Abstract
Transplantation of endothelial progenitor cells (EPCs) is a proven safe and effective method for treatment of cerebral ischemia in animal experiments. However, safety and efficacy need to be determined in clinical trials. We performed a two‐center, randomized, placebo‐controlled phase I/IIa trial with blinded outcome assessment on 18 patients with acute cerebral infarct within the middle cerebral artery territory, and followed for up to 4 years. Autologous ex vivo expanded EPCs were injected intravenously in the EPC group, and patients who received saline or autologous bone marrow stromal cells served as control groups. Mortality of any cause, adverse events, and new‐onset comorbidities were monitored. Changes in neurological deficits were assessed at different time points. We found no toxicity events or infusional or allergic reactions in any treated group. Three patients in the placebo group died during the 4‐year follow‐up. We found that the EPC group had fewer serious adverse events compared with the placebo‐controlled group, although there were no statistical differences in mortality among the three groups. Furthermore, there was no significant difference in neurological or functional improvement observed among the three groups, except for the Scandinavia Stroke Scale score at 3 months between the EPC group and placebo‐controlled group. Autologous transplantation of EPCs appears to improve long‐term safety in acute cerebral infarct patients, supporting the feasibility of this novel method for treatment of ischemic stroke (ClinicalTrials.gov: NCT01468064). Stem Cells Translational Medicine 2019;8:14–21
Keywords: Endothelial progenitor cells, Bone marrow stromal cells, Stroke, Transplantation, Clinical trial
Significance Statement.
This study suggests that autologous transplantation of endothelial progenitor cells improved long‐term safety in patients with acute cerebral infarct, supporting the feasibility of this novel method for treatment of ischemic stroke, and warrants a larger phase II trial.
Introduction
Stroke is associated with persistent disability and is a leading cause of death 1. Recent studies in People's Republic of China suggested a mortality of 3.3%–5.2% in 2 weeks and 9%–9.6% in 3 months after symptom onset. The likelihood of mortality or disability increased to 34.5%–37.1% a year after onset 2. Despite the enormous health impact of stroke, the only approved treatments for acute ischemic stroke, thrombolytic therapies and endovascular recanalization techniques, have a narrow time‐window and only benefit a small subset of patients 3. Because few neuroprotective reagents have been successfully translated “from bench to bedside” in the past several decades, neurorestoration has become a new research hot spot in stroke treatment 4, of which stem cell transplantation has been shown by both animal research and clinical trials to open a novel avenue to promote tissue and function repair after stroke 5, 6.
In addition to hematopoietic stem cells and bone marrow stromal cells (BMSCs), endothelial progenitor cells (EPCs) are another type of stem cell derived from bone marrow that can proliferate and differentiate into mature vascular endothelial cells 7. In the normal central nervous system, EPCs are distinct from endothelial cells, but play an active role in vascular structures 8. After stroke, circulating EPCs can be recruited and participate in cerebral neovascularization 9; however, a critical limitation for the therapeutic application of EPCs is their inherently low number in circulation, which is even smaller in patients with higher cardiovascular risk or aging 10, demonstrating further that transplantation of ex vivo expanded EPCs is an attractive approach to promote restoration after stroke.
In fact, the safety and efficacy of EPC transplantation for stroke have been supported by evidence from numerous laboratories 11, 12, 13, 14. The major mechanism underlying this EPC‐mediated functional recovery may be that EPCs promote endothelial repair and angiogenesis, thus enhancing the neuroplastic effect and resulting in better neurological outcomes 15. Because EPCs can be readily obtained from bone marrow and used for autologous transplantation, virtually eliminating those ethical and immunological considerations, they are believed to be a good graft source for clinical applications. However, no clinical trial for evaluating EPC transfusion in treating ischemic stroke was reported until recently.
Identifying the potential therapeutic effects of EPCs in ischemic stroke will be a challenging but incredibly important breakthrough in neurology that may bring promise for patients with ischemic stroke 15. In this trial, we test the safety and feasibility of autologous transplantation of ex vivo expanded EPCs in patients with acute ischemic stroke. BMSCs are also derived from bone marrow, and have been proved to be safe and effective for treatment of ischemic stroke by animal research and preliminary clinical trials 16, 17, 18. Therefore, autologous transplantation of BMSCs and placebo treatment were employed as controls in this study.
Materials and Methods
Study Design
This study is a two‐center, single‐blinded, randomized, parallel, placebo‐controlled trial that was approved by the Ethics Committee of Zhujiang Hospital and registered with clinicaltrials.gov (NCT01468064). The institutional review board of each participating center approved the treatment protocol before initiation of enrollment. All patients or their legally authorized representatives gave written informed consent. Patients were randomly assigned using layered segment randomization in a 1:1:1 ratio to three groups, and randomization codes were revealed only to the technician responsible for preparing the cells or placebo material. The overall trial profile is shown in Figure 1.
Figure 1.
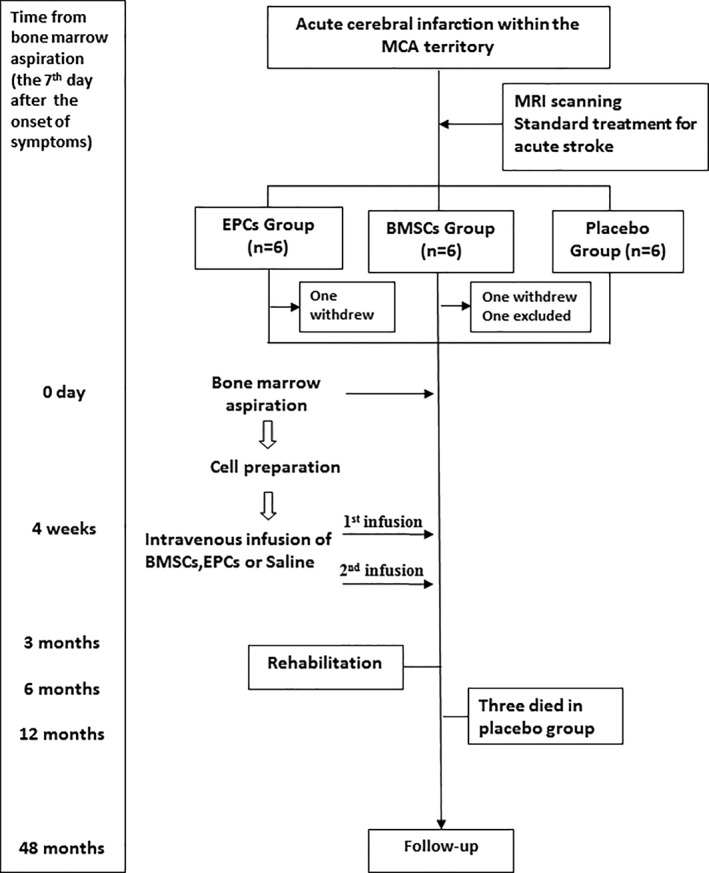
Study profile. Abbreviations: BMSCs, bone marrow stromal cells; EPCs, endothelial progenitor cells; MCA, middle cerebral artery.
Patients
Patients with acute ischemic infarction (within 7 days from symptom onset) who had severe neurological deficits were enrolled from the Zhujiang Hospital of Southern Medical University and Guangdong General Hospital's Nanhai Hospital. Our inclusion criteria were (a) patients aged 18–80 years, within 7 days of onset of symptoms; (b) ischemic lesion within the middle cerebral artery territory as assessed using diffusion‐weighted imaging; (c) a National Institutes of Health Stroke Scale (NIHSS) score ≥7 at day 7 after onset; and (d) signed informed consent. The exclusion criteria were (a) Lacunar syndrome; (b) diagnosis other than ischemic stroke (e.g., intracranial hemorrhage or intracranial tumor); (c) hematological causes of stroke; (d) severe respiratory, hepatic, or renal disorders; (e) presence of severe febrile illness or viral diseases; (f) malignant diseases; (g) presence of autoimmune diseases; (h) positive response of penicillin skin test, or multiple drug allergies; and (i) breast‐feeding or pregnant women.
Patients meeting all of the inclusion and none of the exclusion criteria were approached for consent to participate in the study. All patients received conventional treatment after stroke, and underwent a bone marrow aspiration procedure on the 7th day after symptom onset. Patients were blinded to the grouping. EPCs and BMSCs were expanded ex vivo and then infused intravenously. The placebo‐controlled group did not receive cells transfusion but underwent a bone marrow aspiration procedure and saline transfusion. Neurological assessment, neuroimaging, death of any cause, adverse events, and new onset comorbidities were monitored.
Cell Preparation and Administration
Bone marrow (50 mL) was aspirated from the iliac crest under local anesthesia on the 7th day after symptom onset in each group. Bone marrow mononuclear cells were isolated by centrifugation through a density gradient (Ficoll‐Paque Plus, Pharmacia, Guangzhou, Guangdong Province, China). Cell preparation was performed under good manufacturing practices. For preparation of EPCs, mononuclear cells were seeded on fibronectin (354008, BD Pharmingen)‐coated plastic dishes (1 × 105 cells/cm2) and cultivated in EC basal medium‐2 (EBM‐2, CC‐3162, Lonza) with EGM‐2 MV SingleQuots (CC‐4176, Lonza). BMSCs were prepared and cultured as described previously 16, 17. Nonadherent cells were discarded by removing the medium 4 days later. Once cells reached 80% confluence, they were detached with 0.05% trypsin/0.01% EDTA and sub‐cultured. Cell morphology was observed under a Type CK2 phase‐contrast microscope (Olympus).
Approximately 3–4 weeks after bone marrow aspiration, autologous BMSCs and EPCs were culture‐expanded to reach 3.125 × 106 cells/kg body weight per patient. Among these, 2.5 × 106 cells/kg body weight of autologous EPCs or BMSCs were transplanted into patients intravenously with 250 mL of saline containing 5% autologous serum. The rest of the cells (0.625 × 106 cells/kg body weight) were further expanded for two passages, and thus, another 2.5 × 106 cells/kg body weight of EPCs or BMSCs were again transplanted approximately 1 week after initial boosting. Namely, each cell‐treated patient received a total of 5 × 106 cells/kg body weight of autologous EPCs or BMSCs. This cell amount is equivalent to the dose that was effective in animal studies (1 × 105 to 3 × 106 cells/rat) 6 and in a previous human study (1 × 108 cells/patient) 16, 17. Placebo‐controlled patients received only saline containing 5% autologous serum.
In these cases, the total cell culture time was 33.75 ± 2.75 days (range: 31–37 days) in the BMSC group and 23.60 ± 3.78 days (range: 18–27 days) in the EPC group. And if the cells that reached the first 80% confluence were defined as passage 0, the total population doublings were 5.52 ± 0.22 in the BMSC group and 5.62 ± 0.31 in the EPC group, respectively.
Cell Phenotypes and In Vitro Tube Formation Assay
Cell viability was determined by trypan blue staining at the end of the harvest, and was >98% for every infusion. Cell cultures were tested weekly for sterility, and there was no evidence of bacterial, viral, fungal, or mycoplasmal contamination in any of the flasks. Cell phenotypes were determined by flow cytometry. To identify EPCs, cell binding of UEA‐1 lectin and endocytosis of acetylated low‐density lipoprotein (Dil‐Ac‐LDL), two characteristic features of endothelial lineage cells, were detected as previously described 12.
Tube formation assay was carried out to estimate the in vitro angiogenic capacity of cells. Briefly, 3 × 104 cells were seeded onto 96‐well plates coated with growth factor‐reduced matrigel (70 μL/well; BD Biocoat) and cultured in EBM‐2(Lonza) at 37°C. After incubation for 8 hours, tubes in each well were visualized under an inverted microscope, photographed at ×10 magnification, and analyzed using the Angiogenesis Analyzer for ImageJ software.
Measurements of Safety and Efficacy
All patients were evaluated according to a protocol that included demographic data, medical history, vascular risk factors, laboratory examination, radiological data, and functional scales. The primary endpoint was safety, including adverse events, neurological deterioration (defined as four points or more increase on the NIHSS), and mortality. Patients were followed up at 3, 6, 12, and 48 months after bone marrow aspiration for clinical and functional evaluation. Blood examination of patients including liver, heart, renal function, glycometabolism, lipid metabolism, electrolyte, magnetic resonance scanning, and electrocardiogram were recorded. Death of any cause and adverse effects caused by this study were the primary adverse events monitored. A treatment‐emergent adverse event was defined as any event not present before the initiation of treatment or any event already present that worsened in either intensity or frequency after exposure to study treatment 18.
The secondary endpoints were the difference in neurological or functional outcomes evaluated by the global impairment scales, NIHSS and Scandinavia Stroke Scale (SSS), and the functional scales, Barthel Index (BI) and modified Rankin Scale (mRS). These assessments were evaluated at 0, 3, 6, 12, and 48 months. The assessments were independently conducted by experimental neurologists blind to patient grouping. All assessments of patients were performed through inpatient or outpatient visits to the hospital, or for those who could not visit the hospital, by careful telephone interview.
Statistics
Continuous variables were expressed as the mean ± standard deviation. In addition to descriptive statistics, differences of indicators at baseline between groups were analyzed by one‐way analysis of variance or Kruskal‐Wallis test as appropriate. The incidence of serious adverse events (SAEs) and mortality were analyzed by chi‐square test. Covariance analysis was used to calculate the difference of neurological outcomes of different groups at different time points. Data analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC). Differences were deemed statistically significant at p < .05.
Results
Patient Characteristics
Eighteen patients were recruited and randomly allocated into one of three groups: the BMSC group (n = 6), EPC group (n = 6), and placebo control group (n = 6). Two patients, one from each of the BMSC and EPC groups, declined to receive bone marrow aspiration after enrollment and were excluded from the study. The baseline characteristics of the modified intent‐to‐treat population are shown in Table 1. We found no significant differences between each of the three groups at baseline regarding age, gender, and body mass index, as well as NIHSS, SSS, BI, or mRS score (p > .05).
Table 1.
Baseline characteristics of modified intent‐to‐treat population
| Characteristics | EPCs (n = 5), n (%) | BMSCs (n = 5), n (%) | Placebo (n = 6), n (%) | p value |
|---|---|---|---|---|
| Age, years | 50.80 ± 14.11 | 49.40 ± 10.85 | 52.83 ± 14.95 | .915 |
| Male | 4 (80.0) | 4 (80.0) | 5 (83.3) | .986 |
| Married | 5 (100.0) | 5 (100.0) | 6 (100.0) | |
| Body mass index, kg/m2 | 25.57 ± 3.83 | 23.08 ± 3.66 | 22.33 ± 1.73 | .278 |
| Allergic | 1 (20.0) | 0 (0.0) | 0 (0.0) | .309 |
| Time of cells culture | 40.80 ± 11.43 | 27.00 ± 7.17 | .342 | |
| Risk factors | ||||
| Smoking | 2 (40.0) | 2 (40.0) | 2 (33.3) | .965 |
| Alcohol use | 1 (20.0) | 1 (20.0) | 0 (0.0)) | .504 |
| Hypertension | 3 (60.0) | 4 (80.0) | 1 (16.7) | .09 |
| Dyslipidemia | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Diabetes | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Coronary artery disease | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Atrial fibrillation | 2 (40.0) | 1 (0.0) | 1 (0.0) | .641 |
| COPD | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| TIA | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Stroke mechanisms | ||||
| Atherosclerotic | 3 (60.0) | 4 (80.0) | 5 (83.3) | .641 |
| Cardioembolic | 2 (40.0) | 1 (20.0) | 1 (16.7) | .641 |
| Cryptogenic | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Treatment | ||||
| Thrombolytics | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Drugs | AP(3); AC(2) | AP(3); AC(2) | AP(4); AC(2) | |
| mRS on 7th day | 3.40 ± 0.89 | 4.00 ± 1.22 | 3.67 ± 0.82 | .636 |
| NIHSS on 7th day | 12.20 ± 4.92 | 17.60 ± 5.59 | 15.50 ± 3.02 | .632 |
| Barthel index on 7th day | 39.00 ± 24.60 | 27.00 ± 30.74 | 25.00 ± 20.00 | .203 |
| SSS on 7th day | 28.00 ± 17.38 | 18.20 ± 13.50 | 19.33 ± 10.39 | .483 |
Abbreviations: AC, anticoagulant; AP, antiplatelet agent; BMSCs, bone marrow stromal cells; bpm, beats per minute; COPD, chronic obstructive pulmonary disease; DBP, diastolic blood pressure; EPCs, endothelial progenitor cells; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; SBP, systolic blood; SSS, Scandinavia Stroke Scale; TIA, transient ischemic attack.
Cell Characteristics
As shown in supplemental online Figure 1, the human bone marrow‐derived EPCs expressed HLA‐ABC, CD105, CD90, CD44, and CD29; moderately expressed HLA‐DR, KDR, and CD54; and negatively expressed CD34, CD31, CD45, or CD144. These cells were also shown to endocytose Dil‐Ac‐LDL and bind to UEA‐1, consistent with endothelial lineage cells. As shown in supplemental online Figure 2, the BMSCs expressed HLA‐ABC, CD105, CD90, CD44, and CD29. They moderately expressed HLA‐DR and did not express KDR, CD34, CD31, CD45, or CD144. These cells did not endocytose Dil‐Ac‐LDL or bind to FITC‐UEA‐1 lectin. Moreover, the EPCs formed extensive capillary‐like structures in the tube formation assay, indicating strong angiogenic ability. But the BMSCs did not form extensive capillary‐like structures on matrigel. Quantitatively compared with the EPCs, the BMSCs showed shorter total segment length, indicating lower angiogenic ability than that of the EPCs (supplemental online Fig. 3).
Safety Evaluation
One patient in the BMSC group was diagnosed with intestinal tumors after bone marrow aspiration and before cell infusion. Because the tumor was considered unlikely to be related to the bone marrow aspiration in such a short time, this patient, who died 14 months later, was also excluded from the safety assessment population and the per‐protocol population. No treatment‐emergent adverse events relating to bone marrow aspiration or cell transplantation, such as fever, allergy, increased headache, nausea, constipation, phlebitis, local infection or hematoma, or neurological deterioration, were found in any of the three groups. Profiles of the five patients in the EPC group are shown in supplemental online Table 1. Two patients from this group have complications with atrial fibrillation, which was treated in one patient (patient 10) 6 months after EPC treatment with no recurrence, but remained in the other patient (patient no. 12).
The SAEs during the 4‐year follow‐up are shown in Table 2. No new infectious diseases associated with transplantation occurred in our cohorts. Epilepsy occurred in two patients from the placebo group at the 11th and 12th month, respectively, and they received in‐hospital treatments with antiepileptic drugs. A right lower limb deep vein thrombosis was found in one patient in the BMSC group at the 6th month, who received thrombolysis. A different patient in the placebo group was diagnosed with Parkinson syndrome at the 10th month and received anti‐Parkinson treatment. This patient ultimately died at the 22nd month because of colorectal cancer. Two other patients in the placebo group died from arrhythmia and recurrent stroke at the 4th and 42nd month, respectively. In contrast, all patients in BMSC and EPC groups survived. No event was judged to be attributable to stem cell transplantation. No statistical difference of mortality was found between the arms (Table 3; p = .06); however, the EPC group had lower incidence of SAE compared with the placebo group (Table 4; p = .02).
Table 2.
List of SAEs
| No. | Gender | Age, years | Group | SAE | Diagnostic date | Treatments | Relationship with transplantation | Outcome | Death time |
|---|---|---|---|---|---|---|---|---|---|
| 2 | Male | 37 | Placebo | Seizure | 11th month | Oral drugs | No | Survival | |
| 3 | Male | 45 | Placebo | Seizure | 11th month | Oral drugs | No | Survival | |
| 4 | Male | 32 | BMSCs | Deep vein thrombosis | 6th month | Thrombolysis | No | Survival | |
| 11 | Male | 69 | Placebo | Parkinson's syndrome | 10th month | Oral drugs | No | Dead | 22nd month |
| 16 | Male | 74 | Placebo | Arrhythmia | 3rd month | Hospitalized | No | Dead | 4th month |
| 1 | Female | 47 | Placebo | Recurrent stroke | 42nd month | Hospitalized | No | Dead | 42nd month |
| 11 | Male | 69 | Placebo | Cancer | 20th month | Hospitalized | No | Dead | 22nd month |
Abbreviations: BMSCs, bone marrow stromal cells; SAE, serious adverse events.
Table 3.
Mortality of different groups
| Group | Dead, n (%) | Survival, n (%) | Total, n (%) | Chi‐square | p value |
|---|---|---|---|---|---|
| EPCs | 0 (0.0) | 5 (100.0) | 5 (100.0) | 5.63 | .06 |
| BMSCs | 0 (0.0) | 4 (100.0) | 4 (100.0) | ||
| Placebo | 3 (50.0) | 3 (50.0) | 6 (100.0) |
Abbreviations: BMSCs, bone marrow stromal cells; EPCs, endothelial progenitor cells.
Table 4.
Comparison of incidence of SAEs
| Group | None, n (%) | SAE, n (%) | Total, n (%) | Chi‐square statistic | p value |
|---|---|---|---|---|---|
| EPCs | 5 (100.0) | 0 (0.0) | 5 (100.0) | 8.40 | .02 |
| BMSCs | 3 (75.0) | 1 (25.0) | 4 (100.0) | ||
| Placebo | 1 (16.7) | 5 (83.3) | 6 (100.0) |
Abbreviations: BMSCs, bone marrow stromal cells; EPCs, endothelial progenitor cells; SAE, serious adverse events.
Preliminary Efficacy
Changes of the neurological and functional outcomes evaluated by NIHSS, SSS, BI, and mRS are shown in Figure 2. We found that the NIHSS score of the EPC group at baseline was 12.20 ± 4.92 (95% confidence interval [CI]: 6.09–18.31), which decreased to 5.60 ± 1.54 (95% CI: 5.40–10.22) at 3 months and 3.00 ± 2.35 (95% CI: 0.09–5.91) at 48 months. However, no significant differences were found among the three groups at each time point (p > .05). The SSS score of the EPC group at baseline was 28.00 ± 17.38 (95% CI: 27.89–49.58), and it gradually increased to 47.20 ± 2.96 (95% CI: 38.71–47.93) at 48 months. Except for the 3‐month time point, at which there was a significant difference of SSS score between the EPC group and the placebo group (p < .05), no statistical differences were observed between arms at any other time point (p > .05). In addition, the BI score of the EPC group at baseline was 39.00 ± 24.60 (95% CI: 8.46–69.54), which increased to 84.00 ± 6.78 (95% CI: 59.41–92.02) at 3 months and 94.00 ± 8.94 (95% CI: 82.89–105.11) at 48 months; however, there were also no significant differences between arms (p > .05). The mRS score of the EPC group at baseline was 3.40 ± 0.89 (95% CI: 2.29–4.51). It decreased to 2.00 ± 0.45 (95% CI: 1.57–3.00) at 3 months and to 1.40 ± 1.14 (95% CI: −0.16 to 2.82) at 48 months, but no significant differences were found between arms (p > .05).
Figure 2.
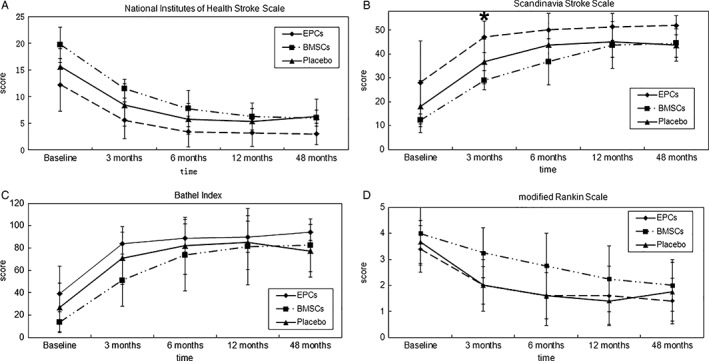
Change of neurological or functional outcome from baseline to 48 months. Gradual improvement of neurological function measured by NIHSS (A), SSS (B), BI (C), and mRS (D) could be observed in the EPCs group. However, no significant difference of the scores at each time point could be observed between arms, except for the SSS score at 3‐month time point between the EPCs group and the placebo‐controlled group. *p < .05. Abbreviations: BMSCs, bone marrow stromal cells; EPCs, endothelial progenitor cells; SSS, Scandinavia Stroke Scale .
Discussion
This study is hitherto the first clinical trial to test the safety and exploratory efficacy of bone marrow‐derived EPCs on patients with ischemic strokes. Our preliminary data indicate that intravenous transplantation of ex vivo expanded autologous EPCs is safe. There were no treatment‐emergent adverse events or delayed toxicity related to intravenous infusion of EPCs during the therapeutic window or within the 4‐year follow‐up period. In addition, neurological function after transplantation of EPCs assessed by NIHSS, SSS, BI, and mRS showed different levels of amelioration compared with baseline assessment, indicating that EPC transplantation at a minimum did not aggravate existing neurological deficits. Furthermore, we also evaluated other SAEs that may be associated with EPC therapy. Arrhythmias, especially supraventricular arrhythmias, were reported to develop in some patients receiving hematopoietic cell transplantation 19, 20. Similarly, seizure may be caused by aberrant innervation from newly formed neural circuits 17. In this study, we found one case of newly occurred arrhythmia and two cases of seizure in the placebo group. In contrast, one case originally complicated with atrial fibrillation in the EPC group was treated 6 months later with no further recurrence. This cure cannot be entirely attributed to EPC treatment, but the treatment did not induce arrhythmia or seizure in our cases. Deep vein thrombosis was found in one patient in the BMSC group at the 6th month, but it is difficult to determine its relationship with BMSC transplantation.
Because of the rare number of EPCs or BMSCs in bone marrow mononuclear cells, a further consideration regarding therapeutic application of these two types of stem cells is the need for ex vivo expansion to obtain a sufficient number of cells, and ex vivo expansion always raises concerns that it may cause cell karyotype instability and increase tumorigenicity. However, in our long‐term study, we observed no increased tumorigenicity after autologous EPC or BMSC treatment. This safety indicator was also confirmed by many animal studies and other trials using expanded bone marrow‐derived stem cells for treatment of ischemic stroke, including allogeneic or gene‐modified cells 17, 18, 21, 22, 23. Direct use of bone marrow mononuclear cells was once thought to be effective for stroke treatment 24, 25; however, a recent multicenter, randomized, controlled study has disproved this approach for the treatment of stroke 26, indicating that ex vivo purification and expansion may be necessary for acquiring therapeutic efficacy.
To determine the exploratory efficacy of EPCs on patients with ischemic stroke, we compared the neurological and functional outcomes measured by NIHSS, SSS, BI, and mRS among the three groups. No significant differences in outcomes were observed among the three groups, except for the SSS score at the 3‐month time point between the EPC and placebo groups. Therefore, it is still not possible to conclude that EPCs or BMSCs improved the neurological or functional outcomes. However, the lower incidence of SAEs in the EPC group not only confirmed safety of the treatment but also suggests a certain degree of efficacy. It improved the quality of life and benefited patient survival. It is also worth noting that all patients in the EPC group survived after long‐term follow‐up, whereas three patients in the placebo group died, although no statistical difference of mortality was found between arms. Taken together, our finding indicates that transplantation of EPCs tended to ameliorate clinical outcome and improve prognosis of patients with stroke.
In fact, improvement of neurological function is important but not exclusive for stroke treatment. As most stroke occurs based on other factors, such as hypertension, atherosclerosis, or diabetes, we consider stroke more a systemic disease, rather than local. In this trial, the route for stem cell transplantation was intravenous systematic infusion. Although there is a concern that only a limited number of cells can reach and reside in the peri‐infarct area, and thus participate in injury repair, intravenous infusion has several unique advantages over stereotactic local injection. First, because many clinical trials have shown that autologous EPC transplantation may confer beneficial effects on several diseases, such as myocardial infarction 27, 28, liver cirrhosis 29, limb ischemia 30, 31, and idiopathic pulmonary arterial hypertension 32, 33, we speculate that many other tissues or organs in addition to the brain may benefit from systematic EPC treatment, and these positive effects all contribute toward the improved prognosis of patients in the EPC group. More attention to the functional improvement of other organs from EPC treatment is necessary in further trials. Second, intravenous infusion is simple and noninvasive, and there were no adverse events related to the procedure in our study. In contrast, SAEs were found in 4 of 11 patients in a study of stereotactic injection of CTX0E03 (an immortalized human neural stem‐cell line) for treatment of chronic ischemic stroke 34. A similar outcome was found in another chronic ischemic stroke study, in which the stereotactic injection of SB623 (a type of modified BMSCs) resulted in all 18 patients experiencing at least one treatment‐emergent adverse event, and 6 patients experienced six serious treatment‐emergent adverse events 18. Third, as mentioned previously, because of surgical and anesthesia risks, it is challenging to design placebo controls for stereotactic injection of stem cells in phase II trials. The surgical procedure raises ethical concerns and may be unacceptable to patients 35, an issue that does not exist for intravenous infusion.
Conclusion
Our results regarding safety, the primary endpoint of our study, suggest that autologous transplantation of EPCs improved long‐term safety in patients with acute cerebral infarct, supporting the feasibility of this novel method for treatment of ischemic stroke. However, the study had limitations, such as a lack of patient‐centered quality of life outcomes. Moreover, because of the small size of the cohorts involved, this trial was also limited in that we could neither identify the neurological or functional benefits of EPCs on ischemic stroke nor determine the pros and cons between EPCs and BMSCs for treatment of stroke, and thus, a larger phase II trial is warranted.
Data Availability
Please contact author for data requests.
Author Contributions
Z.C. and X.J. conceived and designed the trial. Z.C., Y.G., S.T., Z.L., and H.X. contributed to patient enrollment and follow‐up. Y.G., J.F., C.H., and P.H. contributed to the functional assessments. P.C., K.W., J.F., and Y.G. contributed to data analysis. J.F. contributed to the immunofluorescence and flow cytometry detection. J.F. and Z.C. wrote the manuscript with significant input from X.J. and Y.K. All authors have read and approved the final manuscript.
Disclosure of Potential Conflicts of Interest
X.J. received research funding from the government of People's Republic of China. The other authors indicated no potential conflicts of interest.
Supporting information
Supplemental Figure 1. Characteristics of EPCs. (A): Flow cytometry analysis of the phenotype of the bone marrow‐derived EPCs. The blue histogram of each analysis indicates isotype control. (B): The EPCs endocytosed Dil‐Ac‐LDL (red) and bound to FITC‐UEA‐1 lectin (green). Cell nuclei were counterstained with DAPI (blue). Scale bar = 100 μm.
Supplemental Figure 2. Characteristics of BMSCs. (A): Flow cytometry analysis of the phenotype of the BMSCs. (B): The BMSCs did not endocytose Dil‐Ac‐LDL (red) or bind to FITC‐UEA‐1 lectin (green). Cell nuclei were counterstained with DAPI (blue). Scale bar = 100 μm.
Supplemental Figure 3. in vitro tube formation assay. (A): The EPCs formed extensive capillary‐like structures on matrigel, indicating strong angiogenic ability. (B): The BMSCs did not form extensive capillary‐like structures on matrigel. Scale bar = 100 μm. (C): Comparison of the total segment length of EPCs and BMSCs by Angiogenesis Analyzer for ImageJ software. Data from each of nine fields from three patients; *: P < .05 vs. EPCs.
Supplemental Table 1. Profile of EPCs group
Acknowledgments
The study design was approved by the Ethics Committee of Zhujiang Hospital, Southern Medical University. All included patients were informed about the nature of the study and gave their written informed consent.
This study was sponsored by grants from the Special Foundation for Science and Technology Key Plan of Guangdong Province (No. 2011A030400007); The Special Foundation for Science and Technology Key Plan of Guangzhou City (No. 2011Y1–00033‐6); Clinical Research Project of Southern Medical University (No. LC2016PY033); and Project of Outstanding Young Talent Pool of Zhujiang Hospital to Z.C.
Contributor Information
Xiaodan Jiang, Email: jiangxd@smu.edu.cn.
Zhenzhou Chen, Email: czz1020@163.com.
References
- 1. Benjamin EJ, Blaha MJ, Chiuve SE et al. Heart Disease and Stroke Statistics‐2017 Update: A Report From the American Heart Association. Circulation 2017;135(10):e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Society of neurology, Chinese Medical Association. Guidelines for Acute Ischaemic Stroke Diagnosis and Therapy. Chi J Neuro 2015;48:246–257. [Google Scholar]
- 3. Nguyen TN, Babikian VL, Romero R et al. Intra‐arterial treatment methods in acute stroke therapy. Front Neurol 2011;2:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Azad TD, Veeravagu A, Steinberg GK. Neurorestoration after stroke. Neurosurg Focus 2016;40(5):E2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wan H, Li F, Zhu L et al. Update on therapeutic mechanism for bone marrow stromal cells in ischemic stroke. J Mol Neurosci 2014;52(2):177–185. [DOI] [PubMed] [Google Scholar]
- 6. Maria Ferri AL, Bersano A, Lisini D et al. Mesenchymal stem cells for ischemic stroke: Progress and possibilities. Curr Med Chem 2016;23(16):1598–1608. [DOI] [PubMed] [Google Scholar]
- 7. Asahara T, Murohara T, Sullivan A et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997;275:964–967. [DOI] [PubMed] [Google Scholar]
- 8. Galimi F, Summers RG, van Praag H et al. A role for bone marrow‐derived cells in the vasculature of noninjured CNS. Blood 2005;105:2400–2412. [DOI] [PubMed] [Google Scholar]
- 9. Zhang ZG, Zhang L, Jiang Q et al. Bone marrow‐derived endothelial progenitor cells participate in cerebral neovascularization after focal cerebral ischemia in the adult mouse. Circ Res 2002;90:284–288. [DOI] [PubMed] [Google Scholar]
- 10. Hill JM, Zalos G, Halcox JP et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med 2003;348:593–600. [DOI] [PubMed] [Google Scholar]
- 11. Ohta T, Kikuta K, Imamura H et al. Administration of ex vivo‐expanded bone marrow‐derived endothelial progenitor cells attenuates focal cerebral ischemia‐reperfusion injury in rats. Neurosurgery 2006;59:679–686. [DOI] [PubMed] [Google Scholar]
- 12. Chen ZZ, Jiang XD, Zhang LL et al. Beneficial effect of autologous transplantation of bone marrow stromal cells and endothelial progenitor cells on cerebral ischemia in rabbits. Neurosci. Lett. 2008;445:36–41. [DOI] [PubMed] [Google Scholar]
- 13. Geng J, Wang L, Qu M et al. Endothelial progenitor cells transplantation attenuated blood‐brain barrier damage after ischemia in diabetic mice via HIF‐1alpha. Stem Cell Res Ther 2017;8:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bai YY, Peng XG, Wang LS et al. Bone marrow endothelial progenitor cell transplantation after ischemic stroke: An investigation into its possible mechanism. CNS Neurosci Ther 2015;21:877–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li YF, Ren LN, Guo G et al. Endothelial progenitor cells in ischemic stroke: An exploration from hypothesis to therapy. J Hematol Oncol 2015;8:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bang OY, Lee JS, Lee PH et al. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol 2005;57:874–882. [DOI] [PubMed] [Google Scholar]
- 17. Lee JS, Hong JM, Moon GJ et al. A long‐term follow‐up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic stroke. Stem Cells 2010;28:1099–1106. [DOI] [PubMed] [Google Scholar]
- 18. Steinberg GK, Kondziolka D, Wechsler LR et al. Clinical outcomes of transplanted modified bone marrow‐derived mesenchymal stem cells in stroke: A phase 1/2a study. Stroke 2016;47:1817–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Singla A, Hogan WJ, Ansell SM et al. Incidence of supraventricular arrhythmias during autologous peripheral blood stem cell transplantation. Biol Blood Marrow Transplant 2013;19:1233–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tonorezos ES, Stillwell EE, Calloway JJ et al. Arrhythmias in the setting of hematopoietic cell transplants. Bone Marrow Transplant 2015;50:1212–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tang H, Xiang Y, Jiang X et al. Dual expression of hTERT and VEGF prolongs life span and enhances angiogenic ability of aged BMSCs. Biochem Biophys Res Commun 2013;440:502–508. [DOI] [PubMed] [Google Scholar]
- 22. Hess DC, Sila CA, Furlan AJ et al. A double‐blind placebo‐controlled clinical evaluation of MultiStem for the treatment of ischemic stroke. Int J Stroke 2014;9:381–286. [DOI] [PubMed] [Google Scholar]
- 23. Hess DC, Wechsler LR, Clark WM et al. Safety and efficacy of multipotent adult progenitor cells in acute ischaemic stroke (MASTERS): A randomised, double‐blind, placebo‐controlled, phase 2 trial. Lancet Neurol 2017;16:360–368. [DOI] [PubMed] [Google Scholar]
- 24. Moniche F, Gonzalez A, Gonzalez‐Marcos JR et al. Intra‐arterial bone marrow mononuclear cells in ischemic stroke: A pilot clinical trial. Stroke 2012;43:2242–2254. [DOI] [PubMed] [Google Scholar]
- 25. Friedrich MA, Martins MP, Araujo MD et al. Intra‐arterial infusion of autologous bone marrow mononuclear cells in patients with moderate to severe middle cerebral artery acute ischemic stroke. Cell Transplant 2012;21(Suppl 1):S13–S21. [DOI] [PubMed] [Google Scholar]
- 26. Prasad K, Sharma A, Garg A et al. Intravenous autologous bone marrow mononuclear stem cell therapy for ischemic stroke: A multicentric, randomized trial. Stroke 2014;45:3618–3624. [DOI] [PubMed] [Google Scholar]
- 27. Taljaard M, Ward MR, Kutryk MJ et al. Rationale and design of Enhanced Angiogenic Cell Therapy in Acute Myocardial Infarction (ENACT‐AMI): The first randomized placebo‐controlled trial of enhanced progenitor cell therapy for acute myocardial infarction. Am Heart J 2010;159:354–360. [DOI] [PubMed] [Google Scholar]
- 28. Zhu J, Song J, Yu L et al. Safety and efficacy of autologous thymosin beta4 pre‐treated endothelial progenitor cell transplantation in patients with acute ST segment elevation myocardial infarction: A pilot study. Cytotherapy 2016;18:1037–1042. [DOI] [PubMed] [Google Scholar]
- 29. D'Avola D, Fernandez‐Ruiz V, Carmona‐Torre F et al. Phase 1‐2 pilot clinical trial in patients with decompensated liver cirrhosis treated with bone marrow‐derived endothelial progenitor cells. Transl Res 2017;188:80–91 e2. [DOI] [PubMed] [Google Scholar]
- 30. Lara‐Hernandez R, Lozano‐Vilardell P, Blanes P et al. Safety and efficacy of therapeutic angiogenesis as a novel treatment in patients with critical limb ischemia. Ann Vasc Surg 2010;24:287–294. [DOI] [PubMed] [Google Scholar]
- 31. Lasala GP, Silva JA, Gardner PA et al. Combination stem cell therapy for the treatment of severe limb ischemia: Safety and efficacy analysis. Angiology 2010;61:551–556. [DOI] [PubMed] [Google Scholar]
- 32. Zhu JH, Wang XX, Zhang FR et al. Safety and efficacy of autologous endothelial progenitor cells transplantation in children with idiopathic pulmonary arterial hypertension: open‐label pilot study. Pediatr Transplant 2008;12:650–655. [DOI] [PubMed] [Google Scholar]
- 33. Wang XX, Zhang FR, Shang YP et al. Transplantation of autologous endothelial progenitor cells may be beneficial in patients with idiopathic pulmonary arterial hypertension: a pilot randomized controlled trial. J Am Coll Cardiol 2007;49:1566–1571. [DOI] [PubMed] [Google Scholar]
- 34. Kalladka D, Sinden J, Pollock K et al. Human neural stem cells in patients with chronic ischaemic stroke (PISCES): A phase 1, first‐in‐man study. Lancet 2016;388:787–796. [DOI] [PubMed] [Google Scholar]
- 35. Cohen PD, Isaacs T, Willocks P et al. Sham neurosurgical procedures: The patients' perspective. Lancet Neurol 2012;11:1022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Characteristics of EPCs. (A): Flow cytometry analysis of the phenotype of the bone marrow‐derived EPCs. The blue histogram of each analysis indicates isotype control. (B): The EPCs endocytosed Dil‐Ac‐LDL (red) and bound to FITC‐UEA‐1 lectin (green). Cell nuclei were counterstained with DAPI (blue). Scale bar = 100 μm.
Supplemental Figure 2. Characteristics of BMSCs. (A): Flow cytometry analysis of the phenotype of the BMSCs. (B): The BMSCs did not endocytose Dil‐Ac‐LDL (red) or bind to FITC‐UEA‐1 lectin (green). Cell nuclei were counterstained with DAPI (blue). Scale bar = 100 μm.
Supplemental Figure 3. in vitro tube formation assay. (A): The EPCs formed extensive capillary‐like structures on matrigel, indicating strong angiogenic ability. (B): The BMSCs did not form extensive capillary‐like structures on matrigel. Scale bar = 100 μm. (C): Comparison of the total segment length of EPCs and BMSCs by Angiogenesis Analyzer for ImageJ software. Data from each of nine fields from three patients; *: P < .05 vs. EPCs.
Supplemental Table 1. Profile of EPCs group
Data Availability Statement
Please contact author for data requests.


