Abstract
Background:
Cocaine addiction is related to impulsive decision making that is mediated by brain circuitry involved in reward processing and executive functions, such as cognitive control and attentional salience. Resting-state functional connectivity between reward and executive control circuitry is altered among cocaine users, with concomitant deficits in impulsivity and learning. Prior research has examined how select brain regions interact to influence impulsive decision making for drug users; however, research examining interactions between large-scale brain networks and impulsive behavior is limited.
Methods:
The current study compared reward and executive control network resting-state functional connectivity and its relationship to impulsive decision making between cocaine users (n=37) and non-cocaine using control participants (n=35). Participants completed computerized decision-making tasks and a separate resting-state functional magnetic resonance imaging scan. Data underwent independent component, dual regression, and linear regression moderation analyses.
Results:
Higher impulsivity on the Balloon Analogue Risk Task (BART) was associated with inverse resting-state connectivity between the left cognitive control and subgenual anterior cingulate extended reward networks for cocaine users, while the opposite was found for controls. Less impulsivity on the monetary choice questionnaire was associated with stronger positive resting-state connectivity between the attentional salience and striatal core reward networks for controls, while cocaine users showed no association between impulsivity and resting-state connectivity of these networks.
Conclusions:
Cocaine users show aberrant associations between reward-executive control resting-state network coupling and impulsive decision making. The findings support the conclusion that an imbalance between reward and executive control circuitry contributes to impulsivity in drug use.
Keywords: cocaine, connectivity, decision making, fMRI, impulsivity, resting state
1. Introduction
Addiction is characterized by impulsive decision making that can lead to harmful longterm consequences (Verdejo-Garcia et al., 2018). The progression from controlled, voluntary drug use to compulsive drug taking has been described as a shift from flexible prefrontal cortical control to automatic responding driven by corticostriatal circuitry (Everitt et al., 2008). The brain circuits that are implicated in addiction comprise several intrinsic functional neural networks that coordinate decision-making and learning (Laird et al., 2011; Ma et al., 2015). Measuring spontaneous, low-frequency fluctuations in neural activity throughout the brain during rest, commonly called resting-state functional connectivity (rsFC), provides a snapshot of the strength of functional connections between neural networks (Fox and Raichle, 2007). Addiction is associated with altered functional connectivity within corticostriatal networks that manage reward expectancies and learning, as well as executive control networks involved in attentional salience and cognitive control (Ma et al., 2015; Sutherland et al., 2012).
Compared to non-drug-using controls, cocaine users have reduced connectivity within corticostriatal reward circuitry (Gu et al., 2010; Hu et al., 2015), which is predictive of compulsive drug use and relapse (Hu et al., 2015; McHugh et al., 2013). The core and extended reward networks are two distinct functional networks along the mesostriatal and mesolimbic dopamine pathways, respectively. The core reward network is comprised of the thalamus and basal ganglia, including the dorsal and ventral striatum, regions that are often implicated in reactivity to drug use and drug cues (Di Chiara and Bassareo, 2007; Koob and Volkow, 2016). Acute drug administration alters neurotransmitter activity throughout this network and ultimately changes voluntary drug use into the compulsive drug taking behavior that is characteristic of addiction (Koob and Volkow, 2016; Martinez et al., 2007). The core reward network is functionally connected to an extended reward network consisting of the subgenual anterior cingulate cortex (ACC), medial orbitofrontal cortex (OFC), and medial prefrontal cortex (PFC) (Koob and Volkow, 2016). The extended reward network is involved in the valuation and anticipation of drug rewards and drives drug urges and preoccupation with use, especially during withdrawal (Koob and Volkow, 2016; Kuhn and Gallinat, 2011).
Cocaine users also show altered functional connectivity within select cortical brain regions associated with executive control, including the cognitive control and attentional salience networks (Cisler et al., 2013; Kelly et al., 2011; Ray et al., 2015). The bilateral frontoparietal cortices encompassing the dorsolateral PFC (DLPFC) and posterior parietal cortex, mediate cognitive control functions such as inhibitory control, planning, and complex decision making (Laird et al., 2011; Vincent et al., 2008). Functional activation of this network has been shown to be altered for cocaine users compared to controls during inhibitory control tasks such as the Stroop and Go/No-Go tasks (Hester and Garavan, 2004; Worhunsky et al., 2013). The attentional salience network identifies salient external and interoceptive stimuli to inform decision making (Seeley et al., 2007; Sutherland et al., 2012). Cocaine users show aberrant rsFC of key regions in the salience network with other cortical regions involved in decision making compared to controls (Cisler et al., 2013).
Connectivity between reward and cortical executive control networks differentiate cocaine users from control participants (Camchong et al., 2011; Hu et al., 2015; Ray et al., 2015; Wilcox et al., 2011; Wisner et al., 2013). Studies examining functional network interactions in the context of addictive behaviors, however, are still limited. The current study aimed to determine if altered rsFC between large-scale reward and executive control functional networks is predictive of impulsivity for cocaine users on three distinct decision-making tasks. The tasks included the Monetary Choice Questionnaire (MCQ), measuring delay discounting (Kirby et al., 1999), the Iowa Gambling Task (IGT), measuring reward-based learning (Bechara et al., 1994), and the Balloon Analogue Risk Task (BART), measuring risk-taking propensity (Lejuez et al., 2002). These tasks all engage the core and extended reward networks and the cognitive control and attentional salience networks (McClure et al., 2004; Rao et al., 2008). Yet, the function of these networks differs for each task and may help to elucidate specific cocaine-related alterations in functional network connectivity that are associated with impulsive behavior.
The MCQ offers participants a series of choices between smaller, immediate monetary rewards and larger, delayed monetary rewards (Kirby et al., 1999). Prior research shows that both the core and extended reward networks are involved in the choice of immediate rewards (McClure et al., 2004), while the activation of the insular cortex in the attentional salience network has been linked specifically to the choice of delayed rewards (Wittmann et al., 2007). The cognitive control network is engaged during all monetary decisions and activity within the network increases with decision difficulty (McClure et al., 2004). Increased PFC cognitive control network connectivity has been linked to more delay discounting for cocaine users (Camchong et al., 2011) and methamphetamine users showed increased cognitive control network activity during easy monetary choices compared to controls; however, this activation may have been compensatory, since it was not associated with MCQ task performance (Monterosso et al., 2007). Given that stimulant users consistently choose smaller immediate monetary rewards over larger later monetary rewards (Coffey et al., 2003; Heil et al., 2006; Kirby and Petry, 2004), we expected to see higher discounting rates to be associated with a negative correlation, or imbalance, between the reward networks involved in immediate reward choices and the attentional salience network involved in delayed reward choices for cocaine users. Such a finding would lend support for the overvaluation of immediate rewards and undervaluation of delayed rewards in addiction (Bickel et al., 2012).
During the BART, a measure of risk-taking propensity, players accrue 1 cent for each balloon pump, but risk losing their money for that round if the balloon explodes (Lejuez et al., 2002). Risk taking in the form of balloon pumps consistently activates mesostriatal regions in the core reward network as well as the bilateral attentional salience and cognitive control networks (Rao et al., 2008). While some studies show activation of vmPFC and ACC of the extended reward network during balloon pumps (Rao et al., 2008; Weber et al., 2014), deactivations during balloon pumps is more commonly found with fMRI (Fukunaga et al., 2012; Galvan et al., 2013; Schonberg et al., 2012). A prior study correlated rsFC to BOLD activation during the BART and found that less right DLPFC modulation of risk during the BART was linked with increased whole-brain connectivity of a core reward seed network for methamphetamine users, and decreased connectivity of a right cognitive control seed network for controls (Kohno et al., 2014). In this case, stronger links between reward and cognitive control circuitry appear to promote more balloon pumps (i.e., risk) during the task for stimulant users. Therefore, we expected higher risk-taking propensity to be associated with stronger connectivity between the reward and cognitive control networks for cocaine users. Specifically, we expected to see stronger positive connectivity with the core reward network and stronger inverse connectivity with the extended reward network given their direction of task activation.
During the IGT, players choose from four card decks that have varying probabilities of winning and losing money with the end goal of learning which decks are advantageous (Bechara et al., 1994). Expected gains for card choices has been linked with extended reward activity while expected risk and learning from unanticipated outcomes during the IGT has been linked with core reward network activity (Li et al., 2010). Improvement in IGT performance is associated with activation in both the cognitive control and attentional salience networks (Ernst et al., 2002; Li et al., 2010). Stimulant users show deficits in contingency-based learning on the IGT (Bolla et al., 2003; Grant et al., 2000; Verdejo-Garcia et al., 2007a; Verdejo-Garcia et al., 2007b), which has been associated with increased right-sided extended reward (i.e., medial OFC) and decreased right-sided cognitive control (i.e., DLPFC) network activity compared to controls in a study using positron emission tomography (PET) (Bolla et al., 2003). A separate study found that hyperperfusion in the left cognitive control network at rest was linked to worse IGT performance for cocaine users (Tucker et al., 2004). Given these findings, we expected a negative correlation of the extended reward network and cognitive control network to be linked to worse learning on the IGT, which would support hypotheses that addiction is characterized by overvaluation of expected gains and reduced cognitive control (Verdejo-Garcia et al., 2018).
2. Materials and methods
2.1. Participants
The sample included 37 cocaine users and 35 non-cocaine users (controls) who participated in one of two fMRI studies assessing the effects of cocaine and HIV on brain function and decision making. The current analysis included only HIV-negative participants, verified with an OraQuick® rapid HIV test. Cocaine users met the following criteria: ≥3 days of past-month cocaine use or a cocaine-positive urine drug screen, ≥1 year of regular cocaine use, and lifetime cocaine dependence. Current alcohol and marijuana dependence were permitted if cocaine dependence was the principal diagnosis. The control group met the following criteria: no lifetime cocaine use disorder, no history of regular cocaine use, 0 days of cocaine use in the past year, and a cocaine-negative drug screen. Past alcohol and marijuana dependence in full sustained remission were permitted. In all groups, alcohol, marijuana, and nicotine use were permitted, and for all other drugs, individuals were excluded for lifetime dependence, history of >2 years of regular use, and/or a positive drug screen (except for prescribed medications). Additional exclusion criteria were: English non-fluency or illiteracy; <8th grade education; severe learning disability with functional impairment; serious neurological disorders (e.g., multiple sclerosis); lifetime history of severe head trauma with loss of consciousness >30 minutes and persistent functional decline; indicators of severe mental illness or acute psychiatric distress (e.g., schizophrenia, major depressive disorder with suicidal ideation); pregnancy; physical disabilities impeding participation (e.g., blindness); and impaired mental status.
2.2. Procedures
Participants were recruited from the Raleigh-Durham area between October 2011 and May 2015 via advertisements in local newspapers and websites, flyers and brochures at community-based organizations, and participant referrals. After a brief telephone pre-screen, interested individuals completed a comprehensive in-person screening of psychiatric, substance abuse, and medical histories and urine drug and pregnancy testing, as described previously (Meade et al., 2015). Participants also provided a release of information for research staff to request and review their medical records to corroborate self-reported medical history. Eligible participants returned to complete a neurobehavioral assessment that included a neuropsychological battery and computerized decision-making tasks, and an MRI brain scan. Participants were instructed to abstain from using cocaine or other drugs for at least four hours prior to the MRI scan depending on the study protocol. Smokers were allowed to smoke prior to the scan to avoid nicotine withdrawal effects. All procedures were approved by the institutional review boards at Duke University Health System and University of North Carolina at Chapel Hill.
2.3. Computerized decision-making tasks
During the neurobehavioral assessment, participants completed the BART (Lejuez et al., 2002), MCQ (Kirby et al., 1999), and IGT (Bechara et al., 1994). For the BART, participants use a virtual balloon pump to blow up a series of 30 balloons, accruing 1 cent in a temporary bank for each pump. However, if the balloon explodes, all money for that balloon is lost. Risk taking propensity was defined as the average number of pumps on un-popped balloons, with higher scores indicating higher risk-taking propensity.
Participants completed an adapted version of the MCQ during which they made 36 choices between smaller immediate rewards (ranging from $5 to $80) and larger delayed rewards (ranging from $25 to $85 and from 1 to 186 days). An individual’s point of indifference where the perceived value of a smaller immediate reward is equivalent to a larger delayed reward was computed using the following hyperbolic function: Vimmediate= Vdelayed/(1 + kD), in which V is value in dollars, D is delay in days, and k is a free parameter that determines the discount rate. K values ranged from 0.00016 to 4.00 and the natural log of the mean k value was used for analyses. This task was adapted from the original scale to include nine extra items to accommodate a wider range in delay discounting (impulsive) behavior for cocaine users whose scores may be limited by a ceiling effect [see (Towe et al., 2015) for details].
For the IGT, participants selected cards from four decks to accumulate as much money as possible. All cards were followed by feedback that the participant won a specified monetary amount, but some cards were also followed by feedback that the participant lost a specified monetary amount in addition to their win amount. A green bar increased in size on the screen for each win amount and a red bar increased in size for each loss amount. A score of contingency-based learning was determined by the number of cards picked from the negative-expected-value decks subtracted from the number of cards picked from the positive-expected-value decks. Higher scores indicate better contingency-based learning.
2.4. MRI data acquisition
All scans were performed at Duke University Hospital using a 3T GE MR750 (Milwaukee, WI, USA) scanner with an eight-channel head coil. Whole-brain BOLD images were collected using T2*-weighted echo-planar imaging with the following parameters: TR=2s; TE=27ms (study 1) or 25 ms (study 2); FOV=24cm; in-plane matrix size= 64 × 64; and slice thickness= 3.8mm, resulting in 39 axial slices (study 1) or 35 slices (study 2), each with a voxel size of 3.75mm × 3.75mm × 3.8mm, for a total of 150 (study 1) or 156 volumes (study 2). Participants were instructed to view a cross-hair displayed via projector onto a screen within the scanner bore. Tl-weighted structural images used for registration were acquired with the following parameters: TR=8.096ms (study 1) and 8.156ms (study 2); TE=3.18ms; FOV=25.6cm; flip angle=12°; in-plane matrix size=256×256; slice thickness=lmm; and number of slices = 166. There was a similar proportion of participants from each study in the cocaine use and control groups (χ2(1, 72)=0.83, p=.36).
2.5 Data analysis
2.5.1. Standard flvfRI preprocessing.
Image preprocessing and analyses were performed with FSL 5.0.1 (Smith et al., 2004). Images were skull-stripped, corrected for slice timing, registered to high resolution anatomical images (full search, 12 degrees of freedom [DOF]) and to standard MNI-152 space (nonlinear registration, normal search, 12 DOF), spatially smoothed (full-width-half-maximum= 6mm), and intensity normalized (mean= 1000). All scans were shortened to 145 volumes during preprocessing.
Given the sensitivity of resting state analyses to motion artifacts and other forms of signal noise, the data underwent extensive preprocessing. FSL’s MCFLIRT intra-modal motion correction tool was used to correct for motion parameters (Jenkinson et al., 2002). No participants were excluded for relative mean displacement >0.3 mm (M= 0.09 mm, SD= 0.05). The preprocessed functional data were further motion-corrected with independent component analysis-based automatic removal of motion artifacts (ICA-AROMA), which employs an algorithm to identify and regress out signal associated with artifacts, while preserving signal of interest (Pruim et al., 2015). As the final preprocessing step, a high-pass temporal filter of 100 seconds was applied and the filtered, denoised data were registered to MNI standard space. Using FSL’s motion outliers tool (Jenkinson et al., 2012), cocaine users had higher average root mean square intensity differences (refrms) for each run after correction with ICA-AROMA (t(70)=2.77, p=.007). To control for group differences, refrms was included as a covariate in all group-level analyses. Finally, as described in the next section, probabilistic independent component analysis (PICA) was used to isolate artifactual motion-related networks from our networks of interest.
2.5.2. Network identification.
To identify reward and executive control networks, functional runs from all participants were subjected to PICA using MELODIC with multi-session temporal concatenation and automatic component estimation (Beckmann and Smith, 2004). Pre-processing in MELODIC included masking of non-brain voxels, voxel-wise de-meaning of the data, and normalization of the voxel-wise variance. Component maps were thresholded using an alternative hypothesis test based on fitting a Gaussian/gamma mixture model to the distribution of voxel intensities within spatial maps and controlling the local false-discovery rate at p < 0.05. The analysis resulted in 29 independent components (automatic estimation).
2.5.3. Network identification.
Networks of interest were chosen a priori from independent component network (ICN) templates identified by Laird and colleagues (2011). The networks of interest for the current study included the: 1) core reward network located in the bilateral basal ganglia (BG) and thalamus (Laird ICN 3); 2) extended reward network in the subgenual ACC and OFC (Laird ICN 2); 3) attentional salience network in the bilateral anterior insula, frontal opercula, and ACC (Laird ICN 4); and right and left-lateralized fronto-parietal cognitive control networks (Laird ICNs 15 and 18, respectively).
To identify the components derived from our own study sample that best represented the Laird ICN templates of interest, spatial partial correlations were run with FSLUTILS on the 29 components derived from the current sample with the five ICN templates provided by Laird and colleagues (Laird et al., 2011). The sample components with the strongest correlations to each ICN templates of interest were used in all subsequent analyses. Only correlations greater than 0.2 were considered and the strongest correlations ranged from 0.40 to 0.60. See Figure 1 for the final set of ICA components and correlation coefficients with the Laird ICNs of interest.
Figure 1.
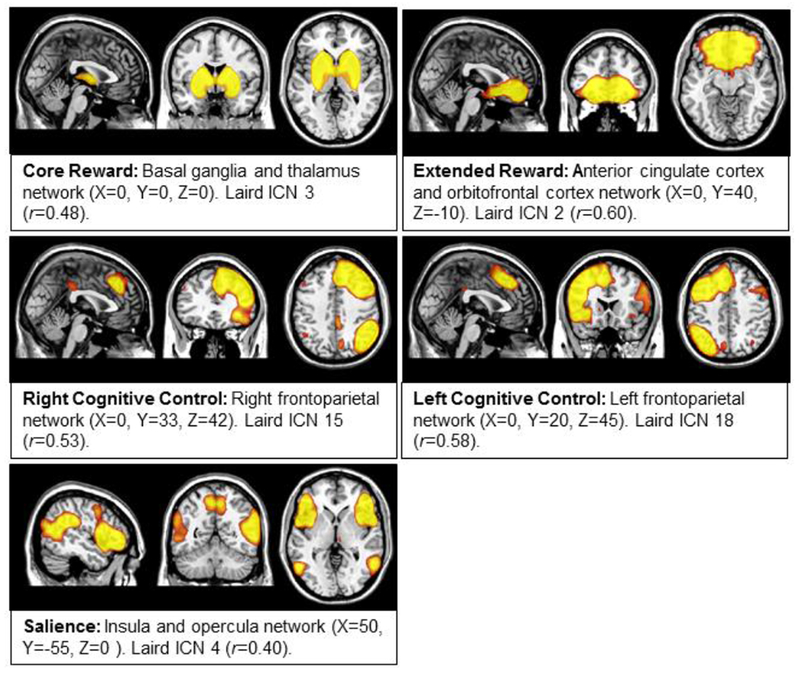
Mean spatial maps derived from independent component analysis (ICA). Components were chosen based on correlations with intrinsic connectivity network templates provided by Laird et al., 2011. All images scaled from z=2.3 through 5.
2.5.4. Dual regression.
Dual regression was conducted to estimate a version of each group-level spatial map and time-course for each participant’s 4D data (Beckmann et al., 2009; Utevsky et al., 2014). In stage one, the group spatial maps were regressed onto each participant’s 4D dataset to give a set of time-courses. In stage two, those time-courses were then regressed onto the same 4D dataset to get a subject-specific set of spatial maps. This analysis resulted in a component time-course and spatial map for each subject and each component. We performed a small-volume correction by restricting the analysis to only include voxels within a component mask derived from the group-ICA analysis that was chosen a priori. To do this, the group-ICA components were thresholded at p≤.05, binarized, and included in the dual-regression script as masks for stages one and two, and only stage one time-courses and stage two spatial maps for the masked components were included in subsequent analyses.
2.5.5. Between-network connectivity.
We used the time-courses from the first stage of the dual-regression analyses to conduct partial correlations on the components for each subject. The time-courses consisted of component connectivity estimates (volume-wise slope in the regression) for each of the 145 volumes for each component. This resulted in a correlation matrix in which each element was the pairwise association between two networks. The resulting correlation coefficients of rsFC between the resting state networks of interest were then transformed to Fisher’s Z statistics to normalize the distributions. Between-network connectivity strength was compared across groups with a series of one-way analyses of covariance with age, gender, years of education, and motion (refrms) as covariates using SPSS Version 22 (IBM Corp, Released 2013).
2.5.6. Moderation analyses.
To determine if cocaine use moderated the association between rsFC and impulsivity, group by rsFC interactions on task scores were examined using Process software in SPSS (Hayes, 2013). We tested for group by rsFC interaction effects on task performance with age, gender, years of education, and motion (refrms) included as covariates.
3. Results
3.1. Participant characteristics
Participant characteristics by group are displayed in Table 1. Of the cocaine users, 95% met criteria for current dependence and 92% reported smoking cocaine as their primary route of administration. They had used cocaine regularly for an average of 17.30 (SD=7.68) years and had used 10.46 (SD=7.57) days out of the past 30. On the day of the fMRI scan, participants reported last using cocaine an average of 3.14 days prior (SD= 3.42) and 26 participants (72%) tested positive for cocaine on the urine screen. Cocaine users were slightly older and less educated. Cocaine users reported significantly more use of other substances, including cigarettes, alcohol to intoxication, and marijuana. For this reason, the cocaine use group is more aptly described as having a cocaine use disorder with polysubstance use. Polysubstance use was not an exclusion criterion to ensure that the results generalize to the general population where polysubstance use is pervasive among cocaine users. Cocaine users had significantly higher discounting rates on the MCQ (Table 1). The groups performed similarly on the BART and IGT.
Table 1.
Participant characteristics by group
| Cocaine Users (N= 37) | Controls (N=35) | Total (N=72) | Test Statistic | |
|---|---|---|---|---|
| Demographics | ||||
| Female Gender, n (%) | 12 (32%) | 13 (37%) | 25 (35%) | χ2(1,72)=0.18 |
| Age, M (SD) | 45.30 (6.23) | 41.29 (9.43) | 43.35 (8.14) | t(70)=2.14* |
| African Americanŧ, n (%) | 33 (89%) | 28 (80%) | 61 (85%) | χ2(l,72)=1.17 |
| Years of Education, M (SD) | 12.65 (2.51) | 13.97 (2.42) | 13.29 (2.54) | t(70)=2.28* |
| Other substance use | ||||
| Current Smokers, n (%) | 25 (68%) | 8 (23%) | 33 (46%) | χ2(1,72)=14.48*** |
| Days of alcohol intoxication in past 30, M (SD) | 9.73 (10.37) | 1.51 (2.96) | 5.74 (8.71) | t(70)=4.52*** |
| Days of marijuana use in past 30, M (SD) | 4.95 (8.98) | 0.31 (1.35) | 2.69 (6.87) | t(70)=3.02** |
| Behavioral task performance | ||||
| BART, M (SD) | 37.94 (14.70) | 37.47 (15.89) | 37.71 (15.18) | t(70)=0.13 |
| IGT, M (SD) | −2.54 (25.37) | 10.57 (38.54) | 3.83 (32.88) | t(70)=−1.71 |
| MCQβ, M (SD) | −2.64 (1.16) | −3.56 (1.90) | −3.09 (1.62) | t(68)=2.45* |
Note: Balloon Analogue Risk Task (BART), Iowa Gambling Task (IGT), Monetary Choice Questionnaire (MCQ)
p<.05,
p<.01,
p<.001
One participant included as African American identified as mixed race.
In k value; Cocaine use group n = 36, Non-drug use group n=34.
3.2. Group comparisons of between-network rsFC
ANCOVAs were used to compare between-network connectivity across cocaine users and controls. There were no significant group differences in between-network connectivity. Average between-network rsFC values are displayed by group in Table 2.
Table 2.
Group comparisons of between-network rsFC
| Cocaine Users, M (SD) | Controls, M (SD) | F-value | |
|---|---|---|---|
| Extended reward network with: | |||
| Attentional salience | −0.11 (0.22) | −0.05 (0.18) | F(1,66)= 3.37 |
| Right cognitive control | 0.01 (0.23) | −0.01 (0.15) | F(1,66)= 0.09 |
| Left cognitive control | −0.09 (0.24) | −0.11 (0.18) | F(1,66)= 0.34 |
| Core reward | 0.06 (0.21) | 0.01 (0.30) | F(1,66)= 1.17 |
| Core reward network with: | |||
| Attentional salience | 0.06 (0.23) | 0.04 (0.26) | F(1,66)= 0.02 |
| Right cognitive control | 0.11 (0.24) | 0.04 (0.19) | F(1,66)= 2.29 |
| Left cognitive control | −0.02 (0.19) | 0.06 (0.21) | F(1,66)= 2.76 |
Note: rsFC= resting state functional connectivity; between-nework rsFC listed as pearson partial correlation coefficient (r) and analyses of variance (ANOVA) conducted on Fischer z transformations with age, gender, years of education, and motion included as covariates
3.3. Moderation analyses
Linear regression analyses examined cocaine use as a moderator between network rsFC and impulsivity on the decision-making tasks. Stronger inverse coupling between the extended reward network and the left cognitive control network was associated with higher risk-taking propensity on the BART for cocaine users but not for control participants. Coupling between the core reward and attentional salience networks was unrelated to impulsivity on the MCQ for cocaine users, but negatively associated with impulsivity for control participants (Table 3, Figure 2). No other group by between-network rsFC interactions were significant.
Table 3.
Significant group by rsFC interaction effects on behavioral task performance
| Cocaine Users | Controls | |||
|---|---|---|---|---|
| r | r | t | B (95% CI) | |
| BART × Extended reward and L Cognitive control | −0.51** | 0.14 | 2.68** | 20.44 (5.20: 35.69) |
| MCT × Core reward and Attentional salience | 0.07 | −0.37* | −2.30* | −1.45 (−3.00: −0.21) |
Note:
p<.05,
p<.01,
p<.001;
rsFC=resting state functional connectivity
Figure 2.
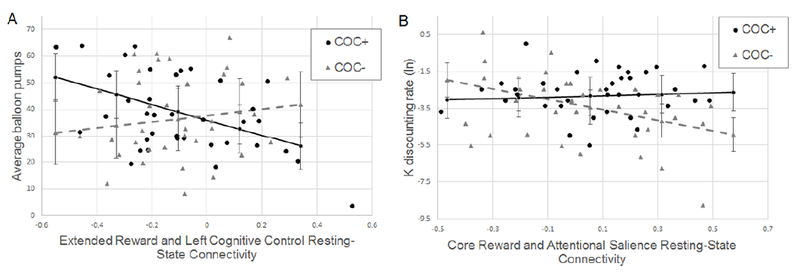
Significant group by between-network connectivity interaction effects on task impulsivity measures. Black circles represent data points from cocaine users (COC+) and gray triangles represent data points from controls (COC−). Lines represent the conditional effects of between-network resting-state connectivity on task impulsivity for cocaine users (black solid line) and controls (gray-dashed line). Error bars display the standard error of the conditional effects. A. Higher average balloon pumps on the Balloon Analogue Risk Task (BART) indicates higher impulsivity. B. Larger, positive natural log (ln) K values on the Monetary Choice Questionnaire (MCQ) indicate higher discounting rates (i.e., impulsivity).
4. Discussion
The current study used a large-scale, functional network approach to examine cocaine-related differences in the association between network rsFC and impulsivity on reward-based decision-making tasks. We examined rsFC between two networks involved in reward processing and expectancies and two cortical executive control networks involved in cognitive control and attentional salience. We found that cocaine users had aberrant associations between network connectivity and impulsivity compared to controls.
For cocaine users in the current study, stronger inverse coupling between the extended reward network and the left cognitive control network was associated with more risk-taking propensity on the BART task compared to controls. This finding supported our hypothesis that stronger reward and cognitive control connectivity would be associated with more balloon pumps (i.e., risk) on the task for cocaine users. This finding is consistent with prior research (Kohno et al., 2014) and models that conceptualize impulsive decision making among drug users as over-active reward function that diminishes or redirects executive control functions toward compulsive behavior (Goldstein and Volkow, 2011; Volkow et al., 2010). Our findings suggest that this imbalance in reward and executive control functions may be measurable using rsFC, not just during task engagement. Risky behavior on the BART has been linked to inverse functional activation of the extended reward and cognitive control networks (Schonberg et al., 2012) and negative connectivity between these networks at rest may predispose cocaine users to make impulsive choices when presented with a risky decision.
This finding was not consistent with our second hypothesis or with prior seed-based connectivity studies that found worse decision making and learning performance with increased positive rsFC between core reward and cognitive control brain regions (Camchong et al., 2011; Hu et al., 2015; Ray et al., 2015; Wilcox et al., 2011). Prior fMRI research has found that “cashing out” (i.e., safe choices), but not pumps (i.e., risky choices), on the BART are linked with activation of the caudate nucleus and hippocampus of the core reward network (Schonberg et al., 2012). The core reward network is more closely linked with reward processing and learning after receipt of the reward, and may be less involved in the pre-decision reward anticipation on tasks like the BART as the extended reward and cognitive control networks. Our divergent results may also be due to our use of data-driven intrinsic networks, rather than a priori seeds; this allowed us to examine the interactions among brain regions within separate functional networks. One of the strengths of ICA is the ability to identify connectivity strength within overlapping networks and to detect and remove signal noise that confounds seed-based connectivity analyses (Beckmann et al., 2005). For these reasons, connectivity within large-scale functional networks may be more predictive of behavior than seed-regions (Menon, 2011) or even task-related activity in affected brain regions (Rowe, 2010).
Our results did not support our hypothesis that inverse connectivity between the extended reward and cognitive control network would be linked with worse learning on the IGT. This was surprising, considering that extended reward and cognitive control inverse connectivity was associated with BART performance for cocaine users in the study. While we did not specify right or left cognitive control networks in our hypotheses since both are linked with decision-making and inhibitory control (Laird et al., 2011), prior fMRI and PET research on IGT and BART performance with stimulant users has implicated right-sided cognitive control network activity involved in reasoning, inhibition, and memory (Bolla et al., 2003; Kohno et al., 2014). Alternatively, we found BART performance to be associated with extended reward and left-sided cognitive control network connectivity, primarily involved in language processing and recall of associative cues (Laird et al., 2011). Compulsive behavior in addiction is, in part, facilitated by associative learning between drug cues and reward expectancies (Di Chiara, 1999) and chronic cocaine users show reduced interhemispheric connectivity of the cognitive control networks (Kelly et al., 2011). Our findings may point to an increasing role of associative recall facilitated by the left cognitive control network during decision making for cocaine users. This is supported by prior research showing cocaine-related increases in left cognitive control network rsFC (Ray et al., 2015). Future research using rsFC and task-based activity may be able to parse out the distinct roles of each hemisphere in impulsivity and addictive behavior.
Although cocaine users demonstrated strong links between rsFC and BART performance, they had an insignificant association between rsFC and intertemporal discounting on the MCT. We hypothesized that inverse connectivity of reward and attentional salience networks would be indicative of network imbalance and related to higher discount rates for cocaine users. Instead, this was true for the control participants. The core reward network is linked with immediate reward valuation while the attentional salience network is linked with delayed reward valuation (McClure et al., 2004; Wittmann et al., 2007). The significant association between resting-state connectivity and intertemporal discounting may be predictive of healthy and expected functional network control over the valuation of delayed over immediate rewards for our control participants who had a wide range of discounting rates. While prior research found that an uncoupling of salience and reward networks was related to higher impulsivity for cocaine users (McHugh et al., 2013; Wisner et al., 2013), our results suggest that imbalance may instead be observed as a dissociation between rsFC and impulsive behavior (Goldstein and Volkow, 2011). Prior studies have found a disconnection between cocaine users’ self-perception and actual task performance (Goldstein et al., 2007; Moeller et al., 2010), which could be described as neural-behavioral dissociation (Goldstein and Volkow, 2011). Neural-behavioral dissociation occurs when autonomic responses fail to predict behavioral outcomes, which is believed to be caused by reduced input from cortical executive control over subcortical regions involved in emotion and conditioned responding (Goldstein and Volkow, 2011). Our results suggest a dissociation between the behavioral responses driven by the core reward network involved in autonomic responses to drug rewards, and the attentional salience network involved in informing behavioral decision making.
The current study adds to the literature by demonstrating how functional connectivity between large-scale networks is associated with impulsive decision making for cocaine users. The strengths of the study include its use of data-driven networks, a relatively large sample size compared to prior research in this area, generalizability to real-world cocaine users, and a focus on reward and executive control networks implicated in impulsive decision making in the context of addiction. The effects of cocaine use in these results are more accurately described as the effects of a cocaine use disorder, in which cocaine use is the primary drug of choice and co-occurs with polysubstance use (i.e., smoking, alcohol, and marijuana) (Wagner and Anthony, 2002). While this may introduce confounding effects from multiple substances, recruiting participants who only use cocaine would have limited our generalizability to the poly-substance cocaine users typically seen in the United States (Wagner and Anthony, 2002).
The study also had several limitations. Age, education, and motion differed across groups and were controlled for in the group analyses. Although results were unchanged with these variables removed from the analysis, differences in demographic characteristics could have contributed to the group effects detected considering their known influence on rsFC (Damoiseaux et al., 2008; Power et al., 2014; Tian et al., 2011). Future research with samples that include more variability on these factors would provide an opportunity to examine their influence on functional connectivity and cognitive outcomes. Given the cross-sectional nature of the current research in this area, it is unclear if changes in rsFC and impulsivity are due to chronic substance use, or predisposing genetic, individual, or environmental factors. In addition, the moderation analyses may have been underpowered to detect small interaction effects (McClelland and Judd, 1993) and we did not correct for multiple comparisons, given that the hypotheses were derived independently and a priori. Finally, the findings should be interpreted with caution in light of recent research showing that the traditional five-minute resting-state fMRI scan may not be as reliable as longer scans (Noble et al., 2017). A priori defined networks, such as the fronto-parietal network, tend to be the most reliable, but test-retest reliability is diminished in subcortical networks due to smaller anatomical regions and potentially unique activity patterns (Noble et al., 2017).
In conclusion, the results of the current study suggest cocaine use disorder is related to altered associations between impulsivity and reward and executive control network rsFC coupling. Given prior links between functional connectivity with drug treatment outcomes and the known plasticity of these connections, developing interventions to manipulate connectivity could potentially improve clinical and behavioral outcomes for cocaine users (Fox et al., 2012; Pierce and Vassoler, 2013). Future research using longitudinal designs and multimodal imaging that incorporates structural and task-based functional data may add to our understanding of the temporal course, directionality, and specificity of these associations to identify intervention targets.
Highlights.
Functional changes in reward and executive brain circuitry occur in addiction
Resting-state connectivity was related to risk-taking propensity for cocaine users
Resting-state connectivity was related to delay discounting for controls
Acknowledgements
We would like to thank David Smith for his suggestion to apply ICA AROMA and his assistance with the process; Syam Gadde for his technical support throughout the analyses; Peter Howells for contributing python scripts; and Sheri L. Towe for data collection and management.
Role of the Funding Source
This project was supported by grants from the National Institute on Drug Abuse: K23-DA028660, R21-DA036450, F32-DA038519.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Bechara A, Damasio AR, Damasio H, Anderson SW, 1994. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition 50, 7–15. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, DeLuca M, Devlin JT, Smith SM, 2005. Investigations into resting-state connectivity using independent component analysis. Philos. Trans. R. Soc. Lond. B Biol. Sci 360, 1001–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann CF, Mackay CE, Filippini N, Smith SM, 2009. Group comparison of resting-state fMRI data using multi-subject ICA and dual regression. Neuroimage 47 (Suppl. 1), 71511–75113. [Google Scholar]
- Beckmann CF, Smith SM, 2004. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans. Med. Imaging 23, 137–152. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Jarmolowicz DP, Mueller ET, Koffarnus MN, Gatchalian KM, 2012. Excessive discounting of delayed reinforcers as a trans-disease process contributing to addiction and other disease-related vulnerabilities: emerging evidence. Pharmacol. Ther 134, 287–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla KI, Eldreth DA, London ED, Kiehl KA, Mouratidis M, Contoreggi C, Matochik JA, Kurian V, Cadet JL, Kimes AS, Funderburk F, Ernst M, 2003. Orbitofrontal cortex dysfunction in abstinent cocaine abusers performing a decision-making task. Neuroimage 19, 1085–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camchong J, MacDonald AW 3rd, Nelson B, Bell C, Mueller BA, Specker S, Lim KO, 2011. Frontal hyperconnectivity related to discounting and reversal learning in cocaine subjects. Biol. Psychiatry 69, 1117–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler JM, Elton A, Kennedy AP, Young J, Smitherman S, Andrew James G, Kilts CD, 2013. Altered functional connectivity of the insular cortex across prefrontal networks in cocaine addiction. Psychiatry Res 213, 39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey SF, Gudleski GD, Saladin ME, Brady KT, 2003. Impulsivity and rapid discounting of delayed hypothetical rewards in cocaine-dependent individuals. Exp. Clin. Psychopharmacol 11, 18–25. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Beckmann CF, Arigita EJ, Barkhof F, Scheltens P, Stam CJ, Smith SM, Rombouts SA, 2008. Reduced resting-state brain activity in the “default network” in normal aging. Cereb. Cortex 18, 1856–1864. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, 1999. Drug addiction as dopamine-dependent associative learning disorder. Eur. J. Pharmacol 375, 13–30. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Bassareo V, 2007. Reward system and addiction: What dopamine does and doesn’t do. Curr. Opin. Pharmacol 7, 69–76. [DOI] [PubMed] [Google Scholar]
- Ernst M, Bolla KI, Mouratidis M, Contoreggi C, Matochik JA, Kurian V, Cadet JL, Kimes AS, London ED, 2002. Decision-making in a risk-taking task: A PET study. Neuropsychopharmacology 26, 682–691. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW, 2008. Review: Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos. Trans. R. Soc. Lond. B. Biol. Sci 363, 3125–3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Halko MA, Eldaief MC, Pascual-Leone A, 2012. Measuring and manipulating brain connectivity with resting state functional connectivity magnetic resonance imaging (fcMRI) and transcranial magnetic stimulation (TMS). Neuroimage 62, 2232–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME, 2007. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci 8, 700–711. [DOI] [PubMed] [Google Scholar]
- Fukunaga R, Brown JW, Bogg T, 2012. Decision making in the Balloon Analogue Risk Task (BART): Anterior cingulate cortex signals loss aversion but not the infrequency of risky choices. Cogn. Affect. Behav. Neurosci 12, 479–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, Schonberg T, Mumford J, Kohno M, Poldrack RA, London ED, 2013. Greater risk sensitivity of dorsolateral prefrontal cortex in young smokers than in nonsmokers. Psychopharmacology 229, 345–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Alia-Klein N, Tomasi D, Zhang L, Cottone LA, Maloney T, Telang F, Caparelli EC, Chang L, Ernst T, Samaras D, Squires NK, Volkow ND, 2007. Is decreased prefrontal cortical sensitivity to monetary reward associated with impaired motivation and self-control in cocaine addiction? Am. J. Psychiatry 164, 43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND, 2011. Dysfunction of the prefrontal cortex in addiction: Neuroimaging findings and clinical implications. Nat. Rev. Neurosci 12, 652–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant S, Contoreggi C, London ED, 2000. Drug abusers show impaired performance in a laboratory test of decision making. Neuropsychologia 38, 1180–1187. [DOI] [PubMed] [Google Scholar]
- Gu H, Salmeron BJ, Ross TJ, Geng X, Zhan W, Stein EA, Yang Y, 2010. Mesocorticolimbic circuits are impaired in chronic cocaine users as demonstrated by resting-state functional connectivity. Neuroimage 53, 593–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF, 2013. Introduction to Mediation, Moderation, and Conditional Process Analysis The Guildford Press, New York, New York. [Google Scholar]
- Heil SH, Johnson MW, Higgins ST, Bickel WK, 2006. Delay discounting in currently using and currently abstinent cocaine-dependent outpatients and non-drug-using matched controls. Addict. Behav 31, 1290–1294. [DOI] [PubMed] [Google Scholar]
- Hester R, Garavan H, 2004. Executive dysfunction in cocaine addiction: Evidence for discordant frontal, cingulate, and cerebellar activity. J. Neurosci 24, 11017–11022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Salmeron BJ, Gu H, Stein EA, Yang Y, 2015. Impaired functional connectivity within and between frontostriatal circuits and its association with compulsive drug use and trait impulsivity in cocaine addiction. JAMA Psychiatry 72, 584–592. [DOI] [PubMed] [Google Scholar]
- IBM Corp, Released 2013. IBM Statistics for Windows, Version 22.0. IBM Corp, Armonk, NY. [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S, 2002. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17, 825–841. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM, 2012. FSL. Neuroimage 62, 782–790. [DOI] [PubMed] [Google Scholar]
- Kelly C, Zuo XN, Gotimer K, Cox CL, Lynch L, Brock D, Imperati D, Garavan H, Rotrosen J, Castellanos FX, Milham MP, 2011. Reduced interhemispheric resting state functional connectivity in cocaine addiction. Biol. Psychiatry 69, 684–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby KN, Petry NM, 2004. Heroin and cocaine abusers have higher discount rates for delayed rewards than alcoholics or non-drug-using controls. Addiction 99, 461–471. [DOI] [PubMed] [Google Scholar]
- Kirby KN, Petry NM, Bickel WK, 1999. Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. J. Exp. Psychol. Gen 128, 78–87. [DOI] [PubMed] [Google Scholar]
- Kohno M, Morales AM, Ghahremani DG, Hellemann G, London ED, 2014. Risky decision making, prefrontal cortex, and mesocorticolimbic functional connectivity in methamphetamine dependence. JAMA Psychiatry 71, 812–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND, 2016. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry 3, 760–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn S, Gallinat J, 2011. Common biology of craving across legal and illegal drugs - A quantitative meta-analysis of cue-reactivity brain response. Eur. J. Neurosci 33, 1318–1326. [DOI] [PubMed] [Google Scholar]
- Laird AR, Fox PM, Eickhoff SB, Turner JA, Ray KL, McKay DR, Glahn DC, Beckmann CF, Smith DV, Fox PM, 2011. Behavioral interpretations of intrinsic connectivity networks. J. Cogn. Neurosci 23, 4022–4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejuez CW, Read JP, Kahler CW, Richards JB, Ramsey SE, Stuart GL, Strong DR, Brown RA, 2002. Evaluation of a behavioral measure of risk taking: The Balloon Analogue Risk Task (BART). J. Exp. Psychol. Appl 8, 75–84. [DOI] [PubMed] [Google Scholar]
- Li X, Lu ZL, D’Argembeau A, Ng M, Bechara A, 2010. The Iowa Gambling Task in fMRI images. Hum. Brain Mapp 31, 410–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Steinberg JL, Moeller FG, Johns SE, Narayana PA, 2015. Effect of cocaine dependence on brain connections: clinical implications. Expert Rev. Neurother 15, 1307–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez D, Narendran R, Foltin RW, Slifstein M, Hwang D, Broft A, Huang Y, Cooper TB, Fischman MW, Kleber HD, Laruelle M, 2007. Amphetamine-induced dopamine release: Markedly blunded in cocaine dependence and predictive of choice to self-administer cocaine Am. J. Psychiatry 164, 622–629. [DOI] [PubMed] [Google Scholar]
- McClelland GH, Judd CM, 1993. Statistical difficulties of detecting interactions and moderator effects. Psychol. Bull 114, 376–390. [DOI] [PubMed] [Google Scholar]
- McClure SM, Laibson DI, Loewenstein G, Cohen JD, 2004. Separate neural systems value immediate and delayed monetary rewards. Science 306, 503–507. [DOI] [PubMed] [Google Scholar]
- McHugh MJ, Demers CH, Braud J, Briggs R, Adinoff B, Stein EA, 2013. Striatal-insula circuits in cocaine addiction: Implications for impulsivity and relapse risk. Am. J. Drug Alcohol Abuse 39, 424–432. [DOI] [PubMed] [Google Scholar]
- Meade CS, Towe SL, Skalski LM, Robertson KR, 2015. Independent effects of HIV infection and cocaine dependence on neurocognitive impairment in a community sample living in the southern United States. Drug Alcohol Depend 149, 128–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, 2011. Large-scale brain networks and psychopathology: A unifying triple network model. Trends Cogn. Sci 15, 483–506. [DOI] [PubMed] [Google Scholar]
- Moeller SJ, Maloney T, Parvaz MA, Alia-Klein N, Woicik PA, Telang F, Wang GJ, Volkow ND, Goldstein RZ, 2010. Impaired insight in cocaine addiction: laboratory evidence and effects on cocaine-seeking behaviour. Brain 133, 1484–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monterosso JR, Ainslie G, Xu J, Cordova X, Domier CP, London ED, 2007. Frontoparietal cortical activity of methamphetamine-dependent and comparison subjects performing a delay discounting task. Hum. Brain Mapp 28, 383–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble S, Spann MN, Tokoglu F, Shen X, Constable RT, Scheinost D, 2017. Influences on the test-retest reliability of functional connectivity MRI and its relationship with behavioral utility. Cereb. Cortex 27, 5415–5429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, Vassoler FM, 2013. Deep brain stimulation for the treatment of addiction: Basic and clinical studies and potential mechanisms of action. Psychopharmacology 229, 487–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL, Petersen SE, 2014. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage 84, 320–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruim RH, Mennes M, van Rooij D, Llera A, Buitelaar JK, Beckmann CF, 2015. ICA-AROMA: A robust ICA-based strategy for removing motion artifacts from fMRI data. Neuroimage 112, 267–277. [DOI] [PubMed] [Google Scholar]
- Rao H, Korczykowski M, Pluta J, Hoang A, Detre JA, 2008. Neural correlates of voluntary and involuntary risk taking in the human brain: An fMRI Study of the Balloon Analog Risk Task (BART). Neuroimage 42, 902–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray S, Gohel S, Biswal BB, 2015. Altered functional connectivity strength in abstinent chronic cocaine smokers compared to healthy controls. Brain Connect 5, 476–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe JB, 2010. Connectivity analysis is essential to understand neurological disorders. Front. Syst. Neurosci 4, pii 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonberg T, Fox CR, Mumford JA, Congdon E, Trepel C, Poldrack RA, 2012. Decreasing ventromedial prefrontal cortex activity during sequential risk-taking: An FMRI investigation of the balloon analog risk task. Front. Neurosci 6, 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD, 2007. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci 27, 2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM, 2004. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23, S208–S219. [DOI] [PubMed] [Google Scholar]
- Sutherland MT, McHugh MJ, Pariyadath V, Stein EA, 2012. Resting state functional connectivity in addiction: Lessons learned and a road ahead. Neuroimage 62, 2281–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L, Wang J, Yan C, He Y, 2011. Hemisphere- and gender-related differences in small-world brain networks: a resting-state functional MRI study. Neuroimage 54, 191–202. [DOI] [PubMed] [Google Scholar]
- Towe SL, Hobkirk AL, Ye DG, Meade CS, 2015. Adaptation of the Monetary Choice Questionnaire to accommodate extreme monetary discounting in cocaine users. Psychol. Addict. Behav 29, 1048–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker KA, Potenza MN, Beauvais JE, Browndyke JN, Gottschalk PC, Kosten TR, 2004. Perfusion abnormalities and decision making in cocaine dependence. Biol. Psychiatry 56, 527–530. [DOI] [PubMed] [Google Scholar]
- Utevsky AV, Smith DV, Huettel SA, 2014. Precuneus is a functional core of the default-mode network. J. Neurosci 34, 932–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Benbrook A, Funderburk F, David P, Jean-Lud C, Bolla KI, 2007a. The differential relationship between cocaine use and marijuana use on decision-making performance over repeat testing with the Iowa Gambling Task. Drug Alcohol Depend 90, 2–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Chong TT, Stout JC, Yucel M, London ED, 2018. Stages of dysfunctional decision-making in addiction. Pharmacol. Biochem. Behav 164, 99–105. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Perales JC, Perez-Garcia M, 2007b. Cognitive impulsivity in cocaine and heroin polysubstance abusers. Addict. Behav 32, 950–966. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL, 2008. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J. Neurophysiol 100, 3328–3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F, Baler R, 2010. Addiction: Decreased reward sensitivity and increased expectation sensitivity conspire to overwhelm the brain’s control circuit. Bioessays 32, 748–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner FA, Anthony JC, 2002. Into the world of illegal drug use: Exposure opportunity and other mechanisms linking the use of alcohol, tobacco, marijuana, and cocaine Am. J. Epidemiol 155, 918–925. [DOI] [PubMed] [Google Scholar]
- Weber MJ, Messing SB, Rao H, Detre JA, Thompson-Schill SL, 2014. Prefrontal transcranial direct current stimulation alters activation and connectivity in cortical and subcortical reward systems: A tDCS-fMRI study. Hum. Brain Mapp 35, 3673–3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox CE, Teshiba TM, Merideth F, Ling J, Mayer AR, 2011. Enhanced cue reactivity and fronto-striatal functional connectivity in cocaine use disorders. Drug Alcohol Depend 115, 137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisner KM, Patzelt EH, Lim KO, MacDonald AW 3rd, 2013. An intrinsic connectivity network approach to insula-derived dysfunctions among cocaine users. Am. J. Drug Alcohol Abuse 39, 403–413. [DOI] [PubMed] [Google Scholar]
- Wittmann M, Leland DS, Paulus MP, 2007. Time and decision making: Differential contribution of the posterior insular cortex and the striatum during a delay discounting task. Exp. Brain Res 179, 643–653. [DOI] [PubMed] [Google Scholar]
- Worhunsky PD, Stevens MC, Carroll KM, Rounsaville BJ, Calhoun VD, Pearlson GD, Potenza MN, 2013. Functional brain networks associated with cognitive control, cocaine dependence, and treatment outcome. Psychol. Addict. Behav 27, 477–488. [DOI] [PMC free article] [PubMed] [Google Scholar]


