Abstract
Key points
Skeletal muscle stem cells, termed satellite cells, play a crucial role in repair and remodelling of muscle in response to exercise
An age‐related decline in satellite cell number and/or function has been hypothesized to be a key factor in the development of sarcopenia and/or the blunted muscle fibre adaptive response to prolonged exercise training in older persons
We report that performing prolonged exercise training improves the acute type II muscle fibre satellite cell response following a single bout of resistance exercise in older men.
The observed improvement in muscle satellite function is associated with an increase in muscle fibre capillarization following exercise training suggesting a possible functional link between capillarization and satellite cell function.
Abstract
Age‐related type II muscle fibre atrophy is accompanied by a fibre type‐specific decline in satellite cell number and function. Exercise training restores satellite cell quantity in older adults; however, whether it can restore the impaired satellite cell response to exercise in older adults remains unknown. Therefore we assessed the acute satellite cell response to a single exercise session before and after prolonged exercise training in older men. Fourteen older men (74 ± 8 years) participated in a 12‐week exercise training programme (resistance exercise performed twice per week, high intensity interval training once per week). Before and after training, percutaneous biopsies from the vastus lateralis muscle were taken prior to and following 24 and 48 h of post‐exercise recovery. Muscle fibre characteristics were evaluated by immunohistochemistry and mRNA expression by RT‐PCR. Whereas no changes were observed in type II muscle fibres, type I muscle fibre satellite cell content increased significantly at 24 and 48 h after a single bout of resistance exercise before the exercise training programme (P < 0.01). Following the exercise training programme, both type I and type II muscle fibre satellite cell content increased significantly at 24 and 48 h after a single bout of resistance exercise (P < 0.05). The greater acute increase in type II muscle fibre satellite cell content at 24 h post‐exercise recovery after training was correlated with an increase in type II muscle fibre capillarization (r = 0.671, P = 0.012). We show that the acute muscle satellite cell response following exercise can be improved by prolonged exercise training in older men.
Keywords: capillary, exercise training, skeletal muscle
Key points
Skeletal muscle stem cells, termed satellite cells, play a crucial role in repair and remodelling of muscle in response to exercise
An age‐related decline in satellite cell number and/or function has been hypothesized to be a key factor in the development of sarcopenia and/or the blunted muscle fibre adaptive response to prolonged exercise training in older persons
We report that performing prolonged exercise training improves the acute type II muscle fibre satellite cell response following a single bout of resistance exercise in older men.
The observed improvement in muscle satellite function is associated with an increase in muscle fibre capillarization following exercise training suggesting a possible functional link between capillarization and satellite cell function.
Introduction
Ageing is accompanied by the progressive loss of skeletal muscle mass and strength, termed sarcopenia (Cruz‐Jentoft et al. 2010). The loss of muscle mass is manifest at the muscle fibre level by type II muscle fibre atrophy (Nilwik et al. 2013), which is accompanied by a type II muscle fibre type‐specific decline in skeletal muscle stem or satellite cell number and function (Dreyer et al. 2006; Verney et al. 2008; McKay et al. 2012; Verdijk et al. 2014; Snijders et al. 2014b). Muscle satellite cells are the only known source to provide additional myonuclei, and therefore they are considered to be indispensable in muscle fibre regeneration, repair and (extensive) growth (Lepper et al. 2011; Egner et al. 2016). Hence, it has been hypothesized that a decreased ability to provide additional myonuclei to allow muscle repair following injury and/or muscle fibre hypertrophy during exercise training may be a key factor in the development of sarcopenia (Snijders et al. 2015).
Resistance exercise training (3–4 sessions per week) is an effective strategy to increase skeletal muscle mass and strength in older adults (Peterson et al. 2010, 2011). In addition, we have previously shown that prolonged resistance exercise training can restore type II muscle fibre satellite cell content to the level of (untrained) healthy young adults (Leenders et al. 2013; Verdijk et al. 2014; Snijders et al. 2017). It remains unknown, however, whether performing exercise training over a prolonged time period is able to enhance type II muscle fibre satellite cell function (i.e. activation in response to exercise) in older adults. The skeletal muscle adaptive response to a single bout of exercise has previously been used as a proxy measure to evaluate muscle satellite cell function in vivo humans (Dreyer et al. 2006; McKay et al. 2012, 2013; Cermak et al. 2013; Snijders et al. 2014a, b ). Percutaneous muscle biopsies are collected before and at multiple time points after a single bout of exercise to assess the acute changes in satellite cell content and activation status during the post‐exercise period. Whereas satellite cell content has been shown to increase substantially in both type I and type II muscle fibres in response to a single bout of resistance exercise in healthy young men, this is not the case in older adults (McKay et al. 2012; Snijders et al. 2014b; Nederveen et al. 2015). Studies have clearly shown an impaired and/or delayed activation and proliferation of satellite cells during the first 48–72 h of post‐exercise in older men (McKay et al. 2012; Snijders et al. 2014b; Nederveen et al. 2015). An adequate muscle microvascular bed is required to allow optimal delivery of oxygen, nutrients and growth factors that are likely necessary to facilitate muscle fibre reconditioning. In line, the acute muscle satellite cell activation response during post‐exercise recovery has been reported to depend on muscle fibre capillarization in young men (Nederveen et al. 2016, 2018). As muscle fibre capillarization declines with advancing age, it has been hypothesized that the impaired acute satellite cell response during post‐exercise recovery may, in part, be due to age‐related reduction in muscle fibre capillarization in older adults (Joanisse et al. 2017).
Mixed results have been reported on the impact of resistance exercise training on skeletal muscle angiogenesis in older adults. Whereas as some do (Frontera et al. 1990; Verdijk et al. 2016), others do not (Hagerman et al. 2000; Snijders et al. 2017) report an increase in muscle fibre capillarization in response to prolonged resistance exercise training in seniors. In contrast, aerobic exercise training has been consistently shown to induce an agiogenic response in older adults (Hepple et al. 1997a; Gavin et al. 2007; Prior et al. 2015). As such, including an aerobic exercise component in a resistance exercise training programme may be of great value for older adults. Moreover, as ageing is not only associated with low muscularity, but also with reduced cardiometabolic health, current guidelines recommend performing both resistance and endurance‐type exercise to support healthy ageing (American College of Sports Medicine et al. 2009; Australian and New Zealand Society for Geriatric Medicine, 2014). However, whether a combined exercise training programme can increase satellite cell content and/or improve the acute muscle satellite cell response during post‐exercise recovery remains unknown. Therefore, in the present study we compared the acute satellite cell response to a single bout of resistance exercise before and after 12 weeks of combined exercise training in healthy older men.
Methods
Ethical approval
All participants were informed of the nature and possible risks of the experimental procedures before their written informed consent was obtained. The study was approved by the Hamilton Health Sciences Integrated Research Ethics Board (approval no. 14‐677), and conformed to the guidelines outlined in the latest version of the Declaration of Helsinki. Participants gave their informed written consent prior to their inclusion to the study. The study was part of a larger project investigating the impact of nutrition and exercise training on skeletal muscle mass/strength in older adults (Bell et al. 2017). This trial was registered at ClinicalTrials.gov (NCT02281331).
Participants
Fourteen healthy older men (age: 74 ± 8 years; weight: 85 ± 14 kg; body mass index (BMI): 28 ± 4 kg m−2) were recruited to participate in a 12‐week progressive combined exercise training programme. All participants had a BMI in the normal–overweight range (between 18.5 and 30.0 kg m−2) and resting blood pressure <140/90 mmHg. All participants had normal cardiac function during a maximal exercise stress test. Exclusion criteria included smoking, diabetes, regular use of non‐steroidal anti‐inflammatory drugs, use of simvastatin and history of chronic illness that would affect the results of the investigation. An oral glucose tolerance test was performed to exclude type 2 diabetes patients from participation. All subjects had not participated in any structured exercise programme in the past year and were living independently.
Exercise programme
Supervised exercise was performed three times a week for a 12‐week period. On Mondays and Fridays participants performed resistance exercise, and on the Wednesdays they performed high intensity interval training (HIIT). A resistance exercise session consisted of a 5 min warm‐up on a cycle ergometer, followed by three sets of four separate exercises in the following order: leg press, chest press or lateral pull‐down, horizontal row or shoulder press, and leg extension. Chest press and horizontal row were only performed on Mondays, and lateral pull‐down and shoulder press were only performed on Fridays (leg press and leg extension were performed at every resistance exercise session). During the first two weeks of the training programme, the workload (based on baseline one‐repetition maximum (1RM) assessment) gradually increased from 65% (10–15 repetitions) of 1RM to 80% 1RM (8–10 repetitions). From week 3 onwards, two sets of eight repetitions were performed at 80% 1RM. The third set of every exercise was performed (at the same intensity as the first two sets) to volitional fatigue, which we defined as the inability to complete an additional repetition with proper form. For every exercise the final set was performed to volitional failure. Resting periods of 1.5 and 3 min were allowed between sets and exercises, respectively. Each session ended with a 5 min cool‐down period on the cycle ergometer. Workload was increased when more than eight repetitions could be performed in the third set of the exercise. In addition, workload intensity was adjusted based on 1RM tests (performed at week 4 and 8). On Wednesdays participants performed HIIT on a cycle ergometer (ISO1000 Upright Bike; SCIFIT, Tulsa, OK) while wearing a heart rate monitor (H7 Heart Rate Sensor; Polar Electro Canada, Lachine, QC, Canada). Following a 3 min warm‐up at 25 W, participants completed 10 × 60 s intervals at a workload which elicited 90% of maximal heartrate, while maintaining a cadence of ≥90 rpm. Workload was adjusted as needed to maintain an average heart rate of ∼90% max. heart rate over the 10 intervals. Intervals were interspersed with 60 s of rest where participants cycled at a self‐selected pace against 25 W. HIIT sessions were concluded with a 5 min cool‐down at 25 W.
Aerobic capacity
Participants performed a test on an electronically braked cycle ergometer (Lode Excalibur Sport V 2.0; Groningen, the Netherlands) while wearing a chest‐strap heart rate monitor. A metabolic cart and online gas collection system (MOXUS Modular Oxygen Uptake Systems; AEI Technologies, Pittsburgh, PA, USA) was used to quantify respiratory gases. Following a 1 min warm‐up at 30 W, the load was increased by 1 W every 4 s. Participants were instructed to maintain a cadence of 60–90 rpm, and tests were terminated if the cadence dropped below 55 rpm for >10 s, or if volitional fatigue was attained. In all subjects, aerobic capacity was performed 6 days before the initial bout of exercise (pre‐acute response assessment) and 6 days before the post‐acute response assessment.
Muscle strength
Muscle strength was assessed using a 1RM strength tests for the following exercises: leg press, chest press, lateral pull‐down, horizontal row, shoulder press and leg extension (HUR, Northbrook, IL, USA). At baseline, proper lifting technique was demonstrated and practiced by participants during a familiarization session, and 1RMs were estimated using the multiple‐repetitions testing procedure. Within 1 week, 1RMs were evaluated as previously described (Verdijk et al. 2009). In all subjects, 1RM assessment was performed 5 days before the initial bout of exercise (pre‐training acute response assessment) and 5 days before the post‐training acute response assessment.
Body composition
Whole body and regional lean soft tissue mass (i.e. fat‐free and bone‐free mass), fat mass and bone mineral content were measured using dual energy X‐ray absorptiometry (DXA; GE‐LUNAR iDXA; Aymes Medical, Newmarket, ON, Canada) following a 10–12 h overnight fast. Regional body compartment analysis was performed in batches by a single investigator who was blinded for assessment time point. In all subject, body composition assessment was performed 1 week before the start and within 1 week following the final exercise session of the exercise training programme
Single bout of resistance exercise
Participants performed a single bout of resistance exercise both prior to (pre‐training acute response) and following 12 weeks (post‐training acute response) of combined exercise training (see Fig. 1 for a schematic representation of the study design). The single bout of resistance exercise for the pre‐training acute response measurements was the first exercise session of the 12‐week combined exercise training programme. To allow for all post‐measurements to take place in the same order as performed at baseline, including 5 days of no strenuous physical activity and/or exercise, the post‐training acute response was performed exactly 2 weeks after the final exercise session of the combined training programme. The single bout of exercise consisted of four sets of 10 repetitions each at 65% of 1RM on leg press, chest press, horizontal row and leg extension. Exercise was performed under personal supervision, all participants were verbally encouraged during the exercise and the final set of each exercise was performed to volitional failure. A resting period of 2 min between sets was allowed. As exercise training is known to change skeletal muscle mass and strength, the single bout of exercise was performed at the same relative intensity both prior to and following combined exercise training. Prior to and following the resistance exercise, a 5 min warm‐up/cool‐down was performed on a cycle ergometer.
Figure 1.
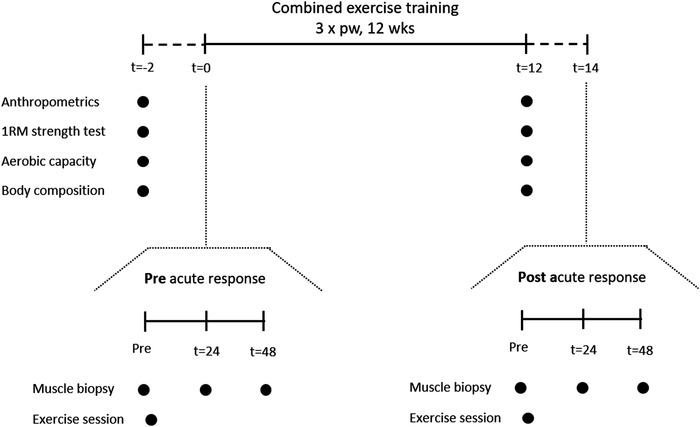
Schematic representation of the experimental design
Muscle biopsy sampling
Percutaneous needle biopsies were taken after an (∼10 h) overnight fast, from the mid‐portion of the vastus lateralis under local anaesthetic using a 5 mm Bergstrom needle adapted for manual suction (Bergstrom, 1975). Subjects had not participated in any physical activity for at least 5 days before the collection of the resting muscle biopsy. The resting biopsy was taken following 30 min of supine rest, and the single bout of exercise was performed immediately after. Consecutive muscle biopsy sampling was alternated between legs. The leg in which the first biopsy was taken was randomly selected. Incisions for the repeated muscle biopsy sampling in the same leg (e.g. pre‐ and 48 h post‐exercise sample) were spaced by at least 3 cm to minimize any effect of the previous biopsy. Upon excision, muscle samples were immediately mounted in optimal cutting temperature (OCT) compound, frozen in liquid nitrogen‐cooled isopentane and stored at −80°C until further analyses
Immunohistochemistry
Muscle cross sections (7 μm) were prepared from unfixed OCT‐embedded samples, allowed to air dry for 30 min and stored at −80°C. Slides were then stained with antibodies against Pax7 (neat; Developmental Studies Hybridoma Bank (DSHB), Iowa City, IA, USA); Myogenic differentiation factor D (MyoD) (anti‐MyoD1; clone 5.8A; 1:50; Dako, Burlington, ON, Canada); A4.951 myosin heavy chain type I (MHCI); slow isoform; 1:1; DSHB); myosin heavy chain type II (MHCII; fast isoform; 1:1000; ab91506; Abcam, Cambridge, MA, USA); CD31 (anti‐CD31; ab28364; 1:30; Abcam); laminin (anti‐laminin; ab11575; Abcam). Secondary antibodies used for Pax7 were (Alexa Fluor 488 or 594, 1:500; Thermo Fisher Scientific, Waltham, MA, USA); MyoD (biotinylated secondary antibody, 1:200; Vector Canada, Burlington, ON, Canada; and streptavidin‐594 fluorochrome, 1:500; Thermo Fisher Scientific); A4.951 (Alexa Fluor 488, 1:500); MHCII (Alexa Fluor 647, 1:500); CD31 (Alexa Fluor 647; 1:500); laminin (Alexa Fluor 488, 647; 1:500). Nuclei were labelled with 4′,6‐diamidino‐2‐phenylindole (DAPI) (1:20000, Sigma‐Aldrich, Oakville, ON, Canada), prior to cover slipping with fluorescent mounting medium (Dako, Burlington, ON, Canada). Slides were viewed with the Nikon Eclipse Ti microscope (Nikon Instruments, Inc., Melville, NY, USA), equipped with a high‐resolution Photometrics CoolSNAP HQ2 fluorescence camera (Nikon Instruments). Images were captured and analysed using the Nikon NIS Elements AR 3.2 software (Nikon Instruments). All images were obtained with a ×20 objective, and ≥200 muscle fibres per subject per time point were included in the analyses for satellite cell content/activation status, fibre cross sectional area (CSA) and perimeter. The activation status of satellite cells was determined via the colocalization of Pax7 and MyoD, Pax7+/MyoD− (bona fide quiescent satellite cell), Pax7+ MyoD+ (activated/proliferating satellite cell) and Pax7−/MyoD+ (differentiating satellite cell). Slides were blinded for both group and time point. The quantification of muscle fibre capillaries was performed on 50 muscle fibres per subject per time point. Based on the work of (Hepple et al. 1997b), quantification was performed for (i) capillary contacts (CC; the number of capillaries around a fibre), (ii) the capillary‐to‐fibre ratio on an individual fibre basis (C/Fi), and (iii) the number of fibres sharing each capillary (i.e. the sharing factor). The capillary‐to‐fibre perimeter exchange index (CFPE) was calculated as an estimate of the capillary‐to‐fibre surface area (Hepple et al. 1997b). Satellite cell distance to nearest capillary was evaluated as described previously (Nederveen et al. 2016). All immunofluorescence analysis were completed in a blinded fashion.
RNA isolation and reverse transcription
RNA was isolated from 15–25 mg of muscle tissue using the Trizol/RNeasy method. All samples were homogenized with 1 ml of Trizol Reagent (Thermo Fisher Scientific), in Lysing Maxtrix D tubes (MP Biomedicals, Solon, OH, USA), with the FastPrep‐24 Tissue and Cell Homogenizer (MP Biomedicals) for a duration of 40 s at a setting of 6 m s−1. Following a 5 min room temperature incubation, 200 μl of chloroform (Sigma‐Aldrich) was added to each sample, mixed vigorously for 15 s, incubated at room temperature for 5 min, and spun at 12,000 g for 10 min at 4°C. The RNA (aqueous) phase was purified using the E.Z.N.A. Total RNA Kit 1 (Omega Bio‐Tek, Norcross, GA, USA) as per the manufacturer's instructions. RNA concentration (ng ml−1) and purity (260/280) was determined with the Nano‐Drop 1000 Spectrophotometer (Thermo Fisher Scientific, Rockville, MD, USA). Samples were reverse transcribed using a high capacity cDNA reverse transcription kit (Thermo Fisher Scientific) in 30 μl reaction volumes, as per the manufacturer's instructions, using an Eppendorf Mastercycler epGradient Thermal Cycler (Eppendorf, Mississauga, ON, Canada) to obtain cDNA for gene expression analysis.
Quantitative real time RT‐PCR
All qPCR reactions were run in duplicate in 25 μl volumes containing Real Time Sybr Green qPCR Master Mix (Qiagen, Valencia, CA, USA), prepared with the epMotion 5075 Eppendorf automated pipetting system (Eppendorf, Mississauga, ON, Canada), and carried out using an Eppendorf Realplex2 Master Cycler epgradient (Eppendorf). Primers are listed in Table 1. Primers were resuspended in 1× TE buffer (10 mm Tris–HCl and 0.11 mm EDTA) and stored at −20°C prior to use. Messenger RNA expression was calculated using the method, and expressed as arbitrary units (AU) (Schmittgen & Livak, 2008). Briefly, C T values were first normalized to the housekeeping gene glyceraldehyde 3‐phosphate dehydrogenase (GAPDH). C T values normalized to GAPDH were expressed as ΔC T. GAPDH expression was not different from pre‐ at any of the post‐intervention time points (data not shown).
Table 1.
Primer sequences for quantitative real‐time PCR analysis
| Gene | Sense primer sequence (5′–3′) | Antisense primer sequence (5′–3′) |
|---|---|---|
| GAPDH | CCTCCTGCACCACCAACTGCTT | GAGGGGCCATCCACAGTCTTCT |
| MYF5 | ATGGACGTGATGGATGGCTG | GCGGCACAAACTCGTCCCCAA |
| MYOD | GGTCCCTCGCGCCCAAAAGAT | CAGTTCTCCCGCCTCTCCTAC |
| MYOG | CAGTGCACTGGAGTTCAGCG | TTCATCTGGGAAGGCCACAGA |
GAPDH, glyceraldehyde 3‐phosphate dehydrogenase; MYF5, myogenic factor‐5; MYOD, myogenic differentiation factor; MYOG, myogenin.
Statistical analyses
Data are expressed as means ± SD. Exercise training‐induced changes over time were analysed using repeated measures ANOVA with time (Pre vs. 12 weeks Post) and fibre type (type I vs. type II) as within subject factors. In the event of a time × fibre type interaction, type I and type II muscle fibres were analysed separately. Additional repeated measure ANOVA were used to analyses the pre‐ and post‐training acute satellite cell response separately, with time (pre vs. 24 h vs. 48 h) and fibre type (type I vs. type II) as within‐subject factors. Pearson correlation analyses was performed between the change in type I and II muscle fibre capillarization parameters with exercise training and the change in the acute type II muscle fibre satellite cell response following the single bout of exercise before and after the exercise training programme. Statistical significance was accepted as P < 0.05. Statistical analysis was completed using SPSS Statistics version 23.0 (IBM Corp., Armonk, NY, USA).
Results
Body composition
No significant difference in whole body and leg lean mass was observed in response to the 12‐week combined exercise training programme. In contrast, whole body and leg fat mass were significantly decreased following exercise training (P = 0.016 and P = 0.024, respectively; Table 2).
Table 2.
Changes in body composition, aerobic capacity and 1RM muscle strength in response to 12 weeks of combined exercise training in older men
| Pre | Post | |
|---|---|---|
| Body composition | ||
| Whole‐body lean mass (kg) | 55.0 ± 7.8 | 55.3 ± 7.7 |
| Whole‐body fat mass (kg) | 27.1 ± 7.1 | 26.3 ± 7.0* |
| Leg lean mass (kg) | 19.3 ± 3.6 | 19.5 ± 3.4 |
| Leg fat mass (kg) | 6.6 ± 1.6 | 6.4 ± 1.7* |
| Aerobic capacity | ||
| Absolute (l min−1) | 2.2 ± 0.5 | 2.4 ± 0.7* |
| Relative (ml min−1 kg) | 25.2 ± 5.1 | 27.3 ± 6.1* |
| Peak aerobic power (W) | 139 ± 39 | 143 ± 42* |
| 1RM muscle strength | ||
| Leg extension (kg) | 27 ± 8 | 35 ± 9* |
| Leg press (kg) | 72 ± 25 | 92 ± 35* |
| Horizontal row (kg) | 28 ± 9 | 29 ± 5 |
| Chest press (kg) | 21 ± 6 | 24 ± 7* |
| Lateral pulldown (kg) | 26 ± 4 | 30 ± 5* |
| Shoulder press (kg) | 24 ± 7 | 27 ± 8 |
Values are means ± SD. *Significantly different compared with Pre (P < 0.05). 1RM: one repetition maximum.
Muscle strength and aerobic capacity
In response to the combined exercise training programme, 1RM muscle strength increased significantly for leg press (+28%; P < 0.001), leg extension (+30%; P < 0.01), chest press (+14%; P < 0.01) and lateral pull down (+15%; P < 0.001, Table 2). No significant change in 1RM muscle strength was observed for the horizontal row and shoulder press (P = 0.068) following exercise training (Table 2). Absolute (+9%; P < 0.05) and relative (+8%; P < 0.05) increased significantly in response to the combined exercise training programme. Likewise, peak aerobic power output increased by 9% following exercise training (P < 0.05, Table 2).
Muscle fibre size and type distribution
At baseline, type II muscle fibre size was significantly lower compared with type I muscle fibres (Table 3, P < 0.01). Type I and type II muscle fibre size tended to increase in response to 12 weeks of combined exercise training (Table 3, main effect of time P = 0.066). A rightward shift was observed in type II muscle fibre size distribution in response to the exercise training programme (Fig. 2). No change was observed in fibre type distribution following 12 weeks of exercise training.
Table 3.
Changes in muscle fibre characteristics in response to 12 weeks of combined exercise training in older men
| Type | Pre | Post | |
|---|---|---|---|
| Muscle fibre size (μm2) | I | 6399 ± 1804 | 7044 ± 1668 |
| II | 5073 ± 1496† | 5495 ± 1137† | |
| Myonuclear content (per fibre) | I | 3.84 ± 0.58 | 3.90 ± 0.60 |
| II | 3.46 ± 0.62† | 3.58 ± 0.60† | |
| Myonuclear domain (μm2) | I | 1641 ± 323 | 1804 ± 343 |
| II | 1455 ± 272† | 1554 ± 316† | |
| Satellite cell content (per fibre) | I | 0.092 ± 0.026 | 0.114 ± 0.041* |
| II | 0.061 ± 0.024† | 0.079 ± 0.025†* | |
| Satellite cell content (% total myonuclei) | I | 2.4 ± 0.7 | 2.9 ± 0.7* |
| II | 1.7 ± 0.7† | 2.2 ± 0.5†* | |
| CC | I | 3.45 ± 0.70 | 3.27 ± 1.4 |
| II | 2.92 ± 0.59† | 3.18 ± 1.4 | |
| C/Fi | I | 1.67 ± 0.30 | 1.89 ± 0.39* |
| II | 1.47 ± 0.29† | 1.67 ± 0.28†* | |
| CFPE (capillaries (1000 μm)−1) | I | 5.55 ± 1.40 | 5.65 ± 1.57 |
| II | 4.84 ± 1.06 | 5.41 ± 0.80 | |
| Satellite cell distance to nearest capillary (μm) | I | 16.1 ± 1.8 | 17.7 ± 5.3 |
| II | 28.9 ± 8.3† | 13.9 ± 3.4* |
Values are means ± SD. †Significantly different compared with type I muscle fibres (P < 0.01). *Significantly different compared with Pre (P < 0.01). CC, capillary contacts; C/Fi, individual muscle fibre capillary‐to‐fibre ratio; CFPE, capillary‐to‐fibre perimeter exchange; I, type I muscle fibres; II, type II muscle fibres.
Figure 2. Muscle fibre size distribution (as a percentage) for type I (A) and type II (B) muscle fibres before (pre) and after (post) the 12‐week combined exercise training programme in healthy older men.
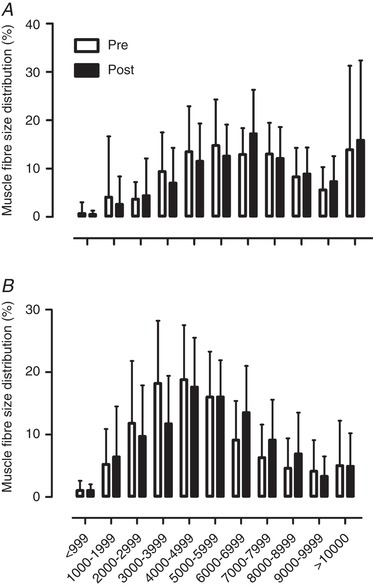
Data are expressed as means ± SD.
Muscle myonuclear content and domain size
Myonuclear content was significantly lower in type II compared with type I muscle fibres, with a difference in myonuclear domain size between fibre types at baseline. After 12 weeks of exercise training, we observed no significant change in myonuclear content and/or domain size (Table 3). As a proxy measure of repairing muscle fibres, the relative number of type I and type II muscle fibres with centrally located myonuclei was evaluated. No significant difference was observed in the relative number of type I or type II repairing muscle fibres in rest, before and after the combined exercise training programme (Table 4). In addition, the relative number of repairing type I and type II muscle fibres did not significantly change at 24 and 48 h after the single bout of exercise before and after the 12 weeks exercise training programme (Table 4).
Table 4.
Change in repairing muscle fibres following a single bout of exercise before (pre‐training acute response) and after (post‐training acute response) 12 weeks of combined exercise training in older men
| Pre‐training acute response | Post‐training acute response | ||||||
|---|---|---|---|---|---|---|---|
| Type | Pre | 24 h | 48 h | Pre | 24 h | 48 h | |
| Repairing muscle fibres (%) | I | 5.7 ± 6.1 | 2.7 ± 4.6 | 1.9 ± 2.8 | 2.6 ± 3.7 | 3.4 ± 3.9 | 5.7 ± 11.8 |
| II | 2.5 ± 2.7 | 2.9 ± 4.9 | 2.1 ± 3.5 | 1.9 ± 2.2 | 3.4 ± 3.5 | 1.7 ± 1.8 | |
Values are means ± SD. Repairing muscle fibres: number of cross‐sectional muscle fibres with centrally positioned myonuclei relative to the total number of cross‐sectional muscle fibres. I, type I muscle fibres; II, type II muscle fibres.
Muscle fibre capillary content
The number of type II muscle fibre CC and C/Fi was significantly lower compared with type I muscle fibres at baseline (P < 0.05; Table 3). No significant difference was observed between type I and type II muscle fibre CFPE index (Table 3). Whereas no significant change in type I and type II muscle fibre CC and CFPE index was observed over time, we found a significant increase in both type I and type II muscle fibre C/Fi in response to the 12‐week combined exercise training programme (P < 0.05; Table 3).
Muscle satellite cell content and activation status
At baseline type II muscle fibre satellite cell content per fibre or expressed as a proportion of total myonuclei was lower compared with type I muscle fibres (P < 0.01, Table 3). Both type I and type II muscle fibre satellite cell content increased significantly in response to 12 weeks of combined exercise training (Table 3). The number of MyoD+ satellite cells per fibre (pre: 0.0068 ± 0.005 vs. post: 0.0092 ± 0.006 MyoD+ satellite cells per fibre) as well as the proportion of MyoD+ satellite cells relative to total number of satellite cells (pre: 10 ± 8 vs. post: 12 ± 8%) was not different in the resting muscle biopsy sample taken before and after 12 weeks of combined exercise training. At baseline, the distance to nearest capillary was significantly greater for type II compared with type I muscle fibre satellite cells (P < 0.05; Table 3). In response to the exercise training programme, satellite cell distance to nearest capillary was significantly reduced for type II muscle satellite cells only (P < 0.01, Table 3)
Pre‐training acute response
We observed a significant time × fibre type interaction (P < 0.01) for muscle fibre satellite cell content during the pre‐training response; as such, type I and type II muscle fibres were analysed separately. The number of type I muscle fibre satellite cells increased from 0.092 ± 0.026 at baseline to 0.132 ± 0.057 at 24 h and 0.126 ± 0.038 satellite cells per fibre at 48 h after the single bout of resistance exercise (main effect of time P < 0.01, Fig. 3 E). In contrast, we observed no change in type II muscle fibre satellite cell content (pre: 0.061 ± 0.024, 24 h: 0.065 ± 0.026 and 48 h: 0.065 ± 0.026 satellite cells per muscle fibre; Fig. 3 E). During the pre‐training acute response, no significant change in the number of MyoD− and MyoD+ satellite cells was observed at 24 and 48 h of post‐exercise recovery (Table 5). Likewise, no change was observed in the proportion of MyoD+ satellite cells during 24 and 48 h of post‐exercise recovery (Pre: 10 ± 8 vs. 24 h: 10 ± 8 vs. 48 h 14 ± 7 % MyoD+ satellite cells; Fig. 4 E). In contrast the number of MyoD+ cells (Pax7−) increased significantly after 24 and 48 h after the single bout of exercise (P < 0.05, Table 5).
Figure 3. Representative images of the fibre type specific satellite cell analyses (A–D) and the acute type I and type II muscle fibre satellite cell response to a single bout of resistance exercise before and after 12 weeks of combined exercise training in healthy older men (E–F).
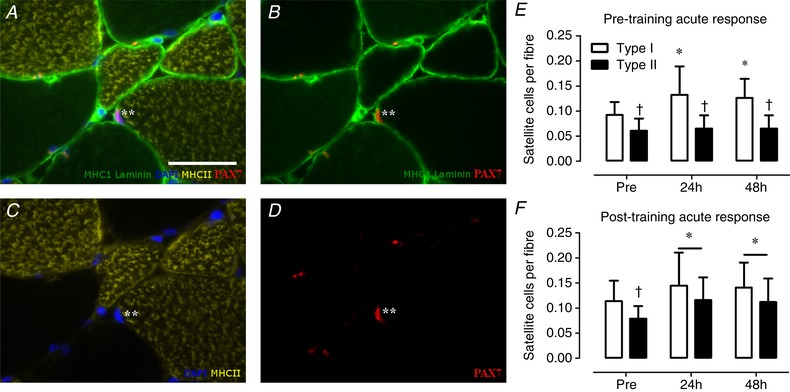
A, MHCI (green), laminin (green), MHCII (yellow), DAPI (blue), Pax7 (red). B, MHCI (green), laminin (green), Pax7 (red). C, MHCII (yellow), DAPI (blue). D, Pax7 (red) only. Asterisk indicates the satellite cell. Scale bar in A: 50 μm. E, type I and type II muscle fibre satellite cell response following a single bout of exercise before 12 weeks of combined exercise training (pre‐training acute response). F, type I and type II muscle fibre satellite cell response following a single bout of exercise after 12 weeks of combined exercise training (post‐training acute response). *Significantly different compared with pre, P < 0.05; †significantly different compared with type I muscle fibre, P < 0.05. Data are expressed as means ± SD. [Color figure can be viewed at wileyonlinelibrary.com]
Table 5.
Change in satellite cell activation status in response to a single bout of resistance exercise before (pre‐training acute response) and after (post‐training acute response) 12 weeks of combined exercise training in older men
| Pre‐training acute response | Post‐training acute response | |||||
|---|---|---|---|---|---|---|
| Pre | 24 h | 48 h | Pre | 24 h | 48 h | |
| Pax7+MyoD− (per fibre) | 0.066 ± 0.022 | 0.086 ± 0.031 | 0.069 ± 0.025 | 0.063 ± 0.028 | 0.100 ± 0.040* | 0.066 ± 0.026** |
| Pax7+MyoD+ (per fibre) | 0.007 ± 0.005 | 0.010 ± 0.008 | 0.010 ± 0.004 | 0.009 ± 0.006 | 0.024 ± 0.019* | 0.026 ± 0.021* |
| Pax7−MyoD+ (per fibre) | 0.014 ± 0.004 | 0.065 ± 0.004* | 0.046 ± 0.021* | 0.037 ± 0.026 | 0.077 ± 0.069 | 0.058 ± 0.055 |
Means ± SD. *Significantly different compared with Pre, P < 0.05. **Significantly different compared with 24 h, P < 0.05.
Figure 4. Representative images of the satellite cell and MyoD colocalization (A–D) and the acute change in satellite cell MyoD colocalization in response to a single bout of resistance exercise before and after 12 weeks of combined exercise training in healthy older men (H–F).
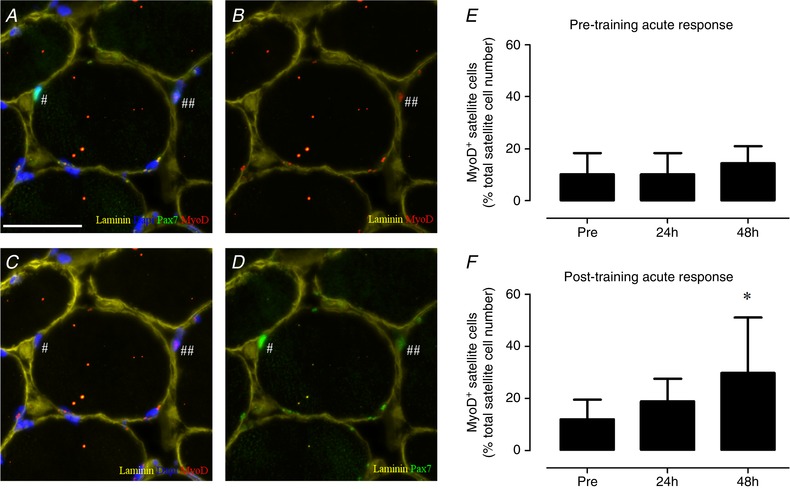
A, laminin (yellow), DAPI (blue), Pax7 (green), MyoD (red). B, laminin (yellow), MyoD (red). C, laminin (yellow), MyoD (red), DAPI (blue). D, laminin (yellow), Pax7 (green). # indicates MyoD− satellite cell (quiescent); ## indicates MyoD+ satellite cell (active). E, the change in MyoD+ satellite cells in response to a single bout of exercise before the combined exercise training programme (pre‐training acute response). F, the change in MyoD+ satellite in response to a single bout of exercise after the combined exercise training programme (post‐training acute response). Scale bar in A: 50 μm. *Significantly different compared with Pre, P < 0.05. Data are expressed as means ± SD. [Color figure can be viewed at wileyonlinelibrary.com]
Post‐training acute response
Following the 12‐week combined exercise training programme, we observed an increase in both type I and type II muscle fibre satellite cell content in response to a single bout of resistance exercise (main effect of time P < 0.01). Type I muscle fibre satellite cell content increased from 0.114 ± 0.041 at baseline to 0.145 ± 0.066 at 24 h and 0.141 ± 0.049 at 48 h of post‐exercise recovery (Fig. 3 F). Type II muscle fibre satellite cells content increased from 0.079 ± 0.024 at baseline to 0.116 ± 0.045 at 24 h and 0.112 ± 0.047 at 48 h of post‐exercise recovery (Fig. 3 F). A significant main effect of time was observed for MyoD− satellite cells (P < 0.05), MyoD+ satellite cells (P < 0.05) and MyoD+ cells (P < 0.05) in response to the single bout of exercise performed after 12 weeks of exercise training (Table 5). Post hoc analyses showed a significant increase in number of MyoD− satellite cells at 24 h (P < 0.05), which returned to baseline at 48 h of post‐exercise recovery (Table 5). The number of MyoD+ satellite cells was significantly increased from baseline at 24 h (P < 0.05) and 48 h (P < 0.05) following the single bout of exercise (Table 5). Similarly, the number of MyoD+ satellite cells expressed relative to the total number of satellite cells was significantly higher at 48 h (30 ± 21%) compared with baseline (12 ± 8%) of the post‐training acute response muscle biopsy sample (P < 0.05; Fig. 4 F). Finally, the number of MyoD+ (Pax7−) cells tended to be increased at 24 h of post‐exercise recovery (P = 0.096; Table 5).
Correlational analyses
Pearson correlation analyses showed a significant positive association between the change (Δ; pre‐ vs. post‐12 weeks of combined exercise training) type II muscle fibre C/Fi and the percentage change in type II muscle fibre satellite cell responsiveness (i.e. increase in SC content at 24 h following a single bout of resistance exercise; pre‐ vs. post‐12 weeks of combined exercise training) in healthy older men (r = 0.671, P < 0.5; Fig. 5 C). In addition, a significant positive correlation was observed between the increase in type I muscle fibre CC and the percentage change in type II muscle fibre satellite responsiveness (i.e. increase in SC content at 48 h following a single bout of resistance exercise; pre‐ vs. post‐12 weeks of combined exercise training; r = 0.779, P < 0.05, Table 6). See Table 6 for an overview of all correlative analyses performed.
Figure 5. Representative images of fibre type‐specific analyses of muscle fibre capillarization (A and B) and scatter plot of its relationship with the improvement in the acute muscle satellite cell response following 12 weeks of combined exercise training in healthy older men (C).
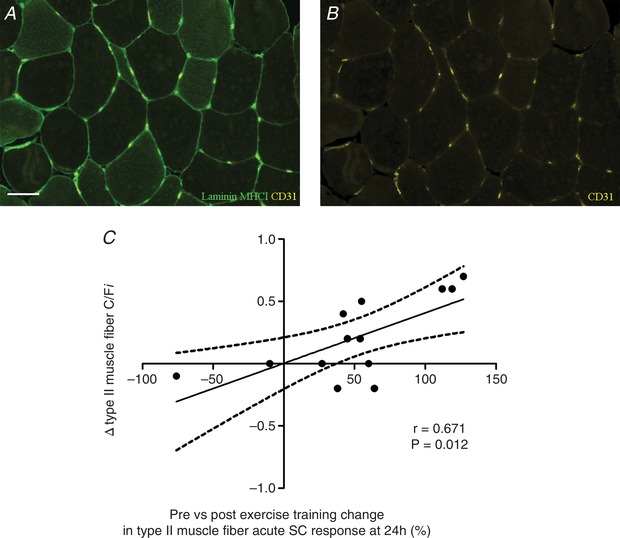
A, laminin (green), MHCI (green), CD31 (yellow). B, CD31 (yellow) only. C, Pearson correlation between Δ (pre‐ vs. post‐12 weeks of combined exercise training) type II muscle fibre capillary to fibre ratio (C/Fi) and the percentage change in type II muscle fibre satellite cell (SC) responsiveness (i.e. increase in SC content at 24 h following a single bout of resistance exercise; pre‐ vs. post‐12 weeks of combined exercise training) in healthy older men. Scale bar in A: 50 μm. [Color figure can be viewed at wileyonlinelibrary.com]
Table 6.
Pearson correlation between the change in acute muscle satellite cell response following a single bout of exercise pre‐ compared with post‐exercise training and change in muscle fibre capillarization
| ΔCC | ΔC/Fi | ΔCFPE index | |||||
|---|---|---|---|---|---|---|---|
| Type I | Type II | Type I | Type II | Type I | Type II | ||
| Pre‐ vs. post‐exercise training change in type II muscle fibre acute satellite cell response at 0–24 h | r | 0.151 | 0.328 | 0.234 | 0.672 | 0.105 | 0.475 |
| P | 0.622 | 0.274 | 0.442 | 0.012 | 0.734 | 0.101 | |
| Pre‐ vs. post‐exercise training change type II muscle fibre acute satellite cell response at 0–48 h | r | 0.779 | 0.510 | 0.136 | 0.003 | 0.388 | 0.104 |
| P | 0.002 | 0.075 | 0.658 | 0.993 | 0.190 | 0.734 | |
| Pre‐ vs. post‐exercise training change type II muscle fibre acute satellite cell response at 24–48 h | r | 0.145 | −0.237 | −0.080 | −0.429 | 0.105 | −0.102 |
| P | 0.636 | 0.436 | 0.795 | 0.144 | 0.732 | 0.740 | |
Significant (P < 0.05) correlations highlighted in bold. Δ, change from pre‐ to post‐exercise training; CC, capillary contacts; C/Fi: individual muscle fibre capillary‐to‐fibre ratio; CFPE, capillary‐to‐fibre perimeter exchange index; r, Pearson correlation coefficient; Type I: type I muscle fibre; Type II, type II muscle fibre.
Myogenic regulatory factors
Pre‐training acute response
In response to the single bout of resistance exercise, no significant change in myogenic factor‐5 (Myf5) , MyoD and/or myogenin mRNA expression was observed over time (Fig. 6 A, C and E).
Figure 6. Acute changes in Myf5 (A and B), MyoD (C and D) and myogenin (E and F) mRNA expression before (Pre) and 24 and 48 h after a single bout of resistance exercise performed before (pre‐training acute response: A, C and E) and after (post‐training acute response: B, D and F) 12 weeks of combined exercise training in healthy older men.
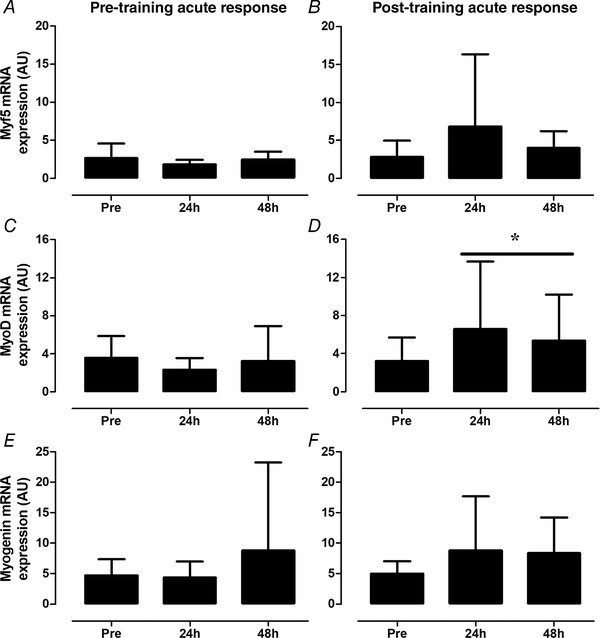
Data are expressed in arbitrary units (AU) calculated using method, with genes of interest normalized to GAPDH. *Significant main effect of time (P < 0.05). Values represent means ± SD.
Post‐training acute response
Following 12 weeks of exercise training, no significant effect of time was found for Myf5 mRNA expression following the single exercise session (Fig. 6 B). In contrast, a significant main effect of time (P < 0.05) was observed for MyoD mRNA expression. Compared with the baseline muscle biopsy sample, MyoD mRNA expression tended to be higher at 24 h (P = 0.080) and 48 h (P = 0.088) after the single bout of resistance exercise (Fig. 6 D). A tendency for a main effect of time was also observed for myogenin mRNA expression (Fig. 6 F; P = 0.069).
Discussion
The present study is the first to show that exercise training can improve the acute muscle satellite cell response following a bout of resistance exercise in healthy older men. In addition, our results indicate that the enhanced muscle satellite cell response post‐training is associated with an improvement in muscle fibre capillarization following exercise training.
Exercise training is an effective strategy to counteract the negative effects of age‐related sarcopenia (Peterson et al. 2010, 2011; Bell et al. 2017). As ageing is known to be associated with reduced cardiometabolic health as well as low muscularity, current guidelines recommend performing both resistance and endurance‐type exercise to support healthy ageing (American College of Sports Medicine et al. 2009; Australian and New Zealand Society for Geriatric Medicine, 2014). Hence, in the present study we employed a combined exercise training programme consisting of twice a week resistance exercise training and once a week HIIT training. Our data show an improvement in aerobic capacity (+8%), increase in 1RM muscle strength (ranging from +14% to +30%) and reduction in fat mass (−3%) following 12 weeks of exercise training. We did not observe a significant increase in whole body or leg lean mass. Muscle fibre size tended (P = 0.066) to increase in response to the exercise training programme, which was further evidenced by a rightward shift in muscle fibre size distribution (Fig. 2). The relatively low volume of resistance exercise training (only twice a week) and/or the potential of an antagonizing effect of performing concurrent aerobic‐type exercise on resistance exercise‐induced muscle growth may explain this lack of significance (Kikuchi et al. 2016). Nevertheless, the significant improvements observed in aerobic capacity and muscle strength following exercise training are important for daily physical function, mobility, mitigation of disease risk, and overall quality of life (Rantanen et al. 2002).
On the level of the muscle fibre, sarcopenia is characterized by a type II muscle fibre atrophy that is accompanied by a type II muscle fibre type‐specific decrease in satellite cell content and reduced activation and expansion of the satellite pool following a single bout of exercise (McKay et al. 2012; Verdijk et al. 2014; Snijders et al. 2014b). Although still debated, it has been suggested that the reduction in muscle satellite cell content and/or function may be a (key) factor in the development of sarcopenia in later life and/or the maladaptive muscle fibre response following exercise training in older adults (Verdijk et al. 2014; Snijders et al. 2015). It is well established that in older adults satellite cell content can be restored to the level of healthy young (untrained) individuals with exercise training (Mackey et al. 2007; Verney et al. 2008; Leenders et al. 2013; Verdijk et al. 2014). However, little is known about whether the delayed increase and impaired activation of the satellite cell pool size following a single bout of exercise is altered following exercise training. In the present study, we attempted to address this issue by comparing the acute muscle satellite cell response to a single bout of resistance exercise before and after 12 weeks of combined exercise training. The activation status of the muscle satellite cell pool was evaluated by co‐staining for MyoD, which plays a key role in muscle satellite cell activation/proliferation (Megeney et al. 1996; Kitzmann et al. 1998). Before the combined exercise training programme, we observed a significant increase in type I and no change in type II muscle fibre satellite cell content at 24 and 48 h after exercise. In addition, we observed no significant change in the number of MyoD− (quiescent), MyoD+ (active) satellite cell and myogenic regulatory factor (i.e. Myf5, MyoD and myogenin) mRNA expression during the post‐exercise period. These results are in line with previous publications from our laboratory in older men (McKay et al. 2012, 2013; Snijders et al. 2014b; Nederveen et al. 2015). Interestingly, we did observe a significant increase in the number of MyoD+ cells (differentiating satellite cells) in response to the single bout of exercise. In combination with the lack of increase in quiescent and active satellite cells at the post‐exercise time points, this may suggest asymmetrical differentiation of the satellite cell pool during post‐exercise recovery. After the combined exercise training programme, we found that baseline type I and type II muscle fibre satellite cell pool size had increased significantly. More importantly, after the 12‐week exercise training programme, type I as well as type II muscle fibre satellite cell content increased significantly at 24 h and 48 h post‐exercise, which was accompanied by a significant increase in the number of MyoD− (quiescent) and MyoD+ (active) satellite cells at 24 and 48 h after the single bout of exercise. Furthermore, we observed a significant upregulation of the mRNA expression of various myogenic regulatory factors at the post‐exercise time points. These results provide convincing evidence that exercise training improved muscle satellite cell content as well as acute muscle satellite cell pool size activation and expansion during post‐exercise recovery in healthy older men.
Adequate vascular perfusion of skeletal muscle is required for the delivery of oxygen, nutrients and growth factors that are likely necessary to facilitate muscle fibre growth following exercise (Fujita et al. 2006; Timmerman et al. 2010; Phillips et al. 2012). An age‐related decline in muscle fibre perfusion has been suggested to be a key contributing factor in the blunted anabolic response in the post‐exercise period (Joanisse et al. 2017). The perfusion of muscle fibres largely relies on the muscle fibre capillary network. Previous studies have shown that muscle fibre capillarization declines with advancing age, with a specific reduction associated with type II muscle fibres (Proctor et al. 1995; Nederveen et al. 2016). Interestingly, we and others have demonstrated that an anatomical relationship exist between muscle satellite cells and the microvascular bed in humans (Christov et al. 2007; Nederveen et al. 2016, 2017, 2018; Snijders et al. 2017). We have shown that type II muscle fibre satellite cells are located significantly further away from their nearest capillary in older compared with young adults. A decline in capillary density per se and/or greater distance between satellite cells and capillaries may be an critical factor in the impaired muscle fibre hypertrophy and/or satellite cell response during resistance exercise training in healthy older men (Snijders et al. 2017). As such, we hypothesized that the age‐related fibre type‐specific reduction in muscle fibre capillarization may prevent optimal satellite cell function in the first days of post‐exercise recovery (Joanisse et al. 2017). Whereas discrepant findings have been reported on the impact of resistance exercise training on angiogenesis in older men, it has been well established that performing prolonged aerobic exercise training increases muscle fibre capillarization in both young and older individuals (Gavin et al. 2007). As our exercise training programme included an aerobic exercise component (once a week HIIT), we assessed whether the enhanced acute post‐exercise muscle satellite cell activation and pool size expansion response following the combined exercise training programme was related to an increase in muscle fibre capillarization. In response to the 12‐week combined exercise training programme, we found a small, but significant, increase (+14%) in type I and type II C/Fi. Interestingly, we observed a significant correlation in variance between the increase in type II muscle fibre C/Fi and the improved acute type II muscle fibre satellite cell response 24 h following the single bout of resistance exercise. Furthermore, we observed a significant reduction in the distance of type II muscle fibre satellite cells to their nearest capillary following the exercise training programme. We speculate that the exercise training‐induced restoration of the acute muscle satellite cell activation/proliferation response following exercise may, in part, be mediated by an improvement in muscle fibre capillarization in older men. However, due to the lack of a non‐exercise control group and additional assessments, we cannot rule out that other mechanisms such as altered signalling (endo‐, exo‐ and/or paracrine) and/or neuromuscular adaptation, which were not assessed in the present study, may also have contributed to improving the acute post‐exercise muscle satellite cell response in older men. Clearly more research is warranted to further establish the underlying mechanisms of the improved muscle satellite cell response observed in the present study. Nevertheless, if our speculation is correct then it would suggest that the development of an exercise prescription that promotes angiogenesis in older adults should be investigated to further improve the efficacy of exercise training to combat the consequences of age‐related sarcopenia.
In conclusion, we show that the acute muscle satellite cell response following a single bout of exercise can be improved by prolonged exercise training in healthy older men. In addition, this improvement in the acute muscle satellite cell response is associated with an increase in muscle fibre capillarization following exercise training suggesting a possible functional link between capillarization and acute muscle satellite cell response during post‐exercise recovery.
Additional information
Competing interests
S.M.P. reports receipt of competitive grant support, travel expenses, and honoraria for speaking received from the US National Dairy Council. No other conflicts of interest, financial or otherwise, are reported by any of the authors.
Author contributions
T.S., S.M.P., K.E.B., G.P., conceived and designed the experiments; T.S., K.E.B., J.P.N., S.W.L., D.A.K., collected samples; T.S. J.P.N., K.E.B., S.W.L., N.M., performed experiments. T.S., K.E.B., J.P.N., G.P., analyzed data; T.S.N. J.P.N., K.E.B., D.A.K., S.M.P., G.P., interpreted results of experiments; T.S., prepared figures; T.S., G.P. drafted manuscript. All authors have read and approved the final version of this manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This research was supported by a funding from the Labarge Optimal Aging Initiative from McMaster University (to GP) and Canadian Institutes of Health Research (CIHR) grant (MOP‐123296) to S.M.P. K.E.B. was supported by CIHR Canada Graduate Scholarship (CGS‐D). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Acknowledgements
The Pax7 hybridoma cells developed by Dr A. Kawakami and the A4.951 developed by Dr H. Blau were obtained from the Developmental Studies Hybridoma Bank, created by the NICHD of the NIH and maintained at The University of Iowa, Department of Biology, Iowa City, IA 52242, USA.
Biography
Tim Snijders holds a BSc in Health Sciences and an MSc in Human Movement Sciences (Maastricht University, the Netherlands). During his PhD he studied the potential role of skeletal muscle satellite cells in different human models of muscle fibre atrophy and hypertrophy (Maastricht University). As a post‐doctoral fellow, he continued his research in exercise physiology at McMaster University (Hamilton, ON, Canada). During this time his studies were focused on the identification of exercise and nutritional intervention strategies to augment skeletal muscle satellite cell function in older adults. He is now an assistant professor at Maastricht University involved in both research and education focused on skeletal muscle fibre perfusion in the muscle adaptive response to exercise training in humans.

Edited by: Michael Hogan & Steven Segal
References
- American College of Sports Medicine , Chodzko‐Zajko WJ, Proctor DN, Fiatarone Singh MA, Minson CT, Nigg CR, Salem GJ & Skinner JS (2009). American College of Sports Medicine position stand. Exercise and physical activity for older adults. Med Sci Sports Exerc 41, 1510–1530. [DOI] [PubMed] [Google Scholar]
- Australian and New Zealand Society for Geriatric Medicine (2014). Australian and New Zealand Society for Geriatric Medicine: Position Statement – Exercise guidelines for older adults. Australas J Ageing 33, 287–294. [DOI] [PubMed] [Google Scholar]
- Bell KE, Snijders T, Zulyniak M, Kumbhare D, Parise G, Chabowski A & Phillips SM (2017). A whey protein‐based multi‐ingredient nutritional supplement stimulates gains in lean body mass and strength in healthy older men: A randomized controlled trial. PLoS One 12, e0181387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergstrom J (1975). Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scand J Clin Lab Invest 35, 609–616. [PubMed] [Google Scholar]
- Cermak NM, Snijders T, McKay BR, Parise G, Verdijk LB, Tarnopolsky MA, Gibala MJ & van Loon LJC (2013). Eccentric exercise increases satellite cell content in type ii muscle fibers. Med Sci Sports Exerc 45, 230–237. [DOI] [PubMed] [Google Scholar]
- Christov C, Chretien F, Abou‐Khalil R, Bassez G, Vallet G, Authier FJ, Bassaglia Y, Shinin V, Tajbakhsh S, Chazaud B & Gherardi RK (2007). Muscle satellite cells and endothelial cells: close neighbors and privileged partners. Mol Biol Cell 18, 1397–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz‐Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinkova E, Vandewoude M, Zamboni M; European Working Group on Sarcopenia in Older People (2010). Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 39, 412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer HC, Blanco CE, Sattler FR, Schroeder ET & Wiswell RA (2006). Satellite cell numbers in young and older men 24 hours after eccentric exercise. Muscle Nerve 33, 242–253. [DOI] [PubMed] [Google Scholar]
- Egner IM, Bruusgaard JC & Gundersen K (2016). Satellite cell depletion prevents fiber hypertrophy in skeletal muscle. Development 143, 2898–2906. [DOI] [PubMed] [Google Scholar]
- Frontera WR, Meredith CN, O'Reilly KP & Evans WJ (1990). Strength training and determinants of VO2max in older men. J Appl Physiol (1985) 68, 329–333. [DOI] [PubMed] [Google Scholar]
- Fujita S, Rasmussen BB, Cadenas JG, Grady JJ & Volpi E (2006). Effect of insulin on human skeletal muscle protein synthesis is modulated by insulin‐induced changes in muscle blood flow and amino acid availability. Am J Physiol Endocrinol Metab 291, E745–E754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavin TP, Ruster RS, Carrithers JA, Zwetsloot KA, Kraus RM, Evans CA, Knapp DJ, Drew JL, McCartney JS, Garry JP & Hickner RC (2007). No difference in the skeletal muscle angiogenic response to aerobic exercise training between young and aged men. J Physiol 585, 231–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman FC, Walsh SJ, Staron RS, Hikida RS, Gilders RM, Murray TF, Toma K & Ragg KE (2000). Effects of high‐intensity resistance training on untrained older men. I. Strength, cardiovascular, and metabolic responses. J Gerontol A Biol Sci Med Sci 55, B336–B346. [DOI] [PubMed] [Google Scholar]
- Hepple RT, Mackinnon SL, Goodman JM, Thomas SG & Plyley MJ (1997a). Resistance and aerobic training in older men: effects on VO2peak and the capillary supply to skeletal muscle. J Appl Physiol (1985) 82, 1305–1310. [DOI] [PubMed] [Google Scholar]
- Hepple RT, Mackinnon SL, Thomas SG, Goodman JM & Plyley MJ (1997b). Quantitating the capillary supply and the response to resistance training in older men. Pflugers Arch 433, 238–244. [DOI] [PubMed] [Google Scholar]
- Joanisse S, Nederveen JP, Snijders T, McKay BR & Parise G (2017). Skeletal muscle regeneration, repair and remodelling in aging: the importance of muscle stem cells and vascularization. Gerontology 63, 91–100. [DOI] [PubMed] [Google Scholar]
- Kikuchi N, Yoshida S, Okuyama M & Nakazato K (2016). The effect of high‐intensity interval cycling sprints subsequent to arm‐curl exercise on upper‐body muscle strength and hypertrophy. J Strength Cond Res 30, 2318–2323. [DOI] [PubMed] [Google Scholar]
- Kitzmann M, Carnac G, Vandromme M, Primig M, Lamb NJ & Fernandez A (1998). The muscle regulatory factors MyoD and myf‐5 undergo distinct cell cycle‐specific expression in muscle cells. J Cell Biol 142, 1447–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leenders M, Verdijk LB, van der Hoeven L, van Kranenburg J, Nilwik R & van Loon LJ (2013). Elderly men and women benefit equally from prolonged resistance‐type exercise training. J Gerontol A Biol Sci Med Sci 68, 769–779. [DOI] [PubMed] [Google Scholar]
- Lepper C, Partridge TA & Fan CM (2011). An absolute requirement for Pax7‐positive satellite cells in acute injury‐induced skeletal muscle regeneration. Development 138, 3639–3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey AL, Esmarck B, Kadi F, Koskinen SO, Kongsgaard M, Sylvestersen A, Hansen JJ, Larsen G & Kjaer M (2007). Enhanced satellite cell proliferation with resistance training in elderly men and women. Scand J Med Sci Sports 17, 34–42. [DOI] [PubMed] [Google Scholar]
- McKay BR, Ogborn DI, Baker JM, Toth KG, Tarnopolsky MA & Parise G (2013). Elevated SOCS3 and altered IL‐6 signaling is associated with age‐related human muscle stem cell dysfunction. Am J Physiol Cell Physiol 304, C717–C728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay BR, Ogborn DI, Bellamy LM, Tarnopolsky MA & Parise G (2012). Myostatin is associated with age‐related human muscle stem cell dysfunction. FASEB J 26, 2509–2521. [DOI] [PubMed] [Google Scholar]
- Megeney LA, Kablar B, Garrett K, Anderson JE & Rudnicki MA (1996). MyoD is required for myogenic stem cell function in adult skeletal muscle. Genes Dev 10, 1173–1183. [DOI] [PubMed] [Google Scholar]
- Nederveen JP, Joanisse S, Seguin CM, Bell KE, Baker SK, Phillips SM & Parise G (2015). The effect of exercise mode on the acute response of satellite cells in old men. Acta Physiol 215, 177–190. [DOI] [PubMed] [Google Scholar]
- Nederveen JP, Joanisse S, Snijders T, Ivankovic V, Baker SK, Phillips SM & Parise G (2016). Skeletal muscle satellite cells are located at a closer proximity to capillaries in healthy young compared with older men. J Cachexia Sarcopenia Muscle 7, 547–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nederveen JP, Joanisse S, Snijders T, Thomas ACQ, Kumbhare D & Parise G (2018). The influence of capillarization on satellite cell pool expansion and activation following exercise‐induced muscle damage in healthy young men. J Physiol 596, 1063–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nederveen JP, Snijders T, Joanisse S, Wavell CG, Mitchell CJ, Johnston LM, Baker SK, Phillips SM & Parise G (2017). Altered muscle satellite cell activation following 16 wk of resistance training in young men. Am J Physiol Regul Integr Comp Physiol 312, R85–R92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilwik R, Snijders T, Leenders M, Groen BB, van Kranenburg J, Verdijk LB & van Loon LJ (2013). The decline in skeletal muscle mass with aging is mainly attributed to a reduction in type II muscle fiber size. Exp Gerontol 48, 492–498. [DOI] [PubMed] [Google Scholar]
- Peterson MD, Rhea MR, Sen A & Gordon PM (2010). Resistance exercise for muscular strength in older adults: a meta‐analysis. Ageing Res Rev 9, 226–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson MD, Sen A & Gordon PM (2011). Influence of resistance exercise on lean body mass in aging adults: a meta‐analysis. Med Sci Sports Exerc 43, 249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips B, Williams J, Atherton P, Smith K, Hildebrandt W, Rankin D, Greenhaff P, Macdonald I & Rennie MJ (2012). Resistance exercise training improves age‐related declines in leg vascular conductance and rejuvenates acute leg blood flow responses to feeding and exercise. J Appl Physiol (1985) 112, 347–353. [DOI] [PubMed] [Google Scholar]
- Prior SJ, Goldberg AP, Ortmeyer HK, Chin ER, Chen D, Blumenthal JB & Ryan AS (2015). Increased skeletal muscle capillarization independently enhances insulin sensitivity in older adults after exercise training and detraining. Diabetes 64, 3386–3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor DN, Sinning WE, Walro JM, Sieck GC & Lemon PW (1995). Oxidative capacity of human muscle fiber types: effects of age and training status. J Appl Physiol (1985) 78, 2033–2038. [DOI] [PubMed] [Google Scholar]
- Rantanen T, Avlund K, Suominen H, Schroll M, Frandin K & Pertti E (2002). Muscle strength as a predictor of onset of ADL dependence in people aged 75 years. Aging Clin Exp Res 14, 10–15. [PubMed] [Google Scholar]
- Schmittgen TD & Livak KJ (2008). Analyzing real‐time PCR data by the comparative C T method. Nat Protoc 3, 1101–1108. [DOI] [PubMed] [Google Scholar]
- Snijders T, Nederveen JP, Joanisse S, Leenders M, Verdijk LB, van Loon LJC & Parise G (2017). Muscle fibre capillarization is a critical factor in muscle fibre hypertrophy during resistance exercise training in older men. J Cachexia Sarcopenia Muscle 8, 267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijders T, Nederveen JP, McKay BR, Joanisse S, Verdijk LB, van Loon LJ & Parise G (2015). Satellite cells in human skeletal muscle plasticity. Front Physiol 6, 283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijders T, Verdijk LB, McKay BR, Smeets JS, van Kranenburg J, Groen BB, Parise G, Greenhaff P & van Loon LJ (2014a). Acute dietary protein intake restriction is associated with changes in myostatin expression after a single bout of resistance exercise in healthy young men. J Nutr 144, 137–145. [DOI] [PubMed] [Google Scholar]
- Snijders T, Verdijk LB, Smeets JS, McKay BR, Senden JM, Hartgens F, Parise G, Greenhaff P & van Loon LJ (2014b). The skeletal muscle satellite cell response to a single bout of resistance‐type exercise is delayed with aging in men. Age (Dordr) 36, 9699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmerman KL, Lee JL, Dreyer HC, Dhanani S, Glynn EL, Fry CS, Drummond MJ, Sheffield‐Moore M, Rasmussen BB & Volpi E (2010). Insulin stimulates human skeletal muscle protein synthesis via an indirect mechanism involving endothelial‐dependent vasodilation and mammalian target of rapamycin complex 1 signaling. J Clin Endocrinol Metab 95, 3848–3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdijk LB, Snijders T, Drost M, Delhaas T, Kadi F & van Loon LJ (2014). Satellite cells in human skeletal muscle; from birth to old age. Age (Dordr) 36, 545–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdijk LB, Snijders T, Holloway TM, van Kranenburg J & van Loon LJ (2016). Resistance training increases skeletal muscle capillarization in healthy older men. Med Sci Sports Exerc 48, 2157–2164. [DOI] [PubMed] [Google Scholar]
- Verdijk LB, van Loon L, Meijer K & Savelberg HH (2009). One‐repetition maximum strength test represents a valid means to assess leg strength in vivo in humans. J Sports Sci 27, 59–68. [DOI] [PubMed] [Google Scholar]
- Verney J, Kadi F, Charifi N, Feasson L, Saafi MA, Castells J, Piehl‐Aulin K & Denis C (2008). Effects of combined lower body endurance and upper body resistance training on the satellite cell pool in elderly subjects. Muscle Nerve 38, 1147–1154. [DOI] [PubMed] [Google Scholar]


