Abstract
Objectives:
Little evidence is available on the association of e-cigarettes with health indices. We investigated the association of e-cigarette use with diagnosed respiratory disorder among adults with data from the Behavioral Risk Factor Surveillance Survey (BRFSS).
Methods:
The 2016 Hawaii BRFSS, a cross-sectional random-dial telephone survey, had 8,087 participants; mean age was 55 years. Items asked about e-cigarette use, cigarette smoking, and being diagnosed by a health professional with (a) asthma or (b) chronic obstructive pulmonary disease. Multivariable analyses tested associations of e-cigarette use with the respiratory variables controlling for smoking and demographic, physical, and psychosocial variables.
Results:
Controlling for the covariates and smoking there was a significant association of e-cigarette use with chronic pulmonary disorder in the total sample (AOR = 2.58, CI 1.36 – 4.89, p < .01) and a significant association with asthma among nonsmokers (AOR = 1.33, CI 1.00 – 1.77, p < .05). The associations were stronger among nonsmokers than among smokers. Results were similar for analyses based on relative risk and absolute risk. There was also a greater likelihood of respiratory disorder for smokers, females, and persons with overweight, financial stress, and secondhand smoke exposure.
Conclusions:
This is the first study to show a significant independent association of e-cigarette use with chronic respiratory disorder. Several aspects of the data are inconsistent with the possibility that e-cigarettes were being used for smoking cessation by persons with existing respiratory disorder. Theoretical mechanisms that might link e-cigarettes use and respiratory symptoms are discussed.
Keywords: e-cigarettes, smoking, asthma, COPD, adults
1. INTRODUCTION
The use of electronic smoking devices (e-cigarettes) has increased in recent years and the prevalence of e-cigarette use is now substantial among both adolescents and adults (Jamal et al., 2017; Wilson & Wang, 2017; Zernike, 2018). Given this prevalence, it is desirable to have knowledge about the health consequences of e-cigarette use (Glantz & Bareham, 2018; Muthy, 2017). Laboratory research using animal models and cell cultures has indicated that e-cigarettes have biological effects that could lead to tissue injury (Chun et al., 2017; St. Helen & Eaton, 2018). For example, e-cigarette vapor has been shown to have cytotoxic effects on lung cells (Lee et al., 2018) and to produce physiological defects usually seen in Chronic Obstructive Pulmonary Disease (COPD; Garcia-Arcos et al., 2016). At present the generalization of these findings from laboratory paradigms to epidemiological samples is unclear. There is limited knowledge on e-cigarette use with clinical or subclinical variables, and reviews have emphasized the importance of research testing such questions (US Department of Health and Human Services, 2016; Stratton et al., 2018). Accordingly, we investigated whether there was an association of e-cigarette use with diagnosed respiratory disorder in a representative sample of adults.
Previous research on asthma has provided evidence of an association with e-cigarette use, primarily from studies of adolescents. For example, a study with high school students in Hawaii found an association of e-cigarette use with asthma that was independent of cigarette smoking and marijuana use as well as several demographic variables (Schweitzer et al., 2017). This concurs with findings from adolescents in Asian countries (Cho & Paik, 2016; Wang et al., 2016) and the US state of Florida (Choi & Bernat, 2016). These results are theoretically consistent with physiological effects observed in laboratory studies (Clapp & Jaspers, 2017). While asthma may begin at earlier ages (Akinbami et al., 2014), the suggestion that e-cigarettes could maintain or exacerbate asthma (Clapp & Jaspers, 2017) led us to predict that an association of e-cigarette use with asthma would be observed among adults.
There is less evidence about chronic respiratory disease but a study with high school students in California found an association of e-cigarette use with reports of bronchitis symptoms (McConnell et al., 2017). Yao et al. (2017) studied a US national sample of current cigarette smokers and found that e-cigarette use was associated with a higher likelihood of wheezing and having multiple colds in the past 30 days, suggesting that e-cigarette use could increase susceptibility to upper respiratory infection. study with an adult sample in Sweden (Hedman et al., 2018) found an association of e-cigarette with respiratory symptoms (e.g., long-standing cough, recurrent wheezing) but did not measure diagnosed respiratory disorder. Hence there is still little epidemiological evidence on chronic respiratory disorder and this has been suggested as a priority for investigation (Stratton et al., 2018). The present research included a measure of chronic pulmonary disorder and based on a body of research from in vitro and animal studies (Chun et al., 2017), we predicted that e-cigarette use would be related to chronic respiratory disorder in adults.
1.1. Study Aims and Hypotheses
To summarize, several lines of research suggest that a relation of e-cigarette use to respiratory disorder might occur among adults. Hence, we investigated whether e-cigarette use is associated with asthma and pulmonary disorder in a representative sample aged 18 years and over and whether e-cigarette use and combustible cigarette smoking could have synergistic effects for respiratory symptomatology (Cho & Paik, 2016). Because respiratory disorder is a multifactorial condition, having several different risk factors that may be correlated, we included measures of known risk factors as controls. For example, e-cigarette use and cigarette smoking tend to be correlated (Dutra et al., 2016; Wills et al., 2015) so it is crucial to control for this potential confound. In addition, asthma and pulmonary symptoms are related to psychosocial factors including financial adversity (Beck et al., 2014; Kopel et al., 2014) and second-hand smoke exposure (McConnell et al., 2017) which also could be related to e-cigarette use. Obesity is a physical risk factor for asthma (Gennuso et al., 1998; von Mutius et al., 2001) so its contribution also needs to be evaluated. Thus the goal of this research was to determine the association of e-cigarette use with asthma and chronic pulmonary disorder controlling for these other factors so as to obtain a more precise test for e-cigarettes. We predicted that in this diverse sample of adults there would be positive associations of e-cigarette use with asthma and with pulmonary disorder. Because of a possibility that effects of e-cigarettes for airway irritation observed in laboratory studies could augment effects of smoking, which is not currently known (Chun et al., 2017), we also tested for an interaction of e-cigarettes and cigarette smoking.
2. METHODS
2.1. Participants and Procedure
This research used data from the Behavioral Risk Factor Surveillance Survey (BRFSS), an annual cross-sectional, random-digit-dialed survey that includes adult participants aged 18 years or older (CDC, 2011). The BRFSS uses a multistage sampling design to select a representative sample of the noninstitutionalized adult population in each of the 50 US states. Since 2011 the methodology has included reaching and interviewing participants on cellular telephones as well as landlines and using a dual-frame survey methodology to improve reliability and representativeness of the data (Hu, Balluz, Battaglia, & Frankel, 2011). The present research uses data from the 2016 BRFSS conducted in the state of Hawaii. This survey had an overall response rate of 43%, a figure that compares favorably with other states and national surveys (CDC, 2016). The final sample had a total of 8,087 participants. Details on sample representativeness for the BRFSS survey procedure and validity of the BRFSS measures for assessing chronic disease are reviewed elsewhere (Pierannunzi et al., 2013; Li et al., 2012). Demonstrations of the generalizability of results from Hawaii to other parts of the have been shown in a number of studies (Soneji et al., 2017; Watkins et al., 2018; Wills et al., 2013) as has generalizability to other countries (Best et al., 2018; Lozano et al., 2017; Treuer et al., 2017).
2.2. Measures
Demographics.
The survey included items on gender (female/male) and age in years (write-in). The item on primary ethnicity asked: “Which one of these groups would you say best represents your race?” Response options included: Alaska ative/American Indian, Black or African-American, Asian (Chinese, Filipino, Japanese, Other Asian), Pacific Islander (Native Hawaiian, Other Pacific Islander), and Caucasian. Educational level (“What is the highest grade or year of school you have completed?”) had five response options (Grades 1–8, 9–11, 12 years, 1–3 years college, 4 years college or more).
E-cigarette use.
A clarifying instruction prior to asking the e-cigarette items stated: “Electronic cigarettes and other ‘vaping’ products include electronic hookahs (e-hookahs), vape pens, e-cigars, and others. These products are battery powered and usually contain nicotine and flavors such as fruit, mint, or candy.” The item on ever e-cigarette use asked, “Have you ever used an electronic cigarette or other electronic ‘vaping’ product in your entire life.” (Yes/No/Not Sure). The item on current use asked, “Do you now use electronic cigarettes or other electronic ‘vaping’ products every day, some days, or not at all.”
Cigarette smoking.
A clarifying instruction prior to the cigarette items stated: “For cigarettes do not include electronic cigarettes (e-cigarettes, NJOY, Bluetip), herbal cigarettes, cigars, cigarillos, little cigars, pipes, bidis, kreteks, water pipes (hookahs), or marijuana.” The basic item on cigarette smoking asked, “Have you smoked at least 100 cigarettes in your entire life?” (Yes/No/Not Sure). Persons who answered Yes to this question were then asked about current smoking: “Do you now smoke cigarettes every day, some days, or not at all.” Persons who indicated current smoking (some days or every day) were asked, “During the past 12 months, have you stopped smoking for one day or longer because you were trying to quit smoking” (Yes/No). Persons answering Yes to lifetime smoking but No to current smoking were asked, “How long has it been since you last smoked a cigarette?” (7 options, within past month to 10 years or more).
Respiratory disorder.
Items followed the lead-in: “Has a doctor, nurse, or other health professional ever told you that you had any of the following.” Items were: “Were you ever told you had asthma?” “Do you still have asthma?” and “Were you ever told you have chronic obstructive pulmonary disease (COPD), emphysema, or chronic bronchitis?” Response options were Yes/No/Not Sure.
Covariates.
Body mass index was assessed with the items, “About how much do you weigh without shoes?” and “About how tall are you without shoes?” (Write-in for both.) This was coded as a four-level variable for overweight status (Underweight, Normal Weight, Overweight, Obese). Exposure to second-hand smoke was assessed with the item: “On how many of the past 7 days did anyone smoke in your home while you were there?” Response options were None, Number of days write-in (1–7), or I was not at home past 7 days. Financial stress was assessed with two items: “How often in the past 12 months would you say you were worried or stressed about: having enough money to pay your rent/mortgage” and “How often in the past 12 months would you say you were worried or stressed about having enough money to having enough money to buy nutritious meals?” Response options Never, Rarely, Sometimes, Usually, Always.
2.3. Data Analysis
Prevalence estimates for substance use and respiratory symptoms were computed with weighted analyses using SAS Proc Surveyfreq, accounting for stratum. For analysis we used binary indices for ever-occurrence of asthma and COPD; for asthma we also computed a three-level index for never had asthma, previous asthma (but not current), and current asthma. For tobacco product use the basic analysis used binary indices (ever used e-cigarettes, smoked cigarettes >100×). A continuous measure for age was recoded to 8 levels (18–29, 30–39, 40–49, 50–59, 60–69, 70–79, 80–89, and 90–99 years). Educational level was recoded to four levels (grade school/some high school, high school graduate, some college, college graduate). For analysis two small ethnic groups (Alaska Native and frican American) were dropped as they caused convergence problems because of small cell sizes in stratified analyses. categorical variable for overweight status was recoded to three levels (under- or normal weight, overweight, obese). For financial stress, raw scores for the two items were summed and the summary score was recoded to a five-level variable (Never, Rarely Stressed, Somewhat Stressed, Often Stressed, Always Stressed).
Preliminary analyses examined the relation of age to measures of respiratory disorder and tobacco use. Zero-order relations of the predictor variables with respiratory disorder, e-cigarette use, and smoking, controlling for age, were analyzed in logistic regression with SAS Surveylogistic. Multivariable analyses for relative risk were performed in logistic regression with a binary index for respiratory disorder (asthma or COPD) as the criterion. E-cigarettes and smoking were the primary predictors. Entered as demographic covariates were age, gender, educational level, and ethnicity (six binary indices for Native Hawaiian, Filipino, Japanese, Chinese, Pacific Islander, and Other Asian contrasted against Caucasian as the reference group). Also entered as covariates were variables for overweight status, second-hand smoke exposure, and financial stress. A four-category variable was constructed for tobacco product usage (1 = Never used e-cigarettes, Nonsmoker; 2 = Smoker, Never used e-cigarettes; 3 = Ever used e-cigarette, Nonsmoker; 4 = Used e-cigarettes and Smoker) and pairwise comparisons of relative risk for asthma or COPD were computed from the model. To test for interaction in prediction of the criterion variables we entered the cross-product of e-cigarette use and smoking after entry of their two main-effect terms and all the covariates. To complement the analyses of relative risk we also report measures of absolute risk for asthma and COPD using the same models (cf. Noordzij et al., 2017); these are presented stratified by age.
There was little missing data for the asthma or COPD items but there were appreciable rates of missing data for some of the covariates. Accordingly, we conducted a full-information analysis using multiple imputation for the predictors (Rubin, 1987) so as to maximize sample size and minimize potential bias. This was performed using the SAS 9.4 Proc MI procedure with 20 imputations based on the Markov Chain Monte Carlo method (Schafer and Graham, 2002). The multivariable analyses were based on unweighted sample sizes of 8,069 for asthma and 8,063 for COPD.
3. RESULTS
3.1. Sample Characteristics
Table 1 reports the sample’s characteristics. The weighted sample was 50% female and mean age was 55 years. Proportions for primary perceived race/ethnicity were 13% Native Hawaiian, 18% Filipino, 20% Japanese, 6% Chinese, 20% Japanese, 3% Other Asian, 3% Other Pacific Islander, and 36% Caucasian. Education level was 9% less than high school, 29% high school graduate, 34% some college, and 28% college graduate.
Table 1.
Frequencies and Percentages for Study Variables
| Variable | Levels | Unweighted frequency |
Weighted Percent |
Weighted frequency |
percent |
|---|---|---|---|---|---|
| Gender | |||||
| Female | 4,314 | 53 | 561,749 | 50 | |
| Male | 3,772 | 47 | 570,279 | 50 | |
| missing | 1 | 125 | |||
| Age (years) | |||||
| 18–29 | 880 | 11 | 221,319 | 20 | |
| 30–39 | 973 | 12 | 217,418 | 19 | |
| 40–49 | 933 | 12 | 156,532 | 14 | |
| 50–59 | 1,496 | 19 | 180,961 | 16 | |
| 60–69 | 1,947 | 24 | 174,450 | 16 | |
| 70–79 | 1,165 | 15 | 103,098 | 9 | |
| 80–89 | 497 | 6 | 51,365 | 5 | |
| 90–99 | 113 | 1 | 14,983 | 1 | |
| missing | 83 | 12,028 | |||
| Race/ethnicity | |||||
| Nat. | 1,096 | 14 | 137,735 | 13 | |
| Hawaiian | |||||
| Filipino | 1,026 | 13 | 194,530 | 18 | |
| Japanese | 1,518 | 19 | 221,102 | 20 | |
| Chinese | 379 | 5 | 63,363 | 6 | |
| Pac. Islander | 185 | 2 | 31,868 | 3 | |
| Other Asian | 160 | 2 | 28,059 | 3 | |
| Caucasian | 3,374 | 43 | 390,153 | 36 | |
| Black | 104 | 1 | 31,019 | 3 | |
| missing | 245 | 2 | 34,322 | ||
| Education | |||||
| Grade school | 293 | 4 | 101,139 | 9 | |
| HS graduate | 2,103 | 26 | 326,062 | 29 | |
| Some college | 2,241 | 28 | 382,107 | 34 | |
| Coll. | 3,428 | 42 | 319,228 | 28 | |
| graduate | |||||
| missing | 22 | 3,617 | |||
| Ever | |||||
| diagnosed | No | 6,727 | 83 | 934,626 | 83 |
| with | Yes | 1,342 | 17 | 195,278 | 17 |
| asthma | missing | 18 | 2,249 | ||
| Have asthma |
No | 7,213 | 90 | 1,006,519 | 89 |
| now | Yes | 832 | 10 | 120,293 | 11 |
| missing | 42 | 5,342 | |||
| Ever diagnosed |
No | 7,615 | 94 | 1,083,315 | 96 |
| with | Yes | 448 | 6 | 45,098 | 4 |
| COPD | missing | 24 | 3,741 | ||
| Ever | |||||
| used | No | 6,427 | 83 | 837,973 | 78 |
| e-cigarettes | Yes | 1,346 | 17 | 239,079 | 22 |
| missing | 314 | 55,101 | |||
| Use | |||||
| e-cigarettes | Not at all | 7,536 | 97 | 1,030,695 | 96 |
| now | Some days | 144 | 2 | 27,143 | 2 |
| Every day | 91 | 1 | 18,997 | 2 | |
| missing | 316 | 55,317 | |||
| Smoked | |||||
| > 100× | No | 4,538 | 58 | 663,946 | 62 |
| Yes | 3,246 | 42 | 414,696 | 38 | |
| missing | 303 | 53,511 | |||
| Smoke | |||||
| cigarettes | Not at all | 6,844 | 88 | 937,239 | 87 |
| now | Some days | 270 | 3 | 44,645 | 4 |
| Every day | 664 | 9 | 96,040 | 9 | |
| missing | 309 | 54,229 | |||
| Weight | |||||
| status | Normal | 3,271 | 43 | 448,025 | 42 |
| Overweight | 2,621 | 34 | 357,240 | 34 | |
| Obese | 1,767 | 23 | 251,883 | 24 | |
| missing | 428 | 75,005 | |||
| Secondhand | |||||
| smoke | None | 6,438 | 92 | 855,432 | 91 |
| exposure | 1–4 days | 185 | 3 | 28,690 | 3 |
| 5–7 days | 393 | 6 | 56,630 | 6 | |
| missing | 1,071 | 191,402 | |||
| Worried | |||||
| about | Never | 3,268 | 53 | 401,932 | 50 |
| finances | Rarely | 1,406 | 23 | 201,913 | 25 |
| during past | Sometimes | 839 | 14 | 121,017 | 15 |
| 12 months | Usually | 381 | 6 | 50,713 | 6 |
| Always | 251 | 4 | 36,036 | 4 | |
| missing | 1,942 | 320,542 | |||
The estimated prevalence in the overall sample was 17% for ever had asthma and 4% for ever had COPD; the weighted N for these analyses was approximately 1,129,000 participants. (For this comparison we report lifetime rates because there was no item corresponding to current COPD.) The estimated prevalence of ever e-cigarette use in the overall sample was 22% and the prevalence of cigarette smoking (smoked > 100 cigarettes) was 38%. For current e-cigarette use, overall prevalence was 2% (some days) and 2% (every day). For current cigarette smoking, overall prevalence was 4% (some days) and 9% (every day).
Asthma and chronic pulmonary disorder were correlated (Supplemental Table 1). For example, the estimated prevalence of COPD was 3% among persons who never had asthma but was 17% among persons who had asthma. However, 48% of persons with COPD had not had asthma and these data supported performing separate analyses for asthma and COPD.
The patterns of data for respiratory symptoms and tobacco use were strongly age-dependent but the direction of the relationship differed across variables (Supplemental Table 2). Reports of asthma tended to decrease with age while COPD increased with age. Cigarette smoking increased in frequency across most of the age groups whereas e-cigarette use was most frequent among the younger age groups. Financial stress showed an almost linear decrease with age. The prevalence of overweight was not linearly age-dependent but there was indication of a quadratic trend. These data supported including age as a covariate for all of the analyses.
E-cigarette use and cigarette smoking were correlated, as is typical in other studies (Bareham & Glantz, 2018; Dutra & Glantz, 2014; Wills et al., 2015). Cross-tabulations for ever-use and current use of e-cigarettes and cigarettes showed significant associations (Supplemental Table 3). For example, 12% of nonsmokers had ever used e-cigarettes compared with 39% for smokers. The cross-tabulation of e-cigarette use and smoking was comparable across most age groups, excepting for 80–99 year groups, where the prevalence of e-cigarette use was low.
3.2. Bivariate Relations for Variables
Logistic regression analyses were used to compute bivariate relations of the study variables with asthma and chronic pulmonary disorder, including age as a covariate (Supplemental Table 4). E-cigarette use and cigarette smoking showed significant positive associations with both asthma and COPD (odds ratios >1) whereas inverse relations were noted for educational level and male gender for asthma (odds ratios < 1). Asthma and COPD were positively associated with overweight status, financial stress, and exposure to secondhand smoke (for COPD). For these analyses the coding compared a given ethnic group with all other groups. Relative to other groups, Native Hawaiians had significantly higher prevalence for asthma and COPD and Caucasians were elevated for COPD, whereas Japanese and Chinese, and Other Asians tended to have lower prevalence for asthma and in two cases for COPD.
Bivariate correlations with e-cigarette use and cigarette smoking controlling for age (Supplemental Table 5) indicated male gender and financial stress were positively associated with smoking and e-cigarette use whereas educational level was inversely associated with these; also there were significant zero-order associations of second-hand smoke exposure and overweight status with these variables. Using the same coding, higher rates of smoking and e-cigarette use were also noted for Native Hawaiians and Caucasians whereas lower rates were noted for Filipinos, Japanese, and Chinese.
3.3. Multivariable Models
In multivariable analyses, measures of e-cigarette use and cigarette smoking were entered together with age and the other covariates, with asthma or chronic pulmonary disorder as the criterion. A quadratic term for age was included in the models. As noted in Analytic Methods, six dummy variables were entered to code ethnicity, with Caucasians as the reference group. As indicated in Table 2, cigarette smoking was positively associated with asthma in the overall sample, AOR = 1.27 (CI = 1.10–1.47), p = .001, but e-cigarette use was marginal, AOR = 1.27 (CI 0.96 – 1.67), p = .10. The interaction was inverse in direction, AOR = 0.78, but did not reach significance. Pairwise comparisons indicated the smoking-only group and the dual-user group had higher rates of asthma relative to the nonuser group (p = .001 and p = .02, respectively) but the e-cigarette only group was marginal (p = 10). The interaction was interpreted by conducting separate analyses for nonsmokers and smokers. These indicated there was a significant association of e-cigarette use with asthma among nonsmokers (AOR = 1.33, CI 1.00 – 1.77, p < .05) but not among smokers (AOR = 0.92, CI 0.73 – 1.15, ns.). Results for the covariates indicated asthma inversely associated with age and positively associated with overweight status, financial stress, Native Hawaiian ethnicity, and female gender. The model for asthma was repeated with age restricted to the lower six age categories (i.e., < 80 years) and with listwise deletion of missing data. he results from these analyses were not substantially different than the ones reported here.
Table 2.
Relative Risk (Odds Ratio and CI) in Multivariate Model for Asthma with Pairwise Comparisons
| Variable | AOR | CI | p |
|---|---|---|---|
| Age | 0.93 | 0.89– 0.97 | .0002 |
| Age**2 | 1.01 | 0.99– 1.03 | .37 |
| E-cigarette use | 1.27 | 0.96– 1.67 | .10 |
| Cigarette smoking | 1.27 | 1.10– 1. 47 | .001 |
| E-cig X smoking | 0.78 | 0.56– 1.09 | .15 |
| Pairwise comparisons | |||
| Dual vs. none | 1.26 | 1.04– 1.53 | .02 |
| Smok vs. none | 1.27 | 1.10– 1.47 | .001 |
| E-cig vs. none | 1.27 | 0.96– 1.67 | .10 |
| Dual vs. e-cig | 1.00 | 0.73– 1.35 | .98 |
| Smok vs. e-cig | 1.00 | 0.74– 1.35 | .98 |
| Dual vs. smok | 0.99 | 0.80– 1.22 | .94 |
| Gender (male) | 0.62 | 0.55– 0.70 | < .0001 |
| Education | 0.94 | 0.88– 1.01 | .10 |
| Financial stress | 1.13 | 1.06– 1.20 | .0003 |
| Overweight status | 1.28 | 1.17– 1.39 | < .0001 |
| Secondhand smoke | 1.00 | 0.87– 1.13 | .95 |
| Native HawaiianA | 1.53 | 1.28– 1.82 | < .0001 |
| Filipino | 0.95 | 0.78– 1.16 | .63 |
| Japanese | 0.97 | 0.81– 1.15 | .71 |
| Chinese | 0.96 | 0.69– 1.33 | .82 |
| Pacific Islander | 0.56 | 0.36– 0.89 | .01 |
| Other Asian | 0.75 | 0.45– 1.24 | .27 |
Note: For usage groups: None = never used e-cigarettes, nonsmoker; E-cig = Only used e-cigarettes, nonsmoker; Smok = Only smoked cigarettes, nonsmoker; Dual = Used e-cigarettes and smoked cigarettes.
Ethnicity contrasts are coded with Caucasians as the reference group.
In the multivariable analysis for chronic pulmonary disorder (Table 3), e-cigarette use and smoking showed significant independent associations with respiratory disorder; the effect size was larger for smoking (AOR = 2.98, CI 2.34 −3.78) than for e-cigarettes (AOR = 2.58, CI 1.36 – 4.89) but both were significant. Pairwise comparisons indicated that relative to the nonuser group there were higher rates of disorder for both the e-cigarette only group (p = .004), the cigarette only group (p = .0001), and the dual user group (p = .0001). The interaction term was significant (AOR = 0.51, CI 0.26 – 1.00, p < .05). Subgroup analyses indicated there was a significant association of e-cigarette use with COPD among nonsmokers (AOR = 2.98, CI 1.51 – 5.88, p < .01), but the association was not significant among smokers (AOR = 1.29, CI 0.94 −1.77, ns.). Covariate effects in the multivariate model indicated COPD positively associated with second-hand smoke exposure, financial stress, female gender, and inversely associated with education and Japanese ethnicity. These analyses were repeated using variant models similar to those used for asthma; results from these analyses were not substantially different from those reported here.
Table 3.
Relative Risk (Odds Ratio and CI) in Multivariate Model for Chronic Pulmonary Disorder with Pairwise Comparisons
| Variable | AOR | CI | p |
|---|---|---|---|
| Age | 1.49 | 1.37– 1.62 | < .0001 |
| Age**2 | 0.97 | 0.94 – 1.00 | .05 |
| E-cigarette use | 2.58 | 1.36 – 4.89 | .004 |
| Cigarette smoking | 2.98 | 2.34 – 3.78 | < .0001 |
| E-cig X smoking | 0.51 | 0.26 – 1.00 | .05 |
| Pairwise comparisons | |||
| Dual vs. none | 3.92 | 2.82 – 5.44 | < .0001 |
| Smok vs. none | 2.98 | 2.34– 3.78 | < .0001 |
| E-cig vs. none | 2.58 | 1.36 – 4.89 | .004 |
| Dual vs. e-cig | 1.52 | 0.81 – 2.87 | .20 |
| Smok vs. e-cig | 1.16 | 0.62 – 2.17 | .65 |
| Dual vs. smok | 1.32 | 0.98 – 1.77 | .07 |
| Gender (male) | 0.71 | 0.58 – 0.88 | .001 |
| Education | 0.88 | 0.79 – 0.98 | .03 |
| Financial stress | 1.19 | 1.08 – 1.31 | .0006 |
| Overweight status | 1.11 | 0.96 – 1.27 | .15 |
| Secondhand smoke | 1.37 | 1.15 – 1.63 | .0003 |
| Native HawaiianA | 1.11 | 0.84 – 1.48 | .47 |
| Filipino | 0.82 | 0.58 – 1.17 | .27 |
| Japanese | 0.62 | 0.46 – 0.83 | .001 |
| Chinese | 0.58 | 0.30 – 1.12 | .11 |
| Pacific Islander | 1.16 | 0.56 – 2.40 | .69 |
| Other Asian | 0.56 | 0.21 – 1.51 | .25 |
Note: For usage groups: None = never used e-cigarettes, nonsmoker; E-cig = Only used e-cigarettes, nonsmoker; Smok = Only smoked cigarettes, nonsmoker; Dual = Used e-cigarettes and smoked cigarettes.
Ethnicity contrasts are coded with Caucasians as the reference group.
Data on the absolute risk for asthma and for pulmonary disorder are presented for each of the usage groups in Tables 4 and 5, respectively. (For concise presentation, persons over 80 are not included.) For asthma, absolute risk was moderate and declined with age (Figure 3). Absolute risk was higher for both the e-cigarette only group and the smoking-only group but the dual user group did not differ significantly from these (Table 3); hence in the figure the lines for these three groups converge. For the COPD item the level of absolute risk was lower and tended to increase with age (Figure 1B). The e-cigarette only group and smoking only group had higher rates than the nonuser group, with the dual-user group having an even higher rate, consistent with the independent contributions noted in the regression analyses (Table 3). Thus the data for absolute risk were consistent with the analyses for relative risk, showing both e-cigarette use and cigarette smoking associated with higher rates of respiratory disorder and the independent contributions being particularly notable for chronic pulmonary disorder.
Table 4.
Absolute Risk from Multivariate Model for Asthma, by Age Group and Usage Group
| Usage group | ||||
|---|---|---|---|---|
| Age (years) | Nonuser | Smok only |
E-cig only |
Dual user |
| 20.0 | 19.0% | 22.9% | 22.8% | 22.8% |
| 22.5 | 18.5% | 22.3% | 22.3% | 22.2% |
| 25.0 | 18.0% | 21.8% | 21.7% | 21.6% |
| 27.5 | 17.5% | 21.3% | 21.2% | 21.1% |
| 30.0 | 17.1% | 20.8% | 20.7% | 20.6% |
| 32.5 | 16.7% | 20.3% | 20.2% | 20.2% |
| 35.0 | 16.3% | 19.9% | 19.8% | 19.7% |
| 37.5 | 16.0% | 19.4% | 19.4% | 19.3% |
| 40.0 | 15.6% | 19.0% | 19.0% | 18.9% |
| 42.5 | 15.3% | 18.7% | 18.6% | 18.5% |
| 45.0 | 15.0% | 18.3% | 18.3% | 18.2% |
| 47.5 | 14.7% | 18.0% | 17.9% | 17.9% |
| 50.0 | 14.5% | 17.7% | l7.6% | 17.5% |
| 52.5 | 14.2% | 17.4% | 17.3% | 17.3% |
| 55.0 | 14.0% | 17.1% | 17.0% | 17.0% |
| 57.5 | 13.7% | 16.8% | 16.8% | 16.7% |
| 60.0 | 13.5% | 16.6% | 16.5% | 16.5% |
| 62.5 | 13.4% | 16.4% | 16.3% | 16.3% |
| 65.0 | 13.2% | 16.2% | 16.1% | 16.1% |
| 67.5 | 13.0% | 16.0% | 15.9% | 15.9% |
| 70.0 | 12.9% | 15.8% | 15.8% | 15.7% |
| 72.5 | 12.7% | 15.6% | 15.6% | 15.5% |
| 75.0 | 12.6% | 15.5% | 15.4% | 15.4% |
| 77.5 | 12.5% | 15.4% | 15.3% | 15.3% |
| 80.0 | 12.4% | 15.3% | 15.2% | 15.1% |
Table 5.
Absolute Risk from Multivariate Model for Chronic Pulmonary Disorder, by Age Group and Usage Group
| Usage group | ||||
|---|---|---|---|---|
| Age (years) | Nonuser | Smok only |
E-cig only |
Dual user |
| 20.0 | 0.4% | 1.2% | 1.0% | 1.5% |
| 22.5 | 0.5% | 1.4% | 1.2% | 1.8% |
| 25.0 | 0.5% | 1.6% | 1.4% | 2.1% |
| 27.5 | 0.6% | 1.8% | 1.6% | 2.4% |
| 30.0 | 0.7% | 2.1% | 1.8% | 2.7% |
| 32.5 | 0.8% | 2.4% | 2.1% | 3.1% |
| 35.0 | 0.9% | 2.7% | 2.4% | 3.5% |
| 37.5 | 1.1% | 3.1% | 2.7% | 4.0% |
| 40.0 | 1.2% | 3.5% | 3.0% | 4.5% |
| 42.5 | 1.4% | 3.9% | 3.4% | 5.1% |
| 45.0 | 1.5% | 4.4% | 3.8% | 5.7% |
| 47.5 | 1.7% | 4.9% | 4.3% | 6.4% |
| 50.0 | 1.9% | 5.5% | 4.8% | 7.1% |
| 52.5 | 2.1% | 6.0% | 5.3% | 7.8% |
| 55.0 | 2.3% | 6.6% | 5.8% | 8.6% |
| 57.5 | 2.6% | 7.3% | 6.4% | 9.4% |
| 60.0 | 2.8% | 8.0% | 7.0% | 10.2% |
| 62.5 | 3.1% | 8.7% | 7.6% | 11.1% |
| 65.0 | 3.4% | 9.4% | 8.2% | 12.0% |
| 67.5 | 3.6% | 10.1% | 8.9% | 12.9% |
| 70.0 | 3.9% | 10.8% | 9.5% | 13.8% |
| 72.5 | 4.2% | 11.6% | 10.2% | 14.7% |
| 75.0 | 4.5% | 12.4% | 10.9% | 15.6% |
| 77.5 | 4.8% | 13.1% | 11.5% | 16.6% |
| 80.0 | 5.1% | 13.9% | 12.2% | 17.5% |
Figure1.
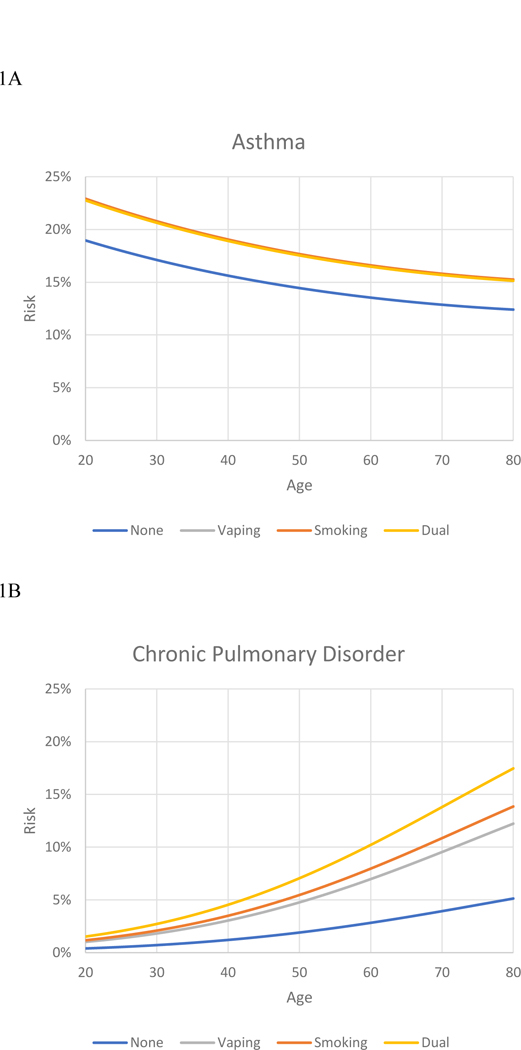
Absolute risk of respiratory disorder, by age, for four tobacco usage groups.
1A. Absolute risk for asthma. 1B. Absolute risk for chronic pulmonary disease.
4. DISCUSSION
The purpose of this research was to study the association of e-cigarette use with two indices of diagnosed respiratory disorder among adults. Findings indicated that e-cigarette use was associated with respiratory disorder independent of cigarette smoking, age, and a range of physical and psychosocial covariates. Results were similar for analyses based on relative risk and absolute risk of asthma and COPD. These results are consistent with several studies showing e-cigarette use related to respiratory symptoms (Cho & Paik, 2016; Hedman et al., 2018; McConnell et al., 2017; Schweitzer et al., 2017); to our knowledge the finding for COPD is novel. The fact that the findings were independent of the covariates means that the results cannot be attributed to a correlation of e-cigarette use with third variables such as gender, education, and financial stress. In addition, the present research delineated racial/ethnic differences in e-cigarette use and respiratory disorder, knowledge which could be utilized to understand and ultimately reduce health disparities.
The primary finding of this research is that e-cigarette use showed a significant association with respiratory disorder among adults. This was true for both asthma and chronic pulmonary disorder. Because the items did not determine exactly when symptoms of asthma or pulmonary disorder originated, there are two possible interpretations of the data. One possible interpretation is that participants began using e-cigarettes when they developed asthma or pulmonary disorder; but it is difficult to think of a reason for why people would do this because e-cigarette vapor has lung irritant properties (e.g., Anderson et al., 2016; Larcombe et al., 2017; Reidel et al., 2018; Wu et al., 2014). As a variant explanation, it could be posited that persons who used e-cigarettes did so to reduce or quit smoking when they developed a respiratory disorder. However, this interpretation would be inconsistent with the data, which showed that the significant relations of e-cigarettes with respiratory disorder occurred primarily among nonsmokers, not among smokers. Moreover, the smoking cessation items indicated that most of the quitters had stopped smoking many years ago and that current e-cigarette use was not related to stopping smoking (data not shown). Thus reverse-causation arguments do not seem very tenable on logical or empirical grounds.
An alternative interpretation is that e-cigarette use contributed to the development or maintenance of respiratory symptoms or the transition from initial symptomatology to chronic disorder. Data from other studies give plausibility to this interpretation because they show e-cigarette use is associated with asthma and bronchitis in high school samples (Choi et al., 2016; McConnell et al., 2017; Schweitzer et al., 2017), ages that precede the development of CO D. While this interpretation has plausibility on the grounds of consistency with other studies and coherence with existing knowledge (Chun et al., 2017; Clapp & Jaspers, 2017), longitudinal research is needed for studying the extent to which e-cigarettes are related to onset of symptomatology or maintenance of existing symptoms.
Biological plausibility is relevant because recent reviews based on controlled laboratory and clinical research have suggested possible mechanisms for a relation of e-cigarettes to respiratory symptoms (Chun et al., 2017; Clapp & Jaspers, 2017). For example, cell studies have implicated e-cigarette aerosol in generation of reactive oxygen species, contributing to oxidative stress that could affect airway cells (Ji et al., 2016; Lerner et al., 2015; Zhao et al., 2018). Other studies have shown cytotoxic and inflammatory effects of e-cigarette vapor for airway cells, effects that could contribute to respiratory symptoms (Larcombe et al., 2017; Lee et al., 2018; Rowell et al., 2017; Wu et al., 2017). Additionally, studies focusing on immune system parameters have provided evidence that exposure to e-cigarette aerosol impairs immune system function, increasing susceptibility to infection (Hwang et al., 2016; Martin et al., 2016; Reidel et al., 2017; Sussan et al., 2015). Whether such mechanisms are involved in observed associations for respiratory disorder needs to be considered in further epidemiological research.
4.1. Study Limitations
While this research used a large representative sample, was based on items that asked about diagnosed disease, and included a number of relevant covariates, some aspects could be noted as limitations. First, the study was cross-sectional and temporal relations between variables cannot be definitively established. Second, the study lacked a detailed measure of smoking history and the survey did not include items on marijuana, which is relevant for asthma (Strongetal., 2018). Though prior studies have shown effects for e-cigarettes independent of marijuana (e.g., Schweitzer et al., 2017), this is a possible limitation and needs to be extended in further research. Third, the respiratory variables were based on self-report, and while validity of BRFSS variables for indexing chronic disease conditions has been previously demonstrated (Li et al., 2012; Pierannunzi et al., 2013), the findings could be confirmed in further research using direct physical examination or biochemical indices. Finally, the sample was from one state and generalization to other areas needs to be studied.
4.2. Conclusions
In summary, this research found an association of e-cigarette use with respiratory disorder in a diverse sample of adults with analyses including demographic and psychosocial controls. Though temporal aspects of the findings need to be clarified, the results are consistent with other studies suggesting a linkage of e-cigarette use with respiratory symptoms at other ages. The fact that findings for respiratory symptoms occurred primarily for nonsmokers argues against several alternative interpretations of the results. Further research is needed to obtain more evidence on whether e-cigarette use may be adding to respiratory disorder in the population among nonsmokers (Glantz & Bareham, 2018; US Department of Health and Human Services, 2016).
Supplementary Material
Highlights.
Few data are available on the association of e-cigarette use with health indices.
We found e-cigarette use associated with asthma and chronic respiratory disorder.
Results were independent of cigarette smoking, physical and psychosocial covariates.
Associations with respiratory disorder were significant primarily among nonsmokers.
Acknowledgments
We thank the staff of the Hawaii Department of Health for assisting with access to the data and providing information for the analysis.
Role of Funding Source
Dr. Wills and Dr. Pagano were supported by grant P30 CA071789 from the National Cancer Institute. The funding agency played no role in analysis and interpretation of the data, preparation of the manuscript, and decision to submit the report for publication. The content is solely the responsibility of the authors and does not necessarily reflect official policy of the U.S. Department of Health and Human Services.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
All authors state that they do not have any financial relationships with any organizations that might have an interest in the submitted work or any other relationships or activities that could appear to have influenced the submitted work.
REFERENCES
- Akinbami LJ, Moorman JE, Simon AE, Schoendorf KC, 2014. Trends in racial disparities for asthma outcomes among children 0 to 17 years, 2001–2010. J. Allergy Clin. Immunol 134, 547–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson C, Majeste A, Hanus J, Wang S 2016. E-cigarette aerosol exposure induces reactive oxygen species, DNA damage, and cell death in vascular endothelial cells. Toxicol Sci 152, 334–340. [DOI] [PubMed] [Google Scholar]
- Beck AF, Huang B, Simmons JM, Moncrief T, Sauers HS, Chen C, et al. , 2014. Role of financial and social hardships in asthma racial disparities. Pediatrics 133, 431–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best C, Haseen F, Currie D, Ozakino G, MacKintos AM, … Haw S, 2018. Relationships between trying an electronic cigarette and subsequent cigarette experimentation in Scottish adolescents: A cohort study. Tob. Control 27, 373–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC, 2011. At a glance: conducting the Behavioral Risk Factor Surveillance System (BRFSS) US Department of Health and Human Services, Centers for Disease Control. [Google Scholar]
- CDC, 2017. BRFSS 2016 Summary Data Quality Report Department of Health and Human [Google Scholar]
- Choi K, Bernat D E-cigarette use among Florida youth with and without asthma. Am. J. Prev. Med 51, 446–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho JH, Paik SY, 2016. Association between electronic cigarette use and asthma among high school students in South Korea. PLoS One 11, e0151022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun LF, Moazed F, Calfee CS, Matthay MA, Gotts JE, 2017. Pulmonary toxicity of e-cigarettes. Amer. J. Physiol. Lung Cell Molec. Physiol 313, L193–L206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp PW, Jaspers I, 2017. Electronic cigarettes: Their constituents and potential links to asthma. Curr. Allergy Asthma Rep 17, 17:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutra LM, Glantz SA, 2014. Electronic cigarettes and conventional cigarette use among US adolescents. JAMA Pediatr 168, 610–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Arcos I, Geraghty P, Baumlin N, et al. , 2016. Chronic electronic cigarette exposure in mice induces features of COPD in a nicotine-dependent manner. Thorax 71, 1119–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennuso J, Epstein LH, Paluch RA, Cerny F, 1998. The relationship between asthma and obesity in urban minority children and adolescents. Arch. Pediatr. Adolesc. Med 152, 1197–2000. [DOI] [PubMed] [Google Scholar]
- Glantz SA, Bareham DW, 2018. E-cigarettes: Use, effects on health, risks, and policy implications. Ann. Rev. Public Health 39, 28.1–28.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedman L, Backman H, Stridsman C, Bosson JA, Lundback M, Lindberg A, et al. , 2018. Association of electronic cigarette use with smoking habits, demographic factors, and respiratory symptoms. JAMA Network Open 1(3):e180789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang JH, Lyes M, Sladewski K, Enany S, McEachern E, Mathew DP, et al. , 2016. Electronic cigarette inhalation alters innate immunity and airway cytokines while increasing the virulence of colonizing bacteria. J. Mol. Med 94, 667–79. [DOI] [PubMed] [Google Scholar]
- Hu SS, Pierannunzi C, Balluz L, 2011. Integrating a multimode design into a national random-digit-dialed telephone survey. Prev. Chron. Dis 8, A145. [PMC free article] [PubMed] [Google Scholar]
- Jamal A, Gentzke A, Hu SS, Cullen KA, Apelberg BJ, Homa DM, King BA, 2017. Tobacco use among middle and high school students: United States, 2011–2016. MMWR Morb. Mortal. Wkly. Rep 66, 597–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji EH, Sun B, Zhao T, Shu S, Chang CH, Messadi D, Xia T, Zhu Y, Hu S, 2016. Characterization of electronic cigarette aerosol and its induction of oxidative stress response in oral keratinocytes. PloS One 11, e0169380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopel LS, Phipatanakul W, Gaffin JM, 2014. Social disadvantage and asthma control in children. Pediatr. Respir. Rev 15, 256–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larcombe AN, Janka MA, Mullins BJ, Berry LJ, Bredin A, Franklin PJ, 2017. The effects of electronic cigarette aerosol exposure on inflammation and lung function in mice. Amer. J. Physiol. Lung Cell Molec Physiol 313, L67–L79. [DOI] [PubMed] [Google Scholar]
- Lee H-W, Park S-H, Weng M-W, et al. , 2018. E-cigarette smoke damages DNA and reduces repair activity in mouse lung, heart, and bladder as well as in human lung and bladder cells. PNAS, 29 January 2018. doi: 10.1073/pnas.1718185115. [DOI] [PMC free article] [PubMed]
- Lerner CA, Sundar IK, Yao H, Gerloff J, Ossip DJ, McIntosh S, et al. , 2015. Vapors produced by electronic cigarettes and e-juices with flavorings induce toxicity, oxidative stress, and inflammatory response in lung epithelial cells and in mouse lung. PLoS One 10, e0116732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Balluz LS, Ford ES, Okoroa CA, Zhao G, Pierannunzi C, 2012. A comparison of prevalence estimates for selected health indicators of chronic diseases or conditions from the Behavioral Risk Factor Survey Surveillance System, the National Health Interview Survey, and the National Health and Nutrition Examination Survey, 2007–2008. Prev. Med 54, 381–387. [DOI] [PubMed] [Google Scholar]
- Lozano P, Barrientos-Guitierrez I, Arollo-Santillan E, Morello P, Mejia R, Sargent JD, Thrasher JF (2017). A longitudinal study of electronic cigarette use and onset of conventional cigarette smoking and marijuana use among Mexican adolescents. Drug Alcohol Depend 180, 427–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin EM, Clapp PW, Rebuli ME, Pawlak EA, Glista-Baker E, Benowitz NL, Fry RC, Jaspers I, 2016. E-cigarette use results in suppression of immune and inflammatory-response genes in nasal epithelial cells similar to cigarette smoke. Am. J. Physiol. Lung Cell Molec. Physiol 311, L135–L144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell R, Barrington-Trimis JL, Wang K, Urman R, Hong H, Unger J, Samet J, Leventhal A, Berhane K, 2017. Electronic cigarette use and respiratory symptoms in adolescents. Am. J. Respir. Crit. Care Med 195, 1043–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthy VH, 2017. E-cigarette use among youth and young adults: A major public health concern. JAMA Pediatr 171, 209–210. [DOI] [PubMed] [Google Scholar]
- von Mutius E, Schwartz J, Neas LM, Dockery D, Weiss ST, 2001. Relation of body mass index to asthma and atopy in children: The National Health and Nutrition Examination Study III. Thorax 56, 835–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noordzij M, van Diepen M, Caskey FC, Jager KJ, 2017. Relative risk versus absolute risk: One cannot be interpreted without the other. Nephrol Dial Transplant 32, ii13–ii18. [DOI] [PubMed] [Google Scholar]
- Pierannunzi C, Hu SS, Balluz L, 2013. A systematic review of publications assessing reliability and validity of the Behavioral Risk Factor Surveillance System (BRFSS), 2004– 2011. BMC Med. Res. Methodol 13, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reidel B, Radicioni G, Clapp P, Ford AA, Abdelwahab S, Rebuli ME, et al. , 2018. E-cigarette use causes a unique innate immune response in the lung involving increased neutrophilic activation and altered mucin secretion. Amer. J. Resp. Crit. Care Med 197, 492–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowell TR, Reeber SL, Lee SL, Harris RA, Nethery RC, Herring AH, Glish GL, Tarran R, 2017. Flavored e-cigarette liquids reduce proliferation and viability in the CALU3 airway epithelial cell line. Amer. J. Physiol. Lung Cell Molec. Physiol 313, L52–L66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin DB, 1987. Multiple imputation for nonresponse in surveys New York, NY: Wiley. [Google Scholar]
- Schafer JL, Graham JW, 2002. Missing data: our view of the state of the art. Psychol. Methods 7, 147–177. [PubMed] [Google Scholar]
- Schweitzer RJ, Wills TA, Tam E, Pagano I, Choi K, 2017. E-cigarette use and asthma in a multiethnic sample of adolescents. Prev. Med 105, 226–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soneji S, Barrington-Trimis J, Wills TA, et al. 2017. E-cigarette use and subsequent cigarette smoking among adolescents and young adults: A systematic review and meta-analysis. JAMA Pediatr 171, 788–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. Helen G, Eaton DL, 2018. Public health consequences of e-cigarettes. JAMA Intern. Med, May 7, 2018. doi: 10.1001/jamainternmed.2018.1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratton K, Kwan LY, Eaton DL, Eds., 2018. Public health consequences of e-cigarettes: A consensus study report of the National Academies of Science, Engineering, and Medicine Washington, DC: National Academies Press. [PubMed] [Google Scholar]
- Strong DR, Myers MG, Pulvers K, Noble M, Brikmanis K, Doran N, 2018. Marijuana use among US tobacco users: Findings from wave 1 of the population assessment of tobacco health (PATH) study. Drug Alcohol Depend 186, 16–22. [DOI] [PubMed] [Google Scholar]
- Sussan TE, Gajghate S, Thimmulappa RK, Ma J, Kim JH, Sudini K, et al. , 2015. Exposure to electronic cigarettes impairs pulmonary anti-bacterial and anti-viral defenses in a mouse model. PLoS One 10, e0116861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treuer JL, Rozema AD, Mathijsen JJP, Oers H, Vink JM, 2017. E-cigarette and waterpipe use in two Dutch adolescent cohorts: Cross-sectional and longitudinal associations with conventional cigarette smoking. Eur. J. Epidemiol, 19 December 2017. doi: 10.1007/s10654017-0345-9. [DOI] [PMC free article] [PubMed]
- U.S. Department of Health and Human Services, 2016. E-Cigarette use among youth and young adults: A report of the Surgeon General U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, Atlanta, GA. [Google Scholar]
- Wang MP, Ho SY, Leung LT, Lam TH, 2016. Electronic cigarette use and respiratory symptoms in Chinese adolescents in Hong Kong. JAMA Pediatr 170, 89–91. [DOI] [PubMed] [Google Scholar]
- Watkins SL, Glantz SA, Chaffee BW, 2018. Association of non cigarette to bacco product use with future cigarette smo king among youth in the population assessment of tobacco and health (PATH) study, 2013–2015. JAMA Pediatr 172, 181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson FA, Wang Y, 2017. Recent findings on the prevalence of e-cigarette use among adults in the U.S. Am. J. Prev. Med 52, 385–390. [DOI] [PubMed] [Google Scholar]
- Wills TA, Bantum EO, Pokhrel PA, et al. , 2013. Dual-process model of substance use: Tests in two diverse populations. Health Psychol 32, 533–542. [DOI] [PubMed] [Google Scholar]
- Wu Q, Jiang D, Minor M, Chu HW, 2014. E-cigarette liquid increases inflammation and virus infection in primary human airway epithelial cells. PLoS One 9, e108342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao T, Max W, Sung H, Glantz SA, Goldberg RL, Wang JB, et al. , 2017. Relationship between spending on electronic cigarettes, 30-day use, and disease symptoms among current adult cigarette smokers in the U.S. PloS One 12, e0187399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zernike K, 2018. Schools struggle with vaping explosion. New York Times, April 2, 2018.
- Zhao S, Zhang Y, Sisler JD, Shaffer J, Leonard SS, Morris AM, Qian Y, Bello D, Demokritou P, 2018. Assessment of reactive oxygen species generated by electronic cigarettes using acellular and cellular approaches. J. Haz. Mater 344, 549–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


