only), exposure to interferon alpha (034/Dose-optimization only), BCR-ABL1 levels, baseline parameters, major molecular response (MMR) at 12 months, line of therapy, duration of prior TKI therapy, and depth of MR at any time. Average daily dose, prior skin rash, and prior autoimmune or lung disease were assessed in the DASISION/034/Dose-optimization pooled population only.
DASISION
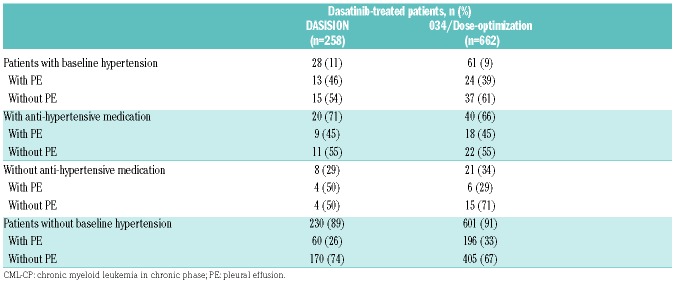
In DASISION, 28 (11%) patients had hypertension, and 13 of 28 (46%) patients with hypertension developed drug-related pleural effusion (Table 2). Of the 13 patients who developed pleural effusion, 9 were taking antihypertensive medications. When the relation between hypertension and pleural effusion was assessed in a multivariate analysis, there was no significant association found (Figure 1A). Pulmonary hypertension was reported in 14 (5%) dasatinib-treated patients, 9 of whom had pleural effusion. One patient with pulmonary hypertension underwent right heart catheterization in order to confirm pulmonary arterial hypertension (PAH); however, the procedure did not support a diagnosis of PAH. Twelve of the 14 pulmonary hypertension diagnoses were drug related.19 In DASISION, the correlation between pulmonary hypertension and pleural effusion was not confirmed.
Table 2.
Hypertension by occurrence of drug-related pleural effusion in dasatinib-treated patients with CML-CP in DASISION and 034/Dose-optimization.
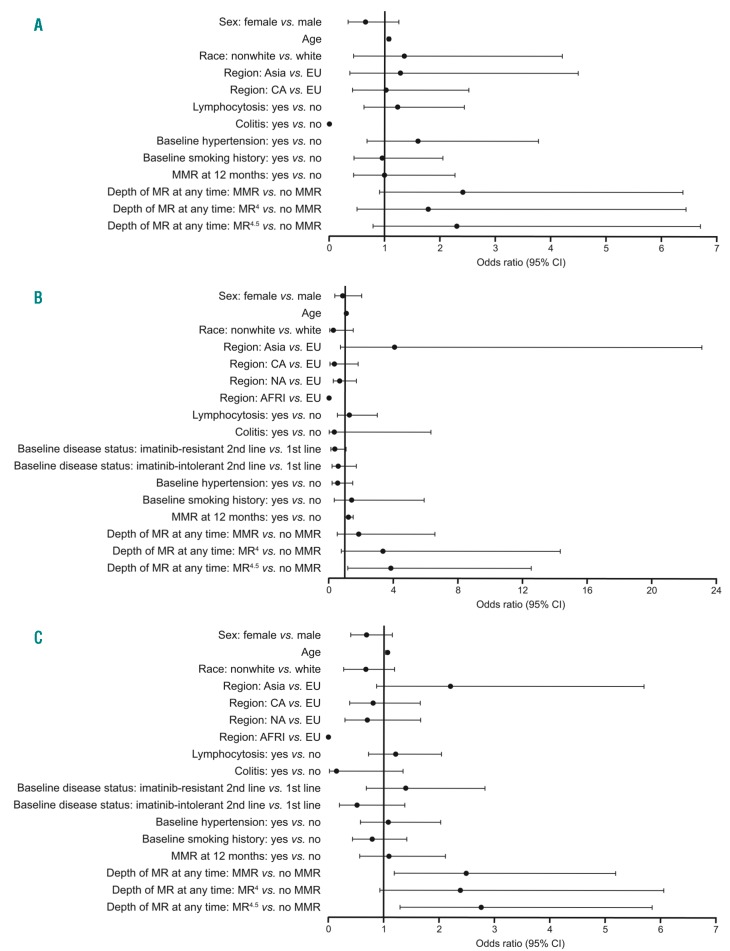
Figure 1.
Retrospective multivariate analysis to determine if there was an association between pleural effusion and potential risk factors. Odds ratios (ORs) with 95% confidence intervals (CIs) are shown for potential predictive variables listed for patients with CML-CP treated with 100 mg QD dasatinib from DASISION (A), 034/Dose-optimization (B), or both DASISION and 034/Dose-optimization (C). AFRI: Africa; CA: Caribbean; CML-CP: chronic myeloid leukemia in chronic phase; EU: European Union; MMR: major molecular response, BCR-ABL1 transcripts ≤0.1% on the International Scale (IS) corresponding to a 3-log reduction from a standardized baseline; MR4: BCR-ABL1 transcripts <0.01% (IS) corresponding to a 4-log reduction from a standardized baseline; MR4.5: BCR-ABL1 transcripts ≤0.0032% (IS) corresponding to a 4.5-log reduction from a standardized baseline; NA: North America; PE: pleural effusion; QD: once daily.
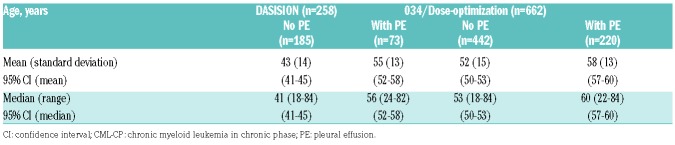
The median age of patients who developed drug-related pleural effusion in DASISION was 56 years, whereas for patients who did not develop pleural effusions it was 41 years (Table 3). Of significance, older age was found to be a risk factor for developing pleural effusion (odds ratio [OR] 1.067; 95% confidence interval [CI] 1.041–1.094; Figure 1A). These results were confirmed using a reduced model multivariate analysis.
Table 3.
Age by occurrence of drug-related pleural effusion in dasatinib-treated patients with CML-CP in DASISION and 034/Dose-optimization.
In DASISION, 29 (11%) patients had lymphocytosis at any time prior to pleural effusion, and 0 patients developed lymphocytosis after. However, lymphocytosis was not found to be a significant risk factor for the development of pleural effusion by multivariate analysis (Figure 1A).
Of the patients receiving 100 mg QD dasatinib in the DASISION trial, 86 (33%) patients had low-risk Euro scores, 124 (48%) had intermediate-risk Euro scores, and 49 (19%) had high-risk Euro scores.16 Using a reduced model multivariate analysis, patients with intermediate-risk Euro scores were not found to be at an increased risk of developing pleural effusion compared with patients with low-risk Euro scores. Similarly, no association was observed between pleural effusion and patients with high-risk Euro scores compared with patients with low-risk Euro scores.
The remaining variables investigated via multivariate analysis were found not to have an association with pleural effusion (Figure 1A).
034/Dose-optimization
Similar to DASISION, no significant association between pleural effusion and hypertension was found for patients in 034/Dose-optimization treated with dasatinib 100 mg QD, the currently approved dose for CML-CP1 (Figure 1B). Twenty-four of 61 (39%) patients from any treatment arm with hypertension developed drug-related pleural effusion (Table 2). Of these 24 patients, 18 were taking antihypertensive medication. Pulmonary hypertension (any grade) was reported in 3 (2%) patients in the 100 mg QD dose group and in 13 (3%) patients in the other dose groups.23 One patient (<1%) in the 100 mg QD dose group reported severe drug-related PAH, confirmed with a right heart catheterization procedure.23 Similar to DASISION, the association between pulmonary hypertension and pleural effusion was not confirmed in these patients.
In 034/Dose-optimization, the median age of all patients who developed drug-related pleural effusion was 60 years; patients who did not develop pleural effusion had a median age of 53 years (Table 3). Advanced age was found to be a significant risk factor for pleural effusion in patients treated with 100 mg QD dasatinib in a multivariate model (OR 1.074; 95% CI 1.035-1.114; Figure 1B). In addition to age, a strong statistical correlation was observed between the depth of MR and the overall incidence of pleural effusion, for those treated with 100 mg QD dasatinib (OR 3.851; 95% CI 1.182–12.552) (Figure 1B). Both age and depth of MR were confirmed as risk factors by a reduced model multivariate analysis.
Lymphocytosis was not found to be associated with the development of pleural effusion in a multivariate analysis of patients treated with 100 mg QD dasatinib (Figure 1B); overall, 17 (10%) patients had lymphocytosis before the onset of pleural effusion versus 0 after.
No other associations were found between pleural effusion and the remaining variables analyzed via multivariate analysis (Figure 1B).
Pooled DASISION and 034/Dose-optimization
To assess the relation between potential prognostic factors and pleural effusion in a larger population, patients treated with 100 mg QD dasatinib from both DASISION (n=258) and 034/Dose-optimization (n=165) were pooled (n=423), and a multivariate analysis was performed (Figure 1C). In this pooled population, patients in MMR (OR 2.482; 95% CI 1.191–5.174) and MR4.5 (OR 2.756; 95% CI 1.30–5.841) had a significantly increased risk of developing pleural effusion compared with patients not in MMR. Increased age also was found to be a significant risk factor (OR 1.069; 95% CI 1.048–1.091).
In the pooled DASISION and 034/Dose-optimization patient population, 40 (9%) patients had a history of lung disease, and 13 (3%) patients had a history of autoimmune disease. When the relationship between pleural effusion and prior lung disease, autoimmune disease, or skin rash was assessed using a reduced model multivariate analysis, no association was found. Average daily dasatinib dose also was not found to be a risk factor using the reduced model.
In the reduced multivariate model, imatinib-intolerant patients receiving second-line dasatinib had a significantly increased risk of developing pleural effusion compared with first-line patients (OR 0.232; 95% CI 0.086-0.623).
Efficacy of patients with pleural effusion
DASISION
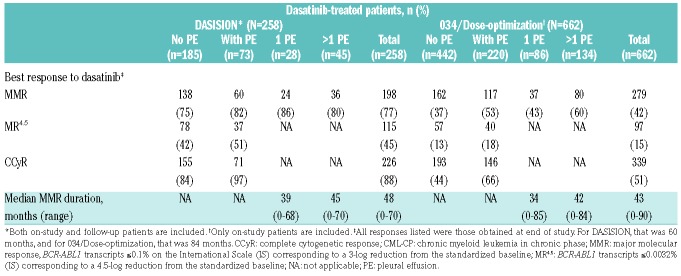
Of 73 dasatinib-treated patients in the DASISION trial with drug-related pleural effusion, 97% achieved CCyR, 82% had MMR, and 51% had MR4.5 (Table 4). These results are comparable to those in patients who did not have drug-related pleural effusion, and reflective of the overall responses for the entire study population.17 Duration of MMR was 39 months for those with 1 drug-related pleural effusion and 45 months for those with >1 (Table 4). Five-year progression-free survival (PFS) was similar for patients with or without drug-related pleural effusion (Online Supplementary Figure S1A). Of patients who experienced drug-related pleural effusion events, 5/73 (7%) progressed, whereas 21/185 (11%) patients who did not experience pleural effusion events progressed. Overall survival (OS) was similar in patients who did or did not experience drug-related pleural effusion as well (Online Supplementary Figure S1B).
Table 4.
Efficacy of dasatinib by occurrence of drug-related pleural effusion in dasatinib-treated patients with CML-CP in DASISION and 034/Dose-optimization.
Many patients did achieve cytogenetic or molecular responses prior to the first occurrence of drug-related pleural effusion, and these responses were often maintained or improved despite dose modifications required to manage the effusion (Online Supplementary Table S2). Sixty-five patients (89% of patients with a drug-related pleural effusion) had CCyR, 35 (48%) had MMR, and 12 (16%) had MR4.5 prior to experiencing their first pleural effusion event. Following the first pleural effusion, 55 patients maintained/improved to CCyR. Similarly, 19 patients maintained/improved to MMR, and 32 patients maintained/improved to MR4.5. One patient went from MMR to BCR-ABL1 0.1-≤1% following their first pleural effusion event. No patient had BCR-ABL1 >10% or lost any cytogenetic response after their first event.
034/Dose-optimization
Of 220 dasatinib-treated patients in 034/Dose-optimization who had drug-related pleural effusion, 66% achieved CCyR, 53% had MMR, and 18% had MR4.5 (Table 4). As seen in the DASISION trial, molecular responses in the 034/Dose-optimization trial for patients who had drug-related pleural effusion events versus patients who did not were similar, although a trend showed slightly higher molecular responses for those who experienced pleural effusions. The duration of MMR was 34 months for those with 1 drug-related pleural effusion, and 42 months for those with >1 (Table 4). At the end of the 034/Dose-optimization 7-year study, PFS for dasatinib-treated patients who experienced drug-related pleural effusion events was similar to PFS for patients who did not experience effusion events (Online Supplementary Figure S2A). Among patients who had drug-related pleural effusion, 93/220 (42%) progressed, compared with 220/442 (50%) patients who did not have pleural effusion but did progress. Seven-year OS in 034/Dose-optimization was similar across patients with and without drug-related pleural effusion (Online Supplementary Figure S2B).
As seen in DASISION, patients in 034/Dose-optimization were able to achieve responses prior to their first event, and these responses were maintained or improved following the pleural effusion in some patients (Online Supplementary Table S3). One hundred and sixteen patients (53% of patients with a drug-related pleural effusion evaluable for efficacy endpoints) had CCyR, 66 (30%) had MMR, and 15 (7%) had MR4.5 prior to experiencing a first case of pleural effusion. Following the effusion, 55 patients maintained or improved to CCyR and 9 patients lost CCyR. Changes in the depth of molecular response were also similar: 55 patients maintained/improved to MMR, and 31 patients maintained/improved to MR4.5. Three patients went from MMR to BCR-ABL1 0.1-≤1% following their first pleural effusion event. Of these patients, all had BCR-ABL1 >0.1% after their first event. Two patients in 034/Dose-optimization were excluded from the efficacy analysis because the date of onset of pleural effusion was not captured.
Discussion
Pleural effusion has been reported in dasatinib-treated patients at any time during the course of treatment, though the severity is generally mild to moderate. Grade 1 effusions are often asymptomatic and may not have been picked up in the absence of routine chest X-rays (only required in the DASISION trial), potentially reducing the incidence of clinically significant effusions in this patient population. However, we cannot comment that Grade 1 pleural effusions would not progress with time. The risk of pleural effusion remains even after long-term dasatinib treatment in effusion-naive patients; thus, maintaining awareness of the risk is important.
As fluid retention events have been reported with most BCR-ABL1-targeted TKIs, it is tempting to attribute the occurrence of pleural effusion to a class effect on fluid overload. However, an immune-mediated mechanism is more likely for dasatinib-related pleural effusion, as exudate containing high lymphocyte counts (predominantly natural killer cells) and chyle accumulate have been reported in pleural fluids and tissue from patients on dasatinib.15,33,34 Pleural effusion developed slightly more often in patients with lymphocytosis than in patients without lymphocytosis (33% with lymphocytosis vs. 26% without lymphocytosis) in the DASISION trial, although this difference was not statistically significant.35 We found that lymphocytosis occurred during therapy, which appears to represent a risk factor for pleural effusion because it preceded pleural effusion events.
Through multivariate analyses, we determined that race, sex, region, exposure to interferon, BCR-ABL1 levels at 3 months, lymphocytosis, colitis, history of autoimmune disease, history of lung disease, history of skin rash, baseline smoking history, MMR at 12 months, average daily dose, line of therapy, baseline Euro risk scores, and duration of prior TKI therapy were not associated with an increased risk of pleural effusion. Other risk factors for pleural effusion previously described include advanced disease, heart disease, and hypercholesterolemia.10,12–14 It is difficult to analyze the association between the incidence of pleural effusion and the depth of molecular response achieved without correcting for the time of dasatinib exposure, given that most patients achieving deep molecular responses are typically on dasatinib longer and would therefore be expected to have a greater risk of developing pleural effusion. To address this, we evaluated MMR at 12 months as a potential risk factor; however, no association was observed. We found second-line patients with previous intolerance of imatinib to be at an increased risk of developing pleural effusion compared with first-line patients, although no association was observed for second-line imatinib-resistant patients. Finally, although effusions can develop in adults at any age, we determined that advanced age was the only significant patient risk factor for pleural effusion, particularly in those treated with 100 mg/day of dasatinib. Additional potential risk factors, such as a history of hypertension, can develop during treatment with dasatinib, and should be considered when evaluating individual patients, though the association between pleural effusion and hypertension was not substantiated in this analysis.
Dasatinib dose, as a potential risk factor for pleural effusion, is of special interest. Wang et al. noted that pleural effusion was associated with trough drug concentrations, indicating that dasatinib pharmacokinetics may play a role in the development of pleural effusion.13 We did not find dasatinib dose to be a risk factor for the development of pleural effusion in the pooled population of patients initially treated with 100 mg QD dasatinib. However, since patients with advanced disease in the pooled population of dasatinib-treated patients with Ph+ leukemia described here were treated with higher doses of dasatinib (up to 240 mg daily) than patients with CML-CP, dasatinib dose may still be associated with the increased rate of Grade 3/4 pleural effusions observed. Also, we reported that pleural effusion (any grade) was observed in a lower percentage of patients in the 100 mg QD arm versus the other dose groups in 034/Dose-optimization. A retrospective study evaluating the toxicity-guided administration of a reduced-dose dasatinib regimen in similar imatinib-resistant/intolerant patients revealed that on/off treatment significantly reduced pleural effusions.36 Furthermore, recent sub-analyses of DASISION revealed that dose reductions for adverse events, including pleural effusion, did not affect dasatinib efficacy,37,38 suggesting that it may be possible to administer lower doses to populations at higher risk for pleural effusion development. Moreover, we found that the achievement of MMR and MR4.5 was found to be correlated with a higher risk of developing pleural effusion. This may be because patients without MMR may have discontinued dasatinib treatment earlier than those with a response. This supports a hypothesis that pleural effusion may be a marker for longevity of treatment: As patients do well on dasatinib and remain on treatment longer, they may be more likely to develop a pleural effusion. Further investigation into the relation between duration of treatment and pleural effusion is warranted.
Clinical data on molecular responses for patients who experienced pleural effusions during treatment with dasatinib are limited, though our observations indicate that patients who experienced pleural effusions while on dasatinib had similar responses to treatment as those who did not develop pleural effusion. A retrospective study examining dasatinib-related pleural effusion in CML patients across 21 hematologic centers in Italy revealed that at the time of the first effusion, 28.6% were in MMR and 37.8% were in MR4.5.39
In summary, pleural effusion is an adverse event seen disproportionately in patients treated with dasatinib; however, the management of pleural effusion by dose reductions does not negatively affect the response rate to dasatinib. Advanced age and longevity of treatment were found to be predictive risk factors for the development of pleural effusion.
Supplementary Material
Acknowledgments
The authors would like to thank the patients and families for making these Bristol-Myers Squibb-sponsored trials possible.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/104/1/93
Funding
This analysis was supported by funding from Bristol-Myers Squibb. The Bristol-Myers Squibb policy on data sharing may be found at: https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html. Professional medical writing and editorial assistance was provided by Samantha L. Dwyer, PhD, and Jessica Franciosi, PhD, of StemScientific, an Ashfield Company, part of UDG Healthcare plc, funded by Bristol-Myers Squibb.
References
- 1.Sprycel (dasatinib) [prescribing information]. Princeton, NJ: Bristol-Myers Squibb Company; 2017. [Google Scholar]
- 2.Kantarjian H, Cortes J, Kim DW, et al. Phase 3 study of dasatinib 140 mg once daily versus 70 mg twice daily in patients with chronic myeloid leukemia in accelerated phase resistant or intolerant to imatinib: 15-month median follow-up. Blood. 2009;113(25):6322–6329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lilly MB, Ottmann OG, Shah NP, et al. Dasatinib 140 mg once daily versus 70 mg twice daily in patients with Ph-positive acute lymphoblastic leukemia who failed imatinib: results from a phase 3 study. Am J Hematol. 2010;85(3):164–170. [DOI] [PubMed] [Google Scholar]
- 4.Saglio G, Hochhaus A, Goh YT, et al. Dasatinib in imatinib-resistant or imatinib-intolerant chronic myeloid leukemia in blast phase after 2 years of follow-up in a phase 3 study: efficacy and tolerability of 140 milligrams once daily and 70 milligrams twice daily. Cancer. 2010; 116(16):3852–3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gleevec (imatinib) [prescribing information]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2017. [Google Scholar]
- 6.Tasigna (nilotinib) [prescribing information]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2017. [Google Scholar]
- 7.Porkka K, Khoury HJ, Paquette RL, Matloub Y, Sinha R, Cortes JE. Dasatinib 100 mg once daily minimizes the occurrence of pleural effusion in patients with chronic myeloid leukemia in chronic phase and efficacy is unaffected in patients who develop pleural effusion. Cancer. 2010; 116(2):377–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mustjoki S, Ekblom M, Arstila TP, et al. Clonal expansion of T/NK-cells during tyrosine kinase inhibitor dasatinib therapy. Leukemia. 2009;23(8):1398–1405. [DOI] [PubMed] [Google Scholar]
- 9.Agrawal V, Doelken P, Sahn SA. Pleural fluid analysis in chylous pleural effusion. Chest. 2008;133(6):1436–1441. [DOI] [PubMed] [Google Scholar]
- 10.Quintas-Cardama A, Kantarjian H, O’Brien S, et al. Pleural effusion in patients with chronic myelogenous leukemia treated with dasatinib after imatinib failure. J Clin Oncol. 2007;25(25):3908–3914. [DOI] [PubMed] [Google Scholar]
- 11.Buettner R, Mesa T, Vultur A, Lee F, Jove R. Inhibition of Src family kinases with dasatinib blocks migration and invasion of human melanoma cells. Mol Cancer Res. 2008;6(11):1766–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang X, Roy A, Hochhaus A, Kantarjian HM, Chen T-T, Shah NP. Differential effects of dosing regimen on the safety and efficacy of dasatinib: retrospective exposure–response analysis of a Phase III study. Clin Pharmacol. 2013;5:85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conchon M, Freitas CM, Rego MA, Braga Junior JW. Dasatinib—clinical trials and management of adverse events in imatinib resistant/intolerant chronic myeloid leukemia. Rev Bras Hematol Hemoter. 2011;33(2):131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Lavallade H, Punnialingam S, Milojkovic D, et al. Pleural effusions in patients with chronic myeloid leukaemia treated with dasatinib may have an immune-mediated pathogenesis. Br J Haematol. 2008; 141(5):745–747. [DOI] [PubMed] [Google Scholar]
- 15.Wang X, Roy A, Hochhaus A, Kantarjian HM, Chen TT, Shah NP. Differential effects of dosing regimen on the safety and efficacy of dasatinib: retrospective exposure-response analysis of phase III study. Clin Pharmacol. 2013;10(5):85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kantarjian H, Shah NP, Hochhaus A, et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2010; 362(24):2260–2270. [DOI] [PubMed] [Google Scholar]
- 17.Kantarjian HM, Shah NP, Cortes JE, et al. Dasatinib or imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: 2-year follow-up from a randomized phase 3 trial (DASISION). Blood. 2012; 119(5):1123–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jabbour E, Kantarjian HM, Saglio G, et al. Early response with dasatinib or imatinib in chronic myeloid leukemia: 3-year followup from a randomized phase 3 trial (DASISION). Blood. 2014;123(4):494–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cortes JE, Saglio G, Kantarjian HM, et al. Final 5-year study results of DASISION: the Dasatinib Versus Imatinib Study in Treatment-Naïve Chronic Myeloid Leukemia Patients Trial. J Clin Oncol. 2016;34(20):2333–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shah NP, Guilhot F, Cortes JE, et al. Long-term outcome with dasatinib after imatinib failure in chronic-phase chronic myeloid leukemia: follow-up of a phase 3 study. Blood. 2014;123(15):2317–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shah NP, Kantarjian HM, Kim DW, et al. Intermittent target inhibition with dasatinib 100 mg once daily preserves efficacy and improves tolerability in imatinib-resistant and -intolerant chronic-phase chronic myeloid leukemia. J Clin Oncol. 2008;26(19):3204–3212. [DOI] [PubMed] [Google Scholar]
- 22.Shah NP, Kim DW, Kantarjian H, et al. Potent, transient inhibition of BCR-ABL with dasatinib 100 mg daily achieves rapid and durable cytogenetic responses and high transformation-free survival rates in chronic phase chronic myeloid leukemia patients with resistance, suboptimal response or intolerance to imatinib. Haematologica. 2010;95(2):232–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shah NP, Rousselot P, Schiffer CA, et al. Dasatinib in imatinib-resistant or -intolerant chronic-phase, chronic myeloid leukemia patients: 7-year follow-up of study CA180-034. Am J Hematol. 2016; 91(9):869–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saglio G, le Coutre P, Cortes J, et al. Safety and tolerability of dasatinib in patients with chronic myeloid leukemia (CML) and Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL): pooled analysis of over 2400 patients. Haematologica. 2014;99(suppl); abstr P884. [Google Scholar]
- 25.Guilhot F, Apperley J, Kim DW, et al. Dasatinib induces significant hematologic and cytogenetic responses in patients with imatinib-resistant or -intolerant chronic myeloid leukemia in accelerated phase. Blood. 2007;109(10):4143–4150. [DOI] [PubMed] [Google Scholar]
- 26.Cortes J, Rousselot P, Kim DW, et al. Dasatinib induces complete hematologic and cytogenetic responses in patients with imatinib-resistant or -intolerant chronic myeloid leukemia in blast crisis. Blood. 2007;109(8):3207–3213. [DOI] [PubMed] [Google Scholar]
- 27.Hochhaus A, Kantarjian HM, Baccarani M, et al. Dasatinib induces notable hematologic and cytogenetic responses in chronic-phase chronic myeloid leukemia after fail ure of imatinib therapy. Blood. 2007; 109(6):2303–2309. [DOI] [PubMed] [Google Scholar]
- 28.Kantarjian H, Pasquini R, Hamerschlak N, et al. Dasatinib or high-dose imatinib for chronic-phase chronic myeloid leukemia after failure of first-line imatinib: a randomized phase 2 trial. Blood. 2007;109(12): 5143–5150. [DOI] [PubMed] [Google Scholar]
- 29.Dasatinib in Imatinib Resistant/Intolerant Chinese CML (Chronic and Advanced Phase) Subjects. [clinicaltrials.gov identifier: NCT00529763]. https://clinicaltrials.gov/ct2/show/NCT00529763?term=00529763&rank=1 Accessed October 11, 2017.
- 30.Trudel GC, Paliwal P, Lainas I. Dasatinib plus SMO antagonist versus dasatinib alone for treating patients (pts) with newly diagnosed Philadelphia chromosome-positive (Ph+) chronic myeloid leukemia in chronic phase (CML-CP): design of CA180–363, a phase II open-label randomized trial. J Clin Oncol. 2012;20(15 suppl):abstr TSP6634. [Google Scholar]
- 31.Talpaz M, Shah NP, Kantarjian H, et al. Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias. N Engl J Med. 2006;354(24):2531–2541. [DOI] [PubMed] [Google Scholar]
- 32.National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) 3.0; 2006. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf Accessed May 11, 2016.
- 33.Bergeron A, Rea D, Levy V, et al. Lung abnormalities after dasatinib treatment for chronic myeloid leukemia: a case series. Am J Respir Crit Care Med. 2007; 176(8):814–818. [DOI] [PubMed] [Google Scholar]
- 34.Cortes JE, Jimenez CA, Mauro M, et al. Pleural effusion in dasatinib-treated patients with chronic myeloid leukemia in chronic phase: identification and management. Clin Lymphoma Myeloma. 2017; 17(2):78–82. [DOI] [PubMed] [Google Scholar]
- 35.Schiffer CA, Cortes J, Saglio G, et al. The association of dasatinib-induced lymphocytosis with treatment outcome in patients with chronic myeloid leukemia (CML). Blood. 2013;122(21):2741. [Google Scholar]
- 36.La Rosée P, Martiat P, Leitner A, et al. Improved tolerability by a modified intermittent treatment schedule of dasatinib for patients with chronic myeloid leukemia resistant or intolerant to imatinib. Ann Hematol. 2013;92(10):1345–1350. [DOI] [PubMed] [Google Scholar]
- 37.Cortes J, Hochhaus A, Kantarjian H, et al. Impact of dose reductions on 5-year efficacy in newly diagnosed patients with chronic myeloid leukemia in chronic phase (CML-CP) from DASISION. Presented at the American Society of Clinical Oncology 2017 Annual Meeting; June 2-6, 2017; Chicago, IL. [Google Scholar]
- 38.Santos FP, Kantarjian H, Fava C, et al. Clinical impact of dose reductions and interruptions of second-generation tyrosine kinase inhibitors in patients with chronic myeloid leukaemia. Br J Haematol. 2010; 150(3):303–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iurlo A, Galimberti S, Abruzzese E, et al. Pleural effusion and molecular response in dasatinib-treated chronic myeloid leukemia patients in a real-life Italian multicenter series. Ann Hematol. 2018;97(1):95–100. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.