Abstract
Introduction:
The U.S. Preventive Services Task Force changed breast cancer screening guidelines in November 2009 for mammograms in women aged 40–49 and 50–74 years. The aim of this study was to assess the impact of the 2009 guideline changes on breast cancer incidence by stage among women aged 40–49 and 50–74 years in the U.S.
Methods:
This was a cross-sectional trend analysis of the impact of 2009 guideline change on breast cancer incidence by stage, using data from the National Program for Cancer Registries and Surveillance, Epidemiology, and End Results Incidence–U.S. Cancer Statistics 2001–2014 database among women aged 40–74 years. Incidence was age adjusted to the U.S. standard population. Data were collected in 2001–2014, released in 2017, and analyzed in 2018.
Results:
Among women aged 40–49 years, the 4-year average annual incidence of breast cancer increased slightly in 2011–2014 for in situ, localized, and distant cancer, but decreased for regional cancer compared with the incidence in 2006–2009. Among women aged 50–74 years, the 4-year average annual incidence of breast cancer increased in 2011–2014 for localized and distant cancer, but decreased for in situ and regional cancer. Joinpoint analyses revealed that annual percentage changes decreased after 2009 for distant cancer among both women aged 40– 49 and 50–74 years. The composition of breast cancer by stage was similar between 2006–2009 and 2011–2014 among both women aged 40–49 and 50–74 years.
Conclusions:
Changes in breast cancer screening by the 2009 U.S. Preventive Services Task Force guidelines had little immediate adverse effects on the stage distribution of breast cancer diagnoses in the U.S. Monitoring the incidence by cancer stages over time is needed.
INTRODUCTION
Breast cancer is the second most prevalent cancer after skin cancers and is the second leading cause of cancer death after lung cancer among women in the U.S.1,2 Life time risk for developing invasive breast cancer among women in the U.S. is 12.3%.1 The American Cancer Society estimates that in 2017 among women in the U.S., there will be 252,710 new cases of invasive breast cancer, 63,410 new cases of in situ breast cancer, and 40,610 breast cancer deaths.3Moreover, racial/ethnic disparities exist for cancer incidence and cause-specific survival: non-Hispanic blacks have the highest proportions of and lowest survival rates from regional (35%) and distant (8%) stage disease.4–7 However, breast cancer mortality can be reduced by early detection of the tumor using mammogram screening.8,9 In November 2009, the U.S. Preventive Services Task Force (USPSTF) changed breast cancer screening guidelines for mammograms from every 1–2 years in women 40 years or older to biennial (i.e., every other year) mammograms in women aged 50–74 years and personalized screening decisions for women aged 40–49 years.10 This screening guideline change is highly controversial. Although it has been reported that annual mammogram rates significantly decreased after the guideline change,11-14 others found minimal or no changes in screening rates.15–17 Collaborative models of breast cancer screening strategies suggested that a reduction in screening frequency would result in less benefit (mortality reduction) but also fewer false-positive results in the biennial mammography as compared with the annual mammography in women aged 50 to 74 years.18–20
It is still unclear whether this increased interval for mammography screening as per the 2009 USPSTF guidelines will lead to increased detection of invasive breast cancer, or changes in the composition of breast cancer by stage. This study aims to assess whether there were differences in breast cancer incidence by stage before and after the 2009 guideline changes among women in the U.S. aged 40–49 and 50–74 years.
METHODS
Study Sample
The authors performed a cross-sectional trend analysis of the impact of the 2009 guideline change on breast cancer incidence, using data from women aged 40–74 years who had a diagnosis of a breast cancer in U.S. Cancer Statistics (USCS), the combined data from the Centers for Disease Control and Prevention’s National Program for Cancer Registries (NPCR) and the National Cancer Institute’s (NCI’s) Surveillance, Epidemiology, and End Results (SEER) Program.21 The USCS 2001–2014 database includes cancer incidence and population data for all 50 states, and the District of Columbia. Hospitals, physicians, and laboratories across the nation report data on demographic characteristics and tumor characteristics to central cancer registries supported by the Centers for Disease Control and Prevention and NCI. The NPCR and SEER Incidence–USCS Public Use Database (2001–2014 database) covered essentially the entire U.S. population between 2001 and 2014. This study was not considered human subjects research by the IRB at The University of Texas Medical Branch, Galveston, Texas.
Measures
The information collected about each incident of cancer diagnosis included demographic characteristics, year of cancer diagnosis, and cancer histology. Stages of breast cancer were classified into four groups comprising in situ, localized, regional, and distant. Ages were grouped into 40–49 and 50–74 years, as screening guidelines differed in those two age groups. This study included in the analyses information about race (non-Hispanic white, non-Hispanic black, Asian/Pacific Islander, and other) and ethnicity (Hispanic or non-Hispanic). Hispanic ethnicity for all cancer cases was identified by the North American Association of Central Cancer Registries Hispanic/Latino Identification Algorithm.22
Statistical Analysis
All analyses were carried out using the SEER*Stat statistical software package, version 8.3.5. Women were divided into the following groups according to age 40–49 and 50–74 years. Breast cancer incidence rates were calculated as cases per 100,000 people and age adjusted to the respective standard population distribution in these age ranges of the 2000 U.S. standard population. The crude incidence rate was the number of new cases of breast cancer (numerator) occurring in a specified population (denominator) in a given year, and the age-adjusted rate was a weighted average of crude rates, where the crude rates are calculated for different age groups and the weights are the proportions of people in the corresponding age groups of the 2000 U.S. standard population. CIs were calculated using the Tiwari method,23 which produces similar confidence limits to the standard normal approximation when the counts are large and the population being studied is similar to the standard population, and is more likely to ensure proper coverage in other cases (e.g., the counts are small). Joinpoint regression models24 were fitted based on annual incidence data of 2006–2014 using the NCI’s Joinpoint Regression Analysis program, version 4.6.0. This analysis program selected the best-fitting log linear regression model to identify the joinpoints (calendar year at diagnosis) when annual percentage changes (APC) differed significantly, allowing for the minimum number of joinpoints necessary to fit the data. APC was calculated as (exp[β]–1) x 100, where the regression coefficient (β) was estimated by fitting a least-squares regression line to the natural logarithm of the rates, using the calendar year as a regressor variable. The number of significant joinpoints is determined by the permutation test. The grid search method was used to fit the segmented regression function and the p-value of each permutation test is estimated using Monte Carlo methods, and the overall asymptotic significance level is maintained through a Bonferroni adjustment.24 The p-value for the comparison of two segmented line regression functions (trends for two time periods) is estimated using the permutation procedure.25 Subgroup analyses were performed in age groups, races/ethnicities, and breast cancer stages. Additionally, 4-year average annual rates were calculated for 2006–2009 and 2011–2014 and compared the differences across age groups and races/ethnicities. Differences in age-adjusted rates were evaluated using RR and the corresponding 95% CI.26 Poisson model of variation was selected for the analyses of breast cancer data as breast cancer is a rare event. Age-adjusted incidences were calculated for each cancer stage when comparing the differences between 2006–2009 and 2011–2014. When stage composition of breast cancer was calculated, the number of cases in each stage was age adjusted to the 2000 U.S. standard population. Chi-square tests were used to compare differences in cancer stage composition between 2006–2009 and 2011–2014. Statistical significances were determined as two-sided p-values <0.05. Data used in this study were collected in 2001–2014, released in 2017, and analyzed in 2018.
RESULTS
The number of cases of breast cancer in 2006–2009 and 2011–2014 among women aged 40–74 years is presented in Appendix Table 1. The 4-year average annual incidence of breast cancer remained similar between 2006–2009 and 2011–2014 among both women aged 40–49 and 50– 74 years (Table 1). Among women aged 40–49 years, the 4-year average annual incidence rate was 202.8 per 100,000 people in 2006–2009 compared with 203.4 per 100,000 in 2011–2014 (p=0.36). Incidence in invasive breast cancer incidence were also similar between 2006–2009 and 2011–2014. Among non-Hispanic white, non-Hispanic black, and Asian or Pacific Islander women aged 40–49 years, the 4-year average annual incidence rate was slightly increased in 2011–2014 compared with 2006–2009. There was a slight increase across all stages of breast cancer except for a decrease in regional breast cancer. Among women aged 50–74 years, the 4-year average annual incidence rate was 395.8 per 100,000 people in 2006–2009 compared with 396.8 per 100,000 in 2011–2014 (p=0.13). Non-Hispanic black and Asian or Pacific Islander women experienced a slight increase in breast cancer incidence from 2006–2009 to 2011–2014. Localized and distant cancers also slightly increased, whereas in situ and regional cancers had a slight decrease.
Table 1.
Age-Adjusted Incidence Rate of Breast Cancer by Stage Among Women Aged 40–74 Years, USCS 2006–2009 and 2011–2014
| Incidence rate, per 100,000 (95% CI) | RR (95% CI) | ||
|---|---|---|---|
| Variable | 2006–2009 | 2011–2014 | |
| Among women aged 40–49 years | |||
| All cases | 202.8 (201.9, 203.7) | 203.4 (202.4, 204.4) | 1.00 (1.00, 1.01) |
| All cases by race/ethnicity | |||
| Hispanic | 152.9 (150.6, 155.1) | 150.1 (148.1, 152.2) | 0.98 (0.96, 1.00) |
| Non-Hispanic white | 213.2 (212.1, 214.4) | 215.5 (214.2, 216.7) | 1.01 (1.00, 1.02) |
| Non-Hispanic black | 195.7 (193.2, 198.2) | 201.8 (199.2, 204.4) | 1.03 (1.01, 1.05) |
| Asian or Pacific Islander | 181.9 (178.1, 185.7) | 195.1 (191.5, 198.8) | 1.07 (1.04, 1.10) |
| Invasive breast cancer | 153.2 (152.4, 154) | 153.1 (152.2, 153.9) | 1.00 (0.99, 1.01) |
| Invasive breast cancer by race/ethnicity | |||
| Hispanic | 118.5 (116.5, 120.4) | 115.2 (113.4, 117) | 0.97 (0.95, 0.99) |
| Non-Hispanic white | 159.4 (158.4, 160.4) | 161.2 (160.2, 162.3) | 1.01 (1.00, 1.02) |
| Non-Hispanic black | 156.8 (154.5, 159.1) | 157.5 (155.3, 159.8) | 1.00 (0.98, 1.03) |
| Asian or Pacific Islander | 131.2 (128, 134.5) | 140.7 (137.6, 143.8) | 1.07 (1.04, 1.11) |
| Stage of breast cancer | |||
| In situ | 49.6 (49.1, 50) | 50.3 (49.9, 50.8) | 1.02 (1.00, 1.03) |
| Localized | 86 (85.4, 86.6) | 88.3 (87.6, 88.9) | 1.03 (1.02, 1.04) |
| Regional | 56.8 (56.3, 57.3) | 54.4 (54, 54.9) | 0.96 (0.95, 0.97) |
| Distant | 7.2 (7, 7.3) | 7.6 (7.4, 7.8) | 1.06 (1.02, 1.10) |
| Among women aged 50–74 years | |||
| All cases | 395.8 (394.8, 396.8) | 396.8 (395.9, 397.8) | 1.00 (1.00, 1.01) |
| All cases by race/ethnicity | |||
| Hispanic | 297 (294, 300) | 299.4 (296.8, 302) | 1.01 (0.99, 1.02) |
| Non-Hispanic white | 411.9 (410.7, 413) | 410.3 (409.2, 411.4) | 1.00 (0.99, 1.00) |
| Non-Hispanic black | 385.9 (383, 389) | 407.6 (404.8, 410.4) | 1.06 (1.05, 1.07) |
| Asian or Pacific Islander | 281.3 (277.3, 285.4) | 305.9 (302.2, 309.6) | 1.09 (1.07, 1.11) |
| Invasive breast cancer | 312 (311.1, 312.8) | 314.5 (313.6, 315.3) | 1.01 (1.00, 1.01) |
| Invasive breast cancer by race/ethnicity | |||
| Hispanic | 237.6 (234.9, 240.3) | 237.9 (235.6, 240.3) | 1.00 (0.99, 1.02) |
| Non-Hispanic white | 324.8 (323.7, 325.8) | 326.6 (325.7, 327.6) | 1.01 (1.00, 1.01) |
| Non-Hispanic black | 303.5 (300.9, 306.2) | 316.5 (314, 319) | 1.04 (1.03, 1.06) |
| Asian or Pacific Islander | 212.6 (209.1, 216.1) | 229.8 (226.6, 233) | 1.08 (1.06, 1.10) |
| Stage of breast cancer | |||
| In situ | 83.8 (83.4, 84.3) | 82.4 (82, 82.8) | 0.98 (0.98, 0.99) |
| Localized | 196.5 (195.8, 197.2) | 206.6 (205.9, 207.3) | 1.05 (1.05, 1.06) |
| Regional | 91.5 (91.1, 92) | 84.1 (83.7, 84.5) | 0.92 (0.91, 0.93) |
| Distant | 16.9 (16.7, 17.2) | 18.0 (17.8, 18.2) | 1.06 (1.05, 1.08) |
Notes: Data were from U.S. Cancer Statistics (USCS), the combined data from the Centers for Disease Control and Prevention (CDC’s) National Program for Cancer Registries (NPCR) and the National Cancer Institute’s (NCI’s) Surveillance, Epidemiology, and End Results (SEER) Program. Boldface indicates statistical significance (p<0.05). Rates are per 1,000,000 and age-adjusted to the 2000 U.S. Standard Population. CIs are 95% for rates (Tiwari method). Differences in age-adjusted 4-year average annual rates between 2011–2014 and 2006–2009 were evaluated using rate ratio (RR) and the corresponding 95% CI. Each RR was derived from a stratified model in a specified population (different age groups, races, or stages).
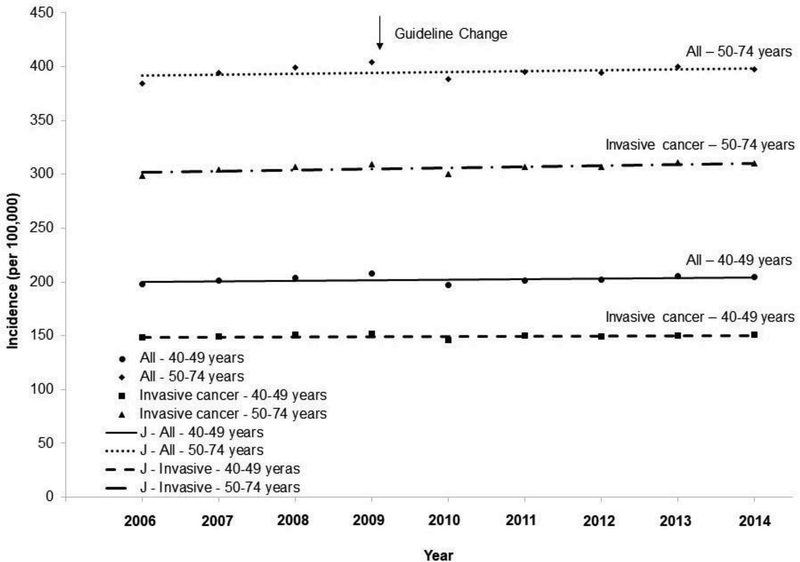
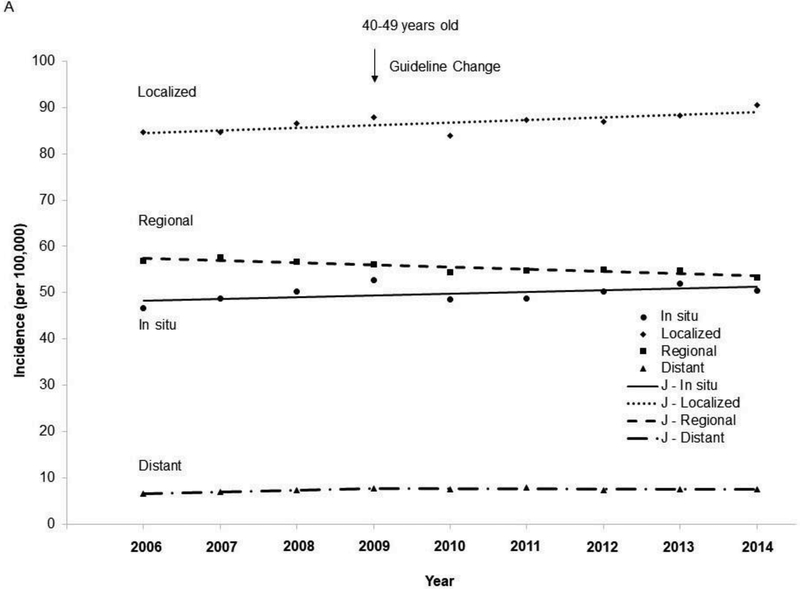
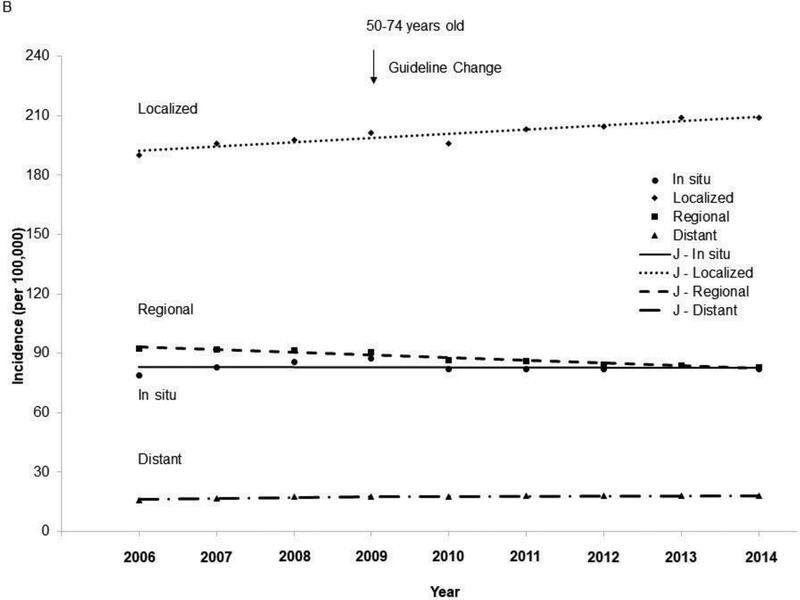
The age-adjusted incidence in invasive breast cancer increased from 2006 to 2014 (APC=0.3, 95% CI=0, 0.7, p=0.043) among women aged 50–74 years (Figure 1), whereas the incidence in invasive breast cancer remained stable among women aged 40–49 years (APC=0.1, 95% CI= – 0.2, 0.5, p=0.41). The incidence of invasive breast cancer increased in Asian/Pacific Islanders aged 40–49 years (Appendix Figure 1) and 50–74 years (Appendix Figure 2), and in non-Hispanic blacks aged 50–74 years. The incidence in localized cancer increased from 2006 to 2014 among both women aged 40–49 years and 50–74 years (Figure 2), whereas the incidence in regional cancer decreased from 2006 to 2014 in both of these age groups. Joinpoint analyses revealed one joinpoint in 2009 for distant breast cancer when the APCs started to decrease after 2009 among both women aged 40–49 and 50–74 years.
Figure 1.
Trends in age-adjusted incidence rate of breast cancer among women aged 40–74 years, 2006–2014.
Notes: Red arrow indicates screening guideline change in 2009 for breast cancer. Lines were joinpoint regression lines. Among women aged 40–49 years: All: 2006–2014 APC=0.2, 95% CI= –0.3, 0.7, p=0.30; Invasive: 2006–2014 APC=0.1, 95% CI= –0.2, 0.5, p=0.41. Among women aged 50–74 years: All: 2006–2014 APC=0.2, 95% CI= –0.2, 0.6, p=0.28; Invasive: 2006–2014 APC=0.3, 95% CI= 0, 0.7, p=0.043.
J -, joinpoint regression line; APC, annual percentage changes;
Figure 2.
Trends in age-adjusted incidence rate of breast cancer by stage among women aged 40–74 years, 2006–2014. (A) Among women 40–49 years of age. (B) Among women 50–74 years of age.
Notes: Red arrow indicates screening guideline change in 2009 for breast cancer. Lines were joinpoint regression lines. Among women aged 40–49 years: In situ: 2006–2014 APC=0.8, 95% CI= –0.3, 1.9, p=0.12; Localized: 2006–2014 APC=0.6, 95% CI=0.1, 1.2, p=0.026; Regional: 2006–2014 APC= –0.8, 95% CI= –1.2, –0.5, p<0.001; Distant: 2006–2009 APC=5.0, 95% CI= –0.2, 10.5, p=0.055; 2009–2014 APC= –0.4, 95% CI= –2.5, 1.7, p=0.63; 2006–2009 vs 2009– 2014, p=0.055. Among women aged 50–74 years: In situ: 2006–2014 APC=0.0, 95% CI= –0.9, 0.8, p=0.93; Localized: 2006–2014 APC=1.1, 95% CI=0.7, 1.5, p<0.001; Regional: 2006–2014 APC= –1.5, 95% CI= –1.9, –1.2, p<0.001; Distant: 2006–2009 APC=2.9, 95% CI=1.4, 4.45, p=0.006; 2009–2014 APC=0.5, 95% CI= –0.1, 1.1, p=0.085; 2006–2009 vs 2009–2014, p=0.015.
J -, joinpoint regression line; APC, annual percentage changes.
Table 2 showed the composition of breast cancer by stage between 2006–2009 and 2011–2014. The distribution of cancer stages remained stable between the two time periods among both women aged 40–49 and 50–74 years. There were also no differences in stage distribution between 2006–2009 and 2011–2014 in Hispanics, non-Hispanic whites, non-Hispanic blacks, and Asian/Pacific Islanders.
Table 2.
Stage Composition of Breast Cancer Among Women Aged 40–74 Years, USCS 2006– 2014
| Variable | 40–49 | 50–74 | ||
|---|---|---|---|---|
| 2006–2009 | 2011–2014 | 2006–2009 | 2011–2014 | |
| Stage of breast cancer, % | ||||
| In situ | 24.8 | 25.1 | 21.6 | 21.1 |
| Localized | 43.1 | 44 | 50.6 | 52.8 |
| Regional | 28.5 | 27.1 | 23.5 | 21.5 |
| Distant | 3.6 | 3.8 | 4.3 | 4.6 |
| Hispanic, % | ||||
| In situ | 23.1 | 23.8 | 20.5 | 21 |
| Localized | 40.5 | 41.3 | 47.8 | 49.7 |
| Regional | 32.2 | 30.7 | 27 | 24.6 |
| Distant | 4.2 | 4.2 | 4.6 | 4.7 |
| Non-Hispanic white, % | ||||
| In situ | 25.6 | 25.4 | 21.5 | 20.6 |
| Localized | 44 | 45.3 | 51.8 | 54.3 |
| Regional | 27.3 | 26 | 22.7 | 20.7 |
| Distant | 3.1 | 3.3 | 4 | 4.3 |
| Non-Hispanic black, % | ||||
| In situ | 20.3 | 22.3 | 21.8 | 22.7 |
| Localized | 39.6 | 39.9 | 43.2 | 45.7 |
| Regional | 34.1 | 31.5 | 28.3 | 25 |
| Distant | 6.1 | 6.3 | 6.7 | 6.6 |
| Asian or Pacific Islander, % | ||||
| In situ | 28.3 | 28.3 | 24.9 | 25.2 |
| Localized | 44.5 | 44.7 | 49.2 | 50.5 |
| Regional | 24.5 | 24.3 | 22.1 | 20.5 |
| Distant | 2.7 | 2.7 | 3.8 | 3.7 |
Notes: Data were from U.S. Cancer Statistics (USCS), the combined data from the Centers for Disease Control and Prevention (CDC’s) National Program for Cancer Registries (NPCR) and the National Cancer Institute’s (NCI’s) Surveillance, Epidemiology, and End Results (SEER) Program. When stage composition of breast cancer was calculated, number of cases in each stage was age-adjusted to the 2000 U.S. Standard Population. Chi-square test was used to compare stage composition between 2006–2009 and 2011–2014, and p-value for each test was >0.05.
DISCUSSION
Using data from the NPCR and SEER Incidence–USCS database, this study evaluated the impact of screening guideline changes for breast cancer on trends in the incidence of breast cancer by stage. The 2001–2014 USCS database includes high-quality population-based cancer incidence data on the entire U.S. population. This study found no differences in the stage distribution of breast cancer before and after the guideline change in 2009 among women aged 40–49 years or 50–74 years. Some minor differences in breast cancer incidence by stage were observed, such as a slightly higher incidence in localized and distant cancer and a lower incidence in regional cancer in 2011–2014 compared with that in 2006–2009 among both the age groups (40–49 years and 50–74 years). However, the APC in distant stage cancer was lower after the 2009 guideline change. Overall, this study found no immediate adverse effects of the USPSTF screening guideline change on breast cancer incidence, though calling for long-term active surveillance of the incidence of breast cancer by stage.
While USPSTF revised their breast cancer screening guidelines in 2009 in terms of screening initiation age and screening interval,10 the American Cancer Society27 and other consensus guidelines28–30 did not endorse these recommendations during this study period. As early as 2007, the American College of Physicians started to recommend screening in women aged 40–59 years based on risk profile and personal choice rather than universal screening every 1–2 years.31,32 Different guidelines vary because of different interpretations of evidence and judgment about the benefits, such as reduced mortality, and the harms, such as false positives and unnecessary workups. Comparative modeling analyses indicate that biennial screening in women aged 50–74 years retained 78.2% of breast cancer mortality reduction of annual screening (25.8% reduction in biennial screening versus 33.0% reduction in annual screening compared with no screening), 84.5% of years of life gained of annual screening (122.4 years of life gained per 1,000 women screened by biennial screening versus 144.8 years by annual screening compared with no screening), and 86.4% of quality-adjusted life years (QALYs) gained of annual screening (86.0 QALYs gained per 1,000 women screened by biennial screening versus 99.5 QALYs by annual screening compared with no screening).18,20 Multiple factors including trust in consensus guidelines, previously established screening patterns, physician financial incentives, and willingness to change preventive care patterns have prevented the full adoption of biennial screening recommendations.11 Nevertheless, since the USPSTF screening guideline changes in 2009, annual mammography rates have declined significantly among U.S. adult women,11,13,14 indicating at least some level of responsiveness to this guideline change.11
Annual screening has 67% more false-positive screening results than biennial screening (1,570 false-positive results per 1,000 women by annual screening versus 940 by biennial screening).19 Longer screening intervals and a late screening initiation age as endorsed by USPSTF would reduce false-positive results and consequent workups. This study found a slightly higher incidence in localized and distant cancer and a lower incidence in regional cancer in 2011–2014 among both women aged 40–49 and 50–74 years. In situ cancer increased slightly in 2011–2014 among women aged 40–49 years, but decreased among women aged 50–74 years. Overall, the cancer incidence and stage distribution were similar between 2006–2009 and 2011–2014 among both women aged 40–49 and 50–74 years. Incidence slightly increased in some stages but decreased in other stages, which resulted in little change in the overall cancer incidence and stage distribution. The slightly increased incidence of metastatic breast cancer calls for close and active monitoring. However, the decreased APCs after 2009 in the incidence in metastatic cancer argues against the adverse contribution of the screening guideline change to the increased incidence. The decreased incidence in regional cancer in 2011–2014 among women aged 40–49 and 50–74 years, increased incidence in in situ cancer among women aged 40–49 years, relatively stable incidence in in situ cancer among women aged 50–74 years, and similar stage distribution between 2006–2009 and 2011–2014 also indicate that the screening guideline change is unlikely to lead to a meaningful increase in distant disease. The increased incidence in distant cancer in 2011–2004 may be the result of a continuously increasing trend in metastatic breast cancer in the past few decades, which was mainly driven by changes in the distribution of risk factors in the U.S. and improved diagnostic imaging.33–35 Fast-growing breast cancer may emerge in less than 24 months and will not be captured by biennial screening.36 A significant proportion (13.8%) of diagnosed breast cancers are interval cancers diagnosed within 24 months after a negative screening mammogram. Kirsh et al.36 compared the stage and grade of breast cancer diagnosed in the interval between mammogram screenings with screen-detected breast cancer, and found that interval cancers missed by biennial screening were higher stages and grades compared with screen-detected cancers. Those fast-growing tumors may need more sensitive screening modalities for early detection. Whether detecting those tumors early will reduce cancer mortality also needs further investigation.
A slight significant increase in breast cancer incidence in 2011–2014 was found among non-Hispanic black and Asian or Pacific Islander women. However, the distribution of cancer stage stratified by race/ethnicity remained the same before and after the screening guideline change. The observed increase in incidence among non-Hispanic black and Asian or Pacific Islander women is likely due to the already increasing incidence within stage. The increased screening utilization among blacks37–39 may contribute to the increased incidence. However, some studies suggest that the observed higher utilization of mammograms in blacks may partly because of self-reported mammogram use tends to overestimate mammogram rates in blacks than in whites.40–41 Among non-Hispanic whites, the incidence in invasive breast cancer increased by about 2 per 100,000 women in 2011–2014 compared with 2006–2009; the statistical significance is likely due to the large sample size. Future studies are needed to examine the detailed impact of the screening guideline change on those racial/ethnic groups and to clarify the underlying contributors to the observed changes in incidence.
Limitations
A major strength of this study is that data were from the NPCR and SEER Incidence–USCS database, which covers the entire U.S. female population aged 40–74 years and enables the generalizability of findings from this study. Data from USCS have high-quality information on breast cancer diagnosis date, stage, and sociodemographic characteristics, allowing for examination of the impact of the screening guideline changes in various subpopulations. This study also has several limitations. Information about mammogram screening usage, screening pattern, and screening modality before breast cancer diagnosis was not available for participants in the USCS database. Additionally, causal inference cannot be concluded from these findings as this study was an ecologic study. Numerous studies have attempted to estimate trends in the underlying incidence of breast cancer in the U.S. and most conclude that there has been a steady increasing trend in the past few decades because of changes in the distribution of risk factors33,34 in the U.S.35,42–44 Such temporal trends may threaten the conclusion of this study if a higher incidence in invasive breast cancer was found after the 2009 screening guideline. However, no changes were found in stage distribution between before and after the guideline changes and a lower APC was found after the guideline changes in distant breast cancer among both women aged 40–49 and 50–74 years.
CONCLUSIONS
Under the context of the 2009 screening guideline changes for breast cancer, this study found no substantial change of incidence rate by stage or stage distribution in 2011–2014 compared to 2006–2009. Active surveillance of breast cancer among the female population in the U.S. is still needed to assure no long-term adverse effects of a longer screening interval or late screening initiation age.
Supplementary Material
ACKNOWLEDGMENTS
Part of the results was presented at the 145th American Public Health Association Annual Meeting and Exposition. Atlanta, GA. November 4–8, 2017.
Dr. Guo is currently supported by the National Cancer Institute of NIH under Award Number K07CA222343, and was supported by a research career development award (K12HD052023: Building Interdisciplinary Research Careers in Women’s Health Program-BIRCWH; Berenson, principal investigator) from the Office of Research on Women’s Health and the Eunice Kennedy Shriver National Institute of Child Health and Human Development at NIH when the study was initially performed. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIH.
The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript, and decision to submit the manuscript for publication.
Fangjian Guo was responsible for study planning, statistical analysis, drafting and revising the manuscript, full access to data, and responsibility for overall content of manuscript. Yong-fang Kuo was responsible for data interpretation and revision of the manuscript. Abbey B. Berenson was responsible for study planning, data interpretation, and drafting and revising the manuscript.
Footnotes
No financial disclosures were reported by the authors of this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.DeSantis CE, Fedewa SA, Goding Sauer A, Kramer JL, Smith RA, Jemal A. Breast cancer statistics, 2015: convergence of incidence rates between black and white women. CA Cancer J Clin. 2016;66(1):31–42. 10.3322/caac.21320. [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society. Breast Cancer Facts & Figures 2015–2016. Atlanta, GA: American Cancer Society, Inc; www.cancer.org/acs/groups/content/@research/documents/document/acspc-046381.pdf. Published 2015. Accessed on June 30, 2016. [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 4.Shi R, Taylor H, McLarty J, Liu L, Mills G, Burton G. Effects of payer status on breast cancer survival: a retrospective study. BMC Cancer. 2015;15:211 10.1186/s12885-015-1228-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sprague BL, Trentham-Dietz A, Gangnon RE, et al. Socioeconomic status and survival after an invasive breast cancer diagnosis. Cancer. 2011;117(7):1542–1551. 10.1002/cncr.25589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Halpern MT, Bian J, Ward EM, Schrag NM, Chen AY. Insurance status and stage of cancer at diagnosis among women with breast cancer. Cancer. 2007;110(2):403–411. 10.1002/cncr.22786. [DOI] [PubMed] [Google Scholar]
- 7.Harper S, Lynch J, Meersman SC, Breen N, Davis WW, Reichman MC. Trends in area-socioeconomic and race-ethnic disparities in breast cancer incidence, stage at diagnosis, screening, mortality, and survival among women ages 50 years and over (1987–2005). Cancer Epidemiol Biomarkers Prev. 2009;18(1):121–131. 10.1158/1055-9965.EPI-08-0679. [DOI] [PubMed] [Google Scholar]
- 8.Liu PH, Wang JD, Keating NL. Expected years of life lost for six potentially preventable cancers in the United States. Prev Med. 2013;56(5):309–313. 10.1016/j.ypmed.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 9.U.S. Preventive Services Task Force. The Guide to Clinical Preventive Services, 2014. www.ahrq.gov/sites/default/files/wysiwyg/professionals/clinicians-providers/guidelines-recommendations/guide/cpsguide.pdf Accessed August 26, 2016.
- 10.U.S. Preventive Services Task Force. Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151(10):716–726. 10.7326/0003-4819-151-10-200911170-00008. [DOI] [PubMed] [Google Scholar]
- 11.Wharam JF, Landon B, Zhang F, Xu X, Soumerai S, Ross-Degnan D. Mammography rates 3 years after the 2009 U.S. Preventive Services Task Force Guidelines changes. J Clin Oncol. 2015;33(9):1067–1074. 10.1200/JCO.2014.56.9848. [DOI] [PubMed] [Google Scholar]
- 12.Qin X, Tangka FK, Guy GP, Howard DH. Mammography rates after the 2009 revision to the United States Preventive Services Task Force breast cancer screening recommendation. Cancer Causes Control. 2017;28(1):41–48. 10.1007/s10552-016-0835-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sprague BL, Bolton KC, Mace JL, et al. Registry-based study of trends in breast cancer screening mammography before and after the 2009 U.S. Preventive Services Task Force recommendations. Radiology. 2014;270(2):354–361. 10.1148/radiol.13131063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharpe RE, Levin DC, Parker L, Rao VM. The effect of the controversial U.S. Preventive Services Task Force recommendations on the use of screening mammography. J Am Coll Radiol. 2013;10(1):21–24. 10.1016/j.jacr.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 15.Wang AT, Fan J, Van Houten HK, et al. Impact of the 2009 U.S. Preventive Services Task Force guidelines on screening mammography rates on women in their 40s. PLoS One. 2014;9(3):e91399 10.1371/journal.pone.0091399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pace LE, He Y, Keating NL. Trends in mammography screening rates after publication of the 2009 U.S. Preventive Services Task Force recommendations. Cancer. 2013;119(14):2518–2523. 10.1002/cncr.28105. [DOI] [PubMed] [Google Scholar]
- 17.Block LD, Jarlenski MP, Wu AW, Bennett WL. Mammography use among women ages 40–49 after the 2009 U.S. Preventive Services Task Force recommendation. J Gen Intern Med. 2013;28(11):1447–1453. 10.1007/s11606-013-2482-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mandelblatt JS, Stout NK, Schechter CB, et al. Collaborative modeling of the benefits and harms associated with different U.S. breast cancer screening strategies. Ann Intern Med. 2016;164(4):215–225. 10.7326/M15-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mandelblatt JS, Cronin KA, Bailey S, et al. Effects of mammography screening under different screening schedules: model estimates of potential benefits and harms. Ann Intern Med. 2009;151(10):738–747. 10.7326/0003-4819-151-10-200911170-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trentham-Dietz A, Kerlikowske K, Stout NK, et al. Tailoring breast cancer screening intervals by breast density and risk for women aged 50 years or older: collaborative modeling of screening outcomes. Ann Intern Med. 2016;165(10):700–712. 10.7326/M16-0476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.NPCR and SEER Incidence – USCS Public Use Databases. www.cdc.gov/cancer/npcr/public-use/index.htm Accessed October 15, 2017.
- 22.NAACCR Race and Ethnicity Work Group. NAACCR Guideline for Enhancing Hispanic/Latino Identification: Revised NAACCR Hispanic/Latino Identification Algorithm [NHIA v2.2.1]. Springfield, IL: North American Association of Central Cancer Registries; September 2011. [Google Scholar]
- 23.Tiwari RC, Clegg LX, Zou Z. Efficient interval estimation for age-adjusted cancer rates. Stat Methods Med Res. 2006;15(6):547–569. 10.1177/0962280206070621. [DOI] [PubMed] [Google Scholar]
- 24.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19(3):335–351. . [DOI] [PubMed] [Google Scholar]
- 25.Kim HJ, Fay MP, Yu B, Barrett MJ, Feuer EJ. Comparability of segmented line regression models. Biometrics. 2004;60(4):1005–1014. 10.1111/j.0006-341X.2004.00256.x. [DOI] [PubMed] [Google Scholar]
- 26.Fay MP, Tiwari RC, Feuer EJ, Zou Z. Estimating average annual percent change for disease rates without assuming constant change. Biometrics. 2006;62(3):847–854. 10.1111/j.1541-0420.2006.00528.x. [DOI] [PubMed] [Google Scholar]
- 27.Smith RA, Andrews KS, Brooks D, et al. Cancer screening in the United States, 2017: a review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin. 2017;67(2):100–121. 10.3322/caac.21392. [DOI] [PubMed] [Google Scholar]
- 28.Bevers TB, Anderson BO, Bonaccio E, et al. NCCN clinical practice guidelines in oncology: breast cancer screening and diagnosis. J Natl Compr Canc Netw. 2009;7(10):1060–1096. 10.6004/jnccn.2009.0070. [DOI] [PubMed] [Google Scholar]
- 29.American College of Obstetricians-Gynecologists. Practice bulletin no. 122: Breast cancer screening. Obstet Gynecol. 2011;118(2 Pt 1):372–382. [DOI] [PubMed] [Google Scholar]
- 30.Mainiero MB, Lourenco A, Mahoney MC, et al. ACR appropriateness criteria breast cancer screening. J Am Coll Radiol. 2013;10(1):11–14. 10.1016/j.jacr.2012.09.036. [DOI] [PubMed] [Google Scholar]
- 31.Qaseem A, Snow V, Sherif K, et al. Screening mammography for women 40 to 49 years of age: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2007;146(7):511–515. 10.7326/0003-4819-146-7-200704030-00007. [DOI] [PubMed] [Google Scholar]
- 32.Armstrong K, Moye E, Williams S, Berlin JA, Reynolds EE. Screening mammography in women 40 to 49 years of age: a systematic review for the American College of Physicians. Ann Intern Med. 2007;146(7):516–526. 10.7326/0003-4819-146-7-200704030-00008. [DOI] [PubMed] [Google Scholar]
- 33.Fedewa SA, Sauer AG, Siegel RL, Jemal A. Prevalence of major risk factors and use of screening tests for cancer in the United States. Cancer Epidemiol Biomarkers Prev. 2015;24(4):637–652. 10.1158/1055-9965.EPI-15-0134. [DOI] [PubMed] [Google Scholar]
- 34.Sauer AG, Siegel RL, Jemal A, Fedewa SA. Updated review of prevalence of major risk factors and use of screening tests for cancer in the United States. Cancer Epidemiol Biomarkers Prev. 2017;26(8):1192–1208. 10.1158/1055-9965.EPI-17-0219. [DOI] [PubMed] [Google Scholar]
- 35.Johnson RH, Chien FL, Bleyer A. Incidence of breast cancer with distant involvement among women in the United States, 1976 to 2009. JAMA. 2013;309(8):800–805. 10.1001/jama.2013.776. [DOI] [PubMed] [Google Scholar]
- 36.Kirsh VA, Chiarelli AM, Edwards SA, et al. Tumor characteristics associated with mammographic detection of breast cancer in the Ontario breast screening program. J Natl Cancer Inst. 2011;103(12):942–950. 10.1093/jnci/djr138. [DOI] [PubMed] [Google Scholar]
- 37.Virk-Baker MK, Martin MY, Levine RS, Wang X, Nagy TR, Pisu M Mammography utilization among Black and White Medicare beneficiaries in high breast cancer mortality US counties. Cancer Causes Control. 2013;24(12):2187–2196. 10.1007/s10552-013-0295-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee JY, Malak SF, Klimberg VS, Henry-Tillman R, Kadlubar S Change in Mammography Use Following the Revised Guidelines from the U.S. Preventive Services Task Force. Breast J. 2017;23(2):164–168. 10.1111/tbj.12703. [DOI] [PubMed] [Google Scholar]
- 39.Ahmed AT, Welch BT, Brinjikji W, et al. Racial Disparities in Screening Mammography in the United States: A Systematic Review and Meta-analysis. J Am Coll Radiol. 2017;14(2):157–165.e159. 10.1016/j.jacr.2016.07.034. [DOI] [PubMed] [Google Scholar]
- 40.Allgood KL, Rauscher GH, Whitman S, Vasquez-Jones G, Shah AM Validating self-reported mammography use in vulnerable communities: findings and recommendations. Cancer Epidemiol Biomarkers Prev. 2014;23(8):1649–1658. 10.1158/1055-9965.EPI-13-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Njai R, Siegel PZ, Miller JW, Liao Y Misclassification of survey responses and black-white disparity in mammography use, Behavioral Risk Factor Surveillance System, 1995-2006. Prev Chronic Dis. 2011;8(3):A59. [PMC free article] [PubMed] [Google Scholar]
- 42.Welch HG, Prorok PC, O’Malley AJ, Kramer BS. Breast-cancer tumor size, overdiagnosis, and mammography screening effectiveness. N Engl J Med. 2016;375(15):1438–1447. 10.1056/NEJMoa1600249. [DOI] [PubMed] [Google Scholar]
- 43.Anderson WF, Katki HA, Rosenberg PS. Incidence of breast cancer in the United States: current and future trends. J Natl Cancer Inst. 2011;103(18):1397–1402. 10.1093/jnci/djr257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harding C, Pompei F, Burmistrov D, Welch HG, Abebe R, Wilson R. Breast cancer screening, incidence, and mortality across U.S. counties. JAMA Intern Med. 2015;175(9):1483–1489. 10.1001/jamainternmed.2015.3043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.