Abstract
Exogenous testosterone therapy can be used to treat testosterone deficiency; however, it has several adverse effects including infertility due to negative feedback on the hypothalamic–pituitary–gonadal (HPG) axis. Leydig stem cell (LSC) transplantation could provide a new strategy for treating testosterone deficiency, but clinical translatability of injecting stem cells inside the testis is not feasible. Here, we explore the feasibility of subcutaneously autografting LSCs in combination with Sertoli and myoid cells to increase testosterone. We also studied whether the grafted LSCs can be regulated by the HPG axis and the molecular mechanism behind this regulation. LSCs were isolated from the testes of 12‐week‐old C57BL/6 mice, and subcutaneously autografted in combination with Sertoli cells and myoid cells. We found that LSCs alone were incapable of self‐renewal and differentiation. However, in combination with Sertoli cells and myoid cells, LSCs underwent self‐renewal as well as differentiation into mature Leydig cells. As a result, the recipient mice that received the LSC autograft showed testosterone production with preserved luteinizing hormone. We found that testosterone production from the autograft was regulated by hedgehog (HH) signaling. Gain of function and loss of function study confirmed that Desert HH (DHH) agonist increased and DHH antagonist decreased testosterone production from autograft. This study is the first to demonstrate that LSCs, when autografted subcutaneously in combination with Sertoli cells and myoid cells, can increase testosterone production. Therefore, LSC autograft may provide a new treatment for testosterone deficiency while simultaneously preserving the HPG axis. Stem Cells Translational Medicine 2019;8:58–65
Keywords: Leydig cell, Hypogonadism, Fertility, Sertoli cell, Myoid cell
Significance Statement.
Leydig stem cell autograft is a novel therapeutic approach to increase serum testosterone while simultaneously preserving testicular function. By optimizing the protocol, a safe and effective treatment can be developed for men with low testosterone who desire fertility preservation. The potential impact of Leydig stem cell autograft as a therapy to increase testosterone could be paradigm shifting for the clinical treatment of low testosterone.
Introduction
Leydig cells (LCs) are the primary source of testosterone in men, and LC dysfunction can lead to testosterone deficiency 1. The current standard of care for men with testosterone deficiency is lifelong exogenous testosterone therapy 2. However, exogenous testosterone therapy results in negative feedback on the hypothalamic–pituitary–gonadal (HPG) axis, inhibiting follicle stimulating hormone (FSH) and luteinizing hormone (LH) production, which results in infertility 3, 4. Additionally, exogenous testosterone therapy is not an appropriate long‐term therapy for men due to side‐effects (gynecomastia, polycythemia, hypertension, acne, and hair loss) and potential risks such as heart attack/stroke/prostate cancer—although controversial 2, 5, 6, 7. Consequently, there is a need to develop different approaches to increasing serum testosterone while simultaneously preserving the HPG axis and fertility.
Leydig stem cells (LSCs) within the testis have the ability to proliferate and differentiate into LCs 8, 9, 10, 11. However, isolating and proliferating LSCs is difficult for three reasons: (a) lack of specific markers, (b) relatively rare population in adult testis (in particular men with low testosterone), and (c) necessity for paracrine signalling from Sertoli and myoid cells. Recently, transplanting stem cells into testis has shown great promise to potentially increase testosterone in men with low testosterone 12. However, clinical translatability of potentially injecting the testis more than once for treatment of low testosterone is impractical. We demonstrate that autografting LSCs (a combination of LCs, Sertoli cells, and peritubular myoid cells) in subcutaneous tissue can increase serum testosterone while simultaneously maintaining the production of FSH and LH. More importantly, we show that the autografted LSCs are modulated by hedgehog (HH) signaling. Thus, this study may provide a new strategy for the treatment of low testosterone.
Materials and Methods
Animals
A total of 27 C57/BL6 (6 weeks old) mice were purchased from Jackson Laboratories. We performed orchiectomy in 24 adult C57/BL6 mice. We subcutaneously autografted LSCs in nine mice and followed them for 30 days and compared them with castrate mice that did not receive an autograft (negative controls), mice that received testosterone pellets (positive controls), mice that received an autograft for 15 days, mice that received CD146+ cells, and mice that received cells treated with vismodegib and SAG (n = 3 mice in each condition). We used recommended dosages of isoflurane and oxygen for anesthesia. The animals were humanely euthanized by cardiac puncture while anesthetized as per recommended protocol. The animal protocol was approved by the Institutional Animal Care and Use Committee of University of Miami Miller School of Medicine, Miami, FL (protocol no. 15‐167).
LSC Isolation from Seminiferous Tubules
The protocol for LSC isolation has been described in ref. 11. Briefly, testes from a 6‐week‐old C57BL/6 mice (Jackson Laboratories, Bar Harbor, ME, USA) were removed and decapsulated. Interstitial cells from testes were dissociated from the seminiferous tubules by treatment with 1 mg/ml trypsin followed by collagenase (collagenase‐D; Roche Molecular Biochemicals, Indianapolis, IN, U.S.A) treatment in Dulbecco's modified Eagle's medium (DMEM) for 10 min at 34°C with shaking. The separated cells were filtered through two layers of 70‐μm pore size nylon mesh, centrifuged at 250 × g, and resuspended in DMEM. The cells were washed with phosphate‐buffered saline (PBS), centrifuged at 250g, and resuspended in phenol red‐free 1:1 DMEM:F12 and plated for cell culture.
LSC Culture in Combination with Sertoli Cells and Myoid Cells
LSCs were cultured in an expansion medium (EM) adapted for embryonic stem cell culture with modification 13. LSCs were maintained in this medium for at least 14 days. To induce differentiation, these cells were replated in a new medium containing differentiation‐inducing factors. Differentiation inducing medium (DIM) included DMEM:F12, 2% fetal bovine serum (FCS), 1% penicillin/streptomycin, 2 ng/ml luteinizing hormone (LH), 1 nM thyroid hormone, 10 ng/ml Insulin‐like growth factor 1 (GF‐1), and the insulin transferrin selenium (ITS) supplement.
Subcutaneous Autograft of LSC in Combination with Sertoli Cells and Myoid Cells
A total of 18 wild‐type adult C57/BL6 mice underwent LSC autograft. Cultured LSCs in combination with Sertoli and myoid cells were removed from culture plates with trypsin, after incubating for 5 min at 37°C; fresh media containing fetal bovine serum was added to stop trypsin activity after 14 days in culture. Then the cells were centrifuged for 5 min at 1500 RPM. Supernatant was replaced with fresh medium followed by cell counting. Desired amount of suspension was transferred to a fresh tube. After centrifuging, the supernatant was discarded and a mixture of PBS and matrigel (1:1) was added to the cell pellet. The cells were loaded into a 0.3‐ml syringe with a 29½‐gauge needle for injection into the subcutaneous tissue of the abdomen in castrate mice. For positive controls, we subcutaneously implanted two testosterone pellets (TESTOPEL, 75 mg/pellet, Endo Pharmaceuticals, Ireland) in three castrated animals for 28 days. Negative controls (castrate mice) received a subcutaneous injection of matrigel+PBS (1:1) without cells. Graft and blood from all animals were examined 28 days after pellet implantation or matrigel injection. LSCs in vitro were treated with vismodegib (Hedgehog inhibitor) and smoothened agonist SAG (Calbiochem, San Diego, CA, USA) at the concentrations of 0.5 μM (in vitro doses were based on a previous report 14) where the dose showed peak signaling activity (among all paracrine factors) with testosterone production with LSCs. To determine the phenotypic outcome, autografts were harvested at 4 weeks, paraffin‐embedded, and labeled with platelet‐derived growth factor receptor alpha (PDGFRA), 3BHSD, SOX9, and 4′,6‐diamidino‐2‐phenylindole (DAPI). In addition, testosterone levels were measured in serum.
Subcutaneous Autograft of Purified LSCs
Castration was performed in a total of three mice. CD146+ stem cells were sorted from total testicular cell population using CD146 MicroBead Kit (MACS, Cat # 130–092‐007, Auburn, CA, USA). Approximately 0.5 × 106 CD146+ cells were subcutaneously autografted (mixed with matrigel in a 1:1 ratio) in the mice. Following 2 weeks standby time, these mice were euthanized and checked for graft viability.
Immunohistochemistry and Fluorescence Staining
For immunohistochemical staining, tissue sections of the graft (both experimental and negative control) were stained with hematoxylin and eosin. A genitourinary pathologist that was blinded to the samples independently verified the presence of LCs under ×10 and ×60 magnification. For fluorescence staining, tissue slides were fixed and blocked, and then slides were stained with antibody against 3β‐Hydroxysteroid dehydrogenase (3BHSD) (sc‐30820, Santa Cruz Biotechnology, Dallas, Texas, USA) followed by Alexa Fluor 488 dye (Thermo Fisher Scientific, Waltham, MA, USA), Platelet‐derived growth factor receptor alpha (PDGFRA) (sc‐338); followed by Alexa Fluor 568 dye, LH receptor (LHR) antibody (sc‐25828); followed by Alexa Fluor 488 dye, SOX‐9 antibody (AB185966, ABCAM, Cambridge, MA, USA); followed by Alexa Fluor 488 dye, alpha smooth muscle actin (αSMA) antibody (AB5694); followed by Alexa Fluor 488 dye, and Promyelocytic leukaemia zinc finger protein (PLZF) antibody (sc‐28319); followed by Alexa Fluor 488 dye, nestin (ab6142); followed by Alexa Fluor 568 dye, smooth muscle Myosin heavy chain 11 (SMHC11) (ab53219); followed by Alexa Fluor 568 dye, Vimentin (ab45939); followed by Alexa Fluor 568 dye, START Domain Containing 4 (STARD4) (ab202060); followed by Alexa Fluor 568 dye at room temperature (RT) for 30 min; and 4',6‐diamidino‐2‐phenylindole (DAPI) (Santa Cruz) for the nucleus. All samples were assessed under a fluorescence microscope at ×60 magnification (Leica Microsystem, Wetzlar, Germany). Images were acquired using MetaMorph version 4.6 (Molecular Devices, Sunnyvale, CA, USA).
Assay of Testosterone, FSH, and LH Concentration
Blood was collected from mice by cardiac puncture immediately following euthanasia. Blood was allowed to clot and then centrifuged, and the serum was collected and stored at −20°C. All hormone assays were measured at the University of Virginia Center for Research and Reproduction Ligand Assay and Analysis Core Laboratory (Charlottesville, VA, USA). Testosterone was measured using a commercially available solid‐phase RIA kit (Coat‐a‐Count Total Testosterone Kit; Siemens Medical Solutions Diagnostics, Tarrytown, NY, USA). Testosterone assay sensitivity = 10 ng/dl; intra‐assay coefficient of variation (CV) = 5.0%; inter‐assay CV = 8.2%. LH and FSH concentrations were quantitated using a Milliplex Map Rat Pituitary Kit (Millipore, Billerica, MA, USA). Samples were run in duplicate. The sensitivity of assay was 2.4 ng/ml for FSH and 0.24 ng/ml for LH; intra‐ and intercoefficients of variations were 6.7% and 16.9% for FSH and 6.9% and 17.2% for LH.
RNA Preparation and Quantitative Real‐Time Polymerase Chain Reaction
Total RNA was extracted from cells using the TRIzol method, and then reverse transcribed to complementary DNA using High‐Capacity cDNA Reverse Transcription Kits (Applied Biosystems, Foster City, CA, USA) according to the manufacturer's protocol. The quantitative reverse transcriptase PCR (RT‐PCR) for indicated genes was performed in TaqMan Universal PCR Master Mix (Applied Biosystems). Quantitation of mRNAs was performed using Applied Biosystems TaqMan Gene Expression Assays according to the manufacturer's protocol. Samples were analyzed using the BIORAD sequence detection system. All PCRs were performed in triplicate, and the specificity of the reaction was determined by melting curve analysis at the dissociation stage. The relative quantitative method was used for the quantitative analysis. The calibrator was the averaged ΔCt from the untreated cells. The endogenous control was glyceraldehyde 3‐phosphate dehydrogenase.
Flow Cytometry
Cells that were harvested from the total testicular tissues and culture were considered. After trypsin treatment, cell number was calculated and a minimum of 1 × 105 cells were considered per tube (n = 3). Cells in tubes were washed with fluorescence‐activated cell sorting (FACS) buffer (2 times). Cells in one tube were fixed with 2% paraformaldehyde (PFA) at this stage; the other two tubes were fixed with BD Cytofix/Cytoperm (Ct No. 554714, San Jose, CA, USA) for 15 min at RT. After washing them two times with perm wash, primary antibodies against PDGFRA, 3BHSD, SOX9, and αSMA were added and cells were incubated for 30 min. Again, cells were washed with perm wash and blocked with Fc receptor block for 20 min, after which secondary antibodies were added and cells were incubated for 30 min. After incubation, cells were washed with FACS buffer (three times), fixed with PFA, and suspended in FACS buffer before analyzing using FACS.
Statistical Analysis and Sample Size Calculation
GraphPad Prism (GraphPad Software) was used for statistical analysis. All data were presented as the means ± SEM. The statistical significance between two groups was estimated by unpaired two‐tailed t test. Multiple group comparisons were performed using a one‐way analysis of variance with least significant difference test. In all cases, p < .05 was considered statistically significant.
Results
Characterization of LSCs
LSCs in combination with Sertoli and myoid cells from castrated adult wild type C57/BL6 mice were maintained in stem cell EM for 14 days. As expected, cells stained intensely for PDGFRA and SOX9 (Fig. 1A) and faintly for 3BHSD. FACS identified that 61% of the cells were LSCs (PDGFRA +), 18.8% were Sertoli cells (SOX9+), and 15.4% were adult LCs (3BHSD) (Fig. 1C). To further assess whether LSCs could be induced to differentiate into 3BHSD‐positive adult LCs (ALCs) in vitro, subconfluent cultures were placed in DIM. After 7 days in DIM, the expression of differentiation marker (3BHSD) increased (Fig. 1B).
Figure 1.
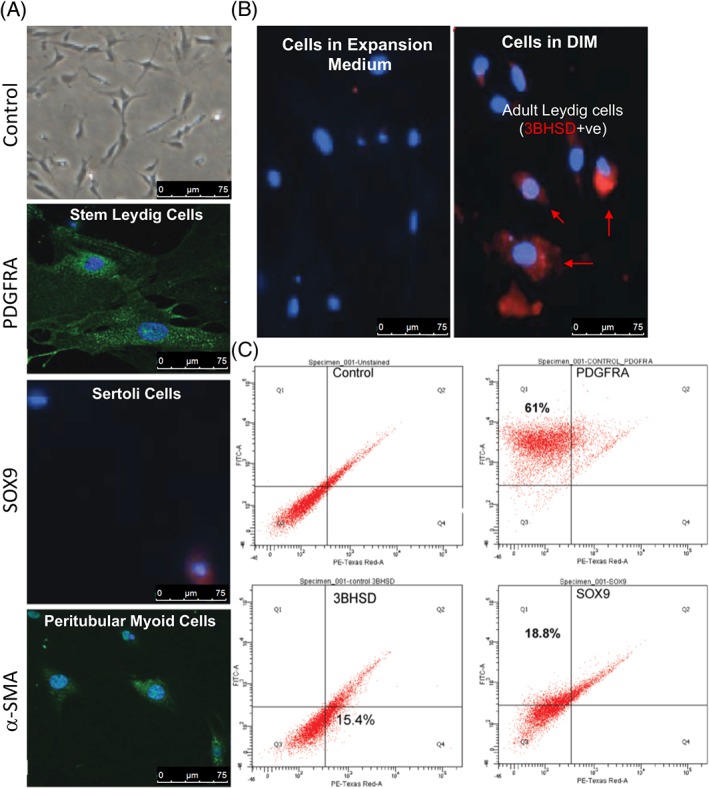
Characterization of isolated Leydig stem cells from adult mouse testes. Spindle‐shaped Leydig stem cells can be maintained in culture as demonstrated with (A) high power photomicrograph. The isolated cells expressed platelet‐derived growth factor receptor alpha (PDGFRA) protein (green) indicative of Leydig stem cells, SOX9 indicative of Sertoli cell population, and alpha smooth muscle actin indicative of peritubular myoid cells. Rabbit IgG served as a negative control using 4′,6‐diamidino‐2‐phenylindole nuclear stain. Scale bar = 75 μm. (B): Figure shows that differentiation could be induced in the cultured cells. The presence of mature Leydig cells was confirmed with cells staining positive (Red) for 3BHSD. Scale bar = 75 μm. (C): Fluorescence‐activated cell sorting, showing population of PDGFRA‐, 3BHSD‐, and SOX9‐positive cells in the culture. Abbreviations: BHSD, beta hydroxysteroid dehydrogenase; DIM, differentiation inducing medium; PDGFRA, platelet‐derived growth factor receptor alpha; αSMA, alpha smooth muscle actin.
Subcutaneous Autograft of LSCs
We prepared LSCs (1 × 106) in combination with myoid cells and Sertoli cells in matrigel (1:1, final volume of 100 μl) and injected them subcutaneously 7 days after castration in six adult wild‐type mice (Fig. 2A, upper image). The animals survived for 1 month, and we monitored the presence of graft every week—no sign of inflammation or trauma at injection site was noticed (Fig. 2A, lower image). After 1 month, the grafts were excised and serum was collected by cardiac puncture (Supporting Information Fig. S1). Structural evaluation of autograft by the pathologist showed the presence of differentiated ALCs as indicated by H&E staining (Fig. 2B) in all the mice along with the matrigel that was used as the vehicle. In contrast, negative control animals that received matrigel alone lacked any ALCs. The presence of differentiation in LCs in autografts was confirmed by immunofluorescence (IF) 8, 11, 12, 13 which stained ALCs positively for 3BHSD, STAR, and LHR (marker of LC) (Fig. 2D). In addition, IF also confirmed the presence of Sertoli cells (SOX9 and Vimentin), LSCs (Nestin), and peritubular myoid cells (αSMA and SMHC) (Fig. 2D). Importantly, no germ cell population was identified (no PLZF expressing cells in the autograft) (Fig. 2D) (Supporting Information Fig. S2). Furthermore, serum testosterone levels were compared between different groups of mice. It was found that castrated mice that did not receive autograft (negative controls) had significantly lower serum testosterone levels (13.20 + 0.37 ng/dl) than the mice which received autograft (22.27 + 1.33 ng/dl) (p < .05). Importantly, mice receiving LSC autograft maintained significantly higher levels of LH (3.00 + 0.63 ng/ml) and FSH (178.30 + 26.56 ng/ml) in comparison with mice that underwent exogenous testosterone administration (LH 0.016 + 0.02 ng/ml, FSH in 51.71 + 12.93 ng/ml) (p < .05) (Fig. 3A).
Figure 2.
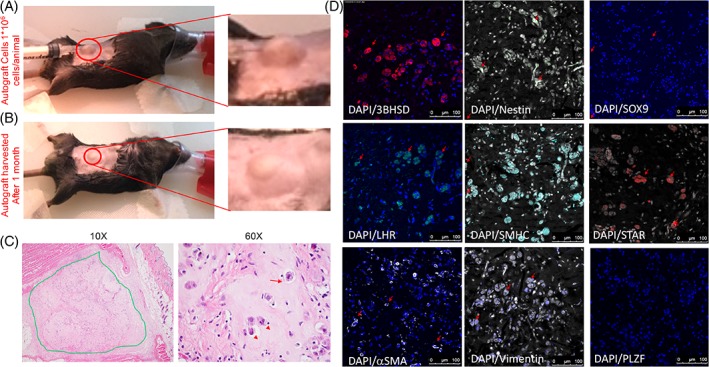
Leydig stem cells injected into subcutaneous tissue (A) (upper image) and autograft identification after 1 month (B) (lower image). (C): H&E staining indicating the presence of mature Leydig cells (LCs) in the autografts. The presence of mature LCs in the autografts which was indicated by H&E staining was then confirmed by immunostaining for the presence of (D) 3BHSD, luteinizing hormone receptor, alpha smooth muscle actin, Nestin, smooth muscle Myosin heavy chain, Vimentin, STAR, SOX9, and PLZF, respectively. Scale = bar 100 μm. Abbreviations: DAPI, 4′,6‐diamidino‐2‐phenylindole; LHR, luteinizing hormone receptor; αSMA, alpha smooth muscle actin; SMHC, smooth muscle myosin heavy chain; STAR, steroidogenic acute regulatory protein.
Figure 3.
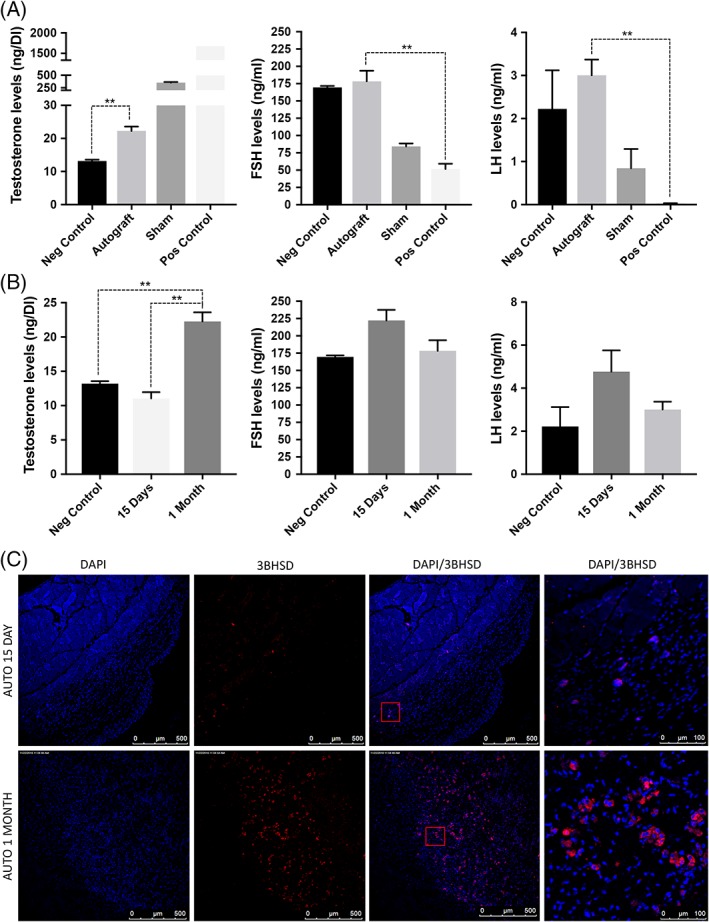
(A): Leydig stem cells function was validated by comparing the production of testosterone, follicle‐stimulating hormone, and luteinizing hormone in negative control (castrated), autograft, sham, and positive control (testopel) using enzyme‐linked immunosorbent assay. (B): To further confirm that it is the stem Leydig cells that undergo differentiation in the grafts, hormone levels were compared after 15 days and 1‐month autograft. (C): Immunostaining showing 3BHSD expressing cells in autografts from 15 days and 1‐month autograft. Significance of differences was calculated using t test; **, p < .01; *, p < .05, and ns (non‐significant) (p > .05). Abbreviations: BHSD, beta hydroxysteroid dehydrogenase; DAPI, 4′,6‐diamidino‐2‐phenylindole; FSH, follicle‐stimulating hormone; LH, luteinizing hormone.
The initial graft had a small but viable ALC population in it. Hence, the question arises whether LSCs within the autograft proliferated and differentiated into ALCs to increase serum testosterone rather than the ALCs that were originally present within the graft. To address this concern, LSCs (1 × 106) in combination with myoid cells and Sertoli cells in matrigel were injected subcutaneously 7 days after castration in adult wild‐type mice. The animals survived for 15 days before the grafts were excised and serum was collected by cardiac puncture. The serum testosterone levels and number of 3BHSD‐positive cells were compared between 15 days and 1 month graft from the above experiment. Results clearly showed an increasing level of serum testosterone from 15 days (11.06 + 1.55 ng/dl) to 1 month graft (22.27 + 1.33 ng/dl) (p < .05) (Fig. 3B). Additionally, the number of cells staining positive for 3BHSD were much higher in 1 month graft (Fig. 3C). These results strengthen the argument that LSCs undergo differentiation to ALCs during the time of autograft.
Next, to determine the relevance of autografting LSCs in combination with Sertoli cells and myoid cells, we isolated and purified CD146+ cells. CD146 is a marker that is frequently found on multipotent progenitor cells, including immature hematopoietic stem and progenitor cells 15. When we autografted 0.5 × 105 purified CD146+ cells, it was found that these cells were incapable of differentiation (Supporting Information Fig. 4). As a result, the autografts failed to remain viable within 2 weeks showing the importance of adjacent testicular cells.
Hedgehog Signaling in Regulation of Subcutaneous Autograft
The HH signaling pathway is closely linked to developmental processes, tissue and stem cell maintenance, cell differentiation processes, cell proliferation, and regenerative responses 16, 17, 18, 19. A study by Jiang Z et al. showed Desert HH (DHH) as one of the most important paracrine factors stimulating the proliferation of the Leydig stem cells (LSCs) in adult rat testis 14. Therefore, we checked the implications of HH signaling in regulating the LSC autograft. This is critical because if true, it indicates the mechanism of biological regulation of the graft in the extra testicular environment. To show this, a mixture of LSCs, myoid cells, and Sertoli cells (total testicular cell population) was treated with SAG (0.5 μM) 14, a HH agonist, and vismodegib (5 μM), a DHH antagonist, for 24 hours (the concentration of vismodegib was standardized by treating the testicular cells with 0, 2.5, 5, and 10 μM of vismodegib and checking its impact on glioma‐associated oncogene (GLI) and smoothened (SMO) expression [data not shown]). This was followed by autografting the treated cells in mice (subcutaneously). One month post‐autograft, mice were euthanized, and grafts and serum were harvested (Fig. 4A). Results revealed that SAG had stimulatory effects on testosterone production as compared with vismodegib (Fig. 4B, 4C). Additionally, 3BHSD, LHR, SOX9, PDGFRA, as well as αSMA expressing cells were identified in grafts from mice which received SAG‐treated cells (Fig. 4D) (Supporting Information Fig. S3). Together, the results implicated the relevance of DHH in regulating LSC autograft.
Figure 4.
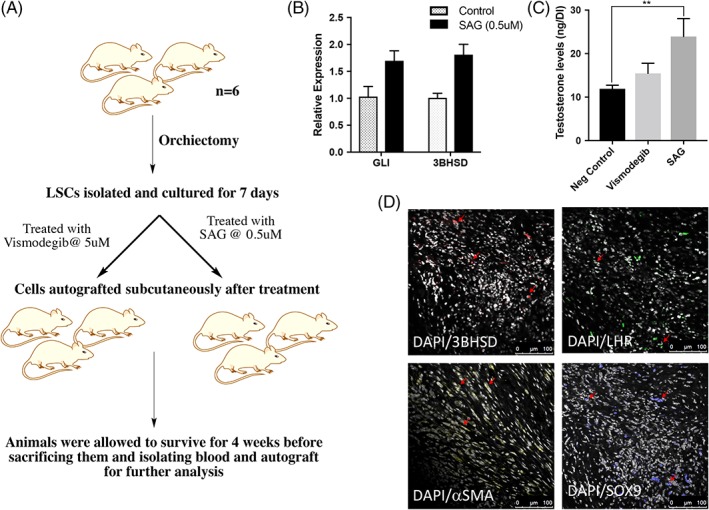
(A): Plan to confirm the implications of hedgehog (HH) signalling on autograft function. (B): Relative expression of GLI and 3BHSD upon SAG (Desert HH [DHH] agonist) treatment. (C): Comparison of testosterone levels in mice which received DHH antagonist (vismodegib) or DHH agonist (SAG). (D): Immunostaining showing cells expressing positive for 3BHSD, platelet‐derived growth factor receptor alpha, alpha smooth muscle actin, SOX9, and luteinizing hormone receptor in grafts from mice which received SAG‐treated Leydig stem cells. Significance of differences was calculated using t test; **, p < .01; *, p < .05, and ns (non‐significant) (p > .05). Abbreviations: BHSD, 3β‐Hydroxysteroid dehydrogenase; DAPI, 4′,6‐diamidino‐2‐phenylindole; LHR, luteinizing hormone receptor; LSC, Leydig stem cell; SAG, smoothened agonist; αSMA, alpha smooth muscle actin.
Discussion
Exogenous testosterone is a contraceptive because when administered exogenously, it blocks the production of FSH and LH, thereby disrupting the HPG axis 17. We determined the feasibility of subcutaneously autografting LSCs (a combination of LCs, Sertoli cells, and peritubular myoid cells). We showed that testosterone production from the subcutaneous autograft is possible, and at low levels of serum testosterone, both FSH and LH production are maintained. We also demonstrated that similar to in vitro studies published by Chen et al. (Add reference), HH signaling regulates testosterone production from the LSCs that are grafted at an ectopic site (outside the testis).
Theoretically, testosterone produced from an ectopic site (autograft) can be considered similar to exogenous testosterone and inhibit FSH and LH production. We hypothesized that LSC autograft will be under the influence of the HPG axis and therefore the production of FSH and LH should not be suppressed. To confirm this hypothesis, we isolated LSCs from whole testes of adult wild‐type mice. LSCs were found to divide in vitro in the presence of factors known to stimulate stem cell renewal and to express 3BHSD when stimulated by a DIM containing LH. We subcutaneously grafted LSCs in combination with Sertoli cells and myoid cells in subcutaneous tissue using matrigel. Four weeks after autograft, we identified the presence of mature ALCs within the graft. We also confirmed testosterone production as well as normal FSH and LH levels in the animals that underwent autograft. This novel experiment is the first demonstration of LSCs being grafted ectopically, differentiating into ALCs, and producing testosterone.
We did not use an auto‐transplantation (transplanting LSCs into the testis) approach because we believe that this approach would not be clinically translatable even though the paracrine effects of testis would be critical for the survival, expansion, and differentiation of LSCs. Therefore, we used an ectopic subcutaneous autograft of a combination of LSCs along with myoid cells and Sertoli cells to verify whether the LH present in the bloodstream from the pituitary gland will differentiate LSCs. Furthermore, to closely mimic the paracrine effects of the testis, we did not sort or isolate the different cell types such as macrophages, fibroblasts, and Sertoli cells. LSCs cannot function optimally when isolated and purified. In fact, when we isolated the LSCs using CD146+ MACS kit and grafted them subcutaneously, the graft failed to survive even after 2 weeks. Therefore, we determined that a combination of peritubular myoid cells and Sertoli cells that include LSCs is critical for LSC graft survival.
There are several strengths and limitations in our study. The study is novel for three major reasons. (a) This is the first study to demonstrate that LSCs can be autografted at an ectopic site and can survive and function up to 4 weeks (viable grafts were reliably obtained in all the nine mice upon 4 weeks autograft). (b) The study demonstrated that the combination of LSC with peritubular myoid cells and Sertoli cells is critical for LSC differentiation and function. (c) HH signaling is critical for LSC survival outside the testis. We also demonstrated that FSH and LH production is not impaired despite increase in serum testosterone from an ectopic site, although a small increase, when compared with castrate animals. However, the study is limited by the short follow‐up, lack of data on the dose of stem cells necessary for optimal testosterone production, and experiments performed in castrate mice. A long follow‐up is necessary to determine whether testosterone levels can be restored to the eugonadal range and the duration that these LSCs will remain viable. What remains to be determined is how many cells are needed and the optimal combination of LSCs with Sertoli and myoid cells to produce the highest testosterone level while simultaneously maintaining FSH and LH. To detect the low levels of testosterone produced by the autograft, we performed the subcutaneous autograft in castrate animals. As expected, in castrate animals, LH levels were higher than expected and may be necessary for LSC differentiation and function. To test whether normal levels of LH is sufficient for LSC differentiation and function within the autograft, we will attempt autograft from one testis in future studies.
Conclusion
In conclusion, our results in this pilot feasibility study demonstrated that LSCs in a subcutaneous autograft can increase serum testosterone without inhibiting circulating LH and FSH. LSC autograft is a novel therapeutic approach to increasing serum testosterone while simultaneously preserving HPG axis in castrate mice. Future studies on the role of LSC subcutaneous graft need to be elucidated in mice with normal testosterone levels.
Author Contributions
H.A.: conception and design, acquisition of data, analysis and interpretation of data, drafting the article, final approval of the completed article; M.S.S.R.Z.: acquisition of data, analysis and interpretation of data; B.N.: acquisition of data; D.L.: interpretation of data and drafting the article; J.M.H.: conception and design, drafting the article; R.R.: conception and design, analysis and interpretation of data, drafting the article, revising manuscript for intellectual content.
Disclosure of Potential Conflicts of Interest
H.A., J.M.H., and R.R. are co‐inventors on provisional U.S. Patent # 32286/51807. J.M.H. discloses a relationship with Vestion Inc. that includes equity, board membership, and consulting, is the Chief Scientific Officer, a compensated consultant, and advisory board member for Longeveron and holds equity in Longeveron; and is also the co‐inventor of intellectual property licensed to Longeveron. All other authors indicated no potential conflicts of interest.
Supporting information
Supplementary Figure 1. Plan showing the procedure followed for subcutaneous autografting Leydig stem cells.
Supplementary Figure 2. Immunostaining showing cells staining positive for (A) 3BHSD, (B) LHR, (C) αSMA, (D) SOX9, (E)PLZF, (F) Nestin, (G) SMHC, (H) Vimentin and (I) STAR respectively, in grafts isolated from mice after one month of autograft. Scale bar 500 and 100 μm.
Supplementary Figure 3. Immunostaining showing cells staining positive for 3BHSD, α‐SMA, LHR and SOX9, respectively, in grafts from mice which received SAG treated LSCs. Scale bar 500 and 100 μm.
Supplementary Figure 4. Immunostaining showing cells staining positive for 3BHSD in grafts from mice which received CD146+ cells only. Scale bar 500 μm.
Acknowledgments
The authors thank Alexander Agoulnik, Florida International University, for mentorship and guidance. This work was supported by the American Urological Association Research Scholar Award and Stanley Glaser Award (to R.R.) as well as NIH grants 1R01 HL137355, 1R01 HL107110, 1R01 HL134558, 5R01 CA136387, and 5UM1 HL113460 and Soffer Family Foundation (to J.M.H.).
References
- 1. Starka L, Raboch J, Neuwirth J. Plasma testosterone levels in men without mature Leydig cells. Cas Lek Cesk 1973;112:166–172. [PubMed] [Google Scholar]
- 2. Afiadata A, Ellsworth P. Testosterone replacement therapy: Who to evaluate, what to use, how to follow, and who is at risk? Hosp Pract 1995;42(2014):69–82. [DOI] [PubMed] [Google Scholar]
- 3. McLachlan RI, O'Donnell L, Meachem SJ et al. Hormonal regulation of spermatogenesis in primates and man: Insights for development of the male hormonal contraceptive, J Androl 23 (2002) 149–162. [PubMed] [Google Scholar]
- 4. Spaliviero JA, Jimenez M, Allan CM et al. Luteinizing hormone receptor‐mediated effects on initiation of spermatogenesis in gonadotropin‐deficient (hpg) mice are replicated by testosterone. Biol Reprod 2004;70:32–38. [DOI] [PubMed] [Google Scholar]
- 5. Tuttelmann F, Damm OS, Luetjens CM et al. Intratesticular testosterone is increased in men with Klinefelter syndrome and may not be released into the bloodstream owing to altered testicular vascularization‐ a preliminary report. Andrology 2014;2:275–281. [DOI] [PubMed] [Google Scholar]
- 6. Li H, Benoit K, Wang W et al. Association between use of exogenous testosterone therapy and risk of venous thrombotic events among exogenous testosterone treated and untreated men with hypogonadism. J Urol 2016;195:1065–1072. [DOI] [PubMed] [Google Scholar]
- 7. Nudleman E, Witmer MT, Kiss S et al. Central serous chorioretinopathy in patients receiving exogenous testosterone therapy. Retina 2014;34:2128–2132. [DOI] [PubMed] [Google Scholar]
- 8. Zang ZJ, Wang J, Chen Z et al. Transplantation of CD51(+) stem Leydig cells: A new strategy for the treatment of testosterone deficiency. Stem Cells 2017;35:1222–1232. [DOI] [PubMed] [Google Scholar]
- 9. Mendis‐Handagama SM, Ariyaratne HB. Differentiation of the adult Leydig cell population in the postnatal testis. Biol Reprod 2001;65:660–671. [DOI] [PubMed] [Google Scholar]
- 10. Lo KC, Lei Z, Rao Ch V et al. De novo testosterone production in luteinizing hormone receptor knockout mice after transplantation of Leydig stem cells. Endocrinology 2004;145:4011–4015. [DOI] [PubMed] [Google Scholar]
- 11. Stanley E, Lin CY, Jin S et al. Identification, proliferation, and differentiation of adult Leydig stem cells. Endocrinology 2012;153:5002–5010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang M, Wang J, Deng C et al. Transplanted human p75‐positive stem Leydig cells replace disrupted Leydig cells for testosterone production. Cell Death Dis 2017;8:e3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ge RS, Dong Q, Sottas CM et al. In search of rat stem Leydig cells: Identification, isolation, and lineage‐specific development. Proc Natl Acad Sci USA 2006;103:2719–2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li X, Wang Z, Jiang Z et al. Regulation of seminiferous tubule‐associated stem Leydig cells in adult rat testes. Proc Natl Acad Sci USA 2016;113:2666–2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jiang MH, Cai B, Tuo Y et al. Characterization of Nestin‐positive stem Leydig cells as a potential source for the treatment of testicular Leydig cell dysfunction. Cell Res 2014;24:1466–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pyczek J, Buslei R, Schult D et al. Hedgehog signaling activation induces stem cell proliferation and hormone release in the adult pituitary gland. Sci Rep 2016;6:24928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hooper JE, Scott MP. Communicating with Hedgehogs. Nat Rev Mol Cell Biol 2005;6:306–317. [DOI] [PubMed] [Google Scholar]
- 18. Machold R, Hayashi S, Rutlin M et al. Sonic hedgehog is required for progenitor cell maintenance in telencephalic stem cell niches. Neuron 2003;39:937–950. [DOI] [PubMed] [Google Scholar]
- 19. Shin K, Lee J, Guo N et al. Hedgehog/Wnt feedback supports regenerative proliferation of epithelial stem cells in bladder. Nature 2011;472:110–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Plan showing the procedure followed for subcutaneous autografting Leydig stem cells.
Supplementary Figure 2. Immunostaining showing cells staining positive for (A) 3BHSD, (B) LHR, (C) αSMA, (D) SOX9, (E)PLZF, (F) Nestin, (G) SMHC, (H) Vimentin and (I) STAR respectively, in grafts isolated from mice after one month of autograft. Scale bar 500 and 100 μm.
Supplementary Figure 3. Immunostaining showing cells staining positive for 3BHSD, α‐SMA, LHR and SOX9, respectively, in grafts from mice which received SAG treated LSCs. Scale bar 500 and 100 μm.
Supplementary Figure 4. Immunostaining showing cells staining positive for 3BHSD in grafts from mice which received CD146+ cells only. Scale bar 500 μm.


