Abstract
Exposure to trichloroethene (TCE), an occupational and ubiquitous environmental contaminant, is associated with the development of several autoimmune diseases, including autoimmune hepatitis (AIH). However, mechanisms contributing to TCE-mediated AIH are not known. Earlier, we have shown that dichloroacetyl chloride (DCAC), one of the reactive metabolites of TCE with strong acylating capability, can elicit an autoimmune response at much lower dose than TCE in female MRL+/+ mice. Furthermore, Kupffer cells (KCs), the liver resident macrophages, are crucial for hepatic homeostasis, but can also participate in the immunopathogenesis of AIH. However, contribution of KCs in TCE-mediated AIH and the underlying mechanisms are not understood. We hypothesized that increased apoptosis and delayed clearance of apoptotic bodies, due to compromised KC function, will result in the breakdown of self-tolerance, autoimmunity, and ultimately AIH. Therefore, using an in vitro model of immortalized mouse KCs, we investigated the contribution of DCAC in TCE-mediated AIH. KCs were treated with different concentrations of DCAC and apoptosis was measured by Annexin V and PI staining. Also, the impact of DCAC on phagocytic potential of KCs was evaluated. Furthermore, markers of inflammasome (NLRP3 and caspase1) were analyzed by real-time PCR and Western blot analysis. DCAC treatment resulted in significantly increased early and late-stage apoptosis, accompanied with inflammasome activation (NLRP3 increases). DCAC treatment resulted in decreased phagocytic function of KCs in a dose-dependent manner, with reduced MFG-E8 levels (phagocytotic function). Furthermore, DCAC exposure led to induction of phos-ERK and phos-AKT signaling. These findings suggest that DCAC induces apoptosis and inflammasome activation, while compromising the phagocytic function of KCs. Our data support that increased apoptosis and impaired KC function by DCAC could be contributory to TCE-mediated AIH.
Introduction
Trichloroethene (trichloroethylene, TCE) is a chlorinated toxic solvent and widely used as industrial degreaser. Exposure to TCE is associated with several autoimmune diseases, including autoimmune hepatitis (AIH) [1–3]. AIH is a global disease in all ages and ethnicities with a female predominance [4]. AIH is characterized by loss of tolerance against self-antigens, where autoreactive T cells lead to progressive liver injury and ultimately failure [5]. However, the precise aetiology of AIH is not fully understood.
It has been shown that TCE exerts its toxic effects primarily in liver and kidney through its metabolites [6]. Earlier, we have demonstrated that exposure to dichloroacetyl chloride (DCAC), one of the metabolites of TCE with strong acylating property, causes induction of autoimmune response, evident from several increased autoantibodies [1, 7, 8]. Kupffer cells (KCs) are key component of hepatic innate immune system through their role in phagocytosis, antigen presentation and cytokine production [9]. KC-mediated phagocytosis is critical for the clearance of the pathogens, antigens or apoptotic bodies to maintain the homeostasis [9]. Compromised phagocytic function of KCs may then cause the accumulation of apoptotic cells/bodies, generation of neoantigens, leading to initiation of inflammatory responses in the liver and subsequently AIH [10]. The dysregulation of immune response in AIH is initiated by presentation of self-antigens to naïve T cells by antigen presentation cells, like dendritic cells (DCs) and KCs [11, 12]. Naïve CD4 T cells then differentiate into Th1, Th2 and Th17 cells, which are associated with the secretion of different cytokines, contributing to the pathogenesis of AIH [13, 14]. Numerous studies have demonstrated that IL17 levels correlate with the hepatic inflammation and fibrosis in patients with AIH [13, 15]. Furthermore, our previous study has shown that long-term TCE exposure (24 or 36 weeks) results in decreased number and function of KCs in the livers of female MRL+/+ mice [2], suggesting that KC function is critical in the pathogenesis of TCE-mediated AIH. However, contribution of DCAC in TCE-mediated KC dysfunction, cytotoxicity and/or AIH and underlying mechanisms are largely unknown.
TCE-mediated AIH is associated with increased oxidative stress, cytokine production and apoptosis in the liver [16, 17]. NOD-like receptor protein 3 (NLRP3) inflammasome is activated by ROS production, resulting in inflammation and programmed cell death in liver disorders, such as viral hepatitis, non-alcoholic fatty liver disease and AIH [18–21]. Activation of NLRP3 may be an important mechanism by which TCE or its metabolites induce an immune response that leads to AIH in MLR+/+ mice. Therefore, to understand the mechanism of TCE-mediated AIH, the present study examined the potential of DCAC, a reactive metabolite of TCE, in inducing inflammasome activation, apoptosis, and impaired phagocytosis of KCs in vitro using murine kupffer cells.
Materials and methods
Reagents
Primary antibodies against AKT, phosphor-AKT (S473), phosphor-AKT (T308), phosphor-ERK, phosphor-mTOR (Ser2448), ERK and NLRP3 were purchased from Cell Signaling Technology (Danvers, MA). Antibodies against MFG-E8 and Caspase1 were purchased from R&D systems (Minneapolis, MN). Immortalized murine kupffer cell line was obtained from EMD Millipore [22], and DCAC was obtained from Sigma-Aldrich (St. Louis, MO). FluoSpheres Carboxylate-Modified Microspheres, size 1.0 μm were purchased from Thermo Fisher Scientific (Waltham, MA).
Apoptosis assays
Kupffer cells (KCs) were cultured with complete RPMI 1640 medium and were used for experiments before passage 5. KCs were plated in 12-well plates overnight and then treated with DCAC (0, 1, 2.5 or 5 mM) for 24 h. Cells were detached using accutase cell detachment solution (BD, Franklin Lakes, NJ), followed by APC-labeled Annexin-V and PI staining. Data were acquired by BD LSR Fortessa, and were analyzed by Flowjo10 software (Franklin Lakes, NJ).
Phagocytosis assays
For phagocytosis analysis, KCs were treated with DCAC as described above. After 24 h, FluoSpheres Carboxylate-Modified Microspheres (200 particles/cell) were added and incubated for 15 min. The supernatant was decanted and the cells were washed with PBS for five times. Fluorescent images were taken from 10 different fields per treatment. The FluoSphere positive cells and the total cells per image were counted to calculate the ratio of FluoSphere positive cells to total cells.
Real time PCR
Total RNA was extracted from cells using RNeasy Mini Kit (Qiagen, Valencia, CA). cDNA was synthesized with iScript™ cDNA synthesis kit (Bio-Rad Laboratories, Hercules, CA). Real-time PCR was conducted according to Wang et al [17]. The mRNA expression of selected genes related to inflammation, inflammasome and phagocytosis were analyzed. Mouse glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as the housekeeping gene. Primer sequences are listed in S1 Table.
Western blot analysis
Protein samples from lysed cells were prepared in RIPA buffer (Cell Signaling Technology, Danvers, MA) supplemented with protease inhibitors (Sigma-Aldrich, St. Louis, MO). Ten μg of protein lysates were subjected to Western blot analyses using antibodies against specific target proteins. Immunoblot images were quantified using Image Studio Lite Ver 5.2 and the intensity was determined and normalized to a respective loading control protein GAPDH.
Statistical analysis
All values are expressed as means ± SEM. Intergroup differences were appropriately assessed by one-way analysis of variance (ANOVA) followed by Tukey-Kramer multiple comparisons test. The p values <0.05 were considered to be statistically significant. * p < 0.05 and ** p < 0.01.
Results
DCAC-induced early and late stage apoptosis in KCs
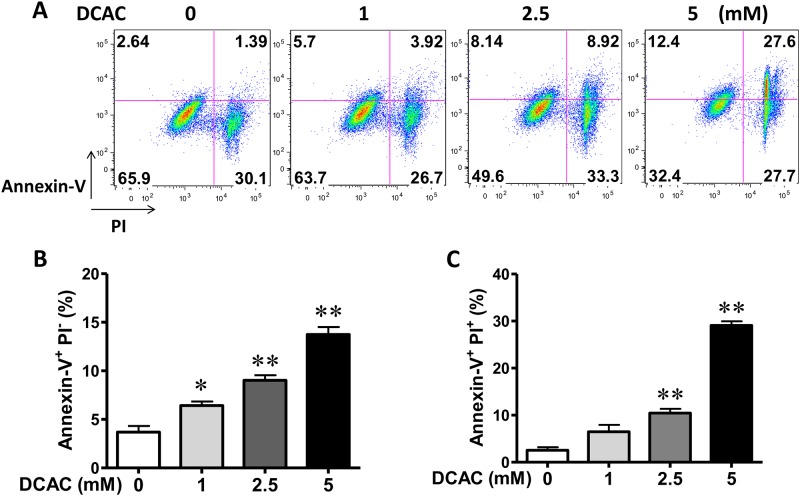
Previous studies have shown that TCE induces autoimmune responses in MLR+/+ mice [1]. To evaluate the contribution of DCAC in TCE-mediated autoimmunity, we investigated the effect of DCAC exposure on cell apoptosis in vitro. Early apoptosis and late apoptosis were quantified using annexin V and propidium iodide staining by FACS, and quantified as Annexin V+ PI- and Annexin V+ PI+ cells (Fig 1A). DCAC treatment resulted in dose-dependent and statistically significant increases in both early- and late-stage apoptosis after 24 h exposure, with higher percentage of Annexin V+PI- and Annexin V+ PI+ cells (Fig 1B and 1C).
Fig 1. Effect of DCAC on apoptosis of KCs.
Cells were exposed to DCAC at concentrations of 0, 1, 2.5 or 5 mM for 24 h. Cell apoptosis was determined by Annex V staining and detected by FACS. (A) Flow cytometry analysis of cell apoptosis. (B) The percentage of early and late-stage apoptotic cells are represented in histogram. n = 3 in each group. *p<0.05; **p<0.01, as compared to the control.
DCAC inhibited the phagocytic activity of KCs
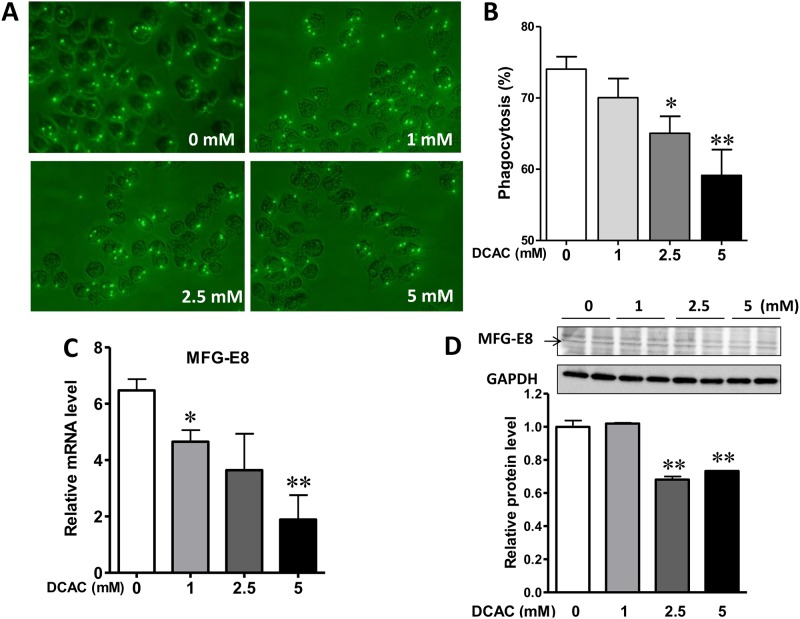
One of the major roles of KCs is to clear the apoptotic bodies from the injured liver, and delayed clearance due to compromised phagocytic function of KCs could lead to activation of hepatic inflammatory responses, eventually eliciting an autoimmune response [23]. Therefore, we examined the effect of DCAC on the phagocytic activity and determined the effect of DCAC on KC function. DCDC exposure led to decreased phagocytic activity, evidenced by reduced number of cells positive for beads (Fig 2A and 2B). Moreover, the number of beads per cell also tended to decrease after DCAC treatment. Milk factor globule EGF factor 8 (MFG-E8), which represents a link between apoptotic cells and phagocytes, enhances clearance of apoptotic cells and suppresses inflammatory responses [24]. We, therefore, also investigated the alterations in MFG-E8 expression in KCs after DCAC exposure. MFG-E8 expression was markedly reduced after DCAC exposure (Fig 2C and 2D). These results indicate that DCAC exposure down-regulates MFG-E8 expression and reduces phagocytic activity.
Fig 2. Effect of DCAC on KC phagocytosis.
Cells were treated with different concentrations of DCAC for 24 h, and then incubated with fluorescent latex beads for 30 min. (A) Representative images of KCs treated with DCAC (0, 1, 2.5 or 5 mM) for 24 h, showing decreased phagocytic activity after DCAC exposure. (B) The KC phagocytosis was measured by counting the bead-internalized KCs from 10 different fields and the percentage of bead-positive cells was calculated, (C) Relative mRNA level of MFG-E8 (phagocytic function), as measured by RT-PCR, and (D) Relative protein level of MFG-E8, as determined by Western blotting. n = 3 in each group. *p<0.05; **p<0.01, as compared to the control.
DCAC modulated iNOS expression and inflammatory cytokines
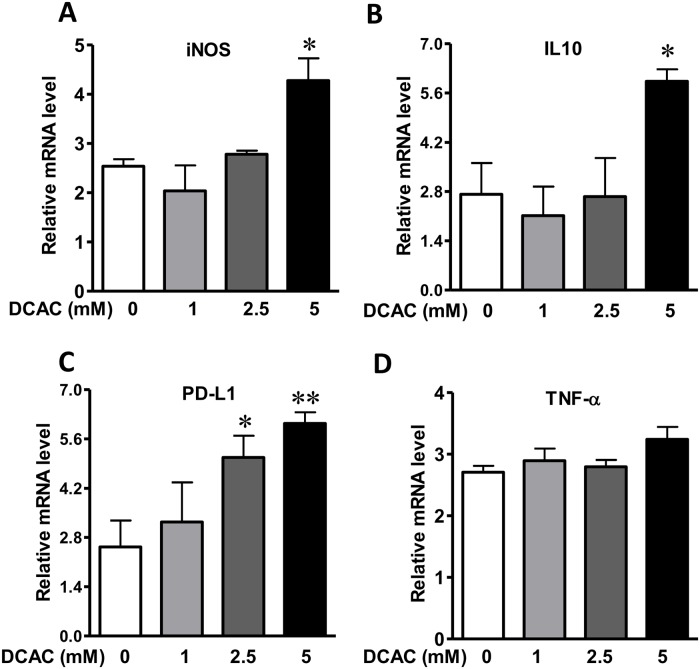
Activated KCs secrete cytokines and chemokines to recruit and activate innate immune cells, which could play a pivotal role in the pathogenesis of AIH [25]. To assess whether DCAC treatment can elicit an inflammatory response in KCs, DCAC pre-treated KCs were stimulated with LPS (100 ng/ml) for 4h. Elevated expression of iNOS and IL10 was observed in KCs treated with higher concentration of DCAC (Fig 3A and 3B), which was also associated with increased caspase1 and NLRP3 expression (Fig 4A and 4B). Moreover, up-regulation of PD-L1 was also observed following DCAC treatment at 2.5 and 5 mM (Fig 3C). However, no significant difference was observed in TNF-α level following DCAC exposure (Fig 3D).
Fig 3. Effect of DCAC on the expression of iNOS, inflammatory cytokines and apoptosis related gene in KCs.
KCs are pre-treated with DCAC for 24 h and cultured with or without LPS (200 ng/ml) for 4 h. qPCR was performed to determine the expression of iNOS and inflammatory cytokines: iNOS (A), IL-10 (B), TNF-α (C) and PD-L1 (D). n = 3 in each group. *p<0.05 vs. the controls.
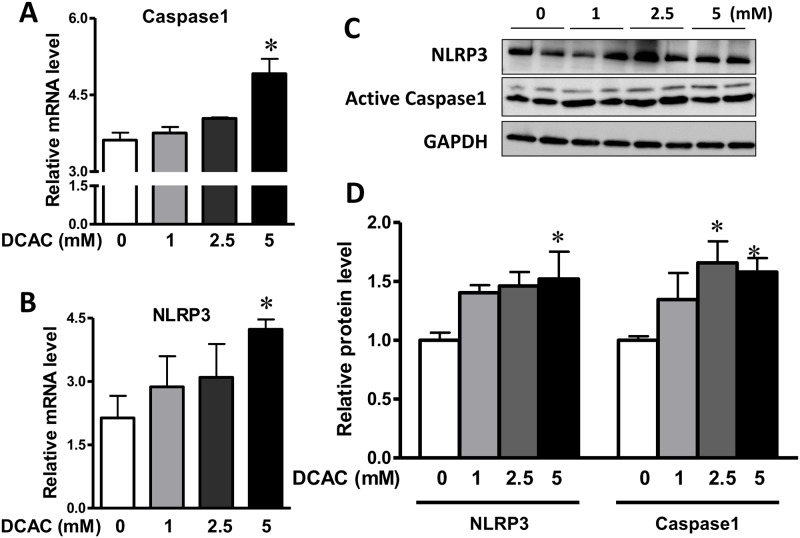
Fig 4. DCAC promoted inflammasome activation in KCs.
KCs were pre-treated with DCAC at concentrations of 0, 1, 2.5 or 5 mM for 24 h. qPCR and Western blot analyses were done for the expression of inflammasome (NLRP3 and caspase1). (A, B) mRNA levels of the caspase1 and NLRP3, as measured by RT-PCR, and (C) Relative protein levels of NLRP3 and caspase1, as determined by Western blotting. n = 3 in each group.*p<0.05 vs. the controls.
DCAC induced inflammasome activation
Inflammasome activation in KCs results in a wide range of immune responses, including cytokine production, immune cell differentiation and programed cell death [20, 26, 27]. We determined the effect of DCAC on inflammasome activity to evaluate whether DCAC-induced apoptosis also involved inflammasome activation. Activated caspase1 and NLRP3 were measured at both mRNA and protein levels after DCAC treatment. Activated caspase 1 was significantly increased with DCAC treatment at 2.5 and 5 mM (Fig 4A and 4C). More importantly, both mRNA and protein levels of NLPR3 showed increasing trends and were significantly higher following exposure to 5 mM DCAC (Fig 4B and 4C).
DCAC up-regulates AKT-mTOR and MAPK signaling pathways
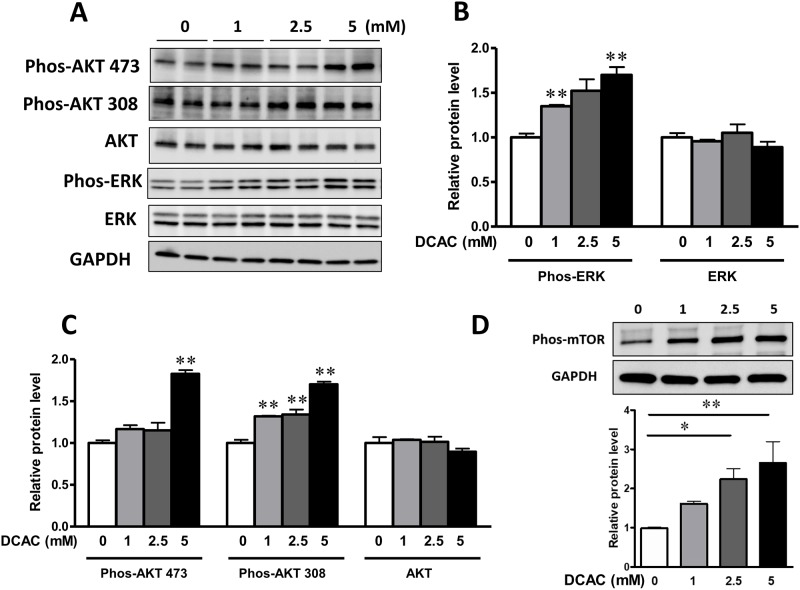
To further investigate the mechanism of DCAC-induced apoptosis and compromised phagocytic KC function, we evaluated the effects of DCAC on reactive oxygen species (ROS) sensitive AKT and ERK phosphorylation. AKT-mTOR activation has been reported as a pro-death signal in insulin-induced programed cell death [28]. In KCs, phosphorylation of phos-AKT Ser-473, phos-AKT Thr-308 and downstream mTOR were significantly increased in response to DCAC treatment (Fig 5A, 5C and 5D). Furthermore, DCAC exposure exhibited significantly increased levels of phospho-ERK (Fig 5A and 5B) among the MAPKs.
Fig 5. DCAC up-regulated the AKT/mTOR and MAPK signaling in KCs.
KCs were pre-treated with DCAC at concentrations of 0, 1, 2.5 or 5 mM for 24 h. (A) Representative images of the cellular levels of phos-AKT S473, phos-AKT T308, total AKT, phos-ERK and ERK, as determined by Western blotting, (B) Relative phos-ERK level, as determined by Western blotting, (C) Relative protein levels of phos-AKT(Ser-473) and phos-AKT(Thr-308), as determined by Western blotting, and (D) Representative images and relative phos-mTOR, as determined by Western blotting. n = 3 in each group. *p<0.05; **p<0.01, as compared to the controls.
Discussion
TCE is an extensively used industrial solvent and a ubiquitous environmental contaminant. TCE exposure has been associated with autoimmune responses in both humans and experimental animals [1, 3, 29]. Long-term exposure to TCE via drinking water has been shown to result in the induction of AIH in MRL+/+ mice, evidenced by increased apoptosis and reduced number and function of KCs [2]. TCE-mediated AIH is also associated with increased hepatic inflammation in MRL+/+ mice [30]. However, mechanism(s) by which TCE leads to induction of AIH is not well understood. Interestingly, DCAC, one of TCE metabolites with strong acylating capacity, induces an autoimmune response in MRL+/+ mice at a much lower concentration than TCE [7], indicating that DCAC formation may be a contributory factor in TCE-induced AIH. In this study, we aimed to further evaluate the contribution of DCAC in TCE-mediated AIH by examining early molecular events in KCs following DCAC treatment in vitro. Our results in this study show that DCAC induces both early and late-stage apoptosis in KCs, inhibits their phagocytic potential, and activates inflammasome as well as MAPK signaling.
Apoptosis plays an important role in various liver diseases [31]. Previous studies have shown that TCE exposure leads to alteration/induction of apoptosis-related genes in hepatocytes [32]. Our data in this study show that DCAC could be an inducer of both early and late-stage apoptosis in KCs. These findings are consistent with previous results and strongly support that TCE exposure induces AIH through increased hepatic apoptosis, which was also evidenced by greater TUNEL staining [2]. Apoptosis is the initial event which could result in non-specific inflammation due to ineffective clearance of the apoptotic bodies by functionally compromised KCs [12, 31, 33]. Our results thus suggest that DCAC-mediated apoptosis in KCs may be a mechanism contributing to TCE-mediated AIH in vivo.
KCs/macrophages are key components of the innate immune system which via phagocytosis, destroy and clear the dead bodies, foreign materials and microbes [34, 35]. KCs are also involved in the immune-mediated liver diseases, and their phagocytic activity is reported to be impaired in TCE-induced hepatitis [2]. The phagocytic activity of peripheral blood mononuclear cells is also significantly impaired in patients with AIH [10]. To determine the direct impact of DCAC treatment on the function of KCs, we evaluated KC’s phagocytic activity and a marker protein (MFG-E8) related to its function. Our data showed that DCAC exposure reduced the phagocytic function of KCs, as evident from inhibition in the phagocytosis of fluorescent latex beads. MFG-E8 is a phosphatidylserine recognition protein, enhancing the phagocytosis of apoptotic cells [36]. MFG-E8 exhibits protective role via attenuating inflammatory responses in systemic lupus erythematosus [37] and reducing ROS in subarachnoid hemorrhage [38]. MFG-E8 deficiency promotes autoimmunity by delaying clearance of dying cells and altering intracellular antigen processing, leading to autoimmune diseases [39]. Our results showed that the expression of MFG-E8 was significantly decreased after DCAC exposure, suggesting that DCAC impaired the phagocytic function of KC via down-regulating MFG-E8, and this could potentially contribute to TCE-mediated AIH.
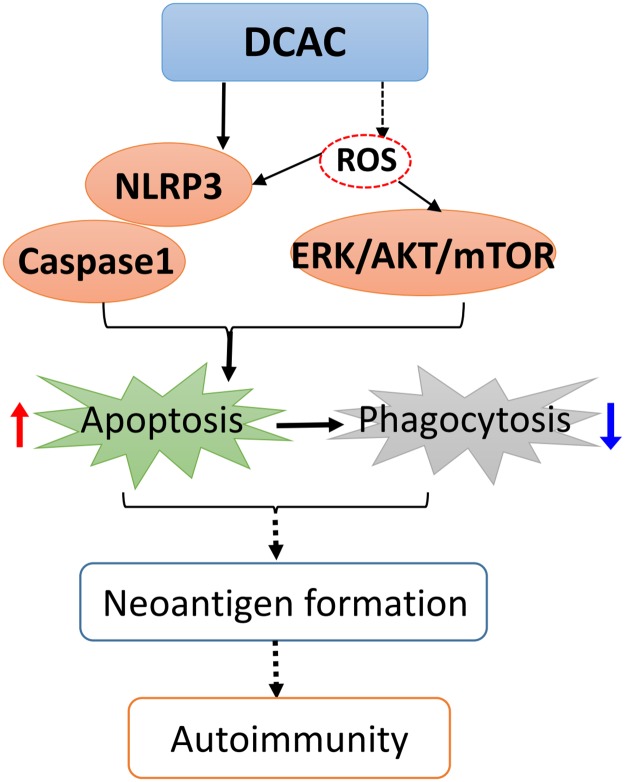
To determine the signaling pathways involved in DCAC-induced apoptosis and impaired phagocytosis, we evaluated the apoptosis related inflammasome activation. Our data show a significant upregulation of NLRP3, a novel regulator of inflammation and cell death, along with active caspase1 after KCs were exposed to DCAC, suggesting the involvement of inflammasome activation during DCAC-induced apoptosis. NLRP3 activation has been reported to induce pyroptosis and apoptosis, and NLRP3-dependent IL-1β production could contribute to the pathogenesis of AIH [40]. Our laboratory previously reported that TCE exposure causes oxidative stress (OS) and also iNOS induction (nitrosative stress) [17, 41, 42]. Furthermore, iNOS knockout MRL+/+ mice show attenuation of TCE-induced autoimmunity [43]. In the present study, the observed increase in iNOS level after DCAC treatment, suggests that DCAC is not only a strong acylating metabolite, but also ROS/reactive nitrogen species (RNS) inducing agent. PD-L1 level in macrophages has been correlated with nitric oxide production and serves as a marker for “primed” inflammatory macrophages [44]. In AIH patients, PD-L1 was co-localized with macrophages and its mRNA expression in the liver was significantly increased [45]. Elevated PD-L1 level was also observed in KCs after DCAC treatment in this study, which is consistent with increased NLRP3 and caspase1 levels. Since increasing evidences provide a link between ROS generation and activation of MAPK signaling pathways [46–48], we, therefore, examined if DCAC also modulated MAPK signaling in KCs. In fact, we observed a dose-dependent induction of phos-ERK, a member of the MAP kinase family of serine/threonine kinases. Our findings are thus consistent with earlier observations that ERK activation could promote intrinsic and/or extrinsic apoptosis in various cell types including KCs [49, 50]. AKT is a metabolic regulator in macrophages activated by intracellular ROS [51, 52], and AKT activation is associated with inflammation and IL-10 production in macrophages [53, 54]. Here, we observed that DCAC exposure increased the expression of phos-AKT Ser-473 and phos-AKT Thr-308, as well as its downstream phos-mTOR. Our findings on DCAC-induced increases in phos-ERK and phos-AKT/mTOR are consistent with elevated ROS/RNS observed in our previous in vivo studies and provide further support to the role OS in TCE-mediated autoimmunity [17, 43]. The key findings on DCAC-induced inflammasome activation, apoptosis and compromised phagocytosis of KCs, leading to Neoantigen formation, and ultimately to AIH are presented in Fig 6.
Fig 6. The plausible mechanism of DCAC-induced autoimmune response.
DCAC can induce inflammasome NLRP3/caspase1 and ROS-related ERK/AKT/mTOR activation in Kupffer cells, leading to increased apoptosis and impaired phagocytosis. Ultimately, delayed clearance of apoptotic bodies due to compromised Kupffer cell function will result in the breakdown of self-tolerance, autoimmunity, and eventually AIH. “------”, established with parent compound TCE.
In summary, our study showed that TCE metabolite DCAC led to increased apoptosis and impaired phagocytic function of KCs. Additionally, we demonstrated that DCAC induced the activation of inflammasome, phos-ERK and phos-AKT/mTOR. Our data thus suggest that increased apoptosis and impaired KC phagocytic function by DCAC could be an important mechanism by which TCE could mediate AIH.
Supporting information
(DOCX)
Acknowledgments
This work was supported by RO1 Grants ES026887 and ES016302 from the National Institute of Environmental Health Sciences (NIEHS), National Institute of Health (NIH), USA. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This work was supported by RO1 Grants ES026887 and ES016302 from the National Institute of Environmental Health Sciences (NIEHS), National Institute of Health (NIH), USA. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
References
- 1.Khan MF, Kaphalia BS, Prabhakar BS, Kanz MF, Ansari GAS. Trichloroethene-Induced Autoimmune-Response in Female Mrl-+/+ Mice. Toxicol Appl Pharm. 1995;134(1):155–60. 10.1006/taap.1995.1179 [DOI] [PubMed] [Google Scholar]
- 2.Kondraganti S, Boor PJ, Khan S, Kaphalia BS, Khan MF, Ansari GAS. Mechanistic Evaluation of Trichloroethene-Mediated Autoimmune Hepatitis-Like Disease In Female MRL+/+ Mice. The Open Toxicology Journal. 2012;5:10. [Google Scholar]
- 3.Gilbert KM, Reisfeld B, Zurlinden TJ, Kreps MN, Erickson SW, Blossom SJ. Modeling toxicodynamic effects of trichloroethylene on liver in mouse model of autoimmune hepatitis. Toxicol Appl Pharmacol. 2014;279(3):284–93. 10.1016/j.taap.2014.07.003 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giorgina Mieli-Vergani DV, Czaja Albert J., Manns Michael P., Krawitt Edward L., Vierling John M., Lohse Ansgar W. & Montano-Loza Aldo J. Autoimmune hepatitis. Nat Rev Dis Primers. 2018;4:18018 10.1038/nrdp.2018.18 . [DOI] [PubMed] [Google Scholar]
- 5.Aizawa Y, Hokari A. Autoimmune hepatitis: current challenges and future prospects. Clin Exp Gastroenter. 2017;10:9–18. 10.2147/Ceg.S101440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lash LH, Putt DA, Parker JC. Metabolism and tissue distribution of orally administered trichloroethylene in male and female rats: identification of glutathione- and cytochrome P-450-derived metabolites in liver, kidney, blood, and urine. J Toxicol Environ Health A. 2006;69(13):1285–309. 10.1080/15287390500360133 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khan MF, Kaphalia BS, Ansari GAS. Time-dependent autoimmune response of dichloroacetyl chloride in female MRL +/+ mice. Immunopharm Immunot. 1997;19(2):265–77. 10.3109/08923979709007662 [DOI] [PubMed] [Google Scholar]
- 8.Cai P, Boor PJ, Khan MF, Kaphalia BS, Ansari GA, Konig R. Immuno- and hepato-toxicity of dichloroacetic acid in MRL(+/+) and B(6)C(3)F(1) mice. J Immunotoxicol. 2007;4(2):107–15. 10.1080/15476910701337225 . [DOI] [PubMed] [Google Scholar]
- 9.Bilzer M, Roggel F, Gerbes AL. Role of Kupffer cells in host defense and liver disease. Liver Int. 2006;26(10):1175–86. 10.1111/j.1478-3231.2006.01342.x . [DOI] [PubMed] [Google Scholar]
- 10.Lin R, Zhang J, Zhou L, Wang BM. Altered function of monocytes/macrophages in patients with autoimmune hepatitis. Mol Med Rep. 2016;13(5):3874–80. 10.3892/mmr.2016.4998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hopp AK, Rupp A, Lukacs-Kornek V. Self-antigen presentation by dendritic cells in autoimmunity. Front Immunol. 2014;5:55 10.3389/fimmu.2014.00055 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Navegantes KC, de Souza Gomes R, Pereira PAT, Czaikoski PG, Azevedo CHM, Monteiro MC. Immune modulation of some autoimmune diseases: the critical role of macrophages and neutrophils in the innate and adaptive immunity. J Transl Med. 2017;15(1):36 10.1186/s12967-017-1141-8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao L, Tang Y, You Z, Wang Q, Liang S, Han X, et al. Interleukin-17 contributes to the pathogenesis of autoimmune hepatitis through inducing hepatic interleukin-6 expression. PLoS One. 2011;6(4):e18909 10.1371/journal.pone.0018909 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Behfarjam F, Sanati MH, Nasseri Moghaddam S, Ataei M, Nikfam S, Jadali Z. Role of Th1/Th2 cells and related cytokines in autoimmune hepatitis. Turk J Gastroenterol. 2017;28(2):110–4. 10.5152/tjg.2017.17501 . [DOI] [PubMed] [Google Scholar]
- 15.Abe M, Hiasa Y, Onji M. T helper 17 cells in autoimmune liver diseases. Clin Dev Immunol. 2013;2013:607073 10.1155/2013/607073 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cai P, Konig R, Boor PJ, Kondraganti S, Kaphalia BS, Khan MF, et al. Chronic exposure to trichloroethene causes early onset of SLE-like disease in female MRL +/+ mice. Toxicol Appl Pharm. 2008;228(1):68–75. 10.1016/j.taap.2007.11.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang G, Ma H, Fan X, Wang J, Ansari GAS, Khan MF. Oxidative Stress and Trichloroethene-Mediated Autoimmunity. Free Radical Bio Med. 2010;49:S144–S. 10.1016/j.freeradbiomed.2010.10.403 [DOI] [Google Scholar]
- 18.Szabo G, Petrasek J. Inflammasome activation and function in liver disease. Nat Rev Gastroenterol Hepatol. 2015;12(7):387–400. 10.1038/nrgastro.2015.94 . [DOI] [PubMed] [Google Scholar]
- 19.Abais JM, Xia M, Zhang Y, Boini KM, Li P. Redox regulation of NLRP3 inflammasomes: ROS as trigger or effector? Antioxid Redox Signal. 2015;22(13):1111–29. 10.1089/ars.2014.5994 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Negash AA, Ramos HJ, Crochet N, Lau DT, Doehle B, Papic N, et al. IL-1beta production through the NLRP3 inflammasome by hepatic macrophages links hepatitis C virus infection with liver inflammation and disease. PLoS Pathog. 2013;9(4):e1003330 10.1371/journal.ppat.1003330 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thomas H. NAFLD: A critical role for the NLRP3 inflammasome in NASH. Nat Rev Gastroenterol Hepatol. 2017;14(4):197 10.1038/nrgastro.2017.21 . [DOI] [PubMed] [Google Scholar]
- 22.Wang Z, Burlak C, Klaunig JE, Kamendulis LM. Development of a cytokine-producing immortalized murine Kupffer cell line. Cytokine. 2014;70(2):165–72. 10.1016/j.cyto.2014.07.251 . [DOI] [PubMed] [Google Scholar]
- 23.Nagata S, Hanayama R, Kawane K. Autoimmunity and the clearance of dead cells. Cell. 2010;140(5):619–30. 10.1016/j.cell.2010.02.014 . [DOI] [PubMed] [Google Scholar]
- 24.Huang W, Wu JY, Yang HQ, Xiong Y, Jiang R, Cui TP, et al. Milk fat globule-EGF factor 8 suppresses the aberrant immune response of systemic lupus erythematosus-derived neutrophils and associated tissue damage. Cell Death and Differentiation. 2017;24(2):263–75. 10.1038/cdd.2016.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ju C, Tacke F. Hepatic macrophages in homeostasis and liver diseases: from pathogenesis to novel therapeutic strategies. Cell Mol Immunol. 2016;13(3):316–27. 10.1038/cmi.2015.104 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Szabo G, Csak T. Inflammasomes in liver diseases. J Hepatol. 2012;57(3):642–54. 10.1016/j.jhep.2012.03.035 . [DOI] [PubMed] [Google Scholar]
- 27.Zannetti C, Roblot G, Charrier E, Ainouze M, Tout I, Briat F, et al. Characterization of the inflammasome in human kupffer cells in response to synthetic agonists and pathogens. J Immunol. 2016;197(1):356–67. 10.4049/jimmunol.1502301 . [DOI] [PubMed] [Google Scholar]
- 28.Wu YT, Tan HL, Huang Q, Ong CN, Shen HM. Activation of the PI3K-Akt-mTOR signaling pathway promotes necrotic cell death via suppression of autophagy. Autophagy. 2009;5(6):824–34. 10.4161/auto.9099 [DOI] [PubMed] [Google Scholar]
- 29.Cooper GS, Makris SL, Nietert PJ, Jinot J. Evidence of autoimmune-related effects of trichloroethylene exposure from studies in mice and humans. Environ Health Perspect. 2009;117(5):696–702. 10.1289/ehp.11782 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gilbert KM, Przybyla B, Pumford NR, Han T, Fuscoe J, Schnackenberg LK, et al. Delineating liver events in trichloroethylene-induced autoimmune hepatitis. Chem Res Toxicol. 2009;22(4):626–32. 10.1021/tx800409r [DOI] [PubMed] [Google Scholar]
- 31.Guicciardi ME, Gores GJ. Apoptosis: a mechanism of acute and chronic liver injury. Gut. 2005;54(7):1024–33. 10.1136/gut.2004.053850 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu X, Liu Y, Lu L, Ke Y, Mao J, Mao K. Altered expression of hepatic metabolic enzyme and apoptosis-related gene transcripts in human hepatocytes treated with trichloroethylene. Hum Exp Toxicol. 2012;31(9):861–7. 10.1177/0960327112444935 [DOI] [PubMed] [Google Scholar]
- 33.Schumann J, Wolf D, Pahl A, Brune K, Papadopoulos T, van Rooijen N, et al. Importance of Kupffer cells for T-cell-dependent liver injury in mice. Am J Pathol. 2000;157(5):1671–83. 10.1016/S0002-9440(10)64804-3 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shao W, Cohen PL. Disturbances of apoptotic cell clearance in systemic lupus erythematosus. Arthritis Res Ther. 2011;13(1):202 10.1186/ar3206 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martin CJ, Peters KN, Behar SM. Macrophages clean up: efferocytosis and microbial control. Curr Opin Microbiol. 2014;17:17–23. 10.1016/j.mib.2013.10.007 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu Y, Tibrewal N, Birge RB. Phosphatidylserine recognition by phagocytes: a view to a kill. Trends Cell Biol. 2006;16(4):189–97. 10.1016/j.tcb.2006.02.003 . [DOI] [PubMed] [Google Scholar]
- 37.Huang W, Wu J, Yang H, Xiong Y, Jiang R, Cui T, et al. Milk fat globule-EGF factor 8 suppresses the aberrant immune response of systemic lupus erythematosus-derived neutrophils and associated tissue damage. Cell Death Differ. 2017;24(2):263–75. 10.1038/cdd.2016.115 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu F, Hu Q, Li B, Manaenko A, Chen Y, Tang J, et al. Recombinant milk fat globule-EGF factor-8 reduces oxidative stress via integrin beta3/nuclear factor erythroid 2-related factor 2/heme oxygenase pathway in subarachnoid hemorrhage rats. Stroke. 2014;45(12):3691–7. 10.1161/STROKEAHA.114.006635 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peng YF, Elkon KB. Autoimmunity in MFG-E8-deficient mice is associated with altered trafficking and enhanced cross-presentation of apoptotic cell antigens. J Clin Invest. 2011;121(6):2221–41. 10.1172/JCI43254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luan J, Zhang X, Wang S, Li Y, Fan J, Chen W, et al. NOD-Like Receptor Protein 3 Inflammasome-Dependent IL-1beta Accelerated ConA-Induced Hepatitis. Front Immunol. 2018;9:758 10.3389/fimmu.2018.00758 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang G, Li H, Khan MF. Differential oxidative modification of proteins in MRL+/+ and MRL/lpr mice: Increased formation of lipid peroxidation-derived aldehyde-protein adducts may contribute to accelerated onset of autoimmune response. Free Radic Res. 2012;46(12):1472–81. 10.3109/10715762.2012.727209 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang G, Pierangeli SS, Papalardo E, Ansari GA, Khan MF. Markers of oxidative and nitrosative stress in systemic lupus erythematosus: correlation with disease activity. Arthritis Rheum. 2010;62(7):2064–72. 10.1002/art.27442 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang G, Wakamiya M, Wang J, Ansari GAS, Khan MF. iNOS null MRL +/+ mice show attenuation of trichloroethene-mediated autoimmunity: contribution of reactive nitrogen species and lipid-derived reactive aldehydes. Free Radical Bio Med. 2015;89:770–6. 10.1016/j.freeradbiomed.2015.10.402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Loke P, Allison JP. PD-L1 and PD-L2 are differentially regulated by Th1 and Th2 cells. Proc Natl Acad Sci USA. 2003;100(9):5336–41. 10.1073/pnas.0931259100 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mataki N, Kikuchi K, Kawai T, Higashiyama M, Okada Y, Kurihara C, et al. Expression of PD-1, PD-L1, and PD-L2 in the liver in autoimmune liver diseases. Am J Gastroenterol. 2007;102(2):302–12. 10.1111/j.1572-0241.2006.00948.x . [DOI] [PubMed] [Google Scholar]
- 46.Shin HJ, Kwon HK, Lee JH, Anwar MA, Choi S. Etoposide induced cytotoxicity mediated by ROS and ERK in human kidney proximal tubule cells. Sci Rep. 2016;6:34064 10.1038/srep34064 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Son Y, Cheong YK, Kim NH, Chung HT, Kang DG, Pae HO. Mitogen-Activated Protein Kinases and Reactive Oxygen Species: How Can ROS Activate MAPK Pathways? J Signal Transduct. 2011;2011:792639 10.1155/2011/792639 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khan MF, Kannan S, Wang J. Activation of transcription factor AP-1 and mitogen-activated protein kinases in aniline-induced splenic toxicity. Toxicol Appl Pharmacol. 2006;210(1–2):86–93. 10.1016/j.taap.2005.08.006 . [DOI] [PubMed] [Google Scholar]
- 49.Peng Y, Sigua CA, Murr MM. Protein kinase C-zeta mediates apoptosis of mouse Kupffer cells via ERK-1/2: A novel mechanism. Surgery. 2011;149(1):135–42. 10.1016/j.surg.2010.04.017 [DOI] [PubMed] [Google Scholar]
- 50.Cagnol S, Chambard JC. ERK and cell death: mechanisms of ERK-induced cell death—apoptosis, autophagy and senescence. FEBS J. 2010;277(1):2–21. 10.1111/j.1742-4658.2009.07366.x . [DOI] [PubMed] [Google Scholar]
- 51.Matta SK, Kumar D. Hypoxia and classical activation limits Mycobacterium tuberculosis survival by Akt-dependent glycolytic shift in macrophages. Cell Death Discov. 2016;2:16022 10.1038/cddiscovery.2016.22 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang Y, Zeigler MM, Lam GK, Hunter MG, Eubank TD, Khramtsov VV, et al. The role of the NADPH oxidase complex, p38 MAPK, and Akt in regulating human monocyte/macrophage survival. Am J Respir Cell Mol Biol. 2007;36(1):68–77. 10.1165/rcmb.2006-0165OC . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhong W, Qian K, Xiong J, Ma K, Wang A, Zou Y. Curcumin alleviates lipopolysaccharide induced sepsis and liver failure by suppression of oxidative stress-related inflammation via PI3K/AKT and NF-kappa B related signaling. Biomed Pharmacother. 2016;83:302–13. 10.1016/j.biopha.2016.06.036 [DOI] [PubMed] [Google Scholar]
- 54.Pengal RA, Ganesan LP, Wei G, Fang H, Ostrowski MC, Tridandapani S. Lipopolysaccharide-induced production of interleukin-10 is promoted by the serine/threonine kinase Akt. Mol Immunol. 2006;43(10):1557–64. 10.1016/j.molimm.2005.09.022 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.