Abstract
Objective:
There are concerns regarding the potential harms in receipt of prenatal chromosome microarray (CMA) results, particularly variants of uncertain significance (VUS). We examined the influence that the return of genomic results had on parental well-being and perceptions of children’s development.
Methods:
Parents (n=138) of 83 children who underwent prenatal chromosomal microarray testing completed questionnaires assessing perception of children’s development, parent-child attachment, parental mood, parenting competence, martial satisfaction, satisfaction with the decision to undergo testing, and attitudes about genetics at age 12 and/or 36 months. Responses were compared between parents who received normal/likely benign results and VUS results.
Results:
Compared to normal/likely benign results, parents who received VUS results rated their child as less competent on the BITSEA scale at 12 (β=−1.65, p=0.04) though not 36 months (p=0.43). There were no differences in parent mood, marital satisfaction, or parenting competence. At 36 months, parents in the VUS group reported less satisfaction with their decision to undergo genetic testing (β=1.51, p=0.02).
Conclusion:
CMA VUS results have limited impact on parental well-being and perception of children’s development. However, the initial diminished perception of child competency and later dissatisfaction with genomic testing indicate the need to assist parents in coping with ambiguous results.
INTRODUCTION
Molecular cytogenetics has advanced over the last decade and allowed for the detection of copy-number variants (CNVs) that were previously unrecognized by conventional cytogenetics. In a previous study, the clinical utility of prenatal chromosome microarray (CMA) testing was evaluated in 4400 individuals undergoing chronic villus sampling (CVS) and amniocentesis for fetal karyotype. In comparison to traditional fetal karyotype, CMA detected additional clinically significant CNVs in 1.7% of the cases [1–3], which led the American College of Obstetricians & Gynecologists (ACOG) to recommend that CMA be made available to every woman undergoing prenatal diagnostic testing [4]. During the first ten years of clinical postnatal use, polymorphic and benign CNVs have been identified and differentiated from pathologic CNVs associated with congenital anomalies [5–7], neurological, and psychiatric diseases [8–16]. Many CNVs were initially classified as variants of uncertain significance (VUS) until large sets of population-based CNV data were available. The delineation of the spectrum of manifestations, frequency and severity of symptoms is still incomplete for many rare CNVs, especially regarding manifestations in adults.
The introduction of CMA into prenatal care aims to increase reproductive autonomy by providing clinically relevant genomic information that can enhance parents’ decision-making capacity by increasing diagnostic yield and allowing for targeted surveillance of associated clinical features to intervene early and improve outcomes [8]. However, there are ethical concerns regarding potential harms caused to parents and their infants in receiving ambiguous VUS prenatal results. Although the frequency of CMA VUS will decrease over time, the overall prevalence of VUS CNVs is currently approximately 1% [17–20]. Furthermore, as the scope of prenatal genomic testing increases and expands to exome/genome sequencing, couples may have the option to receive more genetic information and a significant fraction could be of uncertain significance, especially for patients of non-European ancestry.
Prospective parents receiving VUS results often do not anticipate the psychological and decision-demanding difficulties associated with uncertainty [18]. Studies have described that upon receiving VUS results, parents report feeling a lack of support [21], a lack of preparedness [22], and frustration over the uncertainty and limits in medical knowledge related to VUS [23]. Of particular concern is the potential negative impact on parental psychological well-being and stress [24, 25] that can lead to mood changes, anxiety, and parenting associated distress [26–33]. Emerging research suggests that VUS results are associated with parents anxiously monitoring their infants, often enrolling them in early intervention programs or ongoing medical assessments [33]. The VUS results may modify parents’ perceptions of their child based on the anticipation of a ‘damaged’ child and could alter the parent-child relationship, potentially contributing to altered behavioral development. While research has been conducted on parental perspectives of the prenatal CMA processes and how receipt of CMA results affects parental stress and anxiety (specifically related to child development), little research has investigated the association between the individual genomic results and parental perceptions of children’s behavior, social interaction and health beyond early infancy.
We sought to determine if the receipt of prenatal CMA results, particularly VUS results, alters parents’ perception of their child and/or modifies parental behavior and mood. We surveyed parents from the landmark Wapner et al. prenatal chromosome microarray study [1] when their infants were 12 months and/or 36 months of age and assessed 1) parental perceptions of the infant’s health, behavior, and neurocognitive development; 2) parental perceptions of their parenting competence, the parent-child relationship, and their own well-being; and 3) parental understanding of genetics, tolerance of ambiguity and belief in genetic essentialism—the supremacy of genetics in determining children’s developmental outcomes. We aimed to analyze the data according to the pathogenicity of the CNV result (normal/likely benign or VUS).
METHODS
Participants
Participants with pathogenic/likely pathogenic (mothers and/or fathers of 27 children) or VUS (mothers and/or fathers of 63 children) prenatal results in the Wapner et al. prenatal chromosome microarray study were invited to participate in this ancillary study at the 12-month follow up session of the main CMA study [1]. Parents were enrolled upon mail return of their signed consent, and the study was approved by the Columbia University Institutional Review Board. In addition, patients with contemporaneous pregnancies with the same distribution of indications for chromosome microarray testing as in the Wapner study and normal/likely benign (normal/LB) prenatal CMA results were recruited through the Columbia University Division of Maternal Fetal Medicine and the Center for Prenatal Pediatrics and were invited to participate by their obstetricians and/or genetic counselors at 12 months postpartum. No variants were reclassified during the study, and none of the participants received mixed results.
Study Overview
Mothers and/or fathers completed questionnaires online at 12 months postpartum, 36 months postpartum, or at both time points (Table 1). Twenty children had parent reports from both the mother and father at 12 months and 36 months. Seventeen children had both parent reports at 36 months only. Eight children had both parent reports at 12 months only. The remaining children had either a mother or a father report at 12 months, 36 months, or both.
Table 1:
Total parent reports per child by time point
| Parent Reports per Child | n |
|---|---|
| 12 month only: Mother | 11 |
| 12 month only: Father | 2 |
| 12 month only: Mother + Father | 10 |
| 36 month only: Mother | 11 |
| 36 month only: Father | 1 |
| 36 month only: Mother + Father | 23 |
| 12 month: Mother 36 month: Mother + Father |
2 |
| 12 month: Mother + Father 36 month: Father |
2 |
| 12 month: Mother + Father 36 month: Mother |
6 |
| 12 month and 36 month: Mother + Father | 21 |
| 12 month and 36 month: Mother | 5 |
Participants reported on their age, relationship status, race, ethnicity, and education level at the time of their first survey completion. The following measures were assessed at 12 months and 36 months: Maternal Postnatal Attachment Questionnaire (MPAS), Brief Infant-Toddler Social and Emotional Assessment (BITSEA), the Vulnerable Child Scale (VCS), Profile of Mood States (POMS), Parent Sense of Competence Scale (PSOC), Golombok Rust Inventory of Marital State (GRIMS), Disclosure of Results to Others, Secrecy about Results, Decision Satisfaction Scale, Revised Scale of Ambiguity Tolerance, genetic essentialism, general understanding of genetics and accuracy of understanding of results (both developed for this study). See Table 2 for detailed description of each study instrument used.
Table 2:
Description of Instruments
| Instrument | Description | Reference |
|---|---|---|
| Maternal Postnatal Attachment Scale (MPAS) | A 19-item self-report questionnaire that measures feelings of attachment towards a child. Questions are scored on a 3, 4, or 5-point scale, with a higher score indicating a greater sense of attachment. In additional to a total score, there are subsets of Quality of Attachment, Absence of Hostility, and Pleasure of Interaction. The MPAS has good internal consistency and reliability. | Condon, J. T. and C. J. Corkindale (1998). “The assessment of parent-to-infant attachment: Development of a self-report questionnaire instrument.” Journal of Reproductive and Infant Psychology 16(1): 57–76. |
| Brief Infant-Toddler Social and Emotional Assessment (BITSEA) | A 42-item parent-report measure comprising two scales: 11 items assess socio-emotional competence and 31 items assess problems. Parents rate each item on a 3-point scale (0 = not true/rarely, 1 = somewhat true/sometimes, 2 = very true/always). The range of scores is 0–33 for competence and 33–93 for problems. The BITSEA has demonstrated good construct validity and clinical validity in discriminating children with clinically significant problems from matched control children | Carter, A. S., et al. (2004). “Assessment of young children’s social‐emotional development and psychopathology: recent advances and recommendations for practice.” Journal of Child Psychology and Psychiatry 45(1): 109–134. Briggs-gowan, M. J., et al. (2002). “Brief Infant-Toddler Social and Emotional Assessment (BITSEA) manual, version 2.0.” |
| Vulnerable Child Scale (VCS) | A 16-item parent-report measure of parental perceptions of child vulnerability. Scored of on a four-point scale ranging from 1 to 4 (definitely false, mostly false, mostly true and definitely true). Two items are scored in the reverse direction. Total scores range from 16 to 64, with lower scores reflecting higher perceived vulnerability. The VCS has good validity and internal reliability, with an alpha reported to be .75. | Forsyth, B. W., et al. (1996). “The child vulnerability scale: an instrument to measure parental perceptions of child vulnerability.” J Pediatr Psychol 21(1): 89–101. |
| Profile of Mood States (POMS) | A 65-item self-report measure of six different affective states: Anger/Hostility, Tension/Anxiety, Depression/Dejection, Vigor/Activity, Fatigue/Inertia, and Confusion/Bewilderment. Each item is rated on a scale of 0 (not at all) to 4 (extremely). Scores range from 0 to 260, with higher scores indicating a greater level of distress. The depression subscale consists of 15 items, with a range of scores from 0–60. Internal consistency is reported at 0.63 to 0.96 Cronbach alpha rating. | McNair, D. M. (1971). Manual profile of mood states. Educational & Industrial testing service. |
| Parenting Sense of Competence Scale | A 16-item self-report measure of parental competence in Satisfaction and Efficacy. The satisfaction section examines the parents’ anxiety, motivation and frustration, while the Efficacy section assesses the parents’ competence, capability levels, and problem-solving abilities in their parental role. A higher score indicates a greater sense of parenting competence. | Gibaud-Wallston, J., & Wandersman, L. P. (1978). Parenting sense of competence scale. Lawrence Erlbaum Associates. Chicago |
| Golombok Rust Inventory of Marital States (GRIMS) | A 28-item self-report measure assessing the quality of a relationship between a man and woman who are married or living together. Each item is scored on a four-point Likert scale ranging from 0 to 3 (strongly disagree, disagree, agree, strongly agree). A higher score indicates a more problematic relationship. The GRIMS has good validity and reliability (.90 for women and .92 for men). | Rust, J., et al. (1986). “The golombok rust inventory of marital state (GRIMS).” Sexual and Marital Therapy 1(1): 55–60. |
| Disclosure of Results to Others | A 5-item self-report measure determining to whom, if anyone, an individual disclosed their health results. | Ashida, S., et al. (2009). “Disclosing the disclosure: Factors associated with communicating the results of genetic susceptibility testing for Alzheimer’s disease.” Journal of health communication 14(8): 768–784. |
| Secrecy About Results | A 5-item self-report measure of an individual’s attitude towards disclosing a health diagnosis. Each question is rated on a 6-point Likert scale from 1 (strongly agree) to 6 (strongly disagree). A higher score corresponds to an endorsement of secrecy. The SAR has good validity, with an alpha of .71. | Link, B. G., et al. (1989). “A modified labeling theory approach to mental disorders: An empirical assessment.” American sociological review: 400–423. |
| Decision Satisfaction Scale | A 10-item self-report measure assessing levels of satisfaction about a decision. Each item is answered on a 5-point Likert scale from 1 (strongly disagree) to 5 (strongly agree). A higher score indicates a more positive attitude towards the decision. | *Sainfort F, Booske BC. Measuring postdecision satisfaction. Med Decis Making 2000; 20:51061. AND O’Connor AM. Validation of a decisional conflict scale. Med Decis Making 1995; 15:25–30. |
| Revised Scale for Ambiguity Tolerance (RSAT) | A 20-item self-report measure for the capacity for tolerating ambiguity. Each item is answered with True or False. The questionnaire is scored for high ambiguity tolerance. The RSAT has excellent reliability (.86) and validity. | Mac Donald Jr, A. P. (1970). “Revised scale for ambiguity tolerance: Reliability and validity.” Psychological reports 26(3): 791–798. |
| Genetic Essentialism | A 6-item self-report questionnaire measuring participant’s thoughts about his/her genetic makeup. Each question is rated on a four-point Likert scale (1=strongly disagree; 4=strongly agree). Cronbach’s alpha was 0.76 among N=70 people with epilepsy in a baseline survey. | Klitzman, R., Appelbaum, P. S., Fyer, A., Martinez, J., Buquez, B., Wynn, J., … & Chung, W. K. (2013). Researchers’ views on return of incidental genomic research results: qualitative and quantitative findings. Genetics in Medicine, 15(11), 888–895. AND Phelan, J. C. (2005). Geneticization of Deviant Behavior and Consequences for Stigma: The Case of Mental Illness∗. Journal of Health and Social Behavior, 46(4), 307–322. |
| Genetic Knowledge | Created for purpose of this study. |
Statistical Analysis
Unadjusted between-group comparisons (VUS versus normal/LB) were performed using Wilcoxon’s rank-sum test, Kruskal-Wallis and Fisher’s exact test, as appropriate, to compare demographic characteristics for the participants who completed only the infant behavior questionnaires at one time-point vs those who completed both 12- and 36-month infant behavior questionnaires.
For each outcome, mixed effect regression models separately compared group differences in the parental responses. To account for the potential correlation of a mother and father reporting on the same child, a random intercept was included in the models. In all models, parent gender, race/ethnicity (non-white vs. white), education (bachelor’s or lower vs. master’s or higher), employment status (full time vs. other) and child sex were adjusted as covariates. Group difference in the parental response change over time were compared using mixed effect regression. Each regression included time (12- vs. 36- months), group, and time-by-group interaction as fixed effects. To account for intra-subject and intra-family correlation, nested random effects that allow for varying intercepts for each subject within a family were included in the models. For the significant outcomes, we repeated the same model in the subset of the participants who completed both 12- and 36-month infant behavior questionnaires to ensure the results were not driven by participants with missing values at either time point.
Three parent trait variables (genetic essentialism, genetic knowledge, and tolerance of ambiguity) were examined as moderators in relation to the 17 parent responses; genetic essentialism was significantly associated with 6 and 13 outcomes at 12- and 36-months, respectively, and considered as an independent variable as well as in interactions with group in models.
RESULTS
A total of 158 parents of 94 unique children agreed to participate in this study. Table 3 shows enrollment data of children by diagnostic group and sex. Of those who did not agree to participate in this ancillary study, a greater relative proportion received pathogenic prenatal test results. The clinical indication for undergoing the CMA was known from the Wapner study enrollment. As shown in Table 4, in 55% with pathogenic results, prenatal microarray testing was conducted due to an ultrasound anomaly. In those who received VUS and normal/LB results, the most common reason for prenatal microarray testing was advanced maternal age (42% and 50%, respectively) (Table 4). Because parents of only 11 children with pathogenic results agreed to participate in this study, the pathogenic group was not included in analyses. This reduced the number of parents by 20 so that analyses were based on the 138 parents of 83 unique children from the VUS and normal/LB groups.
Table 3:
Enrollment of Child by Group
| Group | Not Enrolled (%) | Enrolled (%) | N |
|---|---|---|---|
| Pathogenic | 16 (59.3%) | 11 (40.7%) | 27 |
| VUS | 28 (44.4%) | 35 (55.6%) | 63 |
| Normal/Likely benign | 21 (30.4%) | 48 (69.6%) | 69 |
| Total | 65 (40.9%) | 94 (59.1%) | 159 |
Table 4:
Child sex and medical history by group
| Pathogenic | 11/94 (12%) | VUS | 35/94 (37%) | Normal/Likely Benign | 48/94 (51%) |
|---|---|---|---|---|---|
| Child Sex | Child Sex | Child Sex | |||
| Female | 4 (36%) | Female | 16 (46%) | Female | 26 (54%) |
| Male | 7 (64%) | Male | 19 (54%) | Male | 22 (46%) |
| Reason for Microarray Test | Reason for Microarray Test | Reason for Microarray Test | |||
| Ultrasound Anomaly | 6 (55%) | Ultrasound Anomaly | 7 (20%) | Ultrasound Anomaly | 9 (18%) |
| Family History | 1 (9%) | Family History | 3 (9%) | Family History | 3 (6%) |
| Maternal Age | 3 (27%) | Maternal Age | 15 (42%) | Maternal Age | 24 (50%) |
| Positive Serum Screen | 0 (0%) | Positive Serum Screen | 7 (20%) | Positive Serum Screen | 6 (13%) |
| Other | 1 (9%) | Other | 3 (9%) | Other | 6 (13%) |
As represented in Table 5, the majority of participants were married, white/Caucasian, and not Hispanic/Latino. The average age was 38.1 years. There were no CNV-return result group differences in demographic variables (e.g., age, marital status, race, ethnicity, education level, offspring sex) (all ps >0.16).
Table 5:
Demographics of parent participants
| Mean (SD) or % | N | |
|---|---|---|
| Age (years) * | 38.1(5.5) | 138 |
| Gender | ||
| Female | 62% | 80 |
| Male | 42% | 58 |
| Relationship Status | ||
| Married or living as married | 96% | 133 |
| Married, not living together | 1% | 1 |
| Never married | 3% | 4 |
| Race | ||
| American Indian | 1% | 1 |
| Asian/Pacific Islander | 6% | 8 |
| Biracial | 3% | 4 |
| Black/African American | 4% | 6 |
| White/Caucasian | 80% | 111 |
| Other | 6% | 8 |
| Ethnicity | ||
| Hispanic/Latino | 12% | 17 |
| Not Hispanic/Latino | 88% | 121 |
| Education Level | ||
| Bachelors | 28% | 38 |
| Doctoral | 19% | 26 |
| High School Graduate/GED | 1% | 3 |
| Master’s Degree | 36% | 49 |
| Other | 16% | 22 |
at time of first survey completion
Distribution of parent and child outcomes
Table 6 shows average values for parent report of child behavior, parenting competence, attachment, perceived child vulnerability, and parent mood at 12 and 36 months. On average, parents rated their children as competent and without many problems and had minimal levels of distress (See Table 2 for details on assessments used).
Table 6:
Means of parent-report outcome variables at 12 and 36 months
| 12M | 36M | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Maximum | Maximum | ||||||||
| Bitsea: Problem Scale | 91 | 8.3 | 4.5 | 2 | 26 | 101 | 8.9 | 4.1 | 3 | 26 |
| Bitsea: Competency Scale | 91 | 12.0 | 3.2 | 4 | 18.8 | 101 | 15.1 | 2.4 | 7.8 | 19 |
| Parenting Sense of Competency: Subscale 1 | 91 | 38.0 | 5.8 | 21 | 48 | 100 | 36.5 | 6.2 | 14 | 48 |
| Parenting Sense of Competency: Subscale 2 | 91 | 40.2 | 6.5 | 25 | 54 | 100 | 39.5 | 6.9 | 18 | 54 |
| Parenting Sense of Competency: Summary | 91 | 78.2 | 10.6 | 46 | 101 | 100 | 76.1 | 11.6 | 32 | 102 |
| Maternal Postnatal Attachment Scale: Quality of Attachment | 91 | 36.6 | 3.1 | 25 | 42 | 100 | 35.8 | 3.3 | 24 | 42 |
| Maternal Postnatal Attachment Scale: Absence of Hostility | 91 | 17.2 | 1.5 | 11 | 20 | 100 | 16.2 | 1.6 | 11 | 20 |
| Maternal Postnatal Attachment Scale: Pleasurable Interaction | 91 | 22.0 | 2.1 | 16 | 25 | 100 | 21.6 | 2.1 | 16 | 25 |
| Vulnerable Child Scale | 90 | 50 | 5.4 | 35 | 58 | 100 | 51.1 | 5.3 | 32 | 59.7 |
| Profile of Mood States: Tension | 90 | 15.9 | 6.0 | 9 | 37 | 98 | 15.9 | 5.9 | 9 | 41 |
| Profile of Mood States: Depression | 90 | 20.4 | 9.6 | 15 | 62 | 98 | 20.0 | 8.8 | 15 | 70 |
| Profile of Mood States: Anger | 90 | 16.2 | 6.8 | 12 | 45 | 99 | 16.0 | 6.0 | 12 | 43 |
| Profile of Mood States: Fatigue | 90 | 15.6 | 6.2 | 7 | 35 | 98 | 14.6 | 5.6 | 7 | 35 |
| Profile of Mood States: Confusion | 90 | 13.3 | 4.4 | 7 | 29 | 98 | 12.5 | 3.7 | 7 | 29 |
| Profile of Mood States: Vigour | 90 | 23.3 | 6.8 | 9 | 40 | 98 | 23.4 | 7.1 | 8 | 38 |
| Profile of Mood States: Total Mood Disturbances | 90 | 58.1 | 31.6 | 19 | 191 | 98 | 55.6 | 29.8 | 16 | 209 |
| Decision Satisfaction Scale | 90 | 42.7 | 6.3 | 18 | 50 | 98 | 41.9 | 6.9 | 19 | 50 |
Child outcomes by CNV-results returned group status
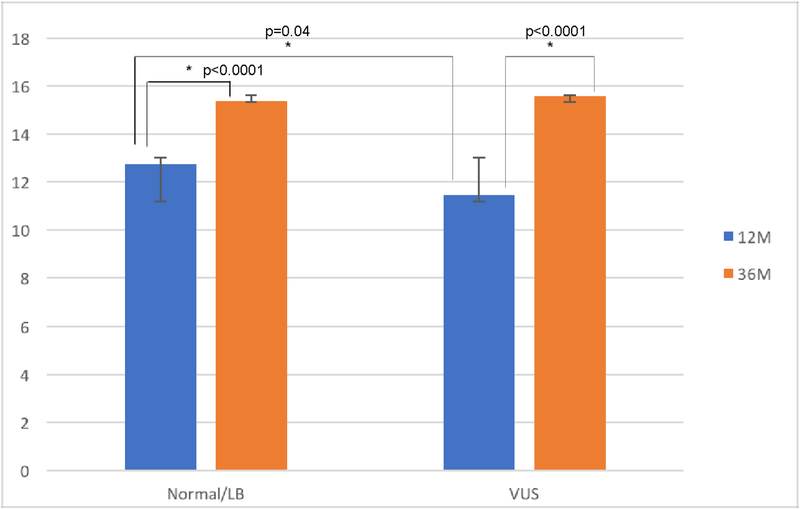
Across six surveys of parental perceptions of child development and attachment to the child, there was one significant main effect of group status related to outcomes. Compared to the normal/LB group, parents in the VUS group rated their child as less competent on the BITSEA scale at 12 months (β=−1.66, p=0.04) though not 36 months (p=0.37) (see Figure 1). For the BITSEA competence scale, the of-concern cutoff point is scores <11, indicating the presence of a socio-emotional competency delay. A lower score reflects less competence [34]. At 12 months, VUS parents’ ratings of their children were at the cutpoint, indicating minimal expected competence (11 on the scale) judged in the clinical range. Data show that health care professionals will refer approximately 7% of these children for further psychosocial evaluation [35].
Figure 1: Scores on parent-report BITSEA competency by group at 12 months and 36 months.
Scores on parent-report of child competency through BITSEA. A higher score indicates greater perceived competency. There is a significant difference in competency scores between VUS and Normal/LB parents at 12 months (p=0.04), though not 36 months (p=0.37). Scores for the normal/LB and VUS groups increased from 12 to 36 months, (β =2.61, p<0.0001, and β =4.12, p<0.0001, respectively), but the VUS group’s scores increased significantly more (p=0.05).
Parent views of child outcomes by CNV-results returned status over time
Both the normal/LB and VUS groups increased in ratings of child competence on the BITSEA from 12 months to 36 months (β =2.61, p<0.0001, and β =4.12, p<0.0001, respectively), though the VUS group showed a significantly larger increase compared to the normal/LB group (β=1.51, p=0.05) (Figure 1).
Parent report of well-being by CNV-return group status
At the 12 and 36-month assessments, there were no group differences in parent mood, marital satisfaction, or parenting sense of competence (all ps> 0.07). However, at 36 months, parents who received VUS compared to normal/LB results reported significantly less satisfaction with their decision to undergo genetic testing (β=−3.25, p=0.02).
Parent trait genetic essentialism
In the group of parents who received VUS results, mothers versus fathers reported significantly higher scores of genetic essentialism (p=0.05). Genetic essentialism was not a significant moderator in any outcomes of child development or parent mood (all ps> 0.11).
DISCUSSION
This study investigated the potential impact of ambiguous versus normal/LB prenatal CMA results on parental perceptions of infants’/toddlers’ emotional and social development, parenting competence, parent attachment, and self-reported parental mood at 12 and 36 months of the child’s age. Our limited significant findings in the context of extensive examination of possible altered parent and child outcomes indicate little effect of CMA VUS results on parents’ perception of their child’s development or their own well-being. Parental report of infant competency was lower in VUS parents compared to the normal/LB group at 12 months, though this difference was not present at 36 months. From 12 to 36 months of age, both groups reported increases in child competence, though the rate of increase was higher for the VUS group. However, our results show that at 36 months, though not at 12 months, parents who received VUS versus normal/LB results were significantly less satisfied with their decision to undergo prenatal CMA testing — perhaps because by that time point, they viewed their children as developing typically and regretted having gone through a period of enhanced surveillance. Traits such as genetic essentialism, genetic knowledge, and tolerance for ambiguity did not moderate the findings.
Previous qualitative studies examining the impact of prenatal CMA testing have shown that parents who receive CMA VUS results often report anxiety about their infant’s development and behavior [19, 20, 33, 36]. Similarly, a recent study investigating parental experience of abnormal fetal ultrasound screenings described that anxiety about the results continued well after the results were proven to be false-positives, and suggest that genetics is not unique in providing ambiguous prenatal information [37]. Driven by fear of the potential bad outcomes that may be associated with an uncertain genetic result, parents may anxiously over examine their infant’s behavior, finding deficits when there are none. One prior study by Briggs-Gowan et al. found that levels of parental worry/anxiety were associated with concerning BITSEA scores on both the social and emotional problem scale and social and emotional competency scale. Most children with worried/anxious parents (62.2%) also had BITSEA scores that fell outside of the normal range [38]. Interestingly, our results showed a lack of differences in self-reported parental mood between parents receiving VUS and normal/LB results. We expected parental mood, specifically parental stress and anxiety from the anticipation of a ‘damaged’ child, to be significantly different between the normal/LB and the VUS groups. This was not the case, and no measure of parental mood was significantly different between any of the groups which may be due to our use of general versus specific stress scales and may benefit from qualitative interview of the impact of prenatal VUS results on parents.
The significant group difference in parental perception of infant competency observed at 12 months was not present at 36 months, a period when both groups of parents increased their judgment of their child’s competence. Consistent with previous studies highlighting parental coping in those who underwent CMA, we suggest that parental perception of infant competency (measured by BITSEA) is less affected by VUS-related vigilance as the infant progresses in development [33]. As additional developmental milestones are reached normally, parents become less anxious, scrutinize their child less, and, therefore, report children as more competent. Despite uncertain genetic results, parents of young children with VUS results remain hopeful about their child’s normal development as the child demonstrates typical levels of competence [39]. Similar to another study, which surveyed parents six months after the receipt of CMA results, in our study, at 36 months, parents who received VUS results were less satisfied with their decision to undergo CMA testing [36]. We suggest this dissatisfaction is due to frustration with the current limitations in our ability to interpret genetic results, the influence of ambiguity on parental psychology and parenting styles promoting “watchful waiting” [36], and parents’ increased sense of child competency as the child gets older. As the child appears more competent, parents may feel regretful of the unnecessary stress and anxiety brought about by the testing process. This experience should be considered as we decide which results to return from prenatal exome and genome sequencing and try to optimize the utility of the test.
Limitations
Our research study has some limitations. As with a large proportion of genetic studies, our cohort of parents was demographically homogenous. The majority of participants were married or living as married, self-reported as white/Caucasian, and had received at least a college-level education. Future research should investigate parental perceptions in other racial and socioeconomic backgrounds, especially given that VUS results are currently more common in other racial/ethnic groups [40]. As providers gained experience with CMA testing, their counseling, explanations, and setting of expectations may have changed over time. We did not record any parental biological markers of stress, which could have been used to further quantify our parental stress and anxiety tests, a subtest of the broader mood state test. We only had 11 children in our pathogenic CNV-returned results group and, therefore, were unable to include this group in statistical analyses. The small sample size of this study is an additional limitation. As a result, further corroboration is needed for our findings.
Future clinical significance
Our minimal positive findings suggest that receipt of CMA VUS results has limited impact on the parental perception of children’s development and behavior by 36 months of age. However, we highlight some potential improvements for the clinical management of prenatal CMA testing based on the perceptions of child competency. Parents receiving VUS results initially perceived their infants as less competent at 12 months. Previous studies suggest that parents of children with VUS results may engage in additional medical and developmental surveillance during early life stages [33]. We suggest that parents receiving VUS results be encouraged to follow up with a pediatric geneticist who can help manage expectations, provide feedback, and alleviate some of the surveillance burden that parents experience. When possible, setting expectations about the large fraction of VUS that are reclassified to likely benign/benign can be helpful. More guidance should be provided to assist parents through coping with ambiguous results, monitoring how parents are coping with the results, how they are perceiving their child’s development, and providing updates on the classification of the CNV as new data are available.
It is somewhat concerning that at 36 months postpartum, parents with VUS results felt greater dissatisfaction with the decision to undergo testing, although the frequency of VUS in the study overall was low. It would be of interest to determine whether decision satisfaction continues to change over time for these parents. Efforts should be made to allow parents to indicate how much detail they would like to receive about CMA and other genomic results, and whether they want to know about inconclusive findings. Education in the pre-test counseling sessions is critical to providing parents with the autonomy, knowledge, and anticipation of possible outcomes to make the right decision for themselves. This educational session can be challenging if coincident with provision of a serious anatomic fetal anomaly and, for some patients, supplementary educational materials including videos or written materials may be helpful.
CMA is the first application of large-scale genomic analysis in the prenatal setting. Large-scale analysis will be offered to more as cell-free CMA testing becomes routinely performed on maternal blood. Much more expansive genomic analysis, including large panels, exome, and genome sequencing, will result in parents facing similar challenges of ambiguity, though with much more data, and the challenges may become even more complex.
What’s already known about this topic?
Studies have examined parental perspectives of prenatal chromosome microarray (CMA) testing, and how the CMA results received affect parental stress, mood and anxiety.
What does this study add?
This study assesses prenatal CMA testing in relation to parental perceptions of child outcomes up to children at age 36 months old. Little research has investigated the impact of individual genomic results on parental perceptions of children’s behavior and health beyond early infancy.
Acknowledgements
We thank the families that participated in this study. Funding was provided by U01 HD055651, P50HG007257 and 1R01HD088105-01A1.
Funding was provided by U01 HD055651, P50HG007257, 1R01HD088105-01A1
Footnotes
There are no conflicts of interest to disclose for any of the authors.
References
- 1.Wapner RJ, et al. , Chromosomal Microarray versus Karyotyping for Prenatal Diagnosis. New England Journal of Medicine, 2012. 367(23): p. 2175–2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hillman SC, et al. , Use of prenatal chromosomal microarray: prospective cohort study and systematic review and meta-analysis. Ultrasound in Obstetrics & Gynecology, 2013. 41(6): p. 610–620. [DOI] [PubMed] [Google Scholar]
- 3.Shaffer LG and Rosenfeld JA, Microarray-based prenatal diagnosis for the identification of fetal chromosome abnormalities. Expert Rev Mol Diagn, 2013. 13(6): p. 601–11. [DOI] [PubMed] [Google Scholar]
- 4.Committee Opinion No.682: Microarrays and Next-Generation Sequencing Technology: The Use of Advanced Genetic Diagnostic Tools in Obstetrics and Gynecology. Obstet Gynecol, 2016. 128(6): p. e262–e268. [DOI] [PubMed] [Google Scholar]
- 5.Richards AA and Garg V, Genetics of Congenital Heart Disease. Current Cardiology Reviews, 2010. 6(2): p. 91–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanna-Cherchi S, et al. , Copy-Number Disorders Are a Common Cause of Congenital Kidney Malformations. The American Journal of Human Genetics, 2012. 91(6): p. 987–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Southard AE, Edelmann LJ, and Gelb BD, Role of Copy Number Variants in Structural Birth Defects. Pediatrics, 2012. 129(4): p. 755–763. [DOI] [PubMed] [Google Scholar]
- 8.Lawson KL and Pierson RA, Maternal Decisions Regarding Prenatal Diagnosis: Rational Choices or Sensible Decisions? Journal of obstetrics and gynaecology Canada : JOGC = Journal d’obstetrique et gynecologie du Canada : JOGC, 2007. 29(3): p. 240–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sebat J, et al. , Strong Association of De Novo Copy Number Mutations with Autism. Science (New York, N.Y.), 2007. 316(5823): p. 445–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stefansson H, et al. , Large recurrent microdeletions associated with schizophrenia. Nature, 2008. 455(7210): p. 232–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weiss LA, et al. , Association between Microdeletion and Microduplication at 16p11.2 and Autism. New England Journal of Medicine, 2008. 358(7): p. 667–675. [DOI] [PubMed] [Google Scholar]
- 12.Heinzen EL, et al. , Rare Deletions at 16p13.11 Predispose to a Diverse Spectrum of Sporadic Epilepsy Syndromes. The American Journal of Human Genetics, 2010. 86(5): p. 707–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mefford HC, et al. , Genome-Wide Copy Number Variation in Epilepsy: Novel Susceptibility Loci in Idiopathic Generalized and Focal Epilepsies. PLoS Genetics, 2010. 6(5): p. e1000962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cooper GM, et al. , A Copy Number Variation Morbidity Map of Developmental Delay. Nature genetics, 2011. 43(9): p. 838–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaminsky EB, et al. , An evidence-based approach to establish the functional and clinical significance of CNVs in intellectual and developmental disabilities. Genetics in medicine : official journal of the American College of Medical Genetics, 2011. 13(9): p. 777–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanders SJ, et al. , Multiple recurrent de novo copy number variations (CNVs), including duplications of the 7q11.23 Williams-Beuren syndrome region, are strongly associated with autism. Neuron, 2011. 70(5): p. 863–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hillman SC, et al. , Additional information from array comparative genomic hybridization technology over conventional karyotyping in prenatal diagnosis: a systematic review and meta-analysis. Ultrasound Obstet Gynecol, 2011. 37(1): p. 6–14. [DOI] [PubMed] [Google Scholar]
- 18.Reiff M, et al. , “What does it mean?”: Uncertainties in understanding results of chromosomal microarray testing. Genetics in medicine : official journal of the American College of Medical Genetics, 2012. 14(2): p. 250–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jez S, et al. , Variants of unknown significance on chromosomal microarray analysis: parental perspectives. Journal of Community Genetics, 2015. 6(4): p. 343–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kiedrowski LA, et al. , Parents’ Perspectives on Variants of Uncertain Significance from Chromosome Microarray Analysis. J Genet Couns, 2016. 25(1): p. 101–11. [DOI] [PubMed] [Google Scholar]
- 21.Wilkins EJ, et al. , “It wasn’t a disaster or anything”: Parents’ experiences of their child’s uncertain chromosomal microarray result. American Journal of Medical Genetics Part A, 2016. 170(11): p. 2895–2904. [DOI] [PubMed] [Google Scholar]
- 22.Werner-Lin A, et al. , Couple’s Narratives of Communion and Isolation Following Abnormal Prenatal Microarray Testing Results. Qual Health Res, 2016. 26(14): p. 1975–1987. [DOI] [PubMed] [Google Scholar]
- 23.Giarelli E and Reiff M, Mothers’ appreciation of chromosomal microarray analysis for autism spectrum disorder. J Spec Pediatr Nurs, 2015. 20(4): p. 244–58. [DOI] [PubMed] [Google Scholar]
- 24.Shuster E, Microarray genetic screening: a prenatal roadblock for life? The Lancet. 369(9560): p. 526–529. [DOI] [PubMed] [Google Scholar]
- 25.Pergament E, Controversies and challenges of array comparative genomic hybridization in prenatal genetic diagnosis. Genet Med, 2007. 9(9): p. 596–9. [DOI] [PubMed] [Google Scholar]
- 26.Clarkeburn H, Parental duties and untreatable genetic conditions. Journal of Medical Ethics, 2000. 26: p. 400–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tercyak KP, et al. , Psychological response to prenatal genetic counseling and amniocentesis. Patient Educ Couns, 2001. 43(1): p. 73–84. [DOI] [PubMed] [Google Scholar]
- 28.Kowalcek I, et al. , Depressive reactions and stress related to prenatal medicine procedures. Ultrasound in Obstetrics and Gynecology, 2002. 19(1): p. 18–23. [DOI] [PubMed] [Google Scholar]
- 29.Lerman C, et al. , Genetic testing: psychological aspects and implications. J Consult Clin Psychol, 2002. 70(3): p. 784–97. [DOI] [PubMed] [Google Scholar]
- 30.Kaiser AS, et al. , Psychological responses to prenatal NTS counseling and the uptake of invasive testing in women of advanced maternal age. Patient Educ Couns, 2004. 54(1): p. 45–53. [DOI] [PubMed] [Google Scholar]
- 31.Allison SJ, Stafford J, and Anumba DOC, The effect of stress and anxiety associated with maternal prenatal diagnosis on feto-maternal attachment. BMC Women’s Health, 2011. 11: p. 33–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reiff M, et al. , “Set in Stone” or “Ray of Hope”: Parents’ Beliefs About Cause and Prognosis After Genomic Testing of Children Diagnosed with ASD. Journal of Autism and Developmental Disorders, 2017. 47(5): p. 1453–1463. [DOI] [PubMed] [Google Scholar]
- 33.Werner-Lin A, et al. , “They Can’t Find Anything Wrong with Him, Yet”: Mothers’ experiences of parenting an infant with a prenatally diagnosed copy number variant (CNV). American Journal of Medical Genetics Part A, 2017. 173(2): p. 444–451. [DOI] [PubMed] [Google Scholar]
- 34.Briggs-Gowan MJ, et al. , The Brief Infant-Toddler Social and Emotional Assessment: screening for social-emotional problems and delays in competence. J Pediatr Psychol, 2004. 29(2): p. 143–55. [DOI] [PubMed] [Google Scholar]
- 35.Kruizinga I, et al. , Screening Accuracy and Clinical Application of the Brief Infant-Toddler Social and Emotional Assessment (BITSEA). PLoS ONE, 2013. 8(8): p. e72602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bernhardt BA, et al. , Women/’s experiences receiving abnormal prenatal chromosomal microarray testing results. Genet Med, 2013. 15(2): p. 139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Larsson A-K, et al. , Parents’ experiences of an abnormal ultrasound examination - vacillating between emotional confusion and sense of reality. Reproductive Health, 2010. 7: p. 10–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Briggs-Gowan MJ and Carter AS, Social-Emotional Screening Status in Early Childhood Predicts Elementary School Outcomes. Pediatrics, 2008. 121(5): p. 957–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Whitmarsh I, et al. , A place for genetic uncertainty: parents valuing an unknown in the meaning of disease. Soc Sci Med, 2007. 65(6): p. 1082–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saulsberry K and Terry SF, The Need to Build Trust: A Perspective on Disparities in Genetic Testing. Genetic Testing and Molecular Biomarkers, 2013. 17(9): p. 647–648. [DOI] [PMC free article] [PubMed] [Google Scholar]