Abstract
Background
A recent study shows that dual antiplatelet therapy with clopidogrel plus aspirin is superior to aspirin monotherapy for minor stroke, which is defined as a National Institutes of Health Stroke Scale (NIHSS)score of ≤3. However, acute mild-moderate ischaemic stroke (4≤NIHSS≤10) still needs aggressive antiplatelet intervention to prevent deterioration and recurrence of stroke. The efficacy and safety of dual antiplatelet therapy versus aspirin monotherapy in the population are not clear. A multicentre clinical trial is designed to evaluate the efficacy and safety of clopidogrel plus aspirin therapy versus aspirin monotherapy within 48 hours of symptom onset of mild-moderate ischaemic stroke.
Methods/Design
The study is a randomised, open-label, multicentre, prospective trial with a target enrolment of 2700 patients from 60 centres in Northeast China. A treatment allocation identification number to each enrolled patient will be provided by a random number generator. The follow-up time for the clopidogrel plus aspirin and aspirin monotherapy groups is 90 days. The primary efficacy endpoint is a stroke progression event, which is defined as ≥4 point increase in the NIHSS score in 48 hours. The second efficacy endpoints include new ischaemic stroke within 90 days, change in the NIHSS score within 14 days, modified Rankin Scale score on day 90 and other vascular or death events within 90 days. The safety endpoints include mucocutaneous haemorrhage, organ haemorrhage and intracranial haemorrhage, adverse events and severe adverse events. χ2 test, t-test (or Mann-Whitney test), survival analysis and Cox proportional hazards models will be conducted. The findings of the study may provide an important evidence for clinical practice for these patients.
Discussion
The trial will be conducted under a rational design and will provide valuable evidence on the appropriate treatment for this population.
Ethics and dissemination
The study was reviewed and approved by the Ethics Committee of the General Hospital of Shen-Yang Military Region (no K(2016) 6).
Trial registration number
NCT02869009; Pre-results.
Keywords: stroke, antiplatelet therapy, clopidogrel plus aspirin, aspirin
Background
At present, thrombolysis and antiplatelets are both considered effective treatment methods for acute ischaemic stroke.1 2 Due to limitations in time window,3 only a minority of patients can receive thrombolysis therapy, whereas most patients need to receive antiplatelet therapy to prevent the progression and recurrence of stroke.4 Studies have found that antiplatelet therapy is superior to anticoagulant therapy in patients with acute ischaemic stroke.5 6 Therefore, the latest guidelines recommend to give priority to treatment with aspirin alone to patients with acute ischaemic stroke who have not received thrombolysis treatment.7 8 In the last decade, it is noteworthy that several studies have reported that compared with aspirin monotherapy, clopidogrel plus aspirin treatment may be more effective for some patients.9–11 Especially, the CHANCE trial has shown that compared with aspirin monotherapy, clopidogrel plus aspirin treatment could reduce the risk of recurrent stroke in patients with transient ischaemic attack (TIA) (ABCD2≥4) or minor ischaemic stroke (National Institutes of Health Stroke Scale (NIHSS) score of ≤3) within 24 hours of symptom onset.12 The most recent POINT trial has reported that in patients with minor ischaemic stroke (NIHSS≤3) or high-risk TIA (ABCD2≥4), those who received a combination of clopidogrel and aspirin had a lower risk of major ischaemic events but a higher risk of major haemorrhage at 90 days than those who received aspirin alone.13 Besides, dual antiplatelet therapy with clopidogrel plus aspirin has received much attention in recent years.14–16
In clinical practice, besides TIA and minor ischaemic stroke (NIHSS≤3), patients with acute mild-moderate ischaemic stroke (4≤NIHSS≤10) still need aggressive intervention to prevent deterioration and recurrence of stroke. Acute ischaemic stroke within 48 hours of symptom onset is prone to deterioration, and about 50% of deterioration has occurred within the time window.17 For these patients, aspirin monotherapy is still recommended by the guidelines.7 8 The FASTER trial has reported that there was a possible signal, but with no clear evidence, to show that dual antiplatelet therapy with clopidogrel plus aspirin might be more suitable for the above population than aspirin monotherapy.18 Our recent single-centre study has also found that in patients with acute mild-moderate ischaemic stroke (NIHSS≤7) within 48 hours of symptom onset, dual antiplatelet therapy with clopidogrel plus aspirin may be superior to aspirin monotherapy.19 Nevertheless, dual-scale clinical trial is still needed to determine the efficacy and safety of clopidogrel plus aspirin therapy versus aspirin monotherapy in the population.
The objective of the Antiplatelet Therapy in Acute Mild-Moderate Ischemic Stroke (ATAMIS) study (https://clinicaltrials.gov; trial registration number: NCT02869009) is to determine the efficacy and safety of clopidogrel plus aspirin therapy versus aspirin monotherapy within 48 hours of symptom onset of mild-moderate ischaemic stroke.
Methods/Design
Study design
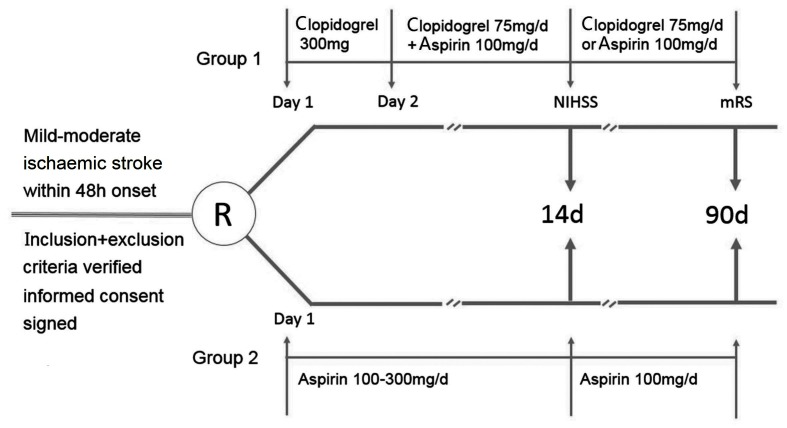
The study is a randomised, open-label, multicentre and prospective trial. All patients meeting the inclusion and exclusion criteria should be invited to the trial, and then the participants will be randomly allocated into groups receiving clopidogrel plus aspirin or aspirin alone. A flow diagram summarising the processes in the present study is shown in figure 1.
Figure 1.
Flow diagram of the study. mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale.
Participants
Participants will be gathered from 60 centres in Northeast China. Participants enrolled in the trial have to meet the inclusion and exclusion criteria. The inclusion and exclusion criteria are listed in table 1.
Table 1.
Inclusion and exclusion criteria of patients
| Inclusion criteria | 1. Age ≥18 years. |
| 2. Acute ischaemic stroke (diagnosed according to the AHA/ASA statement26) that can be randomised within 48 hours of symptom onset. | |
| 3. Neurological deficit: 4≤NIHSS≤10. | |
| 4. First-ever stroke or recurrent stroke without obvious neurological deficit (mRS≤1). | |
| 5. Signed informed consent by the patient or legally authorised representatives. | |
| Exclusion criteria | 1. Intracranial haemorrhage and haemorrhagic cerebral infarction. |
| 2. Thrombolysis for ischaemic stroke. | |
| 3. Allergy to clopidogrel and/or aspirin. | |
| 4. Severe systemic disease (such as severe infection, severe hepatic and renal dysfunction). | |
| 5. Clear indication for anticoagulation (cardiac source of embolism, atrial fibrillation, mechanical cardiac valves, deep venous thrombosis, pulmonary embolism). | |
| 6. History of intracranial haemorrhage. | |
| 7. Planned treatment with non-steroidal anti-inflammatory drugs to affect platelet function. | |
| 8. Anticoagulation within 10 days. | |
| 9. Gastrointestinal bleed or major surgery within 3 months. | |
| 10. Planned or likely revascularisation (any angioplasty or vascular surgery) within the next 3 months. | |
| 11. Planned surgery or intervention to stop antiplatelet therapy. | |
| 12. Ischaemic stroke induced by angiography or surgery. | |
| 13. Pregnancy or childbirth within the previous 4 weeks. | |
| 14. Patients who have been treated with any other investigational drug within 3 months of enrolment. | |
| 15. Severe non-cardiovascular comorbidity with life expectancy <3 months. |
AHA, American Heart Association; ASA, American Stroke Association; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale.
Randomisation and open-label
After eligibility assessment, a randomisation list will be generated with a computer. A treatment allocation identification number to each enrolled patient will be provided by a random number generator. All participants and physicians will not be blinded to treatment assignment, and an open-label design will be chosen for the present study.
Interventions
The clopidogrel plus aspirin group will receive a 300 mg loading dose of clopidogrel, followed by clopidogrel 75 mg/day and aspirin 100 mg/day from day 2 to day 14, and followed by clopidogrel 75 mg/day or aspirin 100 mg/day from day 15 to day 90.
The aspirin group will receive 100–300 mg aspirin from day 1 to day 14, followed by aspirin 100 mg/day from day 15 to day 90.
Relevant concomitant care is both permitted in the two groups during the trial.
Follow-up time
The follow-up time for the two groups is 90 days. The time line of the study is up to December 2019.
Criteria for study withdrawal
Patients may voluntarily withdraw from the trial at any time without giving any reason. If patients’ condition worsens within 90 days, they are probably to receive other therapies, such as anticoagulation and fibrinolysis therapies. Patients who receive other therapies will also be withdrawn from the trial.
Outcomes
The primary efficacy endpoint is a stroke progression event, which is defined as ≥4 point increase in the NIHSS score in 48 hours.
The second efficacy endpoints are as follows: (1) new ischaemic stroke within 90 days; (2) change in the NIHSS score within 14 days; (3) modified Rankin Scale score on day 90; and (4) other vascular or death events within 90 days.
The security endpoints include mucocutaneous haemorrhage, organ haemorrhage and intracranial haemorrhage, adverse events and severe adverse events. Intracranial haemorrhage will be diagnosed and classified according to the Heidelberg Bleeding Classification.20
Quality control
Data checking will be done exactly by a Data Monitoring Committee (DMC). Interim analyses will be conducted three times during the trial. If one therapy shows a statistically significant advantage of efficacy and/or safety over the other therapy, the DMC should recommend stopping the study immediately.
Sample size calculation
Our previous results have indicated that the probability of stroke deterioration in the aspirin group is 6.0%. When the reduction rate of relative risk of the two groups is set as 30%, the power as 0.8 and α as 0.05, the sample size will be 2454. When the dropout rate is set as 10%, the total sample size will be 2699.21 Therefore, the target number of participants is 2700, including 1350 cases in the clopidogrel plus aspirin group and 1350 cases in the aspirin group. PASS V.11.0 has been used to perform sample size calculation.
Data management
We will use the data management system made by MedSci (http://atamis.medsci.cn) to manage the data. The system can automatically generate random number and electronic case report forms for enrolled patients. All of the data will be downloaded and analysed by the General Hospital of Shen-Yang Military Region.
Statistical analysis
Intention-to-treat analysis will be conducted for statistical analysis. Continuous variables that are normally distributed will be presented as mean value and SD. When variables are not normally distributed, they will be presented as median and IQR. Count data will be described with n (%). For comparison of continuous variables, t-test or Mann-Whitney test will be used. χ2 test will be used to compare count data. To compare the survival curve and survival rate of stroke recurrence of the groups, survival analysis will be conducted. Cox proportional hazards models will be used to explore the risk factors of ischaemic stroke recurrence. The O’Brien and Fleming method will be used in the interim analyses to ensure that the p values become gradually less conservative in order to avoid ending the trial prematurely or continuing the trial if there is high risk related to the intervention. P<0.05 in two tails is considered statistically significant. Statistical analysis will be performed using the SPSS V.20.0 software.
Current status
All of the centres have begun recruiting patients, and about 700 subjects have been enrolled so far.
Discussion
An important treatment strategy for patients with acute ischaemic stroke is to prevent deterioration and recurrence of stroke. The CHANCE trial has demonstrated the superiority of the aggressive antiplatelet therapy with clopidogrel plus aspirin to aspirin alone in minor ischaemic stroke (NIHSS≤3) or high-risk TIA in a Chinese population,12 but the SOCRATES trial did not show the positive results by comparing ticagrelor plus aspirin with aspirin alone in an international population.22 One possible explanation for the discrepancy may be that the stroke pathophysiology in the Chinese population differs from that in other populations. In the Chinese population, there are more patients with arteriosclerotic large vessel stroke and patients with large vessel occlusion who might have better efficacy when they receive a combination of clopidogrel and aspirin.23 24 The POINT trial has been conducted among patients with minor ischaemic stroke or those who are at high risk in an international population. The trial has shown the superiority of a combination of clopidogrel and aspirin to aspirin alone in terms of efficacy. However, higher risk of major haemorrhage at 90 days has been found in patients who received clopidogrel plus aspirin.13 Besides TIA and minor ischaemic stroke, new ischaemia or neurological deterioration after mild-moderate ischaemic stroke (4≤NIHSS≤10) will also result in increased disability and mortality.
The ATAMIS trial will be the first study to evaluate the efficacy and safety of clopidogrel-aspirin dual antiplatelet therapy and aspirin monotherapy among patients with acute mild-moderate ischaemic stroke within 48 hours of symptom onset. In the ATAMIS trial, the time window of clopidogrel plus aspirin is 14 days, which is shorter than those of the CHANCE, POINT and SOCRATES trials.12 13 22 We consider that the beneficial effect of dual antiplatelet therapy on the progression or recurrence of stroke may mainly come from early intensive antiplatelet treatment, which quickly acts to inhibit platelet function. Besides, short-term dual antiplatelet may accompany low risk of bleeding events, especially fatal bleeding and intracranial haemorrhage. In the subgroup analysis of the CHANCE trial, Pan et al have reported that the optimal duration of dual antiplatelet therapy is 14 days,25 which is consistent with our views.
As a prospective trial, loss to follow-up bias in this study is inevitable. Despite this limitation, the randomised, multicentre and prospective design of the study has great methodological superiority. For randomisation and multicentre studies, confounding bias and selection bias will be well controlled. For the safety of participants, treatment allocation will be open-label. Once unexpected situation occurs, it can be controlled in time. The scientific calculation of the sample size can guarantee the strong statistical power. Since the statistical method of the study is scientific and advanced, the results will be statistically reliable. In addition, the primary and second endpoints of the study can comprehensively reflect the efficacy and safety of the two therapies. For this reason, the results of the trial will provide significant guidance for clinical practice.
In conclusion, the trial will be conducted under a rational design. Whether the result of the trial is positive or negative, it will provide valuable evidence on the appropriate treatment for this population.
Acknowledgments
The Data Monitoring Committee comprised Yu Cui, Xiaofu Tian and Shuang Wang. The Endpoint Events Assessment Committee comprised Fang Qu, Xiang He and Yanchun Liang. We are really appreciative of all the participants in this study.
Footnotes
Contributors: HC, XL and XW: conceived and designed the study. XH: drafted the manuscript. HC: revised the manuscript. All authors read and approved the final manuscript.
Funding: The work was supported by grants from the Science and Technology Project Plan of Liaoning Province (2014225008).
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Wardlaw JM, Zoppo G, Yamaguchi T. Thrombolysis for acute ischaemic stroke. Cochrane Database Syst Rev 2003;3:CD000213 10.1002/14651858.CD000213 [DOI] [PubMed] [Google Scholar]
- 2. Sandercock P, Gubitz G, Foley P, et al. . Antiplatelet therapy for acute ischaemic stroke. Cochrane Database Syst Rev 2003;2:CD000029 10.1002/14651858.CD000029 [DOI] [PubMed] [Google Scholar]
- 3. Hacke W, Kaste M, Bluhmki E, et al. . Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008;359:1317–29. 10.1056/NEJMoa0804656 [DOI] [PubMed] [Google Scholar]
- 4. Lu X, Huang Y. Thrombolysis treatment of acute ischemic stroke. Chin J Geriatr Heart Brain Vessel [Chinese] 2014;16:1230–2. [Google Scholar]
- 5. The International Stroke Trial (IST): a randomised trial of aspirin, subcutaneous heparin, both, or neither among 19435 patients with acute ischaemic stroke. International Stroke Trial Collaborative Group. Lancet 1997;349:1569–81. 10.1016/S0140-6736(97)04011-7 [DOI] [PubMed] [Google Scholar]
- 6. CAST: randomised placebo-controlled trial of early aspirin use in 20,000 patients with acute ischaemic stroke. CAST (Chinese Acute Stroke Trial) Collaborative Group. Lancet 1997;349:1641–9. [PubMed] [Google Scholar]
- 7. Powers WJ, Rabinstein AA, Ackerson T, et al. . 2018 guidelines for the early management of patients with acute ischemic stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2018;49:e46–e99. 10.1161/STR.0000000000000158 [DOI] [PubMed] [Google Scholar]
- 8. Neurology branch of Chinese Medical Association. Guidelines for the diagnosis and treatment of acute ischemic stroke in China 2014. Chin J Neurol [Chinese] 2015;48:246–57. [Google Scholar]
- 9. Wong KS, Chen C, Fu J, et al. . Clopidogrel plus aspirin versus aspirin alone for reducing embolisation in patients with acute symptomatic cerebral or carotid artery stenosis (CLAIR study): a randomised, open-label, blinded-endpoint trial. Lancet Neurol 2010;9:489–97. 10.1016/S1474-4422(10)70060-0 [DOI] [PubMed] [Google Scholar]
- 10. Li Z, Wang Y, Zhao X, et al. . Treatment effect of clopidogrel plus aspirin within 12 hours of acute minor stroke or transient ischemic attack. J Am Heart Assoc 2016;5:e003038 10.1161/JAHA.115.003038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Keller TT, Squizzato A, Middeldorp S. Clopidogrel plus aspirin versus aspirin alone for preventing cardiovascular disease. Cochrane Database Syst Rev 2007;3:CD005158 10.1002/14651858.CD005158.pub2 [DOI] [PubMed] [Google Scholar]
- 12. Wang Y, Wang Y, Zhao X, et al. . Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med 2013;369:11–19. 10.1056/NEJMoa1215340 [DOI] [PubMed] [Google Scholar]
- 13. Johnston SC, Easton JD, Farrant M, et al. . Clopidogrel and aspirin in acute ischemic stroke and high-risk TIA. N Engl J Med 2018;379:215–25. 10.1056/NEJMoa1800410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kernan WN, Ovbiagele B, Black HR, et al. . Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014;45:2160–236. 10.1161/STR.0000000000000024 [DOI] [PubMed] [Google Scholar]
- 15. Ge F, Lin H, Liu Y, et al. . Dual antiplatelet therapy after stroke or transient ischaemic attack - how long to treat? The duration of aspirin plus clopidogrel in stroke or transient ischaemic attack: a systematic review and meta-analysis. Eur J Neurol 2016;23:1051–7. 10.1111/ene.12982 [DOI] [PubMed] [Google Scholar]
- 16. Wang Z, Xu C, Wang P, et al. . Combined clopidogrel-aspirin treatment for high risk TIA or minor stroke does not increase cerebral microbleeds. Neurol Res 2015;37:993–7. 10.1179/1743132815Y.0000000087 [DOI] [PubMed] [Google Scholar]
- 17. Wasserman JK, Perry JJ, Sivilotti ML, et al. . Computed tomography identifies patients at high risk for stroke after transient ischemic attack/nondisabling stroke: prospective, multicenter cohort study. Stroke 2015;46:114–9. 10.1161/STROKEAHA.114.006768 [DOI] [PubMed] [Google Scholar]
- 18. Kennedy J, Hill MD, Ryckborst KJ, et al. . Fast assessment of stroke and transient ischaemic attack to prevent early recurrence (FASTER): a randomised controlled pilot trial. Lancet Neurol 2007;6:961–9. 10.1016/S1474-4422(07)70250-8 [DOI] [PubMed] [Google Scholar]
- 19. He F, Xia C, Zhang JH, et al. . Clopidogrel plus aspirin versus aspirin alone for preventing early neurological deterioration in patients with acute ischemic stroke. J Clin Neurosci 2015;22:83–6. 10.1016/j.jocn.2014.05.038 [DOI] [PubMed] [Google Scholar]
- 20. von Kummer R, Broderick JP, Campbell BC, et al. . The heidelberg bleeding classification: classification of bleeding events after ischemic stroke and reperfusion therapy. Stroke 2015;46:2981–6. 10.1161/STROKEAHA.115.010049 [DOI] [PubMed] [Google Scholar]
- 21. Hu LP. The application of statistical tri-type theory in trial design. Beijing: People’s military medical publisher, 2006. [Google Scholar]
- 22. Johnston SC, Amarenco P, Albers GW, et al. . Ticagrelor versus aspirin in acute stroke or transient ischemic attack. N Engl J Med 2016;375:35–43. 10.1056/NEJMoa1603060 [DOI] [PubMed] [Google Scholar]
- 23. Gorelick PB, Wong KS, Bae HJ, et al. . Large artery intracranial occlusive disease: a large worldwide burden but a relatively neglected frontier. Stroke 2008;39:2396–9. 10.1161/STROKEAHA.107.505776 [DOI] [PubMed] [Google Scholar]
- 24. Wong LK. Global burden of intracranial atherosclerosis. Int J Stroke 2006;1:158–9. 10.1111/j.1747-4949.2006.00045.x [DOI] [PubMed] [Google Scholar]
- 25. Pan Y, Jing J, Chen W, et al. . Risks and benefits of clopidogrel-aspirin in minor stroke or TIA: Time course analysis of CHANCE. Neurology 2017;88:1906–11. 10.1212/WNL.0000000000003941 [DOI] [PubMed] [Google Scholar]
- 26. Sacco RL, Kasner SE, Broderick JP, et al. . An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013;44:2064–89. 10.1161/STR.0b013e318296aeca [DOI] [PMC free article] [PubMed] [Google Scholar]