Abstract
Because vaccination is an effective means to protect humans from influenza viruses, extensive efforts have been made to develop not only new vaccines, but also for new adjuvants to enhance the efficacy of existing inactivated vaccines. Here, we examined the adjuvanticity of synthetic hemozoin, a synthetic version of the malarial by-product hemozoin, on the vaccine efficacy of inactivated whole influenza viruses in a mouse model. We found that mice immunized twice with hemozoin-adjuvanted inactivated A/California/04/2009 (H1N1pdm09) or A/Vietnam/1203/2004 (H5N1) virus elicited higher virus-specific antibody responses than did mice immunized with non-adjuvanted counterparts. Furthermore, mice immunized with hemozoin-adjuvanted inactivated viruses were better protected from lethal challenge with influenza viruses than were mice immunized with non-adjuvanted inactivated vaccines. Our results show that hemozoin improves the immunogenicity of inactivated influenza viruses, and is thus a promising adjuvant for inactivated whole virion influenza vaccines.
Keywords: influenza virus, vaccine, hemozoin, adjuvant, antibody
Introduction
Despite the worldwide surveillance network of influenza viruses, the incidence and prevalence of influenza are hard to predict, as exemplified by the influenza (H1N1) 2009 pandemic [1, 2]. Vaccination stands on the frontlines of influenza infection control: both live attenuated and inactivated influenza vaccines are currently available [3, 4]. The live attenuated vaccines are more efficient than inactivated vaccines at inducing the mucosal immune responses that play an important role in combating influenza virus infection [5, 6]. However, because of the safety concerns such as the emergence of revertant and/or reassortant viruses, these live vaccines are licensed in a limited number of countries. By contrast, inactivated vaccines have few safety concerns and are globally available. While they efficiently induce humoral immune responses, a high dose (usually 15 μg) of the inactivated vaccine is required to provide adequate immunity [7, 8]. Therefore, there is room for improvement in the current influenza vaccines.
Vaccine is generally assessed on the basis of immunogenicity, safety, and costs [9]. To enhance the immunogenicity of the inactivated vaccines, adjuvants, such as aluminum compounds and salts, have been considered [10]. Adjuvants are defined as immune modulators that are added to inactivated vaccines to boost the immune responses, enable the use of lower amounts of antigens, and thus expand the vaccine supply [10, 11]. Although most of the inactivated influenza vaccines currently used are injected via the intramuscular or subcutaneous routes, previous studies have shown that intranasal vaccinations induce antibodies more effectively than do intramuscular or subcutaneous vaccinations [12–14]. However, the alum compounds that are generally used as adjuvants for intramuscular administration do not enhance the efficacy of intranasal vaccines; therefore, to improve the efficacy of intranasal vaccines, novel intranasal adjuvants are required [15].
Malaria parasites digest hemoglobin in red blood cells, resulting in the production of potentially toxic heme metabolites [16]. To protect themselves from oxidative damage, the parasites polymerize toxic heme enzymatically into a safer insoluble substance, hemozoin [17]. Recently, hemozoin and a chemically identical synthetic version of hemozoin (called β-hematin) have been investigated for their potency as novel adjuvants, and the molecular pathway underlying their immunological function has also been studied. Such studies have demonstrated that purified hemozoin is a non-DNA ligand for Toll-like receptor 9 (TLR9) that may activate innate immune cells via TLR9 [18–20]. This latter point has been a subject of debate, however, because the adjuvant effect of synthetic hemozoin is dependent on MyD88 and not TLR9 [21]. Recently, we reported that hemozoin enhances the protective efficacy of a subcutaneously administered influenza HA split vaccine in a ferret model [22].
We speculated that synthetic hemozoin (hereafter referred to only as hemozoin) could serve as a novel intranasal adjuvant for the inactivated influenza vaccine. Accordingly, here we evaluated the adjuvanticity of hemozoin on the vaccine efficacy of intranasally administered inactivated whole virion influenza vaccines in a murine lethal infection model. The results indicate that hemozoin is a promising adjuvant for inactivated whole virion influenza vaccines.
Materials & Methods
Cells and viruses.
Human embryonic kidney HEK293T cells were maintained in Dulbecco’s modified Eagle medium (Lonza, Basel, Switzerland) supplemented with 10% fetal calf serum (Invitrogen, Carlsbad, CA). Madin-Darby canine kidney (MDCK) cells were maintained in minimum essential medium (MEM) (Invitrogen) supplemented with 5% newborn calf serum (NCS) (Sigma, St. Louis, MO). All cells were maintained in a humidified incubator at 37°C in 5% CO2.
A/California/04/2009 (H1N1; Ca04), which is an early isolate of influenza (H1N1) 2009 pandemic viruses, and mouse-adapted Ca04 (MACa04) [23] viruses were propagated in MDCK cells as previously described [24]. A/Vietnam/1203/2004 (H5N1; VN1203) virus, a representative strain of highly pathogenic avian influenza viruses, was grown in MDCK cells and in 10-day-old embryonated chicken eggs to use as challenge viruses and as vaccine and ELISA antigens, respectively. All work involving live VN1203 virus was carried out at the ABSL-3 laboratory of the Influenza Research Institute, UW-Madison, following the protocol designed by Institutional Animal Care and Use Committee (IACUC).
Inactivated influenza virus and adjuvant.
To inactivate MDCK cell-propagated Ca04 virus and egg-propagated VN1203 virus, formalin (final concentration, 0.1%) was added to the viruses, which were then incubated at 4°C for 1 week. The inactivated viruses were purified through a 10%–50% sucrose density gradient and resuspended in phosphate-buffered saline (PBS) as described previously [25]. Inactivation of Ca04 viruses was confirmed by passaging them twice in MDCK cells and examining their cytopathic effect; inactivation of VN1203 viruses was confirmed by passaging them twice in embryonated chicken eggs followed by hemagglutination assays.
Synthetic hemozoin, was purified from hemin chloride (>98% pure, Fluka) by using the acid-catalyzed method described previously [21] and was re-suspended in endotoxin-free water with no detectable levels of endotoxin. The synthetic hemozoin concentration was calculated in mM (1 mg of hemozoin in 1 ml of water was equal to 1 mM).
Immunization and protection studies.
For the immunization and protection studies with Ca04 virus, six-week-old female BALB/c mice (n=13 per group) were anesthetized with isoflurane and intranasally administered with 50 μl of PBS, 9 mM hemozoin only, inactivated Ca04 only [5×106 plaque-forming unit (PFU), which corresponds to 0.1 μg when the total amount of viral protein was measured by using a BCA protein assay (Thermo Scientific)], or inactivated Ca04 adjuvanted with 9 mM hemozoin, twice with a 2-week interval between the immunizations. Three weeks after the final administration, three mice from each group were euthanized for collection of bronchoalveolar lavage fluid (BALF) and nasal washes. The remaining mice (n=10 per group) were intranasally challenged with 10-fold 50% mouse lethal doses (MLD50) of MACa04 virus. On days 3 and 6 post-challenge, three mice each were euthanized and their lungs were collected, homogenized with MEM containing 0.3% BSA, and examined for virus titers by using plaque assays in MDCK cells. The body weight and survival of the remaining challenged mice (n=4 per group) were monitored daily for 14 days.
For VN1203 virus, four-week-old female BALB/c mice (n=16 per group) were immunized as described above. Two weeks after the last immunization, five mice from each group were euthanized for collection of BALF and nasal washes. The remaining mice (n=11 per group) were challenged with 100 MLD50 of VN1203 virus. On days 3 and 6 post-challenge, three mice each were euthanized and their lungs were collected, homogenized with MEM containing 0.3% BSA, and examined for virus titers by using plaque assays in MDCK cells. The body weight and survival of the remaining challenged mice (n=5 per group) were monitored daily for 14 days.
Detection of virus-specific antibodies.
Virus-specific antibodies in nasal washes, BALF, and serum were detected by using an ELISA as previously described [25–27]. Briefly, 96-well ELISA plate wells were coated with approximately 0.3 μg (in 50 μl) of purified Ca04 or VN1203 virus treated with disruption buffer (0.5 M Tris–HCl [pH 8.0], 0.6 M KCl, and 0.5% Triton X-100) or sarkosyl, respectively. After incubation of the virus-coated plates with the test samples, virus-specific IgA and IgG antibodies in the samples were detected by using anti-mouse IgA and IgG goat antibodies conjugated to horseradish peroxidase (Kirkegaard & Perry Laboratory Inc., Gaithersburg, MD, Rockland), respectively.
Hemagglutination inhibition assay (HI assay).
To detect HI antibodies against Ca04 and VN1203, an HI assay was performed as described previously [28, 29]. Briefly, serum samples were treated with receptor-destroying enzyme (RDE; Denka Seiken Co., Ltd.) by incubating at 37°C for 16–18 h followed by inactivation at 56°C for 30 min. One volume of turkey or horse red blood cells (RBCs) was then added to 20 volumes of serum and the sera were incubated for 1 h on ice with intermittent mixing. The samples were then centrifuged at 900 × g for 5 min, and the supernatants were transferred to new tubes for use in the HI assay. Serially diluted sera (2-fold dilutions) were mixed with 4 HA units of virus antigen and incubated with 0.5% turkey RBCs or 1% horse RBCs to determine the extent of hemagglutination inhibition.
Statistical analysis.
Statistically significant differences in the virus-specific titers (P<0.05 and P<0.01) and the survival rates of the challenged mice (P<0.05) were assessed by use of a one-way ANOVA followed by a Dunnett’s test and Log-rank statistical analysis, respectively.
Results
Hemozoin enhances influenza virus-specific antibody responses in mice.
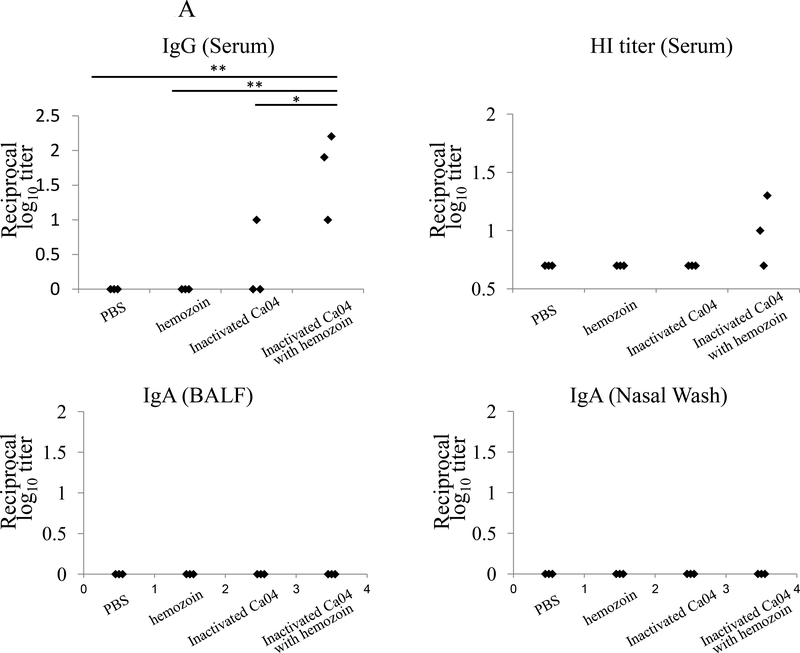
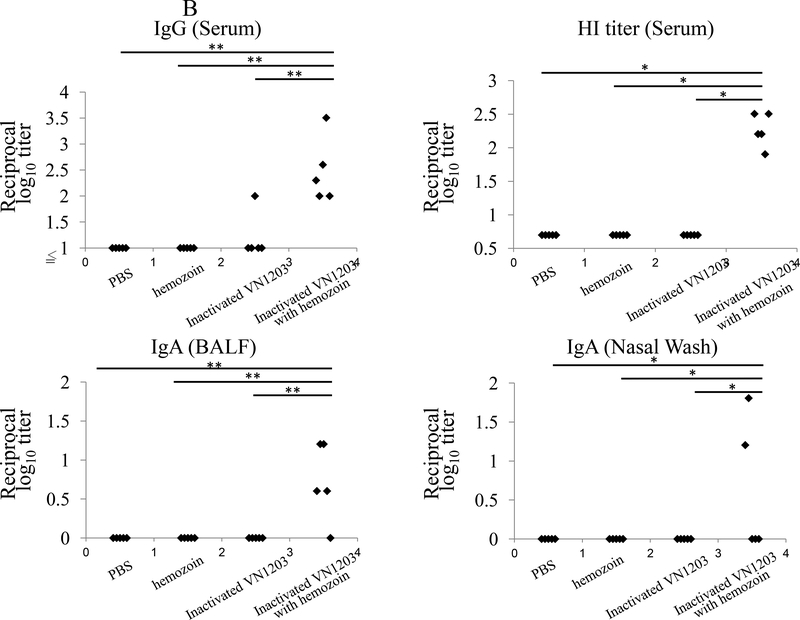
To examine the effect of hemozoin on antibody responses elicited by immunization with inactivated influenza viruses, we intranasally administered BALB/c mice with hemozoin-adjuvanted inactivated virus (Ca04 or VN1203 virus, 5×106 plaque-forming units (PFU), the total amount of viral protein was 0.1 μg) twice with a 2-week interval between the immunizations. At three or two weeks after the final administration, we examined the antibody responses to the administered Ca04 or VN1203 virus by using an ELISA to measure the amount of IgG in the serum and IgA in the BALF and nasal washes (Fig. 1). Neither IgG nor IgA against Ca04 or VN1203 virus was appreciably detected in any samples from the PBS- or hemozoin-administered mice. Under these conditions, although one mouse immunized with non-adjuvanted inactivated Ca04 (Fig. 1A upper panel) and one mouse immunized with non-adjuvanted inactivated VN1203 virus (Fig. 1B upper panel) produced virus-specific IgG in the serum at a detectable level, all of the mice immunized with hemozoin-adjuvanted inactivated Ca04 (n=3) or VN1203 (n=5) virus elicited significantly higher levels of virus-specific IgG in the serum. We also examined the functional properties of the elicited antibodies by using hemagglutination inhibition (HI) assays. For both the Ca04 and VN1203 viruses, greater HI titers were obtained after vaccination with hemozoin-adjuvanted inactivated viruses than with non-adjuvanted inactivated viruses (Fig. 1A&B upper, right panel), although the titer difference for Ca04 virus between the hemozoin group and the control groups was not statistically significant (Fig. 1A upper, right panel). Of note, although the addition of hemozoin did not enhance IgA production in the nasal washes or BALF of the inactivated Ca04 virus-immunized mice, some of the mice immunized with the hemozoin-adjuvanted inactivated VN1203 virus did produce high levels of virus-specific IgA in their nasal washes and BALF (Fig. 1B lower panels). Taken together, these results indicate that hemozoin enhanced the immunogenicity of inactivated influenza viruses, resulting in more efficient production of virus-specific antibodies.
Figure 1. Virus-specific antibody responses in immunized mice.
Virus-specific antibodies were detected by means of ELISA and HI assays with purified Ca04 (A) or VN1203 (B) virus as a viral antigen. IgG antibody titers (upper, left panels) and HI titers (upper, right panels) in serum and IgA antibody titers in the BALF (lower, left panels), and nasal washes (lower, right panels) from mice intranasally mock-immunized with PBS or hemozoin or immunized with non-adjuvanted or hemozoin-adjuvanted inactivated virus were measured. Values represent antibody titers in individual mice (A: n=3, B: n=5). Statistically significant differences (*: P<0.05, **: P<0.01) are indicated.
Hemozoin enhances the efficacy of inactivated influenza vaccine against lethal challenge in mice.
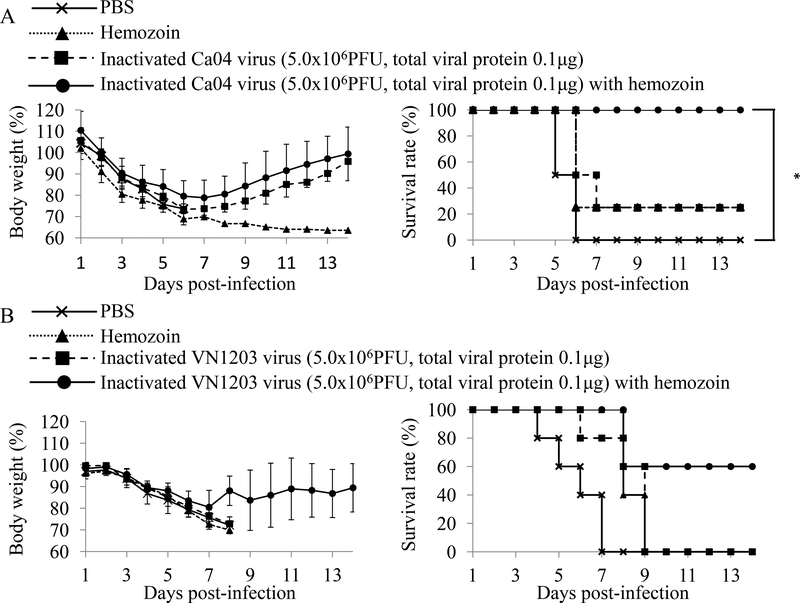
To further assess the adjuvanticity of hemozoin, mice immunized twice with hemozoin-adjuvanted inactivated Ca04 or VN1203 virus were challenged with a lethal dose of MACa04 (10 MLD50) [23] or VN1203 (100 MLD50) virus (Fig. 2). In the MACa04 challenge group, although all of the PBS-administered mice and 75% of the hemozoin-administered or inactivated Ca04 virus-immunized mice died, all of the mice immunized with hemozoin-adjuvanted inactivated Ca04 virus survived (Fig. 2A). Intriguingly, no significant difference was found in Ca04 virus titers in the lungs among the mouse groups tested (Table 1). These results suggest that the adjuvanticity of hemozoin was sufficient to protect mice from lethal challenge with MACa04 virus.
Figure 2. Body weight changes and survival of mice challenged with lethal doses of viruses.
Mice were mock-immunized with PBS or hemozoin, or immunized with non-adjuvanted or hemozoin-adjuvanted inactivated virus twice with a 2-week interval in between the immunizations. Three or four weeks after the final immunization, mice were intranasally challenged with 10 MLD50 of MACa04 virus (A: n=4) or 100 MLD50 of VN1203 virus (B: n=5), respectively. Body weight (left panels) and survival (right panels) were monitored for 14 days after challenge. Values are expressed as mean changes in body weight ± SD (left panels). Statistically significant differences in the survival rate of immunized mice (*: P<0.05) are indicated (A: right panel).
Table 1.
Virus titers in the lungs of immunized mice challenged with mouse-adapted Ca04 virusa.
| Immunization | Day after challenge | Virus titer (mean log10 PFU ± SD/g) in: Lungs |
|---|---|---|
| PBS | 3 | 8.1±0.03 |
| 6 | 6.5±0.3 | |
| Hemozoin | 3 | 8.2±0.03 |
| 6 | 6.6±0.06 | |
| Inactivated Ca04 virus | 3 | 8.1±0.2 |
| 6 | 5.7±1.0 | |
| Hemozoin-adjuvanted inactivated Ca04 virus | 3 | 8.0±0.2 |
| 6 | 6.2±0.4 |
Mice were intranasally immunized twice with the indicated agents (50 μl per mouse) and challenged with 10 MLD50 of MACa04 virus (50 μl per mouse) 3 weeks after the final immunization. Lungs were collected from mice (n = 3) on days 3 and 6 after challenge and examined for virus titers by use of plaque assays in MDCK cells.
For VN1203 virus, all PBS- and hemozoin-administered and inactivated VN1203 virus-immunized mice died following the lethal challenge (Fig. 2B). By contrast, 60% of the mice immunized with hemozoin-adjuvanted inactivated VN1203 virus survived although mice of all groups experienced body weight loss (Fig. 2B). In accordance with the results of the MACa04 virus challenge, the addition of hemozoin to inactivated VN1203 virus immunization did not affect the virus titers in the lungs of VN1203 virus-challenged mice (Table 2). These results suggest that hemozoin enhanced the vaccine efficacy of the inactivated influenza viruses by modulating host responses, but not by directly inhibiting virus replication. Overall, these results suggest that hemozoin is a promising adjuvant for inactivated influenza vaccines.
Table 2.
Virus titers in the lungs of immunized mice challenged with VN1203 virusa.
| Immunization | Day after challenge | Virus titer (mean log10 PFU ± SD/g) in: Lungs |
|---|---|---|
| PBS | 3 | 6.3±0.2 |
| 6 | 6.3±0.2 | |
| Hemozoin | 3 | 6.6±0.2 |
| 6 | 6.3±0.2 | |
| Inactivated VN1203 virus | 3 | 6.7±0.3 |
| 6 | 5.6±0.4 | |
| Hemozoin-adjuvanted inactivated VN1203 virus | 3 | 6.4±0.3 |
| 6 | 6.0±0.4 |
Mice were intranasally immunized twice with the indicated agents (50 μl per mouse) and challenged with 100 MLD50 of VN1203 virus (50 μl per mouse) 4 weeks after the final immunization. Lungs were collected from mice (n = 3) on days 3 and 6 after challenge and examined for virus titers by use of plaque assays in MDCK cells.
Discussion
Here, we examined the effect of an adjuvant candidate, hemozoin, on the vaccine efficacy of inactivated whole virion influenza vaccines against lethal challenge in a mouse model. Significantly better virus-specific antibody responses were induced by hemozoin-adjuvanted inactivated virus than by inactivated viruses (Fig. 1). We further demonstrated that the hemozoin-adjuvanted inactivated viruses protected mice from lethal challenges more efficiently than did their non-adjuvanted counterparts with no effect of virus titers in the lungs (Fig. 2, Tables 1 and 2). These results indicate that hemozoin is a promising candidate as an effective adjuvant for inactivated whole virion influenza vaccines.
We observed significantly higher levels of IgA specific for VN1203 virus in the BALF and nasal washes, and of serum IgG, in mice immunized with hemozoin-adjuvanted inactivated VN1203 virus than in mice immunized with non-adjuvanted inactivated VN1203 virus-immunized mice (Fig. 1B). These results suggest that hemozoin enhanced the mucosal immune responses and may potentially compensate for the well-recognized weakness of inactivated vaccines [30–32]. By contrast, enhanced IgA production by the hemozoin addition was not observed with the Ca04 virus counterparts (Fig. 1A). This contradiction may reflect a difference in immunogenicity between the Ca04 and VN1203 viruses. Further study is required to clarify the mechanisms by which hemozoin promotes IgA responses after immunization with inactivated vaccines. In addition, hemozoin-adjuvanted inactivated virus protected mice better than non-adjuvanted inactivated viruses although virus titers in lungs were similar between animals immunized with and without the adjuvant (Fig. 2, Tables 1 and 2). This finding suggests that hemozoin enhanced the vaccine efficacy of the inactivated influenza viruses by modulating host responses. In the current study, we measured viral loads only in respiratory organs, which are the primary sites of influenza virus replication even for strains that cause systemic infection (e.g., VN1203 virus). A further study to examine the inhibitory effect of hemozoin on systemic spread of influenza viruses may explain the better protection afforded by hemozoin-adjuvanted vaccine.
Although hemozoin is a ligand for TLR9 [18–20], studies using TLR9- or MyD88-deficient mice suggest that the potent adjuvant effect of synthetic hemozoin is mediated not via TLR9, but through MyD88 [21]. In addition, previous studies have demonstrated that hemozoin stimulates innate inflammatory responses, inducing neutrophil recruitment via MyD88 [21, 33]. Thus, one of the possible mechanisms underlying the hemozoin-mediated enhanced efficacy of inactivated influenza vaccine may be that hemozoin induces the balanced Th1/Th2 responses in a MyD88-dependent manner, leading to the improved immunogenicity of the inactivated influenza viruses and to the better protection against lethal challenge with influenza viruses. Of note, one of four mice administered with only hemozoin survived after the lethal challenge with MACa04 virus (Fig. 2A), suggesting that hemozoin itself might have protective effects against influenza virus infection. Additional study is required to clarify the inhibitory effect of hemozoin on influenza virus infection.
In conclusion, here, we demonstrated the potential of hemozoin as a novel whole virion influenza vaccine adjuvant. Because the mechanism by which hemozoin enhances immunogenicity remains unclear, we should continue to evaluate the adjuvanticity of hemozoin in the context of influenza vaccination. In addition, to establish the efficacy of hemozoin as an adjuvant, further studies are needed including studies in an additional animal model such as ferrets.
Highlights.
Hemozoin enhanced the influenza virus-specific antibody responses in mice.
Hemozoin-adjuvanted inactivated influenza viruses protected mice from lethal influenza virus challenge.
Hemozoin is a promising adjuvant for inactivated whole virion influenza vaccines.
Acknowledgements
We thank Dr. Susan Watson for editing the manuscript and Y. Igari and T. Tsukui from Nihon Zenoaq, Co., Ltd for providing synthetic hemozoin. This work was supported, by a Grant-in-Aid for Specially Promoted Research, by the Japan Initiative for the Global Research Network on Infectious Diseases from the Ministry of Education, Culture, Sports, Science, and Technology, Japan, by grants-in-aid from the Ministry of Health, Labour, and Welfare, Japan, by ERATO (Japan Science and Technology Agency), by Strategic Basic Research Programs of Japan Science and Technology Agency, by National Institute of Allergy and Infectious Diseases Public Health Service research grants, and by an NIAID-funded Center for Research on Influenza Pathogenesis (CRIP, HHSN266200700010C). R.U. is supported by JSPS Research Fellowships for young scientists.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Glezen WP. Cold-adapted, live attenuated influenza vaccine. Expert Rev Vaccines 2004. April;3(2):131–9. [DOI] [PubMed] [Google Scholar]
- [2].Neumann G, Kawaoka Y. The first influenza pandemic of the new millennium. Influenza Other Respir Viruses 2011. May;5(3):157–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Nabel GJ, Fauci AS. Induction of unnatural immunity: prospects for a broadly protective universal influenza vaccine. Nat Med 2010. December;16(12):1389–91. [DOI] [PubMed] [Google Scholar]
- [4].Lambert LC, Fauci AS. Influenza vaccines for the future. N Engl J Med 2010. November 18;363(21):2036–44. [DOI] [PubMed] [Google Scholar]
- [5].Rimmelzwaan GF, Fouchier RA, Osterhaus AD. Influenza virus-specific cytotoxic T lymphocytes: a correlate of protection and a basis for vaccine development. Curr Opin Biotechnol 2007. December;18(6):529–36. [DOI] [PubMed] [Google Scholar]
- [6].Cox RJ, Brokstad KA, Ogra P. Influenza virus: immunity and vaccination strategies. Comparison of the immune response to inactivated and live, attenuated influenza vaccines. Scand J Immunol 2004. January;59(1):1–15. [DOI] [PubMed] [Google Scholar]
- [7].Brokstad KA, Cox RJ, Olofsson J, Jonsson R, Haaheim LR. Parenteral influenza vaccination induces a rapid systemic and local immune response. J Infect Dis 1995. January;171(1):198–203. [DOI] [PubMed] [Google Scholar]
- [8].Cox RJ, Brokstad KA, Zuckerman MA, Wood JM, Haaheim LR, Oxford JS. An early humoral immune response in peripheral blood following parenteral inactivated influenza vaccination. Vaccine 1994. August;12(11):993–9. [DOI] [PubMed] [Google Scholar]
- [9].Tetsutani K, Ishii KJ. Adjuvants in influenza vaccines. Vaccine 2012. December 14;30(52):7658–61. [DOI] [PubMed] [Google Scholar]
- [10].Reed SG, Orr MT, Fox CB. Key roles of adjuvants in modern vaccines. Nat Med 2013. December;19(12):1597–608. [DOI] [PubMed] [Google Scholar]
- [11].Dey AK, Srivastava IK. Novel adjuvants and delivery systems for enhancing immune responses induced by immunogens. Expert Rev Vaccines 2011. February;10(2):227–51. [DOI] [PubMed] [Google Scholar]
- [12].van Riet E, Ainai A, Suzuki T, Hasegawa H. Mucosal IgA responses in influenza virus infections; thoughts for vaccine design. Vaccine 2012. August 31;30(40):5893–900. [DOI] [PubMed] [Google Scholar]
- [13].Tumpey TM, Renshaw M, Clements JD, Katz JM. Mucosal delivery of inactivated influenza vaccine induces B-cell-dependent heterosubtypic cross-protection against lethal influenza A H5N1 virus infection. J Virol 2001. June;75(11):5141–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ainai A, Tamura S, Suzuki T, Ito R, Asanuma H, Tanimoto T, et al. Characterization of neutralizing antibodies in adults after intranasal vaccination with an inactivated influenza vaccine. J Med Virol 2012. February;84(2):336–44. [DOI] [PubMed] [Google Scholar]
- [15].Petrovsky N, Aguilar JC. Vaccine adjuvants: current state and future trends. Immunol Cell Biol 2004. October;82(5):488–96. [DOI] [PubMed] [Google Scholar]
- [16].Francis SE, Sullivan DJ Jr., Goldberg DE Hemoglobin metabolism in the malaria parasite Plasmodium falciparum. Annu Rev Microbiol 1997;51:97–123. [DOI] [PubMed] [Google Scholar]
- [17].Arese P, Schwarzer E. Malarial pigment (haemozoin): a very active ‘inert’ substance. Ann Trop Med Parasitol 1997. July;91(5):501–16. [DOI] [PubMed] [Google Scholar]
- [18].Coban C, Ishii KJ, Kawai T, Hemmi H, Sato S, Uematsu S, et al. Toll-like receptor 9 mediates innate immune activation by the malaria pigment hemozoin. J Exp Med 2005. January 3;201(1):19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Parroche P, Lauw FN, Goutagny N, Latz E, Monks BG, Visintin A, et al. Malaria hemozoin is immunologically inert but radically enhances innate responses by presenting malaria DNA to Toll-like receptor 9. Proc Natl Acad Sci U S A 2007. February 6;104(6):1919–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Wu X, Gowda NM, Kumar S, Gowda DC. Protein-DNA complex is the exclusive malaria parasite component that activates dendritic cells and triggers innate immune responses. Journal of immunology 2010. April 15;184(8):4338–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Coban C, Igari Y, Yagi M, Reimer T, Koyama S, Aoshi T, et al. Immunogenicity of whole-parasite vaccines against Plasmodium falciparum involves malarial hemozoin and host TLR9. Cell Host Microbe 2010. January 21;7(1):50–61. [DOI] [PubMed] [Google Scholar]
- [22].Onishi M, Kitano M, Taniguchi K, Homma T, Kobayashi M, Sato A, et al. Hemozoin is a potent adjuvant for hemagglutinin split vaccine without pyrogenicity in ferrets. Vaccine 2014. May 23;32(25):3004–9. [DOI] [PubMed] [Google Scholar]
- [23].Sakabe S, Ozawa M, Takano R, Iwastuki-Horimoto K, Kawaoka Y. Mutations in PA, NP, and HA of a pandemic (H1N1) 2009 influenza virus contribute to its adaptation to mice. Virus Res 2011. June;158(1–2):124–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Yamada S, Hatta M, Staker BL, Watanabe S, Imai M, Shinya K, et al. Biological and structural characterization of a host-adapting amino acid in influenza virus. PLoS Pathog 2010;6(8):e1001034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Uraki R, Kiso M, Iwatsuki-Horimoto K, Fukuyama S, Takashita E, Ozawa M, et al. A novel bivalent vaccine based on a PB2-knockout influenza virus protects mice from pandemic H1N1 and highly pathogenic H5N1 virus challenges. J Virol 2013. July;87(14):7874–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kida H, Brown LE, Webster RG. Biological activity of monoclonal antibodies to operationally defined antigenic regions on the hemagglutinin molecule of A/Seal/Massachusetts/1/80 (H7N7) influenza virus. Virology 1982. October 15;122(1):38–47. [DOI] [PubMed] [Google Scholar]
- [27].Das SC, Hatta M, Wilker PR, Myc A, Hamouda T, Neumann G, et al. Nanoemulsion W805EC improves immune responses upon intranasal delivery of an inactivated pandemic H1N1 influenza vaccine. Vaccine 2012. November 6;30(48):6871–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Jia N, Wang SX, Liu YX, Zhang PH, Zuo SQ, Lin Z, et al. Increased sensitivity for detecting avian influenza-specific antibodies by a modified hemagglutination inhibition assay using horse erythrocytes. J Virol Methods 2008. October;153(1):43–8. [DOI] [PubMed] [Google Scholar]
- [29].Kayali G, Setterquist SF, Capuano AW, Myers KP, Gill JS, Gray GC. Testing human sera for antibodies against avian influenza viruses: horse RBC hemagglutination inhibition vs. microneutralization assays. J Clin Virol 2008. September;43(1):73–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Clements ML, Betts RF, Tierney EL, Murphy BR. Serum and nasal wash antibodies associated with resistance to experimental challenge with influenza A wild-type virus. J Clin Microbiol 1986. July;24(1):157–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Tumpey TM, Renshaw M, Clements JD, Katz JM. Mucosal delivery of inactivated influenza vaccine induces B-cell-dependent heterosubtypic cross-protection against lethal influenza A H5N1 virus infection. J Virol 2001. June;75(11):5141–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Benton KA, Misplon JA, Lo CY, Brutkiewicz RR, Prasad SA, Epstein SL. Heterosubtypic immunity to influenza A virus in mice lacking IgA, all Ig, NKT cells, or gamma delta T cells. J Immunol 2001. June 15;166(12):7437–45. [DOI] [PubMed] [Google Scholar]
- [33].Shio MT, Kassa FA, Bellemare MJ, Olivier M. Innate inflammatory response to the malarial pigment hemozoin. Microbes Infect 2010. November;12(12–13):889–99. [DOI] [PubMed] [Google Scholar]