Abstract
CACNA1C (NM_000719.6) encodes an L-type calcium voltage-gated calcium channel (Cav1.2), and pathogenic variants have been associated with two distinct clinical entities: Timothy syndrome and Brugada syndrome. Thus far, CACNA1C has not been reported as a gene associated with epileptic encephalopathy (EE) and is less commonly associated with epilepsy. We report three individuals from two families with variants in CACNA1C. Patient 1 presented with neonatal onset severe epileptic encephalopathy (NOEE) and was found to have a de novo missense variant in CACNA1C (c.4087G>A (p.V1363M)) on exome sequencing. In family 2, Patient 2 presented with congenital cardiac anomalies and cardiomyopathy and was found to have a paternally inherited splice site variant, c.3717+1_3717+2insA, on a cardiomyopathy panel. Her father, Patient 3, presented with learning difficulties, late-onset epilepsy, and congenital cardiac anomalies. Family 2 highlights variable expressivity seen within a family. This case series expands the clinical and molecular phenotype of CACNA1C-related disorders and highlights the need to include CACNA1C on epilepsy gene panels.
Keywords: CACNA1C, Neonatal onset epileptic encephalopathy (NOEE), Epileptic Encephalopathy (EE), Electroencephalogram (EEG), Exome sequencing (ES)
INTRODUCTION
CACNA1C encodes the alpha-1 subunit (also known as Cav1.2) of the L-type voltage-dependent calcium channel, which consists of 24 transmembrane segments that form the pore for ion transport into the cell. It undergoes extensive alternative splicing and has at least 36 different transcripts that are expressed in heart, brain, lung, and smooth muscle [Napolitano and Antzelevitch, 2011; Hedley et al., 2009]. Pathogenic variants in CACNA1C are known to be associated with vastly different clinical phenotypes. The first, Timothy syndrome, is characterized by multisystem abnormalities including cardiac, limb anomalies, facial dysmorphisms, and neurologic features including autism, seizures, intellectual disability, and hypotonia [Lo-A-Njoe et al., 2005; Napolitano et al., 1993; Reichenbach et al., 1992; Splawski et al., 2004; Gillis et al., 2012; Napolitano and Antzelevitch, 2011]. The second, Brugada syndrome, is characterized by ST elevation in the right precordial leads with symptoms of ventricular arrhythmia, syncope, and sudden death [Brugada et al., 1993; Napolitano et al., 1993; Lo-A-Njoe et al., 2005; Marks et al., 1995b, 1995a; Reichenbach et al., 1992; Splawski et al., 2004; Hedley et al., 2009]. Importantly, CACNA1C’s clinical validity and association with Brugada syndrome was not definitely established when an evidence-based approach was utilized [Hosseini et al., 2018]. Thus far, CACNA1C has not been reported as a gene associated with EE, a severe form of epilepsy associated with developmental delay, cognitive dysfunction, and uncontrolled seizures, nor has it been associated with NOEE, which is characterized by neonatal onset intractable epilepsy with a high seizure burden, severe cognitive and psychomotor delays, failure of neurodevelopment, poor prognosis, and high mortality rate at a young age [Beal et al., 2012; Shellhaas et al., 2017]. We describe the clinical and molecular details of 3 individuals from 2 unrelated families with heterozygous variants in CACNA1C. This includes an infant with a de novo missense variant who presented with NOEE, and a family with variable expressivity, which included tetralogy of Fallot (TOF), cardiomyopathy, and developmental delay in the proband and a father with a ventricular septal defect, late-onset epilepsy and learning disabilities attributed to a splice site variant in CACNA1C.
METHODS
Trio exome sequencing (ES) was done in Patient 1 and her nonaffected parents (Family 1), and a cardiomyopathy panel (combined cardiac sequencing and deletion/duplication panel including 120 genes) was performed on Patient 2 (Family 2). Her father, Patient 3, had targeted testing of the two variants of uncertain significance (VUS) found on panel testing. Informed consent was obtained from all affected individuals or their guardians per the specifications of Seattle Children’s Hospital and University of Washington, including consent to publish photographs.
For the in silico analysis of p.V1363M, seen in Patient 1, topology of the protein encoded by CACNA1C (Cav1.2) was predicted and plotted using Protter [Omasits et al., 2014]. Subsequently, we identified the orthologues of Cav1.2 using reciprocal blast approach in Chordata species in the Uniprot Proteome database. These orthologues were aligned using Clustal Omega [Sievers et al., 2011] and manually inspected to remove sequences with significant gaps. The resulting sequence alignment was visualized using Jalview [Waterhouse et al., 2009]. Finally, we predicted the 3D structure of Cav1.2 using rosettaCM [Song et al., 2013] and Cav1.1 as a template (PDB ID: 5GJV_A). p.V1363M and selected pathogenic variants from ClinVar [Landrum et al., 2016] associated with Timothy syndrome were mapped onto the predicted structure and visualized in Pymol (The PyMOL Molecular Graphics System, Version 2.0 Schrödinger, LLC).
RESULTS
Clinical Report for Patient 1 (Family 1)
She was born at 38 weeks of gestation to a nonconsanguineous 33-year-old mother and a 30-year-old father. Maternal and paternal ancestry was English and Ukrainian respectively. She had a healthy twin brother and an older brother. Apgar scores were 8 and 9 at one and five minutes, respectively. Growth parameters at birth included a weight of 2.4 kg (z-score −1.93), length of 40.6 cm (z-score −3.92), and an occipitofrontal circumference (OFC) of 31.8 cm (z-score −1.84). Following birth, she was hospitalized for two days for evaluation of hypoglycemia (30mg/dL) and multiple congenital anomalies. She was found to have profound axial and appendicular hypotonia, laryngomalacia with stridor, bilateral clubbed feet, bilateral 3–4 finger and 2–3 toe syndactyly, and camptodactyly. Dysmorphic facial features included micrognathia, downturned corners of the mouth, frontal bossing, very scant hair, and bilaterally inverted nipples (Figure 1A). Ophthalmological evaluation demonstrated mild optic nerve hypoplasia. In addition, she had severe gastroesophageal reflux, constipation, and anal stenosis which required sequential dilation. A rectal biopsy ruled out Hirschsprung disease. At 8 months, she required a gastrostomy tube due to severe dysphagia. At 18 months, she lacked progression of developmental milestones and had profound hypotonia and inability to visually track or fixate. In addition, she had severe disturbances of her sleep-wake cycle. Her seizures started within a few hours of birth with numerous subtle tonic seizures as well as prolonged tonic and tonic-myoclonic seizures per day. She was initially treated with zonisamide, with no response. At 3 months, she was diagnosed with epileptic tonic spasms and did not respond to multiple anti-epileptic drugs (AED) including vigabatrin, perampanel, levetiracetam, lacosamide, rufinamide, pyridoxine, folic acid, and pyridoxal 5’-phosphate. She had a small seizure reduction with topiramate and valproic acid. At 7 months, clobazam was started, which significantly reduced her seizure burden from numerous tonic seizures per day to less than 10 per day and improved sleep duration of 8 to 10 hours at night. By 18 months, she still had multiple daily tonic seizures and focal seizures with eye blinking and facial twitching. Her neurologic workup included brain magnetic resonance imaging (MRI) at 7 days and 12 months of age and a proton magnetic resonance spectroscopy at 12 months, all of which were normal. Her first EEG at 5 days of life demonstrated very distinctive features with diffuse excessive fast activities admixed with other frequency activities and subtle tonic seizures. These diffuse fast activities and lack of normal organization have persisted beyond the neonatal period. At 4 months, her EEG demonstrated intermittent diffuse fast activity alternated with diffuse slowing with high voltage occipital sharp wave discharges. Her polysomnography at 5-months of age demonstrated both central and obstructive sleep apnea in the severe range with normal sleep architecture. An echocardiogram (ECHO) and a 24-hour Holter monitor was normal at 13 months. She had an extensive biochemical workup which was normal.
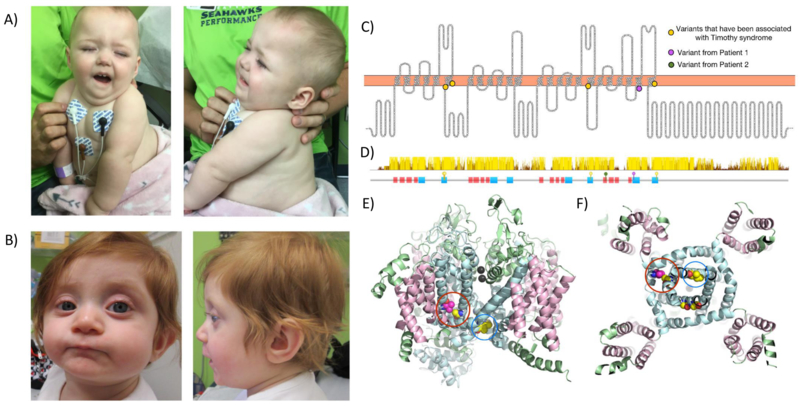
Figure 1.
Photographs of Patient 1 and 2 and topological diagram of CACNA1C. (A) Patient 1 at 13 months of life demonstrating facial dysmorphisms including micrognathia, downturned corners of mouth, flared nares, frontal bossing, and scant hair. (B) Patient 2 at 9months demonstrating low-set posteriorly rotated ears, prominent eyes with shallow orbits, flat nasal bridge, thin vermillion upper lip, and cupping of superior helices. (C) Topology diagram of CACNA1C. Select pathogenic variants from ClinVar associated with Timothy syndrome (A1473G, I1166T, G406R, G402S) are marked as yellow dots. V1363M, seen in Patient 1, is shown as a magenta dot. (D) Sequence conservation (top) and location of predicted transmembrane helices (bottom). The position of disease-causing variants is indicated by yellow (previously reported), magenta (V1363M seen in Patient 1), and green (splice-site variant, c.3717+1_3717+2insA seen in Family 2) pins. (E-F) Side and bottom view (the cytoplasmic side) of the predicted 3D structure of human CACNA1C. The transmembrane helices forming the calcium channel are colored in pale cyan; other transmembrane helices are colored in light pink; the cytoplasmic and periplasmic portion of the protein is colored in pale green. The calcium ions are shown as black spheres. Atoms of the residues that are associated with severe diseases are shown as spheres. V1363M (colored in magenta) is in direct contact with a variant associated with severe neurologic disease, A1473G (colored in yellow).
Diagnostic testing for Patient 1
Her genetic workup included a normal karyotype, chromosomal microarray (CMA), and mitochondrial DNA (mtDNA) analysis. Trio ES revealed a heterozygous de novo variant in CACNA1C (NM_000719.6: c.4087G>A, p.V1363M). The amino acid position is highly conserved, is absent from control databases such as gnomAD, ExAC, and 1000 Genomes [1000 Genomes Project Consortium et al., 2015], and has not been previously reported as a pathogenic variant. Therefore, it was interpreted as a likely pathogenic. Additional findings on ES included a heterozygous, de novo, terminal deletion of MSH2. This deletion involves exons 11–16 ([NM_000251.2] arr[GRCh37] 2p21(47,695,322–47,710,345)x1) of the gene and has been previously reported as pathogenic of hereditary non-polyposis colorectal cancer (HNPCC) (Lynch syndrome) in several families [Lagerstedt-Robinson et al., 2016; Martínez-Bouzas et al., 2007; McPhillips et al., 2005; Romero et al., 2013].
In silico analysis of the CACNA1C p.V1363M variant from Patient 1 and 3D structural prediction
In silico analysis predicts this variant is likely pathogenic, including an elevated GERP score of 4.6, SIFT score of 0 (Damaging), DANN score of 0.9989, and MutationTaster predicts that it is disease causing [Quang et al., 2015; Cooper et al., 2005; Schwarz et al., 2014; Kumar et al., 2009]. Moreover, codon position 1363 is highly conserved across multiple Chordata species as well as 100 vertebrates in PhyloP, PhastCons, and Multiz Alignment (Fig.1D). Taken together, this indicates the functional importance and likelihood of pathogenicity for p.V1363M. Cav1.2 consists of four homologous transmembrane domains, and each domain contains six transmembrane helices (TMH). V1363M localizes in the fifth TMH in domain IV (Figure 1C,D (purple circle). The predicted three-dimensional structure of Cav1.2 shows that (Figure 1E,F) the sixth TMH in each of the four domains form the channel to allow passage of calcium ions, and they appear to be critical for the proper function of Cav1.2. Three pathogenic variants, p.G402S, p.G406R, and p.A1473G in the 6th TMH have been associated with Timothy syndrome. p.G402S and p.G406A were shown to cause reduced channel inactivation, and thus calcium overload in cells [Splawski et al., 2004, 2005]. The fifth TMH in each domain is packed directly against the sixth TMH, and position 1363 is facing towards the sixth TMH. A change from valine to methionine, a larger residue, likely disrupts the protein structure in the sixth TMH, affecting the gating or calcium transmission of Cav1.2.
Clinical report for Patient 2 and 3 (Family 2)
In family 2, patient 2 was born at 39 weeks of gestation to a nonconsanguineous 19-year-old mother and a 24-year-old father (Patient 3) of Irish and German descent. Her birth weight was 3.04kg (z score −0.42) and length was 53cm (z score 1.38). Following delivery, she was found to have an asymptomatic murmur. This worsened following discharge and an ECHO demonstrated TOF which was surgically repaired at 2 months of age. Subsequently, at 4 months of life, she developed acute severe biventricular failure requiring inotropic support and mechanical ventilation. ECHO demonstrated left ventricular noncompaction (LVNC). She has mild dysmorphic features including a flat nasal bridge, low-set ears with cupping of the superior helices, deep-set eyes with shallow orbits, thin vermillion border of the upper lip, and a round face (Figure 1B) and mild bilateral 2–3-4 toe syndactyly. At 8 months, she had mild developmental delays. By 12 months, she spoke 10 words and first walked. Her neurologic exam showed mild diffuse hypotonia. She had an extensive biochemical workup which was normal. Her father, who also carried the splice site variant in CACNA1C, had a congenital ventricular septal defect and aortic valve stenosis which was repaired at age 2.5 months. In addition, he had learning disabilities requiring individualized education program at school and attention-deficit hyperactivity disorder (ADHD). By 18 years, he developed seizures. The clinical semiology consists of feeling numbness on the left side which evolves into generalized tonic-clonic seizures. He was treated with levetiracetam and has 1 to 2 seizures per month. His craniofacial features were similar to his daughters and shared many commonalities to described individuals with Timothy syndrome [Napolitano et al., 1993], including low-set ears, thin vermillion border of the upper lip, premaxillary underdevelopment, and a round face.
Diagnostic Testing for Patient 2 and 3
Patient 2’s CMA was normal. A 120-gene cardiomyopathy panel with sequencing and duplication/deletion analysis revealed three variants in three genes. The first was a heterozygous CACNA1C splice site variant (NM_000719.6: c.3717+1_3717+2insA) that was paternally inherited. This variant has not been previously reported in the literature, is absent from large control population databases, and is predicted to destroy the canonical splice donor site in intron 28, which is invariably a GT at the 5’ end of the intron for normal splicing to occur. Given the phenotypes of Patient 2 and 3 was consistent with Timothy Syndrome, the CACNA1C splice site variant was thought to be likely pathogenic; however, in the absence of functional mRNA studies this remains VUS. The second variant detected on panel testing was a heterozygous splice site variant in ALPK3 (NM_020778.4: c.5105+2dupT). This variant destroys the canonical splice donor site in intron 11 and is predicted to cause abnormal splicing. No additional variants in ALPK3 were detected, including deletion and duplication analysis of the gene. Given inheritance is autosomal recessive, this variant was likely not causing her phenotype, but may be contributing. The third variant was a heterozygous missense variant in TPM1 (NM_001018005.1: c.389T>C, p.I130T). In silico analysis predicts p.I130T is damaging, and the amino acid position is highly conserved across species. It is not present in large control databases and has not been previously reported in the literature. TPM1 is associated with autosomal dominant familial hypertrophic cardiomyopathy, dilated cardiomyopathy, and LVNC. It has not been associated with congenital heart defects. Thus, it was considered a VUS. Targeted testing of TPM1 p.I130T in her father (Patient 3) was negative. Her mother did not undergone targeted testing; therefore, it is uncertain if the variant is de novo or inherited in Patient 2. It is possible that the TPM1 variant was contributing to her cardiomyopathy. In summary, given Patient 2 and 3’s phenotype is most consistent with Timothy syndrome, the splice site variant in CACNA1C was thought to be causative; however, it is classified as a variant of uncertain significance (VUS) in the absence of functional mRNA studies.
DISCUSSION
CACNA1C gene encodes multiple isoforms of the pore-forming alpha-1C subunit of the long lasting (L-type) voltage gated calcium channel (Cav1.2, NM_000719.6) [Takimoto et al., 1997]. Cav1.2 is evolutionally conserved protein that is expressed in the heart, brain, lung and smooth muscles, and is critical for Ca2+ signaling, cellular and neuronal excitability, muscle contraction, and regulation of gene expression [Dai et al., 2009; Hedley et al., 2009]. The variable clinical spectrum summarized in Table 1 [Splawski et al., 2005; Sepp et al., 2017; Corona-Rivera et al., 2015; Etheridge et al., 2011; Splawski et al., 2004; Gillis et al., 2012; Wemhöner et al., 2015; Boczek et al., 2015; Fukuyama et al., 2014; Liu et al., 2017; Antzelevitch et al., 2007] may be accounted for by the numerous isoforms generated by alternatively spliced exons, variable expression of different transcripts in tissue, and the variant’s location in the protein and structural domain of the Cav1.2 channel. For example, in some individuals it is predominantly confined to the heart as seen in Brugada syndrome, while in others there is a predominant neurologic phenotype as seen in Patient 1. In the brain, Cav1.2 is predominantly expressed in the hippocampus, thalamus, cerebral cortex, suprachiasmatic nucleus, and cerebellum [Berger and Bartsch, 2014; Obermair et al., 2004; Nahm et al., 2005]. As the thalamus and hippocampus are important structures for rhythmicity on EEG [Steriade et al., 1993] and the suprachiasmatic nucleus plays a critical role in circadian rhythms, this could be the biologic underpinnings of disturbed circadian rhythm and seizures observed in some individuals with CACNA1C-related disorders. Additional neuropsychiatric impairment such as developmental delay and autism are reported in patients with Timothy Syndrome [Splawski et al., 2005, 2004]. In contrast, seizures are rarely reported in CACNA1C–related disorders with only 3 previously reported individuals prior to our case series [Splawski et al., 2005; Boczek et al., 2015; Gillis et al., 2012]. We expand upon this with an infant presenting with NOEE and an adult with late seizure onset. It is possible that Patient 3’s seizures are unrelated to the splice site variant in CACNA1C; however, given the shared clinical characteristics to individuals with Timothy syndrome and that of his daughter (Patient 2) we hypothesize it may be related. Splicing variants in CACNA1C have been associated with Brugada syndrome [Fukuyama et al., 2014, 2013] and schizophrenia [Purcell et al., 2014], but not been previously reported in patients with Timothy syndrome and epilepsy. As summarized in Table 1, the majority are missense variants; however, given the many different isoforms and importance of splicing on tissue specific expression, we propose this splice site variant and haploinsufficiency can also account for the phenotype in Timothy syndrome. Future mRNA studies are needed to bolster this proposal.
Table 1.
Summary of the clinical and molecular information for our patients and previously reported individuals from the literature grouped by (A) Timothy syndrome phenotype and (B) Brugada syndrome phenotype. GRCh37/hg19 assembly and NM_000719.6 was used as the reference sequence for all variants with the exception of NM_001167625.1 for (c.1216G>A, p.G406R)
| Nucleotide (c.) | Amino Acid (p.) | Exon | Inheritance | Clinical Phenotype | |
|---|---|---|---|---|---|
| A. Timothy Syndrome | |||||
| Patient 1 | 4087G>A | V1363M | 33 | De Novo | Early onset intractable epilepsy, GDD, hypotonia, dysmorphic features, laryngomalacia, syndactyly, camptodactyly, anal stenosis |
| Patient 2 | 3717+1_3717+2insA | N/A | Intron 28 | PI | TOF, severe LVNC and heart failure, syndactyly, dysmorphic features |
| Patient 3 | 3717+1_3717+2insA | N/A | Intron 28 | Unknown | VSD, generalized adult-onset epilepsy, and ID |
| Splawski 2005 | 1216G>A | G406R | 8A | De novo | 13 individuals with classic Timothy syndrome |
| Corona-Rivera 2015 | 1216G>A | G406R | 8A | De novo | Fetal hydrops due to congenital AV block, postnatal prolonged QTc, SCA |
| Sepp 2017 | 1216G>A | G406R | 8A | De novo | No syndactyly with QT prolongation and AV conduction abnormalities |
| Etheridge 2011 | 1216G>A | G406R | 8A | Mosaic | Father (mosaic) with mild phenotype: syndactyly and prolonged QTc and a severely affected child Unrelated child (mosaic) with prolonged QTc, SCA due to VF, syndactyly |
| Splawski 2004, 2005* | 1216G>A* | G406R* | 8* | De novo/ mosaic |
De novo: Severe prolong QT, arrhythmias, sudden cardiac death, and cognitive delays; no syndactyly Mosaic: Two affected sibs with an “unaffected” mother |
| Splaswki 2005 | 1204G>A | G402S | 8 | De novo | Atypical Timothy syndrome, isolated QTc prolongation and arrhythmias, no syndactyly |
| Gillis 2012 | 4418C>G | A1473G | 36 | De novo | Stroke, early onset intractable epilepsy, GDD, joint contractures, dysmorphic facial features, syndactyly, cortical blindness, and myopathy |
| Wemhöner 2015 | 3497T>C | I1166T | 27 | De novo | 1 individual with HCM, syndactyly, ductus arteriosus, SCA |
| Boczek 2015 | 1552C>T | R518C | 12 | Inherited | 3 families with LQTS, HCM, SCA, congenital heart defects |
| B. Brugada Syndrome | |||||
| Antzelevitch 2007 | 116C>T | A39V | 2 | Inherited | SCA, short QT interval |
| 1468G>A | G490R | 10 | Inherited | SCA, short QT interval | |
| Fukuyama 2014 | 1141C>T | P381S | 8 | PI | LQTS |
| 1368G>A | M456I | 9 | Presumed de novo | ||
| 1745C>A | A582D | 13 | MI | ||
| 2573G>A | R858H | 19 | MI | ||
| 5347G>T | G1783C | 42 | De novo | ||
| Wemhöner 2015 | 82G>A | A28T | 2 | PI | LQTS |
| 2578C>G | R860G | 19 | De novo | SCA, LQTS | |
| 3496A>G | I1166V | 27 | Inherited | LQTS, Syncope | |
| 4425C>G | I1475M | 36 | MI | SCA, LQTS | |
| 4486G>A | E1496K | 36 | De novo | SCA, LQTS | |
| Liu 2017 | 5747A>G | Q1916R | 45 | Inherited | SCA due to early repolarization syndrome |
Abbreviations: GDD: Global Developmental delay; Het: Heterozygous; LQTS: Long QT Syndrome; Maternally Inherited: MI; Paternally Inherited: PI; SCA: Sudden cardiac arrest
which leads to an alternatively spliced product.
CONCLUSION
This case series expands the molecular and clinical phenotype for CACNA1C-related disorders to include NOEE with unique clinical-electrical characterization. Patient 1 had many hallmark features of Timothy Syndrome including cutaneous syndactyly, dysmorphic features, and sleep disturbances; however, she lacked cardiac abnormalities. Her presentation differs from previously reported individuals in regard to her severe refractory epilepsy of neonatal onset, lack of neurodevelopment, and recognizable EEG patterns of diffuse fast activity. With this information, we propose the consideration of including the CACNA1C in early-onset epilepsy gene panels. In addition, we report an inherited splice site variant with variable expressivity in the family including congenital heart defects, cardiomyopathy, learning difficulties, epilepsy, and craniofacial features consistent with Timothy syndrome. The clinical phenotype of CACNA1C-related calcium channelopathies is broad and complex. Better understanding of the clinical-electrophysiological features and functional consequences of CACNA1C variants is needed for the development of targeted treatments and novel therapeutics.
ACKNOWLEDGEMENT
We thank the families for participating and sharing their story. We thank Dr. David Baker’s lab at University of Washington for providing the protein structure prediction data.
STUDY FUNDING
Research reported in this publication was supported in part by the National Institute of General Medical Sciences of National Institutes of Health Postdoctoral Training Program in Medical Genetics (5T32GM007454 to J.N.D), and the National Institute of Neurological Disorders and Stroke (NINDS) (K08NS092898 to G.M.M) and Jordan’s Guardian Angels (to G.M.M.)
Footnotes
DISCLOSURE OF CONFLICT OF INTEREST
None of the authors has any conflict of interest to disclose.
ETHICAL PUBLICATION STATEMENT
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines
Web Resources: The URLs for data presented include:
UCSC genome browser: [Di et al., 2009; Hedley et al., 2009; Berger and Bartsch, 2014]
OMIM: http://omim.org/
dbVar: http://www.ncbi.nlm.nih.gov/dbvar/browse/
ClinGen: http://www.ncbi.nlm.nih.gov/projects/dbvar/clingen/
ExAC: http://exac.broadinstitute.org/
gnomAD: http://gnomad.broadinstitute.org
ClinVar: https://www.ncbi.nlm.nih.gov/clinvar/
Uniprot: http://www.uniprot.org
REFERENCES
- 1000 Genomes Project Consortium, Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, Korbel JO, Marchini JL, McCarthy S, McVean GA, Abecasis GR. 2015. A global reference for human genetic variation. Nature 526: 68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antzelevitch C, Pollevick GD, Cordeiro JM, Casis O, Sanguinetti MC, Aizawa Y, Guerchicoff A, Pfeiffer R, Oliva A, Wollnik B, Gelber P, Bonaros EP, Burashnikov E, Wu Y, Sargent JD, Schickel S, Oberheiden R, Bhatia A, Hsu L-F, Haïssaguerre M, Schimpf R, Borggrefe M, Wolpert C. 2007. Loss-of-Function Mutations in the Cardiac Calcium Channel Underlie a New Clinical Entity Characterized by ST-Segment Elevation, Short QT Intervals, and Sudden Cardiac Death. Circulation 115: 442–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beal JC, Cherian K, Moshe SL. 2012. Early-onset epileptic encephalopathies: Ohtahara syndrome and early myoclonic encephalopathy. Pediatr. Neurol 47: 317–323. [DOI] [PubMed] [Google Scholar]
- Berger SM, Bartsch D. 2014. The role of L-type voltage-gated calcium channels Cav1.2 and Cav1.3 in normal and pathological brain function. Cell Tissue Res. 357: 463–476. [DOI] [PubMed] [Google Scholar]
- Boczek NJ, Miller EM, Ye D, Nesterenko VV, Tester DJ, Antzelevitch C, Czosek RJ, Ackerman MJ, Ware SM. 2015. Novel Timothy syndrome mutation leading to increase in CACNA1C window current. Heart Rhythm 12: 211–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugada R, Campuzano O, Sarquella-Brugada G, Brugada P, Brugada J, Hong K. 1993. Brugada Syndrome. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJ, Mefford HC, Stephens K, Amemiya A, Ledbetter N, editors. GeneReviews(®). Seattle (WA): University of Washington, Seattle. [PubMed] [Google Scholar]
- Cooper GM, Stone EA, Asimenos G, NISC Comparative Sequencing Program, Green ED, Batzoglou S, Sidow A. 2005. Distribution and intensity of constraint in mammalian genomic sequence. Genome Res. 15: 901–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corona-Rivera JR, Barrios-Prieto E, Nieto-García R, Bloise R, Priori S, Napolitano C, Bobadilla-Morales L, Corona-Rivera A, Zapata-Aldana E, Peña-Padilla C, Rivera-Vargas J, Chavana-Naranjo E. 2015. Unusual retrospective prenatal findings in a male newborn with Timothy syndrome type 1. Eur. J. Med. Genet 58: 332–335. [DOI] [PubMed] [Google Scholar]
- Dai S, Hall DD, Hell JW. 2009. Supramolecular assemblies and localized regulation of voltage-gated ion channels. Physiol. Rev 89: 411–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etheridge SP, Bowles NE, Arrington CB, Pilcher T, Rope A, Wilde AAM, Alders M, Saarel EV, Tavernier R, Timothy KW, Tristani-Firouzi M. 2011. Somatic mosaicism contributes to phenotypic variation in Timothy syndrome. Am. J. Med. Genet. A 155A: 2578–2583. [DOI] [PubMed] [Google Scholar]
- Fukuyama M, Ohno S, Wang Q, Kimura H, Makiyama T, Itoh H, Ito M, Horie M. 2013. L-type calcium channel mutations in Japanese patients with inherited arrhythmias. Circ. J. Off. J. Jpn. Circ. Soc 77: 1799–1806. [DOI] [PubMed] [Google Scholar]
- Fukuyama M, Ohno S, Wang Q, Shirayama T, Itoh H, Horie M. 2014. Nonsense-mediated mRNA decay due to a CACNA1C splicing mutation in a patient with Brugada syndrome. Heart Rhythm 11: 629–634. [DOI] [PubMed] [Google Scholar]
- Gillis J, Burashnikov E, Antzelevitch C, Blaser S, Gross G, Turner L, Babul-Hirji R, Chitayat D. 2012. Long QT, syndactyly, joint contractures, stroke and novel CACNA1C mutation: expanding the spectrum of Timothy syndrome. Am. J. Med. Genet. A 158A: 182–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedley PL, Jørgensen P, Schlamowitz S, Moolman-Smook J, Kanters JK, Corfield VA, Christiansen M. 2009. The genetic basis of Brugada syndrome: a mutation update. Hum. Mutat 30: 1256–1266. [DOI] [PubMed] [Google Scholar]
- Hosseini SM, Kim R, Udupa S, Costain G, Jobling R, Liston E, Jamal SM, Szybowska M, Morel CF, Bowdin S, Garcia J, Care M, Sturm AC, Novelli V, Ackerman MJ, Ware JS, Hershberger RE, Wilde AAM, Gollob MH, NIH-Clinical Genome Resource Consortium. 2018. Reappraisal of Reported Genes for Sudden Arrhythmic Death: An Evidence-Based Evaluation of Gene Validity for Brugada Syndrome. Circulation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Henikoff S, Ng PC. 2009. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat. Protoc. 4: 1073–1081. [DOI] [PubMed] [Google Scholar]
- Lagerstedt-Robinson K, Rohlin A, Aravidis C, Melin B, Nordling M, Stenmark-Askmalm M, Lindblom A, Nilbert M. 2016. Mismatch repair gene mutation spectrum in the Swedish Lynch syndrome population. Oncol. Rep 36: 2823–2835. [DOI] [PubMed] [Google Scholar]
- Landrum MJ, Lee JM, Benson M, Brown G, Chao C, Chitipiralla S, Gu B, Hart J, Hoffman D, Hoover J, Jang W, Katz K, Ovetsky M, Riley G, Sethi A, Tully R, Villamarin-Salomon R, Rubinstein W, Maglott DR. 2016. ClinVar: public archive of interpretations of clinically relevant variants. Nucleic Acids Res. 44: D862–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Shen Y, Xie J, Bao H, Cao Q, Wan R, Xu X, Zhou H, Huang L, Xu Z, Zhu W, Hu J, Cheng X, Hong K. 2017. A mutation in the CACNA1C gene leads to early repolarization syndrome with incomplete penetrance: A Chinese family study. PloS One 12: e0177532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo-A-Njoe SM, Wilde AA, van Erven L, Blom NA. 2005. Syndactyly and long QT syndrome (CaV1.2 missense mutation G406R) is associated with hypertrophic cardiomyopathy. Heart Rhythm 2: 1365–1368. [DOI] [PubMed] [Google Scholar]
- Marks ML, Trippel DL, Keating MT. 1995a. Long QT syndrome associated with syndactyly identified in females. Am. J. Cardiol 76: 744–745. [DOI] [PubMed] [Google Scholar]
- Marks ML, Whisler SL, Clericuzio C, Keating M. 1995b. A new form of long QT syndrome associated with syndactyly. J. Am. Coll. Cardiol 25: 59–64. [DOI] [PubMed] [Google Scholar]
- Martínez-Bouzas C, Ojembarrena E, Beristain E, Errasti J, Viguera N, Tejada Minguéz M-I. 2007. High proportion of large genomic rearrangements in hMSH2 in hereditary nonpolyposis colorectal cancer (HNPCC) families of the Basque Country. Cancer Lett. 255: 295–299. [DOI] [PubMed] [Google Scholar]
- McPhillips M, Meldrum CJ, Creegan R, Edkins E, Scott RJ. 2005. Deletion Mutations in an Australian Series of HNPCC Patients. Hered. Cancer Clin. Pract 3: 43–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahm S-S, Farnell YZ, Griffith W, Earnest DJ. 2005. Circadian regulation and function of voltage-dependent calcium channels in the suprachiasmatic nucleus. J. Neurosci. Off. J. Soc. Neurosci 25: 9304–9308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napolitano C, Antzelevitch C. 2011. Phenotypical manifestations of mutations in the genes encoding subunits of the cardiac voltage-dependent L-type calcium channel. Circ. Res 108: 607–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napolitano C, Splawski I, Timothy KW, Bloise R, Priori SG. 1993. Timothy Syndrome. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJ, Mefford HC, Stephens K, Amemiya A, Ledbetter N, editors. GeneReviews(®). Seattle (WA): University of Washington, Seattle. [Google Scholar]
- Obermair GJ, Szabo Z, Bourinet E, Flucher BE. 2004. Differential targeting of the L-type Ca2+ channel alpha 1C (CaV1.2) to synaptic and extrasynaptic compartments in hippocampal neurons. Eur. J. Neurosci 19: 2109–2122. [DOI] [PubMed] [Google Scholar]
- Omasits U, Ahrens CH, Müller S, Wollscheid B. 2014. Protter: interactive protein feature visualization and integration with experimental proteomic data. Bioinforma. Oxf. Engl 30: 884–886. [DOI] [PubMed] [Google Scholar]
- Purcell SM, Moran JL, Fromer M, Ruderfer D, Solovieff N, Roussos P, O’Dushlaine C, Chambert K, Bergen SE, Kähler A, Duncan L, Stahl E, Genovese G, Fernández E, Collins MO, Komiyama NH, Choudhary JS, Magnusson PKE, Banks E, Shakir K, Garimella K, Fennell T, DePristo M, Grant SGN, Haggarty SJ, Gabriel S, Scolnick EM, Lander ES, Hultman CM, Sullivan PF, McCarroll SA, Sklar P. 2014. A polygenic burden of rare disruptive mutations in schizophrenia. Nature 506: 185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quang D, Chen Y, Xie X. 2015. DANN: a deep learning approach for annotating the pathogenicity of genetic variants. Bioinforma. Oxf. Engl 31: 761–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenbach H, Meister EM, Theile H. 1992. [The heart-hand syndrome. A new variant of disorders of heart conduction and syndactylia including osseous changes in hands and feet]. Kinderarztl. Prax 60: 54–56. [PubMed] [Google Scholar]
- Romero A, Garre P, Valentin O, Sanz J, Pérez-Segura P, Llovet P, Díaz-Rubio E, de la Hoya M, Caldés T. 2013. Frequency and variability of genomic rearrangements on MSH2 in Spanish Lynch Syndrome families. PloS One 8: e72195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz JM, Cooper DN, Schuelke M, Seelow D. 2014. MutationTaster2: mutation prediction for the deep-sequencing age. Nat. Methods 11: 361–362. [DOI] [PubMed] [Google Scholar]
- Sepp R, Hategan L, Bácsi A, Cseklye J, Környei L, Borbás J, Széll M, Forster T, Nagy I, Hegedűs Z. 2017. Timothy syndrome 1 genotype without syndactyly and major extracardiac manifestations. Am. J. Med. Genet. A 173: 784–789. [DOI] [PubMed] [Google Scholar]
- Shellhaas RA, Wusthoff CJ, Tsuchida TN, Glass HC, Chu CJ, Massey SL, Soul JS, Wiwattanadittakun N, Abend NS, Cilio MR, Neonatal Seizure Registry. 2017. Profile of neonatal epilepsies: Characteristics of a prospective US cohort. Neurology 89: 893–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, Lopez R, McWilliam H, Remmert M, Söding J, Thompson JD, Higgins DG. 2011. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 7: 539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, DiMaio F, Wang RY-R, Kim D, Miles C, Brunette T, Thompson J, Baker D. 2013. High-resolution comparative modeling with RosettaCM. Struct. Lond. Engl 1993 21: 1735–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Splawski I, Timothy KW, Decher N, Kumar P, Sachse FB, Beggs AH, Sanguinetti MC, Keating MT. 2005. Severe arrhythmia disorder caused by cardiac L-type calcium channel mutations. Proc. Natl. Acad. Sci. U. S. A 102: 8089–8096; discussion 8086–8088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Splawski I, Timothy KW, Sharpe LM, Decher N, Kumar P, Bloise R, Napolitano C, Schwartz PJ, Joseph RM, Condouris K, Tager-Flusberg H, Priori SG, Sanguinetti MC, Keating MT. 2004. Ca(V)1.2 calcium channel dysfunction causes a multisystem disorder including arrhythmia and autism. Cell 119: 19–31. [DOI] [PubMed] [Google Scholar]
- Steriade M, McCormick DA, Sejnowski TJ. 1993. Thalamocortical oscillations in the sleeping and aroused brain. Science 262: 679–685. [DOI] [PubMed] [Google Scholar]
- Takimoto K, Li D, Nerbonne JM, Levitan ES. 1997. Distribution, splicing and glucocorticoid-induced expression of cardiac alpha 1C and alpha 1D voltage-gated Ca2+ channel mRNAs. J. Mol. Cell. Cardiol. 29: 3035–3042. [DOI] [PubMed] [Google Scholar]
- Waterhouse AM, Procter JB, Martin DMA, Clamp M, Barton GJ. 2009. Jalview Version 2--a multiple sequence alignment editor and analysis workbench. Bioinforma. Oxf. Engl. 25: 1189–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wemhöner K, Friedrich C, Stallmeyer B, Coffey AJ, Grace A, Zumhagen S, Seebohm G, Ortiz-Bonnin B, Rinné S, Sachse FB, Schulze-Bahr E, Decher N. 2015. Gain-of-function mutations in the calcium channel CACNA1C (Cav1.2) cause non-syndromic long-QT but not Timothy syndrome. J. Mol. Cell. Cardiol 80: 186–195. [DOI] [PubMed] [Google Scholar]