Abstract
Purpose:
Case reports and small prospective trials suggest that administering targeted therapies to patients with advanced cancer and an identified genomic target may be associated with clinical benefit. The TAPUR Study, a phase II, prospective, non-randomized, multi-basket, pragmatic clinical trial aims to identify signals of drug activity when Food and Drug Administration (FDA) approved drugs are matched to pre-specified genomic targets in patients with advanced cancer, outside of approved indications.
Methods:
Patients eligible to participate in TAPUR are ages 12 years and older, with advanced, measurable or evaluable solid tumors, multiple myeloma or B cell non-Hodgkin lymphoma. Eligible participants are matched to any of the sixteen FDA approved study drugs based on protocol specified genomic inclusion and exclusion criteria. Genomic profiling from any Clinical Laboratory Improvement Amendments certified, College of American Pathologists accredited laboratory is acceptable. The treating physician selects the treatment from the available study therapies, or consults with the TAPUR Molecular Tumor Board. Participants are placed into multiple parallel cohorts defined by tumor type, genomic alteration and drug. The primary study endpoint within each cohort is objective response or stable disease of at least 16 weeks duration. Secondary endpoints include safety, progression-free survival and overall survival.
Results:
More than 1000 participants have thus far been registered and more than 800 treated with a TAPUR study drug. Two study cohorts have permanently closed to enrollment due to lack of anti-tumor activity and 12 have expanded to the second stage of enrollment due to promising preliminary activity.
Conclusion:
The TAPUR Study will describe the efficacy and toxicity of the targeted drugs used outside of their approved indications when matched to a somatic genomic variant.
Keywords: precision medicine, pragmatic trial, basket trial, study design, molecular tumor board, genomic target, variant, oncology
Introduction
Evidence is building through reports of clinical trials, case reports, and clinical anecdotes to suggest that patient outcomes may be improved when a targeted agent is matched to a genomic alteration present in a patient’s tumor1–6. Clinical reports to date suggest that 30–80% of advanced solid tumors harbor potentially “actionable” genomic variants7–10.
In a meta-analysis of 570 phase II studies of new anti-cancer agents, Schwaederle and colleagues examined response rate (RR), progression-free survival (PFS), and overall survival (OS) for 32,149 patients who received a personalized treatment strategy versus those that did not11. Multivariable analysis demonstrated that the personalized approach consistently and independently correlated with higher median RR (31% vs 10.5%, P<0.0001), prolonged median PFS (5.9 vs 2.7 months, P<0.0001) and improved OS (13.7 vs 8.9 months, P=0.0001). In a similar approach, Jardim and colleagues analyzed registration trials for agents approved by the U.S. Food and Drug Administration (FDA) between 1998–201312. Analysis of experimental arms in 112 registration trials demonstrated that therapy assigned based on a biomarker selection strategy was associated with higher RRs (48% vs. 23%; P<0.001) and longer PFS (median = 8.3 vs. 5.5 months; P=0.002) and OS (median= 19.3 vs. 13.5 months; p = 0.04). Tsimberidou and colleagues reported results of the MD Anderson Cancer Center experience of genomic profiling of solid tumor patients with advanced disease, the Initiative for Molecular Profiling and Advanced Cancer Therapy (IMPACT) study13. Of 1144 patients analyzed, 40.2% had one or more genomic variants. Of the patients who received a targeted therapy matched to a genomic variant, the median progression-free survival (PFS) and overall survival (OS) times were 3.9 months and 11.4 months respectively compared with 2.2 and 8.6 months for patients who did not receive a matched targeted therapy. The MOSCATO-01 (Molecular Screening for Cancer Treatment Optimization, NCT01566019) trial enrolled patients with treatment-resistant progressive metastatic cancers with lesions accessible to biopsy to perform genomic profiling14. This study compared PFS using therapy based on genomic assessment to the PFS for the most recent therapy on which the patient experienced disease progression. Of 1035 adult patients enrolled, 843 had molecular profiling successfully performed. An actionable variant was identified in 411 patients (49%) and 199 patients actually received a matched therapy. The PFS2/PFS1 ratio was >1.3 in 33% of evaluable patients (63/193) representing 7% of the overall study population. At Indiana University, 43% of advanced cancer patients treated with a genomically guided therapy attained a PFS2/PFS1 ratio >1.3 compared with 5.3% of patients whose treatment was not guided by genomic profiling15. A retrospective matched cohort study conducted by investigators at Intermountain Health reported that patients with advanced cancer treated according to their tumor genomic profile had a median progression-free survival twice as long as a matched control group treated at physician discretion (22.9 vs. 12 weeks)16.
Despite these encouraging findings to support the strategy of matching drugs to a tumor molecular profile, the initial report of a prospective study of this approach has raised questions about its utility. Le Tourneau and colleagues published the results of a randomized phase II trial comparing therapy based on tumor molecular profiling versus physician choice of therapy in patients with refractory cancer17. Approximately 25 genomic targets were assessed in tumor biopsies and could be matched to 11 commercially available targeted drugs provided in the study. The median PFS was 2.3 months for the matched therapy arm and 2.0 months for the physician’s choice arm (p=0.41).
The availability of FDA approved agents targeted against specific genomic alterations along with the widespread availability of tumor genomic profiling tests is fueling off-label prescribing of targeted anti-cancer drugs based on genomic profiling in patients with advanced cancer but with no means or incentive to collect and report the outcomes of patients treated. The American Society of Clinical Oncology (ASCO) decided to sponsor its first clinical trial to attempt to fill this knowledge gap. This article will describe the rationale and design of the TAPUR Study, a large pragmatic precision medicine basket trial with the overarching goal of describing the efficacy and toxicity of targeted anti-cancer drugs used outside of their approved indications for treatment of patients with advanced cancer based on a tumor genomic profile.
Design and Methods
Objectives
The primary objective of TAPUR is to evaluate the anti-tumor activity of commercially available, targeted anti-cancer drugs used outside of their FDA-approved indications for treatment of patients with advanced solid tumors, multiple myeloma, or B cell non Hodgkin lymphoma with a genomic alteration known to be a drug target. Secondary objectives include determination of PFS, OS and safety.
Design
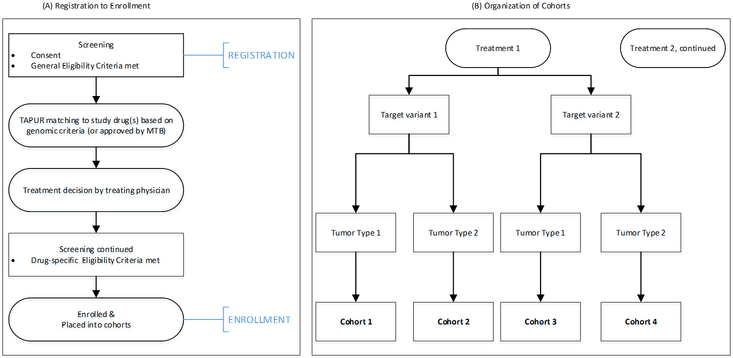
The TAPUR Study is a phase II, prospective, non-randomized, open label clinical trial that aims to define signals of drug activity. The primary study outcome is anti-tumor activity, defined as tumor response at 8 weeks or later or stable disease (SD) at 16 weeks or later from the time of enrollment. For solid tumor patients, response is defined as complete or partial response by RECIST v. 1.118. For patients with non-Hodgkin lymphoma, response is defined according to the Lugano Criteria19, 20. For patients with multiple myeloma, response is defined according to the International Myeloma Working Group (IMWG) Uniform Response Criteria21, 22. All serious adverse events and Grade 3–5 treatment-related adverse events are reported using the National Cancer Institute’s (NCI) Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0 (NCI CTCAE) criteria. The study includes participants as young as 12 years of age, whose tumor harbors a genomic alteration known to be a target of, or to predict sensitivity to, at least one of the sixteen therapeutic options available in the study (Table 1). Patients may not be matched to treatments that are already FDA approved for their cancer type. Eligible participants must have a tumor genomic variant identified on a test performed in a laboratory that has certification under the Clinical Laboratory Improvement Amendments and accreditation by the College of American Pathologists. Patients are matched to at least one of the available study treatments through a set of protocol-defined genomic matching rules or following review of a participant case by the TAPUR Molecular Tumor Board. Participants are then placed into one of the multiple parallel cohorts defined by study drug, genomic alteration, and tumor type as shown in Figure 1. The study utilizes Simon’s optimal two-stage design for cohort analysis.
Table 1:
List of Available TAPUR Study Treatments for both Adults and Pediatric Subjects
| Study Drug | Available to Adult (≥ 18 years)/Pediatric Population |
|---|---|
| Axitinib | Adults and children aged 12–17 |
| Bosutinib | Adults only |
| Cetuximab | Adults and children aged 12–17 |
| Crizotinib | Adults and children aged 16–17 |
| Dasatinib | Adults and children aged 13–17 |
| Erlotinib | Adults and children aged 12–17 |
| Nivolumab plus Ipilimumab | Adults only |
| Olaparib | Adults and children aged 16–17 only |
| Palbociclib | Adults only |
| Pembrolizumab | Adults and children aged 12–17 |
| Regorafenib | Adults and children aged 12–17 |
| Sunitinib | Adults and children aged 12–17 |
| Temsirolimus | Adults and children aged 12–17 |
| Trastuzumab plus Pertuzumab | Adults only |
| Vemurafenib plus Cobimetinib | Adults only |
| Vismodegib | Adults and children aged 12–17 |
Figure 1:
Participant registration, enrollment and cohort assignment process. Panel A displays the process by which participants are registered and enrolled into the study. Panel B displays the organization of the cohorts for analysis which are grouped by treatment, targeted variant and tumor type.
TAPUR was designed independently by ASCO staff and volunteer leaders with input from patient advocates, community-based investigators, the initial collaborating pharmaceutical companies and the ASCO Cancer Research Committee. The TAPUR study protocol was reviewed by the FDA and determined to be exempt from investigational new drug (IND) regulations. The study was registered on ClinicalTrials.gov (NCT02693535) prior to registration of the first participant.
A Data and Safety Monitoring Board (DSMB), consisting of a group of independent experts who are not involved in the conduct of TAPUR, meets bi-annually to monitor the study data and outcomes. The primary functions of the DSMB include assessing the safety and efficacy of study treatments, safeguarding the interests and safety of trial participants, and ensuring that study results are both credible and reported to the medical community in a timely manner. The DSMB reviews all cohorts once Stage 1 enrollment is complete and recommends cohorts for either closure or expansion. For expanded cohorts, the DSMB will review all final cohort analyses and make recommendations regarding release of the cohort data.
Study population and recruitment
TAPUR’s pragmatic approach includes broad eligibility criteria, including participants with Eastern Cooperative Oncology Group Performance Status 0–2, 12 years of age and above, prior malignancies or positive HIV status, as well as previously treated, but clinically stable brain metastases. Major eligibility criteria are summarized in Table 2. TAPUR inclusion/exclusion criteria comport fully with recently published recommendations by ASCO and Friends of Cancer Research for broadening eligibility criteria for cancer clinical trials23. Participating sites include both academic and community centers.
Table 2:
Summary of major inclusion and exclusion criteria for registration*
| Inclusion Criteria |
| 1. Patient (age ≥ 12 years**) with a histologically-proven locally advanced or metastatic solid tumor, multiple myeloma or B cell non-Hodgkin lymphoma who is no longer benefitting from standard anti-cancer treatment or no such treatment is available or indicated. |
| 2. ECOG performance status 0–2 and acceptable organ function defined per protocol. |
| 3. Patients must have measurable or evaluable disease (per RECIST v1.1 for solid tumor, Lugano criteria for non-Hodgkin lymphoma or International Myeloma Working Group criteria for multiple myeloma). |
| 4. Results must be available from a genomic test or immunohistochemistry (IHC) test for protein expression performed in a CLIA-certified, CAP-accredited, and New York State accredited (for labs offering services to residents of NY) laboratory. |
| 5. Have a tumor genomic profile for which treatment with one of the FDA approved targeted anti-cancer treatments included in this study has potential clinical benefit based on the genomic criteria. |
| 6. Ability to understand and the willingness to sign a written informed consent/assent document. |
| Exclusion Criteria |
| 1. Ongoing toxicity ≥ CTCAE grade 2, other than peripheral neuropathy, related to anti-tumor treatment that was completed within 4 weeks prior to registration. Patients with ongoing peripheral neuropathy of ≥ CTCAE grade 3 will be excluded. |
| 2. Patient is receiving any other anti-cancer treatment. |
| 3. Female patients who are pregnant or nursing. Male patients who refuse to practice barrier contraception methods. |
| 4. Patients with primary brain tumors are excluded. Patients with known progressive brain metastases determined by serial imaging or declining neurologic function in the opinion of the treating physician are not eligible. Patients with previously treated brain metastases are eligible, provided that the patient has not experienced a seizure or had a clinically significant change in neurological status within the 3 months prior to registration. All patients with previously treated brain metastases must be clinically stable for at least 1 month after completion of treatment and off steroid treatment for one month prior to study enrollment. |
| 5. Patients with preexisting cardiac conditions, including uncontrolled or symptomatic angina, uncontrolled atrial or ventricular arrhythmias, or symptomatic congestive heart failure are not eligible. |
| 6. Patients with left ventricular ejection fraction (LVEF) known to be < 40% are not eligible. |
| 7. Patients with stroke (including TIA) or acute myocardial infarction within 4 months before the first dose of study treatment are not eligible |
| 8. Patients with any other clinically significant medical condition which, in the opinion of the treating physician, makes it undesirable for the patient to participate in the study or which could jeopardize compliance with study requirements including, but not limited to: ongoing or active infection, significant uncontrolled hypertension, severe psychiatric illness situations, or anticipated or planned anti-cancer treatment or surgery. |
This list includes only major eligibility criteria.
Restrictions apply. Not all therapies are available for patients <18. Refer to Table 1.
Study treatment decision
Once a potential study participant is identified, the clinical site obtains informed consent and registers the participant in the TAPUR electronic data capture (EDC) platform as shown in Figure 1. The TAPUR EDC is a web-based data entry system where all TAPUR study data is entered and treatment matches are surfaced based on genomic profiling test results, according to a pre-specified set of genomic inclusion and exclusion criteria (herein referred to as the “matching rules”). If a study drug is identified and the drug-specific inclusion and exclusion criteria are met, the clinical site completes the drug-specific informed consent process and enrolls the participant into the study. Treatment begins and all protocol-specified evaluations must be performed and data reported in the EDC.
Molecular Tumor Board
Treating physicians may choose to consult with the TAPUR Molecular Tumor Board (MTB) when no or multiple treatment matches surface within the TAPUR matching rules, or if the treating physician would like to propose a treatment match outside of the matching rules. The MTB, comprised of clinical oncologists, molecular pathologists, and patient advocates, reviews the genomic test results, pathology report, and clinical history of submitted cases and identifies potential treatment matches among the TAPUR study treatments. The MTB may also suggest off-study treatments for consideration.
Treatment Schedule and Interval of Evaluations
Study treatments are administered according to the recommended starting dose and schedule described in the package insert of each drug. Adjustments in dosage and scheduling are permitted at the discretion of the treating physician in accordance with the recommended dose modifications contained in the approved prescribing information. Management of side effects is performed according to institutional standards of care, informed by the package insert.
Tumor measurements and radiologic evaluations and evaluation of clinical disease status are performed every 8 weeks following initiation of treatment for the first 16 weeks, and then every 12 weeks if the participant continues treatment, and at the end of study treatment, if possible. Study treatment is continued until progressive disease is documented.
Following progression of disease on any study treatment, participants may be reassessed to determine eligibility for treatment with another study treatment after a minimum of 30 days has elapsed from the last dose of the previous treatment, and any adverse events have resolved to ≤ Grade 2 or stabilized. All general and drug-specific eligibility criteria must be re-confirmed and the participant must sign a new drug-specific consent form.
Adverse Event and Serious Adverse Event Criteria
Adverse and serious adverse events measured by the NCI CTCAE must be reported once the participant has received at least one dose of study treatment. Collection and reporting of qualifying adverse events continues until 30 days after the last dose of study drug. Events not resolved at the end of study treatment require follow-up every 30 days until the event resolves to CTCAE Grade 2 or lower.
TAPUR requires reporting of CTCAE Grade 3–4 adverse events (AE) that are possibly, probably, or definitely related to the study treatment, whether expected or not. All serious adverse events (SAE), regardless of grade, relatedness to study drug, or expectedness, must also be reported, including all deaths.
Endpoints
The primary endpoint is objective response at 8 weeks or later, or stable disease (SD) documented at 16 weeks or later. Participants who have measurable disease present at baseline and have received at least one dose of study treatment are considered evaluable for response. Participants who have lesions present at baseline that are evaluable but do not meet the definition of measurable disease, and receive at least one dose of study treatment, are assessed based on the presence, absence, or unequivocal progression of the lesions. Participants who have no tumor evaluation beyond baseline and are alive with no signs of progressive disease at the time of leaving the study, will be replaced in their cohort with another participant.
Secondary endpoints include Grade 3–5 AEs and SAEs, PFS, response duration and OS. PFS is defined as the time from initiation of treatment to documented disease progression or death from any cause, whichever occurred first. Response duration is defined as time from documentation of PR or CR until objective tumor progression. OS is defined as time from initiation of treatment until death from any cause.
Statistical Considerations
For each cohort, Simon’s optimal two-stage design is utilized. The null hypothesis is that the probability of objective response or stable disease at least 16 weeks duration is 15%, versus the alternative hypothesis that it is at least 35%. Power and type I error rate are assumed to be 85% and 0.10 (one-sided), respectively. Under these assumptions, in the first stage, 10 participants are entered. If two or more participants experience objective response or stable disease of at least 16 weeks duration, an additional 18 participants are enrolled, otherwise the cohort is permanently closed. After 28 participants in the cohort have been observed for the primary outcome, if seven or more participants achieve objective response or stable disease of at least 16 weeks duration, the null hypothesis will be rejected, and we will declare that the study drug is active in the cohort of participants defined by tumor type and genomic alteration. This design has a 54% chance of early stopping if the null hypothesis is true.
Estimates of the response rate, and 95% confidence interval will be presented for each cohort upon closure (at either stage 1 or stage 2)24. The Kaplan-Meier method will be used to estimate the duration of response, PFS and OS distributions.
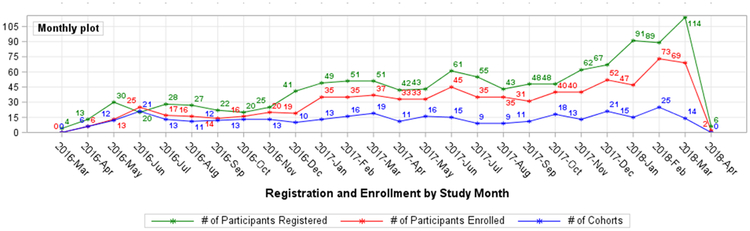
Study Progress
Table 3 summarizes key study milestones. Figure 3 displays participant registration and enrollment by month and the rate of creation of new cohorts. As summarized in Table 4, thus far, 12 cohorts have expanded to the second stage of enrollment and 2 cohorts have been permanently closed after stage I.
Table 3:
TAPUR Study Milestones
| Date | TAPUR Study Milestone |
|---|---|
| March 14, 2016 | Study Launch at 37 clinical site locations with 14 study drugs |
| March 2016 | 1st participant registered (consented) |
| April 2016 | 1st participant enrolled, 2 additional drugs added for total of 16 study drugs |
| May 2016 | Additional drug added for total of 17 study drugs |
| August 2016 | 100th participant registered |
| November 2016 | 100e participant enrolled |
| April 2017 | 33 clinical sites added, for a total of 70 locations |
| June 2017 | 500th participant registered |
| August 2017 | Additional therapy added for 19 study drugs |
| September 2017 | 1st cohort reaches Stage I |
| November 2017 | 500th participant enrolled |
| December 2017 | 43 clinical sites added, for a total of 113 locations |
| February 2018 | 1000th participant registered |
| March 2018 | 10th cohort reaches Stage I |
Table 4:
TAPUR Study initial cohort expansions and closures
| Cohort Status | Study Drug | Tumor Type | Variant |
|---|---|---|---|
| Expansion to Stage II | Cetuximab | Ovarian Cancer | KRAS, NRAS, and BRAF wildtype |
| Cobimetinib + Vemurafenib | Colorectal Cancer | BRAF_V600E/D/K/R mutation | |
| Olaparib | Breast Cancer | Germline or somatic BRCA1/BRCA2 inactivating mutations | |
| Colorectal Cancer | ATM mutation or deletion | ||
| Palbociclib | Head and Neck Cancer | CDKN2A loss or mutation | |
| Soft Tissue Sarcoma | CDK4 amplification | ||
| Malignant neoplasm of bronchus and lung | CDKN2A loss or mutation | ||
| Pembrolizumab | Breast Cancer | High tumor mutational burden | |
| Colorectal Cancer | High tumor mutational burden | ||
| Uterine Cancer | High tumor mutational burden | ||
| Pertuzumab + Trastuzumab | Colorectal Cancer | ERBB2/ERBB3 mutation, amplification or overexpression | |
| Sunitinib | Breast Cancer | FGFR1 mutation or amplification | |
| Closed at Stage I | Palbociclib | Pancreatic Cancer | CDKN2A loss or mutation |
| Malignant Neoplasm of Gallbladder and Bile Ducts |
Discussion
Precision medicine approaches to cancer care have clearly revolutionized the treatment of many cancers that are driven by specific molecular alterations that can be targeted with drugs that inhibit aberrant signaling pathways in tumor cells. Thus the targeting of BCR-ABL alterations in chronic myeloid leukemia, of FLT-3 alterations in acute myeloid leukemia, of EGFR mutations and ALK translocations in non-small cell lung cancer, of BRAF mutations in melanoma and non-small cell lung cancer and of HER2 overexpression in breast and gastric cancer, among others, represent examples of the successful application of precision medicine approaches in clinical oncology that have extended the lives of many patients1, 25–30. Interest in precision medicine has been further fueled by reported responses ranging from modest to exceptional when targeted treatments are selected based on large scale genomic tumor profiling5. The widespread availability of commercial next generation sequencing tests presents opportunities for patients who have exhausted standard treatment options to pursue treatments with investigational agents or approved drugs prescribed outside of their labeled indications.
The TAPUR Study stemmed from the recognition that the rapid dissemination of genomic profiling provides an opportunity to learn from the application of precision cancer medicine in practice while at the same time providing a framework for clinical decision support for clinical oncologists who are struggling to interpret the complex genomic data they now confront in practice. Thus, the study was designed to closely replicate real-world clinical practice with broad eligibility criteria that comport with recent recommendations23, minimum necessary data collection and discretion left to the treating physician regarding the choice of tumor biospecimen to interrogate and the genomic profiling test. However, TAPUR employs standard treatment response and toxicity criteria for assessment of efficacy and toxicity outcomes, structured data collection, protocol-specified evaluation times and a standard Simon two-stage statistical design to assess the primary endpoint as well as an independent DSMB to provide recommendations on cohort expansion and closure. Importantly, the study also provides educational opportunities and clinical decision support tools to treating physicians in the form of protocol-specified genomic matching rules and access to a MTB to assist in interpretation of genomic test reports.
While TAPUR study results are not yet available, preliminary information about the status of certain cohorts suggests that the study can provide meaningful information. The expansion of some cohorts provides confidence that TAPUR can detect signals of drug activity when such activity is known to exist. Thus, the expansion of the olaparib cohort in breast cancer patients whose tumor harbors BRCA 1 or 2 mutations is supported by clinical trial data and the recent FDA approval of olaparib in such patients and the expansion of the trastuzumab-pertuzumab cohort in colorectal cancer patients with HER2-overexpressing tumors is consistent with data recently reported31, 32. Furthermore, the expansion of the pembrolizumab cohorts in breast cancer, uterine and colorectal cancer patients with high tumor mutational burden is consistent with the activity of this class of agents in other tumor types with high tumor mutation loads33. Finally, in view of recent interest in histology-agnostic targeted drug development exemplified by the FDA approval of pembrolizumab in all microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR) advanced solid tumors, it is interesting to note that the TAPUR cohorts of palbociclib in pancreatic cancer and gallbladder/bile duct cancers bearing CDKN2A alterations were closed after the first stage of enrollment while cohorts comprising the same drug and genomic alteration in patients with non-small cell lung cancer and head and neck cancer have been expanded to the second stage of enrollment34.
Many challenges were encountered in the development and launch of TAPUR including defining the genomic matching rules, estimating enrollment rates when the frequency of genomic targets was not well-defined for many tumor types, identifying clinical launch sites that routinely employed genomic profiling, arranging for drug distribution and building an experienced clinical trial management team to operate the study. However, the launch of the TAPUR study by ASCO illustrates both the opportunity for medical professional societies to learn from observing the clinical work of their members and the feasibility of doing so through the development of pragmatic clinical trials.
Figure 2: TAPUR Study Participant Registration, Enrollment and Cohort Creation by Month*.
*April 2018 registration, enrollment, cohort creation in progress at the time of this report.
Acknowledgements
The authors would like to acknowledge all members of the TAPUR Study team for their support and contributions to the TAPUR Study (current and past team members who were with the team for at least 1 year): Kaitlyn R. Antonelli, Cynthia Arias, Linda Bressler, Pharm.D., BCOP, Nicole L. Butler, MPH, Kuo Guo, MS, Molly Holoubek CCRN, Samiha Islam, Linda Miller, MSN, RN, OCN, Shamika Ranasinghe, MS, Brittany M. Rowley, Andrew Lawrence Rygiel, MPH, Katie Wiegand, MEng. The authors would also like to acknowledge the following clinical leads of the supporting pharmaceutical companies that were involved in the development and support of the TAPUR Study: Josefa Briceno, MD, Gregory Curt, MD (AstraZeneca), Sybil Anderson, MD (Bayer), Cynthia Brogdon, PhD (Bristol-Myers Squibb), Allen Melemed, MD (Eli Lilly), Mary Beattie, MD (Genentech), Eric Rubin, MD (Merck), Lynn McRoy, MD; Ronit Simantov, MD (Pfizer).
Funding
This study was funded by AstraZeneca, Bayer, Bristol-Myers Squibb, Eli Lilly, Genentech, Merck, Pfizer.
Footnotes
References
- 1.Kris MG, Johnson BE, Berry LD, et al. : Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. Jama 311:1998–2006, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wagle N, Grabiner BC, Van Allen EM, et al. : Activating mTOR Mutations in a Patient with an Extraordinary Response on a Phase I Trial of Everolimus and Pazopanib. Cancer Discovery 4:546–553, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Solange P, Olivier M, Stefan Z: Dramatic Response Induced by Vemurafenib in a BRAF V600E-Mutated Lung Adenocarcinoma. Journal of Clinical Oncology 31:e341–e344, 2013 [DOI] [PubMed] [Google Scholar]
- 4.Munoz J, Schlette E, Kurzrock R: Rapid response to vemurafenib in a heavily pretreated patient with hairy cell leukemia and a BRAF mutation. J Clin Oncol 31:e351–2, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Moscow JA, Fojo T, Schilsky RL: The evidence framework for precision cancer medicine. Nat Rev Clin Oncol 15:183–192, 2018 [DOI] [PubMed] [Google Scholar]
- 6.Iyer G, Al-Ahmadie H, Schultz N, et al. : Prevalence and co-occurrence of actionable genomic alterations in high-grade bladder cancer. J Clin Oncol 31:3133–40, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cobain EF, Robinson DR, Wu Y-M, et al. : Clinical impact of high-throughput sequencing in patients with advanced cancer: Lessons learned from the Michigan Oncology Sequencing Center. Journal of Clinical Oncology 33:11057–11057, 2015 [Google Scholar]
- 8.Tsimberidou AM, Iskander NG, Hong DS, et al. : Personalized medicine in a phase I clinical trials program: the MD Anderson Cancer Center initiative. Clin Cancer Res 18:6373–83, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MacConaill LE, Garcia E, Shivdasani P, et al. : Prospective enterprise-level molecular genotyping of a cohort of cancer patients. J Mol Diagn 16:660–72, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sukhai MA, Craddock KJ, Thomas M, et al. : A classification system for clinical relevance of somatic variants identified in molecular profiling of cancer. Genet Med 18:128–36, 2016 [DOI] [PubMed] [Google Scholar]
- 11.Fontes Jardim DL, Schwaederle M, Wei C, et al. : Impact of a Biomarker-Based Strategy on Oncology Drug Development: A Meta-Analysis of Clinical Trials Leading to FDA Approval. JNCI: Journal of the National Cancer Institute 107:djv253–djv253, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwaederle M, Zhao M, Lee JJ, et al. : Impact of Precision Medicine in Diverse Cancers: A Meta-Analysis of Phase II Clinical Trials. J Clin Oncol 33:3817–25, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsimberidou A-M, Wen S, Hong DS, et al. : Personalized Medicine for Patients with Advanced Cancer in the Phase I Program at MD Anderson: Validation and Landmark Analyses. Clinical cancer research: an official journal of the American Association for Cancer Research 20:4827–4836, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Massard C, Michiels S, Ferte C, et al. : High-Throughput Genomics and Clinical Outcome in Hard-to-Treat Advanced Cancers: Results of the MOSCATO 01 Trial. Cancer Discov 7:586–595, 2017 [DOI] [PubMed] [Google Scholar]
- 15.Radovich M, Kiel PJ, Nance SM, et al. : Clinical benefit of a precision medicine based approach for guiding treatment of refractory cancers. Oncotarget 7:56491–56500, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haslem DS, Van Norman SB, Fulde G, et al. : A Retrospective Analysis of Precision Medicine Outcomes in Patients With Advanced Cancer Reveals Improved Progression-Free Survival Without Increased Health Care Costs. J Oncol Pract 13:e108–e119, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Le Tourneau C, Delord JP, Goncalves A, et al. : Molecularly targeted therapy based on tumour molecular profiling versus conventional therapy for advanced cancer (SHIVA): a multicentre, open-label, proof-of-concept, randomised, controlled phase 2 trial. Lancet Oncol 16:1324–34, 2015 [DOI] [PubMed] [Google Scholar]
- 18.Eisenhauer EA, Therasse P, Bogaerts J, et al. : New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–47, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Cheson BD, Fisher RI, Barrington SF, et al. : Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol 32:3059–68, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barrington SF, Mikhaeel NG, Kostakoglu L, et al. : Role of imaging in the staging and response assessment of lymphoma: consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J Clin Oncol 32:3048–58, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Durie BGM, Harousseau JL, Miguel JS, et al. : International uniform response criteria for multiple myeloma. Leukemia 20:1467, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Durie BGM, Harousseau JL, Miguel JS, et al. : International uniform response criteria for multiple myeloma. Leukemia 21:1134, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Kim ES, Bruinooge SS, Roberts S, et al. : Broadening Eligibility Criteria to Make Clinical Trials More Representative: American Society of Clinical Oncology and Friends of Cancer Research Joint Research Statement. J Clin Oncol 35:3737–3744, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koyama T, Chen H: Proper inference from Simon’s two-stage designs. Stat Med 27:3145–54, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Druker BJ, Talpaz M, Resta DJ, et al. : Efficacy and Safety of a Specific Inhibitor of the BCR-ABL Tyrosine Kinase in Chronic Myeloid Leukemia. New England Journal of Medicine 344:1031–1037, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Stone RM, Mandrekar SJ, Sanford BL, et al. : Midostaurin plus Chemotherapy for Acute Myeloid Leukemia with a FLT3 Mutation. N Engl J Med 377:454–464, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chapman PB, Hauschild A, Robert C, et al. : Improved Survival with Vemurafenib in Melanoma with BRAF V600E Mutation. New England Journal of Medicine 364:2507–2516, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hyman DM, Puzanov I, Subbiah V, et al. : Vemurafenib in Multiple Nonmelanoma Cancers with BRAF V600 Mutations. N Engl J Med 373:726–36, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Slamon DJ, Leyland-Jones B, Shak S, et al. : Use of Chemotherapy plus a Monoclonal Antibody against HER2 for Metastatic Breast Cancer That Overexpresses HER2. New England Journal of Medicine 344:783–792, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Bang YJ, Van Cutsem E, Feyereislova A, et al. : Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 376:687–97, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Robson M, Im SA, Senkus E, et al. : Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N Engl J Med 377:523–533, 2017 [DOI] [PubMed] [Google Scholar]
- 32.Hainsworth JD, Meric-Bernstam F, Swanton C, et al. : Targeted Therapy for Advanced Solid Tumors on the Basis of Molecular Profiles: Results From MyPathway, an Open-Label, Phase IIa Multiple Basket Study. J Clin Oncol 36:536–542, 2018 [DOI] [PubMed] [Google Scholar]
- 33.Yarchoan M, Hopkins A, Jaffee EM: Tumor Mutational Burden and Response Rate to PD-1 Inhibition. New England Journal of Medicine 377:2500–2501, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Le DT, Uram JN, Wang H, et al. : PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. New England Journal of Medicine 372:2509–2520, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]