Abstract
Background:
The recent NIH mandate to consider sex as a biological variable in preclinical research has focused attention on delineation of sex differences in behavior. To investigate mechanisms underlying sex differences in Δ9-tetrahydrocannabinol (THC) effects, we examined the effects of sex and gonadal hormones on CB1 receptors in cerebellum, hippocampus, prefrontal cortex, and striatum.
Methods:
Adult Sprague-Dawley rats underwent gonadectomy (GDX) or sham-GDX. Half of the GDX females and males received estradiol or testosterone replacement (GDX+H), respectively. All rats were injected with vehicle or 30 mg/kg THC twice daily for 1 week before brain collection. CP55,940-stimulated [35S]GTPγS and [3H]SR141716A saturation binding assays were performed.
Results:
With exception of enhanced receptor activation in the hippocampi of female rats compared to males, vehicle-treated rats exhibited minimal sex differences in CB1 receptor densities or G-protein coupling. Repeated treatment with THC resulted in pronounced CB1 receptor desensitization and downregulation in both sexes in all brain regions with a greater magnitude of change in females.
Conclusions:
These results suggest that sex differences in the density and G-protein coupling of brain CB1 receptors may play a limited role in sex differences in acute THC effects not mediated by the hippocampus. In contrast, sex differences after repeated THC were common, with females (intact, GDX, and GDX+H) showing greater downregulation or desensitization in all four brain regions compared to the respective male groups. This result is consistent with a finding that women tend to progress to tolerance and dependence quicker than men after initiation of cannabis use.
Keywords: CB1 Cannabinoid Receptor, Gonadal Hormones, Sex Differences, Tetrahydrocannabinol
1. Introduction
Historically, most preclinical pharmacological research has been conducted in male animals without regard for potential sex-based variance in drug effects (Beery and Zucker, 2011). To address this sex imbalance in basic research, in 2016 the National Institutes of Health mandated consideration of sex as a biological variable in the review of grant proposals using animal models (Clayton and Collins, 2014). Consequently, research including animals of both sexes is increasing rapidly, highlighting the need to establish sex differences or the lack thereof between male and female rats in their basal, untreated state. To this end, the primary goal of this study was to investigate sex differences in brain cannabinoid type-1 receptors (CB1) receptors in drug naïve male and female rats.
CB1 receptors are part of the body’s endocannabinoid system, which is comprised of two identified receptors (CB1 and CB2) and two predominant endogenous ligands (anandamide and 2-arachidonoylglycerol) along with synthetic and metabolic enzymes that catalyze their synthesis and degradation. Whereas CB2 receptors are located primarily in the periphery, CB1 receptors are the most abundant type of G protein-coupled receptor in mammalian brain and primarily couple to Gαi/o proteins to promote inhibitory signaling (Childers and Breivogel, 1998; Herkenham et al., 1991).Δ9-Tetrahydrocannabinol (THC), the main psychoactive constituent of cannabis, produces its characteristic “high” through activation of CB1 receptors in the brain, and CB1 receptors mediate a wide variety of other emotional and physical effects of cannabinoids. CB1 receptor density varies throughout the brain, and this variation is furthered by region-specific receptor downregulation and desensitization following repeated cannabinoid exposure (Breivogel et al., 2003; Sim et al., 1996). Desensitization in specific brain regions also can be sexdependent: for example, adult male rats show greater desensitization than females in periaqueductal gray (Burston et al., 2010).
In the present study, we compared CB1 receptor density and functional sensitivity between sexes in specific brain regions that are involved in some of THC’s psychoactive effects: cerebellum, striatum, hippocampus, and prefrontal cortex (PFC). In addition, to determine the extent to which gonadal hormones might be responsible for any observed sex differences, we evaluated CB1 receptor density and function in gonadectomized male and female rats (with and without testosterone or estradiol replacement, respectively) and compared results to those obtained in gonadally intact rats of both sexes. Finally, we investigated changes in CB1 receptors induced by repeated dosing with THC in male and female rats with and without gonadectomy and hormone replacement. The effects of sex, gonadal hormones, and THC on tolerance and withdrawal behaviors of these rats has been reported previously (Marusich et al., 2015).
2. Materials and methods
2.1. Subjects
Twenty-four male and twenty-four female Sprague-Dawley rats (Harlan, Dublin, VA), aged 57–63 days on arrival, were pair-housed with a rat of the same sex in polycarbonate cages at 20–22°C under a 12–12 h light/dark cycle (lights on at 6AM). The surgical, behavioral, and pharmacological procedures performed on these rats have been previously reported (Marusich et al., 2015). In brief, all rats underwent either gonadectomy or sham-gonadectomy 6–10 days after arrival. Immediately after surgery, hormone-replacement Silastic® capsules were implanted s.c. between the shoulder blades. These capsules were either blank or contained a 1 mm length of estradiol for females or a 10 mm length of testosterone/100g body weight for males. This hormone replacement regimen has been shown previously to maintain stable testosterone and estradiol levels for up to 10 weeks and 6 weeks, respectively, after surgery/capsule implantation (Wakley et al., 2015).
Ten days after surgery a repeated dosing procedure was initiated. Rats were injected with vehicle (7.8 % Polysorbate 80 NF and 92.2% sterile saline USP) or 30 mg/kg THC twice daily (at approximately 7 am and 3 pm) for 6 days. In the morning of the 7th day, rats were again injected with vehicle or 30 mg/kg THC and were evaluated behaviorally. Previously, we showed that this dosing regimen produced marked tolerance and dependence in intact rats of both sexes (Marusich et al., 2014, 2015). Four h after the last THC or vehicle injection, rats were euthanized and their brains were collected, dissected on ice, and frozen at −80°C until use. All experiments were carried out in accordance with guidelines published in the Guide for the Care and Use of Laboratory Animals (National Research Council, 2011) and were approved by the Institutional Animal Care and Use Committee at RTI International.
2.2. Brain membrane preparation
Cerebellum, prefrontal cortex, hippocampus, and striatum were homogenized by Polytron at 23,000 rpm for 10–15 sec in cold membrane buffer (50 mM Tris–HCl pH 7.4, 3 mM MgCl2, 0.2 mM EGTA and 100 mM NaCl) and then centrifuged at 40,000×g for 10 min at 4°C. To wash away any residual THC that may have remained when tissues were collected (i.e., 4 h after the final THC injection), pellets were re-suspended in membrane buffer, re-homogenized, and centrifuged again. The resulting pellets were homogenized in membrane buffer, protein was quantified by Bradford method, and membranes were preincubated for 10 min at 37°C in 0.003 U/ml adenosine deaminase to remove endogenous adenosine.
2.3. CP55,940-Stimulated [35S]GTPγS and [3H]SR141716A saturation binding assays
Membranes were incubated at 30°C for 1 h at 0.5 ml final volume in membrane buffer with 1 mg/mL BSA. For [35S]GTPγS binding, 0.1–10,000 nM CP55,940, 30 μM GDP, 0.1 nM [35S]GTPγS, and 10 μg membrane protein were added. For saturation binding, 0.06–3.0 nM [3H]SR141716A and 15 μg protein were added. Non-specific binding was determined using either 30 μM unlabeled GTPγS or 5 μM unlabeled SR141716A, respectively. Reactions were terminated by rapid filtration under vacuum through GF/C filter plates followed by 2–3 washes with 2 mL cold rinse buffer (50 mM Tris-HCl, 0.5 mg/mL BSA, pH 7.4). Filter plates were oven-dried for 50 min. Thirty-five μL of Microscint 20 scintillation fluid was added to each well, and bound radioactivity was determined by liquid scintillation spectrophotometry using a Packard Topcount NXT.
2.4. Chemicals
SR141716A, [3H]SR141716A, and CP55,940 were provided by the Drug Supply Program of the National Institute on Drug Abuse (Rockville, MD, USA). Guanosine 5’−3-O(thio)triphosphate (GTPγS), guanosine 5’-diphosphate (GDP), bovine serum albumin (BSA), adenosine deaminase, Tris base, HCl, MgCl2, EGTA, and NaCl were purchased from Sigma Aldrich (St. Louis, MO). [35S]GTPγS, Unifilter GF/C glass fiber filters, and Microscint 20 scintillation cocktail were purchased from PerkinElmer (Waltham, MA).
2.5. Data analysis
Data are presented as mean ± S.E.M. obtained from brain tissue from 4 rats/group. After subtraction of non-specific binding, the [35S]GTPγS net-stimulated binding was calculated as agonist-stimulated minus basal binding. The data were expressed as percent stimulation (%Stim) defined as net-stimulated binding as a percentage of basal binding. Specific [3H]SR141716A binding was calculated by subtracting non-specific binding from total binding values for each concentration and expressed as pmol of bound radioligand per mg of brain membrane protein (pmol/mg). Analyses of saturation binding curves and agonist concentration–effect curves were conducted by iterative non-linear regression using the “One Site – Specific binding” and “log(agonist) vs. response (three parameters)” with Bottom constrained to 0.0, respectively, using Prism 4 (GraphPad Software, San Diego, CA) to obtain Kd, Bmax, logEC50 and Emax values for each sample.
Data for all rats were analyzed by separate three-way ANOVAs (sex × hormonal status × THC/vehicle treatment) for each brain region and for each dependent measure. As expected, THC significantly decreased Bmax and Emax in all brain regions. Subsequently, data for vehicle-treated rats and for THC-treated rats were analyzed by separate two-way ANOVAs (sex × hormone treatment) for each brain region and for each dependent measure. Follow-up analysis of significant (p < 0.05) main effects and interactions was accomplished through use of Tukey post hoc tests (α = 0.05).
3. Results
Figures 1–4 show the results of [3H]SR141761A saturation binding and [35S]GTPγS experiments in brain areas from vehicle- and THC-treated adult male and female rats that were intact, gonadectomized without hormone replacement, or gonadectomized and treated with hormone. In vehicle-treated rats of both sexes, regional differences in Bmax and Emax were evident: Emax showed greater divergence across regions. For both sexes, maximal binding of [3H]SR141716A was observed in the hippocampus (Figure 1, panel A) and least was seen in the PFC (Figure 2, panel A) with a range from 8.8 pmol/mg in gonadectomized (GDX) male and female rats to 3.5 pmol/mg in intact male rats. Whereas maximal CP55,940-induced [35S]GTPγS binding (Emax) was also least in the PFC of both male and female rats (Figure 2, panel C), it was greatest in the cerebellum in rats of both sexes (Figure 3, panel C). Emax values ranged from 64% in intact female PFC (Figure 2, panel C) to 338% in intact female cerebellum (Figure 3, panel C). Kd values for [3H]SR141716A binding in vehicle-treated rat brain tissues were between 0.16 and 0.33 nM in all samples (Figures 1–4, panel B), and EC50 values for CP55,940 stimulation of [35S]GTPγS binding were between −8.5 and −7.9 log M (Figures 1–4, panel D) with little variation across regions.
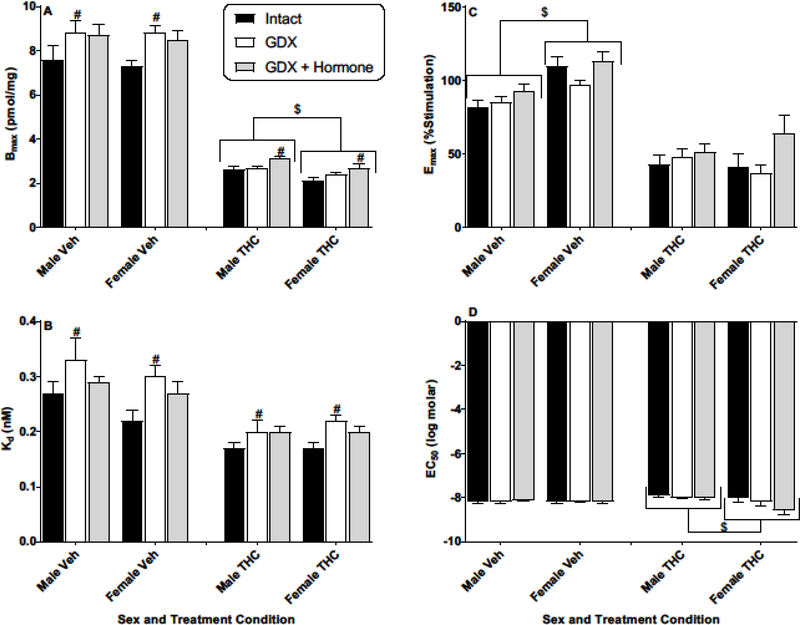
Figure 1.
Effects of sex and hormonal status on [3H]SR141761A saturation binding (Bmax and Kd) and CP55,940-stimulated [35S]GTPγS binding (Emax and EC50) in the hippocampus of vehicle- (Veh) or THC-treated adult rats of both sexes. Rats were gonadally intact (i.e., sham gonadectomy) (filled black bars) or had undergone gonadectomy (GDX) with or without hormone replacement (filled grey bars and unfilled bars, respectively). Each point represents the mean (± SEM) of data for 4 rats in each condition. Based upon curves fitted to the binding values, four dependent measures were calculated: Bmax (panel A), Kd (panel B), Emax (panel C), and EC50 (panel D). Three-way ANOVA showed significant THC-induced decreases in Bmax and Emax for hippocampus (vs. Veh). For 2-way (sex X hormonal status) ANOVAs: $ p<0.05, main effect of sex for designated measure (i.e., different between sexes when collapsed across hormonal condition). # p<0.05, main effect of hormonal condition compared to intact (i.e., hormonal condition produced different results vs intact when collapsed across sex).
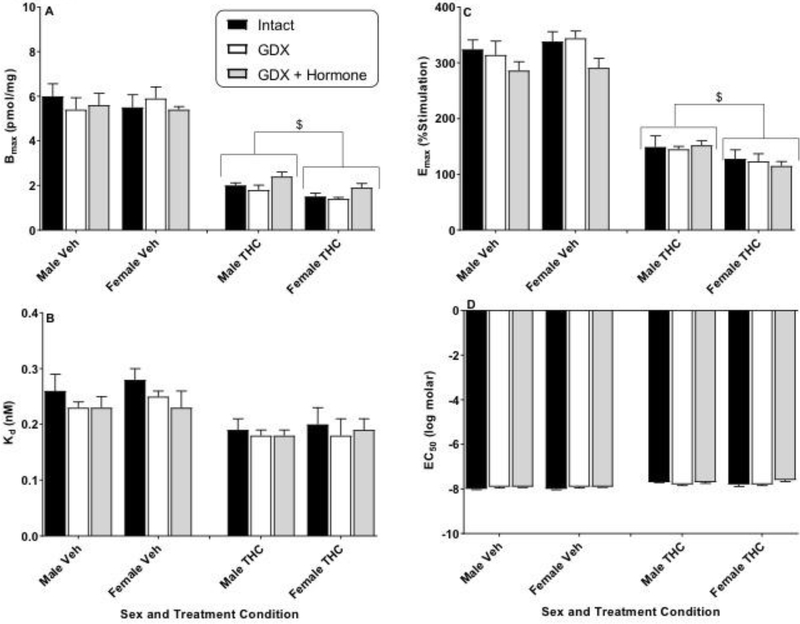
Figure 4.
Effects of sex and hormonal status on [3H]SR141761A saturation binding (Bmax and Kd) and CP55,940-stimulated [35S]GTPγS binding (Emax and EC50) in the striatum of vehicle- (Veh) or THC-treated adult rats of both sexes. Rats were gonadally intact (i.e., sham gonadectomy) (filled black bars) or had undergone gonadectomy (GDX) with or without hormone replacement (filled grey bars and unfilled bars, respectively). Each point represents the mean (± SEM) of data for 4 rats in each condition. Based upon curves fitted to the binding values, four dependent measures were calculated: Bmax (panel A), Kd (panel B), Emax (panel C), and EC50 (panel D). Three-way ANOVA showed significant THC-induced decreases in Bmax and Emax for striatum (vs. Veh). For 2-way (sex X hormonal status) ANOVAs: $ p<0.05, main effect of sex for designated measure (i.e., different between sexes when collapsed across hormonal condition). # p<0.05, main effect of hormonal condition compared to intact (i.e., hormonal condition produced different results vs intact when collapsed across sex).
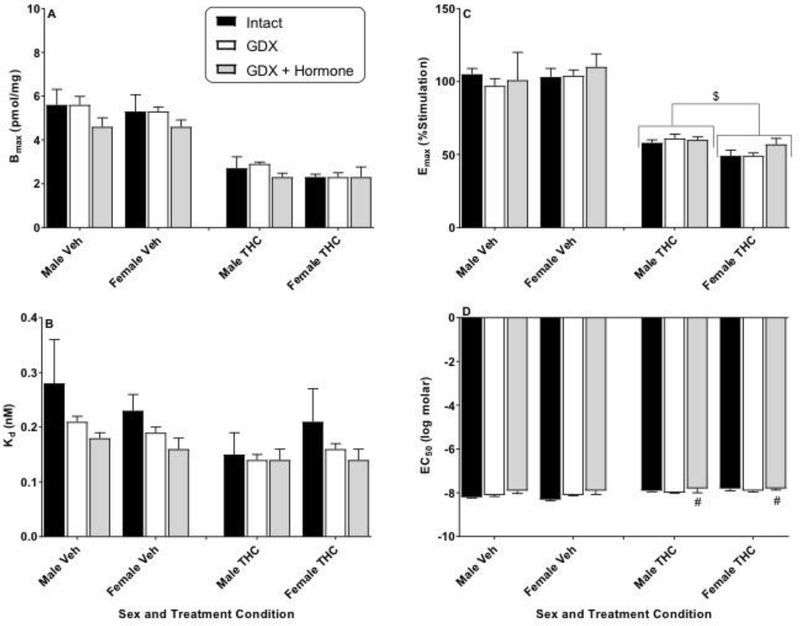
Figure 2.
Effects of sex and hormonal status on [3H]SR141761A saturation binding (Bmax and Kd) and CP55,940-stimulated [35S]GTPγS binding (Emax and EC50) in the prefrontal cortex of vehicle- (Veh) or THC-treated adult rats of both sexes. Rats were gonadally intact (i.e., sham gonadectomy) (filled black bars) or had undergone gonadectomy (GDX) with or without hormone replacement (filled grey bars and unfilled bars, respectively). Each point represents the mean (± SEM) of data for 4 rats in each condition. Based upon curves fitted to the binding values, four dependent measures were calculated: Bmax (panel A), Kd (panel B), Emax (panel C), and EC50 (panel D). Three-way ANOVA showed significant THC-induced decreases in Bmax and Emax for prefrontal cortex (vs. Veh). For 2-way (sex X hormonal status) ANOVAs: $ p<0.05, main effect of sex for designated measure (i.e., different between sexes when collapsed across hormonal condition). # p<0.05, main effect of hormonal condition compared to intact (i.e., hormonal condition produced different results vs intact when collapsed across sex).
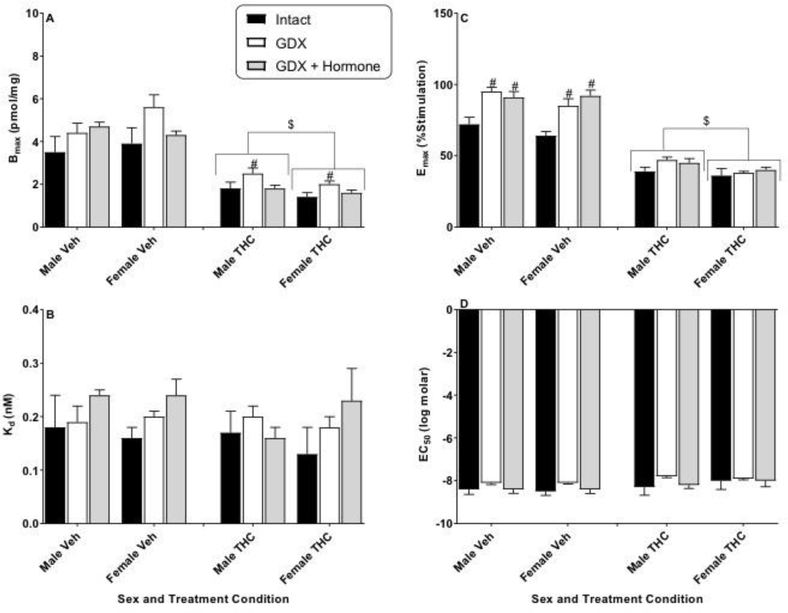
Figure 3.
Effects of sex and hormonal status on [3H]SR141761A saturation binding (Bmax and Kd) and CP55,940-stimulated [35S]GTPγS binding (Emax and EC50) in the cerebellum of vehicle- (Veh) or THC-treated adult rats of both sexes. Rats were gonadally intact (i.e., sham gonadectomy) (filled black bars) or had undergone gonadectomy (GDX) with or without hormone replacement (filled grey bars and unfilled bars, respectively). Each point represents the mean (± SEM) of data for 4 rats in each condition. Based upon curves fitted to the binding values, four dependent measures were calculated: Bmax (panel A), Kd (panel B), Emax (panel C), and EC50 (panel D). Three-way ANOVA showed significant THC-induced decreases in Bmax and Emax for cerebellum (vs. Veh). For 2-way (sex X hormonal status) ANOVAs: $ p<0.05, main effect of sex for designated measure (i.e., different between sexes when collapsed across hormonal condition). Note: y-axis scale for Emax (panel C) is different for cerebellum than for other 3 brain areas (Figures 2–4).
In contrast to the substantial regional differences, effects of gonadectomy/hormone treatment and sex within each region were modest. Figure 1 shows the results of analysis of the hippocampus. Hippocampi taken from vehicle-treated GDX rats without hormone replacement showed significantly greater CB1 receptor density and lower affinity compared to gonadally intact vehicle-treated rats [Figure 1; main effects of hormone status for Bmax (panel A): F(2,18)=4.6, p<0.05 and Kd (panel B): F(2,18)=3.9, p<0.05]. These differences in receptor binding were accompanied by a significant sex difference in CB1 receptor efficacy (but not in potency, EC50; Figure 1, panels C and D) in the hippocampus of vehicle-treated rats, and greater maximal stimulation (Emax) was seen in the hippocampi of females than males [Fig. 1, panel C; main effect of sex: F(1,18)=28.5, p<0.05]. Repeated treatment with THC induced significant downregulation (Bmax) and desensitization (Emax) in the hippocampus [Figure 1, panels A and C; 3-way ANOVA main effect of THC: F(1,36)=793.0, p<0.05 and F(1,36)=134.3, p<0.05 for Bmax and Emax, respectively]. A significant sex difference also emerged for Bmax, with female THCtreated rats showing significantly lower CB1 receptor number (more downregulation) than males [Figure 1, panel A; 2-way ANOVA results, main effect of sex: F(1,18)=17.2, p<0.05] and no change in affinity (Figure 1, panel B). Compared to intact rats, THC-treated GDX rats of both sexes (with hormone replacement) had greater Bmax values [Figure 1, panel A; main effect of hormonal status: F(2,18)=10.4, p<0.05]. While a sex difference was not observed for Emax, THC treatment eliminated the higher Emax values seen in vehicle-treated female rats (Figure 1, panel C), suggesting that the greater downregulation in the hippocampi of female rats may have been accompanied by greater desensitization (as percentage of vehicle as compared to males). CP55,940 also was slightly, but significantly, more potent in stimulating the receptor in female rather than male rats [Figure 1, panel D; 2-way ANOVA results, main effect of sex for EC50: F(1,18)=6.6, p<0.05]. THC treatment did not differentially alter CB1 activation of G-proteins across gonadectomy/hormone status in the hippocampus.
Figure 2 shows analysis of the PFC. Here, significant group differences in maximal CB1 receptor binding (Bmax) or affinity (Kd) were not observed for vehicle-treated rats of either sex regardless of hormonal status (Figure 2, panels A and B, respectively). In contrast, greater maximal stimulation (Emax) was seen in the PFC of vehicle-treated GDX rats (with and without hormone replacement) than of intact rats of both sexes [Figure 2, panel C; main effect of hormone status: F(2,18)=18.9, p<0.05] without accompanying difference in potency (EC50; Figure 2, panel D). Repeated treatment with THC induced significant downregulation (Bmax) and desensitization (Emax) in the PFC [Figure 2, panels A and C, respectively; 3-way ANOVA main effect of THC: F(1,36)=117.0, p<0.05 and F(1,36)=411.8, p<0.05 for Bmax and Emax, respectively]. Further, significant sex differences also emerged for both measures. Despite similar CB1 receptor densities and functioning in vehicle-treated rats of both sexes, female THC-treated rats showed lower CB1 receptor number (more downregulation) and less stimulation (more desensitization) than males in the PFC [Figure 2, panels A and C, respectively; 2-way ANOVA results, main effect of sex: F(1,18)=5.0, p<0.05 and F(1,18)=6.3, p<0.05 for Bmax and Emax, respectively]. Compared to intact rats, THC-treated GDX rats (without hormone replacement) also exhibited greater Bmax values in the PFC regardless of sex [Figure 2, panel A; main effect of hormonal status: F(2,18)=5.7, p<0.05], an effect that was reversed by hormone treatment. THC treatment did not differentially alter CB1 activation of G-proteins across gonadectomy/hormone status in the PFC.
Figure 3 shows the results of analysis of the cerebellum. CB1 receptor binding (panels A and B) and functioning (panels C and D) were similar in the cerebelli of vehicle-treated rats of both sexes regardless of hormonal status. As observed with other brain areas, however, repeated THC treatment produced significant downregulation (Bmax) and desensitization (Emax) in the cerebellum [Figure 3, panels A and C, respectively; 3-way ANOVA main effect of THC: F(1,36)=309.9, p<0.05 and F(1,36)=403.2, p<0.05 for Bmax and Emax, respectively]. Similar to findings in the PFC, the cerebelli of female THC-treated rats showed lower CB1 receptor number (more downregulation) and less agonist-induced stimulation (more desensitization) than males [Figure 3, panels A and C, respectively; 2-way ANOVA results, main effect of sex: F(1,18)=11.4, p<0.05 and F(1,18)=6.4, p<0.05 for Bmax and Emax, respectively]. THC treatment did not differentially alter CB1 receptor density or activation across gonadectomy/hormone status in the cerebellum.
Figure 4 shows results of analysis of the striatum. In vehicle-treated rats, differences across sex and hormonal condition in receptor number or efficacy were not seen in the striatum (Figure 4, panels A and C, respectively). With repeated THC treatment, significant downregulation (Bmax) and desensitization (Emax) emerged in the striatum as for the three other brain areas [Figure 4, panels A and C, respectively; 3-way ANOVA main effect of THC: F(1,36)=121.8, p<0.05 and F(1,36)=140.6, p<0.05 for Bmax and Emax, respectively]. While THC treatment did not differentially affect downregulation for female and male rats as it did in the other three brain areas, a significant sex difference was detected for desensitization in which more desensitization was seen in the striatum of female rats than males [Figure 4, panel C; 2-way ANOVA results, main effect of sex: F(1,18)=11.1, p<0.05]. THC treatment also slightly (but significantly) decreased potency in the striatum for GDX rats of both sexes (with hormone replacement) compared to intact rats (Figure 4, panel D).
4. Discussion
Consistent with the results of previous research (Burston et al., 2010; Sim et al., 1996), regional differences in receptor density and efficacy for G-protein activation were observed in the brains of both male and female rats in the present study. Relative differences in Emax between brain regions were large and agreed with those previously reported with maximal receptor function in the cerebellum being higher than that of other brain regions (Breivogel et al., 2003; Burston et al., 2010). By contrast, receptor densities, measured by maximal [3H]SR141716A binding, exhibited less regional variation. These results agree with previous results showing that the ratio of CB1 receptors to CB1 receptor-activated G-proteins varies widely across brain regions (Breivogel et al., 1997).
Despite the cross-regional differences in receptor function noted above, intact vehicle-treated rats showed few sex differences in basal receptor expression or function within each of the four selected brain regions. A notable exception was enhanced CB1 receptor stimulation in the hippocampi of female rats compared to males. Given the absence of a concomitant sex difference in CB1 receptor numbers (as measured by Bmax), these results suggest increased efficacy of G-protein coupling of hippocampal CB1 receptors in females. Comparison of the present results with those of previous studies is complicated by differences in rat strain (e.g., Long-Evans, Lister, Wistar, and Sprague-Dawley), in procedure used to measure receptor number and function (e.g., autoradiographic analysis in brain sections vs. radioligand binding and agonist-stimulated [35S]GTPγS binding in membrane homogenates, or use of radiolabeled agonist vs. antagonist), and in specific brain regions examined. A few studies reported sex differences in CB1 receptor densities in isolated regions: (a) autoradiographic analysis using labeled agonist revealed lower densities in the amygdala and cingulate areas 1 and 3 of vehicle-treated female compared to male Lister hooded rats (Castelli et al., 2014); (b) lower densities in the mesencephalon of vehicle-treated female compared to male Wistar rats were demonstrated through membrane homogenate binding (Rodriguez de Fonseca et al., 1994); (c) agonist radioligand binding to membranes showed lower CB1 receptor densities in the hypothalamus and higher densities in the amygdala of vehicle-treated female compared to male Sprague-Dawley rats (Riebe et al., 2010). Other studies have failed to find sex differences in CB1 receptor number and/or function in a variety of brain regions in vehicle-treated, intact adult Sprague-Dawley or Long Evans rats (Burston et al., 2010; Silva et al., 2016). The paucity of examination of sex differences in CB1 receptor number and function, as well as the disparate cross-study results described above, highlight the need for additional research in this area using standardized procedures. Nevertheless, review of the extant research suggests that sex differences in brain CB1 receptor number and function in vehicle-treated intact adult rats are not widespread or pronounced, and this conclusion is supported by the present results.
While several studies have examined the effects of gonadectomy on CB1 receptor number and/or function in female rodents, this study represents the first to examine its effects in males. Gonadectomy (with or without hormone replacement) did not alter the overall pattern of minimal sex differences in CB1 receptor density and activation in vehicle-treated rats of either sex. As with intact rats, the most prominent effects of GDX were observed in the hippocampus and PFC. In PFC, GDX rats of both sexes demonstrated increased G-protein activation (Emax) compared to intact rats. While GDX rats also appeared to have a higher receptor density in the PFC compared to intact rats, the effect was not significant (p = 0.075), perhaps because of the relatively small number of rats included in this study or the choice to use the whole PFC rather than specific sub-regions (which may have obscured the ability to detect differences specific to a sub-region).
To the latter point, in untreated rats, GDX females had higher CB1 receptor density and efficacy in cingulate areas 1 and 3 of the PFC than intact females using autoradiographic analysis of [3H]CP55,940 receptor binding and CP55,940 stimulated [35S]GTPγS binding, respectively (Castelli et al., 2014). Whereas recovery of sensitivity in the PFC was observed in GDX females pre-treated with a single injection of estradiol in the previous study, a similar recovery was not seen in this study for either female or male GDX rats treated chronically with estradiol or testosterone, respectively. In the present study, GDX also significantly increased CB1 receptor density (Bmax) in the hippocampi and PFC of males and females relative to intact rats of each sex, as has been reported previously for female rats (Riebe et al., 2010). Again, however, the “chronic” hormone replacement used in the present study did not result in recovery for either sex, although recovery was observed following a single injection of estradiol in the previous study. The hormone replacement regimen we used here has been shown previously to maintain normal reproductive organ (e.g., seminal vesicle or uterine) weight as well as normal levels of sexual behavior (copulatory rate and efficiency for males or lordosis quotient for females) in gonadectomized rats tested 1–1.5 months after surgery+hormone replacement (Stoffel et al., 2003). In ovariectomized females, this estradiol replacement regimen maintains a proestrous- to estrous-like state (Stoffel et al., 2003). Moreover, we have measured serum hormone levels in gonadectomized rats using the same regimen of hormone replacement and found that testosterone and estradiol levels were stable for up to 10 weeks and 6 weeks, respectively, after surgery/capsule implantation (Wakley et al., 2015). Given the disparate findings regarding the effects of hormone replacement between the present and previous studies, further research is needed to determine whether method and/or chronicity of hormone replacement affects various aspects of CB1 receptors.
The relative paucity of sex differences in the number and function of CB1 receptors in vehicle-treated rats belies the frequently observed behavioral differences between responses in males and females induced by acute administration of cannabinoid agonists (Craft et al., 2013; Fattore and Fratta, 2010), some of which have been shown to be modulated by sex hormones (Winsauer et al., 2011). For example, previous studies show that administration of estradiol to GDX female rats increased acute THC-induced antinociception and locomotor activity, and testosterone lessened acute THC effects on motor activity in GDX males (Craft and Leitl, 2008; Wakley et al., 2014a). Pharmacokinetic differences may also play a role in mediation of the greater sensitivity of female rats to THC’s behavioral effects, as females exhibited preferential metabolism of THC to its psychoactive metabolite 11-hydroxy-tetrahydrocannabinol (11-OHTHC), whereas a larger variety of metabolites was observed in males (Tseng et al., 2004; Wiley and Burston, 2014).
By contrast with the relatively small effects of sex and gonadal hormones on CB1 receptor density and function in the brains of vehicle-treated rats, repeated administration of THC induced profound downregulation and desensitization of CB1 receptors in all four brain regions and in both sexes, regardless of hormonal status. These results are consistent with the robust tolerance to THC-induced tetrad effects (i.e., locomotor suppression, antinociception, hypothermia and catalepsy; Marusich et al., 2015) that was exhibited by these rats. In addition, they are consistent with prior investigations in intact male and female rodents (Burston et al., 2010; Sim-Selley, 2003) and in male human subjects (Hirvonen et al., 2012). The present results are novel in that they demonstrate that these THC-induced changes in CB1 receptor number and function occur not only in intact rats of both sexes but also in male and female gonadectomized rats regardless of whether testosterone or estradiol, respectively, are replaced. Sex differences in THC-induced decreases in receptor densities and/or function were observed in all four brain regions with treated males having greater CB1 receptor numbers than females in the cerebellum, hippocampus, and PFC and greater G-protein coupling in the cerebellum, PFC, and striatum. These results contrast with those in vehicle-treated rats (in which sex differences were not observed in any region for Bmax and only in the hippocampus for Emax, with females > males) and suggest that females exhibited greater downregulation than males in the cerebellum, hippocampus, and PFC as well as greater desensitization in all four regions. While female rats have been shown to have greater desensitization than males in the hippocampus, along with the PFC, striatum, and periaqueductal grey after repeated cannabinoid exposure during adolescence (Burston et al., 2010; Rubino et al., 2008; Silva et al., 2015), few studies have examined the effects of repeated THC treatment during adulthood on CB1 receptor number and function in the brains of rats of both sexes (Burston et al., 2010; Wakley et al., 2014a).
In addition to the marked effects of repeated administration of THC on CB1 receptor number and function, repeated THC also significantly increased affinity of [3H]SR141716A binding (Kd) and/or decreased the potency of CP55,940 (EC50) in all brain areas regardless of sex; these effects have not typically been reported in prior studies, perhaps due to their exclusive focus on maximal effect measures (i.e., Bmax and Emax). These results might be related to the decrease in the fraction of the remaining CB1 receptors that are coupled to G-proteins. At any given time, some fraction of G-protein coupled receptors are coupled to a G-protein, and agonists exhibit higher affinity and potency for G-protein coupled receptors that are coupled (Breivogel and Childers, 2000), while inverse agonists may show increased affinity for uncoupled receptors (Bouaboula et al., 1997). Chronic treatment with THC led to desensitization as evidenced by an overall decrease in Emax, and the increase in the EC50 for the agonist CP55,940 most likely indicates a shift of a greater fraction of CB1 into the G-protein uncoupled state. A greater fraction of uncoupled receptors might also explain the slight increase in the affinity (decrease in Kd) seen for the inverse agonist [3H]SR141716A. Hence, enhancement of downregulation and desensitization observed in the hippocampi of THC-treated female rats compared to males conceivably could be related to greater shift in the hippocampi of females towards the G-protein uncoupled state.
Consistent with these findings, sexual dimorphism has also been demonstrated in the effects of chronic THC use in humans. Women tend to progress from initial use to dependence more quickly than men, and male cannabis smokers experience fewer withdrawal symptoms than women (Copersino et al., 2010; Hernandez-Avila et al., 2004; Levin et al., 2010). Development of tolerance to THC’s antinociceptive and locomotor effects may also be greater in female rats compared to male rats (Wakley et al., 2014b; Wiley, 2003), and greater tolerance to THC’s hypothermic effects was observed in the female rats used in the present study (Marusich et al., 2015). Furthermore, intact adult female rats exhibited more signs of THC dependence (Marusich et al., 2014). Subsequent experiments suggested that estradiol and progesterone likely contributed to the greater dependence in female rats, while testosterone may have had a protective effect against dependence in males (Marusich et al., 2015). Sex differences in the acute effects of THC have also been reported with greater sensitivity generally observed in female rats (Craft et al., 2013; Wakley et al., 2014b, 2015). Consequently, the same THC dose that was used for both sexes in the present study represented a functionally higher dose for females than males (Wakley et al., 2015). If so, the greater downregulation and desensitization observed in the brains of female rats may be explained by a difference in the effective dose as opposed to an underlying sex difference in CB1 receptor sensitivity to THC treatment. Alternatively, the effects of higher concentrations of THC’s major psychoactive metabolite 11-OH-THC may also have contributed to the greater downregulation and desensitization observed in female rats (Tseng et al., 2004; Wiley and Burston, 2014).
In summary, the present results partly replicate and add to a growing body of literature that examines the effects of sex and gonadal hormones on the structure and function of brain neurotransmitter systems in preclinical models. Here, we report that the brains of gonadally intact vehicle-treated female and male rats exhibited few differences in CB1 receptor densities in four regions rich in these receptors, but G-protein coupling was greater in the hippocampi of female than male rats. While repeated treatment with THC resulted in pronounced downregulation and desensitization in both sexes, one or both effects were enhanced in female rats in all four brain regions, a result that is consistent with the finding that women tend to progress to tolerance and dependence quicker than men after initiation of cannabis use. Additional research is needed to delineate further the complex interplay between sex differences in the brain and in behavior.
Highlights.
The NIH mandates inclusion of sex as a biological variable in preclinical research.
This study examined sex and hormone differences in CB1 receptors in rats.
Hippocampi of female rats showed enhanced CB1 receptor stimulation versus males.
Magnitude of THC-induced downregulation and desensitization was greater in females.
Sex and hormone differences in CB1 receptors are regionally selective.
Acknowledgements
The authors thank Purvi Patel for technical assistance. This research was supported by grants DA-016644, DA-003672, and DA-040460 from the National Institutes of Health, National Institute on Drug Abuse (NIH/NIDA).
Role of the Funding Source
This research was funded by grants DA-016644, DA-003672, and DA-040460 from the National Institutes of Health, National Institute on Drug Abuse (NIH/NIDA). All drugs were purchased or were provided by the NIDA Drug Supply Program. NIH/NIDA did not have any other role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication. The opinions, findings, and conclusions or recommendations expressed in this publication are those of the authors and do not necessarily reflect those of NIH or NIDA.
Footnotes
Conflict of Interest
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beery AK, Zucker I, 2011. Sex bias in neuroscience and biomedical research. Neurosci. Biobehav. Rev 35, 565–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breivogel CS, Childers SR, 2000. Cannabinoid agonist signal transduction in rat brain: Comparison of cannabinoid agonists in receptor binding, G-protein activation, and adenylyl cyclase inhibition. J. Pharmacol. Exp. Ther 295, 328–336. [PubMed] [Google Scholar]
- Breivogel CS, Scates SM, Beletskaya IO, Lowery OB, Aceto MD, Martin BR, 2003. The effects of delta9-tetrahydrocannabinol physical dependence on brain cannabinoid receptors. Eur. J. Pharmacol 459, 139–150. [DOI] [PubMed] [Google Scholar]
- Breivogel CS, Sim LJ, Childers SR, 1997. Regional differences in cannabinoid receptor/Gprotein coupling in rat brain. J. Pharmacol. Exp. Ther 282, 1632–1642. [PubMed] [Google Scholar]
- Burston JJ, Wiley JL, Craig AA, Selley DE, Sim-Selley LJ, 2010. Regional enhancement of CB1 receptor desensitization in female adolescent rats following repeated Δ9-tetrahydrocannabinol exposure. Br. J. Pharmacol 161, 103–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelli MP, Fadda P, Casu A, Spano MS, Casti A, Fratta W, Fattore L, 2014. Male and female rats differ in brain cannabinoid CB1 receptor density and function and in behavioural traits predisposing to drug addiction: Effect of ovarian hormones. Curr. Pharm. Des 20, 2100–2113. [DOI] [PubMed] [Google Scholar]
- Childers SR, Breivogel CS, 1998. Cannabis and endogenous cannabinoid systems. Drug Alcohol Depend. 51, 173–187. [DOI] [PubMed] [Google Scholar]
- Clayton JA, Collins FS, 2014. Policy: NIH to balance sex in cell and animal studies. Nature 509, 282–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copersino ML, Boyd SJ, Tashkin DP, Huestis MA, Heishman SJ, Dermand JC, Simmons MS, Gorelick DA, 2010. Sociodemographic characteristics of cannabis smokers and the experience of cannabis withdrawal. Am. J. Drug Alcohol Abuse 36, 311319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft RM, Leitl MD, 2008. Gonadal hormone modulation of the behavioral effects of Delta9tetrahydrocannabinol in male and female rats. Eur. J. Pharmacol 578, 37–42. [DOI] [PubMed] [Google Scholar]
- Craft RM, Marusich JA, Wiley JL, 2013. Sex differences in cannabinoid pharmacology: A reflection of differences in the endocannabinoid system? Life Sci. 92, 476–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattore L, Fratta W, 2010. How important are sex differences in cannabinoid action? Br. J. Pharmacol 160, 544–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC, 1991. Characterization and localization of cannabinoid receptors in rat brain: A quantitative in vitro autoradiographic study. J. Neurosci 11, 563–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Avila CA, Rounsaville BJ, Kranzler HR, 2004. Opioid-, cannabis- and alcoholdependent women show more rapid progression to substance abuse treatment. Drug Alcohol Depend. 74, 265–272. [DOI] [PubMed] [Google Scholar]
- Hirvonen J, Goodwin RS, Li CT, Terry GE, Zoghbi SS, Morse C, Pike VW, Volkow ND, Huestis MA, Innis RB, 2012. Reversible and regionally selective downregulation of brain cannabinoid CB1 receptors in chronic daily cannabis smokers. Mol. Psychiatry 17, 642–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin KH, Copersino ML, Heishman SJ, Liu F, Kelly DL, Boggs DL, Gorelick DA, 2010. Cannabis withdrawal symptoms in non-treatment-seeking adult cannabis smokers. Drug Alcohol Depend. 111, 120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusich JA, Craft RM, Lefever TW, Wiley JL, 2015. The impact of gonadal hormones on cannabinoid dependence. Exp. Clin. Psychopharmacol 23, 206–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusich JA, Lefever TW, Antonazzo KR, Craft RM, Wiley JL, 2014. Evaluation of sex differences in cannabinoid dependence. Drug Alcohol Depend. 137, 20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council, 2011. Guide for the care and use of laboratory animals. National Academies Press, Washington, D.C. [Google Scholar]
- Riebe CJ, Hill MN, Lee TT, Hillard CJ, Gorzalka BB, 2010. Estrogenic regulation of limbic cannabinoid receptor binding. Psychoneuroendocrinology 35, 1265–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez de Fonseca F, Cebeira M, Ramos JA, Martin M, Fernandez-Ruiz JJ, 1994. Cannabinoid receptors in rat brain areas: Sexual differences, fluctuations during estrous cycle and changes after gonadectomy and sex steroid replacement. Life Sci. 54, 159–170. [DOI] [PubMed] [Google Scholar]
- Rubino T, Vigano D, Realini N, Guidali C, Braida D, Capurro V, Castiglioni C, Cherubino F, Romualdi P, Candeletti S, Sala M, Parolaro D, 2008. Chronic delta(9)-tetrahydrocannabinol during adolescence provokes sex-dependent changes in the emotional profile in adult rats: Behavioral and biochemical correlates. Neuropsychopharmacology 33, 2760–2771. [DOI] [PubMed] [Google Scholar]
- Silva L, Black R, Michaelides M, Hurd YL, Dow-Edwards D, 2016. Sex and age specific effects of delta-9-tetrahydrocannabinol during the periadolescent period in the rat: The unique susceptibility of the prepubescent animal. Neurotoxicol. Teratol 58, 88–100. [DOI] [PubMed] [Google Scholar]
- Silva L, Harte-Hargrove L, Izenwasser S, Frank A, Wade D, Dow-Edwards D, 2015. Sex-specific alterations in hippocampal cannabinoid 1 receptor expression following adolescent delta-9-tetrahydrocannabinol treatment in the rat. Neurosci. Lett 602, 89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim-Selley LJ, 2003. Regulation of cannabinoid CB1 receptors in the central nervous system by chronic cannabinoids. Crit. Rev. Neurobiol 15, 91–119. [DOI] [PubMed] [Google Scholar]
- Sim LJ, Hampson RE, Deadwyler SA, Childers SR, 1996. Effects of chronic treatment with delta9-tetrahydrocannabinol on cannabinoid-stimulated [35S]GTPgammaS autoradiography in rat brain. J. Neurosci 16, 8057–8066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoffel EC, Ulibarri CM, Craft RM, 2003. Gonadal steroid hormone modulation of nociception, morphine antinociception and reproductive indices in male and female rats. Pain 103, 285–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng AH, Harding JW, Craft RM, 2004. Pharmacokinetic factors in sex differences in Delta 9-tetrahydrocannabinol-induced behavioral effects in rats. Behav. Brain Res 154, 77–83. [DOI] [PubMed] [Google Scholar]
- Wakley AA, McBride AA, Vaughn LK, Craft RM, 2014a. Cyclic ovarian hormone modulation of supraspinal Delta9-tetrahydrocannabinol-induced antinociception and cannabinoid receptor binding in the female rat. Pharmacol. Biochem. Behav 124, 269277. [DOI] [PubMed] [Google Scholar]
- Wakley AA, Wiley JL, Craft RM, 2014b. Sex differences in antinociceptive tolerance to delta-9-tetrahydrocannabinol in the rat. Drug Alcohol Depend. 143, 22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakley AA, Wiley JL, Craft RM, 2015. Gonadal hormones do not alter the development of antinociceptive tolerance to delta-9-tetrahydrocannabinol in adult rats. Pharmacol. Biochem. Behav 133, 111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JL, 2003. Sex-dependent effects of delta 9-tetrahydrocannabinol on locomotor activity in mice. Neurosci. Lett 352, 77–80. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Burston JJ, 2014. Sex differences in Delta(9)-tetrahydrocannabinol metabolism and in vivo pharmacology following acute and repeated dosing in adolescent rats. Neurosci. Lett 576, 51–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winsauer PJ, Daniel JM, Filipeanu CM, Leonard ST, Hulst JL, Rodgers SP, Lassen-Greene CL, Sutton JL, 2011. Long-term behavioral and pharmacodynamic effects of delta-9-tetrahydrocannabinol in female rats depend on ovarian hormone status. Addict. Biol 16, 64–81. [DOI] [PMC free article] [PubMed] [Google Scholar]