Abstract
The alkylation of ketones is taught at basic undergraduate level. In many cases this transformation leads to the formation of a new stereogenic center. However, the apparent simplicity of the transformation is belied by a number of problems. So much so, that a general method for the direct asymmetric alkylation of ketones remains an unmet target. Despite the advancement of organocatalysis and transition-metal catalysis, neither field has provided an adequate solution. Indeed, even use of an efficient and general stoichiometric chiral reagent has yet to be reported. Herein we describe the state-of- the-art in terms of direct alkylation reactions of some carbonyl groups. We outline the limited progress that has been made with ketones, and potential routes towards ultimately achieving a widely applicable methodology for the asymmetric alkylation of ketones.
Keywords: asymmetric, ketones, organocatalysis, transition-metal catalysis, α-alkylation
1. Introduction
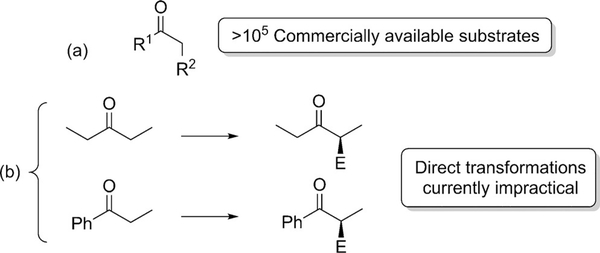
The formation of a new carbon-carbon bond alpha (α) to a carbonyl group is one of the most important reactions in organic synthesis and has contributed significantly to the development of organic chemistry as a whole. In this respect, ketones have found widespread use. Undoubtedly this is due to the diversity of ketone-derived enolate chemistry and the abundance of α-substituted ketones (and derivatives) in biologically active systems, but it is also due to the wide availability of starting materials. Remarkably there are over 105 commercially available ketones of the general structure depicted in Figure 1 a.[1]
Figure 1.
Unreported direct α-alkylation of ketones.
In many cases, α-substitution of carbonyls leads to a new stereogenic center and the asymmetric α-substitution of carbonyls has been the subject of intense discussion. [2] For example we now have methods for the direct α- alkylation of aldehydes [3] and carboxylic acids.[4] Thus it is perhaps surprising that the general, direct asymmetric alkylation (including allylation) of ketones remains elusive. Even the most simple asymmetric alkylation reactions involving ketones have not been reported (Figure 1b).[2c,5]
The α-alkylation of ketones via its enolate seems the obvious choice, but this approach is marred by complications (Figure 2). For example, the geometry of the C—C bond generated during the process should be controlled, as well as the facial approach of the electrophile.[6] For non-symmetrical ketones with two sites for deprotonation, regioselectivity of enolization must also be maintained.
Figure 2.
Complications associated with the asymmetric alkylation of ketones. a) Geometry of enolate; b) Electrophile orbital approach.
In the formation of tertiary stereogenic centers, mild conditions are required in order to avoid the racemization of the newly formed stereogenic center. Undesired reactions such as homolytic couplings and overalkylation are also ever-present problems. Unwanted alkylation at β or γ can also occur.[7]
It should be noted that modifications at the α-position of ketones such as arylation,[8] alkenylation and alkynylation,[9] or alkylating methods that involve a previously modified (e.g. halogenated) α-position[10] will not be discussed here. Rather, we present a compilation of related transformations and efforts towards accomplishing the direct asymmetric α- alkylation of ketones. The word “direct” is, of course, open to interpretation. In this context, we consider it to be a transformation whereby a ketone, unfunctionalized at the α-position, is alkylated in an asymmetric fashion, in one chemical transformation and one pot, without recourse to the formation of, potentially isolable, intermediates. For example, the formation of a chiral auxiliary followed by alkylation and auxiliary cleavage (e.g. Enders’ SAMP/RAMP method), would not qualify as “direct”, even if carried out in one pot. Whereas in situ use of a chiral amine leading to asymmetric iminium alkylation (unreported) would qualify as “direct”. The transformations outlined herein will approach a “direct” transformation to various degrees. In some cases, this will be commented on, in others, opinions on how close the methodologies deviate, will be left to the reader. Examples have been classified depending on the stoichiometric or catalytic system employed.
2. Chiral Lithium Bases
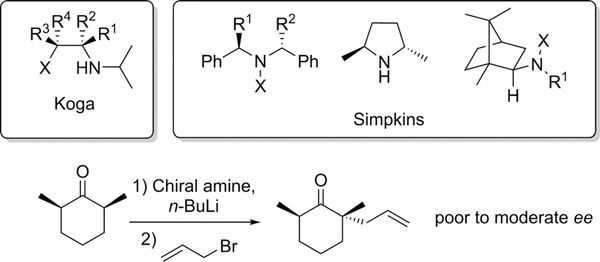
One of the early approaches for the α-alkylation of ketones involved the use of homo chiral lithium bases (HCLAs).[11] In pioneering work, Koga[12] and Simpkins[13] independently reported the use of chiral lithium amide bases (Scheme 1) to desymmetrize substituted cyclohexanones.
Scheme 1.
Chiral amines used and the asymmetric α-alkylation of cyclic ketones.
The chiral base incorporates a stereoelectronic preference for removal of axial protons in rigid systems with a plane of symmetry, and further discriminates between the pair of axial protons to produce the enantiomerically enriched products (Figure 3). Despite the array of chiral amides reported, the application of this methodology has been limited to the desymmetrization of conformationally locked prochiral cyclic ketones. Limited exploitation of the HCLA methodology has emerged but allylation of 2,6-dimethylcyclohexanone, for example, was reported in moderate enantiomeric excess (ee) (Scheme 1).
Figure 3.
Discrimination of axial protons by chiral lithium bases.
The limited substrate scope, which could be accessed by the HCLA methodology restricted widespread uptake.[14]
3. Enolates and Azaenolates
Asymmetric transformations via their corresponding enolates or azaenolates have proven the most effective route to chiral α-substituted ketones in terms of yield and selectivity.[2d] Palomo reported in 2001 a methodology for the asymmetric construction of α-alkylated ketones (Scheme 2).[15] Although far from a direct method, this strategy accomplished the sequential introduction of three alkyl groups by the use of (1R)-(+)-camphor. The preparation of the corresponding (1R)-(+)-camphor enolate allows the introduction of two consecutive alkyl groups. Regeneration of camphor can be achieved with concomitant liberation of the a-branched carboxylic acid or ketone.
Scheme 2.
Alkylation of ketones derived from (1 R)-(+)-camphor.
Use of the (1R)-(+)-camphor derived enolate directs the chemo-, regio- and diastereoselectivity (Figure 4). It selects the enolate configuration and prevents the alkylation at the a’-position. It blocks one of the two possible approaches of R2X to the enolate allowing the second alkylation enantio- selectively. Finally, addition of only one R3X is allowed.
Figure 4.
Role of (1 R)-(+)-camphor at the sequential preparation of α- alkylated ketones.
Azaenolates offer notable advantages over their corresponding enolates: Higher stability, greater reactivity towards electrophiles and better regioselectivity. The first widely used approach involved the SAMP/RAMP methodology.[16] En- ders reported the use of (S)- and (R)-1-amino-2-methoxypyr- rolidine dialkyl hydrazines as chiral auxiliaries for the a- alkylation of hydrazones (Scheme 3). Although this methodology has been widely applied to a number of total syntheses,[2d] it harbors some limitations. The formation of the azaenolate requires exposure to lithium diisopropylamide for long periods (because the hydrazones are weakly acidic) and the alkylation must be conducted at very low temperatures (—78 °C to —110°C), making large-scale applications impractical. Furthermore, the installation and removal (usually O3 or MeI/H3O+) of the auxiliary, limits its step-economy and substrate group tolerance.
Scheme 3.
SAMP/RAMP methodology applied to the a-alkylation of ketones.
More recently, Coltart has reported the use of chiral N- amino cyclic carbamates (Scheme 4).[17] The enhanced acidity of these activated hydrazones allowed a fast deprotonation and avoided the requirement for very low alkylation temperatures. Furthermore, it was possible to control the regiose- lectivity and facilitate the unprecedented a,a-bis-alkylation of ketones.[18] The regiochemical outcome obtained with the N-amino cyclic carbamates is the opposite of that obtained for kinetic LDA-mediated deprotonation of ketones, and the SAMP/RAMP hydrazones. Recently, a racemization-free method to remove the auxiliaries has been reported.[19]
Scheme 4.
Coltart’s chiral N-amino cyclic carbamate hydrazones methodology.
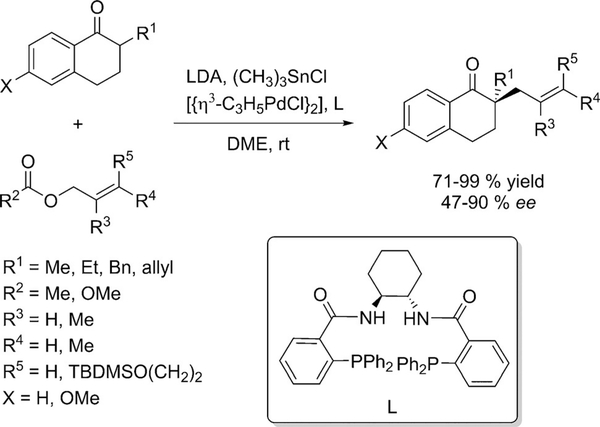
The above methodologies involve the use of chiral auxiliaries and the transfer of chiral information in an intramolecular manner. The first example of the asymmetric a-alkylation of non-chiral hydrazones, via intermolecular transfer of chiral information, was reported by McGlacken.[20] Dimethylhydrazones were successfully alkylated at the a- position using sparteine and other cyclic diamines as chiral ligands, albeit in modest to good enantioselectivities (Scheme 5).
Scheme 5.
Asymmetric α-alkylation of dimethylhydrazones using sparteine as chiral ligand.
4. Metal-Based Catalytic Methods
The formation of α-allylated ketones, especially those bearing quaternary centers, via palladium catalysis has been well documented over the last 20 years.[21] The well-known Tsuji allylation involves the Pd0 mediated decarboxylation of allyl β-ketoesters, or the use of enol derivatives.[22] The enantioselective version of the Tsuji allylation was reported by Stoltz two decades later (Scheme 6).[23]
Scheme 6.
Enantioselective Tsuji allylation reported by Stoltz.
Further studies on Pd-catalyzed intramolecular allylic alkylations of ketones through allyl enol carbonates and allyl β-ketoesters were reported by Trost[24] (Scheme 7 a) and Stoltz[25] (Scheme 7 b) independently. All these methods are very limited in substrate scope and they normally require symmetrical ketones, or unsymmetrical precursors possessing only one available side for allylation.[26]
Scheme 7.
a) Allylic alkylation of ketones through allyl enol carbonates. b) Decarboxylative allylation of β-ketoesters.
The aforementioned examples involved intramolecular allylation, and required a preformed allyl enol carbonate. A seminal report by Trost outlined the asymmetric intermolecular allylation of ketones as early as 1999.[27] The combined action of a Pd complex and a chiral ligand with external allyl donors, allowed the asymmetric alkylation of ketone enolates using six membered rings and 1-tetralones (Scheme 8).
Scheme 8.
Pd-catalyzed asymmetric alkylation of ketone enolates.
Jacobsen expanded the scope of catalytic methods for the asymmetric synthesis of alkylated ketones with the use of Cr/ salen complexes with tributyltin enolates, derived from cyclic and non-cyclic systems (Scheme 9).[28] The tributyltin enolates derived from acyclic systems were prepared as a mixture of E and Z isomers. The Cr/salen complex allowed the incorporation of alkyl groups with other functionalities (which was limited to allylic alkylating agents with previous methodologies). Alkyl halides containing alkynes, alkenes or esters were successfully employed. Good enantioselectivities were achieved, even with tributyltin enolates derived from acyclic ketones.
Scheme 9.
Enantioselective alkylation of tributyltin enolates.
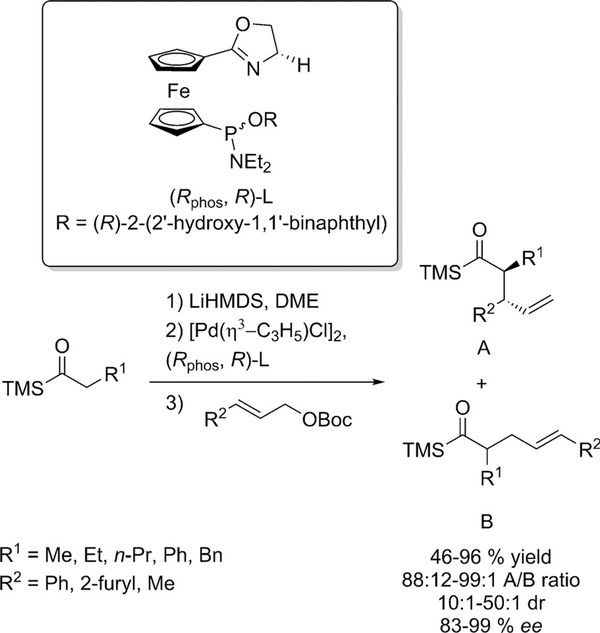
Acylsilanes were reported as suitable nucleophile precursors to perform the asymmetric allylic alkylation of ketones (Scheme 10).[29] Using a Pd catalyst in combination with a ferrocene based ligand, it was possible to achieve the allylation of the acylsilane precursor with high regio-, diastereo- and enantioselectivities to form two new stereogenic centers. The undesired alkylated products were also formed, but in small amount. The corresponding acylsilanes could be further converted to the corresponding α-alkylated ketones without epimerization.
Scheme 10.
Enantioselective allylic allylation of acylsilanes.
Stoltz described in 2010 an enantioselective tandem process that involves palladium-catalyzed decarboxylative enolate formation, followed by conjugate addition and reductive alkylation to ultimately achieve the alkylation of cyclic ketones (Scheme 11).[30]
Scheme 11.
Palladium-catalyzed enolate alkylation cascade.
Later the same group reported the Ir-catalyzed allylic alkylation of β-ketoesters (Scheme 12a).[31] Moreover, the 2- (trimethylsilyl)ethyl β-ketoesters prepared in this manner could then be treated with a source of fluoride (TBAT, tetrabutylammonium difluorotriphenylsilicate) to generate a prochiral enolate and palladium mediated allylation using allyl methylcarbonates, gave the desired a-quaternary ketones in excellent yield and ee (Scheme 12 b).
Scheme 12.
a) Iridium-catalyzed allylic alkylation of β-ketoesters, b) Palladium-catalyzed enantioselective allylic allylation of (trimethylsilyl)- ethyl ester protected enolates.
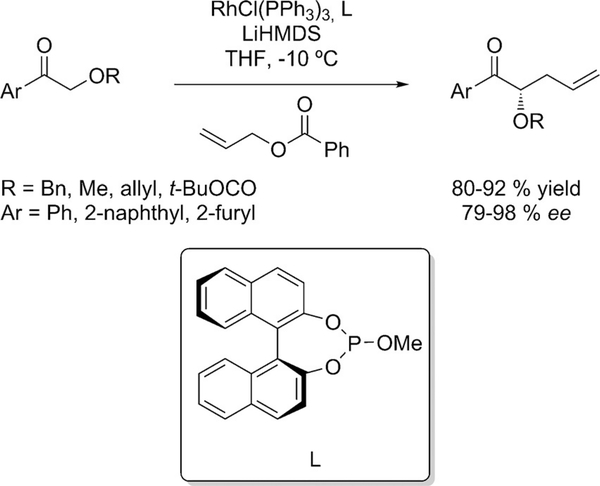
A combination of Wilkinson’s catalyst and a chiral monodentate phosphite was reported by Evans for the allylic alkylation of acyclic α-alkoxy aryl ketones (Scheme 13).[32]
Scheme 13.
Rhodium-catalyzed enantioselective allylic alkylation of acyclic α-alkoxy aryl ketones.
A study with a preformed configurationally defined enolate provided insight into the origin of asymmetric induction. Treatment of the silyl enol ether with methyllithium gave the α-alkylated ketone with the same yield and enantioselectivity as the corresponding aryl ketone, proving that the enolate geometry is not responsible for the formation of the minor enantiomer. The alkylation process is believed to proceed through a saturated 18-electron rhodium allyl complex, which suppresses the direct coordination of the enolate through an outer-sphere process. The π-facial selectivity of the nucleophilic addition determines the enantio selectivity (Figure 5).
Figure 5.
Proposed model for asymmetric induction of rhodium-catalyzed allylic alkylation of acyclic α-alkoxy aryl ketones.
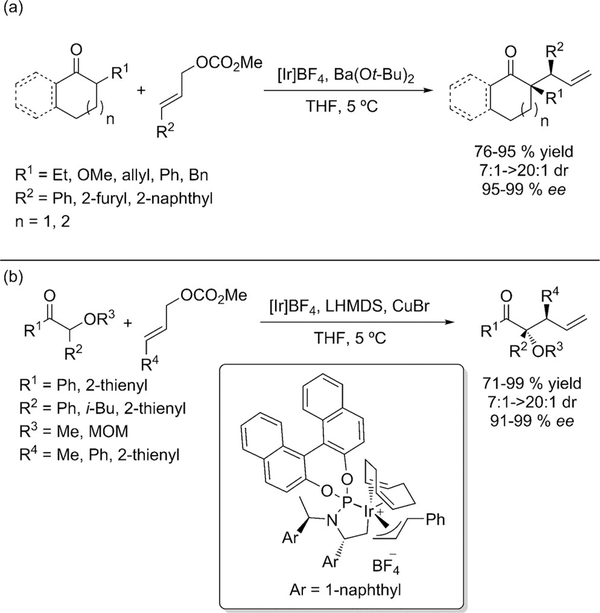
Cyclic ketone enolates were successfully transformed through an asymmetric allylic alkylation using an iridium based catalyst by Hartwig (Scheme 14 a).[33] New quaternary centers were generated with high enantio selectivity. Another more recent report by Hartwig described the diastereo- and enantioselective allylation of acyclic α-alkoxy ketones catalyzed by a copper/iridium system.[34] Upon deprotonation of the α-alkoxy ketone, the geometry of the resulting enolate is fixed by chelation of both oxygen atoms to the copper cation. Iridium catalyzed allylation gave products with vicinal tetrasubstituted and tertiary stereocenters in high yield and excellent d.r. and ee (Scheme 14b). Very recently the protocol was extended for the α-allylation of aryl acetic acid esters by the same group.[35]
Scheme 14.
a) Iridium-catalyzed enantio- and diastereoselective allylation of cyclic ketone enolates, b) iridium-catalyzed enantio- and diastereoselective allylation of a-alkoxy ketones.
A significant advance was reported by List to afford the α- allylation of cyclic ketones (Scheme 15).[36] This is an excellent atom-economic strategy that combines palladium catalysis, enol organocatalysis and CO2-catalyzed activation of allylic alcohol. The corresponding allylated ketones with a-quater- nary centers were obtained in good yields and ee.
Scheme 15.
Asymmetric α-allylation of ketones with CO2 and allylic alcohol.
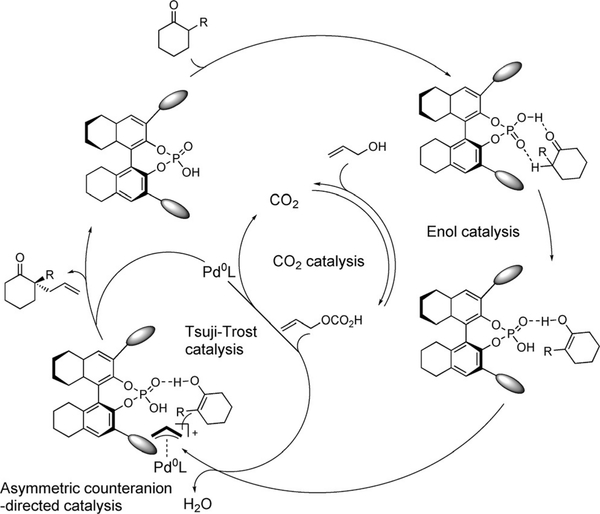
A triple catalytic cycle is proposed by the authors. Initially the chiral phosphoric acid interacts with the ketone enabling reactivity from the thermodynamically more stable enol (Figure 6). Allyl alcohol is activated by CO2 generating the carbonic acid ester, a more reactive p-allyl precursor. In parallel a Tsuji-Trost type catalytic cycle takes place, where the π-allyl-Pd electrophile is generated in an oxidative addition into the carbonic acid ester. Then a [Pd-allyl]+A*— complex is formed upon release of water and CO2 regeneration. The phosphine ligand t-BuXPhos stabilize the Pd complex avoiding the formation of Pd black (only traces of product is obtained in absence of the phosphine), as well as act as a chiral counteranion. Finally, the successive nucleophilic attack of the enol onto the cation, reductive elimination, and release of the chiral acid affords the desired product with regeneration of the phosphoric acid catalyst.
Figure 6.
Proposed model for asymmetric α-allylation of ketones with allylic alcohols.
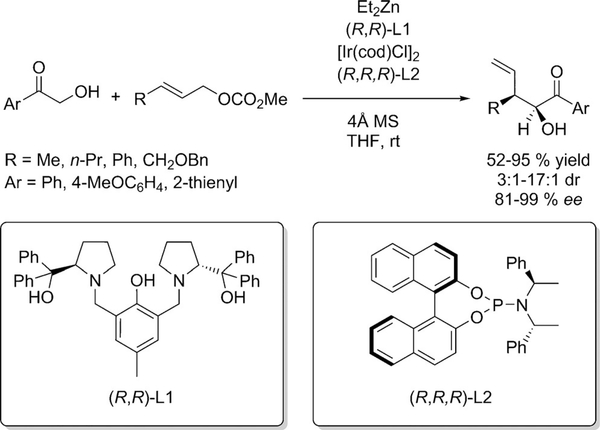
A recent report by Huo et al. describes an excellent example of bimetallic cooperation.[37] The combined action of a chiral Ir complex derived from phosphoramidites and a chiral Zn-ProPhenol complex achieved the α-allylation of unprotected α-hydroxyketones (Scheme 16). The bimetallic system demonstrated exquisite control over the absolute and relative configuration of the newly generated stereogenic centers. Remarkably, all four stereoisomers could be furnished from the same set of starting materials and conditions using different enantiomers of each ligand.
Scheme 16.
Ir/Zn dual catalysis for α-allylation of α-hydroxyketones.
Finally another example of bimetallic catalysis has been described by Lautens.[38] A combination of a Ru-catalyzed C— H activation and a Pd-catalyzed asymmetric allylic alkylation allowed the preparation of 3-allyl-3-aryl oxindoles from aryl a-diazoamides (Scheme 17).
Scheme 17.
Ru/Pd dual catalysis for a route to 3-allyl-3-aryl oxindoles.
5. Organocatalysis
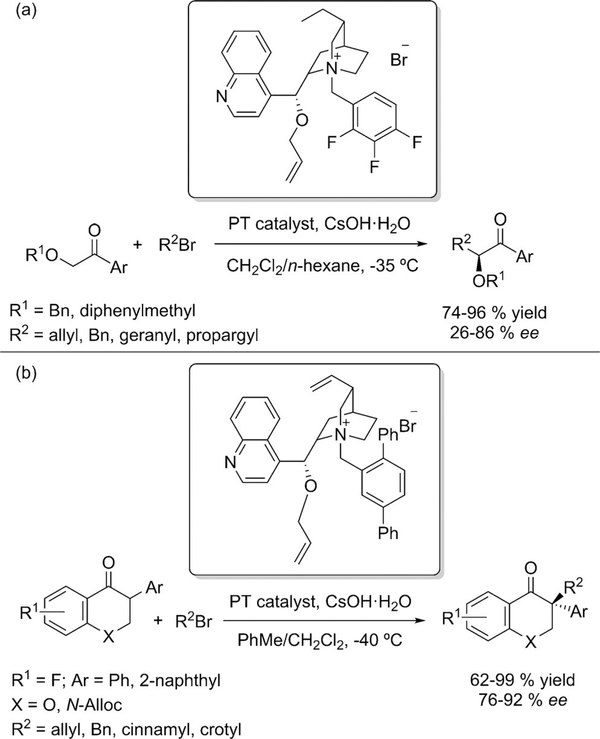
Organocatalysis, as a synthetic methodology, has delivered many advances in the preparation of enantiomerically enhanced α-substituted carbonyls.[3] With ketones specifically however, there are far fewer protocols reported which utilize organocatalytic protocols. However, in 2004 a phase-transfer catalyst was reported for asymmetric glycolate alkylations.[39] A trifluorobenzyl cinchonidinium bromide catalyst was used with allyl, propargyl and benzyl halides as alkylating agents (Scheme 18 a). A similar phase-transfer catalyst was applied for the asymmetric alkylation of substituted isoflavones by Scheidt (Scheme 18b).[40]
Scheme 18.
a) Asymmetric phase-transfer-catalyzed glycolate alkylation; b) Asymmetric phase-transfer-catalyzed alkylation of isoflavones.
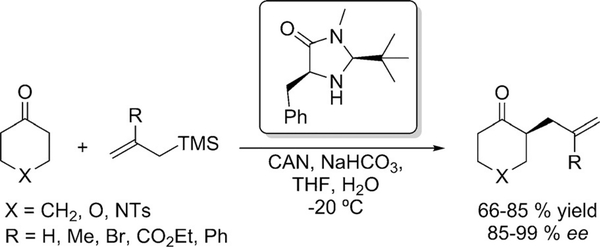
MacMillan reported in 2010 the first example utilizing singly occupied molecular orbital (SOMO) catalysis (Scheme 19).[41] Excellent enantioselectivities were obtainedby this methodology, however the applicability was limited to cyclic and heterocyclic ketones and allyl silanes as alkylating agents. α-Enolation and α-homobenzylation of cyclohexanone were also accomplished.
Scheme 19.
Asymmetric α-alkylation of cyclic ketones via (SOMO) catalysis.
Wang reported a stereoselective Michael addition of α- aryl cyclopentanones to nitroolefins catalyzed by a bifunctional amine-thiourea organocatalyst.[42] A bifunctional orga- nocatalyst bearing multiple hydrogen-bonding donors was able to activate, simultaneously, the α-aryl cyclopentanone and the nitroolefin. Excellent diastereo- and enantioselectivities in the corresponding products was achieved at the quaternary a-position and the adjacent tertiary stereocenter (Scheme 20a). However when a-phenyl cyclohexanone was tried, the yield dropped to 20%. The asymmetric alkylation of cyclohexanones through an asymmetric Pfau–dQAngelo reaction was accomplished by the use of thiourea- and primary amine-based systems by Carter (Scheme 20b) and Kotsuki (Scheme 20c) independently.[43] However, only cyclic substrates were reported.
Scheme 20.
a) Asymmetric Michael addition of α-substituted cyclo- pentanones to nitroolefins. b) Thiourea-catalyzed enantioselective synthesis of a,α-substituted cyclic ketones. c) Primary amine-catalyzed enantioselective synthesis of a,α-substituted cyclic ketones.
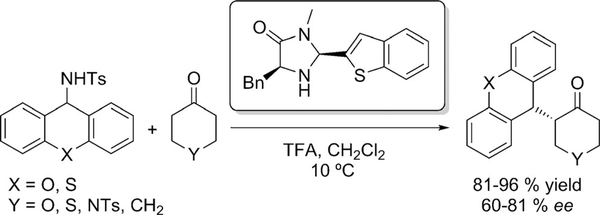
Another interesting approach was disclosed in 2011 by Tian.[44] Using chiral imidazolidinone and trifluoroacetic acid, the α-alkylation of ketones was achieved using N-benzylic sulfonamides as alkylating agents through C—N bond cleavage (Scheme 21). Good enantioselectivities were obtained, but again the methodology was applied on cyclic ketones only.
Scheme 21.
Asymmetric α-alkylation of cyclic ketones using N-benzylic sulfonamides.
Chiral phosphoric acids have showed potential as suitable organocatalysts. Peng reported in 2012 the alkylation of ketones with alcohols in high yields, d.r. and ee.[45] The methodology was applied mainly to cyclic ketones, but three examples of acyclic ketones were also reported with excellent d.r. and ee, but with lower yields (Scheme 22).
Scheme 22.
Asymmetric α-alkylation of ketones by chiral phosphoric acids.
List reported in 2015 the use of chiral phosphoric acids in the enantioselective Michael addition of cyclic ketones to enones (Scheme 23 a).[46] A recent study by Toste on chiral phosphoric acid catalysis has extended the asymmetric α- alkylation of α-substituted cyclic ketones using allenamides (Scheme 23b).[47] The reaction products could be transformed under acidic conditions into their corresponding 1,4- and 1,5- ketoaldehydes derivatives.
Scheme 23.
a) Chiral phosphoric acid-catalyzed addition of cyclic ke tones to enones, b) Asymmetric a-alkylation of a-substituted cyclic ketones with allenamides.
Another example of phase-transfer catalysis was presented by Maruoka (Scheme 24).[48] The asymmetric α-alkylation of 2-arylcyclohexanones was described, but the requirement of an N-methylanilinomethylene blocking group will hamper the applicability.
Scheme 24.
Phase-transfer catalyzed α-alkylation of 2-arylcyclohexa- nones.
Palomo recently described a cinchona-alkaloid derived bifunctional Brϕnsted base catalyst, which was employed in the regio-, diastereo- and enantioselective a-alkylation of α- substituted-β-tetralones with Michael acceptors (Scheme 25).[49] The applicability of the catalyst was also investigated for α-unsubstituted β-tetralones, aromatic ring- fused cycloalkanones with an oxygen heteroatom in the cycle, or larger seven-membered cycloalkanones. In the case of aromatic ring-fused cycloalkanones with an oxygen heteroatom in the cycle the diastereoselectivities obtained were lower in general, probably due to epimerization under basic conditions.
Scheme 25.
Asymmetric α-alkylation of β-tetralones and other related cyclic ketones.
6. Photochemistry
The rapidly developing area of photocatalysis has been applied to the asymmetric α-alkylation of aldehydes.[3e,f] More recently, a few examples have appeared involving the a- alkylation of ketones. Melchiorre’s group[50] extended theirA similar approach was reported by Luo and co-work- ers[52] for the alkylation of β-ketocarbonyls, accessing acyclic quaternary centers (Scheme 27). Again the combination of a chiral primary amine and Ru(bpy)3Cl2 as photocatalyst achieved the corresponding alkylated products with good yields and excellent enantioselectivities. Remarkably, it was possible to apply this system to the challenging 1,3-diketone and β-keto amide acyclic systems.established methodology based on the in situ photogeneration of chiral electron donor-acceptor (EDA) complexes.[3e,f] Using a chiral primary amine in combination with light, they accomplished the alkylation of cyclic ketones with alkyl bromides with high levels of regio-, diastereo- and enantio- selectivity (Scheme 26 a). The EDA complexes were also used for the perfluoroalkylation of β-ketoesters by the same group (Scheme 26b).[51]
Scheme 27.
α-Photoalkylation of β-ketocarbonyls by primary amine and Ru(bpy)3Cl2 catalysis.
Scheme 26.
a) Photo-organocatalyzed a-alkylation of cyclic ketones by a chiral primary amine. b) a) Photo-organocatalyzed enantioselective perfluoroalkylation of β-ketoesters.
A similar approach was reported by Luo and co-workers[52] for the alkylation of β-ketocarbonyls, accessing acyclic quaternary centers (Scheme 27). Again the combination of a chiral primary amine and Ru(bpy)3Cl2 as photocatalyst achieved the corresponding alkylated products with good yields and excellent enantioselectivities. Remarkably, it was possible to apply this system to the challenging 1,3-diketone and β-keto amide acyclic systems.
Meggers reported a chiral Ir-catalyst mediated α-alkylation of 2-acylimidazoles, activated by visible light.[53] Benzyl and phenacyl bromides were used as alkylating reagent, as well as α-silylamines. Recently they reported a combination of a Ru-photoredox catalyst and a Rh-Lewis acid catalyst to afford the α-amination and α-alkylation of ketones with diazo compounds (Scheme 28).[54] The α-alkylation of 2-acylimidazoles was also achieved with good yields and enantioselectivities.
Scheme 28.
Enantioselective α-photoalkylation with diazo compounds.
7. Summary and Outlook
What is clear from this perspective is that a substantial effort has been directed towards the development of the asymmetric alkylation of ketones, and many excellent contributions have emerged, especially over the last decade. What is also clear is that many efforts bypass some of most prominent and simple ketones, for example, 3-pentanone and propiophenone (Figure 1). Considering that even 2-butanone begets the additional problem of regioselectivity, the challenges remain significant. Yet, it is quite remarkable that despite much effort, no general solution to the problem of asymmetric α-alkylation of simple ketones has become available. The available diversity of starting ketones and electrophiles (e.g. halides and even alcohols)[55] would quickly provide a myriad of diverse chemical entities. Extension to the use of simple olefins as enolate reacting partners, would further improve the atom economy and environmental value, but to date no enantioselective variants have been reported using this abundant feedstock.[56] The complexity of the transition states generated during some transition-metal mediated transformations makes enantiocontrol difficult, but some preliminary studies show promise (enantioselectivities are currently moderate),[57] and a recently developed H- borrowing strategy (H-auto-transfer) could have potential for modification to an asymmetric system.[58] Other interesting alternatives to solve this problem is the kinetically controlled enantioselective α-protonation of α-branched ketones.[59] Although many examples have been reported to date, a highly efficient system with a broad scope is still unknown. An asymmetric alkylation catalyzed by enzymes would be a very attractive solution.[60] So far this field has been very limited by two factors: a) the complexity of the enzymes (only a few enzymes have been characterized in vitro) and b) the high cost of S-adenosylmethionine as the usual methyl donor used by the methyltransferases.
Overall, there are few indications that future strategies will involve the formation of a simple enolate followed by addition to an electrophile. Perhaps there is merit in considering this back-to-basics approach, focusing on an alkali metal enolate in a chiral environment. The stereoselectivity of enolization should be surmountable, but perhaps the most daunting problem is control of aggregation states.[61] Much is yet to be learned about the fascinating chemistry of aggregates. Intriguingly, the emerging picture involving lithium or other alkali and alkali earth metals, suggests that chemical behavior is best considered in the realm of coordination chemistry, rather than simplistic concerns of acidity and basicity. Other key obstacles include the post-reaction racemization of the newly formed (tertiary) stereogenic centers. That said, these problems have been overcome previously in other domains, and will not prove impervious to the creativity and tenacity of organic chemists.
Acknowledgements
G.M.G. thanks the Science Foundation Ireland (SFI/12/IP/ 1315, 09/REP/CHS2353 and SFI/12/RC/2275) and the Irish Research Council (RC). A.Z. thanks the National Institutes of Health (NIGMS R01 077379) for support of this work.
Biography
 Rafael Cano obtained his B.Sc. and PH.D. at the University of Alicante. in 2013, he accepted a post-doctoral position with the McClacken group at University College Cork. During this time he undertook research secondments at Merck (Ballydine, Ireland) and at University of Oxford (UK) with Prof. Michael C. Willis. His research is focused on heterogeneous catalysis and asymmetric synthesis.
Rafael Cano obtained his B.Sc. and PH.D. at the University of Alicante. in 2013, he accepted a post-doctoral position with the McClacken group at University College Cork. During this time he undertook research secondments at Merck (Ballydine, Ireland) and at University of Oxford (UK) with Prof. Michael C. Willis. His research is focused on heterogeneous catalysis and asymmetric synthesis.
 Armen Zakarian undertook undergraduate research at the Kochetkov laboratories at Moscow State University under the mentorship of Dr. Borodkin. in 1996 he pursued graduate studies in the Holton group at Florida State University. in 2002 he carried out postdoctoral studies in the Overman group at the University of California, irvine. He joined the Department of Chemistry and Biochemistry at the University of California Santa Barbara in June 2008.
Armen Zakarian undertook undergraduate research at the Kochetkov laboratories at Moscow State University under the mentorship of Dr. Borodkin. in 1996 he pursued graduate studies in the Holton group at Florida State University. in 2002 he carried out postdoctoral studies in the Overman group at the University of California, irvine. He joined the Department of Chemistry and Biochemistry at the University of California Santa Barbara in June 2008.
 Gerard McClacken obtained his Ph.D. at the National University of Ireland, Calway. He then moved to the University of York where his research interests diversified to organometallic transformations with Prof. ian J. S. Fairlamb. A year later, he took up a Molecular Design and Synthesis PostDoctoral Fellowship at Florida State University working with Prof. Robert Holton. He obtained a Lectureship position at University College Cork in 2007.
Gerard McClacken obtained his Ph.D. at the National University of Ireland, Calway. He then moved to the University of York where his research interests diversified to organometallic transformations with Prof. ian J. S. Fairlamb. A year later, he took up a Molecular Design and Synthesis PostDoctoral Fellowship at Florida State University working with Prof. Robert Holton. He obtained a Lectureship position at University College Cork in 2007.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
The ORCID identification number(s) for the author(s) of this article can be found under https://doi.org/10.1002/anie.201703079.
Contributor Information
Dr. Rafael Cano, Department of Chemistry, University College Cork Cork (Ireland) and Analytical and Biological Chemistry Research Facility, University College Cork Cork (Ireland)
Prof. Dr. Armen Zakarian, Department of Chemistry and Biochemistry, University of California Santa Barbara, CA 93106 (USA) zakarian@chem.ucsb.edu
Dr. Gerard P. McGlacken, Department of Chemistry, University College Cork Cork (Ireland) and Analytical and Biological Chemistry Research Facility, University College Cork Cork (Ireland) g.mcglacken@ucc.ie
References
- [1].Donohoe TJ, Pilgrim BS, Jones GR, Bassuto JA, Proc. Natl. Acad. Sci. USA 2012,109, 11605–11608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].a) Smith MB, March Jin March’s Advanced Organic Chemistry, Wiley, New York, 2001; [Google Scholar]; b) Vesely J, Rios R, ChemCatChem 2012, 4, 942–953; [Google Scholar]; c) Reeves CM, Stoltz BM in Asymmetric Synthesis: More Methods and Applications (Eds.: Christmann M, Bräse N), Wiley-VCH, Weinheim, 2012, pp. 1–10; [Google Scholar]; d) Kohler MC, Wengryniuk SE, Coltart DM in Stereoselective Synthesis of Drugs and Natural Products (Eds.: Andrushko V, Andrushko N), Wiley, Hoboken, 2013, pp. 183–213; [Google Scholar]; e) Remes M, Vesely J in Stereoselective Organocatalysis Bond Formation Methodologies and Activation Modes (Ed.: Torres R. Rios), Wiley, New York, 2013, pp. 267–312; [Google Scholar]; f) Liu Y, Han S-J, Liu W-B, Stoltz BM, Acc. Chem. Res 2015,48,740–751; [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Kazmaier U, Org. Chem. Front 2016, 3, 1541–1560. [Google Scholar]
- [3].a) Ibrahem I, Cordova A, Angew. Chem. Int. Ed 2006, 45, 1952–1956; Angew. Chem. 2006, 118, 1986–1990; [DOI] [PubMed] [Google Scholar]; b) Beeson TD, Mastracchio A, Hong J-B, Ashton K, MacMillan DWC, Science 2007, 316, 582–585; [PubMed] [Google Scholar]; c) Wilson JE, Casarez AD, MacMillan DWC, J. Am. Chem. Soc 2009, 131, 11332–11334; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Gomez-Bengoa E, Landa A, Lizarraga A, Mielgo A, Oiarbide M, Palomo C, Chem. Sci 2011, 2, 353–357; [Google Scholar]; e) Arceo E, Jurberg ID, Álvarez-Fernández A, Melchiorre P, Nat. Chem 2013,5,750–756; [DOI] [PubMed] [Google Scholar]; f) Bahamonde A, Melchiorre P, J. Am. Chem. Soc 2016,138, 8019–8030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].a) Ma Y, Stivala CE, Wright AM, Hayton T, Liang J, Keresztes I, Lobkovsky E, Collum DB, Zakarian A, J. Am. Chem. Soc 2013,135,16853–16864; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Lu P, Jackson JJ, Eickhoff JA, Zakarian A, J. Am. Chem. Soc 2015, 137, 656–659; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Yu K, Lu P, Jackson JJ, Nguyen T-AD, Alvarado J, Stivala CE, Ma Y, Mack KA, Hayton TW, Collum DB, Zakarian A, J. Am. Chem. Soc 2017,139, 527–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].a) Evans DA in Asymmetric Synthesis, Vol. 3 (Ed.: Morrison JD), Academic Press, Orlando, 1984, pp. 1–10; [Google Scholar]; b) Enders D in Asymmetric Synthesis, Vol. 3, 1st ed. (Ed.: Morrison JD), Academic Press, Orlando, 1984, pp. 275–339; [Google Scholar]; c) Mekelburger HB, Wilcox CS in Comprehensive Organic Synthesis, Vol. 2 (Eds.: Trost BM, Fleming I), Pergamon, Oxford, 1991, pp. 99–131; [Google Scholar]; d) Heatcock CH in Modern Synthetic Methods, Vol. 6 (Ed.: Scheffold R), Helvetica Chimica Acta, Basel, 1992, pp. 183–213; [Google Scholar]; e) Rizzacasa MA, Perkins M in Stoichiometric Asymmetric Synthesis, Sheffield Academic Press, Sheffield, 2000; [Google Scholar]; f) Carey FA, Sundberg RJ in Advanced Organic Chemistry Part B: Reactions and Synthesis, 5th ed., Springer, New York, 2007. [Google Scholar]
- [6].Cherney AH, Kadunce NT, Reisman SE, J. Am. Chem. Soc 2013,135, 7442–7445. [DOI] [PubMed] [Google Scholar]
- [7].a) Crimmins MT, Nantermet PG, Org. Prep. Proced. Int 1993, 25, 41–81; [Google Scholar]; b) Stoltz BM, Bennett NB, Duquette DC, Goldberg AFG, Liu Y, Loewinger MB, C. M. Reeves in Comprehensive Organic Synthesis, Vol. 3, 2nd ed. (Eds.: Knochel P, Molander GA), Elsevier, Amsterdam, 2014; [Google Scholar]; c) Menon RS, Biju AT, Nair V, Chem. Soc. Rev 2015, 44, 5040–5052. [DOI] [PubMed] [Google Scholar]
- [8].a) Culkin DA, Hartwig JF, Acc. Chem. Res 2003, 36, 234–245; [DOI] [PubMed] [Google Scholar]; b) Johansson CCC, Colacot TJ, Angew. Chem. Int. Ed 2010, 49, 676–707; Angew. Chem. 2010, 122, 686–718; [DOI] [PubMed] [Google Scholar]; c) Bellina F, Rossi R, Chem. Rev 2010,110, 1082–1146. [DOI] [PubMed] [Google Scholar]
- [9].Huang Z, Lim HN, Mo F, Young MC, Dong G, Chem. Soc. Rev 2015, 44, 7764–7786. [DOI] [PubMed] [Google Scholar]
- [10].a) Lou S, Fu GC, J. Am. Chem. Soc 2010, 132, 1264–1266; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Liang Y, Fu GC, J. Am. Chem. Soc 2014,136, 5520–5524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].a) O’Brien P, J. Chem. Soc. Perkin Trans 1 1998, 1439–1457; [Google Scholar]; b) Eames J, Eur. J. Org. Chem 2002, 393–401. [Google Scholar]
- [12].Shirai R, Tanaka M, Koga K, J. Am. Chem. Soc 1986, 108, 543–545. [DOI] [PubMed] [Google Scholar]
- [13].Cain CM, Cousins RPC, Coumbarides G, Simpkins NS, Tetrahedron 1990, 46, 523–544. [Google Scholar]
- [14].For selected examples: Simpkins NS, J. Chem. Soc. Chem. Commun 1986, 88–90;Corey EJ, Gross AW, Tetrahedron Lett. 1984, 25, 495–498;Cousins RPC, Simpkins, Tetrahedron Lett. 1989,30,7241–7244;Sato D, Kawasaki H, Shimada I, Arata Y, Okamura K, Date T, Koga K, J. Am. Chem. Soc 1992,114, 761–763;Bunn BJ, Simpkins NS, J. Org. Chem 1993, 58, 533–534;Coggins P, Gaur S, Simpkins NS, Tetrahedron Lett. 1995, 36, 1545–1548;Lipshutz BH, Wood MR, Lindsley CW, Tetrahedron Lett 1995, 36, 4385–4388;Toriyama M, Sugasawa K, Shindo M, Tokutake N, Koga K, Tetrahedron Lett. 1997, 38, 567–570. For a recent review see:Simpkins NS, Weller MD, Org. React 2013, 79, 317–635.
- [15].Palomo C, Oiarbide M, Mielgo A, González A, García JM, Landa C, Lecumberri A, Linden A, Org. Lett 2001, 3, 3249–3252. [DOI] [PubMed] [Google Scholar]
- [16].Job A, Janeck CF, Bettray W, Petters R, Enders D, Tetrahedron 2002, 58, 2253–2329. [Google Scholar]
- [17].a) Lim D, Coltart DM, Angew. Chem. Int. Ed 2008, 47, 5207–5210; Angew. Chem. 2008, 120, 5285–5288; [DOI] [PubMed] [Google Scholar]; b) Krenske EH, Houk KN, Lim D, Wengryniuk SE, Coltart DM, J. Org. Chem 2010, 75, 8578–8584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wengryniuk SE, Lim D, Coltart DM, J. Am. Chem. Soc 2011,133, 8714–8720. [DOI] [PubMed] [Google Scholar]
- [19].Huynh U, Uddin MN, Wengryniuk SE, McDonald SL, Coltart DM, Tetrahedron 2017, 73, 432–436. [Google Scholar]
- [20].a) McSweeney CM, Foley VM, McGlacken GP, Chem. Commun 2014, 50,14817–14819; [DOI] [PubMed] [Google Scholar]; b) Foley VM, Cano R, McGlacken GP, Tetrahedron: Asymmetry 2016, 27, 1160–1167. [Google Scholar]
- [21].For selected examples of asymmetric allylations of carbonylic compounds see: Janssen JP, Helmchen G, Tetrahedron Lett. 1997,38, 8025–8026;Trost BM, Cramer N, Silverman SM, J.Am. Chem. Soc 2007, 129, 12396–12397;Trost BM, Zhang Y, J. Am. Chem. Soc 2007, 129, 14548–14549;Trost BM, Miller JR, Hoffman CM Jr, J. Am. Chem. Soc 2011, 133, 8165–8167;Chen W, Hartwig JF, J. Am. Chem. Soc 2013,135, 2068–2071;Liu W-B, Reeves CM, Stoltz BM, J. Am. Chem. Soc 2013, 135, 17298–17301;Chen W, Hartwig JF, J. Am. Chem. Soc 2014, 136, 377–382. For a recent review see:Hethcox JC, Shockley SE, Stoltz BM, ACS Catal. 2016, 6, 6207–6213.
- [22].a) Shimizu I, Yamada T, Tsuji J, Tetrahedron Lett. 1980, 21, 3199–3202; [Google Scholar]; b) Tsuji J, Minami I, Shimizu I, Tetrahedron Lett. 1983, 24, 1793–1796; [Google Scholar]; c) Tsuji J, Minami I, Shimizu I, Chem. Lett 1983,12, 1325–1326. [Google Scholar]
- [23].a) Behenna DC, Stoltz BM, J. Am. Chem. Soc 2004, 126, 15044–15045; [DOI] [PubMed] [Google Scholar]; b) Behenna DC, Mohr JT, Sherden NH, Marinescu SC, Harned AH, Tani K, Seto M, Ma S, Novák Z, Krout MR, McFadden RM, Roizen JL, Enquist JA, White DE, Levine SR, Petrova KV, Iwashita A, Virgil SC, Stoltz BM, Chem. Eur J 2011,17, 14199–14223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].a) Trost BM, Xu J, J. Am. Chem. Soc 2005, 127, 2846–2847; [DOI] [PubMed] [Google Scholar]; b) Trost BM, Xu J, J. Am. Chem. Soc 2005,127,17180–17181; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Trost BM, Bream RN, Xu J, Angew. Chem. Int. Ed 2006, 45, 3109–3112; Angew. Chem. 2006,118, 3181–3184. [DOI] [PubMed] [Google Scholar]
- [25].Mohr JT, Behenna DC, Harned AM, Stoltz BM, Angew. Chem. Int. Ed 2005, 44, 6924–6927; Angew. Chem. 2005, 117, 7084–7087. [DOI] [PubMed] [Google Scholar]
- [26].a) Braun M, Laicher F, Meier T, Angew. Chem. Int. Ed 2000, 39, 3494–3497; Angew. Chem. 2000, 112, 3637–3640; [DOI] [PubMed] [Google Scholar]; b) Braun M, Meier T, Synlett 2005, 2968–2972; [Google Scholar]; c) Trost BM, Xu J, Reichle M, J. Am. Chem. Soc 2007, 129, 282–283; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Trost BM, Xu J, Schmidt T, J. Am. Chem. Soc 2008, 130, 11852–11853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Trost BM, Schroeder GM, J. Am. Chem. Soc 1999, 121, 6759–6760. [Google Scholar]
- [28].a) Doyle AG, Jacobsen EN, J. Am. Chem. Soc 2005,127, 62–63; [DOI] [PubMed] [Google Scholar]; b) Doyle AG, Jacobsen EN, Angew. Chem. Int. Ed 2007, 46, 3701–3705; Angew. Chem. 2007,119, 3775–3779. [DOI] [PubMed] [Google Scholar]
- [29].Chen J-P, Ding C-H, Liu W, Hou X-L, Dai L-X, J. Am. Chem. Soc 2010,132, 15493–15495. [DOI] [PubMed] [Google Scholar]
- [30].Streuff J, White DE, Virgil SC, Stoltz BM, Nat. Chem 2010, 2, 192–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].a) Liu W-B, Reeves CM, Virgil SC, Stoltz BM, J. Am. Chem. Soc 2013,135,10626–10629; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Liu W-B, Reeves CM, Stoltz BM, J. Am. Chem. Soc 2013,135,17298–17301; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Reeves CM, Behenna DC, Stoltz BM, Org. Lett 2014,16, 2314–2317. For an application to a natural product synthesis see: [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Enquist JA Jr, Stoltz BM, Nature 2008, 453, 1228–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Evans PA, Clizbe EA, Lawler MJ, Oliver S, Chem. Sci 2012, 3, 1835–1838. [Google Scholar]
- [33].Chen W, Chen M, Hartwig JF, J. Am. Chem. Soc 2014, 136, 15825–15828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Jiang X, Chen W, Hartwig JF, Angew. Chem. Int. Ed 2016,55, 5819–5823; Angew. Chem. 2016,128, 5913–5917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Jiang X, Beiger JJ, Hartwig JF, J. Am. Chem. Soc 2017,139, 87–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Pupo G, Properzi R, List B, Angew. Chem. Int. Ed 2016, 55, 6099–6102; Angew. Chem. 2016,128, 6204–6207. [DOI] [PubMed] [Google Scholar]
- [37].Huo X, He R, Zhang X, Zhang W, J. Am. Chem. Soc 2016, 138,11093–11096. [DOI] [PubMed] [Google Scholar]
- [38].Yamamoto K, Qureshi Z, Tsoung J, Pisella G, Lautens M, Org. Lett 2016,18, 4954–4957. [DOI] [PubMed] [Google Scholar]
- [39].Andrus MB, Hicken EJ, Stephens JC, Org. Lett 2004, 6, 2289–2292. [DOI] [PubMed] [Google Scholar]
- [40].Nibbs AE, Baize A-L, Herter RM, Scheidt KA, Org. Lett 2009,11, 4010–4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Mastracchio A, Warkentin AA, Walji AM, MacMillan DWC, Proc. Natl. Acad. Sci. USA 2010,107, 20648–20651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Dong X-Q, Teng H-L, Tong M-C, Huang H, Tao H-Y, Wang C-J, Chem. Commun 2010, 46, 6840–6842. [DOI] [PubMed] [Google Scholar]
- [43].a) Kang JY, Carter RG, Org. Lett 2012,14, 3178–3181; [DOI] [PubMed] [Google Scholar]; b) Horinouchi R, Kamei K, Watanabe R, Hieda N, Tatsumi N, Nakano, Ichikawa Y, Kotsuki H, Eur. J. Org. Chem 2015,4457–4463; [Google Scholar]; c) Kang JY, Johnston RC, Snyder KM, Cheong PH-Y, Carter RG, J. Org. Chem 2016, 81, 3629–3637. [DOI] [PubMed] [Google Scholar]
- [44].Weng Z-T,Li Y,Tian S-K, J. Org. Chem 2011, 76,8095–8099. [DOI] [PubMed] [Google Scholar]
- [45].Song L, Guo Q-X, Li X-C, Tian J, Peng Y-G, Angew. Chem. Int. Ed 2012, 51, 1899–1902; Angew. Chem. 2012, 124, 1935–1938. [DOI] [PubMed] [Google Scholar]
- [46].Felker I, Pupo G, Kraft P, List B, Angew. Chem. Int. Ed 2015, 54, 1960–1964; Angew. Chem. 2015,127, 1983–1987. [DOI] [PubMed] [Google Scholar]
- [47].Yang X, Toste FD, Chem. Sci 2016, 7, 2653–2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Kano T, Hayashi Y, Maruoka K, J. Am. Chem. Soc 2013,135, 7134–7137. [DOI] [PubMed] [Google Scholar]
- [49].Urruzuno I, Mugica O, Oiarbide M, Palomo C, Angew. Chem. Int. Ed 2017, 56, 2059–2063; Angew. Chem. 2017, 129, 2091–2095. [DOI] [PubMed] [Google Scholar]
- [50].Arceo E, Bahamonde A, Bergonzini G, Melchiorre P, Chem. Sci 2014, 5, 2438–2442. [Google Scholar]
- [51].Wozniak L, Murphy JJ, Melchiorre P, J. Am. Chem. Soc 2015, 137, 5678–5681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Zhu Y, Zhang L, Luo S, J. Am. Chem. Soc 2014,136, 14642–14645. [DOI] [PubMed] [Google Scholar]
- [53].a) Huo H, Shen X, Wang C, Zhang L, Rose P, Chen L-A, Harms K, Marsch M, Hilt G, Meggers E, Nature 2014, 515, 100–103; [DOI] [PubMed] [Google Scholar]; b) Wang C, Zheng Y, Huo H, Rose P, Zhang L, Harms K, Hilt G, Meggers E, Chem. Eur. J 2015, 21, 7355–7359. [DOI] [PubMed] [Google Scholar]
- [54].Huang X, Webster RD, Harms K, Meggers E, J. Am. Chem. Soc 2016,138, 12636–12642. [DOI] [PubMed] [Google Scholar]
- [55].Zhang L, Cui L, Lin X, Li J, Luo S, Cheng J-P, Chem. Eur. J 2010,16, 2045–2049. [DOI] [PubMed] [Google Scholar]
- [56].a) Mo F, Dong G, Science 2014, 345, 68–72; [DOI] [PubMed] [Google Scholar]; b) Tang S, Wu X, Liao W, Liu K, Luo S, Lei A, Org. Lett 2014,16, 3584–3587. [DOI] [PubMed] [Google Scholar]
- [57].Bai D-C, Yu F-L, Wang W-Y, Chen D, Li H, Liu Q-R, Ding C-H, Chen B, Hou X-L, Nat. Commun 2016, 7, 11806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].a) Shen D, Poole DL, Shotton CC, Kornahrens AF, Healy MP, Donohoe TJ, Angew. Chem. Int. Ed 2015, 54, 1642–1645; Angew. Chem. 2015,127,1662–1665; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Huang F, Liu Z, Yu Z, Angew. Chem. Int. Ed 2016, 55, 862–875; Angew. Chem. 2016,128, 872 – 885; [DOI] [PubMed] [Google Scholar]; c) Schlepphorst C, Maji B, Glorius F, ACS Catal. 2016, 6, 4184–4188; [Google Scholar]; d) Peña-López M, Piehl P, Elangovan S, Neumann H, Beller M, Angew. Chem. Int. Ed 2016, 55, 14967–14971; Angew. Chem. 2016,128,15191–15195. [DOI] [PubMed] [Google Scholar]
- [59].Mohr JT, Hong AY, Stoltz BM, Nat. Chem 2009, 1, 359–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Sommer-Kamann C, Fries A, Mordhorst S, Andexer JN, Müller M, Angew. Chem. Int. Ed 2017, 56, 4033–4036; Angew. Chem. 2017,129, 4091–4094. [DOI] [PubMed] [Google Scholar]
- [61].For reviews see: Streitwieser A, J. Mol. Model 2006,12, 673–680;Reich HJ, Chem. Rev 2013, 113, 7130–7178. For selected examples see:Liou LR, McNeil AJ, Ramirez A, Toombes GES, Gruver JM, Collum DB, J. Am. Chem. Soc 2008,130, 4859–4868;Godenschwager PF, Collum DB, J. Am. Chem. Soc 2008, 130, 8726–8732;Gruver JM, Liou LR, McNeil AJ, Ramirez A, Collum DB, J. Org. Chem 2008, 73, 7743–7747;Tallmadge EH, Collum DB, J. Am. Chem. Soc 2015,137,13087–13095;Jin KJ, Collum DB, J. Am. Chem. Soc 2015,137,14446–14455;Tallmadge EH, Jermaks J, Collum DB, J. Am. Chem. Soc 2016,138, 345–355;Ma Y, Mack KA, Liang J, Keresztes I, Collum DB, Zakarian A, Angew. Chem. Int. Ed 2016,55,10093–10097; Angew. Chem. 2016,128, 10247–10251.