Abstract
Mitral valve prolapse (MVP) affects 2.4% of the population and has poorly understood etiology. Recent genetic studies have begun to unravel the complexities of MVP and through these efforts, mutations in the FLNA (Filamin-A) gene were identified as disease causing. Our in vivo and in vitro studies have validated these genetic findings and have revealed FLNA as a central regulator of valve morphogenesis. The mechanisms by which FLNA mutations result in myxomatous mitral valve disease are currently unknown, but may involve proteins previously associated with mutated regions of the FLNA protein, such as the small GTPase signaling protein, R-Ras. Herein, we report that filamin-A is required for R-Ras expression and activation of the Ras-Mek-Erk pathway. Loss of the Ras/Erk pathway correlated with hyperactivation of pSmad2/3, increased extracellular matrix (ECM) production and enlarged mitral valves. Analyses of integrin receptors in the mitral valve revealed that Filamin-A was required for beta1 integrin expression and provided a potential mechanism for impaired ECM compaction and valve enlargement. Our data support Filamin-A as a protein that regulates the balance between Erk and Smad activation and an inability of filamin-A deficient valve interstitial cells to effectively remodel the increased ECM production through a beta1-integrin mechanism. As a consequence, loss of filamin-A function results in increased ECM production and generation of a myxomatous phenotype characterized by improperly compacted mitral valve tissue.
INTRODUCTION:
Mitral valve prolapse (MVP) is one of the most common human diseases, affecting 1 in 40 individuals and is the most common reason for mitral valve surgery(Devereux et al., 1982; Devereux et al., 1986; Duren et al., 1988; Devereux, 1989; Devereux et al., 1989; Avierinos et al., 2002; Grigioni et al., 2008; d’Arcy et al., 2011; Chambers et al., 2013; den Hoed et al., 2013). MVP is characterized by increased proteoglycan and fragmented collagen and elastin, resulting in floppy valves that prolapse into the left atrium and cause mitral regurgitation (MR) (de Vlaming et al., 2012). Serious complications from valve dysfunction include congestive heart failure, and sudden cardiac death. MVP can occur as part of a recognized syndrome, such as Marfan syndrome, or it can occur in isolation, in which case it is called non-syndromic MVP. Mitral valve dysfunction and prolapse in the young population is generally benign but can progress in the aged population to a serious disease with no available non-surgical treatment. The age-dependent nature of MVP has led to the assumption that MVP is not a congenitally-based disease but rather a disease that is acquired over decades. However, based on recent genetic and biological data, we have shown that MVP can indeed be classified as a congenital disease, a finding supported by antenatal echo data in large families with inherited MVP (Kyndt et al., 2007)and mouse knockout data of MVP genes showing developmental valvular anomalies(Sauls et al., 2012; Durst et al., 2015). These findings underscore the importance of establishing the genetic and congenital mechanisms that underlie the initiating causes of mitral valve prolapse.
Mutations in the X-linked FLNA (Filamin-A) gene cause congenital valvular defects and progress to myxomatous mitral valve disease in humans(Kyndt et al., 1998; Kyndt et al., 2007; Lardeux et al., 2011; Le Tourneau et al., 2017). The Filamin family of proteins consist of 3 members: Filamin-A, Filamin-B, and Filamin-C, of which Filamin’s A and B are widely expressed, whereas Filamin-C expression is restricted to cardiac and skeletal muscle. Each of these proteins are large cytoplasmic proteins that function as molecular tethers by interacting with both extracellular matrix (ECM)-bound cell-surface integrin’s and the actin cytoskeleton. In addition to their function as a structural entity within the cell, filamin’s also play a major role in cell signaling. The structural and signaling roles of Filamin proteins are driven through their myriad of protein interactions. To date, Filamin-A is known to interact with over 70 different proteins in cell and tissue dependent contexts to regulate various cell behaviors such as proliferation, differentiation, migration, and ECM compaction(Stossel et al., 2001; Zhou et al., 2007; Zhou et al., 2010). Not surprisingly, global loss of Filamin-A in knockout mice results in embryonic lethality and a host of cardiovascular and skeletal malformations, demonstrating the functional importance of this protein during morphogenesis(Feng et al., 2006).
Mutations in the filamin-A gene cause at least 10 phenotypically unique congenital diseases including: periventricular heteropia(Fox et al., 1998), Melnick-Needles syndrome(Robertson et al., 2003), congenital short bowel syndrome(Oegema et al., 2013; van der Werf et al., 2013), and myxomatous valvular dystrophy(Kyndt et al., 2007; Levine and Slaugenhaupt, 2007; Lardeux et al., 2011; Duval et al., 2014; Le Tourneau et al., 2017). The fact that particular FLNA mutations confer disease specificity argues for cell/tissue-specific interactions that are uniquely impaired in the context of damaging genetic variants. Thus, evaluating the location of mutations that are associated with disease phenotype may inform the mechanistic underpinnings for disease inception. For example, nearly all of the mutations in FLNA that have been reported as causative in non-syndromic myxomatous valvular dystrophy occur within the amino end of the protein. This genetic data suggests a unique interaction between the FLNA amino terminus and various protein interactors to confer valve-specific regulation such that disruption of this interaction would lead to valve defects and the generation of a myxomatous phenotype defined by increased proteoglycans and loss of normal zonal boundary interfaces. R-Ras, a small GTPase was recently identified as interacting within same region in which FLNA mutations have been observed in patients with myxomatous valvular dystrophy(Gawecka et al., 2010; Griffiths et al., 2011). Due to previous reports of R-Ras affecting the balance between Erk and Smad activities in addition to regulating integrin activities in concert with the known interaction between FLNA and beta1 integrin, we tested whether loss of Filamin could impair R-Ras and/or integrin signaling and function(Kretzschmar et al., 1997a; Kretzschmar et al., 1999; D’Addario et al., 2002; Sowa et al., 2002; Lincoln et al., 2006; Krenz et al., 2008; Chen et al., 2009; Gehler et al., 2009; Gawecka et al., 2010; Kim et al., 2010; Griffiths et al., 2011). The overall goal of this study was to utilize genetic data from FLNA patients to inform pathway discovery that would lead to an understanding of the etiology for this common disease.
MATERIALS and METHODS:
Gene-Targeted Mice:
Filamin-A floxed mice were bred with the Tie2 Cre line to obtain conditional KO animals at E17.5. As Filamin-A is X-linked in both mouse and humans, we primarily focused our analyses on male mice, although some female mice have been characterized for phenotypic differences. These mice were reported previously by us and others (Feng et al., 2006; Sauls et al., 2012; Gould et al., 2015; Sauls et al., 2015). All mouse experiments were performed under protocols approved by the Institutional Animal Care and Use Committee, Medical University of South Carolina. Prior to cardiac resection, mice were euthanized in accordance with the Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85–23, revised 1996.)
Immunohistochemistry (IHC):
IHC was performed as we have previously reported (Norris et al., 2010; Sauls et al., 2012; Durst et al., 2015; Sauls et al., 2015). Briefly, Mouse fetuses at embryonic day (E) E17.5 were fixed in 4% paraformaldehyde, embedded in paraffin, and sectioned at 5 μm. Deparaffinized sections were rehydrated through a graded series of ethanols to phosphate buffered saline (PBS-Sigma, St. Louis, MO.). Sections were subjected to antigen unmasking (H-3300; Vector Laboratories, Burlingame, CA) and treated for 1 hr at room temperature with a blocking buffer of PBS (Sigma, St. Louis, MO) containing 5% normal goat serum, (NGS, Cappel, Malvern, PA). Primary antibodies used were: affinity-purified rabbit anti-human Filamin-A monoclonal antibody (Epitomics, Inc) diluted to 1:250, MF20-c (Developmental Studies Hybridoma Bank, Iowa City, IA) diluted 1:50, pSmad3 (Epitomics) diluted 1:100, RRas (Abcam) diluted 1:100, pErk1/2 (Cell signaling) diluted 1:100, beta1 integrin (Abcam) diluted 1:100. Primary antibodies were placed in blocking buffer overnight at 4°C. Following primary antibody incubations, specimens were washed five times in PBS and incubated at room temperature with Alexa Fluor goat α-rabbit 488 and goat α-mouse 568 (Invitrogen, Eugene, OR) diluted 1:100 in PBS. Nuclei were stained with Hoechst dye (1:10,000) (Invitrogen) in PBS for 5 min prior to the final washes in PBS. All samples were cover-slipped using Dabco mounting medium (Sigma). Images of immunostained sections were captured with a Leica DM IRB Microscope System (Leica Microsystems, Inc Exton, PA). Files were transferred to Adobe Photoshop for labeling and figure preparation.
Western analyses:
Single anterior mitral leaflets from E17.5 or P2 control and filamin-A conditional knockout hearts were dissected and used for Western analyses as we have previously reported (Sauls et al., 2015). Dilutions for all primary antibodies were 1:1000. Pairs used were control samples versus conditional knockout and a two-tailed t-test was used to define statistical significance with α=.05
Adhesion assays:
Valve interstitial cells were obtained from sheep mitral valve biopsies and cultured in DMEM/10%FBS/1% Pen/Strep. Cultures were passaged a total of 5 times prior to use. Adhesions assays were performed using adhesion array kits (Chemicon) according to manufacturer’s instructions. Statistical significance was determined using the Student t-test (2-tailed, type 2), with significance (P<.05). Statistical data are presented as the mean + one standard deviation from the mean.
RESULTS:
R-Ras is abundantly expressed in the valve leaflets and is lost in the filamin-A conditional knockout mice.
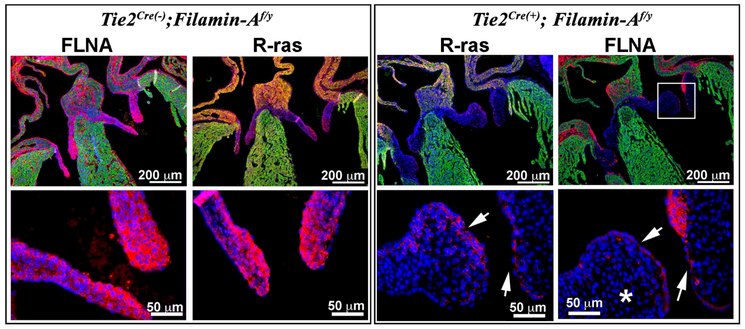
Although R-Ras has been shown to interact with the amino terminus of Filamin-A the consequence of loss of filamin-A on R-Ras signaling has not been evaluated in the developing valves. Due to filamin-A global knockouts resulting in embryonic lethality by E14.5, we took a conditional approach and genetically removed filamin-A from endocardium and endocardial derived cells using a Tie2Cre line. As shown in Figure 1, Tie2Cre- mediated excision of the floxed-Filamin-A allele resulted in near complete loss of filamin-A expression in the mitral and tricuspid valve leaflets, consistent with our previous reports(Sauls et al., 2012; Sauls et al., 2015). Immunohistochemistry (IHC) revealed a near complete loss of R-Ras expression in the mitral and tricuspid valves. Some residual R-Ras staining persisted in the valves, but was concentrated in cells that maintained filamin-A expression. Additional areas within the heart, such as the muscular rim at the base of the atrial septum showed pronounced reduction of R-Ras in the Tie2Cre(+);FilaminAf/y knockout mice. These data show that Filamin-A co-localizes with R-Ras and is required for R-Ras expression in the developing heart.
Figure 1: R-Ras expression is diminished in FLNA cKO valves.
R-Ras is nearly ubiquitous in wild-type animals whereas E17.5 FLNA cKO mice exhibit a near complete loss of R-Ras in mitral leaflets (asterisk). Low levels of R-Ras are persistent in FLNA containing cells (arrows).
Filamin-A regulation of Mek/Erk pathway during mitral valve morphogenesis
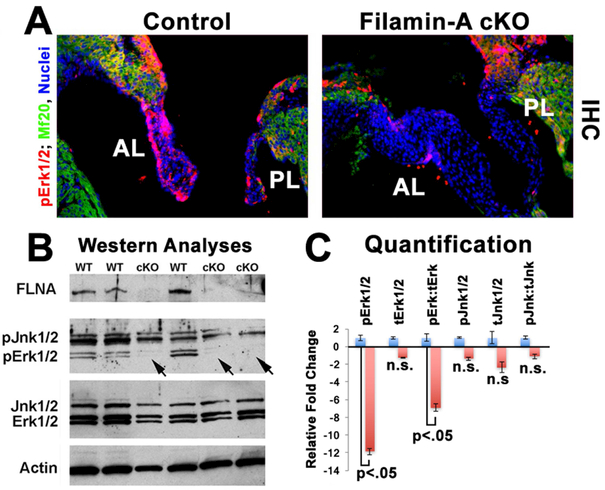
As R-Ras is a major upstream kinase that activates the Mek/Erk pathway, we tested whether loss of Filamin-A (and R-Ras) would negatively affect Erk activation. As shown in Figure 2, activated Erk (pErk1/2) is observed prominently at E17.5 in both endocardium and valve interstitial cells of the anterior and posterior mitral leaflets. IHC for pErk1/2 revealed a near complete loss of Erk activity in the mitral leaflets in the Tie2Cre(+);FilaminAf/y knockout valves. Internal controls for the IHC stainings show robust Erk activity in the atrial myocardium, which is unchanged in the conditional knockout. pErk1/2 activity was quantified by Western analyses of individual E17.5 anterior leaflets, which revealed a statistically significant reduction (11.8 fold) in pErk1/2 activities and roughly seven-fold reduction of pErk when normalized to total Erk1/2 values. Western analyses for Filamin-A on the same blot demonstrate a near complete loss of Filamin-A protein in the conditional knockouts as expected. pJnk1/2 was used as an internal control for parallel kinase cascades and revealed no statistically significant change between conditional knockout and wildtype controls. Combined with data shown in Figure 1, these results support a mechanism whereby Filamin-A is necessary for activation of the Ras/Mek/Erk pathway in the developing cardiac valves.
Figure 2: Erk1/2 activity is blunted in FLNA cKO valves.
Fig. 2: (A) WT and cKO mitral leaflets were immunostained for pErk1/2 (red), MF20 (green) and nuclei (blue). Near complete loss of pErk1/2 is observed in the cKO mice. (B,C) Western blotting and quantification demonstrate that pErk1/2 is significantly diminished whereas total Erk1/2, Jnk1/2, and pJnk1/2 are not significantly (n.s.) changed.
Loss of Filamin-A Results in Hyperactivation of Smad3
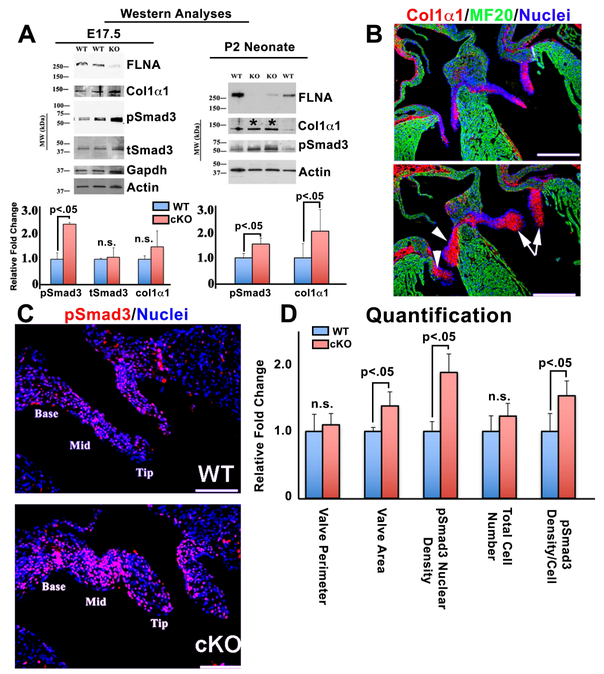
Due to the known inhibitory function of pErk1/2 in suppressing nuclear transport and/or activation of pSmad2/3(Kretzschmar et al., 1999; Sowa et al., 2002), we then examined whether the consequence of loss of pErk1/2 would lead to enhanced pSmad2/3 activity and increased downstream expression of collagen IαI, a known Smad2/3 target gene. Western analyses of single anterior leaflets at E17.5, revealed a statistically significant increase in pSmad3 activation Tie2Cre(+);FilaminAf/y knockout valves (Figure 2A). At this timepoint the increase in collagen I production in the conditional knockout was modest, but trended towards significance (p=.08). However, at the postnatal day 2 (P2) timepoint, Western analyses revealed a statistically significant increase in both pSmad3 and collagen IαI proteins (Figure 2B). This data was further supported by IHC showing increased collagen I throughout the entire mitral and tricuspid valves, no longer being restricted to the developing fibrosa layer (Figure 2C). Coincident with the Western data, IHC revealed a significant increase in pSmad3 activity in the mitral valve with a >50% increase in nuclear density of pSmad3 per cell (Figure 3D). Although this data does not directly prove that loss of pErk1/2 directly affects pSmad2/3 activities, it does demonstrate that loss of filamin-A has a negative effect on Erk activity while positively influencing Smad activation and a correlative increase in collagen expression.
Figure 3: Smad3 activation and collagen I expression increase in FLNA cKO valves.
(A) Western analyses for Smad3 and Collagen 1α1 of individual E17.5 and P2 mitral leaflets from WT and cKO FLNA mice. pSmad3 increases coincident with increased Col 1α1, being statistically significant at postnatal day 2 (P2) (Asterisks). (B) IHC for Col 1α1 (red) at E17.5 showing increased distribution throughout the FLNA cKO valves. (C) pSmad3 (red) staining and (D) quantification of staining showing a 50% increase in pSmad3 density/cell.
Loss of Filamin-A impairs beta1 integrin expression
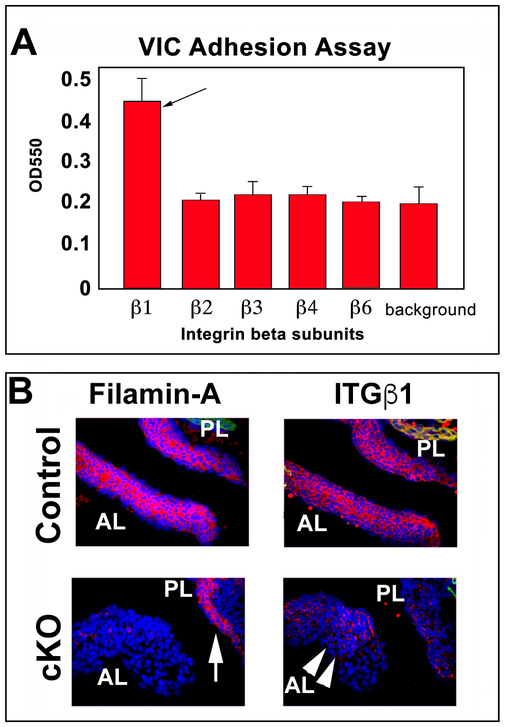
During our analyses, we noted that total cell number does not change in the Tie2Cre(+);FilaminAf/y knockout mitral valves compared to the controls. However, the valve area and volume (previously described) are significantly increased. Thus, the cell density (cells/area) is decreased in the conditional knockout, which we hypothesized was likely due to an increase in ECM production (as evidenced by the increase in collagen I protein) and failure of Filamin-A deficient valve interstitial cells to effectively compact the ECM. Compaction of ECM is largely dependent on integrin receptors that link contractile cytoskeletal forces to the ECM. Recognizing that R-Ras activity had previously been shown as important for integrin expression and activation(Sethi et al., 1999) combined with our data showing loss of R-Ras in the filamin-A knockout mice, led us to test whether integrin expression was modified in the filamin-A knockout valves. To initially test this hypothesis, we assayed which integrins are present on valve interstitial cells and could promote adhesion. As shown in figure 4A and consistent with ours and others previous reports(Latif et al., 2007; Ghatak et al., 2014; Duval et al., 2015), beta1 integrin was the primary subunit that was responsible for valve interstitial cell adhesion, suggesting this integrin subtype as a major contributor to cell-ECM contraction forces. IHC analyses confirmed that beta1 integrin expression in the Tie2Cre(+);FilaminAf/y knockout mitral valves was significantly abrogated compared to the controls and provides a plausible mechanism for altered compaction of the ECM, consistent with our previous reports(Sauls et al., 2012). Additionally, we noted that the beta1 integrin expression was also deficient in the atrial aspect of the posterior mitral leaflet, which retained Filamin-A expression (Figure 4B). This is indicative of a putative paracrine cross-talk mechanism whereby the endocardial-derived mesenchyme can influence protein expression on epicardial-derived cells.
Figure 4: Integrin profile in VICs and Beta1 integrin in Filamin-A cKO mitral leaflets.
(A) Adhesion assays showing beta1 (black arrow) as the primary beta subtype required for adhering VIC’s to the ECM. (B) Diminished β1-integrin (ITGβ1) protein expression (arrow heads) is observed in the FLNA cKO valves at E17.5. Residual filamin-A expression is noted in the posterior leaflet (white arrow), likely due to the epicardial-expressed filamin-A. Blue=nuclei, Red= Filamin-A or ITGβ1; AL, PL= Anterior or Posterior Leaflets, respectively.
DISCUSSION:
The amount of matrix produced during valvulogenesis ultimately defines its final form and function. While various growth factors are known to promote matrix production in the valves, the downstream mechanisms by which matrix synthesis is regulated in response to these signals remains an important, unresolved question. For example, Tgfb-Ligand binding to cognate receptors results in phosphorylation of the carboxyl-tail of Smad2/3 causing its nuclear transport and transcriptional activity(Wieser et al., 1993; Wrana et al., 1994; Massague and Weis-Garcia, 1996). Recent data has also demonstrated that nuclear transport of Smad2/3 can be affected by pErk1/2 activities. Following pErk1/2 phosphorylation of the Smad2/3 linker region, nuclear transport of Smad2/3:Smad4 is suppressed in a cell-type dependent manner(Kretzschmar et al., 1997a; Kretzschmar et al., 1997b; Kretzschmar and Massague, 1998; Calonge and Massague, 1999; Kretzschmar et al., 1999; Sowa et al., 2002; Massague, 2003). Thus, potential opposing regulatory inputs between Erk and growth factor signaling (e.g. Tgfb) to balance transcriptional activities through regulating the amount of Smad2/3 nuclear accumulation. We present data that supports a role for the cytoskeletal protein, Filamin-A in regulating this balancing act between Erk and Smads in the control of matrix synthesis. The amino end of the Filamin-A protein interacts directly with R-Ras to control its kinase activity. Loss of function of filamin-A in the Tie2Cre(+);FilaminAf/y knockout mitral valves exhibit decreased R-Ras and pErk1/2 while increasing pSmad3 and collagen I production. There remains a possibility that loss of filamin-A can lead to increased expression of Tgfb ligand and/or its receptors, which could lead to increased Tgfb signaling and the increase in pSmad2/3 activation. Thus, it is difficult at the current time to define whether the pSmad2/3 levels are a direct consequence of filamin-A interactions or whether they are secondary consequences of altered transcriptional outputs. Nonetheless, these data place filamin-A as a central regulator of ECM synthesis and compaction. Through balancing the amount/levels of Erk and Smad, as well as integrin activities, ECM synthesis and compaction are regulated by Filamin-A (Figure 5). This regulation culminates in remodeling of the mitral leaflets during fetal gestation and is critical for increased mechanical stability and ultimately, proper function. As such, alterations in the balance of these molecular signals would be anticipated in causing valvular heart defects.
Figure 5: Hypothetical model by which FLNA regulates the balance between Erk1/2 and Smad2/3 activation in driving matrix synthesis and compaction.
FLNA bound R-Ras can be activated through various receptor engagements (including TGFβ as depicted) leading to MAPK pathway activation of Erk1/2. pErk1/2 phosphorylates the pSmad2/3 linker region to inhibit nuclear import whereas phosphorylation of the Smad2/3 C-terminus by TGFβ-receptors result in nuclear import. Our data support a process by which pSmad2/3 nuclear import and beta1 integrin expression (possibly through R-Ras activities) are regulated through a filamin-A intermediate to balance the effects of ECM production and ECM compaction/remodeling.
The data presented in this report aim to address fundamental unanswered question in valve development on how ECM synthesis is regulated as well as how the matrix can be assembled into lamellar arrays of fibrous tissue required for normal valve function. Answering these questions is of clinical significance as valvular pathologies (e.g. Mitral Valve Prolapse) are primarily characterized by their alterations in ECM composition and lack of proper organization(Cole et al., 1984; Rabkin et al., 2001; Grande-Allen et al., 2003; Mahimkar et al., 2009). In the context of valvular diseases such as mitral valve prolapse, gene mutations present at conception frequently manifest as clinically relevant diseases later in life. However, we have now begun to appreciate that these genetic lesions lead to altered developmental processes that are not clinically evident at birth but result in increased susceptibility to disease or organ dysfunction as an individual ages. MVP, like most genetic diseases, shows variation in age at onset in families, despite the fact that the affected individuals all carry the same mutation. Mitral valve dysfunction and prolapse has been diagnosed in children and fetuses with Filamin-A mutations, but these mutations can also cause juvenile or adult onset disease(Kyndt et al., 1998; Kyndt et al., 2007). Data in our previous studies as well as in this report show morphogenetic consequences of altering causative mitral valve prolapse genes. Thus, genetic mutations, expressed at the time of valve morphogenesis and in particular cell lineages, leads to developmental defects that, over-time present clinically as MVP. These results define a developmental basis for MVP, and as such MVP should be viewed as a congenital heart defect. Only through the elucidation of relevant developmental pathways can we begin to understand how MVP genes (e.g. Filamin-A) regulates valve morphogenesis, with the goal of determining if these pathways can be modified for patient benefit in the future.
ACKNOWLEDGEMENTS:
We wish to specifically thank Dr. Markwald for his leadership in the field and for his pioneering discoveries, mentorship and friendship over the past decades. All of us in the cardiovascular developmental community (past, present and future) will be forever indebted to him.
Grant Information: The work at MUSC was performed in a facility constructed with support from the National Institutes of Health, Grant Number C06 RR018823 from the Extramural Research Facilities Program of the National Center for Research Resources. Other funding sources: National Heart Lung and Blood Institute: HL131546 (RAN) COBRE GM103444 (RAN), HL127692 (RAN), HL109506 and HL 141917 (RAL and JB), T32HL007260 (DF and KAT); American Heart Association: 17CSA33590067 (RAN), 16PRE30970048 (KAT)
REFERENCES CITED
- Avierinos JF, Gersh BJ, Melton LJ 3rd, Bailey KR, Shub C, Nishimura RA, Tajik AJ, Enriquez-Sarano M. 2002. Natural history of asymptomatic mitral valve prolapse in the community. Circulation 106:1355–1361. [DOI] [PubMed] [Google Scholar]
- Calonge MJ, Massague J. 1999. Smad4/DPC4 silencing and hyperactive Ras jointly disrupt transforming growth factor-beta antiproliferative responses in colon cancer cells. J Biol Chem 274:33637–33643. [DOI] [PubMed] [Google Scholar]
- Chambers JB, Shah BN, Prendergast B, Lawford PV, McCann GP, Newby DE, Ray S, Briffa N, Shanson D, Lloyd G, Hall R, British Heart Valve S. 2013. Valvular heart disease: a call for global collaborative research initiatives. Heart 99:1797–1799. [DOI] [PubMed] [Google Scholar]
- Chen HS, Kolahi KS, Mofrad MR. 2009. Phosphorylation facilitates the integrin binding of filamin under force. Biophys J 97:3095–3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole WG, Chan D, Hickey AJ, Wilcken DE. 1984. Collagen composition of normal and myxomatous human mitral heart valves. Biochem J 219:451–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Addario M, Arora PD, Ellen RP, McCulloch CA. 2002. Interaction of p38 and Sp1 in a mechanical force-induced, beta 1 integrin-mediated transcriptional circuit that regulates the actin-binding protein filamin-A. J Biol Chem 277:47541–47550. [DOI] [PubMed] [Google Scholar]
- d’Arcy JL, Prendergast BD, Chambers JB, Ray SG, Bridgewater B. 2011. Valvular heart disease: the next cardiac epidemic. Heart 97:91–93. [DOI] [PubMed] [Google Scholar]
- de Vlaming A, Sauls K, Hajdu Z, Visconti RP, Mehesz AN, Levine RA, Slaugenhaupt SA, Hagege A, Chester AH, Markwald RR, Norris RA. 2012. Atrioventricular valve development: new perspectives on an old theme. Differentiation 84:103–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Hoed M, Eijgelsheim M, Esko T, Brundel BJ, Peal DS, Evans DM, Nolte IM, Segre AV, Holm H, Handsaker RE, Westra HJ, Johnson T, Isaacs A, Yang J, Lundby A, Zhao JH, Kim YJ, Go MJ, Almgren P, Bochud M, Boucher G, Cornelis MC, Gudbjartsson D, Hadley D, van der Harst P, Hayward C, den Heijer M, Igl W, Jackson AU, Kutalik Z, Luan J, Kemp JP, Kristiansson K, Ladenvall C, Lorentzon M, Montasser ME, Njajou OT, O’Reilly PF, Padmanabhan S, St Pourcain B, Rankinen T, Salo P, Tanaka T, Timpson NJ, Vitart V, Waite L, Wheeler W, Zhang W, Draisma HH, Feitosa MF, Kerr KF, Lind PA, Mihailov E, Onland-Moret NC, Song C, Weedon MN, Xie W, Yengo L, Absher D, Albert CM, Alonso A, Arking DE, de Bakker PI, Balkau B, Barlassina C, Benaglio P, Bis JC, Bouatia-Naji N, Brage S, Chanock SJ, Chines PS, Chung M, Darbar D, Dina C, Dorr M, Elliott P, Felix SB, Fischer K, Fuchsberger C, de Geus EJ, Goyette P, Gudnason V, Harris TB, Hartikainen AL, Havulinna AS, Heckbert SR, Hicks AA, Hofman A, Holewijn S, Hoogstra-Berends F, Hottenga JJ, Jensen MK, Johansson A, Junttila J, Kaab S, Kanon B, Ketkar S, Khaw KT, Knowles JW, Kooner AS, Kors JA, Kumari M, Milani L, Laiho P, Lakatta EG, Langenberg C, Leusink M, Liu Y, Luben RN, Lunetta KL, Lynch SN, Markus MR, Marques-Vidal P, Mateo Leach I, McArdle WL, McCarroll SA, Medland SE, Miller KA, Montgomery GW, Morrison AC, Muller-Nurasyid M, Navarro P, Nelis M, O’Connell JR, O’Donnell CJ, Ong KK, Newman AB, Peters A, Polasek O, Pouta A, Pramstaller PP, Psaty BM, Rao DC, Ring SM, Rossin EJ, Rudan D, Sanna S, Scott RA, Sehmi JS, Sharp S, Shin JT, Singleton AB, Smith AV, Soranzo N, Spector TD, Stewart C, Stringham HM, Tarasov KV, Uitterlinden AG, Vandenput L, Hwang SJ, Whitfield JB, Wijmenga C, Wild SH, Willemsen G, Wilson JF, Witteman JC, Wong A, Wong Q, Jamshidi Y, Zitting P, Boer JM, Boomsma DI, Borecki IB, van Duijn CM, Ekelund U, Forouhi NG, Froguel P, Hingorani A, Ingelsson E, Kivimaki M, Kronmal RA, Kuh D, Lind L, Martin NG, Oostra BA, Pedersen NL, Quertermous T, Rotter JI, van der Schouw YT, Verschuren WM, Walker M, Albanes D, Arnar DO, Assimes TL, Bandinelli S, Boehnke M, de Boer RA, Bouchard C, Caulfield WL, Chambers JC, Curhan G, Cusi D, Eriksson J, Ferrucci L, van Gilst WH, Glorioso N, de Graaf J, Groop L, Gyllensten U, Hsueh WC, Hu FB, Huikuri HV, Hunter DJ, Iribarren C, Isomaa B, Jarvelin MR, Jula A, Kahonen M, Kiemeney LA, van der Klauw MM, Kooner JS, Kraft P, Iacoviello L, Lehtimaki T, Lokki ML, Mitchell BD, Navis G, Nieminen MS, Ohlsson C, Poulter NR, Qi L, Raitakari OT, Rimm EB, Rioux JD, Rizzi F, Rudan I, Salomaa V, Sever PS, Shields DC, Shuldiner AR, Sinisalo J, Stanton AV, Stolk RP, Strachan DP, Tardif JC, Thorsteinsdottir U, Tuomilehto J, van Veldhuisen DJ, Virtamo J, Viikari J, Vollenweider P, Waeber G, Widen E, Cho YS, Olsen JV, Visscher PM, Willer C, Franke L, Global BC, Consortium CA, Erdmann J, Thompson JR, Consortium PG, Pfeufer A, Consortium QG, Sotoodehnia N, Consortium Q-I, Newton-Cheh C, Consortium C-A, Ellinor PT, Stricker BH, Metspalu A, Perola M, Beckmann JS, Smith GD, Stefansson K, Wareham NJ, Munroe PB, Sibon OC, Milan DJ, Snieder H, Samani NJ, Loos RJ. 2013. Identification of heart rate-associated loci and their effects on cardiac conduction and rhythm disorders. Nat Genet 45:621–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux RB. 1989. Diagnosis and prognosis of mitral-valve prolapse. N Engl J Med 320:1077–1079. [DOI] [PubMed] [Google Scholar]
- Devereux RB, Brown WT, Kramer-Fox R, Sachs I. 1982. Inheritance of mitral valve prolapse: effect of age and sex on gene expression. Ann Intern Med 97:826–832. [DOI] [PubMed] [Google Scholar]
- Devereux RB, Kramer-Fox R, Kligfield P. 1989. Mitral valve prolapse: causes, clinical manifestations, and management. Ann Intern Med 111:305–317. [DOI] [PubMed] [Google Scholar]
- Devereux RB, Kramer-Fox R, Webb KH, Hochreiter C, Borer JS. 1986. Long-term follow-up of patients with mitral-valve prolapse. N Engl J Med 314:1119–1120. [DOI] [PubMed] [Google Scholar]
- Duren DR, Becker AE, Dunning AJ. 1988. Long-term follow-up of idiopathic mitral valve prolapse in 300 patients: a prospective study. J Am Coll Cardiol 11:42–47. [DOI] [PubMed] [Google Scholar]
- Durst R, Sauls K, Peal DS, deVlaming A, Toomer K, Leyne M, Salani M, Talkowski ME, Brand H, Perrocheau M, Simpson C, Jett C, Stone MR, Charles F, Chiang C, Lynch SN, Bouatia-Naji N, Delling FN, Freed LA, Tribouilloy C, Le Tourneau T, LeMarec H, Fernandez-Friera L, Solis J, Trujillano D, Ossowski S, Estivill X, Dina C, Bruneval P, Chester A, Schott JJ, Irvine KD, Mao Y, Wessels A, Motiwala T, Puceat M, Tsukasaki Y, Menick DR, Kasiganesan H, Nie X, Broome AM, Williams K, Johnson A, Markwald RR, Jeunemaitre X, Hagege A, Levine RA, Milan DJ, Norris RA, Slaugenhaupt SA. 2015. Mutations in DCHS1 cause mitral valve prolapse. Nature 525:109–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval D, Labbe P, Bureau L, Le Tourneau T, Norris RA, Markwald RR, Levine R, Schott JJ, Merot J. 2015. MVP-Associated Filamin A Mutations Affect FlnA-PTPN12 (PTP-PEST) Interactions. J Cardiovasc Dev Dis 2:233–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval D, Lardeux A, Le Tourneau T, Norris RA, Markwald RR, Sauzeau V, Probst V, Le Marec H, Levine R, Schott JJ, Merot J. 2014. Valvular dystrophy associated filamin A mutations reveal a new role of its first repeats in small-GTPase regulation. Biochim Biophys Acta 1843:234–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Chen MH, Moskowitz IP, Mendonza AM, Vidali L, Nakamura F, Kwiatkowski DJ, Walsh CA. 2006. Filamin A (FLNA) is required for cell-cell contact in vascular development and cardiac morphogenesis. Proc Natl Acad Sci U S A 103:19836–19841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox JW, Lamperti ED, Eksioglu YZ, Hong SE, Feng Y, Graham DA, Scheffer IE, Dobyns WB, Hirsch BA, Radtke RA, Berkovic SF, Huttenlocher PR, Walsh CA. 1998. Mutations in filamin 1 prevent migration of cerebral cortical neurons in human periventricular heterotopia. Neuron 21:1315–1325. [DOI] [PubMed] [Google Scholar]
- Gawecka JE, Griffiths GS, Ek-Rylander B, Ramos JW, Matter ML. 2010. R-Ras regulates migration through an interaction with filamin A in melanoma cells. PLoS One 5:e11269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehler S, Baldassarre M, Lad Y, Leight JL, Wozniak MA, Riching KM, Eliceiri KW, Weaver VM, Calderwood DA, Keely PJ. 2009. Filamin A-beta1 integrin complex tunes epithelial cell response to matrix tension. Mol Biol Cell 20:3224–3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghatak S, Misra S, Norris RA, Moreno-Rodriguez RA, Hoffman S, Levine RA, Hascall VC, Markwald RR. 2014. Periostin induces intracellular cross-talk between kinases and hyaluronan in atrioventricular valvulogenesis. J Biol Chem 289:8545–8561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould RA, Yalcin HC, MacKay JL, Sauls K, Norris RA, Kumar S, Butcher JT. 2015. Cyclic mechanical loading is essential for Rac1 mediated elongation and remodeling of the embryonic mitral valve Currernt Biology In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grande-Allen KJ, Griffin BP, Ratliff NB, Cosgrove DM, Vesely I. 2003. Glycosaminoglycan profiles of myxomatous mitral leaflets and chordae parallel the severity of mechanical alterations. J Am Coll Cardiol 42:271–277. [DOI] [PubMed] [Google Scholar]
- Griffiths GS, Grundl M, Allen Iii JS, Matter ML. 2011. R-Ras interacts with Filamin A to maintain endothelial barrier function. J Cell Physiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigioni F, Tribouilloy C, Avierinos JF, Barbieri A, Ferlito M, Trojette F, Tafanelli L, Branzi A, Szymanski C, Habib G, Modena MG, Enriquez-Sarano M, Investigators M. 2008. Outcomes in mitral regurgitation due to flail leaflets a multicenter European study. JACC Cardiovascular imaging 1:133–141. [DOI] [PubMed] [Google Scholar]
- Kim H, Nakamura F, Lee W, Hong C, Perez-Sala D, McCulloch CA. 2010. Regulation of cell adhesion to collagen via beta1 integrins is dependent on interactions of filamin A with vimentin and protein kinase C epsilon Exp Cell Res In Press. [DOI] [PubMed] [Google Scholar]
- Krenz M, Gulick J, Osinska HE, Colbert MC, Molkentin JD, Robbins J. 2008. Role of ERK1/2 signaling in congenital valve malformations in Noonan syndrome. Proc Natl Acad Sci U S A 105:18930–18935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretzschmar M, Doody J, Massague J. 1997a. Opposing BMP and EGF signalling pathways converge on the TGF-beta family mediator Smad1. Nature 389:618–622. [DOI] [PubMed] [Google Scholar]
- Kretzschmar M, Doody J, Timokhina I, Massague J. 1999. A mechanism of repression of TGFbeta/ Smad signaling by oncogenic Ras. Genes Dev 13:804–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretzschmar M, Liu F, Hata A, Doody J, Massague J. 1997b. The TGF-beta family mediator Smad1 is phosphorylated directly and activated functionally by the BMP receptor kinase. Genes Dev 11:984–995. [DOI] [PubMed] [Google Scholar]
- Kretzschmar M, Massague J. 1998. SMADs: mediators and regulators of TGF-beta signaling. Curr Opin Genet Dev 8:103–111. [DOI] [PubMed] [Google Scholar]
- Kyndt F, Gueffet JP, Probst V, Jaafar P, Legendre A, Le Bouffant F, Toquet C, Roy E, McGregor L, Lynch SA, Newbury-Ecob R, Tran V, Young I, Trochu JN, Le Marec H, Schott JJ. 2007. Mutations in the gene encoding filamin A as a cause for familial cardiac valvular dystrophy. Circulation 115:40–49. [DOI] [PubMed] [Google Scholar]
- Kyndt F, Schott JJ, Trochu JN, Baranger F, Herbert O, Scott V, Fressinaud E, David A, Moisan JP, Bouhour JB, Le Marec H, Benichou B. 1998. Mapping of X-linked myxomatous valvular dystrophy to chromosome Xq28. Am J Hum Genet 62:627–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lardeux A, Kyndt F, Lecointe S, Marec HL, Merot J, Schott JJ, Le Tourneau T, Probst V. 2011. Filamin-a-related myxomatous mitral valve dystrophy: genetic, echocardiographic and functional aspects. J Cardiovasc Transl Res 4:748–756. [DOI] [PubMed] [Google Scholar]
- Latif N, Sarathchandra P, Thomas PS, Antoniw J, Batten P, Chester AH, Taylor PM, Yacoub MH. 2007. Characterization of structural and signaling molecules by human valve interstitial cells and comparison to human mesenchymal stem cells. J Heart Valve Dis 16:56–66. [PubMed] [Google Scholar]
- Le Tourneau T, Le Scouarnec S, Cueff C, Bernstein D, Aalberts JJJ, Lecointe S, Merot J, Bernstein JA, Oomen T, Dina C, Karakachoff M, Desal H, Al Habash O, Delling FN, Capoulade R, Suurmeijer AJH, Milan D, Norris RA, Markwald R, Aikawa E, Slaugenhaupt SA, Jeunemaitre X, Hagege A, Roussel JC, Trochu JN, Levine RA, Kyndt F, Probst V, Le Marec H, Schott JJ. 2017. New insights into mitral valve dystrophy: a Filamin-A genotype-phenotype and outcome study. Eur Heart J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine RA, Slaugenhaupt SA. 2007. Molecular genetics of mitral valve prolapse. Curr Opin Cardiol 22:171–175. [DOI] [PubMed] [Google Scholar]
- Lincoln J, Alfieri CM, Yutzey KE. 2006. BMP and FGF regulatory pathways control cell lineage diversification of heart valve precursor cells. Dev Biol 292:292–302. [DOI] [PubMed] [Google Scholar]
- Mahimkar R, Nguyen A, Mann M, Yeh CC, Zhu BQ, Karliner JS, Lovett DH. 2009. Cardiac transgenic matrix metalloproteinase-2 expression induces myxomatous valve degeneration: a potential model of mitral valve prolapse disease. Cardiovasc Pathol 18:253–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massague J 2003. Integration of Smad and MAPK pathways: a link and a linker revisited. Genes Dev 17:2993–2997. [DOI] [PubMed] [Google Scholar]
- Massague J, Weis-Garcia F. 1996. Serine/threonine kinase receptors: mediators of transforming growth factor beta family signals. Cancer Surv 27:41–64. [PubMed] [Google Scholar]
- Norris RA, Moreno-Rodriguez R, Wessels A, Merot J, Bruneval P, Chester AH, Yacoub MH, Hagege A, Slaugenhaupt SA, Aikawa E, Schott JJ, Lardeux A, Harris BS, Williams LK, Richards A, Levine RA, Markwald RR. 2010. Expression of the familial cardiac valvular dystrophy gene, filamin-A, during heart morphogenesis. Dev Dyn 239:2118–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oegema R, Hulst JM, Theuns-Valks SD, van Unen LM, Schot R, Mancini GM, Schipper ME, de Wit MC, Sibbles BJ, de Coo IF, Nanninga V, Hofstra RM, Halley DJ, Brooks AS. 2013. Novel no-stop FLNA mutation causes multi-organ involvement in males. American journal of medical genetics Part A 161A:2376–2384. [DOI] [PubMed] [Google Scholar]
- Rabkin E, Aikawa M, Stone JR, Fukumoto Y, Libby P, Schoen FJ. 2001. Activated interstitial myofibroblasts express catabolic enzymes and mediate matrix remodeling in myxomatous heart valves. Circulation 104:2525–2532. [DOI] [PubMed] [Google Scholar]
- Robertson SP, Twigg SR, Sutherland-Smith AJ, Biancalana V, Gorlin RJ, Horn D, Kenwrick SJ, Kim CA, Morava E, Newbury-Ecob R, Orstavik KH, Quarrell OW, Schwartz CE, Shears DJ, Suri M, Kendrick-Jones J, Wilkie AO, Group OP-sDCC. 2003. Localized mutations in the gene encoding the cytoskeletal protein filamin A cause diverse malformations in humans. Nat Genet 33:487–491. [DOI] [PubMed] [Google Scholar]
- Sauls K, de Vlaming A, Harris BS, Williams K, Wessels A, Levine RA, Slaugenhaupt SA, Goodwin RL, Pavone LM, Merot J, Schott JJ, Le Tourneau T, Dix T, Jesinkey S, Feng Y, Walsh C, Zhou B, Baldwin S, Markwald RR, Norris RA. 2012. Developmental basis for filamin-A-associated myxomatous mitral valve disease. Cardiovasc Res 96:109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauls K, Toomer K, Williams K, Johnson AJ, Markwald RR, Hajdu Z, Norris RA. 2015. Increased Infiltration of Extra-Cardiac Cells in Myxomatous Valve Disease. J Cardiovasc Dev Dis 2:200–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi T, Ginsberg MH, Downward J, Hughes PE. 1999. The small GTP-binding protein R-Ras can influence integrin activation by antagonizing a Ras/Raf-initiated integrin suppression pathway. Mol Biol Cell 10:1799–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowa H, Kaji H, Yamaguchi T, Sugimoto T, Chihara K. 2002. Activations of ERK1/2 and JNK by transforming growth factor beta negatively regulate Smad3-induced alkaline phosphatase activity and mineralization in mouse osteoblastic cells. J Biol Chem 277:36024–36031. [DOI] [PubMed] [Google Scholar]
- Stossel TP, Condeelis J, Cooley L, Hartwig JH, Noegel A, Schleicher M, Shapiro SS. 2001. Filamins as integrators of cell mechanics and signalling. Nat Rev Mol Cell Biol 2:138–145. [DOI] [PubMed] [Google Scholar]
- van der Werf CS, Sribudiani Y, Verheij JB, Carroll M, O’Loughlin E, Chen CH, Brooks AS, Liszewski MK, Atkinson JP, Hofstra RM. 2013. Congenital short bowel syndrome as the presenting symptom in male patients with FLNA mutations. Genet Med 15:310–313. [DOI] [PubMed] [Google Scholar]
- Wieser R, Attisano L, Wrana JL, Massague J. 1993. Signaling activity of transforming growth factor beta type II receptors lacking specific domains in the cytoplasmic region. Mol Cell Biol 13:7239–7247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrana JL, Attisano L, Wieser R, Ventura F, Massague J. 1994. Mechanism of activation of the TGF-beta receptor. Nature 370:341–347. [DOI] [PubMed] [Google Scholar]
- Zhou AX, Hartwig JH, Akyurek LM. 2010. Filamins in cell signaling, transcription and organ development. Trends Cell Biol 20:113–123. [DOI] [PubMed] [Google Scholar]
- Zhou X, Boren J, Akyurek LM. 2007. Filamins in cardiovascular development. Trends Cardiovasc Med 17:222–229. [DOI] [PubMed] [Google Scholar]