Abstract
Background:
Currently, there are no valid clinical or biological markers to personalize the treatment of depression. Recent evidence suggests that body mass index (BMI) may guide the selection of antidepressant medications with different mechanisms of action.
Methods:
Combining Medications to Enhance Depression Outcomes (CO-MED) trial participants with BMI measurement (n=662) were categorized as normal- or underweight (<25), overweight (25-<30), obese I (30-<35), and obese II+ (≥35). Logistic regression analysis with remission as the dependent variable and treatment arm-by-BMI category interaction as the primary independent variable was used to evaluate if BMI differentially predicted response to escitalopram (SSRI) monotherapy, bupropion-escitalopram combination, or venlafaxine-mirtazapine combination, after controlling for gender and baseline depression severity.
Results:
Remission rates among the three treatment arms differed on the basis of pre-treatment BMI (chi-square=12.80, degrees of freedom=6, p=0.046). Normal- or under-weight participants were less likely to remit with the bupropion-SSRI combination (26.8%) than SSRI monotherapy (37.3%, number needed to treat or NNT=9.5) or venlafaxine-mirtazapine combination (44.4%, NNT=5.7). Conversely, obese II+ participants were more likely to remit with bupropion-SSRI (47.4%) than SSRI monotherapy (28.6%, NNT=5.3) or venlafaxine-mirtazapine combination (37.7%, NNT=10.3). Remission rates did not differ among overweight and obese I participants.
Limitations:
Secondary analysis, higher rates of obesity than the general population.
Conclusions:
Antidepressant selection in clinical practice can be personalized with BMI measurements. Bupropion-SSRI combination should be avoided in normal- or under-weight depressed outpatients as compared to SSRI monotherapy and venlafaxine-mirtazapine combination and preferred in those with BMI≥35.
Keywords: Major Depressive Disorder, Antidepressant medications, Moderator, Body Mass Index, Treatment selection, Obesity
Introduction
Major depressive disorder (MDD) is a widely prevalent and severely disabling illness (Jha et al., 2016; Kessler et al., 2003). Clinical markers, such as baseline symptom severity (Friedman et al., 2012), age of onset (Sung et al., 2013), insomnia (Sung et al., 2015), chronicity (Sung et al., 2012), and atypical or melancholic features (Arnow et al., 2015), have failed to guide selection between presently available antidepressant medications. Head-to-head trials of antidepressant medications within or across different classes have also failed to find any significant differences between these medications (Thase et al., 2005). In clinical practice, selection of antidepressant medications is based either on clinical characteristics (e.g., side-effect profile, previous history of response) or non-clinical factors (e.g., clinician or patient preference, cost) (Gelenberg et al., 2010). This results in a trial-and-error approach, often necessitating multiple attempts to attain adequate symptomatic control (Rush et al., 2006). There is, accordingly, an urgent need to personalize the selection of antidepressant treatment (Trivedi, 2016).
While inflammatory biomarkers present a promising avenue to inform antidepressant selection (Jha et al., 2017a), their utility in clinical practice is restricted by cost and the availability of laboratory services. In contrast, measures of obesity such as body mass index (BMI) can be assessed easily in routine clinical practice and may be a clinically relevant indicator of ongoing inflammation (Shelton et al., 2015b). A recent report from the International Study to Predict Optimised Treatment - in Depression (iSPOT-D) trial found that escitalopram was more effective than venlafaxine in depressed outpatients with normal BMI whereas, venlafaxine monotherapy was more effective than escitalopram in obese II (BMI 35 to <40) and obese III (BMI≥40) depressed outpatients (Green et al., 2017).
This report aims to corroborate and extend the findings of Green et al. (2017) in a sample of convenience using data from the Combining Medications to Depression Outcomes trial (CO-MED) trial which compared escitalopram-plus-placebo (SSRI monotherapy) to bupropion-plus-escitalopram (bupropion-SSRI) and venlafaxine-plus-mirtazapine (venlafaxine-mirtazapine). As bupropion is a dopaminergic noradrenergic antidepressant (Fava et al., 2005), its combination with escitalopram may provide a pharmacological profile similar to that of venlafaxine which was used in iSPOT-D. The aim of this report was to test the hypothesis that pre-treatment BMI differentially predicted antidepressant treatment outcomes. Specifically, it was hypothesized that bupropion-SSRI combination will be more effective than escitalopram monotherapy in depressed patients with BMI≥35 and vice versa in those with normal BMI.
Methods
Study Overview
This report relies on data from the CO-MED trial (Rush et al., 2011) which recruited 665 participants who were randomly assigned to SSRI monotherapy, bupropion-SSRI combination, or venlafaxine-mirtazapine combination after stratification for the site (Rush et al., 2011). The analytic sample for this report includes only those participants with a baseline BMI measurement (n=662). From both primary and psychiatric clinic, participants were recruited using broad inclusion and exclusion criteria to select a diverse participant group (Rush et al., 2011). The inclusion and exclusion criteria are fully listed at (https://clinicaltrials.gov/ct2/show/NCT00590863). Briefly, participants were treatment-seeking patients with chronic or recurrent nonpsychotic major depressive disorder diagnosed by a clinical interview and the MINI International Neuropsychiatric Interview, and with current episode ≥2 months of at least moderate severity (17-item Hamilton Rating Scale at least 16) (Rush et al., 2011).
All study-related procedures or assessments were completed only after obtaining informed consent from participants. The study was reviewed and approved by the institutional review boards at UT Southwestern Medical Center at Dallas, the University of Pittsburgh Data Coordinating Center, each participating regional center, and all relevant clinics. Additionally, the study was monitored by an independent data safety and monitoring board.
Medications
In all three treatment arms, participants were treated using measurement-based care (MBC) approach (Trivedi et al., 2006) where dosage adjustments were based on symptom severity and side-effect, and medications were administered using two types of pills in single-blind fashion(Rush et al., 2011). At the end of acute-phase of CO-MED trial, mean escitalopram and placebo doses were 17.6 mg/day and 1.4 pills/day respectively in SSRI monotherapy arm, mean bupropion SR and escitalopram doses were 324.0 mg/day and 14.0 mg/day respectively in bupropion-SSRI arm, and mean venlafaxine XR and mirtazapine doses were 207.6 mg/day and 25.3 mg/day respectively in venlafaxine-mirtazapine arm (Rush et al., 2011).
Assessments
At baseline, participants provided sociodemographic information. Also at baseline and at all treatment visits, participants filled out 16-item Quick Inventory of Depressive Symptomatology - Self-Report (QIDS-SR) scale (Rush et al., 2003) which was the primary depression symptom severity outcome measure in the CO-MED trial. Remission, the primary outcome of the CO-MED trial, was ascribed if out of the last two consecutive QIDS-SR score at least one was less than 6 while the other was less than 8. This definition of remission was chosen a priori to ensure that one good week did not falsely signal remission (Rush et al., 2011).
Statistical analyses plan
Summary statistics and counts were used to summarize the number of CO-MED trial participants in the following BMI categories: normal- or under-weight (BMI<25), overweight (BMI 25.0–29.9), obese I (30.0–34.9), and obese II+ (≥35), as per previous published report from CO-MED trial (Toups et al., 2013). The proportion of participants with remission during the acute-phase of CO-MED trial in each treatment arm (SSRI monotherapy, bupropion-SSRI, and venlafaxine-mirtazapine) were tabulated according to the BMI categories. The number needed to treat (NNT) between two treatment arms were calculated by subtracting the higher observed remission rate from the lower observed remission rate and dividing 100 by this difference. A logistic regression analysis with remission during the acute-phase as the dependent variable and baseline depression severity, gender, baseline BMI category, treatment arm and treatment arm-by-BMI category interaction was used for the primary outcome. A significant treatment arm-by-BMI category interaction represents that remission rates differed in treatment arms based on baseline BMI category; hence, this was the statistical outcome (independent variable) of interest. To interpret the significant interaction term, logistic regression analyses were repeated after stratification by BMI category. In the above-described logistic regression analyses, BMI as a continuous variable was used with treatment arm-by-BMI being the independent variable of interest. Analyses were repeated after stratification for treatment arm if the interaction was significant. All statistical analyses were conducted with SAS 9.2 with a threshold of significance at p<0.05.
Results
Of the 662 participants with BMI measurement at baseline, 11 were underweight (1.7%), 158 were a normal weight (23.9%), 187 were overweight (28.2%), 133 were obese I (20.1%), and 173 were obese II+ (26.1%). The number of participants in SSRI monotherapy, bupropion-SSRI, and venlafaxine-mirtazapine arms was 224 (33.8%), 219 (33.1%) and 219 (33.1%), respectively. Remission rates in SSRI monotherapy, bupropion-SSRI, and venlafaxine-mirtazapine arms were 86/224 (38.4%), 86/219 (39.3%) and 83/219 (37.9%), respectively. Additionally, remission rates in normal- or under-weight, overweight, obese I, and obese II+ categories were 61/169 (36.1%), 70/187 (37.4%), 59/133 (44.4%), and 65/173 (37.6%), respectively.
In a logistic regression model with remission status as the dependent variable and treatment arm, BMI categories, gender, and baseline depression severity as well as the interaction between treatment arm and BMI categories as the independent variables, there was a significant treatment arm-by-BMI category interaction (chi-square=12.80, degrees of freedom = 6, p=0.046). In subsequent logistic regression analyses, normal- or under-weight participants were less likely to remit with bupropion-SSRI (odds ratio (OR) =0.40, 95% confidence interval (CI) =0.17, 0.93) as compared to venlafaxine-mirtazapine, also see table 1. However, obese II+ participants were more likely to remit with bupropion-SSRI as compared to SSRI monotherapy (OR=2.63, 95% CI=1.20, 5.88). There were no significant differences among treatment arms in overweight (chi-square=1.03, p=0.60) and obese I (chi-square=1.70, p=0.43) BMI categories. When BMI was used as a continuous variable, there was a significant treatment arm-by-BMI interaction in the logistic regression model which included remission status as the dependent variable and treatment arm, BMI, gender, baseline depression severity, and treatment arm-by-BMI interaction as the independent variables. In stratified analyses, higher BMI was associated with greater chances of remission in the bupropion-SSRI arm (OR=1.037, 95% CI=1.025, 1.050) but lower changes of remission in SSRI monotherapy (OR=0.963, 95% CI=0.953, 0.974) and venlafaxine-mirtazapine combination (OR=0.985, 95% CI=0.975, 0.996).
Table 1.
Likelihood of remission based on pre-treatment BMI categories during the acute-phase of CO-MED trial (n=662)
| Odds Ratio | 95% CI | |
|---|---|---|
| Normal- or under-weight (BMI <25) | ||
| SSRI monotherapy vs. Bupropion-SSRI combination | 1.92 | 0.83, 4.46 |
| Venlafaxine-mirtazapine vs. Bupropion-SSRI combination | 2.49 | 1.07, 5.78 |
| Overweight (BMI 25–29.9) | ||
| SSRI monotherapy vs. Bupropion-SSRI combination | 1.09 | 0.53, 2.25 |
| Venlafaxine-mirtazapine vs. Bupropion-SSRI combination | 0.75 | 0.35, 1.59 |
| Obese I (BMI 30–34.9) | ||
| SSRI monotherapy vs. Bupropion-SSRI combination | 1.23 | 0.51, 2.98 |
| Venlafaxine-mirtazapine vs. Bupropion-SSRI combination | 0.71 | 0.30, 1.65 |
| Obese II+ (BMI ≥35) | ||
| SSRI monotherapy vs. Bupropion-SSRI combination | 0.38 | 0.17, 0.83 |
| Venlafaxine-mirtazapine vs. Bupropion-SSRI combination | 0.75 | 0.34, 1.68 |
BMI is body mass index, CI is confidence interval, SSRI is selective serotonin reuptake inhibitor. The three treatment arms in Combining Medications to Enhance Depression Outcomes (CO-MED) trial included escitalopram plus placebo (SSRI monotherapy), sustained release bupropion plus escitalopram (bupropion-SSRI combination), and extended-release venlafaxine plus mirtazapine (venlafaxine-mirtazapine combination). Bolded odds ratio values indicate statistically significant findings as the 95% CI does not include 1.00.
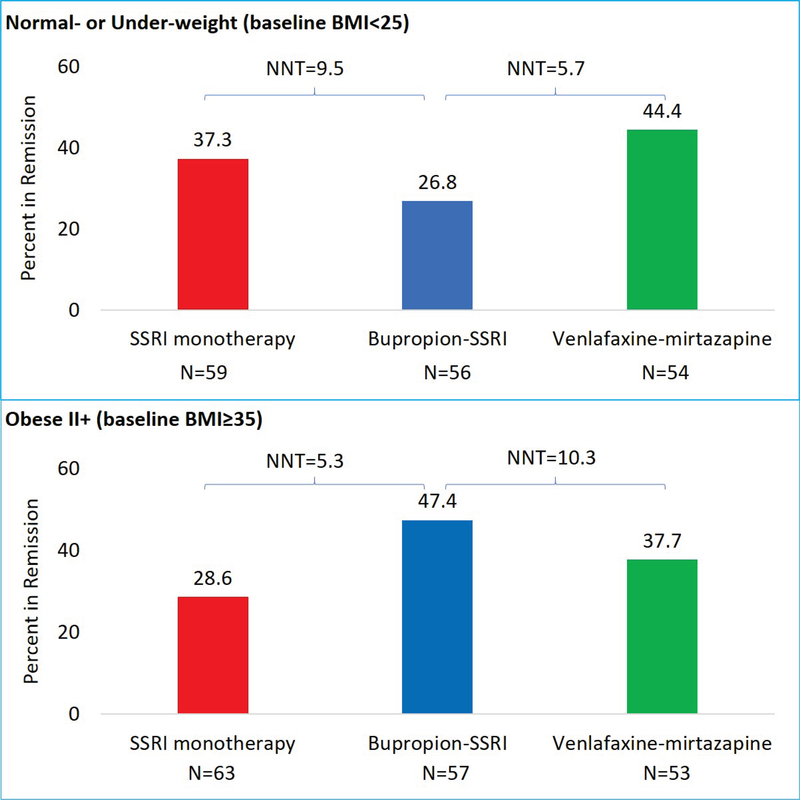
In the normal- or underweight (BMI<25) BMI category, 15/56 (26.8%) participants in bupropion-SSRI arm attained remission as compared to 22/59 (37.3%, NNT=9.5) in SSRI monotherapy and 24/54 (44.4%, NNT=5.7) in venlafaxine-mirtazapine arms (see figure 1). Conversely, in the obese II+ (BMI≥35) category, 27/57 (47.4%) participants in bupropion-SSRI arm attained remission as compared 18/63 (28.6%, NNT=5.3) in SSRI monotherapy and 20/53 (37.7%, NNT=10.3) in venlafaxine-mirtazapine arms.
Figure 1.
Remission rates in normal- or under-weight and obese II+ participants of CO-MED trial BMI is body mass index, SSRI is selective serotonin reuptake inhibitor. This figure presents the observed remission rates in the escitalopram plus placebo (SSRI monotherapy), sustained release bupropion plus escitalopram (bupropion-SSRI), and extended-release venlafaxine plus mirtazapine (venlafaxine-mirtazapine) treatment arms of Combining Medications to Enhance Depression Outcomes (CO-MED) trial. The number needed to treat (NNT) between two treatment arms were calculated by subtracting the higher observed remission rate from the lower observed remission rate and dividing 100 by this difference.
Discussion
In a large sample of depressed outpatients, pre-treatment BMI differentially predicted outcomes to a combination of bupropion-SSRI versus SSRI monotherapy and venlafaxine-mirtazapine combination. While overall remission rates did not differ on the basis of either treatment arm (Rush et al., 2011) or BMI categories (Toups et al., 2013), bupropion-SSRI combination was more effective than SSRI monotherapy in obese II+ (BMI≥35) and less effective than venlafaxine-mirtazapine combination in normal weight or underweight depressed outpatients. It is estimated that if bupropion-SSRI is prescribed in depressed patients with BMI≥35, 5.4 patients will need to be treated for 1 additional remission as compared to SSRI monotherapy. Similarly, in normal weight and underweight depressed patients, 5.7 patients will need to be treated with venlafaxine-mirtazapine for 1 additional remission as compared to the bupropion-SSRI combination. Outcomes did not differ among the three treatment arms in overweight and obese I depressed patients. When BMI was used as a continuous variable, there was a similar pattern of differential outcomes. Higher BMI was associated with greater likelihood of remission in the bupropion-SSRI arm but lower likelihood of remission in SSRI monotherapy and venlafaxine-mirtazapine combination arms.
These findings are consistent with those of Green et al. (2017). Assuming that the combination of bupropion and escitalopram has a similar pharmacological profile as venlafaxine, the finding that bupropion-SSRI was superior to SSRI monotherapy in depressed participants with BMI≥35 is similar to that reported by Green et al. (2017) where venlafaxine was superior to escitalopram in depressed outpatients with BMI≥35. The potential moderator role of BMI could be explained by the role of obesity in inflammation (Shelton et al., 2015a). In patients with MDD, presence of markers of inflammation and blood-brain barrier dysfunction has been shown to favor response to dopaminergic medications (such as bupropion) as compared to predominantly serotonergic antidepressants (such as escitalopram) (Jha et al., 2017b; Jha et al., 2017c; Jha and Trivedi, 2018). Among inflammatory markers, levels of c-reactive protein (CRP) correlate highly with BMI (Jha et al., 2017a); thus, these findings are also consistent with previous reports where depressed patients with elevated levels of CRP responded better for bupropion-SSRI combination or nortriptyline as compared to SSRI monotherapy (Jha et al., 2017a; Uher et al., 2014). Thus, these findings can be easily translated into clinical practice along with or in addition to measures of inflammatory biomarkers (Miller et al., 2017).
This study has several limitations. This is a secondary analysis on a subset of participants in the CO-MED trial. As identifying BMI categories as moderators of treatment outcome was not the primary outcome of the CO-MED trial, there was no a priori test of the power to detect moderator effect of BMI. Additionally, the obesity rate in this sample was higher than the national rates of obesity (Toups et al., 2013). As the definition of remission was based only on self-report measures and the participation in study restricted only to outpatients, the generalizability of these findings do not extend to use of clinician-rated instruments and inpatients with MDD.
To conclude, pre-treatment BMI can personalize the selection of antidepressant medication by avoiding bupropion-SSRI combination in depressed patients with BMI<25 and preferring it in those with BMI≥35.
Highlights:
Antidepressant selection in clinical practice can be personalized with BMI measurements
Outpatients have different reactions to Bupropion-SSRI combination depending upon BMI
Pre-treatment BMI can differentially predict outcomes to a combination of bupropion-SSRI versus SSRI monotherapy and other combinations.
Acknowledgements
The CO-MED trial was funded by NIMH (N01 MH-90003) and received medications at no cost from Forest Pharmaceuticals, GlaxoSmithKline, Organon, and Wyeth Pharmaceuticals. This work was also supported in part through the Center for Depression Research and Clinical Care at UT Southwestern, Hersh Foundation, and Jordan Harris Foundation. The authors thank the clinical staff at each clinical site for their assistance with this project; all of the study participants; and Jeremy Kee for administrative support. The content of this publication does not necessarily reflect the views or policies of the U.S. Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. government. NIMH had no role in the drafting or review of the manuscript or in the collection or analysis of the data.
Footnotes
Conflict of Interest
Drs. Jha and Minhajuddin, as well as Ms. Wakhlu and Ms. Dronamraju, have no potential conflicts of interest. Dr. Greer has received research funding from NARSAD and honoraria and/or consultant fees from H. Lundbeck A/S and Takeda Pharmaceuticals International, Inc. Dr. Trivedi is or has been an advisor/consultant and received fees from Alkermes, AstraZeneca, Cerecor, Eli Lilly & Company, Lundbeck, Naurex, Neuronetics, Otsuka Pharmaceuticals, Pamlab, Pfizer Inc., SHIRE Development and Takeda. In addition, he has received grants/research support from National Institute of Mental Health and National Institute on Drug Abuse.
Previous presentation: No previous presentation of these findings.
References:
- Arnow BA, Blasey C, Williams LM, Palmer DM, Rekshan W, Schatzberg AF, Etkin A, Kulkarni J, Luther JF, Rush AJ, 2015. Depression Subtypes in Predicting Antidepressant Response: A Report From the iSPOT-D Trial. The American journal of psychiatry 172, 743–750. [DOI] [PubMed] [Google Scholar]
- Fava M, Rush AJ, Thase ME, Clayton A, Stahl SM, Pradko JF, Johnston JA, 2005. 15 Years of Clinical Experience With Bupropion HCl: From Bupropion to Bupropion SR to Bupropion XL. Primary Care Companion to The Journal of Clinical Psychiatry 7, 106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman ES, Davis LL, Zisook S, Wisniewski SR, Trivedi MH, Fava M, Rush AJ, Team C-MS, 2012. Baseline depression severity as a predictor of single and combination antidepressant treatment outcome: results from the CO-MED trial. Eur Neuropsychopharmacol 22, 183–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelenberg AJ, Freeman MP, Markowitz JC, Rosenbaum JF, Thase ME, Trivedi MH, Van Rhoads RS, Reus VI, J Raymond DePaulo M Jr,Fawcett JA, 2010. Practice Guideline for the Treatment of Patients With Major Depressive Disorder Third Edition. The American journal of psychiatry 167, 1.20068118 [Google Scholar]
- Green E, Goldstein-Piekarski AN, Schatzberg AF, Rush AJ, Ma J, Williams L, 2017. Personalizing antidepressant choice by sex, body mass index, and symptom profile: An iSPOT-D report. Personalized Medicine in Psychiatry 1, 65–73. [Google Scholar]
- Jha MK, Minhajuddin A, Gadad BS, Greer T, Grannemann B, Soyombo A, Mayes TL, Rush AJ, Trivedi MH, 2017a. Can C-reactive protein inform antidepressant medication selection in depressed outpatients? Findings from the CO-MED trial. Psychoneuroendocrinology 78, 105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha MK, Minhajuddin A, Gadad BS, Greer TL, Mayes TL, Trivedi MH, 2017b. Interleukin 17 selectively predicts better outcomes with bupropion-SSRI combination: Novel T cell biomarker for antidepressant medication selection. Brain, behavior, and immunity 66, 103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha MK, Minhajuddin A, Gadad BS, Trivedi MH, 2017c. Platelet-Derived Growth Factor as an Antidepressant Treatment Selection Biomarker: Higher Levels Selectively Predict Better Outcomes with Bupropion-SSRI Combination. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum (CINP) 20, 919–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha MK, Minhajuddin A, Greer TL, Carmody T, Rush AJ, Trivedi MH, 2016. Early Improvement in Work Productivity Predicts Future Clinical Course in Depressed Outpatients: Findings From the CO-MED Trial. The American journal of psychiatry 173, 1196–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha MK, Trivedi MH, 2018. Personalized Antidepressant Selection and Pathway to Novel Treatments: Clinical Utility of Targeting Inflammation. International journal of molecular sciences 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS, National Comorbidity Survey R, 2003. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA 289, 3095–3105. [DOI] [PubMed] [Google Scholar]
- Miller AH, Trivedi MH, Jha MK, 2017. Is C-reactive protein ready for prime time in the selection of antidepressant medications? Psychoneuroendocrinology. [DOI] [PubMed]
- Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, Markowitz JC, Ninan PT, Kornstein S, Manber R, Thase ME, Kocsis JH, Keller MB, 2003. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry 54, 573–583. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Stewart JW, Nierenberg AA, Fava M, Kurian BT, Warden D, Morris DW, Luther JF, Husain MM, Cook IA, Shelton RC, Lesser IM, Kornstein SG, Wisniewski SR, 2011. Combining medications to enhance depression outcomes (CO-MED): acute and long-term outcomes of a single-blind randomized study. The American journal of psychiatry 168, 689–701. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW, Lebowitz BD, McGrath PJ, Rosenbaum JF, Sackeim HA, Kupfer DJ, Luther J, Fava M, 2006. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. The American journal of psychiatry 163, 1905–1917. [DOI] [PubMed] [Google Scholar]
- Shelton RC, Falola M, Li L, Zajecka J, Fava M, Papakostas GI, 2015a. The pro-inflammatory profile of depressed patients is (partly) related to obesity. Journal of psychiatric research 70, 91–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton RC, Pencina MJ, Barrentine LW, Ruiz JA, Fava M, Zajecka JM, Papakostas GI, 2015b. Association of obesity and inflammatory marker levels on treatment outcome: results from a double-blind, randomized study of adjunctive L-methylfolate calcium in patients with MDD who are inadequate responders to SSRIs. The Journal of clinical psychiatry. [DOI] [PubMed] [Google Scholar]
- Sung SC, Haley CL, Wisniewski SR, Fava M, Nierenberg AA, Warden D, Morris DW, Kurian BT, Trivedi MH, Rush AJ, Team C-MS, 2012. The impact of chronic depression on acute and long-term outcomes in a randomized trial comparing selective serotonin reuptake inhibitor monotherapy versus each of 2 different antidepressant medication combinations. J Clin Psychiatry 73, 967–976. [DOI] [PubMed] [Google Scholar]
- Sung SC, Wisniewski SR, Balasubramani GK, Zisook S, Kurian B, Warden D, Trivedi MH, Rush AJ, Team C-MS, 2013. Does early-onset chronic or recurrent major depression impact outcomes with antidepressant medications? A CO-MED trial report. Psychol Med 43, 945–960. [DOI] [PubMed] [Google Scholar]
- Sung SC, Wisniewski SR, Luther JF, Trivedi MH, Rush AJ, Team CS, 2015. Pre-treatment insomnia as a predictor of single and combination antidepressant outcomes: a CO-MED report. J Affect Disord 174, 157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thase ME, Haight BR, Richard N, Rockett CB, Mitton M, Modell JG, VanMeter S, Harriett AE, Wang Y, 2005. Remission rates following antidepressant therapy with bupropion or selective serotonin reuptake inhibitors: a meta-analysis of original data from 7 randomized controlled trials. The Journal of clinical psychiatry 66, 974–981. [DOI] [PubMed] [Google Scholar]
- Toups MS, Myers AK, Wisniewski SR, Kurian B, Morris DW, Rush AJ, Fava M, Trivedi MH, 2013. Relationship between obesity and depression: characteristics and treatment outcomes with antidepressant medication. Psychosom Med 75, 863–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi MH, 2016. Right patient, right treatment, right time: biosignatures and precision medicine in depression. World psychiatry: official journal of the World Psychiatric Association (WPA) 15, 237–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, Norquist G, Howland RH, Lebowitz B, McGrath PJ, Shores-Wilson K, Biggs MM, Balasubramani GK, Fava M, 2006. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. The American journal of psychiatry 163, 28–40. [DOI] [PubMed] [Google Scholar]
- Uher R, Tansey KE, Dew T, Maier W, Mors O, Hauser J, Dernovsek MZ, Henigsberg N, Souery D, Farmer A, McGuffin P, 2014. An inflammatory biomarker as a differential predictor of outcome of depression treatment with escitalopram and nortriptyline. The American journal of psychiatry 171, 1278–1286. [DOI] [PubMed] [Google Scholar]