Abstract
Purpose
We conducted a retrospective study to evaluate the efficacy and related costs of using two different molecules of granulocyte-colony stimulating factor (G-CSF) (lenograstim – LENO or filgrastim – FIL) as primary prophylaxis of chemotherapy-induced neutropenia in a hematological inpatient setting.
Methods
The primary endpoints of the analysis were the efficacy of the two G-CSFs in terms of the level of white blood cells, hemoglobin and platelets at the end of the treatment and the per capita direct medical costs related to G-CSF prophylaxis.
Results
Two hundred twelve patients (96 LENO, 116 FIL) have been evaluated. The following statistically significant differences have been observed between FIL and LENO: the use of a higher number of vials (11 vs 7; P<0.03) to fully recover bone marrow, a higher grade 3–4 neutropenia at the time of G-CSF discontinuation (29.3% vs 16.7%; P=0.031) and an increased number of days of hospitalization (8 vs 5; P<0.005). A longer hospital stay before discharge was necessary (12 vs 10), which reflects the higher final costs per patient (median treatment cost per cycle 10.706 € for LENO, compared to 12.623 € for FIL).
Conclusion
The use of LENO has been associated with a lower number of days of hospitalization, number of vials and less incidence of grade 3–4 neutropenia at the time of G-CSF discontinuation. LENO seems to be cost-saving when compared with FIL (–15.2%).
Keywords: lenograstim, filgrastim, biosimilar, cost, neutropenia
Introduction
The occurrence of neutropenia is a frequent complication for patients who undergo cytostatic chemotherapy for the treatment of a malignant hematological disease or a solid tumor, and when fever occurs (febrile neutropenia), it is considered as a severe complication.1 The frequency of neutropenia and its severity, as well as the probability of febrile neutropenia, are related to the scheme of chemotherapy used, malignancy and patient-specific factors (gender, age, presence of concomitant diseases and general health status);1,2 in most cases, febrile neutropenia indicates a severe infection.3 Its incidence is between 80% and 90% in patients affected with acute myeloid leukemia and about 50% in patients with solid tumors.4–6 Whether febrile neutropenia is associated with infection or not is one of the principal causes of hospitalization, which can lead to high follow-up costs.7–9 Prophylaxis with recombinant granulocyte-colony stimulating factor (G-CSF) is recommended to prevent febrile neutropenia and associated secondary events such as infections.1,2 Current guidelines recommend primary prophylactic administration of G-CSF during the first chemotherapy cycle (and hence all subsequent cycles) when the risk of febrile neutropenia is over 20%.1,2 The risk assessment should be performed before each cycle, since the risk constellation for febrile neutropenia may change during treatment.1,2
Currently, 4 original G-CSF molecules are commercially available in the European Union (EU) and Italy: filgrastim (FIL), lenograstim (LENO) and pegfilgrastim (PEG), as well as lipegfilgrastim, which is the most recently available preparation; moreover, various filgrastim biosimilars are available, whereas no biosimilars exist for LENO and PEG.10–12
However, the active ingredients differ in their dosage, duration of use, available strength, package size and price.13,14 PEG is administered in a standardized 6 mg dose, once per chemotherapy, an active ingredient amount equivalent to individual doses of 30 million units of daily G-CSF for 11 consecutive days.15–17
LENO, unlike FIL and PEG, is a glycosylated form of G-CSF. Glycosylation increases the receptor affinity of the protein to the corresponding receptor, creates a higher plasma half-life and confers temperature resistance (no need for refrigeration).18 In vitro studies have demonstrated that granulocytes primed with filgrastim show a lower functionality and a less mature phenotype compared with those primed with glycosylated G-CSF.19–21 From a clinical point of view, lenograstim demonstrated a lower incidence of febrile episodes compared to filgrastim; a higher number of patients achieved the target dose of CD34+ cells during mobilization of autologous stem cells and in a slightly shorter time, reducing the duration of neutropenia.22–24 The dose of LENO and FIL is patient specific, which is calculated based on body weight and administered daily until neutrophil counts recover.25 In outpatient treatment, this goal is achieved after an average of 5–6 days for LENO,26 while for some patients, a longer duration of G-CSF has been prescribed (maximum of 10–11 days).25,26 The individual dose required and the frequency of G-CSF administration, in one chemotherapy cycle, have been considered the only determining factors in the cost of G-CSF prophylaxis per cycle.
Assuming that a clinical difference between LENO and FIL could also be observed in the hospital setting, we decided to conduct a retrospective study to evaluate the efficacy and related costs of using LENO or FIL as primary prophylaxis of chemotherapy-induced neutropenia in a hematological inpatient setting.
Patients and methods
In the Hematological Department of Careggi Hospital, Florence (Tuscany, Italy), a retrospective comparative study has been carried out to evaluate two different cohorts of patients: one treated with LENO (from 2009 to 2011) and one with FIL (from 2012 to 2014). From 2009 until the end of 2011, LENO was the only G-CSF available in the hospital, while from January 2012, due to an expenditure restraint, the bio-similar form of FIL replaced the glycosylated G-CSF.
Data sources and resources
Since 2008, medical records have been converted into electronic documents and the following inclusion criteria have been considered to recover patient data in the electronic database: patients affected by hematological malignancies and who were admitted to the hospital for the administration of chemotherapy regimens and required G-CSF prophylaxis. The main exclusion criteria were hospitalization, mobilization of autologous stem cell or other concomitant illness different from chemotherapy administration, which requires G-CSF prophylaxis. Our retrospective database analysis has been conducted in accordance with the Declaration of Helsinki, and informed consent was not required due to the anonymous and retrospective design of the data collection. The analysis has been notified to the ethical committee of Careggi Hospital that received and approved the study.
Endpoint
The primary endpoints of the analysis were the efficacy of the two G-CSFs in terms of the level of white blood cells (WBCs), hemoglobin and platelet at the end of the treatment and the per capita direct medical costs related to G-CSF prophylaxis.
It is therefore important to isolate the variables directly related to the use of the two drugs considered (LENO vs FIL/ biosimilar). In this way, it is possible to assess which of the two alternatives would lead to a lower use of hospital resources. We considered direct medical costs (from 2014) related to G-CSF infusion, including drug cost, the personnel directly involved in the medicine’s infusion and consumables and inpatient stay costs, assuming the hospital authority’s point of view.
The cost per infusion was calculated through a micro-costing approach, which was identified through an interview with a Key Opinion Leader, the input resources needed for a standard infusion and the related costs (VAT inclusive) collected within the hospital accounting service. The only variable cost between the two cohorts was that of the drug. The total cost per infusion was 55.9 € for LENO and 22.3 € for FIL (biosimilar), inclusive of drug cost, consumables and human resources.
Due to lack of data from Careggi Hospital, where the analysis was performed, the cost per day of hospitalization in 2014 was estimated considering the data published by the Italian Ministry of Economy and Finance in relation to the average inpatient day cost in Italy and Tuscany in 2004 discounting the values using the Italian annual average inflation rates (International Monetary Fund, 2016), which were 839 € and 1,031 €, respectively.
The direct medical costs per patient were assessed taking into consideration the number of G-CSF infusions administered and the number of inpatient days after starting the treatment with growth factor until discharge.
The collected and analyzed data do not allow a detailed analysis on the real absorption of resources linked to each individual patient (in terms of outpatient services performed during hospitalization, etc.), therefore different statistical index positions were considered in relation to the variables considered: median values for the number of vials used in the two cohorts (LENO vs FIL/biosimilar) and for inpatient days.
To assess the variability of the results, the same evaluation was conducted by considering the first and third quartiles for the number of days of inpatient stay and the number of vials.
Statistical methods
Demographic characteristics were examined according to qualitative or quantitative data. In general, demographic qualitative data were summarized using absolute frequencies and percentages and analyzed using the chi-squared test, while quantitative data were summarized using medians and ranges and analyzed using an unpaired independent t-test. Comparisons between treatments with respect to the times to bone marrow recovery and times from recovery to hospital discharge were performed using a log-rank test with associated event-free curves reported in graphs using the Kaplan–Meier approach. Comparisons between treatments with respect to the number of vials were performed using the Wilcoxon rank sum test. A two-sided test with a P-value less than or equal to 0.05 was considered statistically significant. Computations were performed using SAS software, version 9.2.
Results
A total of 212 patients have been included in the analysis: 96 treated with LENO (Myelostim) and 116 with FIL (Tevagrastim); the median age was 52 and 54, respectively. Leukemia was the predominant disease, 69.8% and 60.3% in the LENO and FIL cohort, respectively. During hospitalization, nearly all patients received antibiotic and antifungal prophylaxis. The patients’ characteristics are reported in Table 1.
Table 1.
Description of patients’ characteristics
| Variables | Lenograstim | Filgrastim | P-value |
|---|---|---|---|
|
| |||
| Age (years) | |||
| Median (range) | 52 (18–84) | 54 (18–81) | 0.319 |
| Gender | |||
| Female | 52.1 (50/96) | 47.4 (55/116) | 0.547 |
| Male | 47.9 (46/96) | 52.6 (61/116) | |
| Tumor type | |||
| Leukemia | 69.8 (67/96) | 60.3 (70/116) | 0.152 |
| Others | 30.2 (29/96) | 39.7 (46/116) | |
| Previous tumor | |||
| No | 91.7 (88/96) | 89.7 (104/116) | 0.618 |
| Yes | 8.3 (8/96) | 10.3 (12/116) | |
| Pt naive (yes/no) | |||
| No | 64.6 (62/96) | 63.8 (74/116) | 0.905 |
| Yes | 35.4 (34/96) | 36.2 (42/116) | |
| Previous transplant | |||
| No | 93.8 (90/96) | 91.4 (106/116) | 0.5154 |
| Yes | 6.3 (6/96) | 8.6 (10/116) | |
A significantly higher number of FIL vials than LENO vials (11 vs 7; P<0.03) were necessary to obtain the full bone marrow recovery and thus caused an increase in the number of days with high risk for infection in neutropenic patients (8 vs 5; P<0.005). Moreover, a higher number of days before hospital discharge were necessary in patients treated with FIL compared with those treated with LENO (12 vs 10), as reported in Table 2, which reflects the higher final cost per patient.
Table 2.
Description of G-CSF activity
| Variables | Lenograstim | Filgrastim | P-value |
|---|---|---|---|
|
| |||
| G-CSF doses (number of vials) | |||
| Median (range) | 7 (1–64) | 11 (4–49) | 0.028 |
| Days to bone marrow recoverya | |||
| Median (range) | 5 (1–32) | 8 (3–57) | 0.004 |
| Days from recovery to hospital discharge | |||
| Median (range) | 10 (1–73) | 12 (4–61) | 0.695 |
Note:
Neutrophil count >1,000 mcl.
Abbreviation: G-CSF, granulocyte-colony stimulating factor.
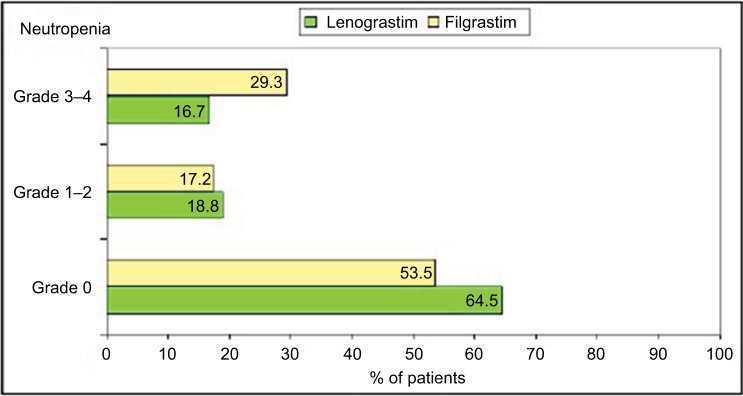
A statistically significant difference has been detected between LENO and FIL in terms of grade 3–4 neutropenia at the time of discontinuation (29.3% vs 16.7%, P=0.031; Figure 1). No differences were observed among patients treated with FIL or LENO in terms of final efficacy (bone marrow recovery; Figure 2). A significant hematological toxicity associated with the type of G-CSF was similar in terms of all grades of WBC, hemoglobin and platelet recovery (P=0.70, P=0.70 and P=0.58, respectively), thus reflecting no differences in the completion of chemotherapy and toxic death.
Figure 1.
Grade of neutropenia at the time of G-CSF discontinuation in lenograstim and filgrastim cohort.
Abbreviation: G-CSF, granulocyte-colony stimulating factor.
Figure 2.
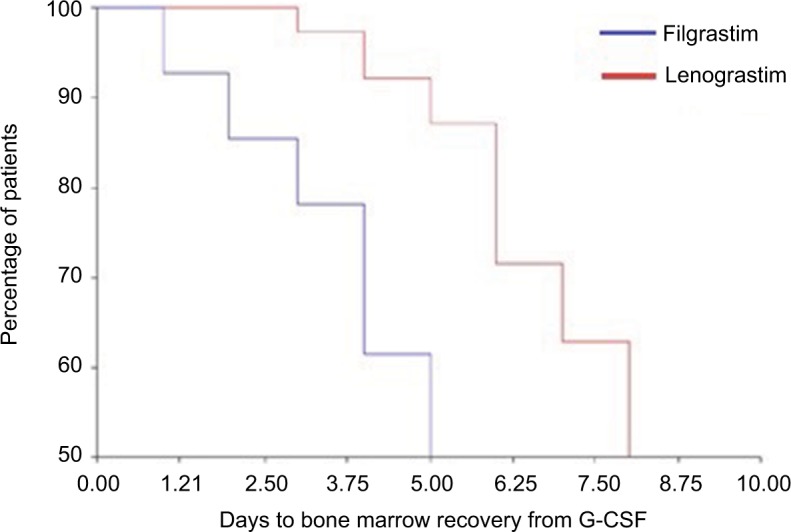
Kaplan–Meier plot of percentage of patients with bone marrow recovery at the time (days) of G-CSF discontinuation (lenograstim or filgrastim).
Abbreviation: G-CSF, granulocyte-colony stimulating factor.
Table 3 shows the average cost of patients treated with FIL/biosimilar and LENO, using the median days of treatment with growth factor at discharge and the number of vials, the cost data of daily hospitalization presented in the patients and methods section, and data obtained using the first and third quartiles.
Table 3.
Cost per patient in the lenograstim and filgrastim groups and percentage difference
| Inpatient day cost setting | Inpatient days and number of vials values | Cost per patient – lenograstim group (€) | Cost per patient – filgrastim group (€) | D% |
|---|---|---|---|---|
|
| ||||
| Tuscany | Median | 10,706 | 12,623 | –15.2 |
| First quartile | 6,468 | 9,462 | –31.6 | |
| Third quartile | 19,349 | 17,870 | +8.3 | |
| Italy | Median | 8,777 | 10,309 | –14.9 |
| First quartile | 5,311 | 7,726 | –31.3 | |
| Third quartile | 15,877 | 14,591 | +8.8 | |
The use of LENO instead of FIL would lead to a decrease of direct medical costs related to inpatient stay and infusions considering median values, with a cost (€) per patient (using inpatient costs data from Tuscany) of 10,706 and of 12,623, respectively, and with a cost (€) per patient (using inpatient costs data from Italy) of 8,777 and of 10,309, respectively. The use of LENO would lead to cost decreases of –15.2% and –14.9%.
The data from the first quartile for the number of vials used per patient and inpatient stay show a further decrease of costs due to the use of LENO, leading to cost decreases of –31.6% and –31.3% (using data from Tuscany and Italy, respectively). The data from the third quartile show an increase of costs of 8.3% and of 8.8% (using data from Tuscany and Italy, respectively).
Discussion
The chemotherapy regimen is one of the primary determinants of the risk of neutropenia, given that some regimens are more myelotoxic than others,3 and this is one of the most relevant causes of morbidity and mortality.8,9 Febrile neutropenia remains a life-threatening medical condition, despite the wide availability of effective antibiotics.8,9
It frequently causes significant hospitalization costs when developed by patients treated with chemotherapy.27,28 These costs include both direct medical costs and indirect costs that are borne by the patient and caregivers.
The administration of G-CSF decreases the incidence of febrile neutropenia and allows the maintenance of a correct dose density and intensity.1,2 It is also important to underline the fact that completion of all planned chemotherapy cycles is essential in order to provide patients with the maximum chance of treatment success,29,30 and febrile neutropenia may cause dose reduction and treatment delay limiting the efficacy of therapy.29,30
It has been clearly established that in patients receiving myelotoxic chemotherapy regimens, prophylaxis with a G-CSF, the primary regulator of granulopoiesis, decreases the occurrence of febrile neutropenia.1,2
Three original G-CSF preparations are commercially available in the EU and Italy: LENO, FIL and its biosimilars, PEG,31 as well as lipegfilgrastim, which is the most recently available preparation.
Despite the fact that all the G-CSFs are reported as similar, considerable evidence has been published indicating that there are chemical, biological and clinical differences between glycosylated and non-glycosylated (pegylated and non-pegylated) molecules.32
According to international guidelines, in settings characterized by a high risk of febrile neutropenia (20% or more), prophylaxis with G-CSF is indicated1,2 starting at 24–48 hours after chemotherapy. The number of injections and the duration of prophylaxis are still a matter of debate, and we know that the onset and the duration of nadir are key points to establish the starting day and duration of G-CSF administration after chemotherapy.3
A shorter schedule seems to decrease the incidence and severity of side effects, is cost saving and more effective.33–36 In addition, a large survey by Falandry et al26 indicates that the required duration of daily G-CSF administration is significantly shorter than recommended in guidelines, namely, 5.5 days, whereas only 9.3% of the patients exceed 7 days. Possible explanations for the discrepancies between guidelines and clinical practice could be that guidelines are often based on studies designed to ascertain the efficacy of G-CSF vs placebo, regardless of proper timing and duration.
In our study, a significantly higher number of FIL vials than LENO vials have been necessary to fully recover bone marrow depletion, with a significant difference between the two G-CSF in terms of grade 3–4 neutropenia at the time of G-CSF discontinuation, 29.3% vs 16.7%, P=0.031. The delay in bone marrow recovery increased the days of hospitalization in patients treated with FIL compared with those treated with LENO (12 days vs 10 days).
Despite the higher cost per vial of LENO with respect to FIL/biosimilar, the median direct medical costs per treatment (drug, infusion and inpatient stay) in patients with neutropenia were lower due to a lower use of vials to resolve neutropenia and due to the reduction of inpatient stay.
The median treatment cost per cycle, in terms of infusions and inpatient stay, amounted to 10.706 € for LENO, compared to 12.623 € for FIL/biosimilar, considering data for inpatient stay from Tuscany (the region where the hospital in which the analysis was performed is located), with a difference of –15.2%. The identification of LENO as a cost-saving G-CSF compared with FIL in Italy is in line with other published cost comparisons.37,38
Conclusion
The use of LENO has been associated with a lower number of days of hospitalization, number of vials and less incidence of grade 3–4 neutropenia at the time of G-CSF discontinuation. LENO seems to be cost-saving compared to FIL.
The economic analysis performed is to be considered conservative due to the lack of quantification of the costs associated with the management of drugs (purchase, storage and handling), which in the case of FIL/biosimilar are likely to be higher due to the need for storage at a low temperature. Future prospective economic evaluations should consider the collection of all direct medical costs and assume a societal perspective to assess indirect costs related to patients and caregivers.
Acknowledgments
The authors thank the nurses of the Hematology Department of AOU Careggi Firenze.
Footnotes
Disclosure
TP is an employee of Italfarmaco SpA; UR declares personal fees from Italfarmaco SpA and Bayer SpA outside the present work. The other authors report no other conflicts of interest in this work.
References
- 1.Aapro MS, Bohlius J, Cameron DA, et al. 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer. 2011;47(1):8–32. doi: 10.1016/j.ejca.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 2.Smith TJ, Khatcheressian J, Lyman GH, et al. 2006 update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guideline. J Clin Oncol. 2006;24(19):3187–3205. doi: 10.1200/JCO.2006.06.4451. [DOI] [PubMed] [Google Scholar]
- 3.Ria R, Reale A, Moschetta M, Dammacco F, Vacca A. Neutropenia and G-CSF in lymphoproliferative diseases. Hematology. 2013;18(3):131–137. doi: 10.1179/1607845412Y.0000000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bodey GP, Buckley M, Sathe YS, Freireich EJ. Quantitative relationships between circulating leukocytes and infection in patients with acute leukemia. Ann Intern Med. 1966;64(2):328–340. doi: 10.7326/0003-4819-64-2-328. [DOI] [PubMed] [Google Scholar]
- 5.Chang HY, Rodriguez V, Narboni G, Bodey GP, Luna MA, Frei-reich EJ. Causes of death in adults with acute leukemia. Medicine. 1976;55(3):259–268. doi: 10.1097/00005792-197605000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Cherpillod A, Pétignat C, Leyvraz S. Infectious complications during chemotherapy for solid tumors. Clin Microbiol Infect. 1997;3:329. [Google Scholar]
- 7.Link H, Maschmeyer G, Meyer P, Study Group of the Paul Ehrlich Society for Chemotherapy Interventional antimicrobial therapy in febrile neutropenic patients. Ann Hematol. 1994;69:231–243. doi: 10.1007/BF01700277. [DOI] [PubMed] [Google Scholar]
- 8.Kuderer NM, Dale DC, Crawford J, Cosler LE, Lyman GH. Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer. 2006;106(10):2258–2266. doi: 10.1002/cncr.21847. [DOI] [PubMed] [Google Scholar]
- 9.Kuderer NM, Dale DC, Crawford J, Lyman GH. Impact of primary prophylaxis with granulocyte colony-stimulating factor on febrile neutropenia and mortality in adult cancer patients receiving chemotherapy: a systematic review. J Clin Oncol. 2007;25(21):3158–3167. doi: 10.1200/JCO.2006.08.8823. [DOI] [PubMed] [Google Scholar]
- 10.Welte K. G-CSF: filgrastim, lenograstim and biosimilars. Expert Opin Biol Ther. 2014;14(7):983–993. doi: 10.1517/14712598.2014.905537. [DOI] [PubMed] [Google Scholar]
- 11.Yang BB, Savin MA, Green M. Prevention of chemotherapy-induced neutropenia with pegfilgrastim: pharmacokinetics and patient outcomes. Chemotherapy. 2012;58(5):387–398. doi: 10.1159/000345626. [DOI] [PubMed] [Google Scholar]
- 12.Bennett CL, Djulbegovic B, Norris LB, Armitage JO. Colony-stimulating factors for febrile neutropenia during cancer therapy. N Engl J Med. 2013;368(12):1131–1139. doi: 10.1056/NEJMct1210890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kubota N, Orita T, Hattori K, Oh-Eda M, Ochi N, Yamazaki T. Structural characterization of natural and recombinant human granulocyte colony-stimulating factors. J Biochem. 1990;107(3):486–492. doi: 10.1093/oxfordjournals.jbchem.a123072. [DOI] [PubMed] [Google Scholar]
- 14.Crawford J, Caserta C, Roila F, ESMO Guidelines Working Group Hematopoietic growth factors: ESMO clinical practice guidelines for the applications. Ann Oncol. 2010;21(Suppl 5):v248–v251. doi: 10.1093/annonc/mdq195. [DOI] [PubMed] [Google Scholar]
- 15.Holmes FA, Jones SE, O’Shaughnessy J, et al. Comparable efficacy and safety profiles of once-per-cycle pegfilgrastim and daily injection filgrastim in chemotherapy-induced neutropenia: a multicenter dose-finding study in women with breast cancer. Ann Oncol. 2002;13(6):903–909. doi: 10.1093/annonc/mdf130. [DOI] [PubMed] [Google Scholar]
- 16.Holmes FA, O’Shaughnessy JA, Vukelja S, et al. Blinded, randomized, multicenter study to evaluate single administration pegfilgrastim once per cycle versus daily filgrastim as an adjunct to chemotherapy in patients with high-risk stage II or stage III/IV breast cancer. J Clin Oncol. 2002;20(3):727–731. doi: 10.1200/JCO.2002.20.3.727. [DOI] [PubMed] [Google Scholar]
- 17.Green MD, Koelbl H, Baselga J, et al. A randomized double-blind multicenter phase III study of fixed-dose single-administration pegfilgrastim versus daily filgrastim in patients receiving myelosuppressive chemotherapy. Ann Oncol. 2003;14(1):29–35. doi: 10.1093/annonc/mdg019. [DOI] [PubMed] [Google Scholar]
- 18.Chamorey AL, Magné N, Pivot X. Impact of glycosylation on the effect of cytokines. A special focus on oncology. Eur Cytokine Netw. 2012;13:154–160. [PubMed] [Google Scholar]
- 19.Ribeiro D, Veldwijk MR, Benner A, et al. Differences in functional activity and antigen expression of granulocytes primed in vivo with filgrastim, lenograstim, or pegfilgrastim. Transfusion. 2007;47(6):969–980. doi: 10.1111/j.1537-2995.2007.01241.x. [DOI] [PubMed] [Google Scholar]
- 20.Kopf B, de Giorgi U, Vertogen B, et al. A randomized study comparing filgrastim versus lenograstim versus molgramostim plus chemotherapy for peripheral blood progenitor cell mobilization. Bone Marrow Transplant. 2006;38(6):407–412. doi: 10.1038/sj.bmt.1705465. [DOI] [PubMed] [Google Scholar]
- 21.Ataergin S, Arpaci F, Turan M, et al. Reduced dose of lenograstim is as efficacious as standard dose of filgrastim for peripheral blood stem cell mobilization and transplantation: a randomized study in patients undergoing autologous peripheral stem cell transplantation. Am J Hematol. 2008;83(8):644–648. doi: 10.1002/ajh.21206. [DOI] [PubMed] [Google Scholar]
- 22.Orciuolo E, Buda G, Marturano E, et al. Lenograstim reduces the incidence of febrile episodes, when compared with filgrastim, in multiple myeloma patients undergoing stem cell mobilization. Leuk Res. 2011;35(7):899–903. doi: 10.1016/j.leukres.2010.10.029. [DOI] [PubMed] [Google Scholar]
- 23.Ria R, Gasparre T, Mangialardi G, et al. Comparison between filgrastim and lenograstim plus chemotherapy for mobilization of PBPCs. Bone Marrow Transplant. 2010;45(2):277–281. doi: 10.1038/bmt.2009.150. [DOI] [PubMed] [Google Scholar]
- 24.Ria R, Reale A, Melaccio A, Racanelli V, Dammacco F, Vacca A. Filgrastim, lenograstim and pegfilgrastim in the mobilization of peripheral blood progenitor cells in patients with lymphoproliferative malignancies. Clin Exp Med. 2015;15(2):145–150. doi: 10.1007/s10238-014-0282-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swanson G, Bergstrom K, Stump E, Miyahara T, Herfindal ET. Growth factor usage patterns and outcomes in the community setting: collection through a practice-based computerized clinical information system. J Clin Oncol. 2000;18(8):1764–1770. doi: 10.1200/JCO.2000.18.8.1764. [DOI] [PubMed] [Google Scholar]
- 26.Falandry C, Campone M, Cartron G, Guerin D, Freyer G. Trends in G-CSF use in 990 patients after EORTC and ASCO guidelines. Eur J Cancer. 2010;46(13):2389–2398. doi: 10.1016/j.ejca.2010.04.031. [DOI] [PubMed] [Google Scholar]
- 27.Gómez H, Hidalgo M, Casanova L, et al. Risk factors for treatment-related death in elderly patients with aggressive non-Hodgkin’s lymphoma: results of a multivariate analysis. J Clin Oncol. 1998;16(6):2065–2069. doi: 10.1200/JCO.1998.16.6.2065. [DOI] [PubMed] [Google Scholar]
- 28.Lyman GH, Lyman CH, Agboola O. Risk models for predicting chemotherapy-induced neutropenia. Oncologist. 2005;10(6):427–437. doi: 10.1634/theoncologist.10-6-427. [DOI] [PubMed] [Google Scholar]
- 29.Bosly A, Bron D, van Hoof A, et al. Achievement of optimal average relative dose intensity and correlation with survival in diffuse large B-cell lymphoma patients treated with CHOP. Ann Hematol. 2008;87(4):277–283. doi: 10.1007/s00277-007-0399-y. [DOI] [PubMed] [Google Scholar]
- 30.Pettengell R, Schwenkglenks M, Leonard R. Impact of Neutropenia in Chemotherapy-European Study Group (INC – EU): neutropenia occurrence and predictors of reduced chemotherapy delivery: results from the INC – EU prospective observational European neutropenia study. Support Care Cancer. 2008;16:1299–1309. doi: 10.1007/s00520-008-0430-4. [DOI] [PubMed] [Google Scholar]
- 31.Guariglia R, Martorelli MC, Lerose R, Telesca D, Milella MR, Musto P. Lipegfilgrastim in the management of chemotherapy-induced neutropenia of cancer patients. Biologics. 2016;10(10):1–8. doi: 10.2147/BTT.S58597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keating GM. Lenograstim: a review of its use in chemotherapy-induced neutropenia, for acceleration of neutrophil recovery following haematopoietic stem cell transplantation and in peripheral blood stem cell mobilization. Drugs. 2011;71(6):679–707. doi: 10.2165/11206870-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 33.Papaldo P, Lopez M, Marolla P, et al. Impact of five prophylactic filgrastim schedules on hematologic toxicity in early breast cancer patients treated with epirubicin and cyclophosphamide. J Clin Oncol. 2005;23(28):6908–6918. doi: 10.1200/JCO.2005.03.099. [DOI] [PubMed] [Google Scholar]
- 34.Hendler D, Rizel S, Yerushalmi R, et al. Different schedules of granulocyte growth factor support for patients with breast cancer receiving adjuvant dose-dense chemotherapy: a prospective nonrandomized study. Am J Clin Oncol. 2011;34(6):619–624. doi: 10.1097/COC.0b013e3181f94716. [DOI] [PubMed] [Google Scholar]
- 35.Wolff AC, Jones RJ, Davidson NE, Jeter SC, Stearns V. Myeloid toxicity in breast cancer patients receiving adjuvant chemotherapy with pegfilgrastim support. J Clin Oncol. 2006;24(15):2392–2394. doi: 10.1200/JCO.2006.05.7174. [DOI] [PubMed] [Google Scholar]
- 36.Potosky AL, Malin JL, Kim B, et al. Use of colony-stimulating factors with chemotherapy: opportunities for cost savings and improved outcomes. J Natl Cancer Inst. 2011;103(12):979–982. doi: 10.1093/jnci/djr152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hadji P, Kostev K, Schröder-Bernhardi D, Ziller V. Cost comparison of outpatient treatment with granulocyte colony-stimulating factors (G-CSF) in Germany. Int J Clin Pharmacol Ther. 2012;50(4):281–289. doi: 10.5414/cp201633. [DOI] [PubMed] [Google Scholar]
- 38.Pfannkuche MS, Glaeske G, Neye H. Kostenvergleiche für Arzneimittel auf der Basis von DDD im Rahmen der Vertragsärztlichen Versorgung. [Advantages and Limitations of the DDD System in the Context of the German statutory Health Insurance System] Gesundh Ökon Qual Manag. 2009;14(1):17–23. German. [Google Scholar]