Abstract
Clostridium difficile is the leading infectious cause of antibiotic-associated diarrhea and colitis. Clostridium difficile infection (CDI) places a heavy burden on the health care system, with nearly half a million infections yearly and an approximate 20% recurrence risk after successful initial therapy. The high incidence has driven new research on improved prevention such as the emerging use of probiotics, intestinal microbiome manipulation during antibiotic therapies, vaccinations, and newer antibiotics that reduce the disruption of the intestinal microbiome. While the treatment of acute C. difficile is effective in most patients, it can be further optimized by adjuvant therapies that improve the initial treatment success and decrease the risk of subsequent recurrence. Lastly, the high risk of recurrence has led to multiple emerging therapies that target toxin activity, recovery of the intestinal microbial community, and elimination of latent C. difficile in the intestine. In summary, CDIs illustrate the complex interaction among host physiology, microbial community, and pathogen that requires specific therapies to address each of the factors leading to primary infection and recurrence.
Keywords: Clostridium difficile infection, emerging therapies, microbiome, microbiota, antibiotics
Graphical abstract
Clostridium difficile is the leading infectious cause of antibiotic-associated diarrhea and colitis. This has driven new research on improving the prevention, primary treatment, and reduction of recurrence of C. difficile infection. This review summarizes current therapy recommendations for C. difficile infection and indicates areas of improvement that new emerging drugs and treatments hope to address.
Introduction
Clostridium difficile Infections
Clostridium difficile is a toxigenic, Gram positive, spore-forming bacterium that can infect the gastrointestinal tract and cause mucosal damage. People can become infected by C. difficile after intestinal microbiota disruption through mechanisms such as the usage of antibiotics. Once infected with C. difficile, clinical manifestations range from asymptomatic colonization to mild diarrhea and colitis to severe fulminant colitis and potentially fatal toxic megacolon. C. difficile causes nearly half a million infections per year in the United States alone1, and costs up to $1.5 billion dollars annually in attributable health care expenses2.
While pseudomembranous colitis was first described in 1893, it was not known to be associated with antibiotic usage until 19743,4. Even then, C. difficile was not known to be the causative agent. C. difficile, first isolated from newborns in 1935, was not identified as a leading cause of antibiotic-associated diarrhea and pseudomembranous colitis until 19783,5. At that time, C. difficile infection (CDI) was seen as a treatable nuisance disease that did not necessitate specific therapy or the development of new treatments6. However, the emergence in the mid-1990s and early 2000s of CDI epidemics caused by strains belonging to the type NAP1/BI/027 led to an increase in incidence and morbidity that galvanized the development for new therapeutics, monitoring, and testing7,8.
The first step of any treatment begins with the correct identification of the disease. While C. difficile is a leading cause of antibiotic-associated diarrhea, it is not the only causative agent. As there are strains of nontoxigenic C. difficile that are incapable of causing disease and a high rate of asymptomatic carriage of toxigenic strains, accurate diagnosis cannot depend solely on identifying C. difficile in the stool. Instead, diagnosis of C. difficile is dependent on two factors: (1) identification of toxigenic C. difficile or its toxins or histopathologic or colonoscopic evidence showing pseudomembranous colitis; and (2) signs of clinical disease such as three or more unformed stools within 24 hours, radiographic evidence of ileus, or toxic megacolon9. There are many diagnostic tests and algorithms for diagnosing C. difficile, each with strengths and weaknesses. Testing can include the following as either single tests or as part of a multi-step algorithm: EIA-based toxin A/B tests, PCR-based nucleic acid amplification tests (NAAT) for tcdB, or glutamine dehydrogenase tests (when paired with one of the prior toxin tests). The various testing algorithms have been summarized in the recent 2018 IDSA/SHEA guidelines9. The main goal is to identify only those patients that require treatment and to classify them in terms of severity potential and post-therapy recurrence risk. A recurrent case of CDI is defined as symptom onset and stool specimen positive for C. difficile 2 to 8 weeks following the last positive specimen during previous treatment of primary CDI. Such classifications help guide therapy and determine which targeted therapeutics to use, since emerging CDI therapies are being developed to specifically address prevention, treatment, and recurrence reduction.
Current therapies are mainly directed at addressing primary CDI with the use of antibiotics such as vancomycin, as well as treating recurrent disease with vancomycin or fidaxomicin. In repeatedly recurrent disease, additional current approaches use antibiotic tapers, antibiotic adjuvants, and fecal microbiota transplants (FMT)9. Newer therapies are being developed and put into practice to reduce initial infection; these include probiotics and vaccines. New treatments can also reduce the risk of recurrence and severe disease with narrow spectrum antibiotics, immunotherapies, and microbial replacement therapies. In this review, we will summarize the current therapy recommendations and indicate areas of improvement that new emerging drugs and treatments hope to address.
How the pathogenesis of C. difficile informs treatment approaches
The pathogenesis of C. difficile infection represents the complex interaction between pathogen, host, and native microbiota (Fig. 1). Spores can be spread by both asymptomatic carriers and symptomatic patients, necessitating the isolation of infected individuals and the appropriate cleaning of the healthcare environment. The initial phase of C. difficile infection occurs when spores enter the gastrointestinal system and local environmental factors trigger germination and growth of vegetative cells. Primary bile acids (e.g., taurocholic acid, cholate) act as germinants during in vitro experiments with glycine as co-germinant10,11. Native members of the microbiota have the capacity to deconjugate and dehydroxylate primary bile acids into secondary bile acids, some of which have shown to be inhibitory to vegetative C. difficile12–14. Antibiotic-mediated alteration of native bacteria in the microbiota can impair primary bile acid conversion to secondary bile acids, leading to an environment that promotes C. difficile sporulation and vegetative growth15.
Figure 1.
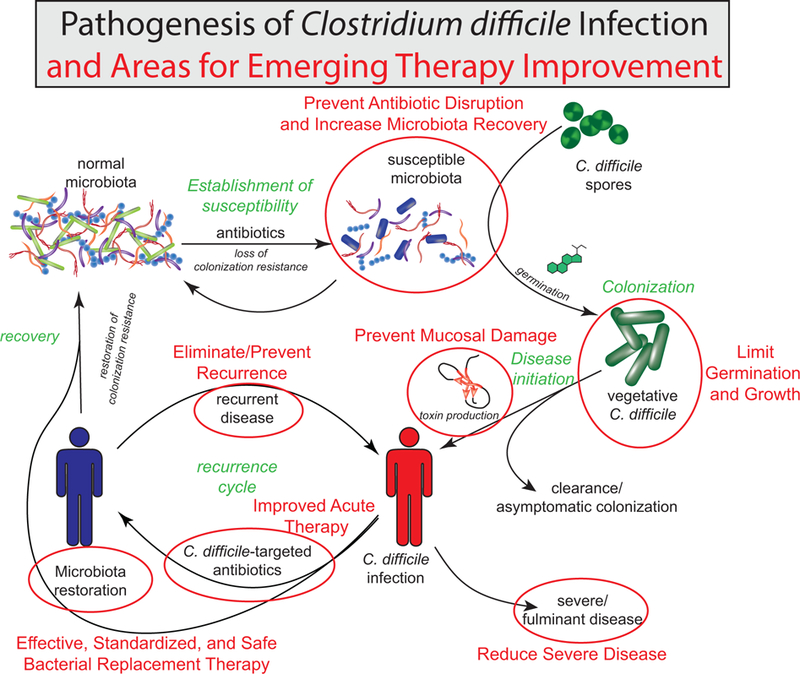
Pathogenesis of Clostridium difficile infection and areas for emerging therapy improvement. Starting with the normal microbiota, antibiotic disruption of the intestinal bacterial community results in a susceptible state, which can lead to colonization with C. difficile. Once germinated, vegetative C. difficile produces a variety of toxins to cause mucosal damage. If the damage is severe, this may lead to severe disease. With effective antibiotic therapy, C. difficile can be reduced and natural colonization resistance can develop over time as the natural microbial community recovers. Reinfection or recurrence may occur before this process is complete. Fecal microbiota transplant may expedite this recovery by directly replacing the missing microbial community members. Areas marked with a red circle are potential areas where new emerging therapies could improve clinical management.
Once C. difficile vegetative cells have grown from the spores in the colon, they produce the toxins TcdA, TcdB, and binary toxin. Specific strains of toxigenic C. difficile produce different levels and subsets of these toxins. For example, certain strains can produce TcdA and TcdB but not the binary toxin. TcdA and TcdB enter the cell by binding to specific cellular receptors found on intestinal epithelial cells16. After endocytosis, acidification of the endosome leads to conformational changes of the toxin that releases the N-terminal glucosyltransferase domain into the cytoplasm. This domain inactivates specific enzymes such as the Rho GTPases through glycosylation. This leads to both cytopathic and cytotoxic downstream effects by altering the cytoskeletal structure, epithelial permeability, and cell-to-cell junctions, as well as by activating the inflammasome and apoptosis17,18. With the destruction of the epithelium, C. difficile infection can cause diarrhea and lead to complicated cases through systemic effects such as sepsis, shock, peritonitis, and bowel perforation as the intestine becomes compromised.
Targeted antibacterial therapy can be used to reduce intestinal C. difficile levels. However, even with successful treatment and clearance, a median of 21.6% of patients will experience recurrent disease with an increased risk to reoccur following each recurrence19. The high recurrence risk highlights the importance of restoring the colonization resistance against C. difficile.
The interactions between host, pathogen, and microbiota in CDI presents multiple opportunities for the development of novel therapies that target specific steps in pathogenesis. For example, emerging therapies can reduce the risk of CDI by lowering the effect of systemic antibiotics, decreasing the levels of primary bile acids, increasing secondary bile acids, and restoring the native microbiota’s ability to convert primary bile acids to secondary bile acids. While there is no universally accepted definition of narrow-spectrum antibiotics, it is accepted in the field that this refers to antibiotics that affect a smaller range of bacterial groups, such as those affecting only Gram positive bacteria. In contrast, broad-spectrum antibiotics are those that affect multiple classes of bacteria, such as those impacting both Gram positive and Gram negative bacteria, including anaerobic bacteria. It has been shown that specific broad-spectrum antibiotics may cause further disruption of the native gut bacteria, leading to increased risk of recurrence. As such, emerging therapies targeting the recovery of native bacteria post-CDI treatment include narrow-spectrum antibiotics that allow for more rapid recovery of native bacteria and bacterial replacement therapies such as specific bacterial communities delivered by enema or oral ingestion. C. difficile cells and toxins can also be targeted by preformed antitoxin and anti-C. difficile antibodies or by anti-C. difficile antibodies natively produced due to vaccination. These therapies have the potential to reduce the risk of CDI, decrease disease severity, and prevent recurrence by targeting spores, vegetative cells, and toxins.
Conventional Management of CDI
Initial management after diagnosis
Once a patient is diagnosed with CDI, any inciting antibiotics should be discontinued if possible20,21. Initial management may also include decreasing proton pump inhibitors and anti-motility agents, which have been associated with the severity of CDI22 and the subsequent risk for recurrence23.
The next step is to determine the severity of the illness, as this will guide the therapeutic approach, with certain treatments recommended based on severity. In the 2018 IDSA/SHEA guidelines, non-severe (mild to moderate) disease is defined as diarrhea occurring with white blood cell count <15,000 cells/mL and serum creatinine <1.5 mg/dL. Severe CDI is defined as CDI with white blood cell count ≥15,000 cells/mL or serum creatinine ≥1.5 mg/dL. Lastly, severe and complicated CDI is defined by systemic signs of infection and evidence of hypotension, ileus, or toxic megacolon9,24.
Current management recommendations
C. difficile is resistant to many antibiotics, including fluoroquinolones and macrolides, with an increased resistance to rifampin seen in the 027 strains25,26. As indicated in the 2018 IDSA/SHEA guidelines, the treatment of CDI is determined by severity and recurrence state. For primary CDI, non-severe disease is treated by vancomycin (125 mg orally 4 times daily for 10 days) or fidaxomcin (200 mg orally 2 times daily for 10 days). If neither is available or tolerated, metronidazole (500 mg orally 3 times daily for 10 days) can be used instead9. For severe CDI, the recommendation is vancomycin (125 mg orally 4 times daily for 10 days) or fidaxomcin (200 mg orally 2 times daily for 10 days). If CDI is severe and complicated, vancomycin (500 mg 4 times daily given orally or by nasogastric tube) can be given with IV metronidazole (500 mg every 8 hours). If ileus is present, vancomycin can be given by rectal enema in addition to the oral vancomycin and IV metronidazole. If surgical intervention is necessary, rectal sparing subtotal colectomy or diverting loop ileostomy with colonic lavage followed by vancomycin flushes are recommended. For recurrent disease, the first recurrence is treated with a standard course of vancomycin (125 mg orally 4 times daily for 10 days) if the previous CDI was treated with metronidazole. If the previous course was not metronidazole, the recurrent case is treated with vancomycin taper (e.g. 125 mg orally 4 times per day for 10–14 days, 2 times per day for a week, 1 per day for a week, and lastly every 2–3 days for 2–8 weeks) or fidaxomicin (200 mg orally 2 times daily for 10 days). For the second or subsequent recurrences, vancomycin taper, vancomycin (125 mg orally 4 times daily for 10 days) followed by rifaximin chaser (400 mg 3 times daily for 20 days), or fidaxomicin therapy (200 mg orally 2 times daily for 10 days) can be used. FMT can be considered for the second or subsequent recurrence, but is not recommended for primary CDI or a first recurrence9. Treatment management and dosing schemes are presented in Figure 2.
Figure 2.

Current clinical management guidelines for Clostridium difficile infection (CDI). This diagram reviews the recommended treatment approaches for primary and recurrent CDI, depending on disease severity based on the 2018 IDSA/SHEA guidelines. Additionally, there is some evidence for fidaxomicin in place of rifaximin as a chaser for recurrent CDI and the EXTEND trial did show evidence for using extended-pulsed fidaxomicin for primary CDI; however, this is not currently reflected in the guidelines9,33,34,137.
* Primary CDI is defined as a new episode of symptoms with no previous positive C. difficile test result within 38 weeks and confirmation of CDI by diagnostic testing.
In the past, metronidazole was recommended for mild to moderate CDI as a cost-effective treatment, while vancomycin was used for metronidazole intolerance and/or if the patient has an increased risk of recurrence. However, recent clinical trials and a meta-analysis have shown that vancomycin is superior to metronidazole for non-severe CDI, with a percentage resolution of 87% compared to 78% for metronidazole. The 2018 IDSA/SHEA guidelines indicate the use of vancomycin or fidaxomicin for non-severe CDI, with metronidazole reserved for cases when the other two drugs are not available or tolerated9.
Fidaxomicin is a narrow-spectrum, nonabsorbable macrocyclic antibiotic that inhibits the bacterial RNA polymerase β subunit (rpoB) and has been found to be noninferior to vancomycin in success rate in multiple Phase III trials, but shows substantially reduced rate of recurrence27. Fidaxomicin has bactericidal activity against C. difficile by inhibiting RNA polymerase and disrupting RNA synthesis28. The narrow-spectrum activity of fidaxomicin potentially maintains the stability of the intestinal microbiota at a higher level, leading to a more transient loss of colonization resistance and more robust recovery of the microbiota after treatment. Fidaxomicin shows similar clinical cure rates with reduced recurrence rates9,29–31. While fidaxomicin is more costly than vancomycin, the treatment may lower the overall cost by reducing recurrence rates if used to treat patients with a high recurrence risk29,32.
Additionally, studies have been done on using alternative fidaxomicin dosing regimens for treating primary and recurrent CDI by looking at clinical cure rate and recurrence reduction. Soriano et al.33 showed in a case study of patients with multiple recurrent CDI that only 2 out of 11 patients (18%) had recurrence when given fidaxomicin in a tapering dose regimen as compared to 3 of 8 patients (38%) that were given only fidaxomicin as a chaser twice daily for 10 days. These patients received these chasing or tapering regimens after a standard CDI antibiotic therapy. This study indicated that fidaxomicin chasers and tapered regimens could help prevent recurrence in patients experiencing multiple recurrent CDI. However, the study was not randomized and had a low sample size, requiring future studies to further evaluate these findings.
EXTEND, a recent randomized, controlled, Phase IIIb/IV trial, compared the effects of extended-pulsed fidaxomicin treatment (200 mg orally given twice daily for days 1–5, once daily every other day from days 7–25) to vancomycin treatment (125 mg orally 4 times a day for 10 days) for primary and recurrent CDI. Of the patients receiving the extended-pulsed fidaxomicin treatment, 124 of 177 (70%) achieved a sustained clinical cure at 30 days after treatment, while in comparison only 106 of 179 (59%) patients receiving the vancomycin treatment achieved a sustained clinical cure at 30 days after treatment (P = 0.030, odds ratio 1.62 [95% CI: 1.04–2.54]). Additionally, the extended-pulsed fidaxomicin treatment resulted in lower recurrence at 90 days (6%) compared to the vancomycin treatment (19%) (P = 0.00073, odds ratio 0.29 [95% CI: 0.14–0.60]). This study showed that the extended-pulsed fidaxomicin treatment was better clinical cure for the patients as a whole, with reduced recurrence34. However, this study was not powered to look directly at this treatment for patients starting with recurrent CDI, and the study also showed that extended-pulsed fidaxomicin treatment may be inferior for severe CDI. Lastly, the study did not compare standard fidaxomicin therapy or extended-pulsed vancomycin therapy to the extended-pulsed fidaxomicin. Future study is needed to look at the effectiveness of this treatment for multiple recurrent CDI and to compare its effectiveness to standard fidaxomicin and extended-pulsed vancomycin therapy.
Lastly, rifaximin is a nonabsorbable rifamycin formula that acts to inhibit bacterial RNA synthesis by binding to bacterial DNA-dependent RNA polymerase. Although rifaximin has a broad spectrum of activity, it appears to only minimally disrupt the intestinal microbiota and has high activity against C. difficile35, but resistance may develop rapidly. Rifaximin has shown potential as an adjuvant to conventional therapy for recurrent disease as a chaser, with one study finding that patients who received rifaximin after conventional therapy experienced recurrence at 15%, and those who received a placebo after conventional therapy experienced a 21% recurrence rate36,37. A small retrospective study of 32 patients treated with rifaximin for recurrent C. difficile infections found the treatment to be safe and with no recurrence of CDI after 12 weeks in 17 patients (53%)38. As such, rifaximin is not used for primary therapy, but can be used as a chaser to vancomycin to reduce recurrent diarrhea9,37,39.
Areas for improvement and targets for emerging therapies
While current therapies lead to successful treatment of mild to moderate disease, there are improvements needed. CDI therapy success requires the resolution of diarrhea with absence of severe abdominal discomfort for more than two consecutive days. Although successful treatment rates are high, as many as 18.9% to 27.3% of patients do not respond to treatment and experience treatment failure40. Additionally, the high severity disease and the significant rate of recurrence are promising targets for emerging therapies.
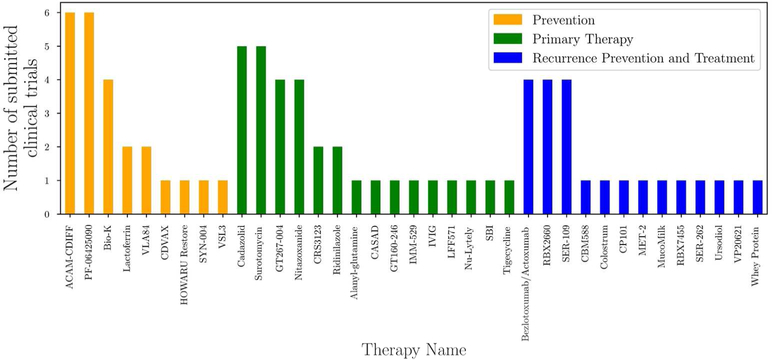
The US National Library of Medicine’s records of clinical trials pertaining to C. difficile infections show a sharp increase in the number of clinical trials since the early 2000s that follows the increase in yearly incidence of inpatient CDI per 100,000 hospitalizations observed from HCUPnet data (Fig. 3)41,42. This highlights the importance of developing new therapies to address the increase in incidence of CDI. While a large portion of the clinical trials are studying topics ranging from optimizing current antibiotic protocols and dosing and FMT, many trials are studying emerging therapies that target prevention, primary therapy, and/or reducing and treating recurrence (Fig. 4). These emerging therapeutics are discussed below and are organized by which phase of pathogenesis they target: prevention, primary therapy, or recurrence (Fig. 1).
Figure 3.

Yearly incidence of US inpatient CDI per 100,000 hospitalizations and the number of first-submission clinical trials by year. The submission of clinical trials increased following the increase in yearly incidence of CDI in the United States. Data on incidence was obtained from HCUPnet and clinical trial data was obtained from ClinicalTrials.gov. Only trials with C. difficile in the condition category were included41,42.
Figure 4.
CDI emerging therapies targeted at prevention, primary therapy, or recurrence prevention and management. Clinical trial data was obtained from ClinicalTrails.gov. Only clinical trials listed on ClinicalTrials.gov was included. If multiple therapeutic aims are being studied for a given drug, we sorted it by the categorization used in this article for the discussion section. Refer to Tables 1–3 for specifics on each therapeutic41.
Prevention of CDI
The high incidence of CDI necessitates the development of strategies aimed at reducing intestinal microbial changes caused by systemic antibiotics, restoring colonization resistance and native bacterial communities, as well as reducing sporulation, colonization, and toxin production by C. difficile. The current emerging therapies are: β-lactamases targeted at reducing systemic β-lactam antibiotic disruption of intestinal bacteria; oral probiotics and bacterial replacement to restore bacteria associated with colonization resistance; vaccination to produce anti-C. difficile antibodies targeting spores, vegetative cells, and toxins; and therapies targeting toxin damage and CDI development once colonized (Table 1).
Table 1.
Emerging therapies targeted at prevention of CDI
| Type | Treatment name |
Adm in. |
Current or last phase completed |
Mechanism | Prevention | Primary therapy |
Recurrence prevention/ treatment |
|---|---|---|---|---|---|---|---|
| β-lactamase | SYN-004 | Oral | Phase II ongoing |
A recombinant β-lactamase from Bacillus licheniformi that acts to reduce the effect of systemic β-lactam antibiotics on the intestinal microbiota. |
X | ||
| Microbial | Bio-K | Oral | Phase III ongoing |
Probiotic containing three bacterial species: Lactobacillus acidophilus (CL1285), Lactobacillus casei (LBC80R), and Lactobacillus rhamnosus (CLR2). |
X | X | |
| Microbial | VSL3 | Oral | Phase II/III completed |
A refrigerated probiotic consisting of 8 different live strains of bacteria: Bifidobacterium breve, Bifidobacterium longum, Bifidobacterium infantis, Lactobacillus acidophilus, Lactobacillus plantarum, Lactobacillus paracasei, Lactobacillus delbrueckii subsp. bulgaricus, and Streptococcus thermophile. |
X | ||
| Microbial | HOWARU Restore |
Oral |
Phase I complete |
Probiotic containing four strains: Lactobacillus acidophilus, Bifidobacterium lactis Bl-04, Bifidobacterium lactis Bi-07, and Lactobacillus paracasei Lpc-37. |
X | ||
| Toxin Vaccine |
VLA84 | IM | Phase II completed |
A recombinant vaccine composed of truncated portions of C. difficile toxins A and B. Given IM, this vaccine may induce anti- toxin antibody production. |
X | ||
| Toxin Vaccine |
ACAM- CDIFF |
IM | Phase III discontinued |
Toxin vaccine of C. difficile toxin A and B. Given IM, this vaccine may induce anti-toxin antibody production. |
X | X | |
| Toxin Vaccine |
PF- 06425090 |
IM | Phase II and III ongoing |
Genetically modified toxin vaccine of C. difficile toxin A and B. Given IM, this vaccine may induce anti-toxin antibody production. |
X | ||
| Oral Vaccine | CDVAX | Oral | Phase I terminated |
An oral vaccine against that utilizes spores from a genetically modified bacterium to produce an oral vaccine may induce strong mucosal immunity. |
X | ||
| Lactoferrin | Lactoferrin | Oral | Phase II ongoing |
May have the potential to reduce the cytotoxic damage of C. difficile toxin B and may delay C. difficile growth and reduces toxin production. |
X |
For each emerging therapy, the administration route, current or last phase completed, and mechanism is listed. Additionally, each drug is marked for the applications it is being studied for in clinical trials, namely: prevention, primary therapy, or recurrence prevention/treatment. Clinical trial information was obtained from ClinicalTrials.gov41.
β-lactamase.
β-lactam antibiotics are associated with increased risk for subsequent CDI. By reducing the amount of active β-lactam antibiotic reaching the intestinal bacteria while preserving systemic drug activity, non-absorbable β-lactamases potentially prevent the loss of natural colonization resistance (Fig. 5). SYN-004 (ribaxamase) is a recombinant β-lactamase manufactured by Synthetic Biologics that is derived from P1A, a β-lactamase isolated from Bacillus licheniformi, and it is formulated for oral dosing that aims to reduce the effects on intestinal microbiota of β-lactam antibiotics that are given systemically. Preclinical trials in dogs showed that SYN-004 was well tolerated, minimally absorbed, and had no measurable effects on the systemic levels of co-administered intravenous ceftriaxone43. These results supported the progression of SYN-004 to clinical trials. Animal studies further showed that SYN-004 was capable of degrading ceftriaxone in the GI tract of dogs and reduced microbial changes in the gut of pigs treated with ceftriaxone44. Two Phase I clinical trials showed that SYN-004 was well tolerated and remained localized to the intestines45. Two Phase II clinical trials confirmed that SYN-004 is capable of degrading systemically administered β-lactam antibiotics that enter the intestines46. A recently completed Phase II clinical trial studied the ability of SYN-004 given orally in a 150 mg dose to prevent CDI in patients with a lower respiratory tract infection receiving IV ceftriaxone (NCT02563106). Results have not yet been posted. One limitation for β-lactamase treatment is that it will only be useful for patients receiving systemic β-lactam antibiotics. Non-β-lactam antibiotics that have a high risk for CDI, such as quinolones and clindamycin, would not be affected by a β-lactamase.
Figure 5.

Emerging therapy therapeutic targets for management of CDI—prevention, primary treatment, and recurrence reduction. Boxes indicate therapies, while arrows indicate the effect of the therapies. Black arrows indicate events and steps in the development of CDI. Starting with healthy microbiota, antibiotic alterations lead to a susceptible state where C. difficile spores can enter and germinate. This leads to colonization and infection. Toxin production can then trigger inflammation and cytotoxic/cytopathic effects on the mucosa, leading to colitis and severe disease. Red boxes and arrows indicate therapies aimed at preventing C. difficile infection. Green boxes and arrows indicate therapies aimed at treating primary CDI and reducing disease severity. Blue boxes and arrows indicate therapies aimed at reducing and treating recurrent CDI. IVIG = intravenous immunoglobulin.
Probiotics.
Probiotics are “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host”47. The aim of probiotics when used for CDI prevention is to restore bacteria associated with colonization resistance that may have been disrupted by systemic antibiotic usage (Fig. 5). While probiotics have the potential to prevent CDI, more research is needed to determine which patients would benefit from probiotic treatment and what the long-term side effects of probiotic administration are48.
Bio-K is a probiotic manufactured by Bio-K Plus International containing three bacterial species: Lactobacillus acidophilus (CL1285), Lactobacillus casei (LBC80R), and Lactobacillus rhamnosus (CLR2). After an outbreak in the 284-bed community hospital Pierre-Le Gardeur (PLGH) in Quebec, every inpatient on antibiotics was prophylactically given Bio-K within 12 hours of the antibiotic prescription. For 10 years, 44,835 inpatients were observed and it was found that rates of CDI dropped from 18 cases per 10,000 patient-days to 2.3 cases per 10,000 patient-days. The rates for C. difficile infections at this hospital were found to be lower than comparable Canadian hospitals. No lactobacillus bacteremia was observed and it was concluded that Bio-K was safe and efficacious49. In a completed Phase III trial, Bio-K prophylaxis after antibiotic usage reduced antibiotic-associated diarrhea and C. difficile-associated diarrhea. Patients either received two capsules per day (Pro-2) or one capsule per day (Pro-1). Patients received the probiotics within 36 hours of antibiotic initiation and continued to receive the probiotics 5 days after antibiotics were concluded. Patients were followed up to 21 days after discontinuation of the probiotic. The incidence rates for antibiotic-associated diarrhea for Pro-2, Pro-1, and placebo were 15.5%, 28.2% and 44.1%, respectively. Rates of Clostridium difficile-associated diarrhea for Pro-2, Pro-1, and placebo were 1.2%, 9.4% and 23.8%, respectively. Additionally, the duration of antibiotic-associated diarrhea was reduced (2.8 days for Pro-2, 4.1 days for Pro-1, and 6.4 days for placebo)50. These results indicate that Bio-K and similar probiotics could be efficacious for the prevention of CDI subsequent to antibiotic exposure.
VSL#3 (VSL3) is a refrigerated probiotic manufactured by Alfasigma that consists of eight different live strains of bacteria, including: Bifidobacterium breve, Bifidobacterium longum, Bifidobacterium infantis, Lactobacillus acidophilus, Lactobacillus plantarum, Lactobacillus paracasei, Lactobacillus delbrueckii subsp. bulgaricus, and Streptococcus thermophiles51. VSL#3 was shown in a Phase II/III trial (NCT00973908) to reduce the rate of antibiotic-associated diarrhea when given as a prophylaxis to average-risk hospitalized patients (0% for VSL#3 versus 11.4% for placebo). In this study, the VSL#3 patients received a VSL#3 sachet twice a day for the duration of their antibiotic course and one week after, while the placebo group received a placebo sachet twice daily instead. The study did not observe any cases of C. difficile-associated diarrhea in either the VSL#3 or placebo groups51. In this study, adverse event rates were found to be similar between the VSL#3 and placebo groups. Further studies are needed to determine which populations of patients would benefit from this prophylactic therapy and to study the ability of VSL#3 to prevent CDIs in high-risk patients. VSL#3 could also be tested for its ability to reduce recurrence.
HOWARU Restore is a four-strain probiotic manufactured by DuPont consisting of Lactobacillus acidophilus, Bifidobacterium lactis Bl-04, Bifidobacterium lactis Bi-07, and Lactobacillus paracasei Lpc-3752. In a randomized dose–response study, patients treated with antibiotics were given either high-dose HOWARU Restore, low-dose HOWARU Restore, or placebo as a preventative measure. Antibiotic-associated diarrhea was lower in the probiotic groups, with rates of 12.5, 19.6, and 24.6% for the high dose consisting of 1.70 × 1010 colony-forming units (CFU), the low dose consisting of 4.17 × 109 CFUs, and the placebo, respectively. Both probiotic groups had reduced levels of C. difficile-associated diarrhea, with rates of 1.8% in the probiotic groups and 4.8% in the placebo group53. No adverse events were found to be related to HOWARU Restore, with the adverse event rate being 4.2%, 4.2% and 7.2% in the high dose, low dose, and placebo groups, respectively. An early Phase I trial (NCT02207140) was recently completed with results pending that studied the effects of HOWARU Restore on healthy elderly patients and the resulting levels of C. difficile and changes in microbial diversity in fecal samples.
Additional clinical trials have been or are being performed looking specifically at the ability of Lactobacillus reuteri probiotics in the prevention of antibiotic-associated diarrhea or C. difficile infection. A Phase III trial was recently completed and another clinical trial is currently recruiting (NCT01295918, NCT02127814). In the completed Phase III trial, patients in the treatment arm were given a chewable tablet containing 1 × 108 CFUs of L. reuteri (produced by BioGaia AB) once per day during their antibiotic treatment and then continued for seven days after their antibiotic treatment was concluded. Results have not yet been posted.
Vaccines.
Asymptomatic carriage of C. difficile is associated with a decreased risk of developing CDI. Additionally, a patient’s levels of antibodies against C. difficile toxins correlates inversely with the development of recurrent disease54,55. As such, the development of vaccinations against C. difficile and its toxins has the potential to prevent not only the initial disease but recurrence as well (Fig. 5). Vaccines have similar advantages and limitations to probiotics. As they are preventative in nature, it is important to determine which patients would benefit from vaccination.
VLA84 (IC84) is a recombinant vaccine developed by Valneva that is composed of truncated portions of C. difficile toxins A and B. In a Phase I trial of 51 adult and 50 elderly patients, VLA84 was well tolerated and induced high levels of antibodies against C. difficile toxins A and B56. A Phase II trial (NCT02316470) was completed in 2015, in which patients were divided into four groups receiving either VLA84 75 µg without Alum (150 patients), VLA84 200 µg without Alum (150 patients), VLA84 200 µg with Alum (150 patients), or placebo (50 patients). The vaccination schedule consisted of intramuscular injections into alternating deltoid regions at day 0, 7, and 28. The results again showed that it induced seroconversion in up to 83% of participants for antibodies against both toxins A and B, and up to 97% against toxin A alone57. If successful, VLA84 and similar vaccinations would be potential preventative treatments that could be given to high-risk populations to protect against future CDIs.
ACAM-CDIFF is a toxin A and B vaccine developed by Sanofi that is given parenterally. It was found to be safe in adults and elderly patients in two Phase I trials (NCT00127803, NCT00214461)58. The optimal dosing was tested in Phase II trials, with one finding that giving a high dose with adjuvant (100 μg antigen + AlOH) at 0–7-30 days resulted in the greatest immune response59. A Phase III trial (NCT01887912) was discontinued and the vaccine research as a whole was discontinued by Sanofi following a discouraging interim analysis60.
PF-06425090 is a vaccine developed by Pfizer composed of genetically modified toxins A and B from C. difficile 61,62. The drug has completed multiple Phase I and a single Phase II trial so far, with another Phase II trial active (NCT02561195) and a Phase III trial (NCT03090191) currently recruiting. The current Phase II trial has three groups: high-dose (200 µg) vaccine, low-dose (100 µg) vaccine, and placebo. In addition, these three groups are split into two vaccination schedules. The vaccine is given in a 3-dose vaccination schedule by 0.5 mL intramuscular injection. The first vaccination schedule is day 1, 8 and 30 and the second schedule is month 0, 1, and 6. Results from this Phase III trial are currently being reported, with an indication that the vaccination is leading to increased anti-toxin A antibody titers, although no statistical analysis has been performed yet. Further study is required to test its efficacy in preventing CDI.
CDVAX is a novel oral vaccine against C. difficile that utilizes spores from a genetically modified bacterium to produce an oral vaccine that may induce strong mucosal immunity. This differs from injectable vaccines as the antigens will be presented from the mucosal side of the GI tract. Recently, a Phase I trial (NCT02991417) for CDVAX vaccination against C. difficile was terminated. More clinical research is needed to test the efficacy and safety of CDVAX 63.
Lactoferrin therapy.
For CDI to develop, C. difficile has to colonize the intestines and produce mucosal damage leading to symptoms such as diarrhea. If therapies are developed to target C. difficile growth, toxin production, and mucosal damage, CDI can be prevented even if the intestinal microbial community is disrupted (Fig. 4). Elevated levels of fecal lactoferrin and increased WBC count has been shown to be associated with increased CDI severity64. Utilizing rat intestinal epithelia cells (IEC-6), Otake et al. showed that lactoferrin has the potential to reduce the cytotoxic damage of C. difficile toxin B65. In an in vitro chemostat gut model, Chilton et al. showed that lactoferrin delays C. difficile growth and reduces toxin production66. There are two currently recruiting clinical trials testing lactoferrin. The first (NCT02626104) is looking at using lactoferrin prophylactically during initial antibiotic usage for pediatric patients to reduce antibiotic-associated diarrhea. In this study, children will receive either 100 mg of oral lactoferrin twice daily for the duration of antibiotic treatment or they will receive a placebo of 100 mg of oral maltodextrin twice daily. The second study (NCT00377078) is looking at the use of lactoferrin to prevent CDIs in long-term care patients with feeding tubes who require broad-spectrum antibiotics. In this study, patients in the treatment arm will be given lactoferrin at a concentration of 5 mg/mL during the enteral feeding system flush cycle, consisting of 600 mL per day. This will be started at the beginning of the first day of antibiotic treatment and continued eight weeks following the last antibiotic dose. The results from this study will hopefully determine if lactoferrin will prove to be an effective treatment worthy of additional study.
Treatment of primary CDI: reducing severity and increasing clinical cure
If the onset of CDI cannot be prevented, additional emerging therapies are needed to reduce mucosal damage, disease severity, and the risk of recurrence. As the highest mortality is associated with severe and complicated CDI, therapies given shortly after the diagnosis of CDI, which aim to reduce the burden of C. difficile cells and toxins, reduce toxin damage at the mucosal barrier, and help restore the microbiota, can potentially reduce the progression to severe and complicated CDI (Table 2).
Table 2.
Emerging therapies targeted at primary management of CDI
| Type | Treatment name |
Admin . |
Current or last phase completed |
Mechanism | Prevention | Primary therapy |
Recurrence prevention/ treatment |
|---|---|---|---|---|---|---|---|
| Antibiotic | Cadazolid | Oral | Phase III completed |
Inhibits protein synthesis and to some extent inhibits DNA synthesis. Inhibits sporulation, toxin production, and is also bactericidal. |
X | ||
| Antibiotic | CRS3123 | Oral | Phase I completed |
Narrow-spectrum antibiotic that acts by inhibiting bacterial methionyl-tRNA synthetase, with high activity against Gram positive bacteria and C. difficile but low activity against Gram negative bacteria. |
X | ||
| Antibiotic | LFF571 | Oral | Phase II completed |
Semisynthetic thiopeptide that acts on Gram positive bacteria by blocking protein synthesis. |
X | ||
| Antibiotic | MCB3837/M CB3681 |
IV | Phase I completed |
Hybrid fluoroquinolone-oxazolidinone that has a water- soluble prodrug formulation termed MCB3837 which can be given by IV. |
X | ||
| Antibiotic | Nitazoxanide | Oral | Phase III completed |
Noncompetitive inhibitor of the pyruvate- ferredoxin/flavodoxin oxidoreductases with anti-C. difficile activity. |
X | ||
| Antibiotic | Ramoplanin | Oral | Phase III, Phase IIb ongoing |
Ramoplanin is a glycolipodepsipeptide which disrupts cell wall biosynthesis by binding to peptidoglycan. It is non- absorbable, binds to spores, and kills vegetative C. difficile cells in vitro. |
X | ||
| Antibiotic | Ridinilazole | Oral | Phase II completed | Narrow-spectrum, nonabsorbable novel antibiotic that potentially impacts cell division, but the mechanism is not fully understood. |
X | ||
| Antibiotic | Surotomycin | Oral | Phase III completed |
Bactericidal cyclic lipopeptide that acts to dissipate the membrane potential of C. difficile. |
X | ||
| Antibiotic | Tigecycline | IV | Intervention al completed |
A glycylcyline which acts by binding to the 30S ribosomal subunit and inhibiting protein translation by blocking tRNA molecules from entering the A site of the ribosome. |
X | ||
| Binder | CASAD | Oral | Phase II Trial terminated |
Sequesters C. difficile toxin A and B with limited off-target protein binding. |
X | ||
| Binder | GT267–004 | Oral | Phase III completed |
A polystyrene binder that may sequester C. difficile toxins A and B. |
X | ||
| Binder | GT160–246 | Oral | Phase II completed |
A high molecular–weight soluble anionic polymer that has been shown in hamsters to reduce mortality from C. difficile infections and in vitro data suggests it can neutralize the activity of toxin A and B. |
X | ||
| Alanyl- glutamine |
Alanyl- glutamine |
Oral | Phase II terminated |
A dipeptide that has been shown to have potential therapeutic effects in reducing C. difficile toxin damage in intestinal epithelial cells. |
X | ||
| Antibody | IVIG | IV | Phase IV terminated |
Intravenous immunoglobulins derived from pooled human serum. |
X | ||
| Antibody | SBI | Oral | Active | Serum-derived bovine immunoglobulins. | X | ||
| Polyclonal antibody |
IMM-529 | Oral | Phase I/II trial ongoing |
Polyclonal antibodies against TcdB, vegetative cells, and spores. |
X | ||
| Bowel prep |
Nu-Lytely | Oral | Phase IV recruiting |
A formulation of the osmotic laxative PEG 3350 and electrolytes that can be used to reduce the luminal load of C. difficile spores and toxins. |
X |
For each emerging therapy, the administration route, current or last phase completed, and mechanism is listed. Additionally, each drug is marked for the applications it is being studied for in clinical trials, namely: prevention, primary therapy, or recurrence prevention/treatment. Clinical trial information was obtained from ClinicalTrials.gov41.
Antibiotics.
One area of interest is the development of new narrow spectrum antibiotics that have less of an effect on native bacteria. This would allow for the native bacteria to recolonize and reestablish during CDI therapy, potentially reducing disease severity and reducing recurrence in the future (Fig. 5). Additionally, some CDI cases are resistant to standard antimicrobial therapy, necessitating the development of novel antibiotic therapies for treating these resistant cases.
Cadazolid is a hybrid fluoroquinolone-oxazolidinone antibiotic produced by Actelion Pharmaceuticals Ltd that acts by inhibiting protein synthesis and to some extent inhibiting DNA synthesis67. In Phase I trials, cadazolid was well tolerated and minimally absorbed, with high concentrations found in the stool68,69. Cadazolid inhibits sporulation, toxin production, and is bactericidal. A Phase II trial (NCT01222702) studied the effectiveness of different doses of cadazolid compared to vancomycin in patients with CDI. The four treatment groups were: 250 mg cadazolid twice daily (20 patients), 500 mg cadazolid twice daily (22 patients), 1000 mg cadazolid twice daily (20 patients), or 125 mg vancomycin four times daily (22 patients). All treatments were given for 10 days. In this study, cadazolid treatment showed lower recurrence rates compared to vancomycin treatment (18–25% compared to 50% recurrence) and cadazolid showed higher sustained clinical cure rates (46.7%–60% compared to 33.3%). There was no evidence for an effect of dosage of cadazolid. In addition, cadazolid treatment was well tolerated70. Two Phase III studies have been completed, with a third currently suspended. The recently completed Phase III trials are being analyzed for efficacy, with one appearing to achieve the primary endpoint and the other failing to meet the primary endpoint of clinical cure rate71. The formal analysis of these two studies will be needed to determine the efficacy of cadazolid for the treatment of CDI.
CRS3123 (REP3123) is a novel narrow-spectrum antibiotic produced by Crestone Inc that acts by inhibiting bacterial methionyl-tRNA synthetase, with high activity against Gram positive bacteria and C. difficile but low activity against Gram negative bacteria72,73. CRS3123 was reported to reduce spore formation and toxin formation in a hamster model74. One Phase I trial found CRS3123 to be well tolerated at multiple doses (100 mg, 200 mg, 400 mg, 800 mg, and 1200 mg) and the indicated safety of the drug supported further research into its efficacy72. Further research is necessary to test the efficacy of CRS3123 for the treatment of CDI.
LFF571 is a semisynthetic thiopeptide produced by Novartis Pharmaceuticals that acts on Gram positive bacteria by blocking protein synthesis75. In a Phase II study (NCT01232595) completed in 2013, LFF571 was found to be minimally absorbed and had high retention in the intestines76. In this study, adults experiencing primary or first recurrent CDI were randomized to receive either LFF571 (200 mg) or vancomycin (125 mg) 4 times daily for 10 days. LFF571 had a noninferior clinical cure rate (90.6%) compared to vancomycin (78.3%), with a potential to have a lower recurrence rate (19% versus 25%)77. LFF571 treatment had more adverse events than vancomycin (76.1% versus 69.2%) but had less adverse events suspected to be related to the treatment (32.6% versus 38.5%).
MCB3681 is a hybrid fluoroquinolone-oxazolidinone produced by Morphochem that has a water-soluble prodrug formulation termed MCB3837 that can be given by IV, making this therapy an option for severe and complicated C. difficile infections when oral therapy fails. It has been shown to have activity against Gram positive bacteria, including C. difficile, but limited activity against Gram negative bacteria such as those native to the human gut78–80. A Phase I study where 12 healthy volunteers were given daily intravenous infusions of 6 mg/kg MCB3837 over 12 hours for five days showed little impact on microbiota and suggested the drug was well tolerated81. Phase II/III trials are being planned currently and the FDA designated MCB3837 as a Qualified Infectious Disease Product (QIDP) for the treatment of CDI82.
Nitazoxanide is believed to be a noncompetitive inhibitor of the pyruvate ferredoxin/flavodoxin oxidoreductases produced by Romark Pharmaceuticals83. Currently, nitazoxanide is FDA approved for treatment of cryptosporidiosis and giardiasis, and is given by tablet orally (500 mg) every 12 hours for 3 days, with possible adverse side-effects of abdominal pain, nausea, headache, or discolored urine84. Nitazoxanide (500 mg twice per day for 7 or 10 days) was shown to have similar clinical cure and recurrence rates for CDI versus vancomycin (125 mg 4 times per day for 10 days) and metronidazole (250 mg 4 times per day for 10 days)85,86. However, nitazoxanide is more expensive than metronidazole, limiting its use for primary CDI87. A Phase III trial (NCT00304356) with results currently being reported is looking at the use of nitrazoxanide (500 mg twice daily) as a treatment for CDI that has failed to be cured by metronidazole or vancomycin.
Ramoplanin is a glycolipodepsipeptide that disrupts cell wall biosynthesis by binding to peptidoglycan 88. It has been shown to be non-absorbable, and to bind to spores and kill vegetative C. difficile cells in vitro. This may indicate a potential use for ramoplanin as a preventative measure to reduce risk of initial disease or reduce recurrence by binding to spores and then killing the ones that germinate89. A Phase II trial comparing treatment using ramoplanin versus vancomycin for CDI showed similar clinical response rates with 400 mg ramoplanin (71% versus 78%) and similar sustained clinical response rates (83% 200 mg ramoplanin, 85.2% 400 mg ramoplanin, 85.7% vancomycin). A Phase III clinical trial has been approved by the FDA.
Ridinilazole (SMT19969) is a narrow-spectrum, non-absorbable novel antibiotic produced by Summit Therapeutics that potentially impacts cell division, but the mechanism is still not fully understood90. A Phase I trial studied the safety of oral doses of up to 2000 mg of ridinilazole. Ridinilazole was shown to have minimal effects on the microbiota and was not measured at high serum levels in this Phase I trial91. A Phase II trial with 100 patients with CDI compared 10-day oral ridinilazole treatment (200 mg every 12 hours) to 10-day oral vancomycin treatment (125 mg every 6 hours). This trial showed that ridinilazole caused a higher sustained response rate compared to vancomycin (67% vs 42%) as well as a reduction in recurrence (14.3% vs. 34.8%)92. There were no adverse events that necessitated discontinuation of ridinilazole, and the adverse events were similar between ridinilazole and vancomycin treatment. An additional Phase II trial (NCT02784002) was completed in 2016 comparing ridinilazole to fidaxomicin; study results are being analyzed.
Surotomycin (CB-183,315) is a bactericidal cyclic lipopeptide originated by Cubist Pharmaceuticals and currently developed by Merck & Co that acts to dissipate the membrane potential of C. difficile93. Surotomycin given to two treatment groups, either at 125 mg twice daily or 250 mg twice daily for 10 days, was shown in a Phase II trial to reduce recurrence of C. difficile infection compared to vancomycin that was given at 125 mg 4 times daily for 10 days94,95. In this Phase II trial, adverse events were similar between both surotomycin arms and the vancomycin arm. However, surotomycin (250 mg given orally twice daily for 10 days) failed to achieve noninferiority versus vancomycin (125 mg given orally four times daily for 10 days) in a recent Phase III trial (NCT01597505), with initial cure rates of 79% versus 83.6% and sustained clinical response rates of 60.6% versus 61.4%96. An additional Phase III trial (NCT01598311) was recently completed studying the effectiveness of surotomycin treatment (285 patients; 250 mg twice daily for 10 days) compared to vancomycin treatment (292 patients; 125 mg vancomycin four times daily for 10 days) for patients with confirmed CDI. The study showed that surotomycin was noninferior to vancomycin for clinical response at the end of the trial (83.4% versus 82.1%), but surotomycin failed to demonstrate superiority to vancomycin in clinical response over time and sustained clinical response97.
Tigecycline is a glycylcyline produced by Pfizer that acts by binding to the 30S ribosomal subunit and inhibiting protein translation by blocking tRNA molecules from entering the A site of the ribosome98. It is FDA approved for treatment of community-acquired pneumonia (100 mg by IV once, with 50 mg IV given every 12 hours thereafter for 7 to 14 days), complicated skin/subcutaneous infection (same dosing but for 5 to 14 days), and complicated abdominal infections (same dosing but for 5 to 14 days)99. Tigecycline has cured CDI in case reports; however, pooled data may indicate an association with higher mortality100,101. An interventional clinical trial (NCT01401023) where patients received standard C. difficile-associated diarrhea treatment of vancomycin or metronidazole with the addition of IV tigecycline (100 mg once, followed by 50 mg IV every 12 hours after) for the duration of hospitalization (approximately 7–14 days) was completed in 2013, with findings on clinical cure not yet reported. A recent retrospective observational study found tigecycline combination therapy with vancomycin ± metronidazole to be safe and effective for the treatment of severe–complicated CDI102.
Toxin Binders.
Toxin binders are molecules that have been shown to sequester toxins, and some have the additional ability to bind to pro-inflammatory factors. While current studies into the use of toxin binders to treat CDI have not proven successful to a large extent, it is important to consider them as they could potentially be effective if the right formulation is discovered. Given orally, these binders enter the lumen and can be used during CDI to reduce the mucosal damage done both by the toxins and by the host immune response. In this way, binders have the potential to reduce disease severity, hopefully lowering the number of cases of severe and complicated CDI cases (Fig. 5). However, these binders could also bind pharmaceuticals, so they should not be co-administered with standard therapy if found to bind to standard drugs21. As this is an important consideration, newer binders aim to reduce pharmaceutical cross-binding.
Calcium aluminosilicate anti-diarrheal (CASAD), developed by Salient Pharmaceuticals, has been shown to sequester C. difficile toxin A and B with limited off-target protein binding103. Similar to other binders, if CASAD can preferentially bind to C. difficile toxins without binding to antibiotics, then this could reduce the severity of disease and potentially reduce recurrence risk. A Phase II trial (NCT01570634) attempting to study the effectiveness of adding CASAD given in 500 mg capsules orally 3 times daily for 14 days to standard CDI treatment was terminated for low enrollment; however, the authors describe the potential of calcium aluminosilicate to bind TNFα, IL-1, IL-6, and IL-10, which could theoretically help reduce the immune response to the infection and decrease fever and leukocytosis. Further research is needed to study the effectiveness and safety of CASAD for CDI treatment.
GT267–004 (tolevamer) is a polystyrene binder developed by Sanofi that is proposed to sequester C. difficile toxins A and B. It was shown in a Phase II trial to be noninferior to vancomycin treatment for mild to moderate CDIs, with the potential to reduce recurrence104. However, the analysis of two Phase III trials showed that tolevamer (563 patients received 9 g loading dose followed by 3 g every 8 hours for 14 days) was inferior to both metronidazole (289 patients received 375 mg every 6 hours for 10 days) and vancomycin (266 patients received 125 mg every 10 hours for 10 days) for clinical cure of CDI (44.2%, 72.7%, and 81.1%, respectively)40. Adverse events were similar across the three treatment groups. A third Phase III trial (NCT00466635) was terminated and it is doubtful that additional development of this compound will be pursued.
GT160–246 is a high molecular–weight soluble anionic polymer produced by Sanofi that has been shown in hamsters to reduce mortality from CDI, and in vitro data suggest GT160–246 can neutralize the activity of toxin A and B105. A GT160–246 Phase I trial showed it to be safe and well tolerated. A Phase II trial (NCT00034294) comparing GT160–246 to vancomycin for the treatment of C. difficile-associated diarrhea was completed, with results not currently posted.
Host response modulation.
One target for reducing disease severity as previously mentioned is regulating the host response in order to reduce host-derived mucosal damage during CDI treatment. Alanyl-glutamine is a dipeptide that has been shown to have potential therapeutic effects in reducing C. difficile toxin damage in intestinal epithelial cells (Fig. 5). Rodrigues et al. showed that alanyl-glutamine reduces apoptosis and increases intestinal cell proliferation in mouse intestinal cells (IEC-6) when exposed to C. difficile toxin B106. Santos et al. additionally showed in IEC-6 cells that alanyl-glutamine treatment reduced TcdA toxin damage and increased RhoA expression, suggesting a potential explanation for the protective effects107. Using intestinal loop models, Warren et al. showed that treatment with ATL 370 (an adenosine A2A receptor agonist) and alanyl-glutamine reduced ileal secretions, apoptosis, mucosal injury, and levels of KC and IL-10 after C. difficile toxin A exposure108. A Phase II clinical trial (NCT02053350) testing the efficacy of alanyl-glutamine as a supplement (44 g orally daily for 10 days) during treatment of CDI was terminated due to low enrollment. Further clinical trials are needed to test the efficacy of alanyl-glutamine in treating primary CDIs and reducing disease severity.
Antibodies.
As disease is caused by the toxins produced from C. difficile, one potential goal during primary therapy is the neutralization of toxins with anti-toxin antibodies. While specific monocolonal and polyclonal antibodies are being studied for their effectiveness in preventing recurrence (as discussed in the recurrence section), intravenous immunoglobulin (IVIG) and serum bovine immunoglobulin (SBI) have been proposed to reduce disease severity by reducing toxin damage (Fig. 5). A Phase IV trial (NCT00177970) looking at IVIG (400 mg/kg infused over 4–6 hours) effectiveness during standard therapy for severe CDI was terminated because it was unable to receive IVIG for free. There is a currently active clinical trial (NCT02730325) studying the effects of giving SBI (10.0 g twice per day) on ulcerative colitis in patients who tested positive for C. difficile and who are on vancomycin. Further study is needed to determine the efficacy of IVIG or SBI during severe CDI. Monoclonal and polyclonal antibodies directed against C. difficile are an alternative that requires further study for their effectiveness during therapy of CDI.
Additionally, IMM-529 is a polyclonal antibody developed by Immuron that has shown cross-reactivity with C. difficile vegetative cells, spores, and toxin B. IMM-529 is currently being tested in a Phase I/II trial (NCT03065374) for the treatment of CDI109. The study aims to determine the safety and tolerability of IMM-529. Patients will receive standard of care treatment for CDI in addition to either IMM-529 (1000 mg orally 3 times daily) or placebo.
Bowel Prep.
One method of reducing C. difficile cell and toxin burden is to flush the luminal content out of the intestines using bowel preps after CDI is diagnosed and before standard therapy (Fig. 5). Nu-Lytely is a formulation of the osmotic laxative PEG 3350 and electrolytes produced by Braintree Laboratories110. A recruiting Phase IV clinical trial (NCT01630096) will study the efficacy of oral lavage by giving Nu-Lytely after the diagnosis of CDI, before antibiotics are started. In this study, patients testing positive for CDI will be assigned to either a control group or the osmotic laxative group in which they will be given the PEG 3350 solution in 8oz volume every 10 minutes until 6 liters are ingested. An additional 2 liters may be ordered if necessary. Both groups will then receive standard of care antibiotic treatments. Further results are needed to determine if oral lavage before standard treatment will reduce disease severity by lowering the C. difficile bacterial and toxin load in the GI tract.
Preventing and treating recurrent CDI
With a median recurrence of 21% and a high rate of re-admissions and cost associated with recurrent CDI, developing novel therapies to prevent recurrence and treat recurrent CDI will have a substantial impact on patient morbidity, as well as on the healthcare burden of CDI111,112. There are two distinct treatment goals concerning recurrent CDI that could be optimized further. The first is reducing initial recurrence in patients experiencing primary CDI, and the second is treating therapy-resistant patients that are experiencing multiple episodes of recurrent CDI. As mentioned in the primary therapy section, many of the emerging antibiotics potentially offer a reduction in recurrence risk following standard therapy, including ridinilazole, LFF571, MCB3681, and ramoplanin. Additionally, probiotics used for prevention could potentially be also used to help recover the natural microbiota following standard therapy (Table 3).
Table 3.
Emerging therapies targeted at recurrent CDI prevention and management
| Type | Treatment name |
Admin . |
Current or last phase completed |
Mechanism | Prevention | Primary therapy |
Recurrence prevention/ treatment |
|---|---|---|---|---|---|---|---|
| Antitoxin antibody |
Bezlotoxumab /actoxumab |
IV | Phase III ongoing |
Human monoclonal antibodies against TcdA (actoxumab) and TcdB (bezlotoxumab) that can prevent toxin damage at the intestinal barrier. |
X | ||
| Oral Bovine antibodies |
Colostrum | Oral | Phase II/III withdrawn |
Colostrum from C. difficile-immunized cows. | X | X | |
| Oral Bovine antibodies |
MucoMilk | Oral | Phase II/III terminated |
A whey protein concentrate 40% (WPC-40) enriched with polyclonal antibodies that is produced from the milk of cows immunized with formaldehyde-inactivated C. difficile cells and C. difficile toxin filtrate. |
X | ||
| Microbial | SER-109 | Oral | Phase III ongoing |
A capsule consisting of bacterial spores derived from screened human donor stool. |
X | ||
| Microbial | SER-262 | Oral | Phase I ongoing |
A manufactured microbial therapeutic. | X | ||
| Microbial | CBM588 | Oral | Phase II withdrawn |
A probiotic consisting of Clostridium butyricum, which lacks toxins associated with C. difficile infections. |
X | ||
| Microbial | MET-2 | Oral | Phase I recruiting |
Consists of a live microbe community derived from healthy donor stools |
X | ||
| Microbial | RBX2660 | Enema | Two Phase II and One Phase III ongoing |
A stool-derived standardized therapy consisting of live bacteria suspension. Suspension is given by retention enema and is derived from healthy donors |
X | ||
| Microbial | RBX7455 | Oral | Phase I ongoing |
A lyophilized oral formulation of RBX2660, which is stable at room temperature. |
X | ||
| Microbial |
CP101 |
Oral |
Phase II ongoing |
Encapsulated lyophilized fecal microbiota derived from human donors. |
X | ||
| Non- Toxigenic Clostridiu m difficile |
VP20621 | Oral | Phase II ongoing |
Consists of spores from the non-toxigenic C. difficile (NTCD) strain M3. |
X |
||
| Secondary bile acid |
Ursodiol | Oral | Phase IV recruiting |
Urodeoxycholic acid is being used as a surrogate for deoxycholic acid, a secondary bile acid. Secondary bile acids have been shown to suppress C. difficile growth in vitro. |
X |
For each emerging therapy, the administration route, current or last phase completed, and mechanism is listed. Additionally, each drug is marked for the applications it is being studied for in clinical trials, namely: prevention, primary therapy, or recurrence prevention/treatment. Clinical trial information was obtained from ClinicalTrials.gov41.
For the second goal, with subsequent recurrence risk increasing after each unsuccessfully treated recurrent episode, it is important to develop novel therapies that specifically treat recurrent-prone CDI. While FMT has been shown to be effective at treating recurrent CDI and preventing further recurrences, human-derived fecal matter is difficult to standardize and has multiple potential risks, including the transfer of infectious material and long-term consequences of inoculating the gut with a foreign fecal material. As such, research is ongoing to develop new agents for treating recurrent CDI. These agents include antibodies directed against C. difficile cells and toxins as well as standardized bacterial replacement cultures and mixtures (Table 3).
IV Antibodies.
Researchers have found an inverse correlation between the development of recurrent disease and anti-toxin antibody levels54,55. Antibodies given intravenously enter the lumen in regions of mucosal damage and help lower the intestinal damage caused by toxins. This may help increase the recovery of the healthy mucosal layer and assist in the recovery of the natural microbiota, leading to restoration of colonization resistance (Fig. 5).
Actoxumab is a human monoclonal antibody against C. difficile toxin A and bezlotoxumab (MK-6072–001) is a human monoclonal antibody against toxin B developed by Merck. Two Phase III clinical trials (MODIFY I and MODIFY II) studied the ability of these two antibodies to reduce the recurrence of CDI in 2655 patients. In these trials, it was shown that the addition of bezlotoxumab (10 mg/kg infusion) to the standard of care antibiotics for primary or recurrent C. difficile infections resulted in a lower rate of recurrence compared to the placebo (MODIFYI: 17% versus 28%; MODIFY II: 16% versus 26%) and a higher sustained clinical cure compared to the placebo (64% versus 54%). The addition of actoxumab (10 mg/kg infusion) alone did not decrease recurrence, and in combination with bezlotoxumab (10 mg/kg infusion of both bezlotoxumab and actoxumab) it did not increase efficacy compared to bezlotoxumab alone113. The adverse event rates were similar among the treatment groups. These results indicate that antibodies targeted against toxin B are a potential therapy for reducing recurrence in high-risk patients. A recent computer model–based analysis has predicted that bezlotoxumab will be cost-effective for the prevention of recurrence in patients receiving standard of care antibiotics for CDI114,115. A new Phase III trial (NCT03182907) studying the effects of bezlotoxumab (10 mg/kg infusion) in addition to standard antibacterial treatment in children with C. difficile infections is currently recruiting. Bezlotoxumab (10 mg/kg infusion) is currently FDA approved for the prevention of recurrent CDI in patients currently on treatment for CDI and who are at high risk for recurrence116.
Polyclonal Oral Antibodies.
Unlike IV antibodies, which enter through the bloodstream, oral antibodies enter the GI lumen directly. If polycolonal antibodies are used, the oral antibodies can be designed to target not only toxins, but also spores and vegetative cells. By reducing the burden of spores and vegetative cells, these treatments can reduce the chance of recurrence. Through neutralization of toxins, these therapies can additionally protect against Clostridium toxin damage, assisting in the restoration of the mucosa and microbiota (Fig. 5).
Colostrum is a type of milk produced during pregnancy that has high levels of antibodies to provide passive immunity to infants. Immunization of pregnant mares and cows with C. difficile proteins results in the production of antibodies that can be obtained from the colostrum and used therapeutically. Colostrum from mares immunized with toxin A and B binding domains was able to block the cytotoxic activity of the C. difficile toxins A and B117. Repeated immunization of a pregnant cow with recombinant mutants of toxin A and B produced hyperimmune bovine colostrum (HBC) that was able to reduce the disease severity of CDI in piglets117. Similarly, HBC was shown to prevent, treat, and reduce recurrence of CDI in mouse models118. A Phase II/III clinical trial (NCT00747071) to test the efficacy and safety of colostrum-derived antibodies for the prevention of CDI was withdrawn. Further clinical trials are needed to assess the ability of colostrum-derived antibodies to prevent, treat, and reduce recurrence of CDI.
MucoMilk is a whey protein concentrate 40% (WPC-40) enriched with polyclonal-antibodies developed by MucoVax that is produced from the milk of cows immunized with formaldehyde-inactivated C. difficile cells and toxin filtrate. As it is given orally, the antibodies will be available on the luminal side and have the potential to target spores, vegetative cells, and toxins. In a hamster model, hamsters infected with C. difficile died when untreated with WPC-40, while hamsters treated with WPC-40 had an 80% to 90% survival, depending on the formulation. In preliminary data, 16 patients with CDI were given WPC-40 three times daily for two weeks following standard antibiotic therapy. The WPC-40 was well tolerated and none of the patients experienced recurrence (median follow-up: 333 days, range: 35 days to 1 year)119. A 60-patient Phase II/III trial (NCT00177775) testing the efficacy and safety of MucoMilk in the prevention of recurrence of C. difficile was completed in 2005 and results are not posted yet. Further study is required to test the benefit of MucoMilk following standard antibiotic therapy to prevent recurrence.
Bacterial Replacement.
While the exact reason for the loss of colonization resistance is unknown, comprehensive bacterial replacement during FMT has shown that restoration of certain components of the microbiota is effective in treating recurrence. Decreased microbial diversity is associated with recurrent disease120. Future research is needed to find the specific community members needed to restore colonization resistance. Emerging therapies are being developed to address this aim with the goal of standardized bacterial replacement therapeutics to restore the natural microbiota (Fig. 5).
SER-109 is a capsule consisting of bacterial spores derived from screened human donor stool. The FDA designated SER-109 as a Breakthrough Therapy and Orphan Drug121. Khanna et al. found that SER-109 potentially prevents CDI recurrence within an 8-week follow-up period in patients experiencing recurrence, presumably through diversification of the gut microbiota and recovery of natural colonization resistance122. A Phase II (ECOSPOR) double-blind, placebo-controlled trial enrolled 89 patients with multiple recurrent CDI to test the safety and efficacy of SER-109 to reduce recurrence of CDI. After patients had completed antibiotic therapy for CDI, they were split into SER-109 (59 patients) and placebo (30 patients) groups. The SER-109 group received one oral dose of SER-109 (1 × 108 bacterial spores) after completion of antibiotics, while the other received the placebo. Results from the Phase II (ECOSPOR) trial indicate that SER-109 did not meet the primary endpoint for reducing CDI recurrence overall. However, in high-risk populations (in this case, those 65 years or older), SER-109 treatment showed reduced recurrence rates (45% versus 80% recurrence risk)123. Two Phase III trials (ECOSPOR III–NCT03183128, ECOSPOR IV–NCT03183141) are currently recruiting.
Seres Therapeutics is also developing another oral microbiome therapeutic called SER-262, which is a manufactured microbial therapeutic that, in contrast to SER-109, is a defined microbial community consisting of the spores of anaerobic, commensal bacteria produced by in vitro fermentation and is not an undefined consortium derived from human stool. SER-262 is currently being tested in a Phase I trial (NCT02830542) for adult patients to prevent recurrent C. difficile infection. In this trial, patients receiving standard of care antibacterial treatment for primary CDI will be assigned to experimental groups with single doses ranging from 1 × 104 to 1 × 108 CFUs or multiple doses ranging from 1 × 107 to 1 × 108 CFUs. This study will examine the safety, tolerability, and the efficacy of SER-262 to prevent CDI recurrence.
CBM588 is a probiotic consisting of Clostridium butyricum produced by Osel that is given by oral administration. C. butyricum lacks toxins associated with CDI and has been shown to be safe124. A Phase II trial (NCT01077245) testing for the ability of CBM588 to reduce recurrence after CDI therapy, in which patients with confirmed CDI will receive standard of care antibacterial therapy in addition to either CBM588 (2g per dose) or placebo twice daily for 42 days, was suspended for lack of enrollment. Future clinical studies need to be performed to test the efficacy of CBM588 in preventing CDI recurrence 125.
MET-2 (Microbial Ecosystem Therapeutics 2) consists of a live microbe community derived from healthy donor stool developed by NuBiyota. A small 20-patient Phase I pilot study (NCT02865616) is currently recruiting to test the safety and efficacy of MET-2 in treating recurrent CDI in patients who have experienced at least two prior C. difficile episodes. In this study, patients who are experiencing a case of recurrent CDI will be given an initial loading dose of MET-2 over 2 days and then a maintenance dose of MET-2 over 8 days. If the patients do not respond to the first loading dose they can be offered a second, higher dose of MET-2. Patients experiencing failure of the second dose may be offered a higher dose given by colonoscopy. Further research will be needed to test its safety and efficacy in preventing recurrence of CDI.
RBX2660 is a stool-derived standardized therapy consisting of a live bacteria suspension developed by Rebiotix. The suspension is given by retention enema and is derived from healthy donors. A Phase II trial in patients with a least two recurrences of CDI found it to be safe and efficacious, with 87.1% of patients (27/31) achieving treatment success after one or two treatments126. Two Phase II trials (NCT02589847, NCT02299570) are currently active but not recruiting, and a Phase III trial (NCT03244644) is currently recruiting to study the efficacy of RBX2660 in the treatment of recurrent CDI. Results for an open-label Phase II trial (Punch™ Open Label) were announced in April 2017, indicating a success rate of 78.8% for RBX2660 compared to the historical control of 51.8% for the prevention of recurrent C. difficile infections127,128. The results of the active clinical trials will help address the relevant efficacy of RBX2660. Rebiotix has also made a lyophilized oral formulation of RBX2660 termed RBX7455, which is stable at room temperature. RBX7455 is currently being tested in a Phase I study for the prevention of recurrent C. difficile infection, and the study was recently expanded129,130.
CP101 is an orally administered capsule containing freeze-dried microbes derived from healthy human donors and was developed by Finch. The FDA has recently granted CP101 the Fast Track designation for the treatment of recurrent CDI131. In a pragmatic cohort study, 49 patients experiencing recurrent CDI were given encapsulated lyophilized fecal microbiota (dosing ranged from 2.5 × 1012 bacteria in 24–27 capsules to 1.25 × 1012 bacteria in 2–3 capsules). This initial study observed an 88% (43/49) clinical success rate defined as no recurrent episodes over 2 months following therapy132. CP101 is currently being tested in a recruiting Phase II clinical trial (NCT03110133) for the treatment of recurrent CDI.
Non-toxigenic C. difficile.
Similar to bacterial replacement, one emerging therapy is utilizing non-toxigenic C. difficile to outcompete toxigenic C. difficile and prevent recurrent disease while the native microbiota recovers (Fig. 5). This therapy may also be useful for prevention of CDI, similar to probiotics. The current clinical trials are looking at using non-toxigenic C. difficile for prevention or recurrence.
VP20621 (NTCD-M3) consists of spores from the non-toxigenic C. difficile (NTCD) strain M3. This strain has been shown to be protective in hamsters against C. difficile challenge. A study in healthy adults showed that VP20261 was well tolerated and resulted in colonization of the GI system following pretreatment with vancomycin133. A Phase II trial sponsored by Shire studied the safety and efficacy of VP20261 for the prevention of C. difficile in patients experiencing initial CDI or a first recurrence. Patients were assigned to four groups receiving oral liquid VP20261 formulation of 104 spores per day for 7 days (43 patients), 107 spores per day for 7 days (44 patients), 107 spores per day for 14 days (42 patients) or placebo for 14 days (44 patients). This study found that VP20261 was able to colonize the GI tract of patients following successful treatment with metronidazole or vancomycin and treatment was well tolerated. Additionally, VP20261 treatment resulted in reduced recurrence of CDI, with 13 of 43 patients experiencing recurrence in the placebo arm and 14 of 125 experiencing recurrence in the VP20261 treatment groups. Of the patients receiving VP20261, recurrence was 2% for patients that were successfully colonized (2 out of 86) compared to a recurrence of 31% for patients not successfully colonized (12 out of 39)134.
Bile acid supplementation.
As particular secondary bile acids have been shown to be inhibitory to vegetative C. difficile, one possible therapy would be supplementation with secondary bile acids during CDI treatment12–14. This could reduce the levels of C. difficile in the gut by suppressing growth and lead to a decreased rate of recurrence. A currently recruiting, Phase IV clinical trial (NCT02748616) will study the effects on the rates of CDI recurrence for urodeoxycholic acid (300 mg) supplementation for two months in total given during and following standard CDI treatment. Urodeoxycholic acid is being used as a surrogate for deoxycholic acid, a secondary bile acid.
Summary of emerging CDI therapies
CDI causes significant morbidity and mortality while also placing a substantial burden on the healthcare system. We currently have effective therapies for primary and recurrent infections, but there is still significant improvement to be had in the areas of prevention, disease severity reduction, recurrence prevention, and treatment of recurrent CDI.
With an incidence reaching nearly 500,000 cases annually in the United States, there is a substantial need for directed CDI prevention therapies for susceptible patients. Already emerging therapies are being utilized in the clinic, with probiotics such as BioK and VSL#3 as examples. The main targets for preventative measures are reducing the initial microbial disruption (β-lactamases or reduced systemic antibiotics), restoring the microbiota with probiotics (BioK, VSL#3), or reducing the ability of C. difficile to grow and thrive by directly targeting it with vaccinations (VLA84, ACAM-CDIFF, PF-06425090, CDVAX) and growth modulators such as lactoferrin. With continued clinical trials and development of preventative therapies, reduction of CDI incidence may soon be in reach, with significant ameliorating effects on the CDI healthcare burden, patient morbidity, and patient mortality.
Once CDI has developed in a susceptible patient, the clinical goals shift to reducing severity, preventing fulminant CDI, obtaining clinical cure, and lowering recurrence risk. Development of effective toxin binders (calcium aluminosilicate, GT267–004, GT160–246) and immune modulators (alanyl-glutamine), which help to reduce the toxin damage caused by C. difficile and restore the mucosa, may prevent severe CDI from developing and aid in the recovery of colonization resistance. Vaccinations, while seen as a method of prevention, may also act to decrease CDI severity by potentially reducing C. difficile organism burden and toxin activity. Bowel prep solutions that reduce luminal toxin levels and C. difficile organism burden may also help prevent severe CDI and increase therapeutic efficacy (Nu-Lytely). The development of antibiotics that are minimally absorbed and reach high luminal concentrations with high activity against C. difficile without broad activity against other native bacteria is promising for the therapy of CDI (cadazolid, CRS3123, LFF571, MCB3681, nitazoxanide, ramoplanin, ridinilazole, surotomycin, tigecycline). The reduced activity against native bacteria may allow for more rapid recovery, potentially leading to lower severity and a decreased recurrence risk.
Even with effective therapy, elevated recurrence risk still persists for months after successful clinical cure. With each recurrence, the risk of subsequent recurrence increases 111. While novel antibiotics are utilized to reduce the risk of CDI recurrence during primary treatment and may have a role in treating recurrent disease, additional therapies are needed to target the restoration of colonization resistance. The emerging use of monoclonal and polyclonal antibodies against C. difficile has shown success in reducing the risk of recurrence when given with standard CDI therapy. Determination of the best toxin motifs and which other C. difficile spore and vegetative cell molecules to target will help increase the effectiveness of toxin neutralization and C. difficile clearance following therapy. These treatments also could potentially be utilized for prevention and during therapy to reduce severity, although directed clinical trials are needed to assess this possibility. Aside from lowering mucosal damage done by toxins and C. difficile, restoration of colonization resistance can be achieved by supplementing the microbiota with bacterial replacement therapies (SER-109, CBM588, MET-2, RBX2600, CP101). Alternatively, or in unison, non-toxigenic C. difficile (VP20261) can be administered in order to compete with toxigenic C. difficile cells and spores that may be remaining in the GI tract or reintroduced during the high-risk few months following CDI therapy.
Future directions: perspectives
While large strides are being taken towards effective prevention, primary treatment, and recurrence reduction, there are still gaps in our knowledge of CDI pathogenesis that would help direct therapy research if elucidated. For the prevention of CDI, research is currently underway in many laboratories and fields to identify the exact species and microbial interactions necessary to produce colonization resistance against C. difficile and what alterations to the GI microbiota lead to an increase in CDI risk. If these questions can be answered, specific bacterial replacement that is purified to only include non-pathogenic bacteria can be used to restore colonization resistance and reduce CDI rates. Additionally, if the specific alterations are known for the increased risk of CDI, such as the loss of keystone species, tests can be developed to monitor patients receiving systemic antibiotics or those at high risk demographically to determine if preventative therapies are needed, reducing cost by targeting the use of these preventative treatments to only those requiring them.
For primary therapy, the direct mechanism leading to the development of severe and complicated CDI needs to be determined to develop targeted therapies for lowering severity. For instance, if mucosal damage is in part caused by immune response, then immune modulators can be utilized to reduce mucosal damage and protect against severe and fulminant CDI. Additionally, if loss of specific members of the microbiota, C. difficile burden, or specific immune states are associated with the development of severe and fulminant CDI, these can be used to predict severity and allow physicians to treat patients prior to reaching the severe or fulminant state of infection.
In our opinion, the first class of emerging therapies that will have substantial impacts on the treatment of CDI is narrow-spectrum, non-absorbable antibiotics. As research has shown that antibiotic treatments that cause large scale disruption of the microbiome are associated with increased recurrence and other adverse outcomes such as sepsis, narrow-spectrum antibiotics such as fidaxomicin and others in development (Table 2) have the potential to reduce microbial disruption during CDI treatment, leading to higher sustained clinical cure, lower recurrence rates, and reduced adverse outcomes135.
While stool products and bacterial replacements have shown considerable promise for treating recurrent CDI, longitudinal and large-scale studies are needed to examine potential long-term side effects of altering the native bacterial community by the addition of therapeutic bacterial communities. In the meantime, the use of narrow-spectrum antibiotics and other preventative measures can reduce recurrent cases without the necessity of bacterial agents such as FMT, filtered stool, or defined bacterial replacement. While the long-term effects are studied, in our opinion clinicians would prefer the development of pharmaceutical grade, FDA-approved filtered stool products and therapeutics, especially those with defined bacterial community structures. We foresee that these types of therapies will become a preferred alternative to FMT if they are shown to be efficacious and safe.
Additionally, well defined bacterial communities with a single agent or limited taxa may be increasingly used as primary prophylaxis for the prevention of primary CDI48. Through this mechanism, the number of primary and recurrent cases of CDI could be further reduced. While vaccines could be another viable preventative measure, they are currently not as effective and more clinical trials will be needed to identify an efficacious and safe vaccine.
An important limiting factor for clinical deployment of preventative and recurrence reduction therapies is the identification of patients who are at high risk of developing primary or recurrent CDI and who would benefit from these therapies. To achieve this risk stratification, large studies are starting to be performed using medical data for the development of predictive models for primary and recurrent CDI risk during a patient’s treatment136. If the risk of primary CDI or recurrence can be accurately predicted by microbial, systemic, or intestinal/colonic markers such as specific bacterial community compositions or immune cytokine levels, then the more expensive but effective therapies, such as bezlotoxumab, can be utilized to protect against future CDI. This will not only increase the effective treatment of recurrent CDI, but also lower the cost to the healthcare system by targeting only those patients predicted to experience primary or recurrent CDI.
We as a community are making immense strides towards effective prevention and management of C. difficile. The impact of these emerging therapies will not only affect CDI, but also other microbial illnesses that are dependent on an interaction between the host, native microbiota, and pathogen.
Acknowledgments
All authors contributed to the writing, generation of the figures and tables, and review of all material in this work. This review was supported by a grant from the National Institute of Allergy and Infectious Diseases U01AI124255 to Vincent B Young. Michael Dieterle was supported in part by NIH grant T32GM007863.
Footnotes
Competing interests
Vincent B. Young has served as a consultant to Vendanta Biosciences, Finch Therapeutics, and Exarca Therapeutics. All other authors declare no competing interests.
References
- 1.Lessa FC, Mu Y, Bamberg WM, et al. Burden of Clostridium difficile Infection in the United States. New England Journal of Medicine 2015;372(9):825–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zimlichman E, Henderson D, Tamir O, et al. Health care-associated infections: a meta-analysis of costs and financial impact on the US health care system. JAMA internal medicine 2013;173(22):2039–2046. [DOI] [PubMed] [Google Scholar]
- 3.Bartlett JG. Historical Perspectives on Studies of Clostridium difficile and C. difficile Infection. Clinical Infectious Diseases 2008;46(Supplement_1):S4–S11. [DOI] [PubMed] [Google Scholar]
- 4.Tedesco FJ, Barton RW, Alpers DH. Clindamycin-associated colitis. A prospective study. Annals of internal medicine 1974;81(4):429–433. [DOI] [PubMed] [Google Scholar]
- 5.Hall IC, O’Toole E. Intestinal flora in new-born infants: With a description of a new pathogenic anaerobe, bacillus difficilis. American Journal of Diseases of Children 1935;49(2):390–402. [Google Scholar]
- 6.Bartlett JG. Treatment of antibiotic-associated pseudomembranous colitis. Reviews of infectious diseases 1984;6 Suppl 1:S235–241. [DOI] [PubMed] [Google Scholar]
- 7.McDonald LC, Killgore GE, Thompson A, et al. An epidemic, toxin gene-variant strain of Clostridium difficile. The New England journal of medicine 2005;353(23):2433–2441. [DOI] [PubMed] [Google Scholar]
- 8.Loo VG, Poirier L, Miller MA, et al. A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. The New England journal of medicine 2005;353(23):2442–2449. [DOI] [PubMed] [Google Scholar]
- 9.McDonald LC, Gerding DN, Johnson S, et al. Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clinical Infectious Diseases 2018:cix1085–cix1085. [DOI] [PMC free article] [PubMed]
- 10.Paredes-Sabja D, Shen A, Sorg JA. Clostridium difficile spore biology: sporulation, germination, and spore structural proteins. Trends in Microbiology 2014;22(7):406–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sorg JA, Sonenshein AL. Bile salts and glycine as cogerminants for Clostridium difficile spores. Journal of bacteriology 2008;190(7):2505–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sorg JA, Sonenshein AL. Chenodeoxycholate is an inhibitor of Clostridium difficile spore germination. Journal of bacteriology 2009;191(3):1115–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ridlon JM, Harris SC, Bhowmik S, Kang DJ, Hylemon PB. Consequences of bile salt biotransformations by intestinal bacteria. Gut microbes 2016;7(1):22–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thanissery R, Winston JA, Theriot CM. Inhibition of spore germination, growth, and toxin activity of clinically relevant C. difficile strains by gut microbiota derived secondary bile acids. Anaerobe 2017;45:86–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Theriot CM, Bowman AA, Young VB. Antibiotic-Induced Alterations of the Gut Microbiota Alter Secondary Bile Acid Production and Allow for Clostridium difficile Spore Germination and Outgrowth in the Large Intestine. mSphere 2016;1(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chandrasekaran R, Lacy DB. The role of toxins in Clostridium difficile infection. FEMS Microbiology Reviews 2017;41(6):723–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Di Bella S, Ascenzi P, Siarakas S, Petrosillo N, di Masi A. Clostridium difficile Toxins A and B: Insights into Pathogenic Properties and Extraintestinal Effects. Toxins 2016;8(5):134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen S, Sun C, Wang H, Wang J. The Role of Rho GTPases in Toxicity of Clostridium difficile Toxins. Toxins (Basel) 2015;7(12):5254–5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deshpande A, Pasupuleti V, Thota P, et al. Risk factors for recurrent Clostridium difficile infection: a systematic review and meta-analysis. Infection control and hospital epidemiology 2015;36(4):452–460. [DOI] [PubMed] [Google Scholar]
- 20.Slimings C, Riley TV. Antibiotics and hospital-acquired Clostridium difficile infection: update of systematic review and meta-analysis. The Journal of antimicrobial chemotherapy 2014;69(4):881–891. [DOI] [PubMed] [Google Scholar]
- 21.Bagdasarian N, Rao K, Malani PN. Diagnosis and treatment of Clostridium difficile in adults: a systematic review. Jama 2015;313(4):398–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rao K, Micic D, Natarajan M, et al. Clostridium difficile ribotype 027: relationship to age, detectability of toxins A or B in stool with rapid testing, severe infection, and mortality. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2015;61(2):233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abou Chakra CN, Pepin J, Sirard S, Valiquette L. Risk Factors for Recurrence, Complications and Mortality in Clostridium difficile Infection: A Systematic Review. PLOS ONE 2014;9(6):e98400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA). Infection control and hospital epidemiology 2010;31(5):431–455. [DOI] [PubMed] [Google Scholar]
- 25.Curry SR, Marsh JW, Shutt KA, et al. High Frequency of Rifampin Resistance Identified in an Epidemic Clostridium difficile Clone from a Large Teaching Hospital. Clinical Infectious Diseases 2009;48(4):425–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.von Muller L, Halfmann A, Herrmann M. [Current data and trends on the development of antibiotic resistance of Clostridium difficile]. Bundesgesundheitsblatt, Gesundheitsforschung, Gesundheitsschutz 2012;55(11–12):1410–1417. [DOI] [PubMed] [Google Scholar]
- 27.Kociolek LK, Gerding DN. Breakthroughs in the treatment and prevention of Clostridium difficile infection. Nature reviews Gastroenterology & hepatology 2016;13(3):150–160. [DOI] [PubMed] [Google Scholar]
- 28.Optimer Pharmaceuticals I. Drug Insert: NDA 201,699 In: Administration USFaD, ed.
- 29.Fehér C, Mensa J. A Comparison of Current Guidelines of Five International Societies on Clostridium difficile Infection Management. Infectious Diseases and Therapy 2016;5(3):207–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cornely OA, Crook DW, Esposito R, et al. Fidaxomicin versus vancomycin for infection with Clostridium difficile in Europe, Canada, and the USA: a double-blind, non-inferiority, randomised controlled trial. The Lancet Infectious diseases 2012;12(4):281–289. [DOI] [PubMed] [Google Scholar]
- 31.Louie TJ, Miller MA, Mullane KM, et al. Fidaxomicin versus Vancomycin for Clostridium difficile Infection. New England Journal of Medicine 2011;364(5):422–431. [DOI] [PubMed] [Google Scholar]
- 32.Bartsch SM, Umscheid CA, Fishman N, Lee BY. Is fidaxomicin worth the cost? An economic analysis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2013;57(4):555–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soriano MM, Danziger LH, Gerding DN, Johnson S. Novel Fidaxomicin Treatment Regimens for Patients With Multiple Clostridium difficile Infection Recurrences That Are Refractory to Standard Therapies. Open Forum Infect Dis 2014;1(2):ofu069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guery B, Menichetti F, Anttila VJ, et al. Extended-pulsed fidaxomicin versus vancomycin for Clostridium difficile infection in patients 60 years and older (EXTEND): a randomised, controlled, open-label, phase 3b/4 trial. The Lancet Infectious diseases 2018;18(3):296–307. [DOI] [PubMed] [Google Scholar]
- 35.Hecht DW, Galang MA, Sambol SP, Osmolski JR, Johnson S, Gerding DN. In Vitro Activities of 15 Antimicrobial Agents against 110 Toxigenic Clostridium difficile Clinical Isolates Collected from 1983 to 2004. Antimicrobial agents and chemotherapy 2007;51(8):2716–2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson S, Schriever C, Patel U, Patel T, Hecht DW, Gerding DN. Rifaximin Redux: treatment of recurrent Clostridium difficile infections with rifaximin immediately post-vancomycin treatment. Anaerobe 2009;15(6):290–291. [DOI] [PubMed] [Google Scholar]
- 37.Garey KW, Ghantoji SS, Shah DN, et al. A randomized, double-blind, placebo-controlled pilot study to assess the ability of rifaximin to prevent recurrent diarrhoea in patients with Clostridium difficile infection. The Journal of antimicrobial chemotherapy 2011;66(12):2850–2855. [DOI] [PubMed] [Google Scholar]
- 38.Mattila E, Arkkila P, Mattila PS, Tarkka E, Tissari P, Anttila VJ. Rifaximin in the treatment of recurrent Clostridium difficile infection. Alimentary pharmacology & therapeutics 2013;37(1):122–128. [DOI] [PubMed] [Google Scholar]
- 39.Salix Pharmaceuticals I. Drug Insert: Reference ID 3469913 In: Administration USFaD, ed.
- 40.Johnson S, Louie TJ, Gerding DN, et al. Vancomycin, metronidazole, or tolevamer for Clostridium difficile infection: results from two multinational, randomized, controlled trials. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2014;59(3):345–354. [DOI] [PubMed] [Google Scholar]
- 41.US National Library of Medicine. clinicaltrials.gov. 2018; https://clinicaltrials.gov/ct2/results?cond=Clostridium+difficile. Accessed January 15th, 2018. [DOI] [PubMed]
- 42.HCUP National Inpatient Sample (NIS). Healthcare Cost and Utilization Project (HCUP). 1993–2014.. www.hcup-us.ahrq.gov/nisoverview.jsp. Accessed January 15th, 2018.
- 43.Kokai-Kun JF, Bristol JA, Setser J, Schlosser M. Nonclinical Safety Assessment of SYN-004: An Oral beta-lactamase for the Protection of the Gut Microbiome From Disruption by Biliary-Excreted, Intravenously Administered Antibiotics. International journal of toxicology 2016;35(3):309–316. [DOI] [PubMed] [Google Scholar]
- 44.Kaleko M, Bristol JA, Hubert S, et al. Development of SYN-004, an oral beta-lactamase treatment to protect the gut microbiome from antibiotic-mediated damage and prevent Clostridium difficile infection. Anaerobe 2016;41:58–67. [DOI] [PubMed] [Google Scholar]
- 45.Roberts T, Kokai-Kun JF, Coughlin O, et al. Tolerability and Pharmacokinetics of SYN-004, an Orally Administered beta-Lactamase for the Prevention of Clostridium difficile-Associated Disease and Antibiotic-Associated Diarrhea, in Two Phase 1 Studies. Clinical drug investigation 2016;36(9):725–734. [DOI] [PubMed] [Google Scholar]
- 46.Kokai-Kun JF, Roberts T, Coughlin O, et al. The Oral beta-Lactamase SYN-004 (Ribaxamase) Degrades Ceftriaxone Excreted into the Intestine in Phase 2a Clinical Studies. Antimicrobial agents and chemotherapy 2017;61(0). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hill C, Guarner F, Reid G, et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nature Reviews Gastroenterology &Amp; Hepatology 2014;11:506. [DOI] [PubMed] [Google Scholar]
- 48.Mills JP, Rao K, Young VB. Probiotics for prevention of Clostridium difficile infection. Current opinion in gastroenterology 2018;34(1):3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maziade PJ, Pereira P, Goldstein EJ. A Decade of Experience in Primary Prevention of Clostridium difficile Infection at a Community Hospital Using the Probiotic Combination Lactobacillus acidophilus CL1285, Lactobacillus casei LBC80R, and Lactobacillus rhamnosus CLR2 (Bio-K+). Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2015;60 Suppl 2:S144–147. [DOI] [PubMed] [Google Scholar]
- 50.Gao XW, Mubasher M, Fang CY, Reifer C, Miller LE. Dose-response efficacy of a proprietary probiotic formula of Lactobacillus acidophilus CL1285 and Lactobacillus casei LBC80R for antibiotic-associated diarrhea and Clostridium difficile-associated diarrhea prophylaxis in adult patients. The American journal of gastroenterology 2010;105(7):1636–1641. [DOI] [PubMed] [Google Scholar]
- 51.Selinger CP, Bell A, Cairns A, Lockett M, Sebastian S, Haslam N. Probiotic VSL#3 prevents antibiotic-associated diarrhoea in a double-blind, randomized, placebo-controlled clinical trial. The Journal of hospital infection 2013;84(2):159–165. [DOI] [PubMed] [Google Scholar]
- 52.DuPont. New Study Results - HOWARU® Restore Formulation. 2012; http://www.danisco.com/about-dupont/news/news-archive/2012/new-study-results-howarur-restore-formulation/. Accessed January 15th, 2018.
- 53.Ouwehand AC, DongLian C, Weijian X, et al. Probiotics reduce symptoms of antibiotic use in a hospital setting: A randomized dose response study. Vaccine 2014;32(4):458–463. [DOI] [PubMed] [Google Scholar]
- 54.Sanchez-Hurtado K, Corretge M, Mutlu E, McIlhagger R, Starr JM, Poxton IR. Systemic antibody response to Clostridium difficile in colonized patients with and without symptoms and matched controls. Journal of medical microbiology 2008;57(Pt 6):717–724. [DOI] [PubMed] [Google Scholar]
- 55.Kyne L, Warny M, Qamar A, Kelly CP. Association between antibody response to toxin A and protection against recurrent Clostridium difficile diarrhoea. Lancet (London, England) 2001;357(9251):189–193. [DOI] [PubMed] [Google Scholar]
- 56.Bezay N, Ayad A, Dubischar K, et al. Safety, immunogenicity and dose response of VLA84, a new vaccine candidate against Clostridium difficile, in healthy volunteers. Vaccine 2016;34(23):2585–2592. [DOI] [PubMed] [Google Scholar]
- 57.Dubischar K, et al. A Phase 2, Dose-Confirmation Immunogenicity and Safety Study of Vla84, A Clostridium difficile Vaccine Candidate, in Adults Aged 50 Years and Older [ASM Microbe 2016 Abstract]. In: 2016.
- 58.Greenberg RN, Marbury TC, Foglia G, Warny M. Phase I dose finding studies of an adjuvanted Clostridium difficile toxoid vaccine. Vaccine 2012;30(13):2245–2249. [DOI] [PubMed] [Google Scholar]
- 59.de Bruyn G, Saleh J, Workman D, et al. Defining the optimal formulation and schedule of a candidate toxoid vaccine against Clostridium difficile infection: A randomized Phase 2 clinical trial. Vaccine 2016;34(19):2170–2178. [DOI] [PubMed] [Google Scholar]
- 60.Sanofi ends development of Clostridium difficile vaccine [press release]. Sanofi.
- 61.Vidunas E, Mathews A, Weaver M, et al. Production and Characterization of Chemically Inactivated Genetically Engineered Clostridium difficile Toxoids. Journal of pharmaceutical sciences 2016;105(7):2032–2041. [DOI] [PubMed] [Google Scholar]
- 62.Donald RGK, Flint M, Kalyan N, et al. A novel approach to generate a recombinant toxoid vaccine against Clostridium difficile. Microbiology 2013;159(Pt 7):1254–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.cdvax. Progress. 2018; http://cdvax.org/progress/. Accessed January 15th, 2018.
- 64.Boone JH, DiPersio JR, Tan MJ, et al. Elevated lactoferrin is associated with moderate to severe Clostridium difficile disease, stool toxin, and 027 infection. European Journal of Clinical Microbiology & Infectious Diseases 2013;32(12):1517–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Otake K, Sato N, Kitaguchi A, et al. The Effect of Lactoferrin and Pepsin-Treated Lactoferrin on IEC-6 Cell Damage Induced by Clostridium Difficile Toxin B. Shock (Augusta, Ga) 2017. [DOI] [PubMed]
- 66.Chilton CH, Crowther GS, Śpiewak K, et al. Potential of lactoferrin to prevent antibiotic-induced Clostridium difficile infection. Journal of Antimicrobial Chemotherapy 2016;71(4):975–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Locher HH, Caspers P, Bruyere T, et al. Investigations of the mode of action and resistance development of cadazolid, a new antibiotic for treatment of Clostridium difficile infections. Antimicrobial agents and chemotherapy 2014;58(2):901–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Baldoni D, Gutierrez M, Timmer W, Dingemanse J. Cadazolid, a novel antibiotic with potent activity against Clostridium difficile: safety, tolerability and pharmacokinetics in healthy subjects following single and multiple oral doses. The Journal of antimicrobial chemotherapy 2014;69(3):706–714. [DOI] [PubMed] [Google Scholar]
- 69.Gehin M, Desnica B, Dingemanse J. Minimal systemic and high faecal exposure to cadazolid in patients with severe Clostridium difficile infection. International journal of antimicrobial agents 2015;46(5):576–581. [DOI] [PubMed] [Google Scholar]
- 70.Louie T, Nord CE, Talbot GH, et al. Multicenter, Double-Blind, Randomized, Phase 2 Study Evaluating the Novel Antibiotic Cadazolid in Patients with Clostridium difficile Infection. Antimicrobial agents and chemotherapy 2015;59(10):6266–6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Maxwell-Scott HG, Goldenberg SD. Existing and investigational therapies for the treatment of Clostridium difficile infection: A focus on narrow spectrum, microbiota-sparing agents. Medecine et maladies infectieuses 2017. [DOI] [PubMed]
- 72.Nayak SU, Griffiss JM, Blumer J, et al. Safety, Tolerability, Systemic Exposure, and Metabolism of CRS3123, a Methionyl-tRNA Synthetase Inhibitor Developed for Treatment of Clostridium difficile, in a Phase 1 Study. Antimicrobial agents and chemotherapy 2017;61(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Citron DM, Warren YA, Tyrrell KL, Merriam V, Goldstein EJ. Comparative in vitro activity of REP3123 against Clostridium difficile and other anaerobic intestinal bacteria. The Journal of antimicrobial chemotherapy 2009;63(5):972–976. [DOI] [PubMed] [Google Scholar]
- 74.Ochsner UA, Bell SJ, O’Leary AL, et al. Inhibitory effect of REP3123 on toxin and spore formation in Clostridium difficile, and in vivo efficacy in a hamster gastrointestinal infection model. The Journal of antimicrobial chemotherapy 2009;63(5):964–971. [DOI] [PubMed] [Google Scholar]
- 75.Leeds JA, Sachdeva M, Mullin S, Dzink-Fox J, LaMarche MJ. Mechanism of Action of and Mechanism of Reduced Susceptibility to the Novel Anti-Clostridium difficile Compound LFF571. Antimicrobial agents and chemotherapy 2012;56(8):4463–4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bhansali SG, Mullane K, Ting LS, et al. Pharmacokinetics of LFF571 and vancomycin in patients with moderate Clostridium difficile infections. Antimicrobial agents and chemotherapy 2015;59(3):1441–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mullane K, Lee C, Bressler A, et al. Multicenter, randomized clinical trial to compare the safety and efficacy of LFF571 and vancomycin for Clostridium difficile infections. Antimicrobial agents and chemotherapy 2015;59(3):1435–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rashid MU, Dalhoff A, Backstrom T, et al. Ecological impact of MCB3837 on the normal human microbiota. International journal of antimicrobial agents 2014;44(2):125–130. [DOI] [PubMed] [Google Scholar]
- 79.Rashid MU, Dalhoff A, Weintraub A, Nord CE. In vitro activity of MCB3681 against Clostridium difficile strains. Anaerobe 2014;28:216–219. [DOI] [PubMed] [Google Scholar]
- 80.Freeman J, Pilling S, Vernon J, Wilcox MH. In Vitro Activities of MCB3681 and Eight Comparators against Clostridium difficile Isolates with Known Ribotypes and Diverse Geographical Spread. Antimicrobial agents and chemotherapy 2017;61(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dalhoff A, Rashid MU, Kapsner T, Panagiotidis G, Weintraub A, Nord CE. Analysis of effects of MCB3681, the antibacterially active substance of prodrug MCB3837, on human resident microflora as proof of principle. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases 2015;21(8):767.e761–764. [DOI] [PubMed] [Google Scholar]
- 82.FDA grants QIDP and Fast Track Designations to MCB3837, Morphochem’s novel intravenous antibacterial to treat C. difficile infections [press release] 2016.
- 83.Hoffman PS, Sisson G, Croxen MA, et al. Antiparasitic Drug Nitazoxanide Inhibits the Pyruvate Oxidoreductases of Helicobacter pylori, Selected Anaerobic Bacteria and Parasites, and Campylobacter jejuni. Antimicrobial agents and chemotherapy 2007;51(3):868–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Product Information: Alinia (R), nitazoxanide tablets and oral suspension.
- 85.Musher DM, Logan N, Bressler AM, Johnson DP, Rossignol JF. Nitazoxanide versus vancomycin in Clostridium difficile infection: a randomized, double-blind study. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2009;48(4):e41–46. [DOI] [PubMed] [Google Scholar]
- 86.Musher DM, Logan N, Hamill RJ, et al. Nitazoxanide for the treatment of Clostridium difficile colitis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2006;43(4):421–427. [DOI] [PubMed] [Google Scholar]
- 87.Drekonja DM. Clostridium difficile infection: current, forgotten and emerging treatment options. Journal of comparative effectiveness research 2014;3(5):547–557. [DOI] [PubMed] [Google Scholar]
- 88.Farver DK, Hedge DD, Lee SC. Ramoplanin: a lipoglycodepsipeptide antibiotic. The Annals of pharmacotherapy 2005;39(5):863–868. [DOI] [PubMed] [Google Scholar]
- 89.Kraus CN, Lyerly MW, Carman RJ. Ambush of Clostridium difficile spores by ramoplanin: activity in an in vitro model. Antimicrobial agents and chemotherapy 2015;59(5):2525–2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Basseres E, Endres BT, Khaleduzzaman M, et al. Impact on toxin production and cell morphology in Clostridium difficile by ridinilazole (SMT19969), a novel treatment for C. difficile infection. The Journal of antimicrobial chemotherapy 2016;71(5):1245–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vickers R, Robinson N, Best E, Echols R, Tillotson G, Wilcox M. A randomised phase 1 study to investigate safety, pharmacokinetics and impact on gut microbiota following single and multiple oral doses in healthy male subjects of SMT19969, a novel agent for Clostridium difficile infections. BMC Infectious Diseases 2015;15:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vickers RJ, Tillotson GS, Nathan R, et al. Efficacy and safety of ridinilazole compared with vancomycin for the treatment of Clostridium difficile infection: a phase 2, randomised, double-blind, active-controlled, non-inferiority study. The Lancet Infectious diseases 2017;17(7):735–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Alam MZ, Wu X, Mascio C, Chesnel L, Hurdle JG. Mode of action and bactericidal properties of surotomycin against growing and nongrowing Clostridium difficile. Antimicrobial agents and chemotherapy 2015;59(9):5165–5170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chesnel L, Sambol S, Gerding D. Treatment of CDAD with oral CB-183 315: Time to recurrence, relapse and reinfection rates compared with vancomycin Vol 182012. [Google Scholar]
- 95.Lee CH, Patino H, Stevens C, et al. Surotomycin versus vancomycin for Clostridium difficile infection: Phase 2, randomized, controlled, double-blind, non-inferiority, multicentre trial. The Journal of antimicrobial chemotherapy 2016;71(10):2964–2971. [DOI] [PubMed] [Google Scholar]
- 96.Boix V, Fedorak RN, Mullane KM, et al. Primary Outcomes From a Phase 3, Randomized, Double-Blind, Active-Controlled Trial of Surotomycin in Subjects With Clostridium difficile Infection. Open Forum Infectious Diseases 2017;4(1):ofw275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Daley P, Louie T, Lutz JE, et al. Surotomycin versus vancomycin in adults with Clostridium difficile infection: primary clinical outcomes from the second pivotal, randomized, double-blind, Phase 3 trial. Journal of Antimicrobial Chemotherapy 2017;72(12):3462–3470. [DOI] [PubMed] [Google Scholar]
- 98.Wyeth Pharmaceuticals Inc. Drug Insert: Reference ID 3379756 In: Administration USFaD, ed2013.
- 99.Product Information: TYGACIL(R) intravenous injection, tigecycline intravenous injection Wyeth Pharmaceuticals Inc (per manufacturer), Philadelphia, PA, 2016. [Google Scholar]
- 100.Tasina E, Haidich AB, Kokkali S, Arvanitidou M. Efficacy and safety of tigecycline for the treatment of infectious diseases: a meta-analysis. The Lancet Infectious diseases 2011;11(11):834–844. [DOI] [PubMed] [Google Scholar]
- 101.Herpers BL, Vlaminckx B, Burkhardt O, et al. Intravenous tigecycline as adjunctive or alternative therapy for severe refractory Clostridium difficile infection. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2009;48(12):1732–1735. [DOI] [PubMed] [Google Scholar]
- 102.Bishop EJ, Tiruvoipati R, Metcalfe J, Marshall C, Botha J, Kelley PG. The Outcome of Patients with Severe and Severe-Complicated Clostridium difficile Infection Treated with Tigecycline Combination Therapy: A Retrospective Observational Study. Internal medicine journal 2018. [DOI] [PubMed]
- 103.Sturino JM, Pokusaeva K, Carpenter R. Effective Sequestration of Clostridium difficile Protein Toxins by Calcium Aluminosilicate. Antimicrobial agents and chemotherapy 2015;59(12):7178–7183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Louie TJ, Peppe J, Watt CK, et al. Tolevamer, a novel nonantibiotic polymer, compared with vancomycin in the treatment of mild to moderately severe Clostridium difficile-associated diarrhea. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2006;43(4):411–420. [DOI] [PubMed] [Google Scholar]
- 105.Kurtz CB, Cannon EP, Brezzani A, et al. GT160–246, a Toxin Binding Polymer for Treatment of Clostridium difficile Colitis. Antimicrobial agents and chemotherapy 2001;45(8):2340–2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rodrigues RS, Oliveira RAC, Li Y, et al. Intestinal Epithelial Restitution After TcdB Challenge and Recovery From Clostridium difficile Infection in Mice With Alanyl-Glutamine Treatment. The Journal of infectious diseases 2013;207(10):1505–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Santos AAQA Braga-Neto MB, Oliveira MR, et al. Glutamine and Alanyl-Glutamine Increase RhoA Expression and Reduce Clostridium difficile Toxin-A-Induced Intestinal Epithelial Cell Damage. BioMed Research International 2013;2013:152052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Warren CA, Calabrese GM, Li Y, et al. Effects of adenosine A(2A )receptor activation and alanyl-glutamine in Clostridium difficile toxin-induced ileitis in rabbits and cecitis in mice. BMC Infectious Diseases 2012;12:13–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Immuron Approved to Commence Clinical Study in C.Difficile Infection (CDI) [press release] 2017.
- 110.Braintree Laboratories I. NuLytely 181 Web Label In: 2013.
- 111.Olsen MA, Yan Y, Reske KA, Zilberberg MD, Dubberke ER. Recurrent Clostridium difficile infection is associated with increased mortality. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases 2015;21(2):164–170. [DOI] [PubMed] [Google Scholar]
- 112.Zilberberg MD, Reske K, Olsen M, Yan Y, Dubberke ER. Risk factors for recurrent Clostridium difficile infection (CDI) hospitalization among hospitalized patients with an initial CDI episode: a retrospective cohort study. BMC Infectious Diseases 2014;14(1):306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wilcox MH, Gerding DN, Poxton IR, et al. Bezlotoxumab for Prevention of Recurrent Clostridium difficile Infection. The New England journal of medicine 2017;376(4):305–317. [DOI] [PubMed] [Google Scholar]
- 114.Prabhu VS, Dubberke ER, Dorr MB, et al. Cost-effectiveness of Bezlotoxumab Compared With Placebo for the Prevention of Recurrent Clostridium difficile Infection. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2018;66(3):355–362. [DOI] [PubMed] [Google Scholar]
- 115.Dieterle MG, Young VB. Reducing Recurrence of C. difficile Infection. Cell 2017;169(3):375. [DOI] [PubMed] [Google Scholar]
- 116.Product Information: ZINPLAVA(TM) IV injection, bezlotoxumab IV injection. Merck Sharp & Dohme Corp (per manufacturer), Whitehouse Station, NJ, 2016. [Google Scholar]
- 117.Artiushin S, Timoney JF, Fettinger M, Fallon L, Rathgeber R. Immunisation of mares with binding domains of toxins A and B of Clostridium difficile elicits serum and colostral antibodies that block toxin binding. Equine veterinary journal 2013;45(4):476–480. [DOI] [PubMed] [Google Scholar]
- 118.Hutton ML, Cunningham BA, Mackin KE, et al. Bovine antibodies targeting primary and recurrent Clostridium difficile disease are a potent antibiotic alternative. Scientific Reports 2017;7:3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.van Dissel JT, de Groot N, Hensgens CM, et al. Bovine antibody-enriched whey to aid in the prevention of a relapse of Clostridium difficile-associated diarrhoea: preclinical and preliminary clinical data. Journal of medical microbiology 2005;54(Pt 2):197–205. [DOI] [PubMed] [Google Scholar]
- 120.Chang JY, Antonopoulos DA, Kalra A, et al. Decreased diversity of the fecal Microbiome in recurrent Clostridium difficile-associated diarrhea. The Journal of infectious diseases 2008;197(3):435–438. [DOI] [PubMed] [Google Scholar]
- 121.Seres Therapeutics I. Clostridium difficile Infection (CDI). http://www.serestherapeutics.com/pipeline/ser-109. Accessed January 15th, 2018.
- 122.Khanna S, Pardi DS, Kelly CR, et al. A Novel Microbiome Therapeutic Increases Gut Microbial Diversity and Prevents Recurrent Clostridium difficile Infection. The Journal of infectious diseases 2016;214(2):173–181. [DOI] [PubMed] [Google Scholar]
- 123.Martin J, Wilcox M. New and emerging therapies for Clostridium difficile infection. Current opinion in infectious diseases 2016;29(6):546–554. [DOI] [PubMed] [Google Scholar]
- 124.Isa K, Oka K, Beauchamp N, et al. Safety assessment of the Clostridium butyricum MIYAIRI 588(R) probiotic strain including evaluation of antimicrobial sensitivity and presence of Clostridium toxin genes in vitro and teratogenicity in vivo. Human & experimental toxicology 2016;35(8):818–832. [DOI] [PubMed] [Google Scholar]
- 125.Osel. Clinical Pipeline. http://oselinc.com/home/clinical-pipeline/. Accessed January 15th, 2018.
- 126.Orenstein R, Dubberke E, Hardi R, et al. Safety and Durability of RBX2660 (Microbiota Suspension) for Recurrent Clostridium difficile Infection: Results of the PUNCH CD Study. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2016;62(5):596–602. [DOI] [PubMed] [Google Scholar]
- 127.Rebiotix Reports Positive Top Line Data from Open-Label Phase 2 Trial of RBX2660 in Recurrent Clostridium difficile [press release] 2017.
- 128.Dubberke Er L, et al. Efficacy and safety of RBX2660 for the prevention of recurrent Clostridium difficile Infection: Results of the PUNCH CD 2 Trial. In. Abstract Presented at ID Week 2016: IDSA; 2016.
- 129.Rebiotix I. RBX7455 Oral Clostridium difficile Prevention. http://www.rebiotix.com/clinical-trials/rbx7455-oral-c-diff-prevention/. Accessed January 15th, 2018.
- 130.Rebiotix Announces Expansion of Phase 1 Trial of the Company’s Oral Capsule Microbiota Product, RBX7455, Following Successful Completion of Initial Study Arms [press release] 2017.
- 131.Healio. FDA fast-tracks Finch’s microbiome capsule for C. difficile. 2018; https://www.healio.com/gastroenterology/infection/news/online/%7Bba5c32ca-10fc-4592-aee9-dc669b59d57b%7D/fda-fast-tracks-finchs-microbiome-capsule-for-c-difficile. Accessed January 15th, 2018.
- 132.Staley C, Hamilton MJ, Vaughn BP, et al. Successful Resolution of Recurrent Clostridium difficile Infection using Freeze-Dried, Encapsulated Fecal Microbiota; Pragmatic Cohort Study. The American journal of gastroenterology 2017;112(6):940–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Villano SA, Seiberling M, Tatarowicz W, Monnot-Chase E, Gerding DN. Evaluation of an oral suspension of VP20621, spores of nontoxigenic Clostridium difficile strain M3, in healthy subjects. Antimicrobial agents and chemotherapy 2012;56(10):5224–5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gerding DN, Meyer T, Lee C, et al. Administration of spores of nontoxigenic Clostridium difficile strain M3 for prevention of recurrent C. difficile infection: a randomized clinical trial. Jama 2015;313(17):1719–1727. [DOI] [PubMed] [Google Scholar]
- 135.Baggs J, Jernigan JA, Halpin AL, Epstein L, Hatfield KM, McDonald LC. Risk of Subsequent Sepsis Within 90 Days After a Hospital Stay by Type of Antibiotic Exposure. Clinical Infectious Diseases 2018;66(7):1004–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Oh J, Makar M, Fusco C, et al. A Generalizable, Data-Driven Approach to Predict Daily Risk of Clostridium difficile Infection at Two Large Academic Health Centers Vol 392018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Johnson S, Gerding DN. Fidaxomicin “Chaser” Regimen Following Vancomycin for Patients With Multiple Clostridium difficile Recurrences. Clinical Infectious Diseases 2013;56(2):309–310. [DOI] [PubMed] [Google Scholar]