Abstract
Introduction:
We evaluated the reliability of measuring muscle thickness with ultrasound in limbs and diaphragms of critically-ill children and determined the sensitivity of these measures to quantify muscle atrophy over time.
Methods:
An expert and trained novice sonographers prospectively measured limb and diaphragm muscle thickness in 33 critically-ill children.
Results:
Expert and novice intra-rater and inter-rater reliability were similar. Intraclass correlations (ICC) and coefficients of variation (CoV) were better in limbs (ICC>0.9, CoV: 3.57% to 5.40%) than diaphragm (ICC>0.8, CoV: novice, 11.88%; expert, 12.28%). Mean relative difference in all muscles was small (1–8%). Limits of agreement of the relative difference were smaller in limb (<13–18%) than diaphragm muscles (<38%).
Discussion:
Muscle thickness is reliably measured using ultrasound by trained examiners in critically-ill children. Our approach detects atrophy >13% in limb and >38% in diaphragm muscles. The smaller detectable change in limb muscles is likely due to their greater thickness.
Keywords: Ultrasound, muscle thickness, children, ICU-acquired weakness, diaphragm, reliability
INTRODUCTION
Intensive care unit acquired weakness (ICU-AW) increases mortality and morbidity in both adults and children 1–3. Identifying ICU-AW in the acute setting is challenging as patients are critically ill. A marker of ICU-AW, muscle atrophy, can be detected using serial ultrasound measurements of muscle thickness performed at the bedside 4. In critically ill adults, muscle atrophy occurs early in the ICU stay, within the first 7 to 10 days 4, 5, and is associated with increased morbidity, mortality, and longer hospital stay 2, 3. Muscle atrophy also occurs in critically ill children 6.
Quadriceps femoris muscles in critically ill adults atrophy by ~30% in the first week of illness 4. Although prior studies in healthy children and children with neuromuscular disease have shown that muscle ultrasound is a reliable technique for measuring limb muscle thickness 7, 8, its use in detecting changes in muscle thickness in critically-ill children over time has only recently been assessed. Highly reliable ultrasound measurement protocols are required to detect levels of atrophy in children similar to those seen in adults because children’s muscles are smaller than those of adults and thus small measurement variability may obscure changes in muscle thickness over time. A prior study of ultrasound reliability to measure quadriceps femoris muscle thickness in critically ill children concluded that a single transverse ultrasound measurement of the quadriceps femoris was insufficiently reliable to detect the anticipated atrophy (<30%) in the muscle thickness 9. A subsequent study found that ultrasound measurement of quadriceps femoris thickness was sufficiently reliable to detect a 5% change in muscle thickness if two repeated measurements obtained from both transverse and longitudinal planes were averaged 6. No studies have assessed the reliability of ultrasound measurements of muscle thickness in other skeletal muscles or in diaphragms of critically-ill intubated children. In this study, we assessed the reliability of ultrasound muscle thickness measurement in upper and lower limb muscles and in the diaphragm of critically-ill children,and compared the reliability of an expert examiner to trained novice examiners.
METHODS
Subjects:
We conducted a prospective observational study with approval from the Washington University in St. Louis School of Medicine institutional research ethics board. Participation required written informed consent from the patients’ legal representatives and, if able, assent from the participant. Between June 2015 and April 2016, we screened all critically ill full-term neonates or children less than 18 years of age who were admitted to the pediatric ICU and intubated, regardless of reason for admission. Children were eligible if they had been intubated for less than 72 hours, were expected to remain intubated for at least 2 days, and were either independently ambulatory or had normal gross motor development (if aged less than 15 months) prior to their hospitalization. Limbs with direct trauma to the imaged structures or other gross anatomical abnormalities that might prevent adequate visualization of the studied muscles were excluded. We also excluded children who had known diagnoses of neuromuscular diseases or who were pregnant. Physical data, including age, sex, height, and weight were obtained for all patients.
Ultrasound measurement protocol:
Ultrasound examinations were performed with a portable ultrasound imaging system (SonoSite Edge II, FujiFilm SonoSite Inc., Bothell, WA) with a 13–6 MHz linear probe. Our ultrasound measurement protocol examined 4 muscles where possible for each child: unilateral biceps brachii/brachialis, quadriceps femoris, tibialis anterior, and the right diaphragm (Figure 1). If the right upper and lower limb could not be imaged because of an overlying structure (eg. intravenous line, external fixator), the contralateral side was imaged instead. The muscles were measured in the supine position, holding arms and legs extended with muscles relaxed. Arms were supinated, knees extended and ankles placed in neutral position. Transverse ultrasound images of the limb muscles were obtained at pre-defined anatomical locations (Suppl Table 1) corresponding to maximal muscle diameter at the muscle belly, and the distance from the bony landmarks recorded for future reference. A surgical pen was used to mark the location for transducer placement on the skin. The transducer was placed perpendicular to the skin with a liberal amount of contact gel, and minimal pressure of the transducer was exerted on the skin to ensure no compression of the muscle. Oblique scanning was avoided by altering the angle of the transducer to achieve the best deep fascia or bone echo. For the diaphragm, ultrasound was performed on the right diaphragm in the intercostal window at the anterior axillary line in cross-sectional view/oblique sagittal plane, to obtain a parallel-appearing image of the superficial and deep aspects of the diaphragm. This was obtained one or two intercostal spaces caudal to the inferior pleural margin.
Figure 1: Sample images of patient 20.
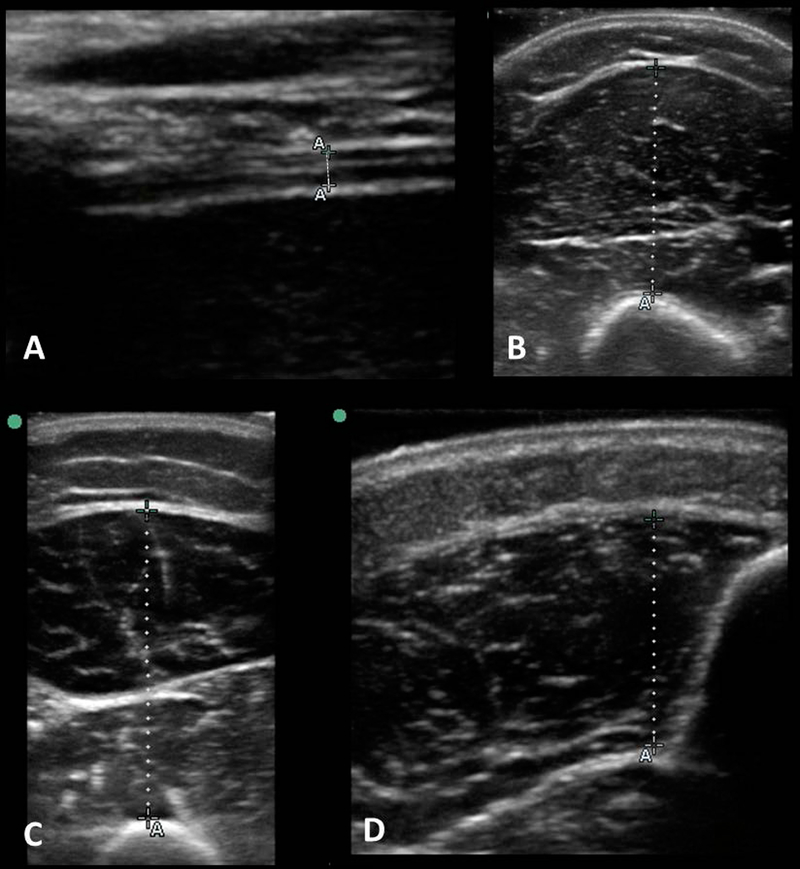
Sample images of patient 20, a 6 year 8 month old intubated male, taken using study ultrasound measurement protocol in 4 muscles, with dotted lines depicting the measured muscle thickness. A: Right Diaphragm: 0.14cm, B: Biceps/brachii: 1.93cm, C: Quadriceps Femoris: 3.15cm, D: Tibialis Anterior: 1.31cm
Diaphragm thickness was estimated as the vertical distance between the pleural and peritoneal layers at the end of expiration, where the respiratory cycle was determined clinically and images timed to match with end-expiration. We used a cine-loop capture, when needed, to scroll through images to capture the minimal diaphragm thickness. An experienced sonographer (CMZ, or ‘trainer’) trained novice sonographers (MS, AD, KN, or ‘trainees’) on the first 10 patients. The novice-acquired data from the first ten patients was for training purposes only and was not included in the study. Training was conducted at the bedside on the ten patients over 3 months. During this time, all evaluators demonstrated competency in measuring muscle thickness. The expert sonographer assessed competency during training at the bedside.
At the start of the study, novice sonographers had no prior experience in muscle imaging or muscle thickness measurements, and only limited exposure to sonography for other indications (ICU procedures, nerve imaging in a few patients).
Each examiner performed 3 consecutive ultrasound measurements of a single transverse plane at the marked anatomic site. During training, we noticed improved reliability between examiners when transducer placement location along the limb was marked by a single examiner, rather than measured independently by each examiner. Thus, for all measurements, we measured and marked a single location on each limb/torso which was used for transducer placement for each subsequent measurement and examiner. The initial marking was placed by the first examiner, regardless of whether expert or novice sonographer. Between measurements, subjects were allowed to move or be repositioned. We standardized our imaging and positioning to capture the muscle so it was positioned directly over the underlying bone or fascia, without angulation, so that the maximal measured thickness extended perpendicular to, and directly overlying, the bony/deep fascia reflection. All images were obtained by waiting for moments when the patient was maximally-relaxed, regardless of the level of consciousness. Not all muscles could be imaged for all patients due to overlying structures (eg. intravenous lines/chest tube).
Intra-observer study:
For each muscle, we obtained three consecutive measures of muscle thickness for each muscle at a single time point. We selected the largest and smallest of the 3 measurements for intra-rater variability analysis. We analyzed and compared the intra-rater reliability of the trainer to the trained novice sonographers (trainees).
Inter-observer study:
Two sonographers performed three consecutive measurements of each muscle one after each other. The time between measurements performed by each sonographer ranged from immediately after to a maximal interval of 12 hours. We averaged the 3 measurements performed by each examiner and analyzed the inter-rater differences between the averaged measures. Not all patients were examined by two examiners due to time and patient care restraints.
Statistics:
Statistical analysis was performed with Microsoft Excel version 14.6.8. A p-value of < 0.05 was considered statistically significant. We assessed reliability by calculating the intraclass correlation coefficient (ICC) using the model for 2-way mixed effects, the coefficients of variation of repeated measures (CoV), and by analysis of Bland-Altman plots. CoV of the repeated measurements was calculated as: (the standard deviation of the inter-rater or intra-rater differences of muscle thickness/the mean thickness)*100% 10. The Intraclass Correlation Coefficient (ICC) was used to determine the degree of agreement as follows: less than 0.21 (poor), 0.21–0.40 (fair), 0.41–0.6 (moderate), 0.61–0.80 (good) and 0.81–1.00 (very good) 11.
As an indicator of the range of error, we calculated the mean and standard deviation of absolute differences between muscle thickness measurements and calculated the mean relative differences (MRD) as the difference between repeated measures/mean muscle thickness * 100%. We defined the limits of agreement as the mean difference ±1.96*SD of the differences between measurements. A single outlier in the inter-rater measures of the quadriceps femoris skewed the limits of agreement analysis and we report this calculation with and without this single data point.
We used Pearson’s correlation coefficient (r) to analyze relationships between the CoV of repeated measurements to body weight and between muscle thickness to age and body weight.
RESULTS
Patient characteristics
In total, we measured muscle thickness in 33 children. Demographic data is provided in Table 1. The main reasons for pediatric ICU admission were respiratory failure, central nervous system pathologies, and trauma.
Table 1:
Demographic data of recruited 33 critically-ill children
| Age (years) | 6.2 (1 week to 17 years) |
| Female [N (%)] | 15 (45) |
| Weight (kg) | 16.0 (3.0 to 70.0) |
| Height (m) | 1.09 (0.49 to 1.66) |
| Diagnoses [N (%)] | |
| Trauma | 5 (15.1) |
| Sepsis | 3 (9.1) |
| Acute abdomen | 2 (6.1) |
| Central nervous system | 8 (24.2) |
| pathologiesAirway/respiratory pathologies | 11 (33.3) |
| Hematology/oncology pathologies | 3 (9.1) |
| Others (hypotension of unknown cause) | 1 (3.0) |
Results are presented as median (range) or as n(%)
Mean (standard deviation) muscle thickness was: quadriceps femoris (2.19±0.89cm), biceps brachii/brachialis (1.56±0.63cm), tibialis anterior (1.16±0.49cm), diaphragm (0.15±0.04cm). Thickness of the limb muscles (biceps brachii/brachialis, tibialis anterior, quadriceps femoris) positively correlated with age and body weight (all p< 0.05). Diaphragm thickness did not correlate with either age or body weight (p>0.05).
Intra-rater reliability of single repeated measures
The expert examiner examined 28 patients. The novice examiners examined 15 patients. Intra-rater reliability was very good for both experienced and novice sonographers (Table 2) and was slightly higher for the limb muscles than the diaphragm. Coefficient of variation was similar for the expert and novice examiners (Table 2) and was lower in the limb muscles than in the diaphragm.
Table 2:
Intra and Inter-rater muscle thickness reliability
| Muscles | Intra-rater | Inter-rater | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Expert | Novice | ||||||||
| N | ICC | CoV (%) | N | ICC | CoV (%) | N | ICC | CoV (%) | |
| Biceps Brachii/Brachialis | 27 | 0.99 | 3.57 | 17 | 0.99 | 4.57 | 15 | 0.98 | 5.26 |
| Quadriceps Femoris | 28 | 0.99 | 3.83 | 17 | 0.99 | 3.79 | 15 | 0.93 | 7.91 (4.81)* |
| Tibialis Anterior | 28 | 0.99 | 4.50 | 16 | 0.99 | 5.40 | 14 | 0.99 | 3.45 |
| Diaphragm | 27 | 0.82 | 12.28 | 17 | 0.84 | 11.88 | 15 | 0.88 | 10.10 |
one outlier removed
N, number of patients assessed; ICC, intraclass correlation coefficient; CoV, coefficient of variation
Intra-rater differences for both the experienced sonographer and the novice sonographers were small and without skew on Bland-Altman plot analysis (Figure 2 and Table 3).
Figure 2: Intra-rater Bland-Altman plots for expert and novice examiner.
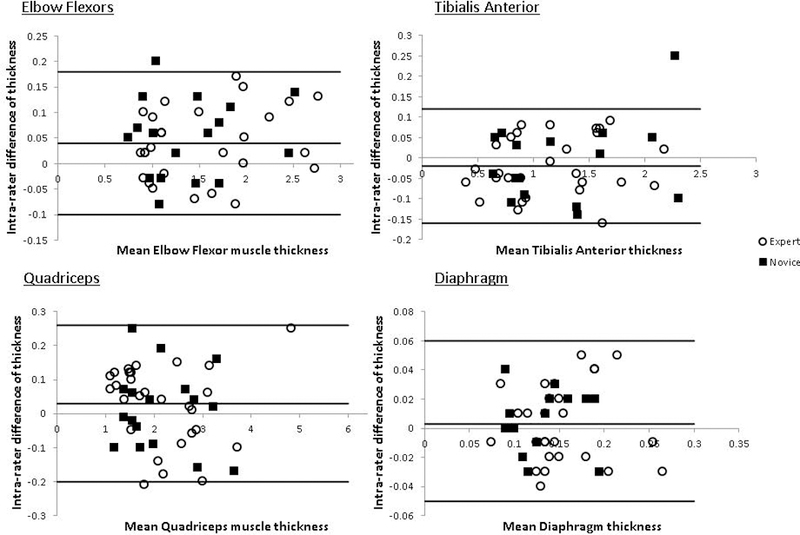
Bland-Altman analysis plots for agreement between the difference between repeated measures (cm) and average muscle thickness (cm). The x-axis shows the average muscle thickness in cm. The y-axis shows the absolute difference between the largest and smallest of the 3-intra-rater repeated measurements in cm. The horizontal lines parallel to the x-axis represent the absolute mean difference and limits of agreement, as defined by the mean±1.96SD of the differences between measurements.
Table 3:
Intra- rater mean and relative mean differences
Expert examiner
| Muscle | N | Mean (SD) muscle thickness (cm) | Mean (SD) Difference (cm) | Lower to Upper Limits (cm) | Mean (SD) Relative difference (%) | Lower to Upper Limits (%) of Mean Relative Difference | |
|---|---|---|---|---|---|---|---|
| Biceps brachii/brachialis | 27 | 1.56 (0.63) | 0.04 (0.07) | −0.10 to 0.18 | 2.40 (4.70) | −6.81 to 11.61 | |
| Quadriceps Femoris | 28 | 2.19 (0.89) | 0.03 (0.12) | −0.20 to 0.26 | 1.98 (5.77) | −9.33 to 13.29 | |
| Tibialis Anterior | 28 | 1.16 (0.49) | −0.02 (0.07) | −0.16 to 0.12 | −2.90 (7.58) | −17.76 to 11.96 | |
| Diaphragm | 27 | 0.15 (0.04) | 0.003 (0.03) | −0.05 to 0.06 | 1.60 (17.50) | −32.70 to 35.90 | |
Inter-rater reliability of averaged measures
Two raters assessed a total of 15 patients. In 13 patients, both an expert and novice sonographer performed the exams. In two patients, the novice sonographers performed the repeated examinations. The inter-rater reliability (Table 2) was slightly higher in the limb muscles than the diaphragm. Similarly, coefficient of variation (Table 2) was slightly lower in limb muscles than in the diaphragm. A single outlier in the inter-rater measures of the quadriceps femoris unduly skewed the variability (CoV for the quadriceps femoris improved to 4.81% following removal of a single outlying value). Inter-rater differences were small and without skew on analysis of Bland-Altman plots (Table 4). The outlier was a full-term infant, who had been more active on one exam than the other, although the measurements were all taken at points where the patient was maximally-relaxed and not moving.
Table 4:
Inter- rater mean and relative mean differences
| Muscle | N | Mean (SD) muscle thickness (cm) | Mean (SD) Difference (cm) | Lower to Upper Limits (cm) | Mean (SD) Relative difference (%) | Lower to Upper Limits (%) of Mean Relative Difference |
|---|---|---|---|---|---|---|
| Biceps brachii/brachialis | 15 | 1.47 (0.57) | −0.03 (0.11) | −0.24 to 0.18 | −1.34 (5.91) | −12.92 to 10.24 |
| Quadriceps Femoris | 14* | 2.14 (0.65) | 0.05 (0.14) | −0.22 to 0.32 | 2.08 (5.64) | −8.97 to 13.13 |
| Tibialis Anterior | 14 | 1.29 (0.55) | 0.02 (0.06) | −0.10 to 0.14 | −1.12 (6.06) | −13.00 to 10.76 |
| Diaphragm | 15 | 0.13 (0.03) | −0.01 (0.02) | −0.04 to 0.02 | −8.35 (11.99) | −31.85 to 15.15 |
one outlier removed
N, number of patients assessed; SD, standard deviation
Muscle thickness reliability and weight
Only the expert intra-rater CoV (%) in the quadriceps femoris showed a relation with patient weight (r=−0.5, p=0.007) (Suppl Fig 1). There was no relationship between the inter-rater CoV (%) and weight for the quadriceps femoris (r=0.35, p=0.21). There was no relationship between either intra- or inter-rater CoV and the biceps brachii/brachialis, tibialis anterior, or diaphragm (p>0.05).
Limits of Agreement Analysis
Expert intra-rater and inter-rater muscle thickness measures showed similar limits of agreement (Table 3 and 4). The mean difference in all muscles was small (≤0.05cm). The limits of agreement between raters was widest in the thickest muscle, the quadriceps femoris, and was narrowest in the thinnest muscle, the diaphragm. The mean relative difference varied between 1–8% and was smaller in the thicker skeletal muscles than in the thinner diaphragm. Similarly, the limits of agreement of the relative difference was smaller in the skeletal muscles (<13–18%) than in the diaphragm (< 38%). Expert and novice intra-rater limits of agreement showed similar results (Table 3).
DISCUSSION
Serial ultrasound examinations of the muscle can identify muscle atrophy and could be used in the acute setting to identify children at risk of developing ICU-AW. One challenge identified in using ultrasound to measure muscle thickness is that in small muscles, as encountered in young children, measurement variability could obscure a relative change in muscle thickness. Prior studies in critically-ill children have shown that reliability of quadriceps femoris muscle thickness measures using ultrasound differs depending on the number of images acquired and the orientation of the probe to the muscle 6, 9 Using our technique, we were able to repeat measurements with ≤ 13% differences between measurements in the quadriceps femoris, biceps brachii/brachialis, and tibials anterior and ≤ 38% differences between measurements in the thinner diaphragm muscle. Our study differs from prior studies in that we imaged multiple muscles rather than only the quadriceps, and evaluated three transverse muscle images rather than one (>30% change) 9 or an average of four measures, two from transverse and two from longitudinal planes (>5% detectable change) 6 from the quadriceps femoris only. It is possible that incorporating both transverse and longitudinal views is more reliable as it is less sensitive to small changes in probe-positioning. Similar to the study by Valla, our methodology required that the location for the transducer placement was marked once on the muscle rather than re-identifying the location for transducer placement between assessments 6. This has ramifications for longitudinal studies, especially if conducted in an outpatient setting, which would require well maintained skin markers for optimizing reliable transducer placement. For our study, in the event that markings were inadvertently washed off, the measured distance of the initial skin marker placement from bony landmarks was recorded and re-used for subsequent measurements.
Our study also shows that it is more difficult to detect a small relative difference in the diaphragm, a much thinner muscle than the skeletal muscles we examined. In a previous study on healthy children, limits of agreement for inter-observer variability for right diaphragmatic thickness was −0.20 to 0.07, and −0.17 to 0.10 for intra-observer variability 12. Despite achieving very small differences between repeated measures in the diaphragm muscle (−0.05 to 0.06cm) in our study, when evaluated as a percentage of muscle thickness, this small variability still resulted in −32 to 38% limits of agreement. Even without operator-dependent variability, the smallest measurable distance of most sonographic machines is 0.01 cm 13, which, in a 0.15cm thick diaphragm, is ~7% of the measurement in the diaphragm.
In previous studies, a significant decrease of ~10 to 13% in muscle thickness of the quadriceps femoris was detected in mechanically-ventilated children 6. In one study, the decrease of end-expiratory diaphragm thickness in mechanically-ventilated children was reported as 13.8%, with an average daily atrophy rate of −3.4% 14, while another study reported a 8.8% decrease in the first two days of intubation, with an average reduction in thickness of 0.68% per day thereafter 15. In ventilated adults, an average decrease in diaphragmatic thickness of 6% per day has been reported 16. Ideally, muscle ultrasound should therefore be reliable enough to detect a relative decrease of muscle thickness of at least 10% in the quadriceps femoris and the diaphragm. The results from our study would suggest that the use of ultrasound may be sufficiently reliable to detect a significant decrease in thickness of the quadriceps femoris, but not the diaphragm.
Muscle thickness reliability is similar in children of different weights. We found that for most measures, the CoV of repeated muscle thickness measures did not vary with weight. Only the expert intra-rater quadriceps femoris muscle CoV varied with weight, and this was mostly due to higher CoV in the smallest children, similar to the prior study by Valla et al 6. Our CoV ranged from 3.5 – 10%, similar to a prior study of ultrasound measured muscle and subcutaneous fat thickness in preterm infants 17.
Skeletal muscle but not diaphragm thickness increased with age and body weight in the children in our study. We found that skeletal muscle thickness increases with body weight, similar to other studies in critically-ill 6 and healthy children 7. Unlike skeletal muscle, diaphragm thickness in our study did not correlate with age or body weight. The average diaphragm thickness in our study (0.15cm) was smaller than in studies of critically ill adults (0.22cm) 18 and healthy adults (0.33cm) 19. It is unclear why the diaphragm would be thinner in children than adults given that we did not find a correlation between diaphragm thickness and weight or age. One reason may be that we only studied intubated children, which may affect diaphragm thickness. Although we modelled our protocol based on prior studies, we may have obtained measurements from slightly different locations than those studies. Another study on intubated children found an initial end-expiratory diaphragm thickness of 0.18 to 0.25 cm 14. A study in healthy, non-intubated children from a much larger study (n=84), demonstrated a correlation between anthropormorphic data, including weight, with diaphragmatic thickness in children < 2 years of age 12.
Our study suggests that trained examiners can sufficiently perform muscle ultrasound assessments even in challenging clinical environments such as the pediatric ICU. We found good intra-class correlation for expert and novice intra-rater measurements for limb muscles (>0.9), similar to prior studies in critically-ill adults 13, 20 and children 6, 9. This is also similar to previous studies in both healthy and diseased children and adults 6, 8, 18. Novice and expert intra-rater intra-class correlation for the diaphragm was also good, but was not quite as high as in skeletal muscle, similar to a prior study in critically-ill adults 18.
Our study had several limitations. Firstly, despite marking sonographic sites with sterile skin marker, these marks occasionally washed off with time. However, we were still able to achieve good reliability by recording the surface measurements from fixed landmarks for the site used for the initial measurement, and using this to approximate the same site for repeat measurement. Secondly, changes of muscle echo-intensity, either due to acute or chronic non-neuromuscular disorders, could render the study technically challenging, due to the difficulty in visualizing the bone echo for an accurate assessment of muscle thickness. Thirdly, some of our patients were not sedated or were actively moving when undergoing the ultrasound study. The increase in muscle tone with movement or increased level of alertness would affect thickness, although sonographers were trained to take the measurements during periods of relaxation in the awake and moving patient. We postulated that this was the cause of the outlier in the inter-rater measures of the quadriceps. Lastly, we excluded children with known diagnoses of neuromuscular diseases, non-ambulatory children and children with significant gross global motor delay, and did not have extremely obese patients in our study cohort. Our results may therefore not be applicable to this patient population.
Our results show that muscle thickness can be reliably measured using ultrasound by trained examiners in children in the ICU. We achieved detectable changes as small as 13% in larger limb muscles and 38% in the diaphragm. Our study highlights the need for a stringent ultrasound protocol if ultrasound is used to detect small changes in muscle thickness over time in the pediatric ICU. It suggests that, for future studies, the reliability of muscle thickness measurements in the quadriceps femoris can be further improved (>5%) by averaging repeated measures obtained from both transverse and longitudinal orientations. To achieve these degrees of reliability, well-marked locations for repeated transducer placement are required. While the quadriceps femoris has been traditionally used for ultrasound studies on muscle atrophy, the biceps brachii/brachialis and tibialis anterior can be similarly reliably measured, although these thinner muscles yielded slightly higher mean relative differences between measurements. Longitudinal studies are required to determine if different limb muscles have different changes in muscle thickness over time in ICU-acquired weakness. In addition, while muscle thickness measurement quantitatively evaluates for muscle atrophy in ICU-acquired weakness, quantitative ultrasound can assess for changes in echo-intensity with increase in fat and fibrous tissue 21, and its use can be investigated in conjunction with muscle thickness measurements for future protocols.
This highlights the need for a stringent ultrasound protocol if ultrasound is used to detect small changes in muscle thickness over time in the pediatric ICU.
Supplementary Material
Novice examiner
| Muscle | N | Mean (SD) muscle thickness (cm) | Mean (SD) Difference (cm) | Lower to Upper Limits (cm) | Mean (SD) Relative difference (%) | Lower to Upper Limits (%) of Mean Relative Difference | |
|---|---|---|---|---|---|---|---|
| Biceps brachii/brachialis | 17 | 1.40 (0.53) | 0.05 (0.08) | −0.10 to 0.20 | 3.96 (6.53) | −8.84 to 16.76 | |
| Quadriceps Femoris | 17 | 2.15 (0.78) | 0.01 (0.12) | −0.22 to 0.24 | 0.71 (5.90) | −10.84 to 12.26 | |
| Tibialis Anterior | 16 | 1.26 (0.57) | −0.01 (0.10) | −0.20 to 0.18 | −1.48 (7.24) | −15.67 to 12.72 | |
| Diaphragm | 17 | 0.13 (0.03) | 0.004 (0.02) | −0.04 to 0.05 | 2.83 (17.87) | −32.19 to 37.86 | |
N, number of patients assessed; SD, standard deviation
Acknowledgments
Part of the material contained within was presented at the 2016 American Association of Neuromuscular and Electrodiagnostic Medicine (AANEM) 63rd annual meeting in New Orleans, USA.
Study funding: Research reported in this publication was supported by the Washington University Institute of Clinical and Translational Sciences grant UL1 TR000448 from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official view of the NIH.
List of abbreviations:
- ICC
intraclass correlation
- CoV
coefficient of variation
- ICU
Intensive Care Unit
- ICU
AW Intensive Care Unit acquired weakness
- MRD
mean relative difference
Footnotes
Ethical Publication Statement: We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Disclosure of Conflicts of Interest: None of the authors has any conflict of interest to disclose.
REFERENCES
- 1.Moisey LL, Mourtzakis M, Cotton BA, Premji T, Heyland DK, Wade CE, et al. Skeletal muscle predicts ventilator-free days, ICU-free days, and mortality in elderly ICU patients. Crit Care 2013;17(5):R206. doi: 10.1186/cc12901. PubMed PMID: 24050662; PubMed Central PMCID: PMC4055977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weijs PJ, Looijaard WG, Dekker IM, Stapel SN, Girbes AR, Oudemans-van Straaten HM, et al. Low skeletal muscle area is a risk factor for mortality in mechanically ventilated critically ill patients. Crit Care 2014;18(2):R12. doi: 10.1186/cc13189. PubMed PMID: 24410863; PubMed Central PMCID: PMC4028783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Field-Ridley A, Dharmar M, Steinhorn D, McDonald C, Marcin JP. ICU-Acquired Weakness Is Associated With Differences in Clinical Outcomes in Critically Ill Children. Pediatr Crit Care Med 2016;17(1):53–7. doi: 10.1097/PCC.0000000000000538. PubMed PMID: 26492063; PubMed Central PMCID: PMC5008971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parry SM, El-Ansary D, Cartwright MS, Sarwal A, Berney S, Koopman R, et al. Ultrasonography in the intensive care setting can be used to detect changes in the quality and quantity of muscle and is related to muscle strength and function. J Crit Care 2015;30(5):1151 e9–14. doi: 10.1016/j.jcrc.2015.05.024. PubMed PMID: 26211979. [DOI] [PubMed] [Google Scholar]
- 5.Puthucheary ZA, Rawal J, McPhail M, Connolly B, Ratnayake G, Chan P, et al. Acute skeletal muscle wasting in critical illness. JAMA 2013;310(15):1591–600. doi: 10.1001/jama.2013.278481. PubMed PMID: 24108501. [DOI] [PubMed] [Google Scholar]
- 6.Valla FV, Young DK, Rabilloud M, Periasami U, John M, Baudin F, et al. Thigh Ultrasound Monitoring Identifies Decreases in Quadriceps Femoris Thickness as a Frequent Observation in Critically Ill Children. Pediatr Crit Care Med 2017;18(8):e339–e47. doi: 10.1097/PCC.0000000000001235. PubMed PMID: 28650903. [DOI] [PubMed] [Google Scholar]
- 7.Scholten RR, Pillen S, Verrips A, Zwarts MJ. Quantitative ultrasonography of skeletal muscles in children: normal values. Muscle Nerve 2003;27(6):693–8. doi: 10.1002/mus.10384. PubMed PMID: 12766980. [DOI] [PubMed] [Google Scholar]
- 8.Zaidman CM, Wu JS, Wilder S, Darras BT, Rutkove SB. Minimal training is required to reliably perform quantitative ultrasound of muscle. Muscle Nerve 2014;50(1):124–8. doi: 10.1002/mus.24117. PubMed PMID: 24218288; PubMed Central PMCID: PMC4102884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fivez T, Hendrickx A, Van Herpe T, Vlasselaers D, Desmet L, Van den Berghe G, et al. An Analysis of Reliability and Accuracy of Muscle Thickness Ultrasonography in Critically Ill Children and Adults. JPEN J Parenter Enteral Nutr 2016;40(7):944–9. doi: 10.1177/0148607115575033. PubMed PMID: 25754437. [DOI] [PubMed] [Google Scholar]
- 10.V S. Evaluation of the standard deviation from duplicate results. Accreditation and Quality Assurance 2008;13:335–7. [Google Scholar]
- 11.Tagliafico A, Martinoli C. Reliability of side-to-side sonographic cross-sectional area measurements of upper extremity nerves in healthy volunteers. J Ultrasound Med 2013;32(3):457–62. PubMed PMID: 23443186. [DOI] [PubMed] [Google Scholar]
- 12.El-Halaby H, Abdel-Hady H, Alsawah G, Abdelrahman A, El-Tahan H. Sonographic Evaluation of Diaphragmatic Excursion and Thickness in Healthy Infants and Children. J Ultrasound Med 2016;35(1):167–75. doi: 10.7863/ultra.15.01082. PubMed PMID: 26679203. [DOI] [PubMed] [Google Scholar]
- 13.Zambon M, Greco M, Bocchino S, Cabrini L, Beccaria PF, Zangrillo A. Assessment of diaphragmatic dysfunction in the critically ill patient with ultrasound: a systematic review. Intensive Care Med 2017;43(1):29–38. doi: 10.1007/s00134-016-4524-z. PubMed PMID: 27620292. [DOI] [PubMed] [Google Scholar]
- 14.Glau CL, Conlon TW, Himebauch AS, Yehya N, Weiss SL, Berg RA, et al. Progressive Diaphragm Atrophy in Pediatric Acute Respiratory Failure. Pediatr Crit Care Med 2018. doi: 10.1097/PCC.0000000000001485. PubMed PMID: 29406380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee EP, Hsia SH, Hsiao HF, Chen MC, Lin JJ, Chan OW, et al. Evaluation of diaphragmatic function in mechanically ventilated children: An ultrasound study. PLoS One 2017;12(8):e0183560. doi: 10.1371/journal.pone.0183560. PubMed PMID: 28829819; PubMed Central PMCID: PMC5567657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grosu HB, Lee YI, Lee J, Eden E, Eikermann M, Rose KM. Diaphragm muscle thinning in patients who are mechanically ventilated. Chest 2012;142(6):1455–60. doi: 10.1378/chest.11-1638. PubMed PMID: 23364680. [DOI] [PubMed] [Google Scholar]
- 17.McLeod G, Geddes D, Nathan E, Sherriff J, Simmer K, Hartmann P. Feasibility of using ultrasound to measure preterm body composition and to assess macronutrient influences on tissue accretion rates. Early Hum Dev 2013;89(8):577–82. doi: 10.1016/j.earlhumdev.2013.02.007. PubMed PMID: 23535172. [DOI] [PubMed] [Google Scholar]
- 18.Sarwal A, Parry SM, Berry MJ, Hsu FC, Lewis MT, Justus NW, et al. Interobserver Reliability of Quantitative Muscle Sonographic Analysis in the Critically Ill Population. J Ultrasound Med 2015;34(7):1191–200. doi: 10.7863/ultra.34.7.1191. PubMed PMID: 26112621. [DOI] [PubMed] [Google Scholar]
- 19.Boon AJ, Harper CJ, Ghahfarokhi LS, Strommen JA, Watson JC, Sorenson EJ. Two-dimensional ultrasound imaging of the diaphragm: quantitative values in normal subjects. Muscle Nerve 2013;47(6):884–9. doi: 10.1002/mus.23702. PubMed PMID: 23625789. [DOI] [PubMed] [Google Scholar]
- 20.Baldwin CE, Bersten AD. Alterations in respiratory and limb muscle strength and size in patients with sepsis who are mechanically ventilated. Phys Ther 2014;94(1):68–82. doi: 10.2522/ptj.20130048. PubMed PMID: 24009347. [DOI] [PubMed] [Google Scholar]
- 21.Witteveen E, Sommers J, Wieske L, Doorduin J, van Alfen N, Schultz MJ, et al. Diagnostic accuracy of quantitative neuromuscular ultrasound for the diagnosis of intensive care unit-acquired weakness: a cross-sectional observational study. Ann Intensive Care 2017;7(1):40. doi: 10.1186/s13613-017-0263-8. PubMed PMID: 28382599; PubMed Central PMCID: PMC5382120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


