Abstract
The previous decade has seen a rapid increase in microglial studies on pain, with a unique focus on microgliosis in the spinal cord after nerve injury and neuropathic pain. Numerous signaling molecules are altered in microglia and contribute to the pathogenesis of pain. Here we discuss how microglial signaling regulates spinal cord synaptic plasticity in acute and chronic pain conditions with different degrees and variations of microgliosis. We highlight that microglial mediators such as pro- and anti-inflammatory cytokines are powerful neuromodulators that regulate synaptic transmission and pain via neuron-glial interactions. We also reveal an emerging role of microglia in the resolution of pain, in part via specialized pro-resolving mediators including resolvins, protectins and maresins. We also discuss a possible role of microglia in chronic itch.
Keywords: Inflammation, itch, microglia, neuroinflammation, nerve injury, neuropathic pain, specialized pro-resolving mediators, spinal cord
Microglia are macrophage-like cells in the central nervous system (CNS) which regulate homeostasis in the brain and spinal cord. During development, microglia originate from erythromyeloid progenitors in the yolk sac and develop in the forming CNS (Crotti and Ransohoff, 2016). Increasing evidence suggests that microglia are a heterogeneous population throughout the CNS and play an active role in maintaining normal physiological conditions, as they sense the cellular environment with their ramified processes and undergo rapid morphological changes in response to mediators such as ATP (Davalos et al., 2005; Hanisch and Kettenmann, 2007). During development microglia dynamically interact with synapses to modify their structures and functions in the healthy brain. For example, microglial processes engulf synapses and induce synaptic pruning during critical developmental stages, which involves the activation of the classic complement cascade (e.g. neuronal C1q and microglial C3 receptors) (Stevens et al., 2007). Microglia are long-lived cells, with a median lifetime of well over 15 months, and half of all microglia survive for the entirety of a mouse’s lifespan (Fuger et al., 2017).
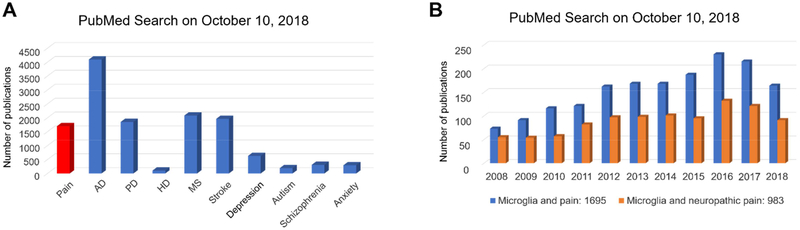
Microglia are emerging as key regulators of brain diseases. These include neurodegenerative diseases like such as Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, multiple sclerosis, stroke, neuropsychiatric diseases for instance depression, and anxiety, and neurodevelopmental diseases such as autism (Crotti and Ransohoff, 2016; Hanisch and Kettenmann, 2007; Mosser et al., 2017; Salter and Stevens, 2017; Zhan et al., 2014). Microglia contribute to the pathogenesis of brain diseases via regulation of neuroinflammation, a localized inflammation of the peripheral and central nervous system (Heppner et al., 2015; Ji et al., 2014; Ransohoff, 2016). A PubMed query reveals hundreds to thousands of publications demonstrating the role of microglia in each of these diseases (Figure 1A).
Figure-1: PubMed search for microglia and neurological diseases.
(A) Number of publications involving microglia in pain, Alzheimer’s disease (AD), Parkinson disease (PD), Huntington disease (HD), multiple sclerosis (MS), stroke, depression, autism, schizophrenia, and anxiety. (B) Number of publications for microglia and pain and microglia and neuropathic pain in last 10 years.
Microglia and Pain
Notably, there are over sixteen hundred publications on microglia and pain, showing a rapid increase in last 10 years (Figure 1B). While the majority of studies on microglia and brain diseases focus on how microglia effect synaptic pruning and neurodegeneration, pain researchers searched for neuromodulators produced by microglia that can rapidly modulate synaptic plasticity, a driving force for the pathogenesis of pain after tissue and nerve injury (Luo et al., 2014; Woolf and Salter, 2000). The injury-induced synaptic plasticity in the spinal cord and brain pain circuits is also termed central sensitization, which sustains chronic pain and causes widespread pain beyond the initial injury site (Ji, 2018; Woolf, 1983). Another striking difference between pain and other neurological diseases is the rapid onset of pain: microglia regulate neuronal and synaptic activities to change pain behavior within minutes to tens of minutes following treatment with microglial activators and inhibitors (Berta et al., 2014; Tsuda et al., 2003b).
Peripheral nerve injury not only results in neuropathic pain, which is characterized by mechanical allodynia, a pain evoked by normally innocuous stimulation such as light touch, but also causes remarkable microgliosis in the spinal cord. Gliosis is a nonspecific reactive change of glial cells in response to injuries and insults and often involves the proliferation or hypertrophy of glial cells. Microgliosis manifests as profound morphological changes, where microglia transition from ramified to amoeboid shapes with enlarged cell bodies and shortened processes. This was first reported in 1975 when Gilmore showed proliferation of non-neuronal cells (also called “neuro-glia) in spinal cords of irradiated, immature rats following transection of the sciatic nerve (Gilmore, 1975). Gilmore and Skinner further reported that sciatic nerve injury in adult rats also resulted in a proliferation of non-neuronal cells in the spinal cord using Nissl and haematoxylin and eosin staining (Gilmore and Skinner, 1979). In 1993, Eriksson and his coworkers demonstrated remarkable microgliosis in the pain-modulating spinal cord and brain stem regions after nerve injury using immunostaining of OX-42, an antibody that recognizes complement receptor CR3 (CD11b) (Eriksson et al., 1993), with others reproducing a profound microglial proliferation, evident within 2 days after nerve injury (Beggs and Salter, 2007; Echeverry et al., 2008; Suter et al., 2007).
Insights into the specific role of microglia in pain were revealed in 2003 when three separate groups showed microglial involvement in neuropathic pain after peripheral nerve injury (Jin et al., 2003; Raghavendra et al., 2003; Tsuda et al., 2003b). Jin et al. reported that spinal nerve ligation evokes phosphorylation of p38 MAP kinase (P-p38, an active form of p38) only in CD11b (OX-42)-expressing spinal microglia but not in GFAP-expressing astrocytes or NeuN-expressing neurons, and furthermore, intrathecal injection of a p38 inhibitor reduced mechanical allodynia, a cardinal feature of neuropathic pain after nerve injury (Jin et al., 2003). Raghavendra et al. showed that minocycline, a non-specific microglial inhibitor, inhibited mechanical hyperalgesia and allodynia in the early phase but not the late phase after spinal nerve transection. This inhibition in the early phase of nerve injury was associated with inhibition of microglial activation in the spinal cord (Raghavendra et al., 2003). Tsuda et al. demonstrated that spinal injection of ATP-activated microglia was sufficient to evoke rapid mechanical allodynia within one hour of treatment. Furthermore, they found (1) nerve injury results in upregulation of the ATP receptor subtype P2X4 exclusively in spinal cord microglia and (2) spinal inhibition of P2X4 attenuated mechanical allodynia (Tsuda et al., 2003b). In 2004, Tsuda et al. also confirmed the role of p38 in spinal microglia for the development of mechanical allodynia after nerve injury (Tsuda et al., 2004). In 2005, Coull et al. demonstrated that spinal microglia produce the brain-derived neurotrophic factor (BDNF) to drive neuropathic pain (Coull et al., 2005).
A major issue in microglia research is the lack of selective pharmacological tools. Grace and collaborators developed Gq- and Gi-coupled DREADDs (Designer Receptor Exclusively Activated by a Designer Drug) for selective activation or inhibition of microglia. DREADDs under a CD68 promoter were intrathecally transfected via an AAV9 vector to target microglia/macrophages. Activation of microglia with Gq (stimulatory) DREADD, which is stimulated by its clozapine-N-oxide (CNO) ligand, is sufficient to induce mechanical allodynia via IL-1 secretion, as this allodynia can be abolished by intrathecal interleukin-1 receptor antagonist. In contrast, nerve injury-induced allodynia is attenuated by intrathecal activation of Gi (inhibitory) DREADD (Grace et al., 2016; Grace et al., 2018). This chemogenetic approach further supports a critical role of spinal microglia in establishing mechanical allodynia.
There are a number of excellent reviews that focus on the role microglia in neuropathic pain (Clark and Malcangio, 2012; Inoue and Tsuda, 2018; McMahon and Malcangio, 2009; Salter and Stevens, 2017; Tsuda et al., 2005). In this review, we offer a more comprehensive view on microglia in various pain states with different degrees of microgliosis. In addition to neuropathic pain with nerve injury, we discuss microgliosis and microglial signaling in inflammatory pain, cancer pain, and drug-induced pain after chemotherapy and chronic opioid exposure. In particular, we present a balanced view of microglia, in which microglia are both detrimental and protective in their effects, contributing to both the pathogenesis and resolution of pain.
Microglia Regulate Neuropathic Pain after Peripheral Nerve Injury
Microglia in the spinal cord horn are strongly activated after peripheral nerve injury (Guan et al., 2016; Tsuda et al., 2005). Spinal microglial activation after nerve injury requires neuronal activity (Wen et al., 2007; Xie et al., 2009). While C-fiber activation is sufficient to elicit spinal microglial activation (Gruber-Schoffnegger et al., 2013; Hathway et al., 2009), activation of large A-fibers is also important to maintain microglial activation (Suter et al., 2009).
Microglial Activators Are Initially Released from Primary Sensory Neurons
Multiple signaling molecules released from damaged primary afferents play a crucial role in the induction and development of spinal microgliosis. Guan et al. show that peripheral nerve injury induces a rapid increase in the expression of colony stimulating factor 1 (CSF1) in injured DRG neurons (Guan et al., 2016; Okubo et al., 2016). The CSF1 released from damaged primary afferents acts on spinal microglia to induce microgliosis and pain behaviors. Spinal injection of CSF1 inhibitor (Okubo et al., 2016) or Cre-mediated deletion of Csf1 in DRG neurons (Guan et al., 2016) both attenuate nerve injury induced microgliosis and mechanical allodynia. Furthermore, intrathecal injection of CSF1 in naïve mice induced mechanical hypersensitivity and microgliosis (Guan et al., 2016).
Sensory neurons also release several chemokines after nerve injury to activate microglia. CCL2 is strongly upregulated in DRG neurons by nerve injury and contribute to neuropathic pain via CCR2 receptor (White et al., 2005). Although CCR2 was implicated in microglial activation in neuropathic pain (Abbadie et al., 2003; Thacker et al., 2009; Zhang et al., 2007), microglial expression of CCR2 in spinal dorsal horn has not been clearly demonstrated in the spinal cord dorsal horn. Instead, CCR2 expression was reported in DRG and spinal cord neurons (Gao et al., 2009; White et al., 2005). It is well documented that CXCL1, released from DRG neurons, induces microgliosis and mechanical allodynia via CX3CR1 (Clark and Malcangio, 2012). Furthermore, liberation of CXCL1 from the DRG cell surface requires cathepsin S (Clark et al., 2009). Additionally, CCL21 is rapidly induced in a small population of DRG neurons and enhances microglial activation and neuropathic pain via CCR7 receptor (Biber et al., 2011).
Neuronal proteases also play an important role in microglia activation. Nerve injury induces a rapid and transient upregulation of metalloproteinase-9 (MMP-9) expression in injured DRG neurons, which contributes to the induction but not maintenance of neuropathic pain (Ji et al., 2009). Intrathecal MMP-9 administration produces neuropathic pain symptoms and microgliosis through IL-1β cleavage. Conversely, MMP-9 inhibition or knockdown reduces microgliosis and mechanical allodynia (Kawasaki et al., 2008a). Caspase 6, a cysteine-aspartic acid protease, is specifically expressed by axonal terminals in the spinal cord and released to the cerebrospinal fluid by neuronal activation (Berta et al., 2014). Nerve injury causes a profound upregulation of caspase-6 mRNA in injured DRG neurons, which contributes to microglial activation and neuropathic pain (Berta et al., 2017; Berta et al., 2016). Extracellular application of caspase-6 triggers a substance TNF release from microglia, leading to TNF-dependent pain following intrathecal injection (Berta et al., 2014).
Following peripheral nerve injury, Neuregulin-1 is also released from primary afferents to activate ErbB receptors on spinal microglia. Intrathecal injection of neuregulin-1 induced microgliosis and development of pain hypersensitivity via phosphorylation of ERK1/2 and Akt in microglia (Calvo et al., 2010).
Up-regulation of Microglial Signaling Molecules after Nerve Injury
Once microglia are activated by the activators released after nerve injury, various microglial signaling molecules are up-regulated in the spinal cord dorsal horn, including cell surface receptors, as well as intracellular and secreted signaling molecules (Table 1).
Table-1:
Microglial signal molecules that are upregulated after peripheral nerve injury and positively contribute to promoting neuropathic pain (mechanical allodynia).
| Upregulation of signaling molecules | Nerve injury models | Contribution to mechanical allodynia | References |
|---|---|---|---|
| Purinergic receptors | |||
| P2X4R | SNT | Yes | Tsuda et al., 2003 |
| P2X7R | SNI | Yes | Kobayashi et al., 2011 |
| P2Y6R | SNI, SNL | Yes | Kobayashi et al., 2012; Barragan-Iglesias et al., 2014 |
| P2Y12R | SNL, SNT | Yes | Tozaki-Saitoh et al., 2008; Kobayashi et al., 2008 |
| P2Y13R | SNI | Yes | Kobayashi et al., 2012; Tatsumi et al., 2015 |
| P2Y14R | SNI | Yes | Kobayashi et al., 2012 |
| Toll-like receptors | |||
| TLR-2 | SNT | Yes | Kim et al., 2007 |
| TLR-4 | SNT | Yes | Tanga et al., 2005 |
| Chemokine receptors | |||
| CX3CR1 | SNL | Yes | Zhuang et al., 2007; Staniland et al., 2010 |
| CCR2 | SNL | Yes | Abbadie et al., 2003 |
| CCR5 | SNT | Yes | Matsushita et al.,2014 |
| Other receptors | |||
| TREM2 | SNT | Yes | Kobayashi et al., 2016 |
| ErbB2 | SNI | Yes | Calvo et al., 2010 |
| TMEM16F | SNL | Yes | Batti et al., 2016 |
| C5aR | SNI | Yes | Griffin et al., 2007 |
| IFN-γR | SNI | Yes | Tsuda et al., 2009 |
| CSF1R | SNI, SNT | Yes | Guan et al., 2016; Okubo et al., 2016 |
| Kinases and enzymes | |||
| p-p38 | SNL,SNT | Yes | Jin et al., 2003; Tsuda et al., 2004 |
| pERK1/2 | SNL | Yes | Zhuang et al., 2005 |
| pERK5 | SNL | Yes | Obata et al., 2007 |
| pSrc | SNT | Yes | Katsura et al., 2006 |
| PLyn | SNT | Yes | Tsuda et al., 2008 |
| Nox-2 | SNT | Yes | Kim et al., 2010 |
| COX-1 | SNT | Yes | Kanda et al., 2013 |
| COX-2 | SCI | Yes | Zhao et al., 2007 |
| 5-LOX | SNI | Yes | Okubo et al., 2010 |
| PGDS | SNT | Yes | Kanda et al., 2013 |
| TXA2S | SNT | Yes | Kanda et al., 2013 |
| LPCAT2 | SNL | Yes | Shindou et al., 2017 |
| Cathepsin S | SNL | Yes | Clark et al., 2007 |
| Transcriptional factors | |||
| IRF8 | SNT | Yes | Masuda et al., 2012 |
| IRF5 | SNT | Yes | Masuda et al., 2014 |
| STAT3 | SNL | Yes | Dominguez et al., 2008 |
| Other proteins | |||
| C1q, C3,C4,C5 | SNL, SNI, CCI | Yes | Griffin et al., 2007 |
| ROS | SNT | Yes | Kim et al., 2010 |
| DAP12 | SNT | Yes | Guan et al., 2016; Kobayashi et al., 2016 |
Abbreviations: CCI, chronic constriction injury; SNI, spared nerve injury; SNL, spinal nerve ligation; SNT, spinal nerve transection
Purinergic Receptors.
ATP is a prominent activator of microglia. There are three subtypes of purinergic receptors, the adenosine P1 receptors, the P2X ion channel receptors, and the G protein-coupled P2Y receptors. Nerve injury up-regulation of P2X4R exclusively occurs in spinal cord microglia. Spinal blockade of P2X4R signaling reduces nerve injury-induced mechanical allodynia, a cardinal feature of neuropathic pain. Furthermore, nerve injury-induced allodynia is abolished in P2×4r knockout mice and intrathecal injection of P2X4R-stimulated microglia can cause allodynia in normal rats (Tsuda et al., 2003a). These results indicate that P2X4R on spinal microglia is a key receptor for establishing mechanical allodynia. Neuronal CCL21 upregulates microglial P2X4 via activation of the CCR7 receptor (Biber et al., 2011). Nerve injury also induces up-regulation of P2X7R in spinal cord microglia (Kobayashi et al., 2011). P2X7R inhibitors reduce mechanical allodynia in neuropathic pain models by intraperitoneal or intrathecal injection. In addition, P2×7r knockout mice have significantly reduced pain hypersensitivities after peripheral nerve injury. Interestingly, P2X7R and P2X4R can form a functional interaction through their physical association in macrophages (Boumechache et al., 2009; Perez-Flores et al., 2015). It will be interesting to study whether this interaction also occurs in spinal microglia and contributes to neuropathic pain. In addition to P2X subtypes, peripheral nerve injury also induced robust increases of P2Y6R, P2Y12R, P2Y13R, and P2Y14R mRNA expression in spinal microglia. The functional blockade of all these P2Y receptors all reduced pain hypersensitivity, indicating that the microglial P2Y receptors and ATP signaling may also play an active role in neuropathic pain (Kobayashi et al., 2012).
Toll-Like Receptor (TLRs).
The TLR family includes 14 subtypes and plays a crucial role in the innate immune response (Liu et al., 2012). The role of TLR4 in nerve injury-induced neuropathic pain has been extensively studied. Tlr4 expression in spinal cord rapidly increased four hours after L5 spinal nerve transection (Tanga et al., 2004). Tlr4-deficient mice show attenuated mechanical allodynia and thermal hyperalgesia after nerve injury (Tanga et al., 2005). Similarly, intrathecal antisense knockdown of spinal TLR4 in wild-type mice inhibits nerve injury-induced microglial activation and cytokine expression and neuropathic pain (Tanga et al., 2005). Furthermore, intrathecal injection of various TLR4 antagonists reverse nerve injury induced mechanical allodynia and thermal hyperalgesia both in mice and rats (Nicotra et al., 2011). These results suggest that TLR4 plays an important role in the initiation and maintenance of spinal microglia activation and neuropathic pain. However, astrocytic expression of TLR4 was shown in spinal dorsal horn under some pathological conditions (Li et al., 2014; Liu et al., 2016). Future studies of conditional deletions of Tlr4 in microglia and astrocytes are required. Tlr2 knockout mice also exhibit reduced neuropathic pain and spinal microglia activation in some nerve injury models (Kim et al., 2007).
Chemokine Receptors.
There receptors are widely expressed in the nervous system and are involved in neuropathic pain. In the spinal cord, the chemokine receptor CX3CR1 is exclusively expressed in microglia and is upregulated after nerve injury (Zhuang et al., 2007). In Cx3cr1 knockout mice, partial sciatic nerve ligation injury-induced spinal microglial activation is suppressed and mechanical allodynia does not occur (Clark et al., 2011; Staniland et al., 2010b).
MAP Kinases (MAPKs).
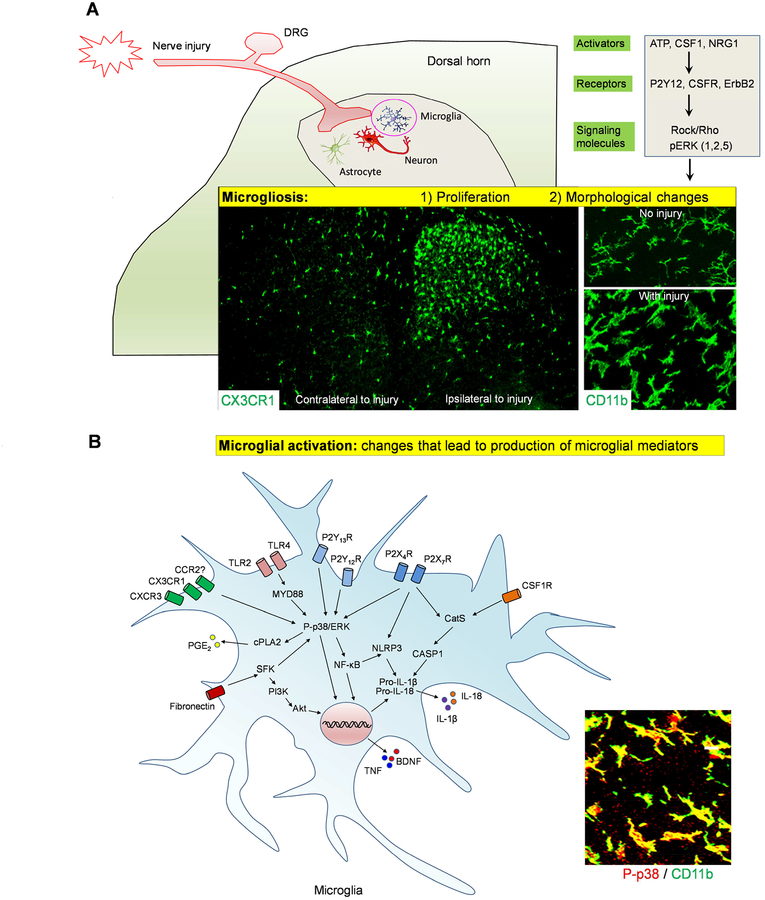
After nerve injury, phosphorylation of p38 MAPK is increased and highly restricted to spinal microglia (Jin et al., 2003; Tsuda et al., 2004). Pharmacological inhibition of p38 MAPK activity or knockdown of the p38α isoform via intrathecal route suppressed the development of mechanical allodynia in various models of neuropathic pain (Jin et al., 2003; Luo et al., 2017; Tsuda et al., 2004). Besides p38 MAPK, phosphorylation of extracellular signal-regulated kinases 1/2 and 5 (ERK1/2 and ERK5), was also found in spinal microglia after nerve injury and both ERK1/2 and ERK5 regulate neuropathic pain (Calvo et al., 2011; Obata et al., 2007; Zhuang et al., 2005). Inhibition of the ERK1/2 pathway and Rho-associated coiled-coil-containing protein kinase (ROCK) reduced microgliosis and pain hypersensitivity after peripheral nerve injury (Calvo et al., 2011; Tatsumi et al., 2015) (Figure 2A).
Figure-2: Nerve injury-induced microgliosis and microglial activation in the spinal cord.
(A) Microgliosis in SDH after nerve injury. (1) Nerve injury (CCI) induces microglial proliferation on the ipsilateral side as revealed in CX3CR1-GFP mice. (2) Nerve injury also induces profound morphological changes of microglia as revealed by CD11b staining with OX-42 antibody. (B) Microglia activation after nerve injury results in the production of microglial mediators, such as TNF, BDNF, IL-1β, and IL-18, which can promote neuropathic pain.
Src-Family Kinases.
Src family has five members in the CNS Src, Lyn, Lck, Yes, and Fyn (Salter and Kalia, 2004). Nerve injury elicits a striking increase in Src phosphorylation in spinal microglia, and activated Src contributes to the development of neuropathic pain (Katsura et al., 2006). ERK is downstream of Src activation and mediates Src-induced chemokine release. Intrathecal injection of Src inhibitor attenuates mechanical allodynia by suppressing ERK activity in spinal microglia (Katsura et al., 2006). Lyn is also upregulated in spinal microglia after nerve injury and activates ERK, leading to increased P2X4R expression in microglia after nerve injury. Moreover, Lyn deletion reduces tactile allodynia after nerve injury (Tsuda et al., 2008).
Proteases:
Peripheral nerve injury induces cathepsin S (CatS) in spinal microglia, and spinal administration of CatS inhibitor is antihyperalgesic and antiallodynic in neuropathic rats (Clark et al., 2007). Interestingly, CatS is a secreted protease, which induces neuropathic pain via cleavage and liberation of CXCL1 (fractalkine), leading to further p38 activation in spinal microglia (Clark et al., 2007; Clark et al., 2009).
Transcription Factors.
Interferon regulatory factor-8 (IRF8) expression is exclusively upregulated in spinal microglia after nerve injury (Masuda et al., 2012). IRF8 activates many genes that are involved in microglial responses and promote neuropathic pain (Masuda et al., 2015; Masuda et al., 2012). Knockdown of IRF8 expression in the spinal cord caused a recovery of tactile allodynia. Furthermore, nerve injury induced neuropathic pain is attenuated in IRF8 knockout mice (Masuda et al., 2012). In addition, IRF8 regulates the expression of IRF1 and IRF5 in microglia. While the IRF8-IRF1 cascade regulates IL-1β expression (Masuda et al., 2015), the IRF8-IRF5 cascade regulates P2X4R expression (Masuda et al., 2014).
Compliment Cascade:
Peripheral nerve injury also upregulates complement components C1q, C3, and C4 in spinal microglia. Notably, C1q and C4 are among the most strongly regulated genes by nerve injury. Furthermore, nerve injury results in upregulations of the terminal complement component C5 and the C5a receptor (C5aR) in spinal microglia, and neuropathic pain is attenuated by deletion of C5 or by C5aR antagonist (Griffin et al., 2007).
Distinct Responses of Microglia: Microgliosis and Microglia Activation
A key aspect of the microglial response to nerve trauma is microgliosis, including microglial morphological changes and proliferation (Figure 2A). Following peripheral nerve injury, in addition to morphological changes, spinal microglia begin to proliferate within 2 to 3 days and reach maximal levels in 4 to 7 days (Echeverry et al., 2008). Normally, microgliosis after nerve injury is a transient and self-limited event, and microglia return to normal levels within a few weeks. Nerve injury induced microgliosis is concomitant with the development of pain hypersensitivity. Accordingly, blocking microgliosis attenuates pain behaviors (Gu et al., 2016; Guan et al., 2016; Sorge et al., 2015). Microglial proliferation is partially controlled by CX3CR1 and P2Y12, and inhibiting the proliferation of microglia effectively alleviated neuropathic pain during the development phase (Gu et al., 2016; Peng et al., 2016). It is important to point out that both spinal microgliosis and neuropathic pain development after nerve injury are age dependent and do not occur during the first several weeks of life due to the masking effect of anti-inflammatory cytokines such as IL-10 (McKelvey et al., 2015).
Resident vs. Migrating Microglia.
Although early studies using radiation revealed a contribution of bone marrow-derived monocytes to microglial proliferation in the spinal cord after nerve injury (Zhang et al., 2007), more recent work using mouse genetic models, such as CCR2(RFP/+):CX3CR1(GFP/+) mice demonstrates that microglial proliferation in the spinal cord after nerve injury resulted from resident microglia but not from infiltrating monocytes (Gu et al., 2016). Further, using parabiosis and mice with genetically labelled microglia, Tashima et al. demonstrated little contribution of circulating bone marrow-derived cells to spinal microglial proliferation after peripheral nerve injury (Tashima et al., 2016). Interestingly, spinal microglia can be ablated selectively using intrathecal injection of Mac-1-saporin, a microglia selective immunotoxin. And they will repopulate rapidly within several days, but monocyte CCR2 signaling is not involved in this microglia repopulation (Yao et al., 2016).
Microgliosis vs. Microglia Activation.
Notably, microgliosis is not always correlated with pain states, and neuropathic pain can be reduced without changes of microgliosis (Ji et al., 2013; Tsuda et al., 2003b). For example, in P2×4-deficient mice nerve injury induced spinal microgliosis is normal, but pain behaviors are inhibited (Ulmann et al., 2008). Thus, microglia could be activated in the absence of microgliosis. It has been widely accepted that activated microglia release bioactive mediators to facilitate pain signaling at both spinal and supraspinal levels to contribute to neuropathic pain (Figure 2B). Importantly, microglial activation via p38 phosphorylation is a common pathway after the activation of cell surface receptors on microglia, such as CX3CR1, P2X4, P2X7, P2Y12, and TLR4 (Clark et al., 2010; Ji et al., 2013; Ji and Suter, 2007; Kobayashi et al., 2008; Trang et al., 2009). Upon activation, p38 MAPK increases the synthesis and release of various microglial mediators, such as TNF-α, IL-1β, IL-6, BDNF, PGE2 and facilitates the development of neuropathic pain (Ji et al., 2013).
Do Microglia Play a Role in Other Pathological Pain Conditions?
While nerve injury induces prominent microgliosis in the spinal cord, microgliosis after other painful insults such as inflammation, arthritis, cancer, and drug treatments (e.g., chemotherapy or chronic opioid exposure) is largely variable in part due to the degree of nerve injury in these insults (Ji et al., 2013). We discuss distinct roles of microglial signaling in these pathological pain conditions, which can be dissociated from microgliosis.
Acute Inflammatory Pain
Microgliosis takes days to manifest after nerve injury. Thus, it was initially believed that microglia exclusively regulate chronic pain such as neuropathic pain with marked microgliosis but has no role in acute inflammatory pain conditions in which microgliosis is not as evident and robust. Emerging evidence suggests that microglial signaling regulates acute inflammatory pain, especially the formalin-induced 2nd phase spontaneous pain, which manifests behaviorally as licking and flinching of the animal’s affected hindpaw. Since this spontaneous pain only lasts for less than 60 min, there is no obvious microgliosis in the spinal cord during this acute pain phase (Berta et al., 2014). Notably, hind paw injection of formalin also causes nerve injury, neuropathic pain, and spinal microgliosis in the late-phase after 1–3 days (Fu et al., 1999). Several lines of pharmacological and genetic evidence point to a role of spinal microglia in regulating formalin-induced 2nd phase spontaneous pain. First, intrathecal minocycline produced a dose-dependent reduction of spontaneous pain in rats and mice (Chen et al., 2018; Hua et al., 2005). Second, activation of p38 in spinal microglia is necessary for spontaneous pain in rats and mice, and this activation regulates microglial synthesis and release of PGE2 and TNF (Ji and Suter, 2007; Svensson et al., 2003a; Svensson et al., 2003b; Taves et al., 2016). Of note, formalin-induced spontaneous pain involves TNF signaling and is substantially attenuated in mice lacking receptors for TNF (TNFR1 or TNFR2) (Zhang et al., 2011). Third, Caspase-6 drives acute inflammatory pain via microglial TNF secretion (Berta et al., 2014). Formalin spontaneous pain is abolished in mice lacking Casp6 or after spinal inhibition of Caspase-6 (Berta et al., 2014). These data suggest that microglia regulate formalin-induced spontaneous pain via transcription-independent mechanisms. Thus, it is not surprising that this pain is not affected in mice lacking the transcription factor IRF5 (PMID 24818655).
Chronic Inflammatory Pain
The role of microglia in chronic inflammatory pain is controversial. P2×4 knockout mice exhibit unaltered inflammatory pain following intraplantar CFA injection (Tsuda et al., 2009). However, Cx3cr1 knockout mice exhibit reduced inflammatory pain following intraplantar zymosan injection (Staniland et al., 2010a). Both intraplantar or intra-articular injections of CFA induce persistent inflammatory pain, but microgliosis is more robust after joint CFA inoculation, suggesting that deep tissue injury is more effective in evoking microglial changes (Ji et al., 2013; Sun et al., 2007). Spinal microgliosis is also evident after collagen-induced poly-arthritis (Nieto et al., 2015). Upregulation and activation of microglial CX3CR1 is essential for arthritic pain (Sun et al., 2007).
Cancer Pain
In rodent models of cancer pain, there is a large variation in spinal microglial reaction, likely due to differences in species/strains and origins of cancer cells. In a rat model of bone cancer pain induced by inoculation of breast cancer cells (Walker 256) into the tibia, there is marked microgliosis in the spinal cord and inhibition of microglia suppresses the bone cancer-induced pain (Yang et al., 2015). Notably, spinal microglia maintain the advanced-phase of bone cancer pain, where a delayed but persistent spinal microglial activation occurs 14 to 21 days after tumor inoculation. Spinal inhibition of microglial signaling by minocycline and inhibitors of P2X7 and p38 effectively reduced established allodynia and hyperalgesia on day 14, whereas pretreatment with minocycline did not block the development of bone cancer pain. Moreover, the P2X7R/p38/IL-18 pathway was shown to maintain bone cancer pain (Yang et al., 2015). In addition, P2X4, P2Y12, and TLR4 also play an activate role in microglial activation and bone cancer pain (Liu et al., 2017; Mao-Ying et al., 2012; Meng et al., 2017). Microglia produce proinflammatory cytokines such as TNF, IL-1b, and IL-18 to elicit central sensitization and facilitate cancer pain (Liu et al., 2017; Mao-Ying et al., 2012; Yang et al., 2015). Strong microgliosis is also found in the trigeminal spinal subnucleus caudalis following inoculation of squamous cell carcinoma cells into the tongue of rats, and this oral cancer pain condition requires P2Y12R (Tamagawa et al., 2016). Microgliosis is less remarkable in murine models of bone cancer (Hald et al., 2009; Honore et al., 2000). Bone cancer pain is not affected in mice by P2×7 deletion or P2X7 antagonists (Hansen et al., 2011). However, follow-up studies from different groups demonstrated moderate microgliosis in the spinal cord using the same bone cancer model (Pevida et al., 2014; Vit et al., 2006). Therefore, microglia may play different roles in different rodent models of cancer pain.
Neuropathic pain after spinal cord injury (SCI)
Compared with nerve injury, the temporal and spatial activation of microglia caused by SCI is much more rapid and extensive. Temporally, the expression of CD11b robustly increases from 2 hours to 180 days post-injury. Spatially, persistent microglial reaction manifests in the entire spinal axis from cervical to lumbar regions (Gwak et al., 2012), as well as supraspinal areas such as the posterolateral nucleus of thalamus, the hippocampus and the anterior cingulate cortex (Galan-Arriero et al., 2014). Intrathecal minocycline not only reduced microgliosis and p38 activation but also suppressed spontaneous and evoked activity in spinal cord neurons and mechanical hypersensitivity in SCI animals (Hains and Waxman, 2006).
Chemotherapy-induced peripheral neuropathy (CIPN)
CIPN is a dose-limiting neurotoxic effect. Although increased spinal microglial activity was reported in rats treated with high-dose of paclitaxel (Peters et al., 2007), other reports failed to show microglial activity increase following the chemotherapy agents paclitaxel (Zheng et al., 2011), oxaliplatin, or bortezomib, despite prominent astrocyte activation in these chemotherapy models (Robinson et al., 2014). Spinal knockdown of p38-a isoform of MAP kinase using potent anti-sense oligodeoxynucleotide reduced mechanical allodynia after nerve injury but not paclitaxel treatment in mice (Luo et al., 2017). Mild microglial activation with TNF and p-p38 upregulation were observed in the spinal cord following vincristine treatment in mice (Shen et al., 2015). Hu et al. demonstrated persistent activation of microglia but not astrocytes in mice for more than 5 weeks following cisplatin-CIPN. Intrathecal injection with minocycline not only alleviated mechanical allodynia but also prevented sensory deficits and loss of epidermal nerve fibers after cisplatin treatment. In spinal microglia, cisplatin induced neuroinflammation via triggering receptor expressed on myeloid cells 2 (TREM2), and functional blockade of TREM2 signaling inhibited CIPN (Hu et al., 2018). Thus, the contribution of microglia to CIPN also depends on chemotherapeutic agents and doses.
Opioid-Induced Tolerance and Hyperalgesia
Long-term administration of opioids not only increases risk of abuse and addiction, but also produces antinociceptive tolerance and paradoxical pain (hyperalgesia) (Ferrini et al., 2013; Mao et al., 1995). Selective ablation of spinal microglia prevented the development but not maintenance of morphine tolerance (Leduc-Pessah et al., 2017). Chronic morphine exposure augmented the expression of P2X4 and P-p38 in spinal microglia, and furthermore, morphine tolerance is abrogated by spinal inhibition of p38 (Cui et al., 2006) or antisense knockdown of P2X4R (Horvath et al., 2010). Differently, Ferrini et al. found that pharmacological blockade or knock-out of P2X4R reversed chronic morphine-induced hyperalgesia, but did not affect morphine analgesic tolerance (Ferrini et al., 2013). Blockade or knockdown of spinal P2X7R attenuated morphine tolerance, microglial activation, and p-p38 and IL-18 levels (Chen et al., 2012; Zhou et al., 2010). Leduc-Pessah et al. revealed a key site (Y382–384) at P2X7R that can be targeted to reduce the development of morphine tolerance (Leduc-Pessah et al., 2017). Pharmacological blockade of TLR4 signaling attenuated the development of analgesic tolerance, hyperalgesia, and opioid withdrawal behavior in rats. Morphine may bind directly to and activate TLR4 by molecular modeling and in vitro cellular assays (Hutchinson et al., 2010; Watkins et al., 2009). However, mice lacking functional TLR4 still developed hyperalgesia with morphine treatment, suggesting that TLR4 is not required for opioid-induced analgesic tolerance or hyperalgesia (Ferrini et al., 2013; Fukagawa et al., 2013). Moreover, chronic morphine treatment prolongs the duration of nerve injury-induced neuropathic pain, and this prolongation requires the activation of NLRP3 (NOD-like receptor protein 3) inflammasome in spinal microglia (Grace et al., 2016). Panx1 hemichannels regulate ATP release from microglia, which is required for morphine withdrawal without affecting opiate analgesia (Burma et al., 2017). Although chronic exposure causes direct activation of microglia, it is still under debate whether microglia express functional opioid receptors. Notably, loss of mu opioid receptor signaling in nociceptors, but not microglia, abrogates morphine tolerance (Corder et al., 2017).
Microglial Mediators Regulate Synaptic Plasticity and Central Sensitization in Spinal Cord Pain Circuits
Spinal Cord Pain Circuits
One of the most important questions regarding microglial control of pain is how microglial mediators regulate synaptic transmission in the pain circuits. In the past 5 years, great progress has been made in dissecting the spinal cord circuits of pain (Braz et al., 2014; Duan et al., 2018; Peirs and Seal, 2016). While earlier studies focused on lamina I projection neurons due to its critical contribution to hyperalgesia (Mantyh et al., 1997; Todd, 2010), recent progress points to important contribution of interneurons, especially lamina II neurons to pain processing. For example, somatostatin-expressing (SOM+) interneurons are essential for mediating mechanical pain (Duan et al., 2014). Transcriptional profiling of SOM+ neurons also reveals distinct subpopulations (Chamessian et al., 2018). These interneurons form a spinal pain circuit by connecting to TRPV1-expressing C-fibers and lamina-I projection neurons (Figure 3A) (Braz et al., 2014; Todd, 2010) and also exhibit robust neural plasticity after nerve injury (Xu et al., 2013b).
Figure-3:
Schematic illustration of microglial regulations of acute and persistent pain in spinal cord pain circuit via multiple mechanisms, including presynaptic mechanisms and post-synaptic mechanisms and regulations of both excitatory and inhibitory synaptic transmission.
Microglia Drive Central Sensitization
Chronic pain is driven by central sensitization, a phenomenon of synaptic plasticity, and increased neuronal responsiveness in central pain circuits after painful insults (Latremoliere and Woolf, 2009). Activation of NMDA receptor (NMDAR), phosphorylation of ERK (pERK), and loss of inhibition are key events that drive central sensitization in spinal cord dorsal horn neurons and pain hypersensitivity after injury (Ji et al., 2003) (Figure 3C–D). Increasing evidence suggests that microglia drive central sensitization via microglial mediators. Microglia are a major source of cytokines including TNF and IL-1β (Hanisch, 2002).
Single-cell PCR analysis reveals that in the spinal cord dorsal horn Tnf mRNA is synthesized by microglia and some astrocytes but not neurons (Berta et al., 2014). TNF is a powerful neuromodulator of synaptic transmission in the spinal cord pain circuit. Notably, TNF acts on synapses at low nM concentrations, in contrast to μM concentrations of classic neurotransmitters such as glutamate (Kawasaki et al., 2008b; Park et al., 2011). Perfusion of TNF very rapidly (i.e. within a minute) increases spontaneous excitatory postsynaptic current (sEPSC) frequency in lamina II neurons via activation of TRPV1 in pre-synaptic terminals, an effect that is abolished in Trpv1 knockout mice (Park et al., 2011). Although TNFR1 is the predominant receptor mediating TNF’s effects in glial cells and neurons, TNFR2 also contributes to TNF-induced central sensitization (Zhang et al., 2011). TNF potentiates the function of NMDA receptors (NMDARs) in lamina II neurons through pERK (Xu et al., 2010). TNF also causes delayed but sustained increase in AMPAR and NMDAR mediated and evoked currents in spinal lamina I neurons (Gruber-Schoffnegger et al., 2013).
In spinal slices, microglia release IL-1β via the activation of TLR4 and P2X7 receptors and p38 (Clark et al., 2010). In dorsal horn neurons, IL-1β increases the phosphorylation of NMDAR and ERK and enhances NMDA currents (Kawasaki et al., 2008b; Zhang et al., 2008). After nerve injury IL-1β enhances the function of presynaptic NMDARs, leading to increased glutamate release and excitatory synaptic transmission (Yan and Weng, 2013).
PGE2, a prominent inflammatory mediator could also be produced from spinal cord microglia following the activation of p38 and plays an active role in central sensitization (Kohno et al., 2008; Svensson et al., 2003b). In addition to direct actions on neurons, cytokines (e.g., TNF and IL-1β) can also act on glial cells and show slow actions in lamina I neurons via secretion of PGE2 (Gruber-Schoffnegger et al., 2013).
Disinhibition as a Common Feature of Microglial Mediators
Disinhibition— the reduction or loss of inhibitory synaptic transmission in the SDH pain circuit— has been strongly implicated in central sensitization (Latremoliere and Woolf, 2009). Disinhibition often occurs after nerve injury when GABA synthesis or potassium/chloride transporter (KCC2) expression are down-regulated (Coull et al., 2003; Moore et al., 2002). Disinhibition in dorsal horn neurons also occurs in acute pain conditions, such as capsaicin-induced hyperalgesia/allodynia, and spinal injection of glycine or GABA receptor antagonists is sufficient to induce rapid allodynia in naïve animals (Lu et al., 2013). Notably, microglial mediators such as cytokines, PGE2, and BDNF modulate inhibitory synaptic transmission in spinal dorsal horn neurons via pre-, post-, and extra-synaptic mechanisms (Ji et al., 2013) (Figure 3D).
Proinflammatory cytokines powerfully modulate inhibitory synaptic transmission at multiple sites in the spinal dorsal horn (Ji et al., 2013). At the pre-synaptic level, IL-1β and IL-6 inhibit the frequency of spontaneous inhibitory postsynaptic currents (sIPSCs) in spinal pain circuit. At post-synaptic sites, IL-1β and IL-6 reduce the sIPSC amplitude. At extra-synaptic sites, GABA and glycine receptor activity (revealed by GABA and glycine induced currents) can be suppressed by IL-1β (Kawasaki et al., 2008b; Yan et al., 2015). Further, TNF rapidly inhibited spontaneous action potentials in GABAergic neurons (Zhang et al., 2010).
Nerve injury releases ATP to activate P2X4R on spinal microglia, causing BDNF release. BDNF acts on TrkB in lamina I neurons and then increases the level of intracellular Cl− by downregulating the neuronal potassium-chloride transporter, KCC2. This change leads to potentiation of synaptic GluN2B-NMDAR currents by Fyn kinase and action potential firing in lamina I neurons (Coull et al., 2005; Hildebrand et al., 2016), resulting in neuropathic pain. However, it is difficult to detect BDNF in spinal microglial cells, although it can be easily labeled in DRG neurons and primary afferents in the spinal cord. Nociceptor-derived BDNF regulates acute and inflammatory but not neuropathic pain (Zhao et al., 2006). Sensory neuron-derived BDNF also contributes to the transition from acute to chronic neuropathic pain (Sikandar et al., 2018). Thus, the cellular locations are still under the investigation.
In addition, PGE2 inhibits glycinergic neurotransmission in spinal cord neurons via post-synaptic GlyR3 and the cAMP/PKA pathway (Harvey et al., 2004). A recent report shows that after inflammation, microglia causes displacement or retraction of inhibitory synapses in the dorsal horn (Kambrun et al., 2018). It will be interesting to learn if this retraction of inhibitory synapses is mediated by pro-inflammatory cytokines.
Regulation of Spinal Cord Long-Term Potentiation (sLTP)
Spinal cord long-term potentiation (sLTP) is an important form of spinal cord synaptic plasticity that contributes to central sensitization and pathological pain (Luo et al., 2014; Sandkuhler, 2000, 2009). Activation of spinal microglia is both sufficient and required for the induction sLTP at C-fiber synapses with spinal lamina I neurons. TNF and IL-1β are individually sufficient and necessary for sLTP induction via redundant pathways in these neurons (Clark et al., 2015; Gruber-Schoffnegger et al., 2013). Spinal TNF modulates sLTP through both TNFR1 and TNFR2 (Liu et al., 2007; Park et al., 2011). Interestingly, caspase-6 contributes to the induction and maintenance of sLTP by triggering TNF release from microglia (Berta et al., 2014). IL-1β triggers sLTP not only in excitatory synapses (Gruber-Schoffnegger et al., 2013) but also in glycinergic synapses on GABAergic neurons in spinal cord slices, as another example of disinhibition (Chirila et al., 2014). Activation of spinal microglial CX3CR1 via CX3CL1/fractalkine is sufficient to elicit sLTP. Specifically, the activation of the CX3CR1 receptor by fractalkine induces the release of IL-1β from microglia, which modulates NMDA signaling in postsynaptic neurons, leading to the release of an eicosanoid messenger, that ultimately enhances presynaptic neurotransmitter release. In contrast to the conventional view, this form of plasticity does not require enhanced neuronal activity to trigger the events leading to synaptic facilitation in lamina I neurons (Clark et al., 2015).
Recently, Sandkuhler and coworkers proposed “gliogenic LTP” that can spread widely in nociceptive pathways (Kronschlager et al., 2016). A fundamental feature of LTP induction in the brain is the requirement for coincident pre- and post-synaptic activity, which is important to restrict LTP expression to activated synapses only (homosynaptic LTP) as well as to define the input specificity of LTP. Gliogenic sLTP can travel long distances via CSF, because this LTP can be induced by glial activation and diffusible messengers, such as D-serine and TNF. Remarkably, transfer of spinal CSF from a donor animal displaying LTP is able to induce LTP in a naïve recipient animal (Kronschlager et al., 2016). Therefore, this diffusible sLTP can affect susceptible synapses at remote sites. Collectively, gliogenic LTP, as well as other forms of diffusible and glial-mediated central sensitization may underlie widespread pain in chronic pain patients, especially in those with overlapping chronic pain conditions (Ji et al., 2018).
Sex Dimorphism in Microglial Signaling in Pain
Clinical pain syndromes such as chronic orofacial pain or temporomandibular disorders occur more frequently in women compared to men. Although 70% of pain clinic patients are women, the majority of preclinical studies have been conducted in male rodents (Mogil, 2012). It is noteworthy that the issue of “Sex As a Biological Variable (SABV)” must be addressed in grant applications to the National Institutes of Health. Despite the estrous cycle in female rodents, females do not show fluctuations of basal mechanical and thermal pain sensitivity when tested at different times during the cycles. Recent evidence shows sex dimorphism in pain processing by immune cells especially microglial cells. Spinal TLR4 regulates inflammatory pain and neuropathic pain in male but not female mice (Sorge et al., 2011), highlighting possible male-dominant microglial signaling in SDH. Furthermore, two studies found equivalent microgliosis in the spinal dorsal horn of male and female mice in response to peripheral nerve injury, accompanied by similar levels of allodynia (Sorge et al., 2015; Taves et al., 2016). However, elimination of spinal microglia with a toxin or spinal inhibition of microglial function with minocycline, P2X4 blocker, or microglial BDNF signaling inhibits mechanical allodynia only in male but not female mice. Also, nerve injury upregulates P2X4 expression in the spinal cord of male mice (Sorge et al., 2015). Interestingly, this male-specific microglial response depends on testosterone, as minocycline did not inhibit allodynia in castrated males but reduced allodynia in testosterone-treated females. It appears that female rodents switch from microglial signaling to T-cell signaling in neuropathic pain and have more T-cells and exhibit increased T-cell marker expression after injury (Sorge et al., 2015).
We also compared nerve injury-induced microglial proliferation and microglial activation markers in both sexes. CCI induced comparable microgliosis and expression of CX3CR1 and IBA-1 in both sexes. However, nerve injury induced p-p38 in spinal microglia predominantly in male mice (Taves et al., 2016). Therefore, only some changes in microglia such as p-p38 but not IBA-1 are sex-dependent. CCI induced comparable synaptic plasticity, i.e. increases in EPSCs in SDH neurons in both sexes. Strikingly, skepinone, a selective p38 inhibitor, not only attenuated CCI-induced mechanical allodynia exclusively in males, but also suppressed CCI-induced synaptic plasticity in males (Taves et al., 2016). Consistently, spinal inhibition of P2XR or p38 signaling was shown to disrupt hyperalgesic priming, a persistent pain condition induced by IL-6 and subsequent challenge with PGE2, in male but not female mice (Paige et al., 2018). Therefore, sex-specific p38 activation and signaling appears to be confined to the spinal cord in inflammatory and neuropathic pain conditions. Notably, delayed microglial activation also contributes to bone cancer pain in female rats inoculated with breast cancer cells (Yang et al., 2015). Thus, sex-dimorphism in microglial regulation of pain depends on pain models and injury conditions (Table 2). Notably, sex dimorphism in microglial signaling was not noticed in mice lacking Cx3cr1 or Tmem16f or after microglia deletion (Batti et al., 2016; Peng et al., 2016; Staniland et al., 2010a), but sex difference was not the focus of these studies the data of both sexes are often combined. Future rigorous studies are necessary to determine the pain conditions and microglial pathways that are sex-dependent.
Table-2.
Sex dimorphism in spinal microglial signaling in pathological pain after peripheral nerve injury (PNI), hind paw injection of formalin and complete Freund’s adjuvant (CFA), and bone cancer. N.T., not tested.
| Sex differences | Male | Female | References | |
|---|---|---|---|---|
| Microgliosis | ||||
| Proliferation | No (PNI) | Yes | Yes | Sorge et al., 2015; Taves et al., 2016 |
| Morphological changes | No (PNI) | Yes | Yes | |
| Iba1/CX3CR1 expression | No (PNI) | Yes | Yes | |
| TMEM16F | No (PNI) | Yes | Yes | Batti et al., 2016 |
|
Microglial ablation (Mac-1-SAP) |
Yes (PNI) | Yes | No | Sorge et al., 2015 |
| Microglia activation & signaling | ||||
| Minocycline (intrathecal) | Yes (PNI, Formalin) | Yes | No | Sorge et al., 2015; Chen et al., 2018 |
| Yes (Bone cancer, SCI) | N.T. | Yes | Chen et al., 2012; Yang et al., 2015 | |
| TLR4 inhibitor | Yes (PNI, CFA) | Yes | No | Sorge et al., 2011 |
| P-p38 expression & p38 inhibitor | Yes (PNI) | Yes | No | Taves et al., 2016 |
| Yes (hyperalgesic priming) | Yes | No | Paige et al., 2018 | |
| Yes (Bone cancer) | N.T. | Yes | Yang et al., 2015 | |
| p38-a knockdown | Yes (PNI) | Yes | No | Luo et al., 2017 |
| Caspase-6 inhibitor | Yes (PNI) | Yes | No | Berta et al., 2017; Chen et al., 2018 |
| P2X4/BDNF expression & inhibitor | Yes (PNI) | Yes | No | Sorge et al., 2015 |
Villa et al. described significant differences in the transcriptome of adult male and female microglia due to perinatal exposure to sex steroids. Strikingly, microglia isolated from adult brains maintain the sex-specific features when put in culture or transplanted in the brain of the opposite sex. Furthermore, female microglia are neuroprotective by restricting focal cerebral ischemia damage (Villa et al., 2018). It is of great interest to assess whether female microglia are also protective against the development of chronic pain. The microbiome also influences prenatal and adult microglia in a sex-specific manner. Antibiotic treatment of adult mice triggered sexually biased microglial responses, revealing both acute and long-term effects of microbiota depletion (Thion et al., 2018).
It is noteworthy that astrocytes also play an important role in the pathogenesis of chronic pain (Ji et al., 2013). Persistent upregulation of astrocytic connexin-43 (Cx43) sustains mechanical allodynia after nerve injury via chemokine release (e.g., CXCL1) (Chen et al., 2014). In contrast to sex dimorphism in microglial signaling, astroglial signaling exhibits no apparent sex-differences, and intrathecal injection of Cx43 blocker and astroglial toxin effectively reduced FSP and mechanical allodynia in both sexes (Chen et al., 2018).
Protective Role of Microglia in Resolution of Neuroinflammation and Pain
Protection of Neuroinflammation in Neurological Diseases
Despite a critical role of microglia in establishing neuroinflammation and pathogenesis of neurological and neuropsychiatric diseases (Ji et al., 2014), these long-lived cells also contribute to homeostasis of the brain and spinal cord and resolution of neuroinflammation (Shemer et al., 2015). For example, microglia-related genes have been implicated in neuropsychiatric or neurological disorders, such as frontotemporal dementia (TREM2), Alzheimer’s disease (CD33), and hereditary diffuse leukencephalopathy (CSF1R) (Erny et al., 2015). In particular, mice lacking Cx3cr1 exhibited greater cognitive dysfunction and increased neuronal death in the chronic phase of traumatic brain injury compared to wild-type mice, despite the detrimental role of microglia in the acute phase where microglial activity correlated with motor deficits and cell death. These distinct behavioral phenotypes in the acute versus chronic phase of brain injury in Cx3cr1–deficient mice may be associated with different microglial phenotypes of these phases (Febinger et al., 2015).
Recent progress has demonstrated that microglial behavior can also be controlled remotely by the gut, as the microbiome can influence both prenatal and adult microglia (Thion et al., 2018). Erny et al (Erny et al., 2015) demonstrated substantial contributions of the host microbiota to microglia homeostasis, as germ-free mice exhibited global defects in microglia and impaired innate immune responses. Eradication of host microbiota altered microglia properties profoundly, and furthermore, the microglial defects could be restored partially by recolonization with a complex microbiota. Intriguingly, bacterial fermentation products such as short-chain fatty acids, which include acetic acid, propionic acid, and butyric acid, are vital for immune cell homeostasis in the colon. They may also regulate microglia homeostasis via short-chain fatty acid receptor FFAR2 (GPR43), which is expressed on splenic Iba1+ myeloid cells but not in microglial cells (Erny et al., 2015).
Rothhammer et al. (Rothhammer et al., 2018) reported an unexpected immunoregulatory role for a ligand-activated transcription factor, the aryl hydrocarbon receptor (AHR), activated by gut-derived metabolites such as tryptophan. Elimination of microglial AHR substantially exacerbated EAE (experimental autoimmune encephalomyelitis), a mouse model of multiple sclerosis, but left immune responses outside the CNS unaltered. This finding suggests that AHR activation in microglia inhibits inflammation in the CNS. When activated in microglia, AHR serves as a transcription factor to regulate the expression of VEGF-B (down-regulation) and TGF-α (up-regulation). While VEGF-B released from microglia enhances the responsiveness of astrocytes, TGF-α dampens astrocyte responsiveness. Thus, AHR-mediated distinct regulation of VEGF-B and TGF in microglia results in the suppression of astrogliosis and neuroinflammation (Wekerle, 2018) (Figure 4).
Figure 4: Schematic for microglial regulation of the resolution of neuroinflammation.
(A) Biosynthesis of SPM families of lipoxins, resolvins, protectins/neuroprotectins, and maresins from DHA and EPA. (B) SPMs receptors. ChemR23 (RvE1 receptor), GPR32 (RvD1 receptor), and GPR18 (RvD2 receptor) are denoted ERV, DRV1, and DRV2, respectively, as with the system of nomenclature for eicosanoid receptors). (C) Microglial signaling via SPM receptors, CB2, and aryl hydrocarbon receptor (AHR), in response to their ligands such as SPMs, cannabinoid, and gut-derived metabolites such as tryptophan (TRP). Activation of these microglial receptors enhances phagocytic activity, reduces the expression of pro-inflammatory mediators (e.g., TNF, IL-1β, and VEGF-B), and increases the production of anti-inflammatory mediators (e.g., IL-10, TGF-α, TGF-β) and SPMs, thus promoting the resolution of neuroinflammation and pathological pain.
Cannabinoid receptor type 2 (CB2) activation induces an anti-inflammatory phenotype of microglia via inducing the expression of MAP kinase phosphatase and subsequent suppression and MAP kinase phosphorylation, as an essential step for the resolution of neuropathic pain (Romero-Sandoval et al., 2009). G protein-coupled receptor kinase 2 (GRK2) is also a regulator of pain resolution, and loss of GRK2 in microglia results in a transition from acute to chronic inflammatory pain (Willemen et al., 2010). Despite recent progress in revealing microglia’s protective actions in neurological disorders, their roles in resolving acute and chronic pain remain to be fully appreciated. Notably, in early life during the first several weeks, microgliosis and neuropathic pain after nerve injury are masked by the anti-inflammatory cytokine IL-10, presumably produced by microglia (McKelvey et al., 2015; Moss et al., 2007).
Specialized Pro-resolving Mediators (SPMs) in Pain Resolution
Using lipid mediator metabololipidomics, proteomics (liquid chromatography-tandem mass spectrometry [LC-MS-MS]), and cell trafficking in self-limited inflammatory exudates, the Serhan lab identified three new families of potent and pro-resolution mediators (Bannenberg et al., 2005; Hong et al., 2003; Serhan et al., 2000b; Serhan et al., 2002) (Figure 4A) coined resolvins (resolution-phase interaction products), protectins/neuroprotectin, and maresins (macrophage mediators in resolving inflammation) (Serhan et al., 2009). Each family is structurally distinct and biosynthesized from separate precursor n-3 fatty acid (FA) substrates, EPA (eicosapentaenoic acid), docosapentaenoic acid (n-3DPA), and DHA (docosahexaenoic acid) (Serhan et al., 2008). Notably, aspirin triggers their biosynthesis, and both acetaminophen and indomethacin also permit some 18-HEPE and 15-HEPE biosynthesis in contrast to the cyclooxygenase-2 (COX-2) inhibitors, which substantially reduce their formation (Serhan et al., 2000a). These findings give a new mechanism for aspirin’s many well-appreciated benefits namely triggering the biosynthesis of endogenous mediators that help to terminate acute inflammation (Gilroy, 2005). In addition, lipoxins biosynthesized from arachidonic acid stop neutrophil infiltration and promote resolution as well as reduce inflammatory and neuropathic pain (Martini et al., 2016; Serhan, 2014; Svensson et al., 2007). Because each member of these families possesses potent pro-resolving and anti-inflammatory actions (recently reviewed in Serhan, 2014, 2017) with special functions in the resolution phase, this super-family is coined “specialized pro-resolving mediators” (SPMs).
The complete stereochemistry and biosynthesis of each major SPM is established (Figure 4A). These major SPM biosynthesis routes are each confirmed with human leukocytes via matching with synthetic compounds, trapping of proposed transient epoxide-containing intermediates and label tracking of precursors and intermediates (Serhan et al., 2015) and are biosynthesized by microglial cells (Serhan et al., 2002) where they reduce cytokine actions on microglia cells. The D-series resolvins (RvDs) are biosynthesized from DHA and DPA, and the E-series resolvins (RvEs) from EPA precursor (Figure 4A). These precursors are enriched in marine oils and enter humans via nutrition or supplementation. NPD1 produced in neural systems is termed neuroprotectin D1 (NPD1) demonstrating potent protective actions in retina, brain, and pain (Asatryan and Bazan, 2017; Bazan et al., 2010) and is biosynthesized by microglial cells and brain (Hong et al., 2003). When biosynthesized in the immune system, this mediator is denoted PD1, reflecting its system of origin.
The stereoselective and low SPM-doses required to stop ongoing inflammation and promote resolution rely on GPCR receptors that transduce intracellular signals. To date, six separate SPM receptors are identified using library screening, labeled-ligands for specific binding (stereospecific nM Kd) and functional cellular responses (Figure 4B). Several of the resolvin and SPM receptors are present on neurons and glial cells in neural tissues (Ji et al., 2011). SPMs, except for NPD1 (Bang et al., 2018), generally do not stimulate increases in intracellular Ca2+ mobilization in leukocytes for signal transduction but rather activate phosphorylation demonstrated using genetically engineered mice and mass cytometry (Chiang et al., 2017; Norris et al., 2017). Each SPM interacts with specific receptors in the pico-nanomolar range with Kd in the nM range. For example, RvE1 specifically binds to Chemerin Receptor 23 (ChemR23, also known as ERV-1) (Arita et al., 2005) and Leukotriene B4 receptor 1 (BLT1) to evoke pro-resolving responses. RvE1 activation of ChemR23 enhances macrophage phagocytosis via phosphoprotein-mediated signaling (Ohira et al., 2010). The RvE1 receptor ERV/ChemR23 is expressed on DRG neurons and reduces pain signals in part via blocking the function of TRPV1 ion channels (Xu et al., 2010). RvD1 binds and activates human GPR32 and shares human and murine LXA4 receptor (ALX/FPR2) to evoke rapid impedance changes. Transgenic mice overexpressing human ALX-FPR2 require less RvD1 to stop inflammation (Krishnamoorthy et al., 2012), RvD2 specifically binds and activates GPR18 with high affinity, regulating sepsis and bacterial clearance (Chiang et al., 2015; Chiang et al., 2017). NPD1/PD1 shows high affinity specific binding on human leukocytes and retinal epithelial cells with 3H-labeled-NPD1 (Marcheselli et al., 2010) that likely represents specific receptors. Notably, GPR37 is expressed by macrophages and required for mediating NPD1-induced phagocytosis. Unlike other SPMs, NPD1 evokes slow but sustained intracellular Ca2+ mobilization in macrophages via GPR37. Functionally, GPR37 is required for the resolution of inflammatory pain (Bang et al., 2018). Receptors for the remaining SPMs such asMaR1 (Serhan et al., 2012) are currently subject of investigations.
Purified cell bulk analysis reveals that microglia express multiple SPM receptors, such as ChemR23 (RvE1 and RvE2 receptor, ERV1), GPR32 (RvD1 receptor, DRV1), and GPR18 (RvD2 receptor) (Thion et al., 2018). Notably, RvD1 and RvE1 were shown to promote resolution of inflammation in microglial cells in vitro (Rey et al., 2016). ChemR23/ERV1 is also expressed by retinal microglia and plays a role in protecting pathological retina angiogenesis by inhibiting TNF production (Connor et al., 2007). In a mouse model of traumatic brain injury, RvE1 treatment increases post-traumatic sleep and normalized microgliosis in the injured brain (Harrison et al., 2015). RvE1 has also been shown to alleviate neuropathic pain by inhibiting microglial activation in intact spinal cord and TNF release in microglia cultures (Xu et al., 2013a). Future studies are necessary to test whether these actions are mediated by the ChemR23 receptor. RvD1 reduces the activation of microglia and improves functional recovery after focal brain damage via the murine ALX/FPR2 receptor (Bisicchia et al., 2018). Spinal treatment of LXA4 attenuated neuropathic pain and spinal microgliosis and TNF expression after spinal cord injury. Furthermore, microglia express ALX/FPR2 which appears to mediate LXA4-induced inhibition of TNF release from microglia (Martini et al., 2016). Kantarci et al. reported decreased levels of SPMs in the hippocampus of mice with Alzheimer’s disease (AD), but a combined treatment of RvE1 and LXA4 decreased neuroinflammation (microglia and astrocyte activation) associated with Aβ pathology in AD mice (Kantarci et al., 2018).
Emerging Role of Microglia in Chronic Itch
Pain and itch (pruritus) are distinct sensations, leading to respective withdrawal response following noxious stimuli and scratching response following pruritic stimuli. Pain is also known to suppress itch, while analgesics such as morphine evokes itch after spinal delivery (LaMotte et al., 2014). Great progress has been made in elucidating itch’s neuronal mechanisms, including peripheral and central mechanisms of itch (Dong and Dong, 2018; Sun and Chen, 2007). Chronic itch is associated with benign skin lesions or cancers such as lymphoma (Han et al., 2018; Yosipovitch and Bernhard, 2013). Distinct mechanisms have been implicated in acute itch and chronic itch(Liu and Ji, 2013). Increasing evidence suggests a role for spinal microglia in the pathogenesis of chronic itch. For example, inhibition of spinal microglial signaling with minocycline and TLR4 antagonist reduced dry skin-evoked chronic itch, without affecting acute itch (Liu et al., 2016). Spinal inhibition of TNF also reduced chronic pruritus (Miao et al., 2018). These studies are still preliminary, and further investigation of microglial signaling in chronic itch in both sexes is necessary.
Clinical Relevance and Future Directions
The clinical relevance of microglial pathophysiology has been challenging to establish, despite the plethora of animal research. The earliest direct indication of microglial activity in human pain states appears to be immunohistochemical data where post-mortem spinal cord from patients with herpetic neuralgia was noted to have microgliosis in the dorsal horns that mapped to the dermatomal region of the rash (Denny-Brown, 1944). Loggia et al were able to verify thalamic glial activation occurred in chronic low back pain patients using positron emission tomography (PET) imaging (Loggia et al., 2015). However, the clinical translation of available microglial modulatory agents has not had robust or consistent results in trials (Curtin et al., 2017; Martinez et al., 2013; Sumitani et al., 2016; Sumracki et al., 2012; Vanelderen et al., 2015; Younger and Mackey, 2009). A pilot study revealed that fibromyalgia symptoms are reduced by low-dose naltrexone (Younger and Mackey, 2009), as low-dose naltrexone was shown to inhibit microglial activation similar to TLR4 inhibitor (Wang et al., 2016). A small trial revealed that minocycline does not decrease intensity of neuropathic pain intensity, but does improve its affective dimension (Sumitani et al., 2016). In a recent clinical study, Curtin tested whether perioperative administration of minocycline would improve pain resolution after standardized hand surgeries in patients with carpal tunnel syndrome, a neuropathic pain condition. Oral administration of minocycline did not reduce time of pain resolution after minor hand surgery. In contrast, there was evidence that minocycline might increase length of pain in those with increased posttraumatic stress disorder symptoms (Curtin et al., 2017), suggesting a possible a role of microglia in promoting pain resolution. In a randomized, double-blind, controlled study, Martinez et al. randomly assigned 100 patients undergoing scheduled lumbar discectomy to placebo and minocycline groups (100 mg orally, twice daily, beginning before surgery and continuing for 8 days). The results showed that perioperative minocycline fails to improve persistent pain after lumbar discectomy. However, exploratory analysis suggested that minocycline might be effective in a subgroup of patients with predominantly deep spontaneous pain at baseline (Martinez et al., 2013). In another randomized, double-blind, placebo-controlled clinical trial, patients with subacute lumbar radicular pain received placebo, amitriptyline 25 mg (which was shown to inhibit spinal p38 activation and neuroinflammation in animal model (Tai et al., 2009), or minocycline (100 mg once a day, n = 20 per group) for 14 days. Minocycline produced no side effects, whereas amitriptyline produced side effects in 10% of patients. Both treatment groups significantly differed from placebo group, but their effect size in pain reduction was small (Vanelderen et al., 2015). There could be several reasons underlying the mixed results and lack of efficacy. First, there are no biomarkers to demonstrate that the treatments i) engage microglia in the spinal cord and brain and ii) alter microglial activity and microglia-mediated neuroinflammation. Second, there could be non-specific (non-microglial) effects of the agents studied, such as minocycline or naltrexone which have pleotropic effects including on metalloproteases and opioid receptors respectively(Ji et al., 2009). Third, there is lack of full understanding of sex dimorphism in microglial pathophysiology in human trials (Berta et al., 2016; Sorge et al., 2015). Fourth, there is lack of appreciation of distinct role of microglia in early vs. late phase clinical pain and a protective role of microglia in the resolution phase (Ji et al., 2009). The lack of robust or consistent effects of microglial modulators clinically may also be due to the study design and heterogeneity of the patient populations, or due to underlying biology. It is noted for example, in postmortem spinal cord tissues from patients with HIV neuropathy, that microgliosis is less obvious than astrogliosis, suggesting perhaps astroglial signaling may be more critical for chronic human pain (Shi et al., 2012).
Microglial biology has been somewhat hampered by the tools and techniques we have to study these powerful but mutable cells. Unlike many of the glial or neuronal cells in the CNS, microglia can rapidly proliferate and change in response to stimuli, while still being maintained for years to decades. New and specific antibodies to identify microglia specifically rather than peripheral macrophages, in combination with new microscopy and tissue preparation methods, should allow for visualizing microglia in vivo or in vitro without disturbing their interactions with surrounding cells and provide better insights into the microglial regulatory framework in pain regulation. With the paucity of specific modulators for microglia, chemogenetic techniques such as DREADDs will also allow for better insights into the role of microglia in various pain states (Grace et al., 2016; Grace et al., 2018). Most exciting, however, is the promise of single-cell based assays that would allow for evaluation of the unique transcriptomes or proteomes of microglia in various physiologic and pathologic states (Ajami et al., 2018; Mathys et al., 2017; Mrdjen et al., 2018). The appreciation that microglia are not a monolithic homogenous cell type, but rather a spectrum of actively changing cells with unique transcriptional and proteomic patterns, should allow us to better understand their role in different pain states (De Biase et al., 2017; Dukhinova et al., 2018; Furube et al., 2018). Application of techniques such as mass cytometry or single-cell sequencing in various models of pain, at various time points, as well as on the resident microglia in pain circuits should help us develop deeper understandings into their spatiotemporal activation parameters.
Although direct targeting of microglial activation and neuroinflammation via inhibitors of microglia, TLRs, ATP receptors, cytokines, and MAP kinases could be effective, these drugs may also produce side effects such as infection and impair the resolution of inflammation, especially after long-term treatment. We should also consider pro-resolution strategies that can control abnormal microglial activation and excessive neuroinflammation as well as promote a return to homeostasis, such as pharmacological approaches (e.g., SPMs and CB2 agonists) (Fig. 4), cell therapies (e.g., bone marrow stem cells), and neuromodulation (e.g., spinal cord stimulation)(Chen et al., 2015; Ji et al., 2018; Sato et al., 2014).
Acknowledgements
This study is supported in part by The National Key Research and Development Program of China (2017YFA0104704) to G.C, China Science Fund (31420103903) to Y.Q.Z and NIH grants R01 NS87988, DE17794, and DE22743 to R.R.J. Y.J.Q is supported by NIH grant T32 GM08600 and the Duke Health Scholars fund. CNS is supported by NIH grant P01GM095467.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbadie C, Lindia JA, Cumiskey AM, Peterson LB, Mudgett JS, Bayne EK, DeMartino JA, MacIntyre DE, and Forrest MJ (2003). Impaired neuropathic pain responses in mice lacking the chemokine receptor CCR2. Proc Natl Acad Sci U S A 100, 7947–7952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajami B, Samusik N, Wieghofer P, Ho PP, Crotti A, Bjornson Z, Prinz M, Fantl WJ, Nolan GP, and Steinman L (2018). Single-cell mass cytometry reveals distinct populations of brain myeloid cells in mouse neuroinflammation and neurodegeneration models. Nat Neurosci 21, 541–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arita M, Bianchini F, Aliberti J, Sher A, Chiang N, Hong S, Yang R, Petasis NA, and Serhan CN (2005). Stereochemical assignment, anti-inflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J Exp Med 201, 713–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asatryan A, and Bazan NG (2017). Molecular mechanisms of signaling via the docosanoid neuroprotectin D1 for cellular homeostasis and neuroprotection. J Biol Chem 292, 12390–12397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang S, Xie YK, Zhang ZJ, Wang Z, Xu ZZ, and Ji RR (2018). GPR37 regulates macrophage phagocytosis and resolution of inflammatory pain. J Clin Invest 128, 3568–3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannenberg GL, Chiang N, Ariel A, Arita M, Tjonahen E, Gotlinger KH, Hong S, and Serhan CN (2005). Molecular circuits of resolution: Formation and actions of resolvins and protectins. J Immunol 174, 4345–4355. [DOI] [PubMed] [Google Scholar]
- Batti L, Sundukova M, Murana E, Pimpinella S, De Castro Reis F, Pagani F, Wang H, Pellegrino E, Perlas E, Di Angelantonio S, et al. (2016). TMEM16F Regulates Spinal Microglial Function in Neuropathic Pain States. Cell reports 15, 2608–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazan NG, Calandria JM, and Serhan CN (2010). Rescue and repair during photoreceptor cell renewal mediated by docosahexaenoic acid-derived neuroprotectin D1. J Lipid Res 51, 2018–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beggs S, and Salter MW (2007). Stereological and somatotopic analysis of the spinal microglial response to peripheral nerve injury. Brain BehavImmun 21, 624–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berta T, Park CK, Xu ZZ, Xie RG, Liu T, Lu N, Liu YC, and Ji RR (2014). Extracellular caspase-6 drives murine inflammatory pain via microglial TNF-alpha secretion. J ClinInvest 124, 1173–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berta T, Perrin FE, Pertin M, Tonello R, Liu YC, Chamessian A, Kato AC, Ji RR, and Decosterd I (2017). Gene Expression Profiling of Cutaneous Injured and Non-Injured Nociceptors in SNI Animal Model of Neuropathic Pain. Scientific reports 7, 9367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berta T, Qadri YJ, Chen G, and Ji RR (2016). Microglial Signaling in Chronic Pain with a Special Focus on Caspase 6, p38 MAP Kinase, and Sex Dependence. J Dent Res 95, 1124–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biber K, Tsuda M, Tozaki-Saitoh H, Tsukamoto K, Toyomitsu E, Masuda T, Boddeke H, and Inoue K (2011). Neuronal CCL21 up-regulates microglia P2X4 expression and initiates neuropathic pain development. EMBO J 30, 1864–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisicchia E, Sasso V, Catanzaro G, Leuti A, Besharat ZM, Chiacchiarini M, Molinari M, Ferretti E, Viscomi MT, and Chiurchiu V (2018). Resolvin D1 Halts Remote Neuroinflammation and Improves Functional Recovery after Focal Brain Damage Via ALX/FPR2 Receptor-Regulated MicroRNAs. Mol Neurobiol 55, 6894–6905. [DOI] [PubMed] [Google Scholar]
- Boumechache M, Masin M, Edwardson JM, Gorecki DC, and Murrell-Lagnado R (2009). Analysis of assembly and trafficking of native P2X4 and P2X7 receptor complexes in rodent immune cells. J Biol Chem 284, 13446–13454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braz J, Solorzano C, Wang X, and Basbaum AI (2014). Transmitting Pain and Itch Messages: A Contemporary View of the Spinal Cord Circuits that Generate Gate Control. Neuron 82, 522–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burma NE, Bonin RP, Leduc-Pessah H, Baimel C, Cairncross ZF, Mousseau M, Shankara JV, Stemkowski PL, Baimoukhametova D, Bains JS, et al. (2017). Blocking microglial pannexin-1 channels alleviates morphine withdrawal in rodents. Nature medicine 23, 355–360. [DOI] [PubMed] [Google Scholar]
- Calvo M, Zhu N, Grist J, Ma Z, Loeb JA, and Bennett DL (2011). Following nerve injury neuregulin-1 drives microglial proliferation and neuropathic pain via the MEK/ERK pathway. Glia 59, 554–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo M, Zhu N, Tsantoulas C, Ma Z, Grist J, Loeb JA, and Bennett DL (2010). Neuregulin-ErbB signaling promotes microglial proliferation and chemotaxis contributing to microgliosis and pain after peripheral nerve injury. J Neurosci 30, 5437–5450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamessian A, Young M, Qadri Y, Berta T, Ji RR, and Van de Ven T (2018). Transcriptional Profiling of Somatostatin Interneurons in the Spinal Dorsal Horn. Scientific reports 8, 6809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Luo X, Qadri MY, Berta T, and Ji RR (2018). Sex-Dependent Glial Signaling in Pathological Pain: Distinct Roles of Spinal Microglia and Astrocytes. Neuroscience bulletin 34, 98–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Park CK, Xie RG, Berta T, Nedergaard M, and Ji RR (2014). Connexin-43 induces chemokine release from spinal cord astrocytes to maintain late-phase neuropathic pain in mice. Brain 137, 2193–2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Park CK, Xie RG, and Ji RR (2015). Intrathecal bone marrow stromal cells inhibit neuropathic pain via TGF-beta secretion. J ClinInvest 125, 3226–3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ML, Cao H, Chu YX, Cheng LZ, Liang LL, Zhang YQ, and Zhao ZQ (2012). Role of P2X7 Receptor-Mediated IL-18/IL-18R Signaling in Morphine Tolerance: Multiple Glial-Neuronal Dialogues in the Rat Spinal Cord. J Pain 13, 945–958. [DOI] [PubMed] [Google Scholar]
- Chiang N, Dalli J, Colas RA, and Serhan CN (2015). Identification of resolvin D2 receptor mediating resolution of infections and organ protection. J Exp Med 212, 1203–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang N, de la Rosa X, Libreros S, and Serhan CN (2017). Novel Resolvin D2 Receptor Axis in Infectious Inflammation. J Immunol 198, 842–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirila AM, Brown TE, Bishop RA, Bellono NW, Pucci FG, and Kauer JA (2014). Long-term potentiation of glycinergic synapses triggered by interleukin 1beta. Proc Natl Acad Sci U S A 111, 8263–8268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AK, Gruber-Schoffnegger D, Drdla-Schutting R, Gerhold KJ, Malcangio M, and Sandkuhler J (2015). Selective activation of microglia facilitates synaptic strength. J Neurosci 35, 4552–4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AK, and Malcangio M (2012). Microglial signalling mechanisms: Cathepsin S and Fractalkine. Exp Neurol 234, 283–292. [DOI] [PubMed] [Google Scholar]
- Clark AK, Staniland AA, and Malcangio M (2011). Fractalkine/CX3CR1 signalling in chronic pain and inflammation. Curr Pharm Biotechnol 12, 1707–1714. [DOI] [PubMed] [Google Scholar]
- Clark AK, Staniland AA, Marchand F, Kaan TK, McMahon SB, and Malcangio M (2010). P2X7-dependent release of interleukin-1beta and nociception in the spinal cord following lipopolysaccharide. J Neurosci 30, 573–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AK, Yip PK, Grist J, Gentry C, Staniland AA, Marchand F, Dehvari M, Wotherspoon G, Winter J, Ullah J, et al. (2007). Inhibition of spinal microglial cathepsin S for the reversal of neuropathic pain. Proc Natl Acad Sci U S A 104, 10655–10660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AK, Yip PK, and Malcangio M (2009). The liberation of fractalkine in the dorsal horn requires microglial cathepsin S. J Neurosci 29, 6945–6954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor KM, SanGiovanni JP, Lofqvist C, Aderman CM, Chen J, Higuchi A, Hong S, Pravda EA, Majchrzak S, Carper D, et al. (2007). Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat Med 13, 868–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corder G, Tawfik VL, Wang D, Sypek EI, Low SA, Dickinson JR, Sotoudeh C, Clark JD, Barres BA, Bohlen CJ, et al. (2017). Loss of mu opioid receptor signaling in nociceptors, but not microglia, abrogates morphine tolerance without disrupting analgesia. Nature Med 23, 164–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, Gravel C, Salter MW, and De Koninck Y (2005). BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature 438, 1017–1021. [DOI] [PubMed] [Google Scholar]
- Coull JA, Boudreau D, Bachand K, Prescott SA, Nault F, Sik A, De Koninck P, and De Koninck Y (2003). Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature 424, 938–942. [DOI] [PubMed] [Google Scholar]
- Crotti A, and Ransohoff RM (2016). Microglial Physiology and Pathophysiology: Insights from Genome-wide Transcriptional Profiling. Immunity 44, 505–515. [DOI] [PubMed] [Google Scholar]
- Cui Y, Chen Y, Zhi JL, Guo RX, Feng JQ, and Chen PX (2006). Activation of p38 mitogen-activated protein kinase in spinal microglia mediates morphine antinociceptive tolerance. Brain Res 1069, 235–243. [DOI] [PubMed] [Google Scholar]
- Curtin CM, Kenney D, Suarez P, Hentz VR, Hernandez-Boussard T, Mackey S, and Carroll IR (2017). A Double-Blind Placebo Randomized Controlled Trial of Minocycline to Reduce Pain After Carpal Tunnel and Trigger Finger Release. J Hand Surg Am 42, 166–174. [DOI] [PubMed] [Google Scholar]
- Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, and Gan WB (2005). ATP mediates rapid microglial response to local brain injury in vivo. NatNeurosci 8, 752–758. [DOI] [PubMed] [Google Scholar]
- De Biase LM, Schuebel KE, Fusfeld ZH, Jair K, Hawes IA, Cimbro R, Zhang HY, Liu QR, Shen H, Xi ZX, et al. (2017). Local Cues Establish and Maintain Region-Specific Phenotypes of Basal Ganglia Microglia. Neuron 95, 341–356 e346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny-Brown DD, R.D. A, and Fitzgerald PJ (1944). Pathologic features of herpes zoster: A note on “geniculate herpes”. Archives of Neurology & Psychiatry 51, 216–231. [Google Scholar]
- Dong X, and Dong X (2018). Peripheral and Central Mechanisms of Itch. Neuron 98, 482–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan B, Cheng L, Bourane S, Britz O, Padilla C, Garcia-Campmany L, Krashes M, Knowlton W, Velasquez T, Ren X, et al. (2014). Identification of spinal circuits transmitting and gating mechanical pain. Cell 159, 1417–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan B, Cheng L, and Ma Q (2018). Spinal Circuits Transmitting Mechanical Pain and Itch. Neuroscience bulletin 34, 186–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dukhinova M, Kopeikina E, and Ponomarev ED (2018). Usage of Multiparameter Flow Cytometry to Study Microglia and Macrophage Heterogeneity in the Central Nervous System During Neuroinflammation and Neurodegeneration. Methods in molecular biology 1745, 167–177. [DOI] [PubMed] [Google Scholar]
- Echeverry S, Shi XQ, and Zhang J (2008). Characterization of cell proliferation in rat spinal cord following peripheral nerve injury and the relationship with neuropathic pain. Pain 135, 37–47. [DOI] [PubMed] [Google Scholar]
- Eriksson NP, Persson JK, Svensson M, Arvidsson J, Molander C, and Aldskogius H (1993). A quantitative analysis of the microglial cell reaction in central primary sensory projection territories following peripheral nerve injury in the adult rat. Exp Brain Res 96, 19–27. [DOI] [PubMed] [Google Scholar]
- Erny D, Hrabe de Angelis AL, Jaitin D, Wieghofer P, Staszewski O, David E, Keren-Shaul H, Mahlakoiv T, Jakobshagen K, Buch T, et al. (2015). Host microbiota constantly control maturation and function of microglia in the CNS. Nature Neurosci 18, 965–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Febinger HY, Thomasy HE, Pavlova MN, Ringgold KM, Barf PR, George AM, Grillo JN, Bachstetter AD, Garcia JA, Cardona AE, et al. (2015). Time-dependent effects of CX3CR1 in a mouse model of mild traumatic brain injury. Journal of neuroinflammation 12, 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrini F, Trang T, Mattioli TA, Laffray S, Del’guidice T, Lorenzo LE, Castonguay A, Doyon N, Zhang W, Godin AG, et al. (2013). Morphine hyperalgesia gated through microglia-mediated disruption of neuronal Cl(−) homeostasis. Nat Neurosci 16, 183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu KY, Light AR, Matsushima GK, and Maixner W (1999). Microglial reactions after subcutaneous formalin injection into the rat hind paw. Brain Res 825, 59–67. [DOI] [PubMed] [Google Scholar]
- Fuger P, Hefendehl JK, Veeraraghavalu K, Wendeln AC, Schlosser C, Obermuller U, Wegenast-Braun BM, Neher JJ, Martus P, Kohsaka S, et al. (2017). Microglia turnover with aging and in an Alzheimer’s model via long-term in vivo single-cell imaging. Nat Neurosci 20, 1371–1376. [DOI] [PubMed] [Google Scholar]
- Fukagawa H, Koyama T, Kakuyama M, and Fukuda K (2013). Microglial activation involved in morphine tolerance is not mediated by toll-like receptor 4. J Anesth 27, 93–97. [DOI] [PubMed] [Google Scholar]
- Furube E, Kawai S, Inagaki H, Takagi S, and Miyata S (2018). Brain Region-dependent Heterogeneity and Dose-dependent Difference in Transient Microglia Population Increase during Lipopolysaccharide-induced Inflammation. Scientific reports 8, 2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galan-Arriero I, Avila-Martin G, Ferrer-Donato A, Gomez-Soriano J, Bravo-Esteban E, and Taylor J (2014). Oral administration of the p38alpha MAPK inhibitor, UR13870, inhibits affective pain behavior after spinal cord injury. Pain 155, 2188–2198. [DOI] [PubMed] [Google Scholar]
- Gao YJ, Zhang L, Samad OA, Suter MR, Yasuhiko K, Xu ZZ, Park JY, Lind AL, Ma Q, and Ji RR (2009). JNK-induced MCP-1 production in spinal cord astrocytes contributes to central sensitization and neuropathic pain. J Neurosci 29, 4096–4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore SA (1975). Proliferation of non-neuronal cells in spinal cords of irradiated, immature rats following transection of the sciatic nerve. Anat Rec 181, 799–811. [DOI] [PubMed] [Google Scholar]
- Gilmore SA, and Skinner RD (1979). Intraspinal non-neuronal cellular responses to peripheral nerve injury. Anat Rec 194, 369–387. [DOI] [PubMed] [Google Scholar]
- Gilroy DW (2005). The role of aspirin-triggered lipoxins in the mechanism of action of aspirin. Prostaglandins Leukot Essent Fatty Acids 73, 203–210. [DOI] [PubMed] [Google Scholar]
- Grace PM, Strand KA, Galer EL, Urban DJ, Wang X, Baratta MV, Fabisiak TJ, Anderson ND, Cheng K, Greene LI, et al. (2016). Morphine paradoxically prolongs neuropathic pain in rats by amplifying spinal NLRP3 inflammasome activation. Proc Natl Acad Sci U S A 113, 3441–3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace PM, Wang X, Strand KA, Baratta MV, Zhang Y, Galer EL, Yin H, Maier SF, and Watkins LR (2018). DREADDed microglia in pain: Implications for spinal inflammatory signaling in male rats. Exp Neurol 304, 125–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin RS, Costigan M, Brenner GJ, Ma CH, Scholz J, Moss A, Allchorne AJ, Stahl GL, and Woolf CJ (2007). Complement induction in spinal cord microglia results in anaphylatoxin C5a-mediated pain hypersensitivity. J Neurosci 27, 8699–8708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber-Schoffnegger D, Drdla-Schutting R, Honigsperger C, Wunderbaldinger G, Gassner M, and Sandkuhler J (2013). Induction of thermal hyperalgesia and synaptic long-term potentiation in the spinal cord lamina I by TNF-alpha and IL-1beta is mediated by glial cells. J Neurosci 33, 6540–6551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu N, Peng J, Murugan M, Wang X, Eyo UB, Sun D, Ren Y, DiCicco-Bloom E, Young W, Dong H, et al. (2016). Spinal Microgliosis Due to Resident Microglial Proliferation Is Required for Pain Hypersensitivity after Peripheral Nerve Injury. Cell reports 16, 605–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Z, Kuhn JA, Wang X, Colquitt B, Solorzano C, Vaman S, Guan AK, Evans-Reinsch Z, Braz J, Devor M, et al. (2016). Injured sensory neuron-derived CSF1 induces microglial proliferation and DAP12-dependent pain. Nat Neurosci 19, 94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwak YS, Kang J, Unabia GC, and Hulsebosch CE (2012). Spatial and temporal activation of spinal glial cells: role of gliopathy in central neuropathic pain following spinal cord injury in rats. Exp Neurol 234, 362–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hains BC, and Waxman SG (2006). Activated microglia contribute to the maintenance of chronic pain after spinal cord injury. J Neurosci 26, 4308–4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hald A, Nedergaard S, Hansen RR, Ding M, and Heegaard AM (2009). Differential activation of spinal cord glial cells in murine models of neuropathic and cancer pain. Eur J Pain 13, 138–145. [DOI] [PubMed] [Google Scholar]
- Han Q, Liu D, Convertino M, Wang Z, Jiang C, Kim YH, Luo X, Zhang X, Nackley A, Dokholyan NV, et al. (2018). miRNA-711 Binds and Activates TRPA1 Extracellularly to Evoke Acute and Chronic Pruritus. Neuron 99, 449–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanisch UK (2002). Microglia as a source and target of cytokines. Glia 40, 140–155. [DOI] [PubMed] [Google Scholar]
- Hanisch UK, and Kettenmann H (2007). Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci 10, 1387–1394. [DOI] [PubMed] [Google Scholar]
- Hansen RR, Nielsen CK, Nasser A, Thomsen SI, Eghorn LF, Pham Y, Schulenburg C, Syberg S, Ding M, Stojilkovic SS, et al. (2011). P2X7 receptor-deficient mice are susceptible to bone cancer pain. Pain 152, 1766–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison JL, Rowe RK, Ellis TW, Yee NS, O’Hara BF, Adelson PD, and Lifshitz J (2015). Resolvins AT-D1 and E1 differentially impact functional outcome, post-traumatic sleep, and microglial activation following diffuse brain injury in the mouse. Brain, behavior, and immunity 47, 131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey RJ, Depner UB, Wassle H, Ahmadi S, Heindl C, Reinold H, Smart TG, Harvey K, Schutz B, Abo-Salem OM, et al. (2004). GlyR alpha3: an essential target for spinal PGE2-mediated inflammatory pain sensitization. Science 304, 884–887. [DOI] [PubMed] [Google Scholar]
- Hathway GJ, Vega-Avelaira D, Moss A, Ingram R, and Fitzgerald M (2009). Brief, low frequency stimulation of rat peripheral C-fibres evokes prolonged microglial-induced central sensitization in adults but not in neonates. Pain 144, 110–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppner FL, Ransohoff RM, and Becher B (2015). Immune attack: the role of inflammation in Alzheimer disease. Nat Rev Neurosci 16, 358–372. [DOI] [PubMed] [Google Scholar]
- Hildebrand ME, Xu J, Dedek A, Li Y, Sengar AS, Beggs S, Lombroso PJ, and Salter MW (2016). Potentiation of Synaptic GluN2B NMDAR Currents by Fyn Kinase Is Gated through BDNF-Mediated Disinhibition in Spinal Pain Processing. Cell Reports 17, 2753–2765. [DOI] [PubMed] [Google Scholar]
- Hong S, Gronert K, Devchand P, Moussignac R-L, and Serhan CN (2003). Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood and glial cells: autacoids in anti-inflammation. J Biol Chem 278, 14677–14687. [DOI] [PubMed] [Google Scholar]
- Honore P, Rogers SD, Schwei MJ, Salak-Johnson JL, Luger NM, Sabino MC, Clohisy DR, and Mantyh PW (2000). Murine models of inflammatory, neuropathic and cancer pain each generates a unique set of neurochemical changes in the spinal cord and sensory neurons. Neuroscience 98, 585–598. [DOI] [PubMed] [Google Scholar]
- Horvath RJ, Landry RP, Romero-Sandoval EA, and DeLeo JA (2010). Morphine tolerance attenuates the resolution of postoperative pain and enhances spinal microglial p38 and extracellular receptor kinase phosphorylation. Neuroscience 169, 843–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu LY, Zhou Y, Cui WQ, Hu XM, Du LX, Mi WL, Chu YX, Wu GC, Wang YQ, and Mao-Ying QL (2018). Triggering receptor expressed on myeloid cells 2 (TREM2) dependent microglial activation promotes cisplatin-induced peripheral neuropathy in mice. Brain Behav Immun 68, 132–145. [DOI] [PubMed] [Google Scholar]
- Hua XY, Svensson CI, Matsui T, Fitzsimmons B, Yaksh TL, and Webb M (2005). Intrathecal minocycline attenuates peripheral inflammation-induced hyperalgesia by inhibiting p38 MAPK in spinal microglia. Eur J Neurosci 22, 2431–2440. [DOI] [PubMed] [Google Scholar]
- Hutchinson MR, Zhang Y, Shridhar M, Evans JH, Buchanan MM, Zhao TX, Slivka PF, Coats BD, Rezvani N, Wieseler J, et al. (2010). Evidence that opioids may have toll-like receptor 4 and MD-2 effects. Brain Behav Immun 24, 83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K, and Tsuda M (2018). Microglia in neuropathic pain: cellular and molecular mechanisms and therapeutic potential. Nat Rev Neurosci 19, 138–152. [DOI] [PubMed] [Google Scholar]
- Ji RR (2018). Neuroinflammation and central sensitization in chronic pain. Anesthesiology In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji RR, Berta T, and Nedergaard M (2013). Glia and pain: Is chronic pain a gliopathy? Pain 154, S10–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji RR, Kohno T, Moore KA, and Woolf CJ (2003). Central sensitization and LTP: do pain and memory share similar mechanisms? Trends Neurosci 26, 696–705. [DOI] [PubMed] [Google Scholar]
- Ji RR, Nackley A, Huh Y, Terrando N, and Maixner W (2018). Neuroinflammation and Central Sensitization in Chronic and Widespread Pain. Anesthesiology 129, 343–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji RR, and Suter MR (2007). p38 MAPK, microglial signaling, and neuropathic pain. MolPain 3, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji RR, Xu ZZ, and Gao YJ (2014). Emerging targets in neuroinflammation-driven chronic pain. Nat Rev Drug Discov 13, 533–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji RR, Xu ZZ, Strichartz G, and Serhan CN (2011). Emerging roles of resolvins in the resolution of inflammation and pain. Trends Neurosci 34, 599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji RR, Xu ZZ, Wang X, and Lo EH (2009). Matrix metalloprotease regulation of neuropathic pain. Trends Pharmacol Sci 30, 336–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin SX, Zhuang ZY, Woolf CJ, and Ji RR (2003). p38 mitogen-activated protein kinase is activated after a spinal nerve ligation in spinal cord microglia and dorsal root ganglion neurons and contributes to the generation of neuropathic pain. J Neurosci 23, 4017–4022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambrun C, Roca-Lapirot O, Salio C, Landry M, Moqrich A, and Le Feuvre Y (2018). TAFA4 Reverses Mechanical Allodynia through Activation of GABAergic Transmission and Microglial Process Retraction. Cell reports 22, 2886–2897. [DOI] [PubMed] [Google Scholar]
- Kantarci A, Aytan N, Palaska I, Stephens D, Crabtree L, Benincasa C, Jenkins BG, Carreras I, and Dedeoglu A (2018). Combined administration of resolvin E1 and lipoxin A4 resolves inflammation in a murine model of Alzheimer’s disease. Experimental neurology 300, 111–120. [DOI] [PubMed] [Google Scholar]
- Katsura H, Obata K, Mizushima T, Sakurai J, Kobayashi K, Yamanaka H, Dai Y, Fukuoka T, Sakagami M, and Noguchi K (2006). Activation of Src-family kinases in spinal microglia contributes to mechanical hypersensitivity after nerve injury. J Neurosci 26, 8680–8690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki Y, Xu ZZ, Wang X, Park JY, Zhuang ZY, Tan PH, Gao YJ, Roy K, Corfas G, Lo EH, et al. (2008a). Distinct roles of matrix metalloproteases in the early- and late-phase development of neuropathic pain. Nat Med 14, 331–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki Y, Zhang L, Cheng JK, and Ji RR (2008b). Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in regulating synaptic and neuronal activity in the superficial spinal cord. J Neurosci 28, 5189–5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Kim MA, Cho IH, Kim MS, Lee S, Jo EK, Choi SY, Park K, Kim JS, Akira S, et al. (2007). A critical role of toll-like receptor 2 in nerve injury-induced spinal cord glial cell activation and pain hypersensitivity. J Biol Chem 282, 14975–14983. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Takahashi E, Miyagawa Y, Yamanaka H, and Noguchi K (2011). Induction of the P2X7 receptor in spinal microglia in a neuropathic pain model. Neurosci Lett 504, 57–61. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Yamanaka H, Fukuoka T, Dai Y, Obata K, and Noguchi K (2008). P2Y12 receptor upregulation in activated microglia is a gateway of p38 signaling and neuropathic pain. J Neurosci 28, 2892–2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Yamanaka H, Yanamoto F, Okubo M, and Noguchi K (2012). Multiple P2Y subtypes in spinal microglia are involved in neuropathic pain after peripheral nerve injury. Glia 60, 1529–1539. [DOI] [PubMed] [Google Scholar]
- Kohno T, Wang H, Amaya F, Brenner GJ, Cheng JK, Ji RR, and Woolf CJ (2008). Bradykinin enhances AMPA and NMDA receptor activity in spinal cord dorsal horn neurons by activating multiple kinases to produce pain hypersensitivity. J Neurosci 28, 4533–4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamoorthy S, Recchiuti A, Chiang N, Fredman G, and Serhan CN (2012). Resolvin D1 receptor stereoselectivity and regulation of inflammation and pro-resolving microRNAs. Am J Pathol 180, 2018–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronschlager MT, Drdla-Schutting R, Gassner M, Honsek SD, Teuchmann HL, and Sandkuhler J (2016). Gliogenic LTP spreads widely in nociceptive pathways. Science 354, 1144–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMotte RH, Dong X, and Ringkamp M (2014). Sensory neurons and circuits mediating itch. NatRevNeurosci 15, 19–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latremoliere A, and Woolf CJ (2009). Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain 10, 895–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leduc-Pessah H, Weilinger NL, Fan CY, Burma NE, Thompson RJ, and Trang T (2017). Site-Specific Regulation of P2X7 Receptor Function in Microglia Gates Morphine Analgesic Tolerance. J Neurosci 37, 10154–10172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Zhang H, Zhang H, Kosturakis AK, Jawad AB, and Dougherty PM (2014). Toll-like receptor 4 signaling contributes to Paclitaxel-induced peripheral neuropathy. J Pain 15, 712–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Yao M, Wang H, Xu L, Zheng Y, Huang B, Ni H, Xu S, Zhou X, and Lian Q (2017). P2Y12 receptor-mediated activation of spinal microglia and p38MAPK pathway contribute to cancer-induced bone pain. J Pain Res 10, 417–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Gao YJ, and Ji RR (2012). Emerging role of Toll-like receptors in the control of pain and itch. Neurosci Bull 28, 131–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Han Q, Chen G, Huang Y, Zhao LX, Berta T, Gao YJ, and Ji RR (2016). Toll-like receptor 4 contributes to chronic itch, alloknesis, and spinal astrocyte activation in male mice. Pain 157, 806–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, and Ji RR (2013). New insights into the mechanisms of itch: are pain and itch controlled by distinct mechanisms? Pflugers Arch 465, 1671–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YL, Zhou LJ, Hu NW, Xu JT, Wu CY, Zhang T, Li YY, and Liu XG (2007). Tumor necrosis factor-alpha induces long-term potentiation of C-fiber evoked field potentials in spinal dorsal horn in rats with nerve injury: the role of NF-kappa B, JNK and p38 MAPK. Neuropharmacology 52, 708–715. [DOI] [PubMed] [Google Scholar]
- Loggia ML, Chonde DB, Akeju O, Arabasz G, Catana C, Edwards RR, Hill E, Hsu S, Izquierdo-Garcia D, Ji RR, et al. (2015). Evidence for brain glial activation in chronic pain patients. Brain 138, 604–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Dong H, Gao Y, Gong Y, Ren Y, Gu N, Zhou S, Xia N, Sun YY, Ji RR, et al. (2013). A feed-forward spinal cord glycinergic neural circuit gates mechanical allodynia. J Clin Invest 123, 4050–4062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo C, Kuner T, and Kuner R (2014). Synaptic plasticity in pathological pain. Trends Neurosci 37, 343–355. [DOI] [PubMed] [Google Scholar]
- Luo X, Fitzsimmons B, Mohan A, Zhang L, Terrando N, Kordasiewicz H, and Ji RR (2017). Intrathecal administration of antisense oligonucleotide against p38alpha but not p38beta MAP kinase isoform reduces neuropathic and postoperative pain and TLR4-induced pain in male mice. Brain, behavior, and immunity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantyh PW, Rogers SD, Honore P, Allen BJ, Ghilardi JR, Li J, Daughters RS, Lappi DA, Wiley RG, and Simone DA (1997). Inhibition of hyperalgesia by ablation of lamina I spinal neurons expressing the substance P receptor. Science 278, 275–279. [DOI] [PubMed] [Google Scholar]
- Mao-Ying QL, Wang XW, Yang CJ, Li X, Mi WL, Wu GC, and Wang YQ (2012). Robust spinal neuroinflammation mediates mechanical allodynia in Walker 256 induced bone cancer rats. Mol Brain 5, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao J, Price DD, and Mayer DJ (1995). Mechanisms of hyperalgesia and morphine tolerance: a current view of their possible interactions. Pain 62, 259–274. [DOI] [PubMed] [Google Scholar]
- Marcheselli VL, Mukherjee PK, Arita M, Hong S, Antony R, Sheets K, Petasis N, Serhan CN, and Bazan NG (2010). Neuroprotectin D1/protectin D1 stereoselective and specific binding with human retinal pigment epithelial cells and neutrophils. Prostaglandins Leukot Essent Fatty Acids 82, 27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez V, Szekely B, Lemarie J, Martin F, Gentili M, Ben Ammar S, Lepeintre JF, Garreau de Loubresse C, Chauvin M, Bouhassira D, et al. (2013). The efficacy of a glial inhibitor, minocycline, for preventing persistent pain after lumbar discectomy: a randomized, double-blind, controlled study. Pain 154, 1197–1203. [DOI] [PubMed] [Google Scholar]
- Martini AC, Berta T, Forner S, Chen G, Bento AF, Ji RR, and Rae GA (2016). Lipoxin A4 inhibits microglial activation and reduces neuroinflammation and neuropathic pain after spinal cord hemisection. J Neuroinflammation 13, 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda T, Iwamoto S, Mikuriya S, Tozaki-Saitoh H, Tamura T, Tsuda M, and Inoue K (2015). Transcription factor IRF1 is responsible for IRF8-mediated IL-1beta expression in reactive microglia. J Pharmacol Sci 128, 216–220. [DOI] [PubMed] [Google Scholar]
- Masuda T, Iwamoto S, Yoshinaga R, Tozaki-Saitoh H, Nishiyama A, Mak TW, Tamura T, Tsuda M, and Inoue K (2014). Transcription factor IRF5 drives P2X4R+-reactive microglia gating neuropathic pain. Nat Commun 5, 3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda T, Tsuda M, Yoshinaga R, Tozaki-Saitoh H, Ozato K, Tamura T, and Inoue K (2012). IRF8 is a critical transcription factor for transforming microglia into a reactive phenotype. Cell Rep 1, 334–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathys H, Adaikkan C, Gao F, Young JZ, Manet E, Hemberg M, De Jager PL, Ransohoff RM, Regev A, and Tsai LH (2017). Temporal Tracking of Microglia Activation in Neurodegeneration at Single-Cell Resolution. Cell reports 21, 366–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKelvey R, Berta T, Old E, Ji RR, and Fitzgerald M (2015). Neuropathic pain is constitutively suppressed in early life by anti-inflammatory neuroimmune regulation. J Neurosci 35, 457–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon SB, and Malcangio M (2009). Current challenges in glia-pain biology. Neuron 64, 46–54. [DOI] [PubMed] [Google Scholar]
- Meng XW, Gao JL, Zuo JL, Wang LN, Liu SL, Jin XH, Yao M, and Namaka M (2017). Toll-like receptor-4/p38 MAPK signaling in the dorsal horn contributes to P2X4 receptor activation and BDNF over-secretion in cancer induced bone pain. Neurosci Res 125, 37–45. [DOI] [PubMed] [Google Scholar]
- Miao X, Huang Y, Liu TT, Guo R, Wang B, Wang XL, Chen LH, Zhou Y, Ji RR, and Liu T (2018). TNF-alpha/TNFR1 Signaling is Required for the Full Expression of Acute and Chronic Itch in Mice via Peripheral and Central Mechanisms. Neurosci bull 34, 42–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogil JS (2012). Sex differences in pain and pain inhibition: multiple explanations of a controversial phenomenon. Nat Rev Neurosci 13, 859–866. [DOI] [PubMed] [Google Scholar]
- Moore KA, Kohno T, Karchewski LA, Scholz J, Baba H, and Woolf CJ (2002). Partial peripheral nerve injury promotes a selective loss of GABAergic inhibition in the superficial dorsal horn of the spinal cord. J Neurosci 22, 6724–6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss A, Beggs S, Vega-Avelaira D, Costigan M, Hathway GJ, Salter MW, and Fitzgerald M (2007). Spinal microglia and neuropathic pain in young rats. Pain 128, 215–224. [DOI] [PubMed] [Google Scholar]
- Mosser CA, Baptista S, Arnoux I, and Audinat E (2017). Microglia in CNS development: Shaping the brain for the future. Prog Neurobiol 149–150, 1–20. [DOI] [PubMed] [Google Scholar]
- Mrdjen D, Pavlovic A, Hartmann FJ, Schreiner B, Utz SG, Leung BP, Lelios I, Heppner FL, Kipnis J, Merkler D, et al. (2018). High-Dimensional Single-Cell Mapping of Central Nervous System Immune Cells Reveals Distinct Myeloid Subsets in Health, Aging, and Disease. Immunity 48, 380–395 e386. [DOI] [PubMed] [Google Scholar]
- Nicotra L, Loram LC, Watkins LR, and Hutchinson MR (2011). Toll-like receptors in chronic pain. Exp Neurol 234, 316–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto FR, Clark AK, Grist J, Chapman V, and Malcangio M (2015). Calcitonin gene-related peptide-expressing sensory neurons and spinal microglial reactivity contribute to pain states in collagen-induced arthritis. Arthritis Rheumatol 67, 1668–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris PC, Libreros S, Chiang N, and Serhan CN (2017). A cluster of immunoresolvents links coagulation to innate host defense in human blood. Sci Signal 10, eaan1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obata K, Katsura H, Mizushima T, Sakurai J, Kobayashi K, Yamanaka H, Dai Y, Fukuoka T, and Noguchi K (2007). Roles of extracellular signal-regulated protein kinases 5 in spinal microglia and primary sensory neurons for neuropathic pain. J Neurochem 102, 1569–1584. [DOI] [PubMed] [Google Scholar]
- Ohira T, Arita M, Omori K, Recchiuti A, Van Dyke TE, and Serhan CN (2010). Resolvin E1 receptor activation signals phosphorylation and phagocytosis. J Biol Chem 285, 3451–3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okubo M, Yamanaka H, Kobayashi K, Dai Y, Kanda H, Yagi H, and Noguchi K (2016). Macrophage-Colony Stimulating Factor Derived from Injured Primary Afferent Induces Proliferation of Spinal Microglia and Neuropathic Pain in Rats. PLoS One 11, e0153375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paige C, Maruthy GB, Mejia G, Dussor G, and Price T (2018). Spinal Inhibition of P2XR or p38 Signaling Disrupts Hyperalgesic Priming in Male, but not Female, Mice. Neuroscience 385, 133–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CK, Lu N, Xu ZZ, Liu T, Serhan CN, and Ji RR (2011). Resolving TRPV1- and TNF-a-mediated spinal cord synaptic plasticity and inflammatory pain with neuroprotectin D1. J Neurosci 31, 15072–15085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirs C, and Seal RP (2016). Neural circuits for pain: Recent advances and current views. Science 354, 578–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Gu N, Zhou L, B.E. U, Murugan M, Gan WB, and Wu LJ (2016). Microglia and monocytes synergistically promote the transition from acute to chronic pain after nerve injury. Nat Commun 7, 12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Flores G, Levesque SA, Pacheco J, Vaca L, Lacroix S, Perez-Cornejo P, and Arreola J (2015). The P2X7/P2X4 interaction shapes the purinergic response in murine macrophages. Biochem Biophys Res Commun 467, 484–490. [DOI] [PubMed] [Google Scholar]
- Peters CM, Jimenez-Andrade JM, Kuskowski MA, Ghilardi JR, and Mantyh PW (2007). An evolving cellular pathology occurs in dorsal root ganglia, peripheral nerve and spinal cord following intravenous administration of paclitaxel in the rat. Brain research 1168, 46–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pevida M, Gonzalez-Rodriguez S, Lastra A, Garcia-Suarez O, Hidalgo A, Menendez L, and Baamonde A (2014). Involvement of spinal chemokine CCL2 in the hyperalgesia evoked by bone cancer in mice: a role for astroglia and microglia. Cell Mol Neurobiol 34, 143–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavendra V, Tanga F, and DeLeo JA (2003). Inhibition of microglial activation attenuates the development but not existing hypersensitivity in a rat model of neuropathy. J Pharmacol Exp Ther 306, 624–630. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM (2016). How neuroinflammation contributes to neurodegeneration. Science 353, 777–783. [DOI] [PubMed] [Google Scholar]
- Rey C, Nadjar A, Buaud B, Vaysse C, Aubert A, Pallet V, Laye S, and Joffre C (2016). Resolvin D1 and E1 promote resolution of inflammation in microglial cells in vitro. Brain, behavior, and immunity 55, 249–259. [DOI] [PubMed] [Google Scholar]
- Robinson CR, Zhang H, and Dougherty PM (2014). Astrocytes, but not microglia, are activated in oxaliplatin and bortezomib-induced peripheral neuropathy in the rat. Neuroscience 274, 308–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Sandoval EA, Horvath R, Landry RP, and DeLeo JA (2009). Cannabinoid receptor type 2 activation induces a microglial anti-inflammatory phenotype and reduces migration via MKP induction and ERK dephosphorylation. Mol Pain 5, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothhammer V, Borucki DM, Tjon EC, Takenaka MC, Chao CC, Ardura-Fabregat A, de Lima KA, Gutierrez-Vazquez C, Hewson P, Staszewski O, et al. (2018). Microglial control of astrocytes in response to microbial metabolites. Nature 557, 724–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter MW, and Kalia LV (2004). Src kinases: a hub for NMDA receptor regulation. Nat Rev Neurosci 5, 317–328. [DOI] [PubMed] [Google Scholar]
- Salter MW, and Stevens B (2017). Microglia emerge as central players in brain disease. Nature medicine 23, 1018–1027. [DOI] [PubMed] [Google Scholar]
- Sandkuhler J (2000). Learning and memory in pain pathways. Pain 88, 113–118. [DOI] [PubMed] [Google Scholar]
- Sandkuhler J (2009). Models and mechanisms of hyperalgesia and allodynia. Physiol Rev 89, 707–758. [DOI] [PubMed] [Google Scholar]
- Sato KL, Johanek LM, Sanada LS, and Sluka KA (2014). Spinal cord stimulation reduces mechanical hyperalgesia and glial cell activation in animals with neuropathic pain. Anesth Analg 118, 464–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN (2014). Pro-resolving lipid mediators are leads for resolution physiology. Nature 510, 92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN (2017). Treating inflammation and infection in the 21st century: new hints from decoding resolution mediators and mechanisms. FASEB J 31, 1273–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Chiang N, and Dalli J (2015). The resolution code of acute inflammation: Novel pro-resolving lipid mediators in resolution. Semin Immunol 27, 200–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Chiang N, and Van Dyke TE (2008). Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. NatRevImmunol 8, 349–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Clish CB, Brannon J, Colgan SP, Chiang N, and Gronert K (2000a). Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J Exp Med 192, 1197–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Clish CB, Brannon J, Colgan SP, Chiang N, and Gronert K (2000b). Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J Exp Med 192, 1197–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Dalli J, Karamnov S, Choi A, Park CK, Xu ZZ, Ji RR, Zhu M, and Petasis NA (2012). Macrophage pro-resolving mediator maresin 1 stimulates tissue regeneration and controls pain. FASEB J 26, 1755–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, and Moussignac R-L (2002). Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter pro-inflammation signals. J Exp Med 196, 1025–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Yang R, Martinod K, Kasuga K, Pillai PS, Porter TF, Oh SF, and Spite M (2009). Maresins: novel macrophage mediators with potent anti-inflammatory and pro-resolving actions. J Exp Med 206, 15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shemer A, Erny D, Jung S, and Prinz M (2015). Microglia Plasticity During Health and Disease: An Immunological Perspective. Trends Immunol 36, 614–624. [DOI] [PubMed] [Google Scholar]
- Shen Y, Zhang ZJ, Zhu MD, Jiang BC, Yang T, and Gao YJ (2015). Exogenous induction of HO-1 alleviates vincristine-induced neuropathic pain by reducing spinal glial activation in mice. Neurobiol Dis 79, 100–110. [DOI] [PubMed] [Google Scholar]
- Shi Y, Gelman BB, Lisinicchia JG, and Tang SJ (2012). Chronic-pain-associated astrocytic reaction in the spinal cord dorsal horn of human immunodeficiency virus-infected patients. J Neurosci 32, 10833–10840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikandar S, Minett MS, Millet Q, Santana-Varela S, Lau J, Wood JN, and Zhao J (2018). Brain-derived neurotrophic factor derived from sensory neurons plays a critical role in chronic pain. Brain 141, 1028–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorge RE, Lacroix-Fralish ML, Tuttle AH, Sotocinal SG, Austin JS, Ritchie J, Chanda ML, Graham AC, Topham L, Beggs S, et al. (2011). Spinal cord Toll-like receptor 4 mediates inflammatory and neuropathic hypersensitivity in male but not female mice. J Neurosci 31, 15450–15454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorge RE, Mapplebeck JC, Rosen S, Beggs S, Taves S, Alexander JK, Martin LJ, Austin JS, Sotocinal SG, Chen D, et al. (2015). Different immune cells mediate mechanical pain hypersensitivity in male and female mice. NatNeurosci 18, 1081–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staniland AA, Clark AK, Wodarski R, Sasso O, Maione F, D’Acquisto F, and Malcangio M (2010a). Reduced inflammatory and neuropathic pain and decreased spinal microglial response in fractalkine receptor (CX3CR1) knockout mice. J Neurochem 114, 1143–1157. [DOI] [PubMed] [Google Scholar]
- Staniland AA, Clark AK, Wodarski R, Sasso O, Maione F, D’Acquisto F, and Malcangio M (2010b). Reduced inflammatory and neuropathic pain and decreased spinal microglial response in fractalkine receptor (CX3CR1) knockout mice. Journal of neurochemistry 114, 1143–1157. [DOI] [PubMed] [Google Scholar]
- Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, Micheva KD, Mehalow AK, Huberman AD, Stafford B, et al. (2007). The classical complement cascade mediates CNS synapse elimination. Cell 131, 1164–1178. [DOI] [PubMed] [Google Scholar]
- Sumitani M, Ueda H, Hozumi J, Inoue R, Kogure T, Yamada Y, and Kogure T (2016). Minocycline Does Not Decrease Intensity of Neuropathic Pain Intensity, But Does Improve Its Affective Dimension. J Pain Palliat Care Pharmacother 30, 31–35. [DOI] [PubMed] [Google Scholar]
- Sumracki NM, Hutchinson MR, Gentgall M, Briggs N, Williams DB, and Rolan P (2012). The effects of pregabalin and the glial attenuator minocycline on the response to intradermal capsaicin in patients with unilateral sciatica. PloS one 7, e38525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S, Cao H, Han M, Li TT, Pan HL, Zhao ZQ, and Zhang YQ (2007). New evidence for the involvement of spinal fractalkine receptor in pain facilitation and spinal glial activation in rat model of monoarthritis. Pain 129, 64–75. [DOI] [PubMed] [Google Scholar]
- Sun YG, and Chen ZF (2007). A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature 448, 700–703. [DOI] [PubMed] [Google Scholar]
- Suter MR, Berta T, Gao YJ, Decosterd I, and Ji RR (2009). Large A-fiber activity is required for microglial proliferation and p38 MAPK activation in the spinal cord: different effects of resiniferatoxin and bupivacaine on spinal microglial changes after spared nerve injury. MolPain 5, 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter MR, Wen YR, Decosterd I, and Ji RR (2007). Do glial cells control pain? Neuron Glia Biol 3, 255–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson CI, Hua XY, Protter AA, Powell HC, and Yaksh TL (2003a). Spinal p38 MAP kinase is necessary for NMDA-induced spinal PGE(2) release and thermal hyperalgesia. Neuroreport 14, 1153–1157. [DOI] [PubMed] [Google Scholar]
- Svensson CI, Marsala M, Westerlund A, Calcutt NA, Campana WM, Freshwater JD, Catalano R, Feng Y, Protter AA, Scott B, et al. (2003b). Activation of p38 mitogen-activated protein kinase in spinal microglia is a critical link in inflammation-induced spinal pain processing. J Neurochem 86, 1534–1544. [DOI] [PubMed] [Google Scholar]
- Svensson CI, Zattoni M, and Serhan CN (2007). Lipoxins and aspirin-triggered lipoxin stop inflammatory pain processing. J Exp Med 204, 245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai YH, Tsai RY, Lin SL, Yeh CC, Wang JJ, Tao PL, and Wong CS (2009). Amitriptyline suppresses neuroinflammation-dependent interleukin-10-p38 mitogen-activated protein kinase-heme oxygenase-1 signaling pathway in chronic morphine-infused rats. Anesthesiology 110, 1379–1389. [DOI] [PubMed] [Google Scholar]
- Tamagawa T, Shinoda M, Honda K, Furukawa A, Kaji K, Nagashima H, Akasaka R, Chen J, Sessle BJ, Yonehara Y, et al. (2016). Involvement of Microglial P2Y12 Signaling in Tongue Cancer Pain. Journal of dental research 95, 1176–1182. [DOI] [PubMed] [Google Scholar]
- Tanga FY, Nutile-McMenemy N, and DeLeo JA (2005). The CNS role of Toll-like receptor 4 in innate neuroimmunity and painful neuropathy. Proc Natl Acad Sci U S A 102, 5856–5861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanga FY, Raghavendra V, and DeLeo JA (2004). Quantitative real-time RT-PCR assessment of spinal microglial and astrocytic activation markers in a rat model of neuropathic pain. Neurochem Int 45, 397–407. [DOI] [PubMed] [Google Scholar]
- Tashima R, Mikuriya S, Tomiyama D, Shiratori-Hayashi M, Yamashita T, Kohro Y, Tozaki-Saitoh H, Inoue K, and Tsuda M (2016). Bone marrow-derived cells in the population of spinal microglia after peripheral nerve injury. Scientific reports 6, 23701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsumi E, Yamanaka H, Kobayashi K, Yagi H, Sakagami M, and Noguchi K (2015). RhoA/ROCK pathway mediates p38 MAPK activation and morphological changes downstream of P2Y12/13 receptors in spinal microglia in neuropathic pain. Glia 63, 216–228. [DOI] [PubMed] [Google Scholar]
- Taves S, Berta T, Liu DL, Gan S, Chen G, Kim YH, Van de Ven T, Laufer S, and Ji RR (2016). Spinal inhibition of p38 MAP kinase reduces inflammatory and neuropathic pain in male but not female mice: Sex-dependent microglial signaling in the spinal cord. Brain, behavior, and immunity 55, 70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thacker MA, Clark AK, Bishop T, Grist J, Yip PK, Moon LD, Thompson SW, Marchand F, and McMahon SB (2009). CCL2 is a key mediator of microglia activation in neuropathic pain states. EurJPain 13, 263–272. [DOI] [PubMed] [Google Scholar]
- Thion MS, Low D, Silvin A, Chen J, Grisel P, Schulte-Schrepping J, Blecher R, Ulas T, Squarzoni P, Hoeffel G, et al. (2018). Microbiome Influences Prenatal and Adult Microglia in a Sex-Specific Manner. Cell 172, 500–516 e516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd AJ (2010). Neuronal circuitry for pain processing in the dorsal horn. NatRevNeurosci 11, 823–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trang T, Beggs S, Wan X, and Salter MW (2009). P2X4-receptor-mediated synthesis and release of brain-derived neurotrophic factor in microglia is dependent on calcium and p38-mitogen-activated protein kinase activation. J Neurosci 29, 3518–3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda M, Inoue K, and Salter MW (2005). Neuropathic pain and spinal microglia: a big problem from molecules in “small” glia. Trends Neurosci 28, 101–107. [DOI] [PubMed] [Google Scholar]
- Tsuda M, Mizokoshi A, Shigemoto-Mogami Y, Koizumi S, and Inoue K (2004). Activation of p38 mitogen-activated protein kinase in spinal hyperactive microglia contributes to pain hypersensitivity following peripheral nerve injury. Glia 45, 89–95. [DOI] [PubMed] [Google Scholar]
- Tsuda M, Shigemoto-Mogami Y, Koizumi S, Mizokoshi A, Kohsaka S, Salter MW, and Inoue K (2003). P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature 424, 778–783. [DOI] [PubMed] [Google Scholar]
- Tsuda M, Tozaki-Saitoh H, Masuda T, Toyomitsu E, Tezuka T, Yamamoto T, and Inoue K (2008). Lyn tyrosine kinase is required for P2X(4) receptor upregulation and neuropathic pain after peripheral nerve injury. Glia 56, 50–58. [DOI] [PubMed] [Google Scholar]
- Ulmann L, Hatcher JP, Hughes JP, Chaumont S, Green PJ, Conquet F, Buell GN, Reeve AJ, Chessell IP, and Rassendren F (2008). Up-regulation of P2X4 receptors in spinal microglia after peripheral nerve injury mediates BDNF release and neuropathic pain. J Neurosci 28, 11263–11268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanelderen P, Van Zundert J, Kozicz T, Puylaert M, De Vooght P, Mestrum R, Heylen R, Roubos E, and Vissers K (2015). Effect of minocycline on lumbar radicular neuropathic pain: a randomized, placebo-controlled, double-blind clinical trial with amitriptyline as a comparator. Anesthesiology 122, 399–406. [DOI] [PubMed] [Google Scholar]
- Villa A, Gelosa P, Castiglioni L, Cimino M, Rizzi N, Pepe G, Lolli F, Marcello E, Sironi L, Vegeto E, et al. (2018). Sex-Specific Features of Microglia from Adult Mice. Cell reports 23, 3501–3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vit JP, Ohara PT, Tien DA, Fike JR, Eikmeier L, Beitz A, Wilcox GL, and Jasmin L (2006). The analgesic effect of low dose focal irradiation in a mouse model of bone cancer is associated with spinal changes in neuro-mediators of nociception. Pain 120, 188–201. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhang Y, Peng Y, Hutchinson MR, Rice KC, Yin H, and Watkins LR (2016). Pharmacological characterization of the opioid inactive isomers (+)-naltrexone and (+)-naloxone as antagonists of toll-like receptor 4. Br J Pharmacol 173, 856–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins LR, Hutchinson MR, Rice KC, and Maier SF (2009). The “toll” of opioid-induced glial activation: improving the clinical efficacy of opioids by targeting glia. Trends PharmacolSci 30, 581–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wekerle H (2018). Brain inflammatory cascade controlled by gut-derived molecules. Nature 557, 642–643. [DOI] [PubMed] [Google Scholar]
- Wen YR, Suter MR, Kawasaki Y, Huang J, Pertin M, Kohno T, Berde CB, Decosterd I, and Ji RR (2007). Nerve conduction blockade in the sciatic nerve prevents but does not reverse the activation of p38 mitogen-activated protein kinase in spinal microglia in the rat spared nerve injury model. Anesthesiology 107, 312–321. [DOI] [PubMed] [Google Scholar]
- White FA, Sun J, Waters SM, Ma C, Ren D, Ripsch M, Steflik J, Cortright DN, LaMotte RH, and Miller RJ (2005). Excitatory monocyte chemoattractant protein-1 signaling is up-regulated in sensory neurons after chronic compression of the dorsal root ganglion. Proc Natl Acad Sci USA 102, 14092–14097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willemen HL, Eijkelkamp N, Wang H, Dantzer R, Dorn GW, Kelley KW, Heijnen CJ, and Kavelaars A (2010). Microglial/macrophage GRK2 determines duration of peripheral IL-1beta-induced hyperalgesia: contribution of spinal cord CX3CR1, p38 and IL-1 signaling. Pain 150, 550–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf CJ (1983). Evidence for a central component of post-injury pain hypersensitivity. Nature 306, 686–688. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, and Salter MW (2000). Neuronal plasticity: increasing the gain in pain. Science 288, 1765–1769. [DOI] [PubMed] [Google Scholar]
- Xie W, Strong JA, and Zhang JM (2009). Early blockade of injured primary sensory afferents reduces glial cell activation in two rat neuropathic pain models. Neuroscience 160, 847–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z-Z, Zhang L, Liu T, Park J-Y, Berta T, Yang R, Serhan CN, and Ji R-R (2010). Resolvins RvE1 and RvD1 attenuate inflammatory pain via central and peripheral actions. Nat Med 16, 592–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu ZZ, Berta T, and Ji RR (2013a). Resolvin E1 inhibits neuropathic pain and spinal cord microglial activation following peripheral nerve injury. J Neuroimmune Pharmacol 8, 37–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu ZZ, Liu XJ, Berta T, Park CK, Lu N, Serhan CN, and Ji RR (2013b). Neuroprotectin/protectin D1 protects against neuropathic pain in mice after nerve trauma. Annals of neurology 74, 490–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X, Jiang E, and Weng HR (2015). Activation of toll like receptor 4 attenuates GABA synthesis and postsynaptic GABA receptor activities in the spinal dorsal horn via releasing interleukin-1 beta. Journal of neuroinflammation 12, 222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X, and Weng HR (2013). Endogenous interleukin-1beta in neuropathic rats enhances glutamate release from the primary afferents in the spinal dorsal horn through coupling with presynaptic NMDA receptors. J BiolChem. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Li H, Li TT, Luo H, Gu XY, Lu N, Ji RR, and Zhang YQ (2015). Delayed Activation of Spinal Microglia Contributes to the Maintenance of Bone Cancer Pain in Female Wistar Rats via P2X7 Receptor and IL-18. JNeurosci 35, 7950–7963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y, Echeverry S, Shi XQ, Yang M, Yang QZ, Wang GY, Chambon J, Wu YC, Fu KY, De Koninck Y, et al. (2016). Dynamics of spinal microglia repopulation following an acute depletion. Scientific reports 6, 22839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yosipovitch G, and Bernhard JD (2013). Clinical practice. Chronic pruritus. NEnglJ Med 368, 1625–1634. [DOI] [PubMed] [Google Scholar]
- Younger J, and Mackey S (2009). Fibromyalgia symptoms are reduced by low-dose naltrexone: a pilot study. Pain Med 10, 663–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan Y, Paolicelli RC, Sforazzini F, Weinhard L, Bolasco G, Pagani F, Vyssotski AL, Bifone A, Gozzi A, Ragozzino D, et al. (2014). Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior. Nat neurosci 17, 400–406. [DOI] [PubMed] [Google Scholar]
- Zhang H, Nei H, and Dougherty PM (2010). A p38 mitogen-activated protein kinase-dependent mechanism of disinhibition in spinal synaptic transmission induced by tumor necrosis factor-alpha. J Neurosci 30, 12844–12855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Shi XQ, Echeverry S, Mogil JS, De Koninck Y, and Rivest S (2007). Expression of CCR2 in both resident and bone marrow-derived microglia plays a critical role in neuropathic pain. J Neurosci 27, 12396–12406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Berta T, Xu ZZ, Liu T, Park JY, and Ji RR (2011). TNF-alpha contributes to spinal cord synaptic plasticity and inflammatory pain: distinct role of TNF receptor subtypes 1 and 2. Pain 152, 419–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang RX, Li A, Liu B, Wang L, Ren K, Zhang H, Berman BM, and Lao L (2008). IL-1ra alleviates inflammatory hyperalgesia through preventing phosphorylation of NMDA receptor NR-1 subunit in rats. Pain 135, 232–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Seereeram A, Nassar MA, Levato A, Pezet S, Hathaway G, Morenilla-Palao C, Stirling C, Fitzgerald M, McMahon SB, et al. (2006). Nociceptor-derived brain-derived neurotrophic factor regulates acute and inflammatory but not neuropathic pain. Mol Cell Neurosci 31, 539–548. [DOI] [PubMed] [Google Scholar]
- Zheng FY, Xiao WH, and Bennett GJ (2011). The response of spinal microglia to chemotherapy-evoked painful peripheral neuropathies is distinct from that evoked by traumatic nerve injuries. Neuroscience 176, 447–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Chen ML, Zhang YQ, and Zhao ZQ (2010). Involvement of spinal microglial P2X7 receptor in generation of tolerance to morphine analgesia in rats. J Neurosci 30, 8042–8047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang ZY, Gerner P, Woolf CJ, and Ji RR (2005). ERK is sequentially activated in neurons, microglia, and astrocytes by spinal nerve ligation and contributes to mechanical allodynia in this neuropathic pain model. Pain 114, 149–159. [DOI] [PubMed] [Google Scholar]
- Zhuang ZY, Kawasaki Y, Tan PH, Wen YR, Huang J, and Ji RR (2007). Role of the CX3CR1/p38 MAPK pathway in spinal microglia for the development of neuropathic pain following nerve injury-induced cleavage of fractalkine. Brain Behav Immun 21, 642–651. [DOI] [PMC free article] [PubMed] [Google Scholar]