Abstract
The objective of this study was to formulate aripiprazole (ARI)-loaded pH-modulated solid dispersions (SD) to enhance solubility, dissolution, and bioavailability via hot-melt extrusion (HME) technology. Kollidon® 12 PF (PVP) and succinic acid (SA) were selected after solubility screenings of various polymers and acidifiers. Several formulations, varying in screw speed and drug/polymer/acidifier ratios, were extruded using an 11 mm twin-screw extruder and were investigated for the effect of these variables. Scanning electron microscopy (SEM), differential scanning calorimetry (DSC), and X-ray diffraction (XRD) were used to perform solid-state characterizations of the pure drug and extrudates. The aqueous solubility and dissolution were evaluated for the pure drug and milled extrudates. Among the prepared formulations, N6 was chosen for in vivo absorption studies. Solid-state characterization demonstrated the transformation of the crystalline ARI to an amorphous state in the formulations. Each formulation showed increased solubility and dissolution compared to the drug powder. The oral bioavailability (Cmax and AUC0–12) of N6 was significantly improved when compared to the pure ARI. This novel study not only discusses the incorporation of acidifiers in SDs but also the preparation of SDs using HME technology as effective techniques to improve drug release and bioavailability.
Keywords: Aripiprazole, Acidifier, Hot-melt extrusion, Solid dispersion, Pharmacokinetics, Oral bioavailability
1. Introduction
It is well known that poorly water-soluble drugs have limited dissolution behavior and consequently, low bioavailability. Also, these types of drugs are often weakly acidic or basic, having a pH dependent solubility (Tran et al., 2010). Converting crystalline drugs into an amorphous form is a common method to improve the dissolution kinetics and bioavailability of such drugs. Highly crystalline compounds have an ordered lattice structure that requires more energy for the dissolution media to disrupt compared the chaotic amorphous form (Fousteris et al., 2013, Konno et al., 2008). However, the disadvantages to amorphous compounds are that they are thermodynamically unstable and might recrystallize. Therefore, studies focusing on the solubilization of poorly-water soluble drugs are becoming more popular in the pharmaceutical industry.
Various techniques are available to enhance the dissolution and bioavailability of poorly water-soluble drugs. Among those techniques that have been successful are nanoparticle formation (Ma and Williams et al., 2018, Yousaf et al., 2016), particle size reduction (Jinno et al., 2006, Loh et al., 2015), salt formation (Grifasi et al., 2015, He et al., 2018), complexation with hydrophilic substances (Chi et al., 2015, Mennini et al., 2016), and solid dispersion (Huang and Dai, 2014, Kim et al., 2016, Rashid et al., 2015, Vasconcelos et al., 2007). Solid dispersions (SD) have shown to be one of the most successful ways to enhance the solubility and therefore, dissolution of drugs.
In short, solid dispersion is defined as a molecular mixture of drug in an inert carrier, often a hydrophilic carrier. The transformation of the crystalline drug to the amorphous form and reduction of particle size for better wettability are the main mechanisms in which a SD is able to improve drug dissolution (Tran et al., 2008, Tran et al., 2010). The polymers in solid dispersions also help to stabilize the amorphous form of drug and prevent recrystallization. The mechanisms responsible for these effects include antiplasticization by the polymers, drug-polymer interactions in the SD, polymeric carrier viscosity and the glass transition temperature of the SD (Andrews et al., 2010, Fousteris et al., 2013, Konno et al., 2008). In this study, the solid dispersions were developed using hot-melt extrusion (HME) technology.
HME is often used to prepare SDs in attempt to overcome the challenge of low bioavailability drugs. HME is a continuous process that operates under defined conditions. The following parameters can be varied in the HME process to achieve a consistent product: screw rotating speed, rate of the feed materials, temperature, and shear (Repka et al., 2011). Raw materials are fed through a heated barrel, containing rotating screws that are divided into different zones for mixing and conveying. The materials are also melted and plasticized as they travel the length of the barrel. Several advantages of using HME technology are short processing time, continuous process, solvent-free, and scalable process (Hwang et al., 2017, Repka et al., 2018). HME has shown to be successful in improving the bioavailability due to the uniform drug dispersion of the final product.
In addition to solid dispersions, modulation of pH by a pH modifier is another effective method to manipulate the release rate of poorly water-soluble drugs to improve bioavailability (Tran et al., 2008, Tran et al., 2009, Tran et al., 2010). In this study, the addition of an acidifier as a pH modifier to the SD was studied to determine the effect it had on the solubility of aripiprazole (ARI). Acidifiers are considered to be an important component to maintaining the micro-environment pH (pHm) for weakly basic drugs. The pH modifier is released upon dissolution and surrounds the drug particles, modulating the pH to improve drug dissolution. Lower pHm are necessary to enhance the drug dissolution for drugs with a higher solubility in more acidic environments (Taniguchi et al., 2014, Tran et al., 2010).
The objective of this study was to formulate solid dispersions via HME technology with an acidifier incorporated as a pH modifier. Aripiprazole (ARI, Figure S1A), a weak alkaline drug with pH-dependent solubility in aqueous solutions (Borbás et al., 2015, Xu et al., 2012), was chosen as the model drug. Since many drugs are pH-dependent, this study provides mechanistic insights on the importance of pH modifiers in the SDs in order to enhance bioavailability. Also, few drugs on the market are now being produced using HME technology. As this method gains popularity in the pharmaceutical industry, more research is needed on the possibility of SDs being produced by HME technology (Kadam and Desai, 2017). This study is innovative in that it incorporates two techniques, hot-melt extrusion and pH modulation, in developing solid dispersions that are successful in improving drug dissolution and bioavailability of pH dependent drugs.
2. Materials and methods
2.1. Materials
Aripiprazole (melting point at 137–142°C) was obtained from Nexconn Pharmatechs Ltd. (Tseung Kwan O, Hong Kong). Phenacetin (internal standard, IS, Figure S1B) was purchased from Tokyo Chemical Industry Co., Ltd (Tokyo, Japan). Acidifiers (adipic acid, citric acid, fumaric acid, malic acid, maleic acid, stearic acid, succinic acid, and tartaric acid) were purchased from Spectrum Quality Products, Inc. (Gardena, CA, USA). Kollidon® 12 PF and Soluplus® were generously supplied by BASF Corporation (Florham Park, NJ, USA). AquaSolve™, HPMCAS-LG, Klucel™ EXF, Benecel™ E15, Benecel™ K15M, and Natrosol™ 250L were gifted by Ashland, Inc. (Lexington, KY, USA). All other chemicals used were reagent grade and were used without further purification.
2.2. Solubility screening of the acidifiers and polymers
The acidifiers and polymers listed above were screened to select the appropriate components for the SDs. The samples contained 15 mL of deionized water and 150 mg of acidifier or polymer to make 1% aqueous solutions. Excess amount of ARI was added to a 2.0 mL microtube containing 1.2 mL of each aqueous solution. The aqueous solutions were vortexed vigorously and placed in a shaking water bath at 100 RPM and 37°C for five days. Then, they were centrifuged for 10 minutes at 25°C and 13200 RPM to separate the undissolved ARI. The supernatant solutions were filtered and diluted with deionized water for quantification of ARI by an UV-Vis spectrophotometer (Genesys 6, Thermo Scientific, USA). The absorbance was measured at 249 nm with a spectrophotometer against deionized water as a blank. All tests were repeated in triplicates.
2.3. Pre-formulation thermal analysis (DSC and TGA)
The miscibility, interaction and thermal stability of ARI, succinic acid (SA), Kollidon® 12 PF (PVP), and physical mixture (PM, 1:1:1 weight ratio) were analyzed using DSC (Perkin Elmer Diamond DSC) and TGA (Perkin Elmer Pyris 1 TGA) instruments. For the DSC, all samples weighed about 5 mg and were sealed in an aluminum pan. An empty aluminum pan was used as a reference. The samples were heated under nitrogen gas for one minute at 25°C and then heated to 200°C at a rate of 10°C per minute. The thermal stability of the components and the PM was evaluated using TGA over the temperature range of 60°C to 220°C. About 3–4 mg of samples were placed in an aluminum crucible and heated at 10°C per minute under a controlled atmosphere of nitrogen. Percent weight loss was plotted against temperature to determine weight loss.
2.4. Preparation of pH-modulated solid dispersions and tablets
ARI (20–40% w/w), PVP (60–80% w/w) and SA (0–10% w/w) were blended using a V-shell blender (Maxiblend, GlobePharma) and extruded at 120°C using a twin-screw extruder (Process 11, Thermo Fisher Scientific) with a standard configuration. The screw speed was varied from 80 to 120 RPM. Then, each extrudate was milled into a fine powder using a laboratory grinder and sieved using a #30 ASTM mesh. For the tablets, MCC (microcrystalline cellulose), Ac-Di-Sol (croscarmellose sodium), and MS (magnesium stearate) were chosen as the excipients in a weight ratio of 90:9:1, respectively. A combined 300 mg of formulation and excipients were compressed to make tablets for the dissolution study (Table S1). Each tablet contained 30 mg of drug, which is comparable to the amount of drug in the commercialized product. The compression for the tablets was 35 kg/cm to maintain a breaking force around 10 kp. A single punch tablet press with 10 mm punches was used to compress the tablets.
2.5. Solubility and dissolution of formulations
The aqueous solubility and % drug release were evaluated for the milled extrudates and the pure drug. For the solubility study, an excess amount of the formulation was added to 1.2 mL of deionized water. The samples were then placed in a sonication machine to ensure complete mixing. The samples were made in triplicates and placed in a shaking water bath at 37°C and 100 RPM for five days. Then, they were centrifuged for 10 minutes at 25°C and 13200 RPM. The absorbance of each sample was evaluated using UV spectroscopy at 249 nm. The dissolution studies were performed using a Hanson SR8-Plus™ Dissolution Test Station. The tablets were placed in 900 mL of distilled water at 37 ± 0.5°C to determine drug release. The paddle method was employed at a rate of 75 RPM. One milliliter of the media was extracted for each tablet at 5, 10, 15, 20, 30, 45, and 60 minutes and an equivalent volume of fresh media was replaced. The absorbance was measured at 249 nm with a spectrophotometer against deionized water as a blank. All tests were repeated in triplicates.
2.6. Design of experiment
MODDE 8.0 was the design of experiment (DoE) software applied to identify the effect of screw speed and drug/polymer/acidifier ratio on solubility and dissolution. It used a 23 full factorial design to provide 11 formulations that varied the drug content from 20% to 40%, the acidifier content from 0% to 10%, and the polymer content from 60% to 80%. The screw speed of the extrusion process was also varied from 80 to 120 RPM. The hot-melt extrusion formulations are shown in Table 1.
Table 1.
Hot-melt extrusion formulations for solid dispersions
| Formulation Name | Screw Speed (RPM) | Drug Content (%) | Acidifier Content (%) | Polymer Content (%) |
|---|---|---|---|---|
| N1 | 80 | 20 | 0 | 80 |
| N2 | 120 | 20 | 0 | 80 |
| N3 | 80 | 40 | 0 | 60 |
| N4 | 120 | 40 | 0 | 60 |
| N5 | 80 | 20 | 10 | 70 |
| N6 | 120 | 20 | 10 | 70 |
| N7 | 80 | 40 | 10 | 50 |
| N8 | 120 | 40 | 10 | 50 |
| N9 | 100 | 30 | 5 | 65 |
| N10 | 100 | 30 | 5 | 65 |
| N11 | 100 | 30 | 5 | 65 |
2.7. Solid-state characterizations
SEM, DSC, and XRD were used to perform solid-state characterizations of the pure drug and optimal formulation. SEM (JSM-5600 SEM) was used to explore the shape and surface morphology of ARI, SA, PVP, and N6 formulation. For DSC, around 5 mg of sample was sealed in an aluminum pan with an empty aluminum pan for comparison. The samples were heated at 25°C for one minute before being heated to 200°C at a rate of 10°C per minute. Nitrogen gas was used in the DSC analysis. The solid state of ARI, SA, PVP, and N6 was determined with a powder X-ray diffraction apparatus (Bruker AXS, Madison, MI), using CuKαradiation at 40kV generator voltage and 40 mA current. The diffraction angles ranged from 5o to 40°(2θ) with a scanning rate at 2°per minute.
2.8. Dissolution of optimal formulation
The dissolution studies were performed using a Hanson SR8-Plus™ Dissolution Test Station. ARI, N6, and N6 tablet blend, containing 30 mg of drug, were placed in 900 mL of deionized water and simulated gastric fluid (SGF) without enzymes at 37 ± 0.5°C to determine the drug release. The paddle method was employed at a rate of 75 RPM. One milliliter of the media was extracted for each tablet at 5, 10, 15, 20, 30, 45, and 60 minutes and an equivalent volume of fresh media was replaced. The absorbance was measured at 249 nm with a spectrophotometer against deionized water or SGF without enzymes as a blank. All tests were repeated in triplicates.
2.9. Liquid chromatography
The chromatographic analysis was performed using Acquity™ Ultra Performance LC, equipped with binary solvent and sample manager (Waters, Milford, MA, USA). The 5 μL aliquots of processed samples were injected into an Acquity UPLC® HSS T3 (1.8 μM, 2.1 X 50 mm, Milford, MA, USA). The binary mobile phase system consisted of reservoir A (0.1% formic acid in Milli Q water) and reservoir B (acetonitrile) which ran on a gradient program with a flow rate of 0.3 mL/min. This was used throughout the analytical run of 4 minutes.
2.10. Mass spectrometry
Quantitation was achieved by MS/MS detection in positive ion mode for analyte and IS (phenacetin) using a Waters Xevo® TQD (Waters, Milford, MA, USA) mass spectrometer, equipped with standard electrospray ionization (ESI). The source parameters viz., source temperature, desolvation temperature, cone gas flow, and desolvation gas were 150°C, 200°C, 25 L/h, and 650 L/h, respectively. Nitrogen was used as the nebulizer and cone gas while argon was used as the collision gas. The ESI conditions were optimized for ARI and IS by performing quadrupole full scan in positive ion detection mode. Analytes were infused directly; the mass spectra for ARI and IS revealed peaks at m/z 450.18 and 180.9, respectively, as protonated molecular ions [M+H]+. The mass spectrometry conditions were optimized, and the compound parameters obtained were used for quantification purposes. The compound parameters viz. cone voltage and collision energy for ARI were 12 and 16 V, respectively. These parameters for IS were 64 and 24 V, respectively. Detection of the ions was performed in the multiple reaction monitoring mode: monitoring the transition of the m/z 450.18 precursor ion to the m/z 287.03 product ion for ARI; 180.9 m/z precursor ion to the m/z 136.88 product ion for the IS. The analytical data were processed by MassLynx software.
2.11. Preparation of calibration standards
The primary stock solution of 1 mg/mL of ARI was prepared by dissolving ARI in methanol. This stock solution was diluted with 80% methanol for working standards of ARI. The working standards were used to develop calibration standards. The working IS solution of 2000 ng/mL was prepared in acetonitrile. To an aliqot of 45 μL of plasma, 5 μL of working standards and 20 μL of IS were spiked to prepare the calibration standard in the range of 5 to 4000 ng/mL.
2.12. Animals
Male Sprague–Dawley rats with body weights of 250 ± 20g were purchased from Envigo and were given time to acclimate. The animals were housed in a room where the temperature and humidity were controlled with a 12 h light/dark cycle. Necessary approval from the Institutional Animal Ethics Committee was obtained to carry out the experiments. The study was conducted per study protocol (Number: 16–017) at the University of Mississippi, School of Pharmacy, USA.
2.13. Pharmacokinetic experiment
The pharmacokinetic study of ARI, N6, and N6 tablet blend was performed in rats. The rats were fasted overnight but were allowed free access to water. Eighteen rats were randomly divided into three groups (n=6). The rats were intragastrically administered a single dose of 20 mg/kg ARI in a 0.5% HPMC suspension. Then, 0.3 mL of blood was sampled from the jugular vein at 5 min, 15 min, 1 h, 2 h, 4 h, 8 h, 12 h, and 24 h after administration. Approximately 0.2 ml of plasma was obtained by centrifuging the blood samples at 8000 RPM for 10 min and 4°C. Plasma samples were kept at −80°C until further analysis.
2.14. Sample Preparation
The protein precipitation extraction trials were performed using organic solvents, acetonitrile and methanol, with and without acidification and alkalinizing to enhance the recovery of analyte from rat plasma. The recovery of ARI and IS from plasma samples was evaluated. The peak area ratio of analyte and IS was considered for calculation. ARI and IS were extracted from plasma using protein precipitation method in which 200 μL of acetonitrile was added to 50 μL plasma samples with analyte and IS and then vortexed for 2 min. These samples were centrifuged at 13000 RPM and 4°C before supernatants were transferred to vials for analysis. The study demonstrated that the recovery of ARI from rat plasma was higher, concentration independent, and reproducible with acidified acetonitrile. The extraction recovery for ARI and IS from rat plasma was 91.70 ± 2.95 % and 94.30 ± 4.12 %, respectively.
2.15. Statistical analyses
Experimental values were calculated as mean ± standard deviation. The plasma concentration–time curve was plotted where each time point represented n=6. The pharmacokinetic parameters were calculated with the mean plasma concentration of rats at each time point. The pharmacokinetic parameters were estimated with non-compartmental analysis by Phoenix® WinNolin™ 6.4 (Cetara®, Princeton, NJ, USA). The area under the concentration–time curve (AUC0–12) was estimated from 0 to 12 h with trapezoidal summations. The statistical analyses were evaluated by a Student’s unpaired t-test with a p-value less than 0.05 as statistically significant.
3. Results and discussion
3.1. Pre-formulation solubility screening
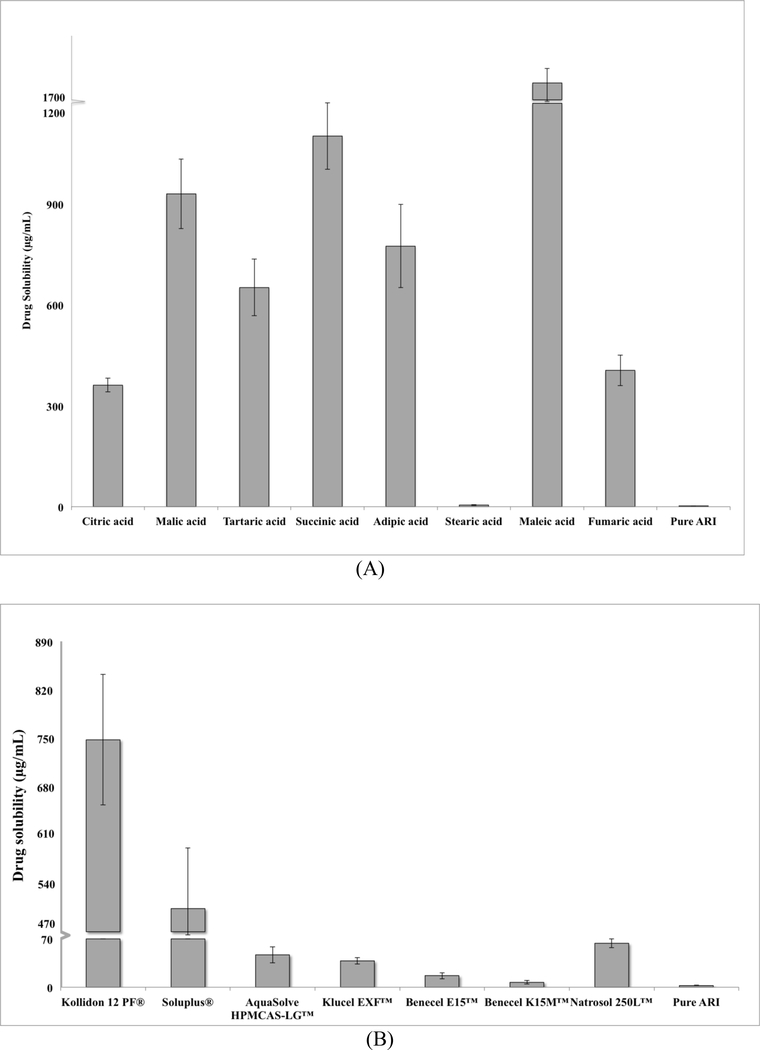
ARI, a weakly basic drug, has been known to have a pH-dependent solubility (Mihajlovic et al., 2012). Preliminary studies showed that ARI had higher levels of drug solubility in more acidic environments (Figure S2). ARI was most soluble at pH values of 1.2 and 4.0 and had very low solubility in pH solutions above 6.8. Appropriate acidifying agents and hydrophilic polymers were screened to improve the solubility and dissolution of ARI. The various polymers and acidifiers were prepared as 1% aqueous solutions to test the drug solubility in each solution. UV spectroscopy was employed to evaluate the solubility of ARI in each polymer and acidifier. The samples were analyzed at 249 nm which was the wavelength used in a study by Silki and Sinha (2018). All of the acidifiers, except for stearic acid, significantly increased the drug solubility compared to the pure drug. Figure 1A showed that ARI had the highest level of drug solubility with maleic acid (1750 ± 390 μg/mL). However, maleic acid was not hot-melt extrudable due to stability issues at low temperatures. Therefore, succinic acid was chosen as the acidifier since ARI had the second highest level of solubility with SA (1100 ± 100 μg/mL). Most of the acidifiers increased the drug solubility above 300 μg/mL whereas most of the polymers were less than 300 μg/mL. The solubility of ARI with the polymers was very dependent on the type of polymer. Figure 1A showed that ARI had the highest amount of solubility in Kollidon® 12 PF (PVP, 750 ± 95 μg/mL), which was chosen as the polymer for this study.
Figure 1.
Solubility of ARI in various (A) acidifiers and (B) polymers
3.2. DSC and TGA of formulation components
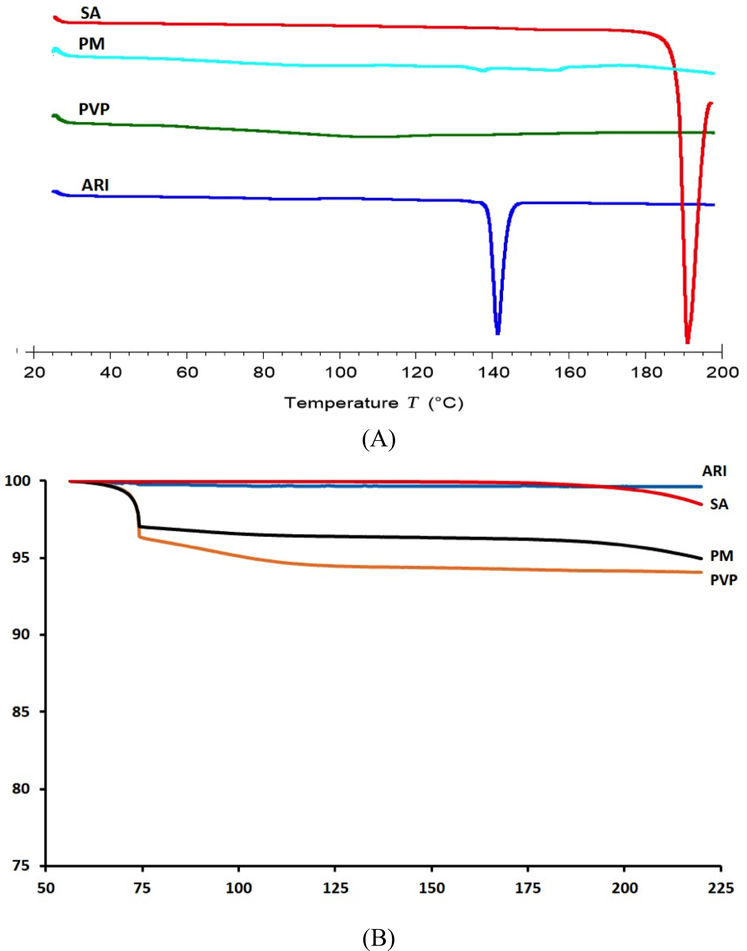
The thermal stabilities of ARI, SA, PVP, and PM were analyzed using DSC and TGA techniques. These techniques can provide information about the decomposition, melting, recrystallization, or change in specific heat capacity for each component (Marasini et al., 2013). The curves for each component in the DSC are shown in Figure 2A. ARI had a peak around 140°C which corresponded to its melting point. PVP displayed a horizontal line over the temperature range analyzed, indicating it is amorphous. Succinic acid had a melting point around 190°C. The physical mixture had a small peak around 140°C, which correlated with the melting temperature of ARI. This peak indicated that as the components in the PM are heated, there are no interactions between the ARI, SA, and PVP. TGA was used to determine the decomposition of the components from 60°C to 220°C. The curves provided useful information for the hot-melt extrusion temperature to avoid decomposition. In Figure 2B, the ARI and SA curves did not contain any moisture nor did they decompose over the temperature range. PVP, a highly hydrophilic polymer, had about a 5 wt% moisture content, which was also evident in the PM curve. The 5% weight loss was the water being evaporated at lower temperatures.
Figure 2.
DSC (A) and TGA (B) curves for ARI, PVP, SA, and PM
3.3. Hot-melt extrusion technology
MODDE 8.0 design of experiment software was employed to develop several formulations that varied in screw speed and drug/acidifier/polymer content to investigate these effects on the solubility and dissolution of ARI. The results from the computation are provided in Table 1. The formulations were extruded at 120°C because this was the lowest temperature at which they could be extruded. Lower temperatures are more likely to a produce a product with more physical stability. Figure S3 illustrated the physical differences in each of the formulations. N1–2 and N5–6 contained 20% of ARI while N3–4 and N7–8 contained 40% of ARI. N1–4 contained no acidifier while N5–8 contained 10% of acidifier. N9–11 all contained 30% ARI and 5% SA. Figure S3 showed that the varying content of ARI, SA, and PVP changed the coloring of the extrudates.
3.4. Solubility and dissolution of the formulations
Hot-melt extrusion is a growing technology in the pharmaceutical industry to develop solid dispersions. With this technology, the SDs are created by dispersing the drug within a hydrophilic polymeric carrier as the components are melted and mixed throughout the length of the heated barrel of the extruder. The SDs improve the drug release in aqueous media, which in turn enhances bioavailability. The SD is able to achieve this improvement by converting the crystalline drug into an amorphous form. The ability of SDs to enhance the bioavailability, especially for poorly water-soluble drugs, is one of its great advantages.
Modulation of pH has been reported as another way to manipulate the drug release for pH-dependent drugs. For weakly basic drugs, such as ARI, including a weak acid as a pH modifier in the solid dispersion can enhance the drug release. The pH modifier achieves this by lowering the micro-environment pH, which is described as the pH of the saturated solution in the environment immediately surrounding the drug particles (Tran et al., 2008). The dissolution media penetrates the tablet, causing the acidifier to release and envelope the drug particles (Tran et al., 2010). The acidifier helps to maintain an optimal pH near the drug particles to improve drug release, which is important for pH dependent drugs.
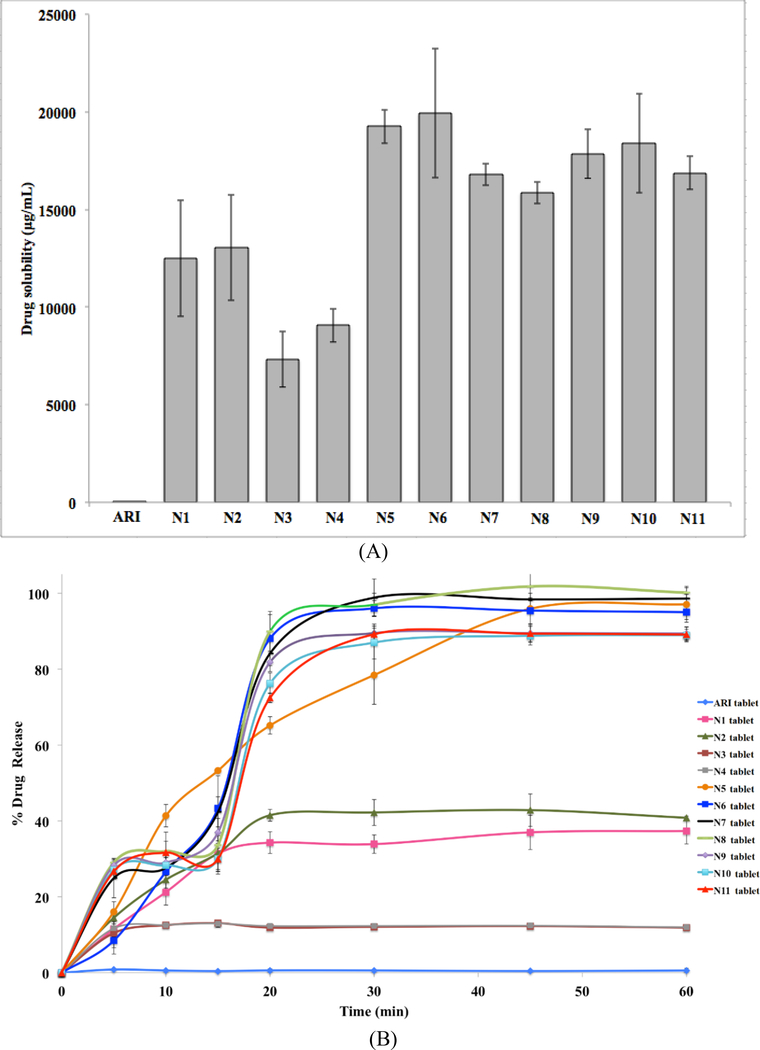
The effects on ARI of the various formulations, with and without acidifier, are shown in Figure 3. All formulations showed improved levels of solubility and drug release compared to the pure drug. However, the varying polymer and acidifier content accounted for fluctuations in the solubility and drug release profiles. In Figure 3, the formulations that contained acidifier (N5- N11) had the highest levels of drug solubility (> 15000 μg/mL) and better drug release profiles (> 90%) compared to the pure drug and other formulations. These formulations also lowered the pH of the dissolution media to around 4.5 to 5.5 compared to 6.75 for the pure drug (Table 2). N5-N8 (10% SA) showed even slightly higher drug release than N9-N11 (5% SA), which was likely due to the increase in acidifier. Although N1-N4 had better solubility (7000–1300 μg/mL) and drug release (15–40%) compared to the pure drug, these formulations did not perform as well as those with acidifier (N5-N11). Therefore, these results proved that the acidifier is successful at lowering the pHm which enhanced the dissolution of the drug.
Figure 3.
Solubility (A) and drug release profile (B) of ARI and formulation tablets
Table 2.
pH of media after dissolution. Each value represents the mean ± S.D. (n=3).
| Formulation | pH |
|---|---|
| pure ARI | 6.74 ± 0.43 |
| N1 | 6.91 ± 0.21 |
| N2 | 6.81 ± 0.09 |
| N3 | 7.14 ± 0.16 |
| N4 | 7.14 ± 0.21 |
| N5 | 4.65 ± 0.07 |
| N6 | 4.66 ± 0.02 |
| N7 | 5.27 ± 0.04 |
| N8 | 5.25 ± 0.06 |
| N9 | 5.51 ± 0.07 |
| N10 | 5.55 ± 0.03 |
| N11 | 5.57 ± 0.07 |
Hydrophilic carriers, like PVP, have proven to be successful in producing amorphous solid dispersions with poorly-water soluble drugs to enhance drug release (Fousteris et al., 2013, Tran et al., 2010). However, the ratio of drug to carrier has shown to have an effect on drug release. The more carrier present, the more a drug is converted into the amorphous form, which leads to greater drug release (Fousteris et al., 2013, Tran et al., 2009). These same results were evident in this study with N1 through N4, which contained no acidifier. Figure 3B showed a distinct gap between the release profiles of N1-N2 and N3-N4 whereas the release profiles of N5 through N11 are relatively similar. This clear difference for N1 through N4 was due to the ratio of drug to polymer. N1 and N2 had 20% ARI and 80% PVP. For N3 and N4, the content was 40% ARI and 60% PVP. The higher drug content SDs might be saturated because there were lower levels of polymer present (Fousteris et al., 2013). Therefore, N1 and N2 were unsaturated SDs with more hydrophilic PVP and had better drug release rates.
All formulations, regardless of their composition improved the solubility and drug release of ARI compared to the pure drug. N1 through N4, which do not contain acidifier, demonstrated the importance of the drug to carrier ratio in the solid dispersions. Higher drug content and therefore, lower polymer content decreased the drug release. However, the ARI/PVP only solid dispersions were not as successful as those formulations with a pH modifier. The acidifier further enhanced the solubility and drug release for N5 through N11 by lowering the pH of the media. These results demonstrate the advantages of acidifier incorporation in solid dispersions.
3.5. Design of Experiment and response surface
The DoE was used to investigate the effects screw speed, drug content, and acidifier content had on the dissolution at 30 minutes and 60 minutes, solubility, and pH. The DoE used the following model equation to calculate these effects:
| (eq. 1). |
The first three terms were the effects of screw speed, drug content, and acidifier content, respectively. The fourth term was the effect of screw speed and drug content while the fifth term was the effect of screw speed and acidifier content. The sixth term was the effect of drug and acidifier content. The statistical effects of these variables are displayed in Table 3. The outputs, yn, were dissolution at 30 minutes, dissolution at 60 minutes, solubility, and pH, respectively. The magnitude of each term determined which variable impacted the output the most. For the dissolution at 30 minutes (y1), the acidifier content (a3×3) had the greatest effect. This was also true for the dissolution at 60 minutes (y2). Since the value was positive for these terms, the drug release rate increased as the acidifier content increased. For the solubility (y3) and pH (y4), the drug content (a2×2) and acidifier content (a3×3), separately, greatly impacted the outputs. For the solubility output and drug content term, the value was negative, indicating that the solubility decreased with increased drug content. The value was positive for the solubility output and acidifier content term, which means the solubility increased with increasing acidifier. The opposite was true for the pH output. The value was positive for the pH output and drug content term, so the pH increased with increasing drug content. However, the pH decreased with increasing acidifier. Since the drug is alkaline and the acidifier was shown to lower the pH, these correlations are valid.
Table 3.
DoE statistical results
| a1x1 | a2x2 | a3x3 | a12x1x2 | a23x2x3 | a13x1x3 | b | R2 | p | |
|---|---|---|---|---|---|---|---|---|---|
| y1 | 3.04 | −3.79 | 33.71 | −3.44 | 0.89 | 9.12 | 66.95 | 0.84 | 0.126 |
| y2 | 0.39 | −5.96 | 36.09 | 0.03 | −0.51 | 7.61 | 69.11 | 0.87 | 0.084 |
| y3 | 254.58 | −1963.66 | 3747.09 | −52.92 | −317.50 | 324.64 | 15178.3 | 0.84 | 0.129 |
| y4 | −0.01 | 0.22 | −1.02 | 0.01 | 0.01 | 0.08 | 5.86 | 0.96 | 0.004 |
The R2 value was an indication of how closely the data fit the model. The closer this value was to 1, the better the data fit the model. Since the R2 values in Table 3 were fairly close to 1, the model was acceptable for the data. The p-value determined if the model was statistically significant or not. Typically, data is considered statistically important if p is less than 0.05. However, this particular software suggested that it is statistically important if p is less than 0.2. Each of the models above had a p-value less than 0.2, so each model was statistically significant.
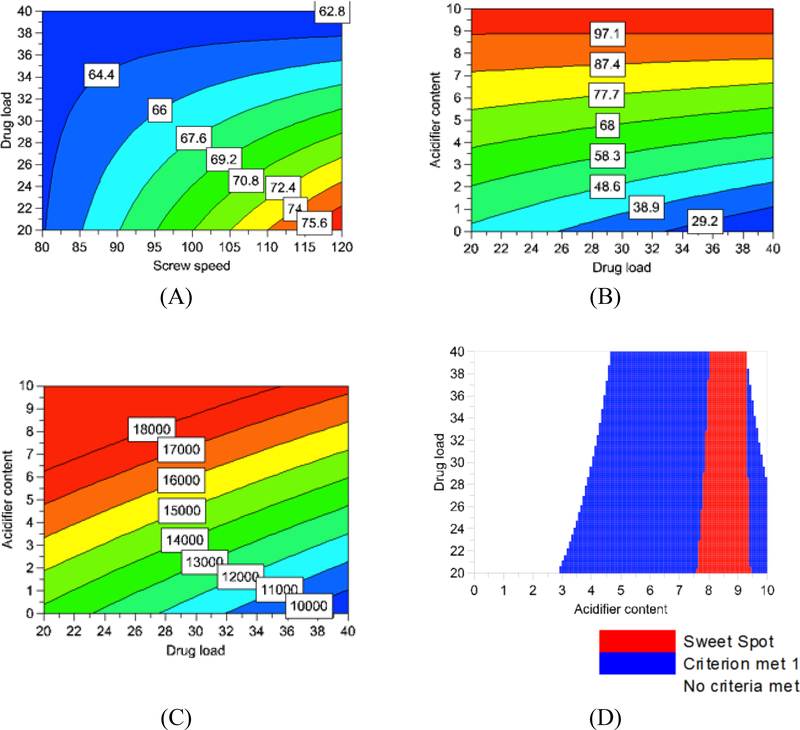
The DoE was also used to produce visual representations of the effects the factors have on the outputs (Figure 4). The screw speed only showed an effect on the dissolution at 30 minutes (Figure 4A). As the screw speed was increased, the drug release rate at 30 minutes also increased. Figure 4B illustrated how the drug release rate at 60 minutes increased with increasing amounts of acidifier. In Figure 4C, the solubility also increased with increasing amounts of acidifier. To produce Figure 4D, the screw speed was set to 100 RPM. The two criteria that had to be met were drug release from 60% to 100% at 30 minutes (criteria 1) and drug release from 90% to 100% at 60 minutes (criteria 2). Figure 4D showed the combinations of acidifier and drug content that met these criteria. The blue areas were the combinations of acidifier and drug content that met criteria one. The red area, or the sweet spot, was the combination of acidifier and drug content that met both criteria.
Figure 4.
Response surfaces from the DoE: (A) Dissolution at 30 minutes (B) Dissolution at 60 minutes (C) Solubility (D) Sweet spot conditions
3.6. Optimal formulation and characterization
Several factors were used to determine the optimal formulation for further studies. As discussed in the previous section, as the drug content decreased, the drug solubility was increased. Therefore, N5 and N6 (20% ARI) showed slightly higher drug solubility in Figure 3A compared to N7-N8 (40% ARI) and N9-N11 (30% ARI). The DoE results also demonstrated that greater acidifier led to greater drug release at 30 and 60 min. N5 through N8 (10% ARI) had comparable drug release profiles in Figure 3B but were all slightly better than N9 through N11 (5% ARI). N5 and N6 were also able to lower the pH of the dissolution media better than the other formulations (Table 2). Figure 4A demonstrated that faster screw speeds led to better drug release within the first 30 minutes of dissolution. Therefore, N6 (120 RPM) was chosen as the optimal formulation instead of N5 (80 RPM) and was further characterized before in vivo studies.
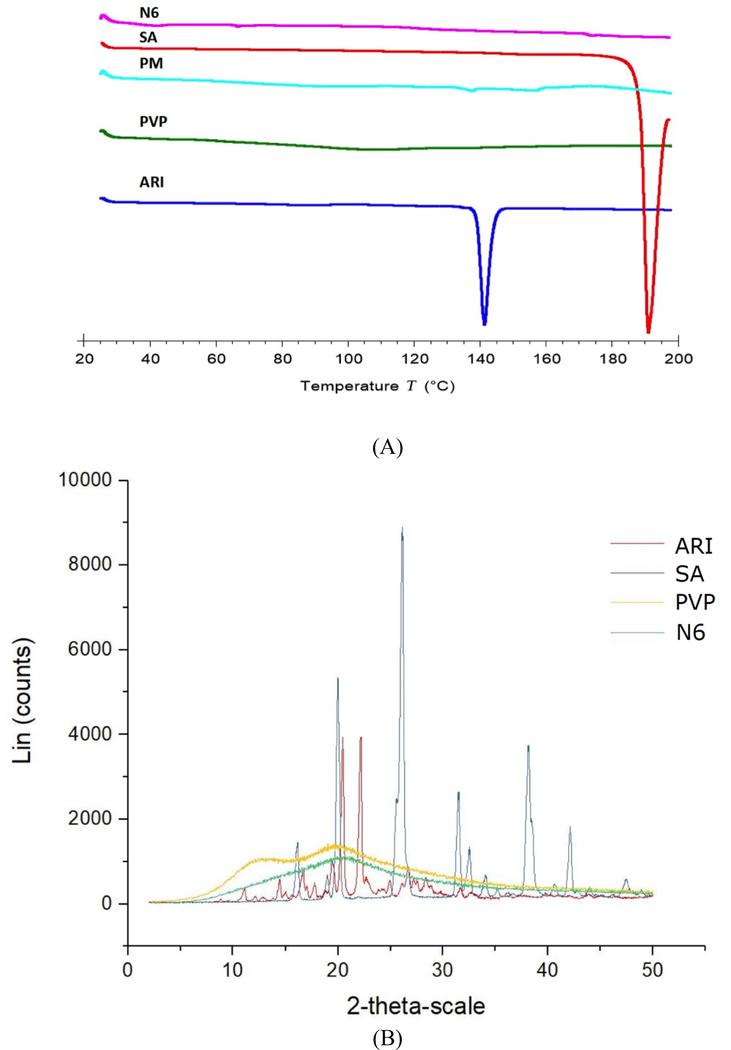
DSC and XRD were applied to the optimal N6 formulation and components for crystallinity analysis. As previously discussed, PVP had an amorphous structure whereas ARI had a melting point around 140°C. Since the drug melting point peak was not present in the DSC curve for N6 (Figure 5A), it can be inferred that the crystalline drug was transformed into the amorphous state. In the DSC curve for the PM, there was a small peak corresponding to the ARI melting point, 140°C. The drug in the PM still maintained some degree of crystallinity. Therefore, it can be concluded that the hot-extrusion process with the polymer changed the crystalline drug to the amorphous form. XRD was used to further confirm the amorphous structure of the optimal N6 formulation. The XRD graph of pure ARI was highly crystalline, having various characteristic peaks. The curve for N6 was smooth with the ARI peaks no longer being present (Figure 5B). These results indicated the drug in the N6 formulation was completely converted into amorphous state. The DSC and XRD graphs proved that the optimal N6 formulation had been transformed into the amorphous state.
Figure 5.
DSC (A) and XRD (B) curves for ARI, SA, PVP, and N6
The DSC and XRD confirmation of ARI being amorphous was valuable as it related to the performance of the drug in the in vitro studies. It is well reported in literature that poorly water-soluble APIs have improved dissolution and bioavailability when present in the amorphous state (Konno et al., 2008). The crystalline state of the API is a highly organized lattice, which has to be disturbed in order to be dissolved, whereas the dissolution energy is minimized for the amorphous state (Fousteris et al., 2013, Konno et al., 2008). However, amorphous materials are thermodynamically unstable and susceptible to recrystallization. Polymers are widely known to inhibit recrystallization and reduce particle size for better wettability (Konno et al., 2008, Tran et al., 2010). Therefore, hydrophilic polymers are commonly used as carriers for hot-melt extrusion to develop amorphous solid dispersions with enhanced dissolution and bioavailability. However, as this study and Tran et al. (2010) demonstrated, the polymer alone in the SD was not capable enough to achieve 100% drug release. The incorporation of the pH modifier to the SD greatly improved the drug release and solubility compared to the formulations without acidifier.
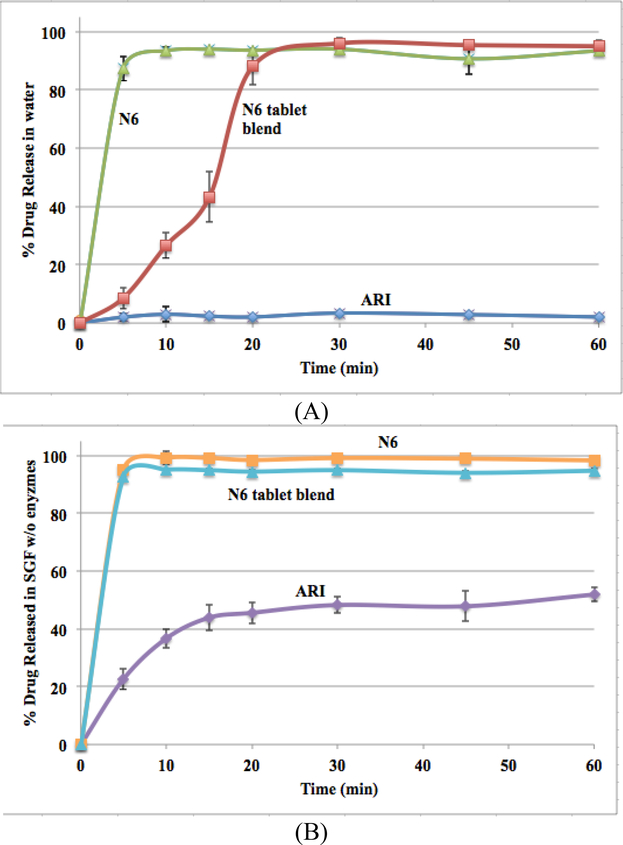
3.7. Dissolution of optimal formulation
The effect of dissolution media pH was investigated for pure N6, N6 tablet blend, and pure ARI for comparison (Figure 6). Pure N6 had a % drug release of approximately 92% and 98% in water and SGF without enzymes, respectively. N6 tablet blend had a % drug release of approximately 95% in both water and SGF without enzymes, respectively. The pure drug had a release around 3% in water but was greatly increased to around 50% in SGF without enzymes. This was due to the pH-dependent solubility of ARI. Although ARI had a much greater drug release rate in the lower pH media compared to water, solid dispersions via HME and the incorporation of the acidifier in the formulation proved to further enhance the drug release rate. There is likely a synergistic effect of both the polymer and the pH modifier improving the drug release kinetics compared to the pure drug.
Figure 6.
Drug release profiles of ARI, N6, and N6 tablet blend in water (A) and SGF without enzymes (B)
3.8. Pharmacokinetic application
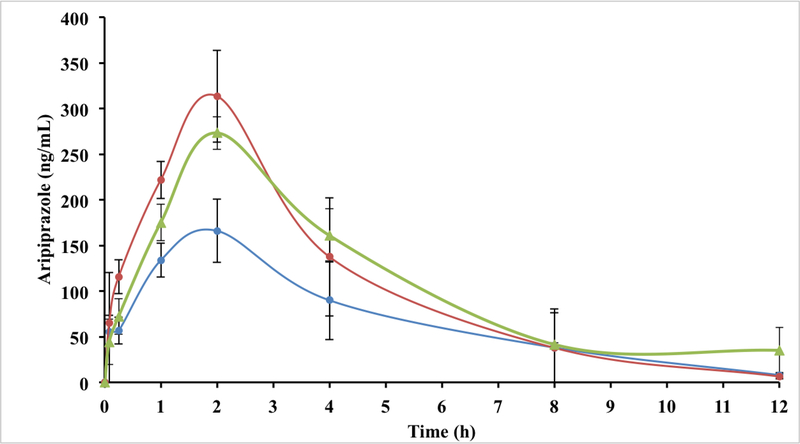
The mean plasma concentrations of ARI, N6, and N6 tablet blend after a single oral dose of 20 mg/kg to male rats were determined at various time points up to 24 h (Figure 7). However, the samples at 24 h were below the limit of quantification and were excluded. The pharmacokinetic parameters of ARI, N6, and N6 tablet blend are provided in Table 4. Statistical significance was considered for p-values less than 0.05. The Cmax for N6 tablet blend, N6, and ARI were 279 ± 10.0, 320 ± 48.0, and 166 ± 34.7 ng/mL, respectively. All three groups reached their Cmax at 2 h. The Cmax values for N6 tablet blend and N6 were not statistically different from each other, but the Cmax values for these two groups were significantly greater than the pure ARI. The AUC0–12 for N6 tablet blend, N6, and ARI were 1329 ± 119, 1302 ± 80.3, and 832 ± 201 ng·h/mL, respectively. The AUC0–12 values for N6 and N6 tablet blend were not significantly different from each other. However, these parameters were significantly greater for both N6 and N6 tablet blend when compared to pure ARI. Table 4 showed that the Cmax and AUC0–12 were increased in N6 and N6 tablet blend compared with ARI. However, the Cmax of N6 tablet blend was slightly lower compared to N6 formulation, which may be due to the excipients in the blend. The t1/2 for N6 tablet blend, N6, and ARI were 8.32 ± 0.21, 4.17 ± 0.39, and 5.26 ± 0.65 h, respectively. The t1/2 for N6 tablet blend was significantly longer than N6 and pure ARI, but there was no statistical difference between the t1/2 for N6 and pure ARI. The high t1/2 for N6 tablet blend may be due to relatively slow absorption and slow elimination of ARI in the N6 tablet blend.
Figure 7.
Mean plasma concentrations of ARI in of N6, N6 tablet blend, and pure ARI (n=6)
Table 4.
Pharmacokinetic parameters of ARI, N6, or N6 tablet blend after a single oral dose of 20 mg/kg to rats. Each value represents the mean ± S.D. (n=6). * P < 0.05, compared to pure ARI; # P < 0.05, compared to N6
| Parameter | ARI | N6 | N6 tablet blend |
|---|---|---|---|
| Cmax (ng/mL) | 166.0 ± 34.7 | 320.0 ± 48.0* | 279.0 ± 10.0* |
| Tmax (h) | 2 | 2 | 2 |
| AUC0–12 (ng h/mL) | 832.0 ± 201.0 | 1302.0 ± 80.3* | 1329.0 ± 119.0* |
| t1/2 (h) | 5.26 ± 0.65 | 4.17 ± 0.39 | 8.32 ± 0.21*, # |
The in vivo study follows the trend of the solubility and dissolution results. The N6 solid dispersions improved drug solubility and drug release and subsequently, performed better than the pure drug in the in vivo study. Tmax and AUC0–12 are related to the rate of absorption and extent of absorption, respectively, while Cmax is related to both (Daravath et al., 2015). Thus, N6 and N6 tablet blend significantly enhanced the Cmax and AUC0–12 compared to the pure ARI, indicating that both with PVP as the carrier in the SD via HME and with the inclusion of a pH modifier were capable of enhancing the bioavailability of ARI.
4. Conclusion
Solid dispersions via HME technology and pH modifiers are two techniques that, separately, have successfully improved drug dissolution behavior and bioavailability for poorly water-soluble drugs with pH dependent solubility. The novelty of this study was the combination of these two techniques to develop solid dispersions that performed better than the pure drug. All formulations demonstrated improved solubility and drug release of the solid dispersions compared to pure ARI. However, the formulations with acidifier (N5-N11) performed much better than the formulations without acidifier (N1-N4). DSC and XRD proved the amorphous state of ARI, which also helps to improve drug dissolution and bioavailability. The in vivo results were similar to the dissolution results in that the solid dispersions improved the bioavailability compared to the pure drug. The combination of SD via HME and the addition of pH modifiers to the SD improved the performance of the drug in both in vitro and in vivo studies.
Supplementary Material
Acknowledgement
This work was supported by Grant Number P20GM104932 from the National Institute of General Medical Sciences (NIGMS), a component of NIH, and a Grant Number 2018R1D1A1B07050598 from the National Research Foundation (NRF) of South Korea.
Abbreviations used
- Ac-Di-Sol
croscarmellose sodium (excipient)
- ARI
aripiprazole
- AUC
area under the concentration-time curve
- BCS
biopharmaceutics classification system
- Cmax
maximum plasma concentration
- DoE
design of experiment
- DSC
differential scanning calorimetry
- ESI
electrospray ionization
- HME
hot-melt extrusion
- HPMC
hydroxypropyl methylcellulose
- IS
internal standard
- MCC
microcrystalline cellulose (excipient)
- MS
magnesium stearate
- pHm
micro-environment pH
- PM
physical mixture
- PVP
Kollidon® 12 PF
- SA
succinic acid
- SEM
scanning electron microscopy
- SD
solid dispersion
- SGF
simulated gastric fluid
- TGA
thermogravimetric analysis
- Tmax
time to reach Cmax
- t1/2
half-life
- XRD
X-ray diffraction
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andrews GP, Abudiak OA, Jones DS, 2010. Physicochemical characterization of hot melt extruded bicalutamide-polyvinylpyrrolidone solid dispersions. Journal of Pharmaceutical Sciences. 99, 1322–1335. 10.1002/jps.21914 [DOI] [PubMed] [Google Scholar]
- Borbás E, Balogh A, Bocz K, Müller J, Kiserdei É, Vigh T, Sinkó B, Marosi A, Halász A, Dohányos Z, Szente L, Balogh GT, Nagy ZK, 2015. In vitro dissolution–permeation evaluation of an electrospun cyclodextrin-based formulation of aripiprazole using μ Flux™. International Journal of Pharmaceutics. 491, 180–189. 10.1016/j.ijpharm.2015.06.019 [DOI] [PubMed] [Google Scholar]
- Chi L, Liu R, Guo T, Wang M, Liao Z, Wu L, Li H, Wu D, Zhang J, 2015. Dramatic improvement of the solubility of pseudolaric acid B by cyclodextrin complexation: preparation, characterization and validation. International Journal of Pharmaceutics. 479, 349–356. 10.1016/j.ijpharm.2015.01.005 [DOI] [PubMed] [Google Scholar]
- Daravath B, Tadikonda RR, Vemula SK, 2015. Formulation and pharmacokinetics of gelucire solid dispersions of flurbiprofen. Drug Development and Industrial Pharmacy. 41, 1254–1262. https://doi.org10.3109/03639045.2014.940963 [DOI] [PubMed] [Google Scholar]
- Fousteris E, Tarantili PA, Karavas E, Bikiaris D, 2013. Poly(vinyl pyrrolidone)-poloxamer-188 solid dispersions prepared by hot melt extrusion. Journal of Thermal Analysis & Calorimetry. 113, 1037–1047. 10.1007/s10973-012-2885-2 [DOI] [Google Scholar]
- Grifasi F, Chierotti MR, Gaglioti K, Gobetto R, Maini L, Braga D, Dichiarante E, Curzi M, 2015. Using salt cocrystals to improve the solubility of niclosamide. Crystal Growth & Design. 15, 1939–1948. 10.1021/acs.cgd.5b00106 [DOI] [Google Scholar]
- He Y, Orton E, Yang D, 2018. The selection of a pharmaceutical salt—the effect of the acidity of the counterion on its solubility and potential biopharmaceutical performance. Journal of Pharmaceutical Sciences. 107, 419–425. 10.1016/j.xphs.2017.10.032 [DOI] [PubMed] [Google Scholar]
- Huang Y, Dai WG, 2014. Fundamental aspects of solid dispersion technology for poorly soluble drugs. Acta Pharmaceutica Sinica B. 4, 18–25. 10.1016/j.apsb.2013.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang I, Kang CY, Park JB, 2017. Advances in hot-melt extrusion technology toward pharmaceutical objectives. Journal of Pharmaceutical Investigation. 47, 123–132. 10.1007/s40005-017-0309-9 [DOI] [Google Scholar]
- Jinno JI, Kamada N, Miyake M, Yamada K, Mukai T, Odomi M, Toguchi H, Liversidge GG, Higaki K, Kimura T, 2006. Effect of particle size reduction on dissolution and oral absorption of a poorly water-soluble drug, cilostazol, in beagle dogs. Journal of Controlled Release. 111, 56–64. 10.1016/j.jconrel.2005.11.013 [DOI] [PubMed] [Google Scholar]
- Kadam A, Desai ND, 2017. Studies on solubility enhancement of poorly soluble NSAID by dual approach of melt granulation and micro-environmental pH modulation. Current Drug Delivery. 14, 1201–1212. 10.2174/1567201814666170413120513 [DOI] [PubMed] [Google Scholar]
- Kim DS, Choi JS, Kim DW, Kim KS, Seo YG, Cho KH, Kim JO, Yong CS, Youn YS, Lim S-J, Jin SG, Cho H-G, 2016. Comparison of solvent-wetted and kneaded l-sulpiride-loaded solid dispersions: powder characterization and in vivo evaluation. International Journal of Pharmaceutics. 511, 351–358. 10.1016/j.ijpharm.2016.07.006 [DOI] [PubMed] [Google Scholar]
- Konno H, Handa T, Alonzo DE, Taylor LS, 2008. Effect of polymer type on the dissolution profile of amorphous solid dispersions containing felodipine. European Journal of Pharmaceutics and Biopharmaceutics. 70, 493–499. 10.1016/j.ejpb.2008.05.023 [DOI] [PubMed] [Google Scholar]
- Loh ZH, Samanta AK, Heng PWS, 2015. Overview of milling techniques for improving the solubility of poorly water-soluble drugs. Asian Journal of Pharmaceutical Sciences 10, 255–274. 10.1016/j.ajps.2014.12.006 [DOI] [Google Scholar]
- Ma X, Williams RO, 2018. Polymeric nanomedicines for poorly soluble drugs in oral delivery systems: an update. Journal of Pharmaceutical Investigation. 45, 61–75. https://link.springer.com/article/10.1007/s40005-017-0372-2 [Google Scholar]
- Marasini N, Tran TH, Poudel BK, Cho HJ, Choi YK, Chi S, Choi H, Yong C, Kim JO, 2013. Fabrication and evaluation of pH-modulated solid dispersion for telmisartan by spray-drying technique. International Journal of Pharmaceutics. 441, 424–432. 10.1016/j.ijpharm.2012.11.012 [DOI] [PubMed] [Google Scholar]
- Mennini N, Maestrelli F, Cirri M, Mura P, 2016. Analysis of physicochemical properties of ternary systems of oxaprozin with randomly methylated-ß-cyclodextrin and l-arginine aimed to improve the drug solubility. Journal of Pharmaceutical and Biomedical Analysis.129, 350–358. 10.1016/j.jpba.2016.07.024 [DOI] [PubMed] [Google Scholar]
- Mihajlovic T, Kachrimanis K, Graovac A, Djuric Z, Ibric S, 2012. Improvement of aripiprazole solubility by complexation with (2-hydroxy) propyl-β-cyclodextrin using spray drying technique. Aaps Pharmscitech, 13, 623–631. 10.1208/s12249-012-9786-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid R, Kim DW, ud Din F, Mustapha O, Yousaf AM, Park JH, Kim JO, Yong CS, Choi HG, 2015. Effect of hydroxypropylcellulose and Tween 80 on physicochemical properties and bioavailability of ezetimibe-loaded solid dispersion. Carbohydrate Polymers. 130, 26–31. 10.1016/j.carbpol.2015.04.071 [DOI] [PubMed] [Google Scholar]
- Repka MA, Bandari S, Kallakunta VR, Vo AQ, McFall H, Pimparade MB, Bhagurkar AM, 2018. Melt extrusion with poorly soluble drugs–An integrated review. International Journal of Pharmaceutics. 535, 68–85. 10.1016/j.ijpharm.2017.10.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repka MA, Shah S, Lu J, Maddineni S, Morott J, Patwardhan K, Mohammed NN, 2011. Melt extrusion: Process to product. Expert Opinion on Drug Delivery. 9, 105–125. 10.1517/17425247.2012.642365 [DOI] [PubMed] [Google Scholar]
- Silki, Sinha VR, 2018. Enhancement of in vivo efficacy and oral bioavailability of aripiprazole with solid lipid nanoparticles. AAPS PharmSciTech. 19, 1264–1273. 10.1208/s12249-017-0944-5 [DOI] [PubMed] [Google Scholar]
- Taniguchi C, Kawabata Y, Wada K, Yamada S, Onoue S, 2014. Microenvironmental pH-modification to improve dissolution behavior and oral absorption for drugs with pH-dependent solubility. Expert Opinion on Drug Delivery. 11, 505–516. 10.1517/17425247.2014.881798 [DOI] [PubMed] [Google Scholar]
- Tran PHL, Tran HTT, Lee BJ, 2008. Modulation of microenvironmental pH and crystallinity of ionizable telmisartan using alkalizers in solid dispersions for controlled release. Journal of Controlled Release. 129, 59–65. 10.1016/j.ijpharm.2008.04.001 [DOI] [PubMed] [Google Scholar]
- Tran TTD, Tran PHL, Lee BJ, 2009. Dissolution-modulating mechanism of alkalizers and polymers in a nanoemulsifying solid dispersion containing ionizable and poorly water-soluble drug. European Journal of Pharmaceutics and Biopharmaceutics. 72, 83–90. 10.1016/j.ejpb.2008.12.009 [DOI] [PubMed] [Google Scholar]
- Tran TTD, Tran PHL, Choi HG, Han HK, Lee BJ, 2010. The roles of acidifiers in solid dispersions and physical mixtures. International Journal of Pharmaceutics. 384, 60–66. 10.1016/j.ijpharm.2009.09.039 [DOI] [PubMed] [Google Scholar]
- Vasconcelos T, Sarmento B, Costa P, 2007. Solid dispersions as strategy to improve oral bioavailability of poor water soluble drugs. Drug Discovery Today. 12, 1068–1075. 10.1016/j.drudis.2007.09.005 [DOI] [PubMed] [Google Scholar]
- Xu Y, Liu X, Lian R, Zheng S, Yin Z, Lu Y, Wu W, 2012. Enhanced dissolution and oral bioavailability of aripiprazole nanosuspensions prepared by nanoprecipitation/homogenization based on acid–base neutralization. International Journal of Pharmaceutics, 438, 287–295. 10.1016/j.ijpharm.2012.09.020 [DOI] [PubMed] [Google Scholar]
- Yousaf AM, Mustapha O, Kim DW, Kim DS, Kim KS, Jin SG, Yong CS, Youn YS, Oh Y-K, Kim JO, Choi HG, 2016. Novel electrosprayed nanospherules for enhanced aqueous solubility and oral bioavailability of poorly water-soluble fenofibrate. International Journal of Nanomedicine. 11, 213–221. 10.2147/IJN.S97496 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.