Abstract
Gorlin-Goltz Syndrome also known as Nevoid Basal Cell Carcinoma Syndrome is an autosomal dominant multisystem disorder. It is characterized by basal cell carcinomas, odontogenic keratocysts, skeletal abnormalities and in a minority of female patients bilateral calcified ovarian fibromas. It is challenging to radiologically assess ovarian fibromas as they have similar imaging patterns to some malignant ovarian lesions. However, it is vitally important to differentiate between benign and malignant lesions to determine patients’ suitability for fertility-sparing surgery. This report describes a case of a 25 year-old patient with Gorlin-Goltz Syndrome and bilateral ovarian fibromas.
Keywords: Gorlin-Goltz syndrome, NBCCS, ovarian fibromas, uterine fibromas, MRI
CASE REPORT
A 25-year-old Caucasian woman underwent gynecological examination for irregular menses. Blood tests were normal apart from a slightly raised prolactin (56 ng/ml; normal ranges 0–23 ng/ml).
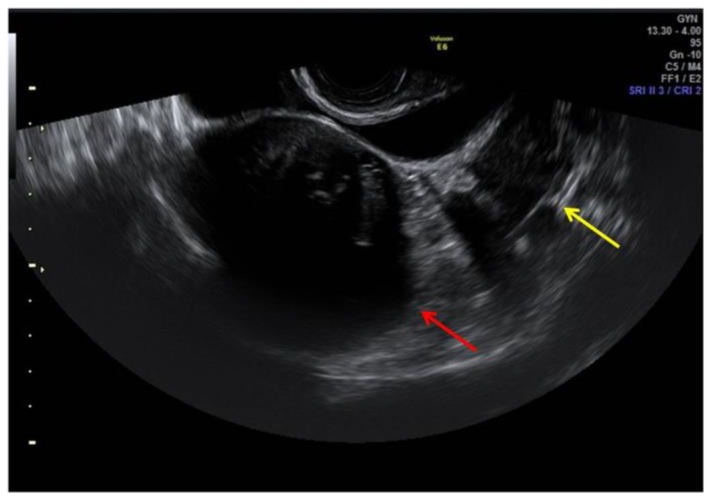
Transrectal ultrasound imaging (US) had highlighted the presence of several uterine and ovarian hypoechoic masses; the largest one between uterus and bladder (Figure 1), measuring 65 × 62 × 59 mm, was adherent to anterior uterine wall, with heterogenous vascularization. Left ovary was appreciable with difficulty due to the presence of a rounded hypoechoic mass measuring 18 × 15 mm. Ovarian follicles were not depictable bilaterally. These findings required further evaluation with Magnetic Resonance Imaging (MRI) of the pelvis.
Figure 1.
25-year-old woman with multiple ovarian and uterine fibromas in Nevoid Basal Cell Carcinoma Syndrome.
Findings: ultrasound imaging highlights the presence of heterogenous hypoechoic uterine round-shaped mass measuring 65 × 62 × 59 mm adherent to the anterior uterine wall (red arrow). Left ovary was appreciable with difficulty due to the presence of a rounded hypoechoic mass measuring 18 × 15 mm (yellow arrow: an ovarian mass). Ovarian follicles were not depictable bilaterally.
Technique: General Electric Voluson E6 (transrectal transducer, 4–9 MHz).
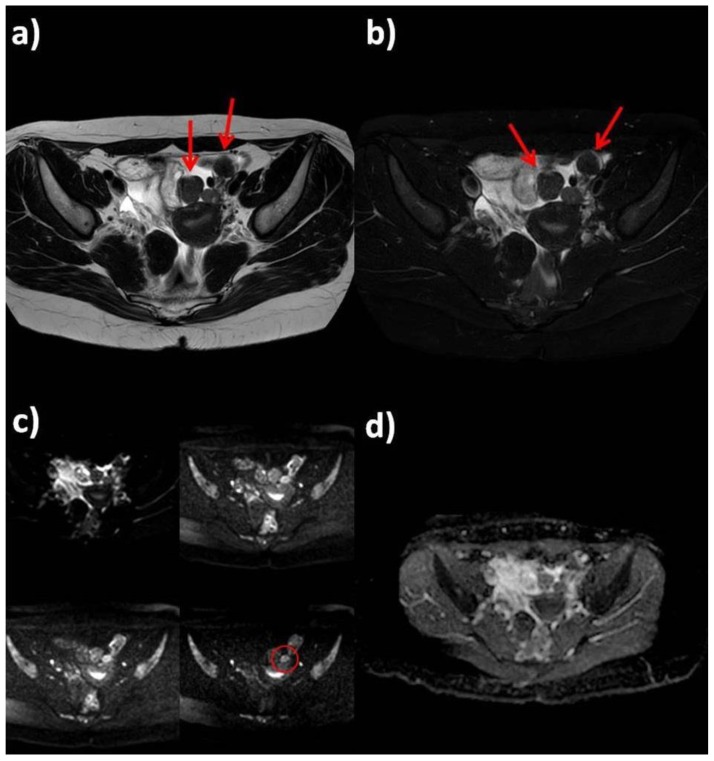
MRI detected multiple round-shaped lesions in both ovaries and uterus, the largest one in the left adnexa (67 × 73 × 55 mm); all the lesions had a similar appearance with a heterogenous signal on the T2-weighted sequences, mild diffusion restriction on diffusion weighted sequence and intense heterogenous contrast-enhancement after Gadolinium administration (Figure 2, Figure 3, Figure 4, Figure 5). The patient also had adjacent lymphadenopathy and intraperitoneal fluid. These findings were suspicious of malignant lesions and a staging computed tomography (CT) examination was performed.
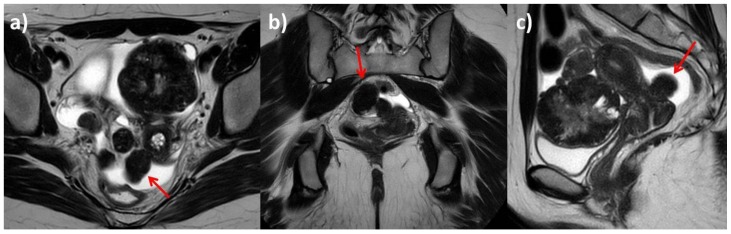
Figure 2.
25-year-old woman with multiple ovarian and uterine fibromas in Nevoid Basal Cell Carcinoma Syndrome.
Findings: Magnetic Resonance shows multiple lesions of the uterus and both ovaries (red arrows). In left adnexal region there are at least four masses, with prevalent hypointense signal in T2-weighted sequences (a: axial T2-weighted, b: axial fat-satured T2-weighted), with little increase in signal intensity on higher b values diffusion images (red circle) and mild heterogenous restricted diffusion on ADC map with ADC coefficient of 0.6 mm2/s (c: Diffusion Weighted sequence with different b values: 0, 330, 660, 1000 s/mm2; d: ADC map).
Technique: Philips Ingenia 1.5 T Magnetic Resonance System. Spin Echo T2-weighted sequences (TR: 4050,55 - TE: 110). Fat-satured Spin Echo T2-weighted sequences (TR: 4718,58, TE 100, TI 160). Diffusion-weighted sequence (TR: 1786,29 - TE: 90,06).
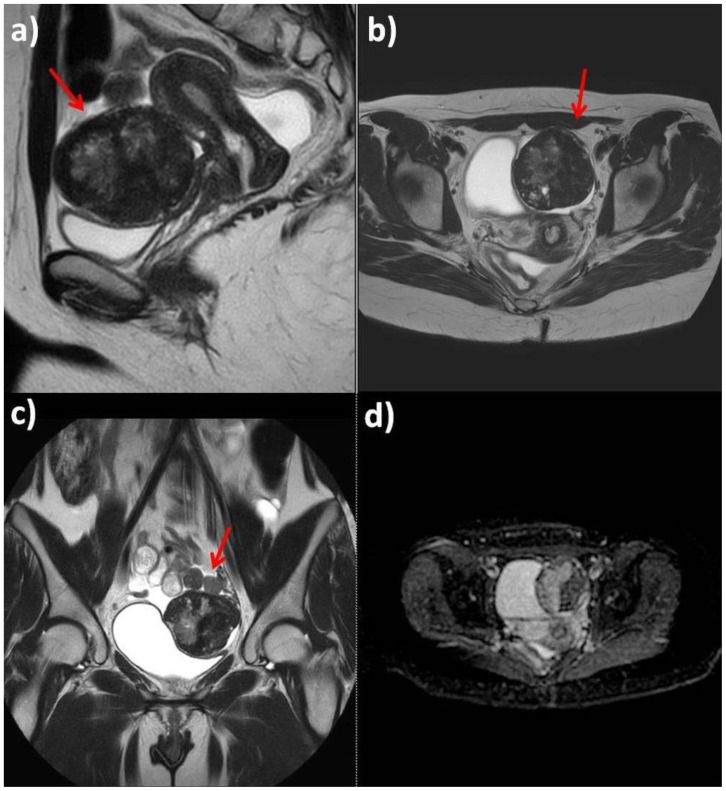
Figure 3.
25-year-old woman with multiple ovarian and uterine fibromas in Nevoid Basal Cell Carcinoma Syndrome.
Findings: On T2-weighted sequences (a: sagittal plane; b: axial plane; c: coronal plane) the largest lesion adherent to the anterior uterine wall shows heterogenous signal (red arrows). ADC map does not show any relevant restricted diffusion areas (d).
Technique: Philips Ingenia 1.5 T Magnetic Resonance System. Spin Echo T2-weighted sequences (TR: 4050,55 - TE: 110); Diffusion-weighted sequence (TR: 1786,29 - TE: 90,06).
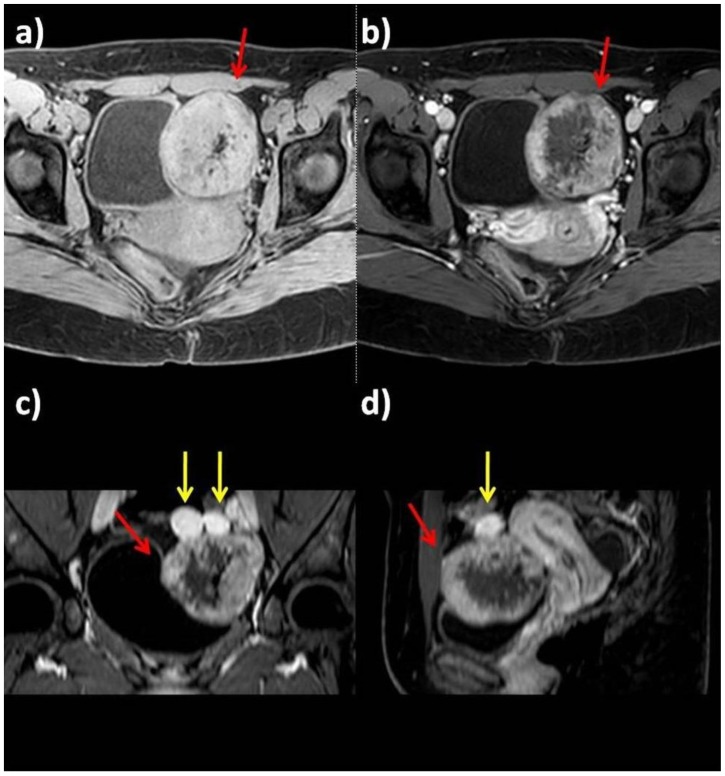
Figure 4.
25-year-old woman with multiple ovarian and uterine fibromas in Nevoid Basal Cell Carcinoma Syndrome.
Findings: On fat-satured T1-weighted sequence before contrast administration, the lesions appear isointense compared to myometrium (a: axial plane before contrast administration); after contrast administration (b: axial plane after contrast administration; c: coronal plane after contrast administration; d: sagittal plane after contrast administration), the masses show heterogenous intense contrast enhancement (red arrows indicate the largest lesion, yellow arrows the smallest masses).
Technique: Philips Ingenia 1.5 T Magnetic Resonance System. Pre and post intravenous contrast administration (contrast agent used: Gadolinium-DTPA 0.2 ml/Kg). Fat-satured Gradient Echo T1-weighted sequence before contrast administration (TR: 4; TE 1,92); fat-satured Gradient Echo T1-weighted sequence after contrast administration (TR: 4; TE 1,92).
Figure 5.
25-year-old woman with multiple ovarian and uterine fibromas in Nevoid Basal Cell Carcinoma Syndrome.
Findings: T2-weighted sequences (a: axial plane; b: coronal plane; c: sagittal plane) show a uterine hypointense pedunculated mass measuring 30 × 24 × 20 mm.
Technique: Philips Ingenia 1.5 T Magnetic Resonance System. Spin Echo T2-weighted sequences (TR: 4050,55 - TE: 110).
CT confirmed the presence of the numerous uterine and ovarian masses, some of which contained calcific foci, but no additional lesions were found in either the thorax or abdomen. A possible differential diagnosis was multiple fibromatosis of the uterus and ovaries, even though some of ovarian masses could not be differentiated radiologically from fibrothecomas or ovarian germ-cell tumors (Figure 6).
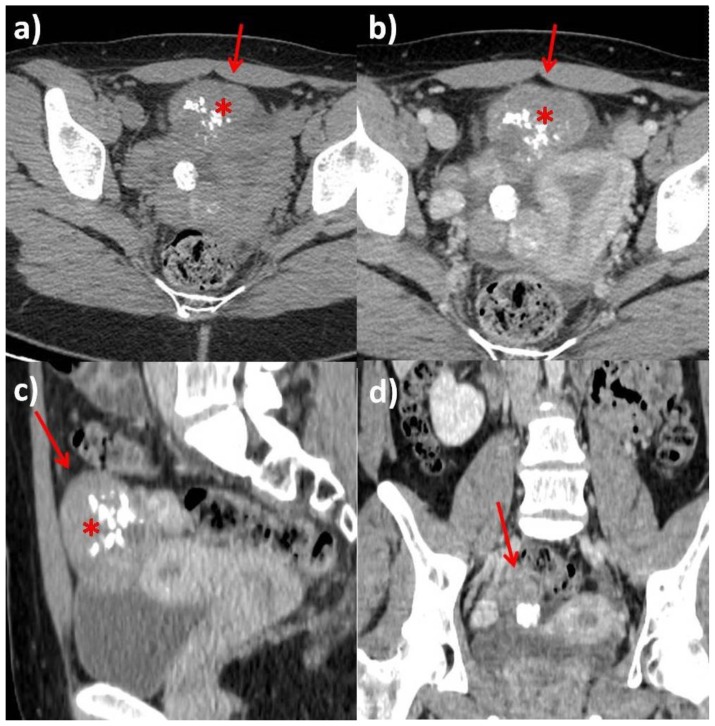
Figure 6.
Computed Tomography. 25-year-old woman with multiple ovarian and uterine fibromas in Nevoid Basal Cell Carcinoma Syndrome.
Findings: Computed Tomography examination on axial (a: before IV contrast administration; b: after contrast administration in venous phase), sagittal (c) and coronal planes (d) demonstrates the presence of numerous uterine and ovarian masses (red arrows), partially calcified (asterisks) on precontrast images which show heterogenous contrast-enhancement. Anteverted uterus, with regular zonal anatomy. Small amount of fluid in Douglas pouch. Ovarian follicles were not depictable bilaterally.
Technique: Computed Tomography General Electric LightSpeed VCT 128-slice, 410 mAs, 120 kV, 2.5 mm slice thickness, 1.25 mm gap, 120 ml Iomeprol 300, DLP 612.48 mGycm.
Upon further questioning, the patient revealed a history of multiple basal cell carcinomas (BCCs), the last one had been excised on the right thigh 4 years prior to presentation, and of an odontogenic cyst, surgically removed 9 years prior to presentation. The patient underwent laparascopic fertility-sparing surgery of the ovarian and uterine masses (Figure 7).
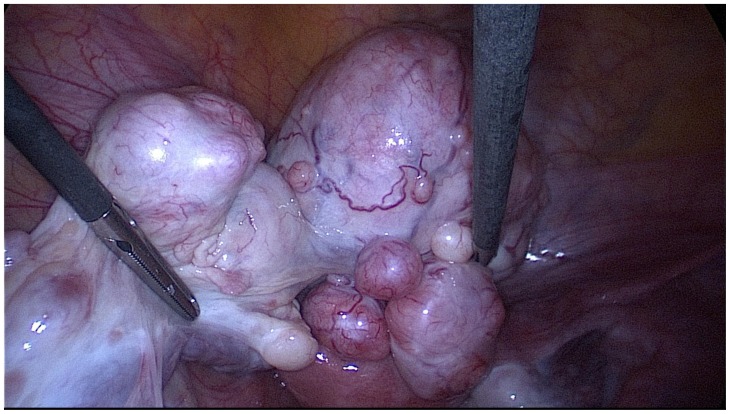
Figure 7.
25-year-old woman with multiple ovarian and uterine fibromas in Nevoid Basal Cell Carcinoma Syndrome.
Findings: laparoscopic image showing the left adnexa with multiple fibroids.
Technique: laparoscopic surgery.
Histological analysis confirmed small aggregates of fibroblast cells without cytologic atipies, consistent with the diagnosis of NBCCS given the patient’s medical history (Figure 8).
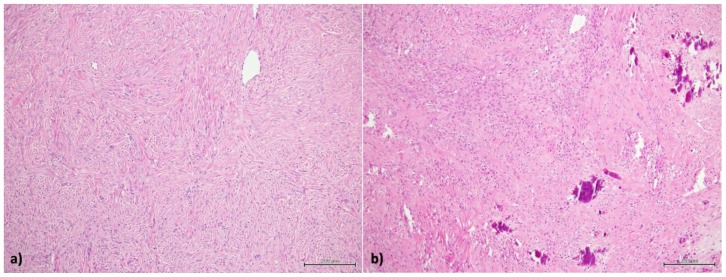
Figure 8.
25-year-old woman with multiple ovarian and uterine fibromas in Nevoid Basal Cell Carcinoma Syndrome.
Findings: a) small aggregates of fibroblast cells without cytologic atipies; b) calcifications in the context of cellular aggregates and fibrous tissue.
Technique: hematoxylin and eosin 10 x.
DISCUSSION
Etiology & Demographics
Gorlin-Goltz Syndrome also known as Nevoid Basal Cell Carcinoma Syndrome (NBCCS) is a rare multisystem disease. It is caused by mutations in the PTCH1 gene and is autosomal dominant with complete penetrance and variable expressivity. This syndrome has several names, but we will use NBCCS, as suggested by Professor Gorlin [1]. Patients and their relatives often use the name “Gorlin Syndrome”, as it does not contain the word “carcinoma”, even if approximately 50 % of patients will develop skin cancers [2].
The estimated prevalence of NBCCS varies from 1/57,000 to 1/256,000 and it commonly appears in adolescence. There is no difference in the prevalence between males and females; however, those affected are predominantly Caucasians [3].
Clinical & Imaging Findings
NBCCS is associated with basal cell carcinomas, odontogenic keratocysts (that generally develop during first to third decades), palmar and/or plantar pits and ectopic calcifications of the falx cerebri [2].
This syndrome is a clinical and radiological diagnosis with the presence of 2 major criteria and 1 minor criterion or 1 major and 3 minor criteria, as suggested by the first classification of Evans et al in 1993 [4], recently updated by the same authors in March 2018 [5] (Table 1), who highlighted that no study has been able to assess which combination of diagnostic criteria represents the best trade-off between sensitivity and specificity. If clinical features are inconclusive, to establish the diagnosis, the identification of heterozygous germline PTCH1 is necessary.
Table 1.
Criteria for diagnosis of Nevoid Basal Cell Carcinoma Syndrome.
| MAJOR CRITERIA | MINOR CRITERIA |
|---|---|
| 1. Lamellar (sheet-like) calcification of the falx or clear evidence of calcification in an individual younger than age 20years | 1. Childhood medulloblastoma (also called primitive neuroectodermal tumor) |
| 2. Odontogenic keratocysts of the jaws proven by histopathology | 2. Lympho-mesenteric or pleural cysts |
| 3. Palmar/plantar pits (≥2) | 3. Macrocephaly (occipitofrontal head circumference >97th centile) |
| 4. Multiple basal cell carcinomas (BCCs) (>5 in a lifetime) or a BCC before age 30 years | 4. Cleft lip/palate |
| 5. First-degree relative with NBCCS | 5. Vertebral/rib anomalies observed on chest x-ray and/or spinal x-ray: bifid/splayed/extra ribs; bifid vertebrae |
| 6. Preaxial or postaxial polydactyly | |
| 7. Ovarian/cardiac fibromas | |
| 8. Ocular anomalies (e.g., cataract, developmental defects, and pigmentary changes of the retinal epithelium) |
Early diagnosis of NBCCS is crucial due to the risk of developing malignancies such as medulloblastoma and aggressive skin cancers. Medulloblastoma is screened for in all patients with NBCCS as early diagnosis can improve outcomes [1].
Clinical history of our patient revealed the presence of 2 major criteria (basal cell carcinomas and odontogenic keratocysts) and 1 minor criteria (ovarian fibromas).
It is difficult to know the frequency of ovarian fibromas in NBCCS as they do not present unless they become multiple, large, calcified or twist on their pedicles. A population-based study, performed in the early 90s, suggests a frequency of 25%. Ovarian fibromas associated with NBCCS are most often bilateral (75%), calcified and nodular, often overlapping medially, eventually erroneously diagnosed as calcified uterine leiomyomas [6]. Ovarian fibromas not associated with this syndrome are often unilateral and calcified only in 10% of cases. Rarely, the tumor may be virilizing or renin secreting [6]. In our case, there was no raise of renin but a mild hyperprolactinemia, which is known to be associated with large uterine fibromas [7].
Sonography is generally used as the first-line imaging technique for the evaluation of ovarian pathologic abnormalities; nonetheless their sonographic features are often non-specific, therefore MRI is often needed for further differentiation [8].
On Ultrasound, fibromas most commonly manifest as solid, hypoechoic masses with sound attenuation. However, the US appearance is variable, and hyperechoic masses with increased through-transmission may be seen [9].
On CT, fibromas manifest as diffuse, slightly hypoattenuating masses; unlike most other solid masses, fibromas show poor, very slow enhancement with administration of contrast material [9].
MR is an excellent imaging modality for studying female pelvis and permits detection and excellent characterization of uterine and ovarian masses [8]. Ovarian fibromas in conventional MR imaging studies are characterized by low to intermediate signal intensity on T1-weighted images and low signal intensity on T2-weighted images caused by densely packed connective tissue.
MRI features of fibromas and fibrothecomas depend on the size of the lesion because the lesions measuring more than 60 mm can mimic malignant ovarian tumors as they present as solid adnexal masses sometimes associated with a pseudocapsule, degenerative changes, oedema, and peripheral subcapsular cystic areas. This explains the varying degree of high intensity portions on T2-weighted images and slow heterogenous enhancement [8, 10]. Only hemorrhage within the lesions would be characterized by the appearance of high signal intensity on T1-weighted images and heterogenous low and high signal mixed intensity on T2-weighted images [11].
On T2-weighted images fibromas and thecomas with a fibrotic component appear as well-circumscribed masses with low signal intensity containing scattered high-signal intensity areas representing oedema or cystic degeneration. The imaging appearance of thecomas without prominent fibrosis is similar to malignant tumors. The prominent lipid component of thecomas could be depicted with chemical-shift MR imaging [9].
The addition of diffusion-weighted imaging (DWI) and perfusion weighted imaging (PWI) to conventional MR might help to diagnose fibrothecomas [10] especially when the ADC map does not show any relevant restricted diffusion areas.
Sometimes ascites and pleural effusions may be present, with large fibromas [8]; the presence of normal ovarian tissue adjacent to an ovarian lesion is a useful morphological feature that can help exclude invasive ovarian malignancy [10]. Therefore, the association of pelvic masses with heterogenous T2-signal and pelvic intraperitoneal fluid do not necessarily imply the presence of a malignant neoplasia, as shown in literature, but it is often related to degenerative aspects in the largest fibromas and fibrothecomas.
Treatment & Prognosis
Treatment of this syndrome must be multidisciplinary and will depend on the systems affected. Prognosis depends on the malignant progression of the lesions; however, life expectancy is no different from that of the general population [3].
Most ovarian fibromas are asymptomatic but when sufficiently large, they present with symptoms related to an abdominal mass, as gastrointestinal or genitourinary symptoms. Rarely, a patient may present with acute symptoms secondary to torsion of the fibroma, as has occurred in other studies. The decision to perform conservative surgery with preservation of ovarian function is problematic. Although ovarian malignancies have not been reported with this syndrome, Abel and Holtz have reported two ovarian fibrosarcomas in a review of 170 adolescents with ovarian tumors [12].
In the absence of gynecological symptoms, it seems wisest to preserve ovarian function in young patients, as there is no reason to sacrifice childbearing capacity. Moreover, fertility does not appear to be affected in NBCCS [13].
Differential Diagnosis
Leiomyomas: the distinction of ovarian fibromas and uterine leiomyomas may be difficult, as they both present as solid hypoechoic masses with ultrasound beam attenuation on US; on CT they are hypoattenuating masses with heterogenous enhancement after intravenous contrast administration; on MRI they both show low-signal either in T1-weighted and T2-weighted images. A careful detection of the pedicle extending toward the uterus, vascular signal voids between the uterus and tumor mass, as well as the evaluation of the relationship between the ipsilateral ovary, can facilitate the diagnosis [10].
Germ-cell tumors: on US germ-cell tumors present as non-specific complex mass with possible calcification; on CT and MRI calcifications, cystic components and small foci of fat are suggestive. Hemorrhage may be present. In this case these calcified solid masses showed no evidence of fat, making germ-cell tumors a less-likely diagnosis [14].
Pelvic metastases: on US they present as mixed echogenicity tumors with vascularity of solid component on Doppler. On CT they present as soft tissue density with areas of cystic necrosis and, after contrast administration, solid components demonstrate heterogenous enhancement. On T1-weighted MRI images they are usually iso to hypointense with variable enhancement after contrast administration and on T2-weighted images they show heterogenous signal of solid component with hyperintensity of cystic component. In this case, the patient had a history of basal cell carcinoma that rarely metastasizes [14]. Metastatic ovarian carcinomas may mimic primary ovarian neoplasm both morphologically and clinically. Furthermore, the radiological features of metastatic ovarian cancer show considerable variability [15].
In conclusion, we presented the case of a young Caucasian woman affected by NBCCS that met three of the diagnostic criteria including BCCs, odontogenic keratocysts and ovarian fibromas. The use of several imaging modalities (ultrasound, MRI and CT) allowed us to formulate a hypothesis of benign uterine and ovarian lesions allowing the patient to have laparoscopic fertility-sparing surgery.
TEACHING POINT
The association of pelvic masses with heterogenous T2-signal and pelvic intraperitoneal fluid do not necessarily imply the presence of a malignant neoplasia but it is often related to degenerative aspects in large fibromas and fibrothecomas. In the differential diagnosis clinical and radiological features must be taken into account globally to identify possible Nevoid Basal Cell Carcinoma Syndrome.
Table 2.
Summary table for Gorlin-Goltz Syndrome with imaging features of associated pelvic fibromas.
| ETIOLOGY | Mutations in the PTCH1 gene |
| INCIDENCE | 1/57,000 – 1/256,000 |
| GENDER RATIO | No difference |
| AGE PREDILECTION | Adolescence |
| RISK FACTORS | No known risk factors |
| TREATMENT | Surgical resection (if possible, fertility-sparing surgery). |
| PROGNOSIS | Good |
| FINDINGS ON IMAGING | Pelvic Fibromas CT: mass effect, possible calcifications. MRI: low to intermediate signal in T1w and low signal in T2w. In DWI no relevant restricted diffusion areas in ADC map. |
Table 3.
Differential diagnosis table for pelvic fibromas.
| US | CT | MRI | |
|---|---|---|---|
| FIBROMAS/LEIOMYOMAS/FIBROTHECOMAS | Variable appearance; generally solid, hypoechoic masses with ultrasound beam attenuation. | Hypoattenuating masses. Calcifications and bilaterality are both uncommon. After IV contrast administration: heterogenous delayed enhancement. |
T1w: low signal. T2w: low signal, sometimes hyperintense areas representing edema or cystic degeneration. T1w after contrast administration: heterogenous enhancement. |
| GERM-CELL TUMORS | Non-specific complex mass with possible calcifications. | Calcifications, cystic components and small foci of fat are suggestive. Hemorrhage may be present. |
Calcifications, cystic components and small foci of fat are suggestive. Hemorrhage may be present. |
| PELVIC METASTASES | Mixed echogenicity tumors with vascularity of solid component on Doppler. | Soft tissue density with areas of cystic necrosis. After contrast administration, solid components demonstrate heterogenous enhancement. | T1: iso to hypointense with variable enhancement after contrast administration. T2: heterogenous signal of solid component with hyperintensity of cystic component. |
ABBREVIATIONS
- BCCs
Basal Cell Carcinomas
- CT
Computed Tomography
- DWI
Diffusion Weighted Imaging
- IV
intravenous
- MR
Magnetic Resonance
- MRI
Magnetic Resonance Imaging
- NBCCS
Nevoid Basal Cell Carcinoma Syndrome
- PWI
Perfusion Weighted Imaging
- US
Ultrasound
REFERENCES
- 1.Kiran NK, Tilak Raj TN, Mukunda KS, Rajashekar Reddy V. Nevoid basal cell carcinoma syndrome (Gorlin-Goltz syndrome) Contemp Clin Dent. 2012;3(4):514–518. doi: 10.4103/0976-237X.107459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lo Muzio L. Nevoid basal cell carcinoma syndrome (Gorlin syndrome) Orphanet J Rare Dis. 2008;3:32. doi: 10.1186/1750-1172-3-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pirschner F, Bastos PM, Contarato GL, Bimbato AC, Filho AC. Gorlin syndrome and bilateral ovarian fibroma. Int J Surg Case Rep. 2012:477–480. doi: 10.1016/j.ijscr.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Evans DG, Ladusans EJ, Rimmer S, Burnell LD, Thakker N, Farndon PA. Complications of the naevoid basal cell carcinoma syndrome: results of a population based study. J Med Genet. 1993;30:460–4. doi: 10.1136/jmg.30.6.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evans DG, Farndon PA. Nevoid Basal Cell Carcinoma Syndrome. GeneReviews® 2018. 2002. Jun 20, [Updated 2018 Mar 29]. Available at: https://www.ncbi.nlm.nih.gov/books/NBK1151/
- 6.Gorlin RJ. Nevoid basal cell carcinoma (Gorlin) syndrome. Genet Med. 2004 Nov-Dec;6(6):530–9. doi: 10.1097/01.gim.0000144188.15902.c4. [DOI] [PubMed] [Google Scholar]
- 7.Sendur SN, Aktoz F, Usubutun A, Tuncer S, Erbas T. Hyperprolactinemia Associated with Uterine Giant Myoma. Endocrine Society’s 98th Annual Meeting and Expo; April 1– 4, 2016; Boston. SUN-486. [Google Scholar]
- 8.Shinagare AB, Meylaerts LJ, Laury AR, Mortele KJ. MRI features of ovarian fibroma and fibrothecoma with histopathologic correlation. AJR. 2012;198:W296–303. doi: 10.2214/AJR.11.7221. [DOI] [PubMed] [Google Scholar]
- 9.Jeong YY, Outwater EK, Kang HK. Imaging evaluation of ovarian masses. Radiographics. 2000;20(5):1445–70. doi: 10.1148/radiographics.20.5.g00se101445. [DOI] [PubMed] [Google Scholar]
- 10.Chung BM, Park SB, Lee JB, Park HJ, Kim YS, Oh YJ. Magnetic resonance imaging features of ovarian fibroma, fibrothecoma, and thecoma. Abdom Imaging. 2015;40:1263–72. doi: 10.1007/s00261-014-0257-z. [DOI] [PubMed] [Google Scholar]
- 11.Kitajima K, Kaji Y, Sugimura K. Usual and Unusual MRI Findings of Ovarian Fibroma: Correlation with Pathologic Findings. Magn Reson Med Sci. 2008;7(1):43–48. doi: 10.2463/mrms.7.43. [DOI] [PubMed] [Google Scholar]
- 12.Abel MR, Holtz F. Ovarian neoplasms in childhood and adolescence. Am J Obstet Gynecol. 1965;93:850. doi: 10.1016/0002-9378(65)90085-2. [DOI] [PubMed] [Google Scholar]
- 13.Seracchioli R, Bagnoli A, Colombo FM, Missiroli S, Venturoli S. Conservative treatment of recurrent ovarian fibromas in a young patient affected by Gorlin syndrome. Human Reproduction. 2001;16(6):1261–1263. doi: 10.1093/humrep/16.6.1261. [DOI] [PubMed] [Google Scholar]
- 14.Fonseca RB, Grzeszczak EF. Case 128: Bilateral ovarian fibromas in nevoid basal cell carcinoma syndrome. Radiology. 2008;246:318–321. doi: 10.1148/radiol.2461041824. [DOI] [PubMed] [Google Scholar]
- 15.Lee SJ, Bae GH, Lee AW, Tong SY, Park YG, Park JS. Clinical characteristics of metastatic tumors to the ovaries. J Korean Med Sci. 2009;24(1):114–119. doi: 10.3346/jkms.2009.24.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]