Abstract
Introduction:
There is no widely accepted testing approach for hepatitis C virus infection in correctional settings, and many U.S. prisons do not provide routine testing. The aim of this study was to determine the most effective hepatitis C virus testing strategy in one U.S. state prison and describe the population with reactive testing.
Methods:
A retrospective analysis was performed using individuals entering the Washington State prison system, which routinely offers hepatitis C virus testing, to compare routine opt-out to current recommendations for risk-based and one-time testing for individuals born between 1945 and 1965. Additionally, liver fibrosis stage was characterized using aspartate aminotransferase to platelet ratio index and Fibrosis-4. Analyses were conducted in 2017.
Results:
Between 2012 and 2016, a total of 24,567 (83%) individuals were tested for the hepatitis C virus antibody and 4,921 (20%) were reactive (test was positive). There were 2,403 (49%) that had hepatitis C virus RNA testing with 1,727 (72%) showing chronic infection. Reactive antibody was more prevalent in individuals born between 1945 and 1965 compared with other years (44% vs 17%); however, most cases (72%) were outside of this cohort. Up to 35% of positive reactive tests would be missed with testing targeted by birth cohort and risk behavior. Of chronically infected individuals, 23% had at least moderate liver fibrosis.
Conclusions:
Targeted testing in the Washington State prison system missed a substantial proportion of hepatitis C virus cases; of those with reactive testing, a sizeable proportion of people had at least moderate liver disease placing them at risk for complications. Routine testing at entry should be considered by U.S. state prisons.
INTRODUCTION
Hepatitis C virus (HCV) infection is the most common blood-borne infection in the U.S.1 HCV seroprevalence in correctional settings is estimated to be 13-fold higher than in the general population, and individuals in the criminal justice system account for approximately 30% of the total U.S. HCV burden.2,3 Among prisons with routine testing HCV seroprevalence has been reported to be as high as 41%.3,4 Despite such high seroprevalence there is no widely accepted approach for HCV testing in U.S. correctional settings. Facilities offering testing often follow a risk-based approach and surveys have shown that approximately 40% of state prison facilities routinely test for HCV.5–8
One of the current barriers to expanding HCV testing in correctional settings is the cost of direct acting antivirals (DAAs). DAAs are very effective and have a cure rate greater than 95%, but they are expensive.5,9 A Rhode Island study estimated that treating all patients with chronic HCV in Rhode Island prisons would require twice the overall healthcare budget.10 Identifying and treating HCV in correctional settings, however, could likely play an important role in the national strategy to eliminate HCV transmission.11 Since first approved in 2013, the price of HCV DAAs has significantly decreased.12 Even without treatment, receiving a diagnosis of HCV might lead to behavior modifications that reduce transmission, but the data are mixed.13 At this time, however, little is known about the yield of different HCV testing strategies in U.S. state prisons and how many infected individuals would be missed using a risk-targeted approach. In addition, the distribution of liver fibrosis and thus the severity of liver disease within prisons have not been well described. Understanding the clinical epidemiology of HCV in prisons is essential to making informed clinical and policy decisions.
The Washington State Department of Corrections (WADOC) routinely offers HCV testing to all individuals at prison entry. The WADOC data are used to compare routine opt-out to targeted testing of individuals born from 1945 to 1965 and with a history of reported drug use. The demographic and clinical characteristics of the individuals with reactive HCV testing provided an estimate of the HCV burden.
METHODS
A de-identified data set of individuals who entered WADOC between 2012 and 2016 was used to compare the yield of two HCV testing strategies: (1) current Centers for Disease Control and Prevention guidance for routine one-time testing of all individuals born between 1945 and 1965,14 as well as targeted testing for individuals who have a history of any drug use (risk-based testing); and (2) routine opt-out testing for all incarcerated individuals without effort to identify those at high-risk (routine testing) as recommended by the U.S. Preventive Services Task Force. The proportion of HCV cases that would have been identified by each strategy was compared and used to describe the demographic and clinical characteristics of those identified with reactive HCV testing. The Boston University Medical Center IRB and WADOC Research Review Committee approved the study.
Study Population
The WADOC includes 12 facilities housing almost 16,000 inmates; ≅8,000 individuals enter the system yearly.15 Approximately half of yearly entries to the system are re-admissions. The average length of stay is ≅15 months for women and ≅25 months for men. In 2010, the WADOC implemented routine opt-out laboratory-based HCV testing along with HIV, hepatitis B virus, and syphilis testing at medical intakes usually occurring within 14 days of prison entry.
Inclusion criteria were as follows: (1) prison entry between January 1, 2012, and July 7, 2016; and (2) ≥14 days spent in prison.
Measures
The yield of risk-based and routine HCV testing was characterized in terms of both the proportion of seropositive tests among individuals tested, and the counts of cases identified and missed by risk-based testing. The demographic characteristics of those with and without reactive HCV testing were compared. Liver fibrosis stage of individuals identified with chronic HCV infection, defined as having a detectable HCV RNA, was described with three approaches to estimate fibrosis stage: (1) the aspartate aminotransferase (AST) to platelet ratio (APRI) using the most sensitive threshold for advanced liver disease, (2) APRI using a more specific threshold for advanced liver disease currently in place at the WADOC, and (3) the Fibrosis-4 (FIB-4) liver fibrosis index.16,17 The distribution of fibrosis stages was described using each of the three estimation techniques, and the burden of moderate to advanced liver fibrosis was compared using each approach. Finally, liver fibrosis stage was evaluated according to ethnic/racial identity and gender.
A de-identified data set compiling information from the WADOC was obtained. All results had previously been uploaded by the contracted laboratory into WADOC’s centralized computer database. Data elements provided for this analysis included demographic information, HCV-related laboratory values, reason for incarceration, sentence duration, and reported history of any drug use. Drug use history was defined by self-report or sentencing for a drug-related offense. HCV-related laboratory information included HCV antibody and RNA tests, liver function tests, and complete blood count including platelet counts.
HCV antibody status was stratified by history of drug use. The following formula was used to calculate APRI scores: ([(AST units per L/AST upper limit of normal international units per L)]/ [platelets 109/L]) × 100. For the most sensitive APRI thresholds, scores <0.7 were classified as normal or mild liver fibrosis (F0–F1); APRI scores between 0.7 and 1.0 corresponded to moderate to severe liver fibrosis (F2–F3); and APRI scores >1.0 were categorized as cirrhosis (F4) or the most advanced liver scarring. An APRI score >1.0 has a sensitivity and specificity of 76% and 72%, respectively, for cirrhosis.17 For APRI calculations with more specific cut offs, <0.5 was used for F0–F1, 0.5–1.5 for F2–F3, and >1.5 for F4. An APRI score >1.5 has a specificity of 95% for liver fibrosis.18 FIB-4 is also a non-invasive measurement of hepatic fibrosis calculated using the following formula: (age in years × AST level units per L)/(platelets 109/L × alanine aminotransferase units per L) ½.16 Less than 1.45 was used for F0–F1, 1.45–3.25 for F2–F3, and >3.25 for F4. A threshold value of <1.45 has a sensitivity of 74% and specificity of 80% for excluding advanced liver fibrosis, whereas a threshold value of >3.25 has a specificity of 98% in confirming advanced disease.
Statistical analysis
First, descriptive statistics were used to compare the HCV seropositivity proportion among individuals tested. The cohort was stratified by age and gender, and means and SDs were used to determine 95% CIs. Chi-square tests were used to compare categories and t-tests for means. The proportion of HCV-infected individuals who would have been missed with targeted testing was determined. In this scenario, only those born 1945–1965 and individuals with a history of drug use were included in the tested group. In all analyses, only the most recent testing for individuals with multiple entries into prison over the time period of interest was included. For individuals with chronic HCV infection and complete laboratory information, liver fibrosis staging was estimated using APRI and FIB-4 scores, and fibrosis staging was compared between different groups. Statistical analysis was performed in 2017 using SAS, version 9.4 and R Core Team, version R 3.4.3 (www.R-project.org/.).
RESULTS
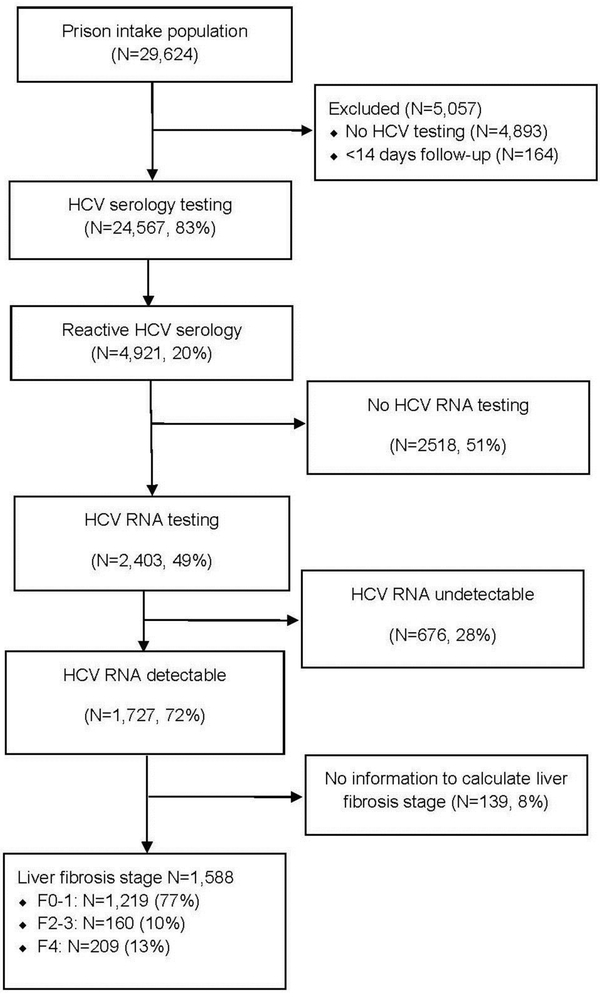
There were 29,624 individuals either newly admitted or re-admitted to the prison within 4 years during the time period evaluated. Individuals (n=24,567, 83%) were tested for HCV at prison entry (Figure 1). Individuals who were not tested were more likely to be younger (mean age 25 vs 36 years, p <0.001) and without a history of identified drug use (35% vs 51%, p <0.001). The cohort for analysis was predominantly male (86%). Sixty-three percent were white, 15% black/African American, 13% Hispanic/Latino, 5% American Indian/Alaska Native, 3% Asian/Pacific Islander, and 1% other (Table 1). Of those who met inclusion criteria, 4,921 (20%) had reactive HCV antibody testing. HCV seropositive individuals were older (mean age 41 vs 35 years, p <0.001) and had a higher proportion of identified drug use (51% vs 38%, p <0.001; Table 1), and a shorter median sentence duration than those without HCV (12.57 months vs 12.77 months, p <0.007). Of those with reactive HCV serology, 2,403 (49%) had confirmatory HCV RNA performed, and of those 1,727 (72%) had chronic HCV infection (Figure 1).
Figure 1.
Study flow diagram.
HCV, hepatitis C virus.
Table 1.
Baseline Characteristics of Entrants to Washington State Department of Corrections Prisons Between 2012–2016
| Characteristics | Reactive HCV antibody |
Non-reactive HCV antibody |
p-value |
|---|---|---|---|
| Full cohort with HCV testing (N=24,567) | |||
| Overall | 4,921 (20) | 19,646 (80) | |
| N | 4,921 | 19,646 | |
| Mean age (SD) | 41 (11) | 35 (10) | <0.001 |
| Men (%) | 4,006 (81) | 17,109 (87) | <0.001 |
| History of drug useb | 2,523 (51) | 7,502 (38) | <0.001 |
| Race/Ethnicity | 0.005 | ||
| White | 3,775 (77) | 11,766 (60) | |
| Black/African American | 384 (8) | 3,231 (16) | |
| Hispanic/Latino | 369 (7) | 2,938 (15) | |
| American Indian/Alaska Native | 327 (7) | 849 (4) | |
| Asian/Pacific Islander | 51 (1) | 679 (3) | |
| Other | 16 | 194 (1) | |
| Individuals born between 1945–1965 with HCV testing (N=3,084) | |||
| Overall | 1367 (44) | 1717 (56) | |
| Mean age (SD) | 55 (4 ) | 55 (5 ) | 0.88 |
| N | 1,367 | 1,717 | |
| Male | 1,199 (88) | 1,487 (87) | 0.13 |
| History of drug use | 652 (48) | 543 (31) | 0.22 |
| Individuals born after 1945–1965 with HCV testing (N=21,483) | |||
| Overall | 3,554 (17) | 17,929 (83) | |
| Mean age (SD) | 37 (8) | 32 (8) | 0.017 |
| N | 3,554 | 17,929 | |
| Male | 2,807 (79) | 15,622 (87) | 0.39 |
| History of drug use | 1,878 (53) | 6,961 (39) | <0.001 |
| Men with HCV testing (N=21,115) | |||
| Overall | 4,006 (19) | 17,109 (81) | |
| Mean age (SD) | 42 (11) | 35 (10) | <0.001 |
| N | 4,006 | 17,109 | |
| History of drug use | 1,964 (49) | 6,286 (37) | <0.001 |
| Women with HCV testing (N=3,452) | |||
| Overall | 915 (27) | 2,537 (73) | |
| Mean age (SD) | 39 (10) | 35 (10) | <0.001 |
| N | 915 | 2,537 | |
| History of drug use | 566 (62) | 1,216 (48) | <0.001 |
Notes: Boldface indicates statistical significance (p<0.05). Proportions were rounded.
Drug use determined by self-report or drug-related offense.
HCV, hepatitis C virus.
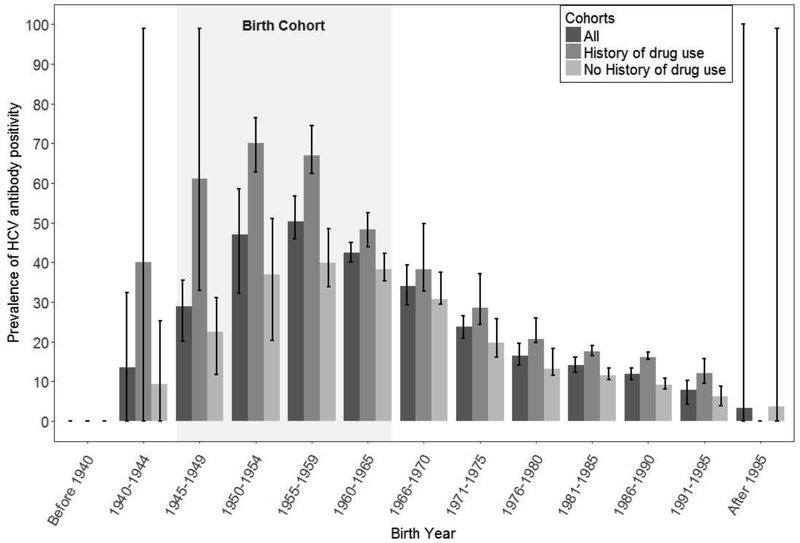
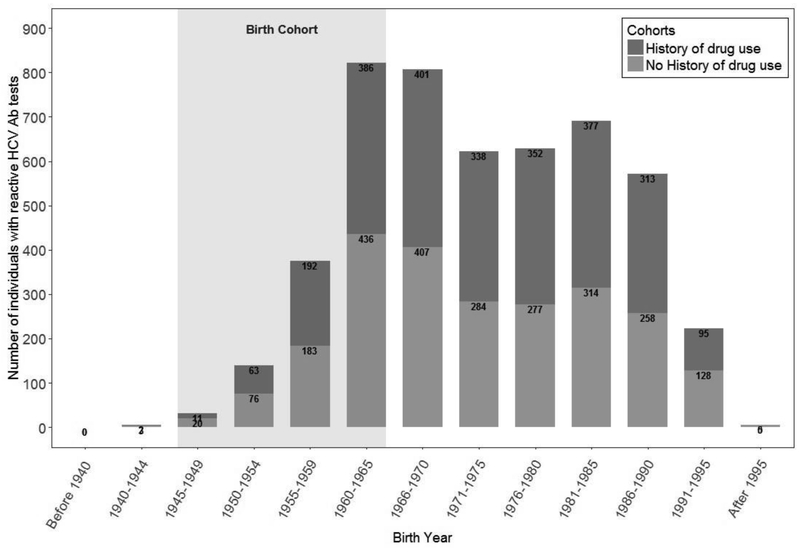
The prevalence of HCV seropositivity was highest among individuals born between 1945 and 1965 (44%), and individuals with a history of identified drug use born after 1965 (53%; Table 1 and Figure 2). In addition, individuals without a history of drug use made up almost half of the identified cases during the time period of this study (Figure 3). Overall, combined risk-based and birth cohort testing would have missed 35% of total HCV seropositive cases identified. Five individuals needed to be tested to identify an HCV case using routine testing, whereas among those born between 1945 and 1965 only three individuals needed to be tested. Among individuals with an identified history of drug use, four needed to be tested.
Figure 2.
Proportion of hepatitis C virus (HCV) antibody seropositivity among entrants to Washington State Department of Corrections (2012–2016) by birth cohort and history of drug use.
Notes: Proportion of hepatitis C virus (HCV) antibody seropositivity among entrants to Washington State Department of Corrections (2012–2016) by birth cohort and history of drug use. This figure shows that the prevalence of HCV was highest among individuals in the birth cohort born between 1945–1965. It also shows that many individuals with reactive HCV antibody did not have an identified history of drug use. The cohort was stratified by age and history of drug use. Each birth cohort included 5 years, and the mean and variance of seropositivity prevalence within each interval was calculated. Means and SDs were used to determine 95% CIs. Each error bar is constructed using a 95% CI of the mean.
Figure 3.
Number of hepatitis C virus (HCV) reactive individuals among entrants to Washington State Department of Corrections Prisons (2012–2016) by birth cohort and history of drug use.
Notes: Number of hepatitis C virus (HCV) reactive individuals among entrants to Washington State Department of Corrections Prisons (2012–2016) by birth cohort and history of drug use. This figure shows that most of the cases with reactive HCV testing were outside of the birth cohort born between 1945–1965. It also shows that many individuals with reactive testing did not have an identified history of drug use.
Although the cohort was mainly composed of individuals who self-identified as white, the highest proportion of HCV antibody positivity (70%) was noted among black/African Americans with birth years between 1950 and 1954 (Appendix Figure 1). This was more than three times the seropositivity proportion of 20% for the overall population. In addition, American Indians/Alaskan Natives made up only 5% of the cohort, but this population had an HCV proportion as high as 60% in the birth cohort born between 1955 and 1959. Furthermore, across most birth cohorts examined American Indians/Alaskan Natives had the highest proportion of HCV seropositivity.
Among individuals with chronic HCV, 1,588 (92%) had the information necessary to calculate an APRI or FIB-4 to estimate liver fibrosis stage. Using FIB-4 or an APRI threshold of 0.7 resulted in similar percentages of people categorized as having at least moderate liver disease (stage F2 or greater, 22% vs 23%, respectively). By contrast, using 0.5 as the lower APRI threshold led to 40% being classified as having stage F2 or greater (Appendix Table 1). FIB-4 and APRI upper threshold of 1.5 led to a smaller proportion of individuals categorized as having advanced liver disease compared with an APRI threshold of 1.0 (4% and 6% vs 13%, respectively), but also led to a larger number who would be considered to have moderate fibrosis that would require additional work-up for disease staging (Appendix Table 1). In addition, among the 1,130 individuals with chronic HCV born outside of the 1945–1965 birth cohort, 23% had at least F2 liver fibrosis staging using an APRI threshold of 0.7. In terms of gender differences, although the cohort was 86% men, women had a higher proportion of reactive HCV testing compared with men (27% vs 19%, p <0.001); however, fewer women than men had significant liver fibrosis (stage F2 or greater, 13% vs 25%, p <0.002) in a bivariate comparison.
DISCUSSION
Although HCV seropositivity was high among individuals born between 1945 and 1965 and among those with an identified history of drug use, a large number of cases were diagnosed in patients without identified risk. Targeted HCV testing missed the majority of cases. The number needed to test when moving from targeted to routine testing (one to three versus one to five) remains small. This is in contrast to other infectious diseases, such as HIV, that require testing a large number of incarcerated individuals to identify a single case.19
From a public health perspective, the data presented here adds to existing evidence supporting the implementation of routine testing of all individuals when entering prison. If coupled with HCV treatment, routine testing would identify and cure many cases, and prevent a substantial burden of future liver disease. Simulation models have demonstrated that curing HCV in correctional settings could also reduce the incidence of HCV transmission in the community when individuals are released.20 Efforts to target HCV testing in this setting would miss many cases and diminish the public health impact of screening this population. Nevertheless, implementing routine screening at prison intake necessitates incorporating HCV testing as part of the workflow and establishing laboratory protocols to efficiently provide test results. These steps are associated with some cost to the prison system including follow-up of positive results.
It is notable that 51% of individuals with reactive HCV antibody testing did not have RNA testing. Although there are current efforts to confirm chronic HCV infection in prisons, prior to the advent of DAAs, HCV RNA was tested mainly in individuals being considered for treatment. Approximately one fifth of individuals with chronic HCV had at least moderate liver fibrosis placing them at risk for complications; however, the proportion labeled as having early or advanced liver disease differs depending on the interpretation of threshold values. Given the typical long interval between HCV infection and the time at which HCV-infected individuals begin experiencing complications, many of the benefits of HCV treatment in prisons would accrue in the community. Payers, such as Medicare and Medicaid, would avoid future spending related to liver complications after prison release. Correctional systems would bear the brunt of the costs because routine HCV testing in prisons could generate a large obligation for correctional systems to pay for treatment of newly diagnosed individuals.2 Currently, even with price reductions over time, HCV treatment remains expensive and given the high prevalence of HCV in prisons, it could threaten to consume the majority of the available pharmaceutical budget.10 Routine testing at prison intake with treatment of all infected individuals requires a substantial commitment of additional resources to correctional health systems.
This study provides new information about the epidemiology of HCV infection in correctional settings. For example, American Indians/Alaska Natives are disproportionally impacted by HCV.21 Although this group made up only 5% of the cohort, it was the ethnic/racial group with the highest proportion of reactive HCV across almost all birth cohorts evaluated (60%). There is little previous information on HCV among American Indians/Alaska Natives and this study provides valuable information on a group that has been understudied. Seventy percent of black/African Americans born in the birth cohort between 1950 and 1954 had reactive HCV serology.
In terms of gender differences, there was a higher proportion of reactive HCV testing among incarcerated women when compared with men.22 This has been attributed to the frequent history of injection drug use and commercial sex work among incarcerated women.22 Although women appear to have a higher proportion of reactive HCV testing, studies have shown that women are more likely to clear the infection.23 There was also a lower proportion of advanced liver fibrosis among women.
This analysis also sheds new light on the implications of treatment restrictions based on liver fibrosis stage and the method that correctional systems use to assess liver fibrosis. Many correctional systems rely on APRI or FIB-4 indices either as the only or as the initial step for disease staging. These non-invasive staging algorithms perform well in ruling out significant fibrosis and ruling in advanced liver disease, but results falling in the mid-range typically require additional evaluation.18 Although raising the threshold for labeling a patient as having advanced disease likely limits the treatment burden for a system, this approach also likely leads to additional spending on staging.
Limitations
Among the limitations to this study, the single state system and retrospective design could impact generalizability. It is uncertain how well these findings generalize to other U.S. prisons. Incarcerated populations vary greatly by state, given differences in state-level laws, prosecutorial priorities, and sentence structures. This study demonstrates, however, that in at least one state, routine testing for HCV in prison identifies many cases missed by targeted testing. It is likely that other prisons not routinely testing for HCV are also missing a substantial number of diagnoses. The HCV seropositivity proportion of 20% shown here is similar to other studies.4,5 The current findings are different from that of a study at the Wisconsin Department of Corrections.24 The latter found that a targeted approach using history of injection drug use and history of liver disease identified 92% of cases. Using the WADOC data set, a targeted approach including history of drugs use and elevated liver function tests would only identify 58% of cases diagnosed with routine testing. Therefore, this targeted approach would miss almost half of all HCV cases. The current study only had access to the most recent HCV testing results. This might have impacted liver disease staging at diagnosis; however, given that liver disease progression spans decades the impact of this approach was probably limited. Furthermore, reasons were not available to explain why HCV serology testing was not performed at entry for some individuals. In some cases, individuals might have already been aware of their diagnosis. HCV RNA and liver fibrosis staging were not performed in all cases. Furthermore, although the definition of identified history of drug use presented here relied on self-identification and conviction on a drug-related offense, it reflects the data by which targeted testing would be based regardless of the accuracy.
CONCLUSIONS
In one mid-sized U.S. state prison system routine opt-out testing identifies a substantial number of HCV cases that would have been missed by targeted testing. Almost a quarter of individuals with chronic HCV had significant liver fibrosis and thus a more urgent need for treatment to prevent complications. As this study determines the population-level health benefits of routine HCV testing in prisons, the implied cost burden to correctional health budgets is also uncovered. Testing and treating HCV in prisons would contribute toward the U.S. national goal of HCV elimination,11 but doing so requires explicit public investment in correctional health budgets. Even if prices fall significantly, an expansion of the workforce including support staff will be necessary in many systems to adequately address the epidemic in a timely manner. Future studies are needed on downstream outcomes after testing, on treatment for prevention during the DAA era, and on the cost associated with implementing different HCV testing approaches.
Supplementary Material
ACKNOWLEDGMENTS
The project was funded by the U.S. Centers for Disease Control and Prevention, National Center for HIV, Viral Hepatitis, STD, and TB Prevention Epidemiologic and Economic Modeling Agreement (5NU38PS004644). Support was also provided by the National Institute of Drug Abuse (K23 DA044085-01, R25 DA035163, R01DA046527, P30 DA040500, 3-R01 DA016017) and the National Institute of Allergy and Infectious Diseases (P30AI042853).
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the Centers for Disease Control and Prevention or NIH.
No financial disclosures were reported by the authors of this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144(10):705–714. 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- 2.Spaulding AS, Kim AY, Harzke AJ, et al. Impact of new therapeutics for hepatitis C virus infection in incarcerated populations. Top Antivir Med. 2013;21(1]:27–35. [PMC free article] [PubMed] [Google Scholar]
- 3.Varan AK, Mercer DW, Stein MS, Spaulding AC. Hepatitis C seroprevalence among prison inmates since 2001: still high but declining. Public Health Rep. 2014;129(2]:187–195. 10.1177/003335491412900213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Larney S, Mahowald MK, Scharff N, Flanigan TP, Beckwith CG, Zaller ND. Epidemiology of hepatitis C virus in Pennsylvania state prisons, 2004–2012: limitations of 1945–1965 birth cohort screening in correctional settings. Am J Public Health. 2014;104(6]:e69–74. 10.2105/AJPH.2014.301943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beckman AL, Bilinski A, Boyko R, et al. New hepatitis C drugs are very costly and unavailable to many state prisoners. Health Aff (Millwood). 2016;35(10]:1893–1901. 10.1377/hlthaff.2016.0296. [DOI] [PubMed] [Google Scholar]
- 6.Beckwith CG, Kurth AE, Bazerman L, et al. Survey of U.S. correctional institutions for routine HCV testing. Am J Public Health. 2015;105(1):68–71. 10.2105/AJPH.2014.302071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spaulding AC, Anderson EJ, Khan MA, Taborda-Vidarte CA, Phillips JA. HIV and HCV in U.S. prisons and jails: the correctional facility as a bellwether over time for the community’s infections. AIDS Rev. 2017;19(3]:134–147. 10.24875/AIDSRev.M17000006. [DOI] [PubMed] [Google Scholar]
- 8.Spaulding AC, Adee MG, Lawrence RT, Chhatwal J, von Oehsen W. Five questions concerning managing hepatitis C in the justice system: finding practical solutions for hepatitis C virus elimination. Infect Dis Clin North Am. 2018;32(2]:323–345. 10.1016/j.idc.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 9.Kowdley KV, Gordon SC, Reddy KR, et al. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med. 2014;370(20]:1879–1888. 10.1056/NEJMoa1402355. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen JT, Rich JD, Brockmann BW, Vohr F, Spaulding A, Montague BT. A budget impact analysis of newly available hepatitis C therapeutics and the financial burden on a state correctional system. J Urban Health. 2015;92(4]:635–649. 10.1007/s11524-015-9953-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Academies of Sciences, Engineering, and Medicine. Eliminating the public health problem of hepatitis B and C in the United States: Phase one report. Washington, DC: The National Academies Press; 2016. 10.17226/23407. [DOI] [PubMed] [Google Scholar]
- 12.Nisen M. AbbVie Wages HCV Drug-Price War on Gilead. BloombergGadfly. August 7, 2017. www.bloomberg.com/gadfly/amp/articles/2017-08-07/abbvie-mavyret-price-threatens-gilead-hepatitis-dominance?utm_source=STAT+Newsletters&utm_campaign=6155ad6ce1-EMAIL_CAMPAIGN_2017_08_07&utm_medium=email&utm_term=0_8cab1d7961-6155ad6ce1-149726369. Accessed 7 August, 2017. [Google Scholar]
- 13.Page K, Morris MD, Hahn JA, Maher L, Prins M. Injection drug use and hepatitis C virus infection in young adult injectors: using evidence to inform comprehensive prevention. Clin Infect Dis. 2013;57(suppl 2:S32–S38. 10.1093/cid/cit300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith BD, Morgan RL, Beckett GA, et al. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945–1965. MMWR Recomm Rep. 2012;61(RR-4):l–32. [PubMed] [Google Scholar]
- 15.Washington State Department of Health. Viral Hepatitis C in Washington State. www.doh.wa.gov/DataandStatisticalReports/DiseasesandChronicConditions/ChronicHepatitisSurveillance. Accessed June 13, 2017.
- 16.Vallet-Pichard A, Mallet V, Nalpas B, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology. 2007;46(1):32–36. 10.1002/hep.21669. [DOI] [PubMed] [Google Scholar]
- 17.Lin ZH, Xin YN, Dong QJ, et al. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: an updated meta-analysis. Hepotology. 2011:53[3]:726–736. 10.1002/hep.24105. [DOI] [PubMed] [Google Scholar]
- 18.Chou R, Wasson N. Blood tests to diagnose fibrosis or cirrhosis in patients with chronic hepatitis C virus infection. Ann Intern Med. 2013;159(5):372 10.7326/0003-4819-159-5-201309030-00021. [DOI] [PubMed] [Google Scholar]
- 19.Bureau of Justice Statistics. HIV in Prisons, 2015-Statistical Tables. www.bjs.gov/content/pub/pdf/hivp15st_sum.pdf. Accessed on November 8, 2017.
- 20.He T, Li K, Roberts MS, et al. Prevention of hepatitis C by screening and treatment in U.S. prisons. Ann Intern Med. 2016;164(2]:84–92. 10.7326/M15-0617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reilley B, Leston J. A tale of two epidemics - HCV treatment among Native Americans and Veterans. N Engl J Med. 2017;377(9):801–803. 10.1056/NEJMp1705991. [DOI] [PubMed] [Google Scholar]
- 22.Macalino GE, Dhawan D, Rich JD. A missed opportunity: hepatitis C screening of prisoners. Am J Public Health. 2005;95(10]:1739–1740. 10.2105/AJPH.2004.056291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grebely J, Page K, Sacks-Davis R, et al. The effects of female sex, viral genotype, and IL28B genotype on spontaneous clearance of acute hepatitis C virus infection. Hepatology. 2014;59(1]:109–120. 10.1002/hep.26639.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stockman LJ, Greer J, Holzmacher R, et al. Performance of risk-based and birth-cohort strategies for identifying hepatatis C virus infection among people entering prison, Wisconsin, 2014. Public Health Rep. 2016;131(4]:544–551. 10.1177/0033354916662212. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.