Abstract
In the glucose-free environment that is the midgut of the tsetse fly vector, the procyclic form of Trypanosoma brucei primarily uses proline to feed its central carbon and energy metabolism. In these conditions, the parasite needs to produce glucose 6-phosphate (G6P) through gluconeogenesis from metabolism of non-glycolytic carbon source(s). We showed here that two phosphoenolpyruvate-producing enzymes, PEP carboxykinase (PEPCK) and pyruvate phosphate dikinase (PPDK) have a redundant function for the essential gluconeogenesis from proline. Indeed, incorporation of 13C-enriched proline into G6P was abolished in the PEPCK/PPDK null double mutant (Δppdk/Δpepck), but not in the single Δppdk and Δpepck mutant cell lines. The procyclic trypanosome also uses the glycerol conversion pathway to feed gluconeogenesis, since the death of the Δppdk/Δpepck double null mutant in glucose-free conditions is only observed after RNAi-mediated down-regulation of the expression of the glycerol kinase, the first enzyme of the glycerol conversion pathways. Deletion of the gene encoding fructose-1,6-bisphosphatase (Δfbpase), a key gluconeogenic enzyme irreversibly producing fructose 6-phosphate from fructose 1,6-bisphosphate, considerably reduced, but not abolished, incorporation of 13C-enriched proline into G6P. In addition, the Δfbpase cell line is viable in glucose-free conditions, suggesting that an alternative pathway can be used for G6P production in vitro. However, FBPase is essential in vivo, as shown by the incapacity of the Δfbpase null mutant to colonise the fly vector salivary glands, while the parental phenotype is restored in the Δfbpase rescued cell line re-expressing FBPase. The essential role of FBPase for the development of T. brucei in the tsetse was confirmed by taking advantage of an in vitro differentiation assay based on the RNA-binding protein 6 over-expression, in which the procyclic forms differentiate into epimastigote forms but not into mammalian-infective metacyclic parasites. In total, morphology, immunofluorescence and cytometry analyses showed that the differentiation of the epimastigote stages into the metacyclic forms is abolished in the Δfbpase mutant.
Author summary
Trypanosoma brucei, the parasite responsible for sleeping sickness in humans, is transmitted by the tsetse fly that primarily uses amino acids for its energy production. In the glucose-free environment encountered between the insect blood meals, T. brucei needs to produce through gluconeogenesis glucose 6-phosphate, a key precursor for several essential metabolic pathways. We have shown here that two key gluconeogenic steps, which produce phosphoenolpyruvate and fructose 6-phosphate, respectively, are performed by redundant enzymes (PPDK and PEPCK for phosphoenolpyruvate production; FBPase and a yet unknown enzyme for fructose 6-phosphate production), which highlights the importance of this metabolic pathway for the insect stages of the parasite. Interestingly, deletion of the parasite FBPase gene abolished both the colonisation of the insect salivary glands and the in vitro differentiation of the epimastigote forms into the mammalian infective form of the parasite. Altogether, these data demonstrate for the first time that gluconeogenesis is essential for development of T. brucei in its insect vector and that early development stages of the parasite present in the tsetse midgut are not affected by the absence of FBPase, probably by developing an alternative yet unknown approach to produce fructose 6-phosphate.
Introduction
Trypanosomes of the Trypanosoma brucei species complex are the etiological agents of Human African Trypanosomiasis, a parasitic disease that affects about 20 countries in sub-Saharan Africa [1]. T. brucei adapts to the different environments encountered in its insect (tsetse fly) and mammalian hosts by remodeling its metabolism. In the glucose-rich environment of mammalian blood, the bloodstream forms of T. brucei rely solely on glucose to produce energy. However, in the glucose-free midgut environment of its insect vector, the procyclic form (PCF) of the parasite develops an elaborated energy metabolism based on amino acids such as proline, which has recently been proved to be essential for the parasite development, at least in the tsetse midgut [2, 3].
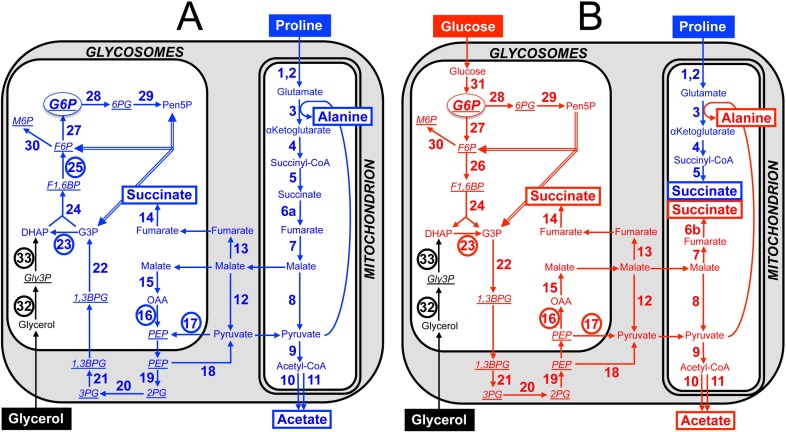
Although glucose is absent from the tsetse midgut lumen between blood meals, the PCF of T. brucei prefers glucose to proline when both carbon sources are available [4]. In these conditions, glucose is converted by aerobic fermentation to the partially oxidised and excreted end products, succinate and acetate [5, 6]. The first seven steps of glycolysis are sequestered within peroxisome-like organelles, called glycosomes [7, 8]. Phosphoenolpyruvate (PEP) is produced in the cytosol, where it is located at a branching point to feed the glycosomal ‘succinate branch’ and the mitochondrial ‘acetate and succinate branches’ (Fig 1B). Both succinate branches are initiated by the glycosomal PEP carboxykinase (PEPCK, EC 4.1.1.49, step 16 in Fig 1) by conversion of PEP into oxaloacetate, which is further converted into malate in the glycosomes (step 15), before being metabolised into succinate in both the glycosomes (steps 13 and 14) and the mitochondrion (steps 6B and 7 in Fig 1B) [9, 10]. PEPCK, together with the glycosomal pyruvate phosphate dikinase (PPDK, EC 2.7.9.1, step 17) [11], are directly involved in the maintenance of the glycosomal ADP/ATP balance, by regenerating the ATP consumed by the first and third glycolytic steps (hexokinase, EC 2.7.1.1, step 31 and phosphofructokinase, PFK, EC 2.7.1.11, step 26,) [12]. Part of PEP is also converted in the cytosol to pyruvate, which enters the mitochondrion to feed the pyruvate dehydrogenase complex (PDH, EC 1.2.4.1, step 9) for acetyl-CoA production, which is further converted into excreted acetate by mitochondrial acetate:succinate CoA-transferase (ASCT, EC 2.8.3.8, step 10) and acetyl-CoA thioesterase (ACH, EC 3.1.2.1, step 11) [13–15]. It is noteworthy that a canonical tricarboxylic acid cycle, with acetyl-CoA being converted into CO2, is not operative in trypanosomes [16]. In glucose-rich condition, proline contributes moderately to central carbon metabolism and is primarily converted into the excreted end product succinate (Fig 1B) [4].
Fig 1. Proline, glucose and glycerol metabolism of the PCF trypanosomes.
Panel A shows a schematic representation of the proline metabolism (blue) in PCF grown in glucose-depleted medium, with glycerol contribution possibly present in the medium (black for the glycerol-specific steps). Panel B corresponds to cells incubated in glucose-rich medium, with contribution of glucose to central carbon metabolism indicated in red. End products excreted from catabolism of proline and glucose are shown in a rectangle, the number corresponding to enzymes under investigation are circled and metabolites analysed by IC-MS/MS are underlined and in italic. For the sake of clarity, the reversible non-oxidative branch of the pentose phosphate pathway (PPP) is represented by double lines and neither the glycerol 3-phosphate (Gly3P)/dihydroxyacetone phosphate (DHAP) shuttle nor the cofactors and nucleotides are shown. Abbreviations: DHAP, dihydroxyacetone phosphate; F1,6BP, fructose 1,6-bisphosphate; 1,3BPG, 1,3-bisphosphoglycerate; F6P, fructose 6-phosphate; G3P, glyceraldehyde 3-phosphate; G6P, glucose 6-phosphate; Gly3P, glycerol 3-phosphate; 6PG, 6-phosphogluconolactone; M6P, mannose 6-phosphate; OAA, oxaloacetate; PEP, phosphoenolpyruvate; 2PG, 2-phosphoglycerate; 3PG, 3-phosphoglycerate; Pen5P, pentose 5-phosphate (ribulose 5-phosphate, ribose 5-phosphate and xylose 5-phosphate). Indicated enzymes are: 1, proline dehydrogenase; 2, pyrroline-5 carboxylate dehydrogenase; 3, L-alanine aminotransferase; 4, α-ketoglutarate dehydrogenase complex; 5, succinyl-CoA synthetase; 6a, succinate dehydrogenase (complex II of the respiratory chain); 6b, mitochondrial NADH-dependent fumarate reductase; 7, mitochondrial fumarase; 8, mitochondrial malic enzyme; 9, pyruvate dehydrogenase complex; 10, acetate:succinate CoA-transferase (ASCT); 11, acetyl-CoA thioesterase; 12, cytosolic malic enzyme; 13, cytosolic fumarase; 14, glycosomal NADH-dependent fumarate reductase; 15, glycosomal malate dehydrogenase; 16, phosphoenolpyruvate carboxykinase (PEPCK); 17, pyruvate phosphate dikinase (PPDK); 18, pyruvate kinase; 19, enolase (ENO); 20, phosphoglycerate mutase; 21, cytosolic phosphoglycerate kinase; 22, glyceraldehyde-3-phosphate dehydrogenase; 23, triose-phosphate isomerase (TIM); 24, aldolase; 25, fructose-1,6-bisphosphatase (FBPase); 26, phosphofructokinase; 27, glucose-6-phosphate isomerase; 28, glucose-6-phosphate dehydrogenase (G6PDH); 29, 6-phosphogluconolactonase, 6-phosphogluconate dehydrogenase, ribose-5-phosphate isomerase and ribulose-5-phosphate epimerase; 30, phosphomannose isomerase; 31, hexokinase; 32, glycerol kinase (GK); 33, NADH-dependent glycerol-3-phosphate dehydrogenase (GPDH).
In contrast, when the PCF are incubated in the absence of glucose, a situation likely encountered by trypanosomes in their different in vivo niches, proline becomes the main carbon source used by the parasite, being consumed up to 6-fold more [4]. In these conditions, proline feeds the whole central carbon and energy metabolism (Fig 1A). For instance, proline-derived succinate produced in the mitochondrion, even in the presence of glucose (steps 1–5 in Fig 1B), is further metabolised and converted into excreted alanine [4]. The absence of glucose also implies that glucose 6-phosphate (G6P), a precursor for essential pathways, such as the pentose phosphate pathway (PPP) and nucleotide sugar biosynthesis [17, 18], needs to be produced by reversal of glycolysis through the so-called gluconeogenesis. Under physiological conditions, all glycolytic enzymes involved in G6P conversion into PEP catalyse a reversible reaction used for glyconeogenesis, except PFK that is replaced by fructose-1,6-bisphosphatase (FBPase, EC 2.7.1.40, step 25), as observed in many organisms including Leishmania major [19]. In addition, the production of the main gluconeogenic precursor, PEP, can theoretically be performed in trypanosomatids by PPDK from pyruvate and/or by PEPCK from oxaloacetate. In the promastigote form of L. major, PPDK and PEPCK both contribute to mannogen biosynthesis through gluconeogenesis from alanine and aspartate, respectively [20]. In contrast, proline has been reported to be incorporated into hexose phosphates through gluconeogenesis in the PCF of T. brucei grown in the absence of glucose, however the contributions of PPDK and PEPCK in these conditions have not been addressed so far [21].
Here, we used a combination of multiple mutations (up to three genes targeted at the same time by knock-out and/or RNAi-mediated knock-down) and metabolomic analyses to study the role of the key gluconeogenic enzymes in the PCF trypanosomes. We showed that PPDK and PEPCK have a redundant essential function for the incorporation of [13C]-enriched proline into gluconeogenic intermediates. In addition, gluconeogenesis from glycerol is abolished in the glycerol kinase (GK, EC 2.7.1.30, step 32) null background. FBPase gene knock-out abolished colonisation of the tsetse fly vector salivary glands by the parasite, as well as in vitro differentiation of the epimastigote forms into the metacyclic form, demonstrating the crucial role of gluconeogenesis, at least during the last part of the trypanosome cyclical development in the tsetse fly.
Results
PPDK and PEPCK are essential for gluconeogenesis from proline
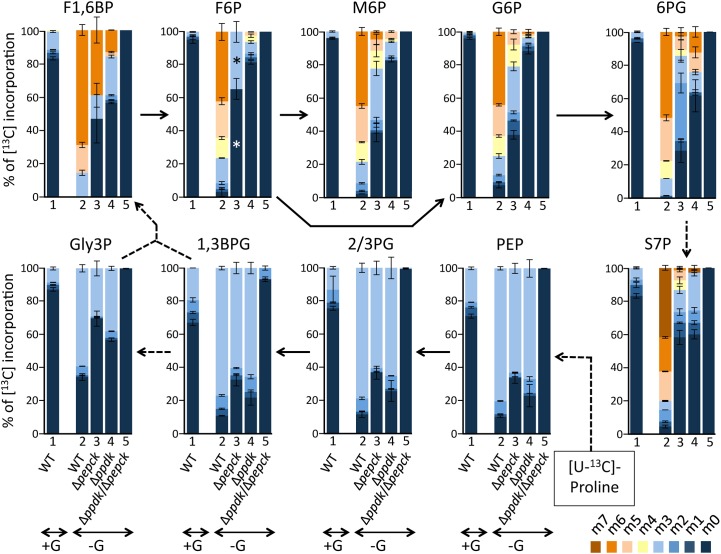
PCF trypanosomes incubated in glucose-free and proline-rich conditions are able to maintain significant levels of (glycolytic) sugar-phosphates, thought at lower extent than cells incubated with glucose and proline (S1 Table). This means that proline is able to support the synthesis of the sugar-phosphates by a gluconeogenesis process. According to the current view of the central carbon metabolism of the PCF trypanosomes, PPDK and PEPCK are the only enzymatic steps that can produce PEP, the precursor of gluconeogenesis, in proline catabolism (steps 16 and 17 in Fig 1A). These two enzymes have been shown to contribute to glucose catabolism, however, their roles in glucose-depleted conditions have not been investigated so far [12, 22]. To investigate this poorly explored gluconeogenic metabolic pathway from proline, we have determined by mass spectrometry the incorporation levels of 13C atoms from uniformly [13C]-enriched proline ([U-13C]-proline) into key gluconeogenic intermediates of parental and knock-out (Δppdk, Δpepck and Δppdk/Δpepck) PCF cell lines. Cells were incubated in PBS containing 2 mM [U-13C]-proline in the presence or absence of the same amounts of non-labelled glucose. Incorporation of 13C into glycolytic intermediates was quantified by IC-MS/MS and the values for selected glycolytic metabolites are shown in Fig 2. Incorporation of 13C atoms into hexose phosphate glycolytic intermediates from [U-13C]-proline is very low in the presence of glucose (3.1% on average), compared to cells incubated in the absence of glucose (94.1% on average). The same labelling pattern was also observed for the intermediates of the pentose phosphate pathways, i.e. 6-phosphogluconolactone and sedoheptulose 7-phosphate. This confirms that proline feeds gluconeogenesis and the pentose phosphate pathway in the absence of glucose, while the presence of glucose down-regulates conversion of proline into metabolite intermediates of these pathways, as we previously observed [21]. Incorporation of at least two 13C atoms into the same hexose phosphates is more reduced in the Δppdk mutant than in the Δpepck mutant (13.8% versus 49.3% on average, respectively), suggesting that PPDK might contribute more than PEPCK to gluconeogenesis from proline (Fig 2). More importantly, this 13C-incorporation is abolished in the Δppdk/Δpepck double mutant (not detectable in Fig 2), although proline consumption is increased by 2-fold [12, 21]. This demonstrates that PPDK and PEPCK are the only enzymes significantly contributing to PEP production from proline catabolism and have a redundant function for the essential production of glycolytic intermediates from proline. These data also confirm that, under the conditions pertaining in the cell, pyruvate kinase (EC 2.7.1.40, step 18 in Fig 1) irreversibly converts PEP into pyruvate, even in the absence of PEPCK and PPDK.
Fig 2. IC-MS/MS analysis of intracellular metabolites after isotopic labelling with [U-13C]-proline.
The EATRO1125.T7T parental (WT), Δpepck, Δppdk and Δppdk/Δpepck cell lines were incubated for 2 h in PBS containing 2 mM [U-13C]-proline with 2 mM glucose (+G, only the parental cells) or without glucose (-G) prior to metabolite extraction. The figure shows enrichment of key glycolytic intermediates at 0 to 6 carbon positions (m0 to m6) with 13C expressed as percentage of all corresponding molecules (MID; Mass Isotopomer Distribution). For abbreviations, see Fig 1: S7P, sedoheptulose 7-phosphate; 2/3PG means that 2-phosphoglycerate (2PG) and 3-phosphoglycerate (3PG) are undistinguished by IC-MS/MS. The asterisks (*) in the F6P column for the Δpepck mutant highlights that the absolute amount of this metabolite being particularly low in this incubation conditions, solely the m0 and m3 isotopomer were detected.
Glycerol is an alternative gluconeogenic carbon source
To explore whether blocking gluconeogenesis from proline affects parasite growth, we developed an SDM79-derived glucose-free medium (SDM79-GlcFree) containing less than 1 μM glucose, as determined by NMR spectrometry and supplemented with 50 mM N-acetyl D-glucosamine, a competitive inhibitor of glucose transport [21]. The growth of the parental EATRO1125.T7T PCF cell line is moderately affected by the absence of glucose with a doubling time increased by ~20% in the SDM79-GlcFree compared to the same medium supplemented with 10 mM glucose (Fig 3A). Similarly, growth of the Δppdk/Δpepck cell line is not compromised in the absence of glucose, suggesting that another gluconeogenic carbon source can be used by the PCF in addition to proline (Fig 3A). The abolition of PEP production from gluconeogenic amino acids (including proline), as demonstrated above, strongly supports the view that glycerol might be a possible alternative to proline that would feed gluconeogenesis in the Δppdk/Δpepck cell line. To address this hypothesis, we have measured by IC-MS/MS the [13C]-incorporation into glycolytic intermediates of the parental PCF incubated with [U-13C]-glycerol. In this experiment, most hexose phosphate glycolytic intermediates (83.2% on average) are fully [13C]-enriched after 2 h of incubation with [U-13C]-glycerol as the only carbon source (S1 Fig).
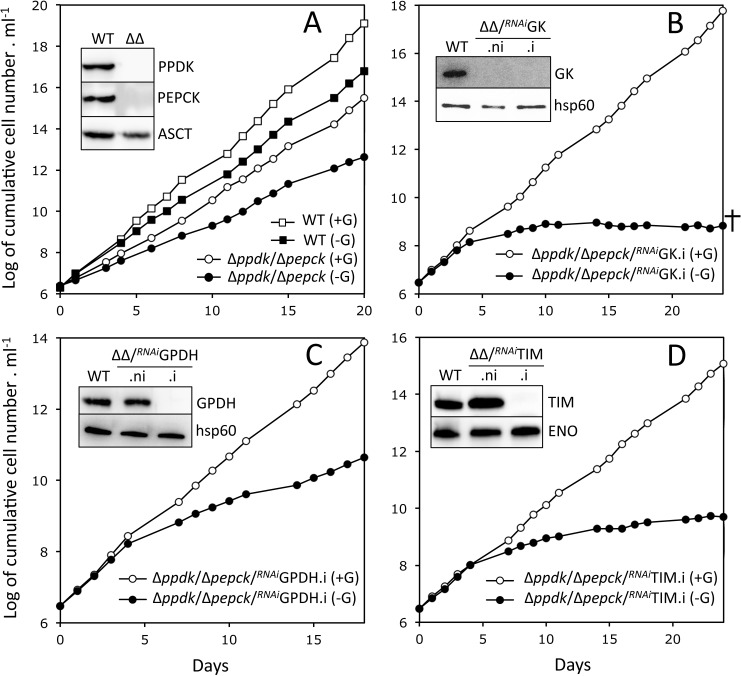
Fig 3. Functional analysis of Δppdk/Δpepck cell lines.
This figure represents growth curves of the Δppdk/Δpepck cell line (panel A) and tetracycline-induced Δppdk/Δpepck/RNAiGK.i, Δppdk/Δpepck/RNAiGPDH.i and Δppdk/Δpepck/RNAiTIM.i triple mutants (panels B-D) grown in SDM79-GlcFree medium containing 10 mM glucose (+G) or not (-G). In glucose-free conditions, the SDM79-GlcFree medium was supplemented with 50 mM N-acetyl-D-glucosamine that inhibits uptake of residual glucose. Cells were maintained in the exponential growth phase (between 106 and 107 cells ml-1). The insets show western blot analyses of the parental (WT), Δppdk/Δpepck (ΔΔ) and tetracycline-induced (.i) and non-induced (.ni) triple mutants with the immune sera indicated in the right margin (ASCT, acetate:succinate CoA-transferase; ENO, enolase). The cross in panel B means that the mutant ultimately dies.
Blocking gluconeogenesis from both proline and glycerol affects growth of the PCF trypanosomes in glucose-free conditions
In order to abolish all possible gluconeogenic pathways, the expression of the key enzymes of glycerol conversion pathway was successively down-regulated in the Δppdk/Δpepck null background, i.e. glycerol kinase (GK, EC 2.7.1.30, Tb927.9.12550-Tb927.9.12630, step 32), glycerol-3-phosphate dehydrogenase (GPDH, EC 1.1.1.8, Tb927.8.3530, step 33) and triose phosphate isomerase (TIM, EC 5.3.1.1, Tb927.11.5520, step 23). We hypothesised that the abolition of gluconeogenesis from both proline and glycerol, in the Δppdk/Δpepck/RNAiGK, Δppdk/Δpepck/RNAiGPDH and Δppdk/Δpepck/RNAiTIM triple mutants, should lead to the death of parasites grown in glucose-free conditions. Indeed, the growth of the tetracycline-induced Δppdk/Δpepck/RNAiGK cell line (Δppdk/Δpepck/RNAiGK.i) in SDM79-GlcFree medium stopped after 10 days of tetracycline induction and cells ultimately died two weeks later, while the addition of glucose restored their growth (Fig 3B). Similarly, the growth of the Δppdk/Δpepck/RNAiTIM.i mutant was strongly affected by the absence of glucose, but this was not lethal to the cells (Fig 3D). However, the growth of the Δppdk/Δpepck/RNAiGPDH.i cells appears to be only moderately affected by the absence of glucose, although GPDH was not detected by western blotting anymore (Fig 3C), probably because the glycerol 3-phosphate/dihydroxyacetone phosphate shuttle could bypass the GPDH step (see Fig 2 in [5] for this pathway).
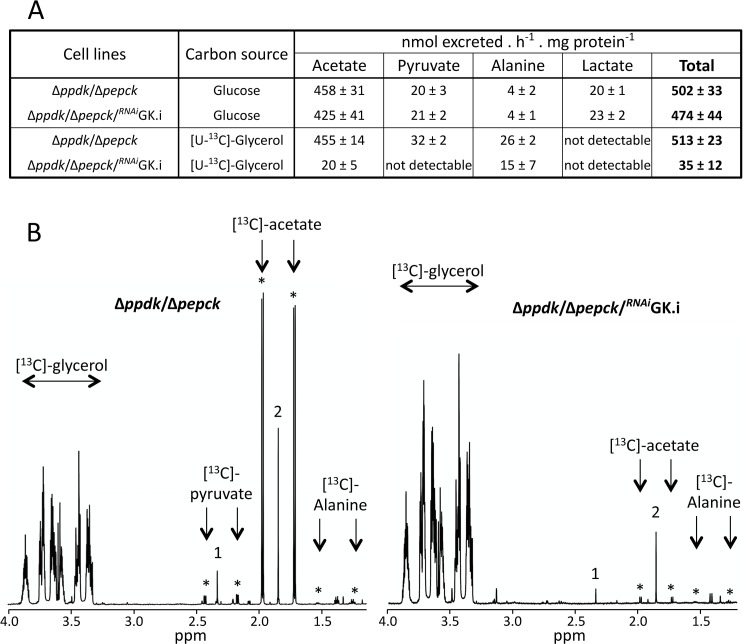
To determine whether the glycerol metabolism is abolished in the Δppdk/Δpepck/RNAiGK.i cell line that shows the strongest growth alteration in SDM79-GlcFree medium, quantitative analyses of 13C-enriched end products excreted from [U-13C]-glycerol metabolism were performed by proton NMR spectrometry [6]. It is noteworthy that (i) the use of 13C-enriched glycerol as carbon source allows us to distinguish between the products excreted from metabolism of [U-13C]-glycerol and an uncharacterised internal carbon source [6, 23], and (ii) the succinate excretion is abolished in the Δppdk/Δpepck/RNAiGK.i mutant (Fig 4), as previously observed for the Δppdk/Δpepck and Δpepck cell lines [12, 22], since the succinate branch is interrupted in the absence of PEPCK (see Fig 1B). The Δppdk/Δpepck/RNAiGK.i mutant shows a 15-fold decrease of the total end products excreted from glycerol breakdown (acetate, alanine, pyruvate and lactate) compared to the parental Δppdk/Δpepck mutant, while glucose metabolism is not affected (Fig 4). This confirms that the growth arrest observed for the Δppdk/Δpepck/RNAiGK.i is due to the abolition of glycerol metabolism that is required to feed gluconeogenesis in the PPDK/PEPCK null background in glucose-free conditions.
Fig 4. Proton (1H) NMR analysis of end products excreted from glucose and [U-13C]-glycerol metabolism.
The Δppdk/Δpepck and tetracycline-induced (.i) Δppdk/Δpepck/RNAiGK cell lines were incubated for 6 h in PBS/NaHCO3 buffer containing 4 mM glucose or 4 mM [U-13C]-glycerol. Panel A shows the quantitative data of excreted end products from glucose or [U-13C]-glycerol metabolism expressed as nmol of acetate, pyruvate, alanine and lactate excreted per h and per mg of protein. Panel B shows one representative NMR spectrum (out of 3 spectra and ranging from 1.2 to 4 ppm) of end products excreted from [U-13C]-glycerol metabolism of the Δppdk/Δpepck and Δppdk/Δpepck/RNAiGK.i cell lines. The resonance massif corresponding to unconsumed [U-13C]-glycerol as indicated (from 3.3 to 3.8 ppm) and resonances indicated by asterisks correspond to [13C]-enriched pyruvate and acetate. The resonances labelled 1 and 2 correspond to non-[13C]-enriched succinate and acetate, respectively, produced from the catabolism of an unknown internal carbon source [6, 23].
FBPase is not essential for PCF trypanosomes in glucose-free in vitro conditions
Gluconeogenesis from glycerol and proline leads to the production of fructose 1,6-bisphosphate (F1,6BP), that is converted into fructose 6-phosphate (F6P) by the fructose-1,6-bisphosphatase (FBPase, EC 3.1.3.11, step 25 in Fig 1A), a well-known gluconeogenic enzyme that is, however, not involved in glycolysis. To confirm the gluconeogenic role of FBPase in PCF, both alleles of the single copy FBPase gene (Tb927.9.8720) were replaced by the blasticidin and puromycin markers (Δfbpase) and FBPase expression was conditionally down-regulated by RNAi (RNAiFBPase). Both the Δfbpase and RNAiFBPase.i cell lines are viable in the absence of glucose, although with a similarly increased doubling time compared to the parental cells (18.4 h and 18.5 h, respectively, compared to 13.7 h) (Fig 5B and 5C). These data suggest that, although the absence of FBPase affects parasite growth in glucose-free conditions, an alternative enzyme or pathway could substitute for the FBPase reaction, in such a way that proline and/or glycerol would contribute to G6P production even in the absence of FBPase. Re-expression of a rescue ectopic copy of the FBPase gene in the Δfbpase background using the tetracycline-inducible pLew100 vector (Δfbpase/FBPase.i cell line) restored the WT growth (Fig 5D).
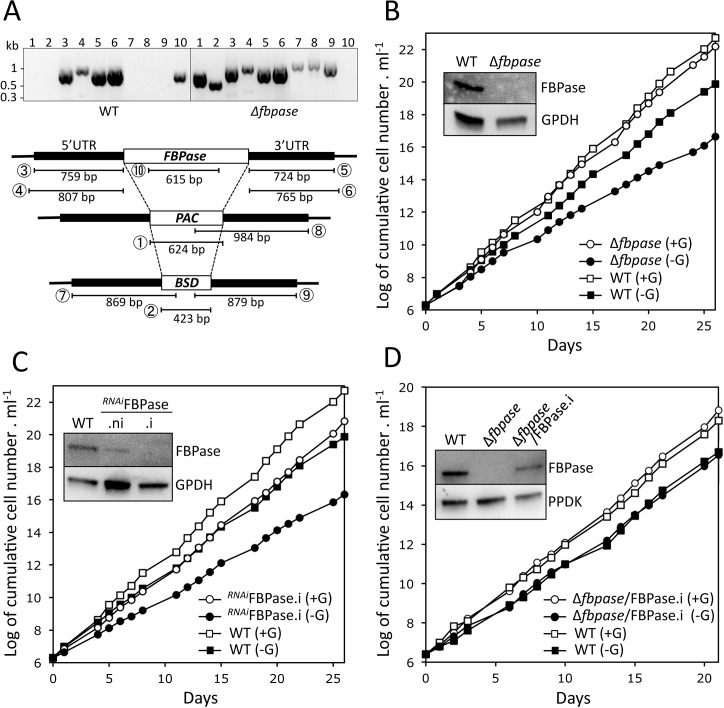
Fig 5. Analysis of FBPase mutants cell lines.
Panel A shows a PCR analysis of genomic DNA isolated from the parental (WT) and Δfbpase cell lines. The 1 to 10 lanes of the gel picture correspond to different PCR products described in the lower panel with circled numbers. As expected, PCR amplification of the FBPase gene (lane 10) was only observed in the parental cell line, while BSD and PAC PCR-products were observed only in the Δfbpase mutant (lanes 1–2 and 7–9). As control, both the 5'- and 3'-untranslated regions (UTRs) are PCR amplified from the parental and Δfbpase samples (lanes 3–6). Panels B-D show the growth curves of the Δfbpase (B), RNAiFPBase.i (C) and Δfbpase/FPBase.i (D) mutant cell lines, together with the parental (WT) cell line, incubated in SDM79-GlcFree medium containing 10 mM glucose (+G) or not (-G). Western blot analyses with the immune sera indicated in the right margin of the parental (WT), Δfbpase, the tetracycline-induced (.i) and non-induced (.ni) RNAiFPBase and/or Δfbpase/FPBase.i mutant are shown in the insets.
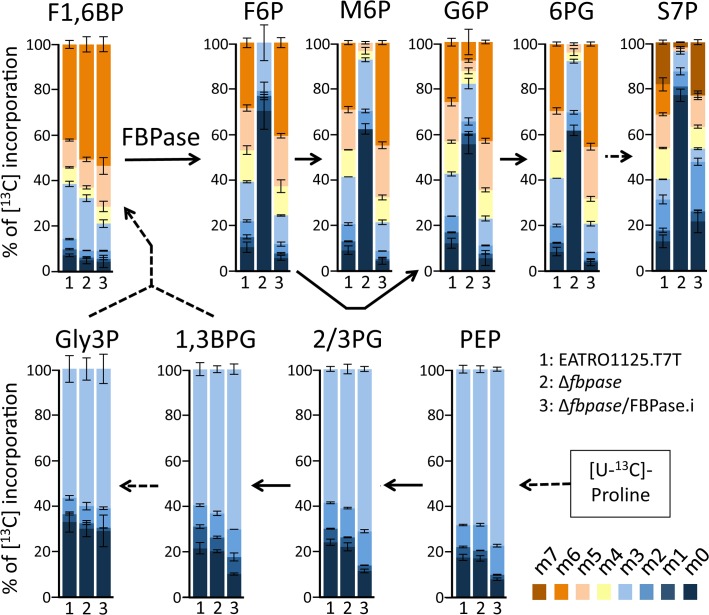
To confirm the role of FBPase in gluconeogenesis, we determined by IC-MS/MS the incorporation levels of [U-13C]-proline into glycolytic intermediates of the WT, Δfbpase and Δfbpase/FBPase.i rescue cell lines (Fig 6). The incorporations of [13C]-enriched atoms from proline into triose phosphates (PEP, 2/3PG, 1,3BPG and Gly3P, see Fig 1 for abbreviations) and F1,6BP are equivalent in the parental, Δfbpase and Δfbpase/FBPase.i cell lines. However, the incorporations of [13C]-enriched atoms in F6P (the product of the reaction catalysed by FBPase), as well as in metabolites produced from F6P, i.e. G6P, mannose 6-phosphate (M6P) and 6-phosphogluconolactone (6PG) and sedoheptulose 7-phosphate (S7P), are strongly reduced in the Δfbpase mutant compared to the parental cell line. Indeed, the relative amounts of non-enriched F6P, G6P, M6P and 6PG and S7P in the Δfbpase cells are increased by 6.5-, 4.6-, 6.9, 7.2- and 5.8-fold, respectively. As expected, incorporation of 13C-carbons into hexose phosphates is restored in the Δfbpase/FBPase.i rescue cell line (Fig 6). These data clearly demonstrate that gluconeogenesis from proline, although strongly reduced, still occurs in the absence of FBPase, which implies the existence of an alternative step to FBPase producing G6P from triose phosphates.
Fig 6. IC-MS/MS analysis of intracellular metabolites of the Δfbpase mutant incubated with [U-13C]-proline.
The parental (EATRO1125.T7T), Δfbpase and Δfbpase/FBPase.i cell lines were incubated for 2 h in PBS containing 2 mM [U-13C]-proline. Enrichment of key glycolytic intermediates at carbon positions 0 to 6 (m0 to m6) is expressed as percentage of all the corresponding molecules. For abbreviations, see Figs 1 and 2.
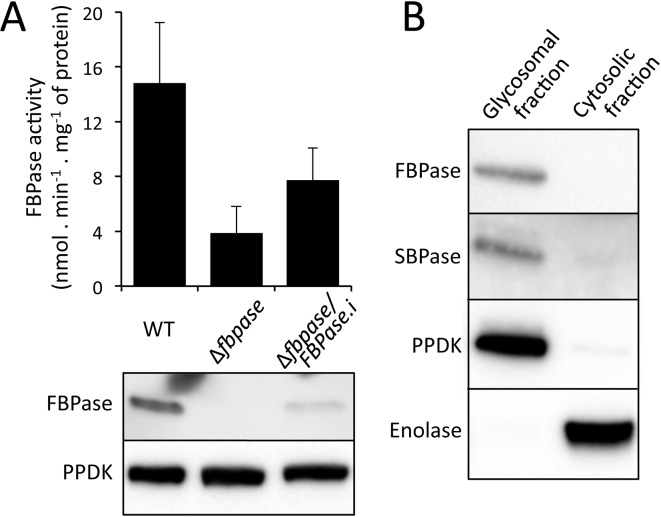
To order to highlight the alternative to FBPase, the FBPase activity was determined on the parental, Δfbpase and Δfbpase/FBPase.i (rescue) cell lines. This enzymatic activity was not detectable on total cellular extracts, but was detectable in the glycosomal fractions of the parental cell line at a low level compared to the glycerol kinase activity (14.8 ±4.5 versus 11,100 ±2,300 nmol min-1 mg-1 of proteins) (Fig 7A). Interestingly, this activity is not abolished in the Δfbpase mutant, although decreased by 3.8-fold. Reintroduction of the FBPase gene in the Δfbpase cell line only partially restored the FBPase activity (7.1 ±2.3 versus 14.8 ±4.5 nmol min-1 mg-1 of proteins), which is consistent with the lower level of FBPase expression in the rescue cell line compared to that of the parental cells (Fig 7A). Altogether, these data strongly support the view that an unknown glycosomal enzyme is responsible for approximately one fourth of the glycosomal FBPase activity. Incidentally, the T. brucei genome contains a single gene potentially coding for a sedoheptulose-1,7-bisphosphatase (SBPase, Tb927.2.5800), which belongs to the same superfamily as the FBPases. Western blot analysis of the glycocomal and cytosolic fractions of the parental trypanosomes with the anti-FBPase and anti-SBPase immune sera demonstrated the glycosomal localisation of these two proteins (Fig 7B), which is in agreement with previously published proteomic analyses of glycosomal fractions [24, 25].
Fig 7. The glycosomal FBPase activity is not abolished in the Δfbpase mutant.
Panel A shows the FBPase activity (top panel) and western blotting analysis (lower panel) of glycosomal fractions of the parental (WT), Δfbpase and Δfbpase/FBPase.i (rescue) cell lines. The FBPase activities were normalised with the glycerol kinase activities. In panel B the glycosomal localisation of FBPase and SBPase was confirmed by western blotting analyses on glycosomal and cytosolic fractions, using control immune sera against glycosomal (PPDK) and cytosolic (enolase) markers.
FBPase is essential for the establishment of mature infections in the insect fly salivary glands
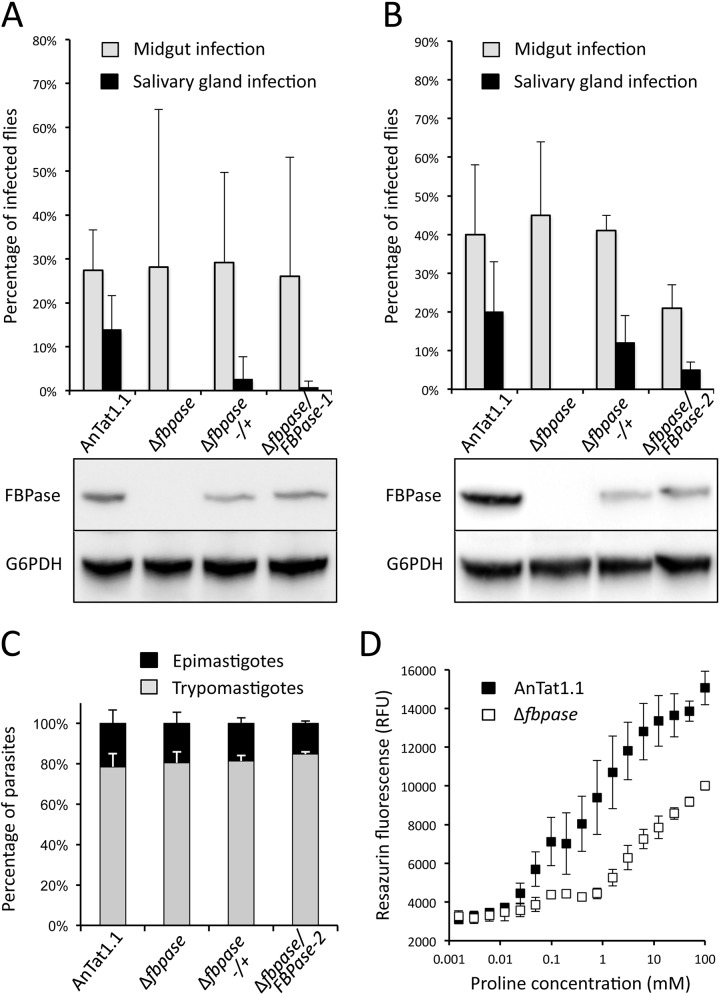
We then attempted to determine whether the absence of FBPase would affect parasite virulence in vivo in their tsetse fly vectors. To perform such experimental infections, a cell line bearing a single FBPase allele (Δfbpase-/+), a FBPase null mutant (Δfbpase), and two distinct constitutive add-back rescue cell lines (Δfbpase/FBPase-1 and 2) were generated in the AnTat1.1E pleomorphic strain [26]. Indeed, this strain has maintained the ability to efficiently colonise salivary glands and to produce infectious metacyclic forms, as opposed to parasites with the EATRO1125 genetic background [27]. Two strategies were developed to produce the Δfbpase/FBPase rescue cell lines, i.e. the addition of a FBPase ectopic copy under the control of the PARP promoter in the pLew100 vector (Δfbpase/FBPase-1), and the in-situ re-insertion of one FBPase gene under the control of its endogenous UTRs (Δfbpase/FBPase-2). Batches of about 50 teneral male tsetse flies were artificially fed through a silicone membrane with cultured PCF parasites of either one of the four cell lines in parallel (the parental wild-type, the Δfbpase-/+, the Δfbpase and the Δfbpase/FBPase-1 or -2 cell lines). Flies were dissected after one month in order to quantify the infection rates per organ, the parasite densities per organ and the proportion of parasite morphotypes as previously described [28]. A total of 784 flies were dissected with similar results obtained with the two series: 338 flies out of 4 biological replicates with the Δfbpase/FBPase-1 panel (Fig 8A) and 446 flies out of 6 replicates with the Δfbpase/FBPase-2 panel (Fig 8B). First, the midgut infection rates were comparable in all conditions (33% on average, ranging from 19% ±27% in Fig 8A to 45% ±19% in Fig 8B for the null mutants). Second, although the salivary gland infection rates were on average 16% for the wild-type cells, 7% for the Δfbpase-/+ parasites and 3% for the Δfbpase/FBPase rescue cell line, no null mutant parasites were seen in any salivary glands and this observation was statistically significant (p = 0.011 by ANOVA Tukey add-hoc post-test at 95% confidence when comparing %SG+ in Δfbpase versus wild-type). This demonstrates that FBPase is essential for the second part of T. brucei cyclical development, i.e. the establishment of mature infections in the insect salivary glands. The lower salivary gland infection rates observed with the Δfbpase/FBPase cell lines could be explained by the additional round of transformation and the longer time spent in culture during the generation of this cell line that would both have affected its virulence. It is also noteworthy that the in-situ rescue approach is apparently more adapted than the use of the pLew100, probably because the PARP promoter used for driving gene expression in the pLew100 vector may be naturally less efficient in the short epimastigotes, attached epimastigotes and/or in the metacyclic stages during the late steps of the cyclical development as compared to the procyclic and mesocyclic midgut stages.
Fig 8. The deletion of the FBPase gene prevents parasites to colonise the tsetse salivary glands.
In this experiment, batches of 50 Glossina morsitans morsitans teneral males were artificially fed with either the parental AnTat1.1E wild-type, the Δfbpase-/+ (one allele deleted), the Δfbpase (null mutant) or the Δfbpase/FBPase-1 and -2 (rescue cell lines) PCF cell lines in culture medium as previously described [28]. Four weeks after the infective meal, a total of 790 flies were dissected to assess the presence of parasites in their midgut and salivary glands by microscopic examination. Midgut and salivary gland infection rates (in % ±SD) are presented for each cell line as the mean of 4 independent biological replicates done with the rescue cell line expressing the FBPase through the pLew100 vector (panel A), and of 6 independent biological replicates done with the rescue cell line expressing the FBPase from an ectopic FBPase copy in the FBPase locus (panel B). The proportion of flies developing a salivary gland infection are: 12/96 for the parental AnTat1.1E, 0/60 for Δfbpase, 3/104 for Δfbpase-/+ and 1/84 for Δfbpase/FBPase-1 in panel A and 33/163 for the parental AnTat1.1E, 0/91 for Δfbpase, 8/65 for Δfbpase-/+ and 6/125 for Δfbpase/FBPase-2 in panel B. Below the graphs are shown western blot analyses of the FBPase expression using the glucose-6-phosphate dehydrogenase (G6PDH) as loading control. (C) The proportions of cells in the different morphotypes found in the proventriculus and midgut were compared after dissection of flies infected with either one the four different cell lines from at least two independent biological replicates. The proportion of trypomastigotes and epimastigotes is indicated in grey and black respectively. 692, 373, 432, and 280, cells isolated from 6, 2, 3 and 2 flies have been analysed for the parental AnTat1.1E, Δfbpase, Δfbpase-/+ and Δfbpase/FBPase-2 cell lines, respectively. Panel D shows growth of the AnTat1.1E and Δfbpase cell lines in the SDM79-GlcFree medium containing from less than 20 μM to 100 mM proline, using the Alamar Blue assay. The proline concentration values on the x axis correspond to the amounts of proline added to the proline-depleted SDM79-GlcFree medium, which contains less than 20 μM proline.
Assuming that (i) the parasite densities in the midgut, estimated by microscopic observations, were similar for all the cell lines and that (ii) no obvious motility defect was detected among these cell lines neither in vitro nor in vivo, we reasoned that the absence of FBPase may have impaired parasite differentiation, especially the first morphotype switch occurring in proventricular parasites. Therefore, parasites were isolated from the anterior midgut and proventriculus of infected flies, and stained with a DNA marker (DAPI) and an axonemal marker Mab25 [29] in order to determine the proportions of cells in each morphotype (trypomastigote versus epimastigote) according to the cell lines (Fig 8C). Nevertheless, no difference was detected between cell lines that were all displaying on average 80% of proventricular parasites in the trypomastigote morphotype and 20% of epimastigotes (280 to 692 cells out of 2 to 6 flies per cell line). This demonstrates again that FBPase is not essential for the trypanosome cyclical development in the midgut, while it is required at least for the initial colonisation of salivary glands.
We reasoned that these differences in behaviour could be the consequence of possible low proline concentrations in the oesophagus and/or the salivary glands of the tsetse, which would not be sufficient for feed gluconeogenesis in the absence of FBPase. To test this hypothesis, we estimated the growth of the parental and Δfbpase cell lines as a function of proline concentrations in the SDM79-GlcFree medium, using the Alamar Blue assay. As expected these two cell lines failed to grow under less than 20 μM proline, this amino acid being the primary carbon source used by the parasite to feed its central carbon metabolism (Fig 8D). Growth of the parental cells is considerably improved by addition of at least 20 μM proline, the parental cells growing only 2-times slower in the presence of 0.5 mM compared to 5 mM proline (concentration in the SDM79-GlcFree medium). In contrast the Δfbpase cells are barely growing in the presence of 0.5 mM proline. This analysis showed that the Δfbpase mutant requires ~30-times more proline to grow at the same speed as the parental cells (Fig 8D), which may provide a rational explanation of the observed difference in behaviour of these two cell lines in vivo if proline concentrations are higher in the midgut compared to the oesophagus and/or the salivary glands. Unfortunately, the concentrations of proline in the tsetse organs are unknown. It is noteworthy that, immunofluorescence analyses using an immune serum against a bona fide glycosomal protein, aldolase, showed no difference in the number of glycosomes per cell between the parental, Δfbpase and Δfbpase/FBPase cell lines (S2 Fig).
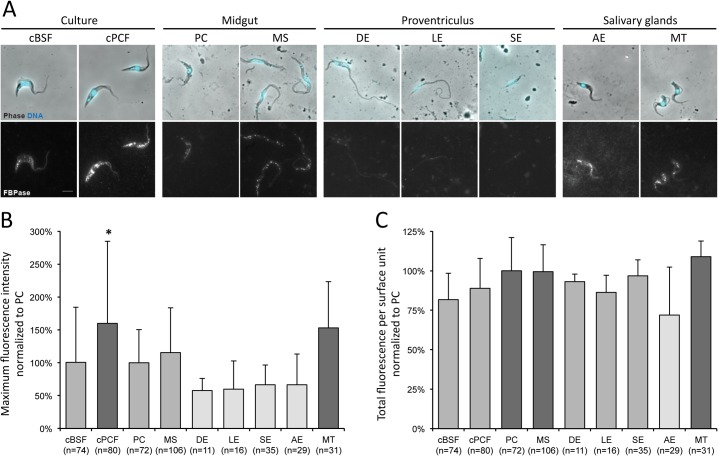
In order to deepen the study of the in vivo role of FBPase, variations of the FBPase expression during the parasite cycle of wild-type trypanosomes were scrutinised by immunofluorescence analysis with an anti-FBPase antibody (Fig 9A). Assuming that the observed fluorescence is directly correlated to the amount of expressed proteins accessible to the antibody, all stages were seen to express FBPase in their glycosomes, yet in variable amounts (Fig 9A). The maximum signal intensities (Fig 9B) and the total amounts of fluorescent signal per surface unit (Fig 9C) were measured in a total of 454 individual cells isolated from more than 12 flies, further normalised to the values obtained on PCF cells from the same fly after removal of the background values, and finally plotted by stage in percentage of the fluorescent signals from PCF. The lowest maximum fluorescent signal intensities (Fig 9B) and total amounts of fluorescence per surface unit (Fig 9C) were observed in proventricular epimastigotes (DE, LE and SE) whereas the highest values were detected in salivary glands metacyclic forms (MT), and the differences between the two groups were statistically significant by ANOVA (p = 0.002, <0.001, <0.0001 and <0.0001 between MT Vs. DE, LE, SE and AE respectively by Tukey ad-hoc post-test on the maximum intensities, and p = 0.002 and <0.0001 between MT Vs. LE and AE for the total fluorescence per surface unit). The naturally lower FBPase expression in proventricular epimastigotes is in accordance with the absence of phenotype of the Δfbpase null mutants in terms of morphotype switching ability (Fig 9A).
Fig 9. FBPase is expressed in all parasite cycle stages yet at variable levels.
(A) Wild-type AnTat1.1E cultured bloodstream (cBSF) and procyclic (cPCF) forms, as well as parasites isolated from infected tsetse flies were fixed in methanol and stained with the anti-FBPase antibody (white) and DAPI (cyan). PC: procyclic trypomastigote, MS: mesocyclic trypomastigote, DE: dividing epimastigote, LE: long epimastigote, SE: short epimastigote, AE: attached epimastigote, and MT: metacyclic trypomastigote. (B-C) A region of interest was drawn on the phase picture of each individual cell and used for the quantification of the fluorescence provided by the anti-FBPase labelling. After background removal, the fluorescent signals were normalised (in %) to that obtained in PCF treated in the same experiment. The maximum fluorescence intensity (B) and total amount of fluorescence (C) corresponding to the FBPase expression levels were plotted according to the parasite stage. The total number of cells analysed from 3 independent experimental infections is indicated below the histogram bars for each stage. The different shades of grey represent groups of stages statistically identical between each other and different from those in the other groups according to a two-tailed ANOVA test with Tukey ad-hoc post-tests at 95% confidence for inter-group comparisons. * indicates the stage statistically different from all others with p<0.005.
FBPase is essential for production of metacyclic forms in proline rich-conditions
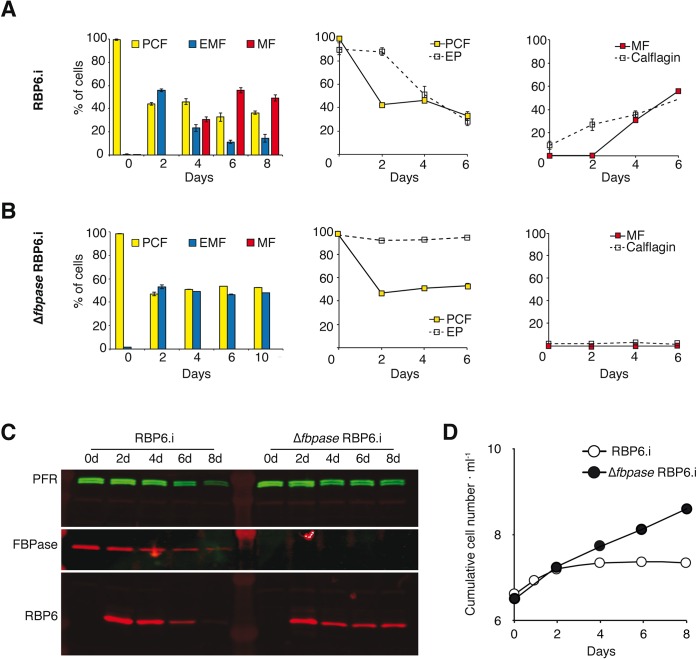
The fly passage phenotype observed may be due to an impairment of the parasite developmental program per se or to defects in migration, and/or to mobility defects during parasite migration in the tsetse and/or to non-adapted interactions with the tsetse environments. To distinguish between these possibilities, we tested the impact of the FBPase deletion in an in vitro model mimicking the parasite development upon inducible over-expression of the RNA binding protein 6 (RBP6), a central regulator of fly-stage differentiation [30]. The stage-specific morphology of induced cells, based on cell size and shape, as well as the relative position of the kinetoplast to the nucleus were monitored over the differentiation kinetics [31]. In PCF trypomastigotes the kinetoplast is at the posterior part of the cell, whereas in epimastigotes (EMF) the kinetoplast migrates to the opposite side of the nucleus and is found at the anterior part of the cell or in close proximity to the nucleus. Metacyclic trypomastigotes (MF) are smaller than EMF and PCF with the kinetoplast at the very end of the rounded posterior tip and a bloodstream form-like flagellar shape. In addition, we used two distinct stage-specific marker proteins for staging, i.e. EP procyclin (EP) for PCF [32] and calflagin for MF [33]. Calflagins are proteins localising to lipid rafts in the flagellar membrane and their amounts in bloodstream forms and MF are ten-fold enriched compared to that in PCF. Four days after the tetracycline-induction of RBP6 over-expression, ~30% of the cells (RBP6.i) had a characteristic MF morphology and after six days MF subpopulation reaches a peak at 55% (Fig 10A, left). Flow cytometry of RBP6.i cultures showed that EP expression was maintained in all cells during the first two days post induction and only then decreased proportionally to the fraction of the remaining PCF population (Fig 10A, middle). EP expression has previously been described in early salivary gland EMF [34]. The fraction of cells expressing calflagin constantly increased over time and attained the same percentage as the MF subpopulation after four and six days (Fig 10A, right). Previous reports showed that EP was not expressed in MF and that calflagins were detectable at basal levels in PCF but significantly up-regulated in MF [35, 36]. Additional evidence for developmental changes in these cultures is provided by RBP6 expression levels that decreased over time of the experiment (Fig 10C) and by a cell proliferation arrest (Fig 10D) in successfully differentiating cultures. The Δfbpase RBP6.i mutant, in which RBP6 was conditionally over-expressed in the Δfbpase background, was analysed in parallel under exactly comparable conditions (Fig 10B). Most importantly, the RBP6 expression levels as determined by quantitative western blotting (Fig 10C) were similar in the Δfbpase and parental backgrounds over the entire time and did not decrease after 8 days. Induced Δfbpase RBP6.i cells developed into EMF after two days but completely failed to differentiate into MF and consequently the fraction of EMF remained stable at around 50% for the duration of the experiment. EP and calflagin expression levels were not different between cell lines all over the time course and the population growth was maintained (Fig 10D). As the EP-positive population did not decrease, it is likely that the developmental blockage occurred in early EMF. Together, we can conclude that FBPase is absolutely essential for development in culture and hence this strong phenotype is cell autonomous.
Fig 10. FBPase is essential for metacyclogenesis in culture.
RBP6-induced differentiation kinetics is shown for the parental EATRO1125.T7T RBP6.i cell line (A) and the Δfbpase RBP6.i mutant (B). RBP6 over-expression was induced at time 0 by addition of 1 μM tetracycline. Developmental stages were morphologically scored by cell size, shape and the relative position of the kinetoplast to the nucleus within the cell (n >100 cells per time point, biological triplicate, SEM). Expression of the stage-specific proteins EP and calflagin was quantified at the single cell level by flow cytometry and gated into two populations with low or high fluorescence. The left panels show histograms of morphotypes with PCF in yellow, EMF in blue and MF forms in red. The middle and right panels present morphotype fractions from the histogram (solid lines, data points colour matched to histogram) together with the fractions of stage marker expression levels (cells with high fluorescence/total number of cells, dashed lines) for EP and calflagin, respectively. (C) Western blot showing the RBP6 over-expression levels upon tetracycline induction of the indicated cell lines. PFR was used as loading control and the Δfbpase knock out was verified by anti-FBPase. (D) Growth curves of the RBP6.i and Δfbpase RBP6.i populations upon induction of RBP6 over-expression. C and D are from a representative replicate of the three experiments shown in A and B.
Discussion
The insect stages of T. brucei live in the midgut, the foregut and the salivary glands of the tsetse fly, which are considered glucose-free environment between the insect blood meals. In this context, trypanosomes need to produce G6P, the precursor of essential pathways, from non-glycolytic carbon sources. Production of hexose phosphates from proline through gluconeogenesis has recently been described for the PCF trypanosomes [21] and was consistent with the previously described metabolic switch toward proline when the parasite is incubated in glucose-depleted conditions [4, 37], as well as the requirement of an active proline metabolism to support development of trypanosomes in the insect midgut [3]. However, the pathway leading to F6P production as well as possible alternative gluconeogenic carbon sources used by the PCF were not addressed so far. Here, we show that two key steps of gluconeogenesis, i.e. production of PEP and production of F6P, are achieved by redundant reactions in PCF trypanosomes grown in vitro. However, the canonical FBPase gluconeogenic enzyme, is essential for the infection of the tsetse fly salivary glands where the differentiation of the attached epimastigote forms into free metacyclic infective forms occurs [35].
Production of the gluconeogenic precursor PEP from proline is performed by two different and complementary T. brucei glycosomal enzymes, i.e. PPDK and PEPCK. Indeed, abolition of G6P production from proline is only observed in the PPDK/PEPCK null background, but not in the Δppdk and Δpepck single mutants. The implication of both the PEPCK and PPDK in gluconeogenesis has also been observed in Leishmania mexicana, however, the redundant effect was not investigated [20]. This redundancy between PPDK and PEPCK has also recently been observed in Arabidopsis thaliana, in which gluconeogenesis is critical to fuel the transition from seed to seedling [38]. To our knowledge, trypanosomatids and plants are the only eukaryotes known for using two distinct routes for the entry of organic acids into gluconeogenesis. In L. mexicana, PEPCK may participate in the entry of aspartate in promastigotes while PPDK is involved in the entry of alanine in amastigotes [20]. It is noteworthy, that this situation does not occur in T. brucei, since alanine and aspartate are not significantly consumed by the procyclic trypanosomes [4, 39, 40]. In A. thaliana PPDK participates in gluconeogenesis by remobilising amino acids such as alanine that give rise to pyruvate, while PEPCK is primarily involved in remobilisation of acetyl-CoA derived from lipids [38]. Interestingly, T. brucei is the only organism known so far to use PPDK in the gluconeogenic or in the glycolytic direction, depending on the carbon source availability. Indeed, we recently showed that in glucose-rich conditions PPDK plays a key role in the maintenance of the glycosomal ATP/ADP balance by converting PEP into pyruvate [12], while here we report that in the absence of glucose PPDK performs the reverse reaction to feed gluconeogenesis.
Important questions remain regarding the maintenance of the glycosomal ADP/ATP and redox balances in the PCF trypanosomes grown in glucose-free conditions. As mentioned above the glycosomal PPDK and PEPCK are critical for the maintenance of the glycosomal ADP/ATP balance by regenerating ATP when the parasite is fed with glucose [12]. However, in the absence of glucose, both enzymes work in the ATP-consuming direction with no glycosomal ATP-generating step in the gluconeogenic pathway. In this context, ATP could be provided to the enzymes involved in the gluconeogenetic pathway by another unknown glycosomal ADP/ATP-dependent enzyme, possibly encoded by one of the many hypothetical genes. Alternatively, glycosomal ATP could be regenerated through a carrier-mediated ADP/ATP exchange with the cytosolic compartment. Such a glycosomal carrier has not been described so far in trypanosomatids, although this hypothesis was previously proposed to explain the maintenance of the glycosomal ADP/ATP balance in the Δppdk/Δpepck mutant grown in glucose-rich conditions [12]. It is important to note that this hypothetical glycosomal ADP/ATP exchange activity should be low enough to prevent the lethal turbo-explosion of the unusual glycolysis developed by trypanosomes [41]. Indeed, trypanosomes lack feedback regulation of the early steps of glycolysis, which is compatible with the glycosomal sequestration of the enzymes within glycosomes. However, partial relocation of the glycosomal enzymes within the cytosol induces accumulation of toxic glycolytic intermediates and ATP depletion by the so-called turbo-explosion. One may consider that a high capacity ATP/ADP exchanger located in the glycosomal membrane would lead to the same negative effect in the presence of glucose, unless the expression of this putative exchanger was controlled by glucose levels.
As expected, the T. brucei FBPase is involved in gluconeogenesis, as shown by a 6.5-fold reduction of F6P production from proline in the Δfbpase mutant, which was restored by re-expressing an ectopic FBPase copy in the Δfbpase background. However, to our surprise, the Δfbpase and RNAiFBPase.i mutants are viable in the glucose-free conditions, while blocking gluconeogenesis from both proline and glycerol in the Δppdk/Δpepck/RNAiGK.i and Δppdk/Δpepck/RNAiTIM.i cell lines compromises growth in the absence of glucose. These data, which are consistent with a residual production of 13C-enriched G6P from [U-13C]-proline in the Δfbpase cell line, imply the existence of an alternative to FBPase for production of hexose phosphates from gluconeogenic carbon source(s). We must also take into account that the alternative to the FBPase reaction is probably not functional in Leishmania major, since FBPase has been shown to be essential for the development of the intracellular amastigote form as well as for the promastigote form growth in the absence of glucose [19]. Interestingly, the T. brucei gene potentially coding for SBPase is not present in the Leishmania genomes. The residual FBPase activity in the glycosomal fraction of the Δfbpase cell line strongly supports the view that the product of this putative T. brucei SBPase is also involved in gluconeogenesis in the PCF trypanosomes. Our interpretation of the data is consistent with the previously characterisation in Cyanobacteria and in yeast of FBPase/SBPase genes showing a dual-function FBPase/SBPase [42–44].
Whatever this unknown alternative pathway is, it seems to be functional in the absence of FBPase in both in vitro cultured PCF and in vivo during the early cyclical development of midgut trypomastigote parasites. It is however not sufficient to allow Δfbpase null mutants to colonise the tsetse fly vector salivary glands, demonstrating the crucial role of gluconeogenesis during this later part of the trypanosome cyclical development. These in vivo data showed that the rise of epimastigotes in the anterior midgut and proventriculus is not affected by the absence of FBPase. However, the absence of salivary gland infection suggests that either the proventricular epimastigotes have lost their capacity of maturation (e.g. to attached epimastigotes) and/or their abilty to migrate/orientate towards the salivary glands through the mouthparts and/or to attach to the salivary gland epithelium. The phenotype obtained in vitro in the RBP6-mediated differentiation model combined to the Δfbpase null mutation is fully consistent with the fly infection data as EMF accumulate and no MF are detectable. Moreover, the development programme arrest in culture rules out that gluconeogenesis is mainly required to meet specific metabolic requirements of the fly alimentary tract micro-environments. A more defined staging of the developmental arrest is not possible in vitro as the EMF accumulating in culture are heterogenous in morphology and do not precisely match the natural morphologies present in the fly. It should also be remembered that the RBP6-induced differentiation is a useful tool yet a clearly “forced” model. Parasite development seems to depend on the expression of an alternative pathway or an enzymatic activity complementing the FBPase gene deletion. Once the identity of this bypass will be unravelled, we will have to test whether this phenotype may be due to a threshold effect of flux through the gluconeogenic pathway.
The growth of the PCF is compromised in glucose-free conditions only if triose phosphate production from proline and glycerol metabolism are together abolished in the Δppdk/Δpepck/RNAiGK.i cell line, since the RNAiGK.i and Δppdk/Δpepck mutants are viable in these growth conditions. This implies that the glycerol conversion pathway is sufficient to feed gluconeogenesis. However, glycerol amounts are in the range of 5–10 μM in standard SDM79 medium (sera contain 50–100 μM glycerol [45, 46]) and should be consumed together with glucose by the PCF cells during preparation of the glucose-free medium. In the absence of glycerol in the medium, it could be produced from the medium compounds, such as phospholipids coming from fetal calf serum by the action of trypanosomal phospholipases [47] or from an endogenous carbon source. Interestingly, we previously reported the presence in PCF trypanosomes of a yet unknown endogenous carbon source detectable by excreted end products from its metabolism, acetate and succinate, in the absence of any extracellular carbon source [6, 23]. We are presently analysing this endogenous carbon source and its possible involvement in gluconeogenesis.
Materials and methods
Trypanosomes and cell cultures
The PCF of T. brucei EATRO1125.T7T (TetR-HYG T7RNAPOL-NEO) and AnTat1.1E [26] were cultivated at 27°C in the presence of 5% CO2 in SDM79 medium containing 10% (v/v) heat-inactivated fetal calf serum and 3.5 mg ml-1 hemin [48] or in a glucose-depleted medium derived from SDM79, called SDM79-GlcFree. This SDM79-GlcFree medium consists of a glucose-depleted SDM79 medium, containing 20% (v/v) heat-inactivated fetal calf serum, in which parental cells were cultured during 72 hours in order to consume the glucose coming from the serum and then diluted with the same volume of glucose-depleted SDM79 medium without serum to finally obtain SDM79-GlcFree. Glucose depletion was verified by NMR spectrometry analyses (with a detection threshold of glucose ≤ 1 μM) and to prevent import of residual glucose, a non-metabolised glucose analogue inhibiting glucose transport (N-acetyl-D-glucosamine, 50 mM) was added in the medium [21]. The same protocol was followed to produce SDM79-GlcFree and proline-depleted medium, except that the starting glucose-depleted SDM79 medium containing 100 μM proline, instead of 5 mM, was diluted with one volume of proline/glucose-depleted SDM79. Considering the rate of proline consumption of PCF trypanosomes, we estimated that the proline-depleted SDM79-GlcFree medium contains less than 20 μM proline. To estimate the cell density by the Alamer Blue assay, cells diluted in 200 μl of proline-depleted SDM79-GlcFree medium in which 1 μM to 100 mM proline was added by serial two-fold dilutions at a 2 x 106 cell density, were incubated for 48 h at 27°C in microplates, before adding 20 μl of 0.49 mM Alamar Blue (Resazurin). Measurement of fluorescence was performed with the microplate reader Fluostar Optima (BMG Labtech) at 550 nm excitation wavelength and 600 nm emission wavelength.
Gene knockout and rescue cell lines
The Δppdk mutant cell line was obtained by replacing both alleles of the PPDK gene (Tb927.11.3120, http://www.genedb.org/genedb/tryp/) by two different plasmids encoding the hygromycin resistance gene and the T7 RNA polymerase gene for the first one, and the neomycin resistance gene and the tetracycline repressor gene under the control of the T7 RNA polymerase promoter for the second one, as described before [49]. Replacement of the PEPCK gene (Tb927.2.4210) by the blasticidin (BSD) and puromycin (PAC) resistance markers via homologous recombination was described before (Δpepck cell line) [22]. The Δppdk/Δpepck double mutant was also described before [12]. Replacement of both alleles of the FBPase gene (Tb927.9.8720) by the blasticidin (BSD) and puromycin (PAC) resistance markers via homologous recombination was performed with DNA fragments containing a resistance marker gene flanked by the FBPase UTR sequences. Briefly, the pGEMt plasmid was used to clone an HpaI DNA fragment containing the BSD or PAC resistance marker gene preceded by the FBPase 5'-UTR fragment (729 bp) and followed by the FBPase 3'-UTR fragment (694 bp). The PAC resistance marker replaced one allele of the FBPase gene via homologous recombination and BSD resistance marker replaced the second allele. The EATRO1125.T7T parental cell line, which constitutively expresses the T7 RNA polymerase gene and the tetracycline repressor under the control of a T7 RNA polymerase promoter for tetracycline inducible expression (TetR-HYG T7RNAPOL-NEO) [50], was used to generate the FBPase knockout cell line. Transfection and selection of drug-resistant clones were performed as reported previously [11]. Transfected cells were selected in SDM79 medium containing hygromycin (25 μg ml-1), neomycin (10 μg ml-1), blasticidin (10 μg ml-1) and/or puromycin (1 μg ml-1). The selected cell line TetR-HYG T7RNAPOL-NEO Δfbpase::BSD/Δfbpase::PAC is called Δfbpase. To produce the add-back Δfbpase/FBPase rescue cell lines, a FBPase ectopic copy under control of the PARP promoter in the pLew100 expression vector, which contains the phleomycin resistance gene (kindly provided by E. Wirtz and G. Cross) [51], was introduced in the Δfbpase null background. The pLew100-FBPase plasmid was generated by introduction the full length FBPase gene in the HindIII and BamHI restriction sites of the vector (Genecust). The Δfbpase/FBPase cell line was selected in SDM79 medium containing phleomycin (5 μg ml-1) in addition to the 4 other antibiotics used to select the Δfbpase cell line.
For experimental infections of Δtsetse flies, the Δfbpase null mutant was generated in the pleomorphic AnTat1.1E PCF strain, using the same approach described above. Then, two distinct strategies were used to produce the add-back Δfbpase/FBPase rescue cell lines in the AnTat1.1E Δfbpase null background. The Δfbpase/FBPase-1 cell line expresses a FBPase ectopic copy under control of the PARP promoter in the pLew100 expression vector as described above for the EATRO1125 Δfbpase/FBPase cell line. The Δfbpase/FBPase-2 cell line was generated by in situ re-insertion of one FBPase gene under the control of its endogenous UTRs. The 3.5 kb DNA fragment for in situ re-insertion consists on the FBPase coding sequence preceded by its 5' intergenic region (722 bp) and followed by its 3' intergenic region (662 bp), the phleomycin resistance gene (375 bp) and a second copy of the FBPase 3' intergenic region. The 3.5 kb DNA fragment flanked by two HpaI restriction sites was introduced into the pBluescript vector (Genecust) and the resulting plasmid was digested by HpaI before transfection of the AnTat1.1E Δfbpase cell line. The Δfbpase/FBPase-1 and Δfbpase/FBPase-2 cell lines were selected in SDM79 medium containing blasticidin (10 μg ml-1), puromycin (1 μg ml-1) and phleomycin (5 μg ml-1).
Inhibition of gene expression by RNAi
Accession numbers of genes targeted by RNAi are as follows; fructose-1,6-bisphosphatase (FBPase, Tb927.9.8720), glycerol kinase (GK, Tb927.9.12550-Tb927.9.12630), glycerol-3-phosphate dehydrogenase (GPDH, Tb927.8.3530) and triosephosphate isomerase (TIM, Tb927.11.5520). RNAi-mediated inhibition of gene expression was performed in the EATRO1125.T7T PCF by expression of stem-loop “sense-antisense” RNA molecules of the targeted sequences [50, 52] using the pLew100 expression vector [51].
To inhibit by RNAi the expression of the FBPase gene, a 597-bp fragment of the FBPase gene (from position 152 to 749) was introduced in the pLew100 vector to produce the pLew-FBPase-SAS plasmid. Briefly, a PCR-amplified 716-bp fragment, containing the antisense FBPase sequence with restriction sites added to the primers, was inserted into the HindIII and BamHI restriction sites of the pLew100 plasmid. The separate 615-bp PCR-amplified fragment containing the sense FBPase sequence was then inserted upstream of the antisense sequence, using HindIII and XhoI restriction sites (XhoI was introduced at the 3'-extremity of the antisense PCR fragment). The resulting plasmid pLew-FBPase-SAS contains a sense and antisense version of the targeted gene fragment, separated by a 89-bp fragment, under the control of a PARP promoter linked to a prokaryotic tetracycline operator. The same strategy was used to produce the pLew-GK-SAS, pLew-GPDH-SAS and pLew-TIM-SAS plasmids designed to inhibit the expression of the GK, GPDH and TIM genes, respectively. The size of the GK, GPDH and TIM targeted sequences are 617 bp (from position 460 to 1077), 622 bp (from position 218 to 840) and 558 bp (from position 67 to 625), respectively. The RNAiFBPase mutant was generated by transfecting the EATRO1125.T7T parental cell line with the pLew-FBPase-SAS plasmid and selection in glucose-rich SDM79 medium containing hygromycin (25 μg ml-1), neomycin (10 μg ml-1) and phleomycin (5 μg ml-1). The triple mutant cell lines (Δppdk/Δpepck/RNAiGK, Δppdk/Δpepck/RNAiGPDH and Δppdk/Δpepck/RNAiTIM) were obtained by introducing the relevant plasmid in the Δppdk/Δpepck cell line, after selection in glucose-rich SDM79 medium containing hygromycin (25 μg ml-1), neomycin (10 μg ml-1), phleomycin (5 μg ml-1), blasticidin (10 μg ml-1) and puromycin (1 μg ml-1). Aliquots were frozen in liquid nitrogen to provide stocks of each line that had not been cultivated long term in medium. Induction of RNAi cell lines was performed by addition of 1 μg ml-1 tetracycline.
Preparation of glycosomal fractions and enzymatic activities
Subcellular fraction enriched in glycosomes was prepared by differential centrifugation as described in [53], after homogenising the cells with silicon carbide as grinding material. Briefly, 2 x 109 cells were washed once in 10 ml of STE (25 mM Tris, 1 mM EDTA, 250 mM sucrose pH 7.8). After centrifugation, the pellet was resuspended in 0.15 ml of homogenisation buffer STE (STE supplemented with ‘Complete EDTA-Free’ protease-inhibitor cocktail, Roche Applied Science, Mannheim, Germany) and ground in a pre-chilled mortar with 1.5 gr of wet-weight silicon carbide per gr of cell pellet. The cells were microscopically checked for at least 90% disruption. The cell lysate was diluted in 7 ml of homogenisation buffer, centrifuged at 1,000 g and then at 5,000 g for 10 min each, at 4°C. The supernatant was centrifuged at 33,000 g for 10 min at 4°C to yield the cytosolic fraction and the glycosome-enriched pellet, which was washed once with 1 ml of STE, centrifuged at 33,000 g for 10 min at 4°C before resuspension in 0.1 ml of STE.
The FBPase activity of aliquots of the glycosomal fractions was measured following the reduction of NADP+ at 340 nm in the presence of 100 mM triethanolamine (pH 7.6), 2 mM MgCl2, 0.1 mM EDTA, 0.3 mM NADP+, 10 mM F1,6BP, 25 μg ml-1 glucose-6-phosphate isomerase and 25 μg ml-1 glucose-6-phosphate dehydrogenase. The glycerol kinase activity was measured at 340 nm via oxidation of NADH according to published procedures [54].
Western blot analyses
Total protein extracts (5 x 106 cells), or glycosomal and cytosolic fractions, of parental (AnTat1.1E, EATRO1125.T7T) or mutant PCF of T. brucei were separated by SDS-PAGE (10%) and immunoblotted on TransBlot Turbo Midi-size PVFD Membranes (Bio-Rad) [55]. Immunodetection was performed as described [55, 56] using as primary antibodies, the rabbit anti-FBPase (1:1,000, gift from P. Michels, Edinburgh, UK), the rabbit anti-SBPase (1:250, gift from P. Michels, Edinburgh, UK), the rabbit anti-GK (1:5,000, gift from P. Michels, Edinburgh, UK), the rabbit anti-G6PDH (1:1,000, gift from P. Michels, Edinburgh, UK), the rabbit anti-TIM (1:1,000, gift from P. Michels, Edinburgh, UK), the rabbit anti-PFR (1:10,000), the rabbit anti-enolase (1:100,000, gift from P. Michels, Edinburgh, UK), the rat anti-PEPCK (1:1,000, gift from T. Seebeck, Bern, Switzerland) [22], the rabbit anti-GPDH (1:1,000) [57], the rabbit anti-hsp60 (1:10,000) [58], the rabbit anti-PPDK (1:1,000) [11], anti-EP mouse monoclonal TBRP1/247 (1:500) (Biozol), anti-calflagin (1:1,000, gift of D. Engman, Chicago, USA [59]) and the rabbit anti-RBP6 (1:1,000, gift from C. Tschudi, New Haven, USA) [30]. Anti-rabbit or anti-rat IgG conjugated to horseradish peroxidase (Bio-Rad, 1:5000 dilution) was used as secondary antibody. Revelation was performed using the Clarity western ECL Substrate as described by the manufacturer (Bio-Rad). Images were acquired and analysed with the ImageQuant LAS 4000 luminescent image analyser.
In vitro differentiation of PCF expressing RBP6
The RBP6 gene was amplified by PCR and cloned via HindIII/BamHI into the pLew100v5b1d vector (pLew100v5 modified with a blasticidin resistance gene BSD). The Δfbpase cell and its parental EATRO1125.T7T cell line were transfected with the pLew100v5-RBP6 linearised with NotI in pools to generate the Δfbpase RBP6 cell line with the genotype TETR::HYG T7RNAP::NEO Δfbpase::BLAS/Δfbpase::PAC RBP6Ti::BLE and RBP6, respectively. In vitro differentiation experiments were done as described in [30] in SDM79 medium without glucose in the presence of 50 mM N-acetyl-D-glucosamine and 10% (v/v) heat-inactivated fetal calf serum. The faster kinetics of differentiation observed in our experiments as compared to that described in [30] is likely due to the different parental backgrounds (Lister 427 29:13 versus EATRO1125.T7T) or to the different media (Cunningham’s medium [60] versus modified SDM79) that were used in the two labs.
Flow cytometry analysis of stage-specific surface marker
2 × 107 cells were harvested and fixed overnight with 2% paraformaldehyde in PBS at 4°C, washed thrice with PBS and resuspended in 500 μl PBS. Cells were incubated with monoclonal mouse anti-procyclin EP (TBRP1/247, 1:500) in 1% BSA. For calflagin detection 3 × 107 cells were permeabilised with 0.2% NP-40 for 5 min prior incubation with polyclonal mouse α-calflagin (1:1,000) [59] in 1% BSA. Alexa Fluor 488-conjugated goat antibodies were used as secondary antibodies. Samples were analysed with a FACSCalibur cell analyser (Becton Dickinson) and data were evaluated with the FlowJo 8.8.6 software.
Mass spectrometry analyses of [13C]-incorporation into cellular metabolites
EATRO1125.T7T parental and mutant cell lines grown in SDM79 medium were washed twice with PBS and resuspended in PBS containing [U-13C]-proline (or [U-13C]-glycerol) with or without the same amount of non-labelled glucose. The cells were incubated for 2 h at 27°C before being collected on filters by fast filtration preparation (2 x 107 cells per filter) for mass spectrometry analysis, as described before [22]. Metabolites were analysed by ion-exchange chromatography coupled with tandem mass spectrometry (IC-MS/MS) using the method described by Bolten et al. [61]. Retention time on the column and multiple reactions monitoring (MRM) transition of each analysed metabolite are shown in Table 1 of [21]. The 13C mass isotopomer distribution of intracellular metabolites was determined from relevant isotopic clusters in the IC-MS/MS analysis, according to Kiefer et al. [62]. 13C mass isotopomer distribution measurements were performed using a triple quadrupole mass spectrometer (4000Qtrap, Applied Biosystems). To obtain [13C]-labeling patterns (13C isotopologues), isotopic clusters were corrected for the natural abundance of isotopes other than 13C, using the in-house software IsoCor (available at MetaSys) [63].
Analysis of excreted end products from glucose and glycerol metabolism by proton NMR
1 x 108 T. brucei PCF were collected by centrifugation at 1,400 g for 10 min, washed once with phosphate-buffered saline (PBS) and incubated in 5 ml of PBS supplemented with 2 g l-1 NaHCO3 (pH 7.4). Cells were maintained for 6 h at 27°C in incubation buffer containing 20 μmol of D-glucose or [U-13C]-glycerol (4 mM). The integrity of the cells during the incubation was checked by microscopic observation. The supernatant was collected and 50 μl of maleate solution in D2O (10 mM) was added as internal reference. H-NMR spectra were performed at 500.19 MHz on a Bruker Avance III 500 HD spectrometer equipped with a 5 mm cryoprobe Prodigy. Measurements were recorded at 25°. Acquisition conditions were as follows: 90° flip angle, 5,000 Hz spectral width, 32 K memory size, and 9.3 sec total recycle time. Measurements were performed with 64 scans for a total time close to 10 min 30 sec. Resonances of the obtained spectra were integrated and metabolites concentrations were calculated using the ERETIC2 NMR quantification Bruker program.
Tsetse fly maintenance, infection and dissection
Tsetse flies (Glossina morsitans morsitans) were maintained, infected and dissected at the Institut Pasteur as previously described [28]. Teneral males were collected 24 h to 48 h post-eclosion and artificially fed through a silicone membrane with 6–9 x 106 parasites ml-1 in SDM79 medium supplemented with 10% FCS for their first meal. Flies were then maintained in Roubaud cages for one month at 26°C and 60% hygrometry and fed twice a week with mechanically defibrinated sheep blood. Flies were starved for at least 48 h before being individually dissected 28 days after ingestion of the infected meal. Salivary glands were first rapidly dissected into a drop of PBS. The whole tsetse alimentary tract was then dissected and arranged lengthways for assessment of parasite presence. The proventriculus and anterior midgut were physically separated from the posterior midgut in a distinct PBS drop. Tissues were dilacerated to allow parasites to spread in PBS, parasites were recovered and treated for further experiments no more than 15 min after dissection.
Immunofluorescence analysis
For immunofluorescence, parasites were rapidly allowed to settle on poly-lysine coated slides until drying. Cells were fixed for 10 sec in methanol at -20°C and re-hydrated in PBS during 10 min. Slides were then incubated for 45 min at 37°C with the anti-FBPase primary antibody diluted at 1:10 in PBS with 0,1% bovine serum albumin. Slides were washed in PBS and incubated for 30 min at 37°C with the appropriate anti-rabbit secondary antibody coupled to an Alexa 488 fluorophore (Invitrogen). Slides were then stained with DAPI for visualisation of their kinetoplast and nuclear DNA contents and mounted under coverslip with Prolong antifade reagent (Invitrogen). IFA experiments were repeated on trypanosomes issued from 2 to 4 flies and from 6 distinct experimental infections. Slides were finally observed with a DMI4000 epifluorescence microscope (Leica) and images were captured with a Horca 03G5 camera (Hamamastu).
Measurements and analyses
Normalisation of signals was carried out by parallel manipulation of min/max signals in ImageJ (NIH). Statistical analyses and plots were performed with XLSTAT 2015.4.01 (Addinsoft) and Excel 2011 (Microsoft), respectively. Statistical analyses include ANOVA with Tukey ad-hoc post-tests for inter-group comparison with 95% confidence.
Supporting information
(PDF)
(PDF)
(XLSX)
Acknowledgments
We thank Paul A. Michels (Edinburgh, Scotland) for providing us with the anti-FBPase, anti-SBPase, anti-GK, anti-enolase, anti-TIM and anti-G6PDH immune sera, as well as Thomas Seebeck (Bern, Switzerland) and C. Tschudi (New Haven, USA) for the anti-PEPCK and anti-RBP6 antibodies, respectively.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. FB's and BR's group were funded by the Agence Nationale de la Recherche (ANR) through GLYCONOV (grant number ANR-15-CE15-0025-01) of the ANR-BLANC-2015 call. FB's group was supported by the Centre National de la Recherche Scientifique (CNRS), the Université de Bordeaux, the ANR through the grants ACETOTRYP (grant number ANR-2010-BLAN-1319-02) of the ANR-BLANC-2010 call, the Laboratoire d’Excellence (LabEx) ParaFrap ANR-11-LABX-0024 and the ParaMet PhD programme of Marie Curie Initial Training Network. BR’s group was supported by the Institut Pasteur, the Institut National de la Santé et de la Recherche Médicale (INSERM). EB is funded by a doctoral fellowship from French National Ministry for Research and Technology (Doctoral School CDV515). MB was funded by the University of Munich and MB and FB were supported by a research cooperation grant of the Franco-Bavarian University Cooperation Center (BFHZ/CCUFB).
References
- 1.Buscher P, Cecchi G, Jamonneau V, Priotto G. Human African trypanosomiasis. Lancet. 2017;390:2397–2409. 10.1016/S0140-6736(17)31510-6 . [DOI] [PubMed] [Google Scholar]
- 2.Bringaud F, Barrett MP, Zilberstein D. Multiple roles of proline transport and metabolism in trypanosomatids. Frontiers in Bioscience 2012;17:349–374. [DOI] [PubMed] [Google Scholar]
- 3.Mantilla BS, Marchese L, Casas-Sanchez A, Dyer NA, Ejeh N, Biran M, et al. Proline metabolism is essential for Trypanosoma brucei brucei survival in the tsetse vector. PLoS Pathog. 2017;13:e1006158 10.1371/journal.ppat.1006158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coustou V, Biran M, Breton M, Guegan F, Riviere L, Plazolles N, et al. Glucose-induced remodeling of intermediary and energy metabolism in procyclic Trypanosoma brucei. J Biol Chem. 2008;283:16342–16354. 10.1074/jbc.M709592200 [DOI] [PubMed] [Google Scholar]
- 5.Bringaud F, Riviere L, Coustou V. Energy metabolism of trypanosomatids: adaptation to available carbon sources. Mol Biochem Parasitol. 2006;149:1–9. 10.1016/j.molbiopara.2006.03.017 [DOI] [PubMed] [Google Scholar]
- 6.Bringaud F, Biran M, Millerioux Y, Wargnies M, Allmann S, Mazet M. Combining reverse genetics and NMR-based metabolomics unravels trypanosome-specific metabolic pathways. Mol Microbiol. 2015;96:917–926. 10.1111/mmi.12990 [DOI] [PubMed] [Google Scholar]
- 7.Opperdoes FR, Borst P. Localization of nine glycolytic enzymes in a microbody-like organelle in Trypanosoma brucei: the glycosome. FEBS Lett. 1977;80:360–364. doi: 0014-5793(77)80476-6. [DOI] [PubMed] [Google Scholar]
- 8.Gualdron-Lopez M, Brennand A, Hannaert V, Quinones W, Caceres AJ, Bringaud F, et al. When, how and why glycolysis became compartmentalised in the Kinetoplastea. A new look at an ancient organelle. Int J Parasitol. 2012;42:1–20. 10.1016/j.ijpara.2011.10.007 [DOI] [PubMed] [Google Scholar]
- 9.Besteiro S, Biran M, Biteau N, Coustou V, Baltz T, Canioni P, et al. Succinate secreted by Trypanosoma brucei is produced by a novel and unique glycosomal enzyme, NADH-dependent fumarate reductase. J Biol Chem. 2002;277:38001–38012. 10.1074/jbc.M201759200 [DOI] [PubMed] [Google Scholar]
- 10.Coustou V, Besteiro S, Riviere L, Biran M, Biteau N, Franconi JM, et al. A mitochondrial NADH-dependent fumarate reductase involved in the production of succinate excreted by procyclic Trypanosoma brucei. J Biol Chem. 2005;280:16559–16570. 10.1074/jbc.M500343200 [DOI] [PubMed] [Google Scholar]
- 11.Bringaud F, Baltz D, Baltz T. Functional and molecular characterization of a glycosomal PPi-dependent enzyme in trypanosomatids: pyruvate, phosphate dikinase. Proc Natl Acad Sci USA. 1998;95:7963–7968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deramchia K, Morand P, Biran M, Millerioux Y, Mazet M, Wargnies M, et al. Contribution of pyruvate phosphate dikinase in the maintenance of the glycosomal ATP/ADP balance in the Trypanosoma brucei procyclic form. J Biol Chem. 2014;289:17365–17378. 10.1074/jbc.M114.567230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Hellemond JJ, Opperdoes FR, Tielens AG. Trypanosomatidae produce acetate via a mitochondrial acetate:succinate CoA transferase. Proc Natl Acad Sci USA. 1998;95:3036–3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riviere L, van Weelden SW, Glass P, Vegh P, Coustou V, Biran M, et al. Acetyl:succinate CoA-transferase in procyclic Trypanosoma brucei. Gene identification and role in carbohydrate metabolism. J Biol Chem. 2004;279:45337–45346. 10.1074/jbc.M407513200 [DOI] [PubMed] [Google Scholar]
- 15.Millerioux Y, Morand P, Biran M, Mazet M, Moreau P, Wargnies M, et al. ATP synthesis-coupled and -uncoupled acetate production from acetyl-CoA by the mitochondrial acetate:succinate CoA-transferase and acetyl-CoA thioesterase in Trypanosoma. J Biol Chem. 2012;287:17186–17197. 10.1074/jbc.M112.355404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Weelden SW, Fast B, Vogt A, Van Der Meer P, Saas J, Van Hellemond JJ, et al. Procyclic Trypanosoma brucei do not use Krebs cycle activity for energy generation. J Biol Chem. 2003;278:12854–12863. 10.1074/jbc.M213190200 [DOI] [PubMed] [Google Scholar]
- 17.Stokes MJ, Guther ML, Turnock DC, Prescott AR, Martin KL, Alphey MS, et al. The synthesis of UDP-N-acetylglucosamine is essential for bloodstream form Trypanosoma brucei in vitro and in vivo and UDP-N-acetylglucosamine starvation reveals a hierarchy in parasite protein glycosylation. J Biol Chem. 2008;283:16147–16161. 10.1074/jbc.M709581200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kovarova J, Barrett MP. The Pentose Phosphate Pathway in Parasitic Trypanosomatids. Trends Parasitol. 2016;32:622–634. 10.1016/j.pt.2016.04.010 [DOI] [PubMed] [Google Scholar]
- 19.Naderer T, Ellis MA, Sernee MF, De Souza DP, Curtis J, Handman E, et al. Virulence of Leishmania major in macrophages and mice requires the gluconeogenic enzyme fructose-1,6-bisphosphatase. Proc Natl Acad Sci USA. 2006;103:5502–5507. 10.1073/pnas.0509196103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodriguez-Contreras D, Hamilton N. Gluconeogenesis in Leishmania mexicana: contribution of glycerol kinase, phosphoenolpyruvate carboxykinase, and pyruvate phosphate dikinase. J Biol Chem. 2014;289:32989–33000. 10.1074/jbc.M114.569434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Allmann S, Morand P, Ebikeme C, Gales L, Biran M, Hubert J, et al. Cytosolic NADPH homeostasis in glucose-starved procyclic Trypanosoma brucei relies on malic enzyme and the pentose phosphate pathway fed by gluconeogenic flux. J Biol Chem. 2013;288:18494–18505. 10.1074/jbc.M113.462978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ebikeme C, Hubert J, Biran M, Gouspillou G, Morand P, Plazolles N, et al. Ablation of succinate production from glucose metabolism in the procyclic trypanosomes induces metabolic switches to the glycerol 3-phosphate/dihydroxyacetone phosphate shuttle and to proline metabolism. J Biol Chem. 2010;285:32312–32324. 10.1074/jbc.M110.124917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Millerioux Y, Ebikeme C, Biran M, Morand P, Bouyssou G, Vincent IM, et al. The threonine degradation pathway of the Trypanosoma brucei procyclic form: the main carbon source for lipid biosynthesis is under metabolic control. Mol Microbiol. 2013;90:114–129. 10.1111/mmi.12351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guther ML, Urbaniak MD, Tavendale A, Prescott A, Ferguson MA. High-confidence glycosome proteome for procyclic form Trypanosoma brucei by epitope-tag organelle enrichment and SILAC proteomics. J Proteome Res. 2014;13:2796–2806. 10.1021/pr401209w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gualdron-Lopez M, Chevalier N, Van Der Smissen P, Courtoy PJ, Rigden DJ, Michels PAM. Ubiquitination of the glycosomal matrix protein receptor PEX5 in Trypanosoma brucei by PEX4 displays novel features. Biochim Biophys Acta. 2013;1833:3076–3092. 10.1016/j.bbamcr.2013.08.008 [DOI] [PubMed] [Google Scholar]
- 26.Le Ray D, Barry JD, Easton C, K. V. First tsetse fly transmission of the "AnTat" serodeme of Trypanosoma brucei. Ann Soc Belg Med Trop. 1977;57:369–381. [PubMed] [Google Scholar]
- 27.Millerioux Y, Mazet M, Bouyssou G, Allmann S, Kiema T-R, Bertiaux E, et al. De novo biosynthesis of sterols and fatty acids in the Trypanosoma brucei procyclic form: carbon source preferences and metabolic flux redistributions. PLoS Pathog. 2018;14:e1007116 10.1371/journal.ppat.1007116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rotureau B, Subota I, Bastin P. Molecular bases of cytoskeleton plasticity during the Trypanosoma brucei parasite cycle. Cell Microbiol. 2011;13:705–716. 10.1111/j.1462-5822.2010.01566.x [DOI] [PubMed] [Google Scholar]
- 29.Pradel LC, Bonhivers M, Landrein N, Robinson DR. NIMA-related kinase TbNRKC is involved in basal body separation in Trypanosoma brucei. J Cell Sci. 2006;119:1852–1863. 10.1242/jcs.02900 [DOI] [PubMed] [Google Scholar]
- 30.Kolev NG, Ramey-Butler K, Cross GA, Ullu E, Tschudi C. Developmental progression to infectivity in Trypanosoma brucei triggered by an RNA-binding protein. Science. 2012;338:1352–1353. 10.1126/science.1229641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rotureau B, Van Den Abbeele J. Through the dark continent: African trypanosome development in the tsetse fly. Front Cell Infect Microbiol. 2013;3:53 10.3389/fcimb.2013.00053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vassella E, Acosta-Serrano A, Studer E, Lee SH, Englund PT, Roditi I. Multiple procyclin isoforms are expressed differentially during the development of insect forms of Trypanosoma brucei. J Mol Biol. 2001;312:597–607. 10.1006/jmbi.2001.5004 [DOI] [PubMed] [Google Scholar]
- 33.Emmer BT, Daniels MD, Taylor JM, Epting CL, Engman DM. Calflagin inhibition prolongs host survival and suppresses parasitemia in Trypanosoma brucei infection. Eukaryot Cell. 2010;9:934–942. 10.1128/EC.00086-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Richardson JP, Jenni L, Beecroft RP, Pearson TW. Procyclic tsetse fly midgut forms and culture forms of African trypanosomes share stage- and species-specific surface antigens identified by monoclonal antibodies. J Immunol. 1986;136:2259–2264. [PubMed] [Google Scholar]
- 35.Rotureau B, Subota I, Buisson J, Bastin P. A new asymmetric division contributes to the continuous production of infective trypanosomes in the tsetse fly. Development. 2012;139:1842–1850. 10.1242/dev.072611 [DOI] [PubMed] [Google Scholar]
- 36.Christiano R, Kolev NG, Shi H, Ullu E, Walther TC, Tschudi C. The proteome and transcriptome of the infectious metacyclic form of Trypanosoma brucei define quiescent cells primed for mammalian invasion. Mol Microbiol. 2017;106:74–92. 10.1111/mmi.13754 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lamour N, Riviere L, Coustou V, Coombs GH, Barrett MP, Bringaud F. Proline metabolism in procyclic Trypanosoma brucei is down-regulated in the presence of glucose. J Biol Chem. 2005;280:11902–11910. 10.1074/jbc.M414274200 [DOI] [PubMed] [Google Scholar]
- 38.Eastmond PJ, Astley HM, Parsley K, Aubry S, Williams BP, Menard GN, et al. Arabidopsis uses two gluconeogenic gateways for organic acids to fuel seedling establishment. Nat Commun. 2015;6:6659 10.1038/ncomms7659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spitznagel D, Ebikeme C, Biran M, NAB N., Bringaud F, Henehan GT, et al. Alanine aminotransferase of Trypanosoma brucei—a key role in proline metabolism in procyclic life forms. FEBS J. 2009;276:7187–7199. 10.1111/j.1742-4658.2009.07432.x [DOI] [PubMed] [Google Scholar]
- 40.Ong HB, Lee WS, Patterson S, Wyllie S, Fairlamb AH. Homoserine and quorum-sensing acyl homoserine lactones as alternative sources of threonine: a potential role for homoserine kinase in insect-stage Trypanosoma brucei. Mol Microbiol. 2014;95:143–156. 10.1111/mmi.12853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haanstra JR, van Tuijl A, Kessler P, Reijnders W, Michels PA, Westerhoff HV, et al. Compartmentation prevents a lethal turbo-explosion of glycolysis in trypanosomes. Proc Natl Acad Sci USA. 2008;105:17718–17723. 10.1073/pnas.0806664105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kuznetsova E, Xu L, Singer A, Brown G, Dong A, Flick R, et al. Structure and activity of the metal-independent fructose-1,6-bisphosphatase YK23 from Saccharomyces cerevisiae. J Biol Chem. 2010;285:21049–21059. 10.1074/jbc.M110.118315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ganapathy U, Marrero J, Calhoun S, Eoh H, de Carvalho LP, Rhee K, et al. Two enzymes with redundant fructose bisphosphatase activity sustain gluconeogenesis and virulence in Mycobacterium tuberculosis. Nat Commun. 2015;6:7912 10.1038/ncomms8912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feng L, Sun Y, Deng H, Li D, Wan J, Wang X, et al. Structural and biochemical characterization of fructose-1,6/sedoheptulose-1,7-bisphosphatase from the Cyanobacterium synechocystis strain 6803. FEBS J. 2014;281:916–926. 10.1111/febs.12657 [DOI] [PubMed] [Google Scholar]
- 45.Jansson PA, Smith U, Lonnroth P. Interstitial glycerol concentration measured by microdialysis in two subcutaneous regions in humans. Am J Physiol. 1990;258:E918–922. 10.1152/ajpendo.1990.258.6.E918 [DOI] [PubMed] [Google Scholar]
- 46.Samra JS, Ravell CL, Giles SL, Arner P, Frayn KN. Interstitial glycerol concentration in human skeletal muscle and adipose tissue is close to the concentration in blood. Clin Sci (Lond). 1996;90:453–456. [DOI] [PubMed] [Google Scholar]
- 47.Belaunzaran ML, Lammel EM, de Isola EL. Phospholipases a in trypanosomatids. Enzyme Res. 2011;2011:392082 10.4061/2011/392082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brun R, Schonenberger M. Cultivation and in vitro cloning or procyclic culture forms of Trypanosoma brucei in a semi-defined medium. Acta Trop. 1979;36:289–292. [PubMed] [Google Scholar]
- 49.Coustou V, Besteiro S, Biran M, Diolez P, Bouchaud V, Voisin P, et al. ATP generation in the Trypanosoma brucei procyclic form: Cytosolic substrate level phosphorylation is essential, but not oxidative phosphorylation. J Biol Chem. 2003;278:49625–49635. 10.1074/jbc.M307872200 . [DOI] [PubMed] [Google Scholar]
- 50.Bringaud F, Robinson DR, Barradeau S, Biteau N, Baltz D, Baltz T. Characterization and disruption of a new Trypanosoma brucei repetitive flagellum protein, using double-stranded RNA inhibition. Mol Biochem Parasitol. 2000;111:283–297. [DOI] [PubMed] [Google Scholar]
- 51.Wirtz E, Leal S, Ochatt C, Cross GA. A tightly regulated inducible expression system for conditional gene knock-outs and dominant-negative genetics in Trypanosoma brucei. Mol Biochem Parasitol. 1999;99:89–101. [DOI] [PubMed] [Google Scholar]
- 52.Ngo H, Tschudi C, Gull K, Ullu E. Double-stranded RNA induces mRNA degradation in Trypanosoma brucei. Proc Natl Acad Sci USA. 1998;95:14687–14692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Opperdoes FR, Borst P, Spits H. Particle-bound enzymes in the bloodstream form of Trypanosoma brucei. Eur J Biochem. 1977;76:21–28. [DOI] [PubMed] [Google Scholar]
- 54.Kralova I, Rigden DJ, Opperdoes FR, Michels PA. Glycerol kinase of Trypanosoma brucei. Cloning, molecular characterization and mutagenesis. Eur J Biochem. 2000;267:2323–2333. [DOI] [PubMed] [Google Scholar]
- 55.Harlow E, Lane D, editors. Antibodies: a laboratory manual: Cold Spring Harbor Laboratory Press; 1988. [Google Scholar]
- 56.Sambrook J, Fritsch EF, Maniatis T, editors. Molecular cloning: a laboratory manual. 2 ed New York: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 57.Denise H, Giroud C, Barrett MP, Baltz T. Affinity chromatography using trypanocidal arsenical drugs identifies a specific interaction between glycerol-3-phosphate dehydrogenase from Trypanosoma brucei and Cymelarsan. Eur J Biochem. 1999;259:339–346. [DOI] [PubMed] [Google Scholar]
- 58.Bringaud F, Peyruchaud S, Baltz D, Giroud C, Simpson L, Baltz T. Molecular characterization of the mitochondrial heat shock protein 60 gene from Trypanosoma brucei. Mol Biochem Parasitol. 1995;74:119–123. [DOI] [PubMed] [Google Scholar]
- 59.Tyler KM, Fridberg A, Toriello KM, Olson CL, Cieslak JA, Hazlett TL, et al. Flagellar membrane localization via association with lipid rafts. J Cell Sci. 2009;122:859–866. 10.1242/jcs.037721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cunningham I. New culture medium for maintenance of tsetse tissues and growth of trypanosomatids. J Protozool. 1977;24:325–329. PubMed [DOI] [PubMed] [Google Scholar]
- 61.Bolten CJ, Kiefer P, Letisse F, Portais JC, Wittmann C. Towards appropriate sampling for metabolome analysis of microorganisms. Anal chem. 2007;79:3843–3849. 10.1021/ac0623888 [DOI] [PubMed] [Google Scholar]
- 62.Kiefer P, Nicolas C, Letisse F, Portais JC. Determination of carbon labeling distribution of intracellular metabolites from single fragment ions by ion chromatography tandem mass spectrometry. Anal Biochem. 2007;360:182–188. 10.1016/j.ab.2006.06.032 [DOI] [PubMed] [Google Scholar]
- 63.Millard P, Letisse F, Sokol S, Portais JC. IsoCor: correcting MS data in isotope labeling experiments. Bioinformatics. 2012;28:1294–1296. 10.1093/bioinformatics/bts127 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(XLSX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.