Abstract
Haplotype-based methods compete with “one-SNP-at-a-time” approaches on being preferred for association studies. Chromosome 6 contains most of the known genetic biomarkers for rheumatoid arthritis (RA) disease. Therefore, chromosome 6 serves as a benchmark for the haplotype methods testing. The aim of this study is to test the North American Rheumatoid Arthritis Consortium (NARAC) dataset to find out if haplotype block methods or single-locus approaches alone can sufficiently provide the significant single nucleotide polymorphisms (SNPs) associated with RA. In addition, could we be satisfied with only one method of the haplotype block methods for partitioning chromosome 6 of the NARAC dataset? In the NARAC dataset, chromosome 6 comprises 35,574 SNPs for 2,062 individuals (868 cases, 1,194 controls). Individual SNP approach and three haplotype block methods were applied to the NARAC dataset to identify the RA biomarkers. We employed three haplotype partitioning methods which are confidence interval test (CIT), four gamete test (FGT), and solid spine of linkage disequilibrium (SSLD). P-values after stringent Bonferroni correction for multiple testing were measured to assess the strength of association between the genetic variants and RA susceptibility. Moreover, the block size (in base pairs (bp) and number of SNPs included), number of blocks, percentage of uncovered SNPs by the block method, percentage of significant blocks from the total number of blocks, number of significant haplotypes and SNPs were used to compare among the three haplotype block methods. Individual SNP, CIT, FGT, and SSLD methods detected 432, 1,086, 1,099, and 1,322 associated SNPs, respectively. Each method identified significant SNPs that were not detected by any other method (Individual SNP: 12, FGT: 37, CIT: 55, and SSLD: 189 SNPs). 916 SNPs were discovered by all the three haplotype block methods. 367 SNPs were discovered by the haplotype block methods and the individual SNP approach. The P-values of these 367 SNPs were lower than those of the SNPs uniquely detected by only one method. The 367 SNPs detected by all the methods represent promising candidates for RA susceptibility. They should be further investigated for the European population. A hybrid technique including the four methods should be applied to detect the significant SNPs associated with RA for chromosome 6 of the NARAC dataset. Moreover, SSLD method may be preferred for its favored benefits in case of selecting only one method.
Introduction
Many researchers associate RA disease with genetic biomarkers through individual SNP studies. Recently, the availability of high genomic density of SNPs allows the application of the haplotype block methods. These methods discover a group of SNPs within an associated block in only one test [1, 2].
The main advantages of haplotype block methods over individual SNP approaches are: (a) the reduction of the association testing dimension by using a single test for a block containing more than one SNP; (b) leading to power preservation and ensuring accepted false-positive rates [3]; (c) acquiring the synergy among SNPs. The main disadvantages of haplotype block methods are: (a) the more haplotypes within a block leads to a higher degree of freedom of the block ending in scaled down power; (b) each partitioning method ends up with haplotype blocks that differ from the others. Therefore, a comparison study should be performed to evaluate all partitioning methods’ performance [1, 4].
Singular SNP approaches achieve inspiring results if there should be an occurrence of monogenetic disorders (for example, sickle cell anemia). Then again, they don't achieve a similar accomplishment in complex diseases. The power of association was studied for individual SNP approaches and haplotype block methods resulting in contradictory findings. This inconsistency may be arisen from the dependency of the method’s performance on the nature of the experimented dataset itself [1, 5].
RA is a chronic autoimmune disease that is prevalent in women more than in men (with a ratio of about 3:1) [6–9]. The MHC (major histocompatibility complex) region extends on the short arm of chromosome 6 (6p21.3) from 26 to 34 Mb (mega base pair) [10]. The MHC region contains the HLA (human leukocyte antigen) region [11] which includes about 50% of the detected biomarkers for RA susceptibility. The association between HLA region and RA disease has been verified in multiethnic populations [12].
There are two objectives of this study. The first objective is comparing the association results of haplotype block methods and individual SNP approach on chromosome 6 of the NARAC dataset. The second objective is selecting the best haplotype block method suitable for the NARAC dataset (if applicable).
Materials and methods
Study population
The NARAC dataset consisted of 2,062 participants (1,493 females and 569 males), 868 RA patients and 1,194 healthy controls. All cases and controls were of European descent. All participants were genotyped on the HumanHap500 v1, Human Hap500 v3, HumanHap300, and HumanHap240 Illumina arrays [13]. The studied genetic variants were 35,574 SNPs included in chromosome 6. After removing 1,452 SNPs, 34,122 SNPs were retained for further analysis. The reasons for excluding the 1,452 SNPs were the biomarker checks: (a) less than 75% genotype percent, (b) less than 0.001 Hardy-Weinberg equilibrium (HWE) P-value or (c) less than 0.001 minor allele frequency (MAF) in the total sample.
Materials
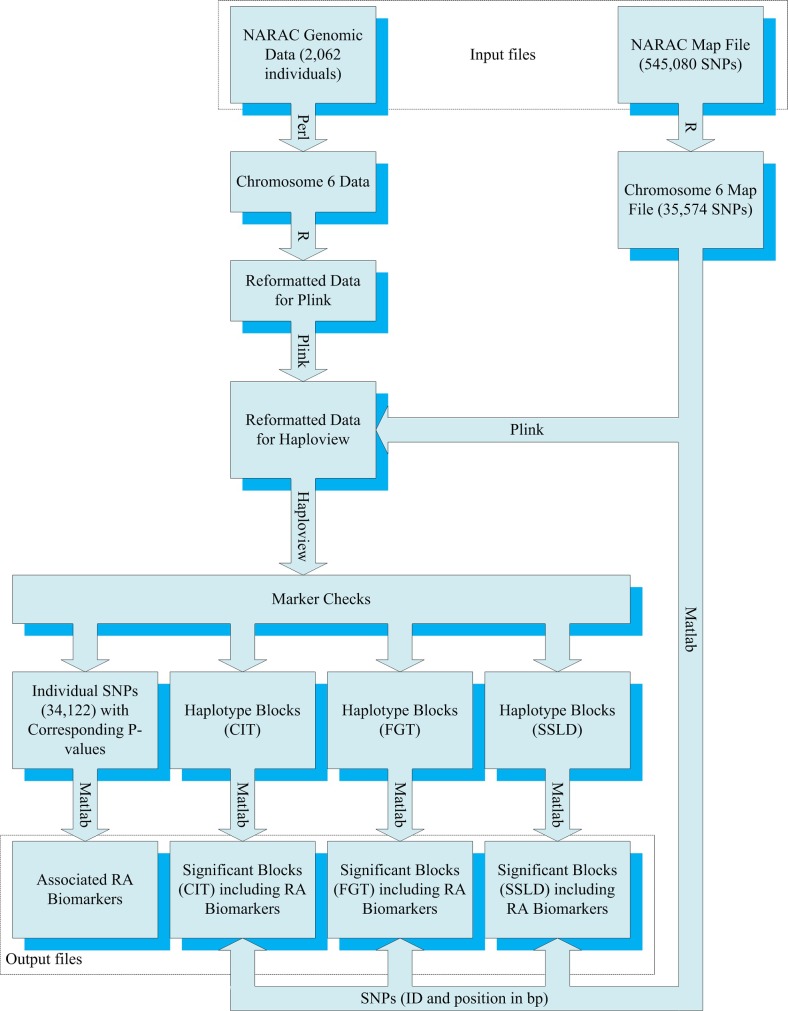
The chromosome 6 data file was extracted from the NARAC data file using the programming language (Perl). The chromosome 6 data file was reformatted to be ready for processing by the program (PLINK) by the statistical package (R 3.1.0). Moreover, the R language was used to extract the chromosome 6 map file from the NARAC map file (SNP ID, physical position, and chromosome number). The chromosome 6 reformatted data and map files were processed by (PLINK 1.07) and (gPLINK 2.05) programs to be ready for processing by the program (Haploview) [14].
The computer program (Haploview 4.2) was used to partition chromosome 6 into successive blocks using CIT, FGT, and SSLD methods and to calculate the corresponding P-value for each haplotype in each block. The default parameters for the three methods were used. In addition, the program (Haploview 4.2) was used to apply the individual SNP approach and to provide the corresponding P-value for each SNP [15]. The distribution of P-values on chromosome 6 for the individual SNP approach was presented using the Integrative Genomics Viewer (IGV 2.3.72) [16, 17]. The significant blocks and the associated SNPs were selected using the programming language (Matlab Release 2010a). Fig 1 showed a block diagram for the whole chromosomic association analysis process.
Fig 1. Experimental design of the proposed system.
Haplotype block methods
The haplotype blocks have been defined through more than one method. Several algorithms for haplotype partitioning have been proposed, among which the CIT, FGT, and SSLD have been implemented in HaploView 4.2. Each method differs greatly from the others in its scope of the definition of the haplotype blocks. Therefore, an objective has been arisen for comparing the results of these methods which was studied through this research paper [18].
Confidence interval test
A haplotype block is defined by the CIT method as a region over which a very small fraction (≤ 5%) of the measures among the informative SNP pairs are in weak linkage disequilibrium (LD). The informative SNP pairs are pairs showing strong LD or weak LD. Biological and artefactual forces in addition to recombination events are the reasons for allowing 5% of weak LD in the haplotype block. These forces could be recurrent mutation, gene conversion, or errors of the genome assembly or genotyping.
Dˊ (normalized deviation) is used to measure the LD between a pair of SNPs. CI is used to assess the reliability of the estimate of Dˊ. Strong LD is defined as the upper limit of the 90% CI is (0.98), and the lower limit is (0.7). In contrast, weak LD is defined as the upper limit of the 90% CI is (0.9), as shown in S1 Fig. The thresholds were obtained by Gabriel et al. Short blocks (2–5 SNPs) were treated with different thresholds for different populations to select the used thresholds [19].
Four gamete test
The FGT is a haplotype block partitioning method that assumes recombination events are not allowed within each block. When the four gametes are identified, a recombination event has been occurred. The rare gamete must be observed at a frequency greater than 0.01 to count a recombination event. The recombination events are only accepted between blocks. The FGT method differs from other haplotype block definitions that it does not require a threshold for Dˊ.
The recombination events interrupt the continuity of the testing process. When a recombination event is observed between the (kth) locus and any preceding locus, the locus (k-1) is considered the ending point of the tested block. The block size could be measured as the distance between the start locus and the end locus. In this situation, the locus (k) is considered the starting point to search for a new block [20].
Solid spine of linkage disequilibrium
The SSLD method defines the haplotype block as a region at which a (spine) of strong LD (Dˊ > 0.8) moving from one allele to the next allele along the legs of the triangle in the LD chart, as shown in S2 Fig. In other words, for each block, there must be a strong LD between (the first SNP and the last SNP) and all the intermediate SNPs. However, the intermediate SNPs should not be in strong LD with each other [15].
Table 1 represents the concept of the SSLD method. Five SNPs are tested, having the same results as in S2 Fig. Here, (SNP2 and SNP3), (SNP3 and SNP4), and (SNP2 and SNP4) are not in strong LD. All other combinations of SNPs are in strong LD. Thus, there is a haplotype block extends from SNP1 to SNP5. Table 2 shows a comparison among the three haplotype block definitions.
Table 1. Example on the solid spine of linkage disequilibrium (SSLD) method.
| SNP# | SNP1 | SNP2 | SNP3 | SNP4 | SNP5 |
|---|---|---|---|---|---|
| SNP1 | - | 0.97 | 0.99 | 0.93 | 0.96 |
| SNP2 | - | 0.18 | 0.67 | 0.98 | |
| SNP3 | - | 0.03 | 0.94 | ||
| SNP4 | - | 0.95 | |||
| SNP5 | - |
Table 2. A comparison among haplotype block definitions.
| Items | FGT | CIT | SSLD |
|---|---|---|---|
| Recombination Event within Block | Not Allowed | ≤ 5% | Allowed only between intermediate SNPs |
| Strong LD | LD is not used | Dˊ upper limit = 0.98 Dˊ lower limit = 0.7 |
Dˊ > 0.8 |
| Weak LD | LD is not used | Dˊ upper limit = 0.9 | Dˊ ≤ 0.8 |
| Morphology in the LD Chart | No recombination event between all SNPs in the block | > 95% Strong LD between all SNPs in the block | Strong LD in the legs of the LD chart |
Testing for associations with disease status
Both the individual SNP associations and the haplotype associations were measured with the aid of the P-values. The statistically significant SNPs were detected using their corresponding P-values after stringent Bonferroni correction for multiple testing (< 0.05/# of tests) [21]. The Bonferroni thresholds were 8.49 × 10−6 for the CIT method, 7.95 × 10−6 for the FGT method, 9.41 × 10−6 for the SSLD method, and 1.47 × 10−6 for the individual SNP approach where the total number of tests were 5,888 for the CIT method, 6,293 for the FGT method, 5,313 for the SSLD method, and 34,122 for the individual SNP approach.
Results
The testing algorithms were applied on Intel Core i7-4720HQ 2.6 GHz system with 16 GB of RAM. S1 Table provided the processing time for each used program. A total working time was 181 minutes.
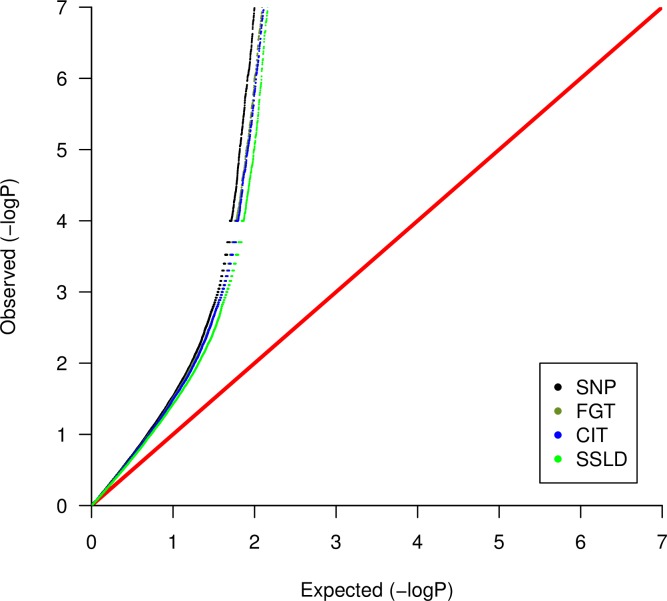
The distribution of the observed P-values for all the used models showed evidence for population stratification (Fig 2) [22, 23]. The reason for this stratification might be the selection of chromosome 6 that contains the HLA region where many highly significant associations occur. The distribution of the P-values across chromosome 6 was presented in Fig 3 for the individual SNP approach. The top SNP (rs660895) in the HLA region (32,685,358 bp) had the lowest P-value (1.03 X 10−113) as previously reported in Arya et al. [24].
Fig 2. Quantile–quantile (Q–Q) plots of the chromosome 6 association results for all the used methods.
Q-Q plot shows the observed distribution of–log10(P-values) on the Y-axis compared to the expected distribution of–log10(P-values) on the X-axis. The red line (X = Y) represents the null distribution. SNP refers to the individual SNP approach.
Fig 3. Manhattan plot for the associations between genotyped SNPs (chromosome 6 of the NARAC dataset) and RA susceptibility using individual SNP approach.
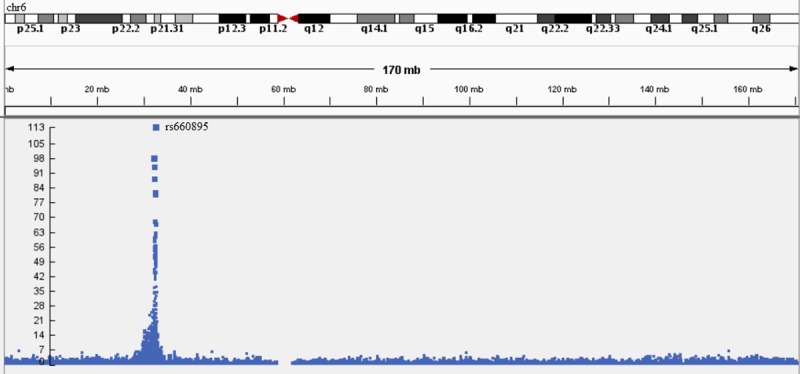
The upper part represents chromosome 6 ideogram and its genomic coordinates. The Y-axis shows–log10(P-values), which represent the strength of association. The larger the point and the higher the point on the scale, the more significant the association with RA susceptibility.
The results related to the haplotype block methods are shown in Table 3. The associated SNPs properties are included in S1 Spreadsheet for the CIT method, S2 Spreadsheet for the FGT method, S3 Spreadsheet for the SSLD method, and S4 Spreadsheet for the individual SNP approach.
Table 3. A comparison among haplotype block methods results.
| Items | CIT | FGT | SSLD |
|---|---|---|---|
| Total number of blocks | 5,888 | 6,293 | 5,313 |
| Maximum block size (bp) | 480,781 | 351,442 | 430,369 |
| Maximum significant block size (bp) | 209,236 | 142,115 | 198,772 |
| Median block size (bp) | 8,456 | 9,582 | 13,943 |
| Median significant block size (bp) | 8,671 | 7,447 | 10,122 |
| Minimum block size (bp) | 6 | 6 | 6 |
| Minimum significant block size (bp) | 25 | 25 | 76 |
| Median number of SNPs within each block | 3 | 4 | 5 |
| Median number of SNPs within each significant block | 5 | 5 | 7 |
| Percentage of uncovered SNPs by the block method | 19.46% | 10.25% | 3.3% |
| Percentage of significant blocks from the total number of blocks | 2.72% | 2.84% | 2.73% |
| Total number of significant haplotypes | 307 | 343 | 302 |
| Total number of significant SNPs | 1,086 | 1,099 | 1,322 |
| Intersection with SNPs detected by individual SNP approach (432 SNPs) | 381 | 387 | 415 |
| Intersection with top 50 SNPs (lowest P-values) detected by the individual SNP approach | 47 | 48 | 50 |
The SSLD significant blocks included more associated SNPs (1,322) than FGT (1,099) and CIT (1,086) blocks. Moreover, the number of the associated SNPs by the individual SNP approach was (432). The SSLD method did the best job of representing the hits of the individual SNP approach with (415) SNPs. While, the CIT method represented (381) SNPs, and the FGT method represented (387) SNPs (S5 Spreadsheet). Interestingly, the SSLD method totally represented the top 50 associated SNPs by the individual SNP approach from (P-values) point of view. In addition, the CIT and FGT methods represented (47) and (48) SNPs respectively from the top 50 SNPs, as shown in Table 4.
Table 4. The top 50 associated SNPs discovered by the individual SNP approach with the corresponding haplotype blocks.
| SNP ID | Position (bp) | Assoc. Allelea | AAFb (Case, Control) | P-value | Gene / Nearest Genes | Haplotype Block (Method, P-value, No. of SNPs in Block) | Haplotype Block Position (bp) (Start, End, Size) | Previously Studied in |
|---|---|---|---|---|---|---|---|---|
| rs1033500 | 32415360 | A | 0.619, 0.393 | 2.1 E-45 | C6orf10 | CIT, 3.49 E-44, 15 | 32415360, 32445664, 30305 | [25] |
| FGT, 3.12 E-44, 8 | 32398932, 32425613, 26682 | |||||||
| SSLD, 1.48 E-97, 26 | 32390832, 32445664, 54832 | |||||||
| rs1980495 | 32454772 | C | 0.511, 0.269 | 3.45 E-52 | C6orf10 / BTNL2 | CIT, 4.08 E-59, 9 | 32454772, 32471794, 17023 | [26, 27] |
| FGT, 4.08 E-59, 9 | 32454772, 32471794, 17023 | |||||||
| SSLD, 1.46 E-81, 3 | 32449376, 32456123, 6748 | |||||||
| rs2076530 | 32471794 | G | 0.699, 0.444 | 2.97 E-59 | BTNL2 | CIT, 4.08 E-59, 9 | 32454772, 32471794, 17023 | [28–32] |
| FGT, 4.08 E-59, 9 | 32454772, 32471794, 17023 | |||||||
| SSLD, 4.62 E-59, 7 | 32462406, 32471794, 9388 | |||||||
| rs2395157 | 32456123 | G | 0.528, 0.276 | 8.58 E-60 | C6orf10 / BTNL2 | CIT, 4.08 E-59, 9 | 32454772, 32471794, 17023 | [33] |
| FGT, 4.08 E-59, 9 | 32454772, 32471794, 17023 | |||||||
| SSLD, 1.46 E-81, 3 | 32449376, 32456123, 6748 | |||||||
| rs2395163 | 32495787 | G | 0.509, 0.203 | 1.61 E-94 | BTNL2 / HLA-DRA | CIT, 5.66 E-95, 11 | 32495758, 32512856, 17098 | [34–39] |
| FGT, 4.41 E-95, 5 | 32491086, 32495787, 4702 | |||||||
| SSLD, 4.86 E-95, 14 | 32491086, 32512837, 21752 | |||||||
| rs2395185 | 32541145 | A | 0.589, 0.315 | 1.01 E-68 | HLA-DRA / HLA-DRB5 | CIT, 6.63 E-131, 11 | 32541145, 32713862, 172718 | [32, 36, 37, 40, 41] |
| FGT, 5.2 E-72, 4 | 32536263, 32678378, 142116 | |||||||
| SSLD, 3.47 E-77, 9 | 32519501, 32678378, 158878 | |||||||
| rs2516049 | 32678378 | G | 0.576, 0.305 | 2.61 E-67 | HLA-DRB1 / HLA-DQA1 | CIT, 6.63 E-131, 11 | 32541145, 32713862, 172718 | [32, 33, 36, 37, 42, 43] |
| FGT, 5.2 E-72, 4 | 32536263, 32678378, 142116 | |||||||
| SSLD, 3.47 E-77, 9 | 32519501, 32678378, 158878 | |||||||
| rs2647012 | 32772436 | G | 0.837, 0.624 | 1.76 E-50 | HLA-DQB1 / HLA-DQA2 | CIT, 3.68 E-54, 31 | 32772436, 32786977, 14542 | [32, 44, 45] |
| FGT, 3.26 E-52, 28 | 32772436, 32778956, 6521 | |||||||
| SSLD, 1.49 E-53, 20 | 32767856, 32777978, 10123 | |||||||
| rs2856717 | 32778286 | G | 0.838, 0.624 | 6.97 E-51 | HLA-DQB1 / HLA-DQA2 | CIT, 3.68 E-54, 31 | 32772436, 32786977, 14542 | [45] |
| FGT, 3.26 E-52, 28 | 32772436, 32778956, 6521 | |||||||
| SSLD, 1.55 E-52, 17 | 32778015, 32787668, 9654 | |||||||
| rs2856725 | 32774716 | A | 0.837, 0.623 | 1.05 E-50 | HLA-DQB1 / HLA-DQA2 | CIT, 3.68 E-54, 31 | 32772436, 32786977, 14542 | [45, 46] |
| FGT, 3.26 E-52, 28 | 32772436, 32778956, 6521 | |||||||
| SSLD, 1.49 E-53, 20 | 32767856, 32777978, 10123 | |||||||
| rs2858305 | 32778442 | A | 0.838, 0.624 | 6.97 E-51 | HLA-DQB1 / HLA-DQA2 | CIT, 3.68 E-54, 31 | 32772436, 32786977, 14542 | [33, 45] |
| FGT, 3.26 E-52, 28 | 32772436, 32778956, 6521 | |||||||
| SSLD, 1.55 E-52, 17 | 32778015, 32787668, 9654 | |||||||
| rs3129871 | 32514320 | C | 0.835, 0.631 | 2.14 E-46 | BTNL2 / HLA-DRA | SSLD, 5.38 E-41, 2 | 32512856, 32514320, 1465 | [31, 46, 47] |
| rs3763309 | 32483951 | A | 0.507, 0.209 | 6.48 E-89 | BTNL2 / HLA-DRA | CIT, 1.99 E-89, 8 | 32483951, 32491201, 7251 | [35–38, 48] |
| FGT, 1.46 E-87, 7 | 32483951, 32488240, 4290 | |||||||
| SSLD, 1.46 E-87, 6 | 32481290, 32487361, 6072 | |||||||
| rs3763312 | 32484326 | A | 0.505, 0.207 | 1.05 E-88 | BTNL2 / HLA-DRA | CIT, 1.99 E-89, 8 | 32483951, 32491201, 7251 | [35, 36, 39, 46] |
| FGT, 1.46 E-87, 7 | 32483951, 32488240, 4290 | |||||||
| SSLD, 1.46 E-87, 6 | 32481290, 32487361, 6072 | |||||||
| rs3817963 | 32476065 | G | 0.534, 0.294 | 1.23 E-54 | BTNL2 | CIT, 1.07 E-54, 3 | 32474399, 32476065, 1667 | [31, 37, 39, 49–52] |
| FGT, 2.51 E-42, 4 | 32476065, 32481676, 5612 | |||||||
| SSLD, 1.54 E-54, 4 | 32474399, 32477466, 3068 | |||||||
| rs3817973 | 32469089 | A | 0.698, 0.440 | 5.48 E-61 | C6orf10 / BTNL2 | CIT, 4.08 E-59, 9 | 32454772, 32471794, 17023 | [31, 32, 39] |
| FGT, 4.08 E-59, 9 | 32454772, 32471794, 17023 | |||||||
| SSLD, 4.62 E-59, 7 | 32462406, 32471794, 9389 | |||||||
| rs3957148 | 32790115 | G | 0.277, 0.094 | 1.88 E-53 | HLA-DQB1 / HLA-DQA2 | CIT, 6.72 E-54, 7 | 32788906, 32790115, 1210 | [53, 54] |
| FGT, 2.32 E-53, 4 | 32789654, 32790286, 633 | |||||||
| SSLD, 6.72 E-54, 7 | 32788906, 32790115, 1210 | |||||||
| rs4424066 | 32462406 | G | 0.698, 0.440 | 1.18 E-60 | C6orf10 / BTNL2 | CIT, 4.08 E-59, 9 | 32454772, 32471794, 17023 | [32, 55] |
| FGT, 4.08 E-59, 9 | 32454772, 32471794, 17023 | |||||||
| SSLD, 4.62 E-59, 7 | 32462406, 32471794, 9389 | |||||||
| rs477515 | 32677669 | A | 0.575, 0.304 | 2.72 E-67 | HLA-DRB1 / HLA-DQA1 | CIT, 6.63 E-131, 11 | 32541145, 32713862, 172718 | [32, 36, 42, 56] |
| FGT, 5.2 E-72, 4 | 32536263, 32678378, 142116 | |||||||
| SSLD, 3.47 E-77, 9 | 32519501, 32678378, 158878 | |||||||
| rs5000634 | 32771542 | G | 0.614, 0.382 | 1.09 E-48 | HLA-DQB1 / HLA-DQA2 | CIT, 2.62 E-81, 3 | 32767856, 32771829, 3974 | [32, 33, 57] |
| FGT, 1.03 E-48, 4 | 32766602, 32771829, 5228 | |||||||
| SSLD, 1.49 E-53, 20 | 32767856, 32777978, 10123 | |||||||
| rs547077 | 32397296 | G | 0.622, 0.397 | 3.98 E-46 | C6orf10 | CIT, 3.14 E-98, 18 | 32332366, 32406350, 73985 | [39] |
| FGT, 1.5 E-98, 15 | 32332366, 32397296, 64931 | |||||||
| SSLD, 1.48 E-97, 26 | 32390832, 32445664, 54833 | |||||||
| rs6457617 | 32771829 | A | 0.803, 0.508 | 2.55 E-82 | HLA-DQB1 / HLA-DQA2 | CIT, 2.62 E-81, 3 | 32767856, 32771829, 3974 | [32, 33, 36, 43, 48, 58–75] |
| FGT, 1.03 E-48, 4 | 32766602, 32771829, 5228 | |||||||
| SSLD, 1.49 E-53, 20 | 32767856, 32777978, 10123 | |||||||
| rs660895 | 32685358 | G | 0.529, 0.192 | 1.03 E-113 | HLA-DRB1 / HLA-DQA1 | CIT, 6.63 E-131, 11 | 32541145, 32713862, 172718 | [33, 35–43, 46, 47, 51, 60, 65, 68, 71, 76–92] |
| FGT, 1.84 E-113, 8 | 32680229, 32713862, 33634 | |||||||
| SSLD, 8.37 E-108, 11 | 32680229, 32760295, 80067 | |||||||
| rs6903608 | 32536263 | A | 0.882, 0.688 | 2.84 E-48 | HLA-DRA / HLA-DRB5 | FGT, 5.2 E-72, 4 | 32536263, 32678378, 142116 | [32, 33, 40, 55] |
| SSLD, 3.47 E-77, 9 | 32519501, 32678378, 158878 | |||||||
| rs6910071 | 32390832 | G | 0.506, 0.195 | 1.25 E-98 | C6orf10 | CIT, 3.14 E-98, 18 | 32332366, 32406350, 73985 | [33, 35–40, 43, 46–48, 50, 65, 68, 71, 79, 86–89, 93, 94] |
| FGT, 1.5 E-98, 15 | 32332366, 32397296, 64931 | |||||||
| SSLD, 1.48 E-97, 26 | 32390832, 32445664, 54833 | |||||||
| rs6932542 | 32488240 | G | 0.769, 0.527 | 1.06 E-56 | BTNL2 / HLA-DRA | CIT, 1.99 E-89, 8 | 32483951, 32491201, 7251 | [33, 95] |
| FGT, 1.46 E-87, 7 | 32483951, 32488240, 4290 | |||||||
| SSLD, 4.87 E-53, 3 | 32487714, 32488240, 527 | |||||||
| rs7192 | 32519624 | C | 0.826, 0.608 | 1.68 E-51 | HLA-DRA | CIT, 2.05 E-25, 3 | 32519501, 32521295, 1795 | [31, 33, 39, 46, 96, 97] |
| FGT, 2.05 E-25, 3 | 32519501, 32521295, 1795 | |||||||
| SSLD, 3.47 E-77, 9 | 32519501, 32678378, 158878 | |||||||
| rs9268528 | 32491086 | G | 0.596, 0.365 | 8.28 E-49 | BTNL2 / HLA-DRA | CIT, 1.99 E-89, 8 | 32483951, 32491201, 7251 | [98–100] |
| FGT, 4.41 E-95, 5 | 32491086, 32495787, 4702 | |||||||
| SSLD, 4.86 E-95, 14 | 32491086, 32512837, 21752 | |||||||
| rs9268542 | 32492699 | G | 0.597, 0.371 | 1.56 E-46 | BTNL2 / HLA-DRA | FGT, 4.41 E-95, 5 | 32491086, 32495787, 4702 | [98, 99] |
| SSLD, 4.86 E-95, 14 | 32491086, 32512837, 21752 | |||||||
| rs9268832 | 32535767 | G | 0.819, 0.597 | 1.82 E-52 | HLA-DRA / HLA-DRB5 | CIT, 8.33 E-54, 2 | 32522251, 32535767, 13517 | [32, 39, 41, 96] |
| FGT, 8.33 E-54, 2 | 32522251, 32535767, 13517 | |||||||
| SSLD, 3.47 E-77, 9 | 32519501, 32678378, 158878 | |||||||
| rs9275224 | 32767856 | G | 0.804, 0.511 | 1.74 E-81 | HLA-DQB1 / HLA-DQA2 | CIT, 2.62 E-81, 3 | 32767856, 32771829, 3974 | [32, 36, 39, 99, 101] |
| FGT, 1.03 E-48, 4 | 32766602, 32771829, 5228 | |||||||
| SSLD, 1.49 E-53, 20 | 32767856, 32777978, 10123 | |||||||
| rs9275312 | 32773706 | G | 0.297, 0.121 | 7.05 E-45 | HLA-DQB1 / HLA-DQA2 | CIT, 3.68 E-54, 31 | 32772436, 32786977, 14542 | [33, 41] |
| FGT, 3.26 E-52, 28 | 32772436, 32778956, 6521 | |||||||
| SSLD, 1.49 E-53, 20 | 32767856, 32777978, 10123 | |||||||
| rs9275371 | 32776274 | G | 0.488, 0.259 | 2.57 E-47 | HLA-DQB1 / HLA-DQA2 | CIT, 3.68 E-54, 31 | 32772436, 32786977, 14542 | [46, 99] |
| FGT, 3.26 E-52, 28 | 32772436, 32778956, 6521 | |||||||
| SSLD, 1.49 E-53, 20 | 32767856, 32777978, 10123 | |||||||
| rs9275374 | 32776504 | A | 0.482, 0.246 | 1.69 E-55 | HLA-DQB1 / HLA-DQA2 | CIT, 3.68 E-54, 31 | 32772436, 32786977, 14542 | [45, 47, 102] |
| FGT, 3.26 E-52, 28 | 32772436, 32778956, 6521 | |||||||
| SSLD, 1.49 E-53, 20 | 32767856, 32777978, 10123 | |||||||
| rs9275383 | 32776824 | A | 0.261, 0.085 | 6.20 E-50 | HLA-DQB1 / HLA-DQA2 | CIT, 3.68 E-54, 31 | 32772436, 32786977, 14542 | [103] |
| FGT, 3.26 E-52, 28 | 32772436, 32778956, 6521 | |||||||
| SSLD, 1.49 E-53, 20 | 32767856, 32777978, 10123 | |||||||
| rs9275388 | 32777062 | G | 0.482, 0.241 | 7.21 E-57 | HLA-DQB1 / HLA-DQA2 | CIT, 3.68 E-54, 31 | 32772436, 32786977, 14542 | [33, 45] |
| FGT, 3.26 E-52, 28 | 32772436, 32778956, 6521 | |||||||
| SSLD, 1.49 E-53, 20 | 32767856, 32777978, 10123 | |||||||
| rs9275390 | 32777134 | G | 0.482, 0.246 | 1.69 E-55 | HLA-DQB1 / HLA-DQA2 | CIT, 3.68 E-54, 31 | 32772436, 32786977, 14542 | [47, 51, 57, 64, 75, 99, 104] |
| FGT, 3.26 E-52, 28 | 32772436, 32778956, 6521 | |||||||
| SSLD, 1.49 E-53, 20 | 32767856, 32777978, 10123 | |||||||
| rs9275393 | 32777417 | A | 0.482, 0.246 | 2.72 E-55 | HLA-DQB1 / HLA-DQA2 | CIT, 3.68 E-54, 31 | 32772436, 32786977, 14542 | [45, 99] |
| FGT, 3.26 E-52, 28 | 32772436, 32778956, 6521 | |||||||
| SSLD, 1.49 E-53, 20 | 32767856, 32777978, 10123 | |||||||
| rs9275406 | 32777933 | A | 0.482, 0.246 | 1.38 E-55 | HLA-DQB1 / HLA-DQA2 | CIT, 3.68 E-54, 31 | 32772436, 32786977, 14542 | [41, 45, 51, 105] |
| FGT, 3.26 E-52, 28 | 32772436, 32778956, 6521 | |||||||
| SSLD, 1.49 E-53, 20 | 32767856, 32777978, 10123 | |||||||
| rs9275407 | 32778015 | A | 0.483, 0.245 | 9.06 E-56 | HLA-DQB1 / HLA-DQA2 | CIT, 3.68 E-54, 31 | 32772436, 32786977, 14542 | [45, 99] |
| FGT, 3.26 E-52, 28 | 32772436, 32778956, 6521 | |||||||
| SSLD, 1.55 E-52, 17 | 32778015, 32787668, 9654 | |||||||
| rs9275408 | 32778088 | G | 0.463, 0.231 | 6.75 E-52 | HLA-DQB1 / HLA-DQA2 | CIT, 3.68 E-54, 31 | 32772436, 32786977, 14542 | - |
| FGT, 3.26 E-52, 28 | 32772436, 32778956, 6521 | |||||||
| SSLD, 1.55 E-52, 17 | 32778015, 32787668, 9654 | |||||||
| rs9275418 | 32778222 | G | 0.482, 0.246 | 1.4 E-55 | HLA-DQB1 / HLA-DQA2 | CIT, 3.68 E-54, 31 | 32772436, 32786977, 14542 | [45, 106–108] |
| FGT, 3.26 E-52, 28 | 32772436, 32778956, 6521 | |||||||
| SSLD, 1.55 E-52, 17 | 32778015, 32787668, 9654 | |||||||
| rs9275424 | 32778554 | G | 0.482, 0.246 | 1.4 E-55 | HLA-DQB1 / HLA-DQA2 | CIT, 3.68 E-54, 31 | 32772436, 32786977, 14542 | [45, 99] |
| FGT, 3.26 E-52, 28 | 32772436, 32778956, 6521 | |||||||
| SSLD, 1.55 E-52, 17 | 32778015, 32787668, 9654 | |||||||
| rs9275425 | 32778852 | A | 0.477, 0.244 | 5.74 E-54 | HLA-DQB1 / HLA-DQA2 | CIT, 3.68 E-54, 31 | 32772436, 32786977, 14542 | [45, 99] |
| FGT, 3.26 E-52, 28 | 32772436, 32778956, 6521 | |||||||
| SSLD, 1.55 E-52, 17 | 32778015, 32787668, 9654 | |||||||
| rs9275427 | 32778893 | A | 0.482, 0.246 | 1.86 E-55 | HLA-DQB1 / HLA-DQA2 | CIT, 3.68 E-54, 31 | 32772436, 32786977, 14542 | [45] |
| FGT, 3.26 E-52, 28 | 32772436, 32778956, 6521 | |||||||
| SSLD, 1.55 E-52, 17 | 32778015, 32787668, 9654 | |||||||
| rs9275428 | 32778956 | G | 0.482, 0.247 | 3.3 E-55 | HLA-DQB1 / HLA-DQA2 | CIT, 3.68 E-54, 31 | 32772436, 32786977, 14542 | [45, 99, 109] |
| FGT, 3.26 E-52, 28 | 32772436, 32778956, 6521 | |||||||
| SSLD, 1.55 E-52, 17 | 32778015, 32787668, 9654 | |||||||
| rs9275439 | 32779499 | G | 0.479, 0.245 | 9.76 E-55 | HLA-DQB1 / HLA-DQA2 | CIT, 3.68 E-54, 31 | 32772436, 32786977, 14542 | [41, 45, 99] |
| FGT, 2.66 E-54, 2 | 32779081, 32779499, 419 | |||||||
| SSLD, 1.55 E-52, 17 | 32778015, 32787668, 9654 | |||||||
| rs9275555 | 32785066 | A | 0.475, 0.222 | 3.04 E-63 | HLA-DQB1 / HLA-DQA2 | CIT, 3.68 E-54, 31 | 32772436, 32786977, 14542 | [45, 46] |
| FGT, 3.19 E-61, 3 | 32785066, 32786977, 1912 | |||||||
| SSLD, 1.55 E-52, 17 | 32778015, 32787668, 9654 | |||||||
| rs9275572 | 32786977 | G | 0.822, 0.599 | 7.07 E-53 | HLA-DQB1 / HLA-DQA2 | CIT, 3.68 E-54, 31 | 32772436, 32786977, 14542 | [40, 45, 110] |
| FGT, 3.19 E-61, 3 | 32785066, 32786977, 1912 | |||||||
| SSLD, 1.55 E-52, 17 | 32778015, 32787668, 9654 | |||||||
| rs9275595 | 32789333 | G | 0.462, 0.218 | 2.59 E-61 | HLA-DQB1/ HLA-DQA2 | CIT, 6.72 E-54, 7 | 32788906, 32790115, 1210 | [39, 40, 45, 104, 111] |
| SSLD, 6.72 E-54, 7 | 32788906, 32790115, 1210 |
aAssoc. Allele: Associated Allele.
bAAF: Associated Allele Frequency.
From Table 4, the intergenic region between HLA-DQB1 and HLA-DQA2 –at the p21.32 band of chromosome 6 (MHC class II)–contained blocks with the largest number of RA-associated biomarkers by the individual SNP approach. Within this region, the SSLD method had two blocks with thirteen (P-value = 1.49 X 10−53, no. of SNPs = 20) and twelve (P-value = 1.55 X 10−52, no. of SNPs = 17) biomarkers. The CIT method was represented by a block (P-value = 3.68 X 10−54, no. of SNPs = 31) having twenty-two biomarkers. The FGT block (P-value = 3.26 X 10−52, no. of SNPs = 28) was detected containing nineteen biomarkers.
Discussion and conclusions
In this study, 34,122 SNPs were used to examine the association with RA susceptibility in the NARAC dataset. The examined SNPs belonged to chromosome 6. The surveyed SNPs on chromosome 6 of the NARAC dataset were dense enough for the application of the haplotype block methods. Four methods were applied to assign the associations (three haplotype block methods and individual SNP approach). The three used haplotype block methods were CIT, FGT, and SSLD. The individual SNP analysis was to point to chromosome regions that are genetically linked to the disease. The haplotype block methods were to further expand from the single SNPs with the strongest signal to the actual causal variants [112].
The aim of this study is to test the NARAC dataset to find out if haplotype block methods or single-locus approach alone can sufficiently provide the significant biomarkers associated with RA. Our research failed to select the best method as each method yielded significant results that were not detected using any of the other methods. S2 Table showed the SNP IDs that were uniquely identified by each method. The individual SNP, CIT, FGT, and SSLD methods exclusively detected 12, 55, 37, and 189 SNPs, respectively. Our findings were in line with Shim et al. conclusion (but they didn’t test the SSLD method) that both the individual SNP approach and the haplotype block methods should be applied side by side to discover the valuable associations in the NARAC dataset [5].
In addition, the 367 SNPs- that were significantly associated with RA susceptibility by the individual SNP approach and the haplotype block methods- represent potent candidates for further investigations (S6 Spreadsheet). The three haplotype block methods were able to detect 916 associated SNPs in common. The SSLD method detected more significant SNPs (1,322) than CIT (1,086), FGT (1,099), and individual SNP (432) methods. This observation could be understood, as the SSLD does not take into account the LD between the intermediate SNPs. Therefore, the SSLD method is the lowest conservative method in including SNPs inside it’s blocks.
The block similarity for the three applied methods were shown in Table 5. The similarity measure represented the intersected SNPs divided by the union SNPs for the two studied methods. The highest block similarity was between the CIT method and the FGT method. While, the lowest block similarity was between the CIT method and the SSLD method. The results showed that the FGT method had the most similarity with the other methods.
Table 5. Block similarity among the used haplotype block methods.
| Block Method | CIT | FGT | SSLD |
|---|---|---|---|
| CIT | 1 | 0.76 | 0.71 |
| FGT | 1 | 0.74 | |
| SSLD | 1 |
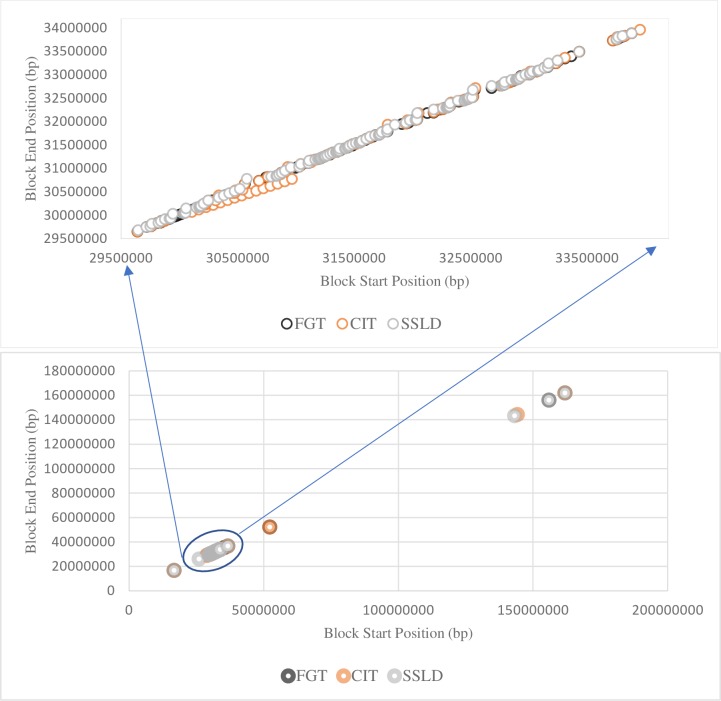
Fig 4 demonstrated the overlapping of the significant blocks resulted from the three haplotype block methods. Nearly, all chromosome 6 regions that were associated with RA were in blocks that were detected by more than one method. Most of the haplotype blocks that showed significant associations with RA disease were in the MHC region or near it (+ 3 Mb). Most of the 916 SNPs that were detected by the three block methods were in the MHC region. These outcomes confirmed the strong association between the MHC region and RA susceptibility.
Fig 4. Comparison of the haplotype blocks obtained by the three methods.
Each circle conistituted a significant haplotype block associated with RA susceptibility. The plot area represented chromosome 6, then zooming in on the MHC region.
For further analysis of the results, a comparison between the properties of the SNPs that were detected by all the methods (intersection) and that were uniquely detected by one method (unique) was shown in Table 6. The properties of all the associated SNPs detected by the corresponding method (reference) were added to Table 6 for more clarification. For all the methods, the P-values of the (intersection) were lower than that of the (reference) and more lower than that of the (unique). This observation confirmed that the 367 SNPs (intersection) represents potent candidates for further investigations. For the CIT and FGT methods, the median block size (bp) of the (unique) was greater than that of the (intersection) and more greater than the (reference). However, this observation might be due to the small number of blocks representing the (unique) in comparison to the (intersection) and the (reference). While, when the number of blocks representing the (unique) (53) was sufficiently comparable to that of the (intersection) (104) and the (reference) (145) in the SSLD method, the median block size (bp) of the (intersection) was greater than that of the (unique) and more greater than that of the (reference).
Table 6. A comparison between the associated SNPs detected with different categories.
| Method | Items | Significant SNPs detected by all methods (intersection) | All significant SNPs detected by a method (reference) | Significant SNPs detected by only one method (unique) |
|---|---|---|---|---|
| CIT | # SNPs | 367 | 1,086 | 55 |
| # blocks | 127 | 160 | 8 | |
| Average P-values | 1.63 E-07 | 7.20 E-07 | 3.33 E-06 | |
| Median Block Size (#SNPs) | 5 | 5 | 7 | |
| Median Block Size (BP) | 9,755 | 8,671 | 17,021 | |
| FGT | # SNPs | 367 | 1,099 | 37 |
| # blocks | 134 | 179 | 12 | |
| Average P-values | 1.37 E-07 | 5.6 E-07 | 7.84 E-07 | |
| Median Block Size (#SNPs) | 6 | 5 | 7 | |
| Median Block Size (BP) | 9,227 | 7,447 | 11,394 | |
| SSLD | # SNPs | 367 | 1,322 | 189 |
| # blocks | 104 | 145 | 53 | |
| Average P-values | 1.86 E-07 | 6.57 E-07 | 9.34 E-07 | |
| Median Block Size (#SNPs) | 9 | 7 | 8 | |
| Median Block Size (BP) | 17,054 | 10,122 | 11,604 | |
| Individual SNP | # SNPs | 367 | 432 | 12 |
| Average P-values | 1.25 E-07 | 1.35 E-07 | 3.26 E-07 |
Some associated SNPs were discovered using all the methods but others were observed by only one method. This finding might be explained by some reasons. For the associations that were observed using individual SNP approach only, it may be that only one SNP represent strong LD with the causal SNP. Therefore, studying haplotypes could decrease the power of association as they consist of several SNPs.
For the associations that were observed using the haplotype block methods only, Individual SNP approach required approximately 83% more tests than the haplotype block methods. Consequently, the Bonferroni correction was more severe for the individual SNP approach. Moreover, the haplotype block methods were able to capture the interactions among many causal SNPs. In addition, haplotypes could capture rare alleles that individual SNPs may not detect. This reason could be clarified as the power to observe associations is maximized when the frequencies of the studied biomarker and the causal SNP are similar.
For the associations that were observed using a haplotype block method but not by the other haplotype block methods, each method differs greatly from the others in its scope of the definition of the haplotype blocks. At last, we conclude that the application of the individual SNP approach and the three haplotype block methods altogether on chromosome 6 of the NARAC dataset will in turn maximize the system’s ability for discovering crucial associations. In case of selecting one method, the SSLD would be the most appropriate method for the NARAC dataset. The SSLD method has valuable advantages such as the highest genomic coverage, the largest minimum, median, and maximum significant block sizes, the biggest number of significant SNPs included in blocks, and the biggest number of associated SNPs discovered exclusively by a method.
The limitations of this study are as follows: a) the effects of population stratification were not accounted for; b) a replication study in other datasets was not performed. In addition, further investigations of other haplotype block methods, such as hidden Markov model [113, 114], dynamic programming-based algorithm [115–119], wavelet decomposition [120], greedy algorithm [121], minimum description length [122, 123], spatial correlation of SNPs [124], sequence kernel association tests [125], and block entropy [126] should be applied and compared to show the effect of the changes in block partitions on the resulting associated biomarkers.
Supporting information
(TIF)
(TIF)
(DOCX)
(DOCX)
Sheet1 “ID” represents SNPs IDs, and each row represents a block. Sheet2 “Bp” represents SNPs physical positions in base pairs, and each row represents a block. Sheet3 “No. of SNPs in Block” represents the number of SNPs in each block. Sheet4 “Start-Stop”–first column represents blocks start physical positions in base pairs. Sheet4 “Start-Stop”–second column represents blocks end physical positions in base pairs. Sheet4 “Start-Stop”–third column represents blocks sizes in base pairs. Sheet5 “Block no.” represents the block numbers (positions) from all blocks partitioned by CIT method. Sheet6 “P-values” represents the P-values of the blocks.
(XLS)
Sheet1 “ID” represents SNPs IDs, and each row represents a block. Sheet2 “Bp” represents SNPs physical positions in base pairs, and each row represents a block. Sheet3 “No. of SNPs in Block” represents the number of SNPs in each block. Sheet4 “Start-Stop”–first column represents blocks start physical positions in base pairs. Sheet4 “Start-Stop”–second column represents blocks end physical positions in base pairs. Sheet4 “Start-Stop”–third column represents blocks sizes in base pairs. Sheet5 “Block no.” represents the block numbers (positions) from all blocks partitioned by FGT method. Sheet6 “P-values” represents the P-values of the blocks.
(XLS)
Sheet1 “ID” represents SNPs IDs, and each row represents a block. Sheet2 “Bp” represents SNPs physical positions in base pairs, and each row represents a block. Sheet3 “No. of SNPs in Block” represents the number of SNPs in each block. Sheet4 “Start-Stop”–first column represents blocks start physical positions in base pairs. Sheet4 “Start-Stop”–second column represents blocks end physical positions in base pairs. Sheet4 “Start-Stop”–third column represents blocks sizes in base pairs. Sheet5 “Block no.” represents the block numbers (positions) from all blocks partitioned by SSLD method. Sheet6 “P-values” represents the P-values of the blocks.
(XLS)
Sheet1 “ID” represents SNPs IDs. Sheet2 “Bp” represents SNPs physical positions in base pairs. Sheet6 “P-values” represents the P-values of the SNPs.
(XLS)
Sheet1 “CIT” represents SNPs IDs detected by both CIT method and Individual SNP Approach. Sheet2 “FGT” represents SNPs IDs detected by both FGT method and Individual SNP Approach. Sheet3 “SSLD” represents SNPs IDs detected by both SSLD method and Individual SNP Approach.
(XLS)
(XLS)
Abbreviations
- BP
Base Pair
- CIT
Confidence Interval Test
- Dˊ
Normalized Deviation
- FGT
Four Gamete Test
- HLA
Human Leukocyte Antigen
- HWE
Hardy-Weinberg Equilibrium
- IGV
Integrative Genomics Viewer
- LD
Linkage Disequilibrium
- MAF
Minor Allele Frequency
- Mb
Mega Base Pair
- MHC
Major Histocompatibility Complex
- NARAC
North American Rheumatoid Arthritis Consortium
- Q-Q
Quantile-Quantile
- RA
Rheumatoid Arthritis
- SNP
Single Nucleotide Polymorphism
- SSLD
Solid Spine of Linkage Disequilibrium
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The authors would like to acknowledge the Genetic Analysis Workshop grant (R01 GM031575) for providing the NARAC dataset. This work is based on data that was gathered with the support of grants from the National Institutes of Health (NO1-AR-2-2263, RO1-AR-44422) and the National Arthritis Foundation.
References
- 1.Saad M. N., Mabrouk M. S., Eldeib A. M. and Shaker O. G., " Identification of rheumatoid arthritis biomarkers based on single nucleotide polymorphisms and haplotype blocks: a systematic review and meta-analysis," J Adv Res, vol. 7, pp. 1–16, 2016. 10.1016/j.jare.2015.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peise E., Fabregat-Traver D. and Bientinesi P., "High performance solutions for big-data GWAS," Parallel Computing, vol. 42, pp. 75–87, 2015. [Google Scholar]
- 3.Clark A. G., "The role of haplotypes in candidate gene studies," Genet Epidemiol, vol. 27, pp. 321–33, December 2004. 10.1002/gepi.20025 [DOI] [PubMed] [Google Scholar]
- 4.Su S. C., Kuo C. C. and Chen T., "Single nucleotide polymorphism data analysis—state-of-the-art review on this emerging field from a signal processing viewpoint," IEEE Signal Process Mag, vol. 24, pp. 75–82, 2007. [Google Scholar]
- 5.Shim H., Chun H., Engelman C. D. and Payseur B. A., "Genome-wide association studies using single-nucleotide polymorphisms versus haplotypes: an empirical comparison with data from the North American Rheumatoid Arthritis Consortium," BMC Proc, vol. 3 Suppl 7, p. S35, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saad M. N., Mabrouk M. S., Eldeib A. M. and Shaker O. G., "Genetic case-control study for eight polymorphisms associated with rheumatoid arthritis," PLoS One, vol. 10, e0131960, 2015. 10.1371/journal.pone.0131960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caliz R., Canet L. M., Lupianez C. B., Canhao H., Escudero A., Filipescu I., et al. , "Gender-specific effects of genetic variants within Th1 and Th17 cell-mediated immune response genes on the risk of developing rheumatoid arthritis," PLoS One, vol. 8, p. e72732, 2013. 10.1371/journal.pone.0072732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.M. N. Saad, M. S. Mabrouk, A. M. Eldeib and O. G. Shaker, "Vitamin D receptor gene polymorphisms in rheumatoid arthritis patients associating osteoporosis," presented at the 7th Cairo International Biomedical Engineering Conference, Cairo, Egypt, 2014.
- 9.Saad M. N., Mabrouk M. S., Eldeib A. M. and Shaker O. G., "Effect of MTHFR, TGFβ1 and TNFB polymorphisms on osteoporosis in rheumatoid arthritis patients," Gene, vol. 568, pp. 124–128, 2015. 10.1016/j.gene.2015.05.037 [DOI] [PubMed] [Google Scholar]
- 10.Kitajima H., Sonoda M. and Yamamoto K., "HLA and SNP haplotype mapping in the Japanese population," Genes Immun, vol. 13, pp. 543–8, October 2012. 10.1038/gene.2012.35 [DOI] [PubMed] [Google Scholar]
- 11.Sekar A., Bialas A. R., de Rivera H., Davis A., Hammond T. R., Kamitaki N., et al. , "Schizophrenia risk from complex variation of complement component 4," Nature, vol. 530, pp. 177–183, 2016. 10.1038/nature16549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perricone C., Ceccarelli F. and Valesini G., "An overview on the genetic of rheumatoid arthritis: a never-ending story," Autoimmun Rev, vol. 10, pp. 599–608, August 2011. 10.1016/j.autrev.2011.04.021 [DOI] [PubMed] [Google Scholar]
- 13.Amos C. I., Chen W. V., Seldin M. F., Remmers E. F., Taylor K. E., Criswell L. A., et al. , "Data for Genetic Analysis Workshop 16 Problem 1, association analysis of rheumatoid arthritis data," BMC Proceedings, vol. 3, pp. 1–4, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Purcell S., Neale B., Todd-Brown K., Thomas L., Ferreira M. A., Bender D., et al. , "PLINK: a tool set for whole-genome association and population-based linkage analyses," Am J Hum Genet, vol. 81, pp. 559–75, September 2007. 10.1086/519795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barrett J. C., Fry B., Maller J. and Daly M. J., "Haploview: analysis and visualization of LD and haplotype maps," Bioinformatics, vol. 21, pp. 263–5, January 2005. 10.1093/bioinformatics/bth457 [DOI] [PubMed] [Google Scholar]
- 16.Robinson J. T., Thorvaldsdottir H., Winckler W., Guttman M., Lander E. S., Getz G., et al. , "Integrative genomics viewer," Nat Biotechnol, vol. 29, pp. 24–6, January 2011. 10.1038/nbt.1754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thorvaldsdottir H., Robinson J. T. and Mesirov J. P., "Integrative Genomics Viewer (IGV): high-performance genomics data visualization and exploration," Brief Bioinform, vol. 14, pp. 178–92, March 2013. 10.1093/bib/bbs017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wall J. D. and Pritchard J. K., "Haplotype blocks and linkage disequilibrium in the human genome," Nat Rev Genet, vol. 4, pp. 587–97, August 2003. 10.1038/nrg1123 [DOI] [PubMed] [Google Scholar]
- 19.Gabriel S. B., Schaffner S. F., Nguyen H., Moore J. M., Roy J., Blumenstiel B., et al. , "The structure of haplotype blocks in the human genome," Science, vol. 296, pp. 2225–9, June 21 2002. 10.1126/science.1069424 [DOI] [PubMed] [Google Scholar]
- 20.Wang N., Akey J. M., Zhang K., Chakraborty R. and Jin L., "Distribution of recombination crossovers and the origin of haplotype blocks: the interplay of population history, recombination and mutation," Am J Hum Genet, vol. 71, pp. 1227–34, November 2002. 10.1086/344398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balding D. J., "A tutorial on statistical methods for population association studies," Nat Rev Genet, vol. 7, pp. 781–91, October 2006. 10.1038/nrg1916 [DOI] [PubMed] [Google Scholar]
- 22.Saxena R., Voight B. F., Lyssenko V., Burtt N. P., de Bakker P. I. W., Chen H., et al. , "Genome-Wide Association Analysis Identifies Loci for Type 2 Diabetes and Triglyceride Levels," Science, vol. 316, pp. 1331–1336, 2007. 10.1126/science.1142358 [DOI] [PubMed] [Google Scholar]
- 23.Wu Y., Fan H., Wang Y., Zhang L., Gao X., Chen Y., et al. , "Genome-wide association studies using haplotypes and individual SNPs in Simmental cattle," PloS one, vol. 9, e109330, 2014. 10.1371/journal.pone.0109330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arya R., Hare E., del Rincon I., Jenkinson C. P., Duggirala R., Almasy L., et al. , "Effects of covariates and interactions on a genome-wide association analysis of rheumatoid arthritis," BMC Proceedings, vol. 3, p. S84, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mo X.-B., Zhang Y.-H. and Lei S.-F., "Genome-Wide Identification of N(6)-Methyladenosine (m(6)A) SNPs Associated With Rheumatoid Arthritis," Frontiers in genetics, vol. 9, pp. 299–299, 2018. 10.3389/fgene.2018.00299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fattal I., Rimer J., Shental N., Molad Y., Gabrielli A., Livneh A., et al. , "Pemphigus vulgaris is characterized by low IgG reactivities to specific self‐antigens along with high IgG reactivity to desmoglein 3," Immunology, vol. 143, pp. 374–380, 2014. 10.1111/imm.12316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim K., Kim H. and Yoon J., "The expression and role of KLF4 in psoriasis," in JOURNAL OF INVESTIGATIVE DERMATOLOGY, 2013, pp. S17–S17. [Google Scholar]
- 28.Orozco G., Eerligh P., Sánchez E., Zhernakova S., Roep B. O., González-Gay M. A., et al. , "Analysis of a Functional BTNL2 Polymorphism in Type 1 Diabetes, Rheumatoid Arthritis and Systemic Lupus Erythematosus," Human Immunology, vol. 66, pp. 1235–1241, 2005/12/01/ 2005. 10.1016/j.humimm.2006.02.003 [DOI] [PubMed] [Google Scholar]
- 29.Steer S., Abkevich V., Gutin A., Cordell H. J., Gendall K. L., Merriman M. E., et al. , "Genomic DNA pooling for whole-genome association scans in complex disease: empirical demonstration of efficacy in rheumatoid arthritis," Genes And Immunity, vol. 8, p. 57, 12/07/online 2006. 10.1038/sj.gene.6364359 [DOI] [PubMed] [Google Scholar]
- 30.Mitsunaga S., Hosomichi K., Okudaira Y., Nakaoka H., Kunii N., Suzuki Y., et al. , "Exome sequencing identifies novel rheumatoid arthritis-susceptible variants in the BTNL2," Journal Of Human Genetics, vol. 58, p. 210, 01/31/online 2013. 10.1038/jhg.2013.2 [DOI] [PubMed] [Google Scholar]
- 31.Wang M., Chen X., Zhang M., Zhu W., Cho K. and Zhang H., "Detecting significant single-nucleotide polymorphisms in a rheumatoid arthritis study using random forests," BMC Proceedings, vol. 3, p. S69, December 15 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang R., Dong J. and Dai Y., "Genome-wide association study of rheumatoid arthritis by a score test based on wavelet transformation," BMC Proceedings, vol. 3, p. S8, 2009/12/15 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chiu Y.-F., Kao H.-Y., Chen Y.-S., Hsu F.-C. and Yang H.-C., "Assessment of gene-covariate interactions by incorporating covariates into association mapping," BMC Proceedings, vol. 3, p. S85, December 15 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu Y., Aryee M. J., Padyukov L., Fallin M. D., Hesselberg E., Runarsson A., et al. , "Epigenome-wide association data implicate DNA methylation as an intermediary of genetic risk in rheumatoid arthritis," Nature Biotechnology, vol. 31, p. 142, 01/20/online 2013. 10.1038/nbt.2487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen L., Zhong M., Chen W. V., Amos C. I. and Fan R., "A genome-wide association scan for rheumatoid arthritis data by Hotelling's T2tests," BMC Proceedings, vol. 3, p. S6, 2009/12/15 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park J., Namkung J., Jhun M. and Park T., "Genome-wide analysis of haplotype interaction for the data from the North American Rheumatoid Arthritis Consortium," BMC Proc, vol. 3(Suppl 7):S34, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Black M. H. and Watanabe R. M., "A principal-components-based clustering method to identify multiple variants associated with rheumatoid arthritis and arthritis-related autoantibodies," BMC Proceedings, vol. 3, p. S129, December 15 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Üstünkar G. and Son Yeşim A., "METU-SNP: An Integrated Software System for SNPComplex Disease Association Analysis," in Journal of Integrative Bioinformatics vol. 8, ed, 2011, p. 204. [DOI] [PubMed] [Google Scholar]
- 39.Taliun D., Gamper J. and Pattaro C., "Efficient haplotype block recognition of very long and dense genetic sequences," BMC Bioinformatics, vol. 15, p. 10, January 14 2014. 10.1186/1471-2105-15-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cho S., Kim H., Oh S., Kim K. and Park T., "Elastic-net regularization approaches for genome-wide association studies of rheumatoid arthritis," BMC Proceedings, vol. 3, p. S25, December 15 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lemire M., "On the association between rheumatoid arthritis and classical HLA class I and class II alleles predicted from single-nucleotide polymorphism data," BMC Proceedings, vol. 3, p. S33, December 15 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jung J., Song J. J. and Kwon D., "Allelic based gene-gene interactions in rheumatoid arthritis," BMC Proceedings, vol. 3, p. S76, 2009/12/15 2009. 10.1186/1753-6561-3-S7-S76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nagai Y. and Imanishi T., "RAvariome: a genetic risk variants database for rheumatoid arthritis based on assessment of reproducibility between or within human populations," Database, vol. 2013, pp. bat073–bat073, 2013. 10.1093/database/bat073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tamiya G., Shinya M., Imanishi T., Ikuta T., Makino S., Okamoto K., et al. , "Whole genome association study of rheumatoid arthritis using 27 039 microsatellites," Human Molecular Genetics, vol. 14, pp. 2305–2321, 2005. 10.1093/hmg/ddi234 [DOI] [PubMed] [Google Scholar]
- 45.Sengupta Chattopadhyay A., Hsiao C.-L., Chang C. C., Lian I.-B. and Fann C. S. J., "Summarizing techniques that combine three non-parametric scores to detect disease-associated 2-way SNP-SNP interactions," Gene, vol. 533, pp. 304–312, 2014/01/01/ 2014. 10.1016/j.gene.2013.09.041 [DOI] [PubMed] [Google Scholar]
- 46.Chanda P., Zhang A., Sucheston L. and Ramanathan M., "A two-stage search strategy for detecting multiple loci associated with rheumatoid arthritis," BMC Proceedings, vol. 3, p. S72, December 15 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alekseyenko A. V., Lytkin N. I., Ai J., Ding B., Padyukov L., Aliferis C. F., et al. , "Causal graph-based analysis of genome-wide association data in rheumatoid arthritis," Biology Direct, vol. 6, p. 25, May 18 2011. 10.1186/1745-6150-6-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Orozco G., Viatte S., Bowes J., Martin P., Wilson A. G., Morgan A. W., et al. , "Novel Rheumatoid Arthritis Susceptibility Locus at 22q12 Identified in an Extended UK Genome-Wide Association Study," Arthritis & Rheumatology, vol. 66, pp. 24–30, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roederer M., Quaye L., Mangino M., Beddall Margaret H., Mahnke Y., Chattopadhyay P., et al. , "The Genetic Architecture of the Human Immune System: A Bioresource for Autoimmunity and Disease Pathogenesis," Cell, vol. 161, pp. 387–403, 2015/04/09/ 2015. 10.1016/j.cell.2015.02.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zheng W. and Rao S., "Knowledge-based analysis of genetic associations of rheumatoid arthritis to inform studies searching for pleiotropic genes: a literature review and network analysis," Arthritis Research & Therapy, vol. 17, p. 202, August 08 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wallace A. D., Wendt G. A., Barcellos L. F., de Smith A. J., Walsh K. M., Metayer C., et al. , "To ERV Is Human: A Phenotype-Wide Scan Linking Polymorphic Human Endogenous Retrovirus-K Insertions to Complex Phenotypes," Frontiers in Genetics, vol. 9, 2018-August-14 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ju J. H., Shenoy S. A., Crystal R. G. and Mezey J. G., "An independent component analysis confounding factor correction framework for identifying broad impact expression quantitative trait loci," PLOS Computational Biology, vol. 13, p. e1005537, 2017. 10.1371/journal.pcbi.1005537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fang H., Knezevic B., Burnham K. L. and Knight J. C., "XGR software for enhanced interpretation of genomic summary data, illustrated by application to immunological traits," Genome Medicine, vol. 8, p. 129, December 13 2016. 10.1186/s13073-016-0384-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goris A., Pauwels I., Gustavsen M. W., van Son B., Hilven K., Bos S. D., et al. , "Genetic variants are major determinants of CSF antibody levels in multiple sclerosis," Brain, vol. 138, pp. 632–643, 2015. 10.1093/brain/awu405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rivera N. V., Ronninger M., Shchetynsky K., Franke A., Nöthen M. M., Müller-Quernheim J., et al. , "High-Density Genetic Mapping Identifies New Susceptibility Variants in Sarcoidosis Phenotypes and Shows Genomic-driven Phenotypic Differences," American Journal of Respiratory and Critical Care Medicine, vol. 193, pp. 1008–1022, 2016. 10.1164/rccm.201507-1372OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rai E. and Wakeland E. K., "Genetic predisposition to autoimmunity–What have we learned?," Seminars in Immunology, vol. 23, pp. 67–83, 2011/04/01/ 2011. 10.1016/j.smim.2011.01.015 [DOI] [PubMed] [Google Scholar]
- 57.Wei W.-H., Loh C.-Y., Worthington J. and Eyre S., "Immunochip Analyses of Epistasis in Rheumatoid Arthritis Confirm Multiple Interactions within MHC and Suggest Novel Non-MHC Epistatic Signals," The Journal of Rheumatology, 2016. [DOI] [PubMed] [Google Scholar]
- 58.Klareskog L., Stolt P., Lundberg K., Källberg H., Bengtsson C., Grunewald J., et al. , "A new model for an etiology of rheumatoid arthritis: Smoking may trigger HLA–DR (shared epitope)–restricted immune reactions to autoantigens modified by citrullination," Arthritis & Rheumatism, vol. 54, pp. 38–46, 2006. [DOI] [PubMed] [Google Scholar]
- 59.MacGregor A. J., Snieder H., Rigby A. S., Koskenvuo M., Kaprio J., Aho K., et al. , "Characterizing the quantitative genetic contribution to rheumatoid arthritis using data from twins," Arthritis & Rheumatism, vol. 43, pp. 30–37, 2000. [DOI] [PubMed] [Google Scholar]
- 60.Ding B., Padyukov L., Lundstrom E., Seielstad M., Plenge R. M., Oksenberg J. R., et al. , "Different patterns of associations with anti-citrullinated protein antibody-positive and anti-citrullinated protein antibody-negative rheumatoid arthritis in the extended major histocompatibility complex region," Arthritis Rheum, vol. 60, pp. 30–8, January 2009. 10.1002/art.24135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cui J., Saevarsdottir S., Thomson B., Padyukov L., van der Helm-Van Mil A. H. M., Nititham J., et al. , "Rheumatoid arthritis risk allele PTPRC is also associated with response to anti–tumor necrosis factor α therapy," Arthritis & Rheumatism, vol. 62, pp. 1849–1861, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Han T.-U., Bang S.-Y., Kang C. and Bae S.-C., "TRAF1 polymorphisms associated with rheumatoid arthritis susceptibility in Asians and in Caucasians," Arthritis & Rheumatism, vol. 60, pp. 2577–2584, 2009. [DOI] [PubMed] [Google Scholar]
- 63.Freudenberg J., Lee H.-S., Han B.-G., Shin H. D., Kang Y. M., Sung Y.-K., et al. , "Genome-wide association study of rheumatoid arthritis in Koreans: Population-specific loci as well as overlap with European susceptibility loci," Arthritis & Rheumatism, vol. 63, pp. 884–893, 2011. [DOI] [PubMed] [Google Scholar]
- 64.Julia A., Ballina J., Canete J. D., Balsa A., Tornero-Molina J., Naranjo A., et al. , "Genome-wide association study of rheumatoid arthritis in the Spanish population: KLF12 as a risk locus for rheumatoid arthritis susceptibility," Arthritis Rheum, vol. 58, pp. 2275–86, August 2008. 10.1002/art.23623 [DOI] [PubMed] [Google Scholar]
- 65.Prasad P., Kumar A., Gupta R., Juyal R. C. and T. B. K, "Caucasian and Asian Specific Rheumatoid Arthritis Risk Loci Reveal Limited Replication and Apparent Allelic Heterogeneity in North Indians," PLOS ONE, vol. 7, p. e31584, 2012. 10.1371/journal.pone.0031584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Snir O., Gomez-Cabrero D., Montes A., Perez-Pampin E., Gómez-Reino J. J., Seddighzadeh M., et al. , "Non-HLA genes PTPN22, CDK6 and PADI4 are associated with specific autoantibodies in HLA-defined subgroups of rheumatoid arthritis," Arthritis Research & Therapy, vol. 16, p. 414, August 20 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rioux J. D., Goyette P., Vyse T. J., Hammarström L., Fernando M. M. A., Green T., et al. , "Mapping of multiple susceptibility variants within the MHC region for 7 immune-mediated diseases," Proceedings of the National Academy of Sciences, vol. 106, pp. 18680–18685, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Delgado-Vega A., Sánchez E., Löfgren S., Castillejo-López C. and Alarcón-Riquelme M. E., "Recent findings on genetics of systemic autoimmune diseases," Current Opinion in Immunology, vol. 22, pp. 698–705, 2010/12/01/ 2010. 10.1016/j.coi.2010.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ritchie M. D., Denny J. C., Crawford D. C., Ramirez A. H., Weiner J. B., Pulley J. M., et al. , "Robust Replication of Genotype-Phenotype Associations across Multiple Diseases in an Electronic Medical Record," The American Journal of Human Genetics, vol. 86, pp. 560–572, 2010/04/09/ 2010. 10.1016/j.ajhg.2010.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li H., Hu Y., Zhang T., Liu Y., Wang Y., Yang T., et al. , "Replication of British Rheumatoid Arthritis Susceptibility Loci in Two Unrelated Chinese Population Groups," Clinical and Developmental Immunology, vol. 2013, p. 6, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lu Q., Cui Y., Ye C., Wei C. and Elston R. C., "Bagging Optimal ROC Curve Method for Predictive Genetic Tests, with an Application for Rheumatoid Arthritis," Journal of Biopharmaceutical Statistics, vol. 20, pp. 401–414, 2010/03/19 2010. 10.1080/10543400903572811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gall B. J., Wilson A., Schroer A. B., Gross J. D., Stoilov P., Setola V., et al. , "Genetic variations in GPSM3 associated with protection from rheumatoid arthritis affect its transcript abundance," Genes And Immunity, vol. 17, p. 139, 01/28/online 2016. 10.1038/gene.2016.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bermejo J. L., Fischer C., Schulz A., Cremer N., Hein R., Beckmann L., et al. , "Representation of genetic association via attributable familial relative risks in order to identify polymorphisms functionally relevant to rheumatoid arthritis," BMC Proceedings, vol. 3, p. S10, December 15 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jeffries N. and Zheng G., "Evaluation of an optimal receiver operating characteristic procedure," BMC Proceedings, vol. 3, p. S56, December 15 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Achour Y., Ben Hamad M., Chaabane S., Rebai A., Marzouk S., Mahfoudh N., et al. , "Analysis of two susceptibility SNPs in HLA region and evidence of interaction between rs6457617 in HLA-DQB1 and HLA-DRB1*04 locus on Tunisian rheumatoid arthritis," Journal of Genetics, vol. 96, pp. 911–918, December 01 2017. [DOI] [PubMed] [Google Scholar]
- 76.Mahdi H., Fisher B. A., Källberg H., Plant D., Malmström V., Rönnelid J., et al. , "Specific interaction between genotype, smoking and autoimmunity to citrullinated α-enolase in the etiology of rheumatoid arthritis," Nature Genetics, vol. 41, p. 1319, 11/08/online 2009. 10.1038/ng.480 [DOI] [PubMed] [Google Scholar]
- 77.Vignal C., Bansal A. T., Balding D. J., Binks M. H., Dickson M. C., Montgomery D. S., et al. , "Genetic association of the major histocompatibility complex with rheumatoid arthritis implicates two non-DRB1 loci," Arthritis & Rheumatism, vol. 60, pp. 53–62, 2009. [DOI] [PubMed] [Google Scholar]
- 78.Yarwood A., Han B., Raychaudhuri S., Bowes J., Lunt M., Pappas D. A., et al. , "A weighted genetic risk score using all known susceptibility variants to estimate rheumatoid arthritis risk," Annals of the Rheumatic Diseases, vol. 74, pp. 170–176, 2015. 10.1136/annrheumdis-2013-204133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Denny J. C., Bastarache L., Ritchie M. D., Carroll R. J., Zink R., Mosley J. D., et al. , "Systematic comparison of phenome-wide association study of electronic medical record data and genome-wide association study data," Nature Biotechnology, vol. 31, p. 1102, 11/24/online 2013. 10.1038/nbt.2749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Solus J. F., Chung C. P., Oeser A., Li C., Rho Y. H., Bradley K. M., et al. , "Genetics of serum concentration of IL-6 and TNFα in systemic lupus erythematosus and rheumatoid arthritis: a candidate gene analysis," Clinical Rheumatology, vol. 34, pp. 1375–1382, August 01 2015. 10.1007/s10067-015-2881-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.González-Recio O., de Maturana E. L., Vega A. T., Engelman C. D. and Broman K. W., "Detecting single-nucleotide polymorphism by single-nucleotide polymorphism interactions in rheumatoid arthritis using a two-step approach with machine learning and a Bayesian threshold least absolute shrinkage and selection operator (LASSO) model," BMC Proceedings, vol. 3, p. S63, December 15 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Arya R., Hare E., del Rincon I., Jenkinson C. P., Duggirala R., Almasy L., et al. , "Effects of covariates and interactions on a genome-wide association analysis of rheumatoid arthritis," BMC Proc, vol. 3(Suppl 7):S84, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Scott I. C., Rijsdijk F., Walker J., Quist J., Spain S. L., Tan R., et al. , "Do Genetic Susceptibility Variants Associate with Disease Severity in Early Active Rheumatoid Arthritis?," The Journal of Rheumatology, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jiang X., Frisell T., Askling J., Karlson E. W., Klareskog L., Alfredsson L., et al. , "To What Extent Is the Familial Risk of Rheumatoid Arthritis Explained by Established Rheumatoid Arthritis Risk Factors?," Arthritis & Rheumatology, vol. 67, pp. 352–362, 2015. [DOI] [PubMed] [Google Scholar]
- 85.Deng F.-Y., Lei S.-F., Zhu H., Zhang Y.-H. and Zhang Z.-L., "Integrative Analyses for Functional Mechanisms Underlying Associations for Rheumatoid Arthritis," The Journal of Rheumatology, 2013. [DOI] [PubMed] [Google Scholar]
- 86.Yarwood A., Viatte S., Okada Y., Plenge R., Yamamoto K., Barton A., et al. , "Loci associated with N-glycosylation of human IgG are not associated with rheumatoid arthritis: a Mendelian randomisation study," Annals of the Rheumatic Diseases, vol. 75, pp. 317–320, 2016. 10.1136/annrheumdis-2014-207210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Simmons C. R., Zou F., Younkin S. G. and Estus S., "Rheumatoid arthritis-associated polymorphisms are not protective against Alzheimer's disease," Molecular Neurodegeneration, vol. 6, p. 33, May 19 2011. 10.1186/1750-1326-6-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xie G., Lu Y., Sun Y., Zhang S. S., Keystone E. C., Gregersen P. K., et al. , "Identification of the NF-kappaB activating protein-like locus as a risk locus for rheumatoid arthritis," Ann Rheum Dis, vol. 72, pp. 1249–54, July 2013. 10.1136/annrheumdis-2012-202076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jalil S. F., Bhatti A., Demirci F. Y., Wang X., Ahmed I., Ahmed M., et al. , "Replication of European Rheumatoid Arthritis Loci in a Pakistani Population," The Journal of Rheumatology, 2013. [DOI] [PubMed] [Google Scholar]
- 90.Hinks A., Marion M. C., Cobb J., Comeau M. E., Sudman M., Ainsworth H. C., et al. , "Brief Report: The Genetic Profile of Rheumatoid Factor–Positive Polyarticular Juvenile Idiopathic Arthritis Resembles That of Adult Rheumatoid Arthritis," Arthritis & Rheumatology, vol. 70, pp. 957–962, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Manning A. K., Ngwa J. S., Hendricks A. E., Liu C.-T., Johnson A. D., Dupuis J., et al. , "Incorporating biological knowledge in the search for gene × gene interaction in genome-wide association studies," BMC Proceedings, vol. 3, p. S81, 2009/12/15 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Das S., Baruah C., Saikia A. K. and Bose S., "Associative role of HLA-DRB1 SNP genotypes as risk factors for susceptibility and severity of rheumatoid arthritis: A North-east Indian population-based study," International Journal of Immunogenetics, vol. 45, pp. 1–7, 2018. 10.1111/iji.12347 [DOI] [PubMed] [Google Scholar]
- 93.Stahl E. A., Raychaudhuri S., Remmers E. F., Xie G., Eyre S., Thomson B. P., et al. , "Genome-wide association study meta-analysis identifies seven new rheumatoid arthritis risk loci," Nat Genet, vol. 42, pp. 508–14, June 2010. 10.1038/ng.582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Viatte S., Flynn E., Lunt M., Barnes J., Singwe-Ngandeu M., Bas S., et al. , "Investigation of Caucasian rheumatoid arthritis susceptibility loci in African patients with the same disease," Arthritis Res Ther, vol. 14, p. R239, 2012. 10.1186/ar4082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Govind N., Choudhury A., Hodkinson B., Ickinger C., Frost J., Lee A., et al. , "Immunochip identifies novel and replicates known, genetic risk loci for rheumatoid arthritis in black South Africans," Molecular medicine (Cambridge, Mass.), vol. 20, pp. 341–349, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lee Y. H., Bae S.-C., Choi S. J., Ji J. D. and Song G. G., "Genome-wide pathway analysis of genome-wide association studies on systemic lupus erythematosus and rheumatoid arthritis," Molecular Biology Reports, vol. 39, pp. 10627–10635, December 01 2012. 10.1007/s11033-012-1952-x [DOI] [PubMed] [Google Scholar]
- 97.Kochi Y., Yamada R., Kobayashi K., Takahashi A., Suzuki A., Sekine A., et al. , "Analysis of single-nucleotide polymorphisms in Japanese rheumatoid arthritis patients shows additional susceptibility markers besides the classic shared epitope susceptibility sequences," Arthritis & Rheumatism, vol. 50, pp. 63–71, 2004. [DOI] [PubMed] [Google Scholar]
- 98.Kirsten H., Al-Hasani H., Holdt L., Gross A., Beutner F., Krohn K., et al. , "Dissecting the genetics of the human transcriptome identifies novel trait-related trans-eQTLs and corroborates the regulatory relevance of non-protein coding loci†," Human Molecular Genetics, vol. 24, pp. 4746–4763, 2015. 10.1093/hmg/ddv194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Brorsson C. A., Onengut S., Chen W.-M., Wenzlau J., Yu L., Baker P., et al. , "Novel association between immune-mediated susceptibility loci and persistent autoantibody positivity in type 1 diabetes," Diabetes, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wolin A., Lahtela E. L., Anttila V., Petrek M., Grunewald J., van Moorsel C. H. M., et al. , "SNP Variants in Major Histocompatibility Complex Are Associated with Sarcoidosis Susceptibility—A Joint Analysis in Four European Populations," Frontiers in Immunology, vol. 8, 2017-April-19 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Herráez D. L., Martínez-Bueno M., Riba L., Torre I. G., Sacnún M., Goñi M., et al. , "Rheumatoid Arthritis in Latin Americans Enriched for Amerindian Ancestry Is Associated With Loci in Chromosomes 1, 12 and 13 and the HLA Class II Region," Arthritis & Rheumatism, vol. 65, pp. 1457–1467, 2013. [DOI] [PubMed] [Google Scholar]
- 102.Okada Y., Yamada R., Suzuki A., Kochi Y., Shimane K., Myouzen K., et al. , "Contribution of a haplotype in the HLA region to anti–cyclic citrullinated peptide antibody positivity in rheumatoid arthritis, independently of HLA–DRB1," Arthritis & Rheumatism, vol. 60, pp. 3582–3590, 2009. [DOI] [PubMed] [Google Scholar]
- 103.Wang K., Baldassano R., Zhang H., Qu H.-Q., Imielinski M., Kugathasan S., et al. , "Comparative genetic analysis of inflammatory bowel disease and type 1 diabetes implicates multiple loci with opposite effects," Human Molecular Genetics, vol. 19, pp. 2059–2067, 2010. 10.1093/hmg/ddq078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liu C., Ackerman H. H. and Carulli J. P., "A genome-wide screen of gene–gene interactions for rheumatoid arthritis susceptibility," Human Genetics, vol. 129, pp. 473–485, May 01 2011. 10.1007/s00439-010-0943-z [DOI] [PubMed] [Google Scholar]
- 105.Zhang L., Pan Q., Wang Y., Wu X. and Shi X., "Bayesian Network Construction and Genotype-Phenotype Inference Using GWAS Statistics," IEEE/ACM Transactions on Computational Biology and Bioinformatics, pp. 1–1, 2018. 10.1109/TCBB.2016.2599867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Eleftherohorinou H., Wright V., Hoggart C., Hartikainen A.-L., Jarvelin M.-R., Balding D., et al. , "Pathway Analysis of GWAS Provides New Insights into Genetic Susceptibility to 3 Inflammatory Diseases," PLOS ONE, vol. 4, p. e8068, 2009. 10.1371/journal.pone.0008068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Feng T. and Zhu X., "Genome-wide searching of rare genetic variants in WTCCC data," Human Genetics, vol. 128, pp. 269–280, September 01 2010. 10.1007/s00439-010-0849-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Schaub M. A., Kaplow I. M., Sirota M., Do C. B., Butte A. J. and Batzoglou S., "A Classifier-based approach to identify genetic similarities between diseases," Bioinformatics, vol. 25, pp. i21–i29, 2009. 10.1093/bioinformatics/btp226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wei W.-H., Viatte S., Merriman T. R., Barton A. and Worthington J., "Genotypic variability based association identifies novel non-additive loci DHCR7 and IRF4 in sero-negative rheumatoid arthritis," Scientific Reports, vol. 7, p. 5261, 2017/07/13 2017. 10.1038/s41598-017-05447-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Matzaraki V., Kumar V., Wijmenga C. and Zhernakova A., "The MHC locus and genetic susceptibility to autoimmune and infectious diseases," Genome Biology, vol. 18, p. 76, April 27 2017. 10.1186/s13059-017-1207-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Andreasi R. B., Khan M., Galuppi E., Govoni M. and Rubini M., "THU0022 Replication analysis of gene-gene interaction between HLA-DQA2 and HLA-DQB2 variants in italian rheumatoid arthritis patients," Annals of the Rheumatic Diseases, vol. 76, pp. 207–207, 2017. [Google Scholar]
- 112.Kim S. A. and Yoo Y. J., "Effects of single nucleotide polymorphism marker density on haplotype block partition," Genomics & Informatics, vol. 14, pp. 196–204, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Daly M. J., Rioux J. D., Schaffner S. F., Hudson T. J. and Lander E. S., "High-resolution haplotype structure in the human genome," Nat Genet, vol. 29, pp. 229–32, October 2001. 10.1038/ng1001-229 [DOI] [PubMed] [Google Scholar]
- 114.Kimmel G. and Shamir R., "A block-free hidden Markov model for genotypes and its application to disease association," J Comput Biol, vol. 12, pp. 1243–60, December 2005. 10.1089/cmb.2005.12.1243 [DOI] [PubMed] [Google Scholar]
- 115.Zhang K., Deng M., Chen T., Waterman M. S. and Sun F., "A dynamic programming algorithm for haplotype block partitioning," Proc Natl Acad Sci U S A, vol. 99, pp. 7335–9, May 28 2002. 10.1073/pnas.102186799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhang K., Qin Z. S., Liu J. S., Chen T., Waterman M. S. and Sun F., "Haplotype block partitioning and tag SNP selection using genotype data and their applications to association studies," Genome Res, vol. 14, pp. 908–16, May 2004. 10.1101/gr.1837404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Katanforoush A., Sadeghi M., Pezeshk H. and Elahi E., "Global haplotype partitioning for maximal associated SNP pairs," BMC Bioinformatics, vol. 10, p. 269, 2009. 10.1186/1471-2105-10-269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zahiri J., Mahdevar G., Nowzari-Dalini A., Ahrabian H. and Sadeghi M., "A novel efficient dynamic programming algorithm for haplotype block partitioning," J Theor Biol, vol. 267, pp. 164–70, November 21 2010. 10.1016/j.jtbi.2010.08.019 [DOI] [PubMed] [Google Scholar]
- 119.Chen W.-P., Hung C.-L. and Lin Y.-L., "Efficient haplotype block partitioning and tag SNP selection algorithms under various constraints," BioMed Research International, vol. 2013, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pugach I., Matveyev R., Wollstein A., Kayser M. and Stoneking M., "Dating the age of admixture via wavelet transform analysis of genome-wide data," Genome Biol, vol. 12, p. R19, 2011. 10.1186/gb-2011-12-2-r19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Patil N., Berno A. J., Hinds D. A., Barrett W. A., Doshi J. M., Hacker C. R., et al. , "Blocks of limited haplotype diversity revealed by high-resolution scanning of human chromosome 21," Science, vol. 294, pp. 1719–23, November 23 2001. 10.1126/science.1065573 [DOI] [PubMed] [Google Scholar]
- 122.Anderson E. C. and Novembre J., "Finding haplotype block boundaries by using the minimum-description-length principle," Am J Hum Genet, vol. 73, pp. 336–54, August 2003. 10.1086/377106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Koivisto M., Perola M., Varilo T., Hennah W., Ekelund J., Lukk M., et al. , "An MDL method for finding haplotype blocks and for estimating the strength of haplotype block boundaries," Pac Symp Biocomput, vol. 8, pp. 502–13, 2003. [DOI] [PubMed] [Google Scholar]
- 124.Pattaro C., Ruczinski I., Fallin D. M. and Parmigiani G., "Haplotype block partitioning as a tool for dimensionality reduction in SNP association studies," BMC Genomics, vol. 9, p. 405, 2008. 10.1186/1471-2164-9-405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ionita-Laza I., Lee S., Makarov V., Buxbaum J. D. and Lin X., "Sequence kernel association tests for the combined effect of rare and common variants," Am J Hum Genet, vol. 92, pp. 841–53, June 6 2013. 10.1016/j.ajhg.2013.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Su S. C., Kuo C. C. and Chen T., "Inference of missing SNPs and information quantity measurements for haplotype blocks," Bioinformatics, vol. 21, pp. 2001–7, May 1 2005. 10.1093/bioinformatics/bti261 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
(TIF)
(DOCX)
(DOCX)
Sheet1 “ID” represents SNPs IDs, and each row represents a block. Sheet2 “Bp” represents SNPs physical positions in base pairs, and each row represents a block. Sheet3 “No. of SNPs in Block” represents the number of SNPs in each block. Sheet4 “Start-Stop”–first column represents blocks start physical positions in base pairs. Sheet4 “Start-Stop”–second column represents blocks end physical positions in base pairs. Sheet4 “Start-Stop”–third column represents blocks sizes in base pairs. Sheet5 “Block no.” represents the block numbers (positions) from all blocks partitioned by CIT method. Sheet6 “P-values” represents the P-values of the blocks.
(XLS)
Sheet1 “ID” represents SNPs IDs, and each row represents a block. Sheet2 “Bp” represents SNPs physical positions in base pairs, and each row represents a block. Sheet3 “No. of SNPs in Block” represents the number of SNPs in each block. Sheet4 “Start-Stop”–first column represents blocks start physical positions in base pairs. Sheet4 “Start-Stop”–second column represents blocks end physical positions in base pairs. Sheet4 “Start-Stop”–third column represents blocks sizes in base pairs. Sheet5 “Block no.” represents the block numbers (positions) from all blocks partitioned by FGT method. Sheet6 “P-values” represents the P-values of the blocks.
(XLS)
Sheet1 “ID” represents SNPs IDs, and each row represents a block. Sheet2 “Bp” represents SNPs physical positions in base pairs, and each row represents a block. Sheet3 “No. of SNPs in Block” represents the number of SNPs in each block. Sheet4 “Start-Stop”–first column represents blocks start physical positions in base pairs. Sheet4 “Start-Stop”–second column represents blocks end physical positions in base pairs. Sheet4 “Start-Stop”–third column represents blocks sizes in base pairs. Sheet5 “Block no.” represents the block numbers (positions) from all blocks partitioned by SSLD method. Sheet6 “P-values” represents the P-values of the blocks.
(XLS)
Sheet1 “ID” represents SNPs IDs. Sheet2 “Bp” represents SNPs physical positions in base pairs. Sheet6 “P-values” represents the P-values of the SNPs.
(XLS)
Sheet1 “CIT” represents SNPs IDs detected by both CIT method and Individual SNP Approach. Sheet2 “FGT” represents SNPs IDs detected by both FGT method and Individual SNP Approach. Sheet3 “SSLD” represents SNPs IDs detected by both SSLD method and Individual SNP Approach.
(XLS)
(XLS)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.