Abstract
Objective:
Among individual depressive symptoms, anhedonia has been reliably associated with activation of the innate immune response. However, it is unclear whether this association extends to T cell cytokines and if gender differentially affects this association.
Method:
Concentrations of T (IL-17, T-helper (Th) 1- and Th2-) and non-T cell cytokines were measured in plasma using the Bioplex Pro™ human cytokine multiplex kit in Combining Medications to Enhance Depression Outcomes (CO-MED) trial participants who provided plasma at baseline (n = 166). Anhedonia was measured with three items of the clinician-rated Inventory of Depressive Symptomatology and depression severity (minus anhedonia item) was measured with Quick Inventory of Depression Severity Self-Report version (modified-QIDS-SR). Separate generalized linear models for anhedonia and modified-QIDS-SR as dependent variables were conducted with IL-17, Th1-, Th2-, and non-T cell- cytokines as primary independent variables and gender, body mass index (BMI), and age as covariates. Exploratory analyses included gender-by-biomarker interactions.
Results:
Higher levels of IL-17 (p=0.032), Th1- (p=0.002), Th2-(p=0.001) and non-T-(p=0.009) cell markers were associated with greater severity of anhedonia controlling for BMI, age, and gender. Gender also had a significant main effect on anhedonia, however, there was a significant gender by immune marker interaction only for IL-17 (p=0.050). Anhedonia severity increased with higher IL-17 in males (r=0.42, p=0.003) but not in females (r=0.09, p=0.336). Only non-T cell markers were associated with the modified-QIDS-SR, and there were no significant gender-specific associations with this variable.
Conclusions:
T and non-T cell-related inflammatory markers were associated with greater severity of anhedonia, while gender moderated the association of IL-17 with anhedonia in patients with major depressive disorder.
Keywords: Anhedonia, inflammation, interleukin 17, depression, gender
1. Introduction
Several lines of evidence implicate dysfunctional immune response in the pathophysiology of major depressive disorder (MDD) (Miller and Raison, 2016). Over one-third of patients treated with interferon alpha (an inflammatory cytokine) develop significant depressive symptoms (Raison et al., 2005). Depressive symptoms, in turn, are associated with higher levels of non-specific markers of inflammation such as c-reactive protein (CRP) (Cepeda et al., 2016). Patients with MDD have higher levels of inflammatory cytokines such as interleukin (IL)-6 in peripheral circulation and in cerebrospinal fluid as compared to controls (Kern et al., 2014; Kohler et al., 2017). Strong supportive evidence for the role of innate immune response in depressive symptoms is provided by the onset of depressive symptoms after injection of lipopolysaccharide (LPS) in animals and human volunteers (Dantzer et al., 2008; Eisenberger et al., 2010). The role of adaptive immunity in depression, however, specifically T cell-mediated immune response, remains unclear. T cell cytokines, such as interleukin 17 (IL-17), have gained recent attention for their potential role in MDD (Beurel and Lowell, 2017). Increased IL-17 has been shown to induce a depressive phenotype in male animals (Beurel et al., 2013), increase with chronic mild stress in female animals treated with vehicle versus fluoxetine (Lu et al., 2017), predict antidepressant treatment response in patients with MDD (Jha et al., 2017a), and is a target for developing novel antidepressants (Griffiths et al., 2017; Jha and Trivedi, 2018; Papp et al., 2016).
The association of T cell cytokines with overall depression severity has been inconsistent. While some studies have found altered levels of T-helper 1 (Th1) and Th2 cytokines (Kohler et al., 2017; Song et al., 2009) in patients with MDD, others have failed to find any significant association of Th1 and Th2 cytokines with depression (Dowlati et al., 2010). Similarly, IL-17 levels were not elevated in patients with MDD as compared to controls (Kohler et al., 2017) and were not significantly associated with overall depressive symptom severity (Jha et al., 2017a). A potential source of these conflicting findings could be the current definition of overall depression severity derived from the sum of nine symptoms that constitute the diagnosis of a major depressive episode. Within the heterogeneous syndromic diagnosis of MDD, anhedonia is an important symptom domain affected by immune activation (Eisenberger et al., 2010; Miller and Raison, 2016; Swardfager et al., 2016). For example, systemic inflammation, as evidenced by increased levels of CRP, is associated with reduced corticostriatal connectivity and greater severity of anhedonia symptoms in patients with MDD (Felger et al., 2016). It is unknown, however, whether IL-17 or other T cell cytokines are associated with anhedonia. Gender-specific differences in the association of inflammatory markers with depression may also account for the aforementioned conflicting findings. Gender-specific differences in immune responses are well-recognized (Klein and Flanagan, 2016). Indeed, autoimmune disorders in males typically involve a pro-inflammatory T-helper (Th1) response whereas those in females are predominantly mediated via autoantibody and Th2 immune responses (Fairweather et al., 2008). Nevertheless, the contribution of gender-specific differences in immune responses to the pathophysiology of MDD remains unclear.
In a recent report, higher CRP was associated with greater depression severity in females but not males (Köhler-Forsberg et al., 2017). This differed from previous reports of stronger association of CRP with depressive symptoms in males as compared to females (Tayefi et al., 2017; Vetter et al., 2013). The inflammatory challenge with lipopolysaccharide injection results in a greater increase in pro-inflammatory cytokines and social disconnection in females as compared to males (Moieni et al., 2015; van Eijk et al., 2007). Similarly, higher IL-1β and TNF-α levels in depressed outpatients were associated with greater depressive symptoms in females but not males (Birur et al., 2017). Use of interferon α (IFN-α) has reportedly been associated with higher rates of depression in females in some (Koskinas et al., 2002) but not all (Bonaccorso et al., 2002; Raison et al., 2005) studies. Nevertheless, few studies have examined the association of T cell cytokines with anhedonia and other depressive symptoms, and there is a paucity of information on whether these relationships are gender-specific.
Thus, the primary aim of this report was to evaluate the association of IL-17 and other T cell and non-T cell cytokines with anhedonia and overall depressive symptom severity (minus the anhedonia item). As exploratory analyses, we also examined the role of gender-specific differences in these associations. To accomplish this aim we used plasma samples collected from a sample of convenience available through the Combining Medications to Enhance Depression Outcomes (CO-MED) trial (Rush et al., 2011).
2. Material and Methods
2.1. Study Overview
Participants of the CO-MED trial who provided plasma specimens (n=166) constitute the analytic sample of this report. As previously described in detail (Rush et al., 2011), CO-MED trial recruited treatment-seeking outpatients with MDD (ascertained by structured interview) from six primary and nine psychiatric care sites who had nonpsychotic chronic (current episode exceeded 2 years) or recurrent depression with current episode ≥2 months and a baseline 17-item Hamilton Rating Scale (HRSD17) ≥16 and not currently taking psychotropic medications. Complete list of exclusion criteria is available at https://clinicaltrials.gov/ct2/show/NCT00590863. Briefly, those with lifetime history of bipolar or psychotic disorders, current substance dependence, or had a general medical condition that is unstable or prohibited the use of study medications were excluded.
Participants (n=665) were randomly assigned after stratification for site to either SSRI monotherapy (escitalopram plus placebo), bupropion-SSRI combination (sustained-release [SR] bupropion plus escitalopram), or venlafaxine-mirtazapine combination (extended-release [XR] venlafaxine plus mirtazapine). Baseline plasma was collected as part of a separate add-on biomarker study that was optional and required an additional consent. All participants in this report provided written informed consent for participation in the main CO-MED trial, along with consent for the biomarker collection. Thus, the number of plasma samples (n=166) collected at baseline was a subset of the total number of CO-MED trial participants (n=665). Participants who did not provide plasma (n=499) at baseline were younger (mean age=44.51 years vs. 42.11, p=0.030) and had lower use of statin medication (20.5% vs 13.6%, p=0.034) when compared to the analytic sample of this report, but did not differ on any other baseline clinical and sociodemographic features as previously reported (Jha et al., 2017b). The trial was reviewed and approved by the Institutional Review Boards at UT Southwestern Medical Center at Dallas, the University of Pittsburgh Data Coordinating Center, each participating regional center, and all relevant clinics.
2.2. Assessments
Participants provided sociodemographic information and filled out the 16-item Quick Inventory of Depressive Symptomatology – Self-Report (QIDS-SR), while clinicians completed the 30-item Inventory of Depressive Symptomatology Clinician-Rated (IDS-C).
Quick Inventory of Depressive Symptomatology Self-Report (QIDS-SR):
This commonly used scale has 16 items, each of which includes four choices that are scored from 0–3. A total score is calculated from nine of these 16 items (consistent with the nine criterion symptom domains of MDD) leading to a range of 0–27 (Rush et al., 2003). The QIDS-SR correlates highly (0.86–0.93) with the 17-item Hamilton Rating Scale for Depression, HRSD17 (Rush et al., 2006). In previous reports, the reported Cronbach’s α of QIDS-SR has ranged from 0.86 to 0.87 (Rush et al., 2006; Rush et al., 2003; Trivedi et al., 2004). In the CO-MED trial, the QIDS-SR served as the primary depression symptom severity outcome measure (Rush et al., 2011). For this report, we created a modified QIDS-SR score by excluding the “interest” item, which reflects the severity of anhedonia, from the total QIDS-SR score resulting in a range of 0–24 for a total score of modified QIDS-SR.
Inventory of Depressive Symptomatology clinician-rated (IDS-C):
Of the 30 items of IDS-C (each item has four choices that are scored from 0–3), 28 items are summed to generate a total score (range 0–84) that correlates very highly (Pearson’s moment correlation equals 0.95) with the HRSD17 (Rush et al., 1996). In previous reports, the Cronbach’s α of IDS-C has ranged from 0.67 to 0.94 (Rush et al., 1996; Trivedi et al., 2004). Anhedonia, as measured by a subscale of three items of IDS-C (range 0–9), was used as the primary outcome for this report. It has been shown to compare favorably to Snaith-Hamilton Pleasure Scale (Snaith et al., 1995) with correlation coefficient of 0.63 (Felger et al., 2016). The Cronbach’s α of the 3-item IDS-anhedonia at baseline of CO-MED trial was 0.55.
2.3. Measurement of inflammatory biomarkers
Plasma samples extracted from CO-MED trial participants (n=166) were transported overnight to the Biologic Core of National Institute of Mental Health Repository and Genomics Resource (NIMH RGR) for storage at −80°C. All samples for this report were obtained from the NIMH RGR core. Biomarker levels were measured in all samples at the same time, blinded to treatment allocation and outcomes by the Microarray Core at UT Southwestern Medical Center using the Bioplex Pro™ human cytokine standard 27-plex kit (Bio-Rad Laboratories, Hercules, CA, USA) with a Bio-plex® 200 instrument that was equipped with Bio-Plex Manager software, version 6.0 (Bio-Rad Laboratory, Hercules, CA, USA). The 27-plex kit measures IL-17, Th1- (interferon gamma or IFN-γ and tumor necrosis factor α or TNF-α), Th2- (IL-4, IL-5, IL-9, and IL-13), and non-Th1/Th2- (IL-1β, IL-1 receptor antagonist, IL-8, IL-6, and macrophage inflammatory protein (MIP) 1 α and β) markers. All measurements were expressed in pg/ml with correction for 4-fold dilution using the standards provided in the kit (Bio-Rad Laboratory, Hercules, CA, USA). The levels of inflammatory biomarkers were interpreted only if the intra- and inter-assay coefficients of variation were less than 10% of detection limits (or precision range) specified by the manufacturer. The upper (UD) and lower (LD) detection limits and well as median, 25th percentile (p25) and 75th percentile (p75) of these immune markers were previously reported (Jha et al., 2017a) and were as follows (all in pg/mL): IL-17 (LD =4.9, UD =12235, median = 96.0, p25 =76.5, p75=125.6), TNF-α (LD =5.8, UD =95484, median =106.2, p35 =91.9, p75 =118.3), IFN-γ (LD =92.6, UD =52719, median =255.6, p25 =180.9, 75 =338.5), IL-4 (LD =2.2, UD =3467, median =8.9, p25 =7.6, p75= 10.6), IL-5 (LD =3.1, UD =7380, median =60.9, p25 =51.4, p75 =76.9), IL-9 (LD=2.1, UD =7989, median =22.1, p25 =15.1, p75= 29.8), IL-13 (LD =3.7, UD =3137, median =13.6, p25 =8.4, p75 =25.6), IL-1β (UD =3.2, LD =3261, median =9.8, p25 =8.0, p75 =11.6, IL-1 receptor antagonist (LD =81.1, UD =70487, median =488.5, p25 =394.1, p75 =648.4), IL-8 (LD =1.9, UD =26403, median =24.0, p25 =19.3, p75 =40.0), IL-6 (LD =2.3, UD =18880, median =15.9, p25 =12.6, p75 =19.3), MIP-1α (LD =1.4, UD =836, median =8.3, p25 =7.2, p75 =10.0), and MIP-1β (LD =2.0, UD =1726, median =82.5, p25 =63.1, p75 =120.4).
2.4. Statistical Analyses
Log-transformation was used for biomarkers that were not normally distributed. As previously described (Jha et al., 2017a), immune markers were grouped into Th1- (IFN-γ factor loading =0.77, TNF-α factor loading =0.77, squared multiple correlations of the variables with each factor =0.69), Th2- (IL-4 factor loading =0.83, IL-5 factor loading =0.74, IL-9 factor loading =0.55, IL-13 factor loading =0.43, squared multiple correlations of the variables with each factor =0.77) and non-Th1/Th2- (IL-1β factor loading =0.85, IL-1 receptor antagonist factor loading =0.73, IL-8 factor loading =0.79, IL-6 factor loading =0.76, MIP-1α factor loading =0.56, MIP-1β factor loading =0.53, squared multiple correlations of the variables with each factor =0.88) factors after confirmatory factor analyses and the factor scores were used in subsequent analyses. As the primary purpose of this report was to test the association of T- and non-T cell markers with anhedonia, separate generalized linear model (GLM) analyses were used with anhedonia as the dependent variable and IL-17, Th1-, Th2- and non-Th1/Th2-factors as the primary independent variables of interest after controlling for gender and body mass index (BMI) and age. As exploratory analyses, these GLM analyses were repeated with gender-by-biomarker interactions, and stratified analyses for each gender (males and females) were conducted to quantify the effect of biomarker after controlling for age and BMI. To replicate the findings of Birur et al. (2017), separate above-specified GLM analyses were repeated with IL-1β and TNF-α in biomarker-by-gender interaction variables for anhedonia and modified QIDS-SR as dependent variables. The strength of association between depressive symptoms and inflammatory biomarkers were estimated using correlation coefficients. To visualize the gender-by-biomarker interaction, individual scores of dependent variables were plotted against the biomarker level. All analyses were conducted with SAS version 9.3 and threshold of significance were set at p <0.05.
3. Results
The mean anhedonia score (range 0–9) for the whole sample (N=166), males (n=49) and females (n=117) was 5.46 (2.04), 4.4(1.9), and 5.9 (2.0) respectively. As shown in Table 1, female participants had a significantly higher level of anhedonia when compared to males (t value=−4.41 (df=92), p<0.001).
Table 1.
Clinical and sociodemographic features based on gender in CO-MED trial participants who provided plasma at baseline (n=166)
| Total | Males | Females | p-value | |||||
|---|---|---|---|---|---|---|---|---|
| Number | 166 | 49 | 117 | |||||
| Categorical variables | N | % | N | % | N | % | χ2 (df) | |
| Race | 0.78 (1) | 0.677 | ||||||
| White | 107 | 64.5 | 34 | 69.4 | 73 | 62.4 | ||
| Black | 46 | 27.7 | 12 | 24.5 | 34 | 29.1 | ||
| Other | 13 | 7.8 | 3 | 6.1 | 10 | 8.5 | ||
| Hispanic ethnicity | 7.58 (1) | 0.006 | ||||||
| No | 139 | 83.7 | 47 | 95.9 | 92 | 78.6 | ||
| Yes | 27 | 16.3 | 2 | 4.1 | 25 | 21.4 | ||
| Education | 0.63 (2) | 0.731 | ||||||
| <12 years | 24 | 14.5 | 7 | 14.3 | 17 | 14.5 | ||
| 12 –15 years | 98 | 59 | 27 | 55.1 | 71 | 60.7 | ||
| >15 years | 44 | 26.5 | 15 | 30.6 | 29 | 24.8 | ||
| Employed at baseline | 73 | 44.0 | 19 | 38.8 | 54 | 46.2 | 0.76 (1) | 0.382 |
| Anxious features present | 120 | 72.3 | 31 | 63.3 | 89 | 76.1 | 2.83 (1) | 0.093 |
| Atypical features present | 35 | 21.1 | 8 | 16.3 | 27 | 23.1 | 0.94 (1) | 0.331 |
| Melancholic features present | 51 | 30.7 | 9 | 18.4 | 42 | 35.9 | 4.99 (1) | 0.026 |
| Suicidal ideation at baseline present | 94 | 56.6 | 27 | 55.1 | 67 | 57.3 | 0.07 (1) | 0.798 |
| Onset of depression before age 18 years | 67 | 40.4 | 18 | 36.7 | 49 | 41.9 | 0.38 | 0.538 |
| Continuous variables | Mean | SD | Mean | SD | Mean | SD | T value (df) | |
| Age in years | 44.5 | 12.0 | 46.5 | 11.2 | 43.7 | 12.2 | 1.42 (97) | 0.160 |
| QIDS-SR | 15.6 | 4.1 | 13.9 | 3.6 | 16.2 | 4.2 | −3.65 (103) | <0.001 |
| Anhedonia severity from IDS-C | 5.5 | 2.0 | 4.4 | 1.9 | 5.9 | 2.0 | −4.41 (92) | <0.001 |
| Episode duration in weeks | 64.5 | 119.3 | 77.1 | 125.7 | 59.2 | 116.7 | 0.85 (84) | 0.396 |
| BMI | 32.0 | 9.1 | 31.2 | 8.5 | 32.3 | 9.4 | −0.80 (99) | 0.427 |
| Number of comorbid psychiatric conditions | 1.3 | 1.7 | 1.6 | 2.0 | 1.1 | 1.6 | 1.46 (73) | 0.148 |
| Number of general medical conditions | 2.1 | 1.6 | 2.3 | 2.0 | 2.0 | 1.5 | 0.82 (71) | 0.417 |
CO-MED is Combining Medications to Enhance Depression Outcomes, QIDS-SR is Quick Inventory of Depressive Symptomatology, Self-Rated version, IDS-C is Inventory of Depressive Symptomatology Clinician-Rated Version, CAST is Concise Associated Symptom Tracking scale, BMI is body mass index.
There was a significant main effect of IL-17 (p=0.032) Th1- (p=0.002), Th2- (p=0.001), and non-Th1/Th2-(p=0.009) factors on severity of anhedonia after controlling for age, gender, and BMI (see Table 2). Of note, there was a main effect of gender on anhedonia in all analyses (all p<0.01). The Pearson’s correlation coefficient between anhedonia and IL-17, Th1-, Th2-, and non-Th1/Th2-factors were 0.18 (p=0.022), 0.22 (p=0.004), 0.23 (p=0.003), and 0.19 (p=0.017) respectively. The association of inflammatory markers after controlling for age, gender, and BMI with modified depressive symptoms (minus anhedonia) was significant only for non-Th1/Th2-factor (p=0.019) but not for IL-17 (p=0.271), Th1- (p=0.580) and Th2-(p=0.422) factors, also see Table 2. The Pearson’s correlation coefficient between depressive symptoms excluding anhedonia and non-Th1/Th2-factors was −0.20 (p=0.009).
Table 2.
Association of T- and non-T cell related inflammatory markers with anhedonia and other depressive and symptoms
| Anhedonia | Modified QIDS-SR* | |||
|---|---|---|---|---|
| F value | p value | F value | p value | |
| Interleukin 17 | ||||
| Age | 0.24 | 0.628 | 1.30 | 0.256 |
| BMI | 1.13 | 0.289 | 0.92 | 0.339 |
| Gender | 18.02 | <0.001 | 11.15 | 0.001 |
| Log of IL-17 | 4.68 | 0.032 | 1.22 | 0.271 |
| Th1-factor | ||||
| Age | 0.76 | 0.386 | 1.18 | 0.278 |
| BMI | 1.43 | 0.234 | 1.28 | 0.259 |
| Gender | 18.89 | <0.001 | 11.16 | 0.001 |
| Th1-factor | 9.72 | 0.002 | 0.31 | 0.580 |
| Th2-factor | ||||
| Age | 0.63 | 0.429 | 1.22 | 0.270 |
| BMI | 0.65 | 0.422 | 1.38 | 0.243 |
| Gender | 20.18 | <0.001 | 11.41 | <0.001 |
| Th2-factor | 10.99 | 0.001 | 0.64 | 0.422 |
| Non-T cell-factor | ||||
| Age | 0.27 | 0.603 | 0.66 | 0.419 |
| BMI | 0.73 | 0.393 | 0.48 | 0.490 |
| Gender | 16.64 | <0.001 | 7.53 | 0.007 |
| Non-Th1/Th2-factor | 7.07 | 0.009 | 5.58 | 0.019 |
Modified QIDS is QIDS-SR (Quick Inventory of Depressive Symptomatology Self-Rated version) minus the anhedonia item, Th1 is T-helper cell type 1, Th2 is T-helper cell type 2, Th1 cytokines include interferon gamma and tumor necrosis factor alpha (TNF-α), Th2 cytokines include interleukin (IL) 4, IL-5, IL-9, and IL-13, non-Th1/Th2 cytokines include IL-1 beta (IL-1β), IL-1 receptor antagonist, IL-8, IL-6, and macrophage inflammatory protein (MIP) 1 alpha and beta.
Exploratory analyses revealed significant biomarker-by-gender interaction on levels of anhedonia for log IL-17 (p=0.050) but not for Th1- (p=0.219), Th2- (p=0.743) and non-Th1/Th2- (p=0.486) factor, as shown in Table 3. There was no significant biomarker-by-gender interaction for depression severity minus anhedonia item (modified-QIDS-SR; Table 3). There was no significant TNF-α-by-gender interaction for anhedonia (F=1.88, p=0.172) and modified-QIDS-SR (F=2.61, p=0.108) as well as no significant IL 1β-by-gender interaction for anhedonia (F=0.81, p=0.370) and modified-QIDS-SR (F=0.22, p=0.640)
Table 3.
Differential association of inflammatory markers with anhedonia and other depressive symptoms based on gender
| Modified QIDS-SR* | Anhedonia | |||
|---|---|---|---|---|
| F value | p value | F value | p value | |
| Interleukin 17 | ||||
| Gender | 3.76 | 0.054 | 5.51 | 0.020 |
| Log of IL-17 | 0.09 | 0.768 | 9.13 | 0.003 |
| Log of IL-17-by-gender | 2.72 | 0.101 | 3.90 | 0.050 |
| Th1 factor | ||||
| Gender | 11.74 | <0.001 | 20.79 | <0.001 |
| Th1 factor | 0.56 | 0.455 | 11.28 | 0.001 |
| Th1 factor-by-gender | 1.39 | 0.239 | 1.52 | 0.219 |
| Th2 factor | ||||
| Gender | 12.09 | <0.001 | 21.70 | <0.001 |
| Th2 factor | 0.78 | 0.379 | 11.81 | <0.001 |
| Th2 factor-by-gender | 1.39 | 0.240 | 0.11 | 0.743 |
| Non-Th1/Th2 factor | ||||
| Gender | 7.84 | 0.006 | 17.93 | <0.001 |
| Non-Th1/Th2 factor | 5.11 | 0.025 | 8.21 | 0.005 |
| Non-Th1/Th2 factor-by-gender | 0.08 | 0.783 | 0.49 | 0.486 |
Modified QIDS is QIDS-SR (Quick Inventory of Depressive Symptomatology Self-Rated version) minus the anhedonia item, Th1 is T-helper cell type 1, Th2 is T-helper cell type 2, Th1 cytokines include interferon gamma and tumor necrosis factor alpha (TNF-α), Th2 cytokines include interleukin (IL) 4, IL-5, IL-9, and IL-13, non-Th1/Th2 cytokines include IL-1 beta (IL-1β), IL-1 receptor antagonist, IL-8, IL-6, and macrophage inflammatory protein (MIP) 1 alpha and beta.
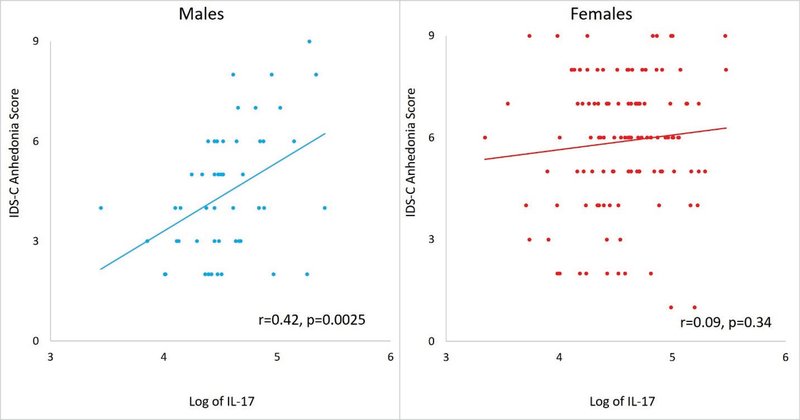
In subsequent analyses stratified by gender, 1-unit higher value of log IL-17 was associated with 2.37 (standard error [SE)=0.76, t value=3.13, p=0.003) a higher score of anhedonia in males even after controlling for age and BMI. Conversely, 1-unit higher value of log IL-17 was not associated with any significant difference in anhedonia for females (est.=0.42, SE=0.45, t value=0.94, p=0.350). As shown in Figure 1, there was a strong correlation between log IL-17 and anhedonia in males (correlation coefficient=0.42) but not in females (correlation coefficient=0.09).
Figure 1.
Higher IL-17 levels were associated with higher IDS-C anhedonia scores in males but not female participants of CO-MED trial (n=166)
IL-17 is interleukin 17, IDS-C is 30-item Inventory of Depressive Symptomatology Clinician-Rated, CO-MED is Combining Medications to Enhance Depression Outcomes, r is Pearson’s correlation coefficient.
4. Discussion
In this large study of depressed outpatients, there was a significant association of IL-17 as well as Th1-, Th2-, and non-Th1/Th2-factors with the severity of anhedonia controlling for age, gender, and BMI. The severity of anhedonia was greater in participants with higher levels of these inflammatory markers. The severity of depressive symptoms excluding anhedonia was associated with non-Th1/Th2 factor only and not with IL-17 or Th1- and Th2-factors. Gender was significantly associated with severity of anhedonia in all analyses and significantly moderated the association of IL-17 and anhedonia (but not of IL-17 and depressive symptoms excluding anhedonia or that of Th1-, Th2- and non Th1/Th2-inflammatory markers and anhedonia or depressive symptoms excluding anhedonia). In males, but not females, higher IL-17 levels were associated with greater severity of anhedonia even after controlling for age and gender. After controlling for age, gender, and BMI, there was a significant association of anhedonia with all biomarkers of inflammation assessed in this report.
The findings of this report further highlight the effect of immune dysfunction on depressive symptoms, specifically on anhedonia. While the severity of depressive symptoms excluding anhedonia was associated only with non-T cell cytokines, we found that severity of anhedonia increased with increased levels of both T- and non-T cell cytokines. Thus, the impact of the immune system appears to be prominent for anhedonia. These findings of a significant association between anhedonia and T- and non--T cell cytokines is consistent with previous reports that have reported small-to-moderate correlation between inflammatory cytokines and severity of anhedonia (Loftis et al., 2008). These are also consistent with previous findings of increased anhedonia severity with higher levels of non-specific markers of inflammation such as CRP (Felger et al., 2016). The greater severity of anhedonia in females than males may reflect different symptomatic profiles of depressive symptoms based on gender where symptoms such as irritability or agitation are seen more often in males and those of anhedonia in females (Amir A. Khan et al., 2002; Kuehner, 2003; Page et al., 2016).
This report adds to the literature regarding gender-specific differential effects of inflammation on the development of depressive symptoms. While the levels of IL-17 did not differ between males and females, the effect of IL-17 on anhedonia was significant only for males but not for females. As shown in Table 1, males and females did not differ on most clinical and sociodemographic variables except for higher rates of Hispanic ethnicity and melancholic features as well as greater severity of self-reported depressive symptoms and anhedonia in females. The lack of gender-specific association of IL-1β and TNF-α in this report differs from a previous report that found both IL-1β and TNF-α levels correlated with depressive symptoms in females but not males (Birur et al., 2017). Potential reasons for this inconsistency include the difference in measures to assess depressive symptom severity, sample size (larger number of depressed participants in the CO-MED trial), and use of single-items to measure symptom domains by Birur et al.
The biological basis for these findings is likely associated with the interaction of the immune and endocrine systems. In animal studies, testosterone has been shown to confer protection against autoimmune disorders mediated by IL-17 (Schwinge et al., 2015). Lower testosterone levels predispose rodents to develop anhedonia following chronic stress (Herrera-Perez et al., 2012) and testosterone substitution prevents the development of anhedonia and depressive symptoms in rodents with low testosterone (Carrier et al., 2015; Herrera-Perez et al., 2012). Studies that include concomitant measures of sex hormones and inflammatory markers are needed to better understand the gender-specific differences in anhedonia based on IL-17 levels. Our data suggest that relatively lower levels of testosterone may contribute to increased IL-17 and anhedonia in these patients. Future flow-cytometry studies measuring Th1, Th2, Th17, and regulatory T (Treg) cells are needed to elucidate pathophysiological mechanisms underlying the role of inflammation and sex hormones in anhedonia. Levels of Treg cells differ in males versus females (Afshan et al., 2012); Treg cells, in turn, control the differentiation of naïve T cells to Th17 cells (Eisenstein and Williams, 2009). Additionally, while the role of IL-17 in disruption of blood-brain barrier is well recognized (Huppert et al., 2010; Kebir et al., 2007), the ratio of Th17 to Th1 cells is considered critical in the infiltration of brain parenchyma by peripheral immune cells and subsequent neuroinflammation (Stromnes et al., 2008).
There are several limitations of this report. This is a secondary analysis using a sample of convenience, thus it may have been inadequately powered to identify gender-specific differences in the association of inflammatory markers and depressive symptoms thus resulting in false negatives (or positives). The generalizability of these findings is also limited due to the higher proportion of females than males (70.5% vs. 29.5%), gender differences in severity of anhedonia, and high illness burden of CO-MED trial participants as the inclusion criteria included either chronic or recurrent MDD, and at least moderate severity of ongoing symptoms. As immune and endocrine systems are a complex interplay of multiple factors, focusing predominantly on IL-17 and using biologically based factor analysis may be insufficient. As the cytokines included in this report can be produced from multiple cell types, the levels of these cytokines may not reflect the number and function of T- and non-T cells, further supporting the need for future flow cytometry studies. Additional limitations include non-adjustment of p-values for multiple comparisons, non-availability of information regarding menstrual phase, smoking history, contraceptive use, and levels of sex steroid hormones, and lack of information regarding factors that may have introduced variability across samples such as the time of the day for plasma collection as well as average time from blood collection to plasma extraction. In light of these limitations, findings from this report are preliminary in nature and warrant future prospective studies.
5. Conclusion
The severity of anhedonia increases with increased levels of T- and non-T cell cytokines. Gender is an important biological factor that moderates the association of IL-17 with anhedonia. While males with higher levels of IL-17 experience greater severity of anhedonia, no such association is evident in females. Additionally, gender did not differentially affect the association of other depressive symptoms with IL-17 or of any depressive symptoms with Th1-, Th2-, and non-Th1/Th2-factors.
Supplementary Material
Highlights.
Elevated levels of T- and non-T-cell markers are associated with greater anhedonia severity.
Gender differentially affects the association of anhedonia with interleukin 17 (IL-17) but not with other T- and non-T-cell markers.
Higher IL-17 levels are associated with greater anhedonia severity in males but not in females.
Acknowledgments
The CO-MED trial was funded by National Institute of Mental Health under contract N01 MH-90003 to the University of Texas Southwestern Medical Center at Dallas (Principal Investigators, M. H. Trivedi, and A.J. Rush). This work was also supported in part through the Center for Depression Research and Clinical Care at UT Southwestern (Principal Investigator: Madhukar H. Trivedi, MD) and Hersh Foundation. The authors thank the clinical staff at each clinical site for their assistance with this project; all of the study participants; and Jeremy Kee, M.A. for administrative support. Forest Pharmaceuticals, GlaxoSmithKline, Organon, and Wyeth Pharmaceuticals provided medications for CO-MED trial at no cost. The content of this publication does not necessarily reflect the views or policies of the U.S. Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. government. NIMH had no role in the drafting or review of the manuscript or in the collection or analysis of the data.
References
- Afshan G, Afzal N, Qureshi S, 2012. CD4+CD25(hi) regulatory T cells in healthy males and females mediate gender difference in the prevalence of autoimmune diseases. Clinical laboratory 58, 567–571. [PubMed] [Google Scholar]
- Khan Amir A., Gardner Charles O., Prescott Carol A., Kendler Kenneth S., 2002. Gender Differences in the Symptoms of Major Depression in Opposite-Sex Dizygotic Twin Pairs. American Journal of Psychiatry 159, 1427–1429. [DOI] [PubMed] [Google Scholar]
- Beurel E, Harrington LE, Jope RS, 2013. Inflammatory T Helper 17 Cells Promote Depression-like Behavior in Mice. Biological Psychiatry 73, 622–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurel E, Lowell JA, 2017. Th17 cells in depression. Brain, behavior, and immunity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birur B, Amrock EM, Shelton RC, Li L, 2017. Sex Differences in the Peripheral Immune System in Patients with Depression. Frontiers in Psychiatry 8, 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaccorso S, Marino V, Biondi M, Grimaldi F, Ippoliti F, Maes M, 2002. Depression induced by treatment with interferon-alpha in patients affected by hepatitis C virus. Journal of Affective Disorders 72, 237–241. [DOI] [PubMed] [Google Scholar]
- Carrier N, Saland SK, Duclot F, He H, Mercer R, Kabbaj M, 2015. The Anxiolytic and Antidepressant-like Effects of Testosterone and Estrogen in Gonadectomized Male Rats. Biological Psychiatry 78, 259–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda MS, Stang P, Makadia R, 2016. Depression Is Associated With High Levels of C-Reactive Protein and Low Levels of Fractional Exhaled Nitric Oxide: Results From the 2007–2012 National Health and Nutrition Examination Surveys. The Journal of clinical psychiatry 77, 1666–1671. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW, 2008. From inflammation to sickness and depression: when the immune system subjugates the brain. Nature reviews. Neuroscience 9, 46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctot KL, 2010. A meta-analysis of cytokines in major depression. Biol Psychiatry 67, 446–457. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Berkman ET, Inagaki TK, Rameson LT, Mashal NM, Irwin MR, 2010. Inflammation-Induced Anhedonia: Endotoxin Reduces Ventral Striatum Responses to Reward. Biological Psychiatry 68, 748–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein EM, Williams CB, 2009. The T(reg)/Th17 cell balance: a new paradigm for autoimmunity. Pediatric research 65, 26r–31r. [DOI] [PubMed] [Google Scholar]
- Fairweather D, Frisancho-Kiss S, Rose NR, 2008. Sex differences in autoimmune disease from a pathological perspective. The American journal of pathology 173, 600–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, Li Z, Haroon E, Woolwine BJ, Jung MY, Hu X, Miller AH, 2016. Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Molecular psychiatry 21, 1358–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths CEM, Fava M, Miller AH, Russell J, Ball SG, Xu W, Acharya N, Rapaport MH, 2017. Impact of Ixekizumab Treatment on Depressive Symptoms and Systemic Inflammation in Patients with Moderate-to-Severe Psoriasis: An Integrated Analysis of Three Phase 3 Clinical Studies. Psychother Psychosom 86, 260–267. [DOI] [PubMed] [Google Scholar]
- Herrera-Perez JJ, Martinez-Mota L, Chavira R, Fernandez-Guasti A, 2012. Testosterone prevents but not reverses anhedonia in middle-aged males and lacks an effect on stress vulnerability in young adults. Hormones and behavior 61, 623–630. [DOI] [PubMed] [Google Scholar]
- Huppert J, Closhen D, Croxford A, White R, Kulig P, Pietrowski E, Bechmann I, Becher B, Luhmann HJ, Waisman A, Kuhlmann CR, 2010. Cellular mechanisms of IL-17-induced blood-brain barrier disruption. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 24, 1023–1034. [DOI] [PubMed] [Google Scholar]
- Jha MK, Minhajuddin A, Gadad BS, Greer TL, Mayes TL, Trivedi MH, 2017a. Interleukin 17 selectively predicts better outcomes with bupropion-SSRI combination: Novel T cell biomarker for antidepressant medication selection. Brain, behavior, and immunity 66, 103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha MK, Minhajuddin A, Gadad BS, Trivedi MH, 2017b. Platelet-Derived Growth Factor as an Antidepressant Treatment Selection Biomarker: Higher Levels Selectively Predict Better Outcomes with Bupropion-SSRI Combination. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum (CINP) 20, 919–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha MK, Trivedi MH, 2018. Personalized Antidepressant Selection and Pathway to Novel Treatments: Clinical Utility of Targeting Inflammation. International journal of molecular sciences 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebir H, Kreymborg K, Ifergan I, Dodelet-Devillers A, Cayrol R, Bernard M, Giuliani F, Arbour N, Becher B, Prat A, 2007. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat Med 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern S, Skoog I, Borjesson-Hanson A, Blennow K, Zetterberg H, Ostling S, Kern J, Gudmundsson P, Marlow T, Rosengren L, Waern M, 2014. Higher CSF interleukin-6 and CSF interleukin-8 in current depression in older women. Results from a population-based sample. Brain, behavior, and immunity 41, 55–58. [DOI] [PubMed] [Google Scholar]
- Klein SL, Flanagan KL, 2016. Sex differences in immune responses. Nature Reviews Immunology 16, 626–638. [DOI] [PubMed] [Google Scholar]
- Köhler-Forsberg O, Buttenschøn HN, Tansey KE, Maier W, Hauser J, Dernovsek MZ, Henigsberg N, Souery D, Farmer A, Rietschel M, McGuffin P, Aitchison KJ, Uher R, Mors O, 2017. Association between C-reactive protein (CRP) with depression symptom severity and specific depressive symptoms in major depression. Brain, behavior, and immunity 62, 344–350. [DOI] [PubMed] [Google Scholar]
- Kohler CA, Freitas TH, Maes M, de Andrade NQ, Liu CS, Fernandes BS, Stubbs B, Solmi M, Veronese N, Herrmann N, Raison CL, Miller BJ, Lanctot KL, Carvalho AF, 2017. Peripheral cytokine and chemokine alterations in depression: a meta-analysis of 82 studies. Acta psychiatrica Scandinavica 135, 373–387. [DOI] [PubMed] [Google Scholar]
- Koskinas J, Merkouraki P, Manesis E, Hadziyannis S, 2002. Assessment of depression in patients with chronic hepatitis: effect of interferon treatment. Digestive diseases (Basel, Switzerland) 20, 284–288. [DOI] [PubMed] [Google Scholar]
- Kuehner C, 2003. Gender differences in unipolar depression: an update of epidemiological findings and possible explanations. Acta psychiatrica Scandinavica 108, 163–174. [DOI] [PubMed] [Google Scholar]
- Loftis JM, Huckans M, Ruimy S, Hinrichs DJ, Hauser P, 2008. Depressive symptoms in patients with chronic hepatitis C are correlated with elevated plasma levels of interleukin-1β and tumor necrosis factor-α. Neuroscience Letters 430, 264–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Ho CS, Liu X, Chua AN, Wang W, McIntyre RS, Ho RC, 2017. Chronic administration of fluoxetine and pro-inflammatory cytokine change in a rat model of depression. PLOS ONE 12, e0186700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Raison CL, 2016. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nature reviews. Immunology 16, 22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moieni M, Irwin MR, Jevtic I, Olmstead R, Breen EC, Eisenberger NI, 2015. Sex differences in depressive and socioemotional responses to an inflammatory challenge: implications for sex differences in depression. Neuropsychopharmacology 40, 1709–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page GG, Opp MR, Kozachik SL, 2016. Sex differences in sleep, anhedonia, and HPA axis activity in a rat model of chronic social defeat. Neurobiology of Stress 3, 105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp KA, Reich K, Paul C, Blauvelt A, Baran W, Bolduc C, Toth D, Langley RG, Cather J, Gottlieb AB, Thaci D, Krueger JG, Russell CB, Milmont CE, Li J, Klekotka PA, Kricorian G, Nirula A, 2016. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. The British journal of dermatology 175, 273–286. [DOI] [PubMed] [Google Scholar]
- Raison CL, Borisov AS, Broadwell SD, Capuron L, Woolwine BJ, Jacobson IM, Nemeroff CB, Miller AH, 2005. Depression during pegylated interferon-alpha plus ribavirin therapy: prevalence and prediction. The Journal of clinical psychiatry 66, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AJ, Bernstein IH, Trivedi MH, Carmody TJ, Wisniewski S, Mundt JC, Shores-Wilson K, Biggs MM, Woo A, Nierenberg AA, Fava M, 2006. An Evaluation of the Quick Inventory of Depressive Symptomatology and the Hamilton Rating Scale for Depression: A Sequenced Treatment Alternatives to Relieve Depression Trial Report. Biological Psychiatry 59, 493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH, 1996. The Inventory of Depressive Symptomatology (IDS): psychometric properties. Psychological medicine 26, 477–486. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, Markowitz JC, Ninan PT, Kornstein S, Manber R, Thase ME, Kocsis JH, Keller MB, 2003. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry 54, 573–583. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Stewart JW, Nierenberg AA, Fava M, Kurian BT, Warden D, Morris DW, Luther JF, Husain MM, Cook IA, Shelton RC, Lesser IM, Kornstein SG, Wisniewski SR, 2011. Combining medications to enhance depression outcomes (CO-MED): acute and long-term outcomes of a single-blind randomized study. The American journal of psychiatry 168, 689–701. [DOI] [PubMed] [Google Scholar]
- Schwinge D, Carambia A, Quaas A, Krech T, Wegscheid C, Tiegs G, Prinz I, Lohse AW, Herkel J, Schramm C, 2015. Testosterone suppresses hepatic inflammation by the downregulation of IL-17, CXCL-9, and CXCL-10 in a mouse model of experimental acute cholangitis. The Journal of Immunology 194, 2522–2530. [DOI] [PubMed] [Google Scholar]
- Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, Trigwell P, 1995. A scale for the assessment of hedonic tone the Snaith-Hamilton Pleasure Scale. The British journal of psychiatry : the journal of mental science 167, 99–103. [DOI] [PubMed] [Google Scholar]
- Song C, Halbreich U, Han C, Leonard B, Luo H, 2009. Imbalance between pro-and anti-inflammatory cytokines, and between Th1 and Th2 cytokines in depressed patients: the effect of electroacupuncture or fluoxetine treatment. Pharmacopsychiatry 42, 182–188. [DOI] [PubMed] [Google Scholar]
- Stromnes IM, Cerretti LM, Liggitt D, Harris RA, Goverman JM, 2008. Differential regulation of central nervous system autoimmunity by T(H)1 and T(H)17 cells. Nat Med 14, 337–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swardfager W, Rosenblat JD, Benlamri M, McIntyre RS, 2016. Mapping inflammation onto mood: Inflammatory mediators of anhedonia. Neuroscience and biobehavioral reviews 64, 148–166. [DOI] [PubMed] [Google Scholar]
- Tayefi M, Shafiee M, Kazemi-Bajestani SMR, Esmaeili H, Darroudi S, Khakpouri S, Mohammadi M, Ghaneifar Z, Azarpajouh MR, Moohebati M, 2017. Depression and anxiety both associate with serum level of hs-CRP: A gender-stratified analysis in a population-based study. Psychoneuroendocrinology 81, 63–69. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Rush AJ, Ibrahim HM, Carmody TJ, Biggs MM, Suppes T, Crismon ML, Shores-Wilson K, Toprac MG, Dennehy EB, Witte B, Kashner TM, 2004. The Inventory of Depressive Symptomatology, Clinician Rating (IDS-C) and Self-Report (IDS-SR), and the Quick Inventory of Depressive Symptomatology, Clinician Rating (QIDS-C) and Self-Report (QIDS-SR) in public sector patients with mood disorders: a psychometric evaluation. Psychological medicine 34, 73–82. [DOI] [PubMed] [Google Scholar]
- van Eijk LT, Dorresteijn MJ, Smits P, van der Hoeven JG, Netea MG, Pickkers P, 2007. Gender differences in the innate immune response and vascular reactivity following the administration of endotoxin to human volunteers*. Critical Care Medicine 35, 1464–1469. [DOI] [PubMed] [Google Scholar]
- Vetter ML, Wadden TA, Vinnard C, Moore RH, Khan Z, Volger S, Sarwer DB, Faulconbridge LF, 2013. Gender differences in the relationship between symptoms of depression and high-sensitivity CRP. International journal of obesity (2005) 37 Suppl 1, S38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.