Abstract
Prenatal exposures to higher levels of maternal cortisol and depression have been linked to a variety of adverse physiological, neurological, and behavioral outcomes, such as dysregulated cortisol production, structural and functional differences in limbic areas of the brain, and greater negative emotionality. This study investigated prospective associations between maternal prepartum depression/cortisol levels and offspring emotional reactivity in 163 mother–child pairs. Women were assessed repeatedly during pregnancy, and later participated in a laboratory visit with their preschool-aged children. Mothers self-reported on depressive symptomatology during pregnancy and provided saliva samples for cortisol assay. Offspring emotional reactivity was assessed through multiple measures, including caregiver reports, cortisol response following a stressor, and laboratory observations of behavior. The findings suggest potential prenatal timing effects, with depression and maternal cortisol measured in the first and second trimesters being more strongly associated with child emotional reactivity. Sex was found to moderate associations between maternal prepartum depression/cortisol and child emotional reactivity, with the general pattern reflecting positive associations in girls, and negative associations in boys.
Keywords: cortisol, depression, emotional reactivity, human, pregnancy
1 |. INTRODUCTION
Maternal depression affects between 8.5% and 20% of women during pregnancy (Gavin et al., 2005; Marcus, 2009; Marcus, Flynn, Blow, & Barry, 2003). Not only does prepartum depression disrupt maternal psychological wellbeing, but may also impair fetal development, with long-term implications for physiological, psychological, cognitive, and behavioral outcomes in the offspring (Davis et al., 2004; Deave, Heron, Evans, & Emond, 2008; Field, Diego, & Hernandez-Reif, 2006; Sohr-Preston & Scaramella, 2006).
Prepartum maternal depression has been hypothesized to exert programming influences on the developing fetus, with mounting evidence suggesting gestational cortisol as a potential pathway underlying this association (Kinsella & Monk, 2009). Depressive symptoms have been linked to the dysregulation of cortisol production in both nonpregnant and pregnant women (Burke, Davis, Otte, & Mohr, 2005; Davis et al., 2007). In addition to these associated changes in maternal stress physiology, the fetus of a depressed mother is also particularly susceptible to elevations in maternal cortisol. During pregnancy, a portion of cortisol crosses the placental barrier, in a process which is regulated by placental enzyme 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2). 11β-HSD2 oxidizes 50%–90% of maternal cortisol as it enters the fetal compartment, turning it into its inactive form (Buss et al., 2012; Glover, O’Connor, & O’Donnell, 2010; Ponder et al., 2011). However, maternal depressive symptoms are associated with the downregulation of 11β-HSD2 (Buss et al., 2012), thereby increasing fetal exposure to elevated levels of circulating maternal cortisol. Prepartum depression and the resulting increase in exposure to gestational cortisol may therefore initiate a cascade of events that shape the stress physiology of the uterine environment, producing consequences for the fetus which persist across development.
Maternal depression and elevated cortisol levels in pregnancy have been hypothesized to influence emotional reactivity in offspring through their impacts on the developing limbic system of the fetal brain and relatedly, the hypothalamic–pituitary–adrenal (HPA) axis (Duthie & Reynolds, 2013). This hypothesis has been supported by findings linking higher levels of maternal prepartum depression and cortisol to elevated offspring cortisol reactivity (Davis, Glynn, Waffarn, and Sandman (2011); Fernandes, Stein, Srinivasan, Menezes, & Ramchandani, 2015; Gutteling, deWeerth, & Buitelaar, 2005; Laurent, Ablow, & Measelle, 2011; O’Connor, Bergman, Sarkar, & Glover, 2013), heightened behavioral reactivity (Davis et al., 2004), lower response threshold toward emotional events (Buss et al., 2012; Tottenham & Sheridan, 2009), and increased irritability and emotional problems (Davis et al., 2004, 2007; Luoma et al., 1998; Zuckerman, Bauchner, Parker, & Cabral, 1990). Collectively, these findings demonstrate that changes in emotional reactivity resulting from prepartum exposures can manifest across both physiological and behavioral dimensions of functioning, thereby highlighting the need to investigate and integrate multiple measures of emotional reactivity outcomes. In the current study, children’s emotional reactivity was assessed through measures of cortisol, laboratory behaviors, and caregiver reports.
The timing of exposure to elevated maternal cortisol and depression may play a critical role in determining fetal vulnerabilities. While fetal exposure to elevated maternal cortisol in late gestation, a time in which cortisol is normatively high, has been shown to be beneficial for fetal cognitive development (Davis & Sandman, 2010), elevated cortisol in the second and early third trimesters of pregnancy has been found to have detrimental consequences for offspring temperament and stress physiology (Davis et al., 2007; O’Connor et al, 2013). Few studies, however, have explored these timing effects across the course of gestation (Davis et al., 2007), and specific timing effects of prepartum maternal depression and elevated cortisol remain unclear. The current study sought to advance the field by examining the impact of the timing and course of maternal depression across pregnancy, as well as potential timing effects of maternal prepartum cortisol elevations, on offspring emotional reactivity outcomes.
Several studies have found sex differences in fetal vulnerability to maternal cortisol and depression (Buss et al., 2012; Sandman, Glynn, & Davis, 2013; Weinstock, 2005). Males tend to exhibit decreased expression of placental 11β-HSD2, suggesting they may be exposed to more maternal cortisol during gestation and perhaps may have an overall greater vulnerability to prenatal stress (Charil, Laplante, Vaillancourt, & King, 2010). Conversely, some studies suggest greater vulnerability among female offspring. For example, Buss and colleagues (2012) found that elevated maternal cortisol levels in early pregnancy predicted larger right amygdala volume and more affective problems in females, but not in males. Sandman et al. (2013) proposed that the greater fetal vulnerability to cortisol among male fetuses and long-term risk for affective problems among females may be explained by a sexually dimorphic viability-vulnerability tradeoff. Similarly, evolutionary adaptation theories have been applied to explain differential impacts of fetal programming in male and female offspring (Glover & Hill, 2012). In line with these theories, a recent study (Braithwaite et al., 2017) found that higher levels of maternal waking cortisol in pregnancy were associated with significantly higher levels of behavioral reactivity in female infants (making them prone to inhibition), and significantly lower levels of behavioral reactivity in male infants (making them prone to aggression), which could serve (in evolutionary terms) as adaptive behavioral strategies in the context of stressful postnatal environments. Given the lack of clear directionality in terms of sex differences throughout the extant literature, this study examined potential sex differences in offspring responses to prepartum exposures in an exploratory manner.
The current study utilized longitudinal, prospective data to examine the impact of varying patterns of maternal depression and cortisol during pregnancy on offspring emotional reactivity outcomes in the preschool period. We conducted multiple assessments of maternal depression and cortisol over the course of pregnancy, and used a data driven approach to identify trajectories of depressive symptoms over gestation. Child emotional reactivity outcomes were assessed through multiple measures, including behavioral and cortisol responses to a set of laboratory frustration tasks, and primary (mother) and alternate caregiver (e.g., father, grandparent, babysitter) reports of child emotional reactivity. We hypothesized that empirically derived trajectory classes of maternal depression across pregnancy would predict child emotional reactivity during the preschool period. We also hypothesized that the timing of increased maternal depressive symptoms and cortisol levels during pregnancy would differentially predict child emotional reactivity. In addition, exploratory analyses were conducted to examine the potential role of child sex as a moderator of the relationships between prepartum exposures and offspring emotional reactivity.
2 |. METHOD
2.1 |. Participants
Participants were drawn from a sample of 178 mother–child dyads, recruited as part of a prospective longitudinal study assessing the long-term cognitive, behavioral, and emotional outcomes of children prenatally exposed to psychotropic medications. Mothers were recruited from the Emory Women’s Mental Health Program (WMHP) at Emory University School of Medicine in the Department of Psychiatry. The WMHP is a mental health treatment center that provides psychiatric care for women. WMHP mothers who agreed to participate in the preschool study also gave us permission to access their prepartum data collected previously collected in the context of separate studies. Prepartum depression data in each trimester was not available for 15 of the mothers within our sample; therefore, our final sample consisted of 163 mother–child dyads. The majority (79.8%) of the mothers in the sample had taken antidepressant, antiepileptic, or antipsychotic medications during pregnancy. Mothers and children excluded from the sample did not differ on maternal age, maternal race, maternal marital status, maternal psychotropic use in pregnancy, self-reports of maternal depression at the preschool follow-up, or on child emotional reactivity measures (all p > .31). See Table 1 for sample demographic information.
TABLE 1.
Sample descriptive information
| Variable | Mean (SD) | |
|---|---|---|
| Child age | 44.0 months (10.6) | |
| Mother age | 37.3 years (4.8) | |
| Mother average BDI score in pregnancy | 9.98 (7.92) | |
| Mother duration of psychiatric illness | 15.2 months (18.9) | |
| Number of siblings | 1.1 (.92) | |
| % (N) | % (N) | |
| Mother | Child | |
| Sex | ||
| Male | 51(85) | |
| Female | 48(78) | |
| Race/ethnicity | ||
| African American | 8.0 (13) | 7.4 (12) |
| Asian | 1.8 (3) | 0 (0) |
| Biracial | .6 (1) | 8.6 (14) |
| Caucasian | 87.0 (140) | 81.6 (133) |
| Hispanic | 2.5 (4) | 1.2 (2) |
| Missing | 1.2 (2) | 1.2 (2) |
| Marital status | ||
| Married or living with someone as if married | 83.4 (136) | |
| Divorced or annulled | 6.7 (11) | |
| Separated | 3.1 (5) | |
| Never married | 5.5 (9) | |
| Missing | 1.2 (2) | |
| Mother’s highest level of education | ||
| Graduated high school or GED | 0.6 (1) | |
| Part college | 9.8 (16) | |
| Graduated 2-year college | 7.4 (12) | |
| Graduated 4-year college | 35.0 (57) | |
| Part graduate/professional school | 3.7 (8) | |
| Completed graduate/professional school | 42.3 (69) | |
| Mother antidepressant use in pregnancy | 64.4 (105) | |
| Mother antiepileptic use in pregnancy | 28.2 (46) | |
| Mother antipsychotic use in pregnancy | 23.3 (38) | |
| Mother tobacco use in pregnancy | 8.6 (24) | |
2.2 |. Procedure
Mothers were initially recruited for participation in the study through the WMHP. Recruited mothers had previously reported on their depression symptoms during their visits to the WMHP over the course of their pregnancy (approximately every 4–6 weeks). A subsample of mothers (n = 50) also provided saliva for cortisol assays during prepartum visits in the second and third trimester. During the preschool study, children and their mothers participated in a 3-hr lab visit. Mothers completed questionnaires about their current symptoms of depression, and mothers and alternate caregivers completed questionnaires about the child’s behavior, which included questions about emotional reactivity. Children also participated in a laboratory-based frustration task and provided two saliva samples: one at the beginning of the lab visit and one following a 20-min postfrustration task delay. All preschool lab visits were completed between 9:30 AM and noon. This study was approved by the Emory University Institutional Review Board. Mothers provided consent for both their own and their children’s participation, and children also provided assent. Alternate caregivers provided consent for their own participation in the study.
2.3 |. Measures
2.3.1 |. Maternal prepartum depression
Maternal depressive symptoms were assessed in 4–6 week intervals throughout pregnancy using the 21-item Beck Depression Inventory (BDI; Beck, Steer, & Garbin, 1988). For this self-report measure, the mother indicated the intensity of each listed symptom over the past 2 weeks. Previous literature demonstrates good internal consistency of the BDI among psychiatric populations, as well as high internal reliability and strong discriminant, concurrent, and construct validity (Beck et al., 1988). Internal consistency for the BDI was also high in our sample (Cronbach α = 0.90). We have previously reported stable performance of the BDI across all trimesters of pregnancy (Ji et al., 2011).
The current study utilized BDI scores over each trimester of pregnancy to assess timing and determine trajectories of maternal depression within the sample. Specifically, BDI scores obtained within each trimester were combined to yield an area under the curve (AUC) measure by trimester, normalized to 40 weeks to account for differences in timing of delivery. AUC relative to ground was calculated using the linear trapezoid method (Pruessner, Kirschbaum, Meinlschmid, & Hellhammer, 2003) and these scores were used in further analyses, as described in detail below.
2.3.2 |. Maternal prepartum cortisol
A subsample of women (n = 50) provided baseline cortisol samples at three time points during pregnancy (at an average of 24, 30, and 36 weeks gestation). Women with prepartum cortisol data did not significantly differ from those without on prepartum depression scores in any trimester or on any child emotional reactivity variables (all p-values > .07). Saliva was collected by passive drool, after mothers acclimated to the lab setting and completed a short set of questionnaires. Visits occurred during regular workday hours (9:00 AM–5:00 PM). Samples were stored at −20°C until assayed for cortisol concentration using a commercially available ELISA kit (Salimetrics). The absolute sensitivity of this kit for saliva cortisol was 0.007, and inter-and intraassay coefficients of variation were 6.7% and <5%, respectively. All standards and samples were run in duplicate by a research assistant blind to maternal depression status and the time point at which the sample was obtained. Cortisol values were subjected to 90% winsorization to remove influential outliers. The mean and SD values (in μg/dL) at each time point were as follows: 24 weeks M = 0.278, SD = 0.155, range = 0.07–0.64; 30 weeks M = 0.288, SD = 0.166, range = 0.09–0.73; 36 weeks M = 0.303, SD = 0.126, range = 0.05–0.54. Waking time, recent food, caffeine intake, and nicotine use were not correlated with cortisol levels (all p-values > .10). The estimated gestational age (in weeks) and time of day were included as covariates in all analyses.
2.3.3 |. Child behavioral response to the laboratory frustration tasks
Two batteries of the preschool version Laboratory Temperament Assessment Battery (Lab-TAB), the “Attractive Toy in a Transparent Box” and “Impossibly Perfect Green Circle” (see Goldsmith, Reilly, Lemery, Longley, & Prescott, 1999 for description) were used to measure behavioral stress reactivity in the preschoolers.
Both Lab-TAB tasks were recorded for later offline behavioral coding. First, each video was divided into 10-s epochs. Then, for each epoch, a trained researcher coded each child along the following dimensions: intensity of anger expression, presence of bodily anger, peak intensity of frustration, intensity of sadness expression, presence of bodily sadness, and peak intensity of gaze aversion. About 10% of videos were recoded for reliability by a Masters level graduate student researcher. Reliability was high (α > 0.8) across all behavioral codes. A summary score was calculated that reflected the number of epochs in which the child displayed an emotional behavioral response. This summary score (M = 5.387, SD = 2.85) was used as the behavioral observation measure of emotional reactivity.
2.3.4 |. Child cortisol response to the laboratory frustration tasks
Salivary cortisol samples were collected (1) following the child’s acclimation to the lab, and (2) after the Lab-TAB frustration tasks, in order to assess the child’s physiological stress reactivity. The collection of the second, poststressor cortisol sample occurred 20 min following the conclusion of both frustration tasks, as cortisol peaks approximately 15–20 min following exposure to a stressor (Kirschbaum & Hellhammer, 1989). Saliva samples were collected by having the child chew on a cotton roll covered in 0.025 g of KoolAid™ to encourage saliva production. Saliva samples were then extracted from the cotton roll using a syringe and were transferred to a plastic vial. Previous literature has shown that salivary cortisol is a common and reliable method of assessing glucocorticoid levels and HPA axis activity in humans (Kirschbaum & Hellhammer, 1989). Its noninvasive method of collection also makes it an appropriate measure for cortisol in young children. Salivary cortisol samples were frozen and stored at −20°C, until they were sent to the Endocrine Core Laboratory at the Yerkes National Primate Research Center to be assayed. Cortisol values were subjected to 90% winsorization to reduce the influence of outliers. Cortisol response to the frustration tasks was then calculated as a difference score between pretask (M = 0.149, SD = 0.141) and posttask (M = 0.130, SD = 0.141) salivary cortisol levels. All analyses examining cortisol response controlled for baseline (pretask) levels. Missing child cortisol data occurred in approximately 17% of the sample, primarily due to refusal or low volume of saliva; maternal prepartum depression and cortisol levels were unrelated to missingness (all p-values > .44).
2.3.5 |. Caregiver ratings of emotional reactivity
Mothers and alternate caregivers (54% fathers, 18% grandmothers, 11% teachers, 4% babysitters, 2% aunts, and 11% missing/other) reported on children’s emotional and behavioral functioning by completing the 100-item 1½–5 Child Behavioral Checklist (CBCL; Achenbach & Rescorla, 2000). For each item, the rater indicated on a 3-point Likert scale if each of the 100 statements was “not true” (0), “somewhat or sometimes true” (1), or “very true or often true” (2) over the past 2 months. The responses were then scored on seven different syndrome subscales, including a 9-item “emotionally reactive scale,” which was the subscale of interest used in analyses for the current study (Sikora, Hall, Hartley, Gerrard-Morris, & Cagle, 2008). Items in the emotionally reactive subscale include the statements describing the child’s behavior, such as “disturbed by any change in routine” and “rapid shifts between sadness and excitement.” The CBCL has been shown to have high test–retest reliability for the emotionally reactive subscale, as well as good criterion-related and construct validity (Achenbach & Rescorla, 2000). Internal consistency on the emotionally reactive subscale was adequate for both maternal CBCL (n = 163; α = 0.66; M = 2.34, SD = 2.08) and the alternate caregiver CBCL (n = 132; α = 0.64; M = 1.89, SD = 1.97) ratings. CBCL ratings were log transformed prior to analyses. Maternal and alternate caregiver ratings were significantly correlated with one another, but the strength of the correlation was low (r = .22, p = .02). In addition, maternal scores were higher, suggesting they observed more of these behaviors overall than did the alternate caregivers. Low agreement across multiple informants is a common phenomenon in ratings of child behavior, given that raters have different opportunities to observe the children (Grietens et al., 2004). Therefore, we examined maternal and alternate caregiver measures separately in our analyses.
2.3.6 |. Statistical analyses plan
Potential confounds were identified prior to hypothesis testing using correlational analyses. Tested variables include maternal factors (age, education, number of children, and Hollingshead rating [a measure of socioeconomic status]), pregnancy factors (number of delivery complications), and child factors (age, sex, race/ethnicity). In addition, health and diet factors which could influence child cortisol measurements (medication use, recent illness, caffeine intake) were tested as potential confounds in cortisol analyses. Factors significantly associated with the dependent variables at the α = 0.05 level of significance included the following, and were controlled for in further analyses for each respective outcome variable. Maternal report of the child’s emotional reactivity was significantly correlated with the total duration of the mother’s psychiatric illness (r(161) = 0.17, p = .03) and maternal tobacco usage during pregnancy (r(160) = .23, p = .003). Alternate caregiver report of the child’s emotional reactivity was significantly associated with maternal age (r(131) = −.21, p = .02), maternal education (r(131) = −.21, p = .02), and number of siblings (r(131) = −.19, p = .03). Child cortisol response was significantly associated with the time of day during which saliva was collected (r(135) = −.19, p = .03). Finally, laboratory observations of emotional reactivity were associated with maternal antipsychotic use during pregnancy (r(161) = .22, p = .005).
Growth mixture modeling (GMM) was conducted using MPlus (Muthén & Muthén, 1998–2017) to empirically identify trajectory classes for maternal depression over the course of pregnancy. Severity of maternal depressive symptoms within each trimester of pregnancy was indicated by AUC scores for each trimester. GMM was then conducted using the AUC BDI data across all three trimesters of pregnancy to identify trajectory classes.
Linear regression models were used to test the hypothesis that the trajectory of maternal depressive symptoms across pregnancy predicts child emotional reactivity. Each outcome variable (cortisol response, behavioral reactivity, and maternal and alternate caregiver report) was evaluated in separate linear regression models. Linear regression models were also used to test timing effects of maternal depressive symptoms during each trimester of pregnancy. AUC for the BDI scores within the first, second, and third trimester of pregnancy were tested as predictors of child emotional reactivity outcomes. Similarly, linear regression models were also conducted to test whether cortisol levels at approximately 24, 30, and 36 weeks predicted the child emotional reactivity variables. Finally, to test the exploratory hypothesis that sex moderates the association between prepartum depressive symptoms and child emotional reactivity, child sex was tested as a moderator in all prediction models.
3 |. RESULTS
3.1 |. Identifying trajectory classes
GMM analyses followed the guidelines laid out in Jung and Wikrama (2008). Various tests of model fit, including Akaike’s information criterion (AIC), Bayesian information criterion (BIC), and the bootstrap likelihood ratio test (BLRT), were used to determine the number of trajectory classes which provided the best fit for the data (see Table 2). Ultimately, the two-class model was selected, as BLRT had a significant p-value (p < .01). In addition, the two-class solution had a relatively low BIC, high entropy, and an adequate number of women in each class to run reliable analyses. Proportions for the latent classes were all also above 0.01, and posterior probabilities for the likelihood of latent class membership within each of the classes were high.
TABLE 2.
Model fit information for GMM of maternal depression
| Model | AIC | BIC | Entropy | BLRT | p-value |
|---|---|---|---|---|---|
| 1-class model | 5698.732 | 5723.571 | — | — | — |
| 2-class model | 5617.539 | 5654.810 | 0.814 | −2843.058 | .0000 |
| 3-class model | 5597.684 | 5650.485 | 0.737 | −2796.769 | .2000 |
AIC, Akaike information criterion; BIC, Bayesian information criterion; BLRT, bootstrap likelihood ratio test. BLRT compares a model with N classes to a model with N-1 classes, so no BLRT values are produced for a 1-class model.
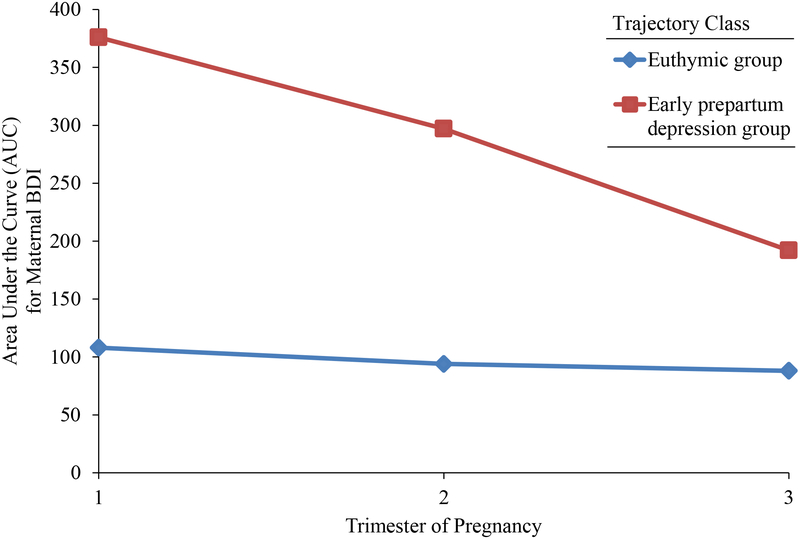
The pattern of depression scores across pregnancy for the two trajectory groups is depicted in Figure 1. Of note, a raw BDI symptom score of 17 or above indicates potentially clinical elevations. The majority of study participants fell into the first trajectory class (n = 127), which had a mean BDI score of 7.11 (SD = 4.34), an average maximum BDI score of 11.39 (SD = 6.09), and a trajectory of relatively low levels of depression throughout pregnancy. Women in the second class (n = 36) followed a trajectory that fell within the clinical range of depression, although symptoms decreased over pregnancy. Their mean pregnancy BDI score was 20.35 (SD = 9.38), and their average maximum BDI score was 30.06 (SD = 9.26). Assessments to these trajectory classes (labeled “euthymic” and “early prepartum depression” for ease of exposition) were used to investigate their association with the child outcomes.
FIGURE 1.
Average trajectories for maternal depression during pregnancy obtained from Growth Mixture Modeling
3.2 |. Trajectory of prepartum maternal depressive symptoms and child emotional reactivity
The first hypothesis was that the trajectories of maternal depression during pregnancy would be associated with child emotional reactivity in the preschool period. This hypothesis was partially supported (see Table 3). Trajectory class was found to have a significant association with cortisol response, such that child cortisol response within the early prepartum depression trajectory class (M = 0.008, SE = −0.016), was significantly higher than child cortisol response within the euthymic trajectory class (M = −0.031, SE = 0.009). There were no significant associations between maternal prepartum depression trajectories and maternal reports of child emotional reactivity and behavioral observations of emotional reactivity, and there was no main effect of trajectory class on alternate caregiver report of child emotional reactivity.
TABLE 3.
Prenatal maternal depression trajectories and child emotional reactivity
| Emotional reactivity measure | F change | p-value | η2 |
|---|---|---|---|
| Maternal report | |||
| Main effect | 3.715 | .056 | 0.024 |
| Gender interaction | 2.041 | .155 | 0.013 |
| Alternate caregiver report | |||
| Main effect | 1.908 | .170 | 0.015 |
| Gender interaction | 4.781 | .031 | 0.037 |
| Males | 0.353 | .555 | 0.005 |
| Females | 7.769 | .0070 | 0.122 |
| Cortisol response | |||
| Main effect | 4.385 | .038 | 0.032 |
| Gender interaction | 0.353 | .554 | 0.003 |
| Behavioral observations | |||
| Main effect | 0.030 | .862 | 0.0002 |
| Gender interaction | 0.535 | .466 | 0.003 |
Bold-faced effects are statistically significant, p < .05.
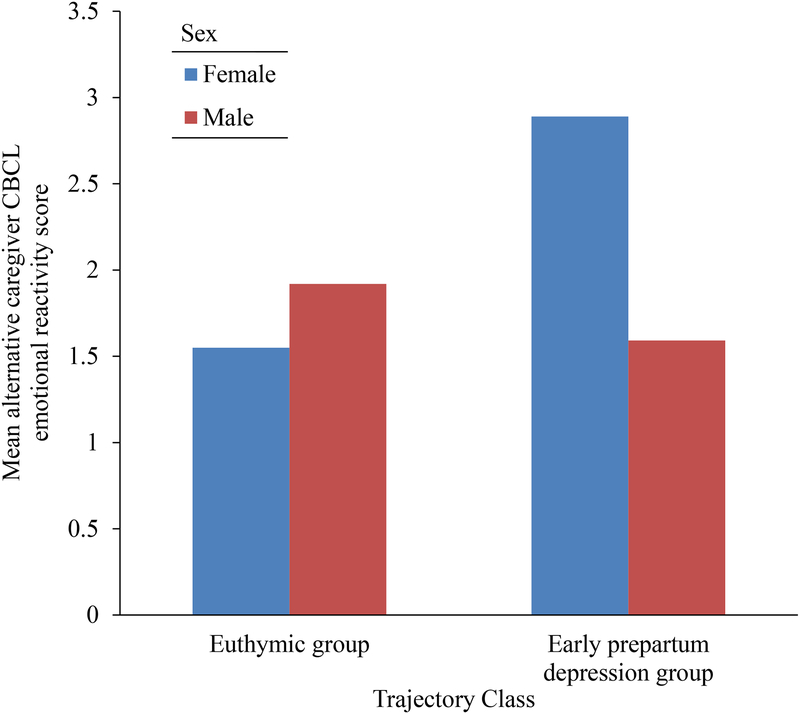
Notably, trajectory classes also interacted with sex to predict alternate caregiver reports of emotional reactivity. Post hoc ANCOVAs separated by sex of the child revealed that for female offspring, the alternate caregiver ratings were significantly higher within the early prepartum depression trajectory class compared to the euthymic group. There was no significant difference in alternate caregiver emotional reactivity ratings among male offspring (see Table 3 and Figure 2).
FIGURE 2.
Mean alternate caregiver CBCL emotional reactivity rating split by class membership and sex of the child
3.3 |. Prepartum maternal depressive symptoms by trimester and child emotional reactivity
The second hypothesis was that the timing of prepartum maternal depression across trimesters would differentially predict child emotional reactivity. To test this hypothesis, regression analyses were conducted examining AUC scores for each trimester of pregnancy separately. Although levels of maternal depression in the second and third trimester were not associated with any measures of child reactivity, several significant effects were noted for first trimester maternal depression (see Table 4). Maternal depression in the first trimester was positively associated with child cortisol response to the stressor tasks. In addition, a sex by first trimester depression interaction was noted in the prediction of alternate caregiver reports of child emotional reactivity. Follow-up regression analyses separated by child sex revealed that higher levels of first trimester maternal depression predicted significantly elevated alternate caregiver reports of emotional reactivity in girls (α = 0.365, t = 2.824, p = .007). In boys, there was no significant association (β = −0.054, t = −0.456, p = .650).
TABLE 4.
First trimester maternal depression and child emotional reactivity
| Emotional reactivity measure | F change | p-value | R2 |
|---|---|---|---|
| Maternal report | |||
| Main effect | 0.398 | .529 | .002 |
| Gender interaction | 1.465 | .228 | .009 |
| Alternate caregiver report | |||
| Main effect | 2.308 | .131 | .016 |
| Gender interaction | 3.930 | .049 | .027 |
| Males | 0.208 | .650 | .003 |
| Females | 7.978 | .007 | .117 |
| Cortisol response | |||
| Main effect | 4.645 | .033 | .013 |
| Gender interaction | 0.033 | .855 | .0001 |
| Behavioral observations | |||
| Main effect | 0.148 | .701 | .001 |
| Gender interaction | 0.924 | .338 | .005 |
Bold-faced effects are statistically significant, p < .05.
3.4 |. The timing of maternal prepartum cortisol and child emotional reactivity.
The third hypothesis was that maternal prepartum levels of cortisol would be associated with higher levels of child emotional reactivity. This hypothesis was partially supported. Maternal cortisol levels at 30 and 36 weeks gestation did not predict any child emotional reactivity outcomes. In contrast, elevated maternal cortisol levels at 24 weeks gestation predicted higher levels of alternate caregiver reports of emotional reactivity (see Table 5). Child sex also significantly moderated the relationship between maternal cortisol at 24 weeks gestation and maternal reports of child emotional reactivity. Regression analyses conducted separately by sex indicated a significant and negative association between maternal prepartum cortisol and emotional reactivity in boys (β = −0.569, t = −2.995, p = .006), and a nonsignificant association between maternal prepartum cortisol and emotional reactivity in girls (β = −0.477, t = 1.313, p = .214).
TABLE 5.
Maternal cortisol at 24 weeks gestation and child emotional reactivity
| Emotional reactivity measure | F change | p-value | R2 |
|---|---|---|---|
| Maternal report | |||
| Main effect | 0.068 | .795 | .002 |
| Gender interaction | 14.327 | .001 | .268 |
| Males | 8.970 | .006 | .259 |
| Females | 1.724 | .214 | .099 |
| Alternate caregiver report | |||
| Main effect | 4.327 | .048 | .098 |
| Gender interaction | 0.023 | 0.52 | .881 |
| Cortisol response | |||
| Main effect | 0.365 | .550 | .005 |
| Gender interaction | 0.573 | .455 | .009 |
| Behavioral observations | |||
| Main effect | 0.237 | .629 | .005 |
| Gender interaction | 0.0002 | .989 | .000004 |
Bold-faced effects are statistically significant, p < .05.
4 |. DISCUSSION
The purpose of this study was to examine associations between child emotional reactivity and prepartum exposures to maternal depressive symptoms and cortisol. The findings suggest that maternal prepartum depressive symptoms confer risk for heightened emotional reactivity in the offspring, as indexed by elevated cortisol in response to an acute stressor. Additionally, we observed a moderation effect of child sex on the association between maternal prepartum depression and alternate caregiver reports of child emotional reactivity, providing some evidence that boys and girls may be differentially susceptible to prepartum exposures. We also observed a timing effect of gestational cortisol on child emotional reactivity, in that maternal cortisol at 24 weeks gestation, but not 30 or 36 weeks, predicted alternate caregiver report of heightened offspring reactivity. The findings suggest that maternal prepartum depressive symptoms and stress physiology have significant implications for their offspring, with impacts that persist well into the postnatal period.
In this study, we investigated both timing effects and trajectories of prepartum depressive symptoms. In trajectory analyses, we were able to successfully identify two distinct trajectory classes of maternal depression within the study sample, reflecting both an early prepartum depressed group as well as a consistently euthymic group. Analyses revealed that children of mothers in the early prepartum depressed group displayed an elevated cortisol response compared to those in the euthymic group, suggesting that the level and/or trajectory of maternal depressive symptoms may have important implications for offspring stress physiology. Analyses of the timing of maternal depression demonstrated that increased severity of symptoms in the first trimester, but not in later pregnancy, is associated with elevated child cortisol response to the stressor tasks in the preschool period. The potential sensitivity of this window of development may be attributed to the early fetal programming of neurological structures that underlie emotional reactivity. While the literature remains mixed on the topic of timing effects (Charil et al., 2010; Davis & Sandman, 2010; Davis et al., 2007), our results suggest that early pregnancy may have particularly significant implications for the development of the offspring HPA axis and the influence of maternal depression.
Although cortisol response in the lab setting was heightened among children prenatally exposed to maternal depression, maternal depression was unrelated to laboratory behavioral observations of child emotional reactivity. This discrepancy in findings across laboratory reactivity measures might be due to the fact that not all emotional reactions are expressed in an overt manner. Methodological factors may also have contributed to our behavioral observation findings. The laboratory task was conducted in a setting that was new to the child, with individuals with whom they were not familiar. These added pressures may have potentially dampened any observable behavioral changes that would otherwise have been displayed. Because both maternal and external caregiver behavioral reports of emotional reactivity were associated with prepartum exposures, our overall study findings suggest that emotional reactivity is likely to be impacted at both physiological and behavioral levels.
Sexual dimorphism in fetal programming was observed in both maternal and alternate caregiver report, but not cortisol response and observational measures of emotional reactivity in a laboratory setting. Maternal and alternate caregiver reports suggest that in girls’ prenatal exposures to elevated maternal depressive symptoms may result in an increase in emotional reactivity, whereas in boys prepartum exposures to elevated maternal cortisol may result in a decrease of emotional reactivity. This finding is consistent with the evolutionary adaptation hypothesis, as well as a recent study showing similar sex specific associations between prepartum cortisol levels and infant emotional reactivity (Braithwaite et al., 2017).
It is important to note that our study sample consisted primarily of mothers with high educations and from predominantly high socioeconomic backgrounds. Given the lack of diversity among study participants, future work should explore whether findings generalize to a sample of individuals from a lower socioeconomic status. In addition, the fact that the mothers were all receiving treatment for their depression is also a unique characteristic of the sample that may limit the generalizability of the findings to mothers with untreated depression. Mean child cortisol levels also did not significantly increase following exposure to the frustration tasks, a phenomenon commonly observed across other studies of stress reactivity in preschool children (Kryski, Smith, Sheikh, Singh, & Hayden, 2011; Tolep & Dougherty, 2014). Given the challenges of eliciting a stress response in laboratory settings, future studies may seek to develop and implement stressor paradigms that elicit individual differences in cortisol. Another potential limitation of the study was the small sample sizes within each trajectory class. While the number of participants within each group was sufficient to conduct study analyses, larger sample sizes would have allowed for higher power to detect group differences in child outcomes. Additionally, only a small subsample of mothers (n = 50) provided saliva samples for cortisol analysis. Results may therefore be interpreted with caution, and future studies are needed to replicate these associations and probe interactions within larger, higher-powered sample sizes.
Despite potential limitations, this study presents many strengths. One major strength of this study is its prospective longitudinal design. This prospective evaluation is perhaps best suited to examine long-term outcomes of fetal programming, and the research questions of interest. Another strength is its use of multiple measures of emotional reactivity, which facilitates a more integrative approach towards evaluating child outcomes by allowing for assessment across various facets of this construct. This study also builds upon prior literature by assessing offspring emotional reactivity in the preschool period. While prior studies have found associations between prepartum depression and offspring emotional and physiological outcomes in infancy (Davis et al., 2004; Kaplan, Evans, & Monk, 2009; Zuckerman et al., 1990), a paucity of research has investigated whether these effects are present in childhood. The current study adds new knowledge by providing evidence that fetal programming effects are maintained well into later development.
The results of the current study may have clinical implications for both depressed mothers and their children. The study findings highlight the importance of having access to effective depression treatment during pregnancy, as prepartum depression can have adverse consequences for both the mother and her child. Early pregnancy was identified as a period in which the fetus may be particularly vulnerable to the effect of maternal depression; therefore, heightened preconception care as well as early prenatal screenings and interventions are likely effective approaches to ameliorate the risks associated with early prenatal exposures. While preventive preconception and prenatal treatments present great promise, future work may also explore the effect of complementary postnatal interventions. Interventions may target the development of adaptive maternal parenting styles, as maternal factors such as sensitive parenting have been shown to modulate the effect of maternal psychological state on offspring cortisol. Potential clinical interventions may also target the behavior of the child directly, with interventions that focus the development of more adaptive emotional regulation strategies.
The findings of this study provide support for the fetal programming hypothesis, highlighting the prepartum period as a window of vulnerability. Additional research is needed to evaluate whether the effect of prepartum exposures extend into adolescence and adulthood. The current study also highlights the importance of further investigating sex differences in fetal programming. The viability–vulnerability tradeoff hypothesis was partially supported, and this emphasizes the merit of examining this relationship at greater depth.
This study expands upon prior work by taking a comprehensive approach in evaluating child emotional reactivity. It provides evidence that the physiological stress regulatory system may be particularly vulnerable to early prepartum exposures. Overall, these findings may have important clinical implications for both depressed mothers and their offspring, identifying children at risk for later emotional dysregulation and highlighting the importance of effective preventive interventions.
ACKNOWLEDGMENTS
This research was supported by NIH (P50ES026071, R01MD009746 and RC1MH088609). Dr. Brennan receives or has received research support from NARSAD, NIH, and the EPA. Dr. Smith receives or has received research support from the American Foundation for Suicide Prevention, Schering Plough Pharmaceuticals, NARSAD, the Conquer Cancer Foundation, NIH, and consulting fees from the Urban Child Institute. Dr. Stowe has received research support from the NIH, GlaxoSmithKline, Pfizer, and Wyeth; served on speakers or advisory boards for Pfizer, Eli Lilly, Wyeth, Bristol-Myers Squibb, and GlaxoSmithKline; and received honoraria from Eli Lilly, GlaxoSmithKline, Pfizer, and Wyeth. Dr. Newport has received research support from Eli Lilly, GlaxoSmithKline, Janssen, the NIH, NARSAD, Takeda Pharmaceuticals, and Wyeth; served on speakers or advisory boards for AstraZeneca, Eli Lilly, GlaxoSmithKline, Janssen, Pfizer, and Wyeth; and received honoraria from Astra-Zeneca, Eli Lilly, GlaxoSmithKline, Pfizer, and Wyeth.
Funding information
National Institutes of Health, Grant/Award Number: P50ES026071 R01MD009746 RC1MH088609; American Foundation for Suicide Prevention; Schering Plough Pharmaceuticals; NARSAD; Conquer Cancer Foundation; GlaxoSmithKline; Pfizer; Wyeth; Eli Lilly; Janssen; Takeda Pharmaceuticals
Footnotes
CONFLICT OF INTEREST
Dr. Winiarski and Ms. Swales declare no conflict of interest.
REFERENCES
- Achenbach TM, & Rescorla LA (2000). Manual for the ASEBA pre-school forms & profiles. Burlington, VT: University of Vermont Research Center for Children, Youth, & Families. [Google Scholar]
- Beck AT, Steer RA, & Garbin MG (1988). Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clinical Psychology Review, 8(1), 77–100. [Google Scholar]
- Braithwaite EC, Pickles A, Sharp H, Glover V, O’Donnell KJ, Tibu F, & Hill J (2017). Maternal prenatal cortisol predicts infant negative emotionality in a sex-dependent manner. Physiology & Behavior, 175, 31–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke HM, Davis MC, Otte C, & Mohr DC (2005). Depression and cortisol responses to psychological stress: A meta-analysis. Psychoneuroendocrinology, 30(9), 846–856. [DOI] [PubMed] [Google Scholar]
- Buss C, Davis EP, Shahbaba B, Pruessner JC, Head K, & Sandman CA (2012). Maternal cortisol over the course of pregnancy and subsequent child amygdala and hippocampus volumes and affective problems. Proceedings of the National Academy of Sciences of the United States of America, 109(20), 1312–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charil A, Laplante DP, Vaillancourt C, & King S (2010). Prenatal stress and brain development. Brain Research Reviews, 65(1), 56–79. [DOI] [PubMed] [Google Scholar]
- Davis EP, Glynn LM, Schetter CD, Hobel C, Chicz-Demet A, & Sandman CA (2007). Prenatal exposure to maternal depression and cortisol influences infant temperament. Journal of the American Academy of Child and Adolescent Psychiatry, 46(6), 737–746. [DOI] [PubMed] [Google Scholar]
- Davis EP, Glynn LM, Waffarn F, Sandman CA (2011). Prenatal maternal stress programs infant stress regulation. Journal of Child Psychology and Psychiatry, 52(2), 119–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EP, & Sandman C (2010). The timing of prenatal exposure to maternal cortisol and psychosocial stress is associated with human infant cognitive development. Child Development, 81(1), 131–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EP, Snidman N, Wadhwa PD, Glynn LM, Schetter CD, & Sandman CA (2004). Prenatal maternal anxiety and depression predict negative behavioral reactivity in infancy. Infancy, 6(3), 319–331. [Google Scholar]
- Deave T, Heron J, Evans J, & Emond A (2008). The impact of maternal depression in pregnancy on early child development. BJOG: An International Journal of Obstetrics and Gynaecology, 115(8), 1043–1051. [DOI] [PubMed] [Google Scholar]
- Duthie L, & Reynolds RM (2013). Changes in the maternal hypothalamic-pituitary-adrenal axis in pregnancy and postpartum: Influences on maternal and fetal outcomes. Neuroendocrinology, 98(2), 106–115. [DOI] [PubMed] [Google Scholar]
- Fernandes M, Stein A, Srinivasan K, Menezes G, & Ramchandani PG (2015). Foetal exposure to maternal depression predicts cortisol responses in infants: Findings from rural South India. Child: Care, Health and Development, 41(5), 677–686. [DOI] [PubMed] [Google Scholar]
- Field T, Diego M, & Hernandez-Reif M (2006). Prenatal depression effects on the fetus and newborn: A review. Infant Behavior & Development, 29(3), 445–455. [DOI] [PubMed] [Google Scholar]
- Gavin NI, Gaynes BN, Lohr KN, Meltzer-Brody S, Gartlehner G, & Swinson T (2005). Perinatal depression: A systematic review of prevalence and incidence. Obstetrics & Gynecology, 106(5), 1071–1083. [DOI] [PubMed] [Google Scholar]
- Glover V, & Hill J (2012). Sex differences in the programming effects of prenatal stress on psychopathology and stress responses: An evolutionary perspective. Physiology & Behavior, 106(5), 736–740. [DOI] [PubMed] [Google Scholar]
- Glover V, O’Connor TG, & O’Donnell K (2010). Prenatal stress and the programming of the HPA axis. Neuroscience and Biobehavioral Reviews, 35(1), 17–22. [DOI] [PubMed] [Google Scholar]
- Goldsmith HH, Reilly J, Lemery KS, Longley S, & Prescott A (1999). The laboratory temperament assessment: Preschool version. Unpublished Measure. [Google Scholar]
- Grietens H, Onghena P, Prinzie P, Gadeyne E, Van Assche V, Ghesquière P, & Hellinckx W (2004). Comparison of mothers’, fathers’, and teachers’ reports on problem behavior in 5-to 6-year-old children. Journal of Psychopathology and Behavioral Assessment, 26(2), 137–146. [Google Scholar]
- Gutteling BM, de Weerth C, & Buitelaar JK (2005). Prenatal stress and children’s cortisol reaction to the first day of school. Psychoneuroendocrinology, 30(6), 541–549. [DOI] [PubMed] [Google Scholar]
- Ji S, Long Q, Newport DJ, Na H, Knight B, Zach EB, … Stowe ZN (2011). Validity of depression rating scales during pregnancy and the postpartum period: Impact of trimester and parity. Journal of Psychiatric Research, 45(2), 213–219. https://10.1016/j.jpsychires.2010.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung T, & Wickrama KAS (2008). An introduction to latent class growth analysis and growth mixture modeling. Social and Personality Psychology Compass, 2(1), 302–317. [Google Scholar]
- Kaplan LA, Evans L, & Monk C (2009). Caregiving on infant biobehavioral regulation: Can prenatal programming be modified? Early Human Development, 84(4), 249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsella M, & Monk C (2009). Impact of maternal stress, depression & anxiety on fetal neurobehavioral development. Clinical Obstetrics and Gynecology, 52(3), 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, & Hellhammer DH (1989). Salivary cortisol in psycho-biological research: An overview. Neuropsychobiology, 22, 150–169. [DOI] [PubMed] [Google Scholar]
- Kryski KR, Smith HJ, Sheikh HI, Singh SM, & Hayden EP (2011). Assessing stress reactivity indexed via salivary cortisol in pre-school-aged children. Psychoneuroendocrinology, 36(8), 1127–1136. [DOI] [PubMed] [Google Scholar]
- Laurent HK, Ablow JC, & Measelle J (2011). Risky shifts: How the timing and course of mothers’ depressive symptoms across the perinatal period shape their own and infant’s stress response profiles. Development and Psychopathology, 23(2), 521–538. [DOI] [PubMed] [Google Scholar]
- Luoma I, Tamminen T, Laippala P, Puura K, Salmelin R, & Almqvist F (1998). Longitudinal study of maternal depressive symptoms and child well-being. Journal of the American Academy of Child & Adolescent Psychiatry, 40(12), 1367–1374. [DOI] [PubMed] [Google Scholar]
- Marcus SM (2009). Depression during pregnancy: Rates, risks and consequences. The Canadian Journal of Clinical Pharmacology, 16(1), 15–22. [PubMed] [Google Scholar]
- Marcus SM, Flynn HA, Blow FC, & Barry KL (2003). Depressive symptoms among pregnant women screened in obstetrics settings. Journal of Women’s Health, 12(4), 373–380. [DOI] [PubMed] [Google Scholar]
- Muthén LK, & Muthén BO (1998–2017). Mplus user’s guide (8th ed.). Los Angeles, CA: Muthén & Muthén. [Google Scholar]
- O’Connor TG, Bergman K, Sarkar P, & Glover V (2013). Prenatal cortisol exposure predicts infant cortisol response to acute stress. Developmental Psychobiology, 55(2), 145–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponder KL, Salisbury A, Mcgonnigal B, Laliberte A, Lester B, & Padbury JF (2011). Maternal depression and anxiety are associated with altered gene expression in the human placenta without modification by antidepressant use: Implications for fetal programming. Developmental Psychobiology, 53(7), 711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, & Hellhammer DH (2003). Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology, 28(7), 916–931. [DOI] [PubMed] [Google Scholar]
- Sandman CA, Glynn LM, & Davis EP (2013). Is there a viability-vulnerability tradeoff? Sex differences in fetal programming. Journal of Psychosomatic Research, 75(4), 327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikora DM, Hall TA, Hartley SL, Gerrard-Morris AE, & Cagle S (2008). Does parent report of behavior differ across ADOS-G classifications: Analysis of scores from the CBCL and GARS. Journal of Autism and Developmental Disorders, 38(3), 440–448. [DOI] [PubMed] [Google Scholar]
- Sohr-Preston SL, & Scaramella LV (2006). Implications of timing of maternal depressive symptoms for early cognitive and language development. Clinical Child and Family Psychology Review, 9(1), 65–83. [DOI] [PubMed] [Google Scholar]
- Tolep MR, & Dougherty LR (2014). The conundrum of the laboratory: Challenges of assessing preschool-age children’s salivary cortisol reactivity. Journal of Psychopathology and Behavioral Assessment, 36(3), 350–357. [Google Scholar]
- Tottenham N, Sheridan MA (2009). A review of adversity, the amygdala and the hippocampus: A consideration of developmental timing. Frontiers in Human Neuroscience, 3(68), 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock M (2005). The potential influence of maternal stress hormones on development and mental health of the offspring. Brain, Behavior, and Immunity, 19(4), 296–308.https://10.1016/j.bbi.2004.09.006 [DOI] [PubMed] [Google Scholar]
- Zuckerman B, Bauchner H, Parker S, & Cabral H (1990). Maternal depressive symptoms during pregnancy and newborn irritability. Developmental and Behavioral Pediatrics, 11(4), 190–194. [PubMed] [Google Scholar]