Abstract
Background
The inflammasome plays an important role in the inflammatory innate immune response after central nervous system (CNS) injury. Inhibition of the inflammasome after traumatic brain injury (TBI) results in improved outcomes by lowering the levels of caspase-1 and interleukin (IL)-1b. We have previously shown that inflammasome proteins are elevated in the cerebrospinal fluid (CSF) of patients with TBI and that higher levels of these proteins were consistent with poorer outcomes after TBI when compared to patients that presented these inflammasome proteins at lower levels.
Methods and findings
Here we extend our work by analyzing serum from 21 TBI patients and CSF from 18 TBI patients compared to 120 serum samples and 30 CSF samples from no-TBI donor controls for the expression of caspase-1, apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC), interleukin(IL)-1b and IL-18. Analysis was carried out using the Ella Simple Plex system (Protein Simple) to determine the sensitivity and specificity of inflammasome proteins as biomarkers of TBI. Receiver operator characteristic (ROC) curves, confidence intervals and likelihood ratios for each biomarker was determined. ROC curves, confidence intervals, sensitivity and specificity for each biomarker examined revealed that caspase-1 (0.93 area under the curve (AUC)) and ASC (0.90 AUC) in serum and ASC (1.0 AUC) and IL-18 (0.84 AUC) in CSF are promising biomarkers of TBI pathology. Importantly, higher protein levels (above 547.6 pg/ml) of ASC (0.91 AUC) were consistent with poorer outcomes after TBI as determined by the Glasgow Outcome Scale-Extended (GOSE).
Conclusion
These findings indicate that inflammasome proteins are excellent diagnostic and predictive biomarkers of TBI.
Introduction
A biomarker is a surrogate indicator of biological processes occurring in an individual. Biomarkers provide information about the pathology of a disease or condition, as well as the response to a pathogen or treatment. In the context of traumatic brain injury (TBI), biomarkers have the potential to be used as diagnostic markers of injury severity, as well as markers of response to treatment (monitoring biomarkers) or even as predictors of outcomes after trauma (predictive biomarkers).
Recently, the Brain Trauma Indicator has been authorized by the FDA to evaluate concussion in adults. This indicator is obtained by measuring the levels of ubiquitin C-terminal hydro-lase–L1 (UCH-L1) and glial acidic fibrillary protein (GFAP) in blood within 12 hours after trauma [1–3]. Other promising biomarkers have been suggested for diagnosis, monitoring and prognosis of traumatic brain injury (TBI) such as microtubule-associated protein-2 (MAP2, neuron-specific enolase (NSE), myelin basic protein (MBP), tau, s100β and neurofilament heavy chain protein (NF-H) [4–6]. However, these proteins are yet to be approved for the clinical care of TBI patients. In addition, heart fatty acid binding protein (H-FABP), when combined with interleukin (IL)-10, S100β and GFAP, has been shown to be elevated in patients positive for brain damage as determined by a head CT scan [7].
The innate immune response is a significant contributor to inflammation after TBI that is regulated by inflammasomes. To date several inflammasomes have been shown to play a role in the inflammatory response in the nervous system. For instance, the first inflammasome to be described in the nervous system was the NLRP1 inflammasome that is activated in neurons after spinal cord injury [8], brain injury [9] and stroke [10]. Moreover, the NLRP2 inflammasome in astrocytes has been shown to be involved in the inflammatory response after ATP stimulation [11]. The most studied inflammasome is the NLRP3 inflammasome and is is present in microglia and astrocytes [12–14]. Additionally, the NLRC4 inflammasome is present in astrocytes [14], and the AIM-2 inflammasome in neurons [15]. Inflammasome activation involves sensing of a particular trigger by cytosolic receptors such as NOD-like receptors (NLRs) or AIM-2 like receptors (ALRs). This activation process is triggered following the formation of large multiprotein complex called the inflammasome [16]. These inflammasome complexes undergo oligomerization that then serves as a platform to activate inflammatory caspases. However, some differences remain in that NLRP1 and NLRC4 can activate caspase-1 without ASC oligomerization, but ASC substantially improves the production of IL-1β. Thus, it is likely that inflammasomes that do not rely on ASC for assembly are less stable and as a result the inflammatory response mounted is weaker than it would be in the presence of ASC [17, 18]. Inflammasomes process inflammatory cytokines such as interleukin(IL)-1β and IL-18 through the activation of caspase-1 [18]. We have previously shown that inflammasomes contribute to the pathology of TBI and that inhibition of the inflammasome results in decreased inflammation and improved histopathological outcomes after brain injury [9]. Moreover, inflammasome proteins are secreted after injury in extracellular vesicles isolated from serum [19] and CSF [20] that add to the systemic inflammatory response after trauma [18, 21].
In this study, we provide receiver operator characteristic (ROC) curves with associated confidence intervals (CI) following analyses of serum and CSF samples from patients with TBI and from no-TBI control donors. In addition, we determine the sensitivity and specificity of inflammasome proteins to examine the potential of the inflammasome signaling proteins caspase-1, ASC, IL-1β and IL-18 as biomarkers of TBI.
Methods
Participants
Serum and CSF samples from patients with TBI and samples from no-TBI control donors were used in this study. Twenty-one TBI serum and 18 CSF samples were obtained after informed consent. Informed consent was obtained from a family member or proxy according to the University of Miami Miller School of Medicine IRB protocol # 20030154. All subjects were admitted to the Neurological Intensive Care Unit and/or the Ryder Trauma Center at Jackson Memorial Hospital (Table 1). Samples from normal donors (120 samples) were purchased from BioreclamationIVT. The normal donor group consisted of samples obtained from 60 male and 60 female donors in the age range of 20 to 70 years old (Table 2). Thirty control CSF samples were obtained from BioreclamationIVT. TBI Samples were collected three times a day for the first 5 days after patients arrived to the hospital. Samples were analyzed for the 1st, 2nd collection (Day 1) as well as 4th and 6th collections (Day 2). Later time points were analyzed but the data was not shown to be significant (data not shown).
Table 1. Subjects with TBI.
| Age | Gender | Race | Mechanism of Injury | Injury | GCS at resus | GOSE | Outcome |
|---|---|---|---|---|---|---|---|
| 81 (Deceased) | Female | White | Blunt injury | Contusion, IVH, tSAH | 3 | 1 | Deceased |
| 26 | Male | Unknown | Gunshot wound | Contusion, tSAH, | 3 | 3 | Unfavorable |
| 29 | Male | Black | s/p MCC with helmet | Contusion, DAI | 3 | 6 | Favorable |
| 69 (Deceased) | Male | Black | Blunt injury | SDH, tSAH | 8 | 1 | Deceased |
| 23 | Male | White | MVA w/blunt injury | SDH, t SAH | 3 | 2 | Unfavorable |
| 22 | Male | Black | GSW to back of head | LSDH, contusion | 4 | 8 | Favorable |
| 70 | Male | White | Blunt injury/assault | SDH | 10 | 3 | Unfavorable |
| 21 (Deceased) | Male | White | Blunt injury pedestrian struck by car w/head injury | SDH | 3 | 1 | Deceased |
| 22 | Male | White | Blunt injury punched to head and fell | SDH | 4 | 6 | Moderate |
| 80 (Deceased) | Male | White | Penetrating injury, GSW to posterior head | Contusion, IVH | 3 | 1 | Deceased |
| 71 | Female | White | Blunt injury. MVC | 3 | 1 | Deceased | |
| 51 (Deceased) | Male | Black | Blunt injury, hit by car while biking | tSAH, contusion, IVH | 3 | 1 | Deceased |
| 39 (Deceased) | Male | Unknown | MVA w/ blunt injury | tSAH, IVH | 3 | 1 | Deceased |
| 18 | Female | White | Blunt injury | SDH, contusion | 5 | 7 | Favorable/Rehab |
| 23 (Deceased) | Male | White | MCA w/blunt injury | Contusions, DAI, tSAH | 3 | 1 | Deceased |
| 21 | Male | Black | GSW to head w/ penetrating injury | tSAH, Contusion | 5 | 4 | Unfavorable |
| 63 | Male | White | Blunt injury | tSAH, SDH, Contusion | 7 | 1 | Deceased |
| 21 | Male | White | MVA w/blunt injury | Contusion, DAI, tSAH | 5 | 3 | Unfavorable |
| 45 | Male | White | MVC blunt injury | SDH, contusions, tSAH, IVH | 8 | 2 | Unfavorable |
| 51 | Male | White | Blunt injury | EDH, SDH, tSAH | 3 | 7 | Favorable/Rehab |
| 22 | Male | White | MVC w/blunt injury | tSAH, SDH, contusion | 3 | 4 | Unfavorable |
s/p: status-post; MCC: Motor cycle crash; MVA: Motor Vehicle Accident; GSW: Gunshot wound; MVC: Motor Vehicle accident; MCA: Motor cycle accident; IVH: Intraventricular bleeding; tSAH: traumatic Subarachnoid bleeding; DAI: Diffuse Axonal Injury; SDH: Subdural hematoma; LSDH: Left Subdural hematoma; GCS at resus: Glasgow Coma Scale at resuscitation; GOSE: Glasgow Outcome Scale (Extended).
Table 2. Summary of control and subjects with TBI.
| Control | TBI | |
|---|---|---|
| Sample Size | 120 | 21 |
| Gender | ||
| Male | 60 | 18 |
| Female | 60 | 3 |
| Age Range | 20–70 y/o | 21–81 y/o |
| Race | ||
| White: 28 | White: 13 | |
| Black: 65 | Black: 5 | |
| Hispanic: 27 | Unknown: 2 | |
| ASC Levels | ||
| Range | 105.6–458.3 pg/ml | 138.5–1720 pg/ml |
| Mean | 236.6 pg/ml | 629 pg/ml |
| Caspase-1 Levels | ||
| Range | 0.952–2.703 pg/ml | 0.918–20.55 pg/ml |
| a | 1.436 pg/ml | 4.540 pg/ml |
| IL-18 Levels | ||
| Range | 40.5–422.7 pg/ml | 43.72–587.7 pg/ml |
| Mean | 213.5 pg/ml | 218.625 pg/ml |
| IL-1beta Levels | ||
| Range | 0.413–3.276 pg/ml | 0.4–4.674 pg/ml |
| Mean | 1.214 pg/ml | 1.0618 pg/ml |
y/o: years old.
Simple Plex Assay
Analysis of inflammasome protein concentration in serum and CSF samples were performed using the Ella System (Protein System) as described in [22, 23].
Biomarker analyses
Data obtained by the Simple Plex assay were analyzed with Prism 7 software (GraphPad). Analyses were carried after removing outliers, and the area under the ROC curve and the 95% confidence interval (CI) were determined. The p-value of significance used was <0.05. Sensitivity and specificity of each biomarker was obtained for a range of different cut-off points. Samples that resulted in values outside the level of detection of the assay were not included in the analyses [22, 23]. Multiple group comparisons were carried out using a Kruskal-Wallis test ANOVA followed by Dunn’s multiple comparisons test. Paired comparisons were performed using a two-tailed t-test. Normality was evaluated using the D'Agostino and Pearson normality test. Difference in sample size throughout the study is due to the removal of outliers and because the protein concentration of some samples was out of the range of detection of the assay for some of the analytes and was not evaluated. The box plot shows the 5th and 95th percentiles, and the line inside the box represents the median.
Results
ASC and caspase-1 are elevated in the serum of patients after TBI
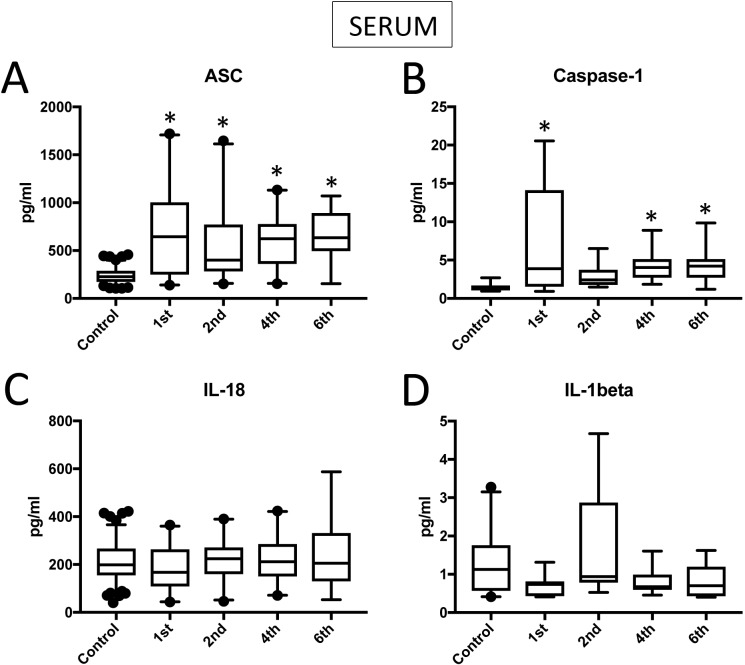
We analyzed serum samples (S1 Table) from TBI patients and compared them to serum from healthy/control individuals using a Simple Plex assay (Protein Simple) for the protein expression levels of ASC (Fig 1A), caspase-1 (Fig 1B), IL-18 (Fig 1C) and IL-1β (Fig 1D). Samples were collected three times a day for five days. Data shown corresponds to the 1st, 2nd 4th and 6th collection. We determined that the protein levels of ASC were higher in TBI samples (1st collection: 693.5+/-108.5 pg/ml, 2nd: 562.6+/-84.51 pg/ml, 4th: 601.7+/-64.4 pg/ml, 6th: 658.2+/-79.69 pg/ml) when compared to samples from subjects without TBI (control, 236.6+/-7.758 pg/ml) (Fig 1A). Thus, indicating that inflammasome proteins are elevated in serum after TBI.
Fig 1. Inflammasome proteins are elevated in the serum of TBI patients.
Protein levels in pg/ml of ASC (A), caspase-1 (B), IL-18 (C) and IL-1β (D) in serum samples from patients with TBI and healthy donors (controls). ASC: N = 120 control, 20 TBI. Caspase-1: N = 11 control, 19 TBI. IL-18: N = 120 control, 21 TBI. IL-1β: N = 25 control, 10 TBI. Box and whiskers are shown for the 5th and 95th percentile. * p < 0.05.
ASC and Caspase-1 are promising serum biomarkers of TBI
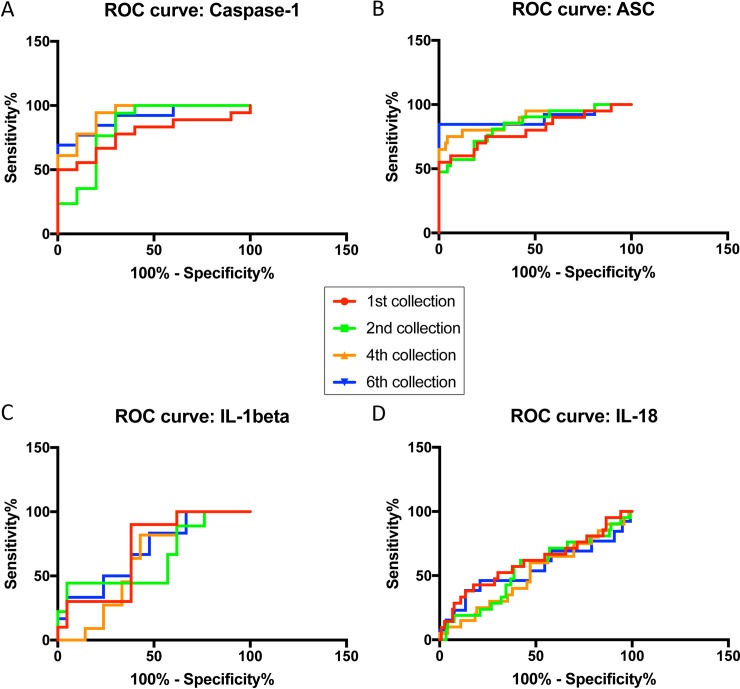
To determine if these inflammasome signaling proteins are reliable biomarkers for TBI in serum, we determined the area under the curve (AUC) for caspase-1 (Fig 2A), ASC (Fig 2B), IL-1β (Fig 2C) and IL-18 (Fig 2D). AUC values were highest for caspase-1 and ASC (Fig 2) with 0.93 (4th collection) and 0.90 (6th collection), respectively (Table 3).
Fig 2.
ROC curves for caspase-1 (A), ASC (B), IL-1β (C) and IL-18 (D) from serum samples of TBI patients and healthy donors.
Table 3. ROC analysis results for inflammasome signaling proteins in serum including area, standard error (STD. ERROR), 95% confidence interval (CI) and p-value for collections 1st, 2nd, 4th and 6th.
| Caspase-1 Serum | AREA | STD. ERROR | 95% C.I. | P VALUE |
|---|---|---|---|---|
| 1st Collection | 0.78 | 0.08772 | 0.6058 to 0.9497 | 0.01 |
| 2nd Collection | 0.83 | 0.0479 | 0.8395 to 1.027 | 0.005 |
| 4th Collection | 0.93 | 0.1407 | 0.8353 to 0.8869 | 0.0002 |
| 6th Collection | 0.91 | 0.06065 | 0.7888 to 1.027 | 0.001 |
| ASC Serum | ||||
| 1st Collection | 0.80 | 0.06472 | 0.6762 to 0.9299 | <0.0001 |
| 2nd Collection | 0.84 | 0.05026 | 0.7425 to 0.9395 | <0.0001 |
| 4th Collection | 0.89 | 0.04898 | 0.7931 to 0.9851 | <0.0001 |
| 6th Collection | 0.90 | 0.0697 | 0.759 to 1.032 | <0.0001 |
| IL-1beta Serum | ||||
| 1st Collection | 0.7 | 0.0965 | 0.5109 to 0.8891 | 0.0759 |
| 2nd Collection | 0.64 | 0.1182 | 0.4085 to 0.8719 | 0.2304 |
| 4th Collection | 0.6234 | 0.09765 | 0.432 to 0.8148 | 0.2582 |
| 6th Collection | 0.6984 | 0.1162 | 0.4707 to 0.9261 | 0.1448 |
| IL-18 Serum | ||||
| 1st Collection | 0.61 | 0.07475 | 0.4593 to 0.7524 | 0.1227 |
| 2nd Collection | 0.55 | 0.07064 | 0.4082 to 0.6851 | 0.4966 |
| 4th Collection | 0.51 | 0.0713 | 0.372 to 0.6515 | 0.8666 |
| 6th Collection | 0.55 | 0.1015 | 0.3532 to 0.7509 | 0.5387 |
Furthermore, the cut-off point for caspase-1 was 1.943 pg/ml with 94% sensitivity and 89% specificity (Table 4). For ASC, the cut-off point was 451.3 pg/ml with 85% sensitivity and 99% specificity (Table 4); whereas values for caspase-1 showed 100% sensitivity and the cut-off point was 1.679 pg/ml with 77.78% specificity. For ASC, the cut-off point was 153.4 pg/ml and a 19% specificity. In the case of caspase-1, for 100% specificity, the cut-off point was 2.717 pg/ml with 78% sensitivity. For ASC with 100% specificity, the cut-off point was 462.4 pg/ml with 85% sensitivity. Thus, these findings indicate that caspase-1 and ASC are reliable serum biomarkers for TBI.
Table 4. ROC analysis results for caspase-1 and ASC in serum including cut-off point in pg/ml, sensitivity and specificity, as well as positive and negative likelihood ratios (LR+/LR-).
| Caspase-1 Serum | Cut-off point (pg/ml) | Sensitivity (%) | Specificity (%) | LR + | LR - |
|---|---|---|---|---|---|
| 1st Collection | > 1.439 | 83 | 67 | 2.50 | 0.25 |
| 2nd Collection | > 1.531 | 94 | 78 | 4.24 | 0.08 |
| 4th Collection | > 1.943 | 94 | 89 | 8.50 | 0.06 |
| 6th Collection | > 1.947 | 85 | 89 | 7.62 | 0.17 |
| ASC Serum | |||||
| 1st Collection | > 210 | 85 | 43 | 1.50 | 0.35 |
| 2nd Collection | > 275 | 81 | 72 | 2.91 | 0.26 |
| 4th Collection | > 339.4 | 80 | 88 | 6.57 | 0.23 |
| 6th Collection | > 451.3 | 85 | 99 | 97.26 | 0.16 |
ASC and IL-18 are elevated in the CSF of patients after TBI
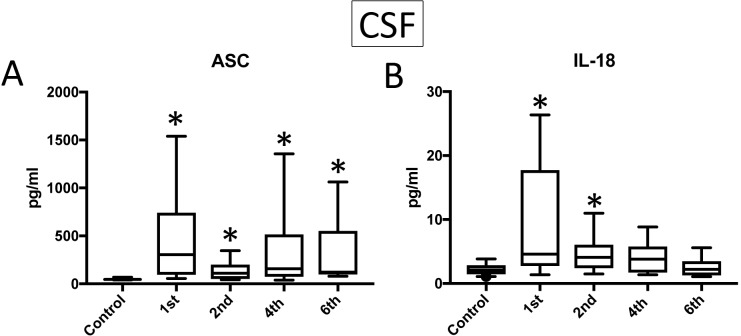
We then analyzed CSF samples (S2 Table) from TBI patients and compared them to CSF from no-TBI control individuals for the protein expression of ASC (Fig 3A) and IL-18 (Fig 3B). The protein levels of ASC and IL-18 were higher in TBI samples (ASC 1st: 473.8+/-129.4 pg/ml, 2nd: 132.8+/-24.45 pg/ml, 4th: 362.1+/-106.3 pg/ml, 6th: 325.4+/-124.4 pg/ml, IL-18: 1st: 9.164+/-2.169, 2nd: 4.626+/-0.7595 pg/ml, 4th: 4.169+/-0.7801 pg/ml) when compared to control samples (ASC: 48.52+/-2.259 pg/ml, IL-18: 2.246+/-0.1971). Moreover, the protein concentration of caspase-1 and IL-1β in the CSF was below the level of detection of the assay. Thus, we were unable to carry out any statistical analysis for these two proteins in the present study. These findings are consistent with previous reports indicating a role for the inflammasome in the pathology of TBI [20, 24].
Fig 3. Inflammasome proteins are elevated in the CSF of TBI patients.
Protein levels in pg/ml of ASC (A) and IL-18 (B) in CSF samples from patients with TBI and healthy donors (controls). ASC: N = 21 control, 15 TBI. IL-18: N = 24 control, 16 TBI. Box and whiskers are shown for the 5th and 95th percentile. * p < 0.05.
ASC and IL-18 are reliable CSF biomarkers of TBI
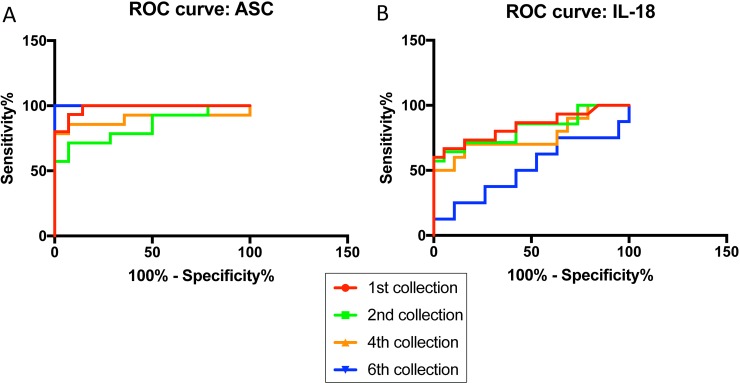
Next, we determined whether ASC and IL-18 are reliable biomarkers in CSF for TBI. The area under the curve (AUC) for ASC (Fig 4A) was 1.0 for the 6th collection (Table 5), and for IL-18 (Fig 4B) the AUC was 0.84 for the 1st collection (Table 5), indicating that ASC is a more reliable biomarker than IL-18 in CSF after TBI.
Fig 4.
ROC curves for ASC (A) and IL-18 (B) from CSF samples of TBI patients and healthy donors.
Table 5. ROC analysis results for ASC and IL-18 in CSF including area, standard error (STD. ERROR), 95% confidence interval (CI) and p-value for collections 1st, 2nd, 4th and 6th.
| ASC CSF | AREA | STD. ERROR | 95% C.I. | P VALUE |
|---|---|---|---|---|
| 1st Collection | 0.981 | 0.0195 | 0.9427 to 1.019 | <0.0001 |
| 2nd Collection | 0.8418 | 0.07661 | 0.6917 to 0.992 | 0.0021 |
| 4th Collection | 0.898 | 0.07262 | 0.7556 to 1.04 | 0.0003 |
| 6th Collection | 1 | 0 | 1 to 1 | 0.0001 |
| IL-18 CSF | ||||
| 1st Collection | 0.8404 | 0.0731 | 0.6971 to 0.9836 | 0.0008 |
| 2nd Collection | 0.8195 | 0.07969 | 0.6634 to 0.9757 | 0.002 |
| 4th Collection | 0.7632 | 0.1061 | 0.552 to 0.9711 | 0.9711 |
| 6th Collection | 0.5132 | 0.1344 | 0.2498 to 0.7765 | 0.9154 |
The cut-off point for ASC was 74.33 pg/ml with 100% sensitivity and 100% specificity (Table 6). For IL-18, the cut-off point was 2.722 pg/ml with 80% sensitivity and 68% specificity (Table 6). In the case of IL-18, for 100% specificity, the cut-off point was 3.879 pg/ml with 60% sensitivity, and for 100% sensitivity, the cut-off point was 1.358 pg/ml with 16% specificity. Thus, these findings indicate that ASC and IL-18 are promising biomarkers in CSF for TBI.
Table 6. ROC analysis results for ASC and IL-18 in CSF including cut-off point in pg/ml, sensitivity and specificity, as well as positive and negative likelihood ratios (LR+/LR-).
| ASC CSF | Cut-off point (pg/ml) | Sensitivity (%) | Specificity (%) | LR + | LR - |
|---|---|---|---|---|---|
| 1st Collection | > 55.11 | 100 | 85.71 | 7 | 0 |
| 2nd Collection | > 50.25 | 78.57 | 64.29 | 2.20 | 0.33 |
| 4th Collection | > 64.58 | 85.71 | 92.86 | 12 | 0.15 |
| 6th Collection | > 74.33 | 100 | 100 | 0 | |
| IL-18 CSF | |||||
| 1st Collection | > 2.722 | 80 | 68.42 | 2.53 | 0.29 |
| 2nd Collection | > 2.221 | 85.71 | 57.89 | 2.04 | 0.25 |
| 4th Collection | > 3.055 | 70 | 84.21 | 4.43 | 0.36 |
| 6th Collection | > 1.707 | 75 | 36.84 | 1.19 | 0.68 |
ASC is elevated in the serum of patients with unfavorable outcomes after TBI
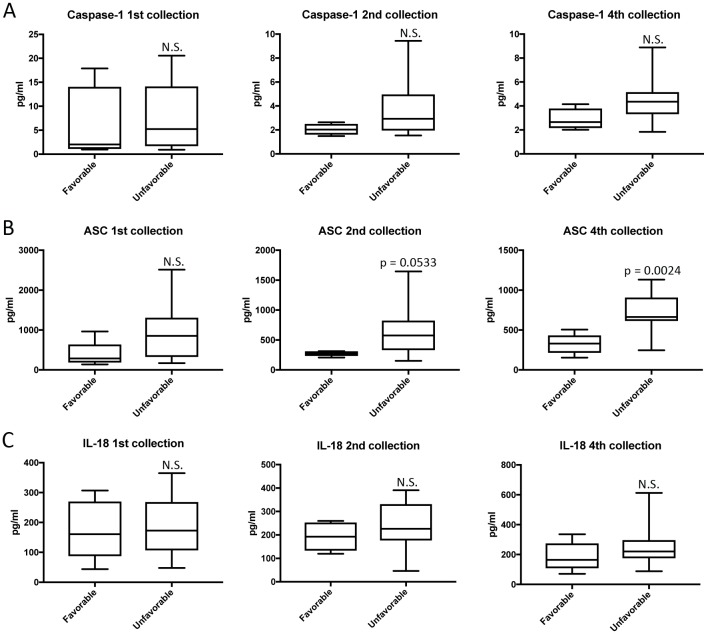
We then separated the TBI patients according to their clinical outcomes (S3 Table); either favorable or unfavorable outcomes based on the Glasgow Outcome Scale-Extended (GOSE) in which patients with a score of 6 to 8 were considered to have favorable outcomes and those with a score of 1 to 4 were considered to have unfavorable outcomes (Table 1). We found that the protein level of ASC was higher in the serum of TBI patients with unfavorable outcomes (2nd: 652.9+/-100.9pg/ml, 4th: 719.2+/-68.14 pg/ml) when compared to the samples obtained from patients with favorable outcomes (2nd: 273.7+/-19.41 pg/ml, 4th: 327.6+/-53.84 pg/ml) (Fig 5B), whereas the caspase-1 (Fig 5A) and IL-18 (Fig 5C) levels were not statistically different between the two groups.
Fig 5. Inflammasome proteins as predictive biomarkers of TBI.
Protein levels in pg/ml of caspase-1 (A), ASC (B), and IL-18 (C) in serum samples from patients with TBI. Groups were divided into favorable and unfavorable outcomes based on the GOSE. p-value of significance is shown above each box plot. Box and whiskers are shown for the 5th and 95th percentile. Caspase-1: N = 4 favorable and 16 unfavorable ASC: N = 5 favorable and 16 unfavorable; and IL-18: N = 5 favorable and 16 unfavorable.
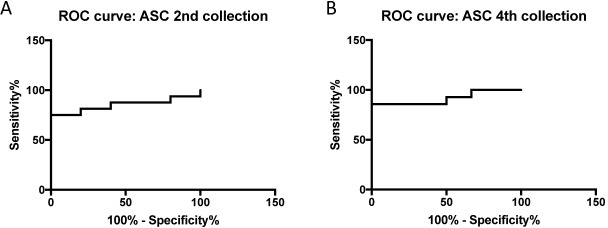
ASC is a promising predictive biomarker of TBI in serum
To determine if ASC in serum can be used as predictive biomarkers of TBI, we determined the AUC for ASC at the 2nd (Fig 6A) and 4th collection (Fig 6B). The AUC for ASC was 0.9167 in the 4th collection with a CI between 0.7914 and 1.042 (Table 7). Furthermore, the cut-off point was 547.6 pg/ml with 86% sensitivity and 100% specificity (Table 8). Thus, these findings indicate that ASC is an excellent predictive biomarker of TBI.
Fig 6. ROC curves for ASC outcomes (Favorable vs. Unfavorable) for the 2nd and 4th collection.
Table 7. ROC analysis results for ASC in serum for Favorable vs Unfavorable outcomes, including area, standard error (STD. ERROR), 95% confidence interval (CI) and p-value for collections 1st, 2nd and 4th.
| ASC Serum (GOSE) | AREA | STD. ERROR | 95% C.I. | P VALUE |
|---|---|---|---|---|
| 1st Collection | 0.7625 | 0.1133 | 0.544 to 0.9846 | 0.0829 |
| 2nd Collection | 0.85 | 0.08355 | 0.6862 to 1.014 | 0.0208 |
| 4th Collection | 0.9167 | 0.06391 | 0.7914 to 1.042 | 0.0039 |
Table 8. ROC analysis results for ASC in serum for Favorable vs Unfavorable outcomes, including cut-off point in pg/ml, sensitivity and specificity, as well as positive and negative likelihood ratios (LR+/LR-) for collections 1st, 2nd and 4th.
| ASC Serum (GOSE) | CUT-OFF POINT (pg/ml) | SENSITIVITY (%) | SPECIFICITY (%) | LR + | LR - |
|---|---|---|---|---|---|
| 1st Collection | > 353.7 | 75 | 80 | 3.75 | 0.31 |
| 2nd Collection | > 311.2 | 81.25 | 80 | 4.06 | 0.23 |
| 4th Collection | > 547.6 | 85.71 | 100 | 0.14 |
Discussion
TBI affects approximately 10 million people worldwide and has become a major cause of death and disability [25]. TBI severity is categorized as mild, moderate or severe. Signs and symptoms of TBI may include loss of consciousness, amnesia, nausea, dizziness, headaches, cognitive decline, structural brain damage and presence of other neurological symptoms [26]. The level of consciousness after TBI is often used to determine the severity of trauma in the Glasgow Coma Scale (GCS) score. This score is combined with brain radiographic imaging, other neurological findings and fluid biomarkers to assess the clinical status of the patient[27]. Fluid biomarkers can be obtained from CSF, serum or plasma. Potential biomarkers for the care of patients with TBI include total tau (T-Tau), amyloid-β (Aβ), UCH-L1, NSE, spectrin-α chain, S100B and GFAP [26]. Although there appears to be a variety of candidate proteins that may be potentially used as biomarkers, many studies have only looked at protein levels across patient cohorts including controls. Many of the studies aiming at identify biomarkers of TBI have failed to use the adequate statistical analyses that are needed to determine the suitability of a protein as a biomarker. These analyses include determination of the AUC, sensitivity, specificity and likelihood ratio as well as positive and negative predictive values [23]. Currently, there is only one approved FDA test for blood-based biomarkers in the United States, involving GFAP and UCH-L1, [28] which was approved this year.
In the present study, we show that ASC and IL-18 are reliable biomarkers for TBI in CSF with AUC values of 1.0 and 0.84, respectively. Most importantly, since obtaining CSF is a very invasive procedure, our finding that inflammasome proteins are elevated in serum, opens the possibility of using a less invasive test such as a blood test for biomarker analysis. In serum, we found that the AUC values for ASC was 0.90 and for caspase-1, 0.93. Thus, of the inflammasome proteins that we tested in this study, caspase-1 and ASC are the best candidate biomarkers in serum for the care of patients with brain injury. Thus, the development of a clinically validated brain biomarker test involving inflammasome proteins has the potential to alter diagnostic approaches and development of clinical care procedures of TBI subjects.
The importance of inflammasome regulation in TBI secondary injury processes has been recently reported. The NLRP1 inflammasome plays a significant role in the inflammatory response after TBI[9], and inhibition of NLRP1 signaling by antibody neurtralization results in decreased inflammation and improved histopathology after brain injury [9]. Additionally, the NLRP3 inflammasome is activated after brain injury [29, 30], and inhibition of the NLRP3 inflammasome results in decreased inflammation and improved neurological function in a mouse model of brain injury [31, 32]. The AIM2 inflammasome has been associated with the inflammasome-mediated cell death mechanism of pyroptosis, thus affecting blood brain barrier permeability after trauma [15, 33]. Inflammasome proteins are elevated in the CSF of patients with TBI, and high levels of these proteins correlate with poor outcomes in the chronic stages after injury [24]. Lastly, hyperbaric oxygen therapy, [34] hypothermia, [35] omega-3 fatty acids, [36] resveratrol[37] and propofol [38] provide beneficial effects after TBI by lowering inflammasome activation. Thus, innate immune inflammatory processes regulated by inflammasomes play an integral role in pathological processes following TBI.
Standards for Reporting Diagnostic accuracy studies (STARD) guidelines were used in this study. However, our study has several limitations. For example, the sample collection time points from patients after trauma was not uniform. As a result, the second collection of samples from patients varied after trauma. In addition, we were unable to collect CSF from all patients, and as a result the number of CSF samples are lower when compared to the number of serum samples. In addition, most samples collected in this study were obtained from subjects with severe TBI. Thus, future studies are needed to evaluate the prognostic potential of these biomarker in patients with mild TBI. Moreover, patients enrolled in this study suffered from isolated head trauma (i.e. gunshot wound) or had polytrauma (i.e. motorvehicle accident). As a result, it is likely that the effects of trauma on serum levels of inflammasome proteins are not entirely related to brain injury but to tissue damage originating in other organs such as lung [19], spleen or liver. Lastly, our study involved Whites and Blacks but did not contain Hispanics or other races in the TBI cohort.
We have recently shown that inflammasome proteins may be used as biomarkers of multiple sclerosis, stroke and depression [23, 39, 40]. However, patients in each disease category present different levels of inflammasome proteins and these levels may vary depending on disease severity. Thus, it is likely that these proteins may be used to monitor disease inflammatory progression or severity. The specificity of inflammasome proteins as biomarkers may be altered by changing the cut-off point to reflect a different sensitivity since sensitivity and specificity are inversely correlated such that as specificity increases, sensitivity decreases. In this study, we chose to set cut-off points that provided high sensitivity and the highest possible specificity for all analytes. Therefore, for clinical purposes the sensitivity should be set at the highest level and the specificity may be increased by the addition of other clinical findings such as imaging biomarkers, symptoms or other fluid-based biomarkers.
In conclusion, in this study we show that the inflammasome protein ASC may be use as a biomarker of TBI in serum and CSF, whereas caspase-1 may be used as a serum biomarker and IL-18 as a biomarker in CSF. In future studies, we will analyze these biomarkers in larger cohorts of patients with more stratified and specific types of brain injury. Importantly, the identification of these proteins as biomarkers is also indicative of their potential as therapeutic targets for the treatment of inflammation after trauma.
Supporting information
(PDF)
(PDF)
(PDF)
Acknowledgments
This work was supported by a STTR grant (4R42NS086274-012) from the NINDS/NIH to RWK and WDD and by a public grant from Fondo de Investigación Sanitaria (FIS PI16/00737) to JPB.
Data Availability
The data underlying the results presented in the study are found in the supporting information of this manuscript.
Funding Statement
This work was supported by a STTR grant (4R42NS086274-012) from the NINDS/NIH to RWK: https://www.nih.gov/; and by a public grant from Fondo de Investigación Sanitaria (FIS PI16/00737) to JPB: https://portalfis.isciii.es/es/Paginas/inicio.aspx. No funding bodies had any role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Yokobori S, Gajavelli S, Mondello S, Mo-Seaney J, Bramlett HM, Dietrich WD, et al. Neuroprotective effect of preoperatively induced mild hypothermia as determined by biomarkers and histopathological estimation in a rat subdural hematoma decompression model. J Neurosurg. 2013;118(2):370–80. 10.3171/2012.10.JNS12725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mondello S, Palmio J, Streeter J, Hayes RL, Peltola J, Jeromin A. Ubiquitin carboxy-terminal hydrolase L1 (UCH-L1) is increased in cerebrospinal fluid and plasma of patients after epileptic seizure. BMC Neurol. 2012;12:85 10.1186/1471-2377-12-85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mondello S, Jeromin A, Buki A, Bullock R, Czeiter E, Kovacs N, et al. Glial neuronal ratio: a novel index for differentiating injury type in patients with severe traumatic brain injury. J Neurotrauma. 2012;29(6):1096–104. 10.1089/neu.2011.2092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strathmann FG, Schulte S, Goerl K, Petron DJ. Blood-based biomarkers for traumatic brain injury: evaluation of research approaches, available methods and potential utility from the clinician and clinical laboratory perspectives. Clin Biochem. 2014;47(10–11):876–88. 10.1016/j.clinbiochem.2014.01.028 . [DOI] [PubMed] [Google Scholar]
- 5.Yokobori S, Hosein K, Burks S, Sharma I, Gajavelli S, Bullock R. Biomarkers for the clinical differential diagnosis in traumatic brain injury—a systematic review. CNS Neurosci Ther. 2013;19(8):556–65. 10.1111/cns.12127 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zetterberg H, Smith DH, Blennow K. Biomarkers of mild traumatic brain injury in cerebrospinal fluid and blood. Nat Rev Neurol. 2013;9(4):201–10. 10.1038/nrneurol.2013.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lagerstedt L, Egea-Guerrero JJ, Bustamante A, Rodriguez-Rodriguez A, El Rahal A, Quintana-Diaz M, et al. Combining H-FABP and GFAP increases the capacity to differentiate between CT-positive and CT-negative patients with mild traumatic brain injury. PLoS One. 2018;13(7):e0200394 10.1371/journal.pone.0200394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Rivero Vaccari JP, Lotocki G, Marcillo AE, Dietrich WD, Keane RW. A molecular platform in neurons regulates inflammation after spinal cord injury. J Neurosci. 2008;28(13):3404–14. 10.1523/JNEUROSCI.0157-08.2008 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Rivero Vaccari JP, Lotocki G, Alonso OF, Bramlett HM, Dietrich WD, Keane RW. Therapeutic neutralization of the NLRP1 inflammasome reduces the innate immune response and improves histopathology after traumatic brain injury. J Cereb Blood Flow Metab. 2009;29(7):1251–61. 10.1038/jcbfm.2009.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abulafia DP, de Rivero Vaccari JP, Lozano JD, Lotocki G, Keane RW, Dietrich WD. Inhibition of the inflammasome complex reduces the inflammatory response after thromboembolic stroke in mice. J Cereb Blood Flow Metab. 2009;29(3):534–44. 10.1038/jcbfm.2008.143 . [DOI] [PubMed] [Google Scholar]
- 11.Minkiewicz J, de Rivero Vaccari JP, Keane RW. Human astrocytes express a novel NLRP2 inflammasome. Glia. 2013;61(7):1113–21. 10.1002/glia.22499 . [DOI] [PubMed] [Google Scholar]
- 12.Goldmann T, Tay TL, Prinz M. Love and death: microglia, NLRP3 and the Alzheimer's brain. Cell Res. 2013;23(5):595–6. 10.1038/cr.2013.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schnaars M, Beckert H, Halle A. Assessing beta-amyloid-induced NLRP3 inflammasome activation in primary microglia. Methods Mol Biol. 2013;1040:1–8. 10.1007/978-1-62703-523-1_1 . [DOI] [PubMed] [Google Scholar]
- 14.Freeman L, Guo H, David CN, Brickey WJ, Jha S, Ting JP. NLR members NLRC4 and NLRP3 mediate sterile inflammasome activation in microglia and astrocytes. J Exp Med. 2017;214(5):1351–70. 10.1084/jem.20150237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adamczak SE, de Rivero Vaccari JP, Dale G, Brand FJ, 3rd, Nonner D, Bullock MR, et al. Pyroptotic neuronal cell death mediated by the AIM2 inflammasome. J Cereb Blood Flow Metab. 2014;34(4):621–9. 10.1038/jcbfm.2013.236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heneka MT, McManus RM, Latz E. Inflammasome signalling in brain function and neurodegenerative disease. Nat Rev Neurosci. 2018;19(10):610–21. 10.1038/s41583-018-0055-7 . [DOI] [PubMed] [Google Scholar]
- 17.Cai X, Xu H, Chen ZJ. Prion-Like Polymerization in Immunity and Inflammation. Cold Spring Harb Perspect Biol. 2017;9(4). 10.1101/cshperspect.a023580 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Rivero Vaccari JP, Dietrich WD, Keane RW. Activation and regulation of cellular inflammasomes: gaps in our knowledge for central nervous system injury. J Cereb Blood Flow Metab. 2014;34(3):369–75. 10.1038/jcbfm.2013.227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kerr N, De Rivero Vaccari JP, Abbassi S, Kaur H, Zambrano R, Wu S, et al. Traumatic Brain Injury-Induced Acute Lung Injury: Evidence for Activation and Inhibition of a Neural-Respiratory-Inflammasome Axis. J Neurotrauma. 2018. 10.1089/neu.2017.5430 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Rivero Vaccari JP, Brand F 3rd, Adamczak S, Lee SW, Perez-Barcena J, Wang MY, et al. Exosome-mediated inflammasome signaling after central nervous system injury. J Neurochem. 2016;136 Suppl 1:39–48. 10.1111/jnc.13036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Rivero Vaccari JP, Dietrich WD, Keane RW. Therapeutics targeting the inflammasome after central nervous system injury. Transl Res. 2016;167(1):35–45. 10.1016/j.trsl.2015.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brand FJ 3rd, Forouzandeh M, Kaur H, Travascio F, de Rivero Vaccari JP. Acidification changes affect the inflammasome in human nucleus pulposus cells. J Inflamm (Lond). 2016;13(1):29 10.1186/s12950-016-0137-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keane RW, Dietrich WD, de Rivero Vaccari JP. Inflammasome Proteins As Biomarkers of Multiple Sclerosis. Front Neurol. 2018;9:135 10.3389/fneur.2018.00135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adamczak S, Dale G, de Rivero Vaccari JP, Bullock MR, Dietrich WD, Keane RW. Inflammasome proteins in cerebrospinal fluid of brain-injured patients as biomarkers of functional outcome: clinical article. J Neurosurg. 2012;117(6):1119–25. 10.3171/2012.9.JNS12815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hyder AA, Wunderlich CA, Puvanachandra P, Gururaj G, Kobusingye OC. The impact of traumatic brain injuries: a global perspective. NeuroRehabilitation. 2007;22(5):341–53. . [PubMed] [Google Scholar]
- 26.Blennow K, Brody DL, Kochanek PM, Levin H, McKee A, Ribbers GM, et al. Traumatic brain injuries. Nat Rev Dis Primers. 2016;2:16084 10.1038/nrdp.2016.84 . [DOI] [PubMed] [Google Scholar]
- 27.Teasdale G, Maas A, Lecky F, Manley G, Stocchetti N, Murray G. The Glasgow Coma Scale at 40 years: standing the test of time. Lancet Neurol. 2014;13(8):844–54. 10.1016/S1474-4422(14)70120-6 . [DOI] [PubMed] [Google Scholar]
- 28.Bazarian JJ, Biberthaler P, Welch RD, Lewis LM, Barzo P, Bogner-Flatz V, et al. Serum GFAP and UCH-L1 for prediction of absence of intracranial injuries on head CT (ALERT-TBI): a multicentre observational study. Lancet Neurol. 2018. 10.1016/S1474-4422(18)30231-X . [DOI] [PubMed] [Google Scholar]
- 29.Irrera N, Pizzino G, Calo M, Pallio G, Mannino F, Fama F, et al. Lack of the Nlrp3 Inflammasome Improves Mice Recovery Following Traumatic Brain Injury. Front Pharmacol. 2017;8:459 10.3389/fphar.2017.00459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu HD, Li W, Chen ZR, Hu YC, Zhang DD, Shen W, et al. Expression of the NLRP3 inflammasome in cerebral cortex after traumatic brain injury in a rat model. Neurochem Res. 2013;38(10):2072–83. 10.1007/s11064-013-1115-z . [DOI] [PubMed] [Google Scholar]
- 31.Xu X, Yin D, Ren H, Gao W, Li F, Sun D, et al. Selective NLRP3 inflammasome inhibitor reduces neuroinflammation and improves long-term neurological outcomes in a murine model of traumatic brain injury. Neurobiol Dis. 2018;117:15–27. 10.1016/j.nbd.2018.05.016 . [DOI] [PubMed] [Google Scholar]
- 32.Ismael S, Nasoohi S, Ishrat T. MCC950, the Selective Inhibitor of Nucleotide Oligomerization Domain-Like Receptor Protein-3 Inflammasome, Protects Mice against Traumatic Brain Injury. J Neurotrauma. 2018;35(11):1294–303. 10.1089/neu.2017.5344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ge X, Li W, Huang S, Yin Z, Xu X, Chen F, et al. The pathological role of NLRs and AIM2 inflammasome-mediated pyroptosis in damaged blood-brain barrier after traumatic brain injury. Brain Res. 2018. 10.1016/j.brainres.2018.06.008 . [DOI] [PubMed] [Google Scholar]
- 34.Geng F, Ma Y, Xing T, Zhuang X, Zhu J, Yao L. Effects of Hyperbaric Oxygen Therapy on Inflammasome Signaling after Traumatic Brain Injury. Neuroimmunomodulation. 2016;23(2):122–9. 10.1159/000445689 . [DOI] [PubMed] [Google Scholar]
- 35.Tomura S, de Rivero Vaccari JP, Keane RW, Bramlett HM, Dietrich WD. Effects of therapeutic hypothermia on inflammasome signaling after traumatic brain injury. J Cereb Blood Flow Metab. 2012;32(10):1939–47. 10.1038/jcbfm.2012.99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin C, Chao H, Li Z, Xu X, Liu Y, Bao Z, et al. Omega-3 fatty acids regulate NLRP3 inflammasome activation and prevent behavior deficits after traumatic brain injury. Exp Neurol. 2017;290:115–22. 10.1016/j.expneurol.2017.01.005 . [DOI] [PubMed] [Google Scholar]
- 37.Zou P, Liu X, Li G, Wang Y. Resveratrol pretreatment attenuates traumatic brain injury in rats by suppressing NLRP3 inflammasome activation via SIRT1. Mol Med Rep. 2018;17(2):3212–7. 10.3892/mmr.2017.8241 . [DOI] [PubMed] [Google Scholar]
- 38.Ma J, Xiao W, Wang J, Wu J, Ren J, Hou J, et al. Propofol Inhibits NLRP3 Inflammasome and Attenuates Blast-Induced Traumatic Brain Injury in Rats. Inflammation. 2016;39(6):2094–103. 10.1007/s10753-016-0446-8 . [DOI] [PubMed] [Google Scholar]
- 39.Kerr N, Garcia-Contreras M, Abbassi S, Mejias NH, Desousa BR, Ricordi C, et al. Inflammasome Proteins in Serum and Serum-Derived Extracellular Vesicles as Biomarkers of Stroke. Front Mol Neurosci. 2018;11:309 10.3389/fnmol.2018.00309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Syed SA, Beurel E, Loewenstein DA, Lowell JA, Craighead WE, Dunlop BW, et al. Defective Inflammatory Pathways in Never-Treated Depressed Patients Are Associated with Poor Treatment Response. Neuron. 2018;99(5):914–24 e3. 10.1016/j.neuron.2018.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(PDF)
Data Availability Statement
The data underlying the results presented in the study are found in the supporting information of this manuscript.