Abstract
Key points
The control of locomotion is thought to be generated by activating groups of muscles that perform similar actions, which are termed muscle synergies.
Here, we investigated if muscle synergies are controlled at the level of the spinal cord.
We did this by comparing muscle activity in the legs of cats during stepping on a treadmill before and after a complete spinal transection that abolishes commands from the brain.
We show that muscle synergies were maintained following spinal transection, validating the concept that muscle synergies for locomotion are primarily controlled by circuits of neurons within the spinal cord.
Abstract
Locomotion is thought to involve the sequential activation of functional modules or muscle synergies. Here, we tested the hypothesis that muscle synergies for locomotion are organized within the spinal cord. We recorded bursts of muscle activity in the same cats (n = 7) before and after spinal transection during tied‐belt locomotion at three speeds and split‐belt locomotion at three left–right speed differences. We identified seven muscles synergies before (intact state) and after (spinal state) spinal transection. The muscles comprising the different synergies were the same in the intact and spinal states as well as at different speeds or left–right speed differences. However, there were some significant shifts in the onsets and offsets of certain synergies as a function of state, speed and left–right speed differences. The most notable difference between the intact and spinal states was a change in the timing between the knee flexor and hip flexor muscle synergies. In the intact state, the knee flexor synergy preceded the hip flexor synergy, whereas in the spinal state both synergies occurred concurrently. Afferent inputs also appear important for the expression of some muscle synergies, specifically those involving biphasic patterns of muscle activity. We propose that muscle synergies for locomotion are primarily organized within the spinal cord, although their full expression and proper timing requires inputs from supraspinal structures and/or limb afferents.
Keywords: Locomotion, spinal cord, muscle synergies
Key points
The control of locomotion is thought to be generated by activating groups of muscles that perform similar actions, which are termed muscle synergies.
Here, we investigated if muscle synergies are controlled at the level of the spinal cord.
We did this by comparing muscle activity in the legs of cats during stepping on a treadmill before and after a complete spinal transection that abolishes commands from the brain.
We show that muscle synergies were maintained following spinal transection, validating the concept that muscle synergies for locomotion are primarily controlled by circuits of neurons within the spinal cord.
Introduction
A central issue in the control of mammalian locomotion is how the central nervous system (CNS) controls the multiple degrees of freedom inherent to the body (Bernstein, 1967). Stated differently, how are the many joints, muscles and spinal neurons controlled to produce smooth and efficient locomotion? A proposed concept argues that the CNS is organized into functional units, also termed modules, primitives or synergies, whereby a common signal activates a group of muscles to perform a synergistic action (reviewed in Grillner, 1981; Tresch et al. 1999; d'Avella and Bizzi, 2005; Bizzi et al. 2008; Tresch and Jarc, 2009; Giszter and Hart, 2013; Ting et al. 2015; Giszter, 2015). Thus, instead of controlling thousands of motor units or spinal neurons, commands sent to a small number of modules activate a group of muscles, or muscle synergies, to produce a specific biomechanical action. A flexible modulation of muscle synergies generates goal‐directed and task‐appropriate locomotion.
A frequently used technique to validate the concept of muscle synergies in locomotor studies and other types of movements is by recording the electrical activity of muscles (EMG, electromyography) and applying statistical analyses. Here, we use the definition of a muscle synergy to include synchronously active muscles (d'Avella and Bizzi, 2005; Krouchev et al. 2006; Drew et al. 2008; Tresch and Jarc, 2009; Kargo et al. 2010; Markin et al. 2012). Studies have shown that locomotion in cats and humans can be explained by a small number of muscle synergies (Ivanenko et al. 2004; Krouchev et al. 2006; Dominici et al. 2011; Markin et al. 2012).
A fundamentally important question in locomotor control is where muscle synergies for locomotion are potentially organized. Dominici et al. (2011) showed that muscle synergies for locomotion were similar in rats, cats, macaques and guineafowls, as well as in humans at different developmental stages, indicating a phylogenetically conserved neural mechanism. Thus, despite considerable differences in the cerebral cortex of these species, identified muscle synergies were similar, suggesting that they are organized at lower levels of the CNS. It is well established that the basic pattern of locomotion is generated by a neuronal network within the spinal cord, termed the central pattern generator (CPG), which has been extensively studied in several vertebrates, from fish to mammals, including non‐human primates (reviewed in (Grillner, 1981; McCrea and Rybak, 2008; Grillner and Jessell, 2009; Kiehn, 2011, 2016). Several lines of evidence suggest that the mammalian spinal cord is organized into functional modules (Tresch and Bizzi, 1999; Lemay and Grill, 2004; Hagglund et al. 2013; Caggiano et al. 2016) that provide the basic building blocks for locomotion (Bellardita and Kiehn, 2015).
The goal of the present study was to test whether the concept of muscle synergies applies to the spinal control of locomotion in an adult mammalian system. We tested this concept by characterizing hindlimb muscle synergies before and after spinal transection in the same adult cats in different locomotor conditions. We hypothesized that muscle synergies controlling adult mammalian locomotion are organized within the spinal cord.
Methods
Ethical approval
We performed experimental procedures according to the policies and directives of the Canadian Council on Animal Care, and the Animal Care Committee of the Université de Sherbrooke approved the experimental protocol (Protocol 273‐15). We carried out experiments on four female and three male cats that weighed between 3.0 and 4.7 kg and were at least 9 months of age at the time of experimentation. Before and after experiments, cats were housed and fed in a dedicated room within the animal care facility of the Faculty of Medicine and Health Sciences at the Université de Sherbrooke. The experiments comply with the ARRIVE guidelines (Kilkenny et al. 2010) and principles of animal research established by The Journal of Physiology (Grundy, 2015).
Implantation of electrodes to record muscle activity
We implanted electrodes to record hindlimb muscle activity (EMG, electromyography) in intact cats under aseptic conditions in an operating room with sterilized equipment, as described previously (Hurteau et al. 2017, 2018; Frigon et al. 2015). Before surgery, induction was made with an intramuscular injection of Butorphanol (0.4 mg kg−1), Acepromazine (0.1 mg kg−1), Glycopyrrolate (0.01 mg kg−1) and ketamine/diazepam (0.11 ml kg−1 in a 1:1 ratio). We shaved the cats using electric clippers and cleaned the skin using chlorhexidine soap. Gaseous anaesthesia (isoflurane 1.5–3%) was initiated with a mask and then with an endotracheal tube. We adjusted isoflurane concentration by monitoring cardiac/respiratory rates and by applying pressure to the paw to detect limb withdrawal. A rectal thermometer monitored body temperature during surgery and we maintained it between 35 and 37°C using a water‐filled heating pad placed under the animal and an infrared lamp ∼50 cm over it.
We directed pairs of Teflon‐insulated multistrain fine wires (AS633; Cooner Wire, Chatsworth, CA, USA) subcutaneously from two head‐mounted 34‐pin connectors (Omnetics, Minneapolis, MN, USA) and tied them into the belly of selected hindlimb muscles for bipolar recordings, with 1–2 mm of insulation removed from each wire. We verified electrode placement during surgery by electrically stimulating each muscle through its appropriate head connector channel.
At the end of surgery, we injected an antibiotic (Convenia, 0.1 ml kg−1) subcutaneously and taped a transdermal fentanyl patch (25 mcg h−1) to the back of the animal 2–3 cm rostral to the base of the tail. We also injected buprenorphine (0.01 mg kg−1), a fast‐acting analgesic, subcutaneously at the end of the surgery and ∼7 h later. After surgery, we placed the cats in an incubator and closely monitored them until they regained consciousness and could stand. Five days after surgery, we removed the fentanyl patch. At the conclusion of the experiments, cats received a lethal dose of pentobarbital through the cephalic vein.
Spinal transection and locomotor training
After collecting data in the intact state (see below), a complete spinal transection was made at low thoracic levels. The surgical conditions and drugs were the same as in the previous section. The skin was incised over the 12th and 13th thoracic vertebrae, and after carefully setting aside muscle and connective tissue, a small dorsal laminectomy was made. The dura was removed and xylocaine (lidocaine hydrochloride, 2%) was applied topically followed by two to three injections within the spinal cord, which was then transected with surgical scissors. Hemostatic material (Spongostan) was inserted within the gap, muscles and skin were sewn back and the opening was closed in anatomical layers.
After spinal transection, the cat's bladder was manually expressed one to two times daily. One week after spinal transection, we started training the cats five times a week to recover hindlimb locomotion, with each training session lasting 20–30 min. Initially, training consisted of two experimenters moving the hindlimbs over the moving treadmill belt to reproduce locomotion, with one of the experimenters holding the tail for support. The forelimbs were placed on a fixed platform located 1 cm above the belt and a Plexiglas separator prevented the hindlimbs from crossing. After a few days of training, hindlimb stepping movements could be elicited by manually stimulating the skin of the perineal region. Over the course of a few additional weeks, cats recovered full weight‐bearing hindlimb locomotion with consistent plantar placement. During data collection, an experimenter held the tail to provide equilibrium.
Data collection and analysis
We collected EMG and kinematic data before (intact state) and after cats recovered hindlimb locomotion following complete spinal transection (spinal state) during tied‐belt (equal left–right speeds) and split‐belt (unequal left–right speeds) locomotion. In the intact state, cats performed quadrupedal locomotion, while in the spinal state, they performed bipedal hindlimb locomotion with the forelimbs on a stationary platform. Cats performed nine different locomotor conditions: tied‐belt locomotion with both sides stepping at 0.4, 0.7 and 1.0 m s−1 and split‐belt locomotion with the slow limb stepping at 0.4 m s−1 and the fast limb stepping at 0.5, 0.7 and 1.0 m s−1. Both the left and right limbs were used as the slow and fast limbs during split‐belt locomotion. We collected data from 10–15 consecutive step cycles in each locomotor condition.
The EMG signals were pre‐amplified (10×, custom‐made system), band‐pass filtered (30–1000 Hz), and amplified (100–5000×) using a 16‐channel amplifier (AM Systems Model 3500, Sequim, WA, USA). As we implanted more than 16 muscles per cat, we obtained data in each locomotor condition twice, one for each connector. The EMG data were digitized (5000 Hz) with a National Instruments card (NI 6032E, Austin, TX, USA), acquired with custom‐made acquisition software and stored on computer. An experimenter (E.D.) determined the onsets and offsets of EMG bursts from the raw waveforms by visual inspection using a custom‐made program. The onsets and offsets of some muscles, particularly those displaying two clear bursts per cycles (i.e. biphasic bursts), became clearer at faster speeds and/or with larger left–right speed differences. As such, a few passes were made to clearly define the onsets and offsets of the EMG bursts.
The left and right sides were captured on video using two cameras (Basler AcA640‐100 gm) at 60 frames s−1 with a spatial resolution of 640 by 480 pixels. Video images were acquired using a custom‐made program (Labview) and synchronized with EMG data. Videos were analysed off‐line at 60 frames s−1 using custom‐made software. By visual inspection, we determined limb contact as the first frame where the hindpaw made visible contact with the treadmill surface and limb liftoff as the frame with the most caudal displacement of the hindlimb. Cycle duration was measured from successive contacts of the same limb.
Identification of muscle synergies by cluster analysis
Each recorded muscle had one or two bursts of EMG activity within a locomotor cycle and was obtained from at least two cats. Each cycle was normalized to limb contact. In order to group and compare muscles from both limbs, muscles recorded in the left and right hindlimbs were normalized to step cycle of the left and right hindlimbs, respectively. Table 1 summarizes the muscles included in the analysis of muscle synergies along with their main action and a few studies that have described the same EMG bursts during cat locomotion. Excellent summaries of EMG bursts during cat locomotion can also be found elsewhere (Rossignol, 1996; Rasmussen et al. 1978; Yakovenko et al. 2002).
Table 1.
Muscles used to characterize muscle synergies and their main function at the hip, knee and/or ankle
| Muscles | Abbreviation | Main function | EMG studies of cat locomotion |
|---|---|---|---|
| Biceps femoris anterior | BFa | Hip extensor | English & Weeks (1987), Chanaud et al. (1991), Trank & Smith (1996), Markin et al. (2012) |
| Biceps femoris posterior | BFp | Hip extensor/knee flexor | English & Weeks (1987), Chanaud et al. (1991), Markin et al. (2012) |
| Caudofemoralis | CF | Hip extensor | Pratt et al. (1991), Brown et al. (1998) |
| Flexor digitorum longus | FDL | Ankle extensor | Belanger et al. (1996), Trank & Smith (1996), Krouchev et al. (2006) |
| Flexor hallucis longus | FHL | Ankle extensor | Belanger et al. (1996), Trank & Smith (1996) |
| Iliopsoas | IP | Hip flexor | Belanger et al. (1996), Trank & Smith (1996), Markin et al. (2012) |
| Lateral gastrocnemius | LG | Ankle extensor/knee flexor | Belanger et al. (1996), Trank & Smith (1996), Krouchev et al. (2006) |
| Medial gastrocnemius | MG | Ankle extensor/knee flexor | Belanger et al. (1996), Trank & Smith (1996), Krouchev et al. (2006) |
| Peroneus longus | PLo | Ankle extensor | Abraham & Loeb (1985), Hensbergen & Kernell (1992) |
| Plantaris | PLA | Ankle extensor | Abraham & Loeb (1985), Trank & Smith (1996) |
| Sartorius anterior | SrtA | Hip flexor/knee extensor | Hoffer et al. (1987 a, b), Pratt & Loeb (1991), Trank & Smith (1996) |
| Sartorius medialis | SrtM | Hip flexor | Hoffer et al. (1987 a), Pratt & Loeb (1991), Trank & Smith (1996), Markin et al. (2012) |
| Semimembranosus | Sm | Hip extensor/knee flexor | Pratt et al. (1991, 1996), Smith et al. (1998) |
| Semitendinosus | St | Hip extensor/knee flexor | Chanaud et al. (1991), Smith et al. (1993), Trank & Smith (1996), Krouchev et al. (2006) |
| Soleus | SOL | Ankle extensor | Belanger et al. (1996), Trank & Smith (1996), Markin et al. (2012) |
| Tensor fascia lata | TFL | Hip extensor/knee flexor | Chanaud et al. (1991), Pratt et al. (1991) |
| Tibialis anterior | TA | Ankle flexor | Chanaud et al. (1991), Perell et al. (1993), Trank & Smith (1996), Krouchev et al. (2006) |
| Vastus lateralis | VL | Knee extensor | Loeb et al. (1985), Hoffer et al. (1987 b), Trank & Smith (1996), Krouchev et al. (2006) |
| Vastus medialis | VM | Knee extensor | Loeb et al. (1985), Hoffer et al. (1987 b) |
To identify muscle synergies, we used a cluster analysis, where synergies are defined based on synchronous periods of EMG activity, as described in previous studies (Krouchev et al. 2006; Drew et al. 2008; Yakovenko et al. 2011; Markin et al. 2012). First, onsets and offsets of a given EMG burst were determined and normalized to step cycle in relation to limb contact. They were then represented graphically with the onsets and offsets on the x‐ and y‐axes, respectively (Fig. 1 A). Thus, each EMG burst was represented by a data point on a scatterplot. Data points from different cycles generated a cluster around a mean value (Fig. 1 B). For each EMG burst, the mean of the onsets and offsets was calculated along with their standard deviations: σonset and σoffset. Next, the radius (R) was measured as the square root of the sum of the square of σonset and of σoffset (1).
| (1) |
Figure 1. Characterization of muscle synergies with cluster analysis.
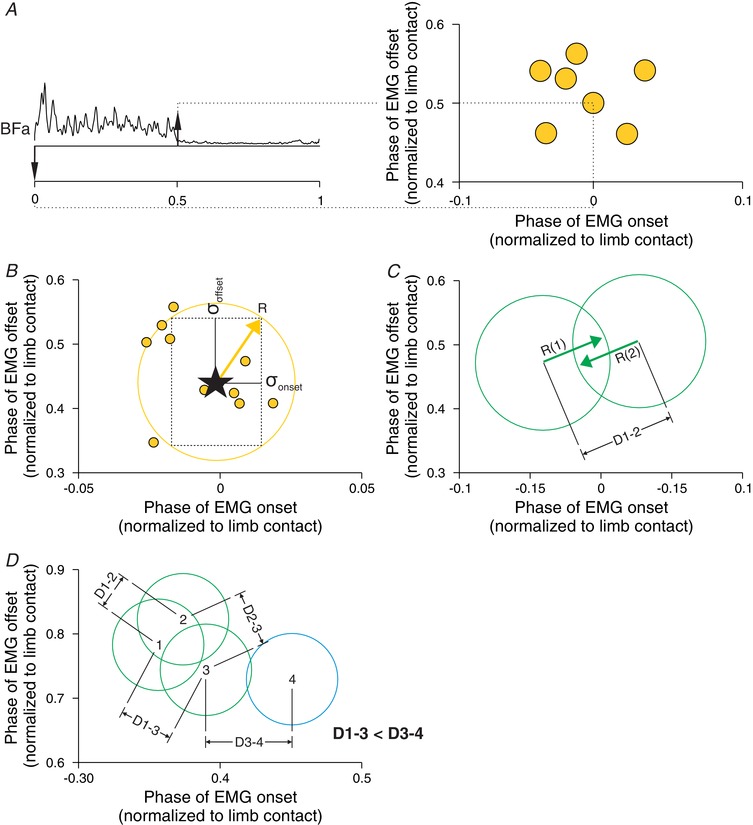
A, the onsets and offsets of individual EMG bursts were tagged manually (left panel) and represented on a scatterplot (right panel). B, for each muscle, the mean of the onsets and offsets was calculated (star) along with their standard deviations: σonset and σoffset. The radius (R) of the circle representing the cluster was measured as the square root of the sum of the square of σonset and of σoffset. C, we then calculated D, which is the distance between different clusters. D, to determine if different EMG bursts belong to the same synergy, we compared D between clusters.
The R value delimits the clustering of the EMG bursts by drawing a circle around the data points with the centre representing the mean of the onsets and offsets (Fig. 1 B). The R values were measured and representative circles were drawn for each EMG burst (Fig. 1 C). To mathematically determine if two EMG bursts belonged to the same synergy, we first selected an EMG burst of reference that had the earliest onset. We then compared this EMG burst of reference to the EMG burst with the next onset. We then calculated the distance (D) between each cluster (i.e. the centre of the circles).
| (2) |
In eqn (2), X 1 and X 2 represent the mean of the onsets of EMG bursts 1 and 2, respectively, and Y 1 and Y 2 represent the mean of the offsets of EMG bursts 1 and 2, respectively. Then, we determined if the sum of R 1 and R 2, the radii of the clusters of EMG burst 1 and 2, were smaller than the distance between EMG bursts (D 1–2) (Fig. 1 C). If the sum of the radii was smaller than D, then mathematically these EMG bursts were categorized as potentially belonging to the same muscle synergies.
In our classification, an EMG burst from a given muscle could only belong to a single synergy. If the clusters of different EMG bursts overlapped, we assigned them as potentially belonging to the same synergy. This was the first separation of potential muscle synergies. Some EMG bursts were initially assigned to several potential synergies. Thus, in Fig. 1 D, clusters 1, 2 and 3 as well as 3 and 4 potentially belong to the same synergy. To determine if overlapping clusters belonged to the same muscle synergy, we proceeded with triplet comparisons. In the example, based on D values, clusters 2 and 3 belong with cluster 1 because D 1–2 < R 1 + R 2 and D 1–3 < R 1 + R 3. We then determined if clusters 2 and 3 belonged in the same synergy by determining if D 2–3 < R 2 + R 3. If these criteria were met, then clusters 1, 2 and 3 belonged to the same synergy. In the example, clusters 3 and 4 also overlapped. To determine if cluster 3 is part of the synergy with clusters 1 and 2 or with cluster 4, we used another triplet comparison. We kept the smallest distance between clusters 1, 2 and 3 (D 1–3 in this case) and compared it with the distance between clusters 3 and 4 (D 3−4). We know that D 1–3 < R 1 + R 3 and that D 3–4 < R 3 + R 4. However, D 1–4 > R 1 + R 4 and as such, clusters 1 and 4 could not be included in the same synergy. As D 1–3 was smaller than D 3–4, then cluster 3 belonged in the synergy with cluster 1 and not cluster 4, which would be part of a different synergy. These triplet comparisons were made sequentially with all overlapping clusters until each EMG burst was included in a single synergy.
Statistics
To determine if there were significant shifts in the onsets and offsets of muscle synergies in the different locomotor conditions, we performed a mixed linear model. This type of model is effective when analysing repeated measurements (state, speed) and can accommodate incomplete data sets, as different muscles were available for analysis on an individual cat basis. In the mixed linear model, cats (n = 7) were treated as a random effect. We performed statistical analyses for tied‐belt and split‐belt locomotion at three speeds and left–right speed differences, respectively. For split‐belt locomotion, data obtained in the slow and fast limbs were treated separately. In each condition, we performed a two‐factor (state, speed) mixed linear model on the onsets and offsets of each muscle synergy. Analyses were made with SPSS Statistics 24.0 (IBM Corp., Armonk, NY, USA) and the results were statistically significant at P < 0.05. All residual values were validated.
Results
Hindlimb muscle synergies in the intact and spinal states
To identify muscle synergies, we applied a cluster analysis to hindlimb EMG activity in seven cats, before and after spinal transection. Twenty‐five periods of EMG activity from 19 hindlimb muscles (six of these had biphasic EMG activity) were recorded from two separate connectors in different locomotor conditions. For all muscles, we normalized EMG activity to limb contact. For example, we normalized the EMG activities of the left and right vastus lateralis (VL) to contact of the left and right hindlimbs, respectively. This allowed including muscles from the two limbs and from different locomotor episodes in the cluster analysis. The number of muscle synergies identified varied slightly between cats (4 to 7), depending on the number of high quality EMG signals and because of slight inter‐animal variations in EMG onsets and offsets.
In the examples shown in Figs 2 and 3 from Cat 5, we identified six muscle synergies with the cluster analysis before and after spinal transection, three corresponding to limb contact/stance phase (S1–S3) and three to limb liftoff/swing phase (S5–S7). Note that S4, another stance‐related synergy found in other cats, was not present in Cat 5. In the intact state, the first synergy (S1) consisted of the ankle extensors lateral gastrocnemius (LG), medial gastrocnemius (MG), soleus (SOL) and plantaris (PLA) that discharged synchronously starting before limb contact and ending prior to liftoff. A second synergy (S2) that consisted of the second burst of the semitendinosus (St) and biceps femoris posterior (BFp) muscles (knee flexors/hip extensors) had a slightly delayed onset compared to the first synergy, with short bursts ending just before limb contact. A third synergy (S3) included the knee (vastus lateralis; VL) and hip (biceps femoris anterior (BFa), semimembranosus (Sm) and caudofemoralis (CF)) extensors, which had a delayed onset compared to the first and second synergies with an offset similar to S1. A fourth synergy (S5) consisted of the main knee flexor burst of the St and BFp muscles, with an onset prior to liftoff and an offset around mid‐swing. A fifth synergy (S6) consisted of the hip flexors sartorius anterior (SrtA) and iliopsoas (IP) starting before liftoff and ending around limb contact. A sixth synergy (S7) consisted of the ankle flexor tibialis anterior (TA), with a delayed onset compared to S5 and S6 and an offset around limb contact. The hindlimb muscle synergies we identified in the intact state were consistent with those reported in intact cats during treadmill locomotion (Krouchev et al. 2006; Markin et al. 2012).
Figure 2. Hindlimb muscle synergies during tied‐belt locomotion at 0.7 m s−1 in one intact cat.
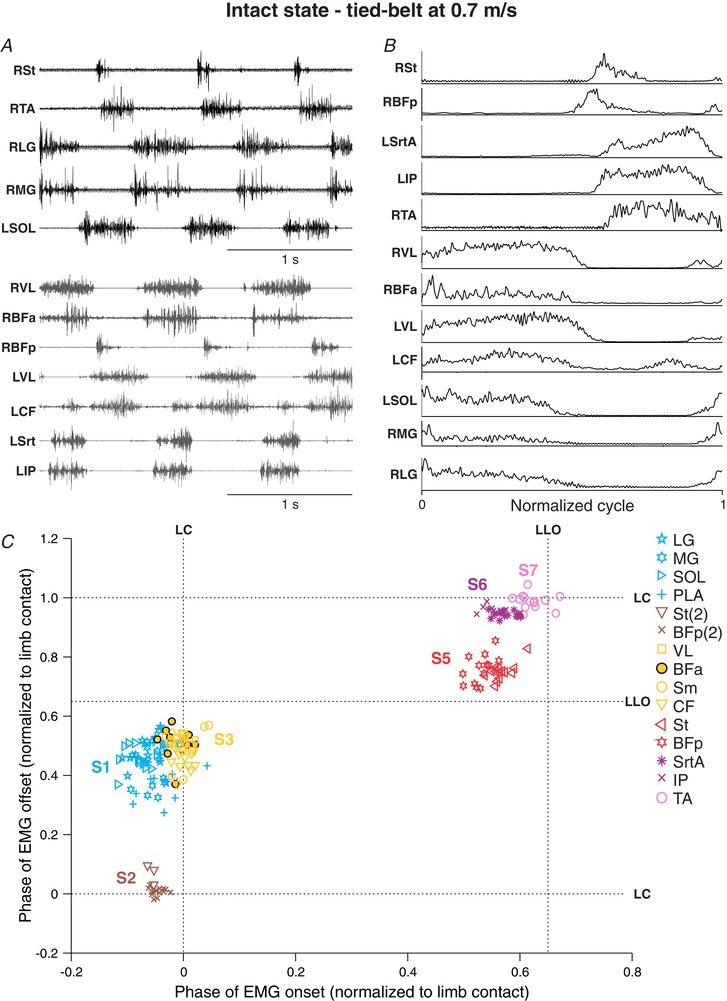
A, raw EMG waveforms obtained from 12 hindlimb muscles from two different locomotor episodes (black and grey EMGs). B, the EMGs were then averaged, rectified, and normalized to limb contact. C, the onsets and offsets of individual EMG bursts were measured and represented on a scatterplot. Each data point represents an EMG burst from one step cycle. The different muscle synergies (S1–S7) were determined by cluster analysis. Note that S4 is not present in this cat. For muscle abbreviations, see Table 1. Data are from Cat 5.
Figure 3. Hindlimb muscle synergies during tied‐belt locomotion at 0.7 m s−1 in one spinal cat.
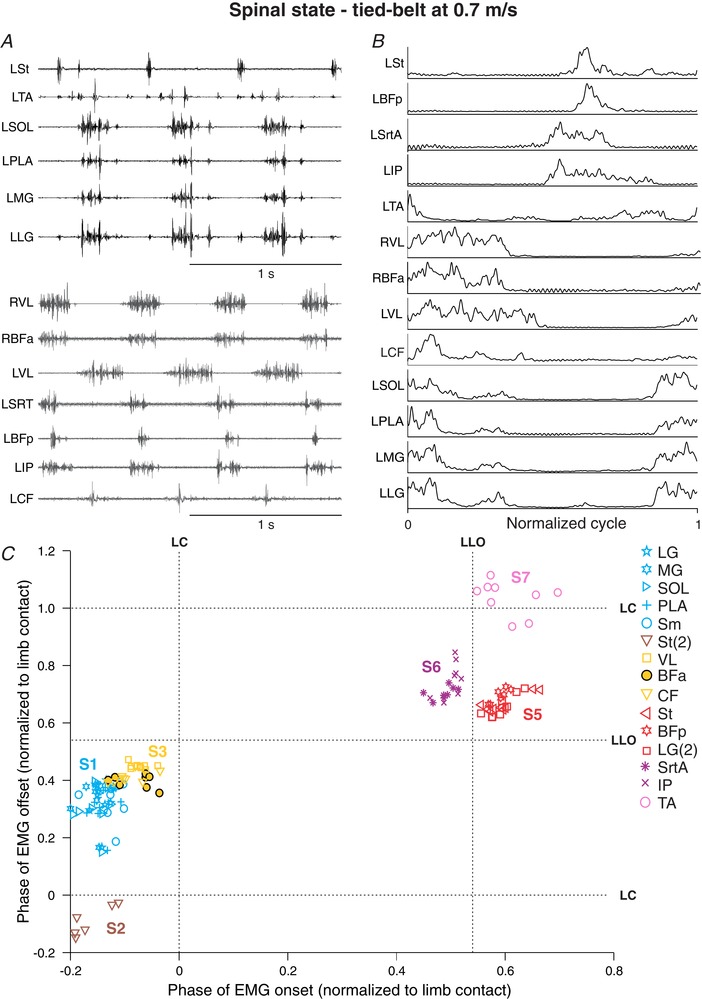
A, raw EMG waveforms obtained from 13 hindlimb muscles from two different locomotor episodes (black and grey EMGs). B, the EMGs were then averaged, rectified, and normalized to limb contact. C, the onsets and offsets of individual EMG bursts were measured and represented on a scatterplot. Each data point represents an EMG burst from one step cycle. The different muscle synergies (S1–S7) were determined by cluster analysis. Note that S4 is not present in this cat. For muscle abbreviations, see Table 1. Data are from Cat 5.
After spinal transection (i.e. the spinal state), hindlimb muscle synergies were maintained, although their timing in relation to one another and to limb contact/liftoff could change (Fig. 3 C). For instance, S1–S3 were shifted to the left in relation to limb contact, indicating that their onsets occurred earlier in the normalized cycle. The Sm muscle, a knee flexor and hip extensor, also changed from S3 in the intact state (Fig. 2 C) to S1 in the spinal state, having synchronous activity with ankle extensors. The most striking change occurred in the relation between S5 (St, BFp) and S6 (SrtA, IP). The hip flexor synergy S6 had an earlier onset than the knee flexor synergy S5, the latter starting after liftoff. Other than the Sm muscle changing synergies and the absence of a second burst in BFp in the spinal state, the number of synergies in the intact and spinal states was the same in this cat, as were the muscles that comprised these synergies.
Figure 4 summarizes synergies of all muscles obtained at 0.7 m s−1 during tied‐belt locomotion in the intact and spinal state for the group. Overall, we identified seven muscle synergies in both states. The first synergy (S1) consisted of ankle extensors, with the exception of Sm a hip extensor/knee flexor. The second synergy (S2) consisted of the second burst of St and BFp around limb contact. In the spinal state, S2 had a slightly earlier onset than S1. The third synergy (S3) consisted of hip and knee extensors, with an onset and offset slightly delayed compared to S1. The fourth synergy (S4), which was not present in Cat 5 (Figs 2 and 3) consisted of the second burst of the hip extensor tensor fascia lata (TFL) and the hip flexor/knee extensor SrtA, starting during early stance and ending during mid‐stance. The fifth synergy (S5) consisted of knee flexors (St, BFp, Sm and the second LG burst) that also extend the hip. The sixth synergy (S6) consisted of hip flexors (SrtA, SrtM, IP) and the hip extensor TFL that stabilizes the hip during locomotion (Chanaud et al. 1991). The seventh synergy (S7) consisted of the ankle flexor TA. Although this muscle appears to synchronize with S7 for the group when averaged, on an individual cat basis it was separate from S6, with an earlier or later onset and offset depending on the animal. The seven synergies identified in the intact state (Fig. 4 A) were present in the spinal state (Fig. 4 B), although there were some shifts in timing between certain synergies and in relation to limb contact and liftoff, most notably S6 preceding S5 in the spinal state.
Figure 4. Hindlimb muscle synergies during tied‐belt locomotion at 0.7 m s−1 in the intact and spinal states for the group.
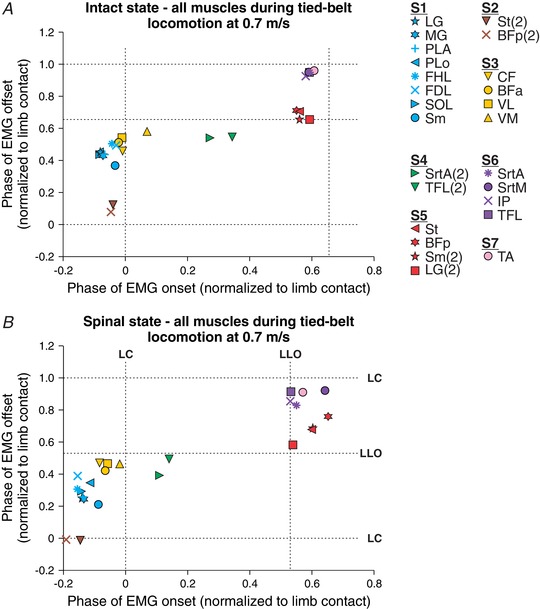
All EMG bursts obtained in the intact (A) and the spinal (B) states were classified into one of seven muscle synergies using the cluster analysis. Each data point is the average from 2–7 cats. The dashed vertical and horizontal lines show limb contact (LC) and limb liftoff (LLO, swing onset).
Hindlimb muscle synergies at different speeds in the intact and spinal states
It was argued that recording and evaluating EMGs in various behavioural conditions strengthens the hypothesis that muscle synergies are the main functional units of locomotor control (d'Avella and Bizzi, 2005; Tresch and Jarc, 2009). We therefore characterized hindlimb muscle synergies at different speeds during tied‐belt locomotion and at different left–right speeds during split‐belt locomotion (see next section). An increase in speed requires modulation of EMG activity. For instance, extensor burst duration is reduced with increasing speed while flexor burst duration remains relatively the same (reviewed in Gossard et al. 2011). We characterized hindlimb muscle synergies at three different speeds in the intact (Fig. 5 A) and spinal (Fig. 5 B) states for the group. Different symbols (circles, triangles, squares) and colours represent different speeds and synergies, respectively. A specific coloured symbol represents a different muscle at a given speed. For example, there are eight light blue squares for S1, each representing a different muscle (see Fig. 4 legend for the muscles comprising each synergy).
Figure 5. Hindlimb muscle synergies during tied‐belt locomotion at three different speeds in the intact and spinal states for the group.
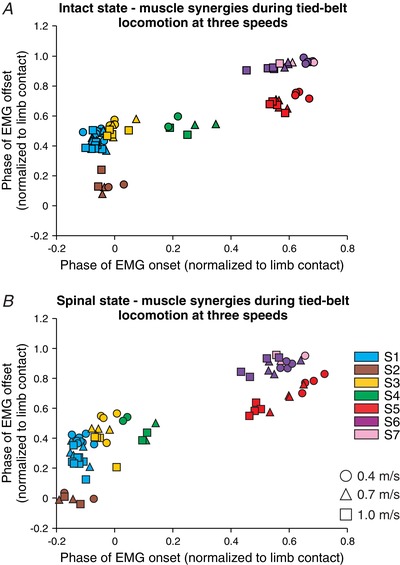
The seven hindlimb muscle synergies are shown at three different speeds in the intact (A) and the spinal (B) states. Each data point represents a different EMG burst. Different symbols (circles, triangles, squares) and colours represent different speeds and synergies, respectively. Each data point is the average from 2–7 cats.
To determine speed‐ and state‐dependent effects on the onsets and offsets of muscle synergies during tied‐belt locomotion, we ran a two‐factor mixed linear model (see Methods) on S1–S6. We did not have enough data points for the TA muscle to run statistical tests on S7. The muscles comprising the different synergies remained the same at different speeds, although there were some significant speed‐dependent shifts in the onsets and/or offsets of synergies. For instance, S1 (P = 0.04), S2 (P = 0.03), S5 (P = 0.0003) and S6 (P = 0.00002) had a significantly earlier onset in relation to limb contact with increasing speed but not S3 (P = 0.47) and S4 (P = 0.24). The offset also occurred significantly earlier in relation to limb contact with increasing speed for S1 (P = 0.01), S3 (P = 0.009), S4 (P = 0.02) and S5 (P = 0.01), but not S2 (P = 0.82) and S6 (P = 0.17).
Although the muscles comprising each synergy remained the same following spinal transection, there were some significant state‐dependent changes in the onsets and/or offsets of some synergies. For instance, S1 (P = 0.00000004), S2 (P = 0.0009), S3 (P = 0.0002), S4 (P = 0.001) and S6 (P = 0.002) had significantly earlier onsets in relation to limb contact but not S5 (P = 0.36). The offsets of S1 (P = 0.00002), S2 (P = 0.04), S3 (P = 0.08), S4 (P = 0.01) and S6 (P = 0.0000002) also occurred significantly earlier with no change in the offset of S5 (P = 0.47). Thus, the change in the timing between the knee flexor synergy S5 and the hip flexor synergy S6 is explained by an earlier onset and offset of S6 in the normalized step cycle without a change in S5.
Hindlimb muscle synergies at different left–right speeds in the intact and spinal states
Adjusting to split‐belt locomotion requires predictable adjustments in cycle and phase durations, as well as modulation of EMG burst durations in the limbs stepping on the slow and fast belts in intact and spinal cats (Forssberg et al. 1980; Halbertsma, 1983; Frigon et al. 2013, 2015, 2017). We characterized hindlimb muscle synergies in the slow and fast limbs in the intact and spinal states. In the examples shown in Fig. 6, six muscle synergies were identified in Cat 5 during split‐belt locomotion with the slow and fast limbs stepping at 0.4 m s−1 and 0.7 m s−1, respectively, in the intact and spinal states. Small differences were found between the slow and fast limbs. For example, in the intact state, the separation between S6 (SrtA, IP) and S7 (TA) was more evident in the fast limb compared to the slow limb (Fig. 6 A). In the spinal state, the change in relation between S6 and S7 was observed in the slow and fast limbs (Fig. 6 B). However, overall, the same synergies were found in the slow and fast limbs in both states, indicating that they are maintained after spinal transection.
Figure 6. Hindlimb muscle synergies during split‐belt locomotion in the intact and spinal states in one cat.
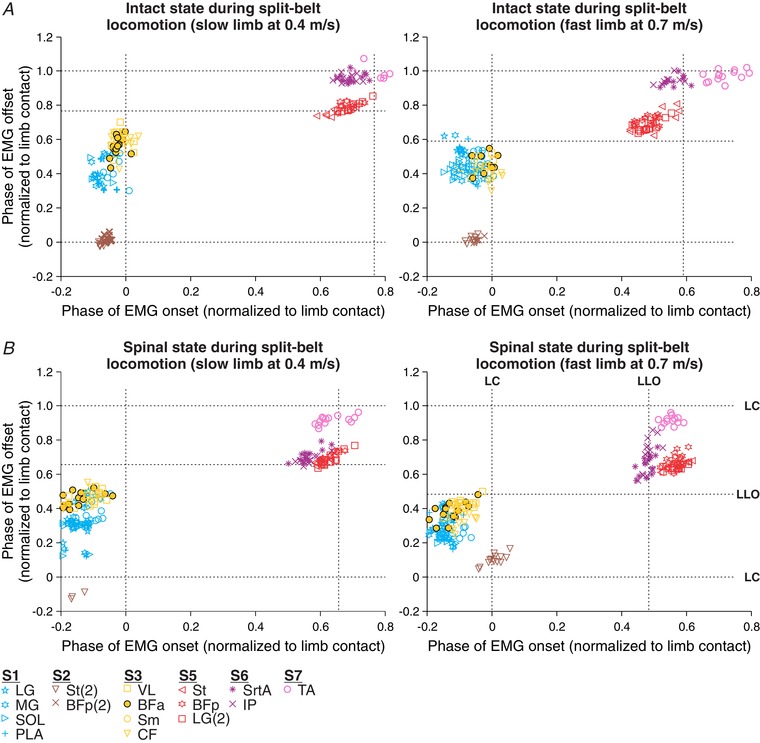
Shown are muscle synergies obtained during split‐belt locomotion with the slow (left panels) and fast (right panels) limbs stepping at 0.4 and 0.7 m s−1, respectively, in the intact (A) and the spinal (B) state. The onsets and offsets of individual EMG bursts were measured and represented on a scatterplot. Each data point represents an EMG burst from one step cycle. The different muscle synergies (S1–S7) were determined by cluster analysis. Note that S4 is not present in this cat. For muscle abbreviations, see Table 1. Data are from Cat 5.
Figure 7 summarizes the synergies of all muscles obtained during split‐belt locomotion with the slow and fast limbs stepping at 0.4 and 0.7 m s−1, respectively, in the intact and spinal states for the group. Overall, seven muscle synergies were identified in the slow and fast limbs in the intact (Fig. 7 A) and spinal (Fig. 7 B) states. The number and type of muscle synergies were the same as those identified during tied‐belt locomotion (Fig. 4), although there could be some differences in onsets and offsets between the slow and fast limbs and between states, as described below.
Figure 7. Hindlimb muscle synergies during split‐belt locomotion in the intact and spinal states for the group.
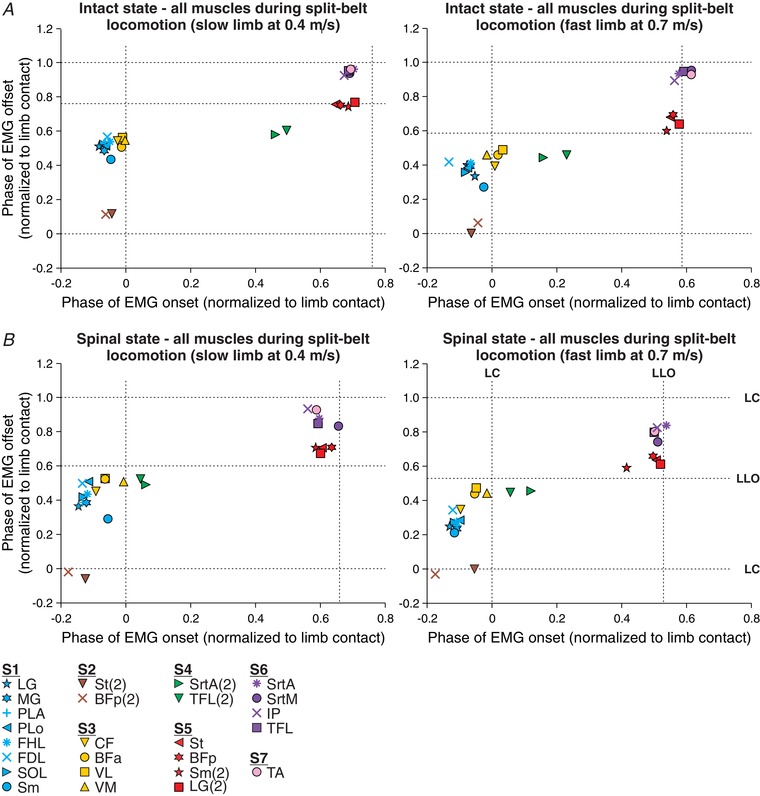
Shown are muscle synergies obtained during split‐belt locomotion with the slow (left panels) and fast (right panels) limbs stepping at 0.4 and 0.7 m s−1, respectively, in the intact (A) and the spinal (B) state. All EMG bursts obtained were classified into one of seven muscle synergies using the cluster analysis. Each data point is the average from 2–7 cats. The dashed vertical and horizontal lines show limb contact (LC) and limb liftoff (LLO, swing onset).
To determine speed‐ and state‐dependent effects on the onsets and offsets of muscle synergies during split‐belt locomotion, we ran a two‐factor mixed linear model (see Methods) on S1–S6 in the slow and fast limbs. As with tied‐belt locomotion, we did not have enough data points for the TA muscle to run statistical tests on S7 during split‐belt locomotion. We characterized hindlimb muscle synergies at three different speeds of the fast limb while the slow limb was stepping at 0.4 m s−1 in the intact (Fig. 8 A) and spinal (Fig. 8 B) states. As in Fig. 5, different symbols (circles, triangles, squares) and colours represent different speeds (of the fast limb) and synergies, respectively. In the slow limb, which stepped at 0.4 m s−1, there were no significant effects of increasing the speed of the fast limb for S1 (P = 0.45), S2 (P = 0.97), S3 (P = 0.43), S4 (P = 0.91), S5 (P = 0.56) or S6 (P = 0.56). Similarly, increasing the speed of the fast limb had no significant effects on the offsets of S1 (P = 0.52), S2 (P = 0.74), S3 (P = 0.84), S4 (P = 0.35), S5 (P = 0.63) or S6 (P = 0.63) of the slow limb.
Figure 8. Hindlimb muscle synergies during split‐belt locomotion at three different speeds of the fast limb in the intact and spinal states for the group.
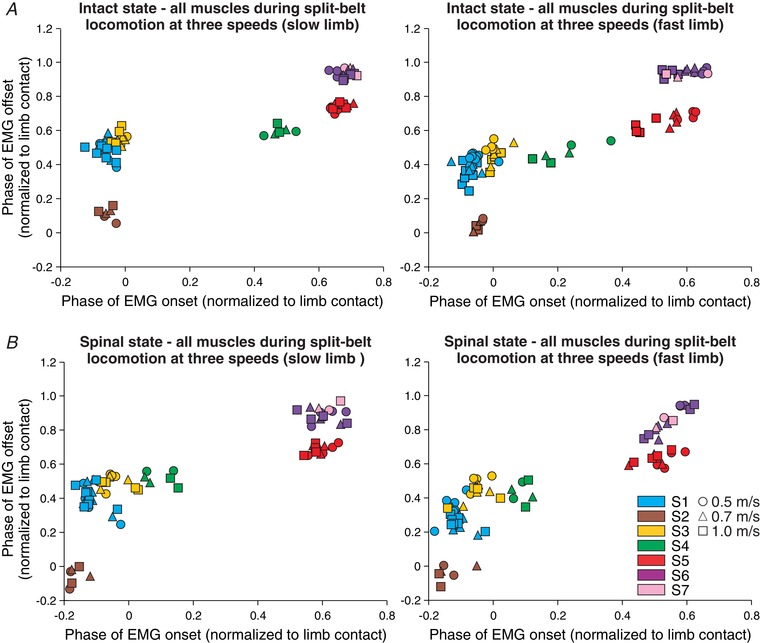
The seven hindlimb muscle synergies are shown at three different speeds of the fast limb in (A) the intact and (B) the spinal states. Each data point represents a different EMG burst. Different symbols (circles, triangles, squares) and colours represent different speeds and synergies, respectively. Each data point is the average from 2–7 cats.
In the fast limb, which stepped at 0.5, 0.7 and 1.0 m s−1, increasing its speed generated a significantly earlier onset for S5 (P = 0.002) and S6 (P = 0.002) with no significant changes in S1 (P = 0.24), S2 (P = 0.60), S3 (P = 0.54) and S4 (P = 0.47). Increasing the speed of the fast limb had no significant effects on the offsets of its synergies: S1 (P = 0.23), S2 (P = 0.78), S3 (P = 0.28), S4 (P = 0.65), S5 (P = 0.81) and S6 (P = 0.66). Thus, increasing the speed of only one limb during split‐belt locomotion produces fewer significant shifts in the onsets and offsets of muscle synergies compared to increasing the speed of both limbs concurrently during tied‐belt locomotion.
Although the muscles that compose each synergy also remained the same following spinal transection during split‐belt locomotion, there were some significant state‐dependent changes in the onsets and/or offsets of some synergies. For instance, in the slow limb, S1 (P = 0.00000002), S2 (P = 0.002), S3 (P = 0.0001), S4 (P = 0.01), S5 (P = 0.02) and S6 (P = 0.0000004) had significantly earlier onsets in relation to limb contact after spinal transection. However, only S1 (P = 0.03) and S2 (P = 0.007) of the slow limb had a significantly earlier offset after spinal transection, with no changes in S3 (P = 0.61), S4 (P = 0.84), S5 (P = 0.87) and S6 (P = 0.66). In the fast limb, S1 (P = 0.0000001), S3 (P = 0.00000005), S4 (P = 0.03) and S6 (P = 0.01) had significantly earlier onsets in relation to limb contact after spinal transection but not S2 (P = 0.06) and S5 (P = 0.61). The change in state had no significant change in the offsets of synergies of the fast limb: S1 (P = 0.19), S2 (P = 0.06), S3 (P = 0.17), S4 (P = 0.30), S5 (P = 0.16) and S6 (P = 0.35).
Discussion
We characterized muscle synergies during locomotion in the same animals before (intact state) and after (spinal state) spinal transection, showing that muscle synergies at different speeds during tied‐belt and split‐belt locomotion were maintained in the spinal state, although there were some significant shifts in the onsets and/or offsets of some synergies. Our results validate the concept that muscle synergies controlling hindlimb locomotion in an adult mammalian system are organized within the spinal cord.
Hindlimb locomotion requires a small number of muscle synergies
The cat or human leg has approximately 50 muscles and several are compartmentalized with distinct innervations. Despite the high number of muscles, locomotion can be explained by five to seven hindlimb/leg muscle synergies in cats and humans (Ivanenko et al. 2004; Krouchev et al. 2006; Dominici et al. 2011; Markin et al. 2012). In the present study, we characterized 25 periods of EMG activity from 19 hindlimb muscles during the step cycle, showing that seven muscle synergies were sequentially activated during the locomotor cycle in the intact and spinal states. The synergies corresponded to limb contact preparation (S2), stance (S1, S3 and S4), lifting the leg upwards at the stance‐to‐swing transition (S5), moving the limb forward during swing (S6) and flexing the ankle during swing (S7). The muscle synergies and their functional roles during the step cycle are summarized in Fig. 9. Although additional synergies might be present with more muscles implanted, we believe that this would simply have added more muscles per synergy and not additional muscle synergies, as the main behavioural features of the step cycle were represented by at least one synergy (Krouchev et al. 2006).
Figure 9. Summary figure of hindlimb muscle synergies during the step cycle.
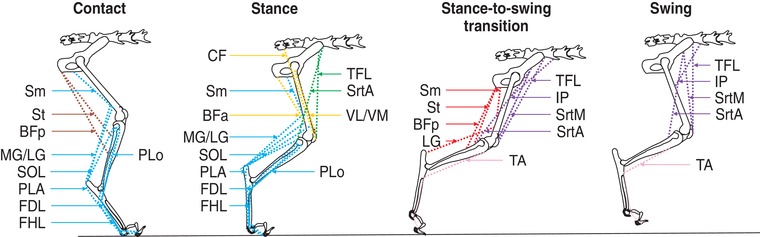
The different hindlimb muscles and their origins and insertions are shown. Each synergy is represented by a different colour. The image of the hindlimb was adapted from Markin et al. (2012).
The hindlimb muscle synergies described in the present study were similar to those obtained in intact cats at a single treadmill speed (Krouchev et al. 2006; Markin et al. 2012). One difference with those studies is that hip extensors, such as BFa and gluteus medius were grouped with ankle extensors in one synergy and the knee extensor VL in another. In our study, stance‐related synergies were divided into an ankle extensor synergy (S1) that also included Sm and a hip extensor/knee extensor synergy (S3). The S1 synergy was activated before S3, consistent with stabilizing the ankle at limb contact before activating knee and hip extensor muscles. Although we found mathematical overlap of clusters belonging to EMG bursts of hip and ankle extensors, our analysis before and after spinal transection in the different locomotor conditions allowed us to separate hip and ankle extensor muscles in two separate synergies. In other words, the different locomotor conditions allowed us to more effectively delineate burst onsets and offsets. The Sm, a hip extensor, is an interesting case. Markin et al. (2012) also separated hip and ankle extensor synergies based on deletion data obtained during fictive locomotion but noted that the bursting pattern in the combined Sm–BFa nerve behaved differently from ankle extensor nerves. Similar fictive locomotor data using individual Sm and BFa nerves are required to confirm their convergent or divergent firing patterns and whether they belong to separate muscle synergies. The S4 synergy, which includes the second burst of the hip extensor TFL and the hip flexor/knee extensor SrtA, most likely serves to increase stiffness of the hip joint from early to mid‐stance. Interestingly, the S4 synergy was not present in some cats (e.g. Cat 5 in Fig. 2). Although we can only speculate, we believe that the absence of synergies in some cats is due to biomechanical differences in the locomotor pattern between animals. A thorough biomechanical analysis coupled to muscle synergy analysis is required to address this issue.
We showed that the seven muscle synergies were maintained at three different speeds during tied‐belt locomotion in the intact and spinal states (Fig. 5). Not surprisingly, there were some significant shifts in the onsets and offsets of muscle synergies in relation to limb contact with increasing speed. This is because the duration of the stance phase, or extensor activity, is reduced with increasing speed while the duration of the swing phase, or flexor activity, remains relatively unchanged (reviewed in Gossard et al. 2011). Studies in healthy humans during locomotion have also shown that leg muscle synergies remained consistent at different speeds, although new synergies could be added during running as additional muscles were recruited (Ivanenko et al. 2004, 2006; Yokoyama et al. 2017). We did not investigate muscle synergies in different gaits, such as walking, trotting and galloping because galloping is difficult to safely obtain on a treadmill in intact cats and are not readily quantifiable in spinal cats due to the lack of forelimb movements.
Instead, we characterized muscle synergies during split‐belt locomotion. An increase in speed in the fast limb requires asymmetric adjustments in the durations of the stance and swing phases in the slow and fast limbs (Frigon et al. 2013, 2017), imposing a different challenge to the neural control system compared to a bilateral speed increase. We found that muscle synergies remained consistent during split‐belt locomotion in both the slow and fast limbs with increasing left–right speed differences. Therefore, the presence of the same muscle synergies during tied‐belt and split‐belt locomotion are consistent with muscle synergies as a robust neural control strategy.
Hindlimb muscle synergies for locomotion are organized within the spinal cord
In the different locomotor conditions, the muscles comprising the different synergies remained the same before and after spinal transection. This indicates that the control of muscle synergies when adjusting to speed, at least over the range studied, and to basic challenges in left–right coordination imposed by split‐belt locomotion, is organized within the spinal cord. Other studies that compared hindlimb muscle activity in the same cats before and after spinal transection during locomotion also showed that the temporal structure of EMG bursts was largely the same in the intact and spinal states (Belanger et al. 1996; Frigon and Rossignol, 2008).
It has been proposed that muscle synergies are controlled by spinal interneurons organized into functional units, or modules, and that these are targets of inputs from limb afferents and supraspinal structures (Dominici et al. 2011; Markin et al. 2012; Bizzi and Cheung, 2013). It was further proposed that the spinal modules controlling muscle synergies are part of the spinal locomotor CPG (McCrea and Rybak, 2008; Markin et al. 2012; Giszter and Hart, 2013). To determine if muscle synergies were generated centrally, Markin et al. (2012) compared synergies obtained during real locomotion in intact cats and during fictive locomotion evoked by electrical stimulation of the mesencephalic locomotor region in curarized decerebrate cats. They showed that muscle synergies during real and fictive locomotion were similar, concluding that they are primarily organized within the spinal cord, although supraspinal contributions from brainstem or other subcortical structures cannot be excluded in the decerebrate preparation.
The main difference observed by Markin et al. (2012) was the absence of biphasic EMG bursts during fictive locomotion in complex bifunctional muscles, such as BFp, St and Sm, which were prominent during real locomotion. In the present study, we had six muscles that showed a clear second burst during the step cycle that were included in three separate muscle synergies: S2, S4 and S5 (Figs 4 and 7). The fact that these muscle synergies were present in the intact and spinal states is consistent with an important role of limb afferents in their expression. We propose that, during locomotion, sensory feedback regulates the spinal modules controlling muscle synergies and that some afferent inputs are necessary for the full repertoire of muscle synergies. The flexible control of muscle synergies by sensory feedback allows cats to adjust to different speeds and left–right speed differences during locomotion. Although how limb afferents interact with spinal modules controlling muscle synergies for locomotion remains to be investigated, we recently proposed a model showing how sensory feedback related to loading, hip extension and hip flexion interact with spinal CPGs to coordinate the left and right sides during split‐belt locomotion (Frigon et al. 2017). Limb afferents were shown to regulate muscle synergies for adjusting limb trajectories in intact and spinal frogs (Kargo and Giszter, 2000, 2008; Cheung et al. 2005; Kargo et al. 2010).
It is important to note that our results do not exclude the existence of functional modules controlling hindlimb muscle synergies in supraspinal structures or the control of spinal modules by supraspinal pathways (Krouchev et al. 2006; Drew et al. 2008; Yakovenko et al. 2011; Roh et al. 2011; Yakovenko and Drew, 2015). Descending commands from supraspinal pathways are undoubtedly critical for goal‐oriented behaviours, activating and combining spinal locomotor modules to generate appropriate movements, particularly during precision walking, such as when avoiding an obstacle. Moreover, a supraspinal contribution appears important for the proper timing of the knee flexor and hip flexor synergies at swing onset. In the intact state, the knee flexor synergy S5 started before liftoff and preceded the hip flexor synergy S6, allowing the limb to be elevated before swinging forward (Fig. 4 A). However, in the spinal state, the knee flexor synergy started at or after swing onset (Fig. 4 B). The change in the timing between knee flexor and hip flexor EMG at swing onset is thought to explain paw drag after spinal lesions (Belanger et al. 1996; Jiang and Drew, 1996). One important limitation of the cluster analysis in identifying muscle synergies is that it only considers covariations of muscle burst onsets and onsets in assigning membership to a synergy. The weighting of individual muscles, or the balance between muscles, within a synergy might change according to task demands or between states (intact versus spinal). Other methods of identifying muscles synergies, such as non‐negative matrix factorization (Cheung et al. 2005), could provide additional insights on this issue.
Concluding remarks
We propose, as others have (Tresch et al. 1999; Hart and Giszter, 2004; d′Avella and Bizzi, 2005; Hart and Giszter, 2010; Roh et al. 2011; Bizzi and Cheung, 2013; Ting et al. 2015), that the organization of hindlimb muscle synergies within the spinal cord simplifies the neural control of locomotion, allowing motor cortical areas to be concerned with higher level functions, such as precision walking. Consistent with recent findings in spinal cord‐injured rodents (Wenger et al. 2016), spinal muscle synergies could be an important physiological target to restore meaningful locomotor movements after neurological injury.
Additional information
Competing interests
The authors declare no competing financial interests.
Author contributions
All experiments were performed in the laboratory of Dr Frigon at the Université de Sherbrooke. E.D., J.H., A.D., M.‐F.H. and A.F. contributed to data acquisition and revised the work critically for important intellectual content. E.D. and A.F. contributed to data analysis and in drafting the work. A.F. contributed to the conception and design of the work. All authors have read and approved the final version of this manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
The present research was funded by a Discovery Grant (RGPIN‐2016‐03790) from the Natural Sciences and Engineering Research Council of Canada (NSERC), a Project Team Grant (182035) from the Fonds de Recherche du Québec – Natures et Technologies and by the Arthur C. Guyton Award for Excellence in Integrative Physiology and the Beverly Petterson Bishop Award for Excellence in Neuroscience from The American Physiological Society to A. Frigon. M.‐F. Hurteau was supported by a doctoral scholarship from NSERC.
Acknowledgements
We thank P. Drapeau (Rossignol and Drew labs) for data acquisition and analysis software.
Biography
Etienne Desrochers obtained a bachelor's degree in mechanical engineering from the Université de Sherbrooke followed by a master's degree in physiology in the laboratory of Prof. Alain Frigon at the same institution. He now works at Royer as an innovation engineer, supervising footwear design for optimal performance. Using his background in motor control, he ensures that footwear respect basic biomechanical principles. In doing so, he is able to combine his passions for the human body, engineering and physics.

Edited by: Janet Taylor & Dario Farina
Linked articles: This article is highlighted in a Perspectives article by Giszter. To read this article, visit http://doi.org/10.1113/JP277310.
References
- Abraham LD & Loeb GE (1985). The distal hindlimb musculature of the cat. Patterns of normal use. Exp Brain Res 58, 583–593. [PubMed] [Google Scholar]
- Belanger M, Drew T, Provencher J & Rossignol S (1996). A comparison of treadmill locomotion in adult cats before and after spinal transection. J Neurophysiol 76, 471–491. [DOI] [PubMed] [Google Scholar]
- Bellardita C & Kiehn O (2015). Phenotypic characterization of speed‐associated gait changes in mice reveals modular organization of locomotor networks. Curr Biol 25, 1426–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein N (1967). The Co‐ordination and Regulation of Movements Pergamon Press, Oxford. [Google Scholar]
- Bizzi E & Cheung VC (2013). The neural origin of muscle synergies. Front Comput Neurosci 7, 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizzi E, Cheung VC, d'Avella A, Saltiel P & Tresch M (2008). Combining modules for movement. Brain Res Rev 57, 125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown IE, Satoda T, Richmond FJ & Loeb GE (1998). Feline caudofemoralis muscle. Muscle fibre properties, architecture, and motor innervation. Exp Brain Res 121, 76–91. [DOI] [PubMed] [Google Scholar]
- Caggiano V, Cheung VC & Bizzi E (2016). An optogenetic demonstration of motor modularity in the mammalian spinal cord. Sci Rep 6, 35185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanaud CM, Pratt CA & Loeb GE (1991). Functionally complex muscles of the cat hindlimb. V. The roles of histochemical fiber‐type regionalization and mechanical heterogeneity in differential muscle activation. Exp Brain Res 85, 300–313. [DOI] [PubMed] [Google Scholar]
- Cheung VC, d'Avella A, Tresch MC & Bizzi E (2005). Central and sensory contributions to the activation and organization of muscle synergies during natural motor behaviors. J Neurosci 25, 6419–6434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Avella A & Bizzi E (2005). Shared and specific muscle synergies in natural motor behaviors. Proc Natl Acad Sci U S A 102, 3076–3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominici N, Ivanenko YP, Cappellini G, d'Avella A, Mondi V, Cicchese M, Fabiano A, Silei T, Di PA, Giannini C, Poppele RE & Lacquaniti F (2011). Locomotor primitives in newborn babies and their development. Science 334, 997–999. [DOI] [PubMed] [Google Scholar]
- Drew T, Kalaska J & Krouchev N (2008). Muscle synergies during locomotion in the cat: a model for motor cortex control. J Physiol 586, 1239–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English AW & Weeks OI (1987). An anatomical and functional analysis of cat biceps femoris and semitendinosus muscles. J Morphol 191, 161–175. [DOI] [PubMed] [Google Scholar]
- Forssberg H, Grillner S, Halbertsma J & Rossignol S (1980). The locomotion of the low spinal cat. II. Interlimb coordination. Acta Physiol Scand 108, 283–295. [DOI] [PubMed] [Google Scholar]
- Frigon A, Desrochers E, Thibaudier Y, Hurteau MF & Dambreville C (2017). Left–right coordination from simple to extreme conditions during split‐belt locomotion in the chronic spinal adult cat. J Physiol 595, 341–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigon A, Hurteau MF, Thibaudier Y, Leblond H, Telonio A & D'Angelo G (2013). Split‐belt walking alters the relationship between locomotor phases and cycle duration across speeds in intact and chronic spinalized adult cats. J Neurosci 33, 8559–8566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigon A & Rossignol S (2008). Adaptive changes of the locomotor pattern and cutaneous reflexes during locomotion studied in the same cats before and after spinalization. J Physiol 586, 2927–2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigon A, Thibaudier Y & Hurteau MF (2015). Modulation of forelimb and hindlimb muscle activity during quadrupedal tied‐belt and split‐belt locomotion in intact cats. Neuroscience 290, 266–278. [DOI] [PubMed] [Google Scholar]
- Giszter SF (2015). Motor primitives—new data and future questions. Curr Opin Neurobiol 33, 156–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giszter SF & Hart CB (2013). Motor primitives and synergies in the spinal cord and after injury—the current state of play. Ann N Y Acad Sci 1279, 114–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossard JP, Sirois J, Noue P, Cote MP, Menard A, Leblond H & Frigon A (2011). Chapter 2 – The spinal generation of phases and cycle duration. Prog Brain Res 188, 15–29. [DOI] [PubMed] [Google Scholar]
- Grillner S (1981). Control of locomotion in bipeds, tetrapods, and fish In Handbook of Physiology, section I, The Nervous System, vol. II, Motor Control, ed. Brooks VB, pp. 1179–1236. American Physiological Society, Bethesda, MD, USA. [Google Scholar]
- Grillner S & Jessell TM (2009). Measured motion: searching for simplicity in spinal locomotor networks. Curr Opin Neurobiol 19, 572–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy D (2015). Principles and standards for reporting animal experiments in The Journal of Physiology and Experimental Physiology . J Physiol 593, 2547–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagglund M, Dougherty KJ, Borgius L, Itohara S, Iwasato T & Kiehn O (2013). Optogenetic dissection reveals multiple rhythmogenic modules underlying locomotion. Proc Natl Acad Sci U S A 110, 11589–11594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbertsma JM (1983). The stride cycle of the cat: the modelling of locomotion by computerized analysis of automatic recordings. Acta Physiol Scand Suppl 521, 1–75. [PubMed] [Google Scholar]
- Hart CB & Giszter SF (2004). Modular premotor drives and unit bursts as primitives for frog motor behaviors. J Neurosci 24, 5269–5282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart CB & Giszter SF (2010). A neural basis for motor primitives in the spinal cord. J Neurosci 30, 1322–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensbergen E & Kernell D (1992). Task‐related differences in distribution of electromyographic activity within peroneus longus muscle of spontaneously moving cats. Exp Brain Res 89, 682–685. [DOI] [PubMed] [Google Scholar]
- Hoffer JA, Loeb GE, Sugano N, Marks WB, O'Donovan MJ & Pratt CA (1987. a). Cat hindlimb motoneurons during locomotion. III. Functional segregation in sartorius. J Neurophysiol 57, 554–562. [DOI] [PubMed] [Google Scholar]
- Hoffer JA, Sugano N, Loeb GE, Marks WB, O'Donovan MJ & Pratt CA (1987. b). Cat hindlimb motoneurons during locomotion. II. Normal activity patterns. J Neurophysiol 57, 530–553. [DOI] [PubMed] [Google Scholar]
- Hurteau MF, Thibaudier Y, Dambreville C, Chraibi A, Desrochers E, Telonio A & Frigon A (2017). Non‐linear modulation of cutaneous reflexes with increasing speed of locomotion in spinal cats. J Neurosci 37, 3896–3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurteau MF, Thibaudier Y, Dambreville C, Danner SM, Rybak I & Frigon A (2018). Intralimb and interlimb cutaneous reflexes during locomotion in the intact cat. J Neurosci 38, 4104–4122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanenko YP, Poppele RE & Lacquaniti F (2004). Five basic muscle activation patterns account for muscle activity during human locomotion. J Physiol 556, 267–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanenko YP, Poppele RE & Lacquaniti F (2006). Spinal cord maps of spatiotemporal alpha‐motoneuron activation in humans walking at different speeds. J Neurophysiol 95, 602–618. [DOI] [PubMed] [Google Scholar]
- Jiang W & Drew T (1996). Effects of bilateral lesions of the dorsolateral funiculi and dorsal columns at the level of the low thoracic spinal cord on the control of locomotion in the adult cat. I. Treadmill walking. J Neurophysiol 76, 849–866. [DOI] [PubMed] [Google Scholar]
- Kargo WJ & Giszter SF (2000). Rapid correction of aimed movements by summation of force‐field primitives. J Neurosci 20, 409–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kargo WJ & Giszter SF (2008). Individual premotor drive pulses, not time‐varying synergies, are the units of adjustment for limb trajectories constructed in spinal cord. J Neurosci 28, 2409–2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kargo WJ, Ramakrishnan A, Hart CB, Rome LC & Giszter SF (2010). A simple experimentally based model using proprioceptive regulation of motor primitives captures adjusted trajectory formation in spinal frogs. J Neurophysiol 103, 573–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehn O (2011). Development and functional organization of spinal locomotor circuits. Curr Opin Neurobiol 21, 100–109. [DOI] [PubMed] [Google Scholar]
- Kiehn O (2016). Decoding the organization of spinal circuits that control locomotion. Nat Rev Neurosci 17, 224–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne WJ, Cuthill IC, Emerson M & Altman DG (2010). Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol 8, e1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krouchev N, Kalaska JF & Drew T (2006). Sequential activation of muscle synergies during locomotion in the intact cat as revealed by cluster analysis and direct decomposition. J Neurophysiol 96, 1991–2010. [DOI] [PubMed] [Google Scholar]
- Lemay MA & Grill WM (2004). Modularity of motor output evoked by intraspinal microstimulation in cats. J Neurophysiol 91, 502–514. [DOI] [PubMed] [Google Scholar]
- Loeb GE, Hoffer JA & Pratt CA (1985). Activity of spindle afferents from cat anterior thigh muscles. I. Identification and patterns during normal locomotion. J Neurophysiol 54, 549–564. [DOI] [PubMed] [Google Scholar]
- Markin SN, Lemay MA, Prilutsky BI & Rybak IA (2012). Motoneuronal and muscle synergies involved in cat hindlimb control during fictive and real locomotion: a comparison study. J Neurophysiol 107, 2057–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrea DA & Rybak IA (2008). Organization of mammalian locomotor rhythm and pattern generation. Brain Res Rev 57, 134–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perell KL, Gregor RJ, Buford JA & Smith JL (1993). Adaptive control for backward quadrupedal walking. IV. Hindlimb kinetics during stance and swing. J Neurophysiol 70, 2226–2240. [DOI] [PubMed] [Google Scholar]
- Pratt CA, Buford JA & Smith JL (1996). Adaptive control for backward quadrupedal walking V. Mutable activation of bifunctional thigh muscles. J Neurophysiol 75, 832–842. [DOI] [PubMed] [Google Scholar]
- Pratt CA, Chanaud CM & Loeb GE (1991). Functionally complex muscles of the cat hindlimb. IV. Intramuscular distribution of movement command signals and cutaneous reflexes in broad, bifunctional thigh muscles. Exp Brain Res 85, 281–299. [DOI] [PubMed] [Google Scholar]
- Pratt CA & Loeb GE (1991). Functionally complex muscles of the cat hindlimb. I. Patterns of activation across sartorius. Exp Brain Res 85, 243–256. [DOI] [PubMed] [Google Scholar]
- Rasmussen S, Chan AK & Goslow GE Jr (1978). The cat step cycle: electromyographic patterns for hindlimb muscles during posture and unrestrained locomotion. J Morphol 155, 253–269. [DOI] [PubMed] [Google Scholar]
- Roh J, Cheung VC & Bizzi E (2011). Modules in the brain stem and spinal cord underlying motor behaviors. J Neurophysiol 106, 1363–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol S (1996). Neural control of stereotypic limb movements In Handbook of Physiology, Section 12, Exercise: Regulation and Integration of Multiple Systems, ed. Rowell LB. & Sheperd JT, 173–216. Oxford University Press, New York. [Google Scholar]
- Smith JL, Carlson‐Kuhta P & Trank TV (1998). Forms of forward quadrupedal locomotion. III. A comparison of posture, hindlimb kinematics, and motor patterns for downslope and level walking. J Neurophysiol 79, 1702–1716. [DOI] [PubMed] [Google Scholar]
- Smith JL, Chung SH & Zernicke RF (1993). Gait‐related motor patterns and hindlimb kinetics for the cat trot and gallop. Exp Brain Res 94, 308–322. [DOI] [PubMed] [Google Scholar]
- Ting LH, Chiel HJ, Trumbower RD, Allen JL, McKay JL, Hackney ME & Kesar TM (2015). Neuromechanical principles underlying movement modularity and their implications for rehabilitation. Neuron 86, 38–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trank TV & Smith JL (1996). Adaptive control for backward quadrupedal walking VI. Metatarsophalangeal joint dynamics and motor patterns of digit muscles. J Neurophysiol 75, 678–679. [DOI] [PubMed] [Google Scholar]
- Tresch MC & Bizzi E (1999). Responses to spinal microstimulation in the chronically spinalized rat and their relationship to spinal systems activated by low threshold cutaneous stimulation. Exp Brain Res 129, 401–416. [DOI] [PubMed] [Google Scholar]
- Tresch MC & Jarc A (2009). The case for and against muscle synergies. Curr Opin Neurobiol 19, 601–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tresch MC, Saltiel P & Bizzi E (1999). The construction of movement by the spinal cord. Nat Neurosci 2, 162–167. [DOI] [PubMed] [Google Scholar]
- Wenger N, Moraud EM, Gandar J, Musienko P, Capogrosso M, Baud L, Le Goff CG, Barraud Q, Pavlova N, Dominici N, Minev IR, Asboth L, Hirsch A, Duis S, Kreider J, Mortera A, Haverbeck O, Kraus S, Schmitz F, DiGiovanna J, van den Brand R, Bloch J, Detemple P, Lacour SP, Bezard E, Micera S & Courtine G (2016). Spatiotemporal neuromodulation therapies engaging muscle synergies improve motor control after spinal cord injury. Nat Med 22, 138–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakovenko S & Drew T (2015). Similar motor cortical control mechanisms for precise limb control during reaching and locomotion. J Neurosci 35, 14476–14490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakovenko S, Krouchev N & Drew T (2011). Sequential activation of motor cortical neurons contributes to intralimb coordination during reaching in the cat by modulating muscle synergies. J Neurophysiol 105, 388–409. [DOI] [PubMed] [Google Scholar]
- Yakovenko S, Mushahwar V, VanderHorst V, Holstege G & Prochazka A (2002). Spatiotemporal activation of lumbosacral motoneurons in the locomotor step cycle. J Neurophysiol 87, 1542–1553. [DOI] [PubMed] [Google Scholar]
- Yokoyama H, Ogawa T, Shinya M, Kawashima N & Nakazawa K (2017). Speed dependency in alpha‐motoneuron activity and locomotor modules in human locomotion: indirect evidence for phylogenetically conserved spinal circuits. Proc Biol Sci 284, 20170290. [DOI] [PMC free article] [PubMed] [Google Scholar]


